- 1Department of Neurology, King Abdulaziz Medical City, Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia
- 2King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 3College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
Background: Giant cell arteritis (GCA) is a granulomatous vasculitis in older individuals that primarily affects medium-to-large vessels. Owing to the involvement of the ophthalmic arteries, GCA can lead to severe ischemic complications, including vision loss. Optic perineuritis (OPN) is characterized by inflammation around the optic nerve sheath and is a rare manifestation of GCA with diagnostic and therapeutic challenges.
Case presentation: This case study reports a 75-year-old female presenting with subacute constitutional symptoms of fever and poor appetite with bilateral eye pain and visual disturbance. The patient had elevated inflammatory markers, specifically an erythrocyte sedimentation rate of 120 mm/h, with imaging findings consistent with bilateral OPN and temporal artery biopsy-proven GCA. Treatment with high-dose dexamethasone, followed by oral prednisolone and tocilizumab, led to symptomatic improvement in vision stabilization.
Conclusion: This case highlights the importance of recognizing OPN as a possible manifestation of GCA. Although cases of OPN are mostly idiopathic, it can rarely represent the first manifestation of GCA. Therefore, prompt diagnosis through brain imaging and temporal artery biopsy is essential, and aggressive treatment with steroids is crucial for managing GCA-associated OPN to prevent irreversible vision loss.
1 Introduction
Giant cell arteritis (GCA) is the predominant primary systemic vasculitis in adults, mostly affecting individuals aged 50 and older (1). In a notable proportion (25–50% of cases), GCA induces ocular manifestations through inflammatory constriction of the ophthalmic and short posterior ciliary arteries that supply blood to the optic nerve and nerve head (2). The most common cause is arteritic anterior ischemic optic neuropathy, which primarily arises from optic disc ischemia, followed by arteritic posterior ischemic optic neuropathy caused by retrobulbar injury (3, 4). Less frequent etiologies include central retinal and cilioretinal artery occlusion. However, visual impairments attributable to optic perineuritis (OPN) are exceptionally rare, with only a limited number of cases documented in the literature. The significance of this case lies in its presentation of isolated bilateral OPN in a 75-year-old woman with GCA and no disc edema, a manifestation seldom reported and often challenging to diagnose. This case contributes to the growing recognition of atypical ocular complications in GCA, underscoring the need for increased clinical awareness and early detection.
2 Case description
A 75-year-old female with a history of pulmonary hypertension, dyslipidemia, bronchiectasis, and grade II diastolic heart failure presented to the emergency department. She had a 3-week history of bilateral eye pain followed by blurred vision, a documented fever at home of 38 degrees, new onset persistent mild-to-moderate holocephalic headache, and poor appetite. The patient initially visited multiple hospitals and was diagnosed with glaucoma and sinusitis. The patient underwent minimally invasive laser surgery and was discharged on oral augmentin without any reportable improvement.
The patient did not have any other neurological symptoms, such as recurrent headache attacks, double vision, weight loss, painful eye movements, limb numbness or weakness, joint pain, or jaw claudication. Upon arrival at the emergency department, the patient had a low-grade fever of 38.2 degrees with normal blood pressure and heart rate. Her initial neurological examination was nonfocal, with no meningeal signs or encephalitis. Ophthalmological examination revealed bilateral early cataracts and a small optic disc on both sides with a cup-to-disc ratio of 0.3, normal color vision, and no evidence of a relative afferent pupillary defect. Her visual acuity was 20/60 on both sides, with no evidence of disc edema. Her macula was healthy, and the intraocular pressure was low; therefore, the anti-glaucoma drops were discontinued. Systemic examination was unremarkable, and the temporal artery was palpable on both sides, with no tenderness.
Initial laboratory tests showed leukocytosis of 13,000 cells, a hemoglobin level of 11 gram/dL, an erythrocyte sedimentation rate (ESR) of 120 mm/h, and a C-reactive protein (CRP) level of 141 mg/L (normal lab value <3). Serum electrolyte, liver, and renal function test results were normal. Non-contrast brain computed tomography (CT) did not reveal acute or chronic central nervous system insults or space-occupying lesions. Meningitis was suspected, considering the history of headache with fever, and a lumbar puncture was performed, which was unremarkable with normal cerebrospinal fluid protein, glucose, cell count, culture, and viral multiplex. The patient was started on empirical Piperacillin/Tazobactam (Tazocin®) and admitted for further investigation as a case of fever of unknown origin.
Her systemic workup was negative for brucellosis, tuberculosis, cytomegalovirus, Epstein–Barr virus, hepatitis sexology, human immunodeficiency virus, and malaria. No organisms were identified in blood, urine, or respiratory cultures. Moreover, vasculitis workup, including anti-nuclear antibodies, anti-double-stranded antibodies, Anti-Sjögren’s-syndrome-related antigen A (Ro) and Anti-Sjögren’s-syndrome-related antigen B (La), antineutrophilic cytoplasmic antibodies, complement levels, cyclic citrullinated peptide antibodies, and rheumatoid factor levels, were within normal ranges. Serum folate, vitamin B12, thyroid-stimulating hormone, and parathyroid hormone levels were normal. Chest and abdominal CT revealed no suspicious infectious or inflammatory processes.
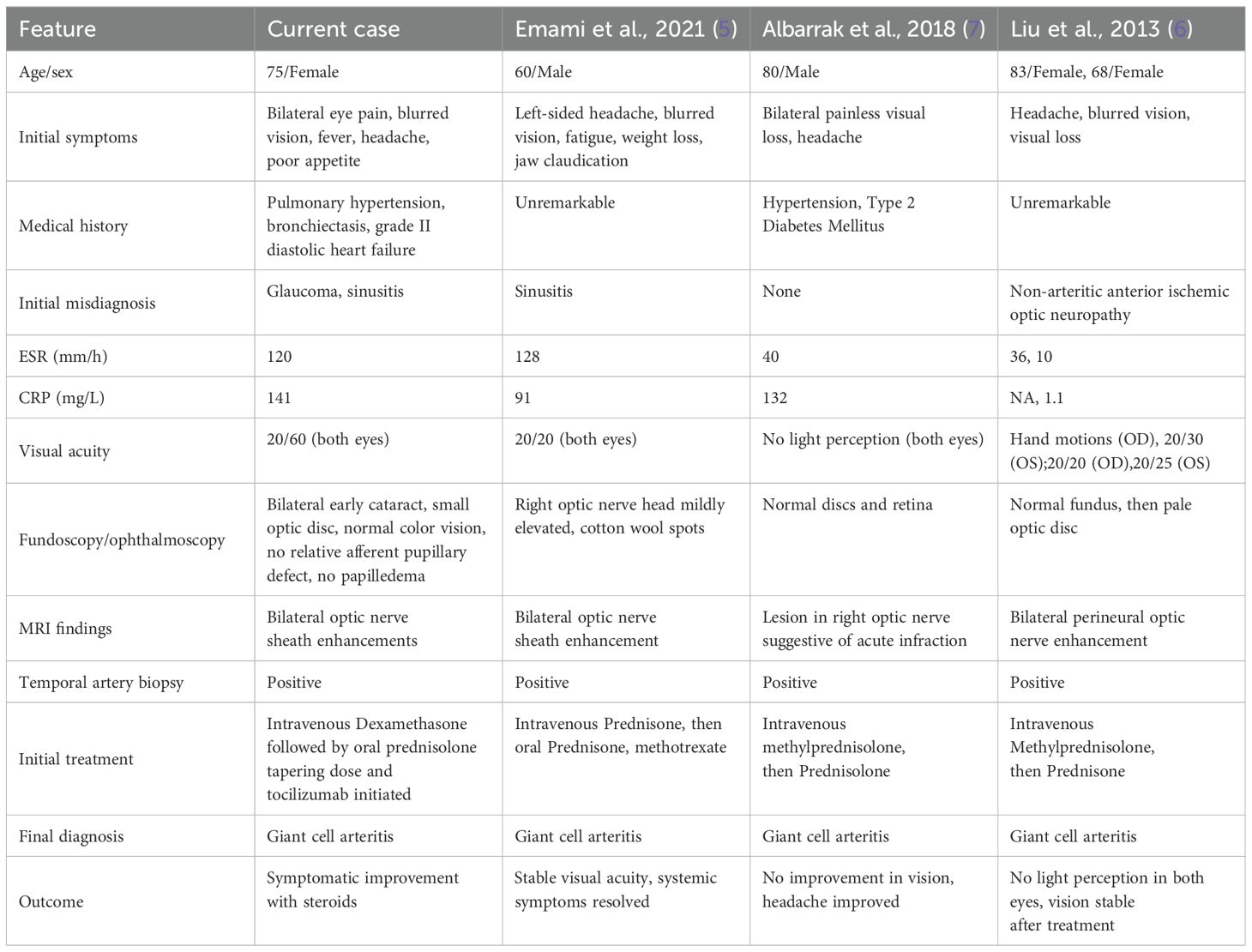
Brain and contrast-orbital magnetic resonance imaging (MRI) showed bilateral optic nerve sheath enhancements sparing the optic nerve extending to the chiasm and pituitary stalk without intracranial, meningeal, or parenchymal abnormalities (Figure 1). Findings of ophthalmic imaging studies are shown in Figures 2, 3. Serum aquaporin 4 and myelin oligodendroglial cell antibodies for neuromyelitis optica spectrum disorder, and myelin oligodendrocyte glycoprotein
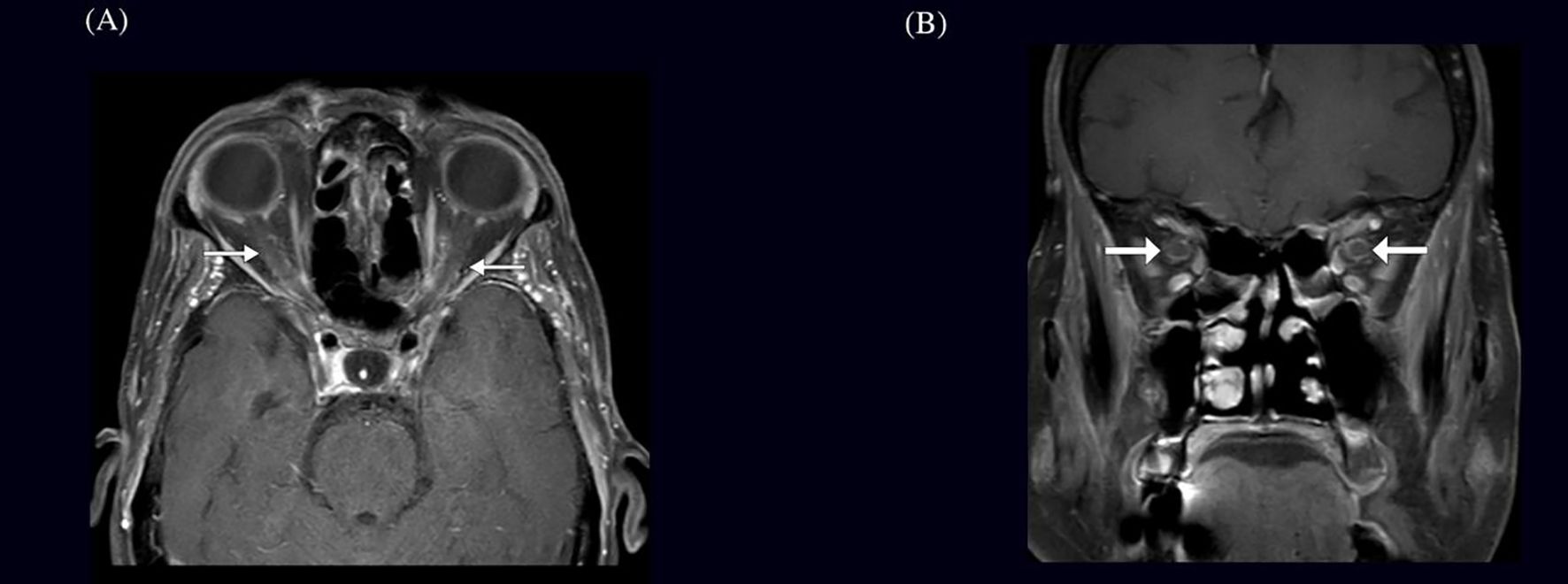
Figure 1. MRI findings in a patient with bilateral optic nerve perineuritis (A) Axial and (B) Coronal brain MRI showing post-contrast T1-weighted image with fat suppression demonstrating bilateral optic nerve sheath enhancement, characteristic of perineuritis.
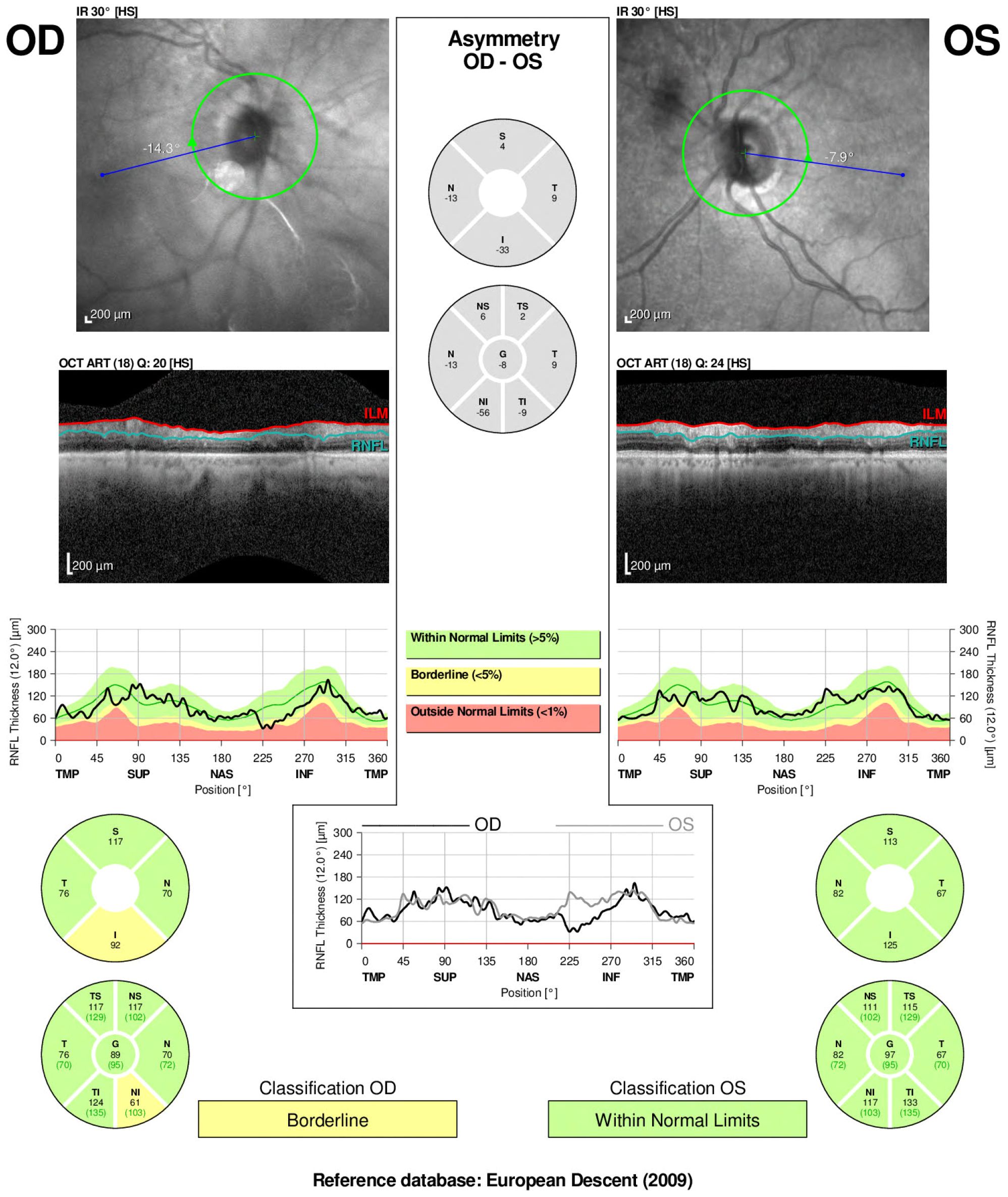
Figure 2. Optical coherence tomography (OCT) and retinal nerve fiber layer (RNFL) analysis of both eyes in a patient with optic perineuritis done 3 weeks after start on symptoms. Infrared imaging and cross-sectional OCT scans demonstrate optic disc appearance and RNFL thickness. The right eye (OD) shows borderline RNFL thinning, particularly in the nasal and inferior quadrants, whereas the left eye (OS) remains within normal limits.
Figure 3. Humphrey Visual Field (HVF) 30–2 threshold tests of both eyes The right eye (OD) shows a mean deviation (MD) of −14.85 dB and visual field index (VFI) of 59%, with dense superior and peripheral field loss, particularly nasally, consistent with optic nerve dysfunction. The left eye (OS) demonstrates a mean deviation of −11.01 dB and VFI of 83%, with similar, though less severe, nasal and arcuate defects.
were negative; therefore, GCA was suspected, and a right temporal artery biopsy was performed.
The patient was started on empirical intravenous dexamethasone 60 mg three times on the same day as the procedure, and the biopsy results were positive for temporal artery luminal narrowing with intimal thickening, internal elastic layer disruption, lymphoblastic histiocytic aggregates, and dystrophic calcification, consistent with GCA (Figure 4). Two days after steroid treatment initiation, the patient reported marked improvement in headache and eye pain.
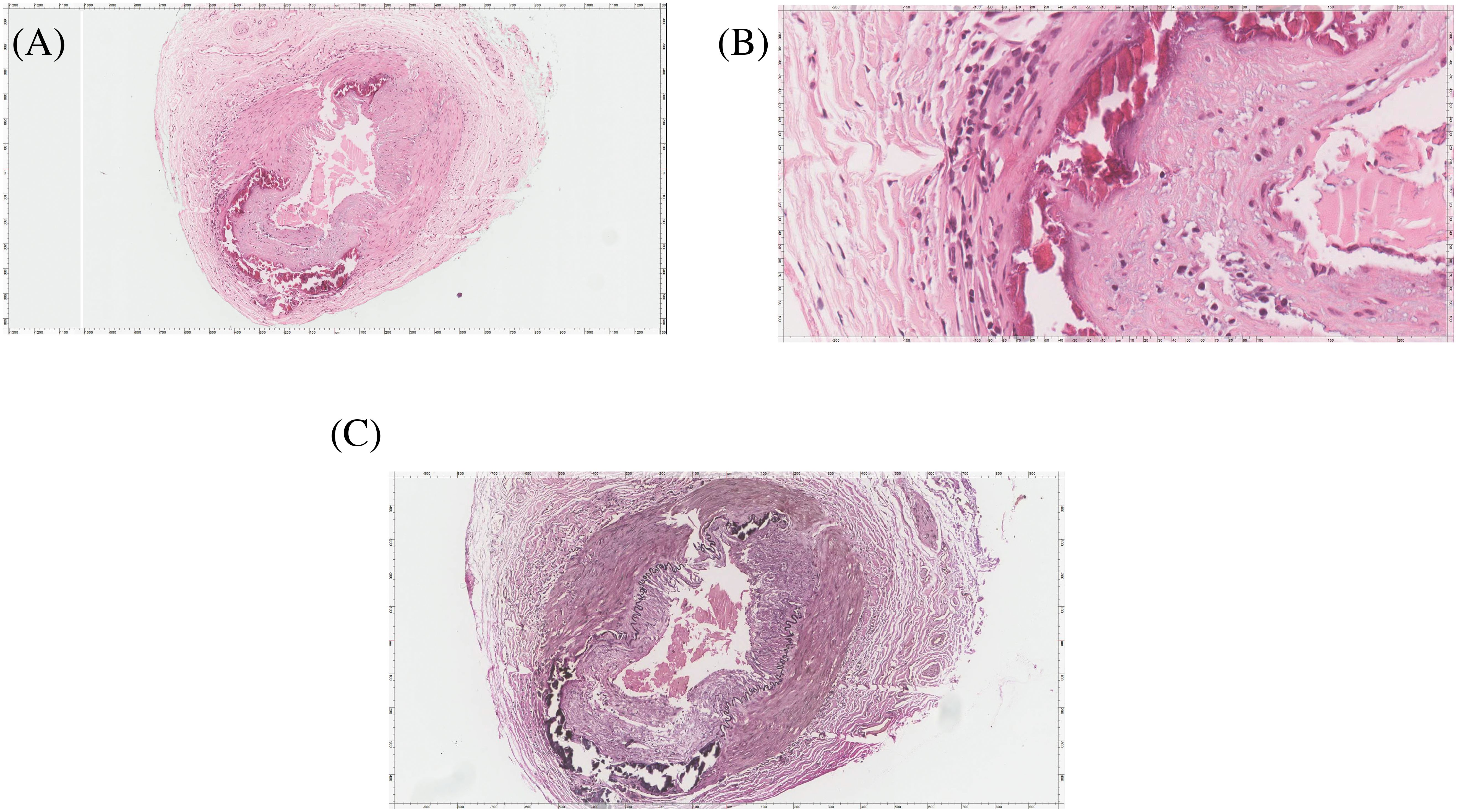
Figure 4. The temporal artery biopsy. (A) HE stained slide shows a muscular artery with lumen narrowing, intimal thickening, disruption of internal elastica, lymphoplasma histiocytic aggregates, and dystrophic calcification. (B) High magnification image highlighting the lymphoplasma histiocytic aggregates and dystrophic calcification. The disruption of internal elastica is best seen with the Elastic Verhoeff Van Gieson Stain in (C).
The patient was discharged on oral prednisolone 60 mg/day, with complete symptom resolution. Ten days after discharge, the patient was evaluated in a rheumatology clinic where weekly tocilizumab injections were started, and her prednisolone dose was tapered gradually with a plan to stay at 20 mg once daily. Two months later, the patient visited the follow-up clinic and was completely asymptomatic with a stable visual acuity of 20/40 in both eyes.
3 Discussion
Giant cell arteritis (GCA) is a systemic vasculitis of large and medium-sized vessels primarily affecting individuals over the age of 50. Its classic ophthalmologic manifestation, arteritic anterior ischemic optic neuropathy (AAION), is typically associated with disc edema due to ischemia of the optic nerve head. However, optic perineuritis (OPN) an inflammatory process confined to the optic nerve sheath is a rarely reported ocular manifestation of GCA, and even more uncommon when it presents bilaterally without disc edema.
Our patient represents a unique presentation of biopsy-proven GCA, manifesting as bilateral optic perineuritis without optic disc swelling or relative afferent pupillary defect. Magnetic resonance imaging confirmed optic nerve sheath enhancement sparing the optic nerve proper, consistent with OPN. This imaging profile, coupled with systemic inflammatory markers and constitutional symptoms, raised suspicion for GCA, which was subsequently confirmed histopathologically.
This case aligns with a small but growing body of literature documenting GCA-associated OPN (Table 1). Emami et al. described a 60-year-old African male with biopsy-proven GCA and bilateral optic nerve sheath enhancement. Although that patient demonstrated mild optic disc swelling and cotton wool spots, his visual acuity was preserved and systemic inflammatory markers were markedly elevated features shared with our case (5). Liu et al. also described a patient with GCA who exhibited bilateral optic nerve sheath enhancement on MRI without evidence of disc edema, underscoring the potential for GCA to present with isolated perineural inflammation (6)
Gold and Galetta documented a patient with sequential optic perineuritis followed by GCA diagnosis, suggesting that OPN may precede or even signal the onset of systemic vasculitis (8). Similarly, Pappolla et al. presented a case where bilateral OPN was the initial manifestation of GCA, emphasizing that perineural inflammation can occur without classic features such as disc edema or visual field defects (9).
The mechanism underlying OPN in GCA remains uncertain. One theory posits inflammation of the perineural pial vessels or vasa nervorum, in contrast to AAION where posterior ciliary artery occlusion is central. MRI findings in OPN typically reveal sheath enhancement with preserved axonal structure, as seen in our patient, and may reflect an early or isolated inflammatory process prior to ischemic damage (10).
This case shows the critical need for a structured diagnostic approach in elderly patients presenting with optic perineuritis, particularly when disc edema is absent. The presence of bilateral optic nerve sheath enhancement on MRI, systemic inflammatory markers, and constitutional symptoms should prompt strong consideration of GCA even in the absence of visual acuity loss or classic cranial features. Differential diagnoses such as infectious optic neuritis, sarcoidosis, neuromyelitis optica, and orbital inflammation must be excluded through targeted serological and radiologic evaluations. Temporal artery biopsy remains the gold standard, although high-resolution imaging modalities like black-blood MRI and vascular ultrasound have proven valuable in detecting inflammation in less accessible arteries like the occipital branch (11, 12). Management should be prompt and aggressive: intravenous corticosteroids followed by oral tapering, supplemented by tocilizumab or other steroid-sparing agents when indicated (11). Early recognition and intervention are essential to prevent irreversible visual sequelae and systemic complications.
OPN is a rare and unusual neuro-ophthalmic presentation of GCA. A high degree of suspicion can lead to timely diagnosis, prompt treatment and prevention of vision loss, as OPN typically responds well to corticosteroid therapy, as evidenced by our patient’s rapid improvement in visual symptoms and headache following high-dose steroids and tocilizumab.
4 Patient perspective
When I developed this eye pain and blurred vision in one eye, the first thing I thought about was raised intraocular pressure (glaucoma). When I visited a private center, the doctor there started me on anti-glaucoma drops for suspected glaucoma. A few days later, the vision continued to worsen and involved the other eye, along with a new-onset headache. I visited another doctor who told me it might be sinusitis. I was given antihistamines and a nasal decongestant, but again, there was no improvement. Finally, after two weeks, I visited your hospital, and the workup revealed giant cell arteritis. I was started on steroid therapy. I was afraid of losing my vision permanently; however, I am very grateful and thankful for all of your efforts, and I am doing fine right now with very good visual acuity.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JM: Conceptualization, Data curation, Writing – review & editing, Writing – original draft, Investigation, Validation. AA: Validation, Writing – review & editing, Investigation, Writing – original draft, Data curation, Conceptualization. MA: Writing – review & editing, Writing – original draft, Validation, Conceptualization, Data curation, Investigation. YA: Validation, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank the patient for their willingness to share their story. We are also grateful to Dr. Hassan Altayeb Mohammed Ahmed, Neuroradiologist, for providing high-quality imaging that significantly contributed to this work. Our appreciation extends to Dr. Fahd Al Sufiani, Neuropathologist and Anatomical Pathologist, for supplying the histopathology slides and offering valuable insights into their interpretation. We also thank Dr. Nawaf A. Alhussaini for his assistance in acquiring and accessing the ophthalmic imaging.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guittet L, de Boysson H, Cerasuolo D, Morello R, Sultan A, Deshayes S, et al. Whole-country and regional incidences of giant cell arteritis in French continental and overseas territories: A 7-year nationwide database analysis. ACR Open Rheumatol. (2022) 4:753–9. doi: 10.1002/acr2.11472
2. Opri&-Belinski D, Cobilinschi CO, and Săulescu I. Current perspectives in giant cell arteritis: Can we better connect pathogenesis and treatment? Medicina (B Aires). (2024) 60:400. doi: 10.3390/medicina60030400
3. Hickman SJ. Optic perineuritis. Curr Neurol Neurosci Rep. (2016) 16:16. doi: 10.1007/s11910-015-0617-2
4. Hayreh SS, Podhajsky PA, and Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. (1998) 125:509–20. doi: 10.1016/S0002-9394(99)80192-5
5. Emami S, Howarth D, and Margolin E. Giant cell arteritis presenting as bilateral optic perineuritis in an african man. J Neuroophthalmol. (2021) 41:e149–52. doi: 10.1097/WNO.0000000000000951
6. Liu TY and Miller NR. Giant cell arteritis presenting as unilateral anterior ischemic optic neuropathy associated with bilateral optic nerve sheath enhancement on magnetic resonance imaging. J Neuroophthalmol. (2015) 35:360–3. doi: 10.1097/WNO.0000000000000269
7. Albarrak AM, Mohammad Y, Hussain S, Husain S, and Muayqil T. Simultaneous bilateral posterior ischemic optic neuropathy secondary to giant cell arteritis: a case presentation and review of the literature. BMC Ophthalmol. (2018) 18:317. doi: 10.1186/s12886-018-0994-9
8. Gold DM and Galetta SL. An inflammatory milieu: Optic perineuritis, retroperitoneal fibrosis, and giant cell arteritis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e642. doi: 10.1212/NXI.0000000000000642
9. Pappolla A, Silveira F, Norscini J, Miquelini L, and Patrucco L. Bilateral optic perineuritis as initial presentation of giant cell arteritis. Neurologist. (2019) 24(1):1–3. doi: 10.1097/NRL.0000000000000206
10. Purvin V, Kawasaki A, and Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol. (2001) 119:1299–306. doi: 10.1001/archopht.119.9.1299
11. Harimohan H, Bilagi R, and Huynh Q. Giant cell arteritis and the use of ultrasound in its diagnosis. Cureus. (2025) 17:e77283. doi: 10.7759/cureus.77283
12. Guggenberger K, Riedling L, Kern DM, Werner RA, Vogt ML, Fröhlich M, et al. Isolated inflammatory involvement of the occipital artery in giant cell arteritis and polymyalgia rheumatica: findings from a retrospective analysis and the critical role of MRI in diagnosis. Rheumatol Int. (2025) 45(1):27. doi: 10.1007/s00296-024-05765-4
Keywords: optic perineuritis, vision loss, giant cell arteritis, brain imaging, case report
Citation: Madkhali J, Aba Alkhail AB, Aldriweesh MA and Al Malik Y (2025) Bilateral optic perineuritis: a rare manifestation of giant cell arteritis - case report and literature review. Front. Ophthalmol. 5:1598302. doi: 10.3389/fopht.2025.1598302
Received: 24 March 2025; Accepted: 16 June 2025;
Published: 04 July 2025.
Edited by:
Sachin Kedar, Emory University, United StatesReviewed by:
Abhishek Mahesh Appaji, BMS College of Engineering, IndiaNagham Al-Zubidi, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Madkhali, Aba Alkhail, Aldriweesh and Al Malik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed B. Aba Alkhail, QWhtZWRhYmFhbGtoYWlsMUBnbWFpbC5jb20=
 Jnadi Madkhali
Jnadi Madkhali Ahmed B. Aba Alkhail
Ahmed B. Aba Alkhail Mohammed A. Aldriweesh
Mohammed A. Aldriweesh Yaser Al Malik1,2,3
Yaser Al Malik1,2,3