Abstract
Understanding hypoxia/hyperoxia exposure requires either a high-altitude research facility or a chamber in which gas concentrations are precisely and reproducibly controlled. Hypoxia-induced conditions such as hypoxic-ischemic encephalopathy (HIE), obstructive or central apneas, and ischemic stroke present unique challenges for the development of models with acute or chronic hypoxia exposure. Many murine models exist to study these conditions; however, there are a variety of different hypoxia exposure protocols used across laboratories. Experimental equipment for hypoxia exposure typically includes flow regulators, nitrogen concentrators, and premix oxygen/nitrogen tanks. Commercial hypoxia/hyperoxia chambers with environmental monitoring are incredibly expensive and require proprietary software with subscription fees or highly expensive software licenses. Limitations exist in these systems as most are single animal systems and not designed for extended or intermittent hypoxia exposure. We have developed a simple hypoxia chamber with off-the-shelf components, and controlled by open-source software for continuous data acquisition of oxygen levels and other environmental factors (temperature, humidity, pressure, light, sound, etc.). Our chamber can accommodate up to two mouse cages and one rat cage at any oxygen level needed, when using a nitrogen concentrator or premixed oxygen/nitrogen tank with a flow regulator, but is also scalable. Our system uses a Python-based script to save data in a text file using modules from the sensor vendor. We utilized Python or R scripts for data analysis, and we have provided examples of data analysis scripts and acquired data for extended exposure periods (≤7 days). By using FLOS (Free-Libre and open-source) software and hardware, we have developed a low-cost and customizable system that can be used for a variety of exposure protocols. This hypoxia/hyperoxia exposure chamber allows for reproducible and transparent data acquisition and increased consistency with a high degree of customization for each experimenter’s needs.
Introduction
Chronic and acute hypoxia exposure has been used in the past to study many different diseases, including hypoxic-ischemic encephalopathy (HIE) (Lacaille et al., 2019), intermittent sleep apnea (Toth and Bhargava, 2013; Arias-Cavieres et al., 2020; Lebek et al., 2020; Arias-Cavieres et al., 2021), ischemic stroke (Tsuji et al., 2013) and epilepsy (Toth and Bhargava, 2013; Lebek et al., 2020). Many studies use plethysmography (whole body and head-out) to assess short-term hypoxia and acquire other physiological data (Toth and Bhargava, 2013; Lebek et al., 2020). However, commercial plethysmography systems are comparatively expensive, typically require proprietary software for recording and monitoring pressure, volume, and flow, and may be difficult to set up without extensive technical support. Investigators focused on hyperoxia- or hypoxia-induced pathologies (neonatal HIE and stroke for example) often utilize simple hypoxia exposure systems that have rudimentary recording and environmental control (Yager, 2004). Experiments using hypoxia/hyperoxia exposure in animal models have used a variety of exposure times, oxygen concentrations, and other variables—contributing to high variability in neurological injury and making it difficult to determine the optimal hypoxia exposure settings for a given disease model. Because of this variability, there are significant challenges to reproducing experiments from different laboratories. In our laboratory, we have focused on HIE in preterm infants and have found it challenging to develop a reproducible model that will facilitate development of new HIE treatment, as have others (Rumajogee et al., 2016).
Our goal in this paper is to make our inexpensive, highly customizable open-source hypoxia chamber available to other investigators. Our chamber is useful across multiple experimental applications including: simulation of high altitudes, hypoxic ischemic stroke, sleep apnea, and other models requiring precise monitoring and control of the environment. Researchers studying HIE commonly use two types of exposures: long-chronic exposure or short-term hypoxia exposure (Vannucci and Vannucci, 2005; Lacaille et al., 2019). These exposures, however, also require animals’ homeostasis to be regulated by the use of external environment regulation. For example, the Rice-Vanucci Model pups undergo 10% oxygen exposure for one to 2 hours. They must also be kept at a constant temperature to reduce experimental variability (Vannucci and Vannucci, 2005). In Lacaille et al. (2019), long-term hypoxia requires monitoring and regulation to help stabilize the animals through a 7-day chronic hypoxia exposure (Lacaille et al., 2019). However, inconsistencies in factors other than oxygen level result in outcomes with a range of severity. To help reduce this variability in outcome, regulating and recording environmental variables continuously—such as temperature and humidity—can reduce data variability (Li et al., 2009; Summa et al., 2012). For example, the temperature should be monitored during hypoxia exposure as previous work shows that mice at 22°C ambient temperature had low plasma triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C) levels that increased during intermittent hypoxia while mice at an ambient temperature of 30°C had high plasma TG and LDL-C levels that did not increase during intermittent hypoxia (Jun et al., 2013). Thus, these investigators concluded that ambient temperature affects lipid metabolism during hypoxia exposure (Jun et al., 2013).
Humidity is also an important variable to quantify when performing hypoxia testing. In moist air, mice’s metabolic rate was significantly lower than in dry air since evaporating the air’s moisture requires heat and would increase the energy needed for respiratory and metabolic processes (Phillips et al., 1950; Xiao et al., 2020). As a result, they found that, in dry air, less oxygen was required for respiratory and metabolic processes, which delayed the effects of hypoxia compared to in more humid air (Phillips et al., 1950). Additionally, others have found that heat stress and humidity can affect lactation in rodents (Xiao et al., 2020). Alterations to animal lactation further demonstrate the critical need to monitor and regulate temperature and humidity. The relationship between chamber oxygen pressure and arterial oxygen levels (Ogawa et al., 2019) still requires further investigation; however, barometric pressure may also play a role in batch variability.
Sound monitoring within the chamber is another key variable that should be monitored to minimize the possibility of hearing loss or sound-induced stress. Exposure to auditory stress during pregnancy in mice affected reabsorption and pup survival (Jafari et al., 2017). Likewise, light levels and day/night cycles should be monitored while the mice undergo intermittent or chronic hypoxia testing as, when combined with dim light at night, mice are more likely to exhibit anxiety and depression-like behavior (Aubrecht et al., 2013). Other studies have shown that, during hypoxia exposure, dams show depressive-like behaviors that are only observed in female rats (Kanekar et al., 2015). Thus if the mice are exposed to dim light at night during the hypoxia runs, other depression-like behaviors can serve as a confounding variable.
Monitoring oxygen levels is key for assuring reproducible hypoxia exposure, since the concentration of oxygen in the chamber must be reduced (Wenger et al., 2015) and tightly controlled. Wenger and others suggest that it can require hours to achieve the desired hypoxic conditions, but hypoxia levels can be rapidly altered by a brief opening of the hypoxia chamber to disrupt the chamber’s hypoxic conditions (Wenger et al., 2015). Others have found that exhaling breath with high levels of volatile organic compounds (VOC) occurs during reduced oxygen levels (Harshman et al., 2015). By ensuring that VOC concentration is similar across hypoxia runs, it is possible to ensure that hypoxic conditions do not vary between hypoxia exposures.
To reduce the cost and provide maximum customization in our chamber design, we have provided a detailed overview of our chamber and supporting software that relies upon inexpensive off-the-shelf components allowing investigators high-resolution monitoring and control over temperature, humidity, and oxygen levels without the need for proprietary software or custom fabricated equipment. We developed a system based on Free/Libre Open-Source (FLOS) principles so others can use the system for greater reproducibility experiments by labs of any size and funding status. By creating a system to monitor and modulate these environmental factors, we can unify these surgical models and their hypoxia exposures (Henderson et al., 2018). Our goal here is to create an inexpensive system for controlled gas exposure that will lower the bar of entry for other investigators interested in understanding physiological changes induced by hypoxia/hyperoxia.
Methods
Chamber Components
Exposure to hypoxia during a surgical procedure elicits a stress response from the animals exposed. Therefore, to reduce and quantify variability in environmental factors, we assembled a comprehensive set of tools to monitor environmental variables and maintain them within desired ranges for a given experiment. A complete list of the parts and components of the chamber are included in the Supplemental Material and Table 1. Additionally, the Python code, PCB file, 3D files and instructions for chamber assembly can be accessed on github.com (https://github.com/drcgw/hypox-chamber.git).
TABLE 1
| Cage Aspect | Item | Description | Company | Part Number | Variable, Accuracy, Percision | Quantity Used | Quantity per Unit | Cost Per Unit | |
|---|---|---|---|---|---|---|---|---|---|
| Cage Structure | |||||||||
| Glass Tank | Aqueon Glass Aquarium Tank - 20 Gallon | PetCo | — | Chamber | 1 | 1 | $ 49.99 | ||
| Glass Tinting | One-way window film | Amazon | — | Visual Stimulus Reduction | 1 | 1 | $ 11.99 | ||
| Acrylic Sheet | Chamber top - 1/4 x 31.5 x 14.25 in | ACME Plastics | — | Chamber Lid | 1 | 1 | $ 45.00 | ||
| Junction Box | Steel Junction Box, 254 x 254 x 101.6 mm | Newark | 40T7867 | Electrical Input | 1 | 1 | $ 31.60 | ||
| Cable Management | 3.35 inch black cable straps | Amazon | B0881FW8TQ | Electrical Management | Variable | 50 | $ 9.99 | ||
| Airflow | |||||||||
| Bulkhead | 1/2 in female PVC bulkhead fitting | Amazon | — | Airflow - In | 2 | 2 | $ 12.49 | ||
| PVC Tubing | 1/2 in FPT T joint | Home Depot | — | Airflow - In | 3 | 1 | $ 1 - 3 | ||
| — | 1/2 in MPT Valve | Home Depot | — | Airflow - In | 2 | 1 | $ 1 - 3 | ||
| — | 1/2 in MPT Adapters (1/4, 5 and 10 in) | Home Depot | — | Airflow - In | 8 | 1 | $ 1 - 3 | ||
| — | 1/2 in MPT/FPT Elbow | Home Depot | — | Airflow - In | 6 | 1 | $ 1 - 3 | ||
| Brass Tubing | 1/4 in Bulkhead | Amazon | — | Airflow - Out | 2 | 2 | $ 12.89 | ||
| — | 1/4 in R-angle Elbow | Amazon | — | Airflow - Out | 2 | 2 | $ 14.59 | ||
| — | 1/4 in Control Valve | Amazon | — | Airflow - Out | 2 | 2 | $ 14.99 | ||
| Electrical | |||||||||
| Sensor PCB* | Custom PCB boards for sensor attachment | OSH Park | Sensor Mount | 5 | 5 | $ 102.50 | |||
| Raspberry Pi** | Raspberry Pi 4B, 2 GB RAM | Adafruit | 4292 | Microprocessor | 1 | 1 | $ 45.00 | ||
| PiCamera | Raspberry Pi Camera 2 | Adafruit | 3099 | Image (jpg) (8 megapixel) | 1 | 1 | $ 29.95 | ||
| Power Supply | Adjustable DC Powersupply 110 V - 220 V, Output: 0 - 24 V, 20 A 480 W | Amazon | B08GFSVHLS | Power (Percision: 0.1 V) | 1 | 1 | $ 35.99 | ||
| MCP9808 | High accuracy I2°C Temperature Sensor | Adafruit | 1782 | Temperature (Accuracy: ± 0.25°C, Percision: ± 0.0625°C) | 3 | 1 | $ 4.95 | ||
| BH1750 | Light Sensor I2°C | Adafruit | 4681 | Light (Lux) | 2 | 1 | $ 4.50 | ||
| BME280 | Temperature, Humidity & Pressure sensor I2°C | Adafruit | 2652 | Temperature (± 1.0°C), Humidity (± 3%), Pressure (± 1.0 hPa) | 2 | 1 | $ 14.95 | ||
| CCS811 | VOC and eCO2 Sensor | Adafruit | 3566 | CO2 & VOC | 2 | 1 | $ 19.95 | ||
| ADS1115 | 4 Channel 16 bit Analog to Digital Converter | Adafruit | 1085 | Converter (Percision 8-860 SPS) | 1 | 1 | $ 14.95 | ||
| Voltage Regulator | 3.3 V 800 mA Voltage regulator | Adafruit | 2165 | Voltage (Accuracy: ± 1%) | 5 | 1 | $ 1.25 | ||
| 0.1 uF Capacitor | 0.1 uF capacitor, max of 50 V | Adafruit | 753 | Voltage (Accuracy: -20%/ + 80%) | 5 | 10 | $ 1.95 | ||
| 10 uF Capacitor | 10 uF capacitor, max 50°V low-fequency | Adafruit | 2195 | Voltage (± 20%) | 5 | 10 | $ 1.95 | ||
| Power Jack | Gravitech DC Power Connector 2.1 mm x 5.5 mm | Mouser | 992-CON-SOCJ-2155 | Voltage | 5 | 1 | $ 1.00 | ||
| D-Sub 15 Female | D-Sub High Density DBHD15, Female | Amazon | CNR15HDM-F-10PACK | Connector | 8 | 10 | $ 12.88 | ||
| VGA Cables | UGREEN VGA, 3 meter, male to male coaxial | Amazon | B00OZL3HLO | Cable | 4 | 1 | $ 7.99 | ||
| USB Cables | Micro-USB Cable, R-angle joint | Amazon | B09C5P7YFN | Cable | 1 | 3 | $ 9.99 | ||
| USB Cables | USB-C Cable, R-angle joint | Amazon | B09CG9LZSR | Cable | 1 | 2 | $ 9.99 | ||
| DC Power Cable | DC Power Pigtail, 3ft, Male 5.5 mm x 2.1 mm plug | Amazon | B08PYWN3T7 | Cable | 4 | 2 | $ 9.99 | ||
| DC Cable Extender | 6 ft DC Extention Cord, male to female | Amazon | B074WJZNZD | Cable | 3 | 4 | $ 13.99 | ||
Major components and prices included in the chamber creation as of June 2022.
Can be replace with breadboards and jumper cables.
Can also be run with Raspberry Pi 3B, 4B - 1 GB, 4B - 4 GB or 4B - 8 GB.
Additional hardware and custom parts can be found online at: https://github.com/drcgw/hypox-chamber.git and in the Supplemental Material.
Systems Recording
Temperature calibration was achieved by periodically taking temperature measurements with MCP9808, BME280, and/or BME680 sensors. Depending on the needs of the researcher we have designed the chamber to have some redundancy. The MCP9808 is a high precision (+0.0625°C) temperature sensor with a range between -40 and 125°C and accuracy of ±0.25 °C (Ada, 2021). In contrast the BME280 and BME680 are able to detect multiple variables including: temperature, humidity, pressure and total volatile organic compounds (Only BME680) but lose precision and accuracy. The accuracy and range of the BME280s sensors are: relative humidity ±3% between 0 and 100%, barometric pressure ±1 hPa between 300 and 1,100 hPa and temperature ±1.0 °C between -40 and 85 °C (Ada et al., 2015). The accuracy of the BME680s sensors are the same as the BME280 with the addition of a heated metal oxide resistor to look at total volatile organic compounds (tVOC) (Ada and Rembor, 2017). The BME280/680 sensors also have limitations regarding how many of each sensor type can be attached due to limited I2C addresses available for each sensor. The MCP9808 has eight possible addresses whereas the BME280 and BME680 only contain two I2C addresses which are duplicated for the two sensor boards (Ada and Montoya, 2017). For our chamber we utilize both MCP9808 and BME280 despite the overlapping temperature variable, we utilize the MCP9808’s precision to calibrate the BME280 however, utilization of any of these three sensors will also allow for complete data collection. After these measurements were taken, the PiCamera was programmed to take a picture of a mercury thermometer located within the chamber (Figure 1A). After collecting the data, these images were used to determine the actual temperature in the chamber. The actual temperature in the chamber was then compared with the sensor readings to determine the precision of the sensors and calibrate the temperature sensors accordingly. Using a mercury thermometer for chamber monitoring is not practical because it violates animal health and well-being requirements and lab safety regulations.
FIGURE 1
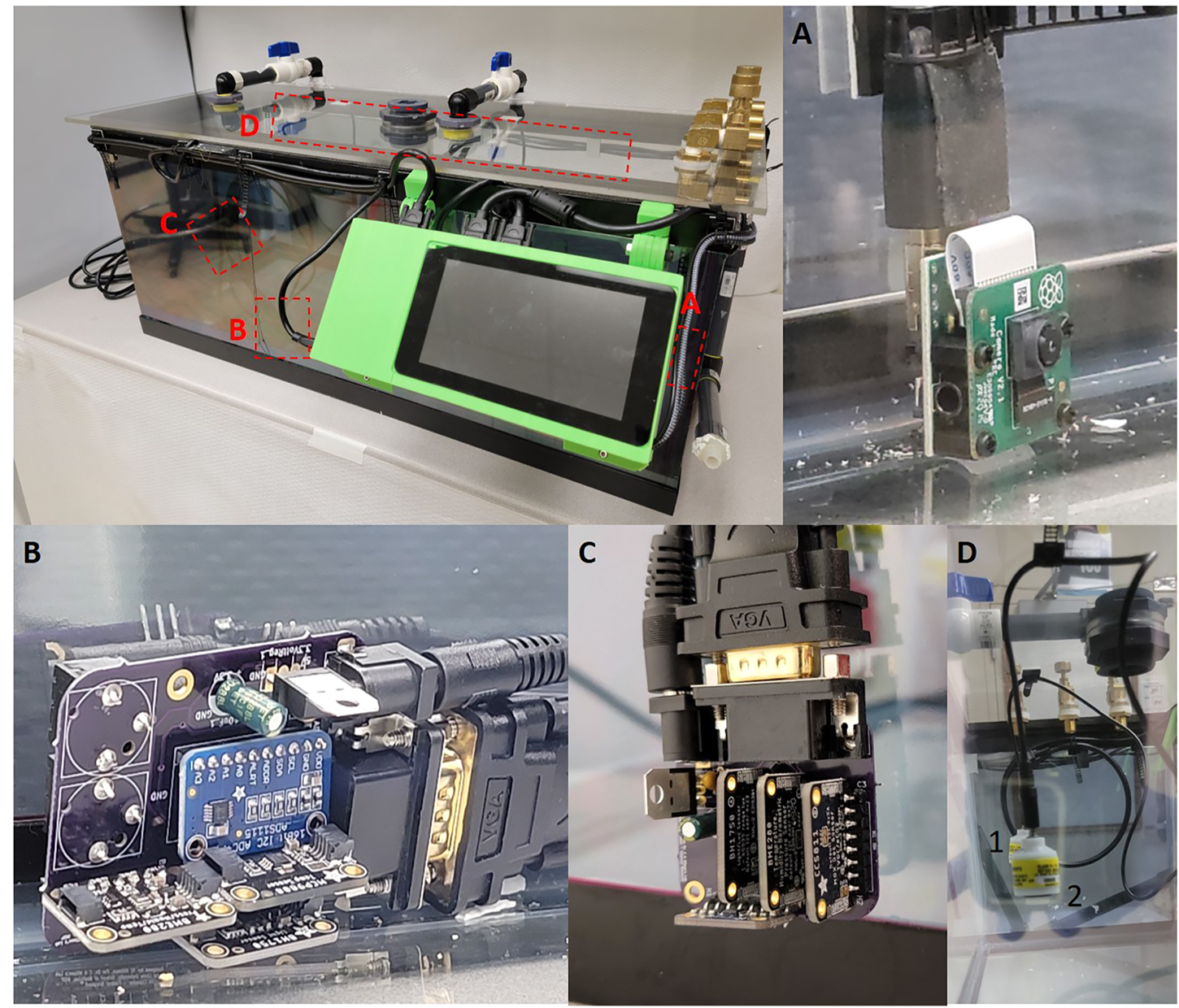
(A) Raspberry Pi Camera version two attached to the Raspberry Pi via a HDMI cable. (B) Central sensor PCB board with ADS1115, MCP9808, BME280 and BH1750 sensors, the rear of the board contains two Aux jacks which allow for Teledyne sensor attachment. (C) External sensor breakout which contains MCP9808, BME280, BH1750 and CCS811. (D) Teledyne oxygen sensors hung from the roof of the chamber utilizing cable management clips. Two sensors are held by cables clips on the sealing, identified by the one and two identifiers.
Oxygen detection and calibration was done by pulling the voltage from a Teledyne R17A oxygen sensor using an analog-to-digital converter (ADS1115). The Teledyne R17A has a functional range of 0–100% oxygen at 25°C with a resolution of 0.1% oxygen (Model R-17A, 2022). The voltage being recorded by the ADS1115 gets converted using the oxygen calibration data. Oxygen calibration was achieved by connecting a Teledyne R17A oxygen sensor to a hose fitting attached to a flow regulator to generate oxygen concentrations of 30, 15, 10, and 5% oxygen to develop a standard curve. Additionally, if a flow regulator is not available, the oxygen sensor can also be calibrated with premix tanks (10 & 5%) and room air (20.9%), although room oxygen is unreliable. To generate a standard curve using oxygen tanks, we attach one of the oxygen sensors to a manifold and tubing connected to tanks with different gas mixtures and expose the sensor to a given O2 concentration for 10 min (Teledyne recommendation). The oxygen calibration script will take a series of samples every 10 s and then average the voltages to get a voltage value for the concentration being exposed. Repeat the process for at least three O2 concentrations then create a standard curve to be used in percentage calculations. Once we established the calibration curve, the oxygen sensor’s output was measured and calculated based on the standard curve to determine the actual oxygen concentration (Figure 1D).
Light calibration is essential for quantifying light/dark cycle and understanding changes in physiology related to diurnal cycling. Obtaining the light by measuring lux allows us to determine if precise day/night cycles are adhered to within our animal care facility, again as a way to control the chamber environment and reduce treatment variability. To detect light the system utilizes adafruit BH1750 sensor boards (Siepert, 2021). The sensor board has a range from 0.11 lux minimum to 100,000 lux minimum with a noise reduction of 50–60 Hz. The sensor variation is ±20% with minimal infrared interference (Siepert, 2021). Additionally, the chamber walls were treated with a one-way mirror reflective coating to help minimize animals’ perception of handlers during the hypoxia exposures (Figure 2). Any variant of one way mirror film can be used to treat the chamber walls.
FIGURE 2
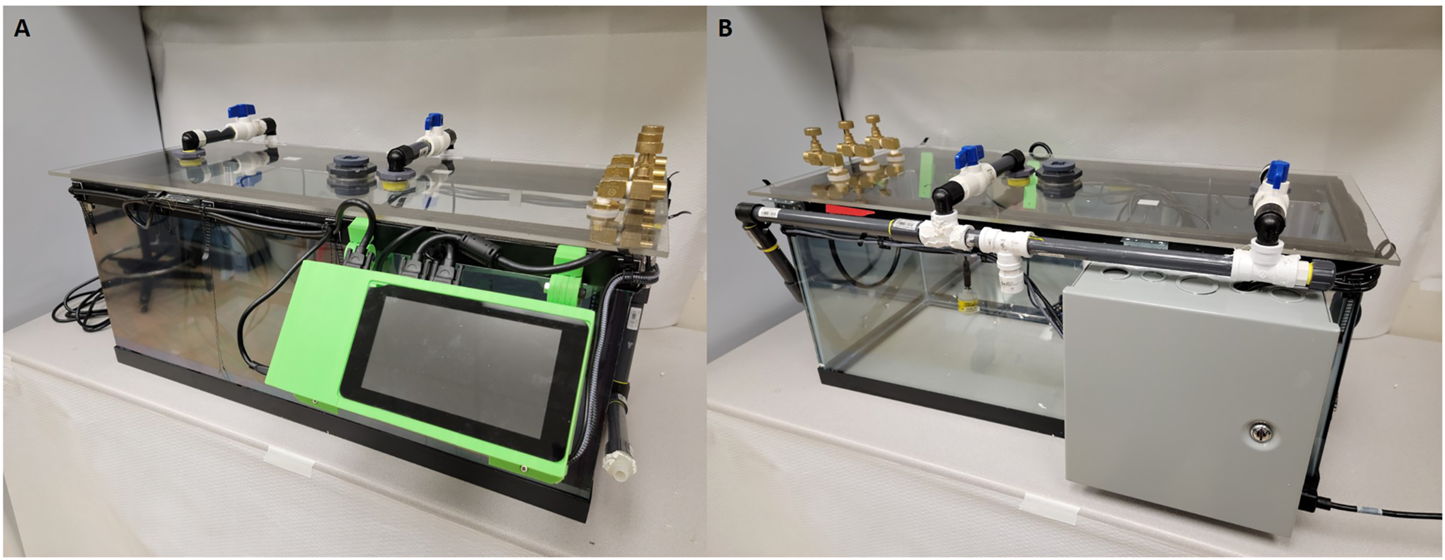
Images of current cage design including updated sensors and revised power supply. (A) Front view of the chamber with a 7 inch Raspberry Pi touch screen and visible airflow systems. (B) Rear view of the chamber with power supply container and airflow input tubes visible.
Data Acquisition
Each sensor has its own function written in a master Python program which specifies the data recording resolution and interval that each sensor measures. These values are assigned to variables and it is easy to calculate calibration curves and obtain precise, calibrated measurements for each experimental condition measured by the on-board sensors. Our data acquisition cycle for the chamber is episodic every (10 min), using a function that saves the start and end times from the Raspberry Pi system clock and then calculates the time required to collect all the data and average over the 10 min epoch. The sampling rate can be readily adjusted within the Python program by changing the sampling interval. The data is acquired and stored throughout each 10 min acquisition period. All data is saved as comma-separated ASCII text files (.CSV) with values for raw, unprocessed data, and processed data with specific measurements. Storing data in a text file allows a great deal of flexibility since data analysis can be performed using Python, R, MATLAB or other analysis software. The caveat is, of course, that text files take up more disk space than binary files. It would be easy to add a compression function to make the files smaller but that is not currently included in our code as we use gzip or bzip2 to batch compress data files after checking them. The sensors are mounted on custom PCB boards (Central, Top, and External), which connect all the sensors back to the RaspberryPi via VGA cables (Figures 1B, C) which provide excellent shielding in a compact form factor.
The chamber is designed with a breakout PCB board to convert the 40 pinouts to four VGA connectors allowing for four different sensor PCB breakout boards to be attached depending on researcher needs.
Results and Analysis
Chamber monitoring allows us to record and monitor the environmental conditions within the chamber during extended hypoxia exposure, providing the ability to make real-time adjustments to the chamber’s environment. During preliminary tests of the chamber, graphical time-series plots were generated to compare temperature, humidity, oxygen levels (Figure 3). Python and R were used to analyze and summarize chamber temperature and oxygen level data and identify changes in environmental conditions. We utilized the Raspbian operating system (based on Debian GNU/Linux), which provides remote access to the chamber software/hardware using secure shell (ssh). With the Python script collating reported values in a text file, custom analysis can be done using a variety of programs, including Python, R, Excel, LibreOffice, GraphPad Prism, etc. according to the user’s preferences.
FIGURE 3
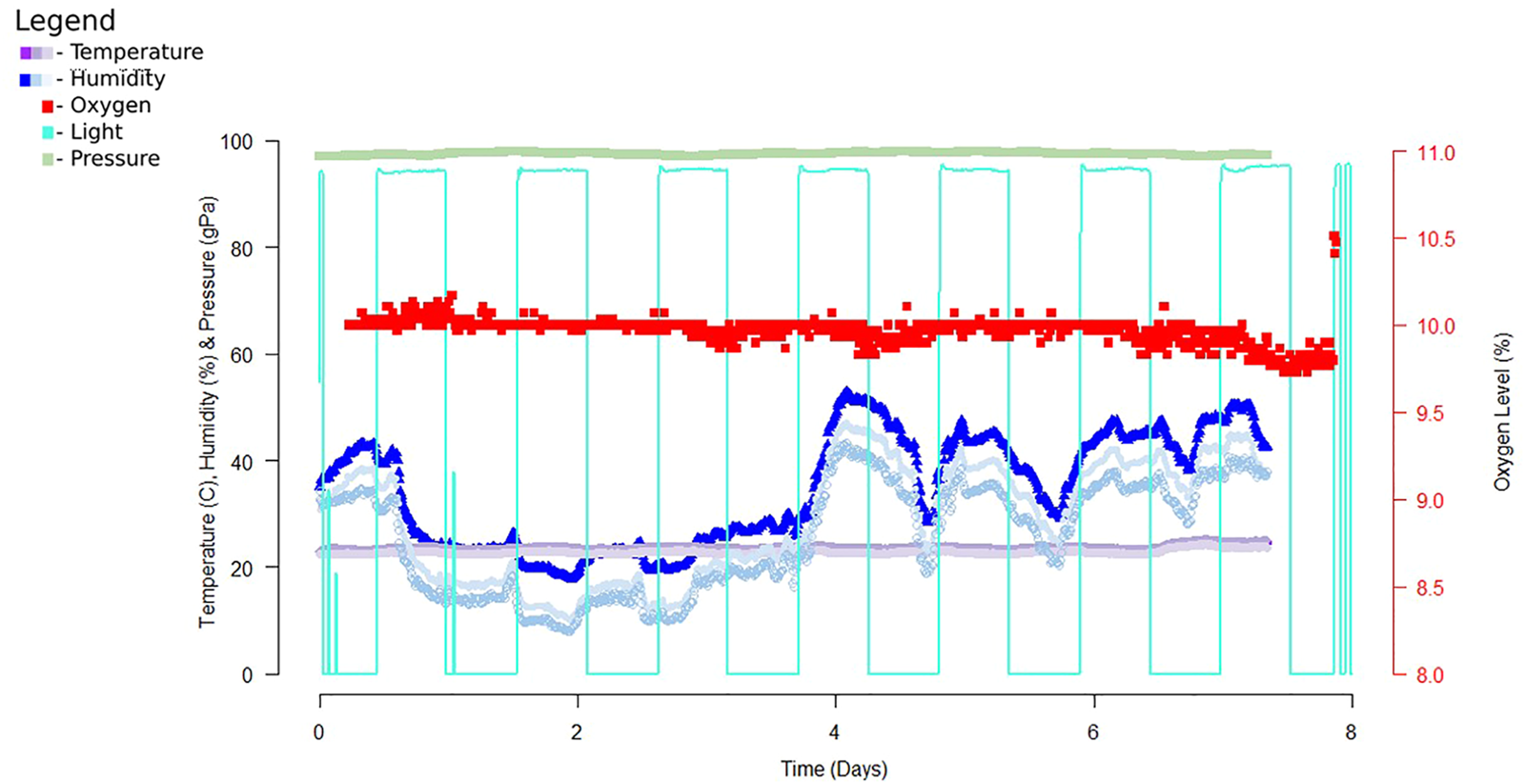
Example data from a 7-day exposure of the chamber. Data was recorded using a Python script then analyzed with R studio. The data recorded above reports data collected from early experiments utilizing DHT22, ADS1115 and Teledyne oxygen sensor, TSL2591, and MPL3115A2. For this dataset we recorded data every 10 minutes for the entire 7 day exposure time. Since completion of this sampling we replaced these sensors with sensors of higher accuracy and precision.
In our analysis, we focused on identifying time points where oxygen deviated from our tolerance of 10 ± 0.5% O2. Deviations from our target oxygen levels were compared to other environmental variables to determine if a change in the environment was altering oxygen levels. In order to get accurate readings, oxygen concentration was determined by two-point calibration of the sensor using a vendor-supplied tank with a custom oxygen mix. The data collected provides a post-exposure view of the environmental condition of the chamber. As shown in Figure 2, the chamber allows for a controlled environment that can house multiple cages, for consistent exposure across several animals simultaneously, allowing us to reduce variability between exposure groups.
Control of Environment Variables
Environmental regulation is important in any experiment and being able to record multiple environmental variables can facilitate reproducibility and reliability of data. During early experiments, we found that a consistent temperature and oxygen levels led to higher survival rates in our experimental animals. This observation was supported by previous studies showing that a cold environment can lead to increased stress and affect the immune system (Meyer et al., 2007; Hankenson et al., 2018). During our hypoxia exposures, the animals have already undergone an immune challenge, so the addition of temperature stress can increase the stress on experimental animals in a given protocol. Due to this, we wished to monitor and control as chamber environment variables as possible. Environmental stress during development has been attributed to psychological stress in rodents as well, so minimizing this stress is critical in most studies (Takuma et al., 2011). The animals’ environment has also been shown to affect their immune response when exposed to an inflammatory challenge (e.g. a “two hit” protocol or more complicated exposure paradigm); thus it is important to provide a controlled environment for experiments in which animals are exposed to stressors (Mueller et al., 2018).
Finally, our goal has been to provide an affordable chamber with supporting hardware and software for high-resolution data acquisition and reproducible results for experiments requiring O2/CO2/N2 gas and monitoring of other environmental variables. By monitoring the environment for experimental animals, we provide a platform for more reproducible and cost-effective experiments. Alternatives that are currently used include plethysmography chambers, both head-out and whole-body, for monitoring breathing during hypoxia/hyperoxia exposure. Our open-source system provides a more flexible foundation for rigor, transparency, and reproducibility. Commercial exposure systems can be very expensive and the investigator is often limited by their budget for multiple animal exposures. Our system lowers the cost of multiple animal exposures significantly while providing high-resolution data from the suite of sensors we have specified. We are not suggesting that our system replaces more sophisticated commercial exposure systems but, instead, provides a cost-effective alternative. Commercial plethysmography systems can provide a more extensive array of sensors and data acquisition (Ortega-Porcayo et al., 2014), and we will continue to develop and expand upon our open-source system. Finally, the ability of an open-source system allows for high customizability of the apparatus for each researcher’s needs. Collaboration between labs utilizing this system can help us improve the chamber and control system while also allowing for new laboratories to develop their own custom systems (Supplementary Table S1).
Future Directions
The hypoxia chamber we describe has the ability to monitor and acquire data at high resolution and provide a way to standardize exposure protocols inexpensively by virtue of FLOS hardware and software. The chamber hardware and software are in constant development and we look forward to feedback for the development of additional features. An additional feature that would enhance the flexibility of our system would be to add a feedback controlled humidifier to control humidity in the chamber. A movable camera would also provide enhanced monitoring of experimental animals and a way to assess behavior and health of the animals. We also could add sound frequency and pressure measurements within the chamber. The addition of a white-noise sound suppression system to reduce the effect of environmental noise within the chamber and could mask environmental noise that might be a stressor. Including more sophisticated temperature control may be useful for some investigators and that should be possible with minimal changes to the current control circuitry and software. Finally, we intend to develop a graphical user interface (GUI) front-end to the control program to make it easier for non-technical users to use. While the chamber has several improvements that are in progress, the current system allows investigators with limited budgets to develop flexible exposure protocols and easily acquire environmental data. Even a minimal monitoring set-up will allow for a more uniform exposure system across typical hypoxia-based studies (Vannucci and Vannucci, 2005; Gunn and Bennet, 2009; Lacaille et al., 2019).
Conclusion
We have described a low-cost hypoxia exposure chamber that allows for high-resolution data monitoring and reporting of environmental status. Our goal in developing this chamber and associated control systems, was to provide a highly reproducible, FLOS system to facilitate low-cost physiology experiments. Use of FLOS software and hardware allows for improvements, customization, and tailoring of the chamber and software for specific experimental conditions as needed in each laboratory. We hope that, by providing this system, we can stimulate collaboration and continuous development of the chamber hardware and software. We designed the system to have a usable, minimum set of sensors acquiring temperature, humidity, light, pressure, tVOC and oxygen levels. In the future, we hope to include real-time movement monitoring to make the system more attractive to a wider range of investigators as these factors have a significant impact on experimental animals (Mueller et al., 2018). We will continue to develop this system and seek input from other investigators to maximize the system’s versatility and usefulness for open, reproducible science. Our hope is that other investigators will benefit from our work and contribute to our on-going efforts to improve and expand upon the chamber hardware and software.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/drcgw/hypox-chamber.
Ethics statement
The animal study was reviewed and approved by the Loma Linda University Institutional Animal Care and Use Committee.
Author contributions
TH and CW conceived of the chamber and the ideas presented in the manuscript. RI and TH wrote the first draft of the manuscript, developed code, assembled the chamber, and collected all data presented in the manuscript. CW performed final edits.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.891005/full#supplementary-material
References
1
AdaL. (2021). Adafruit MCP9808 Precision I2C Temperature Sensor Guide.
2
AdaL.DiColaT.BearnesB.NeedellG.HerradaE.MontoyaJ. (2015). Adafruit BME280 Humidity + Barometric Pressure + Temperature Sensor Breakout.
3
AdaL.MontoyaJ. (2017). I2C Addresses! the List. Available at: https://learn.adafruit.com/i2c-addresses/the-list (Accessed June 2022).
4
AdaL.RemborK. (2017). Adafruit BME680. Available at: https://learn.adafruit.com/adafruit-bme680-humidity-temperature-barometic-pressure-voc-gas (Accessed June 2022).
5
Arias-CavieresA.FontehA.Castro-RiveraC. I.GarciaA. J. (2021). Intermittent Hypoxia Causes Targeted Disruption to NMDA Receptor Dependent Synaptic Plasticity in Area CA1 of the hippocampus. Exp. Neurol.344, 113808. 10.1016/j.expneurol.2021.113808
6
Arias-CavieresA.KhuuM. A.NwakuduC. U.BarnardJ. E.DalginG.GarciaA. J. (2020). A HIF1a-dependent Pro-oxidant State Disrupts Synaptic Plasticity and Impairs Spatial Memory in Response to Intermittent Hypoxia. ENeuro7. ENEURO.0024-20.2020. 10.1523/ENEURO.0024-20.2020
7
AubrechtT. G.WeilZ. M.MagalangU. J.NelsonR. J. (2013). Dim Light at Night Interacts with Intermittent Hypoxia to Alter Cognitive and Affective Responses. Am. J. Physiology-Regulatory, Integr. Comp. Physiology305, R78–R86. 10.1152/ajpregu.00100.2013
8
GunnA. J.BennetL. (2009). Fetal Hypoxia Insults and Patterns of Brain Injury: Insights from Animal Models. Clin. Perinatology36, 579–593. 10.1016/j.clp.2009.06.007
9
HankensonF. C.MarxJ. O.GordonC. J.DavidJ. M. (2018). Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp. Med.68, 425–438. 10.30802/AALAS-CM-18-000049
10
HarshmanS. W.GeierB. A.FanM.RinehardtS.WattsB. S.DrummondL. A.et al (2015). The Identification of Hypoxia Biomarkers from Exhaled Breath under Normobaric Conditions. J. Breath. Res.9, 047103. 10.1088/1752-7155/9/4/047103
11
HendersonP.IslamR.BachmanP.PineauJ.PrecupD.MegerD. (2018). Deep Reinforcement Learning that Matters. Proc. AAAI Conf. Artif. Intell.32. 10.1609/aaai.v32i1.11694
12
JafariZ.FarajiJ.Mirza AghaB.MetzG. A. S.KolbB. E.MohajeraniM. H. (2017). The Adverse Effects of Auditory Stress on Mouse Uterus Receptivity and Behaviour. Sci. Rep.7, 4720. 10.1038/s41598-017-04943-8
13
JunJ. C.ShinM.-K.YaoQ.DeveraR.Fonti-BevansS.PolotskyV. Y. (2013). Thermoneutrality Modifies the Impact of Hypoxia on Lipid Metabolism. Am. J. Physiology-Endocrinology Metabolism304, E424–E435. 10.1152/ajpendo.00515.2012
14
KanekarS.BogdanovaO. V.OlsonP. R.SungY.-H.D'AnciK. E.RenshawP. F. (2015). Hypobaric Hypoxia Induces Depression-like Behavior in Female Sprague-Dawley Rats, but Not in Males. High Alt. Med. Biol.16, 52–60. 10.1089/ham.2014.1070
15
LacailleH.VacherC.-M.BakalarD.O’ReillyJ. J.SalzbankJ.PennA. A. (2019). Impaired Interneuron Development in a Novel Model of Neonatal Brain Injury. ENeuro6, 0300–0318. 10.1523/ENEURO.0300-18.2019
16
LebekS.HegnerP.SchachC.ReuthnerK.TafelmeierM.MaierL. S.et al (2020). A Novel Mouse Model of Obstructive Sleep Apnea by Bulking Agent-Induced Tongue Enlargement Results in Left Ventricular Contractile Dysfunction. PloS One15, e0243844. 10.1371/journal.pone.0243844
17
LiQ.CheungC.WeiR.HuiE. S.FeldonJ.MeyerU.et al (2009). Prenatal Immune Challenge Is an Environmental Risk Factor for Brain and Behavior Change Relevant to Schizophrenia: Evidence from MRI in a Mouse Model. PLOS ONE4, e6354. 10.1371/journal.pone.0006354
18
MeyerU.YeeB. K.FeldonJ. (2007). The Neurodevelopmental Impact of Prenatal Infections at Different Times of Pregnancy: the Earlier the Worse?Neuroscientist13, 241–256. 10.1177/1073858406296401
19
Model R-17A (2022). R-17A Automotive Oxygen Sensor. Available at: https://www.teledyne-ai.com/Products/Oxygen-Sensors/Pages/R17A.aspx (Accessed June 2022).
20
MuellerF. S.PoleselM.RichettoJ.MeyerU.Weber-StadlbauerU. (2018). Mouse Models of Maternal Immune Activation: Mind Your Caging System!. Brain, Behav. Immun.73, 643–660. 10.1016/j.bbi.2018.07.014
21
OgawaT.FujiiN.KurimotoY.NishiyasuT. (2019). Effect of Hypobaria on Maximal Ventilation, Oxygen Uptake, and Exercise Performance during Running under Hypobaric Normoxic Conditions. Physiol. Rep.7, e14002. 10.14814/phy2.14002
22
Ortega-PorcayoL. A.Cabrera-AldanaE. E.Arriada-MendicoaN.Gómez-AmadorJ. L.Granados-GarcíaM.Barges-CollJ. (2014). Operative Technique for En Bloc Resection of Upper Cervical Chordomas: Extended Transoral Transmandibular Approach and Multilevel Reconstruction. Asian Spine J.8, 820–826. 10.4184/asj.2014.8.6.820
23
PhillipsN. E.SaxonP. A.QuimbyF. H. (1950). Effect of Humidity and Temperature on the Survival of Albino Mice Exposed to Low Atmospheric Pressure. Am. J. Physiology-Legacy Content161, 307–311. 10.1152/ajplegacy.1950.161.2.307
24
RumajogeeP.BregmanT.MillerS. P.YagerJ. Y.FehlingsM. G. (2016). Rodent Hypoxia–Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol.7, 57. 10.3389/fneur.2016.00057
25
SiepertB. (2021). Adafruit BH1750 Ambient Light Sensor.
26
SummaK. C.VitaternaM. H.TurekF. W. (2012). Environmental Perturbation of the Circadian Clock Disrupts Pregnancy in the Mouse. PLOS ONE7, e37668. 10.1371/journal.pone.0037668
27
TakumaK.AgoY.MatsudaT. (2011). Preventive Effects of an Enriched Environment on Rodent Psychiatric Disorder Models. J. Pharmacol. Sci.117, 71–76. 10.1254/jphs.11r07cp
28
TothL. A.BhargavaP. (2013). Animal Models of Sleep Disorders. Comp. Med.63, 91–104.
29
TsujiM.OhshimaM.TaguchiA.KasaharaY.IkedaT.MatsuyamaT. (2013). A Novel Reproducible Model of Neonatal Stroke in Mice: Comparison with a Hypoxia-Ischemia Model. Exp. Neurol.247, 218–225. 10.1016/j.expneurol.2013.04.015
30
VannucciR. C.VannucciS. J. (2005). Perinatal Hypoxic-Ischemic Brain Damage: Evolution of an Animal Model. Dev. Neurosci.27, 81–86. 10.1159/000085978
31
WengerR.KurtcuogluV.ScholzC.MartiH.HoogewijsD. (2015). Frequently Asked Questions in Hypoxia Research. Hypoxia (Auckl)3, 35–43. 10.2147/HP.S92198
32
XiaoY.KronenfeldJ. M.RenquistB. J. (2020). Feed Intake-dependent and -independent Effects of Heat Stress on Lactation and Mammary Gland Development. J. Dairy Sci.103, 12003–12014. 10.3168/jds.2020-18675
33
YagerJ. Y. (2004). Animal Models of Hypoxic-Ischemic Brain Damage in the Newborn. Seminars Pediatr. Neurology11, 31–46. 10.1016/j.spen.2004.01.006
Summary
Keywords
hypoxia, ischemia, neonate, stroke, inflammation, open-source
Citation
Hillman TC, Idnani R and Wilson CG (2022) An Inexpensive Open-Source Chamber for Controlled Hypoxia/Hyperoxia Exposure. Front. Physiol. 13:891005. doi: 10.3389/fphys.2022.891005
Received
07 March 2022
Accepted
08 June 2022
Published
12 July 2022
Volume
13 - 2022
Edited by
Ovidiu Constantin Baltatu, Anhembi Morumbi University, Brazil
Reviewed by
Richard James Wilson, University of Calgary, Canada
Thorsten Schwerte, University of Innsbruck, Austria
Updates
Copyright
© 2022 Hillman, Idnani and Wilson.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher G. Wilson, cgwilson@llu.edu
This article was submitted to Integrative Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.