- 1Neuroscience Axis, CHU de Québec Research Center (CHUL), Québec City, QC, Canada
- 2Department of Psychiatry and Neuroscience, Faculty of Medicine, Laval University, Québec City, QC, Canada
Background
Stroke constitutes a major cause of death and disability of the adults in the industrialized world. Ischemic stroke accounts for the majority of cases (Dirnagl, 2012). Interruption of the blood supply triggers the ischemic cascade leading to cell death and inflammation (Dirnagl et al., 1999; Lo, 2008). Our understanding for the molecular mechanisms underlying neuronal death has tremendously advanced in the last decade, leading to the development of several neuroprotective agents (Moskowitz et al., 2010). Although neuroprotection was successful in experimental studies, it failed to achieve clinical benefits in acute stroke patients (Gladstone et al., 2002; Lo, 2008). Currently, tissue plasminogen activator (tPA)-induced thrombolysis is the only Food and Drug Administration (FDA)-approved treatment used in clinics to restore the cerebral blood flow (CBF) (Wardlaw et al., 2014). Nonetheless, less than 5% of stroke patients can benefit from thrombolysis, as tPA should be administered within a narrow therapeutic time window of 4.5 h after onset (Wang et al., 2004). Endovascular mechanical embolectomy has emerged as a therapeutic option when thrombolysis is unsuccessful or cannot be applied (Smith et al., 2005).
Beyond neuroprotection in the acute phase, there is a growing interest in neurorestoration that aims to promote brain remodeling in the post-acute phase (Gladstone and Black, 2000; Gladstone et al., 2002). The interest has emerged from the overwhelming experimental and clinical findings suggesting that the brain is actively trying to recover after stroke by repairing itself (Chopp et al., 2009). The neurorestorative processes include coordinated neurogenic and angiogenic responses, which aim to improve functional recovery (Ohab et al., 2006; Zhang et al., 2008; Chopp et al., 2009; Chen et al., 2014). Until now, no clinically validated neurorestorative approach exists, and cognitive/motor rehabilitation remains the only approach used in clinics in the post-stroke phase.
Evidence from clinical trials suggests that saving neurons alone after stroke may not be sufficient to develop clinically viable therapies (Gladstone et al., 2002; Lo, 2008). Neuronal survival narrowly depends upon integrity of the microvasculature, which regulates oxygen and nutrients delivery. The functional interaction between the neuronal and vascular systems is governed by the neurovascular unit, which comprises sealed endothelial cells forming the blood-brain barrier (BBB), pericytes, astrocytes, microglia, and neurons (Hermann and ElAli, 2012). As such, it is well established that any clinically viable therapies must succeed to restore the neurovascular unit by stabilizing the microvasculature, while limiting neuronal loss and stimulating neuronal plasticity. Moreover, pre-clinical investigations use essentially healthy animals, which do not adequately translate the clinical setup in which stroke patients usually present several vascular risk factors, namely atherosclerosis associated to hyperlipidemia (Gladstone et al., 2002; ElAli et al., 2011). This aspect must be adequately and systemically addressed in the pre-clinical context.
Hyperlipidemia: An Acknowledged—Yet Not Fully Understood—Risk Factor in Stroke Patients
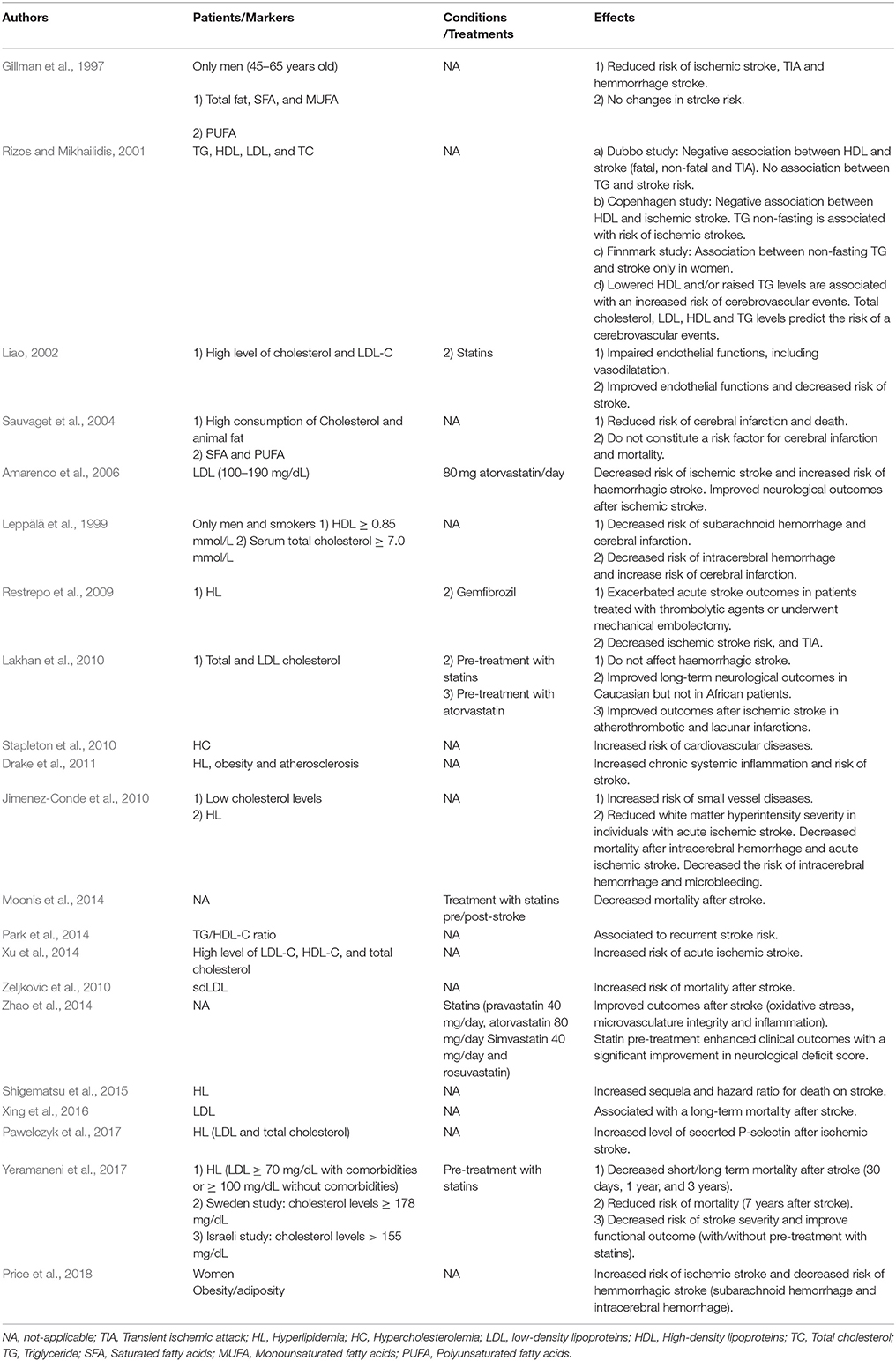
Hyperlipidemia is caused by an excessive uptake of high-cholesterol diet leading to high levels of blood lipids. Importantly, in registries and clinical trials up to 60% of documented patients have high levels of blood lipids including cholesterol (ElAli et al., 2011). Elevated cholesterol levels (>7.0 mmol/L) are associated to an increased risk of stroke incidence (Leppälä et al., 1999). In addition to extracranial atherosclerosis, hyperlipidemia promotes cervical or coronary atherosclerosis, which predisposes to atherothrombotic and cardioembolic stroke (Ayata et al., 2013). Though hyperlipidemia is well established as a prevalent risk factor for stroke incidence, clinical investigations showed controversial results to how it influences stroke acute and post-acute outcomes. Some clinical investigations have reported a protective effect of hyperlipidemia in stroke patients, essentially via reduction of the mortality rates (Jimenez-Conde et al., 2010; Shigematsu et al., 2015). Hyperlipidemic stroke patients tend to have reduced white matter hyperintensity (WMH) volume (Jimenez-Conde et al., 2010). Severity of the WMH has been reported to predict infarct progression upon stroke leading to poor clinical outcomes (Arsava et al., 2009). Importantly, abnormal cholesterol profile in obese individuals, namely the elevated levels of low-density lipoproteins (LDL) and the reduced levels of high-density lipoproteins (HDL), has been proposed to cause white matter abnormalities (Cohen et al., 2011). This suggests that the profile of the lipoproteins is the factor to consider not only the absolute elevated levels of total blood lipids. Indeed, in a recent study it was demonstrated that LDL level was associated with long-term mortality after stroke (Xing et al., 2016). On the other hand, some other studies have shown that hyperlipidemia negatively impacts acute stroke outcomes in patients who were treated with thrombolytic agents or underwent mechanical embolectomy (Restrepo et al., 2009). Interestingly, administration of cholesterol-lowering drugs was reported to ameliorate the neurological outcomes of stroke patients (Amarenco et al., 2006). It is important to mention that most of these studies have evaluated the impact of hyperlipidemia on the long-term mortality after stroke, and important data, such as the size and location of the initial lesions were not systemically evaluated. Furthermore, it is very probable that hyperlipidemic patients might have been treated with cholesterol-lowering drugs, such as statins, before stroke incidence. For instance, several studies have indicated that patients receiving statins exhibited less severe structural injury and had better neurological outcomes (Lakhan et al., 2010). Furthermore, patients who have received statins just after stroke were more likely to be discharged home vs. patients already on statins before stroke (Moonis et al., 2014). Both group of patients were also more likely to be discharged home than those patients who did not receive statin therapy at all (Moonis et al., 2014). However, a recent study has shown that hyperlipidemia is associated to a lower risk of short- and long-term mortality after stroke irrespectively of statin use (Yeramaneni et al., 2017), thus further fuelling the controversy. Nonetheless, it is well recognized that independently of its cholesterol lowering effects, statins provide tissue protection via improvement of the microvasculature integrity, attenuation of the inflammation, and reduction of the oxidative stress (Liao, 2002; Zhao et al., 2014). Therefore, the pleotropic cholesterol lowering-independent protective effects of statins on the integrity of the microvasculature in hyperlipidemic patients should be systemically evaluated in the clinical trials in order to better interpret data (Table 1).
Impact of Hyperlipidemia on the Neurovascular Unit: Lessons From Animal Studies
In contrast to the clinical setting, there is a consensus as to the detrimental effect of hyperlipidemia in experimental stroke studies. In rodents, hyperlipidemia has been demonstrated to exacerbate the ischemic damage through endothelial cell injury, oxidative stress, inflammation and neuronal loss (ElAli et al., 2011; Ayata et al., 2013; Deng et al., 2014; Cao et al., 2015). Moreover, it has been shown that the ischemic damage in hyperlipidemic mice is directly associated to duration of the blood supply interruption (Maysami et al., 2015). It is noteworthy to mention that in the majority of these experimental studies, hyperlipidemia was mostly induced in transgenic mice lacking key genes implicated in the metabolism and transport of lipids, namely apolipoprotein E (ApoE) (Zhang et al., 1992; Zechariah et al., 2013a,b). The advantage of these mice is that they develop atherosclerosis following short-term exposure to high-fat diet, usually up to 8 weeks. In ApoE knockout (ApoE−/−) mice fed with a high-fat diet, hyperlipidemia has been shown to exacerbate neuronal death and loss upon ischemic stroke induction (ElAli et al., 2011; Ayata et al., 2013; Herz et al., 2014). However, in wildtype littermates fed with the same high-fat diet for the same period of time - which are often used as controls - hyperlipidemia has not significantly influenced neuronal death and loss (ElAli et al., 2011; Ayata et al., 2013; Herz et al., 2014). These results suggest that depletion of the ApoE gene might increase neuronal vulnerability to the ischemic insult independently upon the effects of hyperlipidemia. Indeed, ApoE and its receptors were demonstrated to cooperatively regulate common mechanisms essential to neuronal survival in the adult brain (Beffert et al., 2006). As such, studies involving ApoE−/− mice, or other lipoprotein receptors, should be interpreted with some caution, especially when it comes to neuronal death and loss.
Hyperlipidemia has been clearly demonstrated to exacerbate vascular damage in transgenic and wildtype mouse strains. It has been shown to alter neurovascular coupling, reduce resting CBF, impair the physiologic cerebral vasodilator reflexes, and worsen cerebral perfusion deficits upon cerebral ischemia in mice even before atherosclerosis appearance in cervical or intracranial arteries (Ayata et al., 2013). Moreover, hyperlipidemia in wildtype mice, and not ApoE−/− mice, has been reported to increase BBB permeability, and promote the formation of brain oedema upon cerebral ischemia in the acute phase via mechanisms implicating lipid peroxidation, matrix metalloproteinases (MMPs) activation, and RhoA over-activation (ElAli et al., 2011; Deng et al., 2014). Furthermore, hyperlipidemia has been shown to trigger an inflammatory response at the brain microvasculature associated to endothelial cell activation, and facilitate infiltration of the circulating immune cells into the brain, as well as platelet activation and adhesion to the injured endothelial cells (Stapleton et al., 2010). Indeed, positron emission tomography (PET) imaging combined to post-mortem histochemical analysis has demonstrated that hyperlipidemia is associated to microglial cell activation and over-expression of several vascular adhesion molecules on injured endothelial cells (Drake et al., 2011). These observations suggest that the detrimental effects of hyperlipidemia are mainly associated to microvasculature dysfunction, an aspect that is often neglected in the clinical setting.
It has been proposed that therapeutic angiogenesis, which aims to increase vascular density within the lesion site, should enhance the CBF and attenuate the ischemic damage (Jean LeBlanc et al., 2017). In experimental studies, therapeutic angiogenesis induced by vascular endothelial growth factor (VEGF) has been shown to promote structural and functional neurological recovery after stroke (Hermann and Zechariah, 2009). Indeed, local VEGF administration has been shown to promote the formation of functional brain microvasculature by stimulating the crosstalk between endothelial cells and pericytes (Zechariah et al., 2013a), and consequently reduced the ischemic insult severity by enhancing the CBF and stabilizing the energy state of the brain (Zechariah et al., 2013a). Importantly, hyperlipidemia has abolished the VEGF-hemodynamic improvements by disrupting the interaction between endothelial cells and pericytes (Zechariah et al., 2013b). Based on these observations, it is conceivable to postulate that hyperlipidemia could decisively influence the efficacy of strategies that aim at promoting neuroprotection and/or neurorestoration (Supplementary Table 1).
Conclusion and Perspectives
One of the priorities in the field should be elucidating the exact role of hyperlipidemia in stroke patient outcomes. One promising avenue is the detailed profile of lipoprotein subclasses. A recent study has demonstrated that the elevated levels of a specific LDL particle—small, dense LDL (sdLDL)—and not total LDL, constitute a strong predictor of stroke incidence, and most importantly is associated to an increased risk of mortality (Zeljkovic et al., 2010; Xu et al., 2014). Therefore, it might be more appropriate to investigate dyslipidemia with an emphasis on lipoprotein subclasses and their association with stroke severity and outcomes. Another major challenge in stroke therapies remains in the translational potential of pre-clinical investigations. Clinical studies were based on pre-clinical investigations obtained in healthy animals that do not have comorbid conditions (Gladstone et al., 2002; ElAli et al., 2011). As such, it is important now to systemically include animals that present at least one comorbid condition, such as hyperlipidemia, to increase the translational potential of the experimental findings. However, induction of hyperlipidemia itself is crucial aspect. It is important to avoid using transgenic animals lacking genes that could influence neuronal vulnerability to ischemic insult. As an alternative, it would be more appropriate to use non-transgenic animals fed with high-fat diet for longer time periods. Moreover, it is important to evaluate the effects of different varieties of high-fat diets. This point is important, as fat in the diets used in pre-clinical study is often prepared from well-defined and specific sources, whereas in humans the source of fat in diets is highly diversified. To sum up, it is conceivable to propose that to achieve breakthroughs in stroke therapies, pre-clinical investigations should systemically evaluate how major risk factors, including hyperlipidemia, influence structural and functional recovery while validating new neuroprotective or neurorestorative strategies.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery Grant Program), and an establishment grant from the Fonds de recherche du Québec—Santé (FRQS).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.00488/full#supplementary-material
References
Amarenco, P., Bogousslavsky, J., Callahan, A. III., Goldstein, L. B., Hennerici, M., Rudolph, A. E., et al. (2006). High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 6, 549–559. doi: 10.1016/j.jvs.2006.10.008
Arsava, E. M., Rahman, R., Rosand, J., Lu, J., Smith, E. E., Rost, N. S., et al. (2009). Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 16, 1403–1410. doi: 10.1212/WNL.0b013e3181a18823
Ayata, C., Shin, H. K., Dileköz, E., Atochin, D. N., Kashiwagi, S., Eikermann-Haerter, K., et al. (2013). Hyperlipidemia disrupts cerebrovascular reflexes and worsens ischemic perfusion defect. J. Cereb. Blood Flow Metab. 6, 954–962. doi: 10.1038/jcbfm.2013.38
Beffert, U., Nematollah-Farsian, F., Masiulis, I., Hammer, R. E., Yoon, S. O., Giehl, K. M., et al. (2006). ApoE receptor 2 controls neuronal survival in the adult brain. Curr. Biol. 24, 2446–2452. doi: 10.1016/j.cub.2006.10.029
Cao, X. L., Du, J., Zhang, Y., Yan, J. T., and Hu, X. M. (2015). Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp. Brain Res. 10, 2753–2765. doi: 10.1007/s00221-015-4269-x
Chen, J., Venkat, P., Zacharek, A., and Chopp, M. (2014). Neurorestorative therapy for stroke. Front. Hum. Neurosci. 8:382. doi: 10.3389/fnhum.2014.00382
Chopp, M., Li, Y., and Zhang, Z. G. (2009). Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40, S143–S145. doi: 10.1161/STROKEAHA.108.533141
Cohen, J. I., Cazettes, F., and Convit, A. (2011). Abnormal cholesterol is associated with prefrontal white matter abnormalities among obese adults: a diffusion tensor imaging study. Neuroradiol. J. 6, 854–861. doi: 10.1177/197140091102400604
Deng, J., Zhang, J., Feng, C., Xiong, L., and Zuo, Z. (2014). Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice†. Cardiovasc. Res. 4, 473–484. doi: 10.1093/cvr/cvu154
Dirnagl, U. (2012). Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann. N.Y. Acad. Sci. 1268, 21–25. doi: 10.1111/j.1749-6632.2012.06691.x
Dirnagl, U., Iadecola, C., and Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397. doi: 10.1016/S0166-2236(99)01401-0
Drake, C., Boutin, H., Jones, M. S., Denes, A., McColl, B. W., Selvarajah, J. R., et al. (2011). Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 6, 1113–1122. doi: 10.1016/j.bbi.2011.02.008
ElAli, A., Doeppner, T. R., Zechariah, A., and Hermann, D. M. (2011). Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 42, 3238–3244. doi: 10.1161/STROKEAHA.111.615559
Gillman, M. W., Cupples, L. A., Millen, B. E., Ellison, R. C., and Wolf, P. A. (1997). Inverse association of dietary fat with development of ischemic stroke in men. JAMA 278, 2145–2150. doi: 10.1001/jama.1997.03550240035030
Gladstone, D. J., and Black, S. E. (2000). Enhancing recovery after stroke with noradrenergic pharmacotherapy: a new frontier? Can. J. Neurol. Sci. 27, 97–105. doi: 10.1017/S0317167100052173
Gladstone, D. J., Black, S. E., and Hakim, A. M., and Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery. (2002). Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33, 2123–2136. doi: 10.1161/01.STR.0000025518.34157.51
Hermann, D. M., and ElAli, A. (2012). The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci. Signal 5:re4. doi: 10.1126/scisignal.2002886
Hermann, D. M., and Zechariah, A. (2009). Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J. Cereb. Blood Flow Metab. 10, 1620–1643. doi: 10.1038/jcbfm.2009.100
Herz, J., Hagen, S. I., Bergmüller, E., Sabellek, P., Göthert, J. R., Buer, J., et al. (2014). Exacerbation of ischemic brain injury in hypercholesterolemic mice is associated with pronounced changes in peripheral and cerebral immune responses. Neurobiol. Dis. 62, 456–468. doi: 10.1016/j.nbd.2013.10.022
Jean LeBlanc, N., Guruswamy, R., and ElAli, A. (2017). Vascular endothelial growth factor isoform-B stimulates neurovascular repair after ischemic stroke by promoting the function of pericytes via vascular endothelial growth factor receptor-1. Mol. Neurobiol. 55, 3611–3626. doi: 10.1007/s12035-017-0478-6
Jimenez-Conde, J., Biffi, A., Rahman, R., Kanakis, A., Butler, C., Sonni, S., et al. (2010). Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke 3, 437–442. doi: 10.1161/STROKEAHA.109.563502
Lakhan, S. E., Bagchi, S., and Hofer, M. (2010). Statins and clinical outcome of acute ischemic stroke: a systematic review. Int. Arch. Med. 3:22. doi: 10.1186/1755-7682-3-22
Leppälä, J. M., Virtamo, J., Fogelholm, R., Albanes, D., and Heinonen, O. P. (1999). Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke 12, 2535–2540. doi: 10.1161/01.STR.30.12.2535
Liao, J. K. (2002). Beyond lipid lowering: the role of statins in vascular protection. Int. J. Cardiol. 1, 5–18. doi: 10.1016/S0167-5273(02)00195-X
Lo, E. H. (2008). A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 14, 497–500. doi: 10.1038/nm1735
Maysami, S., Haley, M. J., Gorenkova, N., Krishnan, S., McColl, B. W., and Lawrence, C. B. (2015). Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. J. Neuroinflammation 12:140. doi: 10.1186/s12974-015-0359-8
Moonis, M., Kumar, R., Henninger, N., Kane, K., and Fisher, M. (2014). Pre and post-stroke use of statins improves stroke outcome. Indian J. Community Med. 4, 214–217. doi: 10.4103/0970-0218.143021
Moskowitz, M. A., Lo, E. H., and Iadecola, C. (2010). The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198. doi: 10.1016/j.neuron.2010.07.002
Ohab, J. J., Fleming, S., Blesch, A., and Carmichael, S. T. (2006). A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006
Park, J. H., Lee, J., and Ovbiagele, B. (2014). Non-traditional serum lipid variables and recurrent stroke risk. Stroke 45, 3269–3274. doi: 10.1161/STROKEAHA.114.006827
Pawelczyk, M., Kaczorowska, B., and Baj, Z. (2017). The impact of hyperglycemia and hyperlipidemia on plasma P-selectin and platelet markers after ischemic stroke. Arch. Med. Sci. 13, 1049–1056. doi: 10.5114/aoms.2017.65816
Price, A. J., Wright, F. L., Green, J., Balkwill, A., Kan, S. W., Yang, T. Y. O., Floud, S., et al. (2018). Differences in risk factors for 3 types of stroke. UK prospective study and meta-analyses. Neurology 90, e298–e306. doi: 10.1212/WNL.0000000000004856
Restrepo, L., Bang, O. Y., Ovbiagele, B., Ali, L., Kim, D., Liebeskind, D. S., et al. (2009). Impact of hyperlipidemia and statins on ischemic stroke outcomes after intra-arterial fibrinolysis and percutaneous mechanical embolectomy. Cerebrovasc. Dis. 4, 384–390. doi: 10.1159/000235625
Rizos, E., and Mikhailidis, G. P. (2001). Are high density lipoprotein (HDL) and triglyceride levels relevant in stroke prevention? Cardiovasc. Res. 5, 199–207. doi: 10.1016/S0008-6363(01)00383-2
Sauvaget, C., Nagano., J., Hayashi, M., and Yamada, M. (2004). Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke 35, 1531–1537. doi: 10.1161/01.STR.0000130426.52064.09
Shigematsu, K., Watanabe, Y., Nakano, H., and Kyoto Stroke Registry Committee. (2015). Influences of hyperlipidemia history on stroke outcome; a retrospective cohort study based on the Kyoto Stroke Registry. BMC Neurol. 15:44. doi: 10.1186/s12883-015-0297-1
Smith, W. S., Sung, G., Starkman, S., Saver, J. L., Kidwell, C. S., Gobin, Y. P., et al. (2005). Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36, 1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d
Stapleton, P. A., Goodwill, A. G., James, M. E., Brock, R. W., and Frisbee, J. C. (2010). Hypercholesterolemia and microvascular dysfunction: interventional strategies. J. Inflamm. 7:54. doi: 10.1186/1476-9255-7-54
Wang, X., Tsuji, K., Lee, S. R., Ning, M., Furie, K. L., Buchan, A. M., et al. (2004). Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 35, 2726–2730. doi: 10.1161/01.STR.0000143219.16695.af
Wardlaw, J. M., Murray, V., Berge, E., and del Zoppo, G. J. (2014). Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 7:CD000213. doi: 10.1002/14651858.CD000213.pub3
Xing, Y., An, Z., Yu, N., Zhao, W., Ning, X., and Wang, J. (2016). Low density lipoprotein cholesterol and the outcome of acute ischemic stroke: results of a large hospital-based study. Eur. Neurol. 76, 195–201. doi: 10.1159/000450604
Xu, T., Zhang, J. T., Yang, M., Zhang, H., Liu, W. Q., Kong, Y., et al. (2014). Dyslipidemia and outcome in patients with acute ischemic stroke. Biomed. Environ. Sci. 2, 106–110. doi: 10.3967/bes2014.023
Yeramaneni, S., Kleindorfer, D. O., Sucharew, H., Alwell, K., Moomaw, C. J., Flaherty, M. L., et al. (2017). Hyperlipidemia is associated with lower risk of poststroke mortality independent of statin use: a population-based study. Int. J. Stroke 2, 152–160. doi: 10.1177/1747493016670175
Zechariah, A., ElAli, A., Doeppner, T. R., Jin, F., Hasan, M. R., Helfrich, I., et al. (2013a). Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke 6, 1690–1697. doi: 10.1161/STROKEAHA.111.000240
Zechariah, A., ElAli, A., Hagemann, N., Jin, F., Doeppner, T. R., Helfrich, I., et al. (2013b). Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler. Thromb. Vasc. Biol. 7, 1561–1567. doi: 10.1161/ATVBAHA.112.300749
Zeljkovic, A., Vekic, J., Spasojevic-Kalimanovska, V., Jelic-Ivanovic, Z., Bogavac-Stanojevic, N., Gulan, B., et al. (2010). LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis 2, 548–554. doi: 10.1016/j.atherosclerosis.2009.11.040
Zhang, R. L., Zhang, Z. G., and Chopp, M. (2008). Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology 55, 345–352. doi: 10.1016/j.neuropharm.2008.05.027
Zhang, S. H., Reddick, R. L., Piedrahita, J. A., and Maeda, N. (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 5081, 468–471. doi: 10.1126/science.1411543
Keywords: stroke recovery, neurovascular unit, hyperlipidemias, neurorestoration, neuroprotection
Citation: Menet R, Bernard M and ElAli A (2018) Hyperlipidemia in Stroke Pathobiology and Therapy: Insights and Perspectives. Front. Physiol. 9:488. doi: 10.3389/fphys.2018.00488
Received: 30 January 2018; Accepted: 17 April 2018;
Published: 15 May 2018.
Edited by:
Vincent Pialoux, Claude Bernard University Lyon 1, FranceReviewed by:
Jun Zhang, Texas Tech University Health Sciences Center, United StatesCopyright © 2018 Menet, Bernard and ElAli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayman ElAli, YXltYW4uZWwtYWxpQGNyY2h1ZGVxdWViZWMudWxhdmFsLmNh
 Romain Menet
Romain Menet Maxime Bernard1,2
Maxime Bernard1,2 Ayman ElAli
Ayman ElAli