- 1Laboratory of Bio-Pesticide Creation and Application of Guangdong Province, College of Agriculture, South China Agricultural University, Guangzhou, China
- 2Beijing Genomic Institute, Shenzhen, China
- 3School of Life Sciences, Central China Normal University, Wuhan, China
The development of resistance by Plutella xylostella to almost all insecticides is of significant concern all over the world. Entomopathogenic fungi such as Isaria fumosorosea have been used as an alternative to insecticides. However, the knowledge of miRNA-regulated reactions against entomopathogenic fungi is still in its infant stage. In the present study, P. xylostella was challenged with I. fumosorosea at four different time points (12, 18, 24, and 36 h) including a control, to build miRNA libraries by Illumina sequencing. The results of differential expression analysis exhibited that 23 miRNAs were differentially expressed, compared to control, in all treatments. It is worth mentioning, of these, some conserved miRNAs such as miR-2, miR-9a, miR-745, miR-7b, and miR-2767, known to play critical roles in host-pathogen interaction, were also identified. Furthermore, differentially expressed miRNAs were validated by RT-qPCR. Our results provide an essential information for further functional studies of the interaction between I. fumosorosea and P. xylostella at the post-transcriptional level.
Introduction
The diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), is recognized as a major invasive pest of Brassica crops worldwide. The annual control and damage costs for this pest has reached approximately at 4–5 billion dollars globally (Zalucki et al., 2012). The use of chemicals is considered as the major tool for suppressing P. xylostella populations, however, this pest quickly evolves insecticide resistance (Shakeel et al., 2017a). The growing concern of insecticide resistance coupled with their harmful effects on the environment has drawn the attention of worldwide researchers toward the development of alternative control strategies (Shakeel et al., 2017a). Therefore, the use of biological control agents, such as entomopathogenic fungi, has received an increased attention. There are several benefits of employing fungal biological control agents, including a decreased impact on the environment, less chance of resistance development, and decreased no-target effects (Lai and Su, 2011; Fan et al., 2012; Smalling et al., 2013). A number of entomopathogenic fungi have been isolated and used to control several insect pests, including P. xylostella (Altre et al., 1999; Leemon and Jonsson, 2008; Bukhari et al., 2011). Amongst them, Isaria fumosorosea has received attention to be used as a potential fungal biological control agent and has been used in various mycopesticides worldwide (Zimmermann, 2008).
The field of immunology, one of the fascinating facets of biology, has always attracted researchers to elucidate the mechanisms, molecular and cellular, involved in sensing and neutralizing the infectious foreign agents (Imler, 2014). All multicellular organisms have developed a potent and diversified immune system to protect themselves from infectious microorganisms. Insects represent by far the most numerous and diverse group of multicellular organisms. Although insects lack adaptive immunity, specialized defense system of vertebrates, they do have innate immunity that is consisted of cellular and humoral immune responses (Hultmark, 1993). The cellular innate immune response is mainly mediated by hemocytes and comprises phagocytosis, encapsulation, and nodulation (Lavine and Strand, 2002). The insect humoral reactions involve clotting, melanization, and production of potent antimicrobial peptides (Hoffmann and Reichhart, 2002).
MicroRNAs, small non-coding RNA molecules of 18–24 nucleotides in length, are vital regulators of gene expression at the post-transcriptional level in metazoans (Nehammer et al., 2015). In eukaryotes, gene expression is regulated by miRNAs via specific base-pairing with the 3′ untranslated regions (UTRs) of corresponding target genes (Bartel, 2009). There is an increasing number of reports that miRNAs play vital roles in many physiological processes, including development, apoptosis, cell division and differentiation, and immune challenge (Brennecke et al., 2003; Stark et al., 2003; Leaman et al., 2005; Asgari, 2011). While there is a well-established information available about the role of miRNAs in vertebrate development, knowledge is limited about their roles in insect host-pathogen interactions (Hussain and Asgari, 2014). Although the role of insect miRNAs against viruses is recognized, there is no report, until now, according to our information, on miRNA-regulated reactions against entomopathogenic fungi such as I. fumosorosea.
Previously, our results of RNA-Seq and differentially expressed gene expression (DGE) analysis of destruxin A and I. fumosorosea treated P. xylostella exhibited that most of the immunity-related genes were up-regulated in response to destruxin A injection, whereas I. fumosorosea has the ability to suppress the immune system of P. xylostella (Shakeel et al., 2017c; Xu et al., 2017). Therefore, given the fact that miRNAs play important role in host-pathogen interaction, herein, we aimed to explore the response of P. xylostella miRNAs to I. fumosorosea, and to determine how the abundance of differential expression of known and novel miRNAs changes following an infection and whether it varies at different times of infection. To achieve these results, we profiled miRNA expression in P. xylostella infected with I. fumosorosea at 12, 18, 24, and 36 h time points with a control using small RNA deep sequencing.
Materials and Methods
Insect Stock
The susceptible population of P. xylostella was maintained under insecticide free conditions for 10 generations in the Engineering Research Centre of Biological Control, Ministry of Education, South China Agricultural University (SCAU). The insects were kept at 60–70% relative humidity and at 25 ± 1°C under a 16:8 h light: dark cycle.
Fungal Strain and Samples Collection
Stain IfB01 of I. fumosorosea (China Center for Type Culture Collection access number: CCTCC M 2012400) was grown on potato dextrose agar (PDA) at 26°C. The conidia were prepared as described previously (Huang et al., 2010). Healthy third instar larvae of P. xylostella were selected and treated with 1 × 107 spores/ml suspension and then surviving larvae (50) were collected at 12, 18, 24, and 36 h, post-treatment. The control group larvae were treated with sterile deionized water containing 0.05% Tween-80 and the samples were collected at 0 h post-treatment.
RNA Extraction, Small RNA Library Construction, and Sequencing
Trizol Total RNA Isolation Kit (Takara, Japan) was used to extract total RNA from normal and treated larval samples following manufacturer's instructions. The concentrations of RNA were assessed using Nanodrop (Bio-Rad, USA) and its integrity was determined on Agilent 2100 Bioanalyzer (Agilent, USA). The small RNA libraries were constructed from each time-point of infection using a TruSeq small RNA sample preparation kit (Illumina). Briefly, RNAs were firstly ligated with 3′ adapter and after size fraction ligated to 5′ adapter. The small RNA fractions were then used for reverse transcription following PCR. The final ligation PCR products, after purification, were sequenced using Illumina Genome Analyzer (San Diego, CA, USA) at the Beijing Genomics Institute (BGI, Shenzhen, China).
Bioinformatics Analysis of Small RNA Sequences
To screen clean reads, raw data reads were filtered to remove low-quality, 5′ primer contaminants, without 3′ primers and insert tag, and sequences fewer than 18 nucleotides. The remaining high-quality reads were initially mapped to P. xylostella genome (GCA_000330985.1) using Bowtie software (Langmead and Salzberg, 2012), and then annotated into different classes to remove rRNA, scRNA, snoRNA, snRNA, and tRNA using Rfam database. Finally, the unannotated clean sequences were used to predict novel miRNAs using the miRDeep2 software.
Differential Expression Analysis of miRNAs
The expression of miRNAs was compared between treatment and control to identify differentially expressed miRNAs. First, the expression of miRNA in the five libraries was normalized to transcripts per million (TPM). If the normalized expression of the miRNA was 0, it was modified to 0.01 to enable calculation. If the normalized expression of the miRNA was less than 1 in all libraries, it was ignored to compare for low expression. The normalization formula was:
The normalized data were then used to calculate fold-change values and P-values, and a scatter plot of the fold-change values was generated. Fold-change was calculated as;
Fold-change = log2 (Treatment/Control).
The P-value was calculated by the following equation:
Where x represents small RNA total clean reads in the control, y represents total clean reads in the treatment, N1 represents the normalized expression of a miRNA in library control, and N2 represents the normalized expression of the same miRNA in library treatment. The corrected P-value corresponds to differential gene expression test using Bonferroni method (Abdi, 2007).
miRNA Target Prediction and Functional Analysis
The potential mRNA targets of differentially expressed miRNAs were predicted and analyzed using three different programs, such as RNAhybrid, miRanda, and TargetScan following already established criteria for target prediction (Allen et al., 2005; Schwab et al., 2005). To get more reliable results, we selected those mRNA targets which were predicted by all three programs. Additionally, functional annotation of all the predicted target genes was conducted by using Gene Ontology (GO) database and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, with the threshold set at a corrected P-value ≤ 0.05.
RT-qPCR Validation
Real-time quantitative PCR (RT-qPCR) is the method of choice for analyzing expression of genes and to confirm the results of RNA-Sequencing (Shakeel et al., 2017b). Thus, to confirm the results of sRNA-Seq in the current study, RT-qPCR analysis was conducted to ensure the expression levels of miRNAs displayed by Illumina sequencing results and 10 miRNAs were selected. RT-qPCR was performed on a Bio-Rad iQ2 optical system (Bio-Rad) using SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA) following the instructions of the manufacturer. The U6 snRNA was used as an internal control. The reaction program was set as 95°C for 30 s, 40 cycles of 95°C for 5 s, and 55°C for 10 s with a dissociation curve generated from 65 to 95°C to ensure the purity of PCR products (Shakeel et al., 2015). Each experiment was replicated in triplicate. Finally, data analysis was performed using 2−ΔΔCT method (Livak and Schmittgen, 2001).
Results and Discussion
Overview of Small RNA Dataset
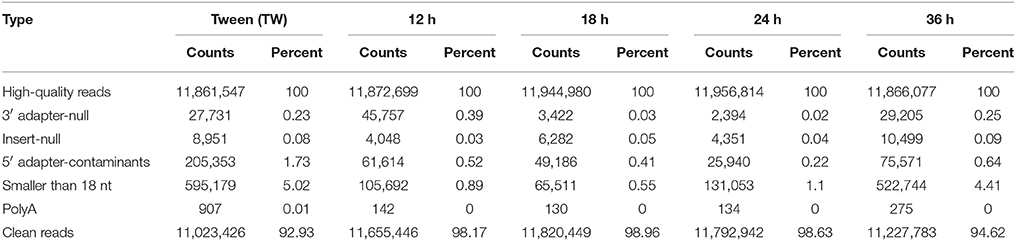
To identify miRNAs in I. fumosorosea challenged P. xylostella, we constructed five small RNA libraries (Tween (TW), 12, 18, 24, and 36 h) using high-throughput Illumina sequencing platform. In total, 11,861,547; 11,872,699; 11,944,980; 11,956,814, and 11,866,077 raw reads were obtained, respectively. After low-quality sequences, adaptors, and sequences less than 18 nucleotides were discarded, 92.93, 98.17, 98.96, 98.63, and 94.62% clean reads were obtained in TW, 12, 18, 24, and 36 h, respectively, for further analysis (Table 1).
The small RNA size distribution in the five libraries showed that most of the sRNAs ranged from 18 to 30 nt, with 28 nt being the most abundant following 22, and 23 nt (Figure 1) in the five libraries. The two peaks observed at 22 and 28 nt, in the present study, represent a typical length of miRNAs and piwi-interacting RNAs, respectively. Our findings are in consistency with the typical size of miRNAs and piwi-interacting RNAs in previous reports (Wei et al., 2009; Etebari et al., 2013; Xu et al., 2015; Li et al., 2016). Among the clean reads, 85.10% sRNAs were common between 12 and 18 h, 85.31% sRNAs between 24 and 12 h, 83.28% sRNAs between 24 and 18 h, 83.92% sRNAs between 24 and 36 h, 84.55% sRNAs between 24 h and TW, 84.89% sRNAs between 36 and 12 h, 83.91% sRNAs between 36 and 18 h, 86.44% sRNAs between 36 h and TW, 85.77% sRNAs between TW and 12 h, and 84.18% sRNAs between TW and 18 h, respectively (Supplementary Figure 1).
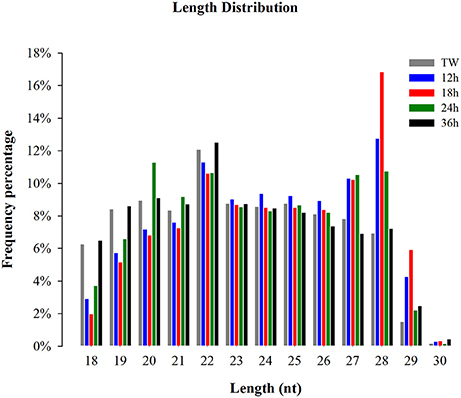
Figure 1. Size distribution of small RNA reads in the libraries of Plutella xylostella. Different colors represent different libraries. X-axis represents small RNA length distribution and Y-axis represents frequency percentage. Tween (TW) was used as a control.
Genome Mapping and Small RNA Annotation
Of the clean reads, 6,784,087, 6,965,570, 7,410,628, 7,023,256, and 6,510,880 reads from control, 12, 18, 24, and 36 h accounted for 61.54, 59.76, 62.69, 59.55, and 57.99%, respectively, and were mapped to the genome of P. xylostella (Supplementary Table 1). The annotation of sRNAs was carried out by following priority rule of rRNA etc.; (GenBank>Rfam) > known miRNA > repeat > exon > intron (Calabrese et al., 2007). The clean reads were categorized into miRNA, rRNA, snRNA, snoRNA, tRNA, and unannotated (unann). The composition of the sRNA classes in each library is displayed in Supplementary Figure 2.
Identification of Known and Novel miRNAs
After successful mapping of clean reads against P. xylostella genome, the mapped miRNA sequences were matched to miRNAs reported by Etebari and Asgari (2016). Our analysis initially identified, based on sequence similarity, in total, 191 mature miRNAs. Then, precursor sequences of these mature miRNAs were aligned to those reported by Etebari and Asgari (2016), and 102 highly confident precursor miRNAs, which produced 172 of 194 mature miRNAs. Our analysis indicated that precursor miRNA sequences of the remaining 22 conserved miRNAs were not detectable in the current assembly of P. xylostella genome. After removing those known miRNAs with read count < 10 in all libraries, remaining 116 known miRNAs with precursor sequences (Supplementary Table 2), and 15 miRNAs without precursor sequences (Supplementary Table 3) were retained for further analysis. The remaining sequences that were not matched to conserved miRNAs were used to predict novel miRNAs by using miRDeep2 program (Friedländer et al., 2012). The prediction of novel miRNAs analysis predicted 42 potential novel miRNAs from all the libraries (Supplementary Table 4) following the standard criteria of novel miRNA prediction with a miRDeep score >1, randfold P-value < 0.05, and MFE < −19 kcal/mol.
It is worth mentioning that a low copy number of miR-1, a conserved miRNA, was detected after parasitization in a previous report (Etebari et al., 2013), however, in the present study, miR-1 was the most abundant miRNA following pxy-let-7-5p, pxy-miR-184-3p, pxy-miR-10-3p, and miR-31-5p (Table 2). The abundant and common expression of these conserved miRNAs indicates that these miRNAs might play crucial roles in P. xylostella. Our results are in consistency with previous reports where a high expression of these miRNAs was observed in other insect small RNA libraries (Cai et al., 2010; Cristino et al., 2011; Liu et al., 2012). Bantam, a most abundantly expressed miRNA, plays multiple roles in insects such as apoptosis inhibition, cell proliferation and stem cell stem cell maintenance, and immunity in Drosophila melanogaster (Smibert and Lai, 2010; Fullaondo and Lee, 2012). Although a high copy number of bantam was observed in our study, however, its up-regulation after infection was less than 1-fold. Let-7, a highly conserved miRNA, has also been reported to play an important role in immunity, for example, it binds to 3' UTR of antimicrobial peptide diptericin to repress translation of this protein in D. melanogaster (Garbuzov and Tatar, 2010). Interestingly, we found that few miRNAs like miR-2755, miR-10, and miR-31 showed high expression in all treatments. A higher expression of these miRNAs after fungal treatment indicates that these miRNAs might play important roles in defending P. xylostella against pathogens.
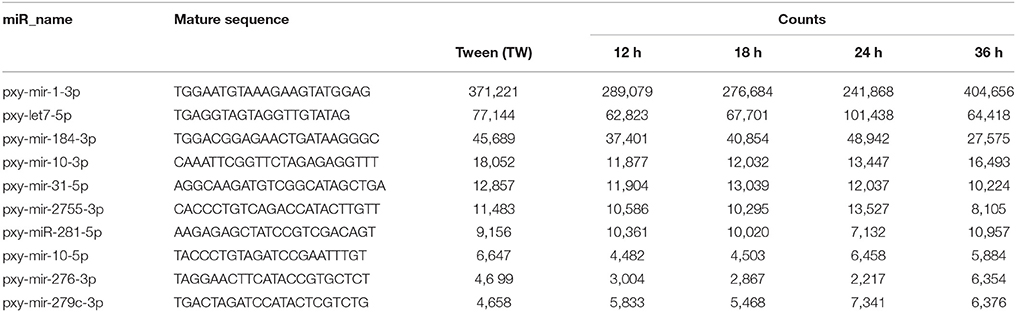
Table 2. Top 10 most abundant miRNAs commonly expressed in the five libraries of Plutella xylostella.
The novel miRNA analysis identified 42 potential novel miRNAs in P. xylostella after infection (Supplementary Table 4). Among novel miRNAs, pxy-novel-miR-26 was the abundantly expressed miRNA following pxy-novel-miR-1, pxy-novel-miR-33, and pxy-novel-miR-35 (Supplementary Table 4).
I. fumosorosea Responsive miRNAs
The differential abundance of host miRNAs, a common observation in host-pathogen systems, changes at different infection stages following an infection (Asgari, 2011). In the present study, to find out the I. fumosorosea responsive miRNAs, a differential expression analysis was performed using the sequencing results (Figure 2). The differential expression analysis exhibited that 13, 12, 16, and 5 known miRNAs were differentially expressed in 12, 18, 24, and 36 h, respectively, compared to control (Supplementary Table 5). The top five differentially expressed known miRNAs are presented in Table 3. Furthermore, 12, 19, 13, and 11 novel miRNAs were differentially expressed, in 12, 18, 24, and 36 h, respectively, compared to control (Supplementary Table 6).
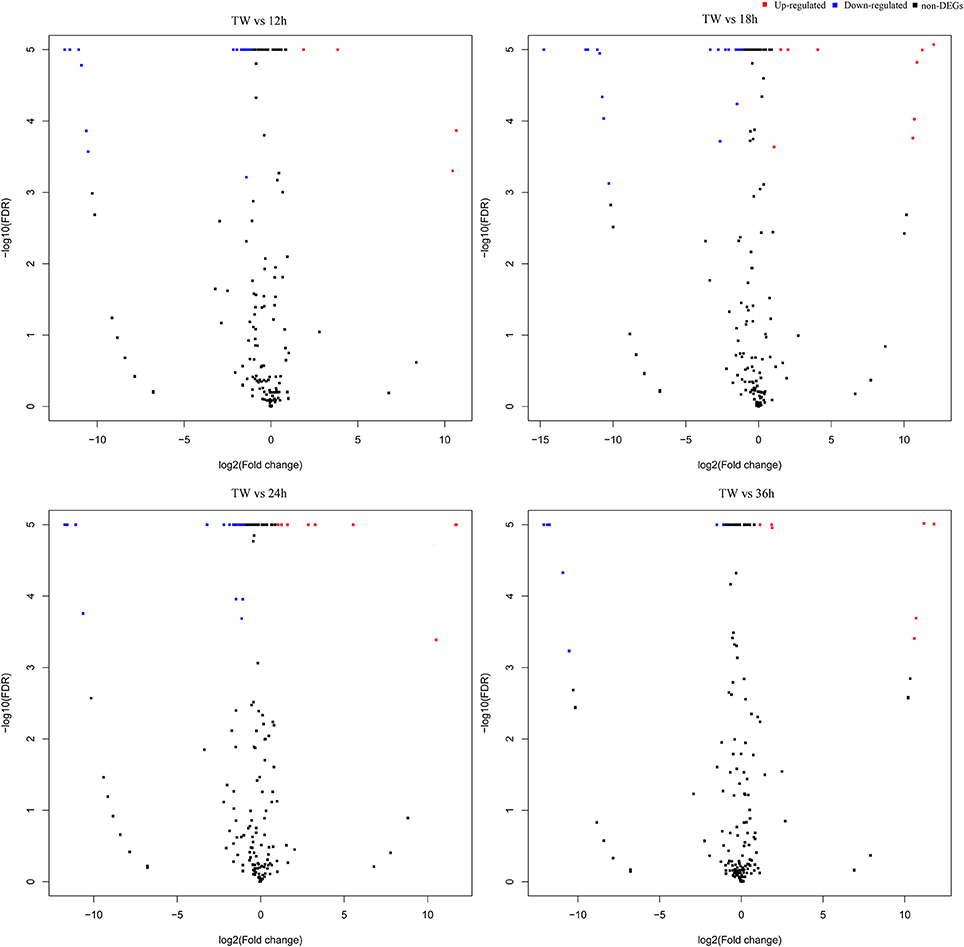
Figure 2. Volcano plot of differentially expressed microRNAs in Plutella xylostella post-infection. The volcano plots represent differentially expressed miRNAs at different time points (12, 18, 24, and 36 h) post-infection compared to control.
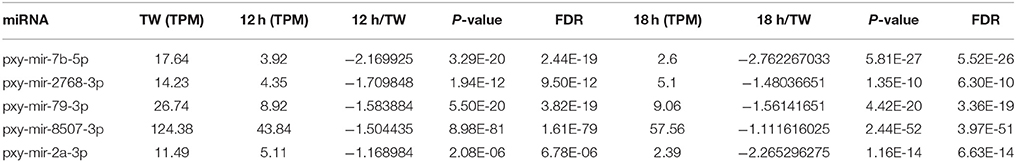
Table 3. Five common differentially expressed miRNAs at 12 and 18 h compared to Tween (TW) in Plutella xylostella.
Interestingly, in the present study, we found that the expression of few conserved miRNAs like miR-2, miR-9, miR-279, miR-745, miR-7b, and miR-2767 was changed following the infection of I. fumosorosea. Our findings suggest that these miRNAs might play very important roles in P. xylostella immunity to I. fumosorosea. In accordance to our study, previous reports also suggested the important roles of these conserved miRNAs in the immunity of different insects against different pathogens, such as bacteria-injected larvae of Manduca sexta and Diadegma semiclausum parasitized P. xylostella resulted in differential expression of miR-2, miR-9, and miR-279, indicating the role of these miRNAs in immunity against bacteria and parasite, respectively (Zhang et al., 2012; Etebari et al., 2013). It is of note that miR-9 has been predicted to play an essential role in signal recognition in M. sexta, and in toll pathway in Drosophila melanogaster (Fullaondo and Lee, 2012). It is worth mentioning that the read number of most of the miRNAs dropped after infection and, overall, only 3 miRNAs (miR-282,−2796, and −34) were up-regulated while 20 miRNAs were down-regulated in all the treatments compared to control. Previously, it has been reported that when Galleria mellonella was infected with entomopathogenic fungi, Metarhizium anisopliae, at larval stage, only one miRNA (miR-210b) showed differential expression (Mukherjee and Vilcinskas, 2014), whereas, in our study, 23 miRNAs were differentially expressed, however, miR-210b was not detected in our small RNA libraries.
Validation of Differentially Expressed miRNAs by RT-qPCR
To validate small RNA sequencing results, 10 randomly selected miRNAs were analyzed by RT-qPCR (Figure 3). The results exhibited that the trend of the expression level of the selected miRNAs showed consistency with sequencing results except for a few miRNAs like pxy-miR-2a-3p, pxy-miR-2b-3p, and pxy-miR-274-5p.
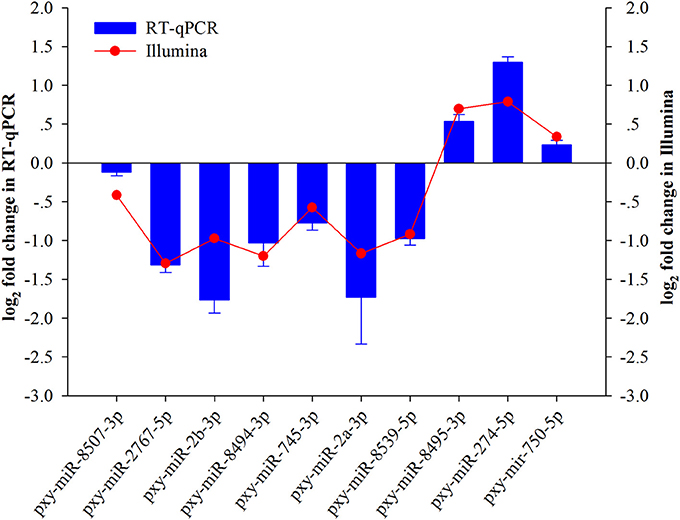
Figure 3. Validation of expression of ten miRNAs achieved by RT-qPCR and sRNA-Seq in Plutella xylostella after Isaria. fumosorosea infection. Error bars represent ± SD from three independent experiments. U6 snRNA was used as an internal control.
Prediction and Annotation of miRNA Target Genes
To better understand the function of differentially expressed miRNAs, putative target genes were predicted using the genome of P. xylostella using RNAhybrid, miRanda, and TargetScan software. Our target prediction results indicated that 30,930 common spots were detected between RNAhybrid and TargetScan, 30,818 between RNAhybrid and miRanda, and 31,942 between TargetScan and miRanda. When the target prediction results of all three software were combined, 30,699 common spots were detected and were selected for further analysis (Figure 4).
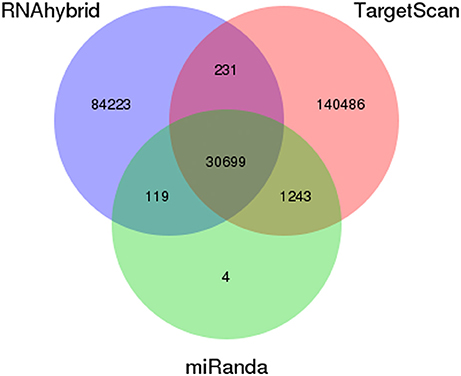
Figure 4. Prediction of potential target genes in all libraries. Venn diagram show the number of miRNA targets and their overlapping spots predicted by the three programs (RNAhybrid, miRanda, and TargetScan).
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analysis
The GO enrichment analysis was performed to classify the functions of miRNA target genes. The putative target genes were classified into three major categories, biological process, cellular component, and molecular function, of GO classification. Our results exhibited that cellular process, cell part, and catalytic activity were the most enriched categories in the biological process, cellular component, and molecular function, respectively, at all-time points of treatment (Supplementary Figure 3). Similar to our findings, previously, target genes of Ostrinia. furnacallis in response to Bacillus thuringiensis and Wolbachia- responsive miRNAs in Tetranychus urticae were also categorized into the cellular process, cell part, and catalytic activity (Rong et al., 2014; Xu et al., 2015).
To find out particular signaling pathways of the putative miRNA target genes, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed. The most enriched categories were transport and catabolism, signal transduction, and cancers in cellular processes, environmental information processing, and human diseases, respectively, at all-time points of infection (Supplementary Figure 4).
Conclusion
In conclusion, using high-throughput sRNA sequencing, we screened out I. fumosorosea responsive immunity-related miRNAs in P. xylostella. Based on our knowledge, this is the first study about immunity-related miRNA profiles of P. xylostella in response to I. fumosorosea. The major finding of this study is the identification of conserved immunity-related differentially expressed miRNAs such as miR-2, miR-9, miR-92, miR-745, and miR-2767. Our findings provide an essential information for further functional studies of the interaction between I. fumosorosea and P. xylostella at the post-transcriptional level.
Ethics Statement
Our work confirms to the legal requirements of the country in which it was carried out.
Author Contributions
Conceived and designed the experiments: FJ, MS, and XiaoxX. Performed the experiments: JX and XiaoxX. Analyzed the data: MS, XiaojX, JY, XZ, and JX. Contributed reagents, materials, analysis tools: SL and SW. Wrote the manuscript: MS, JX, and XiaoxX. Revised the manuscript: MS, FJ, and XY.
Funding
This work was supported by grant from The National Natural Science Foundation of China (31371989, 31572069), Science and Technology Program of Guangzhou China (201509010023) and Department of Science and Technology of Guangdong China (2014A020208106).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank BGI-Shenzhen for assisting in the sequencing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2017.01054/full#supplementary-material
References
Abdi, H. (2007). “Bonferroni and Šidák corrections for multiple comparisons,” in Encyclopedia of Measurement and Statistics, ed N. Salkind (Thousand Oaks, CA; London: SAGE Publications), 103–107.
Allen, E., Xie, Z., Gustafson, A. M., and Carrington, J. C. (2005). microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121, 207–221. doi: 10.1016/j.cell.2005.04.004
Altre, J. A., Vandenberg, J. D., and Cantone, F. A. (1999). Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J. Invertebr. Pathol. 73, 332–338. doi: 10.1006/jipa.1999.4844
Asgari, S. (2011). Role of microRNAs in insect host–microorganism interactions. Front. Physiol. 2:48. doi: 10.3389/fphys.2011.00048
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B., and Cohen, S. M. (2003). Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36. doi: 10.1016/S0092-8674(03)00231-9
Bukhari, T., Takken, W., and Koenraadt, C. J. (2011). Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit. Vectors 4:23. doi: 10.1186/1756-3305-4-23
Cai, Y., Yu, X., Zhou, Q., Yu, C., Hu, H., Liu, J., et al. (2010). Novel microRNAs in silkworm (Bombyx mori). Funct. Integr. Genomics 10, 405–415. doi: 10.1007/s10142-010-0162-7
Calabrese, J. M., Seila, A. C., Yeo, G. W., and Sharp, P. A. (2007). RNA sequence analysis defines Dicer's role in mouse embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 104, 18097–18102. doi: 10.1073/pnas.0709193104
Cristino, A. S., Tanaka, E. D., Rubio, M., Piulachs, M.-D., and Belles, X. (2011). Deep sequencing of organ-and stage-specific microRNAs in the evolutionarily basal insect Blattella germanica (L.) (Dictyoptera, Blattellidae). PLoS ONE 6:e19350. doi: 10.1371/journal.pone.0019350
Etebari, K., and Asgari, S. (2016). Revised annotation of Plutella xylostella microRNAs and their genome-wide target identification. Insect Mol. Biol. 25, 788–799. doi: 10.1111/imb.12263
Etebari, K., Hussain, M., and Asgari, S. (2013). Identification of microRNAs from Plutella xylostella larvae associated with parasitization by Diadegma semiclausum. Insect Biochem. Mol. Biol. 43, 309–318. doi: 10.1016/j.ibmb.2013.01.004
Fan, Y., Borovsky, D., Hawkings, C., Ortiz-Urquiza, A., and Keyhani, N. O. (2012). Exploiting host molecules to augment mycoinsecticide virulence. Nat. Biotechnol. 30, 35–37. doi: 10.1038/nbt.2080
Friedländer, M. R., Mackowiak, S. D., Li, N., Chen, W., and Rajewsky, N. (2012). miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40, 37–52. doi: 10.1093/nar/gkr688
Fullaondo, A., and Lee, S. Y. (2012). Identification of putative miRNA involved in Drosophila melanogaster immune response. Dev. Comp. Immunol. 36, 267–273. doi: 10.1016/j.dci.2011.03.034
Garbuzov, A., and Tatar, M. (2010). Hormonal regulation of Drosophila microRNA let-7 and miR-125 that target innate immunity. Fly 4, 306–311. doi: 10.4161/fly.4.4.13008
Hoffmann, J. A., and Reichhart, J. M. (2002). Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 3, 121–126. doi: 10.1038/ni0202-121
Huang, Z., Ali, S., Ren, S.-X., and Wu, J.-H. (2010). Effect of Isaria fumosoroseus on mortality and fecundity of Bemisia tabaci and Plutella xylostella. Insect Sci. 17, 140–148. doi: 10.1111/j.1744-7917.2009.01299.x
Hultmark, D. (1993). Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 9, 178–183. doi: 10.1016/0168-9525(93)90165-E
Hussain, M., and Asgari, S. (2014). MicroRNAs as mediators of insect host–pathogen interactions and immunity. J. Insect Physiol. 70, 151–158. doi: 10.1016/j.jinsphys.2014.08.003
Imler, J. L. (2014). Overview of Drosophila immunity: a historical perspective. Dev. Comp. Immunol. 42, 3–15. doi: 10.1016/j.dci.2013.08.018
Lai, T., and Su, J. (2011). Assessment of resistance risk in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest Manag. Sci. 67, 1468–1472. doi: 10.1002/ps.2201
Langmead, B., and Salzberg, S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. doi: 10.1038/nmeth.1923
Lavine, M. D., and Strand, M. R. (2002). Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 32, 1295–1309. doi: 10.1016/S0965-1748(02)00092-9
Leaman, D., Chen, P. Y., Fak, J., Yalcin, A., Pearce, M., Unnerstall, U., et al. (2005). Antisense-mediated depletion reveals essential and specific functions of microRNAs in Drosophila development. Cell 121, 1097–1108. doi: 10.1016/j.cell.2005.04.016
Leemon, D. M., and Jonsson, N. N. (2008). Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J. Invertebr. Pathol. 97, 40–49. doi: 10.1016/j.jip.2007.07.006
Li, X., Zhang, F., Coates, B., Zhang, Y., Zhou, X., and Cheng, D. (2016). Comparative profiling of microRNAs in the winged and wingless English grain aphid, Sitobion avenae (F.) (Homoptera: Aphididae). Sci. Rep. 6:35668. doi: 10.1038/srep35668
Liu, F., Peng, W., Li, Z., Li, W., Li, L., Pan, J., et al. (2012). Next-generation small RNA sequencing for microRNAs profiling in Apis mellifera: comparison between nurses and foragers. Insect Mol. Biol. 21, 297–303. doi: 10.1111/j.1365-2583.2012.01135.x
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mukherjee, K., and Vilcinskas, A. (2014). Development and immunity-related microRNAs of the lepidopteran model host Galleria mellonella. BMC Genomics 15:705. doi: 10.1186/1471-2164-15-705
Nehammer, C., Podolska, A., Mackowiak, S. D., Kagias, K., and Pocock, R. (2015). Specific microRNAs regulate heat stress responses in Caenorhabditis elegans. Sci. Rep. 5:8866. doi: 10.1038/srep08866
Rong, X., Zhang, Y. K., Zhang, K. J., and Hong, X. Y. (2014). Identification of Wolbachia-responsive microRNAs in the two-spotted spider mite, Tetranychus urticae. BMC Genomics 15:1122. doi: 10.1186/1471-2164-15-1122
Schwab, R., Palatnik, J. F., Riester, M., Schommer, C., Schmid, M., and Weigel, D. (2005). Specific effects of microRNAs on the plant transcriptome. Dev. Cell 8, 517–527. doi: 10.1016/j.devcel.2005.01.018
Shakeel, M., Farooq, M., Nasim, W., Akram, W., Khan, F. Z. A., Jaleel, W., et al. (2017a). Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: a review. Environ. Sci. Pollut. Res. 24, 14537–14550. doi: 10.1007/s11356-017-8996-3
Shakeel, M., Rodriguez, A., Tahir, U. B., and Jin, F. (2017b). Gene expression studies of reference genes for quantitative real-time PCR: an overview in insects. Biotechnol. Lett. doi: 10.1007/s10529-017-2465-4. [Epub ahead of print].
Shakeel, M., Xu, X., Xu, J., Zhu, X., Li, S., Zhou, X., et al. (2017c). Identification of immunity-related genes in Plutella xylostella in response to fungal peptide destruxin A: RNA-Seq and DGE analysis. Sci. Rep. 7:10966. doi: 10.1038/s41598-017-11298-7
Shakeel, M., Zhu, X., Kang, T., Wan, H., and Li, J. (2015). Selection and evaluation of reference genes for quantitative gene expression studies in cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 18, 123–130. doi: 10.1016/j.aspen.2015.01.001
Smalling, K. L., Kuivila, K. M., Orlando, J. L., Phillips, B. M., Anderson, B. S., Siegler, K., et al. (2013). Environmental fate of fungicides and other current-use pesticides in a central California estuary. Marine Poll. Bull. 73, 144–153. doi: 10.1016/j.marpolbul.2013.05.028
Smibert, P., and Lai, E. C. (2010). A view from Drosophila: multiple biological functions for individual microRNAs. Semin. Cell Dev. Biol. 21, 745–753. doi: 10.1016/j.semcdb.2010.03.001
Stark, A., Brennecke, J., Russell, R. B., and Cohen, S. M. (2003). Identification of Drosophila microRNA targets. PLoS Biol. 1:e60. doi: 10.1371/journal.pbio.0000060
Wei, Y., Chen, S., Yang, P., Ma, Z., and Kang, L. (2009). Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol. 10:R6. doi: 10.1186/gb-2009-10-1-r6
Xu, J., Xu, X., Shakeel, M., Li, S., Wang, S., Zhou, X., et al. (2017). The entomopathogenic fungi Isaria fumosorosea plays a vital role in suppressing the immune system of Plutella xylostella: RNA-Seq and DGE analysis of immunity-related genes. Front. Microbiol. 8:1421. doi: 10.3389/fmicb.2017.01421
Xu, L.-N., Ling, Y.-H., Wang, Y.-Q., Wang, Z.-Y., Hu, B.-J., Zhou, Z.-Y., et al. (2015). Identification of differentially expressed microRNAs between Bacillus thuringiensis Cry1Ab-resistant and-susceptible strains of Ostrinia furnacalis. Sci. Rep. 5:15461. doi: 10.1038/srep15461
Zalucki, M. P., Shabbir, A., Silva, R., Adamson, D., Shu-Sheng, L., and Furlong, M. J. (2012). Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J. Econ. Entomol. 105, 1115–1129. doi: 10.1603/EC12107
Zhang, X., Zheng, Y., Jagadeeswaran, G., Ren, R., Sunkar, R., and Jiang, H. (2012). Identification and developmental profiling of conserved and novel microRNAs in Manduca sexta. Insect Biochem. Mol. Biol. 42, 381–395. doi: 10.1016/j.ibmb.2012.01.006
Keywords: microRNAs, immunity, innate, Plutella xylostella, Isaria fumosorosea, host pathogen interactions
Citation: Xu J, Xu X, Li S, Wang S, Xu X, Zhou X, Yu J, Yu X, Shakeel M and Jin F (2017) Genome-Wide Profiling of Plutella xylostella Immunity-Related miRNAs after Isaria fumosorosea Infection. Front. Physiol. 8:1054. doi: 10.3389/fphys.2017.01054
Received: 01 September 2017; Accepted: 01 December 2017;
Published: 14 December 2017.
Edited by:
Bin Tang, Hangzhou Normal University, ChinaReviewed by:
Jalal Jalali Sendi, University of Gilan, IranRan Wei, University of Western Ontario, Canada
Sassan Asgari, The University of Queensland, Australia
Copyright © 2017 Xu, Xu, Li, Wang, Xu, Zhou, Yu, Yu, Shakeel and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Shakeel, ZmFpemFuZWFiaXdhcWFzQHNjYXUuZWR1LmNu
Fengliang Jin, amZsYmFuZ0BzY2F1LmVkdS5jbg==
†These authors have contributed equally to this work.
 Jin Xu
Jin Xu Xiaoxia Xu1†
Xiaoxia Xu1† Xianqiang Zhou
Xianqiang Zhou Jialin Yu
Jialin Yu Xiaoqiang Yu
Xiaoqiang Yu Muhammad Shakeel
Muhammad Shakeel Fengliang Jin
Fengliang Jin