- 1Department of Pharmacognosy, Faculty of Pharmacy, Minia University, Minia, Egypt
- 2Department of Pharmacognosy, Faculty of Pharmacy, Deraya University, New Minia, Egypt
- 3Department of Pharmacognosy, Faculty of Pharmacy, Nahda University, Beni-Suef, Egypt
- 4Department of Pharmacognosy, Faculty of Pharmacy, Almaaqal University, Basra, Iraq
- 5Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt
Natural products (NPs) are significant for drug development. They appear in consistently low amounts in natural medicines (Sturm and Seger, 2012). Now, the advancement of adequate and selective techniques for the extraction and isolation of those bioactive NPs is vital (Rosén et al., 2009). Despite serious increases in extraction-separation procedures, the isolation of NPs from marine creatures, microorganisms, or plants is still a challenging task (Rosén et al., 2009). Hybrid techniques like LC-MS or LC-NMR meant on-line structure elucidation remained possible and showed impressive illustrations of NPs’ recognition without early isolation. However, in many examples, the requirement to show the isolated NPs is still needed (Sturm and Seger, 2012). Detailed chemical designs involving stereochemistry of recent NPs often require separated, highly purified, isolated compounds (Sturm and Seger, 2012). Furthermore, assessing biological activity in vitro and/or in vivo must be determined after purification to remove conflict with turbulent compounds (Sturm and Seger, 2012). Writing rules for the quality management of medicinal herbal plants principally hinges on compound separation with significant purity (Sturm and Seger, 2012). The classic isolation system of NPs involves recognition, selection, and formation of the biological equipment. Descent with different solvents from limited to stronger polarity.
Bioassay-guided isolation schemes associating data on the chemical surveys of crude extracts and portions with their enterprise data for in vitro bioassays observed in micro-scale automatically shortened the duration for target detection (Molyneux et al., 2007). Fundamentally, it has proven to be a distinct step to pick up from a natural source to an intense isolate. However, some crucial steps must be recognized, such as correct natural source recognition, awareness of conversions during preparation and/or extraction, or previously recognized compounds’ de-replication at the fractionation scheme basic stage (Molyneux et al., 2007).
Most research intending to isolate substantial amounts of pure isolated compounds still employs the wide use of liquid chromatographic techniques as MPLC, VLC, and HPLC (van Beek et al., 2009). Taking advantage of enhanced separation efficiencies allows the involvement of slight particle diameter and distinct selectivity. Solid-phase extraction, basically set up as a purification technique before GC or HPLC analysis, is accepted as a technique for rapid fractionation of raw extracts or for trapping pure isolates that are eluted after HPLC separation brought to capillary NMR test for de novo design elucidation (van Beek et al., 2009). The main aim of this article is to summarize the various extraction and isolation techniques of NPs. It chiefly focused on the analytical procedures that incorporate the extraction methods and isolation of NPs between conventional and modern techniques.
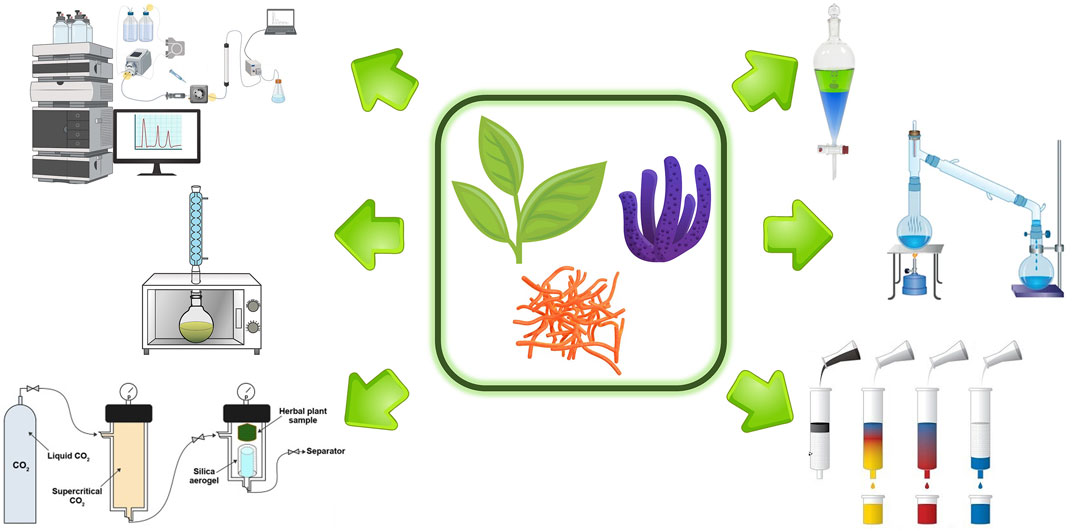
Figure 1 summarizes the different isolation methods that are currently applied to natural products during the drug discovery pipeline.
Extraction Methods
Extracting NPs from their natural source involves many issues. These issues include the stability and polarity of the solvent and extractives, the volatility, toxicity, purity, and viscosity of the extractive solvent (Maltese et al., 2009), artefact development possibility during the process of extraction, and the bulk material output to be extracted (Maltese et al., 2009).
Classical Solvent Extraction
Most isolation operations still employ basic extraction programs with water, organic solvents of various polarities, and their mixes (Bucar et al., 2013). These processes involve infusion, decoction, maceration, percolation, Soxhlet, and reflux extraction. Infusion is achieved by suspending the material into solvent over a short period. In decoction, the material is boiled to dissolve its chemicals. Consequently, this is suitable only for extracting water-soluble and heat-stable constituents. Maceration is brought out at the chamber with heat (also known as digestion), or without it in a closed container by immersing the material in the extracted solvent with or without consequent stirring (Bucar et al., 2013). It benefits under moderate descending circumstances, but suffers from excessive solvent usage, lengthy extraction phases, and poor extraction crops. Extraction output is enhanced by percolation, in which the pre-wet material is loaded in a package which provides the regularly adjusted elimination of the excerpt via a pipe at the basement and continuous fresh solvent from top; percolation suffers from excessive solvent utilization and tedious extraction stages. Another attractive extraction method is using Soxhlet apparatus, as it diminishes solvent utilization. It effectively recycles a limited volume of solvent to dissolve a greater extent of material. Reflux is an extraction process carried out at a stable heat with repeatable solvent evaporation and condensation for a particular time without the loss of solvent. Soxhlet and reflux are efficient, simple to handle, and cost effective. However, Thermo-labile isolates are impaired during the eradication process using heat (Bucar et al., 2013).
Ultrasound Assisted Extraction
In the UAE, the material is generally put in a glass vessel, soaked in the extraction solvent, and placed in an ultrasonic bath. It reduces the time of extraction and promotes the yields owing to automatic stress, which leads to cavitation with cellular disruption (Bucar et al., 2013). UAE can also be used with mixtures of immiscible solvents, such as hexane with methanol/water. But the process creates heat, so Thermo-labile isolates are impaired during the eradication process (Bucar et al., 2013).
Microwave Assisted Extraction
Today, extraction using either diffused microwaves in sealed techniques or focused microwaves in open procedures are accepted techniques. MAE has been modified into various forms, such as nitrogen-protected microwave-assisted extraction (NPMAE), vacuum microwave-assisted extraction (VMAE), dynamic microwave-assisted extraction (DMAE), or ultrasonic microwave-assisted extraction (UMAE) (Bucar et al., 2013).
Ionic Liquids Assisted Extraction
In recent periods, utilization of ILs for MAE, UAE, or basic extraction has been receiving increased attention. ILs are organic salts which, when in the liquid state, interact with an organic/inorganic anion and an organic cation. ILs can dissolve a large area of polar/non-polar NPs, have a low vapor pressure, exhibit a great thermal establishment, and have low combustibility. Another aspect of ILs that is still inadequately studied is their biodegradability and their impact on the habitat if applied at industry; both require further study (Bucar et al., 2013).
Accelerated Solvent Extraction
In correlation to most alternative extraction procedures which require an added step to split the standing non-dissolved matter from the liquid excerpt, on-line filtration through the automated descent process of ASE is introduced. The technique is used with solid/semisolid samples (1–100 g scale), and can handle solvents at raised heat and pressure (Bucar et al., 2013).
Supercritical Fluid Extraction
Changing organic extraction solvents with technologies for extraction-which less harmful to the environment—can be viewed as an encouraging force to increase demand of supercritical fluid extraction. An outline of the procedure, with extraction contracts and operations in NPs’ extraction, is given by Nahar and Sarker 2012 (Nahar and Sarker, 2012). Application of organic solvent modifiers in SFE extends its solvating efficiencies to medium-polar, and polar NPs, and expands the scope of NPs classes available to SFE. Using this technique, ecological complications related to organic solvent descents are reduced to a limited extent (Bucar et al., 2013).
Solid Phase Extraction
Extraction, which can adsorp analyte or undesirable contaminations in a solid phase, achieved a prominent character in NPs extracts purification, owing to its assimilation into computerized specimen formation and isolation processes. Most applications employ SPE, which uses a broad field of stationary phases with differing chemistry as reversed-phase material, silica gel, ion-exchange resins, and HILIC stationary phases in pre-loaded plastic or glass columns.
Distillation
Volatiles, as essential oils, have till now been gained through distillation procedures, although working at raised temperatures can result in chemical transformations. New improvements in distillation methodology have resulted in microwave steam distillation, which uses microwaves to raise the interruption of glands and cells at the same time for steam crossing through the plant cell material and obtaining the essential oil (Bucar et al., 2013).
Isolation of Natural Products Using Different Chromatography Techniques
A broad range of chromatographic methods is applicable for better fractionation and finished NPs’ purification. The choice generally revolves around the purity stage of the extract or fraction, and the decisive purpose of the isolated NPs.
Preparative Planar Chromatography
Due to its low cost and easy usage in isolation of NPs, PPC has been an often-employed procedure, although the number of applications is less compared to column chromatography (Marston, 2011). An interesting aspect of PPC is the broad field of chemical disclosure techniques characteristic for NPs, which can be achieved on a limited area of the plate, leaving most compounds consistent and convenient for isolation. To reduce the uncontrolled flow rates for the classical TLC mobile phase, forced-flow procedures such as over-pressured layer chromatography or centrifugal planar chromatography have been established, permitting elution and on-line disclosure of compounds (Marston, 2011).
Column Chromatographic
Vacuum liquid chromatography (VLC) (Reid and Sarker, 2012), flash chromatography (FC) (Reid and Sarker, 2012), hydrophilic interaction chromatography (HILIC) (Reid and Sarker, 2012), low-pressure liquid chromatography (LPLC) (Reid and Sarker, 2012), medium-pressure liquid chromatography (MPLC) (Cheng et al., 2010), and high-performance (high-pressure) liquid chromatography (HPLC) (Alpert, 1990) are used.
Chiral Chromatographic
After chiral compounds’ isolation, generally a process to detect absolute configuration is required. For enantio-separation at an analytical scale, GC, HPLC, SFC, or CE have universally been applied. However, HPLC is used most frequently. This separation procedure provides insulating enantiomers directly with chiral stationary or mobile-phase, or indirectly with chiral derivatization agents’ additives (Dalgliesh, 1952).
Preparative Gas Chromatography
PGC is an interesting choice for the isolation of volatile oils. Mostly, loaded columns with greater sample capacity but lower peak resolution are established. However, there have been an expanding number of outstanding operations of thick-phase film full-bore capillaries with capillary GC apparatus during the last years (Zuo et al., 2012).
Conclusion
Recently, several significant improvements have been made in the NPs isolation field. A rising number of techniques have been built up by chromatographic hyphenation and spectroscopic means with the objective of elucidating structures of the known compounds without the need for isolation. MAE and UAE strategies are accepted as systems that increase extraction potency and reduce the descent stage. ILs are a further impending field in the natural products field and could improve the interest compounds. SPE broadened its utilization towards fractionation procedures like VLC. But one extremely interesting outcome in SPE is the selective isolation of objective compounds by molecularly engraved stationary phases. Chiral separations are also applied at preparations scale, taking the chiral character of many NPs into interest. HILIC is currently experiencing a significant expansion in applications for NPs isolation, producing an added mechanism of separation related to normal and reversed-phase chromatography. Although pure compounds’ isolation from raw matrices is still challenging and we are far from one-step isolation methods, the analysis of extremely selective processes of extraction to fractionation and purification will improve the collection of a final purified compound.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. URA: conceptualization; AMS and AHE Writing the original draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alpert, A. J. (1990). Hydrophilic-interaction Chromatography for the Separation of Peptides, Nucleic Acids and Other Polar Compounds. J. Chromatogr. A 499, 177–196. doi:10.1016/s0021-9673(00)96972-3
Bucar, F., Wube, A., and Schmid, M. (2013). Natural Product Isolation - How to Get from Biological Material to Pure Compounds. Nat. Prod. Rep. 30 (4), 525–545. doi:10.1039/C3NP20106F
Cheng, Y., Liang, Q., Hu, P., Wang, Y., Jun, F. W., and Luo, G. (2010). Combination of Normal-phase Medium-Pressure Liquid Chromatography and High-Performance Counter-current Chromatography for Preparation of Ginsenoside-Ro from Panax Ginseng with High Recovery and Efficiency. Sep. Purif. Technol. 73 (3), 397–402. doi:10.1016/j.seppur.2010.04.029
Dalgliesh, C. (1952). The Optical Resolution of Aromatic Amino-Acids on Paper Chromatograms. J. Chem. Soc. (Resumed) 756, 3940–3942.
Maltese, F., van der Kooy, F., and Verpoorte, R. (2009). Solvent Derived Artifacts in Natural Products Chemistry. Nat. Prod. Commun. 4 (3), 447–454. doi:10.1177/1934578x0900400326
Marston, A. (2011). Thin-layer Chromatography with Biological Detection in Phytochemistry. J. Chromatogr. A 1218 (19), 2676–2683. doi:10.1016/j.chroma.2010.12.068
Molyneux, R. J., Mahoney, N., Kim, J. H., and Campbell, B. C. (2007). Bioassay-Directed Isolation and Identifi Cation of Antiafl Atoxigenic Constituents of Walnuts. J. Agric. Food Chem. 60, 435–452. doi:10.1201/9781420006889.ch16
Nahar, L., and Sarker, S. D. (2012). Supercritical Fluid Extraction in Natural Products Analyses. Methods Mol. Biol. 864, 43–74. doi:10.1007/978-1-61779-624-1_3
Reid, R. G., and Sarker, S. D. (2012). Isolation of Natural Products by Low-Pressure Column Chromatography. Methods Mol. Biol. 864, 155–187. doi:10.1007/978-1-61779-624-1_7
Rosén, J., Gottfries, J., Muresan, S., Backlund, A., and Oprea, T. I. (2009). Novel Chemical Space Exploration via Natural Products. J. Med. Chem. 52 (7), 1953–1962. doi:10.1021/jm801514w
Sturm, S., and Seger, C. (2012). Liquid Chromatography-Nuclear Magnetic Resonance Coupling as Alternative to Liquid Chromatography-Mass Spectrometry Hyphenations: Curious Option or Powerful and Complementary Routine Tool? J. Chromatogr. A 1259, 50–61. doi:10.1016/j.chroma.2012.05.032
van Beek, T. A., Koleva,, , Dapkevicius, I. I., Exarchou, A., Jeurissen, V., Suzanee, M., et al. (2009). Recent Developments in the Rapid Analysis of Plants and Tracking Their Bioactive Constituents. Phytochem. Rev. 8 (2), 387–399. doi:10.1007/s11101-009-9125-9
Keywords: natural products, extraction, isolation, methods, purification
Citation: Abdelmohsen UR, Sayed AM and Elmaidomy AH (2022) Natural Products’ Extraction and Isolation-Between Conventional and Modern Techniques. Front. Nat. Prod. 1:873808. doi: 10.3389/fntpr.2022.873808
Received: 11 February 2022; Accepted: 26 April 2022;
Published: 19 May 2022.
Edited by:
Tsong-Long Hwang, Chang Gung University, TaiwanReviewed by:
Mohamed El-Shazly, Ain Shams University, EgyptCopyright © 2022 Abdelmohsen, Sayed and Elmaidomy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Usama Ramadan Abdelmohsen, dXNhbWEucmFtYWRhbkBtdS5lZHUuZWc=
 Usama Ramadan Abdelmohsen
Usama Ramadan Abdelmohsen Ahmed M. Sayed
Ahmed M. Sayed Abeer H. Elmaidomy
Abeer H. Elmaidomy