- 1The First Clinical Medical College of Gansu University of Chinese Medical, Lan Zhou, China
- 2Cerebrovascular Disease Center of Gansu Provincial People's Hospital, Lan Zhou, China
- 3Key Laboratory of Cerebrovascular Diseases in Gansu Province, Lan Zhou, China
- 4Day Treatment Center II of Gansu Provincial Maternity and Child-Care Hospital, Lan Zhou, China
- 5The First School of Clinical Medicine of Lanzhou University, Lan Zhou, China
Respirable fine particulate matter (PM2.5) has been one of the most widely publicized indicators of pollution in recent years. Epidemiological studies have established a strong association between PM2.5, lung disease, and cardiovascular disease. Recent studies have shown that PM2.5 is also strongly associated with brain damage, mainly cerebrovascular damage (stroke) and neurological damage to the brain (changes in cognitive function, dementia, psychiatric disorders, etc.). PM2.5 can pass through the lung–gas–blood barrier and the “gut–microbial–brain” axis to cause systemic oxidative stress and inflammation, or directly enter brain tissue via the olfactory nerve, eventually damaging the cerebral blood vessels and brain nerves. It is worth mentioning that there is a time window for PM2.5-induced brain damage to repair itself. However, the exact pathophysiological mechanisms of brain injury and brain repair are not yet fully understood. This article collects and discusses the mechanisms of PM2.5-induced brain injury and self-repair after injury, which may provide new ideas for the prevention and treatment of cerebrovascular and cerebral neurological diseases.
Introduction
In recent years, with the expansion of urban scale and the increasingly serious air pollution, more and more attention has been paid to the impact of PM2.5 on human health. According to the Global Burden of Disease Study in 2017, about 4.9 million people died from air pollution worldwide, and respirable fine particulate matter (PM2.5) accounted for about 60% of deaths (GBD 2017 Risk Factor Collaborators, 2018), making air pollution a global problem affecting human health. The polluted air is a mixture rich in harmful components, such as respirable fine particles, carbon monoxide, lead, nitrogen dioxide, ozone, and sulfur dioxide, of which respirable fine particles (PM2.5) are one of the most concerning environmental pollution indicators. PM2.5 is particulate matter with a kinetic equivalent diameter of less than or equal to 2.5 microns in the atmosphere. It is rich in a large amount of toxic and harmful substances and has the characteristics of long residence time in the atmosphere and long transportation distance. It has a significant influence on human health and air quality (Brook et al., 2010). The main sources of PM2.5 are natural and anthropogenic sources, with anthropogenic sources accounting for the major part (Kim et al., 2015). Numerous studies have shown that PM2.5 can cause not only respiratory diseases (allergic airway inflammation, asthma, and chronic obstructive pulmonary disease) (Choi et al., 2018; Weinmayr et al., 2018), but also diseases outside the respiratory system, such as cardiovascular disease, diabetes, childhood obesity, and cancer (Desikan, 2017; Mao et al., 2017; Maji et al., 2018; Wu et al., 2021).
PM2.5 enters the lung tissue at the end of the respiratory tract through the central airway after being filtered by nasal hairs, and the accumulated PM2.5 stimulates oxidative stress and inflammation in the lung, and a large amount of inflammatory factors enter the blood through the air–blood barrier and cause systemic inflammation and brain tissue damage (Costa et al., 2017). In addition, PM2.5 can also directly cross the olfactory nerve and cause damage to the blood–brain barrier (Oberdörster et al., 2004), or PM2.5 can cause dysbiosis of the intestinal flora and cause brain damage through the “gut–brain axis” (Shou et al., 2019). The changes in the brain after PM2.5 exposure mainly lie in the damage to cerebral blood vessels, the damage of cranial nerves, and the formation of brain tumors. The cerebrovascular damage is mainly in terms of stroke, while the neurological damage is mainly in terms of altered cognitive function, dementia, and psychiatric disorders (Kioumourtzoglou et al., 2016; Qiu et al., 2017). PM2.5 has been demonstrated to be a potentially important variable risk factor for stroke, especially in low- and middle-income countries (Feigin et al., 2016). The psychiatric and neurological symptoms caused by neurological damage to the brain often lead to disability, reduce the number of years of survival, largely affect the quality of life and workforce, and increase the burden on families. PM2.5-induced neurological brain injury has been extensively studied in recent years. Kioumourtzoglou et al. (2016) conducted a large-scale, multi-site epidemiological study in the northeastern U.S. and found that cities with long-term PM2.5 exposure increased the risk of Alzheimer's disease and Parkinson's disease in local residents. In addition, PM2.5 exposure may also increase the risk of brain tissue atrophy and aging and lead to damage to brain white matter. Although this study has various limitations, such as region and ethnicity, it still provides a basis for further exploration of large epidemiological studies. PM2.5 is widely present in the air, and a comprehensive understanding of the relationship between PM2.5 and brain injury may provide new ideas for the prevention and treatment of brain injury, and a review of the relationship between PM2.5 and brain injury and the pathogenesis is necessary to discuss.
PM2.5 exposure-induced brain outcomes
Cerebrovascular damage
Currently, the research on cerebral vascular damage induced by PM2.5 is mainly manifested in vascular endothelial cell dysfunction, intracranial atherosclerosis, and stroke (ischemic stroke and hemorrhagic stroke). Long et al. studied the effect of PM2.5 on vascular endothelial function in ApoE-/- mice exposed to PM2.5 many times. The results showed that NO is the initial factor of oxidative stress induced by PM2.5 exposure, and oxidative stress leads to inflammation and vascular dysfunction (Long et al., 2020). In addition, Hu et al. (2021) found through in vivo and in vitro experiments that PM2.5 exposure leads to a significant relationship between NLRP3 inflammasome activation and vascular endothelial dysfunction. Vascular endothelial dysfunction is the initial pathological change of atherosclerosis, and PM2.5 aggravating intracranial atherosclerosis has been confirmed in animal experiments. A study by Guan et al. found that exposure of adult Sprague-Dawley rats to PM2.5 for 12 weeks significantly aggravated intracranial atherosclerosis (Guan et al., 2017). O'Donnell et al. (2011) through the analysis of 9,202 hospitalized patients with acute ischemic stroke, it was found that the correlation between PM2.5 and the risk of ischemic stroke varied with different causes of stroke, and the correlation between stroke caused by large artery atherosclerosis was the strongest. A meta-analysis of 20 relevant studies by Scheers et al. (2015) found that for each 5-ug/m3 increase in PM2.5 concentration in Europe and North America, the hazard ratios for incident stroke and stroke mortality were 1.064 (1.021–1.109) and 1.125 (1.007 −1.256). A study by Leiva et al. (2013) found an association between PM2.5 exposure and stroke admissions, with each 10-ug/m3 increase in PM2.5 concentration associated with a 1.29% increase in the risk of emergency admission for cerebrovascular disease. Zhang R. et al. (2018) found that elevated PM2.5 levels were associated with increased ischemic and hemorrhagic stroke mortality [0.23% (95% CI, 0.04–0.42%), 0.37% (95% CI, 0.07–0.67%)], whereas elevated PM10 levels were only associated with ischemic stroke mortality. Yang et al. (2021) further demonstrated that PM2.5 should be considered an additional risk factor for ischemic stroke by conducting a meta-analysis of the literature on the relationship between PM2.5 and ischemic stroke in Taiwan, China, and that short-, medium-, and long-term exposure to ambient PM2.5 increases the risk of ischemic stroke. Compared with ischemic stroke, the findings of PM2.5 associated with hemorrhagic stroke are inconsistent across countries. Most studies in Western countries have shown that PM2.5 is significantly associated with ischemic stroke, but not with hemorrhagic stroke; while studies in Asian countries have shown an association between PM2.5 and hemorrhagic stroke. For example, a Japanese study also reported a stronger effect of PM pollution on hemorrhagic stroke [1.041 (95% confidence interval: 1.011–1.072)] than on ischemic stroke (Yorifuji et al., 2011), while studies in the United States and European countries have shown that PM pollution is associated with an ischemic stroke rather than hemorrhagic stroke (Wellenius et al., 2015). In conclusion, PM2.5 is closely related to cerebrovascular disease, and controlling air pollution may be a way to reduce cerebrovascular morbidity and mortality.
Brain nerve damage
The damage of PM2.5-induced cranial nerves is mainly manifested in neurological and psychiatric diseases caused by neurotoxic effects, such as Alzheimer's disease, Parkinson's disease, cognitive impairment, depression, anxiety, and autism. There are currently studies in animal experiments and clinical trials. Ku et al. (2017) conducted a study using the Morris water maze to test the spatial learning and memory ability of C57BL/6 mice after inhaling PM2.5 for 4 weeks. The results showed that the spatial learning and memory ability of mice decreased significantly after inhaling PM2.5. A study by Nephew et al. (2020) found that long-term exposure to traffic-related PM in rats and their offspring during pregnancy and lactation reduces social behavior, increases anxiety, impairs cognition, reduces levels of inflammation and growth factors (associated with behavioral changes), and undermines the neurointegrity of young male offspring. This further proves that PM is closely related to mental disorders. There is also experimental evidence that PM2.5 exposure can lead to early Alzheimer's disease (AD)-related pathology in transgenic AD mice, leading to AD-related molecular and cellular changes, such as mitochondrial dysfunction, synaptic defects, axonal growth impairment, nerve cell death, glial cell activation, neuroinflammation and neurovascular dysfunction, and increased levels of amyloid β (Aβ) and tau phosphorylation. These changes are the basis of cognitive impairment associated with AD (Wang L. et al., 2020). A 2016 study published in Environmental HealthPerspectives showed that long-term exposure to PM2.5 increases the risk of dementia, Alzheimer's disease, and Parkinson's disease in humans (Kioumourtzoglou et al., 2016). A large body of epidemiological evidence suggests that PM2.5 exposure in the elderly may lead to memory impairment (Fonken et al., 2011), and exposure to high levels of PM2.5 is strongly associated with increased risk of neuropathic dysfunction, cognitive decline, and accelerated brain aging (Power et al., 2011; Wellenius et al., 2015), and studies by Elder et al. (2006) have demonstrated that PM2.5 is a major cause of learning and memory impairment. Sunyer et al. (2015) recruited 2,715 children aged 7–10 years in 39 schools in Barcelona in a prospective study, which showed that children attending schools with high traffic pollution showed less cognitive improvement, which further demonstrates the toxic effect of PM2.5 in terms of brain neurological damage. The damage to the brain nerves seriously affects the quality of life of the organism, and since the number of neurodegenerative diseases is increasing year by year in recent years with the increase of global aging, it is necessary to carry out an in-depth study of PM2.5 as one of the risk molecules that induce brain nerve damage.
Brain tumor formation
Epidemiological evidence on the relationship between PM2.5 and brain tumors is scarce, but based on PM2.5-induced neuroinflammation and oxidative stress, this may also contribute to brain tumor formation. Jørgensen et al. (2016) conducted a study on the relationship between long-term exposure to air pollution and the risk of brain tumors and found that the number of brain tumors was weakly positively correlated with PM2.5, NO2, and NOx. Meningeal tumors were more strongly associated with PM2.5 and NO2 than tumors located in the brain, and benign tumors were more strongly associated with malignant tumors. Weichenthal et al. (2020) conducted a cohort study on the spatial differences of environmental ultrafine particulate matter in 1.9 million Canadian adults and found that every 10,000/cm3 increase in the environmental ultrafine particulate matter was positively correlated with the incidence of brain tumors. Ultrafine particulate matter in the environment may be a risk factor for the development of brain tumors in adults. However, McKean-Cowdin et al. (2009) failed to find an association between brain tumor mortality (n = 1,284) and residential exposure to PM2.5 and PM10 or NO2 in a large US cancer prevention study cohort. Whether there is a correlation between PM2.5 and brain tumor formation is still controversial, and more research is needed to discover the link between PM2.5 and brain tumors in the future.
Underlying mechanisms of brain injury
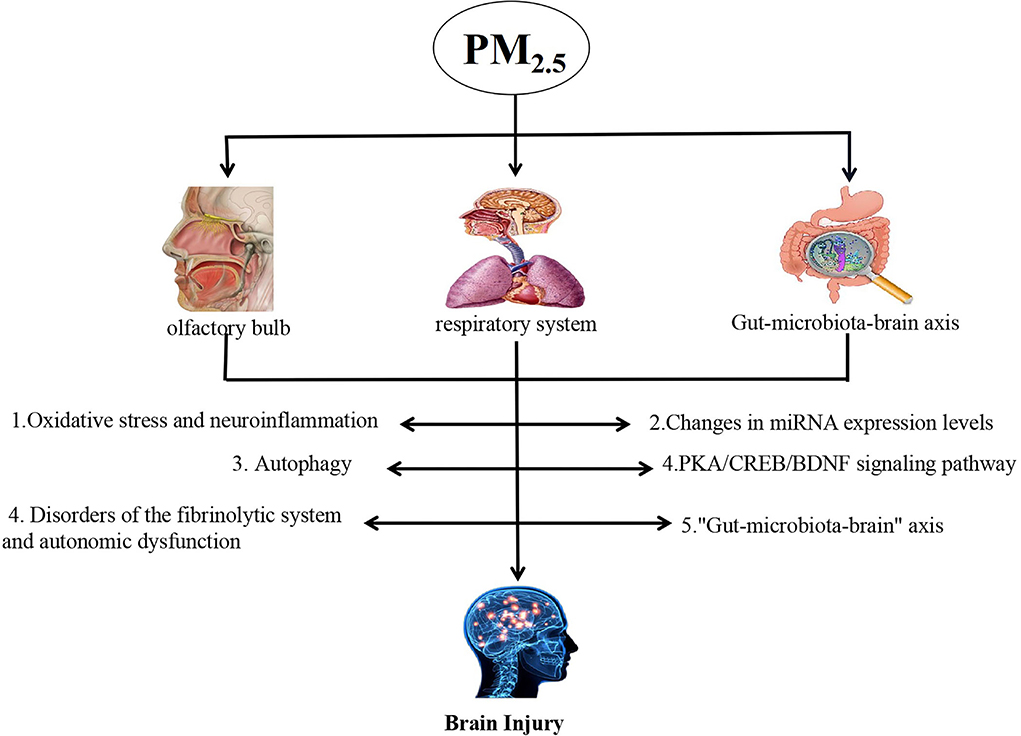
PM2.5 can cause brain damage in a variety of ways, but the specific mechanism of inducing brain damage has not been fully clarified so far. Now, the existing research mechanism is discussed (shown in Figure 1).
Oxidative stress and neuroinflammation
ROS-mediated MAPK signaling pathway PM2.5-induced oxidative stress is considered to be the key molecular mechanism of PM2.5-mediated toxicity. Oxidative stress is caused by an imbalance between reactive oxygen species (ROS) production and antioxidant mechanisms. Exposure of cells to ROS or ROS-generating systems can activate various cellular signaling pathways (Eckers and Klotz, 2009). MAPKs are a group of protein serine/threonine kinases that are activated in response to various extracellular stimuli and mediate signal transduction from the cell surface to the nucleus, including c-Jun NH2-terminal kinase (JNK), extracellular signal regulation Kinase (ERK), and p38 MAPK (Davis, 2000). ROS can lead to the continuous activation of p38 MAPK through various pathways, such as activation of MAPK kinase and inhibition of MAPK phosphatase (Talwar et al., 2017). Rui et al. (2016) found that PM2.5 induced phosphorylation of N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and protein kinase B (AKT) of Jun by exposing human umbilical vein cell line EA.hy926 to PM2.5, and activated nuclear factor kappa B (NF-κB). ROS may act as a signal molecule to trigger the expression of ICAM-1 and VCAM-1 by activating the ERK/AKT/NF-κB-dependent pathway, and further promoting the adhesion of monocytes to endothelial cells. PM2.5 exposure can also induce oxidative stress through activation of the MAPK/AP-1 cascade, leading to upregulation of angiotensin II type 1 receptor (AT1R) and vascular endothelial cell dysfunction (Xu et al., 2019).
PI3K/AKT/FoxO1 pathway PI3K/Akt is an important signaling pathway related to oxidative stress, and the upregulation of this pathway will lead to an increase in the production of reactive oxygen species in the body (Morimoto et al., 2013). FoxO1 is a member of the FoxO family, which is an important transcription factor that can regulate various physiological and pathological processes, such as oxidative stress, inflammatory response, energy metabolism, and apoptosis. However, AKT can inactivate FoxO1 phosphorylation, rendering its regulatory role ineffective. Song et al. (2021) found that long-term exposure to PM2.5 in rats increased the mRNA expression of PI3K and AKT in brain tissue, and decreased the mRNA expression of FoxO1. Brain injury induced by PM2.5 may be related to the activation of the PI3K/AKT/FoxO1 pathway.
Inflammatory genes can be activated under the drive of oxidative stress, producing a large number of inflammatory mediators (tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-17, and IL-10) involved in the systemic chronic inflammation reaction. Studies have found that PM2.5 can also activate the nuclear factor-κB signaling pathway, and then induce the high expression of inflammatory mediators, such as IL-6, IL-8, IL-1β, and tumor necrosis factor-α, causing extensive pulmonary inflammatory lesions (He et al., 2017; Wang Y. et al., 2020). These inflammatory mediators become the external stimulus transduction signals required for the activation of the nuclear factor-κB pathway, which further activates the nuclear factor-κB signaling pathway, induces an inflammatory cascade, and aggravates neuroinflammation (Zhang Y. et al., 2018). PM2.5 exposure can also activates the NLRP3 inflammasome, induces increased production of IL-1β and IL-18, causes systemic and neuroinflammation, and leads to endothelial and target organ damage (Gao et al., 2022).
Changes in miRNA expression levels
MicroRNAs (miRNAs) are a group of non-coding RNA molecules of 18–25 nucleotides that are increasingly recognized as key regulators of proteins at the translational level (Bartel, 2009). It is also a potent regulator of nervous system development, function, and disease. Alterations in miRNA expression levels are closely associated with cognitive degeneration and neurodegenerative diseases, including AD (Pichler et al., 2017). A study (Chao et al., 2017) found that fetal mice exposed to PM2.5 increased the expression levels of miR-6315, miR-3588, and miR-466b-5p in the cerebral cortex. These genes were positively correlated with PKn2 (astrocyte migration), Gorab (neuritogenesis), and Mobp (which induces experimental allergic encephalomyelitis). PM2.5 also decreased the expression of miR-338-5p and let-7e-5p, both genes involved in intellectual development. In addition, PM2.5 exposure decreased the expression of MiR99b-5p, miR-92b-5p, and miR-99a-5p in the hippocampus and inhibited the learning and motor coordination of fetal mice. β-Site amyloid precursor protein (APP) cleaving enzyme 1 (β-secretase, BACE1) is a key enzyme that catalyzes the production of amyloid β (A β) peptides from APP. The activation of BACE1 is a sign of early cognitive impairment and plays an important role in the gradual transformation into AD. Previous studies (Ku et al., 2017) have found that PM2.5 inhalation impairs synaptic and cognitive function mediated by NF-κB p65-induced downregulation of miR-574-5p against BACE1. Ma et al. (2022) found that the high expression of MicroRNA-29b (miR-29b) may regulate apoptosis and oxidative stress, which is an important factor leading to neuronal injury after cerebral ischemia.
Autophagy
Autophagy is a general term for the degradation of intracellular substances through lysosomes after cells are stimulated. Moderate autophagy in the early stage of the body has a protective effect, while over-activated autophagy will aggravate the damage to the body's function. In the rat model of focal cerebral ischemia (MCAO), early autophagy has neuroprotective effects, while overactivated autophagy aggravates neurological deficits (Gao et al., 2012). Wang et al. (2021) found in rotenone-treated PC12 cells in vivo and in vitro studies on PM2.5 and neurodegenerative diseases, exposure to PM2.5 can reduce the LC3II/LC3I ratio and the expression level of Atg5, and increase the mammalian target of rapamycin (mTOR) expression levels, suggesting that PM2.5 exposure inhibited autophagy. Autophagy in the substantia nigra of Parkinson's mice following PM2.5 inhalation in vitro changes consistent with cellular models. However, Ren et al. (2021) found an increase in the level of Lc3 and a decrease in the level of p62 in brain tissue after PM2.5 exposure in evaluating the effect of PM2.5 on brain injury in mice, indicating that PM2.5 exposure increased the level of autophagy. Moreover, after exposure to PM2.5, the level of AMPK increased and the level of MTOR decreased, suggesting that PM2.5 may induce autophagy by activating the AMPK/mTOR pathway.
PKA/CREB/BDNF signaling pathway
Protein kinase A (PKA) is an important regulator of various cellular processes. As the major upstream kinase of cAMP response element binding protein (CREB), PKA can phosphorylate CREB, thereby promoting the recruitment of transcription factor components to its promoter, including the brain-derived neurotrophic factor (BDNF) gene. PKA can promote the transcription of BDNF and expression, thereby exerting neuroprotective effects (Leal et al., 2017). The PKA/CREB/BDNF signaling pathway is responsible for promoting neuronal survival, regulating synaptic morphology, and enhancing synaptic transmission efficiency (Zhong et al., 2018). Liu et al. (2019) found that neonatal rats' early exposure to PM2.5 can lead to synaptic damage and emotional and cognitive impairment, possibly through the CREB/BDNF signaling pathway. Liu et al. (2021) found upregulation by PM2.5 in cultured hippocampal neurons (DIV3) or downregulation of the PKA/CREB/BDNF signaling pathway can alleviate or aggravate PM2.5-induced neuronal damage to varying degrees, further demonstrating that PKA/CREB/BDNF pathway may play an important role in PM2.5-mediated neurodevelopmental toxicity.
Disorders of the fibrinolytic system and autonomic dysfunction
PM2.5 can lead to changes in coagulation and fibrinolytic system homeostasis. PM2.5 induces overexpression of tissue factor (TF), which activates the receptor and cofactor for coagulation factor VIIa (F VIIa), and the formation of the TF/FVIIa complex initiates the exogenous coagulation pathway and promotes blood hypercoagulation, which is thought to be a key pathway for thrombin production in vivo. Moreover, the inflammatory reaction mediated by PM2.5 can change the balance between tissue plasminogen activator and plasminogen activator inhibitor-1, resulting in the imbalance of fibrinolysis (Rückerl et al., 2014), which greatly increases the risk of thrombosis.
PM2.5 induces autonomic dysfunction, and this impairment appears to be attributable to stimulation of the HPA axis (hypothalamic–pituitary–adrenal axis) following hypothalamic inflammation induced by PM2.5 exposure in this setting. The activated central nervous system amplifies or modulates systemic cardiometabolic responses by stimulating the hypothalamic-pituitary-adrenal (HPA) axis, manifested as corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and corticotropin Increased secretion of alcohol (Li et al., 2017). Ultimately, it disrupts cardiovascular and cerebrovascular homeostasis, damages vascular function, affects hemodynamics, and increases blood pressure.
“Gut–microbiota–brain” axis
The gut microbiota is the normal microbiota present in the gut. It is closely linked to human metabolism, gut homeostasis, immune development (Lynch and Pedersen, 2016), and brain development processes and behavior (Mayer et al., 2015; Morais et al., 2021). A stable and diverse intestinal flora is optimal for maintaining health. Alterations or dysbiosis of the gut microbiota can trigger a variety of diseases, such as inflammatory bowel disease (Fasano, 2020), celiac disease (Odenwald and Turner, 2017), metabolic syndrome (Fan and Pedersen, 2021), diabetes (Sorini et al., 2019), colon cancer (Fidelle et al., 2020), autism, anxiety, depression, and neurodegenerative diseases (Rutsch et al., 2020; Zhu et al., 2020). The link between gut flora and the physiological functions of the brain is described as the “gut–microbe–brain” axis (Lombardi et al., 2018). It has been shown that disruption or interruption of any of the communication pathways between these microbes and the brain may trigger an inflammatory response in the organism. Pathogenic microbes release metabolites and molecules that trigger cytokines in the host and cause inflammation in the central nervous system, greatly contributing to the development and progression of brain disease (Zhu et al., 2020). PM2.5 can enter the gastrointestinal tract either directly or indirectly. Some studies have shown that exposure to PM through inhalation can alter the composition of the gut microbiota across the gastrointestinal tract, increasing the permeability of the intestinal barrier and increasing the chances of pathogens, such as bacteria, crossing the intestinal mucosa and entering the circulatory system (Mutlu et al., 2018). PM2.5 exposure can induce the secretion of inflammatory cytokines from intestinal epithelial cells through the damaged intestinal barrier from the peripheral system to the central nervous system, leading to neuroinflammation. Recent evidence has demonstrated a relationship between microbiome changes and the pathophysiology of AD (Köhler et al., 2016) and an association between microbial populations and the development of Alzheimer's disease has been found (Vogt et al., 2017).
Self-repair after brain injury
Self-repair after PM2.5-induced brain injury is also an important issue of concern. Previous studies have found that after continuous exposure of C57BL/6 male mice to high levels of PM2.5 for 4, 8, and 12 weeks, the mice exhibited an injury–repair–imbalance response that ultimately led to depressive behavior and upregulation of pro-inflammatory cytokines (Liu et al., 2018). Ji et al. (2019), in a study of the effects and subsequent recovery at the end of PM2.5 exposure, found that inflammatory cytokines and chemokines were persistently elevated in mice after 4 weeks of PM2.5 exposure, but these effects all returned to normal levels within 2 weeks after cessation of exposure. A study by Ren et al. (2020) also confirmed that PM2.5-induced damage to the organism could repair itself after cessation of exposure, but their further study found that this type of repair could not be considered a true repair. This is because significant changes were observed between groups when re-exposed to the same dose of PM2.5, which instead increased the susceptibility of target organs. Song et al. (2021) also found in a study on the mechanisms of PM2.5-induced brain damage that there may be antioxidant, anti-inflammatory, fibrinolytic, and other partial physiological processes in the organism during 2–4 months of PM2.5 exposure in rats, but this finding needs to be further investigated. Therefore, it is necessary to evaluate PM2.5-induced injury with increasing exposure time, which can help to identify early biological indicators and clarify potential time points of repair or deterioration in the injury process, which will provide important information in the prevention of PM2.5-induced brain injury in the future.
Conclusion
Nowadays, as the problem of air pollution around the world intensifies, exposure to PM2.5 in air pollution has also become a problem that threatens human health and safety. The results of animal experiments and epidemiological experiments at home and abroad have shown that exposure to PM2.5 can cause serious respiratory and cardiovascular diseases, as well as damage to the nervous system in many ways. The existing damage mechanisms mainly include oxidative stress and neuroinflammatory pathways, changes in miRNA expression levels, autophagy, PKA/CREB/BDNF signaling pathways, and disorders of the HPA axis. The “gut-microbe-brain” axis is also a research hotspot in recent years. The damage after exposure to PM2.5 is not a single mechanism, it is a chain reaction. The body's self-repair after PM2.5-induced brain injury is also worthy of further study. Identifying potential repair or deterioration time points in the process of injury and early prevention and control can greatly reduce the degree of brain damage. However, due to the complexity and diversity of PM components in the environment and the unclear toxicity, it brings challenges to the research of PM2.5-induced brain injury. Further clarification of the induced brain injury and the unresearched mechanism is our future efforts.
Author contributions
WL participated in the design of the study, collected and analyzed the literature, and drafted and revised the manuscript. GL, ZX, YZha, BL, YZho, and YM analyzed the literature. EC designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely appreciate the support of primary literature data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell. 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Brook, R. D., Rajagopalan, S., Pope, C. A. III., Brook, J. R., Bhatnagar, A., Diez-Roux, A. V., et al. (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 121, 2331–2378. doi: 10.1161/CIR.0b013e3181dbece1
Chao, M. W., Yang, C. H., Lin, P. T., Yang, Y. H., Chuang, Y. C., Chung, M. C., et al. (2017). Exposure to PM2.5 causes genetic changes in fetal rat cerebral cortex and hippocampus. Environ. Toxicol. 32, 1412–1425. doi: 10.1002/tox.22335
Choi, J., Oh, J. Y., Lee, Y. S., Min, K. H., Hur, G. Y., Lee, S. Y., et al. (2018). Harmful impact of air pollution on severe acute exacerbation of chronic obstructive pulmonary disease: particulate matter is hazardous. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 1053–1059. doi: 10.2147/COPD.S156617
Costa, L. G., Cole, T. B., Coburn, J., Chang, Y. C., Dao, K., and Roqué, P. J. (2017). Neurotoxicity of traffic-related air pollution. Neurotoxicology. 59, 133–139. doi: 10.1016/j.neuro.2015.11.008
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell. 103, 239–252. doi: 10.1016/S0092-8674(00)00116-1
Desikan, A. (2017). Outdoor air pollution as a possible modifiable risk factor to reduce mortality in post-stroke population. Neural. Regen. Res. 12, 351–353. doi: 10.4103/1673-5374.202917
Eckers, A., and Klotz, L. O. (2009). Heavy metal ion-induced insulin-mimetic signaling. Redox. Rep. 14, 141–6. doi: 10.1179/135100009X392610
Elder, A., Gelein, R., Silva, V., Feikert, T., Opanashuk, L., Carter, J., et al. (2006). Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 114, 1172–8. doi: 10.1289/ehp.9030
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fasano, A. (2020). All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 9, F1000 Faculty Rev-69. doi: 10.12688/f1000research.20510.1
Feigin, V. L., Roth, G. A., Naghavi, M., Parmar, P., Krishnamurthi, R., Chugh, S., et al. (2016). Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 15, 913–924. doi: 10.1016/S1474-4422(16)30073-4
Fidelle, M., Yonekura, S., Picard, M., Cogdill, A., Hollebecque, A., Roberti, M. P., et al. (2020). Resolving the paradox of colon cancer through the integration of genetics, immunology, and the microbiota. Front. Immunol. 11, 600886. doi: 10.3389/fimmu.2020.600886
Fonken, L. K., Xu, X., Weil, Z. M., Chen, G., Sun, Q., Rajagopalan, S., et al. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry. 16, 987–95, 973. doi: 10.1038/mp.2011.76
Gao, L., Jiang, T., Guo, J., Liu, Y., Cui, G., Gu, L., et al. (2012). Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS ONE. 7, e46092. doi: 10.1371/journal.pone.0046092
Gao, L., Qin, J. X., Shi, J. Q., Jiang, T., Wang, F., Xie, C., et al. (2022). Fine particulate matter exposure aggravates ischemic injury via NLRP3 inflammasome activation and pyroptosis. CNS Neurosci. Ther. 28, 1045–1058. doi: 10.1111/cns.13837
GBD 2017 Risk Factor Collaborators (2018). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392, 1923–1994. doi: 10.1016/S0140-6736(18)32225-6
Guan, L., Geng, X., Shen, J., Yip, J., Li, F., Du, H., et al. (2017). PM2.5 inhalation induces intracranial atherosclerosis which may be ameliorated by omega 3 fatty acids. Oncotarget. 9, 3765–3778. doi: 10.18632/oncotarget.23347
He, M., Ichinose, T., Yoshida, S., Ito, T., He, C., Yoshida, Y., et al. (2017). PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J Appl. Toxicol. 37, 1203–1218. doi: 10.1002/jat.3482
Hu, T., Zhu, P., Liu, Y., Zhu, H., Geng, J., Wang, B., et al. (2021). PM2.5 induces endothelial dysfunction via activating NLRP3 inflammasome. Environ. Toxicol. 36, 1886–1893. doi: 10.1002/tox.23309
Ji, X., Yue, H., Ku, T., Zhang, Y., Yun, Y., Li, G., et al. (2019). Histone modification in the lung injury and recovery of mice in response to PM2.5 exposure. Chemosphere. 220, 127–136. doi: 10.1016/j.chemosphere.2018.12.079
Jørgensen, J. T., Johansen, M. S., Ravnskjær, L., Andersen, K. K., Bräuner, E. V., Loft, S., et al. (2016). Long-term exposure to ambient air pollution and incidence of brain tumours: The Danish Nurse Cohort. Neurotoxicology. 55, 122–130. doi: 10.1016/j.neuro.2016.06.003
Kim, K. H., Kabir, E., and Kabir, S. (2015). A review on the human health impact of airborne particulate matter. Environ Int. 74, 136–143. doi: 10.1016/j.envint.2014.10.005
Kioumourtzoglou, M. A., Schwartz, J. D., Weisskopf, M. G., Melly, S. J., Wang, Y., Dominici, F., et al. (2016). Long-term PM2.5 Exposure and Neurological Hospital Admissions in the Northeastern United States. Environ. Health Perspect. 124, 23–29. doi: 10.1289/ehp.1408973
Köhler, C. A., Maes, M., Slyepchenko, A., Berk, M., Solmi, M., Lanctôt, K. L., et al. (2016). The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in alzheimer's disease. Curr. Pharm. Des. 22, 6152–6166. doi: 10.2174/1381612822666160907093807
Ku, T., Li, B., Gao, R., Zhang, Y., Yan, W., Ji, X., et al. (2017). NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol. 14, 34. doi: 10.1186/s12989-017-0215-3
Leal, G., Bramham, C. R., and Duarte, C. B. (2017). BDNF and Hippocampal Synaptic Plasticity. Vitam Horm. 104, 153–195. doi: 10.1016/bs.vh.2016.10.004
Leiva, G. M. A., Santibañez, D. A., Ibarra, E. S., Matus, C. P., and Seguel, R. A. (2013). five-year study of particulate matter (PM2.5) and cerebrovascular diseases. Environ. Pollut. 181, 1–6. doi: 10.1016/j.envpol.2013.05.057
Li, H., Cai, J., Chen, R., Zhao, Z., Ying, Z., Wang, L., et al. (2017). Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 136, 618–627. doi: 10.1161/CIRCULATIONAHA.116.026796
Liu, J., Liu, B., Yuan, P., Cheng, L., Sun, H., Gui, J., et al. (2021). Role of PKA/CREB/BDNF signaling in PM2.5-induced neurodevelopmental damage to the hippocampal neurons of rats. Ecotoxicol. Environ. Saf. 214, 112005. doi: 10.1016/j.ecoenv.2021.112005
Liu, J., Yang, C., Yang, J., Song, X., Han, W., Xie, M., et al. (2019). Effects of early postnatal exposure to fine particulate matter on emotional and cognitive development and structural synaptic plasticity in immature and mature rats. Brain Behav. 9, e01453. doi: 10.1002/brb3.1453
Liu, X., Qian, X., Xing, J., Wang, J., Sun, Y., Wang, Q., et al. (2018). Particulate matter triggers depressive- like response associated with modulation of inflammatory cytokine homeostasis and brain- derived neurotrophic factor signaling pathway in mice. Toxicol. Sci. 164, 278–288. doi: 10.1093/toxsci/kfy086
Lombardi, V. C., De Meirleir, K. L., Subramanian, K., Nourani, S. M., Dagda, R. K., Delaney, S. L., et al. (2018). Nutritional modulation of the intestinal microbiota; future opportunities for the prevention andtreatment of neuroimmune and neuroinflammatory disease. J. Nutr. Biochem. 61, 1–16. doi: 10.1016/j.jnutbio.2018.04.004
Long, M. H., Zhu, X. M., Wang, Q., Chen, Y., Gan, X. D., Li, F., et al. (2020). PM2.5 exposure induces vascular dysfunction via NO generated by iNOS in lung of ApoE-/- mouse. Int. J. Biol. Sci. 16, 49–60. doi: 10.7150/ijbs.36073
Lynch, S. V., and Pedersen, O. (2016). The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379. doi: 10.1056/NEJMra1600266
Ma, Y. H., Deng, W. J., Luo, Z. Y., Jing, J., Pan, P. W., Yao, Y. B., et al. (2022). Inhibition of microRNA-29b suppresses oxidative stress and reduces apoptosis in ischemic stroke. Neural Regen. Res. (2022) 17, 433–439. doi: 10.4103/1673-5374.314319
Maji, S., Ghosh, S., and Ahmed, S. (2018). Association of air quality with respiratory and cardiovascular morbidity rate in Delhi India. Int. J. Environ. Health Res. 28, 471–490. doi: 10.1080/09603123.2018.1487045
Mao, G., Nachman, R. M., Sun, Q., Zhang, X., Koehler, K., Chen, Z., et al. (2017). Individual and joint effects of early-life ambient exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ. Health Perspect. 125, 067005. doi: 10.1289/EHP261
Mayer, E. A., Tillisch, K., and Gupta, A. (2015). Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938. doi: 10.1172/JCI76304
McKean-Cowdin, R., Calle, E. E., Peters, J. M., Henley, J., Hannan, L., Thurston, G. D., et al. (2009). Ambient air pollution and brain cancer mortality. Cancer Causes Control. 20, 1645–51. doi: 10.1007/s10552-009-9412-1
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Morimoto, H., Iwata, K., Ogonuki, N., Inoue, K., Atsuo, O., Kanatsu-Shinohara, M., et al. (2013). ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 12, 774–786. doi: 10.1016/j.stem.2013.04.001
Mutlu, E. A., Comba, I. Y., Cho, T., Engen, P. A., Yazic,i, C., Soberanes, S., et al. (2018). Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 240, 817–830. doi: 10.1016/j.envpol.2018.04.130
Nephew, B. C., Nemeth, A., Hudda, N., Beamer, G., Mann, P., Petitto, J., et al. (2020). Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environ. Res. 183, 109242. doi: 10.1016/j.envres.2020.109242
Oberdörster, G., Sharp, Z., Atudorei, V., Elder, A., Gelein, R., Kreyling, W., et al. (2004). Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 16, 437–445. doi: 10.1080/08958370490439597
Odenwald, M. A., and Turner, J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21. doi: 10.1038/nrgastro.2016.169
O'Donnell, M. J., Fang, J., Mittleman, M. A., Kapral, M. K., and Wellenius, G. A. (2011). Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 22, 422–31. doi: 10.1097/EDE.0b013e3182126580
Pichler, S., Gu, W., Hartl, D., Gasparoni, G., Leidinger, P., Keller, A., et al. (2017). The miRNome of Alzheimer's disease: consistent downregulation of the miR-132/212 cluster. Neurobiol. Aging. 50, 167.e1–167.e10. doi: 10.1016/j.neurobiolaging.2016.09.019
Power, M. C., Weisskopf, M. G., Alexeeff, S. E., Coull, B. A., Spiro, A. I. I. I., and Schwartz, J. (2011). Traffic-related air pollution and cognitive function in a cohort of older men. Environ. Health Perspect. 119, 682–7. doi: 10.1289/ehp.1002767
Qiu, H., Sun, S., Tsang, H., Wong, C. M., Lee, R. S., Schooling, C. M., et al. (2017). Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology. 88, 1709–1717. doi: 10.1212/WNL.0000000000003903
Ren, F., Xu, X., Xu, J., Mei, Y., Zhang, J., Wang, X., et al. (2021). Compound essential oils relieve oxidative stress caused by PM2.5 exposure by inhibiting autophagy through the AMPK/mTOR pathway. Environ. Toxicol. 36, 1765–1774. doi: 10.1002/tox.23297
Ren, H., Lu, J., Ning, J., Su, X., Tong, Y., Chen, J., et al. (2020). Exposure to fine particulate matter induces selfrecovery and susceptibility of oxidative stress and inflammation in rat lungs. Environ. Sci. Pollut. Res. Int. 27, 40262–40276. doi: 10.1007/s11356-020-10029-2
Rückerl, R., Hampel, R., Breitner, S., Cyrys, J., Kraus, U., et al. (2014). Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ. Int. 70, 32–49. doi: 10.1016/j.envint.2014.05.013
Rui, W., Guan, L., Zhang, F., Zhang, W., and Ding, W. (2016). PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J. Appl. Toxicol. 36, 48–59. doi: 10.1002/jat.3143
Rutsch, A., Kantsj,ö, J. B., and Ronchi, F. (2020). The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11, 604179. doi: 10.3389/fimmu.2020.604179
Scheers, H., Jacobs, L., Casas, L., Nemery, B., and Nawrot, T. S. (2015). Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke. 46, 3058–3066. doi: 10.1161/STROKEAHA.115.009913
Shou, Y., Huang, Y., Zhu, X., Liu, C., Hu, Y., and Wang, H. (2019). A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer's disease. Ecotoxicol. Environ. Saf. 174, 344–352. doi: 10.1016/j.ecoenv.2019.02.086
Song, L., Pan, K., Du, X., Jiang, S., Zeng, X., Zhang, J., et al. (2021). Ambient PM2.5-induced brain injury is associated with the activation of PI3K/AKT/FoxO1 pathway. Environ. Sci. Pollut. Res. Int. 28, 68276–68287. doi: 10.1007/s11356-021-15405-0
Sorini, C., Cosorich, I., Lo Conte, M., De Giorgi, L., Facciotti, F., Lucian,ò, R., et al. (2019). Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 116, 15140–15149. doi: 10.1073/pnas.1814558116
Sunyer, J., Esnaola, M., Alvarez-Pedrerol, M., Forns, J., Rivas, I., López-Vicente, M., et al. (2015). Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 12, e1001792. doi: 10.1371/journal.pmed.1001792
Talwar, H., Bauerfeld, C., Bouhamdan, M., Farshi, P., Liu, Y., Samavati, L., et al. (2017). MKP-1 negatively regulates LPS-mediated IL-1β production through p38 activation and HIF-1α expression. Cell Signal. 34, 1–10. doi: 10.1016/j.cellsig.2017.02.018
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer's disease. Sci. Rep. 7, 13537. doi: 10.1038/s41598-017-13601-y
Wang, L., Wei, L. Y., Ding, R., Feng, Y., Li, D., Li, C., et al. (2020). Predisposition to alzheimer's and age-related brain pathologies by PM2.5 exposure: perspective on the roles of oxidative stress and TRPM2 channel. Front. Physiol. 11, 155. doi: 10.3389/fphys.2020.00155
Wang, Y., Li, C., Zhang, X., Kang, X., Li, Y., Zhang, W., et al. (2021). Exposure to PM2.5 aggravates Parkinson's disease via inhibition of autophagy and mitophagy pathway. Toxicology. 30, 456. doi: 10.1016/j.tox.2021.152770
Wang, Y., Li, D., Song, L., and Ding, H. (2020). Ophiopogonin, D., attenuates PM2.5-induced inflammation via suppressing the AMPK/NF-κB pathway in mouse pulmonary epithelial cells. Exp. Ther. Med. 20, 139. doi: 10.3892/etm.2020.9268
Weichenthal, S., Olaniyan, T., Christidis, T., Lavigne, E., Hatzopoulou, M., Van Ryswyk, K., et al. (2020). Within-city spatial variations in ambient ultrafine particle concentrations and incident brain tumors in adults. Epidemiology. 31, 177–183. doi: 10.1097/EDE.0000000000001137
Weinmayr, G., Pedersen, M., Stafoggia, M., Andersen, Z. J., Galassi, C., Munkenast, J., et al. (2018). Particulate matter air pollution components and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts of Air Pollution Effects. (ESCAPE). Environ. Int. 120, 163–171. doi: 10.1016/j.envint.2018.07.030
Wellenius, G. A., Koutrakis, P., and Wang, Y. (2015). Ambient air pollution and depressive symptoms in older adults: Wellenius et al. respond. Environ. Health Perspect. 123, A114–A115. doi: 10.1289/ehp.1409657R
Wu, X., Zhu, B., Zhou, J., Bi, Y., Xu, S., and Zhou, B. (2021). The epidemiological trends in the burden of lung cancer attributable to PM2.5 exposure in China. BMC Public Health. 21, 737. doi: 10.1186/s12889-021-10765-1
Xu, X., Xu, H., Qimuge, A., Liu, S., Wang, H., Hu, M., et al. (2019). MAPK/AP-1 pathway activation mediates AT1R upregulation and vascular endothelial cells dysfunction under PM2.5 exposure. Ecotoxicol. Environ. Saf. 170, 188–194. doi: 10.1016/j.ecoenv.2018.11.124
Yang, C. P., Li, C. Y., Huang, W. J., Yu, H. L., Yang, C. C., Lu, M. C., et al. (2021). Short-, mid-, and long-term associations between PM2.5 and stroke incidence in Taiwan. J. Occup. Environ. Med. 63, 742–751. doi: 10.1097/JOM.0000000000002222
Yorifuji, T., Kawachi, I., Sakamoto, T., and Doi, H. (2011). Associations of outdoor air pollution with hemorrhagic stroke mortality. J. Occup. Environ. Med. 53, 124–6. doi: 10.1097/JOM.0b013e3182099175
Zhang, R., Liu, G., Jiang, Y., Li, G., Pan, Y., Wang, Y., et al. (2018). Acute effects of particulate air pollution on ischemic stroke and hemorrhagic stroke mortality. Front. Neurol. 9, 827. doi: 10.3389/fneur.2018.00827
Zhang, Y., Wang, S., Zhu, J., Li, C., Zhang, T., Liu, H., et al. (2018). Effect of atmospheric PM2.5 on expression levels of NF-κB genes and inflammatory cytokines regulated by NF-κB in human macrophage. Inflammation. 41, 784–794. doi: 10.1007/s10753-018-0732-8
Zhong, Y., Chen, J., Li, L., Qin, Y., Wei, Y., Pan, S., et al. (2018). PKA-CREB-BDNF signaling pathway mediates propofol-induced long-term learning and memory impairment in hippocampus of rats. Brain Res. 15, 64–74. doi: 10.1016/j.brainres.2018.04.022
Keywords: respirable fine particulate matter (PM2.5), brain injury, mechanism, review, self-repair of brain injury
Citation: Li W, Lin G, Xiao Z, Zhang Y, Li B, Zhou Y, Ma Y and Chai E (2022) A review of respirable fine particulate matter (PM2.5)-induced brain damage. Front. Mol. Neurosci. 15:967174. doi: 10.3389/fnmol.2022.967174
Received: 14 June 2022; Accepted: 09 August 2022;
Published: 07 September 2022.
Edited by:
Daniele Dell'Orco, University of Verona, ItalyReviewed by:
Chenju Yi, Seventh Affiliated Hospital, Sun Yat-sen University, ChinaNyzil Massey, Iowa State University, United States
Copyright © 2022 Li, Lin, Xiao, Zhang, Li, Zhou, Ma and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erqing Chai, MjQzODkwMjEwNkBxcS5jb20=
 Wei Li
Wei Li Guohui Lin4
Guohui Lin4 Yichuan Zhang
Yichuan Zhang