Abstract
Skeletal muscle contracts either by shortening or lengthening (concentrically or eccentrically, respectively); however, the two contractions substantially differ from one another in terms of mechanisms of force generation, maximum force production and energy cost. It is generally known that eccentric actions generate greater force than isometric and concentric contractions and at a lower metabolic cost. Hence, by virtue of the greater mechanical loading involved in active lengthening, eccentric resistance training (ECC RT) is assumed to produce greater hypertrophy than concentric resistance training (CON RT). Nonetheless, prevalence of either ECC RT or CON RT in inducing gains in muscle mass is still an open issue, with some studies reporting greater hypertrophy with eccentric, some with concentric and some with similar hypertrophy within both training modes. Recent observations suggest that such hypertrophic responses to lengthening vs. shortening contractions are achieved by different adaptations in muscle architecture. Whilst the changes in muscle protein synthesis in response to acute and chronic concentric and eccentric exercise bouts seem very similar, the molecular mechanisms regulating the myogenic adaptations to the two distinct loading stimuli are still incompletely understood.
Thus, the present review aims to, (a) critically discuss the literature on the contribution of eccentric vs. concentric loading to muscular hypertrophy and structural remodeling, and, (b) clarify the molecular mechanisms that may regulate such adaptations.
We conclude that, when matched for either maximum load or work, similar increase in muscle size is found between ECC and CON RT. However, such hypertrophic changes appear to be achieved through distinct structural adaptations, which may be regulated by different myogenic and molecular responses observed between lengthening and shortening contractions.
Lengthening vs. shortening contractions
It is known that skeletal muscle can contract either by shortening or lengthening (concentrically or eccentrically, respectively). During concentric contractions the muscle shortens and exerts a force, which is transmitted via the tendon to the joint, enables movement to occur and causes a change in joint angle. Eccentric contractions occur in everyday motor activities and usually are responsible for two important characteristics in natural locomotion. Eccentric contractions allow the dissipation of mechanical energy during body deceleration (Konow and Roberts, 2015; e.g., descending stairs/walking downhill, in which the quadriceps and plantar flexors muscles generate force while lengthening, to exert a breaking action against downward movement and to maintain balance), but they also allow the conversion of kinetic energy into elastic energy of tendons (Hoppeler, 2014). Such energy is then regained during limb support, resulting in less muscle work and energy required in locomotion.
Eccentric and concentric contractions fundamentally differ one from the other from a mechanical, metabolic and neural control point of view. Moreover, recent evidence obtained in our laboratory shows that distinct differences in terms of muscle morphological adaptations to resistive training exist between eccentric and concentric contractions.
Eccentric contractions can produce greater force than concentric contractions through different mechanisms of force generation
The two contraction types involve different mechanisms of force generation at the contractile protein level; this constitutes one of the main reasons for the greater force production during active lengthening compared to shortening. Muscle force development is the result of the interaction between the contractile filaments. Maximum force is produced when the overlap of the myosin and actin filaments enables the formation of the maximum number of cross-bridges, which occurs at the optimum sarcomere length (Gordon et al., 1966; Huxley and Simmons, 1971). The force developed by a muscle not only depends on sarcomere length and cross-bridges formation, but also on the velocity of shortening (Hill, 1938) or lengthening (Katz, 1939). During shortening contractions in vitro, the force generated is always lower than in isometric contractions (for the same level of muscle activation). This occurs because, the quicker the movement, the lesser the number of cross-bridges formed (Huxley, 1957) and higher rate of cross-bridges detachment (Rome et al., 1999). The greater the velocity of contraction, the shorter the time in which myosin can bind to actin. Moreover, during fast movements, the S2 complex of myosin molecule (i.e., the flexible fragment of the myosin tail close to the globular head—Rayment et al., 1993; VanBuren et al., 1994) will not be fully extended, resulting in compression of the S2 complex and in a lower pulling force applied by the thick filament on actin (Figure 1A). When velocity of movement approaches 0, then, not only will a larger number of cross-bridges be attached but also myosin S2 complexes will be fully stretched and able to pull onto the actin filaments to produce bigger values of force (Figure 1B; Huxley and Simmons, 1971; Jones et al., 2004).
Figure 1
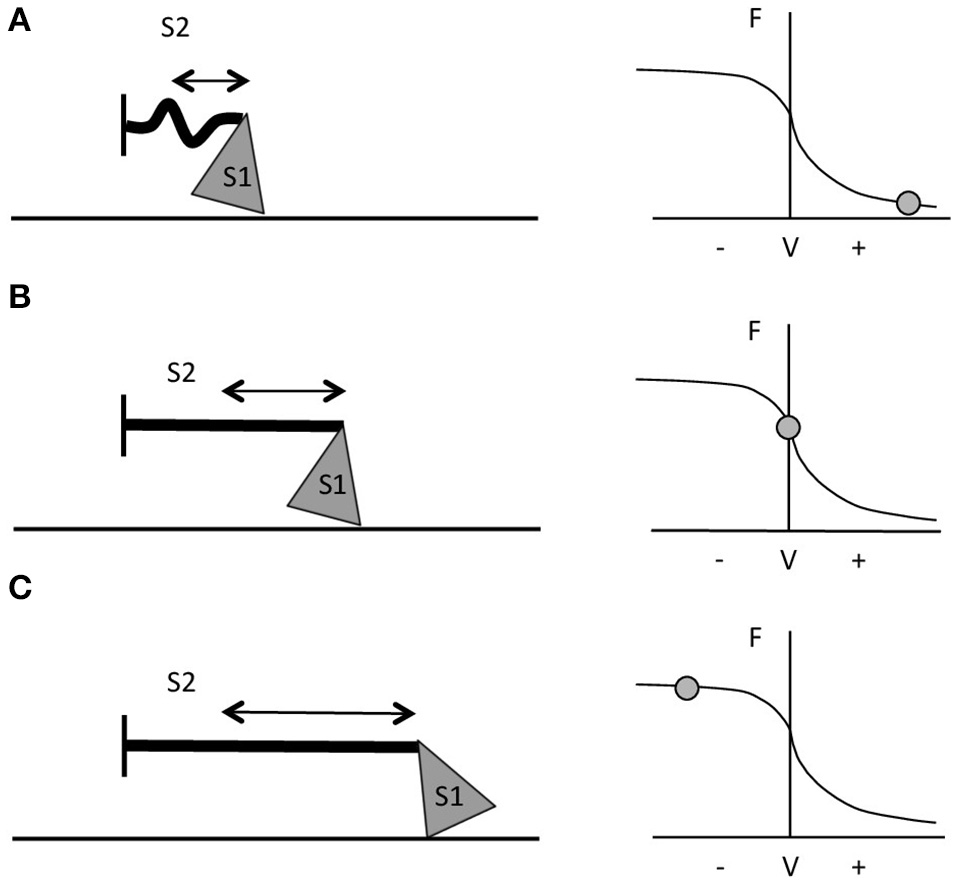
Schematic representation of myosin S1 and S2 segments behavior during different contractions and at different respective portions of the F-V curve. (A) During fast shortening muscle actions, S2 complex will not fully stretch, hence myosin will apply lower pulling force onto actin (i.e., the thinner straight line in the figure). (B) During slower shortening contractions, up to when the shortening velocity would be equal to 0 (i.e., isometric contractions), the S2 segment will be fully stretched and therefore myosin will be able to apply greater pulling force onto the thin filament. (C) During lengthening actions, myosin S2 complex will be able to stretch even further (figure adapted from Jones et al., 2004).
In vitro, with the increase of lengthening velocity, the force developed rises until it reaches a plateau at a value close to 1.8 times the maximum isometric force (Katz, 1939; Lombardi and Piazzesi, 1990). In vivo, the eccentric muscle force is lower than the eccentric force obtained in vitro (about 1.2 times the maximum isometric force) and this is probably due to neural inhibition (Amiridis et al., 1996; Babault et al., 2001; Beltman et al., 2004); nevertheless, it is still greater than the one generated by either isometric or concentric contractions (Westing et al., 1988; Aagaard et al., 2000). According to Huxley's model, the greater force produced during active lengthening could be due to a further greater stretch of S2 portions of myosin, occurring first at slow velocity of stretching (Figure 1C). As the lengthening becomes faster, fewer myosin heads will be able to bind to actin, but a good number of these will just remain in the attached position; if these cross bridges are stretched even more, they will inevitably be forced to detach. The proposed mechanism is that these thick filament heads would be able to re-bind to the thin filament very quickly, which maybe representing the reason why muscles are able to develop high forces during lengthening contractions and at lower energetic cost (Lombardi and Piazzesi, 1990; Jones et al., 2004).
The observation that the force generated during active muscle lengthening is substantially greater than the values obtained during isometric and shortening contractions suggests a potential contribution of some structural scaffold to the force developed (as myofibrils would be forcibly lengthened beyond actin-myosin optimal overlap zone; Herzog et al., 2008; Herzog, 2014). The work by Nishikawa (Nishikawa et al., 2012) suggests that the giant protein titin could be involved in muscle contraction mechanics, acting as an internal spring able to store and release elastic potential energy. It has been demonstrated that, during the cross-bridges cycle, actin rotates as myosin translates (Morgan, 1977). The theory proposes titin behaving as a “winding filament,” which is activated by Ca++ release and winds upon the actin filament when the latter is rotated by myosin translation in cross-bridges (Monroy et al., 2012); thus, titin will “actively” participate to the generation of force of a muscle through its stiffening when winded up onto actin during active lengthening contractions (please see Hessel et al., 2017 for further illustrations).
Eccentric contractions are more efficient than concentric contractions
Fundamental differences exist between eccentric and concentric contractions in terms of energy cost. Early observations by Bigland-Ritchie and Woods (Bigland-Ritchie and Woods, 1976) showed that for the same movement (cycling) speed, the energy cost of positive work (shortening) is about six-fold greater than that of negative work (lengthening). Thus, eccentric contractions have been advocated as particularly suitable for recovering muscle mass and strength in elderly and clinical populations (Hoppeler, 2014; Mitchell et al., 2017). The features of metabolic and energetic costs of ECC compared to CON contractions have been recently reviewed by Professor Hoppeler (Hoppeler, 2016).
Eccentric contractions present different neural control strategies compared to concentric contractions
Concentric and eccentric contractions seem to considerably differ also in terms of neural drive (Duchateau and Enoka, 2008). EMG amplitude is typically greater during shortening than during lengthening contractions (Tesch et al., 1990; Westing et al., 1991; Amiridis et al., 1996; Kellis and Baltzopoulos, 1998; Aagaard et al., 2000; Komi et al., 2000) and voluntary activation can be lower during lengthening contractions (Amiridis et al., 1996; Babault et al., 2001; Beltman et al., 2004). During maximal voluntary contractions, it seems well established that there is a deficit in voluntary activation in eccentric contractions. Indeed, due to the greater force capacity of muscle during lengthening contractions, fewer motor units are recruited and the discharge rate is lower during lengthening contractions compared with shortening contractions (Duchateau and Enoka, 2016). However, the mechanisms responsible for the activation deficit of eccentric contractions are only partly understood. While some authors argue that this inhibition may originate from excessive tension applied to the tendon complex due to excitation of the Golgi tendon organ, leading to reduced motor neurone responsiveness to incoming descending inputs (Westing et al., 1991; Aagaard et al., 2000); a decline in output from the motor cortex or an increase in presynaptic inhibition of facilitation from the periphery are also likely to be involved (Duchateau and Enoka, 2016).
The contribution of chronic concentric vs. eccentric loading to muscle hypertrophy
ECC resistance training, when performed at high intensities, is associated with greater increases in both total and eccentric strength compared to CON RT (Roig et al., 2009; Isner-Horobeti et al., 2013; Baroni et al., 2015). However, while a substantial wealth of literature exists on hypertrophic responses to concentric and eccentric training (Jones and Rutherford, 1987; Higbie et al., 1996; Seger et al., 1998; Blazevich et al., 2007; Reeves et al., 2009; Moore et al., 2012), the mechanisms regulating these changes have not yet been fully elucidated, especially in humans.
There is still some confusion regarding the singular contribution of both contractions to muscular hypertrophy. Lengthening contractions have the potential to generate greater muscle force than isometric and shortening ones (Katz, 1939; Westing et al., 1991; Cook and McDonagh, 1995); for this reason (i.e., the possibility to train with greater loads), the general consensus is that eccentric exercise may have the potential to promote larger increases in muscle size and strength compared to concentric and isometric training (Roig et al., 2009). However, other authors (Wernbom et al., 2007; Hyldahl and Hubal, 2013) have suggested that if the two types of loading are performed at same intensity and/or work volume, then it is difficult to establish which is the best training mode, as significant hypertrophy is reached in either case. The most relevant studies that compared ECC vs. CON training and the correspondent increase in muscle size are presented in the following subsections.
The investigations that have been included in this table are those that compared eccentric only vs. concentric only loading paradigms within a young population. Many investigations differed one from the other on what has been considered/chosen as index of muscle hypertrophic adaptations to RT. Therefore, studies have been grouped in 6 different categories, depending on the different index (and thus method of assessment) of hypertrophy: muscle girth (Table 1.1), muscle anatomical cross-sectional area (ACSA; obtained from either CT or MRI scans) (Table 1.2), muscle thickness (obtained from ultrasound scans; Table 1.3), muscle volume (obtained from MRI scans; Table 1.4), fiber type II CSA (obtained from muscle biopsies and microscope analysis; Table 1.5), and thigh muscle fat free mass/lean mass (obtained by region of interest analyses from DXA; Table 1.6).
Table 1.1
| Studies | Population & muscle group | Intensity/duration | Matching | Results | Interpretation |
|---|---|---|---|---|---|
| Komi and Buskirk, 1972 | Young—Elbow Flexors | MVC—7 Wks | No | ECC only increase | Muscle Girth: ECC> CONC in 2 studies (elbow flexors, pectoralis major). Similar responses in 2 out of 4 studies using Isokinetic or Isotonic loading |
| Duncan et al., 1989 | Young men—Quadriceps | MVC—6 Wks | No | No sig changes for ECC and CON | |
| Ben-Sira et al., 1995 | Young women—Quadriceps | 65% 1RM CON—10 Wks | No | Similar ECC and CON increase | |
| Coratella and Schena, 2016 (*) | Young men—Pectoralis Major | CON = 85% 1RM, ECC = 120% CON 1RM—6 wks | Matched for load and training volume | ECC only increase |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in muscle girth within a young healthy population.
, Investigation performed on already trained subjects.
Table 1.2
| Studies | Population & muscle group | Intensity/duration | Matching | Results | Interpretation |
|---|---|---|---|---|---|
| Jones and Rutherford, 1987 | Young men—Quadriceps | 80% CON 1RM—12 Wks | No | Similar ECC and CON increase | 1- Muscle ACSA ECC> CONC: clear differences in only 2 studies out of 10. 2- Similar responses in 8/10 studies using Isokinetic or Isotonic loading. 3- The two loading modalities seem to promote regional hypertrophic responses. 4- When matched for work or load: Similar ECC and CON ACSA increase |
| Higbie et al., 1996 | Young women—Quadriceps | MVC—10 Wks | No | ECC > CON | |
| Smith and Rutherford, 1995 | Young men—Quadriceps | ECC load = 35% grater than CON load | ~ Load Match (ECC overload) | Similar ECC and CON increase distal ACSA | |
| Seger et al., 1998 | Young men—Quadriceps | MVC—10 Wks | No | Similar ECC and CON increase ACSA Mid, Distal ACSA: ECC > CON | |
| Vikne et al., 2006 | Young men—Elbow Flexors | MVC—12 Wks | No | ECC only increase | |
| Blazevich et al., 2007 | Young men—Quadriceps | MVC—10 Wks | No | Similar ECC and CON increase | |
| Moore et al., 2012 | Young men—Elbow Flexors | MVC—9 Wks | Total External Work | Similar ECC and CON increase | |
| Farup et al., 2014 | Young men—Quadriceps | %60-80 1RM CON, 120% 1RM CON for ECC group—12 Wks | ~ Load Match (ECC overload) | Similar ECC and CON increase | |
| Rahbek et al., 2014 | Young men—Quadriceps | 1RM = training load Progressive (please see reference) 12 wks | No | ECC and CON similar increase % ACSA, greater increase in the Whey supplement group vs. control | |
| Franchi et al., 2014 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training mode—10 Wks | Matched for max relative load and theoretical equivalent neural activation | CON > ECC Mid ACSA ECC > CON distal ACSA Similar proximal ACSA |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in muscle ACSA within a young healthy population.
Table 1.3
| Studies | Population & muscle group | Intensity/duration | Matching | Results | Interpretation |
|---|---|---|---|---|---|
| Blazevich et al., 2007 | Young men—Quadriceps | MVC—10 Wks | No | Similar ECC and CON increase in whole Quadriceps and VL and VM | Muscle Volume: Similar responses between ECC and CON to either Isokinetic or Isotonic RET for whole Quadriceps, VL and VM |
| Franchi et al., 2014 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training mode—10 Wks | Matched for max relative load and theoretical equivalent neural activation | Similar ECC and CON increase in VL Volume |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in muscle volume within a young healthy population.
Table 1.4
| Studies | Population & muscle group | Intensity/duration | Matching | Result | Interpretation |
|---|---|---|---|---|---|
| Farthing and Chilibeck, 2003 | Young men and women—Elbow Flexors | MVC—8 Wks | No | ECC > CON (at different sites and velocities) | Muscle Thickness: ECC> CONC in only one study (elbow flexors and with different velocities), but similar responses between ECC and CON in 5 out of 6 studies using Isokinetic or Isotonic loading, investigating quadriceps, VL, VM, BFLh and supraspinatus muscles |
| Blazevich et al., 2007 | Young men—Quadriceps | MVC—10 Wks | No | Similar VL and VM ECC and CON increase | |
| Cadore et al., 2014 | Young men—Quadriceps | MVC—6 Wks | No | Similar ECC and CON increase | |
| Franchi et al., 2015 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training mode—4 Wks | Matched for max relative load and theoretical equivalent neural activation | Similar VL ECC and CON increase | |
| Timmins et al., 2016a | Young men—Biceps Femoris | MVC—6 Wks | No | No significant changes for ECC and CON | |
| Kim et al., 2015 | Young men and women—Supraspinatus | MVC—8 Wks | No | Similar ECC and CON increase |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in muscle thickness within a young healthy population.
Table 1.5
| Studies | Population & muscle group | Intensity/duration | Matching | Result | Interpretation |
|---|---|---|---|---|---|
| Mayhew et al., 1995 | Young men and women—Quadriceps | 90% maximal CON power—4 Wks | No | CON > ECC | Muscle fiber type II CSA: ECC> CONC in 3 studies out of 5 Similar responses between ECC and CON in 1 study and CON > ECC in 1 study. |
| Hortobágyi et al., 1996 | Young women—Quadriceps | MVC—12 Wks | No | ECC > CON | |
| Seger et al., 1998 | Young men—Quadriceps | MVC—10 Wks | No | No differences between ECC and CON | |
| Hortobágyi et al., 2000 | Young men and women—Quadriceps | MVC—12 Wks | No | ECC > CON | |
| Vikne et al., 2006 | Young—Elbow Flexors | MVC—12 Wks | No | ECC only increase |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in fiber type II CSA within a young healthy population.
Table 1.6
| Studies | Population & muscle group | Intensity/duration | Matching | Results | Interpretation |
|---|---|---|---|---|---|
| Nickols-Richardson et al., 2007 | Young Women—Quadriceps/Biceps fem—Elbow flexors/extensors | MVC—20 Wks | No | No differences in fat free mass for ECC and CON | Muscle FFM/LM: Similar responses between ECC and CON to either Isokinetic or Isotonic RET for thigh fat free mass and lean mass. |
| Hawkins et al., 1999 | Young women—Quadriceps | MVC—18 Wks | No | Similar ECC and CON increase in Lean Mass | |
| English et al., 2014 | Young men—Quadriceps & Triceps Surae | ECC performed at 0/33/66/100 or 138 % CON load (5 training groups)—8 Wks | Only one ECC group was load matched (138% CON load) | Similar ECC and CON increase in Lean Mass | |
| Franchi et al., 2015 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training—4 Wks | Matched for max relative load and theoretical equivalent neural activation | Similar ECC and CON increase in Lean Mass |
Studies investigating ECC only vs. CON only RT and the contribution to the increase in lean mass/fat free mass within a young healthy population.
Muscle girth
When considering muscle girth as an index of muscle hypertrophy in response to ECC vs. CON RT, a total of four studies have been identified. Two of these studies used Isokinetic RT modality (one investigating elbow flexors—Komi and Buskirk, 1972, the other focusing on knee extensors, Duncan et al., 1989), while the other two utilized isotonic RT (one investigating the knee extensors—Ben-Sira et al., 1995–and the other using a bench-press exercise, focusing on pectoralis major girth—Coratella and Schena, 2016). The results are quite conflicting, as only two out of four studies showed superiority of ECC RT. It is noteworthy to mention that these two studies focused on upper body muscles, whereas no significant differences were found between ECC and CON RT in terms of muscle girth increase in knee extensor muscles, irrespective of the modality of exercises (i.e., Isokinetic or Isotonic) adopted. Therefore, it is also important to acknowledge the possible variation that exists among different muscle groups in the responses to similar training modalities.
Muscle ACSA
When considering muscle ACSA as index of muscle hypertrophy in response to ECC vs. CON RT, a total of ten studies have been identified: eight investigated knee extensor muscles while just two focused on the elbow flexors group. Only two studies (Higbie et al., 1996; Vikne et al., 2006- on knee extensors and elbow flexors, respectively) clearly support a superiority of ECC loading in muscle hypertrophy, whereas the other eight investigations presented inconclusive evidence of a superiority of one mode over the other.
Seger et al. (1998) reported that ECC RT produced greater increase in ACSA compared to CON RT, but this was only found at the distal portion of the quadriceps while CON RT resulted in greater increase of mid-belly CSA compared to ECC loading (although this difference was not statistically significant, see Figure 2). However, potentially, if the two sites had been considered together as a sum of ACSA across consecutive axial scans, (as in Higbie et al., 1996), the differences between ECC and CON in terms of “whole” hypertrophic (and not regional) response might have shown another outcome.
Figure 2
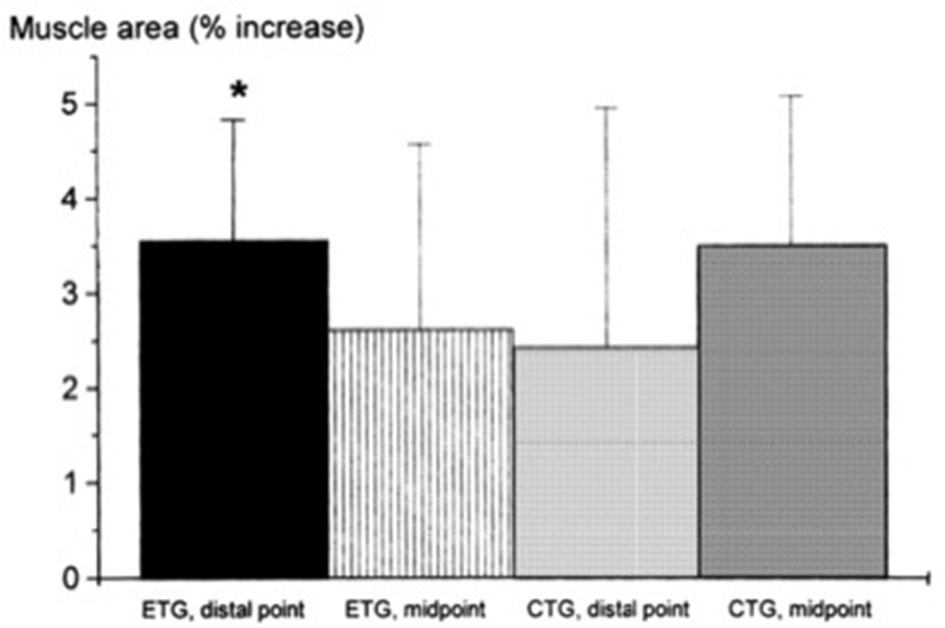
From Seger et al. (1998): preferential distal significant hypertrophy after ECC RT. If the two sites had been considered together as a sum of ACSA across consecutive axial scans, (as in Higbie et al., 1996), the differences between ECC and CON in terms of “whole” hypertrophic (and not regional) response might have shown a different outcome (ECC ~ CON hypertrophy). *P < 0.05.
Nonetheless, other investigations underlined the importance of regional growth (Narici et al., 1989; Smith and Rutherford, 1995; Blazevich et al., 2007). We previously showed that the different contractile stimuli might lead to regional hypertrophy, as presented in Figure 3 (taken from Franchi et al., 2014, where an average of 5 CSA was taken from each vastus lateralis length portion). From this study, it appears that ECC RT induces greater changes in CSA toward the distal portion of the VL muscle, whereas CON RT results in a greater mid-belly associated growth.
Figure 3
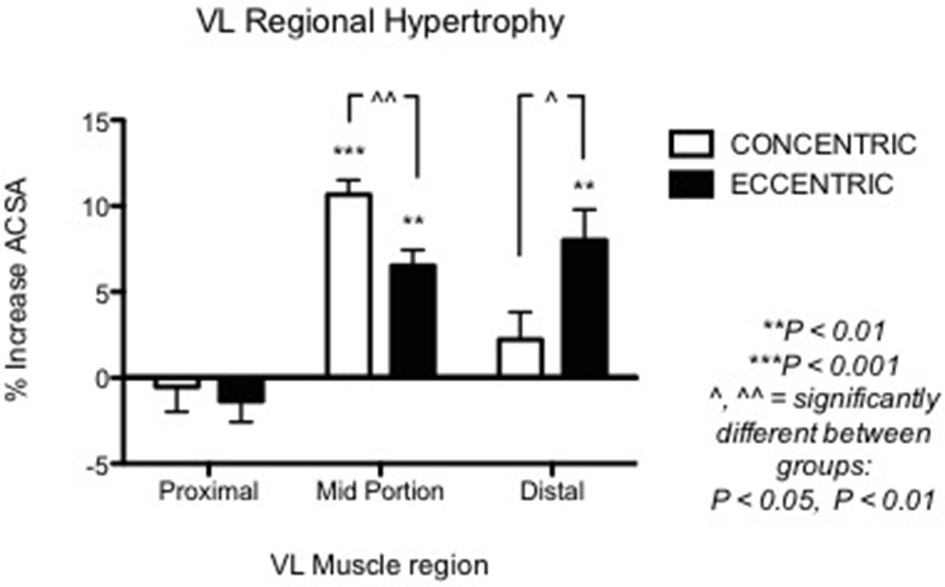
From Franchi et al. (2014): VL Regional hypertophic adaptations to ECC vs. CON RT programs are shown, ECC resulting in greater distal hypertrophy while CON presents larger mid muscle belly increase of CSA.
In conclusion, the results of the studies that used muscle ACSA as hypertrophy index suggest that there is still not enough evidence for ECC RT to lead to greater muscle growth than CON RT: in addition, when the two training modes are performed while matched either for work or load, changes in muscle size are very similar.
Muscle volume
When considering muscle volume as an index of muscle hypertrophy in response to ECC vs. CON RT, a total of only two studies were found. Neither of the investigations presented significant differences between the loading typologies in the increase of muscle volume after training. It is intriguing to highlight that, in the study from our lab (Franchi et al., 2014), even if different regional morphological patterns of muscle hypertrophy were reported between ECC vs. CON RT (distal vs. mid-belly hypertrophy, respectively), these did not result in significant differences in whole muscle volume. This implies that, if only the peak of ACSA at mid-thigh (scientifically well accepted) would have been used as index of hypertrophy (ECC RT vs. CON RT mid ACSA increase = 7 vs. 11%), the study in question would have potentially provided slight different outcomes than the ones reported (ECC RT vs. CON RT whole volume increase = 6 vs. 8%).
Muscle thickness
When considering muscle thickness as index of muscle hypertrophy in response to ECC vs. CON RT, a total of six studies were identified. Five out of the six examined studies failed in finding significant differences between loading paradigms on knee extensors, biceps femoris long head or supraspinatus muscles. One study (Farthing and Chilibeck, 2003) showed that, for the elbow flexors, at the mid and distal site, high velocity ECC RT resulted in higher increase of MT compared to high and slow velocity CON RT. However, when slow velocity ECC RT was compared to slow velocity CON RT, no differences were found in MT change between exercise programs. Nonetheless, at the proximal site, the study showed a substantial superiority of High Velocity ECC RT in producing greater changes in MT.
In conclusion, the results of the studies that used MT increase as hypertrophy index suggest that no clear differences could be found between ECC and CON RT.
Muscle fiber type II CSA
When considering muscle fiber type II CSA as index of muscle hypertrophy in response to ECC vs. CON RT, a total of five studies were considered. Data on type II muscle fibers only have been reported as it has been showed the preferential recruitment (and consequent hypertrophy) of these fibers in eccentric actions (Fridén et al., 1983; Hortobágyi et al., 1996). Indeed, it appears that ECC loading may also favor the increase of type II fiber CSA, as three studies out of five showed clear superiority of lengthening contractions as stimulus for such adaptations. Furthermore, there seems to be a close connection between ECC RT, performed at higher intensities/velocities of movement, and fiber type II increase in CSA (and percentage too). This connection was the object of previous works, which investigated the effect of ECC training, performed in isolated manner (Paddon-Jones et al., 2001; Shepstone et al., 2005) or as overload phase in mixed-model designs (Hather et al., 1991; Hortobágyi et al., 2000; Friedmann-Bette et al., 2010). All studies found a significant increase in either of CSA or distribution of type II fibers in human skeletal muscle in response to ECC RT. Conversely, just one study (Mayhew et al., 1995) have observed greater fiber type II CSA change after CON compared to ECC RT (both contractions performed at 90% of Max CON power).
Muscle lean mass/fat free mass
When considering muscle lean mass/fat free mass as index of muscle hypertrophy in response to ECC vs. CON RT, a total of four studies were found. None of the investigations presented significant differences between the loading typologies in the increase of muscle volume after training.
Mixed concentric/eccentric loading and muscle hypertrophy
While the present review is specifically focused on ECC only vs. CON only RT adaptations, it is important to briefly illustrate the adaptations in muscle hypertrophy in response to modes of RT that use a combination of concentric and eccentric contractions. Previous studies have compared the increase in muscle size (mainly in terms of type I and type II fiber CSA) in response to CON only RT vs. the combination of CON/ECC RT performed with isotonic (Hather et al., 1991) or isokinetic devices (Colliander and Tesch, 1990; Hortobágyi et al., 2000). The results are mixed, as no differences in type I CSA increase were found between loading modes (Colliander and Tesch, 1990) but the combination of CON/ECC RT resulted in greater type II fiber CSA compared to CON only (Hather et al., 1991; Hortobágyi et al., 2000). However, Friedmann-Bette et al. (2010) showed that, when comparing two different CON/ECC RT programs (group 1 = same load displaced between phases vs. group 2 = ECC overload, with ECC load ~1.9 of CON one), only CON/ECC overload RT program resulted in significant fiber type II CSA increase. It therefore appears that, when combining both contraction phases in an exercise regime, the best results for muscle growth are obtained by training paradigms that present some sort of ECC overload. In fact, the highest rate of whole muscle hypertrophy increase in humans is observed after flywheel training (Lundberg et al., 2013; Tesch et al., 2017), which provides accommodate resistance in the full range of motion of the CON phase, with ECC overload (Tesch et al., 2017). Flywheel training has shown to lead to very early hypertrophic and architectural adaptations (visible by MRI-assessed ACSA and ultrasound-derived fascicle length from 21 days after the start of the protocol; Seynnes et al., 2007). Although it is not within the scope of this manuscript to further present mixed CON/ECC loading strategies, it is intriguing to acknowledge that differences exist between muscular adaptations to ECC only or CON only and the combination of both types of contractions (with or without ECC overload) in training regimes.
The contribution of chronic concentric vs. eccentric loading to changes in muscle architecture
This review aimed to critically discuss the evidence for eccentric and concentric training to lead to different morphological adaptations. Perhaps, a significant difference between the two training modes lies in the contraction-specific regional hypertrophy that has been previously observed (Seger et al., 1998; Franchi et al., 2014; Douglas et al., 2016), where greater eccentric-associated distal growth is juxtaposed to a more pronounced mid-belly hypertrophy after concentric training.
Nonetheless, while similar “whole muscle” growth is often observed as a result of both types of RT, what really seems to differ between ECC and CON loading are the mechanisms of structural remodeling by which the hypertrophic responses are achieved. Our lab has recently shown that different architectural adaptations (measured by using B-mode ultrasound technique, Figure 4) can be found between ECC vs. CON contractions: ECC results in a markedly greater increase in fascicle length (Lf) while CON promotes greater changes in pennation angle (PA), likely reflecting the differential addition of sarcomeres either in series or in parallel, respectively (Reeves et al., 2009; Franchi et al., 2014, 2015) (Figures 5, 7). Therefore, muscle growth is achieved with both loading modalities, but the mechanisms of structural remodeling are contraction-specific (Franchi et al., 2016a). Although, a good number of investigations have focused on architectural responses to ECC RT only, so far, only five studies have compared isolated CON vs. isolated ECC in terms of structural remodeling. Those studies are presented in Table 2.
Figure 4
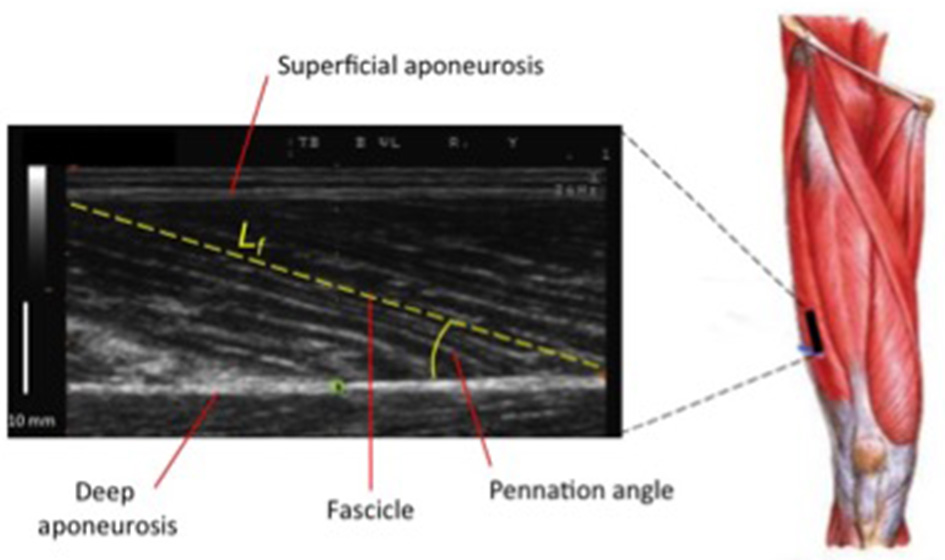
Ultrasound scan of VL muscle: Lf (fascicle length) and PA (pennation angle) represent two of the major features of muscle architecture.
Figure 5
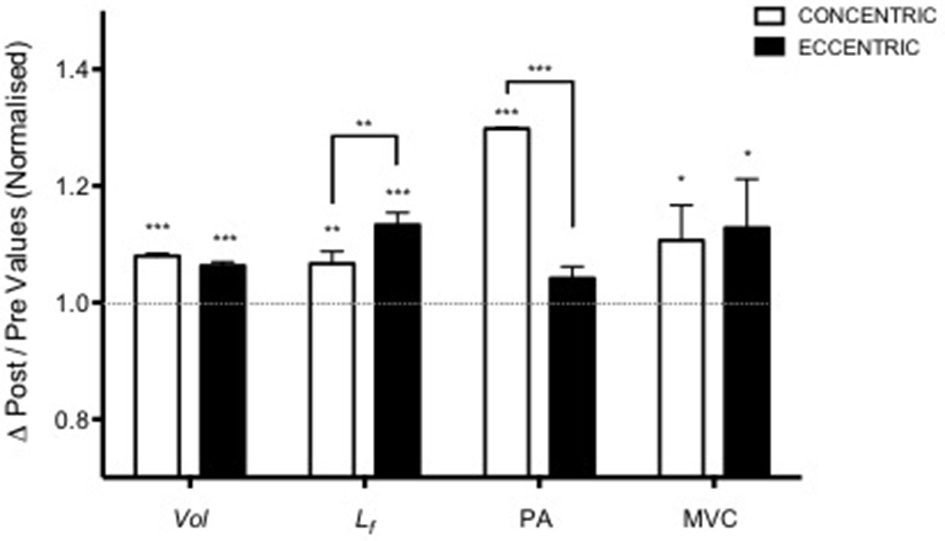
Adapted from Franchi et al. (2014). Contraction-dependent muscle growth in response to eccentric and concentric resistive training in young males. Similar hypertrophy is achieved through two different patterns of structural re-assembly (*P < 0.05, **P < 0.001, ***P < 0.0001). Y = 1 represent the baseline value, data are normalized to pre-exercise values (Vol, Volume; Lf, fascicle length; PA, pennation angle; MVC, maximum voluntary contraction).
Figure 6
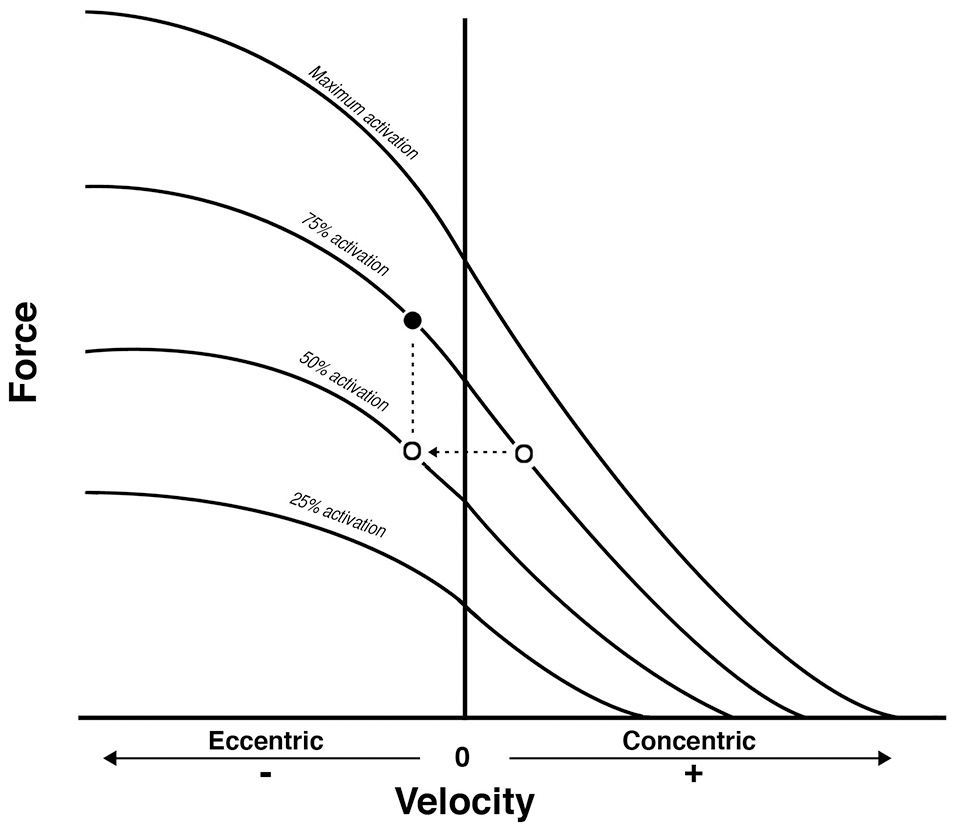
From Reeves et al. (2009): conceptual diagram that illustrates what could theoretically happen when displacing the same external load (empty dots) between concentric and eccentric phases in Conventional RT. As a consequence, the eccentric contraction belongs to a different force-velocity curve of lower neural activation. Because a fundamental requirement of the force-velocity relationship is that all the point of force and velocity should belong to a curve of same neural activation, then the filled dot represent the level of external load that should be adopted to meet such requirement: something that is not occurring in conventional resistance training.
Figure 7
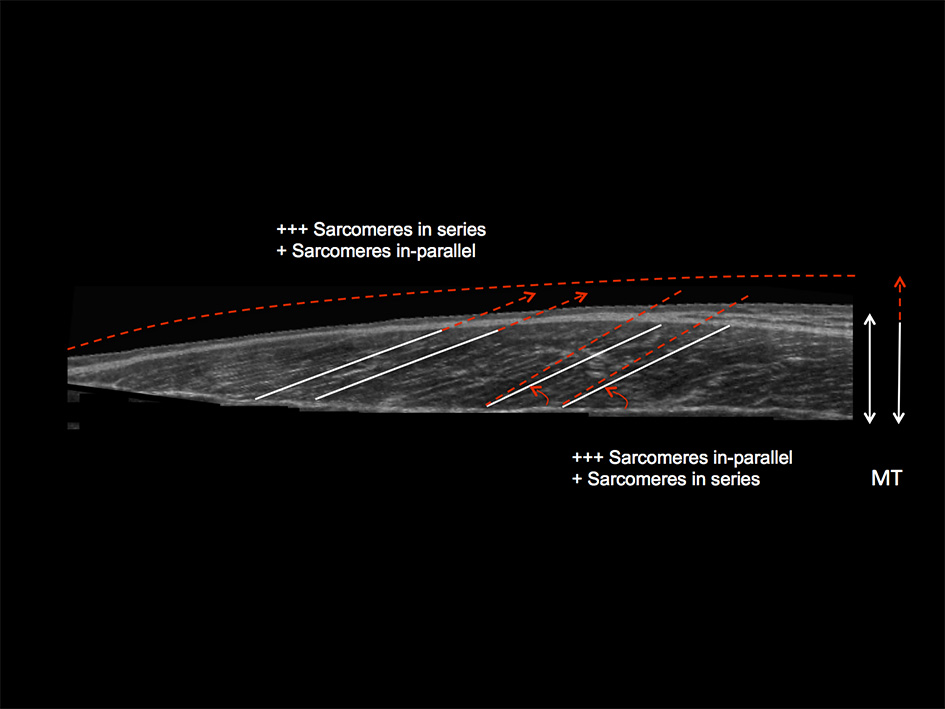
Schematic diagram illustrating the distinct contraction-specific hypertophic patterns in response to chronic ECC vs. CON RT in human vastus lateralis (image acquired by using extended field of view ultrasound technique): a similar increase in MT can indeed be reached either through a preferential addition of sarcomeres in-parallel (usually occurring after CON RT) with an increase in PA, or through a preferential addition of sarcomeres in-series (usually occurring after ECC RT), which is represented by an increase in Lf (Franchi et al., 2014, 2015). +++ = the preferential addition of either sarcomeres in-series or in-parallel (dependent on the contraction mode used) compared to + = likely to happen as a marginal response. The white lines highlight the initial pre-training scenario, whereas the red dotted lines represent a post-training hypertrophic state.
Table 2
| Studies | Population & muscle group | Intensity/duration | Matching | Results | Interpretation |
|---|---|---|---|---|---|
| Blazevich et al., 2007 | Young men—Quadriceps | MVC—10 Wks | No | Similar Lf, PA and CSA increase in both groups | Muscle Architecture: 4 studies out of 5 show clear distinct architectural adaptations in reponse to either CON or ECC training. CON RET induces a greater increase of PA compared to ECC RET, whereas ECC loading is the predominant stimulus to achieve greater changes in Lf. Only 1 study found ECC and CON to achieve similar architectural remodeling patterns (Blazevich et al., 2007) |
| Reeves et al., 2009 | Older men—Quadriceps | %80 5RM ECC and CON, respective to ECC or Conventional training mode—14 Wks | Matched for max relative load | ECC > CON on Lf increase (20% vs. 8%); CON > ECC on PA increase (35% vs. 5%); Similar increase in MT (~12%) | |
| Franchi et al., 2014 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training mode—10 Wks | Matched for max relative load and theoretical equivalent neural activation | ECC > CON on Lf increase (12% vs. 5%); CON > ECC on PA increase (30% vs. 5%); Similar increase in VOL (~7%) | |
| Franchi et al., 2015 | Young men—Quadriceps | %80 1RM ECC and CON, respective to the training mode—4 Wks | Matched for max relative load and theoretical equivalent neural activation | ECC > CON on Lf increase (5% vs. 2%); CON > ECC on PA increase (7% vs. 3%); Similar increase in MT (~8%) | |
| Timmins et al., 2016a | Young men—Biceps Femoris | MVC—6 Wks | No | ECC > CON on Lf increase (16% vs.—11%); CON > ECC on PA increase (20% vs.—7%); No significant increase in MT in both groups |
Summary of studies investigating the architectural adaptations to ECC only vs. CON only loading protocols.
Thus, when considering the results reported in Table 2, the prevalent observation is that the two contractions induce distinct structural adaptations. Since changes in architecture can influence the functional properties of muscles (Narici, 1999; Lieber and Fridén, 2000; Narici et al., 2016), a thorough understanding of skeletal muscle remodeling during hypertrophic states is then fundamental. Conventional (Narici et al., 1996; Alegre et al., 2006), concentric and eccentric loading can result in changes of Lf (Blazevich et al., 2007): however, eccentric-only RT induces greater increase in Lf of the VL muscle than conventional (Reeves et al., 2009) and concentric-only RT (Franchi et al., 2014, 2015). Similar findings have been observed in the biceps femoris muscle (Timmins et al., 2016a). By contrast, Blazevich et al. (2007) found both contractions to induce a similar and significant increase in Lf; however, in this study, the two different loading paradigms were not matched for work, relative load or neural drive. As previously discussed by Reeves et al. (2009) during conventional RT (typically consisting of consecutive concentric and eccentric phases), because the same absolute load is displaced between CON and ECC phases, motor units must be de-recruited to enable lowering of the training load (eccentric phase). Hence in the eccentric phase of conventional RT, muscle activation and relative loading, are considerably lower than those achieved in the concentric phase (Figure 6). Therefore, to guarantee that the ECC component of resistance training would not result under-loaded, Reeves et al. (2009) and Franchi et al. (2014) increased the eccentric training load by about 20% in order to obtain the same level of neural drive (EMG), to meet the fundamental requirement of the force-velocity relation that each value of force and velocity along this curve should belong to the same level of neural activation (Bigland and Lippold, 1954). Such a controlled study design allowed the authors to better compare the two loading modalities.
In relation to the muscle morphological adaptations to CON and ECC training, Blazevich and colleagues suggested that training at greater than normal joint range of motion may explain the similar structural remodeling in response to the two loading modes, instead of the contraction type. This hypothesis seems supported by other studies (McMahon et al., 2014; Guex et al., 2016), which found a greater increase in fascicle length of VL when training at greater ROM of the knee joint. Nonetheless, Noorkõiv et al. (2014) showed that isometric training at longer muscle lengths (i.e., thus almost re-producing an ECC contraction scenario) can induce a substantial increase in Lf. Thus, regarding the findings from Blazevich's study (2007) the authors raise the point of whether more pronounced mechanical stretch might have been applied to single sarcomeres (and thus fascicles) during large ROM CON RT at higher joint knee angle (counting the full leg extension as anatomical zero = 0 degrees) and if this could have affected serial sarcomeres distribution and ultimately the architectural adaptations presented by the aforementioned studies.
There is a substantial bulk of literature showing Lf increases after eccentric-only (Duclay et al., 2009; Potier et al., 2009; Baroni et al., 2013) or isoinertial exercise (Seynnes et al., 2007), reinforcing the concept that muscle longitudinal growth may be intimately related to lengthening contractions. The changes in Lf observed after these loading interventions theoretically imply the addition of sarcomeres in series. It is known from previous animal work that skeletal muscle responds to passive and intermittent stretch by adding new sarcomeres in-series (Williams and Goldspink, 1971; Holly et al., 1980; Goldspink, 1985; Williams et al., 1988; Williams, 1990), a trend observed after exercise regimes/overload (Goldspink, 1999; Proske and Morgan, 2001). Greater addition of serial sarcomeres was found in rats after downhill compared to uphill running (Lynn and Morgan, 1994; Butterfield et al., 2005): thus, it has been suggested that more contractile material placed in series (i.e., reflecting the increase in Lf) can be regarded as a “protective” mechanisms after eccentric exercise induced muscle damage (Morgan and Talbot, 2002), also because of the increase of maximal force produced at longer muscle lengths (Timmins et al., 2016a).
Conversely, an increase of PA has been regarded as a strategy for the muscle to pack more contractile material along the deep tendinous aponeurosis (Gans, 1982; Kawakami et al., 1993): thus, hypertrophy of pennate muscles can be often accompanied by a substantial increase of PA (Ema et al., 2016). However, PA appears to markedly increase after concentric and conventional RT (in which the concentric contraction stimulus is the most dominant, as the intensity is usually based on CON 1RM %), whereas none/small and often non-significant changes are observed in response to ECC loading programmes (Baroni et al., 2015).
Taken together, these results strongly suggest that skeletal muscle is able to respond to different mechanical stimuli by following distinct remodeling patterns, reflecting divergent strategies for the addition of new sarcomeric material in its structural re-assembly (Narici et al., 2016) (Figure 7). This also results in important implications in rehabilitation settings, because different contractions can therefore influence the shift of the optimum length-tension relationship, with notable repercussions on performance and injury prevention (Brughelli and Cronin, 2007; Timmins et al., 2016b). In fact, the addition of serial sarcomeres appears to directly impact the maximum velocity of shortening of muscle fibers (in cat semitendinosus muscle; Bodine et al., 1982): thus, eccentric contractions, while favoring the increase in Lf without presenting significant changes in PA, may have profound influence on muscle performance (Hoppeler, 2014).
Molecular and metabolic responses to acute and chronic concentric vs. eccentric loading
Skeletal muscle hypertrophy in response to exercise is the result of the addition of new contractile material, regulated by different molecular mechanisms, involving translational enhancement of muscle protein synthesis (Hoppeler et al., 2011; Atherton and Smith, 2012; Hoppeler, 2016). A small number of studies investigated the molecular mechanisms of muscle hypertrophy in response to concentric vs. eccentric exercise, particularly in humans; the existing work focused on the acute rather than on the chronic molecular responses, and only few investigations reported architectural and morphological adaptations over chronic resistive loading interventions (Blazevich et al., 2007; Franchi et al., 2014, 2015; Timmins et al., 2016a).
For this reason, there is currently no clear consensus as to what mechanisms regulate the structural remodeling of skeletal muscle with ECC vs. CON RT.
This section shall examine the possible molecular mechanisms regulating the responses to ECC and CON loading, from the animal and human data available up to present date, with the objective to better clarify if specific molecular alterations could explain such distinct structural adaptations.
Muscle protein synthesis after acute and chronic concentric vs. eccentric loading
The distinct remodeling patterns of ECC vs. CON RT observed in regional hypertrophy and muscle architecture point to a contraction-specific muscle growth that may be regulated by distinct whole and regional muscle anabolism. A total of four human investigations were identified and none of them found any notable differences in MPS between the two loading modalities. Phillips et al. (1997) reported similar increase in mixed protein synthesis at 3, 24, and 48 h after single CON and ECC exercise bouts (not matched for work). Likewise, Cuthbertson et al. (2006) found no differences in myofibrillar fractional synthetic rates (FSR) following 12 min step up (CON) or step down (ECC). When the loading paradigms were matched for work, myofibrillar MPS presented greater increase at 4.5 h after ECC RT compared to CON RT, with no differences found after 8.5 h (Moore et al., 2005). We recently explored the potential differences in anabolic response between ECC and CON loading and at different vastus lateralis muscle sites (at the middle of the muscle belly—MID—and at the distal portion, close to the knee myotendinous junction—MTJ; Franchi et al., 2015). The rationale for these measurements being that different architectural adaptations could be governed by contraction-specific responses in MPS; in addition, in a previous study of similar design, distinct patterns of regional hypertrophy were found, as distal growth was associated to ECC RT whereas CON RT seemed to favor increase of mid muscle ACSA (Franchi et al., 2014). Not only ECC and CON (matched for relative maximum load) did not differ in the quantity of myofibrillar FSR (measured over 4 weeks period, using deuterium-oxide tracing technique), but, surprisingly, no differences were observed between muscle sites. Interestingly, Fujita et al. (2007) showed that de novo sarcomere assembly in C2C12 myotubes can occur without the requirement for newly synthesized proteins. Collectively, these data suggest that, during muscle structural remodeling, assembly of sarcomeres may be independent of the quantity of new contractile material (i.e., proteins) synthesized. Hence, the different structural adaptations in response to ECC and CON cannot be determined merely through quantifying longer-term MPS responses. In further support of this concept, previous work by Garma et al. (2007) showed that, in rodent muscle, under conditions of equivalent volumes of force integral, both concentric and eccentric loading programmes (i.e., and isometric too) presented near identical activation of processes leading to anabolic response. Similar increase of total mRNA content, IGF-1 mRNA and protocollagen 3 were observed, as well as a decrease in myostatin mRNA. Lastly, in a more recent study on rats, mTORC1 signaling was not modulated by the contraction mode, rather the key factor seemed to be the force-time integral (Ato et al., 2016).
Exercise-induced muscle damage (EIMD)
Some research suggests that EIMD may play an important role in mediating muscle growth, speculating that the associated inflammatory response and protein turnover could be critical for increase in muscle size (Evans and Cannon, 1991). As EIMD is found predominantly after ECC exercise bouts, but not CON (Fridén et al., 1986; Faulkner and Brooks, 1993), this has prompted researchers to postulate that ECC RT could lead to higher hypertrophic responses: nevertheless, as showed in Section The Contribution of Chronic Concentric vs. Eccentric Loading to Muscle Hypertrophy of the present review, even at high training intensities, many studies cannot clearly affirm which training mode results in greater long-term hypertrophy. Moreover, it is widely known that EIMD decreases since after the first exercise bout (McHugh, 2003; i.e., the repeated bout effect phenomenon) because of several factors, including neural adaptations and structural remodeling of the extra-cellular matrix (ECM; Hyldahl et al., 2017). Thus, the real contribution of EIMD in distinct long-term hypertrophic adaptations to ECC vs. CON RT remains to be determined.
Satellite cells activity after acute and chronic concentric vs. eccentric loading
If MPS and EIMD do not fully explain the contraction-specific skeletal muscle remodeling patterns, further answers may be found in the satellite cells activity in response to ECC vs. CON RT.
During muscle repair following EIMD and during subsequent hypertrophic adaptations, the role of satellite cells is central for muscle growth (Fry et al., 2017). Satellite cells are stem cell situated between the basal lamina and the sarcolemma of skeletal muscle fibers (Mauro, 1961), which, upon activation, can proliferate, and ultimately fuse with existing fibers, thus leading to an increase in muscle fibers size (Harridge, 2007). It is noteworthy to mention that greater satellite cells activity has been observed after a single bout of ECC exercise (with consequent EIMD showed) compared to load-intensity matched CON (with no damage; Hyldahl et al., 2014). When we consider RT programs overtime, chronic CON RT only has lead to an increase in satellite cells pool, compared to chronic ECC RT (Farup et al., 2014). Thus, these data suggest that distinct contractions can differently modulate satellite cells activity over RT periods.
A recent study highlighted the very close relationship between myogenic progenitor cells (MPCs) and the ECM that surrounds muscle fibers in response to hypertrophic stimuli (Fry et al., 2017). Fry and colleagues showed that when MPCs are activated by mechanical overload, they proliferate and will release exosomes containing different micro RNAs (miRNA) into the ECM-niche. Such cross talk leads to a reduction of collagen mRNA expression in the fibrogenic cell, and thus appears to regulate ECM deposition during muscle structural remodeling. Conversely, the absence of activated satellite cells results in excessive ECM deposition and attenuated myofibre growth (Fry et al., 2017). Furthermore, in a recent investigation, a reduced cohort of satellite cells did not prevent serial sarcomeres addition after chronic stretch in mice soleus, but resulted in significantly altered muscle morphology (reduced fiber CSA with concomitant fibrosis and ECM hypertrophy; Kinney et al., 2017). This proves that satellite cells play a crucial role in controlling ECM and muscle structural remodeling.
Evidences of enhanced extra-cellular matrix remodeling in response to eccentric contractions
The previous sub-section has pointed out that the cross-talk between mechanical stimuli, satellite cells activation and ECM remodeling could be differently modulated after ECC vs. CON contractions.
A recent human study by Hyldahl et al. (2015) investigated the ECM remodeling contribution to the repeated bout effect, the physiological protective muscular adaptations that usually occurs after a single bout of ECC RT.
The present study presented two major results. Firstly, a global transcriptomic analysis showed that ECM gene networks alteration following an ECC RT bout (compared to the non-exercised leg) were related to early deadhesion (Tenascin C highly activated 2 days post-exercise), force loss and muscle damage, suggesting that these acute responses represented possible source of muscular adaptations in the repeated bout effect process. Furthermore, the ECM collagen genes, both fibrillar (type I and III) and basal lamina (IV) types, showed a prolonged response to one single bout of lengthening contractions, being markedly expressed even after 27 days from the exercise session, suggesting a possible delayed remodeling activity of ECM. In addition, the TGF-β/Smad signaling pathway was found highly activated at the same time point, supporting the above mentioned gene expression data, as TGF-β is regarded as an up-regulator of mechanical loading-derived collagen synthesis (Verrecchia et al., 2001). The present data support the contention that ECM may have a strong role in muscle remodeling and structural assembly after lengthening contractions. However, in this study, no responses to shortening contraction were investigated. Nevertheless, in a previous study on rats (Heinemeier et al., 2007), myostatin mRNA was found decreased the most after ECC compared to CON exercise, together with a larger increase of MGF (Mechano-Growth Factor), TGF-β-1 (transforming Growth Factor β-1, a marker of collagen expression) and CTGF (Connective Tissue Growth Factor) mRNA values in response to ECC training. Collagen type I α1 also presented a larger increase after ECC and Isometric exercise compared to CON; these data suggest once again that extra-cellular matrix-related remodeling could be one of the keys to interpret differences in muscle structural re-assembly in response to lengthening vs. shortening contractions (Mackey and Kjaer, 2016).
Different gene responses and cellular signaling pathways after acute and chronic concentric vs. eccentric loading
The only study that investigated the gene expression profile of concentric vs. eccentric exercise (unilateral stepping up and down of a small jump box) (Kostek et al., 2007) found that fifty-one genes were differently expressed after 3 h from ECC vs. CON RT. These genes were mostly related to protein turnover, cellular stress and sarcolemmal-structural remodeling. Differences in single genes expression (FBXO32, SIX1, CSRP3 and MUSTN1) were detected between the distinct loading modalities utilized: the four genes targeted, uniquely regulated by lengthening contractions, may play specific roles in skeletal muscle as regulating protein turnover, being potentially responsible for fiber-type change and involved in mechano-transduction processes. Thus, these data seem to support the idea that ECC and CON contractions/training modalities trigger distinct and unique gene networks.
When focusing on skeletal muscle signaling, some interesting findings are worth being reported on the cellular responses to either ECC or CON contractions. Wretman et al. (2001) observed that, in isolated rat muscle, greater increases in phosphorylation of p38 and ERK 1/2 mitogen-activated protein kinases (MAPKs) were induced by ECC compared to CON stimulus. These results were later confirmed by our lab on humans (Franchi et al., 2014), as we observed a greater activation of p38, ERK ½ and p90RSK after 30 min of ECC RT compared to CON. Intriguingly, ERK ½ expression was previously shown to regulate CON vs. ECC growth pathways in cardiomyocytes, suggesting MAPKs are involved in regulating architectural remodeling processes in both cardiac and skeletal muscle (Kehat et al., 2011). Thus, it is interesting to underline that the MAPKs signaling pathways are indeed differently regulated by distinct contractions.
Martineau and Gardiner (2001), observed that MAPKs activation was quantitatively related to tension, with ECC providing the greater stimulus compared to shortening muscle actions. It is well established that mechanical tension is one of the crucial factors influencing muscle growth (Goldberg et al., 1975; Fry, 2004) and also that eccentric contractions are associated with a greater development of tension compared to concentric contractions (Katz, 1939). Interestingly, a successive study, in which the force × time integral (FTI) was manipulated by changing the force of contraction, demonstrated that p38 and FAK phosphorylation correlate with the FTI (Rahnert and Burkholder, 2013) indicating a possible intimate relationship between those targets and the nature of the mechanical stimulus applied on to the muscle. This observation, lead us to investigate the role of FAK activation (at the y-397 tyrosine site) between different contractions (lengthening vs. shortening) and at distinct muscle sites (mid muscle vs. MTJ): a preliminary data analysis showed that y-397FAK activation was greater after a single ECC RT session compared to CON (Franchi et al., 2016b). Moreover, such activation appeared to be muscle site-dependent (MTJ > MID), supporting the regionality/heterogeinity of hypertrophic and structural remodeling adaptations to distinct loading modes.
In conclusion, it appears that when both contraction types are applied to the muscle with matched high intensity and work, similar response in protein synthesis can be observed, with a small potential difference only at very early time points (4.5 h after an exercise bout- Moore et al., 2005). Nonetheless, chronically assessed protein synthetic response (i.e., evaluated within a period of 4 weeks) does not highlight any significant differences between ECC vs. CON (Franchi et al., 2015). Distinct satellite cells activity, ECM-related gene expression and cellular signaling have been observed both in animals and humans, suggesting that ECC may trigger greater connective tissue/structural remodeling. Although, further research is needed to clarify the link between morphological and molecular adaptations, these phenomena could be linked/regulated to/by a greater activation of the MAPK family and mechano-transductors pathways (Wretman et al., 2001; Franchi et al., 2014, 2016b).
Conclusions
Although, ECC RT has been usually associated to greater increases in muscle mass compared to CON RT, the present review clearly illustrated that the findings presented in the literature are too varied to clearly affirm which training mode leads to greater long-term muscle growth. Furthermore, when both exercises paradigms are matched for either maximum load or work, the hypertrophic responses are very similar. What appears to be different is how this increase in muscle size is reached, as distinct contraction-specific adaptations in muscle architecture are found. In addition, different molecular and myogenic mechanisms have found distinctly activated after ECC vs. CON exercise bouts, suggesting that these responses could be underlying the structural remodeling patterns previously described: further investigations are needed to establish the strength of such potential micro-to-macro connections. At present time, the aforementioned scenarios are clearly described for a young population, whereas this is yet to be fully elucidated in older individuals and in clinical settings.
Statements
Author contributions
MF, NR, and MN contributed altogether to the conception and writing up process of this manuscript.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/K019104/1].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aagaard P. Simonsen E. B. Andersen J. L. Magnusson S. P. Halkjaer-Kristensen J. Dyhre-Poulsen P. (2000). Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J. Appl. Physiol.89, 2249–2257.
2
Alegre L. M. Jiménez F. Gonzalo-Orden J. M. Martín-Acero R. Aguado X. (2006). Effects of dynamic resistance training on fascicle length and isometric strength. J. Sports Sci.24, 501–50810.1080/02640410500189322
3
Amiridis I. G. Martin A. Morlon B. Martin L. Cometti G. Pousson M. et al . (1996). Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur. J. Appl. Physiol. Occup. Physiol.73, 149–156. 10.1007/BF00262824
4
Atherton P. J. Smith K. (2012). Muscle protein synthesis in response to nutrition and exercise. J. Physiol.590, 1049–1057. 10.1113/jphysiol.2011.225003
5
Ato S. Makanae Y. Kido K. Fujita S. (2016). Contraction mode itself does not determine the level of mTORC1 activity in rat skeletal muscle. Physiol. Rep.4:e12976. 10.14814/phy2.12976
6
Babault N. Pousson M. Ballay Y. Van Hoecke J. (2001). Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J. Appl. Physiol.91, 2628–2634.
7
Baroni B. M. Geremia J. M. Rodrigues R. De Azevedo Franke R. Karamanidis K. Vaz M. A. (2013). Muscle architecture adaptations to knee extensor eccentric training: rectus femoris vs. vastus lateralis. Muscle Nerve48, 498–506. 10.1002/mus.23785
8
Baroni B. M. Pinto R. S. Herzog W. Vaz M. A. (2015). Eccentric resistance training of the knee extensor muscle: training programs and neuromuscular adaptations. Isokinet. Exerc. Sci.23, 183–198. 10.3233/IES-150580
9
Beltman J. G. M. Sargeant A. J. van Mechelen W. de Haan A. (2004). Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. J. Appl. Physiol.97, 619–626. 10.1152/japplphysiol.01202.2003
10
Ben-Sira D. Ayalon A. Tavi M. (1995). The effect of different types of strength training on concentric strength in women. J. Strength Cond. Res. 9. 10.1519/1533-4287(1995)009<0143:TEODTO>2.3.CO;2
11
Bigland B. Lippold O. (1954). The relation between force, velocity and integrated electrical activity in human muscles. J. Physiol. 123, 214–224.
12
Bigland-Ritchie B. Woods J. J. (1976). Integrated electromyogram and oxygen uptake during positive and negative work. J. Physiol.260, 267–277. 10.1113/jphysiol.1976.sp011515
13
Blazevich A. J. Cannavan D. Coleman D. R. Horne S. (2007). Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J. Appl. Physiol.103, 1565–1575. 10.1152/japplphysiol.00578.2007
14
Bodine S. C. Roy R. R. Meadows D. A. Zernicke R. F. Sacks R. D. Fournier M. et al . (1982). Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J. Neurophysiol.48, 192–201.
15
Brughelli M. Cronin J. (2007). Altering the length-tension relationship with eccentric exercise : implications for performance and injury. Sports Med.37, 807–826. 10.2165/00007256-200737090-00004
16
Butterfield T. A. Leonard T. R. Herzog W. (2005). Differential serial sarcomere number adaptations in knee extensor muscles of rats is contraction type dependent. J. Appl. Physiol.99, 1352–135810.1152/japplphysiol.00481.2005
17
Cadore E. L. González-Izal M. Pallarés J. G. Rodriguez-Falces J. Häkkinen K. Kraemer W. J. et al . (2014). Muscle conduction velocity, strength, neural activity, and morphological changes after eccentric and concentric training. Scand. J. Med. Sci. Sports24, e343–e352. 10.1111/sms.12186
18
Colliander E. B. Tesch P. A. (1990). Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol. Scand.140, 31–39. 10.1111/j.1748-1716.1990.tb08973.x
19
Cook C. S. McDonagh M. J. (1995). Force responses to controlled stretches of electrically stimulated human muscle-tendon complex. Exp. Physiol.80, 477–490. 10.1113/expphysiol.1995.sp003862
20
Coratella G. Schena F. (2016). Eccentric resistance training increases and retains maximal strength, muscle endurance, and hypertrophy in trained men. Appl. Physiol. Nutr. Metab. 41, 1184–1189. 10.1139/apnm-2016-0321
21
Cuthbertson D. J. Babraj J. Smith K. Wilkes E. Fedele M. J. Esser K. et al . (2006). Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am. J. Physiol. Endocrinol. Metab.290, E731–E738. 10.1152/ajpendo.00415.2005
22
Douglas J. Pearson S. Ross A. McGuigan M. (2016). Chronic adaptations to eccentric training: a systematic review. Sport. Med. 47, 917–941. 10.1007/s40279-016-0628-4
23
Duchateau J. Enoka R. M. (2008). Neural control of shortening and lengthening contractions: influence of task constraints. J. Physiol.586, 5853–5864. 10.1113/jphysiol.2008.160747
24
Duchateau J. Enoka R. M. (2016). Neural control of lengthening contractions. J. Exp. Biol.219, 197–204. 10.1242/jeb.123158
25
Duclay J. Martin A. Duclay A. Cometti G. Pousson M. (2009). Behavior of fascicles and the myotendinous junction of human medial gastrocnemius following eccentric strength training. Muscle Nerve39, 819–827. 10.1002/mus.21297
26
Duncan P. W. Chandler J. M. Cavanaugh D. K. Johnson K. R. Buehler A. G. (1989). Mode and speed specificity of eccentric and concentric exercise training. J. Orthop. Sports Phys. Ther. 11, 70–75.
27
English K. L. Loehr J. A. Lee S. M. C. Smith S. M. (2014). Early-phase musculoskeletal adaptations to different levels of eccentric resistance after 8 weeks of lower body training. Eur. J. Appl. Physiol. 114, 2263–2280. 10.1007/s00421-014-2951-5
28
Ema R. Akagi R. Wakahara T. Kawakami Y. (2016). Training-induced changes in architecture of human skeletal muscles: current evidence and unresolved issues. J. Phys. Fit. Sport. Med.5, 37–46. 10.7600/jpfsm.5.37
29
Evans W. J. Cannon J. G. (1991). The metabolic effects of exercise-induced muscle damage. Exerc. Sport Sci. Rev.19, 99–125. 10.1249/00003677-199101000-00003
30
Farthing J. P. Chilibeck P. D. (2003). The effects of eccentric and concentric training at different velocities on muscle hypertrophy. Eur. J. Appl. Physiol. 89, 578–586. 10.1007/s00421-003-0842-2
31
Farup J. Rahbek S. K. Vendelbo M. H. Matzon A. Hindhede J. Bejder A. et al . (2014). Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand. J. Med. Sci. Sports24, 788–798. 10.1111/sms.12083
32
Faulkner J. A. Brooks S. V. Opiteck J. A. (1993). Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys. Ther.73, 911–921. 10.1093/ptj/73.12.911
33
Franchi M. V. Atherton P. J. Reeves N. D. Flück M. Williams J. Mitchell W. K. et al . (2014). Architectural, functional, and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol.210, 642–654. 10.1111/apha.12225
34
Franchi M. V. Atherton P. J. Maganaris C. N. Narici M. V. (2016a). Fascicle length does increase in response to longitudinal resistance training and in a contraction-mode specific manner. Springerplus5, 94. 10.1186/s40064-015-1548-8
35
Franchi M. V. Ruoss S. Quinlan J. I. Atherton P. J. Smith K. Narici M. V. et al . (2016b). Load-induced tyrosine 397 phosphorylation of focal adhesion kinase with resistance training is muscle region and contraction-type specific, in Abstract from the European College of Sports Science Conference 2016.
36
Franchi M. V. Wilkinson D. J. Quinlan J. I. Mitchell W. K. Lund J. N. Williams J. P. et al . (2015). Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle. Physiol. Rep.3, e12593–e12593. 10.14814/phy2.12593
37
Fridén J. Sfakianos P. N. Hargens A. R. (1986). Muscle soreness and intramuscular fluid pressure: comparison between eccentric and concentric load. J. Appl. Physiol.61, 2175–2179.
38
Fridén J. Sjöström M. Ekblom B. (1983). Myofibrillar damage following intense eccentric exercise in man. Int. J. Sports Med.4, 170–176. 10.1055/s-2008-1026030
39
Friedmann-Bette B. Bauer T. Kinscherf R. Vorwald S. Klute K. Bischoff D. et al . (2010). Effects of strength training with eccentric overload on muscle adaptation in male athletes. Eur. J. Appl. Physiol.108, 821–836. 10.1007/s00421-009-1292-2
40
Fry A. C. (2004). The role of resistance exercise intensity on muscle fibre adaptations. Sports Med.34, 663–679. 10.2165/00007256-200434100-00004
41
Fry C. S. Kirby T. J. Kosmac K. McCarthy J. J. Peterson C. A. (2017). Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell20, 56–69. 10.1016/j.stem.2016.09.010
42
Fujita H. Nedachi T. Kanzaki M. (2007). Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp. Cell Res.313, 1853–1865. 10.1016/j.yexcr.2007.03.002
43
Gans C. (1982). Fiber architecture and muscle function. Exerc. Sport Sci. Rev.10, 160–207. 10.1249/00003677-198201000-00006
44
Garma T. Kobayashi C. Haddad F. Adams G. R. Bodell P. W. Baldwin K. M. (2007). Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J. Appl. Physiol. 102, 135–143. 10.1152/japplphysiol.00776.2006
45
Goldberg A. L. Etlinger J. D. Goldspink D. F. Jablecki C. (1975). Mechanism of work-induced hypertrophy of skeletal muscle. Med. Sci. Sports7, 185–98.
46
Goldspink G. (1985). Malleability of the motor system: a comparative approach. J. Exp. Biol.115, 375–91.
47
Goldspink G. (1999). Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J. Anat.194(Pt 3), 323–334. 10.1046/j.1469-7580.1999.19430323.x
48
Gordon A. M. Huxley A. F. Julian F. J. (1966). The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol.184, 170–192. 10.1113/jphysiol.1966.sp007909
49
Guex K. Degache F. Morisod C. Sailly M. Millet G. P. (2016). Hamstring architectural and functional adaptations following long vs. short muscle length eccentric training. Front. Physiol.7:340. 10.3389/fphys.2016.00340
50
Harridge S. D. R. (2007). Plasticity of human skeletal muscle: gene expression to in vivo function. Exp. Physiol.92, 783–797. 10.1113/expphysiol.2006.036525
51
Hather B. M. Tesch P. A. Buchanan P. Dudley G. A. (1991). Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol. Scand.143, 177–185. 10.1111/j.1748-1716.1991.tb09219.x
52
Hawkins S. A. Schroeder E. T. Wiswell R. A. Jaque S. V. Marcell T. J. Costa K. (1999). Eccentric muscle action increases site-specific osteogenic response. Med. Sci. Sports Exerc. 31, 1287–1292.
53
Heinemeier K. M. Olesen J. L. Haddad F. Langberg H. Kjaer M. Baldwin K. M. et al . (2007). Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol.582(Pt 3), 1303–1316. 10.1113/jphysiol.2007.127639
54
Herzog W. (2014). Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J. Appl. Physiol.116, 1407–1417. 10.1152/japplphysiol.00069.2013
55
Herzog W. Leonard T. R. Joumaa V. Mehta A. (2008). Mysteries of muscle contraction. J. Appl. Biomech.24, 1–13. 10.1123/jab.24.1.1
56
Hessel A. L. Lindstedt S. L. Nishikawa K. C. (2017). Physiological mechanisms of eccentric contraction and its applications: a role for the giant titin protein. Front. Physiol.8:70. 10.3389/fphys.2017.00070
57
Higbie E. J. Cureton K. J. Warren G. L. Prior B. M. (1996). Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J. Appl. Physiol.81, 2173–2181.
58
Hill A. V. (1938). The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. B Biol. Sci.126, 136–195. 10.1098/rspb.1938.0050
59
Holly R. G. Barnett J. G. Ashmore C. R. Taylor R. G. Molé P. A. (1980). Stretch-induced growth in chicken wing muscles: a new model of stretch hypertrophy. Am. J. Physiol.238, C62–C71.
60
Hoppeler H. (2014). Eccentric Exercise: Physiology and Application in Sport and Rehabilitation. Taylor & Francis.
61
Hoppeler H. (2016). Moderate load eccentric exercise; a distinct novel training modality. Front. Physiol.7:483. 10.3389/fphys.2016.00483
62
Hoppeler H. Baum O. Lurman G. Mueller M. (2011). Molecular mechanisms of muscle plasticity with exercise. Compr. Physiol.1, 1383–1412. 10.1002/cphy.c100042
63
Hortobágyi T. Dempsey L. Fraser D. Zheng D. Hamilton G. Lambert J. et al . (2000). Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J. Physiol.524(Pt 1), 293–304. 10.1111/j.1469-7793.2000.00293.x
64
Hortobágyi T. Hill J. P. Houmard J. A. Fraser D. D. Lambert N. J. Israel R. G. (1996). Adaptive responses to muscle lengthening and shortening in humans. J. Appl. Physiol. 80, 765–772.
65
Huxley A. F. (1957). Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem.7, 255–318.
66
Huxley A. F. Simmons R. M. (1971). Proposed mechanism of force generation in striated muscle. Nature233, 533–538. 10.1038/233533a0
67
Hyldahl R. D. Chen T. C. Nosaka K. (2017). Mechanisms and mediators of the skeletal muscle repeated bout effect. Exerc. Sport Sci. Rev.45, 24–33. 10.1249/JES.0000000000000095
68
Hyldahl R. D. Hubal M. J. (2013). Lengthening our perspective: morphological, cellular and molecular responses to eccentric exercise. Muscle Nerve. 49, 155–170. 10.1002/mus.24077
69
Hyldahl R. D. Nelson B. Xin L. Welling T. Groscost L. Hubal M. J. et al . (2015). Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J.29, 2894–2904. 10.1096/fj.14-266668
70
Hyldahl R. D. Olson T. Welling T. Groscost L. Parcell A. C. (2014). Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol.5:485. 10.3389/fphys.2014.00485
71
Isner-Horobeti M.-E. Dufour S. P. Vautravers P. Geny B. Coudeyre E. Richard R. (2013). Eccentric exercise training: modalities, applications and perspectives. Sports Med.43, 483–512. 10.1007/s40279-013-0052-y
72
Jones D. A. Round J. M. de Haan A. (2004). Skeletal Muscle from Molecules to Movement: A Textbook of Muscle Physiotherapy for Sport, Exercise and Physiotherapy. Churchill Livingstone.
73
Jones D. A. Rutherford O. M. (1987). Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J. Physiol.391, 1–11. 10.1113/jphysiol.1987.sp016721
74
Katz B. (1939). The relation between force and speed in muscular contraction. J. Physiol.45–64. 10.1113/jphysiol.1939.sp003756
75
Kawakami Y. Abe T. Fukunaga T. (1993). Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J. Appl. Physiol.74, 2740–2744.
76
Kehat I. Davis J. Tiburcy M. Accornero F. Saba-El-Leil M. K. Maillet M. et al . (2011). Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ. Res.108, 176–183. 10.1161/CIRCRESAHA.110.231514
77
Kellis E. Baltzopoulos V. (1998). Muscle activation differences between eccentric and concentric isokinetic exercise. Med. Sci. Sports Exerc.30, 1616–1623. 10.1097/00005768-199811000-00010
78
Kim S. Y. Ko J. B. Farthing J. P. Butcher S. J. (2015). Investigation of supraspinatus muscle architecture following concentric and eccentric training. J. Sci. Med. Sport18, 378–382. 10.1016/j.jsams.2014.05.007
79
Kinney M. C. Dayanidhi S. Dykstra P. B. McCarthy J. J. Peterson C. A. Lieber R. L. (2017). Reduced skeletal muscle satellite cell number alters muscle morphology after chronic stretch but allows limited serial sarcomere addition. Muscle Nerve55, 384–392. 10.1002/mus.25227
80
Komi P. V. Buskirk E. R. (1972). Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics15, 417–434.
81
Komi P. V. Linnamo V. Silventoinen P. Sillanpää M. (2000). Force and EMG power spectrum during eccentric and concentric actions. Med. Sci. Sports Exerc.32, 1757–1762. 10.1097/00005768-200010000-00015
82
Konow N. Roberts T. J. (2015). The series elastic shock absorber: tendon elasticity modulates energy dissipation by muscle during burst deceleration. Proc. Biol. Sci.282:20142800. 10.1098/rspb.2014.2800
83
Kostek M. C. Chen Y.-W. Cuthbertson D. J. Shi R. Fedele M. J. Esser K. A. et al . (2007). Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol. Genomics31, 42–52. 10.1152/physiolgenomics.00151.2006
84
Lieber R. L. Fridén J. (2000). Functional and clinical significance of skeletal muscle architecture. Muscle Nerve23, 1647–1666. 10.1002/1097-4598(200011)23:11<1647::AID-MUS1>3.0.CO;2-M
85
Lombardi V. Piazzesi G. (1990). The contractile response during steady lengthening of stimulated frog muscle fibres. J. Physiol.431, 141–171. 10.1113/jphysiol.1990.sp018324
86
Lundberg T. R. Fernandez-Gonzalo R. Gustafsson T. Tesch P. A. (2013). Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J. Appl. Physiol.114, 81–89. 10.1152/japplphysiol.01013.2012
87
Lynn R. Morgan D. L. (1994). Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J. Appl. Physiol. 77, 1439–1444.
88
Mackey A. L. Kjaer M. (2016). Connective tissue regeneration in skeletal muscle after eccentric contraction-induced injury. J. Appl. Physiol.122, 533–540. 10.1152/japplphysiol.00577.2016
89
Martineau L. C. Gardiner P. F. (2001). Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol.91, 693–702.
90
Mauro A. (1961). Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol.9, 493–495. 10.1083/jcb.9.2.493
91
Mayhew T. P. Rothstein J. M. Finucane S. D. Lamb R. L. (1995). Muscular adaptation to concentric and eccentric exercise at equal power levels. Med. Sci. Sports Exerc. 27, 868–873.
92
McHugh M. P. (2003). Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand. J. Med. Sci. Sports13, 88–97. 10.1034/j.1600-0838.2003.02477.x
93
McMahon G. E. Morse C. I. Burden A. Winwood K. Onambélé G. L. (2014). Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J. Strength Cond. Res.28, 245–255. 10.1519/JSC.0b013e318297143a
94
Mitchell W. K. Taivassalo T. Narici M. V. Franchi M. V. (2017). Eccentric Exercise and the Critically Ill Patient. Front. Physiol.8:120. 10.3389/fphys.2017.00120
95
Monroy J. A. Powers K. L. Gilmore L. A. Uyeno T. A. Lindstedt S. L. Nishikawa K. C. (2012). What is the role of titin in active muscle?Exerc. Sport Sci. Rev.40, 73–78. 10.1097/JES.0b013e31824580c6
96
Moore D. R. Phillips S. M. Babraj J. A. Smith K. Rennie M. J. (2005). Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am. J. Physiol. Endocrinol. Metab.288, E1153–E1159. 10.1152/ajpendo.00387.2004
97
Moore D. R. Young M. Phillips S. M. (2012). Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur. J. Appl. Physiol.112, 1587–1592. 10.1007/s00421-011-2078-x
98
Morgan D. L. Talbot J. A. (2002). The addition of sarcomeres in series is the main protective mechanism following eccentric exercise. J. Mech. Med. Biol.2, 421–431. 10.1142/S0219519402000423
99
Morgan R. S. (1977). Actin rotates as myosin translates. J. Theor. Biol.67, 769–771. 10.1016/0022-5193(77)90261-2
100
Narici M. (1999). Human skeletal muscle architecture studied in vivo by non-invasive imaging techniques: functional significance and applications. J. Electromyogr. Kinesiol.9, 97–103. 10.1016/s1050-6411(98)00041-8
101
Narici M. V. Binzoni T. Hiltbrand E. Fasel J. Terrier F. Cerretelli P. (1996). In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. 496(Pt 1), 287–297.
102
Narici M. V. Roi G. S. Landoni L. Minetti A. E. Cerretelli P. (1989). Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur. J. Appl. Physiol. Occup. Physiol.59, 310–319. 10.1007/BF02388334
103
Narici M. Franchi M. Maganaris C. (2016). Muscle structural assembly and functional consequences. J. Exp. Biol.219, 276–284. 10.1242/jeb.128017
104
Nickols-Richardson S. M. Miller L. E. Wootten D. F. Ramp W. K. Herbert W. G. (2007). Concentric and eccentric isokinetic resistance training similarly increases muscular strength, fat-free soft tissue mass, and specific bone mineral measurements in young women. Osteoporos. Int. 18, 789–796. 10.1007/s00198-006-0305-9
105
Nishikawa K. C. Monroy J. A. Uyeno T. E. Yeo S. H. Pai D. K. Lindstedt S. L. (2012). Is titin a “winding filament”? A new twist on muscle contraction. Proc. Biol. Sci.279, 981–990. 10.1098/rspb.2011.1304
106
Noorkõiv M. Nosaka K. Blazevich A. J. (2014). Neuromuscular adaptations associated with knee joint angle-specific force change. Med. Sci. Sport. Exerc.46, 1525–1537. 10.1249/MSS.0000000000000269
107
Paddon-Jones D. Lonergan A. Abernethy P. Leveritt M. (2001). Adaptation to chronic eccentric exercise in humans: the influence of contraction velocity. Eur. J. Appl. Physiol.85, 466–471. 10.1007/s004210100467
108
Phillips S. M. Tipton K. D. Aarsland A. Wolf S. E. Wolfe R. R. (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol.273, E99–E107.
109
Potier T. G. Alexander C. M. Seynnes O. R. (2009). Effects of eccentric strength training on biceps femoris muscle architecture and knee joint range of movement. Eur. J. Appl. Physiol.105, 939–944. 10.1007/s00421-008-0980-7
110
Proske U. Morgan D. L. (2001). Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 537(Pt 2), 333–345. 10.1111/j.1469-7793.2001.00333.x
111
Rahbek S. K. Farup J. MÃýller A. B. Vendelbo M. H. Holm L. Jessen N. et al . (2014). Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids46, 2377–2392. 10.1007/s00726-014-1792-1
112
Rahnert J. A. Burkholder T. J. (2013). High-frequency electrical stimulation reveals a p38-mTOR signaling module correlated with force-time integral. J. Exp. Biol.216, 2619–2631. 10.1242/jeb.080705
113
Rayment I. Holden H. M. Whittaker M. Yohn C. B. Lorenz M. Holmes K. C. et al . (1993). Structure of the actin-myosin complex and its implications for muscle contraction. Science261, 58–65. 10.1126/science.8316858
114
Reeves N. D. Maganaris C. N. Longo S. Narici M. V (2009). Differential adaptations to eccentric versus conventional resistance training in older humans. Exp. Physiol.94, 825–833. 10.1113/expphysiol.2009.046599
115
Roig M. O'Brien K. Kirk G. Murray R. McKinnon P. Shadgan B. et al . (2009). The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br. J. Sports Med.43, 556–568. 10.1136/bjsm.2008.051417
116
Rome L. C. Cook C. Syme D. A. Connaughton M. A. Ashley-Ross M. Klimov A. et al . (1999). Trading force for speed: why superfast crossbridge kinetics leads to superlow forces. Proc. Natl. Acad. Sci. U.S.A.96, 5826–5831. 10.1073/pnas.96.10.5826
117
Seger J. Y. Arvidsson B. Thorstensson A. (1998). Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur. J. Appl. Physiol. Occup. Physiol.79, 49–57.
118
Seynnes O. R. de Boer M. Narici M. V (2007). Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J. Appl. Physiol.102, 368–373. 10.1152/japplphysiol.00789.2006
119
Shepstone T. N. Tang J. E. Dallaire S. Schuenke M. D. Staron R. S. Phillips S. M. (2005). Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J. Appl. Physiol.98, 1768–1776. 10.1152/japplphysiol.01027.2004
120
Smith R. C. Rutherford O. M. (1995). The role of metabolites in strength training. I. A comparison of eccentric and concentric contractions. Eur. J. Appl. Physiol. Occup. Physiol.71, 332–336. 10.1007/BF00240413
121
Tesch P. A. Fernandez-Gonzalo R. Lundberg T. R. (2017). Clinical applications of iso-inertial, eccentric-overload (YoYoTM) resistance exercise. Front. Physiol.8:241. 10.3389/fphys.2017.00241
122
Tesch P. A. Thorsson A. Colliander E. B. (1990). Effects of eccentric and concentric resistance training on skeletal muscle substrates, enzyme activities and capillary supply. Acta Physiol. Scand.140, 575–580. 10.1111/j.1748-1716.1990.tb09035.x
123
Timmins R. G. Ruddy J. D. Presland J. Maniar N. Shield A. J. Williams M. D. et al . (2016a). Architectural changes of the biceps femoris long head after concentric or eccentric training. Med. Sci. Sport Exerc.48, 499–508. 10.1249/MSS.0000000000000795
124
Timmins R. G. Shield A. J. Williams M. D. Lorenzen C. Opar D. A. (2016b). Architectural adaptations of muscle to training and injury: a narrative review outlining the contributions by fascicle length, pennation angle and muscle thickness. Br. J. Sports Med.50, 1467–1472. 10.1136/bjsports-2015-094881
125
VanBuren P. Waller G. S. Harris D. E. Trybus K. M. Warshaw D. M. Lowey S. (1994). The essential light chain is required for full force production by skeletal muscle myosin. Proc. Natl. Acad. Sci. U.S.A.91, 12403–12407. 10.1073/pnas.91.26.12403
126
Verrecchia F. Chu M.-L. Mauviel A. (2001). Identification of novel TGF- /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem.276, 17058–17062. 10.1074/jbc.M100754200
127
Vikne H. Refsnes P. E. Ekmark M. Medbø J. I. Gundersen V. Gundersen K. (2006). Muscular performance after concentric and eccentric exercise in trained men. Med. Sci. Sports Exerc. 38, 1770–1781. 10.1249/01.mss.0000229568.17284.ab
128
Wernbom M. Augustsson J. Thomeé R. (2007). The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med.37, 225–264. 10.2165/00007256-200737030-00004
129
Westing S. H. Cresswell A. G. Thorstensson A. (1991). Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur. J. Appl. Physiol. Occup. Physiol.62, 104–108. 10.1007/BF00626764
130
Westing S. H. Seger J. Y. Karlson E. Ekblom B. (1988). Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur. J. Appl. Physiol. Occup. Physiol.58, 100–104. 10.1007/BF00636611
131
Williams P. E. (1990). Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann. Rheum. Dis.49, 316–317. 10.1136/ard.49.5.316
132
Williams P. E. Catanese T. Lucey E. G. Goldspink G. (1988). The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J. Anat.158, 109–114.
133
Williams P. E. Goldspink G. (1971). Longitudinal growth of striated muscle fibres. J. Cell Sci. 9, 751–767.
134
Wretman C. Lionikas A. Widegren U. Lännergren J. Westerblad H. Henriksson J. (2001). Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J. Physiol.535, 155–164. 10.1111/j.1469-7793.2001.00155.x
Summary
Keywords
eccentric exercise, concentric exercise, eccentric contraction, muscle architecture, muscle remodeling, muscle hypertrophy, muscle signaling, mechanotransduction
Citation
Franchi MV, Reeves ND and Narici MV (2017) Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 8:447. doi: 10.3389/fphys.2017.00447
Received
27 February 2017
Accepted
13 June 2017
Published
04 July 2017
Volume
8 - 2017
Edited by
Kimberly Huey, Drake University, United States
Reviewed by
Kevin Andrew Murach, University of Kentucky, United States; Sandra K. Hunter, Marquette University, United States
Updates
Copyright
© 2017 Franchi, Reeves and Narici.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martino V. Franchi Martino.Franchi@balgrist.ch
This article was submitted to Exercise Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.