Abstract
Inflammation is a well-organized protective response to pathogens and consists of immune cell recruitment into areas of infection. Inflammation either clears pathogens and gets resolved leading to tissue healing or remains predominantly unresolved triggering pathological processes in organs. Periodontal disease (PD) that is initiated by specific bacteria also triggers production of inflammatory mediators. These processes lead to loss of tissue structure and function. Reactive oxygen species and oxidative stress play a role in susceptibility to periodontal pathogenic bacterial infections. Periodontal inflammation is a risk factor for systemic inflammation and eventually cardiovascular disease (CVD). This review discusses the role of inflammation in PD and its two way association with other health conditions such as diabetes and CVD. Some of the mechanisms underpinning the links between inflammation, diabetes, CVD and PD are also discussed. Finally, we review available epidemiological data and other reports to assess possible links between oral health and CVD.
Introduction
Periodontal disease (PD) is a chronic inflammatory disorder characterized by the destruction of the periodontium, or the supporting tissues of the teeth (gingival tissue, periodontal ligament, and alveolar bone). PD is highly prevalent, and approximately 50% of adults 30 years and older and 70% of adults 65 or older have a form of the disease (Eke et al., 2012). Clinically, the failure to treat PD leads to loss of teeth (Schultz-Haudt et al., 1954; Hajishengallis et al., 2011; Abusleme et al., 2013). Central to PD is dysregulation of the resolution of inflammation, resulting in characteristic chronic and progressive destruction.
Inflammation is a programmed signaling event initiated to protect organisms upon infection and/or injury. In general, infection-injury stimuli lead to release of pathogen or danger associated molecular patterns (PAMPs or DAMPs) followed by their binding to respective receptors in the host cells. Although there are multiple contributing factors to PD, one of the increasingly well-characterized triggers is the colonization of the oral cavity by pathogenic bacteria and their subsequent penetration into local epithelial lining (Darveau, 2010; Abusleme et al., 2013). This initiates an inflammatory cascade characterized by increased expression of various inflammatory mediators and adhesion molecules that collectively mobilize and recruit polymorphonuclear neutrophils (PMN), macrophages, natural killer (NK), dendritic cells (DC) etc. into the affected tissue. Under normal conditions, neutrophils and macrophages phagocytose the microbial organisms after which they undergo apoptosis at the inflamed site (Fox et al., 2010). The clearance of apoptotic cells facilitates a switch from a pro- to an anti-inflammatory macrophage phenotype (Fadok et al., 1998; Michlewska et al., 2009) and initiates the onset of the resolution of inflammation, a coordinated signaling process that restores tissue integrity and function. However, failure to switch off the inflammation cascade once the pathogenic stimulus is removed, leads chronic inflammation (i.e., an uncontrolled inflammatory response that can culminate into damage to the host tissue) and is the hallmark of several inflammatory disorder related pathologies. In PD specifically, the inflammatory response becomes chronic when pathogenic bacteria continue to propagate and cannot be controlled by the acute immune response, resulting in unresolved inflammation, destruction of local bone and soft tissue, and fibrosis (Cochrane, 2008).
Importantly, reports have consistently highlighted a role for periodontal inflammation in acceleration of various vascular pathologies and other systemic implications (Humphrey et al., 2008; Ogrendik, 2013; Ketabi et al., 2016). The destruction of local epithelium by PD pathogens can result in release of local inflammatory mediators from the periodontal pocket into the systemic circulation thus facilitating immune cell recruitment elsewhere. Also, bacteria can either indirectly (within immune cells that have ingested them) or directly circulate in the bloodstream (Lockhart et al., 2009). Therefore, under conditions where there is disposition (e.g., genetic, lifestyle) toward cardiovascular disease (CVD), the bacterial components and systemic inflammatory mediators can potentially accelerate plaque formation. To this point, PD pathogens have been detected in distant tissues and organs, particularly in the cardiovascular system (Okuda et al., 2001; Kozarov et al., 2006; Nakano et al., 2006; Pessi et al., 2013; Moreno et al., 2017). The relationship between PD and systemic diseases such as CVD has been increasingly well-characterized. Importantly, two classic meta-analyses demonstrated the correlation between PD and CVD, highlighting PD as a potential risk factor for CVD processes such as coronary artery disease (Janket et al., 2003; Khader et al., 2004). Additionally, recent evidence suggests a major role for reactive oxygen species (ROS) and proteolytic enzymes (bacteria- and host-derived) in PD and CVD such as atherosclerosis (Chistiakov et al., 2016).
CVD is an umbrella term for a number of linked pathologies, commonly defined as coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, rheumatic and congenital heart diseases, and venous thromboembolism (Lara-Pezzi et al., 2012; Mandviwala et al., 2016). The associated risk factors include ethnicity, age, and family history of CVD, dyslipidemia, hypertension, tobacco smoke, excess body weight, physical inactivity, and diabetes mellitus. It is well established that these classic risk factors interact with cellular immune-inflammatory signaling processes to lead to endothelial dysfunction and atheromatous plaque development (Lopez-Candales et al., 2017; Lazzerini et al., 2019). Thus chronic inflammation plays a crucial role in the long-term progression of atherosclerosis. About 35–50% of the world population suffers from periodontitis as reported by World Health Organization (Petersen and Ogawa, 2012); therefore understanding any correlation or link between PD and CVD is a question that has tremendous importance given the high incidences of both diseases. This review summarizes pathophysiology of PD and examines the possibility of its link with CVD.
Periodontal Disease
The inflammation of tissues in gingivitis and periodontitis is caused by a host of bacteria (Schultz-Haudt et al., 1954). The bacterial species present in the gingival margin are Porphyromonas gingivalis, Treponema denticola, and Tannnerella forsythia, all of which are Gram negative. Also present are Gram positive bacteria like Streptococcus sanguis, Streptococcus oralis, Streptococcus mutans, Actinomyces naeslundii, and Actinomyces odontolyticus (Abusleme et al., 2013). This is followed by appearance of secondary bacteria such as Fusobacterium nucleatum (Kolenbrander et al., 1989). Aggregates of bacterial colonies form and Gram-positive and Gram-negative bacilli become embedded in the extracellular matrix (Gibbons, 1989). Indeed more than 700 bacterial species have been reported to be detected in dental plaques (Moore, 1987; Gao et al., 2018). Bacterial species normally act as symbiotic communities with the host but shifts of the oral microbiome often associated with “poor” host health can lead to dysbiosis, an imbalance that is responsible for the development of microbe-related PD (Socransky et al., 1998; Darveau, 2010).
Several of these bacteria are also present in healthy individuals; however it is their relative abundance due to with poor oral hygiene (Hoare et al., 2019), tobacco consumption etc. that drives the selection and prevalence of pathogenic bacteria in subgingival margin that lead to the onset of periodontitis (Schultz-Haudt et al., 1954; Socransky et al., 1998). Increased oxidative stress with smoking, lifestyle diseases and aging also plays a role; indeed a strong association between oxidative stress and PD has been reported (Chapple and Matthews, 2007). This occurs either due to diminution of antioxidants or an exaggerated inflammatory response post periodontal infection. This is described in the next section. Bacterial plaque formation leads to increases in PAMPs causing a rise in local inflammation, causing increased flow of gingival crevicular fluid (GCF). This in turn provides protein rich nutrients that increase the proliferation of the Gram-negative bacteria. The dental plaque biofilm of bacteria in the periodontal crevice induces clinical signs of inflammation. The progression of PD is driven primarily by the proliferation of P. gingivalis which facilitates increase in harmful microbiota. The next step is the secondary bacteria F. nucleatum’s role in the subgingival biofilm as this bacterium interacts with other bacterial species found in the biofilm. F. nucleatum serves as a bridge between the early colonizers like Streptococcus sp. and the late colonizers like P. gingivalis. The innate immune response is the recruitment of PMN and the NK cells that is driven by the subgingival bacterial community present in the periodontal pocket. As the microorganisms are abundant, PMN recruitment and phagocytosis is followed by extensive PMN apoptosis or necrosis. A cytokine rich proinflammatory environment consists of tumor necrosis factor (TNF)-α, interleukins (IL)-1, IL-4, IL-10, interferon (IFN-γ) and transforming growth factor (TGF-β). These signaling molecules stimulate the activation of enzymes and transcription factors that in turn recruit more immune cells and degrade the surrounding tissues by maintaining a continual loop of local inflammation (Cekici et al., 2014; Figure 1). This is also accompanied by an adaptive immune response as antigen uptake and processing is carried out by DC and presented to naive T cells. DCs direct CD4+ T cells to differentiate to T-cell subsets such as T helper cells types 1, 2, and 17, and regulatory T cells (Song et al., 2018). CD4+ T cells produce the bone resorption promoting cytokine, Receptor activator of nuclear factor-κB (RANK-L; Tang et al., 2007) leading to bone loss.
FIGURE 1
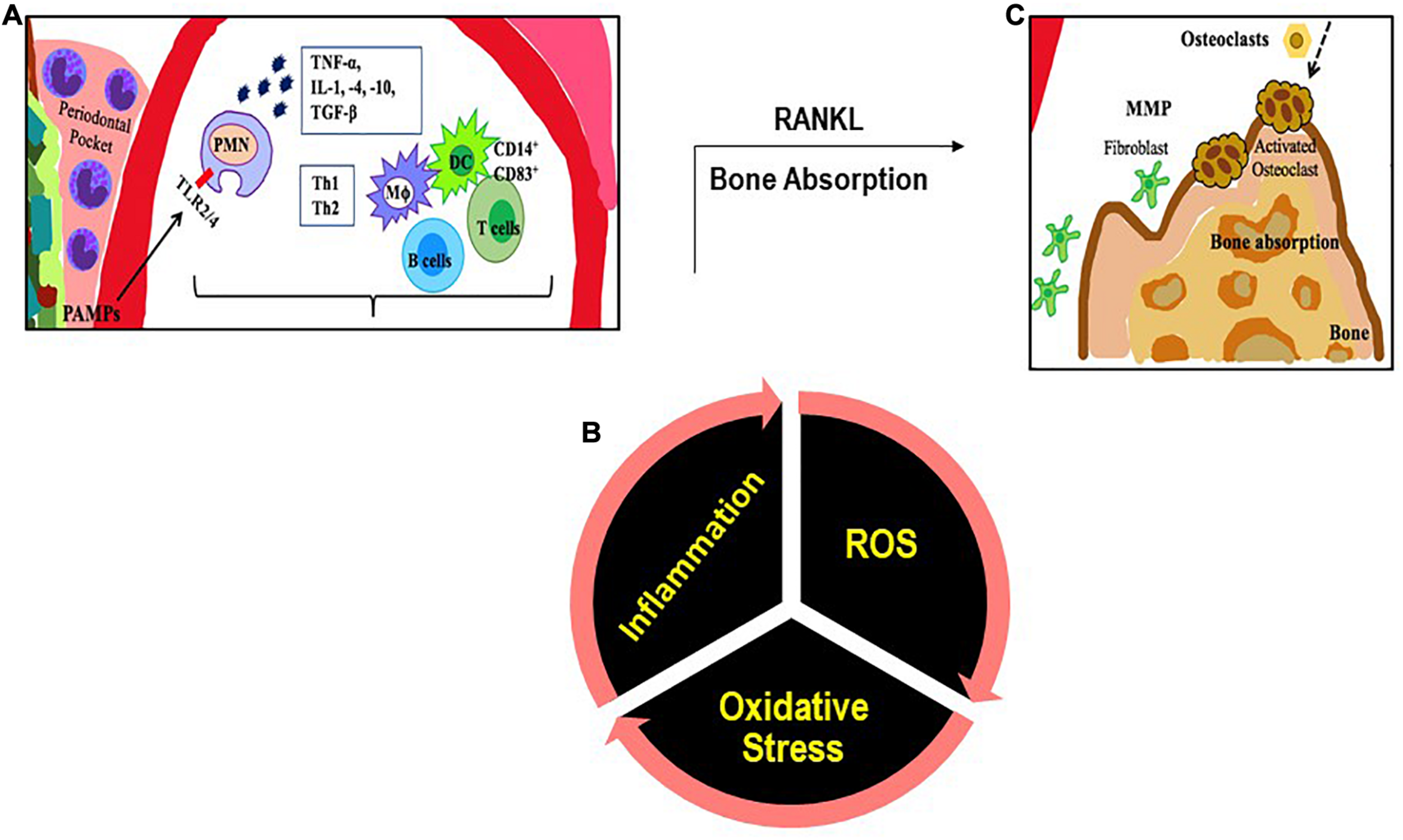
An illustration of the inflammatory immune response with PD. (A) PAMPs (like LPS) are recognized by TLRs. The PAMP-TLR interaction triggers a proinflammatory signaling cascade that drives a chemokine and cytokine rich environment into which multiple immune cells [macrophages (MΦ), T and B lymphocytes, dendritic cells (DC)] are recruited. (B) Multiple immune cells produce ROS. (C) T cells produce the cytokine RANKL that participates in bone resorption.
Oxidative Stress in PD and CVD
Reactive oxygen species and the resultant oxidative stress plays an important role in onset and progression of PD (Chapple and Matthews, 2007). This occurs via multiple mechanisms. First, is the ROS production that occurs with periodontal infection, as inflammatory cells are recruited to the infection site with chronic or aggressive periodontitis. Numerous reports have shown that PMN in the population diagnosed with PD generate significantly more ROS (upon stimulation) as compared to PMN of healthy controls (Aboodi et al., 2011; White et al., 2014; Ling et al., 2016). While this is indicative of a hyper-reactive phenotype of neutrophils in the PD affected, it also suggests that high oxidative stress arising from the excessive ROS could increase local gingival oxidative stress which in turn would drive more inflammation. Alteration of the gingival crevicular environment increase susceptibility to periodontal pathogenic bacteria. Second, is the antioxidant status of PD affected individuals; several studies have shown that the low levels of anti-oxidants (that may be associated with high levels of ROS) in the GCF activated the local periodontal inflammation and caused oxidative injury and destruction of the tissue (Tsai et al., 2005; Chapple and Matthews, 2007; Konopka et al., 2007). Figure 2 shows the feed forward loop of infection induced ROS and oxidative stress that in turn drives more inflammation and changes the local gingival tissue environment making it more susceptible to infection. Lifestyle diseases also play a part in this ROS oxidative stress inflammation cascade.
FIGURE 2
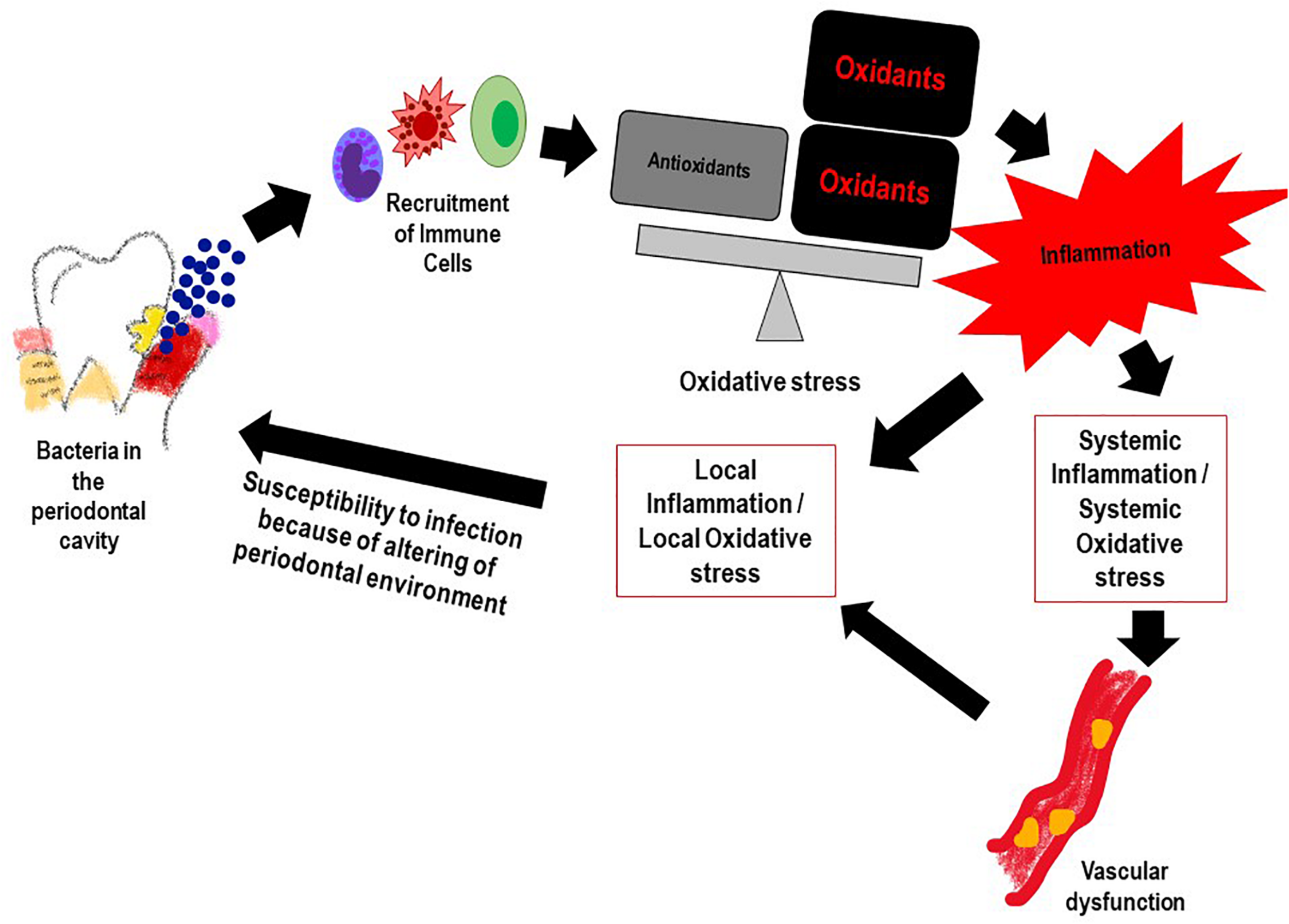
The feed forward mechanism of the “Infection-ROS-Inflammation” triad that seems to accelerate susceptibility to infection, inflammation and oxidative stress.
As is well established, inflammation and oxidative stress are pivotal events that lead to CVD (Mandviwala et al., 2016; Cervantes Gracia et al., 2017) and seem to be the common link between the onset of tissue destruction in periodontitis and systemic inflammation (Wang et al., 2017). Indeed several lifestyle and age related conditions associated with CVD (such as diabetes, hypertension etc.) that lead to high oxidative stress (as assessed by markers of ROS and lipid peroxidation) can also increase susceptibility to PD (Dhadse et al., 2010). When periodontitis susceptible patients are exposed to the bacterial antigen, their main two immune responses in the form of neutrophil recruitment and proteolytic enzymes production further release ROS at the gingival site, thus perpetuating oxidative stress and tissue damage (Scott and Krauss, 2012; Cortes-Vieyra et al., 2016). As periodontitis progresses, periodontal inflammation produces ROS that diffuses into the blood stream (Sobaniec and Sobaniec-Lotowska, 2000; Tomofuji et al., 2007). As a result, various moieties in the blood get oxidized and induce an oxidative stress on other organs via the circulating blood causing circulating oxidative stress (Yagi, 1987; Tomofuji et al., 2007; Figure 3). Thus, it can be inferred that the bacteria present in the periodontal pocket suppress detoxification of ROS by consuming the antioxidants present in the pockets of the oral cavity (Ekuni et al., 2009). The consequence of the lowered levels of antioxidants enables ROS to enter into systemic circulation from the periodontal tissues (Wang et al., 2017).
FIGURE 3
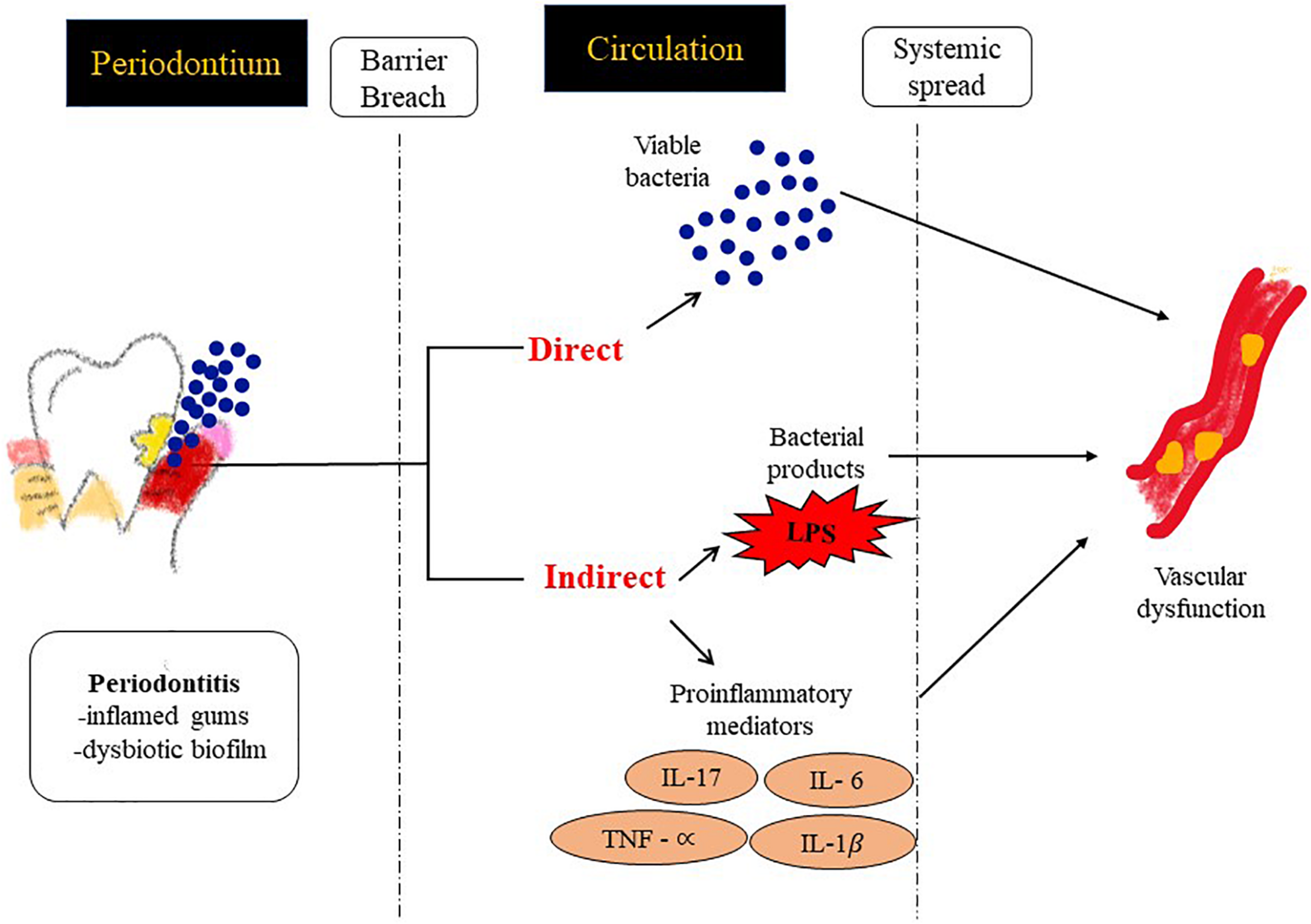
Possible mechanisms by which PD contributes to inflammation at distal sites and thus drives an atherosclerotic phenotype. (1) Direct: vascular infection by periodontal bacterial pathogens and (2) Indirect: facilitating the passage of inflammatory modulators into the systemic circulation.
Regular smoking, diabetes mellitus, insufficient and appropriate nutrition, and aging have all been mentioned as risk factors for both PD and CVD (Yanbaeva et al., 2007; Graves and Kayal, 2008; Dhadse et al., 2010). All the above lifestyle diseases have been known to increase the circulating oxidative stress; indeed increase in levels of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) locally and systemically with PD and with CVD have been observed, thus suggesting an association between local and systemic oxidative stress diseases (Celec et al., 2005; Guentsch et al., 2008; Hendek et al., 2015). Similarly, levels of antioxidants such as SOD and glutathione decrease in the (GCF) and saliva due to smoking in both, healthy individuals and patients with periodontitis (Guentsch et al., 2008; Agnihotri et al., 2009).
Risk Factors (PD and Vascular Inflammation)
Vascular inflammation involves the onset of a signaling cascade that is triggered by endothelial signaling which leads to increase in cellular adhesions molecules, cytokines and chemokines. This leads to recruitment and adherence of immune cells. The atherogenic process starts with endothelial dysfunction and the accumulation of several plasma low density lipoproteins (LDL) in the subendothelial space. The accumulation of LDL correlates with classical risk factors, such as smoking, hypertension, and metabolic dysregulation in obesity and diabetes mellitus (Gimbrone et al., 2000). As these risk factors are largely associated with PD too, it is reasonable to conclude that common biochemical signaling pathways play a role in vulnerability to both CVD and PD. All the major risk factors associated with PD either activate pathogen initiated inflammation signals (bacteria like P. gingivalis and A. actinomycetemcomitans B. forsythus, P. intermedia, P. micros, and F. nucleatum (Lovegrove, 2004; Saito et al., 2008) or a life style (diabetes mellitus, obesity, aging, smoking, vascular disease) driven inflammatory cascade (Jensen et al., 1991; Grossi and Genco, 1998; Cohen, 2000; Merchant et al., 2003; Humphrey et al., 2008; Hujoel, 2009; Kumar et al., 2011; Nakamura et al., 2011; Ozcaka et al., 2011). Nutrition and oral health are closely linked. This is because oxidative stress and antioxidant balance which drives ROS induced inflammation signals, can be regulated by diets rich in antioxidants (Muniz et al., 2015). Diets that lead to obesity such as high carbohydrates and sugars have been implicated in dental caries and PD as these drive plaque formation and accelerate inflammation thus causing dental tissue oxidative damage and decay (Hujoel, 2009). Changes in dietary intake influence the extent of PD. As seen in the dietary intake of adolescents between 11 and 18 years old, the decrease in consumption of raw fruits and non-potato vegetables with concomitant increase in uptake of soft drinks, led to increased PD (Chaffee and Weston, 2010).
Indeed when all these risk factors are controlled, both PD and CVD show improvement (Table 1; Higashi et al., 2008; Bokhari et al., 2012; Montenegro et al., 2019; Lobo et al., 2020).
TABLE 1
| Citation | Aims | Patients | Cohorts | Outcomes |
| Elter et al., 2006 | Effect of periodontal treatment on endothelium-dependent flow-mediated dilation and serum inflammatory biomarkers in patients with PD | Twent-two male patients with PD | All patients underwent periodontal treatment (scaling and root planning, periodontal flap surgery if indicated, and extraction of hopeless teeth) | Periodontal treatment resulted in significant improvements in flow-mediated dilation and decreased serum IL-6 (and a trend toward reduction in CRP) |
| D’Aiuto et al., 2006 | Effects of intensive periodontal treatment on serum inflammatory biomarkers, serum lipid levels, and blood pressure in patients with PD | Forty patients with severe PD | - Experimental group: subject to intensive periodontal treatment (defined as standard treatment plus adjunctive use of a locally delivered antimicrobial) - Control group: standard periodontal treatment (scaling and root planning) | Intensive periodontal treatment resulted in significant reduction in IL-6, CRP, total cholesterol, and systolic blood pressure at 2-month follow-up |
| Tonetti et al., 2007 | To investigate the effects of periodontal treatment on parameters of endothelial function in patients with PD | Hundred and twnety patients with severe PD | - Experimental group: subject to intensive PD treatment - Control group: subject to community-based periodontal care | Intensive periodontal treatment resulted in reduced brachial artery flow, reduced levels of CRP and IL-6, and elevated endothelial-activation markers.- Intensive treatment was associated with reduced indexes of periodontal disease severity and significantly better endothelial function at 6 month follow-up |
| Higashi et al., 2008 | To evaluate endothelial function in patients with hypertension and PD | Sixty-four patients with hypertension (26 with PD and 38 without PD) | - Experimental group: subject to periodontal treatment - Control group: subject to community-based periodontal care | Periodontal treatment resulted in decreased serum CRP and IL-6 at 24-week follow-up Periodontal treatment resulted in reduced acetylcholine-stimulated vasodilation Delivery of a nitric oxide synthase inhibitor before and after PD treatment resulted in similar acetylcholine-stimulated vasodilation, suggesting role of nitric oxide bioavailability in mechanism of endothelial dysfunction in patients with PD |
| Higashi et al., 2009 | To evaluate endothelial function and the effects of periodontal treatment on patients with CAD and PD | 101 patients with CAD (48 with PD and 53 without PD) | - Experimental group: subject to periodontal treatment - Control group: subject to community-based periodontal care | - Periodontal treatment resulted in decreased serum CRP and IL-6 at follow-up - Periodontal treatment resulted in reduced acetylcholine-stimulated vasodilation - Delivery of a nitric oxide synthase inhibitor before and after PD treatment resulted in similar acetylcholine-stimulated vasodilation, suggesting role of nitric oxide bioavailability in mechanism of endothelial dysfunction in patients with PD |
| Offenbacher et al., 2009 | Impact of periodontal treatment on serum CRP levels, clinical PD parameters, and cardiovascular endpoints in patients with PD | Three hundred three patients with PD and previous history of CVD | - Experimental group: subject to periodontal treatment (scaling and root planning - Control group: not subject to periodontal treatment | Periodontal treatment resulted in a significant improvement in PD status at 6 months as assessed by reduction of probing depth, but no difference in attachment levels, bleeding upon probing, or extent of subgingival calculus Periodontal treatment resulted in significant decrease of the odds of being in the high-risk (>3 mg/L) CRP group at 6 months, with obesity nullifying such effect |
| Vidal et al., 2009 | Effects of non-surgical PD treatment on plasma levels of inflammatory markers (interleukin [IL]-6, C-reactive protein [CRP], and fibrinogen) in patients with severe PD and refractory arterial hypertension | Twenty-two patients with severe PD and refractory arterial hypertension | - Experimental group: subject to periodontal treatment at start of trial - Control group: subject to periodontal treatment delayed 3-months from start of trial | Periodontal treatment resulted in significant reduction in markers of PD severity (probing, probing depth, and clinical attachment loss) Periodontal treatment resulted in significant reduction of fibrinogen, CRP, and IL-6 |
| Wehmeyer et al., 2013 | Impact of periodontal treatment PD parameters and inflammatory biomarkers in patients with end-stage renal disease and PD | Three hundred forty-two dialysis patients with moderate/severe chronic PD | - Experimental group: subject to intensive periodontal treatment - Control group: subject to intensive periodontal treatment following study completion at 6 months | Intensive periodontal treatment resulted in significantly improved measures of periodontal health at 3 months, but only PD remained significantly different at 6 months No significant difference between the groups inflammatory biomarkers (serum albumin or high-sensitivity interleukin 6) at any time point |
| Bokhari et al., 2012 | Effect of non-surgical periodontal treatment on systemic C-reactive protein, fibrinogen and white blood cells in coronary heart disease patients with PD | Three hundred seventeen patientswith angiographically proven coronary heart disease | - Experimental group: subject to on-surgical periodontal treatment (scaling, root planning and oral hygiene instructions) - Control group: not subject to periodontal treatment | Non−surgical periodontal treatment resulted in significant reduction of systemic levels of inflammatory markers (CRP, fibrinogen and WBCs) at 2-month follow-up |
| Caula et al., 2014 | Influence of non−surgical mechanical PD treatment on c-reactive protein serum level, erythrocyte sedimentation rate (ESR), and lipid profile in patients with severe chronic periodontitis | Sixty-four patients with severe chronic PD | - Experimental group: Began non-surgical PD treatment - Control group: Withheld PD treatment during study period | PD treatment resulted in a significant reduction of ESR and triglycerides at 2 months PD treatment resulted in significant reduction in median values of C−reactive protein, ESR, total cholesterol, and triglycerides after 6 months |
| Zhou et al., 2017 | To investigate if intensive periodontal treatment can lower blood pressure levels and endothelial microparticles (EMPs) in patients with prehypertension and PD without antihypertensive medication | Hundred and seven patients with prehypertension and PD | - Experimental group: Subject to intensive periodontal treatment - Control group: Subject to community−based periodontal care | Intensive periodontal treatment resulted in reduced clinical PD parameters, reduced systolic and diastolic blood pressures, and reduced endothelial microparticles |
| Montenegro et al., 2019 | To assess the effect of periodontal treatment on clinical PD parameters and levels of cardiovascular risk biomarkers in stable coronary artery disease (CAD) patients (CRP, glycated hemoglobin, lipids, IL−1β, IL−6, IL−8, IL−10, IFN−γ and TNF−α) | 88 patients with stable coronary artery disease and periodontitis | - Experimental group: Subject to non-surgical periodontal treatment - Control group: Subject to one session of plaque removal | Periodontal treatment resulted in significantly better periodontal parameters after 3 months without significant differences in blood biomarkers In patients with baseline high levels of CRP, periodontal treatment resulted in lower levels of CRP, IL−6 and IL−8 |
| Lobo et al., 2020 | To investigate the impact of periodontal treatment on the endothelial function of patients with a recent ST-segment elevation myocardial infarction (STEMI), specifically looking at variation of flow-mediated vasodilation (FMD) in the brachial artery, inflammatory biomarkers, and adverse CVD events | Forty-eight patients with PD and with recent admission for STEMI | - Experimental group: subject to periodontal treatment within 2 weeks of STEMI - Control group: not subject to periodontal treatment | Periodontal treatment significantly improved endothelial function of the brachial artery without adverse clinical effects over a period of 6 months Inflammatory biomarkers and cardiovascular events were not significantly different between both groups |
Clinical trials investigating relationship between periodontal disease and cardiovascular disease.
PD and CVD: Is There a Link?
It is not clear if there is a direct and common thread between PD and CVD; however the fact that people with PD have a two or three times higher risk of a cardiovascular event (stroke, heart attack etc.) seems to point to a cluster of shared risk factors between the two (Sanz et al., 2020). The “inflammation” link seems to be a key contributor to both.
For instance, when infected with P. gingivalis, the host innate immune system responds by activating inflammation consisting of the NLRP3 inflammasome (pro-inflammatory IL-1β, IL-18) (Lamkanfi and Dixit, 2009; Xue et al., 2015). Patients with chronic PD and aggressive PD expressed significantly higher levels of NLRP3 in gingiva (Xue et al., 2015; Ran et al., 2017). In wild type mice with P. gingivalis infection, the increase in expression of NLRP3 inflammation cascade in the gingival tissue was matched with a concomitant increase in caspase-1 activity in the macrophages found in peritoneum; this was not observed in NLRP3 deficient mice (Yamaguchi et al., 2017). This suggests that the NLRP3 inflammasome activated in periodontitis has effects on the systemic organs. NLRP3 inflammasome has also been shown to be highly expressed and activated with systemic vascular disease (Satoh et al., 2014) although it is not clear whether NLRP3 from P. gingivalis (Yamaguchi et al., 2017) is directly involved. However, excess ROS, glucose, ATP, ceramides, sphingosine, cholesterol crystals, uric acid and oxidized LDL (all of which are associated with CVD) have been known to activate NLRP3 inflammasome (Duewell et al., 2010; Jiang et al., 2012; Luheshi et al., 2012).
Epidemiologic data till date that suggest an association between PD and CVD (Humphrey et al., 2008; Blaizot et al., 2009; Tonetti, 2009) have monitored PD via indices of clinical attachment level, pocket depth, bleeding on probing and decayed-missing-filled teeth and CVD by degree and number of obstructed coronary arteries, observed an association between PD and CVDs (Ketabi et al., 2016). Table 1 shows the clinical trials which investigated the relationship between the two diseases. While risk factors as discussed in the earlier section play a crucial role in the onset of CVD, increasing number of CVD patients do not harbor the classical risk factors. Low grade infection such as in periodontal infection could be a potential cause for CVD in these cases; indeed several studies show that PD as a risk factor for CVD and, in particular, atherosclerosis (Bartova et al., 2014; Toregeani et al., 2016).
Potential links between PD and CVD could be via two mechanisms (Figure 3).
-
1.
Systemic Inflammation: Systemic inflammatory markers such as C-reactive protein (CRP), IL-6 etc. have shown direct correlation with specific indices of CVD such as carotid-intima media thickness, or MI (myocardial infarct size) (Ali et al., 2006). Chronic periodontal infection is characterized by elevation of CRP and inflammatory cytokines in the systemic circulation (Loos et al., 2000), so it is possible that systemic inflammation in patients with PD can potentially accelerate endothelial dysfunction, plaque buildup and CHD events.
-
2.
Vascular Infection: There have been reports that identify bacterial species in blood after dental procedures suggesting gingiva as a portal via which oral bacterial pathogens can enter the systemic circulation (Bahrani-Mougeot et al., 2008; Lockhart et al., 2009). As a result bacteremia of dental origin seems to play a role in the appearance of bacterial endocarditis (Mang-de la Rosa et al., 2014) and periodontal bacterial components colonize human atheromatous plaques (Haraszthy et al., 2000; Fiehn et al., 2005).
Conclusion
While both PD and CVD have manifestations of classic inflammation, a causative link between them has not been established. However, oxidative stress arising from lifestyle diseases play a crucial role in progression of both PD and CVD, indicating that host influence in terms of an imbalance between ROS production and endogenous antioxidant levels can increase susceptibility in individuals.
Understanding how oxidative stress and inflammation overlap in PD and CVD for high risk and older populations is of great public health importance because of the high prevalence of PD. Although both these pathologies arise from the same risk factors and show a similar systemic inflammation profile, it is not clear how these diseases intersect. Therefore it cannot be concluded that therapeutic periodontal interventions can prevent heart disease or stroke. Nevertheless, controlling the overall inflammation status by implementing a good periodontal maintenance program could presumably control the progression of CVD in periodontitis patients.
Statements
Author contributions
OP drafted the article and prepared the figures. PA drafted the article. MM drafted the article and prepared the table. SC developed the concept and outline, drafted the article, and formalized the final version. All authors approved the submission.
Funding
Funding was provided by a Colgate Palmolive Grant (A-2019-592-OC) to SC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aboodi G. M. Goldberg M. B. Glogauer M. (2011). Refractory periodontitis population characterized by a hyperactive oral neutrophil phenotype.J. Periodontol.82726–733. 10.1902/jop.2010.100508
2
Abusleme L. Dupuy A. K. Dutzan N. Silva N. Burleson J. A. Strausbaugh L. D. et al (2013). The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation.ISME J.71016–1025. 10.1038/ismej.2012.174
3
Agnihotri R. Pandurang P. Kamath S. U. Goyal R. Ballal S. Shanbhogue A. Y. et al (2009). Association of cigarette smoking with superoxide dismutase enzyme levels in subjects with chronic periodontitis.J. Periodontol.80657–662. 10.1902/jop.2009.080545
4
Ali Y. S. Rembold K. E. Weaver B. Wills M. B. Tatar S. Ayers C. R. et al (2006). Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness.Atherosclerosis187186–190. 10.1016/j.atherosclerosis.2005.09.003
5
Bahrani-Mougeot F. K. Paster B. J. Coleman S. Ashar J. Barbuto S. Lockhart P. B. (2008). Diverse and novel oral bacterial species in blood following dental procedures.J. Clin. Microbiol.462129–2132. 10.1128/jcm.02004-07
6
Bartova J. Sommerova P. Lyuya-Mi Y. Mysak J. Prochazkova J. Duskova J. et al (2014). Periodontitis as a risk factor of atherosclerosis.J. Immunol. Res.2014:636893.
7
Blaizot A. Vergnes J. N. Nuwwareh S. Amar J. Sixou M. (2009). Periodontal diseases and cardiovascular events: meta-analysis of observational studies.Int. Dent. J.59197–209.
8
Bokhari S. A. Khan A. A. Butt A. K. Azhar M. Hanif M. Izhar M. et al (2012). Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial.J. Clin. Periodontol.391065–1074. 10.1111/j.1600-051x.2012.01942.x
9
Caula A. L. Lira-Junior R. Tinoco E. M. Fischer R. G. (2014). The effect of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial.J. Clin. Periodontol.41875–882. 10.1111/jcpe.12290
10
Cekici A. Kantarci A. Hasturk H. Van Dyke T. E. (2014). Inflammatory and immune pathways in the pathogenesis of periodontal disease.Periodontology200057–80. 10.1111/prd.12002
11
Celec P. Hodosy J. Celecova V. Vodrazka J. Cervenka T. Halcak L. et al (2005). Salivary thiobarbituric acid reacting substances and malondialdehyde–their relationship to reported smoking and to parodontal status described by the papillary bleeding index.Dis. Markers21133–137. 10.1155/2005/693437
12
Cervantes Gracia K. Llanas-Cornejo D. Husi H. (2017). CVD and oxidative stress.J. Clin. Med.6:22. 10.3390/jcm6020022
13
Chaffee B. W. Weston S. J. (2010). Association between chronic periodontal disease and obesity: a systematic review and meta-analysis.J. Periodontol.811708–1724. 10.1902/jop.2010.100321
14
Chapple I. L. Matthews J. B. (2007). The role of reactive oxygen and antioxidant species in periodontal tissue destruction.Periodontology2000160–232. 10.1111/j.1600-0757.2006.00178.x
15
Chistiakov D. A. Orekhov A. N. Bobryshev Y. V. (2016). Links between atherosclerotic and periodontal disease.Exp. Mol. Pathol.100220–235. 10.1016/j.yexmp.2016.01.006
16
Cochrane (2008). New cochrane systematic reviews — cochrane oral health group.J. Evid. Based Dent. Pract.8258–260. 10.1016/j.jebdp.2008.09.001
17
Cohen D. W. (2000). Periodontal medicine in the next millennium.Intl. J. Periodontics Restorative Dent.206–7.
18
Cortes-Vieyra R. Rosales C. Uribe-Querol E. (2016). Neutrophil functions in periodontal homeostasis.J. Immunol. Res.2016:1396106.
19
D’Aiuto F. Parkar M. Nibali L. Suvan J. Lessem J. Tonetti M. S. (2006). Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial.Am. Heart. J.151977–984. 10.1016/j.ahj.2005.06.018
20
Darveau R. P. (2010). Periodontitis: a polymicrobial disruption of host homeostasis.Nat. Rev. Microbiol.8481–490. 10.1038/nrmicro2337
21
Dhadse P. Gattani D. Mishra R. (2010). The link between periodontal disease and cardiovascular disease: how far we have come in last two decades?J. Indian Soc. Periodontol.14148–154. 10.4103/0972-124x.75908
22
Duewell P. Kono H. Rayner K. J. Sirois C. M. Vladimer G. Bauernfeind F. G. et al (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals.Nature4641357–1361. 10.1038/nature08938
23
Eke P. I. Dye B. A. Wei L. Thornton-Evans G. O. Genco R. J. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010.J. Dent. Res.91914–920. 10.1177/0022034512457373
24
Ekuni D. Tomofuji T. Sanbe T. Irie K. Azuma T. Maruyama T. et al (2009). Periodontitis-induced lipid peroxidation in rat descending aorta is involved in the initiation of atherosclerosis.J. Periodontal Res.44434–442. 10.1111/j.1600-0765.2008.01122.x
25
Elter J. R. Hinderliter A. L. Offenbacher S. Beck J. D. Caughey M. Brodala N. et al (2006). The effects of periodontal therapy on vascular endothelial function: a pilot trial.Am. Heart. J.151:47.
26
Fadok V. A. Bratton D. L. Konowal A. Freed P. W. Westcott J. Y. Henson P. M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta. PGE2, and PAF.J. Clin. Invest.101890–898. 10.1172/jci1112
27
Fiehn N. E. Larsen T. Christiansen N. Holmstrup P. Schroeder T. V. (2005). Identification of periodontal pathogens in atherosclerotic vessels.J. Periodontol.76731–736. 10.1902/jop.2005.76.5.731
28
Fox S. Leitch A. E. Duffin R. Haslett C. Rossi A. G. (2010). Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease.J. Innate Immunity2216–227. 10.1159/000284367
29
Gao L. Xu T. Huang G. Jiang S. Gu Y. Chen F. (2018). Oral microbiomes: more and more importance in oral cavity and whole body.Protein Cell9488–500. 10.1007/s13238-018-0548-1
30
Gibbons R. J. (1989). Bacterial adhesion to oral tissues: a model for infectious diseases.J. Dent. Res.68750–760. 10.1177/00220345890680050101
31
Gimbrone M. A. Jr. Topper J. N. Nagel T. Anderson K. R. Garcia-Cardena G. (2000). Endothelial dysfunction, hemodynamic forces, and atherogenesis.Ann. N. Y. Acad. Sci.902230–239. 10.1111/j.1749-6632.2000.tb06318.x
32
Graves D. T. Kayal R. A. (2008). Diabetic complications and dysregulated innate immunity.Front. Biosci.131227–1239. 10.2741/2757
33
Grossi S. G. Genco R. J. (1998). Periodontal disease and diabetes mellitus: a two-way relationship.Ann. Periodontol.351–61. 10.1902/annals.1998.3.1.51
34
Guentsch A. Preshaw P. M. Bremer-Streck S. Klinger G. Glockmann E. Sigusch B. W. (2008). Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: effect of smoking and periodontal treatment.Clin. Oral Investig.12345–352. 10.1007/s00784-008-0202-z
35
Hajishengallis G. Liang S. Payne M. A. Hashim A. Jotwani R. Eskan M. A. et al (2011). Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement.Cell Host Microbe10497–506. 10.1016/j.chom.2011.10.006
36
Haraszthy V. I. Zambon J. J. Trevisan M. Zeid M. Genco R. J. (2000). Identification of periodontal pathogens in atheromatous plaques.J. Periodontol.711554–1560. 10.1902/jop.2000.71.10.1554
37
Hendek M. K. Erdemir E. O. Kisa U. Ozcan G. (2015). Effect of initial periodontal therapy on oxidative stress markers in gingival crevicular fluid, saliva, and serum in smokers and non-smokers with chronic periodontitis.J. Periodontol.86273–282. 10.1902/jop.2014.140338
38
Higashi Y. Goto C. Hidaka T. Soga J. Nakamura S. Fujii Y. et al (2009). Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease.Atherosclerosis206604–610. 10.1016/j.atherosclerosis.2009.03.037
39
Higashi Y. Goto C. Jitsuiki D. Umemura T. Nishioka K. Hidaka T. et al (2008). Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients.Hypertension51446–453. 10.1161/hypertensionaha.107.101535
40
Hoare A. Soto C. Rojas-Celis V. Bravo D. (2019). Chronic Inflammation as a Link between periodontitis and carcinogenesis.Mediators Inflamm.2019:1029857.
41
Hujoel P. (2009). Dietary carbohydrates and dental-systemic diseases.J. Dent. Res.88490–502. 10.1177/0022034509337700
42
Humphrey L. L. Fu R. Buckley D. I. Freeman M. Helfand M. (2008). Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis.J. Gen. Intern. Med.232079–2086. 10.1007/s11606-008-0787-6
43
Janket S. J. Baird A. E. Chuang S. K. Jones J. A. (2003). Meta-analysis of periodontal disease and risk of coronary heart disease and stroke.Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod.95559–569. 10.1067/moe.2003.107
44
Jensen J. A. Goodson W. H. Hopf H. W. Hunt T. K. (1991). Cigarette smoking decreases tissue oxygen.Arch. Surg.1261131–1134. 10.1001/archsurg.1991.01410330093013
45
Jiang Y. Wang M. Huang K. Zhang Z. Shao N. Zhang Y. et al (2012). Oxidized low-density lipoprotein induces secretion of interleukin-1beta by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation.Biochem. Biophys. Res. Commun.425121–126. 10.1016/j.bbrc.2012.07.011
46
Ketabi M. Meybodi F. R. Asgari M. R. (2016). The association between periodontal disease parameters and severity of atherosclerosis.Dent. Res. J.13250–255. 10.4103/1735-3327.182185
47
Khader Y. S. Albashaireh Z. S. Alomari M. A. (2004). Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: a meta-analysis.J. Periodontol.751046–1053. 10.1902/jop.2004.75.8.1046
48
Kolenbrander P. E. Andersen R. N. Moore L. V. (1989). Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and selenomonas sputigena with strains from 11 genera of oral bacteria.Infect. Immun.573194–3203. 10.1128/iai.57.10.3194-3203.1989
49
Konopka T. Krol K. Kopec W. Gerber H. (2007). Total antioxidant status and 8-hydroxy-2’-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients.Arch. Immunol. Ther. Exp.55417–422. 10.1007/s00005-007-0047-1
50
Kozarov E. Sweier D. Shelburne C. Progulske-Fox A. Lopatin D. (2006). Detection of bacterial DNA in atheromatous plaques by quantitative PCR.Microbes Infect.8687–693. 10.1016/j.micinf.2005.09.004
51
Kumar P. S. Matthews C. R. Joshi V. De Jager M. Aspiras M. (2011). Tobacco smoking affects bacterial acquisition and colonization in oral biofilms.Infect. Immun.794730–4738. 10.1128/iai.05371-11
52
Lamkanfi M. Dixit V. M. (2009). Inflammasomes: guardians of cytosolic sanctity.Immunol. Rev.22795–105. 10.1111/j.1600-065x.2008.00730.x
53
Lara-Pezzi E. Dopazo A. Manzanares M. (2012). Understanding cardiovascular disease: a journey through the genome (and what we found there).Dis. Models Mech.5434–443. 10.1242/dmm.009787
54
Lazzerini P. E. Hamilton R. M. Boutjdir M. (2019). Editorial: cardioimmunology: inflammation and immunity in cardiovascular disease.Front. Cardiovasc. Med.6:181.
55
Ling M. R. Chapple I. L. Matthews J. B. (2016). Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy.J. Clin. Periodontol.43652–658. 10.1111/jcpe.12575
56
Lobo M. G. Schmidt M. M. Lopes R. D. Dipp T. Feijo I. P. Schmidt K. E. S. et al (2020). Treating periodontal disease in patients with myocardial infarction: a randomized clinical trial.Eur. J. Intern. Med.7176–80. 10.1016/j.ejim.2019.08.012
57
Lockhart P. B. Brennan M. T. Thornhill M. Michalowicz B. S. Noll J. Bahrani-Mougeot F. K. et al (2009). Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia.J. Am. Dent. Assoc.1401238–1244. 10.14219/jada.archive.2009.0046
58
Loos B. G. Craandijk J. Hoek F. J. Wertheim-Van Dillen P. M. Van Der Velden U. (2000). Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients.J. Periodontol.711528–1534. 10.1902/jop.2000.71.10.1528
59
Lopez-Candales A. Hernandez Burgos P. M. Hernandez-Suarez D. F. Harris D. (2017). Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome.J. Nat. Sci.3:e341.
60
Lovegrove J. M. (2004). Dental plaque revisited: bacteria associated with periodontal disease.J. N. Z. Soc. Periodontol.20047–21.
61
Luheshi N. M. Giles J. A. Lopez-Castejon G. Brough D. (2012). Sphingosine regulates the NLRP3-inflammasome and IL-1beta release from macrophages.Eur. J. Immunol.42716–725. 10.1002/eji.201142079
62
Mandviwala T. Khalid U. Deswal A. (2016). Obesity and cardiovascular disease: a risk factor or a risk marker?Curr. Atheroscler. Rep.18:21.
63
Mang-de la Rosa M. R. Castellanos-Cosano L. Romero-Perez M. J. Cutando A. (2014). The bacteremia of dental origin and its implications in the appearance of bacterial endocarditis.Med. Oral Patol. Oral Cir. Bucal19e67–e74.
64
Merchant A. T. Pitiphat W. Ahmed B. Kawachi I. Joshipura K. (2003). A prospective study of social support, anger expression and risk of periodontitis in men.J. Am. Dent. Assoc.1341591–1596. 10.14219/jada.archive.2003.0104
65
Michlewska S. Dransfield I. Megson I. L. Rossi A. G. (2009). Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha.FASEB J.23844–854. 10.1096/fj.08-121228
66
Montenegro M. M. Ribeiro I. W. J. Kampits C. Saffi M. A. L. Furtado M. V. Polanczyk C. A. et al (2019). Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: preliminary findings of 3 months.J. Clin. Periodontol.46321–331. 10.1111/jcpe.13085
67
Moore W. E. (1987). Microbiology of periodontal disease.J. Periodontal Res.22335–341. 10.1111/j.1600-0765.1987.tb01595.x
68
Moreno S. Parra B. Botero J. E. Moreno F. Vasquez D. Fernandez H. et al (2017). Periodontal microbiota and microorganisms isolated from heart valves in patients undergoing valve replacement surgery in a clinic in Cali, Colombia.Biomedica37516–525. 10.7705/biomedica.v37i4.3232
69
Muniz F. W. Nogueira S. B. Mendes F. L. Rosing C. K. Moreira M. M. De Andrade G. M. et al (2015). The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: a systematic review.Arch. Oral. Biol.601203–1214. 10.1016/j.archoralbio.2015.05.007
70
Nakamura Y. Tagusari O. Seike Y. Ito Y. Saito K. Miyamoto R. et al (2011). Prevalence of periodontitis and optimal timing of dental treatment in patients undergoing heart valve surgery.Interact. Cardiovasc. Thorac. Surg.12696–700. 10.1510/icvts.2010.255943
71
Nakano K. Inaba H. Nomura R. Nemoto H. Takeda M. Yoshioka H. et al (2006). Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens.J. Clin. Microbiol.443313–3317. 10.1128/jcm.00377-06
72
Offenbacher S. Beck J. D. Moss K. Mendoza L. Paquette D. W. Barrow D. A. et al (2009). Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease.J. Periodontol.80190–201. 10.1902/jop.2009.080007
73
Ogrendik M. (2013). Rheumatoid arthritis is an autoimmune disease caused by periodontal pathogens.Intl. J. Gen. Med.6383–386. 10.2147/ijgm.s45929
74
Okuda K. Ishihara K. Nakagawa T. Hirayama A. Inayama Y. (2001). Detection of Treponema denticola in atherosclerotic lesions.J. Clin. Microbiol.391114–1117. 10.1128/jcm.39.3.1114-1117.2001
75
Ozcaka O. Bicakci N. Pussinen P. Sorsa T. Kose T. Buduneli N. (2011). Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis.Oral Dis.1768–76. 10.1111/j.1601-0825.2010.01705.x
76
Pessi T. Karhunen V. Karjalainen P. P. Ylitalo A. Airaksinen J. K. Niemi M. et al (2013). Bacterial signatures in thrombus aspirates of patients with myocardial infarction.Circulation127:e1-6.
77
Petersen P. E. Ogawa H. (2012). The global burden of periodontal disease: towards integration with chronic disease prevention and control.Periodontology6015–39. 10.1111/j.1600-0757.2011.00425.x
78
Ran S. Liu B. Gu S. Sun Z. Liang J. (2017). Analysis of the expression of NLRP3 and AIM2 in periapical lesions with apical periodontitis and microbial analysis outside the apical segment of teeth.Arch. Oral. Biol.7839–47. 10.1016/j.archoralbio.2017.02.006
79
Saito Y. Fujii R. Nakagawa K. I. Kuramitsu H. K. Okuda K. Ishihara K. (2008). Stimulation of Fusobacterium nucleatum biofilm formation by Porphyromonas gingivalis.Oral Microbiol. Immunol.231–6. 10.1111/j.1399-302x.2007.00380.x
80
Sanz M. Marco Del, Castillo A. Jepsen S. Gonzalez-Juanatey J. R. D’aiuto F. et al (2020). Periodontitis and cardiovascular diseases: consensus report.J. Clin. Periodontol.47268–288.
81
Satoh M. Tabuchi T. Itoh T. Nakamura M. (2014). NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin.Clin. Sci.126233–241. 10.1042/cs20130043
82
Schultz-Haudt S. Bibby B. G. Bruce M. A. (1954). Tissue-destructive products of gingival bacteria from nonspecific gingivitis.J. Dent. Res.33624–631. 10.1177/00220345540330050601
83
Scott D. A. Krauss J. (2012). Neutrophils in periodontal inflammation.Front. Oral Biol.1556–83. 10.1159/000329672
84
Sobaniec H. Sobaniec-Lotowska M. E. (2000). Morphological examinations of hard tissues of periodontium and evaluation of selected processes of lipid peroxidation in blood serum of rats in the course of experimental periodontitis.Med. Sci. Monit.6875–881.
85
Socransky S. S. Haffajee A. D. Cugini M. A. Smith C. et al (1998). Microbial complexes in subgingival plaque.J. Clin. Periodontol.25134–144. 10.1111/j.1600-051x.1998.tb02419.x
86
Song L. Dong G. Guo L. Graves D. T. (2018). The function of dendritic cells in modulating the host response.Mol. Oral Microbiol.3313–21. 10.1111/omi.12195
87
Tang D. Shi Y. Kang R. Li T. Xiao W. Wang H. et al (2007). Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1.J. Leukoc. Biol.81741–747. 10.1189/jlb.0806540
88
Tomofuji T. Ekuni D. Yamanaka R. Kusano H. Azuma T. Sanbe T. et al (2007). Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats.J. Periodontol.781999–2006. 10.1902/jop.2007.070056
89
Tonetti M. S. (2009). Periodontitis and risk for atherosclerosis: an update on intervention trials.J. Clin. Periodontol.36(Suppl. 10), 15–19. 10.1111/j.1600-051x.2009.01417.x
90
Tonetti M. S. D’aiuto F. Nibali L. Donald A. Storry C. Parkar M. et al (2007). Treatment of periodontitis and endothelial function.N. Engl. J. Med.356911–920.
91
Toregeani J. F. Nassar C. A. Nassar P. O. Toregeani K. M. Gonzatto G. K. Vendrame R. et al (2016). Evaluation of periodontitis treatment effects on carotid intima-media thickness and expression of laboratory markers related to atherosclerosis.Gen. Dent.6455–62.
92
Tsai C. C. Chen H. S. Chen S. L. Ho Y. P. Ho K. Y. Wu Y. M. et al (2005). Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis.J. Periodontal Res.40378–384. 10.1111/j.1600-0765.2005.00818.x
93
Vidal F. Figueredo C. M. Cordovil I. Fischer R. G. (2009). Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension.J. Periodontol.80786–791. 10.1902/jop.2009.080471
94
Wang Y. Andrukhov O. Rausch-Fan X. (2017). Oxidative stress and antioxidant system in periodontitis.Front. Physiol.8:910.
95
Wehmeyer M. M. Kshirsagar A. V. Barros S. P. Beck J. D. Moss K. L. Preisser J. S. et al (2013). A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients With ESRD: results of an exploratory study.Am. J. Kidney Dis.61450–458. 10.1053/j.ajkd.2012.10.021
96
White P. Cooper P. Milward M. Chapple I. (2014). Differential activation of neutrophil extracellular traps by specific periodontal bacteria.Free Radic. Biol. Med.75(Suppl. 1):S53.
97
Xue F. Shu R. Xie Y. (2015). The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry.Arch. Oral. Biol.60948–958. 10.1016/j.archoralbio.2015.03.005
98
Yagi K. (1987). Lipid peroxides and human diseases.Chem. Phys. Lipids45337–351. 10.1016/0009-3084(87)90071-5
99
Yamaguchi Y. Kurita-Ochiai T. Kobayashi R. Suzuki T. Ando T. (2017). Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease.Inflamm. Res.6659–65. 10.1007/s00011-016-0992-4
100
Yanbaeva D. G. Dentener M. A. Creutzberg E. C. Wesseling G. Wouters E. F. (2007). Systemic effects of smoking.Chest1311557–1566. 10.1378/chest.06-2179
101
Zhou Q. B. Xia W. H. Ren J. Yu B. B. Tong X. Z. Chen Y. B. et al (2017). Effect of intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: a randomized controlled trial.J. Periodontol.88711–722. 10.1902/jop.2017.160447
Summary
Keywords
vascular inflammation, cardiovascular disease, dental plaque, risk factors, oxidative stress, antioxidants, NLRP3 inflammasome, periodontal disease (PD)
Citation
Paul O, Arora P, Mayer M and Chatterjee S (2021) Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 11:609614. doi: 10.3389/fphys.2020.609614
Received
24 September 2020
Accepted
21 December 2020
Published
14 January 2021
Volume
11 - 2020
Edited by
John D. Imig, Medical College of Wisconsin, United States
Reviewed by
Dorina Lauritano, University of Milano-Bicocca, Italy; Tomoko Kurita-ochiai, Nihon University School of Dentistry at Matsudo, Japan
Updates
Copyright
© 2021 Paul, Arora, Mayer and Chatterjee.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shampa Chatterjee, shampac@pennmedicine.upenn.edu
This article was submitted to Vascular Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.