- Department of Endocrinology, Key Laboratory of Endocrinology, Translational Medicine Center, Ministry of Health, Peking Union Medical College, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Adverse early-life exposures program increased risk of chronic metabolic diseases in adulthood. However, the effects of genistein supplementation in early life on metabolic health in later life are largely unclear. Our objective was to investigate whether maternal genistein intake could mitigate the deleterious influence of a maternal high-fat diet on glucose and lipid metabolism in offspring and to explore the role of gut microbiota in mediating the transgenerational effects. C57BL/6 female mice were fed either a high-fat diet (HF), high-fat diet with genistein (0.6 g/kg diet) (HFG) or normal control diet (C) for 3 weeks before pregnancy and throughout pregnancy and lactation. The male offspring had ad libitum access to normal chow diet from weaning to 24 weeks of age. Then the content of inguinal subcutaneous adipose tissue (SAT) and epididymal visceral adipose tissue (VAT) were weighed. Glucose tolerance test (GTT), the level of serum insulin and lipid profiles were analyzed. The caecal contents were collected for 16S rDNA sequencing. The results showed that maternal genistein intake could significantly reduce blood glucose levels during GTT, fasting insulin levels, VAT mass and serum triglyceride levels as well as increase high-density lipoprotein cholesterol in adult male offspring. Significant decrease of germs from the Tenericutes phylum and enrichment of Rikenella as well as SCFA (short-chain fatty acid)-producing bacteria, including Alloprevotella, Odoribacter, and Clostridium XlVa, in offspring of genistein fed dams might play crucial roles in the improvement of glucose and lipid metabolism. Overall, early-life genistein intake attenuated the harmful effects of maternal HF on metabolism in adult offspring and the protective effects were associated with the alterations in gut microbiota, which provides new evidence and targets for mitigate the poor effects of adverse early-life exposures on metabolic health in later life.
Introduction
Type 2 diabetes mellitus (T2DM) and obesity arise at the interface of genetics and environmental factors, including physical inactivity, smoking and nutrition intake. Interestingly, a large number of human studies and experimental animal models demonstrated that adverse environmental exposures experienced by their parents during intrauterine or early postnatal life significantly influenced health of the next generations (Dong et al., 2019; Lund et al., 2019; Zhang et al., 2019). More specifically, poor early-life exposures robustly increased the risks of developing chronic metabolic diseases in adult life, including glucose intolerance, T2DM and obesity (Csongova et al., 2018; Kearney et al., 2018; Wen et al., 2018; Yu et al., 2018; Chang et al., 2019). This association has been conceptualized by the fetal programming hypothesis and the developmental origins of health and disease, which propose that environmental stimuli during critical windows of development programmed permanent changes in later life (Aris et al., 2018; Velazquez et al., 2019). Although epigenetics, microbiome, and metabolome were all considered the potential mechanisms, the specific mechanism of the “fetal programming” is still largely unclear (Wankhade et al., 2016). Substantial research and our previous studies both showed that early-life overnutrition-maternal high-fat diet during pregnancy and lactation significantly increased susceptibility to glucose intolerance, insulin resistance and lipid disorders in adult offspring (Zheng et al., 2016; Huang et al., 2017; Thompson et al., 2019). Therefore, early-life might be a critical window for preventing the transmission of metabolic diseases across generation and reducing the prevalence of T2DM. Effective measures should be taken to reset the trajectories of chronic metabolic disease.
In recent years, bioactive food components have gained increasing attention in academia. Genistein, which is one of the most important ingredients in soy isoflavone, is structurally similar with 17β-estradiol and is also known as a phytoestrogen. The beneficial effects of genistein on cardiovascular disease (Shi et al., 2019), cancer (Lepri et al., 2018), bone metabolic disease (Ye et al., 2018) and Alzheimer’s disease (Devi et al., 2017) have been extensively researched. And the safety of genistein intake was also verified in both animals and humans (Gilbert and Liu, 2013). In terms of metabolic diseases, several large epidemiological studies in American, Japan, and Chinese have shown that the intake of soy isoflavone is negatively correlated with the risk of T2DM, especially in obese population (Nanri et al., 2010; Odegaard et al., 2011; Ding et al., 2016). Subsequently, growing numbers of clinical research and animal models confirmed the benefits of genistein on glucose intolerance, insulin sensitivity and lipid metabolic disorders (Gilbert and Liu, 2013; Liu et al., 2017). Evidence has indicated that the potential mechanisms deciphering the protective effects of genistein against metabolic disturbances included directly acting on β-cell to enhance proliferation, promoting secretion of insulin and inhibiting apoptosis, regulating glucose and lipid metabolism in liver, epigenetic modifications, modulating cAMP/PKA signaling pathway as well as modifying gut microbiota (Gilbert and Liu, 2013). However, studies exploring the effects of genistein intake on transgenerational metabolic health are lacking.
There is a community of 1000 or more species of bacteria that is 10 times more than human cells colonizing in gastrointestinal tract, which has multiple functions for the body. During the last few decades, emerging research has focused on the role of gut microbiota in metabolic diseases and showed that gut microbiota dysbiosis was intertwined with various disorders, such as glucose intolerance, insulin sensitivity and disturbances of serum lipid profiles (Mithieux, 2018). Recently, with the propose of “in utero colonization hypothesis,” increasing studies have demonstrated that gut microbiota might play a key role in the effects of adverse early-life exposures on metabolism in later life (Soderborg et al., 2016; Zhou and Xiao, 2018). And recent evidence has indicated the association between genistein intake and changes of gut microbiota (Lopez et al., 2018). However, the effects of genistein intake experienced by mothers on gut microbiota and the relationship between metabolic alterations and gut microbial communities in adult offspring are unclear.
Therefore, we aimed to explore whether maternal genistein intake for 3 weeks before pregnancy, and throughout pregnancy and lactation could mitigate the deleterious effects of the high-fat diet on glucose and lipid metabolism in adult offspring. In addition, to investigate the associated mechanisms, the changes of gut microbiota and the relationship between metabolic parameters and altered bacteria were also detected in our study.
Materials and Methods
Animals and Study Design
We obtained four-week-old C57BL/6 female mice from the National Institutes for Food and Drug Control (Beijing, China; SCXK-2014-0013). Mice were kept under SPF (specific pathogen free) conditions (room temperature at 22 ± 2°C; 12 h light/dark cycle). Mice had ad libitum access to food and sterile water throughout the study. After 1 week of environmental adaptation, dams were randomly assigned to three groups and were fed a high-fat diet (HF, n = 8), high-fat diet with genistein (CAS: 466-72-0, G0272, TCI Development Co., Ltd.) (0.6 g/kg diet) (HFG, n = 7) or normal control diet (AIN-93G) (C, n = 8) for 3 weeks. Previous studies have confirmed the metabolic protects of genistein at this dose (Al-Nakkash et al., 2012; Simperova et al., 2016). The ingredients are shown in Supplementary Table S1. The high-fat diet included 60% (kcal%) fat, whereas the control diet contained 15.8% fat.
After 3 weeks of intervention, females were mated to eight-week-old normal C57BL/6 males. The dams were checked for postcopulatory plugs every morning after mating, and the appearance of a plug was taken as d 0.5 of pregnancy. The pregnant females continued on their respective diets throughout the pregnancy and lactation. The litter size was all culled to 5 pups to ensure that there was no nutritional bias between litters. Offspring were weaned at 3 weeks of age. At weaning, all male offspring (n = 7–8 per group) were given ad libitum access to a normal chow diet until 24 weeks of age. The body weights of both the mothers and offspring were measured once per week. At the end of the experiment, one male offspring from different litters (n = 7–8 per group) were euthanized. Blood samples were collected from the intraorbital retrobulbar plexus after 10 h of fasting from anesthetized mice, and the inguinal subcutaneous adipose tissue (SAT) and epididymal visceral adipose tissue (VAT) were removed and weighed; the caecal contents were quickly removed, snap frozen in dry ice, and then stored at -80°C for further analysis. All the procedures were approved by the Animal Care and Use Committee of the Peking Union Medical College Hospital (Beijing, China, SYXC-2014-0029). All the animal operations were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Oral Glucose Tolerance Tests (OGTT)
The male offspring were fasted for 6 h and given a glucose load (2 g/kg of body weight) by gavage. Blood glucose (BG) levels were measured at 0, 30, 60, and 120 min after the gavage from tail vein blood using a Contour TS glucometer (ACCU-CHEK Mobile, Beijing, China). The area under the curve (AUC) of the OGTT was calculated as previously described (Zheng et al., 2014).
Serum Biochemical Parameters Measurement
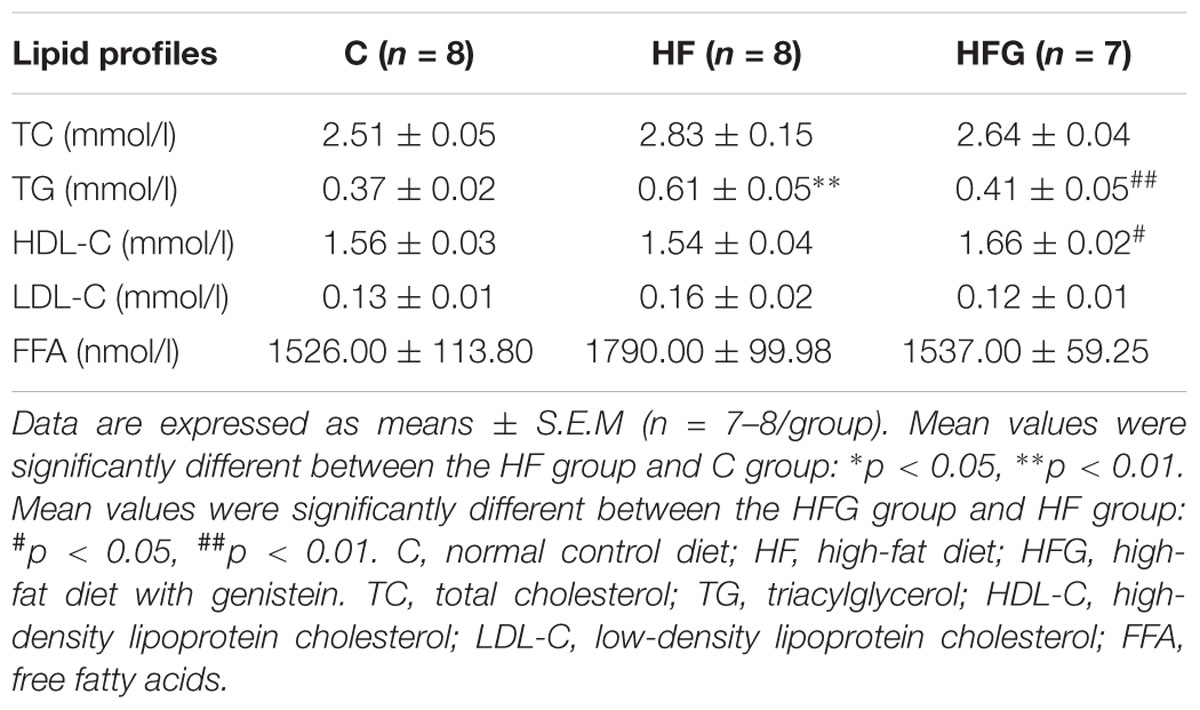
The blood samples collected from male offspring at 24 weeks of age were centrifuged at 3000 × g for 10 min at 4°C, and the serum was stored in aliquots at -80°C. The serum insulin concentrations were measured using an ELISA kit (80-INSMSU-E01, Salem, NH, United States). Insulin sensitivity was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR). The HOMA-IR was calculated as previously described (Zheng et al., 2014). Serum total cholesterol (TC), triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and free fatty acids (FFA) were measured by routine automated laboratory methods.
Gut Microbiota Analysis
Microbial DNA was extracted from the caecal contents using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s protocols. The V3-V4 regions of the 16S rRNA genes were amplified using the primers 341F 5’-CCTACGGGRSGCAGCAG-3′and 806R, 5′-GGACTACVVGGGTATCTAATC-3′. Amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) and quantified using Qubit®2.0 (Invitrogen, United States). The tags were sequenced on the Illumina HiSeq platform (Illumina, Inc., CA, United States).
After merging paired-end reads, reads were performed by quality filtering. High quality reads were clustered into operational taxonomic units (OTUs) with the 97% similarity using UPARSE software (version 7.0.1001) (Edgar, 2013), and representative sequences for each OTU were screened using QIIME software (version 1.7.0, Quantitative Insights into Microbial Ecology) (Caporaso et al., 2010). Then, the GreenGene Database (DeSantis et al., 2006) was used to annotate taxonomic information based on the RDP classifier version 2.2 algorithm (Wang et al., 2007). Alpha and beta diversity analysis were achieved by QIIME software (Version 1.7.0) and R software (Version 2.15.3). For alpha diversity, Chao1, Simpson and the Shannon index were analyzed. For beta diversity, principal coordinates analysis (PCoA) plots and analysis of similarities (ANOSIM) were performed using unweighted UniFrac. In addition, linear discriminant analysis (LDA) of the effect size (LEfSe) was used to determine differences among the groups.
Statistical Analysis
The data were expressed as mean ± standard error of the mean (S.E.M). The statistics were analyzed by one-way ANOVA and two-way ANOVA, with Turkey post hoc analyses. The differences of the relative abundance of gut microbiota were analyzed by Kruskal–Wallis test, with Benjamini and Hochberg post hoc analyses. Correlation analyses between the relative abundance of bacterial taxa at genus levels and metabolic parameters were performed by Spearman correlation coefficient test. A p value < 0.05 was considered statistically significant. Prism version 7.0 (GraphPad Software Inc., San Diego, CA, United States) was used for statistical analysis.
Results
The Effects of Maternal Genistein Intake on Glucose Tolerance and Insulin Sensitivity
Consistent with higher body weight in male offspring of the dams fed a HF diet compared with that of C fed dams (p < 0.05) at 4 weeks of age (Figure 1A), they had significant glucose intolerance at this time (Figure 1B). After fed a normal chow diet, there was no significant difference of body weight among the three groups throughout the study. However, at the end of the study, there was significantly impaired glucose tolerance in the adult offspring of the HF fed dams (p < 0.0001), which was almost normalized in mice of dietary genistein fed dams (p < 0.0001) (Figure 1B). As shown in Figure 2, the blood glucose levels were higher at 30 min (p < 0.0001), 60 min (p < 0.0001) and 120 min (p < 0.001) (Figure 2A) and the AUC was significantly larger (p < 0.0001) (Figure 2B) for offspring of HF group than that of C group at 24 weeks of age. Male offspring in HFG group showed a dramatic improvement in glucose metabolism with lower blood glucose levels at 30 min (p < 0.0001), 60 min (p < 0.0001) and 120 min (p < 0.001) and smaller AUC (p < 0.0001) than that in HF group. Moreover, to determine whether maternal genistein influenced insulin sensitivity in adult offspring, we examined the fasting serum insulin levels. It indicated that serum insulin concentrations (p < 0.05) and HOMA-IR (p < 0.05) of offspring in the HF group were significantly higher than that from C group. Maternal dietary genistein prevented the deleterious effects on insulin sensitivity induced by HF, with significantly lower insulin levels (p < 0.05) and HOMA-IR(p < 0.05) (Figures 2C,D).
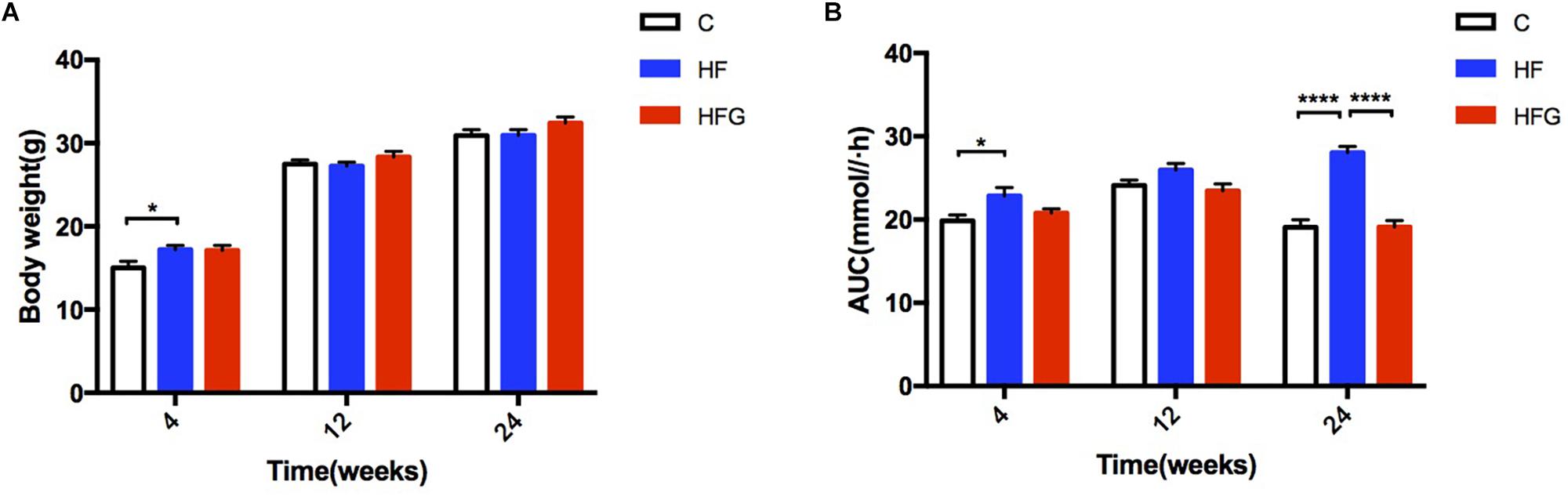
Figure 1. Body weight and AUC of OGTT for male offspring from 4 to 24 weeks. (A) Body weight, and (B) AUC. C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein. Data are expressed as means ± S.E.M. (n = 7–8/group). Mean values were significantly different between the groups: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
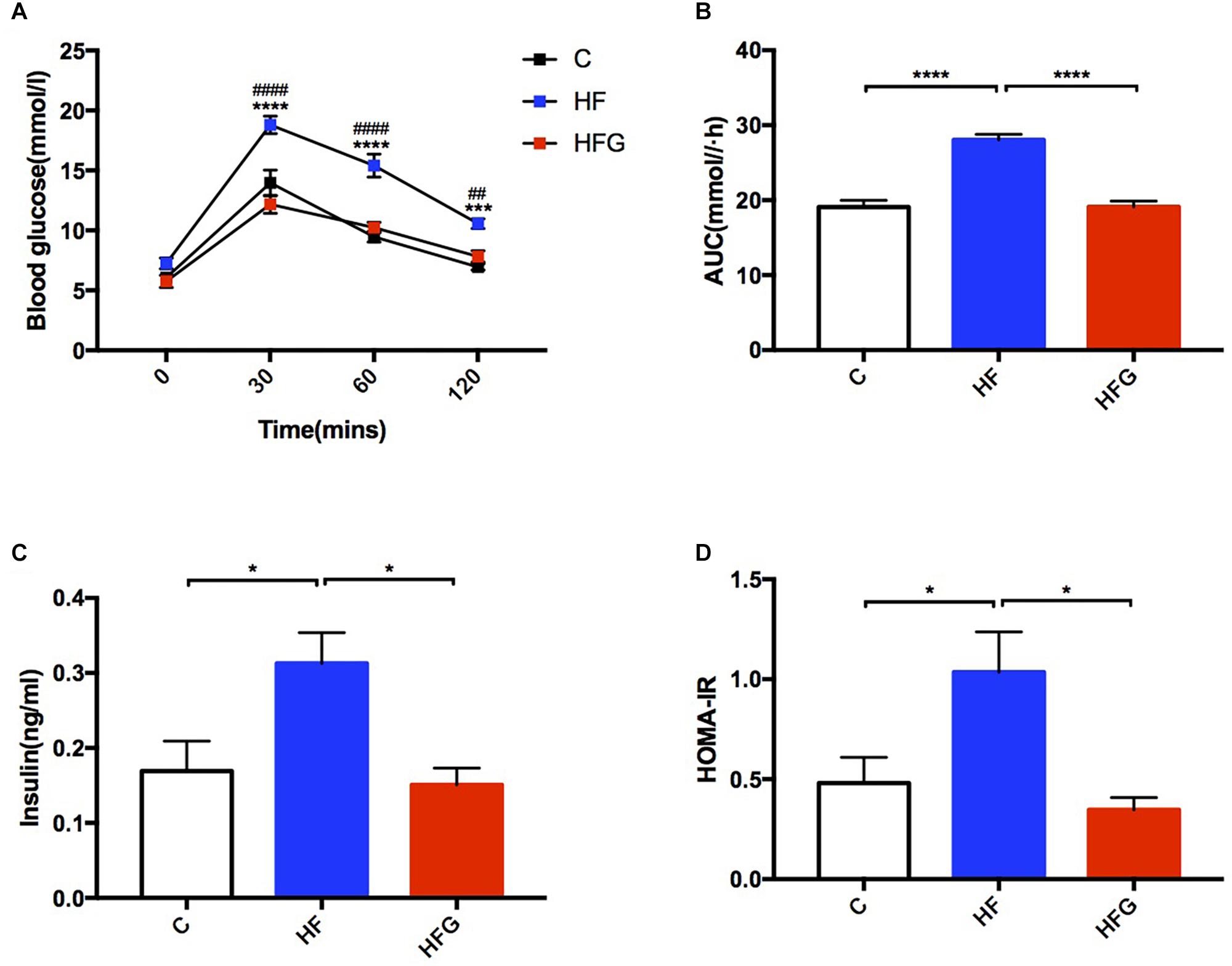
Figure 2. Glucose metabolism of the male offspring at 24 weeks of age. (A) OGTT; (B) AUC; (C) serum insulin levels; (D) HOMA-IR. C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein. OGTT, oral glucose tolerance test; AUC, area under the curve; HOMA-IR, the homeostasis model assessment of insulin resistance. Data are expressed as means ± S.E.M. (n = 7–8/group). Mean values were significantly different between C group and the HF group during OGTT: ∗; Mean values were significantly different between HFG group and the HF group during OGTT: #. Mean values were significantly different between the groups: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Maternal Dietary Genistein Counteracted Lipid Metabolic Disorders Induced by Early Life High-Fat Diet in Adult Mice
In addition to glucose metabolism, we assessed the effects of maternal dietary genistein on serum lipid profiles and fat mass in adult offspring. The serum lipids of adult offspring among the three groups were summarized in Table 1. Maternal genistein feeding did not alter the levels of TC, LDL-C and FFA in offspring at 24 weeks of age. The levels of serum TG (p < 0.01) were significantly higher in offspring of HF fed dams than that from C group, whereas maternal genistein feeding resulted in a dramatic improvement in the serum TG (p < 0.01) in male offspring. Furthermore, the levels of HDL-C were also higher in mice of the HFG group compared with that of HF group. Although maternal dietary genistein did not influence the body weight of the adult offspring, we further detected the effects of maternal genistein intake on fat mass in offspring by weighing the adipose tissue. As shown in Figure 3, the SAT and VAT were both significantly increased in offspring of HF fed dams than that of C fed dams (p < 0.05, p < 0.01). Maternal dietary genistein dramatically decreased the visceral fat mass induced by maternal HF in adult offspring (p < 0.01).
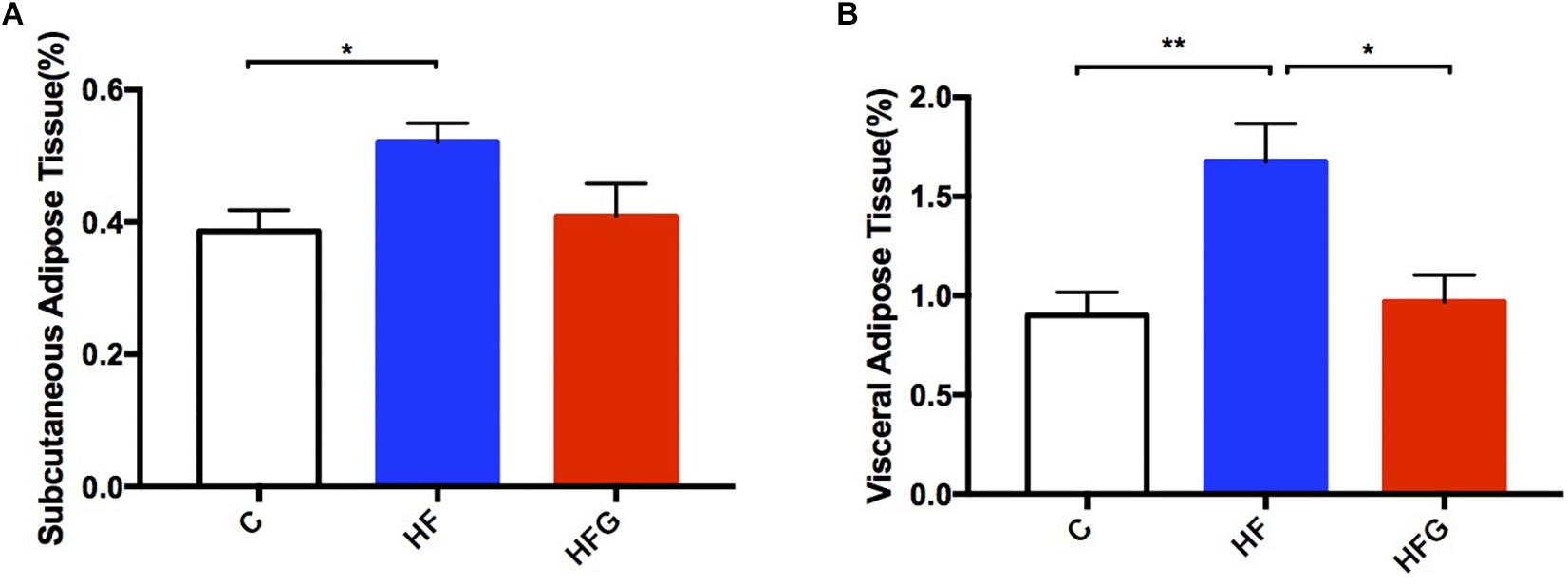
Figure 3. Fat mass of the male offspring at 24 weeks of age. (A) Subcutaneous adipose tissue; and (B) visceral adipose tissue. C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein. Data are expressed as means ± S.E.M. (n = 7–8/group). Mean values were significantly different between the groups: ∗p < 0.05, ∗∗p < 0.01.
The Changes of Gut Microbiota in Adult Offspring
To explore the mechanism that maternal dietary genistein prevented metabolic disorders caused by HF and the changes of gut microbiota, we performed 16S rDNA sequencing for caecal contents in adult offspring among the three groups. The amplicon sequencing data have been submitted to the sequence read archive (SRA) database (accession number PRJNA529750). Firstly, we assessed the OTUs among the three groups to identify the shared and unique species. Venn diagram showed that there were 296 shared OTUs, 15 unique OTUs in offspring of C fed dams, 16 OTUs in mice of HF fed dams, as well as 15 OTUs in offspring of HFG fed dams (Figure 4A). Then we evaluated the composition of the gut microbiota in offspring. At the phylum level, the Bacteroidetes, Firmicutes, Verrucomicrobia and Probacteraia were the most dominant, whereas the relative abundance of the four phyla were not significantly different among the three groups (Figure 4B). The phylum Tenericutes was significantly increased in mice of the HF fed dams compared with that in the C group (q < 0.01) (Figure 4D). And maternal genistein feeding could dramatically decreased the abundance of this phyla (q < 0.01). Figure 4C showed the relative abundance of top 20 species at the genus level among the three groups. The Anaeroplasma (q < 0.05), Eubacterium (q < 0.05) and Enterorhabdus (q < 0.05) were all significantly increased in male offspring of HF fed dams compared with that of C fed dams. The abundance of Barnesiella (q < 0.01), Anaeroplasma(q = 0.087), Eubacterium (q = 0.080) and Enterorhabdus (q < 0.01) were distinctly decreased, whereas the Alloprevotella (q < 0.05), Clostridium XIVa (q = 0.053) and Odoribacter (q < 0.001) were increased in offspring of HFG fed dams compared with that in the HF group. Significantly different species at the genus level among the three groups were summarized in a heatmap (Figure 4E).
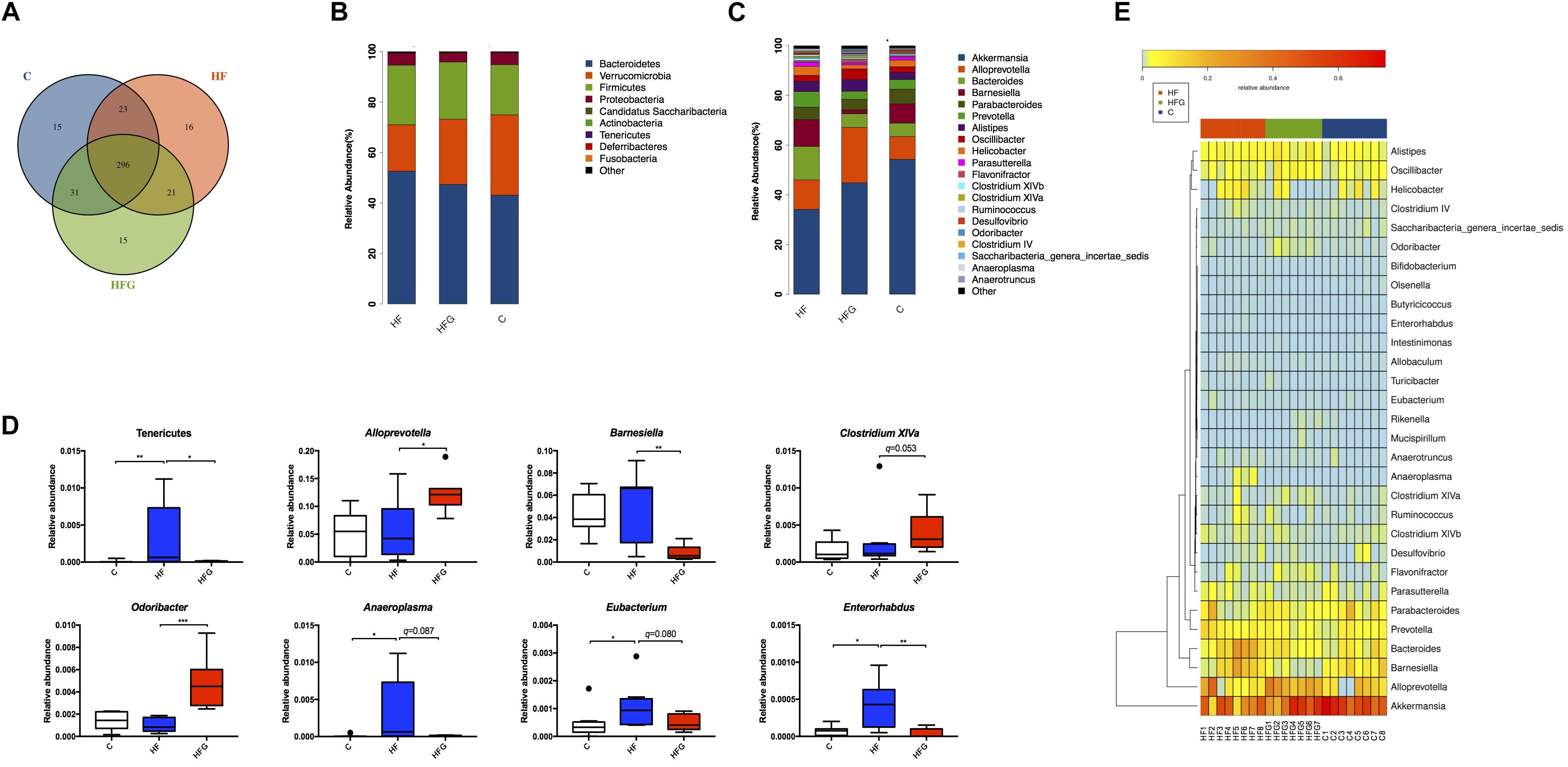
Figure 4. The changes of gut microbiota in adult offspring. (A) Venn diagram of the OUTs; (B) relative abundance of the top ten phyla; (C) relative abundance of the top twenty genera; (D) Significantly different germs at the phylum and genus levels; and (E) Heat map analysis of the different genera among the three group. C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein. Data are expressed as means ± S.E.M. (n = 7–8/group). Mean values were significantly different between the groups: ∗q < 0.05, ∗∗q < 0.01.
Then we analyzed the gut microbial structure in mice among the three groups. Alpha diversity analysis revealed that there was a parallel community richness (Chao 1) and diversity (Simpson and Shannon index) among the groups (Supplementary Table S2 and Supplementary Figure S1). Principal coordinate analysis (PCoA) on unweighted UniFrac distances was done to characterize the differences among groups. As shown in Figure 5A, the intestinal microbiota was dramatically separated in adult offspring among the three groups. ANOSIM analysis demonstrated that the differences among the groups were significant (R = 0.467, p = 0.001) (Figure 5B). Figure 6 lists the significantly different microbiota among the groups at the phylum, class, order, family and genus level. The genus Clostridium XVIII and genus Olsenella were significantly enriched in the C group. The abundance of genus Barnesiella, genus Enterorhabdus, genus Anaeroplasma from the phylum Tenericutes and genus Eubacterium were distinctly increased in mice of the HF fed dams. Maternal dietary genistein significantly enriched the genus Alloprevotella from family Prevotellaceae, genus Odoribacter, genus Clostridium XlVa, genus Rikenella and genus Mucispirillum from phylum Deferribacteres in adult offspring. Thus, maternal genistein feeding changed the composition and abundance of gut microbial communities in male offspring.
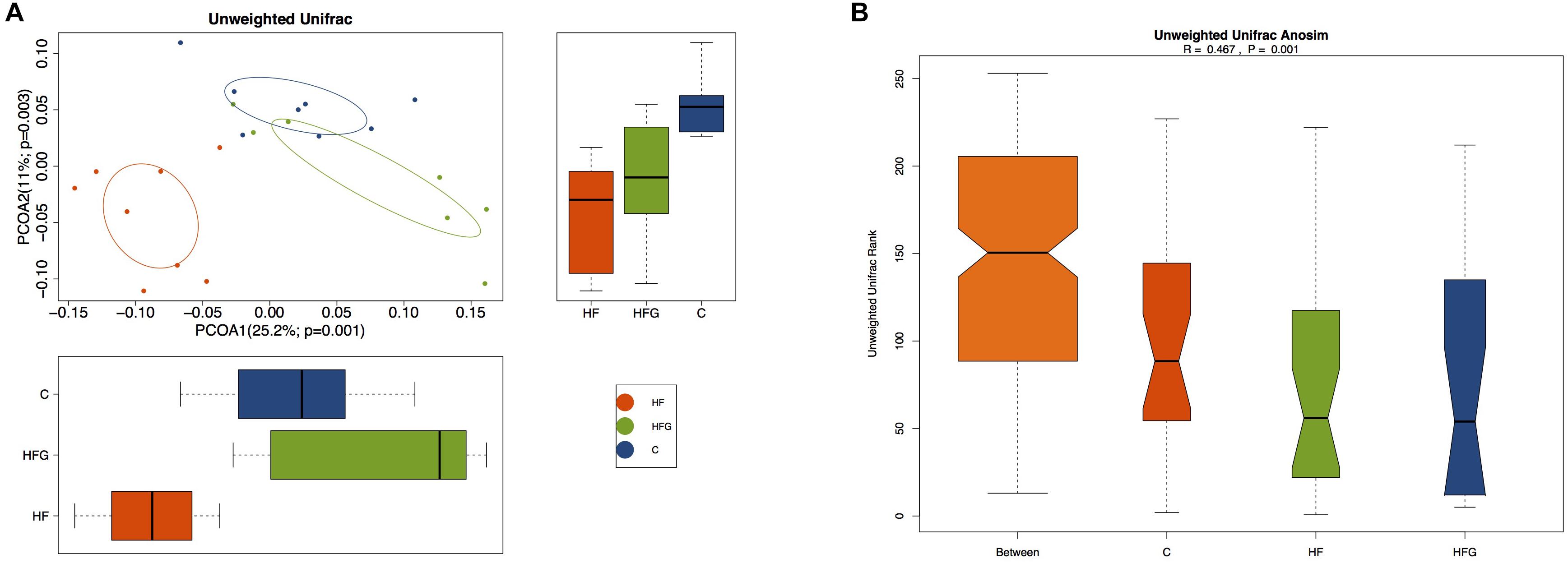
Figure 5. Beta-diversity analysis of the gut microbiota in the adult male offspring. (A) PCoA plots of gut communities; and (B) Unweighted Unifrac ANOSIM analysis between the three groups (n = 7–8/group). C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein.
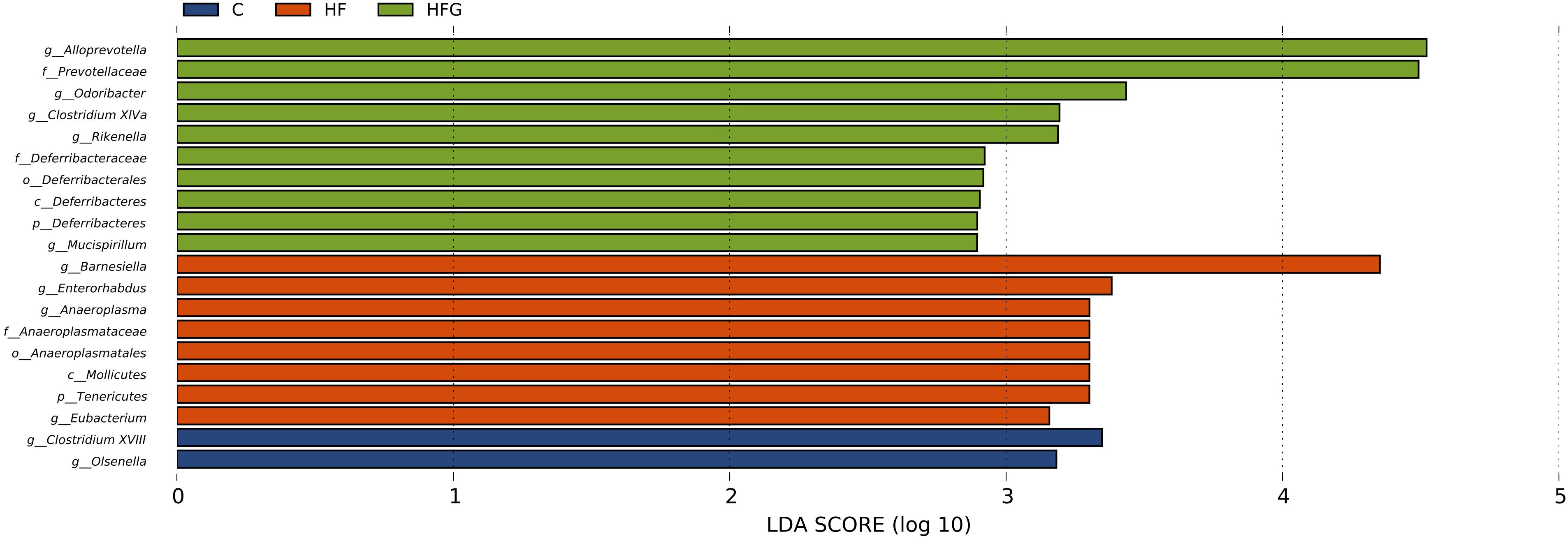
Figure 6. The LEfSe analysis of the different gut microbiota from the phylum level down to the genus level (n = 7–8/group). C, normal control diet; HF, high-fat diet; HFG, high-fat diet with genistein.
Correlation Analyses Between Gut Microbiota and Metabolic Parameters
In order to analysis the relationship between gut microbiota and glucose and lipid metabolism in adult offspring, we performed a correlation analysis between the changed metabolic parameters and significantly different microbiota at the genus level (Figure 7). The blood glucose level during the OGTT and AUC were positively correlated with the relative abundance of Eubacterium, Enterorhabdusv and Anseroplasma, but were negatively correlated with the abundance of Odoribacter, Mucispirillum, Rikenella and Clostridium XVIII (p < 0.05 or p < 0.01). In light of the insulin sensitivity, fasting insulin levels (p < 0.01) and HOMA-IR (p < 0.01) were both positively correlated with the genus Anaeroplasma. In addition to glucose metabolism, we found that the serum TG was positively correlated with the abundance of Enterorhabdus (p < 0.05) and Anseroplasma (p < 0.01), but were negatively correlated with the Rikenella (p < 0.01) and Clostridium XVIII (p < 0.01). The VAT mass also had significantly positive correlation with the relative abundance of Eubacterium (p < 0.05), Enterorhabdusv (p < 0.01) and Anseroplasma (p < 0.05), whereas had negative correlation with Rikenella (p < 0.05) and Clostridium XVIII (p < 0.01). By contrast, the serum HDL-C level was negatively correlated with Enterorhabdusv (p < 0.05) and Barnesiella (p < 0.01), but was positively correlated withAlloprevotella (p < 0.01).
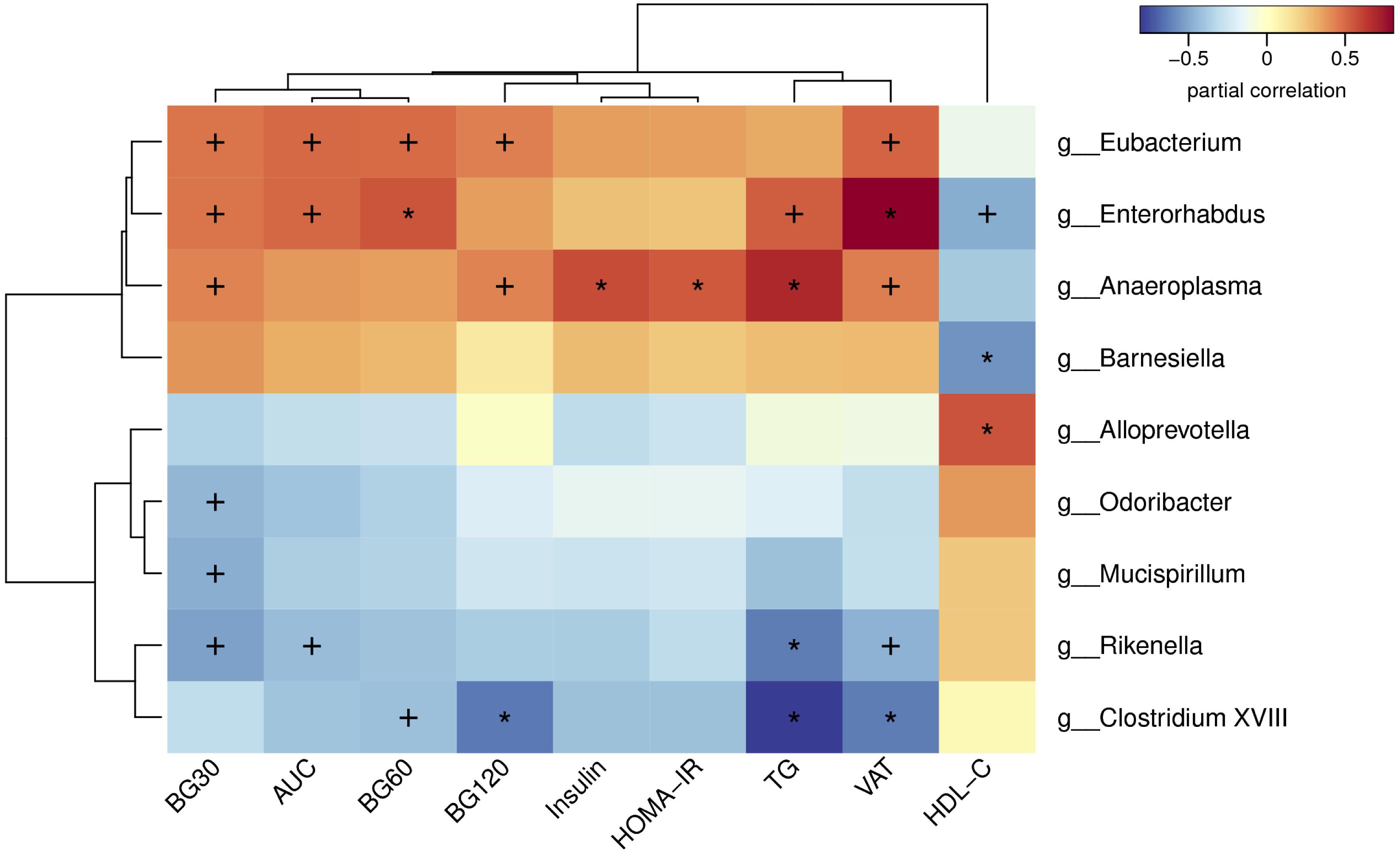
Figure 7. Heatmap of spearman correlation analysis between the altered genera and glucose and lipid metabolic parameters. BG30, blood glucose level at 30 min of OGTT; BG60, blood glucose level at 60 min of OGTT; BG120, blood glucose level at 120 min of OGTT; AUC, area under the curve of OGTT; HOMA-IR, the homeostasis model assessment of insulin resistance; TG, triglyceride; VAT, visceral adipose tissue mass; HDL-C, high-density lipoprotein cholesterol. Values were significantly correlated between the genera and glucose and lipid metabolic parameters: ∗p < 0.05; +p < 0.01.
Discussion
During the last few decades, a multitude of studies have demonstrated that adverse early-life exposures were associated with the prevalence of obesity and diabetes. Consistent with published works (Zheng et al., 2014; Cuthbert et al., 2017), our present study also showed that maternal high-fat diet for 3 weeks prior to pregnancy and throughout pregnancy and lactation resulted in significant glucose intolerance, insulin resistance and disorders of serum lipid profiles in adult male offspring. Thus, early life might be a critical window for fighting against the rapid increase of metabolic disorders. It has been commonly accepted that genistein has beneficial effects on glucose homeostasis and lipid metabolism (Gilbert and Liu, 2013). However, research on the influence of early-life genistein intake on metabolism in later life is limited. In the current study, we found that maternal dietary genistein during pre-pregnancy, pregnancy and lactation could significantly counteract the deleterious effects induced by high-fat diet on glucose tolerance, insulin sensitivity and lipid metabolism in adult offspring. Our previous studies and other experimental animal models only detected the protective effects of maternal genistein feeding on metabolism in early life of offspring (Zhang et al., 2015; Han et al., 2017; Zhou et al., 2018). To the best of our knowledge, this is the first study showing the benefits of genistein supplementation in early life on improving metabolic health in adult offspring.
Recent evidence has shown that gut microbiota might play important roles in linking adverse early-life environments and metabolic disorders in later life (Codagnone et al., 2019). Given the crucial role of gut microbiota in metabolic health and the relationship between genistein intake and gut microbial community, we hypothesized that there were alterations in intestinal microbiota in adult mice experienced by early-life genistein exposures. The results revealed that maternal high-fat diet resulted in significant dysbiosis of gut microbial composition and structure, whereas maternal genistein feeding counteracted these changes.
Our present studies showed that early-life over-nutrition (high-fat diet) significantly increased the relative abundance of the phylum Tenericutes as well as the genus Barnesiella, genus Enterorhabdus, genus Anaeroplasma from the phylum Tenericutes in male offspring, which were positively correlated with the levels of blood glucose during OGTT, AUC of OGTT, insulin and TG, but negatively correlated with HDL-C. Similarly, Yan et al. (2016) demonstrated that the Tenericutes were higher in type 2 diabetic rats than lean controls at the phylum level. Furthermore, the phylum Tenericutes have been shown to be increased in HFD fed mice and are related with obesity-associated metabolic parameters (Lecomte et al., 2015). n - 3 PUFA deficiency has been shown to be associated with development and progression of a multitude of chronic metabolic disorders. The relationship between n - 3 deficient diets and elevated Tenericutes phylum and Anaeroplasma has been preliminary confirmed (Robertson et al., 2017). The Enterorhabdus and Barnesiella and were also be implicated in the development of T2DM, obesity and non-alcoholic fatty liver disease in humans or mice (Le Roy et al., 2013; Yang et al., 2015). More specifically, low-birth-weight mice experienced by nutrition restriction during late pregnancy with accelerated postnatal growth also leaded to significant increase of Enterorhabdus and Barnesiella in infants (Wang et al., 2016). Thus, the dramatically elevated Tenericutes, Barnesiella, Enterorhabdus and Anaeroplasma might played vital roles in deciphering the deleterious metabolic effects of maternal high-fat diet on adult offspring. Therefore, decreasing the abundances of those species could bring some metabolic benefits. It has been reported that prebiotic, such as inulin and xylooligosaccharide, which had beneficial effects on metabolic health, could decrease the abundance of Tenericutes and Enterorhabdus (Yang et al., 2015; Zhang et al., 2018; Li et al., 2019). In this study, we found that maternal genistein intake could significantly reduce the abundance of Tenericutes, Barnesiella, Enterorhabdus and Anaeroplasma in adult offspring, which might play crucial roles in mediating the transgenerational metabolic benefits of dietary genistein.
Maternal dietary genistein intake significantly enriched the Alloprevotella, Odoribacter and Clostridium XlVa at the genus level in adult offspring, which were all confirmed to be short chain fatty acid (SCFA) producers (Pryde et al., 2002; Gomez-Arango et al., 2016; Shang et al., 2017; Shi et al., 2018). A large number of studies has illustrated that the abundance of these bacteria were lower in obese or type 2 diabetic rats or humans (Schwiertz et al., 2010; Gomez-Arango et al., 2016; Jung et al., 2016). By contrast, improvement of glucose and lipid metabolism by polyunsaturated fatty acids (Li et al., 2018), berberine fumarate (Cui et al., 2018), dietary fucoidan (Shang et al., 2017), oral hydroxysafflor yellow A (Liu et al., 2018), a leucine-deprived diet (Wei S. et al., 2018), a galacto-oligosaccharide-rich diet (Cheng et al., 2018) or a traditional Chinese medicine prescription-Xiexin Tang (Wei X. et al., 2018) were all positively correlated with the abundance of these bacterial species. SCFAs (mainly acetate and butyrate), which were produced by fermentation of indigestible dietary components by specific gut microbiota, appeared to promote epithelial restitution and have anti-inflammation activity (Sturm and Dignass, 2008). The main roles of SCFAs were to regulate glucose homeostasis and insulin sensitivity in the liver, skeletal muscle and adipose tissue, possibly though inhibiting intracellular lipolysis to decrease the lipid overflow and ectopic accumulation of fat and regulating chronic inflammation by activating anti-inflammatory Treg cells or directly decreasing the production of proinflammatory cytokines and chemokines (Tremaroli and Backhed, 2012; Canfora et al., 2015). Similarly, in our present study, the enrichment of these three SCFA-producing genera in adult mice of HFG fed dams were negatively correlated with the unfavorable glucose and lipid metabolic parameters. It might be that their metabolites SCFAs possessed the metabolic protective effect. However, the specific mechanisms of the altered bacteria regulating metabolism still need further exploration.
In addition, the genus Rikenella from the family Rikenellaceae was also significantly enriched in adult offspring of genistein fed dams. Although the concrete function of Rikenella remained largely unclear, the opposite relationship between the abundance of Rikenella or Rikenellaceae with body weight or fat mass in mice and humans has been demonstrated in several studies (Oki et al., 2016; Li et al., 2017). Our previous research also showed that maternal dietary genistein intake could significantly increase the abundance of Rikenella in early life of offspring (Zhou et al., 2018). Correlation analysis indicated that improved glucose and lipid metabolic parameters were significantly associated with elevated Rikenella in mice of genistein fed dams. Thus, our studies demonstrated that the genus Rikenella might be a crucial factor in mediating the beneficial effects of maternal genistein intake in offspring metabolism throughout the life.
In conclusion, maternal dietary genistein intake significantly mitigated the adverse effects of high-fat diet on glucose and lipid metabolism in adult male offspring. In the mean time, the changes of gut microbiota might play a crucial role in the metabolic benefits. To the best of our knowledge, this is the first study to show the important role of gut microbiota in linking maternal genistein supplementation and metabolic protects in adulthood. However, there are still several limitations in this study. First, we only investigated the effects of maternal genistein feeding on metabolism in adult male offspring. The female offspring is still worth to be researched in future. In addition, only relationship between the gut microbiota and metabolism was analyzed in this study, while the causality is in request for further exploration. Finally, the specific mechanism by which maternal genistein intake regulates metabolism and gut microbiota in offspring is still unclear and needs further exploration. Overall, our study provides new evidence and methods for offsetting the poor effects of adverse early-life exposures on metabolic health in later life.
Data Availability
The datasets for this manuscript are not publicly available because the datasets supporting the conclusions of this manuscript are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to XX, eGlhb3hoMjAxNEB2aXAuMTYzLmNvbQ==.
Ethics Statement
All the procedures were approved by the Animal Care and Use Committee of the Peking Union Medical College Hospital (Beijing, China, SYXC-2014-0029). All the animal operations were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Author Contributions
XX and JZ designed the experiments. LZ and MD performed the experiments. LZ analyzed the data and drafted the manuscript. XX and QZ reviewed the manuscript. All authors approved the submitted version of the manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81170736, 81570715, 81870579, and 81870545).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very grateful to Realbio Genomics Institute (Shanghai, China) for their technical support with 16S rDNA sequences.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00985/full#supplementary-material
References
Al-Nakkash, L., Martin, J. B., Petty, D., Lynch, S. M., Hamrick, C., Lucy, D., et al. (2012). Dietary genistein induces sex-dependent effects on murine body weight, serum profiles, and vascular function of thoracic aortae. Gend. Med. 9, 295–308. doi: 10.1016/j.genm.2012.07.001
Aris, I. M., Fleisch, A. F., and Oken, E. (2018). Developmental origins of disease: emerging prenatal risk factors and future disease risk. Curr. Epidemiol. Rep. 5, 293–302. doi: 10.1007/s40471-018-0161-0
Canfora, E. E., Jocken, J. W., and Blaak, E. E. (2015). Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591. doi: 10.1038/nrendo.2015.128
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chang, R. C., Wang, H., Bedi, Y., and Golding, M. C. (2019). Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 12:9. doi: 10.1186/s13072-019-0254-0
Cheng, W., Lu, J., Lin, W., Wei, X., Li, H., Zhao, X., et al. (2018). Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 9, 1612–1620. doi: 10.1039/c7fo01720k
Codagnone, M. G., Spichak, S., O’Mahony, S. M., O’Leary, O. F., Clarke, G., Stanton, C., et al. (2019). Programming bugs: microbiota and the developmental origins of brain health and disease. Biol. Psychiatry 85, 150–163. doi: 10.1016/j.biopsych.2018.06.014
Csongova, M., Gurecka, R., Koborova, I., Celec, P., Domonkos, E., Ulicna, O., et al. (2018). The effects of a maternal advanced glycation end product-rich diet on somatic features, reflex ontogeny and metabolic parameters of offspring mice. Food Funct. 9, 3432–3446. doi: 10.1039/c8fo00183a
Cui, H. X., Hu, Y. N., Li, J. W., and Yuan, K. (2018). Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid. Med. Cell Longev. 2018:8930374. doi: 10.1155/2018/8930374
Cuthbert, C. E., Foster, J. E., and Ramdath, D. D. (2017). A maternal high-fat, high-sucrose diet alters insulin sensitivity and expression of insulin signalling and lipid metabolism genes and proteins in male rat offspring: effect of folic acid supplementation. Br. J. Nutr. 118, 580–588. doi: 10.1017/s0007114517002501
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. doi: 10.1128/aem.03006-05
Devi, K. P., Shanmuganathan, B., Manayi, A., Nabavi, S. F., and Nabavi, S. M. (2017). Molecular and therapeutic targets of genistein in Alzheimer’s Disease. Mol. Neurobiol. 54, 7028–7041. doi: 10.1007/s12035-016-0215-6
Ding, M., Pan, A., Manson, J. E., Willett, W. C., Malik, V., Rosner, B., et al. (2016). Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 70, 1381–1387. doi: 10.1038/ejcn.2016.117
Dong, J., Cong, Z., You, M., Fu, Y., Wang, Y., Wang, Y., et al. (2019). Effects of perinatal di (2-ethylhexyl) phthalate exposure on thyroid function in rat offspring. Environ. Toxicol. Pharmacol. 67, 53–60. doi: 10.1016/j.etap.2019.01.012
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Gilbert, E. R., and Liu, D. (2013). Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 4, 200–212. doi: 10.1039/c2fo30199g
Gomez-Arango, L. F., Barrett, H. L., McIntyre, H. D., Callaway, L. K., Morrison, M., Dekker Nitert, M., et al. (2016). Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68, 974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910
Han, A., Won, S. B., and Kwon, Y. H. (2017). Different effects of maternal low-isoflavone soy protein and genistein consumption on hepatic lipid metabolism of 21-day-old male rat offspring. Nutrients 9:E1039. doi: 10.3390/nu9091039
Huang, Y. H., Ye, T. T., Liu, C. X., Wang, L., Chen, Y. W., and Dong, Y. (2017). Maternal high-fat diet impairs glucose metabolism, beta-cell function and proliferation in the second generation of offspring rats. Nutr. Metab. 14:67. doi: 10.1186/s12986-017-0222-2
Jung, M. J., Lee, J., Shin, N. R., Kim, M. S., Hyun, D. W., Yun, J. H., et al. (2016). Chronic repression of mtor complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 6:30887. doi: 10.1038/srep30887
Kearney, M., Perron, J., Marc, I., Weisnagel, S. J., Tchernof, A., and Robitaille, J. (2018). Association of prenatal exposure to gestational diabetes with offspring body composition and regional body fat distribution. Clin. Obes. 8, 81–87. doi: 10.1111/cob.12237
Le Roy, T., Llopis, M., Lepage, P., Bruneau, A., Rabot, S., Bevilacqua, C., et al. (2013). Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 62, 1787–1794. doi: 10.1136/gutjnl-2012-303816
Lecomte, V., Kaakoush, N. O., Maloney, C. A., Raipuria, M., Huinao, K. D., Mitchell, H. M., et al. (2015). Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 10:e0126931. doi: 10.1371/journal.pone.0126931
Lepri, S. R., Sartori, D., Semprebon, S. C., Baranoski, A., Coatti, G. C., and Mantovani, M. S. (2018). Genistein affects expression of cytochrome P450 (CYP450) genes in hepatocellular carcinoma (hepg2/c3a) cell line. Drug Metab. Lett. 12, 138–144. doi: 10.2174/1872312812666180709150440
Li, K., Zhang, L., Xue, J., Yang, X., Dong, X., Sha, L., et al. (2019). Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 10, 1915–1927. doi: 10.1039/c8fo02265h
Li, R., Wang, H., Shi, Q., Wang, N., Zhang, Z., Xiong, C., et al. (2017). Effects of oral florfenicol and azithromycin on gut microbiota and adipogenesis in mice. PLoS One 12:e0181690. doi: 10.1371/journal.pone.0181690
Li, T. T., Liu, Y. Y., Wan, X. Z., Huang, Z. R., Liu, B., and Zhao, C. (2018). Regulatory efficacy of the polyunsaturated fatty acids from microalgae spirulina platensis on lipid metabolism and gut microbiota in high-fat diet rats. Int. J. Mol. Sci. 19:E3075. doi: 10.3390/ijms19103075
Liu, J., Yue, S., Yang, Z., Feng, W., Meng, X., Wang, A., et al. (2018). Oral hydroxysafflor yellow a reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res. 134, 40–50. doi: 10.1016/j.phrs.2018.05.012
Liu, Y., Li, J., Wang, T., Wang, Y., Zhao, L., and Fang, Y. (2017). The effect of genistein on glucose control and insulin sensitivity in postmenopausal women: a meta-analysis. Maturitas 97, 44–52. doi: 10.1016/j.maturitas.2016.12.004
Lopez, P., Sanchez, M., Perez-Cruz, C., Velazquez-Villegas, L. A., Syeda, T., Aguilar-Lopez, M., et al. (2018). Long-term genistein consumption modifies gut microbiota, improving glucose metabolism, metabolic endotoxemia, and cognitive function in mice fed a high-fat diet. Mol. Nutr. Food Res. 62:e1800313. doi: 10.1002/mnfr.201800313
Lund, I. O., Moen Eilertsen, E., Gjerde, L. C., Roysamb, E., Wood, M., Reichborn-Kjennerud, T., et al. (2019). Is the association between maternal alcohol consumption in pregnancy and pre-school child behavioural and emotional problems causal? Multiple approaches for controlling unmeasured confounding. Addiction 114, 1004–1014. doi: 10.1111/add.14573
Mithieux, G. (2018). Gut microbiota and host metabolism: what relationship. Neuroendocrinology 106, 352–356. doi: 10.1159/000484526
Nanri, A., Mizoue, T., Takahashi, Y., Kirii, K., Inoue, M., Noda, M., et al. (2010). Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J. Nutr. 140, 580–586. doi: 10.3945/jn.109.116020
Odegaard, A. O., Koh, W. P., Butler, L. M., Duval, S., Gross, M. D., Yu, M. C., et al. (2011). Dietary patterns and incident type 2 diabetes in chinese men and women: the singapore chinese health study. Diabetes Care 34, 880–885. doi: 10.2337/dc10-2350
Oki, K., Toyama, M., Banno, T., Chonan, O., Benno, Y., and Watanabe, K. (2016). Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 16:284. doi: 10.1186/s12866-016-0898-x
Pryde, S. E., Duncan, S. H., Hold, G. L., Stewart, C. S., and Flint, H. J. (2002). The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217, 133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x
Robertson, R. C., Seira Oriach, C., Murphy, K., Moloney, G. M., Cryan, J. F., Dinan, T. G., et al. (2017). Deficiency of essential dietary n-3 PUFA disrupts the caecal microbiome and metabolome in mice. Br. J. Nutr. 118, 959–970. doi: 10.1017/s0007114517002999
Schwiertz, A., Taras, D., Schafer, K., Beijer, S., Bos, N. A., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195. doi: 10.1038/oby.2009.167
Shang, Q., Song, G., Zhang, M., Shi, J., Xu, C., Hao, J., et al. (2017). Dietary fucoidan improves metabolic syndrome in association with increased akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 28, 138–146. doi: 10.1016/j.jff.2016.11.002
Shi, C., Zhu, Y., Niu, Q., Wang, J., Wang, J., and Zhu, W. (2018). The changes of colonic bacterial composition and bacterial metabolism induced by an early food introduction in a neonatal porcine Model. Curr. Microbiol. 75, 745–751. doi: 10.1007/s00284-018-1442-z
Shi, Y. N., Zhang, X. Q., Hu, Z. Y., Zhang, C. J., Liao, D. F., Huang, H. L., et al. (2019). Genistein protects h9c2 cardiomyocytes against chemical hypoxia-induced injury via inhibition of apoptosis. Pharmacology 103, 282–290. doi: 10.1159/000497061
Simperova, A., Al-Nakkash, L., Faust, J. J., and Sweazea, K. L. (2016). Genistein supplementation prevents weight gain but promotes oxidative stress and inflammation in the vasculature of female obese ob/ob mice. Nutr. Res. 36, 789–797. doi: 10.1016/j.nutres.2016.03.011
Soderborg, T. K., Borengasser, S. J., Barbour, L. A., and Friedman, J. E. (2016). Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia 59, 895–906. doi: 10.1007/s00125-016-3880-0
Sturm, A., and Dignass, A. U. (2008). Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 14, 348–353.
Thompson, M. D., Derse, A., Ferey, J., Reid, M., Xie, Y., Christ, M., et al. (2019). Transgenerational impact of maternal obesogenic diet on offspring bile acid homeostasis and non-alcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 16, E674–E686.
Tremaroli, V., and Backhed, F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249. doi: 10.1038/nature11552
Velazquez, M. A., Fleming, T., and Watkins, A. J. (2019). Periconceptional environment and the developmental origins of disease. J. Endocrinol. 242, T33–T49. doi: 10.1530/JOE-18-0676
Wang, J., Tang, H., Wang, X., Zhang, X., Zhang, C., Zhang, M., et al. (2016). The structural alteration of gut microbiota in low-birth-weight mice undergoing accelerated postnatal growth. Sci. Rep. 6:27780. doi: 10.1038/srep27780
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/aem.00062-07
Wankhade, U. D., Thakali, K. M., and Shankar, K. (2016). Persistent influence of maternal obesity on offspring health: mechanisms from animal models and clinical studies. Mol. Cell Endocrinol. 435, 7–19. doi: 10.1016/j.mce.2016.07.001
Wei, S., Zhao, J., Wang, S., Huang, M., Wang, Y., and Chen, Y. (2018). Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon 4:e00830. doi: 10.1016/j.heliyon.2018.e00830
Wei, X., Tao, J., Xiao, S., Jiang, S., Shang, E., Zhu, Z., et al. (2018). Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 8:3685. doi: 10.1038/s41598-018-22094-2
Wen, J., Hong, Q., Wang, X., Zhu, L., Wu, T., Xu, P., et al. (2018). The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 8:365. doi: 10.1038/s41598-017-18770-4
Yan, X., Feng, B., Li, P., Tang, Z., and Wang, L. (2016). Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J. Diabetes Res. 2016:2093171. doi: 10.1155/2016/2093171
Yang, J., Summanen, P. H., Henning, S. M., Hsu, M., Lam, H., Huang, J., et al. (2015). Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front. Physiol. 6:216. doi: 10.3389/fphys.2015.00216
Ye, C. F., Pan, Y. M., and Zhou, H. (2018). Regulation of vitamin D receptor and genistein on bone metabolism in mouse osteoblasts and the molecular mechanism of osteoporosis. J. Biol. Regul. Homeost Agents 32, 497–505.
Yu, J., Yang, J., Luo, Y., Mengxue, Y., Li, W., Yang, Y., et al. (2018). The adverse effects of chronic low-dose exposure to nonylphenol on type 2 diabetes mellitus in high sucrose-high fat diet-treated rats. Islets 10, 1–9. doi: 10.1080/19382014.2017.1404211
Zhang, Q., Yu, H., Xiao, X., Hu, L., Xin, F., and Yu, X. (2018). Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 6:e4446. doi: 10.7717/peerj.4446
Zhang, X., Dong, Y., Sun, G., Hasan, A. A., Tian, M., Zeng, S., et al. (2019). Paternal programming of liver function and lipid profile induced by a paternal pre-conceptional unhealthy diet: potential association with altered gut microbiome composition. Kidney Blood Press Res. 44, 133–148. doi: 10.1159/000497487
Zhang, Y. B., Yan, J. D., Yang, S. Q., Guo, J. P., Zhang, X., Sun, X. X., et al. (2015). Maternal genistein intake can reduce body weight in male offspring. Biomed. Environ. Sci. 28, 769–772. doi: 10.3967/bes2015.107
Zheng, J., Xiao, X., Zhang, Q., Yu, M., Xu, J., Qi, C., et al. (2016). The effects of maternal and post-weaning diet interaction on glucose metabolism and gut microbiota in male mice offspring. Biosci. Rep. 36:e00341. doi: 10.1042/bsr20160103
Zheng, J., Xiao, X., Zhang, Q., Yu, M., Xu, J., and Wang, Z. (2014). Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the PPAR pathway in the early life of offspring. Int. J. Mol. Sci. 15, 14967–14983. doi: 10.3390/ijms150914967
Zhou, L., and Xiao, X. (2018). The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci. Rep. 38:BSR20171234. doi: 10.1042/bsr20171234
Keywords: maternal genistein intake, glucose metabolism, lipid metabolism, gut microbiota, adult life, male offspring, high-fat diet
Citation: Zhou L, Xiao X, Zhang Q, Zheng J and Deng M (2019) Maternal Genistein Intake Mitigates the Deleterious Effects of High-Fat Diet on Glucose and Lipid Metabolism and Modulates Gut Microbiota in Adult Life of Male Mice. Front. Physiol. 10:985. doi: 10.3389/fphys.2019.00985
Received: 24 April 2019; Accepted: 15 July 2019;
Published: 30 July 2019.
Edited by:
Kesia Palma-Rigo, State University of Maringá, BrazilReviewed by:
Rodrigo Mello Gomes, Universidade Federal de Goiás, BrazilPaulo Cezar De Freitas Mathias, State University of Maringá, Brazil
Copyright © 2019 Zhou, Xiao, Zhang, Zheng and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinhua Xiao, eGlhb3hoMjAxNEB2aXAuMTYzLmNvbQ==
 Liyuan Zhou
Liyuan Zhou Xinhua Xiao*
Xinhua Xiao*