Background
Cannabinoids are a class of natural compounds that are found in the endocannabinoid system and the cannabis plant. Synthetic cannabinoids have also emerged, often discussed as new psychoactive substances (NPS), however, these cannabinoid analogues are additionally used as tool compounds and as potential novel therapeutic agents.
The interest in cannabinoid science has grown steadily in recent years, particularly with the legalisation of medicinal cannabis in many jurisdictions around the world. It has become abundantly clear that cannabinoids interact with a host of different receptors and proteins, both within the endocannabinoid system and outside of it (Figure 1) (Pertwee, 2008; Billakota et al., 2019; Ghovanloo and Ruben, 2021). The interactions between these compounds and various molecular targets affect and alleviate a wide variety of physiological processes underlying pain, mood changes, and seizure disorders. Indeed, with the identification of treatment-resistant disorders, and growing public health problems such as the opioid crisis (Krausz et al., 2021), the need to investigate cannabinoids across various disciplines is increasingly urgent; they have already revealed some surprising potential to treat otherwise intractable health problems.
FIGURE 1
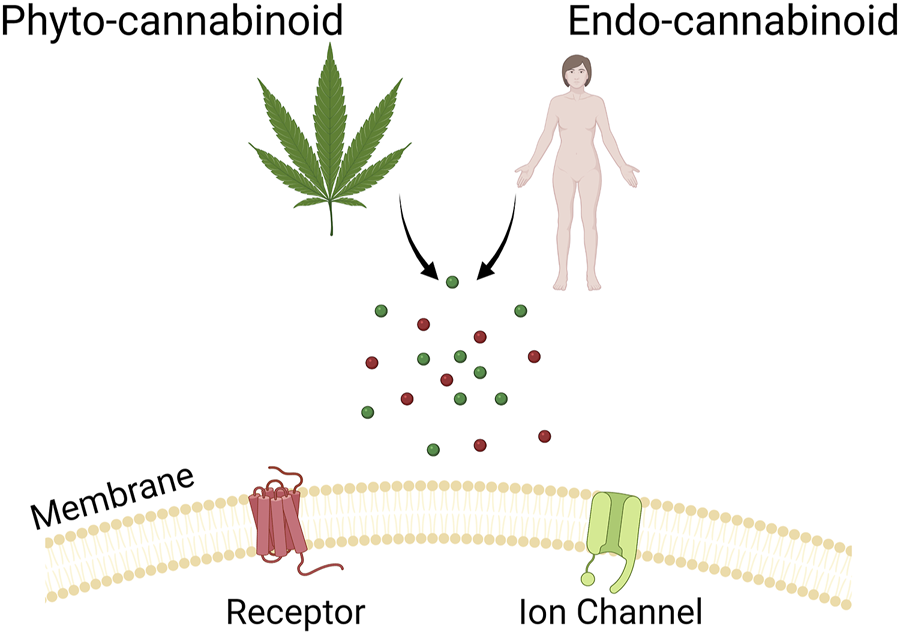
Shows a cartoon representation of the theme of this Research Topic.
Cannabidiol (CBD) is among the most promising cannabinoids and has shown clinically proven efficacy against epileptic disorders (Devinsky et al., 2017; Devinsky et al., 2018). The intrigue surrounding this compound arises from anecdotal reports of its efficacy against many other disorders and is likely related to the large number of its pharmacological targets. In contrast to ∆9-tetrahydrocannabinol (THC), which has low nanomolar affinity and partial agonist effects on endocannabinoid (CB) receptors, CBD has limited binding at CB receptors and complex effects with inverse agonist/antagonist and negative allosteric modulatory effects at CB receptors being described (Thomas et al., 2007; Laprairie et al., 2015). Thus, its mechanism of efficacy, especially against seizure-related disorders, is mainly attributed to targets independent of CB receptors. This concept is at the core of why CBD, as well as other cannabinoids, are increasingly studied against different molecular targets ranging from ion channels to receptors, and even the bio-membrane itself (Wade et al., 2004; Ross et al., 2008; De Petrocellis et al., 2012; Ghovanloo et al., 2018; 2021; 2022c; Harding et al., 2018; Fouda et al., 2020; Orvos et al., 2020; Sait et al., 2020; Zhang and Bean, 2021; Zhang et al., 2022). Because each of these targets has an important role in many physiological processes and disease mechanisms, cannabinoids have therapeutic potential for disorders that originate throughout the body including, but perhaps not limited to, excitable tissues: muscles and nerves (Devane et al., 1988; Elsohly, 2007; Ghovanloo and Ruben, 2021). Indeed, looking beyond CBD and THC, other “minor” plant cannabinoids have been more recently explored for their medicinal potential with preclinical research showing a diversity of potential therapeutic applications including anti-inflammatory, anti-cancer, anti-seizure, anxiolytic, and anti-emetic activities (Anderson et al., 2019; 2021b; 2021a; Assareh et al., 2020; Rock et al., 2021; Colvin et al., 2022; Lowin et al., 2023). However, the modes of action of these cannabis constituents remain to be determined.
Our goal in this Research Topic was to disseminate up-to-date knowledge of cannabinoid science pertaining to activity at different targets, with implications for physiology and the treatment of medical conditions.
Articles published in Research Topic
A total of five papers appeared in our Research Topic, including both literature reviews and original research articles.
The first paper published this Research Topic by Oz et al. provides a literature review on the effects of cannabinoids on ligand-gated ion channels. This review paper focuses on how various cannabinoids, phytocannabinoids, endocannabinoids, and synthetic cannabinoids, modulate the activity of nicotinic, 5-HT3, glycine, and GABAA receptors (Oz et al.).
In the second paper, Schmiedhofer et al. contribute a systematic review on the interactions of cannabinoids with Cys-loop receptors. This paper begins with an overview of cannabinoids followed by an in-depth description of interactions with Cys-loops, which have important molecular consequences. The paper summarizes pharmacological and medicinal implications of cannabinoid/Cys-loop interactions (Schmiedhofer et al.).
The third paper by Ghovanloo et al. is a mini-review on the interactions of two important non-psychoactive compounds, cannabigerol (CBG) and CBD, with voltage-gated sodium (Nav) channels. This paper starts with an overview of CBD’s mechanism of action on Nav channels, and then describes how CBG may have therapeutic potential to treat neuropathic pain, in part, via activity on Nav channels (Ghovanloo et al.; Ghovanloo et al., 2022b).
The fourth paper by Milligan et al. is an original research article that builds on the idea that Nav channels are critical to cannabinoid activity. This paper investigates the inhibitory effects of several lesser studied cannabinoids on Nav channels, including cannabigerolic acid (CBGA), cannabidivarinic acid (CBDVA), cannabichromenic acid (CBCA), and cannabichromene (CBC). These compounds were recently identified as having anti-seizure activity in preclinical epilepsy models, however their modes of action are unclear (Anderson et al., 2019; Anderson et al., 2021a; Anderson et al., 2021b). This paper highlights the importance of gaining a better understanding of the molecular actions of all cannabinoids on not only Nav channels, but other important signalling proteins (Milligan et al.).
Finally, in the fifth paper, an intriguing original research article by Harman et al. provides insights into how MEPIRAPIM-derived synthetic cannabinoids inhibit T-type calcium (Cav3) channels with divergent effects in seizure models. This paper provides a novel avenue for the development of future anti-seizure therapeutics through targeting T-type channels using a synthetic cannabinoid scaffold without CB receptor activity (Harman et al.).
In summary, this Research Topic presents a series of papers focused on various therapeutic and molecular investigations of cannabinoids. Despite much research in this area, there are many questions that remain unanswered, including the synergistic effects of cannabinoids. We hope that this Research Topic inspires an even greater interest in cannabinoid science.
Statements
Author contributions
M-RG wrote the manuscript, PR and JA edited and revised the manuscript. All authors approved the manuscript for publication. All authors contributed to the article and approved the submitted version.
Funding
M-RG is a Banting Fellow at Yale and is supported by the Canadian Institutes of Health Research (CIHR)/Grant No. 471896.
Acknowledgments
We thank all authors, reviewers, and the staff of the Editorial Office for the successful publication of this Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AndersonL. L.AmetovskiA.Lin LuoJ.Everett-MorganD.McGregorI. S.BanisterS. D.et al (2021a). Cannabichromene, related phytocannabinoids, and 5-Fluoro-cannabichromene have anticonvulsant properties in a mouse model of dravet syndrome. ACS Chem. Neurosci.12, 330–339. 10.1021/ACSCHEMNEURO.0C00677/SUPPL_FILE/CN0C00677_SI_001.PDF
2
AndersonL. L.HeblinskiM.AbsalomN. L.HawkinsN. A.BowenM. T.BensonM. J.et al (2021b). Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol.178, 4826–4841. 10.1111/BPH.15661
3
AndersonL. L.LowI. K.BanisterS. D.McGregorI. S.ArnoldJ. C. (2019). Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of dravet syndrome. J. Nat. Prod.82, 3047–3055. 10.1021/ACS.JNATPROD.9B00600/ASSET/IMAGES/LARGE/NP9B00600_0007.JPEG
4
AssarehN.GururajanA.ZhouC.LuoJ. L.KevinR. C.ArnoldJ. C. (2020). Cannabidiol disrupts conditioned fear expression and cannabidiolic acid reduces trauma-induced anxiety-related behaviour in mice. Behav. Pharmacol.31, 591–596. 10.1097/FBP.0000000000000565
5
BillakotaS.DevinskyO.MarshE. (2019). Cannabinoid therapy in epilepsy. Curr. Opin. Neurol.32, 220–226. 10.1097/wco.0000000000000660
6
ColvinE. K.HudsonA. L.AndersonL. L.KumarR. P.McGregorI. S.HowellV. M.et al (2022). An examination of the anti-cancer properties of plant cannabinoids in preclinical models of mesothelioma. Cancers (Basel)14, 3813. 10.3390/CANCERS14153813
7
De PetrocellisL.OrlandoP.MorielloA. S.AvielloG.StottC.IzzoA. A.et al (2012). Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol.204, 255–266. 10.1111/j.1748-1716.2011.02338.x
8
DevaneW. A.DysarzF. A.JohnsonM. R.MelvinL. S.HowlettA. C. (1988). Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol.34, 605–613. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2848184 (Accessed February 11, 2019).
9
DevinskyO.CrossJ. H.LauxL.MarshE.MillerI.NabboutR.et al (2017). Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med.376, 2011–2020. 10.1056/NEJMoa1611618
10
DevinskyO.PatelA. D.CrossJ. H.VillanuevaV.WirrellE. C.PriviteraM.et al (2018). Effect of cannabidiol on drop seizures in the lennox–gastaut syndrome. N. Engl. J. Med.378, 1888–1897. 10.1056/nejmoa1714631
11
ElsohlyM. A. (2007). Marijuana and the cannabinoids. 10.1007/978-1-59259-947-9
12
FoudaM. A.GhovanlooM.-R.RubenP. C. (2020). Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br. J. Pharmacol.177, 2932–2946. 10.1111/bph.15020
13
GhovanlooM.-R.ChoudhuryK.BandaruT. S.FoudaM. A.RayaniK.RusinovaR.et al (2021). Cannabidiol inhibits the skeletal muscle nav1.4 by blocking its pore and by altering membrane elasticity. J. Gen. Physiol.153, e202012701. 10.1085/jgp.202012701
14
GhovanlooM.-R.EstacionM.Higerd-RusliG. P.ZhaoP.Dib-HajjS.WaxmanS. G. (2022b). Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability. Br. J. Pharmacol.179, 4010–4030. 10.1111/bph.15833
15
GhovanlooM.-R.GoodchildS. J.RubenP. C. (2022c). Cannabidiol increases gramicidin current in human embryonic kidney cells: An observational study. PLoS One17, e0271801. 10.1371/journal.pone.0271801
16
GhovanlooM.-R.RubenP. C. (2021). Cannabidiol and sodium channel pharmacology: General overview, mechanism, and clinical implications. Neuroscientist28, 318–334. 10.1177/10738584211017009
17
GhovanlooM.-R.ShuartN. G.MezeyovaJ.DeanR. A.RubenP. C.GoodchildS. J. (2018). Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem.293, 16546–16558. 10.1074/jbc.RA118.004929
18
HardingS. D.SharmanJ. L.FaccendaE.SouthanC.PawsonA. J.IrelandS.et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res.46, D1091–D1106. 10.1093/nar/gkx1121
19
KrauszR. M.WestenbergJ. N.ZiafatK. (2021). The opioid overdose crisis as a global health challenge. Curr. Opin. Psychiatry34, 405–412. 10.1097/YCO.0000000000000712
20
LaprairieR. B.BagherA. M.KellyM. E. M.Denovan-WrightE. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol.172, 4790–4805. 10.1111/bph.13250
21
LowinT.Tigges-PerezM. S.ConstantE.PongratzG. (2023). Anti-inflammatory effects of cannabigerol in rheumatoid arthritis synovial fibroblasts and peripheral blood mononuclear cell cultures are partly mediated by TRPA1. Int. J. Mol. Sci.24, 855. 10.3390/IJMS24010855
22
OrvosP.PásztiB.TopalL.GazdagP.ProrokJ.PolyákA.et al (2020). The electrophysiological effect of cannabidiol on hERG current and in Guinea-pig and rabbit cardiac preparations. Sci. Rep.10, 16079. 10.1038/s41598-020-73165-2
23
PertweeR. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol.153, 199–215. 10.1038/sj.bjp.0707442
24
RockE. M.LimebeerC. L.PertweeR. G.MechoulamR.ParkerL. A. (2021). Therapeutic potential of cannabidiol, cannabidiolic acid, and cannabidiolic acid methyl ester as treatments for nausea and vomiting. Cannabis cannabinoid Res.6, 266–274. 10.1089/CAN.2021.0041
25
RossH. R.NapierI.ConnorM. (2008). Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem.283, 16124–16134. 10.1074/jbc.M707104200
26
SaitL. G.SulaA.GhovanlooM.-R.HollingworthD.RubenP. C.WallaceB. A. (2020). Cannabidiol interactions with voltage-gated sodium channels. Elife9, 585933–e58617. 10.7554/eLife.58593
27
ThomasA.BaillieG. L.PhillipsA. M.RazdanR. K.RossR. A.PertweeR. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br. J. Pharmacol.150, 613–623. 10.1038/sj.bjp.0707133
28
WadeD. T.MakelaP.RobsonP.HouseH.BatemanC. (2004). Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler.10, 434–441. 10.1191/1352458504ms1082oa
29
ZhangH. X. B.BeanB. P. (2021). Cannabidiol inhibition of murine primary nociceptors: Tight binding to slow inactivated states of Nav1.8 channels. J. Neurosci.41, 6371–6387. 10.1523/JNEUROSCI.3216-20.2021
30
ZhangH. X. B.HeckmanL.NidayZ.JoS.FujitaA.ShimJ.et al (2022). Cannabidiol activates neuronal Kv7 channels. Elife11, e73246. 10.7554/ELIFE.73246
Summary
Keywords
cannabinoid, endocannabinnoid, phytocannabinoid, ion channel, receptor, biomembrane, voltage-gated sodium and calcium channels, CB and CB 1 2
Citation
Ghovanloo M-R, Arnold JC and Ruben PC (2023) Editorial: Cannabinoid interactions with ion channels, receptors, and the bio-membrane. Front. Physiol. 14:1211230. doi: 10.3389/fphys.2023.1211230
Received
24 April 2023
Accepted
02 May 2023
Published
09 May 2023
Volume
14 - 2023
Edited and reviewed by
Christoph Fahlke, Helmholtz Association of German Research Centres (HZ), Germany
Updates
Copyright
© 2023 Ghovanloo, Arnold and Ruben.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad-Reza Ghovanloo, reza.ghovanloo@yale.edu; Jonathon C. Arnold, jonathon.arnold@sydney.edu.au; Peter C. Ruben, pruben@sfu.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.