A commentary on
Expression of the Wnt signaling system in central nervous system axon guidance and regeneration
by Hollis, E. R. II, and Zou, Y. (2012). Front. Mol. Neurosci. 5:5. doi: 10.3389/fnmol.2012.00005
Research in recent years challenged what we once thought of as the canonical function of signaling proteins to reveal new and surprising mechanisms of action. Morphogens, like Wnts, BMPs, or SHHs, long thought to be the factors that provide positional information to specify cell fate and proliferation have turned out to be as important for synaptogenesis, axonal growth, and guidance during both development of the nervous system as well as during post-injury axonal regeneration in the adult. Establishment of axonal connections can be subdivided into outgrowth, pathfinding – often over long distances – and target recognition. Wnt and Shh receptor/ligand systems form longitudinal gradients along the entire developing spinal cord, thus making them ideal candidates to push (in the case of Wnt) or pull (Shh) axons along the anterior–posterior axis of the spinal cord. They are potentially perfectly situated players for the organization and specification of corticospinal (CST) and sensory axons (Lyuksyutova et al., 2003; Bourikas et al., 2005; Liu et al., 2005; Keeble et al., 2006; Wolf et al., 2008; Li et al., 2009) as nicely summarized in this Frontiers in Molecular Neuroscience Special Topic article by Hollis and Zou (2012). In particular, the Wnt–Ryk signaling axis first promotes the outgrowth of CST axons then pushes them posteriorly along a decreasing Wnt gradient by repulsion during development. The different effects are mediated by specific Wnt receptor and ligand combinations (Li et al., 2009). This push effect may be of relevance for spinal cord injuries in adults, where axonal injury can sever the trajectories of corticospinal tract and/or the sensory input to the brain causing long-term disability. Although improvement in regeneration of ascending and descending pathways occurs when myelin and other inhibitors (including re-expressed axonal growth repellents) are blocked (for review, e.g.: Blesch and Tuszynski, 2002; Buchli and Schwab, 2005; Filbin, 2006; Harel and Strittmatter, 2006) the results are not completely satisfactory. Could the developmental functions of Wnt be reconstituted in the adult to promote corticospinal axon regeneration? Wnt receptor/ligand systems including the Wnt–Ryk “pushing” pathway are actually recapitulated after spinal cord injury (Liu et al., 2008; Miyashita et al., 2009). However, their upregulation does not promote effective axonal regeneration and the problem appears to be the scar tissue.
One discrepancy between successful axonal outgrowth and guidance during development and failed axonal regeneration in the adult seems to be a consequence of the inability of the re-expressed Wnt ligands and receptors to “push” the axons over or around the scar tissue. In fact, ligand concentration is highest around the injury, which is in front of the regenerating, descending axons, and not behind them as it is in development (Liu et al., 2008). It fails to create the potentially pro-regenerative wave pushing axon growth from behind. Thus, the big problem is still for axons to grow over or through, or even circumnavigate the lesion site. If they could manage it they would have a nice high Wnt signal at their back “again” and maybe propelled forward without much further ado? In fact, blocking Ryk receptors after spinal cord injury stopped degeneration and promoted the sprouting of corticospinal axon collaterals within the injured spinal cord tissue but only up to the scar tissue and not further (Liu et al., 2008). External supply of Wnt3a, which attracts CST axons promoted functional recovery of the injured animals (Yin et al., 2008; Suh et al., 2011). This might suggest that applying a block for Ryk or applying Wnt3a to overcome repulsion with attraction would be only needed for a very limited time at the beginning of recovery phase but could result in a more long-lasting benefit for axonal regeneration (Figure 1).
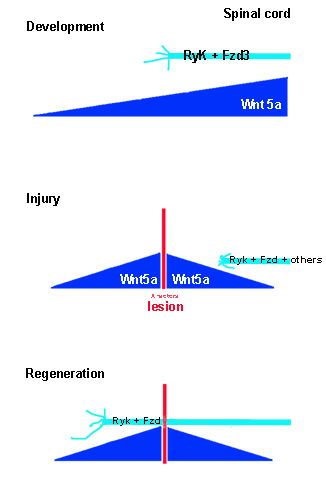
Figure 1. A simple model of how regenerating CST axon could be propelled down the posterior spinal cord when sprouting axons would managed to cross the lesion.
However, the influence of Wnt3a on axon regrowth might be secondary. Yin et al. (2008) showed that even a single injection of Wnt3 into the lesioned spinal cord also directs spinal cord precursor cells into neuronal rather than astrocytic fate. Similarly, transplanted fibroblasts that continuously secreted Wnt3a enhanced not only axonal regeneration and functional improvement after a spinal cord injury, but also limited the inhibitory glial environment (Suh et al., 2011). All together, more neurons than astrocytes (in short-term experiments; Yin et al., 2008) as well as new oligodendrocytes were generated (in long-term experiments; Suh et al., 2011). Thus, the re-shaping of the inhibitory environment in both short and long-term applications of Wnt3a could be the primary reason for axonal regeneration. More experiments are needed to decide whether short or long-term applications of Wnt3a may be more beneficial for functional recovery also considering that transplanting cells might cause very different problems after regeneration.
Since many factors, including Wnts and other ligands as well as other axon guidance proteins and growth inhibitory factors are upregulated in injured adult nervous tissue and many inhibit axon regrowth over the lesion site (Harel and Strittmatter, 2006; Fouad and Tse, 2008), the obstacle to axonal regeneration is not completely solved by adding growth promoting Wnts or Ryk inhibitors. However, short-term or long-term application of growth promoting Wnts (e.g., Wnt3a) together with the re-expression of Wnt repellents (e.g., Wnt5a) may mimic development (Liu et al., 2008; Yin et al., 2008; Miyashita et al., 2009; Suh et al., 2011) and thus could potentially be exploited to improve recovery.
Acknowledgments
I want to thank J. Clarke and B. Knöll for helpful comments and very enjoyable discussions.
References
Blesch, A., and Tuszynski, M. H. (2002). Spontaneous and neurotrophin-induced axonal plasticity after spinal cord injury. Prog. Brain Res. 137, 415–423.
Bourikas, D., Pekarik, V., Baeriswyl, T., Grunditz, A., Sadhu, R., Nardo, M., and Stoeckli, E. T. (2005). Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat. Neurosci. 8, 297–304.
Buchli, A. D., and Schwab, M. E. (2005). Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann. Med. 37, 556–567.
Filbin, M. T. (2006). Recapitulate development to promote axonal regeneration: good or bad approach? Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1565–1574.
Fouad, K., and Tse, A. (2008). Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol. Res. 30, 17–27.
Harel, N. Y., and Strittmatter, S. M. (2006). Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 7, 603–616.
Hollis, E. R. II, and Zou, Y. (2012). Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Front. Mol. Neurosci. 5, 5. doi: 10.3389/fnmol.2012.00005
Keeble, T. R., Halford, M. M., Seaman, C., Kee, N., Macheda, M., Anderson, R. B., Stacker, S. A., and Cooper, H. M. (2006). The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 26, 5840–5848.
Li, L., Hutchins, B. I., and Kalil, K. (2009). Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 29, 5873–5883.
Liu, Y., Shi, J., Lu, C. C., Wang, Z. B., Lyuksyutova, A. I., Song, X. J., and Zou, Y. (2005). Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8, 1151–1159.
Liu, Y., Wang, X., Lu, C. C., Kerman, R., Steward, O., Xu, X. M., and Zou, Y. (2008). Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 28, 8376–8382.
Lyuksyutova, A. I., Lu, C. C., Milanesio, N., King, L. A., Guo, N., Wang, Y., Nathans, J., Tessier-Lavigne, M., and Zou, Y. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988.
Miyashita, T., Koda, M., Kitajo, K., Yamazaki, M., Takahashi, K., Kikuchi, A., and Yamashita, T. (2009). Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J. Neurotrauma 26, 955–964.
Suh, H. I., Min, J., Choi, K. H., Kim, S. W., Kim, K. S., and Jeon, S. R. (2011). Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir. (Wien) 153, 1003–1010.
Wolf, A. M., Lyuksyutova, A. I., Fenstermaker, A. G., Shafer, B., Lo, C. G., and Zou, Y. (2008). Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J. Neurosci. 28, 3456–3467.
Citation: Wizenmann A (2012) Regenerating Wnts. Front. Mol. Neurosci. 5:43. doi: 10.3389/fnmol.2012.00043
Received: 21 February 2012; Accepted: 20 March 2012;
Published online: 09 April 2012.
Copyright: © 2012 Wizenmann. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence:YXdpemVubWFubkBhbmF0b20udW5pLXR1ZWJpbmdlbi5kZQ==
