- Muscle Biophysics Lab, Department of Kinesiology, University of Massachusetts, Amherst, MA, United States
Elevated levels of the metabolic by-products, including acidosis (i.e., high [H+]) and phosphate (Pi) are putative agents of muscle fatigue; however, the mechanism through which they affect myosin’s function remain unclear. To elucidate these mechanisms, we directly examined the effect of acidosis (pH 6.5 vs. 7.4), alone and in combination with elevated levels of Pi on the force-generating capacity of a mini-ensemble of myosin using a laser trap assay. Acidosis decreased myosin’s average force-generating capacity by 20% (p < 0.05). The reduction was due to both a decrease in the force generated during each actomyosin interaction, as well as an increase in the number of binding events generating negative forces. Adding Pi to the acidic condition resulted in a quantitatively similar decrease in force but was solely due to an elimination of all high force-generating events (>2 pN), resulting from an acceleration of the myosin’s rate of detachment from actin. Acidosis and Pi also had distinct effects on myosin’s steady state ATPase rate with acidosis slowing it by ∼90% (p > 0.05), while the addition of Pi under acidic conditions caused a significant recovery in the ATPase rate. These data suggest that these two fatigue agents have distinct effects on myosin’s cross-bridge cycle that may underlie the synergistic effect that they have muscle force. Thus these data provide novel molecular insight into the mechanisms underlying the depressive effects of Pi and H+ on muscle contraction during fatigue.
Introduction
Muscle fatigue from intense contractile activity is due, in large part, to the accumulation metabolic by-products, primarily hydrogen ions (H+) and phosphate (Pi), inhibiting myosin’s ability to generate force and motion (Allen et al., 2008; Fitts, 2008; Debold et al., 2016). However, the molecular basis of this effect is still unclear, in part because the effect of Pi and H+ myosin’s force and motion-generating capacity have not been directly observed.
During fatigue Pi levels can exceed 15 mM (Cady et al., 1989) and are thought to inhibit force by accelerating myosin’s detachment from actin (Pate and Cooke, 1988, 1989; Takagi et al., 2004). A widely accepted model posits that Pi rebinds actomyosin in the ADP-bound state (AM.ADP), reverses myosin’s powerstroke, and induces detachment, restoring myosin to the pre-powerstroke state (Pate and Cooke, 1988; Takagi et al., 2004). However, this model is based, primarily, on the effects of Pi single fiber contractile properties (Hibberd et al., 1985a,b; Pate and Cooke, 1988, 1989; Dantzig et al., 1992; Debold et al., 2004; Caremani et al., 2008), which represent the average behavior of billions of myosin molecules, making it difficult to determine how Pi affects a single actomyosin cross-bridge. Indeed, our recent findings demonstrating that Pi increases velocity at low pH in the motility assay challenge the notion of Pi binding to actomyosin reversing the powerstroke, as do findings demonstrating the strain dependence of the effects of Pi (Caremani et al., 2008, 2013, 2015, Linari et al., 2010). The Pi-induced increase in velocity at low pH led us to propose that the rebinding of Pi to actomyosin does not reverse the powerstroke, but instead induces myosin’s detachment in a post-powerstroke state (Debold et al., 2011, 2013)
However, we have only examined the effect of Pi on force at neutral pH (7.4) and not in combination with acidosis, which occurs in muscle fatigue (Cady et al., 1989). Under acidic conditions Pi is still thought to rebind to the AM.ADP state and induce detachment, but acidosis is thought to prolong the AM.ADP lifetime (Debold et al., 2008) increasing the vulnerability to Pi rebinding, and thus a potential mechanism for the synergistic effect these ions have on the force-generating capacity of muscle (Nosek et al., 1987; Nelson et al., 2014). However, this phenomenon has not been directly examined at the molecular level.
The molecular mechanisms underlying the depressive effects of acidosis (i.e., low pH) are also unclear, in part, because its role in the reduction of force remains highly controversial (Fitts, 2016; Westerblad, 2016), with some suggesting that it prevents rather than causes the loss of force during fatigue (Pedersen et al., 2004). Early work in muscle fibers suggested that a fatiguing level of acidosis depressed force by ∼50% and unloaded shortening velocity by ∼30% (Chase and Kushmerick, 1988; Cooke et al., 1988). But these observations were made well below mammalian physiological temperatures and subsequent observations at 30°C revealed that acidosis reduced force by only ∼10–20% (Pate et al., 1995; Westerblad et al., 1997; Knuth et al., 2006), leading some authors to conclude that acidosis plays little or no role in fatigue. However, the effect on unloaded shortening velocity appears to be much less temperature-sensitive, with a decrease from pH 7.0 to 6.2 causing reductions of 20–30% at both 15 and 30°C (Knuth et al., 2006). Indeed, the decrease in velocity leads to a 35–40% reduction in peak fiber power; a measure which is more relevant for understanding muscle fatigue (Knuth et al., 2006), but the molecular basis of these effects have not been elucidated.
Methods
Proteins
Fast skeletal muscle myosin (Margossian and Lowey, 1982; Debold et al., 2011) and actin were purified from (Pardee and Spudich, 1982) chicken pectoralis muscle as previously described. For the mini-ensemble laser trap assay, actin was labeled with 50% TRITC/phalliodin and 50% biotin/phalliodin. Animal tissue was obtained in accordance with the policies of the National Institutes of Health using a protocol approved by the Institutional Animal Care and Use Committee at the University of Massachusetts.
Mini-Ensemble Laser Trap Assay
Myosin was loaded into a nitrocellulose coated flow-cell at 25 μg ml-1 in a high salt buffer (300 mM KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, and 1 mM dithiothreitol). The trapping buffer included fluorescently labeled actin and silica beads in a low salt buffer (60 mM KCl, 25 mM imidazole, 1 mM EGTA, and 4 mM MgCl2) with 100 μM MgATP, at pH 7.4 or 6.5 and either 0 or 15 mM added Pi. Total ionic strength was kept constant 90 mM by varying KCl. Two 1 μm neutravidin-coated silica beads were trapped in time-shared optical traps and subsequently attached to a single actin filament (Debold et al., 2013). Once attached to actin, the trapped beads were separated to add 3–4 pN pretension to the filament. The trap stiffness was ∼0.02 pN/nm, which combined with the measures of displacement determined the force generated with each actomyosin interaction (see Supplementary Materials and Debold et al., 2013). At the myosin concentration used, the geometry of the assay indicates that ∼10, randomly oriented, myosin molecules were available to interact with the single actin filament (Debold et al., 2013).
ATPase Assay
The effect of acidosis and Pi on myosin’s steady state rate ATP hydrolysis was determined using an NADH-linked assay solution with the heavy meromyosin (HMM) fragment of whole myosin at 30°C, using established methods (De La Cruz et al., 2000). HMM was dialyzed into the appropriate low salt buffer under control conditions (20 mM KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, and 1 mM dithiothreitol at pH 7.4). 10 mM Pi was used instead of the 15 mM used in the laser trap assay in order to keep the ionic strength sufficiently low for the ATP hydrolysis rate to approach saturation at the actin concentrations used (0–50 μM). Previous evidence demonstrates that Pi exerts ∼70% of its effect on muscle force between 0 and 10 mM, with only minor additional decrement (<5%) caused by 15 mM (Tesi et al., 2000), thus the differences between the two different levels of Pi used are likely minimal.
Results
The mini-ensemble of myosin molecules stochastically interacted with the actin filament to produce a range of low and high force-generating events (Figure 1). We identified the peak force and lifetime of these events with a customized event-detection algorithm (see Supplementary Materials and Longyear et al., 2017). Decreasing the pH from 7.4 to 6.5 significantly (p < 0.05) decreased the average peak force by ∼20% in the mini-ensemble laser trap assay. Examination of the distribution of events revealed this was caused by a decrease in the frequency of high force-generating events (events > 2 pN), as well as an increase in the frequency of negative force-generating events (events occurring opposite of the predominate direction). The addition of Pi at pH 6.5 caused a similar depression in force, but was largely due to the elimination of higher force-generating events, accompanied by a decrease in the frequency of negative force events.
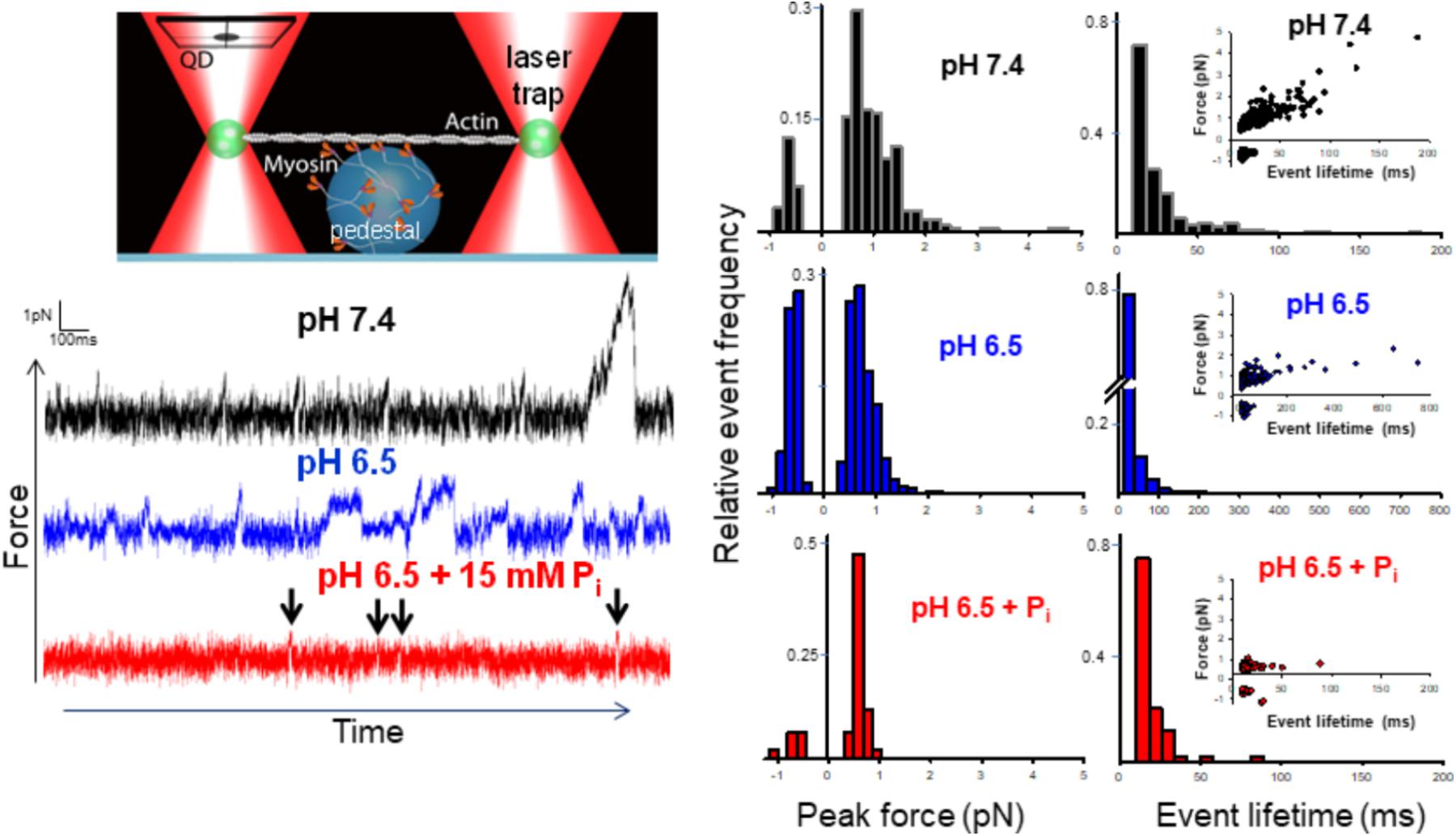
FIGURE 1. Effect of acidosis and phosphate on myosin’s force-generating capacity. Schematic of the mini-ensemble of myosin molecules in a laser trap assay (on large sphere), showing a single actin filament pulled taut between two optically trapped 1 um silica beads, top left. Raw displacement vs. time records with actomyosin binding events appearing as reductions in the variance and displacements from the mean signal under each condition indicated (see section “Methods”). Determination of the stiffness of the laser trap (0.02 pN/nm) enabled converting these displacements into forces. An automated routine written MatLab was used to determine the peak force for each event (see section “Methods” and Supplementary Materials). Scored events were used to construct histograms and plotted as a function of the total number of events in a condition (middle set of panels). Then median forces were 0.78 ± 0.27 (pH 7.4, black), 0.63 ± 0.13 (pH 6.5, blue)∗, and 0.59 ± 0.06 pN (pH 6.5 + 15 mM Pi, red)∗ for control, acidosis, and acidosis with added Pi respectively, ∗indicates significantly (p < 0.05) lower than control. The lifetime of each event was also determined using the same automated routine (see section “Methods” and Supplementary Materials) and histograms constructed for each condition (right panels). Insets show peak force plotted as a function of event lifetime. Median event lifetimes were 18 ± 1, 15 ± 1∗, and 15 ± 0.5∗ ms for control (black), acidosis (blue), and acidosis + Pi respectively (red) (∗indicates significantly different from control, p < 0.05). The data shown represent between 329, 623, and 87 binding events for control, acidosis, and acidosis + Pi respectively.
Event lifetimes appeared exponentially distributed (Figure 1), and in the case of the control were linearly related to the forces generated (Figure 1, far right panels). Decreasing the pH to 6.5 caused a reduction in the number of high force-generating events, but also caused the slope of the force vs. duration to decrease significantly (0.0273 ± 0.0015 vs. 0.0044 ± 0.0005, p < 0.05). This finding indicates that the binding events lasted longer at pH 6.5 despite generating less force. This suggests that acidosis slows the rate of detachment for the longer duration events. By contrast, the addition of Pi at pH 6.5 caused a pronounced reduction in longer event lifetimes with all but two binding events lasting for <50 ms, suggesting an accelerated rate of detachment.
To gain insight into the effect acidosis and Pi might have on steps of the cross-bridge cycle occurring off actin we measured the effect on the steady state ATPase rate in solution (Figure 2). Acidosis strongly depressed actin-activated Vmax, causing a 60% reduction at pH 6.8 and ∼90% reduction at pH 6.5 (Figure 2). In contrast, the addition of 10 mM Pi at low pH significantly increased myosin’s ATPase rate. The effect was small at pH 7.4 but became more pronounced as the pH was decreased (Figure 2). Despite the recovery it should be noted that at pH 6.5 in the presence of Pi the ATPase remained significantly slower than the control at pH 7.4.
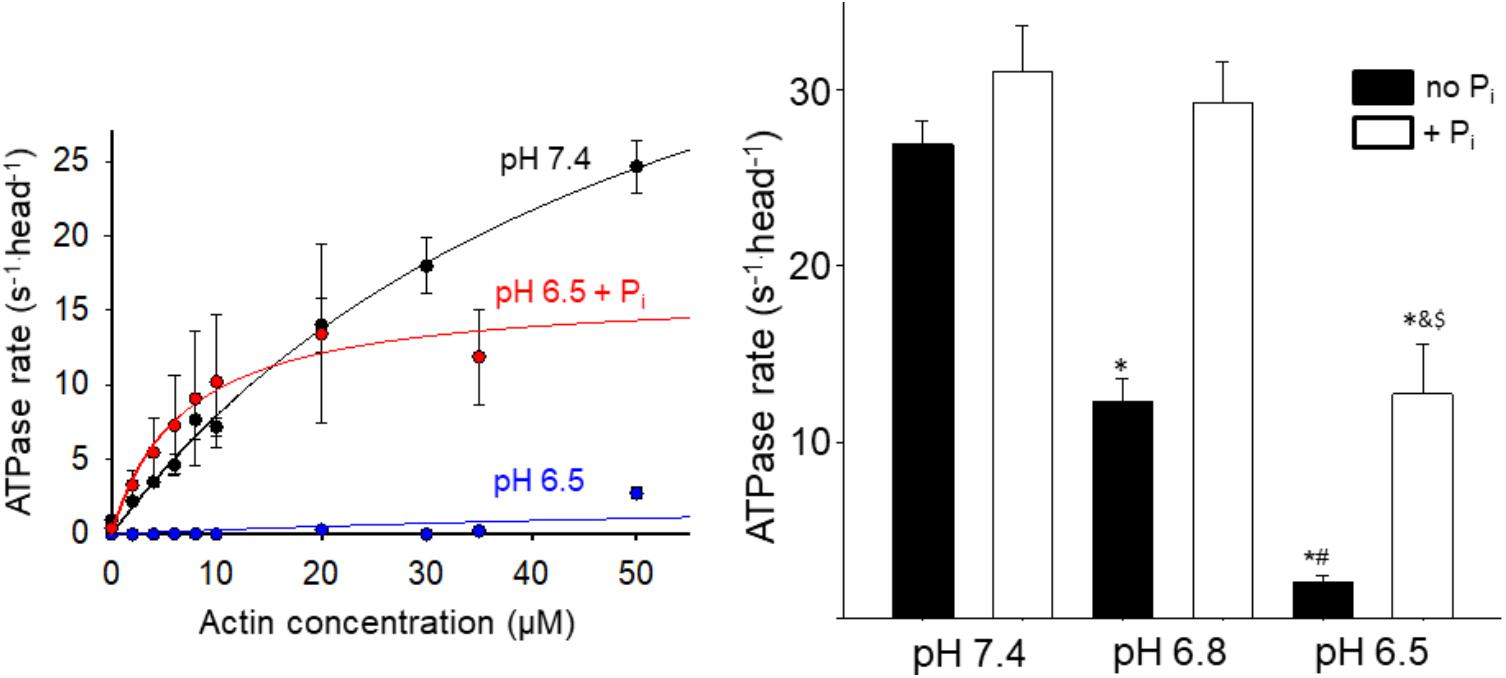
FIGURE 2. Effect of acidosis and phosphate on steady state ATPase. The rate of product release from HMM as a function of actin concentration was determined using an NADH-linked assay (left graph, see Supplementary Materials for details), under control (pH 7.4 no added Pi), acidic (pH 6.5), and acidic plus Pi (pH 6.5 + 10 mM Pi) conditions. Each point represents the mean ± SEM between 7 and 33 measures at each actin concentration. The maximum rates obtained under each condition (including pH 6.8 and pH 6.8 + Pi) are plotted in the graph on the right side. Bars represent mean ± SEM for the concentration of actin yielding the highest rate. ∗Indicates significantly (p < 0.05) different from control, #indicates significantly different from pH 6.8 no Pi, &indicates significantly different from pH 6.5 no Pi, and $indicates significantly different from pH 6.8 + Pi.
Discussion
Both acidosis alone and in combination with Pi significantly reduced myosin’s force-generating capacity; however, the mechanism underlying this effect appears to be driven by distinct mechanisms. Acidosis decreased force by decreasing the number of high force-generating events and increasing the frequency of negative forces, while the addition of Pi reduced force by eliminating all high force/long duration binding events (Figure 1).
The decrease in high force-generating events under acidic conditions suggests that less myosin heads were strongly bound during a given interaction, indicative of slowing myosin’s weak-to-strong binding transition. This suggests that one or more of the steps of myosin’s cross-bridge cycle that occur off actin are slowed by acidosis. One possibility is that myosin’s putative rate-limiting step, Pi-release (Lymn and Taylor, 1971), which occurs closely or concomitantly with strong-binding (Takagi et al., 2004), is slowed by acidosis. This possibility is consistent with the acidosis-induced reduction of the myosin steady state ATPase rate (Figure 2).
The increase in negative force events under acidic conditions is consistent with prior observations demonstrating that acidosis increases the frequency of non-productive single actomyosin interactions (Debold et al., 2008). Because beads in a laser trap experience Brownian motion, non-productive actomyosin interactions cause the frequency of negative displacement binding events to increase (Debold et al., 2008). Myosin’s powerstroke is thought to be re-primed with the hydrolysis of ATP, off actin (Steffen et al., 2003), therefore an increase in non-productive interactions may indicate that acidosis slows the rate of ATP hydrolysis by myosin.
The decrease in the slope of the force vs. event lifetime relationship with acidosis (Figure 1) indicates that at any given force, actomyosin binding events lasted longer than at pH 7.4. This observation is consistent with acidosis prolonging single actomyosin interactions, which has been attributed to a slowing rate of ADP-release (Debold et al., 2008, 2012). This mechanism, therefore, provides a molecular mechanism for the acidosis-induced decrease in unloaded shortening velocity observed in the motility assay (Debold et al., 2008, 2011), and in skinned muscle fibers (Knuth et al., 2006).
In contrast to the effects of acidosis alone, increasing Pi at low pH reduced force solely by reducing the event lifetime (Figure 1). Indeed, the combined effects of acidosis and Pi on force are nearly identical to the effect of Pi alone (Debold et al., 2013), where virtually all of the high force/long duration events are eliminated by elevated Pi. This suggests that any effect of acidosis to prolong the actomyosin interaction is opposed by the Pi-induced detachment from an AM.ADP state (Hibberd et al., 1985b; Webb et al., 1986). Indeed, the prolongation of the AM.ADP state by acidosis may extend the lifetime of the state to which Pi rebinds to induce detachment. Thus, acidosis may make actomyosin more vulnerable to the rebinding of Pi, which may provide a molecular basis for the synergistic effects Pi and H+ have on muscle force (Nelson et al., 2014).
Another key question is what happens to myosin following this Pi-induced detachment from actin? The conventional view posits that the rebinding of Pi to AM.ADP induces a reversal of the powerstroke before myosin detaches from actin; thus myosin is restored to a pre-powerstroke AM.ADP Pi state (Pate and Cooke, 1988; Takagi et al., 2004). However, this view fails to account for the Pi-induced increase in actin filament velocity at low pH which we observed in the motility assay (Debold et al., 2011). Therefore, we proposed an alternative model in which Pi induces detachment from a post-powerstroke state through a branch in the normal cross-bridge cycle (Debold et al., 2011, 2013). This model is able to accurately reproduce the Pi-induced increase in velocity at low pH (Debold et al., 2011), suggesting that myosin detaches from actin in a post-powerstroke state, and completes it ATPase off of actin. Indeed, the effect of acidosis and Pi on myosin’s ATPase rate (Figure 2) is strikingly similar to the effect on velocity providing further support for our model (Debold et al., 2011, 2012). However, this similarity may be coincidental as velocity, in situ, is thought to be limited by the ADP-release (Nyitrai et al., 2006), while the ATPase rate, in solution, is believed to be limited by Pi-release (Lymn and Taylor, 1971); rates which are at least an order of magnitude different. In our model, Pi increases velocity at low pH by accelerating detachment from a post-powerstroke state (Debold et al., 2011). The ATPase data in the present study suggest that, under acidic conditions, once myosin is detached from actin by Pi it completes product release faster off actin than it does when strongly bound to actin (Figure 2). Thus acidosis may have a greater effect on ADP-release when myosin is strongly bound to actin than once it has been detached by Pi.
Conclusion
Elevated levels of the fatigue agents, Pi and H+, directly inhibit myosin’s force-generating capacity; however, this seems to arise from distinctly different molecular mechanisms. Acidosis appears to slow both myosin’s weak-to-strong binding transition and its rate of detachment from actin, while Pi reduces force by accelerating detachment from actin. The Pi-induced acceleration in detachment may mitigate some of the effects of acidosis on velocity and the hydrolysis rate, but this likely comes at the expense of force generation. Thus these data may provide a molecular basis for the putative synergistic effects of Pi and H+ on muscle force in muscle fibers (Nosek et al., 1987; Nelson et al., 2014) and during fatigue (Cady et al., 1989).
Author Contributions
ED conceived the idea, collected and analyzed the data, and wrote the manuscript. MW collected data and helped to analyze the data and was involved in the preparation of the manuscript.
Funding
This study was supported by American Heart Association (Award ID: AHA GIA #14GRNT20450002).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.00862/full#supplementary-material
References
Allen, D. G., Lamb, G. D., and Westerblad, H. (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88, 287–332. doi: 10.1152/physrev.00015.2007
Cady, E. B., Jones, D. A., Lynn, J., and Newham, D. J. (1989). Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J. Physiol. 418, 311–325. doi: 10.1113/jphysiol.1989.sp017842
Caremani, M., Dantzig, J., Goldman, Y. E., Lombardi, V., and Linari, M. (2008). Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys. J. 95, 5798–5808. doi: 10.1529/biophysj.108.130435
Caremani, M., Melli, L., Dolfi, M., Lombardi, V., and Linari, M. (2013). The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 591, 5187–5205. doi: 10.1113/jphysiol.2013.257410
Caremani, M., Melli, L., Dolfi, M., Lombardi, V., and Linari, M. (2015). Force and number of myosin motors during muscle shortening and the coupling with the release of the ATP hydrolysis products J. Physiol. 3313–3332. doi: 10.1113/JP270265
Chase, P. B., and Kushmerick, M. J. (1988). Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys. J. 53, 935–946. doi: 10.1016/S0006-3495(88)83174-6
Cooke, R., Franks, K., Luciani, G. B., and Pate, E. (1988). The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J. Physiol. 395, 77–97. doi: 10.1113/jphysiol.1988.sp016909
Dantzig, J. A., Goldman, Y. E., Millar, N. C., Lacktis, J., and Homsher, E. (1992). Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 451, 247–278. doi: 10.1113/jphysiol.1992.sp019163
De La Cruz, E. M., Sweeney, H. L., and Ostap, E. M. (2000). ADP inhibition of myosin V ATPase activity. Biophys. J. 79, 1524–1529. doi: 10.1016/S0006-3495(00)76403-4
Debold, E. P., Beck, S. E., and Warshaw, D. M. (2008). The effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am. J. Physiol. Cell Physiol. 295, C173–C179. doi: 10.1152/ajpcell.00172.2008
Debold, E. P., Dave, H., and Fitts, R. H. (2004). Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am. J. Physiol. Cell Physiol. 287, C673–C681. doi: 10.1152/ajpcell.00044.2004
Debold, E. P., Fitts, R. H., Sundberg, C. W., and Nosek, T. M. (2016). Muscle Fatigue from the Perspective of a Single Crossbridge. Med. Sci. Sports Exerc. 48, 2270–2280. doi: 10.1249/MSS.0000000000001047
Debold, E. P., Longyear, T. J., and Turner, M. A. (2012). The effects of phosphate and acidosis on regulated thin-filament velocity in an in vitro motility assay. J. Appl. Physiol. 113, 1413–1422. doi: 10.1152/japplphysiol.00775.2012
Debold, E. P., Turner, M., Stout, J. C., and Walcott, S. (2011). Phosphate enhances myosin-powered actin filament velocity under acidic conditions in a motility assay. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1401–R1408. doi: 10.1152/ajpregu.00772.2010
Debold, E. P., Walcott, S., Woodward, M., and Turner, M. A. (2013). Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of Myosin molecules. Biophys. J. 105, 2374–2384. doi: 10.1016/j.bpj.2013.09.046
Fitts, R. H. (2008). The cross-bridge cycle and skeletal muscle fatigue. J. Appl. Physiol. 104, 551–558. doi: 10.1152/japplphysiol.01200.2007
Fitts, R. H. (2016). The role of acidosis in fatigue: pro perspective. Med. Sci. Sports Exerc. 48, 2335–2338. doi: 10.1249/MSS.0000000000001043
Hibberd, M. G., Dantzig, J. A., Trentham, D. R., and Goldman, Y. E. (1985a). Phosphate release and force generation in skeletal muscle fibers. Science 228, 1317–1319. doi: 10.1126/science.3159090
Hibberd, M. G., Webb, M. R., Goldman, Y. E., and Trentham, D. R. (1985b). Oxygen exchange between phosphate and water accompanies calcium- regulated ATPase activity of skinned fibers from rabbit skeletal muscle. J. Biol. Chem. 260, 3496–3500.
Knuth, S. T., Dave, H., Peters, J. R., and Fitts, R. H. (2006). Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J. Physiol. 575, 887–899. doi: 10.1113/jphysiol.2006.106732
Linari, M., Brunello, E., Reconditi, M., Fusi, L., Caremani, M., Narayanan, T., et al. (2015). Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528, 276–279. doi: 10.1038/nature15727
Linari, M., Caremani, M., and Lombardi, V. (2010). A kinetic model that explains the effect of inorganic phosphate on the mechanics and energetics of isometric contraction of fast skeletal muscle. Proc. Biol. Sci. 277, 19–27. doi: 10.1098/rspb.2009.1498
Longyear, T., Walcott, S., and Debold, E. P. (2017). The molecular basis of thin filament activation: from single molecule to muscle. Sci. Rep. 7:1822. doi: 10.1038/s41598-017-01604-8
Lymn, R. W., and Taylor, E. W. (1971). Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10, 4617–4624. doi: 10.1021/bi00801a004
Margossian, S. S., and Lowey, S. (1982). Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 85, 55–71. doi: 10.1016/0076-6879(82)85009-X
Nelson, C. R., Debold, E. P., and Fitts, R. H. (2014). Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am. J. Physiol. Cell Physiol. 307, C939–C950. doi: 10.1152/ajpcell.00206.2014
Nosek, T. M., Fender, K. Y., and Godt, R. E. (1987). It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 236, 191–193. doi: 10.1126/science.3563496
Nyitrai, M., Rossi, R., Adamek, N., Pellegrino, M. A., Bottinelli, R., and Geeves, M. A. (2006). What limits the velocity of fast-skeletal muscle contraction in mammals? J. Mol. Biol. 355, 432–442. doi: 10.1016/j.jmb.2005.10.063
Pardee, J. D., and Spudich, J. A. (1982). Purification of muscle actin. Methods Cell Biol. 24, 271–289. doi: 10.1016/S0091-679X(08)60661-5
Pate, E., Bhimani, M., Franks-Skiba, K., and Cooke, R. (1995). Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J. Physiol. 486, 689–694. doi: 10.1113/jphysiol.1995.sp020844
Pate, E., and Cooke, R. (1988). A model of crossbridge action: the effects of ATP, ADP and Pi. J. Muscle Res. Cell Motil. 10, 181–196. doi: 10.1007/BF01739809
Pate, E., and Cooke, R. (1989). Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 414, 73–81. doi: 10.1007/BF00585629
Pedersen, T. H., Nielsen, O. B., Lamb, G. D., and Stephenson, D. G. (2004). Intracellular acidosis enhances the excitability of working muscle. Science 305, 1144–1147. doi: 10.1126/science.1101141
Steffen, W., Smith, D., and Sleep, J. (2003). The working stroke upon myosin-nucleotide complexes binding to actin. Proc. Natl. Acad. Sci. U.S.A. 100, 6434–6439. doi: 10.1073/pnas.1231998100
Takagi, Y., Shuman, H., and Goldman, Y. E. (2004). Coupling between phosphate release and force generation in muscle actomyosin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1913–1920. doi: 10.1098/rstb.2004.1561
Tesi, C., Colomo, F., Nencini, S., Piroddi, N., and Poggesi, C. (2000). The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys. J. 78, 3081–3092. doi: 10.1016/S0006-3495(00)76845-7
Webb, M. R., Hibberd, M. G., Goldman, Y. E., and Trentham, D. R. (1986). Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle. Evidence for Pi binding to a force-generating state. J. Biol. Chem. 261, 15557–15564.
Westerblad, H. (2016). Acidosis is not a significant cause of skeletal muscle fatigue. Med. Sci. Sports Exerc. 48, 2339–2342. doi: 10.1249/MSS.0000000000001044
Keywords: muscle fibers and skeletal, myosins, phosphates, fatigue, acidosis
Citation: Woodward M and Debold EP (2018) Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms. Front. Physiol. 9:862. doi: 10.3389/fphys.2018.00862
Received: 23 March 2018; Accepted: 18 June 2018;
Published: 10 July 2018.
Edited by:
Kenneth S. Campbell, University of Kentucky, United StatesReviewed by:
Brett Colson, University of Arizona, United StatesGabriella Piazzesi, Università degli Studi di Firenze, Italy
Copyright © 2018 Woodward and Debold. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edward P. Debold, ZWRlYm9sZEBraW4udW1hc3MuZWR1
 Mike Woodward
Mike Woodward Edward P. Debold
Edward P. Debold