- 1CNRS UPR 3212, Institut des Neurosciences Cellulaires et Intégratives, Centre National de la Recherche Scientifique and University of Strasbourg, Strasbourg, France
- 2SMPMS, Mass Spectrometry Facilities of the CNRS UPR 3212, Institut des Neurosciences Cellulaires et Intégratives, Centre National de la Recherche Scientifique, Strasbourg, France
Morphine remains the gold standard painkiller available to date to relieve severe pain. Morphine metabolism leads to the production of two predominant metabolites, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). This metabolism involves uridine 5′-diphospho-glucuronosyltransferases (UGTs), which catalyze the addition of a glucuronide moiety onto the C3 or C6 position of morphine. Interestingly, M3G and M6G have been shown to be biologically active. On the one hand, M6G produces potent analgesia in rodents and humans. On the other hand, M3G provokes a state of strong excitation in rodents, characterized by thermal hyperalgesia and tactile allodynia. Its coadministration with morphine or M6G also reduces the resulting analgesia. Although these behavioral effects show quite consistency in rodents, M3G effects are much more debated in humans and the identity of the receptor(s) on which M3G acts remains unclear. Indeed, M3G has little affinity for mu opioid receptor (MOR) (on which morphine binds) and its effects are retained in the presence of naloxone or naltrexone, two non-selective MOR antagonists. Paradoxically, MOR seems to be essential to M3G effects. In contrast, several studies proposed that TLR4 could mediate M3G effects since this receptor also appears to be essential to M3G-induced hyperalgesia. This review summarizes M3G’s behavioral effects and potential targets in the central nervous system, as well as the mechanisms by which it might oppose analgesia.
Introduction
Over the last decade, chronic pain has become one of the top health burdens threatening economic and healthcare systems (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017). Opiates, such as morphine and its derivatives, remain the most potent painkillers available at the hospital. However, their use and efficiency are limited by mild (i.e., nausea, constipation) to severe side effects, including analgesic tolerance, opioid use disorders and ultimately respiratory depression, which can lead to death (Trescot et al., 2008; Koller et al., 2019; Bachmutsky et al., 2020). Among side effects, analgesic tolerance corresponds to the decreased effect of opioid-induced analgesia following repeated administrations (Trescot et al., 2008; Weinsanto et al., 2018; Gabel et al., 2022). Consequently, dose escalation is required to relieve pain, although it might result in greater risks of severe side effects. In addition, opiate efficiency and side effects are influenced by numerous factors, including sex, age (van Crugten et al., 1997; Fullerton et al., 2021; Gabel et al., 2022), comorbidities (Gupta et al., 2018), additional drug treatments and pain types (Vellucci, 2012; Hopkins et al., 2019), resulting in complex patient care (Turk, 1996; Vellucci, 2012). In particular, morphine has been extensively used to decipher the mechanisms involved in opiate-induced analgesia, tolerance and opioid use disorders.
Morphine’s effects are mediated mainly through the activation of mu opioid receptors (MORs) located in cerebral structures involved in the descending controls of pain, including the periaqueductal gray matter (PAG), the rostral ventromedial medulla (RVM) and the spinal cord. Upon activation, these receptors induce the hyperpolarization of MOR-expressing neurons, resulting in the inhibition of nociceptive signal transmission (for review, see Lau and Vaughan, 2014). From a pharmacokinetic point of view, after administration, morphine undergoes sequential pharmacological processes, consisting of absorption, distribution, metabolism, and excretion (ADME). Following intestinal absorption, morphine reaches the liver and enters the hepatocytes, wherein a major part of its metabolism occurs. Hence, morphine bioavailability is relatively low in humans (Hasselstrom and Sawe, 1993; Lotsch et al., 1999; Lloret-Linares et al., 2016), with only 25–35% of morphine reaching the circulation and even less being distributed within the central nervous system (CNS). Indeed, the blood-brain barrier (BBB) restrains CNS access to xenobiotics and, to a more general extent, hydrophilic compounds. The BBB is a selectively semipermeable barrier composed of adjacent endothelial cells, astrocyte end feet, and pericytes (Ballabh et al., 2004). Although morphine crosses endothelial cell membranes due to a certain degree of lipophilicity, its BBB permeability relies on the P-glycoprotein (P-gp) drug transporter, which drives morphine from endothelial cells back into the blood (Schaefer et al., 2017). Therefore, the effectiveness and duration of the analgesic effect of morphine are partially modulated by both morphine metabolism and the permeability of the BBB. Pharmacodynamic processes are also key elements affecting morphine’s effects. However, they are beyond the scope of the present review and have already been discussed elsewhere (for review, see Al-Hasani and Bruchas, 2011; Williams et al., 2013).
Morphine Metabolism
Glucuronidation of Morphine
Morphine metabolism involves mainly hepatic glucuronidation by uridine 5′-diphospho-glucuronosyltransferase (UGT) phase II enzymes. Glucuronidation occurs at the C3-OH and C6-OH positions, leading to two active metabolites: morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) (Lotsch, 2005). However, to a much lesser extent, other morphine metabolites (5%) can be found in the blood and urine and include normorphine or morphine sulfates (Yeh et al., 1977; Hand et al., 1987a; Cone et al., 2008; Laux-Biehlmann et al., 2013). In addition, 10% of morphine is excreted in its intact form due to its intrinsic hydrophilicity (Yeh, 1975). Pharmacokinetic studies of morphine in humans have shown blood terminal half-life average values of 2–3 h in healthy patients (Hasselstrom and Sawe, 1993). However, significant variations, ranging from less than 1 h up to 7 h (Webster et al., 1976; Sawe, 1986), have been reported based on the route of administration (e.g., more prolonged for oral vs. intravenous) and individual physiology (e.g., age, gender, comorbidities, cotreatments).
UDP-Glucuronosyltransferases
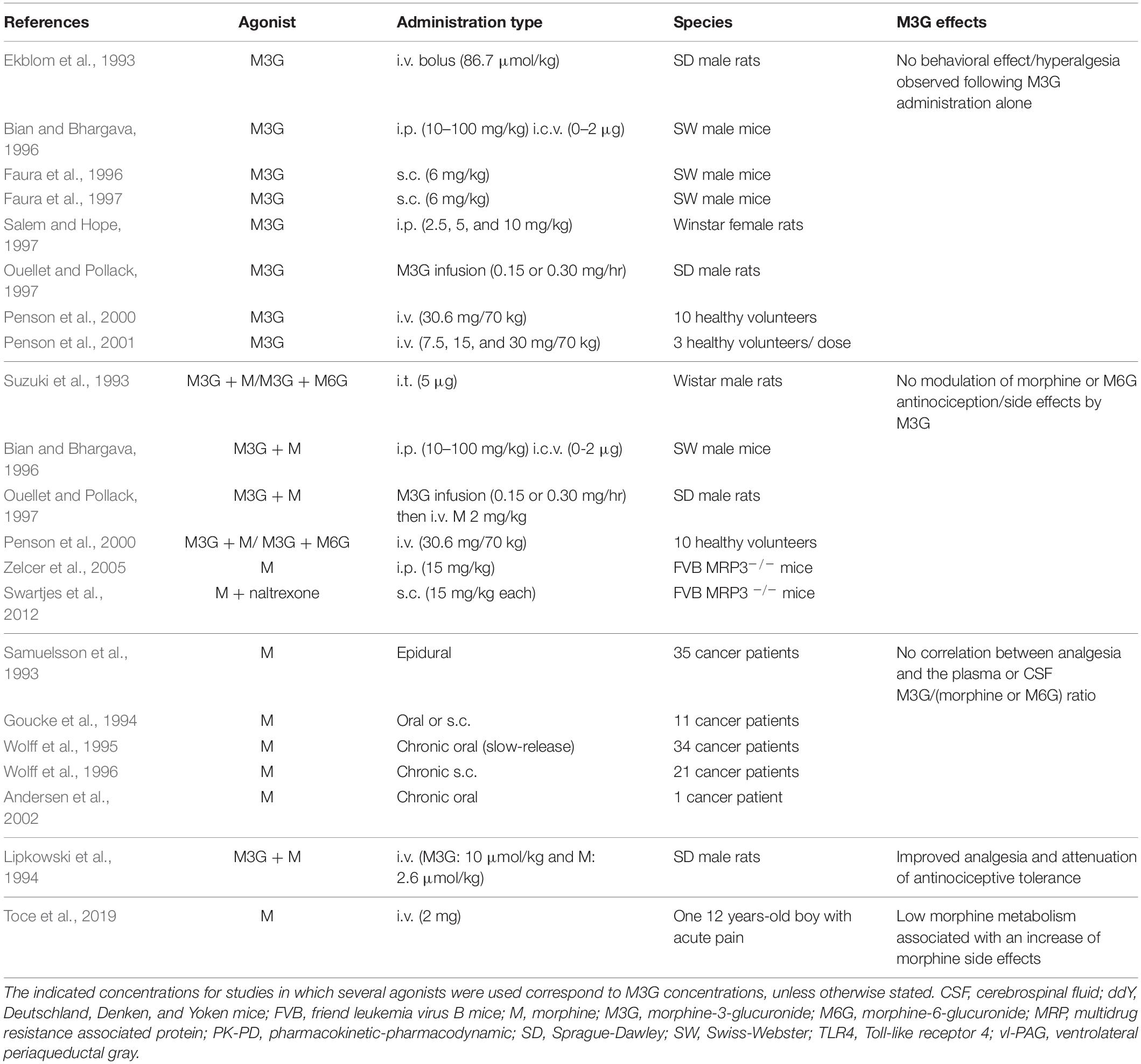
UGTs are transmembrane glycoproteins located in the smooth endoplasmic reticulum (ER; Figure 1). These proteins are composed of approximately 550 amino acids (around 49 kDa) and represent a superfamily of enzymes divided into two groups: UGT1A and UGT2B (for review, see Meech et al., 2019). Studies using human liver microsomes have established that several UGTs are involved in morphine glucuronidation, including UGT1A1, UGT1A3, UGT1A6, UGT1A8, UGT2B1, and UGT2B7 (Stone et al., 2003; Nair et al., 2015). Among them, the UGT2B7 is considered as the main enzyme involved in morphine metabolism. These enzymes catalyze the conjugation of a nucleophilic aglycone moiety (acceptor substrate; i.e., morphine) to the glycosyl group of a nucleotide sugar (donor; i.e., uridine diphosphate glucuronic acid, UDPGA). The main transporters involved in morphine transport across cell membranes are organic cation transporter member 1 (OCT1; Figure 1; Tzvetkov et al., 2013; Meyer et al., 2019), OCT2 (Imaoka et al., 2021) and the organic anion transporter polypeptide 2B1 (OATP2B1) (Yang Z. Z. et al., 2016). UGTs are found in the ER lumen, and only 20 amino acids remain in the cytosolic side, with a di-lysine (KK) motif being responsible for their membrane retention (Jackson et al., 1990). The luminal amino-terminal part of the protein carries the substrate-binding domain, whereas the carboxy-terminal part binds the cosubstrate UDP-glucuronic acid (UDPGA). It requires that both morphine and UDPGA be transported inside the ER. While the transport of UDPGA relies on several ER transporters, such as UGTrel7 (UDP-galactose transporter-related protein 7) (Muraoka et al., 2001; Kobayashi et al., 2006; Rowland et al., 2015) or UGTrel1 (Ondo et al., 2020), there is currently no identified transporter for aglycones and conjugated compounds in general or for morphine in particular. Once morphine glucuronides are transported back into the cytosol, plasma membrane efflux multidrug resistance-associated protein 2 and 3 transporters (MRP2 and MRP3) (Zelcer et al., 2005; Lloret-Linares et al., 2016), located on the basolateral side of hepatocytes, allow for their release outside the cell. Then, M3G and M6G are likely to be taken up and released into the bloodstream by endothelial cells via a probenecid-sensitive transport system (Xie et al., 2000). From the bloodstream, they reach the kidneys to be excreted in urine.
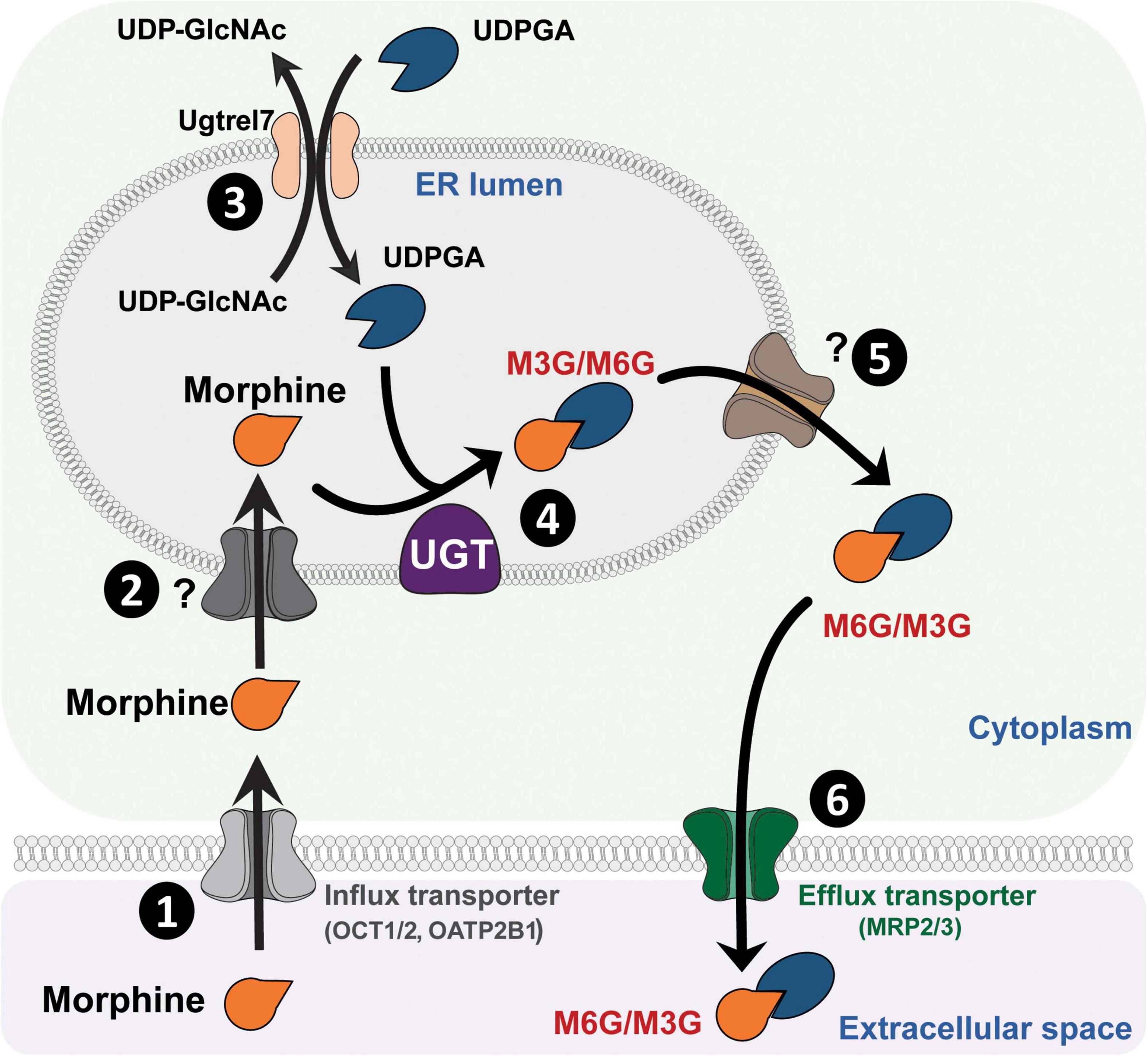
Figure 1. Glucuronidation process of morphine. (1) Morphine is first transferred from the extracellular space into the cytoplasm through active transporters such as OCT1 or OATP2B1. (2) Then, morphine enters the lumen of the ER by way of an unknown transporter. (3) Concomitantly, UDPGA is transported across the ER membrane in exchange for UDP-GlcNac, due to UGTrel transporters, such as UGTrel7. (4) Inside the lumen, UGT catalyzes the addition of a glucuronide moiety belonging to UDPGA onto morphine to form M3G and M6G. (5) Metabolites are then transferred into the cytoplasm by unknown transporters. (6) Finally, they are released into the extracellular space through active transporters such as MRP2 or MRP3. It is worth noting that a significant proportion of morphine that enters the cytoplasm can be directly released into the extracellular space via P-gp. M3G, morphine-3-glucuronide; M6G, morphine-6-glucuronide; MRP, multidrug resistance-associated protein; OATP2B1, organic anion transporter polypeptides 2B1; OCT1, organic cation transporter 1; P-gp, P-glycoprotein; ER, endoplasmic reticulum; UDPGA, uridine diphosphate glucuronic acid; UDP-GlcNac, UDP-N-acetylglucosamine; UGT, UDP-glucuronosyltransferase.
UGTs have been widely conserved across evolution from bacteria to plants and mammals (King et al., 2000; Bock, 2016). However, notable differences exist in enzyme expression and morphine metabolism between species (Oda et al., 2015). For instance, major disparities in morphine metabolism have been reported between humans and rodents. In particular, morphine has a shorter terminal half-life in C57BL/6 mice than in humans (i.e., 30 min vs. few hours in humans) (Handal et al., 2002). In humans, M3G and M6G represent 90 and 10% of all glucuronidated metabolites, respectively (Hasselstrom and Sawe, 1993). Alternatively, mice exclusively convert morphine into M3G due to the lack of UGT2B7 expression. Indeed, UGT2B7 seems to be required for M6G production, as witnessed in several in vitro studies using human and rodent microsomes (Lotsch and Geisslinger, 2001; Stone et al., 2003; for review, see Court, 2005). One hypothesis could be that the piperidine ring bearing the tertiary amine N17 disturbs the interaction between other UGTs and the C6-OH position of morphine. Thus, it might significantly decrease the glucuronidation probability at this position. Nevertheless, a baculovirus-Sf9 cell system for expressing UGTs, established by Kurita et al. (2017), demonstrated that UGT2B36 is the main M3G-forming enzyme in male FVB mice. In addition, although UGT1A1 and UGT2B1 individually did not form M3G in one particular study, heterodimers of these isoforms produced high levels of M3G (Miyauchi et al., 2020). In summary, although a few reports have suggested that rodents which lack UGT2B7 (Shelby et al., 2003; Buckley and Klaassen, 2007) might produce low levels of M6G (Nagano et al., 2000; Togna et al., 2013; Yang et al., 2016a,b; Yang Z. Z. et al., 2016), the general consensus is that they cannot synthesize such metabolites (Oguri et al., 1990; Kuo et al., 1991; Salem and Hope, 1997; Zelcer et al., 2005; Dahan and Lotsch, 2015; Allette et al., 2017; Gabel et al., 2022).
Since the beginning of the 1970s, the predominant metabolites of morphine, M3G and M6G, have been shown to be biologically active. On the one hand, M6G binds to MOR with high affinity and produces potent analgesia. On the other hand, M3G has been described as having pronociceptive properties that could counteract morphine and M6G analgesia. This review focuses on what is known about M3G behavioral effects, its potential targets in the CNS and the mechanisms underlying its properties. M6G effects are beyond the scope of this review and have already been discussed elsewhere (for review, see Lotsch and Geisslinger, 2001).
Morphine-3-Glucuronide
Behavioral Observations
Rodents
The predominant morphine metabolite, M3G, is devoid of analgesic effects whether it is injected through the s.c. or i.c.v. routes or directly into the PAG (Shimomura et al., 1971; Pasternak et al., 1987). However, the first M3G administrations in rats have elicited neuroexcitatory effects that could oppose morphine and M6G antinociception (Table 1). For instance, i.t. or i.c.v. administration of M3G induces robust behavioral excitation in rodents characterized by spontaneous agitation, hyperalgesia and allodynia, epileptic episodes and death following high doses of M3G (Labella et al., 1979; Woolf, 1981; Yaksh et al., 1986; Yaksh and Harty, 1988; Bartlett et al., 1994a; Bian and Bhargava, 1996). Following these observations, M3G’s pronociceptive effects were evaluated after direct injection or when coadministered with morphine or M6G. In rodents, i.p., s.c. and i.t. injections of M3G alone clearly induce thermal hyperalgesia and mechanical allodynia (Juni et al., 2006; Komatsu et al., 2009, 2016; Lewis et al., 2010; Due et al., 2012; Arout et al., 2014; Allette et al., 2017; Roeckel et al., 2017; Blomqvist et al., 2020). Additionally, morphine and M6G analgesic effects are markedly reduced by M3G (Smith et al., 1990; Qian-Ling et al., 1992; Ekblom et al., 1993; Faura et al., 1996, 1997; Gardmark et al., 1998). Hence, whether it is injected alone or with morphine or M6G, several studies have demonstrated that M3G has pronociceptive properties in rodents (Table 1).
Interestingly, Smith and Smith (1995) observed that, when morphine is infused continuously in rats, the higher the plasmatic metabolic ratio M3G/morphine is, the lower the antinociception is, independently of the M3G or morphine plasmatic concentrations. Similar observations were made in the extracellular cortical fluid following s.c. administration of morphine (Barjavel et al., 1995). Consequently, M3G was proposed to counteract morphine-induced analgesia and to produce neuroexcitatory effects responsible for some morphine side effects (Gong et al., 1991; Smith and Smith, 1995; Faura et al., 1997; Roeckel et al., 2017; Blomqvist et al., 2020).
Although a considerable number of studies have indicated that M3G possesses pronociceptive properties, some studies did not observe pronociceptive effects following M3G administration or when it was coadministered with morphine or M6G (Table 2). For instance, Ouellet and Pollack (1997) observed that, when M3G was infused for 12 h in rats, there was no hyperalgesia or modulation of morphine analgesia (Suzuki et al., 1993). In another study, it was even noted that the i.v. coadministration of morphine and M3G improved morphine analgesia (Lipkowski et al., 1994).
Interestingly, in a MRP3–/– mouse model, the antinociception and hyperalgesia induced by an injection of morphine remained intact (Swartjes et al., 2012). In these mice, although morphine is still metabolized into M3G, M3G has been shown to remain trapped in hepatocytes due to the lack of the MRP3 efflux transporter. Therefore, plasma levels of M3G were extremely low in these transgenic animals compared to control animals (Zelcer et al., 2005; Swartjes et al., 2012). These data indicate that hyperalgesia may occur without significant contribution of hepatic M3G. However, it is worth noting that, although M3G is not found in the blood of these animals, morphine might be directly metabolized into M3G within the CNS and could still elicit its central effects (Gabel et al., 2022).
Humans
In humans, there have been few reports of the pronociceptive effects of M3G (Table 1). Smith and collaborators observed in 14 cancer patients improved pain relief, which was corroborated by a decrease in the M3G/(morphine + M6G) ratios. These results indirectly suggest a pronociceptive role of M3G by reducing morphine analgesia (Smith et al., 1999). In a pharmacokinetic-pharmacodynamic study involving 50 patients with moderate to severe pain, M3G effects seemed to oppose morphine analgesia (Mazoit et al., 2007). Several case reports have also suggested that M3G might play a role in morphine’s side effects such as morphine-induced hyperalgesia and seizures following high dose of morphine. However, these observations have shown considerable heterogeneity and do not demonstrate a pronociceptive role of M3G in humans (Morley et al., 1992; Sjogren et al., 1993, 1998; Rozan et al., 1995; Hagen and Swanson, 1997; Kronenberg et al., 1998).
Contrastingly, several reports have not observed any correlation between analgesia and plasmatic concentrations of M3G or the metabolic M3G/(morphine + M6G) ratios (Table 2; Samuelsson et al., 1993; Goucke et al., 1994; Wolff et al., 1995; Andersen et al., 2002; Toce et al., 2019). In addition, there have been two studies, published by the same group, in which healthy volunteers were administered M3G to evaluate its effects in humans (Penson et al., 2000, 2001). The first study was a randomized, double-blind, placebo-controlled trial in which M3G was infused in 10 healthy volunteers. Analgesia was assessed with numerical and visual analog scales in a submaximal ischemic pain model. No M3G-induced hyperalgesia or dysphoria was observed. In addition, the coadministration of M3G along with morphine or M6G did not affect analgesia (Penson et al., 2000). In the second study, which was blinded, but not controlled, three concentrations of M3G were used, but no effect was observed (Penson et al., 2001). These two studies are extremely valuable, although the number of subjects used was relatively small for obvious reasons.
Potential Origin of the Behavioral Effect
Considered together, the few studies in humans are matter of debate, whereas, in rodents, reports have shown much more consistency toward the pronociceptive effects of M3G, even though these effects are not always observed. The origin of the behavioral effect of M3G might rely on its glucuronide moiety. Indeed, M3G is not the only “3-glucuronide” metabolite displaying pronociceptive effects. Several studies published by Lewis et al. (2013, 2015) showed that estradiol-3-glucuronide, as well as ethyl-glucuronide, produces hyperalgesia after i.t. administration. Interestingly, glucuronic acid injected alone also triggered a similar effect, demonstrating the importance of the glucuronide moiety in the pronociceptive effects of these molecules (Lewis et al., 2013).
Supporting this idea, other 3-glucuronide metabolites of morphine-derived compounds, such as normorphine, noroxymorphone and hydromorphone, display pronociceptive properties (Yaksh and Harty, 1988; Smith et al., 1997; Wright et al., 2001). Consistently, Peckham and Traynor (2006) showed robust sex differences in analgesia only with morphine derivatives that are conjugated into a 3-glucuronide metabolite. Importantly, these observations were not related to binding affinity, ability to activate the MOR or lipophilicity. We also recently observed that sex differences in morphine analgesia could have their origin in morphine metabolism. Indeed, morphine metabolism is higher in the female brain, resulting in higher levels of M3G in pain-related brain regions (Gabel et al., 2022).
Pharmacological Targets
Mu Opioid Receptor
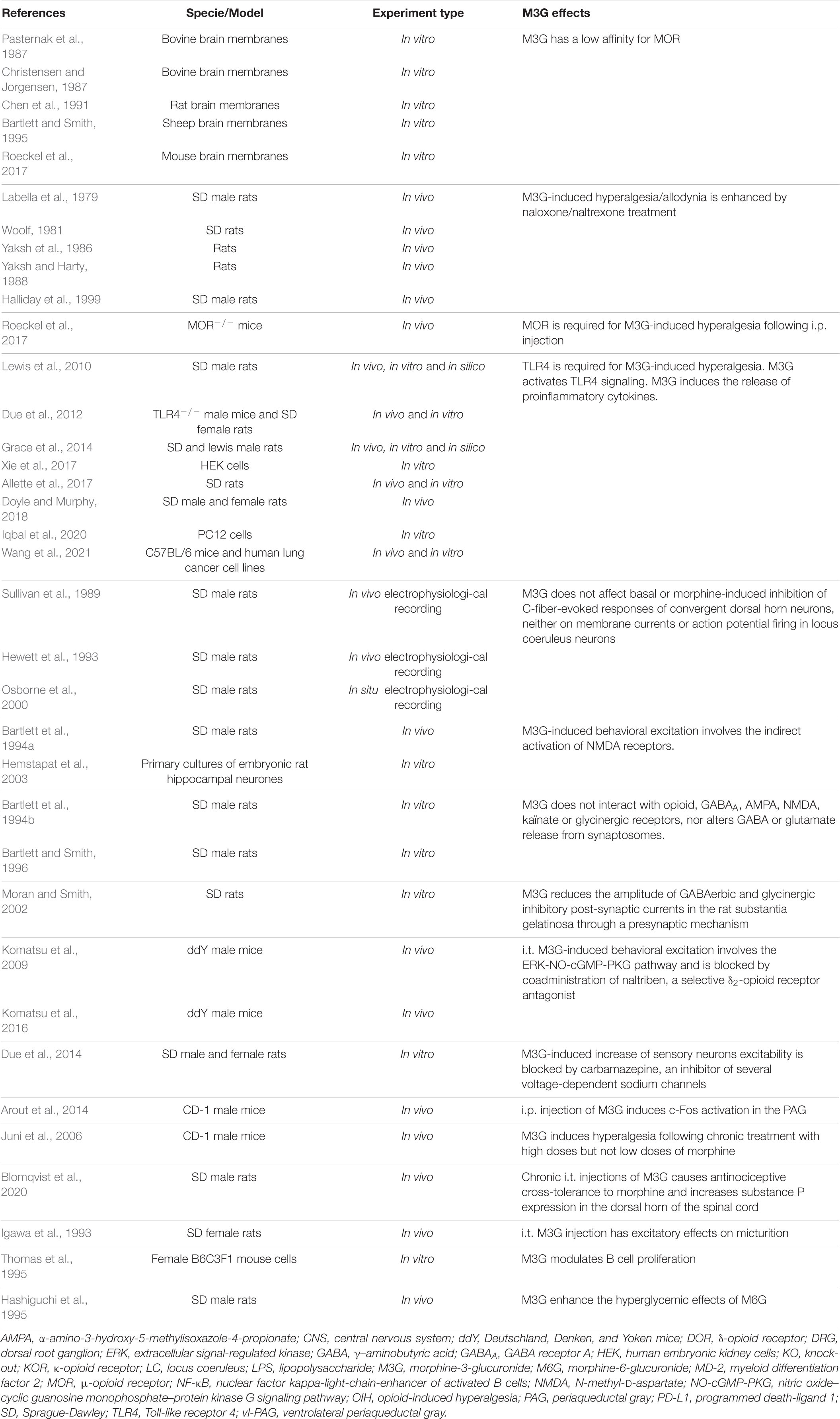
The molecular mechanisms underlying the effects of M3G remain a matter of debate (Table 3). On the one hand, one study published observations in MOR–/– mice suggesting its requirement for M3G pronociceptive effects (Roeckel et al., 2017). In this valuable study, i.p. administration of M3G induces thermal hyperalgesia and tactile allodynia in WT but not MOR–/– animals. In addition, M3G binds MOR on brain membranes from WT mice, although with low affinity (∼1.4 μM), and induces a weak Gi-dependent activity but no β-arrestin2 recruitment (Figure 2). This activity is not observed neither in brain membranes from MOR–/– mice, nor in the presence of naloxone (Roeckel et al., 2017).
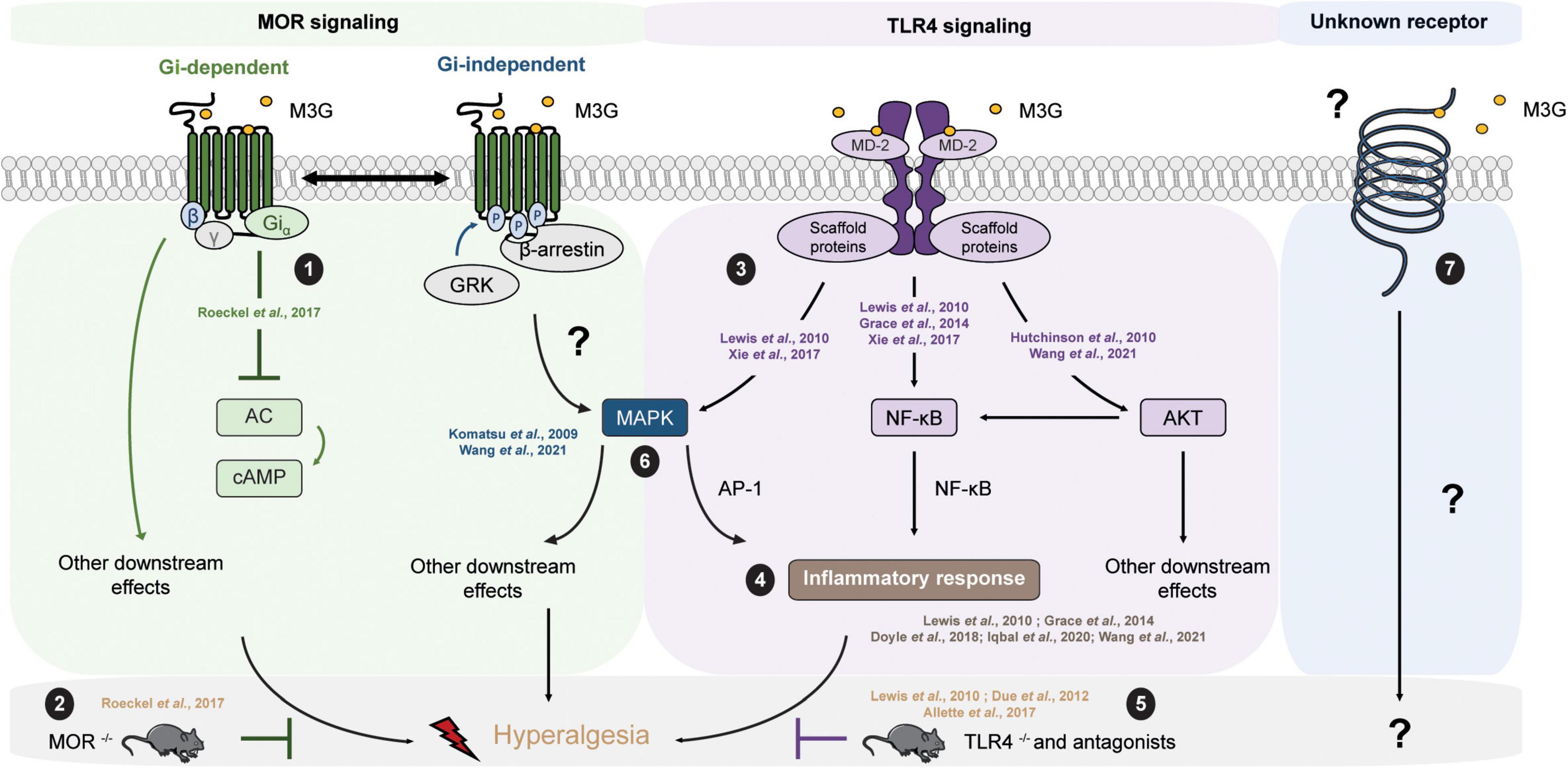
Figure 2. M3G known and possible intracellular pathways resulting in hyperalgesia. M3G administration causes hyperalgesia in rodents. (1) M3G has low affinity for MOR and has been shown to induce a weak MOR Gi-dependent signaling, although it does not seem to stimulate β-arrestin recruitment. (2) In a MOR– /– mouse strain, M3G hyperalgesia is abolished. (3) M3G can bind MD-2 and has been shown to induce the activation of the MAPK, NF-κB and AKT pathways in TLR4 signaling studies. (4) M3G has been described to cause the release of proinflammatory cytokines known to be powerful modulators of nociception counteracting morphine-induced antinociception. (5) M3G-induced hyperalgesia is also abolished in a TLR4– /– mouse strain. (6) Interestingly, both MOR and TLR4 signaling involves the MAPK pathway. This pathway is involved in morphine-induced hyperalgesia as well as in proinflammatory cytokine release following TLR4 activation. Antagonism of the MAPK pathway components results in inhibition of M3G-induced hyperalgesia. MOR-TLR4 crosstalk might thus be involved in M3G-induced hyperalgesia. (7) An interesting alternative assumption suggests the existence of a yet unknown receptor that could mediated M3G effects.
On the other hand, M3G showed only low (>μM) affinity for MOR in several binding studies employing radio-labeled molecules, such as [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) or naloxone (Labella et al., 1979; Christensen and Jorgensen, 1987; Pasternak et al., 1987; Coimbra-Farges et al., 1990; Chen et al., 1991; Roeckel et al., 2017). It was even proposed that the apparent affinity of M3G for MORs results from residual morphine contamination in M3G stock solutions (Bartlett and Smith, 1995). In addition, several in vivo studies have demonstrated that M3G’s pronociceptive effects persist in the presence of naloxone or naltrexone, two non-selective antagonists of MORs, whether they are injected systematically or directly into the CNS (Labella et al., 1979; Woolf, 1981; Yaksh et al., 1986; Yaksh and Harty, 1988; Halliday et al., 1999). Altogether, these pieces of evidence indicate that M3G might not bind to MOR, although one study suggested that this receptor appears to be mandatory for M3G effects.
TLR4
One interesting hypothesis suggests the existence of an alternative non-opioid receptor that could mediate M3G effects (Table 3). More precisely, in silico studies have indicated that M3G is able to bind the Toll-like receptor 4 (TLR4) and myeloid differentiation factor 2 (MD-2) complex through an interaction with the lipopolysaccharide (LPS) binding pocket of MD-2 (Hutchinson et al., 2010; Lewis et al., 2010; Grace et al., 2014) (for a review of opioid interactions with TLR4, see Gabr et al., 2021). TLR4 downstream signaling involves the activation of 3 parallel intracellular pathways: the NF-κB, the MAPK and the PI3K/AKT pathway. In agreement with the initial reports, it has been shown in vitro that the reporter cell line HEK-Blue™ hTLR4 exhibits significant activation upon M3G stimulation, which is inhibited by LPS from Rhodobacter sphaeroides (LPS-RS), a selective TLR4 antagonist (Lewis et al., 2010; Xie et al., 2017). This reporter cell line expresses the human TLR4 and a reporter gene under the control of a promoter inducible by NF-κB and AP-1, two transcription factors involved in TLR4 signaling cascade and proinflammatory cytokines release. In addition, it has been shown that the PI3K/AKT pathway, the third TLR4 intra-cellular signaling pathway, is also activated following M3G stimulation (Figure 2; Hutchinson et al., 2010; Wang et al., 2021). In human cancer cell lines, the activation of the AKT pathway by M3G results in upregulation of programmed death ligand 1 (PD-L1), which promotes tumor growth (Wang et al., 2021). It is, however, worth noting that, although Wang et al. (2021) observed activation of the AKT and NF-κB pathways in the A549 cell line (a human lung cancer cell line), they did not observe activation of the MAPK pathway in their model.
In vivo, M3G-induced hyperalgesia following i.p. administration in rodents is abolished by administration of TLR4 antagonists, as well as in a TLR4–/– mouse model (Figure 2; Due et al., 2012; Allette et al., 2017). Consistently, M3G seems to display proinflammatory properties through upregulation of NF-κB and proinflammatory cytokines, including interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNFα), such that it was proposed to be involved in the modulation of morphine properties (Figure 2; Lewis et al., 2010; Grace et al., 2014; Doyle and Murphy, 2018; Iqbal et al., 2020; Wang et al., 2021). These interesting findings take into account that a considerable number of studies have described the immunomodulatory effects of morphine and M3G (Wybran et al., 1979; Shavit et al., 1986; Freier and Fuchs, 1994; Thomas et al., 1995; Wang et al., 2012; Eisenstein, 2019). Considered together, these data suggest that TLR4 could be responsible for the inflammation triggered by M3G, which would thwart morphine’s analgesic effects.
Several studies have implicated TLR4 in dampening morphine antinociceptive effects or in some side effects, such as antinociceptive tolerance (Hutchinson et al., 2010; Liu et al., 2011; Wang et al., 2012, 2020, 2021; Eidson and Murphy, 2013; Bai et al., 2014; Grace et al., 2014; Eidson et al., 2017; Thomas et al., 2022). For instance, a recent study revealed that antinociceptive tolerance was prevented in TLR2 and TLR4 null mutants, but not in MyD88–/– animals (Thomas et al., 2022). Since several studies suggested that TLR4 could be the receptor mediating M3G effects, M3G has been proposed to play a role in morphine side effects, especially in antinociceptive tolerance (Juni et al., 2006; Blomqvist et al., 2020). However, one should note that two studies invalidate the implication of TLR4 in morphine’s effects (Fukagawa et al., 2013; Mattioli et al., 2014). The TLR4 mutant mouse strain C3H/HeJ, which expresses a non-functional TLR4, a TLR4–/– mouse strain on a C57BL/6 background and the B10ScNJ mouse strain, which has a spontaneous mutation that completely removes the TLR4 coding sequence, were used. In the first study, after repeated injection of morphine, CD11b (a marker of microglial activation) mRNA expression was increased in the spinal cord of control mice. Minocycline, a microglial inhibitor, attenuated the development of morphine tolerance in these mice. Conversely, in the C3H/HeJ mutant mouse strain and in a TLR4–/– mouse strain, neither the increase of CD11b mRNA expression, nor the antinociceptive tolerance was affected by TLR4 invalidation (Fukagawa et al., 2013). In the second study, neither acute antinociceptive response to a single dose of morphine, nor the development of antinociceptive tolerance was affected by TLR4 invalidation in the C3H/HeJ and B10ScNJ mouse strains (Mattioli et al., 2014). These results suggest that, in these models, TLR4 is not involved in the modulation of the antinociceptive effect of morphine, in its side effects or in the microglial activation observed during morphine tolerance. This evidence is interesting and provides insight into the complexity of M3G physiology.
Mu Opioid Receptor – TLR4 Crosstalk
On the one hand, M3G-induced hyperalgesia is abolished in a MOR–/– mouse model (Roeckel et al., 2017). On the other hand, the same effect was observed in a TLR4–/– mouse model (Due et al., 2012). This piece of evidence raises the possibility that the hyperalgesia observed following M3G administration might depend on the cross-talk between MORs and TLR4s within the CNS (Figure 2), for which both receptors are mandatory (for review, see Zhang et al., 2020). To support this idea, both receptors are expressed in microglia, astrocytes and even neurons under pathological conditions (Aicher et al., 2000; Lehnardt et al., 2003; Calvo-Rodriguez et al., 2017; Maduna et al., 2018; Zhang et al., 2018; Nam et al., 2019). Secondly, the mitogen-activated protein kinase (MAPK) pathway is recruited following both MOR and TLR4 stimulation. This pathway seems to be involved in morphine-induced hyperalgesia, as well as in the inflammatory response following TLR4 activation (Zhang et al., 2020). Finally, different studies have reported that M3G effects were abolished in presence of MAPK pathway inhibitors (Figure 2; Komatsu et al., 2009; Wang et al., 2021). Taken together, the MAPK pathway represents an interesting target to assess to better understand M3G effects.
Several studies have also suggested that, although M3G alone does not induce hyperalgesia, its coadministration with morphine decreases analgesia (Ekblom et al., 1993; Faura et al., 1996, 1997). In these studies, relatively low concentrations of M3G were injected through the i.p. route, while most of the studies in which direct hyperalgesia was observed injected high concentrations of M3G directly into the CNS (Table 1). Hence, it could be possible that, following CNS administration, M3G reaches sufficient CNS concentrations to activate both MOR and TLR4 on its own and produce hyperalgesia, although it has a low apparent affinity for MOR. In contrast, after peripheral injection of low dose of M3G alone, M3G would not reach sufficient CNS concentrations for MOR activation even though TLR4 might be activated. The presence of morphine along with M3G would then allow MOR and TLR4 activation and thus hyperalgesia. Nonetheless, this hypothesis remains to be investigated. Interestingly, in humans, M3G plasmatic and cerebrospinal fluid (CSF) concentrations following morphine administration show significant variation according to administration types, doses and patients (Hand et al., 1987b; Osborne et al., 1990; Hasselstrom and Sawe, 1993; Goucke et al., 1994; Westerling et al., 1995; Wolff et al., 1995, 1996; Hoffman et al., 1997; Christrup et al., 1999; Smith et al., 1999; Sarton et al., 2000; Meineke et al., 2002). For instance, after i.v. injection of 5 mg of morphine in healthy volunteers, M3G maximal plasmatic concentration reaches approximatively 100 nM, whereas it reaches 2 μM after a 30 min infusion of 0.5 mg/kg of morphine in neurosurgical patients (Hasselstrom and Sawe, 1993; Meineke et al., 2002). In the CSF, M3G concentrations range approximatively from 4 nM in patients that were given 30 mg of morphine orally to 0.7 μM in patients receiving chronic oral morphine therapy (Hand et al., 1987b; Goucke et al., 1994; Wolff et al., 1995, 1996; Smith et al., 1999). Depending on dose and treatment duration, M3G might reach the required CNS concentrations to induce MOR and TLR4 activation.
It is also worth noting that, although numerous studies have proposed pieces of evidence that TLR4 is involved in M3G’s effects, there is few data regarding the direct binding of M3G to TLR4. In a biophysical binding assay, M3G has been shown to bind the accessory protein MD-2 with a relatively low dissociation constant of approximatively 1.5 μM (Grace et al., 2014). However, there is no study in which radiolabeled molecules were used to investigate whether M3G can bind TLR4 or not. Therefore, one should consider an additional assumption that suggests the existence of an alternative receptor that could trigger a TLR4-dependent signaling pathway (Figure 2). In addition, to our knowledge, TLR4/MOR heteromers have not yet been described, although such association might participate in the complex response to M3G.
Modulation of Neuronal Activity
Since the early 1990s, several studies have investigated the effects of M3G on the modulation of neuronal activity (Table 3). Consistent with the TLR4 assumption, M3G increases the excitability of nociceptive dorsal root ganglion neurons in a similar manner as LPS, and this effect seems to rely on TLR4 (Due et al., 2012, 2014; Allette et al., 2017). The implication of NaV currents has subsequently been reported in this phenomenon using carbamazepine, a known inhibitor of several NaV channels (Due et al., 2012, 2014). Concomitantly, one study showed higher c-Fos levels within the PAG following s.c. co-administration of naltrexone and M3G, rather than naltrexone and morphine (Arout et al., 2014).
Ionotropic N-methyl-D-aspartate (NMDA) glutamatergic receptors also appear to be involved in M3G’s effects. First, M3G did not induce any excitation when embryonic cultured hippocampal neurons were preincubated with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, an NMDA receptor antagonist), showing the requirement of this receptor in the excitatory effects of M3G in vitro. This inhibition is not observed with naloxone and seems to rely on the indirect recruitment of NMDA receptors (Hemstapat et al., 2003). Moreover, behavioral excitation triggered by M3G administration was attenuated in rats pretreated with LY274614, another NMDA receptor antagonist, or when antagonists were coinjected with M3G (Bartlett et al., 1994a; Komatsu et al., 2009). Komatsu et al. (2009) have performed i.t. injections of M3G together with different antagonists, and they postulated that the phosphorylation of extracellular signal-regulated kinases (ERKs) follows the activation of the NO-cGMP-PKG pathway in response to NMDA receptor activation and that this mechanism could be responsible for an increase in neuronal excitability after M3G administration. Later, the same group showed that both nociceptive responses induced by M3G and ERK activation might be triggered via δ2-opioid receptors (DOR2) activated by Leu-enkephalin (Komatsu et al., 2016).
These data are, to a certain extent, consistent with M3G having no affinity for NMDA receptors and not being able to modulate glutamate release from whole-brain synaptosomes (Bartlett et al., 1994b; Bartlett and Smith, 1996). M3G fails to affect evoked excitatory postsynaptic currents obtained from patch-clamp recordings in neurons of the substantia gelatinosa, yet it decreases the amplitude of inhibitory postsynaptic currents in a dose-dependent manner. This effect is insensitive to naloxone and seems to stem from a presynaptic mechanism, resulting in the disinhibition of substantia gelatinosa neurons, although the identity of the recorded neurons remains unknown (Moran and Smith, 2002). This study seems to note that M3G could modulate the inhibitory systems in the spinal cord. However, it is worth noting that M3G fails to modulate γ-aminobutyric acid (GABA) release from whole-brain synaptosomes, although local suppression of GABA release, for instance, in the spinal cord, should not be excluded (Bartlett and Smith, 1996). Other reports have made this puzzling situation even more complex. Indeed, some in vivo pieces of evidence have suggested that M3G has no effect on the C-fiber-evoked responses of dorsal horn nociceptive neurons following i.t. pretreatment in anesthetized rats (Sullivan et al., 1989; Hewett et al., 1993; Osborne et al., 2000). Overwhelmingly, the current consensus is that M3G might modulate neuronal activity through a non-opioidergic pathway, but considerable efforts are still needed to clarify the exact underlying mechanism. Finally, M3G has also been shown to modulate several peripheral functions such as micturition and glycemia regulation following M6G administration (Igawa et al., 1993; Hashiguchi et al., 1995).
Summary
With these outcomes considered together, M3G is able to induce both hyperalgesia and allodynia in rodents and could thus oppose morphine antinociception, although the relevance of its effects in humans is debated. M3G might act on TLR4 or both TLR4 and MOR, as well as on an additional receptor not yet characterized (Figure 3). Such a multimodal mechanism might explain the heterogeneity observed between studies and the difficulty of drawing conclusions regarding M3G neuronal effects.
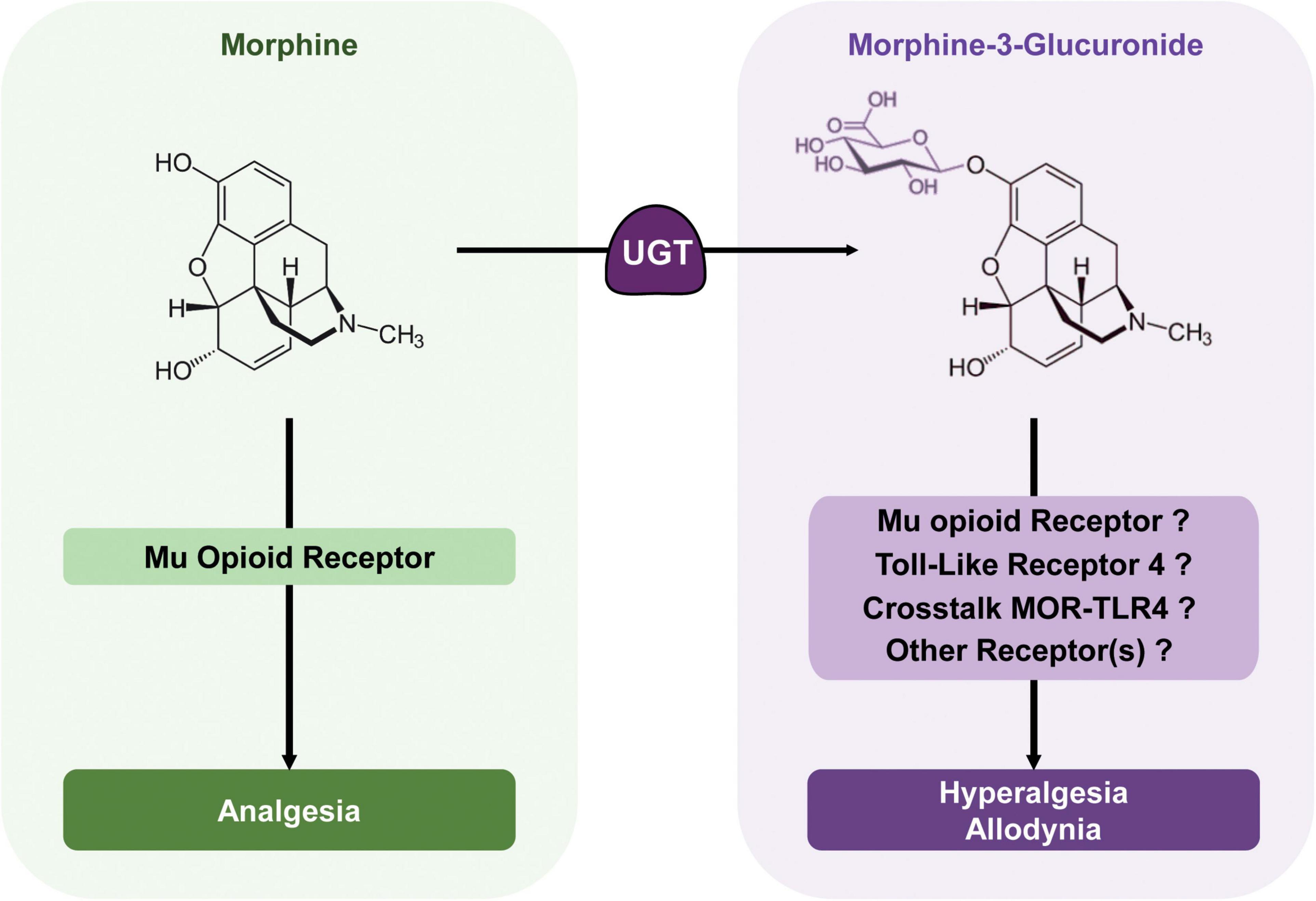
Figure 3. Morphine metabolic balance participates to the modulation of morphine analgesia. Morphine acts mainly on MORs to produce potent analgesia. It is metabolized by UGTs into its predominant metabolite; M3G. In rodents, M3G has been described as inducing thermal hyperalgesia and tactile allodynia, which might oppose morphine analgesia, although in humans, the relevance of these effects remains a matter of debate. M3G has been proposed to bind to TLR4, and this receptor appears to be required for M3G-induced hyperalgesia. At the same time, one study has shown that MORs are also required for M3G effects, although M3G seems to have no or little affinity for these receptors. Considered together, the crosstalk between these receptors could be key to M3G effects, whereas another receptor could also be involved.
Author Contributions
FG, VH, and YG: writing – original draft. FG, VH, YG, and A-KB: writing – review and editing. YG: funding acquisition. FG and YG: supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by INSERM, CNRS, University of Strasbourg (Unistra) and French Ministère Délégué à la Recherche et à l’Enseignement Supérieur (Ph.D. fellowship to FG and VH). We thank the following research programs of excellence for their support: FHU Neurogenycs, French National Research Agency (ANR) through the Programme d’Investissement d’Avenir (FG, VH, and A-KB; contract ANR-17-EURE-0022, EURIDOL graduate school of pain).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADME, absorption-distribution-metabolism-excretion; BBB, blood-brain barrier; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; CNS, central nervous system; CSF, cerebrospinal fluid; DAMGO, [D-Ala2, N-MePhe4, Gly-ol]-enkephalin; DOR2, δ2-opioid receptors; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinases; GABA, γ-aminobutyric acid; i.c.v, intracerebroventricular; IL-1β, interleukine 1β; IL-6, interleukine 6; i.p., intraperitoneal; i.t., intrathechal; LPS, lipopolysaccharide; LPS-RS, LPS from Rhodobacter sphaeroides; M3G, morphine-3-glucuronide; M6G, morphine-6-glucuronide; MAPK, mitogen-activated protein kinase; MD-2, myeloid differentiation factor 2; MOR, μ-opioid receptor; MRP, multidrug resistance-associated protein; NF-κB, nuclear factor κB; NMDA, ionotropic N-methyl-D-aspartate; OATP, organic anion transporter polypeptides; OCT, organic cation transporter; PAG, periaqueductal gray; P-gp, P-glycoprotein; RVM, rostral ventromedial medulla; s.c., subcutaneous; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor α; UDPGA, uridine diphosphate glucuronic acid; UDP-GlcNac, UDP-N-acetylglucosamine; UGTrel7, UDP-galactose transporter-related protein 7; UGT, uridine 5′-diphospho-glucuronosyltransferases.
References
Aicher, S. A., Sharma, S., Cheng, P. Y., Liu-Chen, L. Y., and Pickel, V. M. (2000). Dual ultrastructural localization of mu-opiate receptors and substance p in the dorsal horn. Synapse 36, 12–20. doi: 10.1002/(SICI)1098-2396(200004)36:1<12::AID-SYN2>3.0.CO;2-E
Al-Hasani, R., and Bruchas, M. R. (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115, 1363–1381. doi: 10.1097/ALN.0b013e318238bba6
Allette, Y. M., Kim, Y., Randolph, A. L., Smith, J. A., Ripsch, M. S., and White, F. A. (2017). Decoy peptide targeted to Toll-IL-1R domain inhibits LPS and TLR4-active metabolite morphine-3 glucuronide sensitization of sensory neurons. Sci. Rep. 7:3741. doi: 10.1038/s41598-017-03447-9
Andersen, G., Christrup, L. L., Sjogren, P., Hansen, S. H., and Jensen, N. H. (2002). Changing M3G/M6G ratios and pharmacodynamics in a cancer patient during long-term morphine treatment. J. Pain Symptom Manag. 23, 161–164. doi: 10.1016/s0885-3924(01)00398-0
Arout, C. A., Caldwell, M., McCloskey, D. P., and Kest, B. C. (2014). Fos activation in the periaqueductal gray following acute morphine-3beta-D-glucuronide or morphine administration. Physiol. Behav. 130, 28–33. doi: 10.1016/j.physbeh.2014.02.056
Bachmutsky, I., Wei, X. P., Kish, E., and Yackle, K. (2020). Opioids depress breathing through two small brainstem sites. Elife 9:e52694. doi: 10.7554/eLife.52694
Bai, L., Zhai, C., Han, K., Li, Z., Qian, J., Jing, Y., et al. (2014). Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci. Bull. 30, 936–948. doi: 10.1007/s12264-014-1483-7
Ballabh, P., Braun, A., and Nedergaard, M. (2004). The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. doi: 10.1016/j.nbd.2003.12.016
Barjavel, M. J., Scherrmann, J. M., and Bhargava, H. N. (1995). Relationship between morphine analgesia and cortical extracellular fluid levels of morphine and its metabolites in the rat: a microdialysis study. Br. J. Pharmacol. 116, 3205–3210. doi: 10.1111/j.1476-5381.1995.tb15125.x
Bartlett, S. E., and Smith, M. T. (1995). The apparent affinity of morphine-3-glucuronide at mu1-opioid receptors results from morphine contamination: demonstration using HPLC and radioligand binding. Life Sci. 57, 609–615. doi: 10.1016/0024-3205(95)00311-s
Bartlett, S. E., and Smith, M. T. (1996). Effects of morphine-3-glucuronide and morphine on the K+-evoked release of [3H]-glutamic acid and [14C]-gamma-aminobutyric acid from rat brain synaptosomes. Life Sci. 58, 447–454. doi: 10.1016/0024-3205(95)02310-0
Bartlett, S. E., Cramond, T., and Smith, M. T. (1994a). The excitatory effects of morphine-3-glucuronide are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life Sci. 54, 687–694. doi: 10.1016/0024-3205(94)00552-4
Bartlett, S. E., Dodd, P. R., and Smith, M. T. (1994b). Pharmacology of morphine and morphine-3-glucuronide at opioid, excitatory amino acid. GABA and glycine binding sites. Pharmacol. Toxicol. 75, 73–81. doi: 10.1111/j.1600-0773.1994.tb00327.x
Bian, J. T., and Bhargava, H. N. (1996). Effects of morphine-3-glucuronide on the antinociceptive activity of peptide and nonpeptide opioid receptor agonists in mice. Peptides 17, 1415–1419. doi: 10.1016/s0196-9781(96)00215-x
Blomqvist, K. J., Viisanen, H., Ahlstrom, F. H. G., Jokinen, V., Sidorova, Y. A., Suleymanova, I., et al. (2020). Morphine-3-glucuronide causes antinociceptive cross-tolerance to morphine and increases spinal substance P expression. Eur. J. Pharmacol. 875:173021. doi: 10.1016/j.ejphar.2020.173021
Bock, K. W. (2016). The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: animal-plant arms-race and co-evolution. Biochem. Pharmacol. 99, 11–17. doi: 10.1016/j.bcp.2015.10.001
Buckley, D. B., and Klaassen, C. D. (2007). Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab. Dispos. 35, 121–127. doi: 10.1124/dmd.106.012070
Calvo-Rodriguez, M., de la Fuente, C., Garcia-Durillo, M., Garcia-Rodriguez, C., Villalobos, C., and Nunez, L. (2017). Aging and amyloid beta oligomers enhance TLR4 expression, LPS-induced Ca(2+) responses, and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflammation 14:24. doi: 10.1186/s12974-017-0802-0
Chen, Z. R., Irvine, R. J., Somogyi, A. A., and Bochner, F. (1991). Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 48, 2165–2171. doi: 10.1016/0024-3205(91)90150-a
Christensen, C. B., and Jorgensen, L. N. (1987). Morphine-6-glucuronide has high affinity for the opioid receptor. Pharmacol. Toxicol. 60, 75–76. doi: 10.1111/j.1600-0773.1987.tb01724.x
Christrup, L. L., Sjogren, P., Jensen, N. H., Banning, A. M., Elbaek, K., and Ersboll, A. K. (1999). Steady-state kinetics and dynamics of morphine in cancer patients: is sedation related to the absorption rate of morphine? J. Pain Symptom Manag. 18, 164–173. doi: 10.1016/s0885-3924(99)00068-8
Coimbra-Farges, R., Puget, A., Monsarrat, B., Moisand, C., and Meunier, J. C. (1990). Morphine metabolism in the naturally morphine-tolerant afghan pika: a preliminary study. Life Sci. 46, 663–669. doi: 10.1016/0024-3205(90)90135-e
Cone, E. J., Caplan, Y. H., Moser, F., Robert, T., and Black, D. (2008). Evidence that morphine is metabolized to hydromorphone but not to oxymorphone. J. Anal. Toxicol. 32, 319–323. doi: 10.1093/jat/32.4.319
Court, M. H. (2005). Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Methods Enzymol. 400, 104–116. doi: 10.1016/S0076-6879(05)00007-8
Dahan, A., and Lotsch, J. (2015). Morphine is not a prodrug. Br. J. Anaesth. 114, 1005–1006. doi: 10.1093/bja/aev125
Doyle, H. H., and Murphy, A. Z. (2018). Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol. Behav. 187, 32–41. doi: 10.1016/j.physbeh.2017.11.030
Due, M. R., Piekarz, A. D., Wilson, N., Feldman, P., Ripsch, M. S., Chavez, S., et al. (2012). Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J. Neuroinflammation 9:200. doi: 10.1186/1742-2094-9-200
Due, M. R., Yang, X. F., Allette, Y. M., Randolph, A. L., Ripsch, M. S., Wilson, S. M., et al. (2014). Carbamazepine potentiates the effectiveness of morphine in a rodent model of neuropathic pain. PLoS One 9:e107399. doi: 10.1371/journal.pone.0107399
Eidson, L. N., and Murphy, A. Z. (2013). Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J. Neurosci. 33, 15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013
Eidson, L. N., Inoue, K., Young, L. J., Tansey, M. G., and Murphy, A. Z. (2017). Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology 42, 661–670. doi: 10.1038/npp.2016.131
Eisenstein, T. K. (2019). The role of opioid receptors in immune system function. Front. Immunol. 10:2904. doi: 10.3389/fimmu.2019.02904
Ekblom, M., Gardmark, M., and Hammarlund-Udenaes, M. (1993). Pharmacokinetics and pharmacodynamics of morphine-3-glucuronide in rats and its influence on the antinociceptive effect of morphine. Biopharm. Drug Dispos. 14, 1–11. doi: 10.1002/bdd.2510140102
Faura, C. C., Olaso, J. M., Cabanes, C. G., and Horga, J. F. (1996). Lack of morphine-6-glucuronide antinociception after morphine treatment. Is morphine-3-glucuronide involved? Pain 65, 25–30. doi: 10.1016/0304-3959(95)00198-0
Faura, C. C., Olaso, M. J., and Horga, J. F. (1997). Morphine-3-glucuronide prevents tolerance to morphine-6-glucuronide in mice. Eur. J. Pain 1, 161–164. doi: 10.1016/s1090-3801(97)90074-4
Freier, D. O., and Fuchs, B. A. (1994). A mechanism of action for morphine-induced immunosuppression: corticosterone mediates morphine-induced suppression of natural killer cell activity. J. Pharmacol. Exp. Ther. 270, 1127–1133.
Fukagawa, H., Koyama, T., Kakuyama, M., and Fukuda, K. (2013). Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J. Anesth. 27, 93–97. doi: 10.1007/s00540-012-1469-4
Fullerton, E. F., Rubaharan, M., Karom, M. C., Hanberry, R. I., and Murphy, A. Z. (2021). Advanced age attenuates the antihyperalgesic effect of morphine and decreases mu-opioid receptor expression and binding in the rat midbrain periaqueductal gray in male and female rats. Neurobiol. Aging. 98, 78–87. doi: 10.1016/j.neurobiolaging.2020.10.020
Gabel, F., Hovhannisyan, V., Andry, V., and Goumon, Y. (2022). Central metabolism as a potential origin of sex differences in morphine antinociception but not in the induction of antinociceptive tolerance in mice. Br. J. Pharmacol. 1–19. doi: 10.1111/bph.15792
Gabr, M. M., Saeed, I., Miles, J. A., Ross, B. P., Shaw, P. N., Hollmann, M. W., et al. (2021). Interaction of Opioids with TLR4-Mechanisms and Ramifications. Cancers 13:5274. doi: 10.3390/cancers13215274
Gardmark, M., Karlsson, M. O., Jonsson, F., and Hammarlund-Udenaes, M. (1998). Morphine-3-glucuronide has a minor effect on morphine antinociception. Pharmacodynamic modeling. J. Pharm. Sci. 87, 813–820. doi: 10.1021/js980056f
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2
Gong, Q. L., Hedner, T., Hedner, J., Bjorkman, R., and Nordberg, G. (1991). Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur. J. Pharmacol. 193, 47–56. doi: 10.1016/0014-2999(91)90199-z
Goucke, R. C., Hackett, P. L., and Ilett, K. F. (1994). Concentrations of morphine, morphine-6-glucuronide and morphine-3-glucuronide in serum and cerebrospinal fluid following morphine administration to patients with morphine-resistant pain. Pain 56, 145–149. doi: 10.1016/0304-3959(94)90088-4
Grace, P. M., Ramos, K. M., Rodgers, K. M., Wang, X., Hutchinson, M. R., Lewis, M. T., et al. (2014). Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience 280, 299–317. doi: 10.1016/j.neuroscience.2014.09.020
Gupta, K., Nagappa, M., Prasad, A., Abrahamyan, L., Wong, J., Weingarten, T. N., et al. (2018). Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open 8:e024086. doi: 10.1136/bmjopen-2018-024086
Hagen, N., and Swanson, R. (1997). Strychnine-like multifocal myoclonus and seizures in extremely high-dose opioid administration: treatment strategies. J. Pain Symptom Manag. 14, 51–58. doi: 10.1016/S0885-3924(97)00001-8
Halliday, A. J., Bartlett, S. E., Colditz, P., and Smith, M. T. (1999). Brain region-specific studies of the excitatory behavioral effects of morphine-3-glucuronide. Life Sci. 65, 225–236. doi: 10.1016/s0024-3205(99)00239-8
Hand, C. W., Blunnie, W. P., Claffey, L. P., McShane, A. J., McQuay, H. J., and Moore, R. A. (1987a). Potential analgesic contribution from morphine-6-glucuronide in CSF. Lancet 2, 1207–1208. doi: 10.1016/s0140-6736(87)91341-9
Hand, C. W., Moore, R. A., McQuay, H. J., Allen, M. C., and Sear, J. W. (1987b). Analysis of morphine and its major metabolites by differential radioimmunoassay. Ann. Clin. Biochem. 24(Pt 2), 153–160. doi: 10.1177/000456328702400205
Handal, M., Grung, M., Skurtveit, S., Ripel, A., and Morland, J. (2002). Pharmacokinetic differences of morphine and morphine-glucuronides are reflected in locomotor activity. Pharmacol. Biochem. Behav. 73, 883–892. doi: 10.1016/s0091-3057(02)00925-5
Hashiguchi, Y., Molina, P. E., and Abumrad, N. N. (1995). Modulation of metabolic effects of morphine-6-glucuronide by morphine-3-glucuronide. Brain Res. Bull. 38, 325–329. doi: 10.1016/0361-9230(95)00104-m
Hasselstrom, J., and Sawe, J. (1993). Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin. Pharmacokinet. 24, 344–354. doi: 10.2165/00003088-199324040-00007
Hemstapat, K., Monteith, G. R., Smith, D., and Smith, M. T. (2003). Morphine-3-glucuronide’s neuro-excitatory effects are mediated via indirect activation of N-methyl-D-aspartic acid receptors: mechanistic studies in embryonic cultured hippocampal neurones. Anesth. Analg. 97, 494–505. doi: 10.1213/01.ANE.0000059225.40049.99
Hewett, K., Dickenson, A. H., and McQuay, H. J. (1993). Lack of effect of morphine-3-glucuronide on the spinal antinociceptive actions of morphine in the rat: an electrophysiological study. Pain 53, 59–63. doi: 10.1016/0304-3959(93)90056-U
Hoffman, M., Xu, J. C., Smith, C., Fanelli, C., Pascal, V., Degaetano, C., et al. (1997). A pharmacodynamic study of morphine and its glucuronide metabolites after single morphine dosing in cancer patients with pain. Cancer Invest. 15, 542–547. doi: 10.3109/07357909709047595
Hopkins, R. E., Bui, T., Magliano, D., Arnold, C., and Dooley, M. (2019). Prescriber education interventions to optimize opioid prescribing in acute care: a systematic review. Pain Physician 22, E551–E562.
Hutchinson, M. R., Zhang, Y., Shridhar, M., Evans, J. H., Buchanan, M. M., Zhao, T. X., et al. (2010). Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 24, 83–95. doi: 10.1016/j.bbi.2009.08.004
Igawa, Y., Westerling, D., Mattiasson, A., and Andersson, K. E. (1993). Effects of morphine metabolites on micturition in normal, unanaesthetized rats. Br. J. Pharmacol. 110, 257–262. doi: 10.1111/j.1476-5381.1993.tb13802.x
Imaoka, T., Huang, W., Shum, S., Hailey, D. W., Chang, S. Y., Chapron, A., et al. (2021). Bridging the gap between in silico and in vivo by modeling opioid disposition in a kidney proximal tubule microphysiological system. Sci. Rep. 11:21356. doi: 10.1038/s41598-021-00338-y
Iqbal, S., Parker, L. M., Everest-Dass, A. V., Moh, E. S. X., Sayyadi, N., Hutchinson, M. R., et al. (2020). Lipopolysaccharide and Morphine-3-glucuronide-induced immune signalling increases the expression of polysialic acid in PC12 cells. Mol. Neurobiol. 57, 964–975. doi: 10.1007/s12035-019-01791-7
Jackson, M. R., Nilsson, T., and Peterson, P. A. (1990). Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 9, 3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x
Juni, A., Klein, G., and Kest, B. (2006). Morphine hyperalgesia in mice is unrelated to opioid activity, analgesia, or tolerance: evidence for multiple diverse hyperalgesic systems. Brain Res. 1070, 35–44. doi: 10.1016/j.brainres.2005.11.054
King, C. D., Rios, G. R., Green, M. D., and Tephly, T. R. (2000). UDP-glucuronosyltransferases. Curr. Drug Metab. 1, 143–161.
Kobayashi, T., Sleeman, J. E., Coughtrie, M. W., and Burchell, B. (2006). Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem J. 400, 281–289. doi: 10.1042/BJ20060429
Koller, G., Schwarzer, A., Halfter, K., and Soyka, M. (2019). Pain management in opioid maintenance treatment. Expert Opin. Pharmacother. 20, 1993–2005. doi: 10.1080/14656566.2019.1652270
Komatsu, T., Katsuyama, S., Nagase, H., Mizoguchi, H., Sakurada, C., Tsuzuki, M., et al. (2016). Intrathecal morphine-3-glucuronide-induced nociceptive behavior via Delta-2 opioid receptors in the spinal cord. Pharmacol. Biochem. Behav. 140, 68–74. doi: 10.1016/j.pbb.2015.10.010
Komatsu, T., Sakurada, S., Kohno, K., Shiohira, H., Katsuyama, S., Sakurada, C., et al. (2009). Spinal ERK activation via NO-cGMP pathway contributes to nociceptive behavior induced by morphine-3-glucuronide. Biochem. Pharmacol. 78, 1026–1034. doi: 10.1016/j.bcp.2009.06.106
Kronenberg, M. F., Laimer, I., Rifici, C., Saltuari, L., Bramanti, P., Moriggl, U., et al. (1998). Epileptic seizure associated with intracerebroventricular and intrathecal morphine bolus. Pain 75, 383–387. doi: 10.1016/s0304-3959(97)00173-5
Kuo, C. K., Hanioka, N., Hoshikawa, Y., Oguri, K., and Yoshimura, H. (1991). Species difference of site-selective glucuronidation of morphine. J. Pharmacobiodyn. 14, 187–193. doi: 10.1248/bpb1978.14.187
Kurita, A., Miyauchi, Y., Ikushiro, S., Mackenzie, P. I., Yamada, H., and Ishii, Y. (2017). Comprehensive characterization of mouse UDP-glucuronosyltransferase (ugt) belonging to the Ugt2b subfamily: identification of Ugt2b36 as the predominant isoform involved in morphine glucuronidation. J. Pharmacol. Exp. Ther. 361, 199–208. doi: 10.1124/jpet.117.240382
Labella, F. S., Pinsky, C., and Havlicek, V. (1979). Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res. 174, 263–271. doi: 10.1016/0006-8993(79)90849-7
Lau, B. K., and Vaughan, C. W. (2014). Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol. 29, 159–164. doi: 10.1016/j.conb.2014.07.010
Laux-Biehlmann, A., Mouheiche, J., Veriepe, J., and Goumon, Y. (2013). Endogenous morphine and its metabolites in mammals: history, synthesis, localization and perspectives. Neuroscience 233, 95–117. doi: 10.1016/j.neuroscience.2012.12.013
Lehnardt, S., Massillon, L., Follett, P., Jensen, F. E., Ratan, R., Rosenberg, P. A., et al. (2003). Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. U.S.A. 100, 8514–8519. doi: 10.1073/pnas.1432609100
Lewis, S. S., Hutchinson, M. R., Frick, M. M., Zhang, Y., Maier, S. F., Sammakia, T., et al. (2015). Select steroid hormone glucuronide metabolites can cause toll-like receptor 4 activation and enhanced pain. Brain Behav. Immun. 44, 128–136. doi: 10.1016/j.bbi.2014.09.004
Lewis, S. S., Hutchinson, M. R., Rezvani, N., Loram, L. C., Zhang, Y., Maier, S. F., et al. (2010). Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience 165, 569–583. doi: 10.1016/j.neuroscience.2009.10.011
Lewis, S. S., Hutchinson, M. R., Zhang, Y., Hund, D. K., Maier, S. F., Rice, K. C., et al. (2013). Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain. Brain Behav. Immun. 30, 24–32. doi: 10.1016/j.bbi.2013.01.005
Lipkowski, A. W., Carr, D. B., Langlade, A., Osgood, P. F., and Szyfelbein, S. K. (1994). Morphine-3-glucuronide: silent regulator of morphine actions. Life Sci. 55, 149–154. doi: 10.1016/0024-3205(94)90106-6
Liu, L., Coller, J. K., Watkins, L. R., Somogyi, A. A., and Hutchinson, M. R. (2011). Naloxone-precipitated morphine withdrawal behavior and brain IL-1beta expression: comparison of different mouse strains. Brain Behav. Immun. 25, 1223–1232. doi: 10.1016/j.bbi.2011.03.016
Lloret-Linares, C., Miyauchi, E., Luo, H., Labat, L., Bouillot, J. L., Poitou, C., et al. (2016). Oral morphine pharmacokinetic in obesity: the role of P-Glycoprotein, MRP2, MRP3, UGT2B7, and CYP3A4 jejunal contents and obesity-associated biomarkers. Mol. Pharm. 13, 766–773. doi: 10.1021/acs.molpharmaceut.5b00656
Lotsch, J., and Geisslinger, G. (2001). Morphine-6-glucuronide: an analgesic of the future? Clin. Pharmacokinet. 40, 485–499. doi: 10.2165/00003088-200140070-00001
Lotsch, J., Weiss, M., Ahne, G., Kobal, G., and Geisslinger, G. (1999). Pharmacokinetic modeling of M6G formation after oral administration of morphine in healthy volunteers. Anesthesiology 90, 1026–1038. doi: 10.1097/00000542-199904000-00016
Maduna, T., Audouard, E., Dembele, D., Mouzaoui, N., Reiss, D., Massotte, D., et al. (2018). Microglia express mu opioid receptor: insights from transcriptomics and fluorescent reporter mice. Front. Psychiatry 9:726. doi: 10.3389/fpsyt.2018.00726
Mattioli, T. A., Leduc-Pessah, H., Skelhorne-Gross, G., Nicol, C. J., Milne, B., Trang, T., et al. (2014). Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One 9:e97361. doi: 10.1371/journal.pone.0097361
Mazoit, J. X., Butscher, K., and Samii, K. (2007). Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth. Analg. 105, 70–78. doi: 10.1213/01.ane.0000265557.73688.32
Meech, R., Hu, D. G., McKinnon, R. A., Mubarokah, S. N., Haines, A. Z., Nair, P. C., et al. (2019). The UDP-Glycosyltransferase (UGT) superfamily: new members, new functions, and novel paradigms. Physiol. Rev. 99, 1153–1222. doi: 10.1152/physrev.00058.2017
Meineke, I., Freudenthaler, S., Hofmann, U., Schaeffeler, E., Mikus, G., Schwab, M., et al. (2002). Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br. J. Clin. Pharmacol. 54, 592–603. doi: 10.1046/j.1365-2125.2002.t01-1-01689.x
Meyer, M. J., Neumann, V. E., Friesacher, H. R., Zdrazil, B., Brockmoller, J., and Tzvetkov, M. V. (2019). Opioids as substrates and inhibitors of the genetically highly variable organic cation transporter OCT1. J. Med. Chem. 62, 9890–9905. doi: 10.1021/acs.jmedchem.9b01301
Miyauchi, Y., Kurita, A., Yamashita, R., Takamatsu, T., Ikushiro, S., Mackenzie, P. I., et al. (2020). Hetero-oligomer formation of mouse UDP-glucuronosyltransferase (UGT) 2b1 and 1a1 results in the gain of glucuronidation activity towards morphine, an activity which is absent in homo-oligomers of either UGT. Biochem. Biophys. Res. Commun. 525, 348–353. doi: 10.1016/j.bbrc.2020.02.075
Moran, T. D., and Smith, P. A. (2002). Morphine-3beta-D-glucuronide suppresses inhibitory synaptic transmission in rat substantia gelatinosa. J. Pharmacol. Exp. Ther. 302, 568–576. doi: 10.1124/jpet.102.035626
Morley, J. S., Miles, J. B., Wells, J. C., and Bowsher, D. (1992). Paradoxical pain. Lancet 340:1045.
Muraoka, M., Kawakita, M., and Ishida, N. (2001). Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 495, 87–93. doi: 10.1016/s0014-5793(01)02358-4
Nagano, E., Yamada, H., and Oguri, K. (2000). Characteristic glucuronidation pattern of physiologic concentration of morphine in rat brain. Life Sci. 67, 2453–2464. doi: 10.1016/s0024-3205(00)00825-0
Nair, P. C., Meech, R., Mackenzie, P. I., McKinnon, R. A., and Miners, J. O. (2015). Insights into the UDP-sugar selectivities of human UDP-glycosyltransferases (UGT): a molecular modeling perspective. Drug Metab. Rev. 47, 335–345.
Nam, M. H., Han, K. S., Lee, J., Won, W., Koh, W., Bae, J. Y., et al. (2019). Activation of astrocytic mu-opioid receptor causes conditioned place preference. Cell Rep. 28, 1154–1166. doi: 10.1016/j.celrep.2019.06.071
Oda, S., Fukami, T., Yokoi, T., and Nakajima, M. (2015). A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab. Pharmacokinet. 30, 30–51. doi: 10.1016/j.dmpk.2014.12.001
Oguri, K., Hanioka, N., and Yoshimura, H. (1990). Species differences in metabolism of codeine: urinary excretion of codeine glucuronide, morphine-3-glucuronide and morphine-6-glucuronide in mice, rats, guinea pigs and rabbits. Xenobiotica 20, 683–688. doi: 10.3109/00498259009046884
Ondo, K., Arakawa, H., Nakano, M., Fukami, T., and Nakajima, M. (2020). SLC35B1 significantly contributes to the uptake of UDPGA into the endoplasmic reticulum for glucuronidation catalyzed by UDP-glucuronosyltransferases. Biochem. Pharmacol. 175:113916. doi: 10.1016/j.bcp.2020.113916
Osborne, P. B., Chieng, B., and Christie, M. J. (2000). Morphine-6 beta-glucuronide has a higher efficacy than morphine as a mu-opioid receptor agonist in the rat locus coeruleus. Br. J. Pharmacol. 131, 1422–1428. doi: 10.1038/sj.bjp.0703697
Osborne, R., Joel, S., Trew, D., and Slevin, M. (1990). Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin. Pharmacol. Ther. 47, 12–19. doi: 10.1038/clpt.1990.2
Ouellet, D. M., and Pollack, G. M. (1997). Effect of prior morphine-3-glucuronide exposure on morphine disposition and antinociception. Biochem. Pharmacol. 53, 1451–1457. doi: 10.1016/s0006-2952(97)00086-5
Pasternak, G. W., Bodnar, R. J., Clark, J. A., and Inturrisi, C. E. (1987). Morphine-6-glucuronide, a potent mu agonist. Life Sci. 41, 2845–2849. doi: 10.1016/0024-3205(87)90431-0
Peckham, E. M., and Traynor, J. R. (2006). Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J. Pharmacol. Exp. Ther. 316, 1195–1201. doi: 10.1124/jpet.105.094276
Penson, R. T., Joel, S. P., Bakhshi, K., Clark, S. J., Langford, R. M., and Slevin, M. L. (2000). Randomized placebo-controlled trial of the activity of the morphine glucuronides. Clin. Pharmacol. Ther. 68, 667–676. doi: 10.1067/mcp.2000.111934
Penson, R. T., Joel, S. P., Clark, S., Gloyne, A., and Slevin, M. L. (2001). Limited phase I study of morphine-3-glucuronide. J. Pharm. Sci. 90, 1810–1816. doi: 10.1002/jps.1131
Qian-Ling, G., Hedner, J., Bjorkman, R., and Hedner, T. (1992). Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain 48, 249–255. doi: 10.1016/0304-3959(92)90065-J
Roeckel, L. A., Utard, V., Reiss, D., Mouheiche, J., Maurin, H., Robe, A., et al. (2017). Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci. Rep. 7:10406. doi: 10.1038/s41598-017-11120-4
Rowland, A., Mackenzie, P. I., and Miners, J. O. (2015). Transporter-mediated uptake of UDP-glucuronic acid by human liver microsomes: assay conditions, kinetics, and inhibition. Drug Metab. Dispos. 43, 147–153. doi: 10.1124/dmd.114.060509
Rozan, J. P., Kahn, C. H., and Warfield, C. A. (1995). Epidural and intravenous opioid-induced neuroexcitation. Anesthesiology 83, 860–863. doi: 10.1097/00000542-199510000-00027
Salem, A., and Hope, W. (1997). Role of morphine glucuronide metabolites in morphine dependence in the rat. Pharmacol. Biochem. Behav. 57, 801–807. doi: 10.1016/s0091-3057(96)00471-6
Samuelsson, H., Hedner, T., Venn, R., and Michalkiewicz, A. (1993). CSF and plasma concentrations of morphine and morphine glucuronides in cancer patients receiving epidural morphine. Pain 52, 179–185. doi: 10.1016/0304-3959(93)90129-D
Sarton, E., Olofsen, E., Romberg, R., den Hartigh, J., Kest, B., Nieuwenhuijs, D., et al. (2000). Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology 93, 1245–1254. doi: 10.1097/00000542-200011000-00018
Sawe, J. (1986). High-dose morphine and methadone in cancer patients. Clinical pharmacokinetic considerations of oral treatment. Clin. Pharmacokinet. 11, 87–106. doi: 10.2165/00003088-198611020-00001
Schaefer, C. P., Tome, M. E., and Davis, T. P. (2017). The opioid epidemic: a central role for the blood brain barrier in opioid analgesia and abuse. Fluids Barriers CNS 14:32. doi: 10.1186/s12987-017-0080-3
Shavit, Y., Terman, G. W., Lewis, J. W., Zane, C. J., Gale, R. P., and Liebeskind, J. C. (1986). Effects of footshock stress and morphine on natural killer lymphocytes in rats: studies of tolerance and cross-tolerance. Brain Res. 372, 382–385. doi: 10.1016/0006-8993(86)91149-2
Shelby, M. K., Cherrington, N. J., Vansell, N. R., and Klaassen, C. D. (2003). Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab. Dispos. 31, 326–333. doi: 10.1124/dmd.31.3.326
Shimomura, K., Kamata, O., Ueki, S., Ida, S., Oguri, K., Yoshimura, H., et al. (1971). Analgesic effect of morphine glucuronides. Tohoku J. Exp. Med. 105, 45–52. doi: 10.1620/tjem.105.45
Sjogren, P., Dragsted, L., and Christensen, C. B. (1993). Myoclonic spasms during treatment with high doses of intravenous morphine in renal failure. Acta Anaesthesiol. Scand. 37, 780–782. doi: 10.1111/j.1399-6576.1993.tb03809.x
Sjogren, P., Thunedborg, L. P., Christrup, L., Hansen, S. H., and Franks, J. (1998). Is development of hyperalgesia, allodynia and myoclonus related to morphine metabolism during long-term administration? Six case histories. Acta Anaesthesiol. Scand. 42, 1070–1075. doi: 10.1111/j.1399-6576.1998.tb05378.x
Smith, G. D., and Smith, M. T. (1995). Morphine-3-glucuronide: evidence to support its putative role in the development of tolerance to the antinociceptive effects of morphine in the rat. Pain 62, 51–60. doi: 10.1016/0304-3959(94)00228-7
Smith, G. D., Prankerd, R. J., and Smith, M. T. (1997). Biochemical synthesis, purification and preliminary pharmacological evaluation of normorphine-3-glucuronide. Life Sci. 61, 95–104. doi: 10.1016/s0024-3205(97)00364-0
Smith, M. T., Watt, J. A., and Cramond, T. (1990). Morphine-3-glucuronide–a potent antagonist of morphine analgesia. Life Sci. 47, 579–585. doi: 10.1016/0024-3205(90)90619-3
Smith, M. T., Wright, A. W., Williams, B. E., Stuart, G., and Cramond, T. (1999). Cerebrospinal fluid and plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in patients before and after initiation of intracerebroventricular morphine for cancer pain management. Anesth. Analg. 88, 109–116.
Stone, A. N., Mackenzie, P. I., Galetin, A., Houston, J. B., and Miners, J. O. (2003). Isoform selectivity and kinetics of morphine 3- and 6-glucuronidation by human udp-glucuronosyltransferases: evidence for atypical glucuronidation kinetics by UGT2B7. Drug Metab. Dispos. 31, 1086–1089. doi: 10.1124/dmd.31.9.1086
Sullivan, A. F., McQuay, H. J., Bailey, D., and Dickenson, A. H. (1989). The spinal antinociceptive actions of morphine metabolites morphine-6-glucuronide and normorphine in the rat. Brain Res. 482, 219–224. doi: 10.1016/0006-8993(89)91184-0
Suzuki, N., Kalso, E., and Rosenberg, P. H. (1993). Intrathecal morphine-3-glucuronide does not antagonize spinal antinociception by morphine or morphine-6-glucuronide in rats. Eur. J. Pharmacol. 249, 247–250. doi: 10.1016/0014-2999(93)90441-j
Swartjes, M., Mooren, R. A., Waxman, A. R., Arout, C., van de Wetering, K., den Hartigh, J., et al. (2012). Morphine induces hyperalgesia without involvement of mu-opioid receptor or morphine-3-glucuronide. Mol. Med. 18, 1320–1326. doi: 10.2119/molmed.2012.00244
Thomas, J. H. L., Lui, L., Abell, A., Tieu, W., Somogyi, A. A., Bajic, J. E., et al. (2022). Toll-like receptors change morphine-induced antinociception, tolerance and dependence: studies using male and female TLR and signalling gene KO mice. Brain Behav. Immun. 102, 71–85. doi: 10.1016/j.bbi.2022.02.001
Thomas, P. T., Bhargava, H. N., and House, R. V. (1995). Immunomodulatory effects of in vitro exposure to morphine and its metabolites. Pharmacology 50, 51–62. doi: 10.1159/000139266
Toce, M. S., Kim, H., Chung, S., and Krauss, B. S. (2019). Prolonged central apnoea after intravenous morphine administration in a 12-year-old male with a UGT1A1 loss-of-function polymorphism. Br. J. Clin. Pharmacol. 85, 258–262. doi: 10.1111/bcp.13779
Togna, A. R., Antonilli, L., Dovizio, M., Salemme, A., De Carolis, L., Togna, G. I., et al. (2013). In vitro morphine metabolism by rat microglia. Neuropharmacology 75, 391–398. doi: 10.1016/j.neuropharm.2013.08.019
Trescot, A. M., Helm, S., Hansen, H., Benyamin, R., Glaser, S. E., Adlaka, R., et al. (2008). Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician 11(2 Suppl), S5–S62.
Turk, D. C. (1996). Clinicians’ attitudes about prolonged use of opioids and the issue of patient heterogeneity. J. Pain Symptom Manag. 11, 218–230. doi: 10.1016/0885-3924(95)00188-3
Tzvetkov, M. V., dos Santos Pereira, J. N., Meineke, I., Saadatmand, A. R., Stingl, J. C., and Brockmoller, J. (2013). Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem. Pharmacol. 86, 666–678. doi: 10.1016/j.bcp.2013.06.019
van Crugten, J. T., Somogyi, A. A., Nation, R. L., and Reynolds, G. (1997). Concentration-effect relationships of morphine and morphine-6 beta-glucuronide in the rat. Clin. Exp. Pharmacol. Physiol. 24, 359–364. doi: 10.1111/j.1440-1681.1997.tb01202.x
Wang, H., Zhang, Y., Ma, X., Wang, W., Xu, X., Huang, M., et al. (2020). Spinal TLR4/P2X7 receptor-dependent NLRP3 inflammasome activation contributes to the development of tolerance to morphine-induced antinociception. J. Inflamm. Res. 13, 571–582. doi: 10.2147/JIR.S266995
Wang, K., Wang, J., Liu, T., Yu, W., Dong, N., Zhang, C., et al. (2021). Morphine-3-glucuronide upregulates PD-L1 expression via TLR4 and promotes the immune escape of non-small cell lung cancer. Cancer Biol. Med. 18, 155–171. doi: 10.20892/j.issn.2095-3941.2020.0442
Wang, X., Loram, L. C., Ramos, K., de Jesus, A. J., Thomas, J., Cheng, K., et al. (2012). Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. U.S.A. 109, 6325–6330. doi: 10.1073/pnas.1200130109
Webster, G. W., Shuster, L., and Eleftheriou, B. E. (1976). Morphine analgesia in mice of different ages. Exp. Aging Res. 2, 221–233. doi: 10.1080/03610737608257178
Weinsanto, I., Laux-Biehlmann, A., Mouheiche, J., Maduna, T., Delalande, F., Chavant, V., et al. (2018). Stable isotope-labelled morphine to study in vivo central and peripheral morphine glucuronidation and brain transport in tolerant mice. Br. J. Pharmacol. 175, 3844–3856. doi: 10.1111/bph.14454
Westerling, D., Persson, C., and Hoglund, P. (1995). Plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide after intravenous and oral administration to healthy volunteers: relationship to nonanalgesic actions. Ther. Drug Monit. 17, 287–301. doi: 10.1097/00007691-199506000-00013
Williams, J. T., Ingram, S. L., Henderson, G., Chavkin, C., von Zastrow, M., Schulz, S., et al. (2013). Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254. doi: 10.1124/pr.112.005942
Wolff, T., Samuelsson, H., and Hedner, T. (1995). Morphine and morphine metabolite concentrations in cerebrospinal fluid and plasma in cancer pain patients after slow-release oral morphine administration. Pain 62, 147–154. doi: 10.1016/0304-3959(94)00268-J
Wolff, T., Samuelsson, H., and Hedner, T. (1996). Concentrations of morphine and morphine metabolites in CSF and plasma during continuous subcutaneous morphine administration in cancer pain patients. Pain 68, 209–216. doi: 10.1016/s0304-3959(96)03102-8
Woolf, C. J. (1981). Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res. 209, 491–495. doi: 10.1016/0006-8993(81)90176-1
Wright, A. W., Mather, L. E., and Smith, M. T. (2001). Hydromorphone-3-glucuronide: a more potent neuro-excitant than its structural analogue, morphine-3-glucuronide. Life Sci. 69, 409–420. doi: 10.1016/s0024-3205(01)01133-x
Wybran, J., Appelboom, T., Famaey, J. P., and Govaerts, A. (1979). Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J. Immunol. 123, 1068–1070.
Xie, N., Gomes, F. P., Deora, V., Gregory, K., Vithanage, T., Nassar, Z. D., et al. (2017). Activation of mu-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain Behav. Immun. 61, 244–258. doi: 10.1016/j.bbi.2016.12.002
Xie, R., Bouw, M. R., and Hammarlund-Udenaes, M. (2000). Modelling of the blood-brain barrier transport of morphine-3-glucuronide studied using microdialysis in the rat: involvement of probenecid-sensitive transport. Br. J. Pharmacol. 131, 1784–1792. doi: 10.1038/sj.bjp.0703759
Yaksh, T. L., and Harty, G. J. (1988). Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J. Pharmacol. Exp. Ther. 244, 501–507.
Yaksh, T. L., Harty, G. J., and Onofrio, B. M. (1986). High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology 64, 590–597. doi: 10.1097/00000542-198605000-00008
Yang, Z. Z., Li, L., Wang, L., Xu, M. C., An, S., Jiang, C., et al. (2016). SiRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: a mechanism for acute morphine tolerance suppression. Sci. Rep. 6:33338. doi: 10.1038/srep33338
Yang, Z., Li, L., Hu, H., Xu, M., Gu, J., Wang, Z. J., et al. (2016a). Reverse of acute and chronic morphine tolerance by lithocholic acid via down-regulating UGT2B7. Front. Pharmacol. 7:404. doi: 10.3389/fphar.2016.00404
Yang, Z., Wang, L., Xu, M., Gu, J., Yu, L., and Zeng, S. (2016b). Simultaneous analysis of gemfibrozil, morphine, and its two active metabolites in different mouse brain structures using solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry with a deuterated internal standard. J. Sep. Sci. 39, 2087–2096. doi: 10.1002/jssc.201600088
Yeh, S. Y. (1975). Urinary excretion of morphine and its metabolites in morphine-dependent subjects. J. Pharmacol. Exp. Ther. 192, 201–210.
Yeh, S. Y., Gorodetzky, C. W., and Krebs, H. A. (1977). Isolation and identification of morphine 3- and 6-glucuronides, morphine 3,6-diglucuronide, morphine 3-ethereal sulfate, normorphine, and normorphine 6-glucuronide as morphine metabolites in humans. J. Pharm. Sci. 66, 1288–1293. doi: 10.1002/jps.2600660921
Zelcer, N., van de Wetering, K., Hillebrand, M., Sarton, E., Kuil, A., Wielinga, P. R., et al. (2005). Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc. Natl. Acad. Sci. U.S.A. 102, 7274–7279. doi: 10.1073/pnas.0502530102
Zhang, P., Yang, M., Chen, C., Liu, L., Wei, X., and Zeng, S. (2020). Toll-Like receptor 4 (TLR4)/Opioid receptor pathway crosstalk and impact on opioid analgesia. Immune function, and gastrointestinal motility. Front. Immunol. 11:1455. doi: 10.3389/fimmu.2020.01455
Keywords: morphine, metabolism, M3G, hyperalgesia, MOR – mu opioid receptor, TLR4 – toll-like receptor 4
Citation: Gabel F, Hovhannisyan V, Berkati A-K and Goumon Y (2022) Morphine-3-Glucuronide, Physiology and Behavior. Front. Mol. Neurosci. 15:882443. doi: 10.3389/fnmol.2022.882443
Received: 23 February 2022; Accepted: 26 April 2022;
Published: 12 May 2022.
Edited by:
Ana Reynders, UMR 7288 Institut de Biologie du Développement de Marseille (IBDM), FranceReviewed by:
Daniel Morgan, Marshall University, United StatesHeather Knych, University of California, Davis, United States
Copyright © 2022 Gabel, Hovhannisyan, Berkati and Goumon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yannick Goumon, eWdvdW1vbkB1bmlzdHJhLmZy
 Florian Gabel
Florian Gabel Volodya Hovhannisyan
Volodya Hovhannisyan Abdel-Karim Berkati
Abdel-Karim Berkati Yannick Goumon
Yannick Goumon