Abstract
Harmonia axyridis is an important natural predator used in the biological control of insect pests. Vitellogenin (Vg) supplementation to artificial diet can improve fecundity of H. axyridis, however, the effects of Vg on physiology of H. axyridis at the molecular level is unclear. This study investigated the effects of Vg on the physiology (digestive enzyme activities) and transcriptome patterns by feeding H. axyridis adults with treatment (artificial diet with Vg supplement) and control (artificial diet supplemented with bovine serum albumin (BSA). The transcriptome sequencing yielded 43.94 Gb of clean data, and 3,946 differentially expressed genes (DEGs) – including 93 upregulated and 3,853 downregulated genes between the treatment and control. Six DEGs related to development and digestive enzyme were chosen for quantitative real-time PCR (qRT-PCR) to validate the accuracy of the RNA-seq results and confirmed that the transcriptome analysis yielded reliable results. The Vg supplement has increased activities of digestive enzymes and related genes expression in H. axyridis. The transcript level of digestive enzyme genes (apolipoprotein D and phosphoenolpyruvate carboxykinase) were much higher in adults fed on diet supplemented with Vg compared with that of the control.
Introduction
Harmonia axyridis (Pallas) is an omnivorous predator that was widely introduced in North America and Europe as early as 1916 (Gordon, 1985; Adriaens et al., 2003). This polyphagous predator feeds on aphids, coccids (Mcclure, 1987; Hodek and Honěk, 2009), lepidopteran eggs (Ferran et al., 1984) and other insects (Koch et al., 2003). Due to its large predation, good adaptability and strong fecundity, this insect is widely used for biological control purposes (De Bach and Rosen, 1991; Ferran et al., 1996; Yasuda et al., 2000). To reduce the use of and adverse effects of chemical pesticides, insect pest control by natural enemy insects has received widespread attention. Mass rearing is important for the commercial production of beneficial insects used in the biological control of insect pests and has become a promising industry (Lenteren et al., 1997; Greany and Carpenter, 1998). However, a natural prey diet is unable to sustain a population of H. axyridis over an entire year because the environmental conditions might limit the supply of prey. It is thus necessary to implement an artificial diet to rear this beneficial insect as supplemental predators for the effective field control of pests. Coudron et al. (2002) indicated that the cost of rearing Podisus maculiventris on an artificial larval diet is similar as that associated with the use of natural prey. Zhang et al. (2015) studied an artificial diet that could be used as larval food in the mass rearing of H. axyridis. Similarly, other researchers also reported three artificial diets used to rear H. axyridis (Castro-Guedes et al., 2016).
The digestive enzymes are important in insects for food digestion and nutrient absorption. In insects, the food is ingested through the food canal into the alimentary canal, where it is further digested and absorbed by enzymes such as trehalase and lipase. Trehalase is very important for the use of sugar, energy production and macromolecular biosynthesis in insects (Friedman, 1978). Also, lipase plays an important role in the lipid metabolism and absorption, and related to the growth and development of insects (Chapman, 1998; Dottorini et al., 2007). The nutrients digested by enzymes play a crucial role in the development and reproduction of the insects. Tufail and Takeda (2008) reported that Vg is major nutrition source for the embryo development. Many researchers have showed that Vg is related with growth and development of occytes and egg production (Zeng et al., 1997). The amount and quality of food directly influence various biological aspects of Coccinellidae (Dixon, 2000), and so a lack of nutrients could affect their growth and development (Hodek et al., 2012). In fact, food permits the complete development and reproduction of Coccinellids. The nutrients in an artificial diet also impact the degree of ovarian maturation and the regulatory mechanisms for oogenesis (Adams, 2000). One goal in mass insect rearing is to use cost-effective artificial diets. Cheng et al. (2017) found that the artificial diet for adults was superior to any previously developed formulas and might thus have potential for improving the artificial diet used for the mass rearing of Chrysopa septempunctata. These studies reveal that nutrient addition is beneficial to the metabolism and optimization of the artificial diet and thus affects the physiology and reproduction of H. axyridis. However, most current artificial diets for H. axyridis result in low fecundity, and the methods used to evaluate their diet are limited in terms of their biological category, thus, the other alternative method is necessary to test the effects of the diet formulation on insect performance, such as diet optimization by measuring a few preselected biochemical and physiological parameters. In addition, the techniques of comparative transcriptomics may be used to help effectively evaluate the artificial diets for mass rearing of insects. Based on a study showing that nutrition affects gene expression patterns (Yocum et al., 2006), the study of these patterns not only allows the exploration of the insects’ response to changes in their food stream but also offers information on diet limitations. Zou et al. (2013) compared the transcriptome of Arrma chinensis fed an artificial diet with that of Antheraea pernyi pupae and found that many metabolic pathways related to nutrition were upregulated in the diet-fed A. chinensis. Some diet-regulating genes play an important role in reproduction and longevity as found by Alaux et al. (2011) in that nutrition influences genes related to longevity and the production of some antimicrobial peptides. Chen and Hsiao (1984) found that a delay in oviposition or an increased developmental rate might decrease the intrinsic rate. Liu et al. (2015) cloned the complete Vg cDNA sequence from a Neuropteran species, C. septempunctata Wesmael, and studied the vitellogenn functions, they found that RNAi mediated by injection of dsRNA depleted CsVg transcripts, significantly reduced egg production and decreased egg hatching rate. Du and Zeng (2016) analyzed time-series RNA-seq data from the H. axyridis adult ovary to identify development-related genes. Zhang T. et al. (2017) further explored the effects of Vg fragment on development and reproduction H. axyridis, they found that the Vg fragment significantly increased the egg production of H. axyridis. The influence of artificial diet supplemented with Vg on physiology and the transcriptome of H. axyridis may be used to further understand the action of nutrients on H. axyridis at molecular level and improve artificial diet for this insect. Therefore, we have studied the effects of Vg supplement in an artificial diet on H. axyridis using comparative transcriptome. We hypothesized that the addition of Vg supplement could increase H. axyridis enzyme related gene expression levels, and then increase enzyme activities and improve nutritional digestion and absorption and therefore increase reproduction of H. axyridis. The main goals of this study were to understand the influence of Vg supplement on H. axyridis physiology (enzyme activities) and egg reproduction, assess the molecular-level effects of Vg on H. axyridis using RNA-seq technology. The study will obtain the abdomen transcriptomes of H. axyridis adults fed diet supplemented with Vg (treatment) or BSA (control) on the fifth day after oviposition and performed an analysis of generate accurate data, and identify nutritional and development-related genes. The information obtained from the transcriptome-wide study of mRNA in the H. axyridis abdomen may be used to improve the artificial diet for the growth and reproduction of H. axyridis.
Results
Transcriptome Sequencing and Read Assembly
The transcriptome sequencing of the H. axyridis abdomen yielded 43.94 Gb of clean data. Libraries of controls samples (C1 and C2) and the libraries treatments samples (T01, T02, and T03) were collected (Only 5 samples, two BSA controls (C1 and C2) and three Vg treatments (T1, T2, and T3), were analyzed by DEGs (Anders and Huber, 2010), C3, one of control samples was degraded and not included for analysis). Five libraries containing clean reads (C1: 20,632,283; C2: 29,972,500; T01: 31,985,871; T02: 35,389,508; T03: 30,517,849) were generated for analysis after cleaning and checking. The percentages of GC contents were analyzed in five libraries (C1: 39.88%; C2: 40.13%; T01: 39.45%; T02: 39.81%; T03: 39.53%). The five libraries of percentage ≥ Q30 were 87.54, 91.43, 90.68, 91.37, and 91.43%, respectively (Table 1). After assembly, the total numbers of 107,939 transcripts and 75,717 unigenes were obtained, and the N50s of the transcripts and unigenes were 1,922 and 1,400 bp, respectively. Means length of the transcripts and unigenes were 1009.31 and 767.48 bp (Table 2). The raw transcriptome reads were deposited in the NCBI SRA database (accession number: SRP104023).
TABLE 1
| Samples | Clean reads | Clean data | GC Content (%) | %=Q30 |
| C1 | 20,632,283 | 6,095,457,898 | 39.88% | 87.54% |
| C2 | 29,972,500 | 8,908,858,910 | 40.13% | 91.43% |
| T01 | 31,985,871 | 9,446,577,644 | 39.45% | 90.68% |
| T02 | 35,389,508 | 10,479,909,588 | 39.81% | 91.37% |
| T03 | 30,517,849 | 9,012,385,962 | 39.53% | 91.43% |
Statistics of the sequencing data.
Samples, name of the sample; clean reads, total number of paired-end reads in the clean data; GC content, percentage of G and C bases among the total bases; and % ≥ Q30, percentage of bases with quality value ≥ 30.
TABLE 2
| Length range | Transcript | Unigene |
| 200-300 | 32,454 (30.07%) | 29,864 (39.44%) |
| 300-500 | 21,231 (19.67%) | 16,784 (22.17%) |
| 500-1000 | 21,932 (20.32%) | 14,055 (18.56%) |
| 1000-2000 | 17,264 (15.99%) | 8,644 (11.42%) |
| 2000 + | 15,058 (13.95%) | 6,370 (8.41%) |
| Total Number | 107,939 | 75,717 |
| Total Length | 108,944,081 | 58,111,525 |
| N50 Length | 1,922 | 1,400 |
| Mean Length | 1009.31 | 767.48 |
Sequencing data statistics.
Annotation of Unigenes
For functional annotation, we received 27,992 unigenes annotated using Blastx tool against NCBI Integrated Nr databases (Nr), 14,025 unigenes were annotated in the GO annotation databases, 14,803 unigenes were annotated in the KEGG annotation databases, 23,524 unigenes were annotated in the Pfam annotation database, 10,535 unigenes were annotated in the COG, and 22.803 unigenes were annotated in the KOG annotation databases, respectively. A total of 36,668 unigenes were annotated based on the selection of parameters with an E-value ≤ 1e-10 (Table 3).
TABLE 3
| #Anno_Database | Annotated_ Number | 300=length < 1000 | Length = 1000 |
| COG_Annotation | 10535 | 4001 | 4655 |
| GO_Annotation | 14025 | 5320 | 6430 |
| KEGG_Annotation | 14803 | 5918 | 6325 |
| KOG_Annotation | 22803 | 8884 | 9388 |
| Pfam_Annotation | 23524 | 9275 | 10690 |
| Swissprot_Annotation | 28316 | 11364 | 12289 |
| eggNOG_Annotation | 33884 | 13860 | 12085 |
| Nr_Annotation | 27992 | 11158 | 12266 |
| All_Annotated | 36668 | 15183 | 12738 |
Statistics of the unigene annotations.
Analysis of Differentially Expressed Genes (DEGs)
The comparison of control (C2 and C2) with treatment (T01, T02, and T03) identified 3,946 DEGs, including 93 upregulated genes and 3,853 downregulated genes (Table 4). The MA plot clearly shows the overall distribution of gene expression abundance and the various differences between the two sets of sample groups. Each black point represents a non-significant difference in gene expression, each red point indicates an upregulate expressed gene, and each green point indicates a downregulate expressed gene. Abscissa: log2 (FPKM) represents the logarithm of the mean value of the expressions in the two sample groups. Ordinate: log2 (FC) represents the logarithm of the multiple of gene expression difference between the two sample groups (Figure 1). All of the obtained DEGs were combined into one DEG database (Supplementary Dataset File S1).
TABLE 4
| DEG_Set | All_DEG | Upregulated | Downregulated |
| C1_C2_vs_T01_T02_T03 | 3946 | 93 | 3853 |
Number of differentially expressed genes.
The threshold value for significance in the comparison of the control (C1 and C2) with the treatment (T01, T02, and T03) was FPKM fold change > 2 and FDR < 0.05.
FIGURE 1
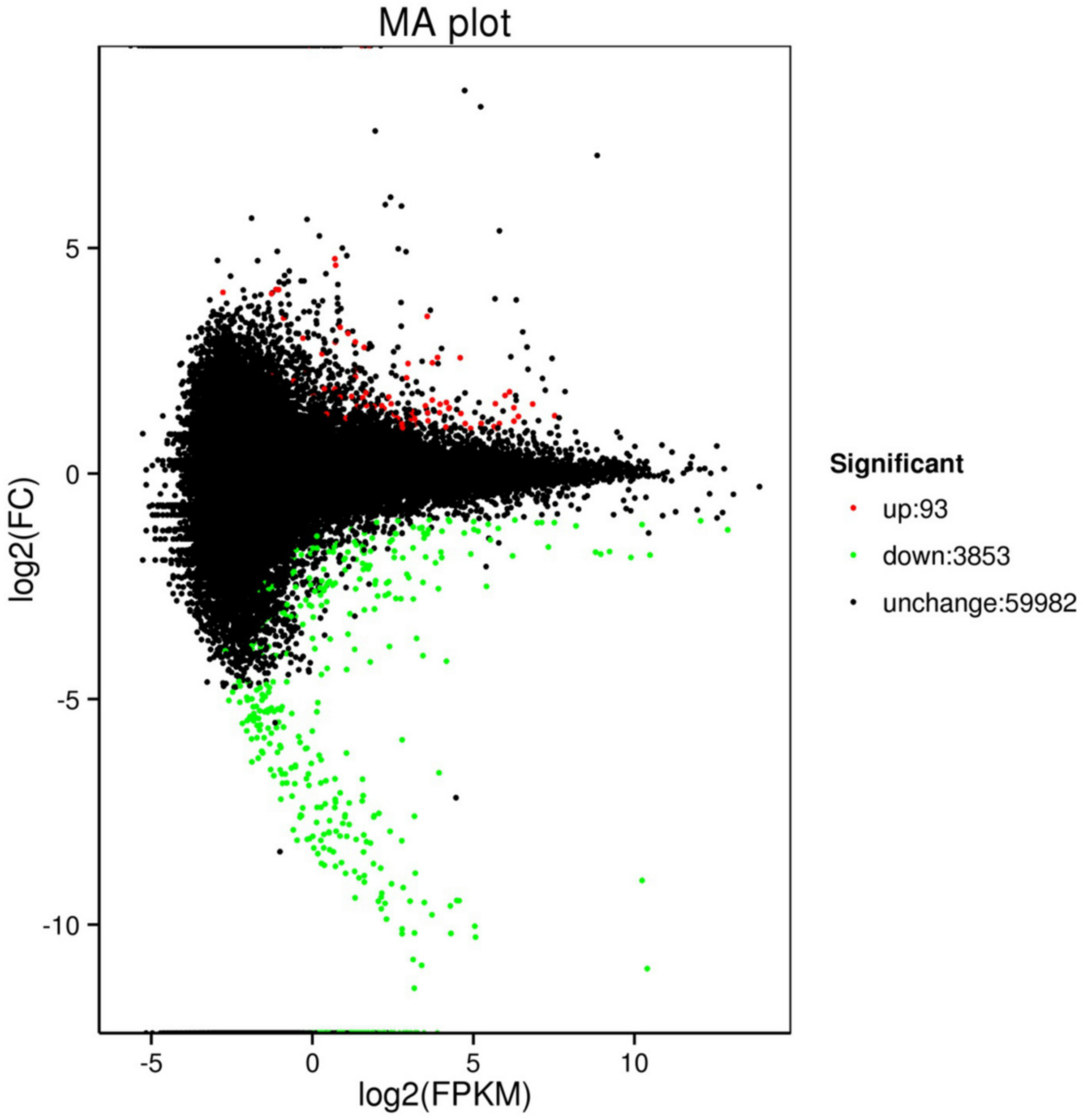
Scatter diagrams representing the comparisons of the genome-wide expression profiles of control and treatment. Note: Each green point represents a decrease in gene expression, each red point represents an increase in gene expression, and each black point represents no significant difference in gene expression.
Functional Annotation and Enrichment Analysis of DEGs
The DEGs enrichment was classified into 56 different groups belonging to three main categories of GO annotation: they are biological process (22901 unigenes), cellular component (6700 unigenes) and molecular function (8896 unigenes) (Figure 2 and Supplementary Dataset Files S2–S4). Within the biological process, the three most common categories were metabolic process, cellular process and single-organism process. The COG database can be used for the direct classification of gene products, and 1,471 sequences had a COG classification. Among the 25 COG categories, the cluster for “General function prediction only” constituted the largest group (336, 22.84%), followed by “Posttranslational modification, protein turnover, chaperones” (233, 15.84%), “Translation, ribosomal structure and biogenesis” (209, 14.21%), “Energy production and conversion” (134, 9.11%) and “Signal transduction mechanisms” (127, 8.63%). In contrast, “Extracellular structures” (0, 0%), “Nuclear structure” (0, 0%), “Cell motility” (5, 0.34%) and “RNA processing and modification” (7, 0.48%) represented the smallest groups (Figure 3).
FIGURE 2
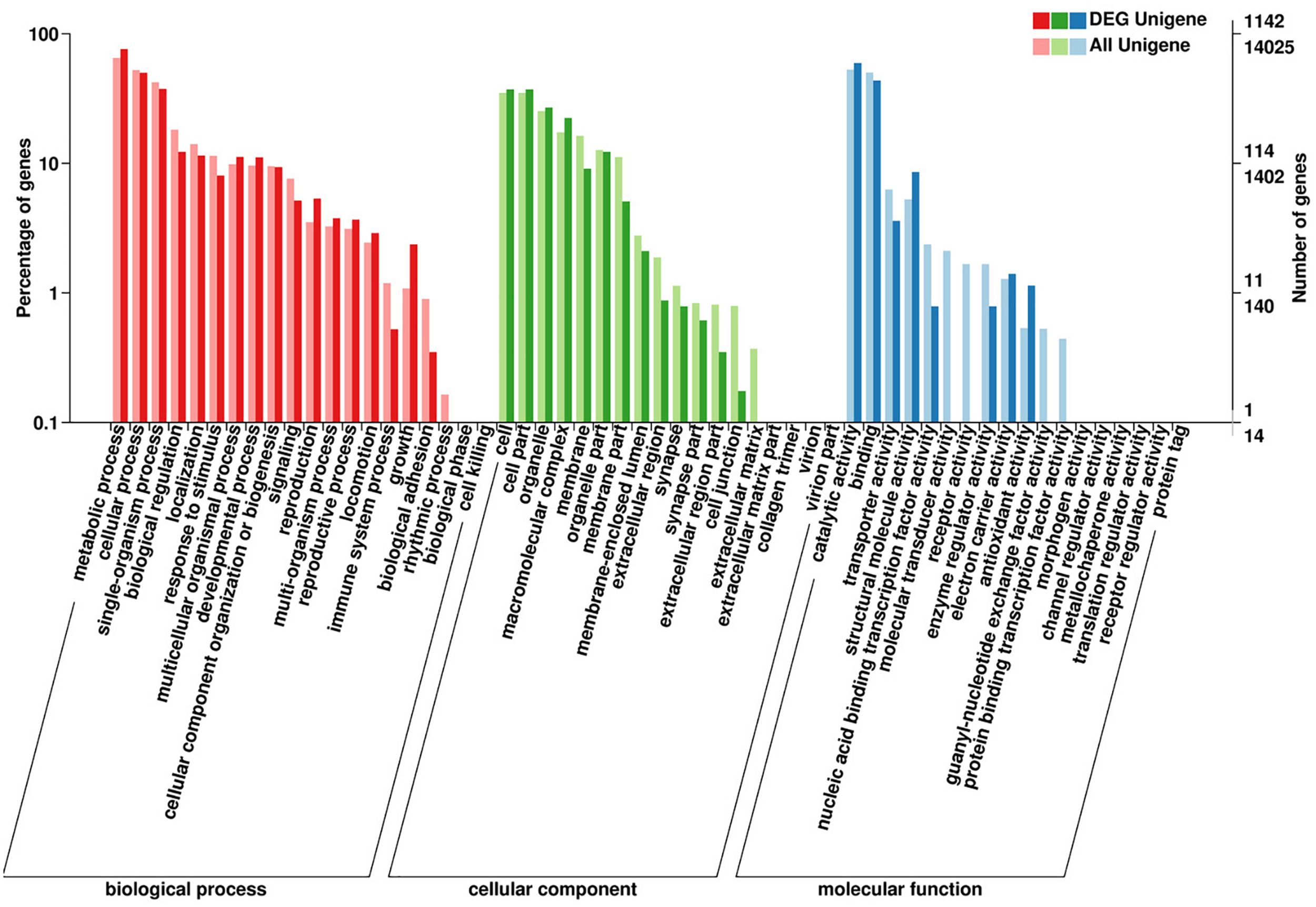
Summary of the annotations of differentially expressed genes in H. axyridis. The GO classifications according to involvement in biological processes, cellular components and molecular functions are shown.
FIGURE 3
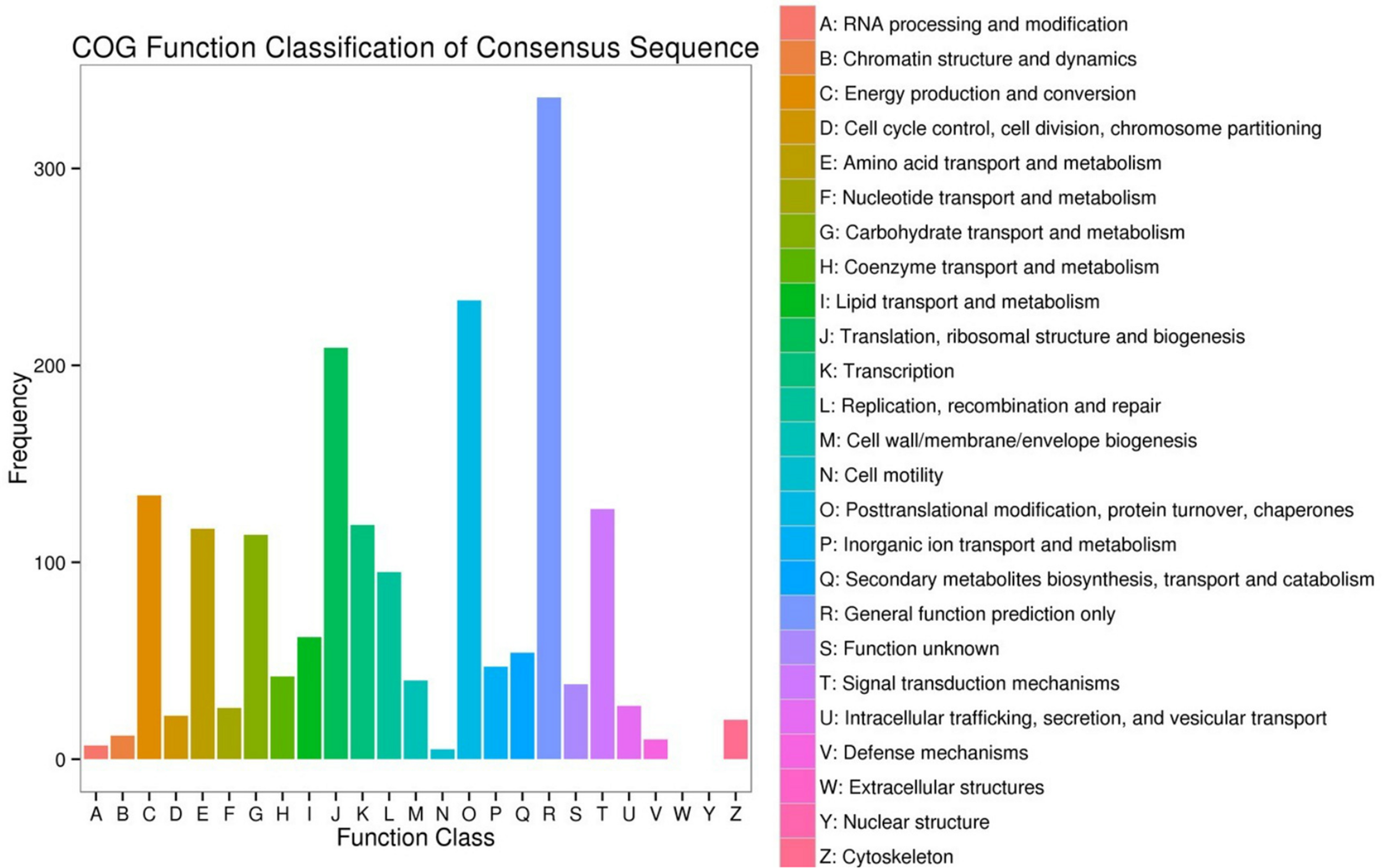
Clusters of Orthologous Groups (COG) functional classification. The 1,471 sequences were classified into 25 COG categories.
Quantitative Real-Time PCR Validation of the DEGs Results
To validate the DEGs determined from the transcriptome results, six reproduction and nutrition-related genes were selected from the results for qRT-PCR analysis. The six pairs of primers were designed to validate the RNA-seq data (Table 5). β-actin was used as an internal control for normalization. The thresholds were significantly differences in six genes expression (FDR < 0.05; FPKM > 2). The qRT-PCR results of six genes were confirmed the expression pattern with a trend of upregulation. Hexamerin 3 precursor and apolipophorins showed the upregulated significantly with the up-trend, and the other genes were slightly upregulated in the raise expression in the qRT-PCR results. The expression levels of six target genes were consistent with the qRT-PCR and RNA-seq data (Figure 4). Thus, qRT-PCR analysis confirmed that the transcriptome analysis identified the correct change direction and yielded reliable results.
TABLE 5
| Gene ID | Homologous function in Nr | Primer (5′-3′) | Product size | Expression level | Accession no. |
| β-actin | CTATGTCGGAGCCATCACT | 112 | |||
| AGCAGTTGTAGCTTCTCCGT | |||||
| c47903.graph_c0 | pleiotrophin isoform X1 | ACGAAGACCATCCAGAAG | 129 | up | MH341578 |
| TTCACAAGAGGCATCACT | |||||
| c57764.graph_c0 | hexamerin 3 precursor | CCAAGTAGACGCCAAGTA | 132 | up | MH341579 |
| CCGCCATATACACCAGAT | |||||
| c48800.graph_c1 | collagen alpha-1(IV) chain | GGCGTTCCTGGTATTATTG | 189 | up | MH341580 |
| GGTGTAGTCTTCCTGTTCT | |||||
| c59088.graph_c0 | apolipophorins | CAATGCGAAGACAGAAGTT | 158 | up | MH341581 |
| CGAAGTGAATATCTACAGTATGG | |||||
| c49776.graph_c0 | apolipoprotein D | CGACCTCAGTTAATATGTTAGTAA | 190 | up | MK886791 |
| GATGTAGATTGTTGCTTCAGA | |||||
| c56676.graph_c1 | phosphoenolpyruvate carboxykinase | TAACTCAAGGTAAGAACAC | 158 | up | MK886792 |
| AAAGTATCATCAAATAACATTAGA |
Sequences of the primers used for the qRT-PCR analysis of six randomly selected genes.
FIGURE 4
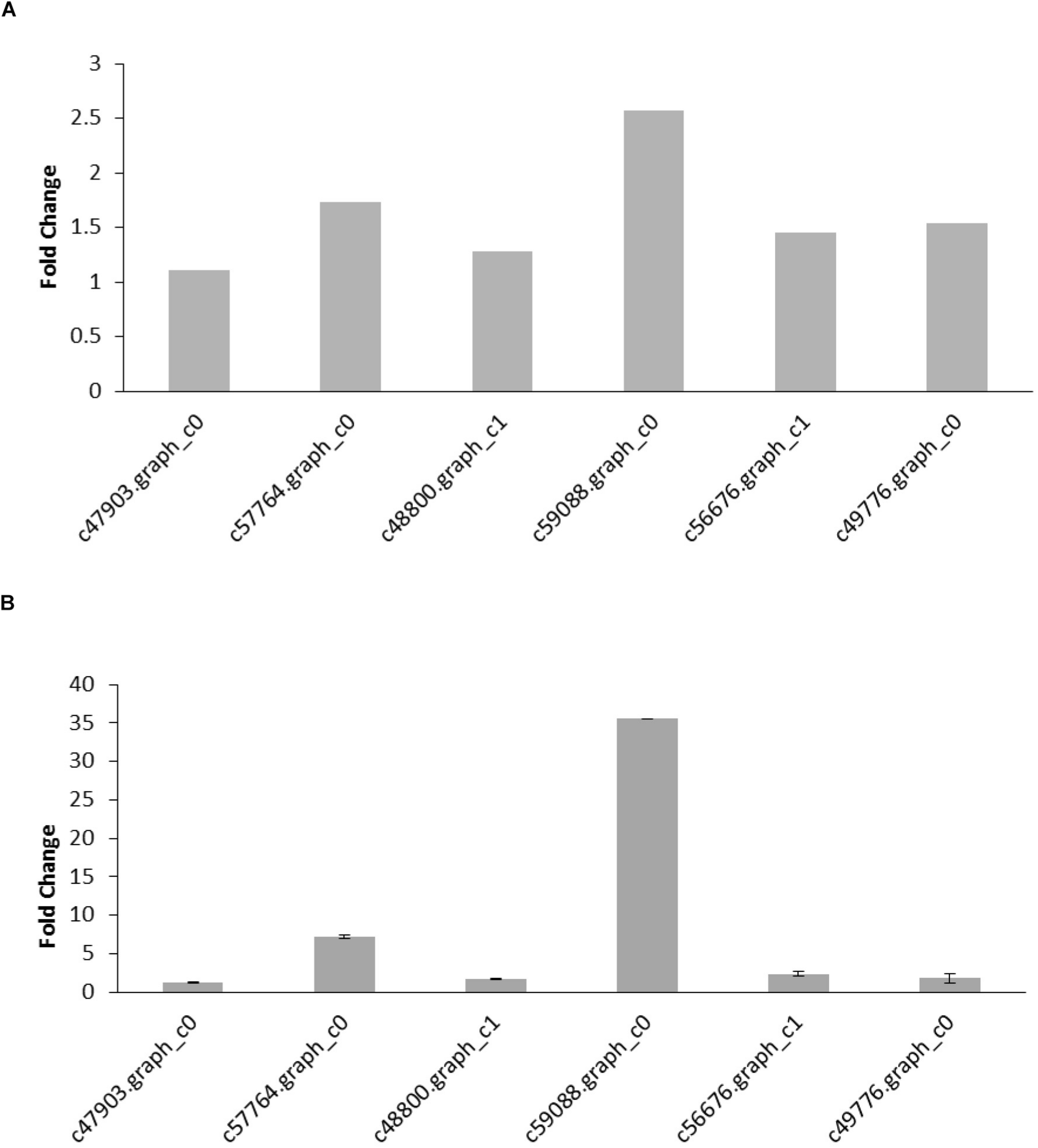
(A) DEGs identified from the transcriptome analysis. The fold change of the gene was calculated as a log2 value shown on y-axis. (B) qRT-PCR results of gene expression. The values are expressed as the means ± SEs of three independent biological replicates.
Effects of Vg Supplement on Physiology and Related Genes of H. axyridis
The effects of the Vg supplement on physiology (enzyme activities and reproduction) of H. axyridis were investigated. The lipase and trehalase activities during the female adult stage and on the fifth day after female oviposition after fed with the Vg (treatment) and BSA (control)-supplemented artificial diets were determined, respectively. The activities of the treatment groups were markedly different from those obtained from the control group for the lipase (F = 641.666; df = 16, 23; p < 0.0001) and trehalase (F = 155.867; df = 16, 23; p < 0.0001) (Figure 5) during the female adult stage. Similarly, the activities of lipase (F = 99.219; df = 2, 8; p < 0.0001) and trehalase (F = 40.227; df = 2, 8, p < 0.0001) in the treatment groups were significant differences on the fifth day after oviposition compared with the control group (Figure 6). The results from this study indicated that Vg supplement had effect on H. axyridis reproduction. The data showed that the total eggs in the groups treated with 60 μg/mL of Vg fragment were significantly higher than those groups treated with 60 μg/mL of BSA (F = 6.991; df = 3, 23; P = 0.002) (Figure 7) during the 1 month period. The total eggs in the groups treated with 30 μg/mL of Vg fragment were also much higher (1.67 times) than those in the groups treated with 30 μg/mL of BSA (Figure 7).
FIGURE 5
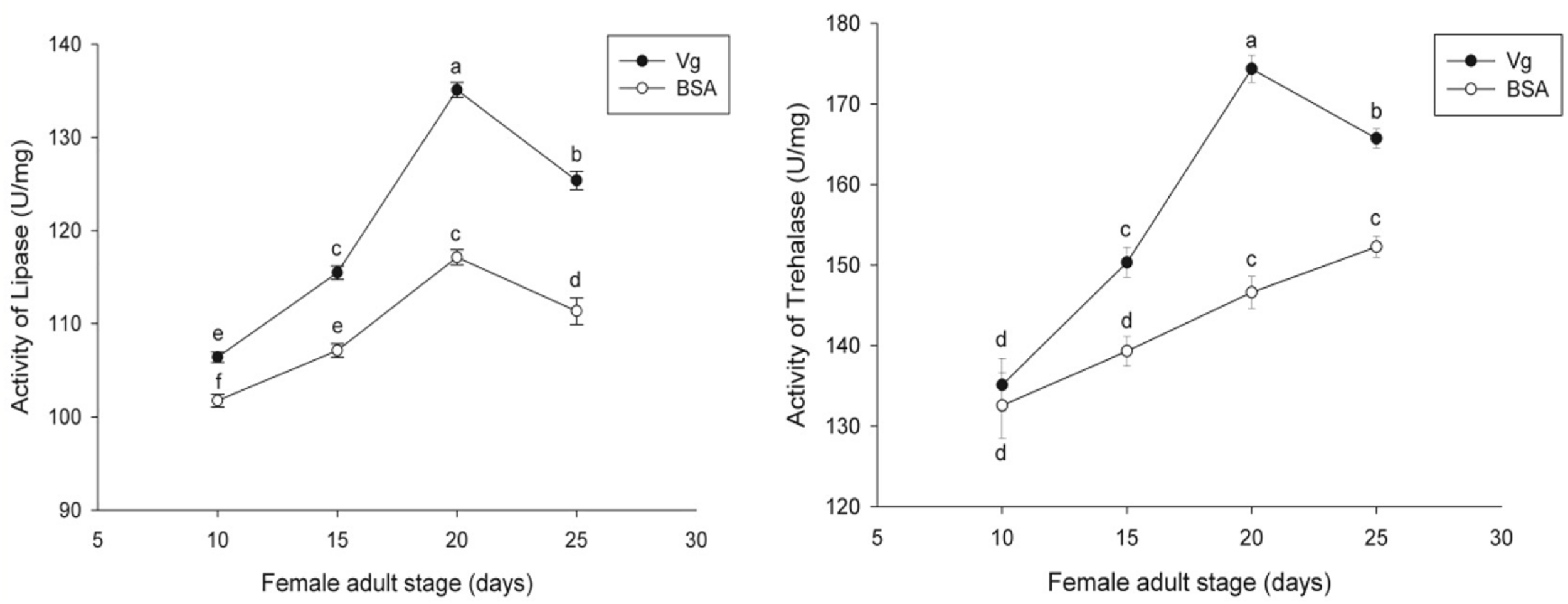
H. axyridis lipase and trehalase activity during the female adult stage after treatment with the Vg- and BSA-supplemented artificial diets. The significance of the differences in the enzyme activities were analyzed by SPSS. The enzyme activities as shown as the means ± SEs and were found to be significantly different (P < 0.0001).
FIGURE 6
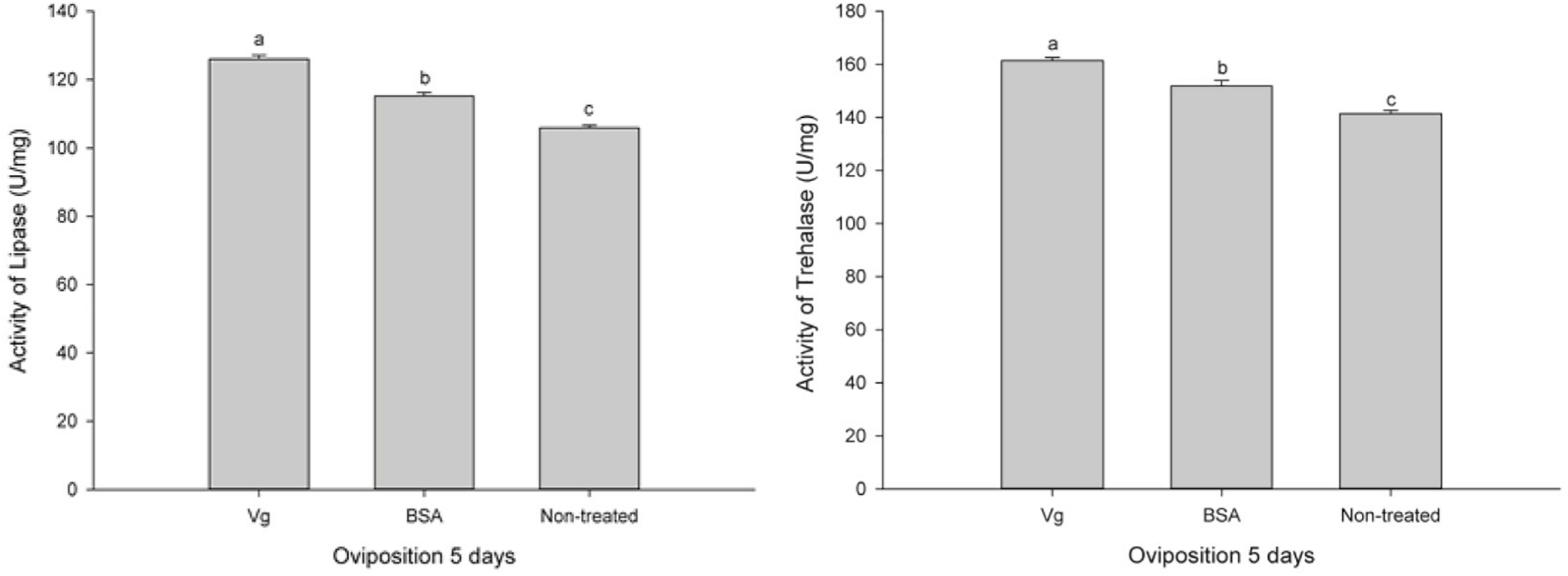
H. axyridis lipase and trehalase activities on the fifth day after female oviposition after treatment with the Vg- and BSA-supplemented artificial diets. The significance of the differences in the enzyme activities were analyzed by SPSS. The enzyme activities as shown as the means ± SEs and were found to be significantly different (P < 0.0001).
FIGURE 7
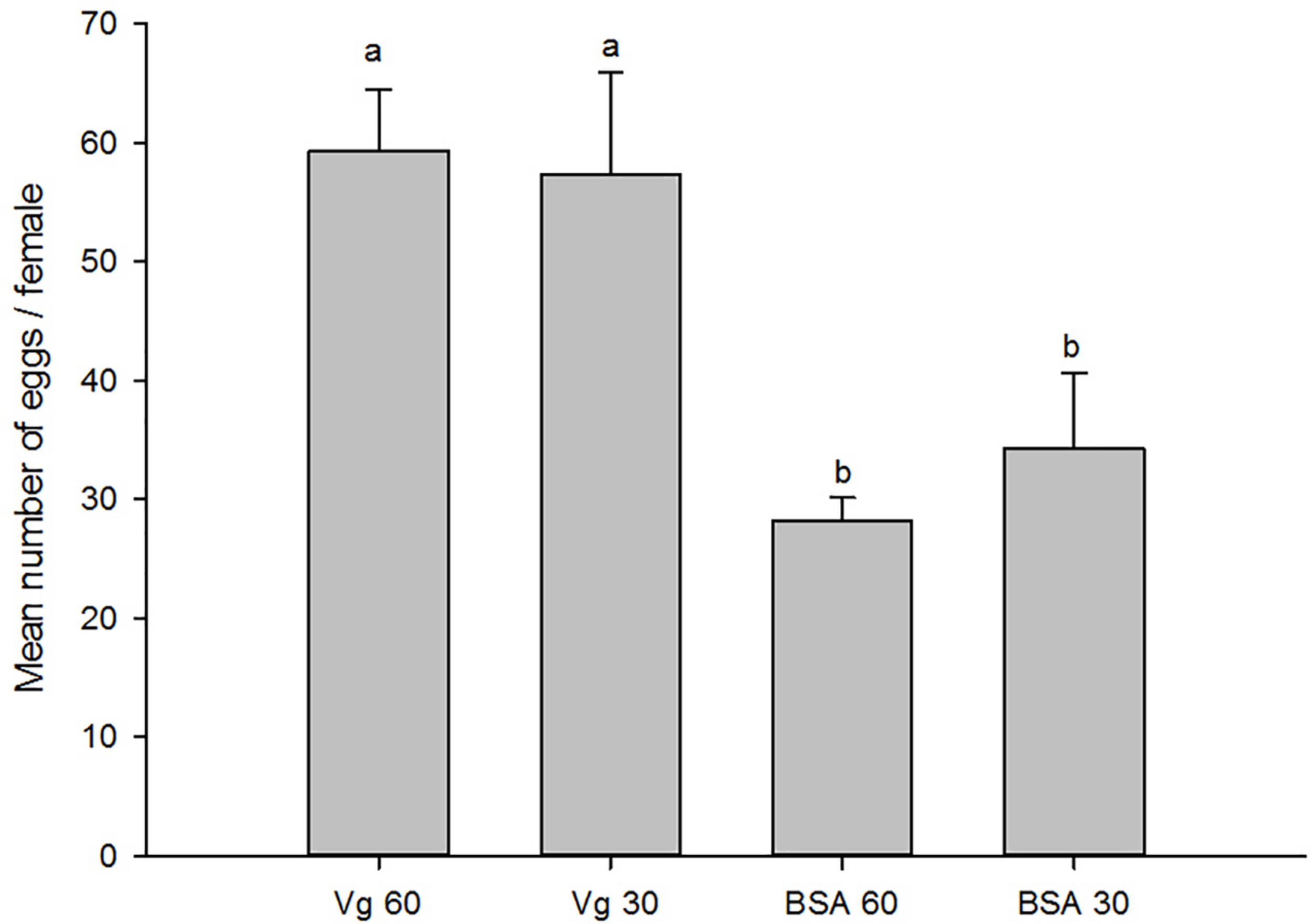
Total H. axyridis egg production in groups of the Vg- and BSA-supplemented artificial diets during 1 month period. The data were analyzed by SPSS. The egg production was shown as the means ± SEs.
The effects of the Vg supplement on physiology related gene transcription levels of H. axyridis were also determined. The 3 growth and development-related and 2 enzyme-related genes were identified using the unigenes annotation databases.
The gene id, homologous function in Nr, full length, product size, E-value and accession number are summarized (Table 6). According to the nr annotations, these genes belong to the following groups: two genes belong to heat shock protein gene group, one gene is in serine protein group, another gene is related to hydrolase group, one gene belongs to transport and catabolism gene group.
TABLE 6
| Gene ID | Homologous function in Nr | Full length | Product size | E-value | Accession no. |
| c52950. graph_c0 | Serine protease 33 | Yes | 1812 | 2.28E-10 | MN241440 |
| c49623. graph_c0 | heat shock protein 70 B2-like | Yes | 1970 | 3.45E-14 | MN241441 |
| c52099. graph_c0 | heat shock protein 90 | Yes | 926 | 4.32E-17 | MN241442 |
| c47043. graph_c1 | Abhydrolase domain-containing protein 4-like isoform X1 | Yes | 2197 | 9.35E-05 | MN241443 |
| c53557. graph_c1 | ATP-binding cassette sub-family A member 3-like (Tribolium castaneum) | Yes | 805 | 0.009619 | MN241444 |
Development and enzyme-related differentially expressed genes were shown in nr.
Discussion
The nutritional composition of food is a key factor that influences the development and reproduction of insect species (Bonte et al., 2010). An assessment of predator insect survival and reproductive performance indicates whether an artificial diet has potential for use in the mass rearing of the insect (Xie et al., 2017). Previous studies showed that adult reproductive performance was unfavorably affected by restricted food supply during the larval stages (Xie et al., 2015a, b). Also, Niijima et al. (1977) developed an artificial diet for H. axyridis, they found that an artificial diet does not assist in egg production; however, it does sustain adult H. axyridis. In the present study, we used different supplement samples mixed with a successful artificial diet (Zhang S. et al., 2017) to compare the differences between the Vg and BSA proteins effects through a transcriptome analysis, the gene expression profiles between insects fed the Vg and BSA were compared by Illumina sequencing to obtain insights into the enzyme activities, nutritional absorption, energy metabolism and reproduction of H. axyridis. The Illumina sequencing of five samples yielded 43.94 Gb of clean data, and the data were assembled into 75,717 unigenes. Functional annotation results were obtained for 36,668 of the assembled unigenes. The gene structures were analyzed using a unigene library, and 4,637 SSR markers were obtained from an SSR analysis. Based on the RNA-seq results, approximately 62 DEGs in the H. axyridis abdomen were related to reproductive development. A previous study suggested that the Vg fragment can increase the reproduction of H. axyridis (Zhang T. et al., 2017). These 62 DEGs could provide new insights for our understanding of the nutrigenomics of H. axyridis affected by components of artificial diet such as Vg supplement.
Furthermore, the results from this study revealed that different insect artificial diet components showed the influence on gene regulation. We selected six DEGs related to nutrition and development, which found different gene expression levels due to different component in artificial diet. The mRNA levels of these genes were notably higher in insects fed the artificial diet with Vg-supplement than in those fed the diet with BSA. The six genes are pleiotrophin isoform X1, collagen alpha-1(IV) chain, hexamerin 3 precursor, apolipophorins, Phosphoenolpyruvate carboxykinase and Apolipoprotein D. The c47903.graph_c0 was annotated as pleiotrophin isoform X1 and might have participated in the development; previous studies showed that pleiotrophin is involved in the migratory processes of neurons during brain development (Nobuaki and Masaharu, 1998). The c48800.graph_c1 was annotated as collagen alpha-1(IV) chain and is similar to collagen alpha-1 (X), which is a major avian eggshell membrane (ESM) structural constituent (Cordeiro and Hincke, 2016). The c57764.graph_c0 was annotated as a hexamerin 3 precursor; the hexamerins of insects belong to a growing protein superfamily that also includes the arthropod hemocyanins and prophenoloxidases and the hexamerin receptors discovered in Diptera (Beintema et al., 1994; Burmester and Scheller, 1996; Telfer and Kunkel, 2003). The hexamerins of hexapods are derived from hemocyanins with whom they share a similar protein structure, whereas insect hexamerins serve as storage proteins (Terwilliger et al., 1999; Burmester, 2002). Levenbook and Bauer (1984) investigated hexamerin function during metamorphosis, and Burmester (1999) recognized the inter-order hexamerin relationships and the assignment of distinct hexamerin classes conserved within single insect orders. The c59088.graph_c0 was annotated as apolipophorins and participates in lipid transport vehicles; the fat body serves as the site of apolipophorin I (apoLp-I), apolipophorin II (apoLp-II) and lipid synthesis and well as the site of lipid storage and lipoprotein assembly. Depending on the insect species, lipophorins are released as high-density lipoproteins into the haemolymph (Prasad et al., 1986; Venkatesh et al., 1987; Capurro and Bianchi, 1990; Weers et al., 1992; Heusden et al., 1998). Phosphoenolpyruvate carboxykinase (PEPCK,c56676.graph_c1) has been indicated to catalyze a rate-limiting step in lactate gluconeogenesis in hepatocytes from fasted rats (Rognstad, 1979). Our results revealed that the expression of PEPCK, an enzyme belonging to the lyase family that participates in the gluconeogenesis metabolic pathway, was upregulated. Specifically, PEPCK converts oxaloacetate into phosphoenolpyruvate and carbon dioxide (Chakravarty et al., 2005; Méndez-Lucas et al., 2013). Gluconeogenesis is used by humans and many other animals to maintain blood glucose levels and avoid low levels of glucose (hypoglycemia), and it is used for glycogen degradation (glycogenolysis). PEPCK may indirectly enhance glucose levels and provide nutrients to adult insects. Apolipoprotein D (ApoD, c49776.graph_c0) is belongs to the lipocalin family (Sánchez et al., 2002),which is a secreted glycoprotein with many putative functions including lipid transport. The Vg protein may stimulate nutritional-related gene expression, followed by more protease activities and the utilization of lipids and proteins in H. axyridis. This study showed that an artificial diet supplemented with Vg protein could be more effective for rearing H. axyridis.
Similar to all oviparous animals, insects supply their eggs with proteins, carbohydrates, and other resources to provide sustenance for the developing embryo. Trehalase serves as an energy reserve in insects, and changes in nutrition, physiological and environmental conditions lead to different concentrations of trehalase in the insect haemolymph. If insect development and life activities require energy, the stored trehalase can be converted into glucose (Elbein et al., 2003). Zeng and Cohen (2001) found that certain foods can induce enzyme activity, and other researchers have reported that food quality affects insect biochemistry (Shapiro and Legaspi, 2006). Vg is a major protein in insect eggs (Tufail and Takeda, 2008), and the promotion of Vg synthesis indicates an increase in protein requirements. The data from this study showed that upregulate gene was related to enzyme activities, and artificial diets with different components might stimulate related gene expression, which increases enzyme activities. In insects, food is digested and absorbed by enzymes such as trypsin (Zeng and Cohen, 2000; Zeng et al., 2002). The results from this study support our hypothesis and the presence of Vg supplement in artificial diets alter the enzyme activity of H. axyridis, and then increase the digestion of nutrients in the artificial diet, and subsequently increase the reproduction of H. axyridis.
Materials and Methods
Insect Rearing and Treatment
Throughout all of its developmental stages, the first colony of H. axyridis, was collected from the suburbs of Beijing and reared in a growth chamber (RXZ, Ningbo, China) with a climate-controlled incubator maintained at 25 ± 1°C with a 16-h light: 8-h dark photoperiod and 70 ± 5% relative humidity. The artificial diet to feed H. axyridis included major components of pork liver, eggs, sugar, yeast and so on (the artificial diet contains protein, sugar and lipid, which constitute approximately 10.9, 1.95, and 1.72% of the feed, respectively (Zhang T. et al., 2017). The full-length H. axyridis Vg cDNA was cloned by RT-PCR. A Willerbrand factor type D (VWD) domain were expressed and purified by the clone Vg gene was characterized. Vg fragment contains specific Vg conserved domain (VWD) and can express specific protein related amino-acides. The insects belonging to the treatment groups were fed with Vg supplement (developed and expressed by this research), and the control groups were fed with BSA supplement (purchased from JiangChen, Beijing, China). Abdomen samples were collected 5 days after oviposition. The six samples of control with three replications (C1, C2, and C3) and the treatment with three replications (T01, T02, and T03) were collected and send out for analysis by BioMarker (China, Beijing). The Illumina Solexa deep-sequencing technology was used for the transcriptome sequencing of the H. axyridis abdomen samples.
cDNA Library Construction and Illumina Sequencing for Transcriptome Analysis
Five days after oviposition, the total RNA from H. axyridis adult females was extracted using the Tranzol reagent according to the manufacturer’s instructions. The control and treatment group samples were collected from the first abdominal ganglia to the tail end. Genomic DNA was digested with DNase I (Transgene, Beijing, China), and the RNA integrity was determined by agarose gel electrophoresis, which revealed clear bands for 18S and 28S. For ligation to sequence adapters, the cDNA was purified and repaired, and A bases were added to the 3′-ends.
Unigene Annotation
Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1997) software was used to compare the unigenes to the Integrated Nr database (Deng et al., 2006), Swiss-Prot (Apweiler et al., 2004), Gene Ontology (GO) (Ashburner et al., 2000), Clusters of Orthologous Groups (COG) (Tatusov et al., 2000), eukaryotic Orthologous Groups (KOG) (Koonin et al., 2004), eggNOG4.5 (Huerta-Cepas et al., 2016), Kyoto Encyclopedia of Genes, and Genomes (KEGG) (Kanehisa et al., 2004) were used to annotate the unigenes using KOBAS2.0 (Xie et al., 2011). The amino acid sequences of the unigenes were predicted, and annotation information for the unigenes was then obtained through comparison with the Protein family (Pfam) (Finn et al., 2014) database using HMMER software (Eddy, 1998). Gene function annotation was performed using the following databases: Nr1, COG2, Pfam3, KOG4, Swiss-Prot5, KEGG (see text footnote 5), and GO6.
DGE Analysis
The DESeq (Anders and Huber, 2010) was used to analyse the differential expression between the control and treatment groups in this study to obtain the expression gene sets from above groups. During the differential expression analysis, the Benjamini-Hochberg method was adopted to correct the p-value obtained by the original hypothesis test, namely, the FDR (False Discovery Rate), which was used as the key indicator for screening the differentially expressed genes. In the screening process, fragments per kilobase of exon model per million mapped reads (FPKM) fold change > 2 and a false discovery rate (FDR) < 0.05 were used as the thresholds to identify significant differences in gene expression.
Real-Time Quantitative RT-PCR
The primers for the target and reference genes were designed using the online primer3 program (version 4.0.0)7 and are listed in Table 5. The total RNA from female adults was isolated and treated with RNase-free DNase I at 37°C for 30 min using the DNase I kit (Takara, Dalian, China). A reaction volume of 20 μl, which contained 200 nM of each forward and reverse primer, cDNA produced from 2 μg of total RNA, 8 μl of nuclease-free water, and 10 μl of 2X iTaq universal SYBR Green Supermix (BIO-RAD, CA, United States) was used. The real-time PCR analysis of 200 ng of total RNA was performed using a 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, United States). The qPCR reaction conditions were as follows: 95°C for 2 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The levels of the target genes were compared with those of the housekeeping gene β-actin using the 2–ΔΔCt qPCR method. The means and standard errors for each time point were obtained from the average of four independent samples. The individual animals were randomized into two treatment groups, and three biological replications of each treatment were performed.
Effects of Vg Supplement on Physiology and Related Genes of H. axyridis
The effects of the Vg supplement on physiology (enzyme activities and reproduction) of H. axyridis were investigated. The lipase and trehalase activities and the related gene transcription levels of H. axyridis were also determined. The trehalase activity was measured using a trehalase ELISA kit (SU-B97117, Collodi, Quanzhou, China). Five days after spawning, the Vg protein- and BSA protein-fed female adults were weighed and individually grounded in liquid nitrogen. Every 100 mg of insect tissue was suspended in 1 ml of sample diluent. All standards and samples were added in duplicate to a microtiter plate. The wash buffer (20X) was diluted with 19 volumes of deionized or distilled water, and 50 μl of the sample was added to the appropriate wells. The blank wells did not contain any standard or sample. One hundred microliters of enzyme conjugate was added to the standard and sample wells (not to the blank wells). The plate was then covered with adhesive strips, incubated at 37°C for 60 min and washed four times, and 50 μl each of substrate A and B were added to each well. The plate was then gently mixed and incubated at 37°C for 15 min. If the color in the wells was green or not uniform, the plate was gently tapped to ensure thorough mixing. Fifty microliters of stop solution was then added each well, and the color changed from blue to yellow. Within 15 min, the optical density (OD) was read at 450 nm using a microtiter plate reader (Flexstation 3, CA, United States). The levels of lipase and trehalase activity were detected using the Insect Lipase (Trehalase) ELISA kit (SU-B97122, Collodi, Quanzhou, China), according to the procedures described for the measurement of activity. The effects of the Vg on H. axyridis egg production determined as follows: the Vg supplement in different concentrations (30 and 60 μg/mL) were added into the artificial diet to feed H. axyridis adults in the treatment groups. The control groups were supplemented with equivalent concentrations of BSA proteins (Guangda, Beijing, China). The total eggs in the treatment and control groups within 1-month period by each pair of adults were recorded. The experimental design was total randomized with six replications.
Statistical Analysis
The differential gene expression between the control and treatment groups in this study was analyzed by DESeq (Anders and Huber, 2010). The qRT-PCR results, enzyme activity measurements and egg production data were analyzed by SPSS. The data are shown as the means ± SEs of three independent biological replicates.
Statements
Author contributions
JM and FZ designed the study. TZ and YH performed the experiments. JZ and LZ analyzed the data. TZ, GZ, and FZ wrote the main text of the manuscript and prepared all the figures. FZ and TZ revised the manuscript. All authors reviewed the manuscript.
Funding
This work was sponsored by the Fundamental Research Funds for the Central Universities (2572018AA09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a shared affiliation, though no other collaboration, with several of the authors TZ, JM, FZ, and LZ at the time of review.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.01148/full#supplementary-material
Footnotes
1.^ https://ftp.ncbi.nih.gov/blast/db/
2.^ http://www.ncbi.nlm.nih.gov/COG/
4.^ https://ftp.ncbi.nih.gov/pub/COG/KOG
5.^ http://www.genome.jp/kegg/
References
1
Adams T. S. (2000). Effect of diet and mating status on ovarian development in a predaceous stink bug Perillus bioculatus (Hemiptera: Pentatomidae).Ann. Entomol. Soc. Am.93529–535.10.1603/0013-8746(2000)093[0529:eodams]2.0.CO;2
2
Adriaens T. Branquart E. Maes D. (2003). E multicoloured Asian ladybird Harmonia axyridis Pallas (Coleoptera: Coccinellidae), a threat for nativeaphid predators in Belgium?Belg. J. Zool.133195–196.
3
Alaux C. Dantec C. Parrinello H. Le Conte Y. (2011). Nutrigenomics in honey bees: digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees.BMC. Genomics.12:498. 10.1186/1471-2164-12-496
4
Altschul S. F. Madden T. L. Schäffer A. A. Zhang J. Zhang Z. Miller W. et al (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.Nucleic. Acids. Res.253389–3402. 10.1093/nar/25.17.3389
5
Anders S. Huber W. (2010). Differential expression analysis for sequence count data.Genome. Biol.11:R106. 10.1186/gb-2010-11-10-r106
6
Apweiler R. Bairoch A. Wu C. H. Barker W. C. Boeckmann B. Ferro S. et al (2004). UniProt: the universal protein knowledgebase.Nucleic. Acids. Res.32D115–D119.
7
Ashburner M. Ball C. A. Blake J. A. Botstein D. Butler H. Cherry J. M. et al (2000). Gene ontology: tool for the unification of biology.Nat. Genet.2525–29.
8
Beintema J. J. Stam W. T. Hazes B. Smidt M. P. (1994). Evolution of arthropod hemocyanins and insect storage proteins (hexamerins).Mol. Biol. Evol.11493–503.
9
Bonte M. Samih M. A. Clercq P. D. (2010). Development and reproduction of Adalia bipunctata, on factitious and artificial foods.Bio. Control.55485–491. 10.1007/s10526-010-9266-1
10
Burmester T. (1999). Evolution and function of the insect hexamerins.Eur. J. Entomol.96213–225.
11
Burmester T. (2002). Origin and evolution of arthropod hemocyanins and related proteins.J. Comp. Physiol. B.17295–107. 10.1007/s00360-001-0247-7
12
Burmester T. Scheller K. (1996). Common origin of arthropod tyrosinase, arthropod hemocyanin, insect hexamerin, and dipteran arylphorin receptor.J. Mol. Evol.42713–728. 10.1007/bf02338804
13
Capurro M. D. L. Bianchi A. G. D. (1990). Larval Musca domestica, lipophorin biosynthesis.Comp. Biochem. Phys. B97655–659. 10.1016/0305-0491(90)90103-z
14
Castro-Guedes C. F. D. Almeida L. M. D. Moura M. O. (2016). Effect of different diets on biology, reproductive variables and life and fertility tables of Harmonia axyridis (Pallas) (Coleoptera. Coccinellidae).Rev. Bras. Entomol.60260–266. 10.1016/j.rbe.2016.03.003
15
Chakravarty K. Cassuto H. Reshef L. Hanson R. W. (2005). Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-c.Crit. Rev. Biochem. Mol.40129–154. 10.1080/10409230590935479
16
Chapman R. F. (1998). The Insect: Structure and Function.Cambridge: Cambridge University Press.
17
Chen C. N. Hsiao W. F. (1984). Influence of food and temperature on life history traits and population parameters of Spodoptera litura.Plant Protect. Bull.26219–229.
18
Cheng Y. Zhi J. R. Li F. L. Li W. H. Zhou Y. H. (2017). Improving the artificial diet for adult of seven spotted ladybird beetle Coccinella septempunctata L. (Coleoptera: Coccinellidae) with orthogonal design.B Entomol. Res.108337. 10.1017/S0007485317000797
19
Cordeiro C. M. Hincke M. T. (2016). Quantitative proteomics analysis of eggshell membrane proteins during chick embryonic development.Proteomics.13011–25. 10.1016/j.jprot.2015.08.014
20
Coudron T. A. Wittmeyer J. Kim Y. (2002). Life history and cost analysis for continuous rearing of Podisus maculiventris (Say) (Heteroptera: Pentatomidae) on a zoophytophagous artificial diet.J. Econ. Entomol.951159–1168. 10.1603/0022-0493-95.6.1159
21
De Bach P. Rosen D. (1991). Biological Control by Natural Enemies.Cambridge: Cambridge University Press.
22
Deng Y. Y. Li J. Q. Wu S. F. Zhu Y. P. Chen Y. W. He F. C. (2006). Integrated nr database in protein annotation system and its localization.Comput. Eng.3271–72.
23
Dixon A. F. G. (2000). Insect Predator–Prey Dynamics: Ladybird Beetles and Biological Control.Cambridge: Cambridge University Press.
24
Dottorini T. Nicolaides L. Ranson H. Rogers D. W. Crisanti A. Catteruccia F. (2007). A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior.PNAS10416215–16220. 10.1073/pnas.0703904104
25
Du W. Zeng F. (2016). Identification of development related genes in the ovaries of adult Harmonia axyridis (Pallas) lady beetles using a time-series analysis by RNA-seq.Sci. Rep.6:39109. 10.1038/srep39109
26
Eddy S. R. (1998). Profile hidden markov models.Bioinformatics14755–763.
27
Elbein A. D. Pan Y. T. Pastuszak I. Carroll D. (2003). New insights on trehalose: a multifunctional molecule.Glycobiology1317–27.
28
Ferran A. Boelpaepe M. O. C. D. Schanderl H. Larroque M. M. (1984). Les aptitudes trophiques et reproductrices des femelles de Semiadalia undecimnotata [Col.: Coccinellidae].Bio. Control.29157–170. 10.1007/bf02372105
29
Ferran A. Niknam H. Kabiri F. Picart J. L. Deherce C. Brun J. et al (1996). The use of Harmonia axyridis larvae (Coleoptera: Coccinellidae) against Macrosiphum rosae (Hemiptera: Sternorrhyncha: Aphididae) on rose bushes.Eur. J. Entomol.9359–67.
30
Finn R. D. Bateman A. Clements J. Coggill P. Eberhardt R. Y. Eddy S. R. et al (2014). Pfam: the protein families database.Nucleic. Acids. Res.42D222–D230. 10.1093/nar/gkt1223
31
Friedman S. (1978). Trehalose regulation, one aspect of metabolic homeostasis.Annu. Rev. Entomol.23389–407. 10.1146/annurev.en.23.010178.002133
32
Gordon R. D. (1985). The Coccinellidae (Coleoptera) of America North of Mexico.J. New. York. Entomol. S931–912.
33
Greany P. D. Carpenter J. E. (1998). Culture Medium for Parasitic and Predaceous Insects. US. Partent No:5799607.
34
Heusden M. C. V. Thompson F. Dennis J. (1998). Biosynthesis of Aedes aegypti lipophorin and gene expression of its apolipoproteins.Insect. Biochem. Molec.28733–738. 10.1016/s0965-1748(98)00068-x
35
Hodek I. Honěk A. (2009). Scale insects, mealybugs, whiteflies and psyllids (Hemiptera. Sternorrhyncha) as prey of ladybirds.Biol. Control.51232–243. 10.1016/j.biocontrol.2009.05.018
36
Hodek I. Van Emden H. F. Honěk A. (2012). Ecology and Behavious of the Ladybird Beetles (Coccinellidae).Hoboken: Wiley Online Library.
37
Huerta-Cepas J. Szklarczyk D. Forslund K. Cook H. Heller D. Walter M. C. et al (2016). egg NOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences.Nucleic. Acids. Res.44D286–D293.
38
Kanehisa M. Goto S. Kawashima S. Okuno Y. Hattori M. (2004). The KEGG resource for deciphering the genome.Nucleic. Acids. Res.32D277–D280.
39
Koch H. M. Drexler H. Angerer J. (2003). An estimation of the daily intake of di (2-ethylhexyl) phthalate (DEHP) and other phthalates in the general population.Int. J. Hyg. Envir. Heal.20677–83. 10.1078/1438-4639-00205
40
Koonin E. V. Fedorova N. D. Jackson J. D. Jacobs A. R. Krylov D. M. Makarova K. S. et al (2004). A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes.Genome. Biol.5:R7.
41
Lenteren J. C. V. Roskam M. M. Timmer R. (1997). Commercial massproduction and pricing of organisms for biological control of pests in Europe.Biol. Control.10143–149. 10.1006/bcon.1997.0548
42
Levenbook L. Bauer A. (1984). The fate of the larval storage protein calliphorin during adult development of Calliphora vicina.Insect. Biochem.1477–86. 10.1016/0020-1790(84)90086-6
43
Liu C. Y. Mao J. J. Zeng F. R. (2015). Chrysopa septempunctata (Neuroptera: Chrysopidae) vitellogenin functions through effects on egg production and hatching.J. Econ. Entomol.1082779. 10.1093/jee/tov229
44
Mcclure M. S. (1987). Potential of the Asian predator, Harmonia axyridis pallas (Coleoptera: Coccinellidae), to control Matsucoccus resinosae bean and godwin (Homoptera: Margarodidae) in the United States.Environ. Entomol.16224–230. 10.1093/ee/16.1.224
45
Méndez-Lucas A. Duarte J. Sunny N. E. Satapati S. He T. T. Fu X. R. et al (2013). PEPCK-M expression in mouse liver potentiates, not replaces. PEPCK-C mediated gluconeogenesis.J. Hepatol.59105–113. 10.1016/j.jhep.2013.02.020
46
Niijima K. Nishimura R. Matsuka M. (1977). “Rearing of larvae using a chemically defined diet and fractions of drone honeybee powder,” in Nutritional Studies of an Aphidophagous Coccinellid, Harmonia Axyridis, ed.NiijimaK. (Machida: Bulletin of the Faculty of Agriculture Tamagawa University), 45–51.
47
Nobuaki M. Masaharu N. (1998). Involvement of receptor-like protein tyrosine phosphatase ζ/RPTPβ and its ligand pleiotrophin/heparin-binding growth- associated molecule (HB-GAM) in neuronal migration.J. Cell. Biol.142203–216. 10.1083/jcb.142.1.203
48
Prasad S. V. Fernando-Warnakulasuriya G. J. Sumida M. Law J. H. Wells M. A. (1986). Lipoprotein biosynthesis in the larvae of the tobacco hornworm, Manduca sexta.Biol. Chem.3617174–17176.
49
Rognstad R. (1979). Rate-limiting steps in metabolic pathways.J Biol Chem.2541875–1882.
50
Sánchez D. Ganfornina M. D. Martínez S. (2002). Expression pattern of the lipocalin Apolipoprotein D during mouse embryogenesis.Mech. Dev.110225–229. 10.1016/s0925-4773(01)00578-0
51
Shapiro J. P. Legaspi J. C. (2006). Assessing biochemical fitness of predator Podisus maculiventris (Heteroptera: Pentatomidae) in relation to food quality: Effects of five species of prey.Ann. Entomol. Soc. Am.99321–326.10.1603/0013-8746(2006)099[0321:abfopp]2.0.CO;2
52
Tatusov R. L. Galperin M. Y. Natale D. A. Koonin E. V. (2000). The COG database: a tool for genome-scale analysis of protein functions and evolution.Nucleic. Acids. Res.2833–36. 10.1093/nar/28.1.33
53
Telfer W. H. Kunkel J. G. (2003). The function and evolution of insect storage hexamers.Annu. Rev. Entomol.36205–228. 10.1146/annurev.ento.36.1.205
54
Terwilliger N. B. Dangott L. Ryan M. (1999). Cryptocyanin, a crustacean molting protein: Evolutionary link with arthropod hemocyanins and insect hexamerins.Proc. Natl. Acad. Sci. U.S.A.962013–2018. 10.1073/pnas.96.5.2013
55
Tufail M. Takeda M. (2008). Molecular characteristics of insect vitellogenins.J. Insect. Physiol.541447–1458. 10.1016/j.jinsphys.2008.08.007
56
Venkatesh K. Lenz C. J. Bergman D. K. Chippendale G. M. (1987). Synthesis and release of lipophorin in larvae of the southwestern corn borer, Diatraea grandiosella: An in vitro study.Insect. Biochem.171173–1111.
57
Weers P. M. Horst D. J. V. D. Marrewijk W. J. V. Eijnden M. V. D. Doorn J. M. V. Beenakkers A. M. (1992). Biosynthesis and secretion of insect lipoprotein.J. Lipid. Res.33485–491.
58
Xie C. Mao X. Z. Huang J. J. Ding Y. Wu J. M. Dong S. et al (2011). KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases.Nucleic. Acids. Res.39316–322. 10.1093/nar/gkr483
59
Xie J. Clercq P. D. Pan C. Li H. S. Zhang Y. H. Pang H. (2015a). Larval nutrition-induced plasticity affects reproduction and gene expression of the ladybeetle, Cryptolaemus montrouzieri.BMC. Evol. Biol.15:276. 10.1186/s12862-015-0549-0
60
Xie J. Clercq P. D. Zhang Y. Wu H. Pan C. Pang H. (2015b). Nutrition-dependent phenotypes affect sexual selection in a ladybird.Sci. Rep.5:13111. 10.1038/srep13111
61
Xie J. Wu H. Pang H. De C. P. (2017). An artificial diet containing plant pollen for the mealybug predator Cryptolaemus montrouzieri.Pest. Manag. Sci.73514–545. 10.1002/ps.4309
62
Yasuda H. Takagi T. Kogi K. (2000). Effect of conspecific and heterospecific larval tracks on the oviposition behavior of the predatory ladybird. Harmonia axyridis (Coleoptera: Coccinellidae).Eur. J. Entomol.97551–553. 10.14411/eje.2000.085
63
Yocum G. D. Coudron T. A. Brandt S. L. (2006). Differential gene expression in Perillus bioculatus nymphs fed a suboptimal artificial diet.J. Insect. Physiol.52586–592. 10.1016/j.jinsphys.2006.02.006
64
Zeng F. Cohen A. C. (2000). Comparison of alpha-amylase and protease activities of a zoophytophagous and two phytozoophagous Heteroptera.Com.Biochem. Phys. A126101–106. 10.1016/s1095-6433(00)00193-8
65
Zeng F. Cohen A. C. (2001). Induction of elastase in a zoophytophagous heteropteran. Lygus hesperus (Hemiptera: Miridae).Eur. J. Entomol.94146–151.10.1603/0013-8746(2001)094[0146:ioeiaz]2.0.CO;2
66
Zeng F. Shu S. Park Y. Ramaswamy S. B. (1997). Vitellogenin and egg production in the moth, Heliothis virescens.Arch. Insect Biochem. Physiol.34287–300. 10.1002/(sici)1520-6327(1997)34:3<287::aid-arch4>3.0.co;2-s
67
Zeng F. Zhu Y. C. Cohen A. C. (2002). Molecular cloning and partial characterization of a trypsin-like protein in salivary glands of Lygus hesperus (Hemiptera: Miridae).Insect. Biochem. Molec.32455–464. 10.1016/s0965-1748(01)00123-0
68
Zhang S. Mao J. Zeng F. (2015). Effects of an artificial diet with non-insect ingredient on biological characteristics of Harmonia axyridis (Pallas).Chin. J. Biol. Contr.3135–40.
69
Zhang S. Zhang T. T. Zhang G. C. (2017). “Techniques for mass rearing of lady bird beetles,” in Theory and Practice of Mass Rearing of Insects and Predatory Mites, ed.ZengF. R. (Beijing: Science Press), 196–199.
70
Zhang T. Zhang G. Zeng F. Mao J. Lang H. Liu F. (2017). Molecular cloning of the vitellogenin gene and the effects of vitellogenin protein expression on the physiology of Harmonia axyridis (Coleoptera: Coccinellidae).Sci. Rep.7:13926. 10.1038/s41598-017-14339-3
71
Zou D. Coudron T. A. Liu C. Zhang L. Wang M. Chen H. (2013). Nutrigenomics in Arma chinensis: transcriptome analysis of Arma chinensis fed on aArtificial diet and Chinese Oak Silk Moth Antheraea pernyi Pupae.Plos. One.8:e60881. 10.1371/journal.pone.0060881
Summary
Keywords
artificial diets, ingredients, transcriptome analysis, reproduction, nutritional, development
Citation
Zhang T, He Y, Zeng J, Zhang L, Zeng F, Mao J and Zhang G (2019) Search for Nutritional Fitness Traits in a Biological Pest Control Agent Harmonia axyridis Using Comparative Transcriptomics. Front. Physiol. 10:1148. doi: 10.3389/fphys.2019.01148
Received
21 January 2019
Accepted
26 August 2019
Published
18 September 2019
Volume
10 - 2019
Edited by
Youjun Zhang, Institute of Vegetables and Flowers (CAAS), China
Reviewed by
LinQuan Ge, Yangzhou University, China; Bin Tang, Hangzhou Normal University, China
Updates
Copyright
© 2019 Zhang, He, Zeng, Zhang, Zeng, Mao and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanrong Zeng, zengfr@caas.net.cnJianjun Mao, maojianjun0615@126.comGuocai Zhang, zhang640308@126.com
†These authors have contributed equally to this work
This article was submitted to Invertebrate Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.