Abstract
A rhythmic expression of clock genes occurs within the cells of multiple organs and tissues throughout the body, termed “peripheral clocks.” Peripheral clocks are subject to entrainment by a multitude of factors, many of which are directly or indirectly controlled by the light-entrainable clock located in the suprachiasmatic nucleus of the hypothalamus. Peripheral clocks occur in the gastrointestinal tract, notably the epithelia whose functions include regulation of absorption, permeability, and secretion of hormones; and in the myenteric plexus, which is the intrinsic neural network principally responsible for the coordination of muscular activity in the gut. This review focuses on the physiological circadian variation of major colonic functions and their entraining mechanisms, including colonic motility, absorption, hormone secretion, permeability, and pain signalling. Pathophysiological states such as irritable bowel syndrome and ulcerative colitis and their interactions with circadian rhythmicity are also described. Finally, the classic circadian hormone melatonin is discussed, which is expressed in the gut in greater quantities than the pineal gland, and whose exogenous use has been of therapeutic interest in treating colonic pathophysiological states, including those exacerbated by chronic circadian disruption.
Introduction
Biological rhythms that persist on a roughly 24-h cycle under stable environmental conditions, can be synchronized by external cues (zeitgebers), and retain constancy across varying physiological temperatures are classified as circadian (Aschoff, 1981). However, most studies on daily rhythms in colonic function do not rigorously test these criteria, which makes their findings suggestive but not definitive of circadian rhythmicity. In this review, such instances will be categorized under “daily rhythms,” while evidence meeting circadian criteria will be explicitly identified. In discussing genes and proteins, the review primarily draws on mouse data, using “Clock” for gene or messenger RNA, and “CLOCK” for the protein.
Circadian rhythms are present in mammals, tuning cell and organ processes to the ambient 24-h light-dark cycle, optimising and coordinating bodily functions including feeding (Segers and Depoortere, 2021), defecation (Duboc et al., 2020), and urination (Noh et al., 2011). Controlling the body’s rhythmicity is a hierarchical system comprised of multiple functionally overlapping circadian oscillators. At the top of the hierarchy is the main light-entrainable clock of the circadian system, which lies within the suprachiasmatic nucleus (SCN) of the hypothalamus containing around 20,000 neurons (Ralph et al., 1990; Hastings et al., 2018; Yan et al., 2020). A ∼ 24-h circadian cycle must be reset by a daily cue (zeitgeber) to be synchronized with external environmental time (Duffy and Czeisler, 2009). Light is the primary zeitgeber for the SCN. In mammals, the SCN is principally entrained by retinal melanopsin-expressing non-visual photoreceptors (intrinsically-photosensitive retinal ganglion cells) that detect the light environment (Schlangen and Price, 2021) and signal via the retinohypothalamic tract. The SCN signals to other parts of the brain via projections to local circadian clocks of the brain centres that control cognition, mood, behavioural rhythms such as sleep-wakefulness and feeding-fasting, and autonomic and neuroendocrine circadian rhythms (Hastings et al., 2018).
Cell rhythmicity in the SCN involves a core molecular oscillator referred to as the transcription-translation feedback loop (TTFL). See Table 1 for the expanded names of TTFL components. The TTFL may be considered an interaction between positive transactivating elements through CLOCK/BMAL1 and negative transinhibiting elements through PER/CRY (Lowrey and Takahashi, 2011). The core mammalian TTFL pacemaking loop involves nuclear transcription of the Clock and Bmal1 genes, followed by post translational cytosolic heterodimer formation of CLOCK-BMAL1 protein complexes (Reppert and Weaver, 2002). Succeeding nuclear translocation of CLOCK-BMAL1 drives daytime expression of Per1/2 and Cry1/2 through E box enhancers. The formation and increasing levels of subsequent PER-CRY protein complexes (with Ck1δ; Cao et al., 2023) inhibit Per and Cry expression via CLOCK-BMAL1 E box dissociation (Hastings et al., 2018; Cao et al., 2021), possibly driving CLOCK-BMAL1 to act at other DNA sites (Koch et al., 2022). A decrease in Per and Cry mRNA levels and proteasomal degradation of PER-CRY complexes (Hastings et al., 2018) lead to a disinhibition that enables the next CLOCK/BMAL1-driven cycle (Lowrey and Takahashi, 2011). Genomic and proteomic regulation of Per and Cry takes ∼24 h. In mouse SCN, PER shows large circadian fluctuations in abundance (Yamaguchi et al., 2003), whilst BMAL1, CLOCK and CRY protein levels are more constantly expressed showing lower amplitude circadian rhythmicity (von Gall et al., 2003; Maywood et al., 2013; Yang et al., 2020). The core loop comprising BMAL1-CLOCK and PER-CRY drives ancillary, interlocking TTFLs through proteins RORα/β, and REV-ERBα/β that stabilize the core loop period and amplitude (Cho et al., 2012), and through DBP and NFIL3 (Takahashi, 2017). Together these transcription factors also drive rhythmic expression of other genes via their respective promotors (i.e., clock-controlled genes outside the TTFL), thus coupling the molecular oscillator to cell functions (Takahashi, 2017).
TABLE 1
| Clock, CLOCK | Circadian locomotor output cycles kaput |
|---|---|
| Bmal1, BMAL1 | brain and muscle ARNT (aryl hydrocarbon receptor nuclear translocator)-like protein 1 (also known as Mop3) |
| Per1/2, PER1/2 | period 1, period 2 |
| Cry1/2, CRY1/2 | cryptochrome 1, cryptochrome 2 |
| Csnk1d, CK1 | casein kinase 1 delta |
| Rorα/β, RORα/β | retinoic acid receptor-related orphan receptor alpha/beta |
| Rev-erbα/β, REV-ERBα/β | reverse-erythroblastosis virus alpha/beta (also known as NR1D1/NR1D2) |
| Dbp, DBP | D site albumin promoter binding protein |
| Nfil3, NFIL3 | nuclear factor, interleukin 3, regulated |
Gene, protein, and expanded names of components of the transcription-translation feedback loop.
Remarkably, the core TTFL also operates in the cells of peripheral tissues and organs (termed “peripheral clocks”) such as in the gut, liver, bladder, adipose tissue and skeletal muscle (Labrecque and Cermakian, 2015; Reinke and Asher, 2016; Basinou et al., 2017; Hastings et al., 2018). Thus, the same molecular oscillator underlies the rhythmic output of vastly different gene sets, depending on the tissue/cell type (Partch et al., 2014). The cell specificity of oscillator controlled outputs is achieved in part by components of the TTFL binding other transcription factors and nuclear receptors to suppress or enhance a cell specific transcription program (Patke et al., 2020). In addition, the output of the molecular oscillator can be differentiated by variations in genome and chromatin access in a cell/tissue specific manner (Patke et al., 2020). It is worth noting that core clock proteins interact with histone acetyltransferases to induce chromatin states that allow transcription to take place and that this process involves regulation by the histone deacetylase, SIRT1; a protein sensor of energy status (Takahashi, 2017). This contributes to a mechanism by which feeding behaviour and diet composition can modify the molecular oscillator (for review, see Sato and Sassone-Corsi, 2022).
Peripheral clocks drive rhythmic expression of different gene sets in a cell specific manner. In addition, where identical non-clock genes are rhythmically expressed in different organs/cell types of the mouse, their peak expression timing nevertheless differed in phase by many hours, or indeed were antiphase (Zhang et al., 2014). Yet core clock gene phases were more aligned, each peaking within a window of ∼3 h across multiple tissues, indicating significant divergence in regulation of the non-clock genes between cell types (Zhang et al., 2014). The acrophase of Bmal1 in mouse stomach and colon was similarly within 3 h of the SCN, but Per2 diverged by up to ∼10 h (Hoogerwerf et al., 2007). The question thus arises as to the mechanisms coupling/entraining and maintaining phase relationships between central and peripheral clocks (for review, including intercellular coupling within tissues, see Astiz et al., 2019;Finger et al., 2020;Pilorz et al., 2020). In the case of the colon, the major candidate links to the SCN include neural inputs from the parasympathetic and sympathetic divisions of the autonomic nervous system, circulating hormonal factors, and the rhythmicity of feeding behaviours (see schematic diagram, Figure 1). Evidence for the roles of these mechanisms in maintaining rhythmicity of colonic functions and clock gene expression is discussed throughout this review. More generally, the SCN clock regulates the oscillation of peripheral clocks directly by neural signalling through sympathetic and parasympathetic nerves, and hormonal signalling via pineal and adrenal glands (Dickmeis, 2009; Ohdo, 2010; Richards and Gumz, 2012; Astiz et al., 2019), and indirectly through its influence on behaviours like sleep-wake cycles and feeding (Dibner et al., 2010). For the gut and liver peripheral clocks, one of the most important SCN-driven mechanisms is the temporal control of feeding, since food intake is a significant entraining cue (Damiola et al., 2000; Stephan, 2001; Stokkan et al., 2001; Stephan, 2002). Food intake entrains the circadian rhythm of clock genes in the gut, while those in the liver may be entrained via insulin secretion which subsequently regulates Per1/2 expression (Finger et al., 2020; Zhang et al., 2020; Taleb and Karpowicz, 2022). Inversion of feeding times in mice results in an inversion of peripheral clocks in the gut, but not the SCN (Hoogerwerf et al., 2007). Indeed, peripheral and local oscillators outside the SCN that can control general activity rhythms are implied by experiments showing that non-photic cues such as timed food access (the food-entrainable oscillator; FEO) and methamphetamine administration (methamphetamine sensitive circadian oscillator; MASCO) can restore rhythmicity after SCN disruption, but little is known of their anatomical substrates (Mistlberger, 1994; Menaker et al., 2013; Pendergast and Yamazaki, 2018; Mistlberger, 2020; Taufique et al., 2022).
FIGURE 1
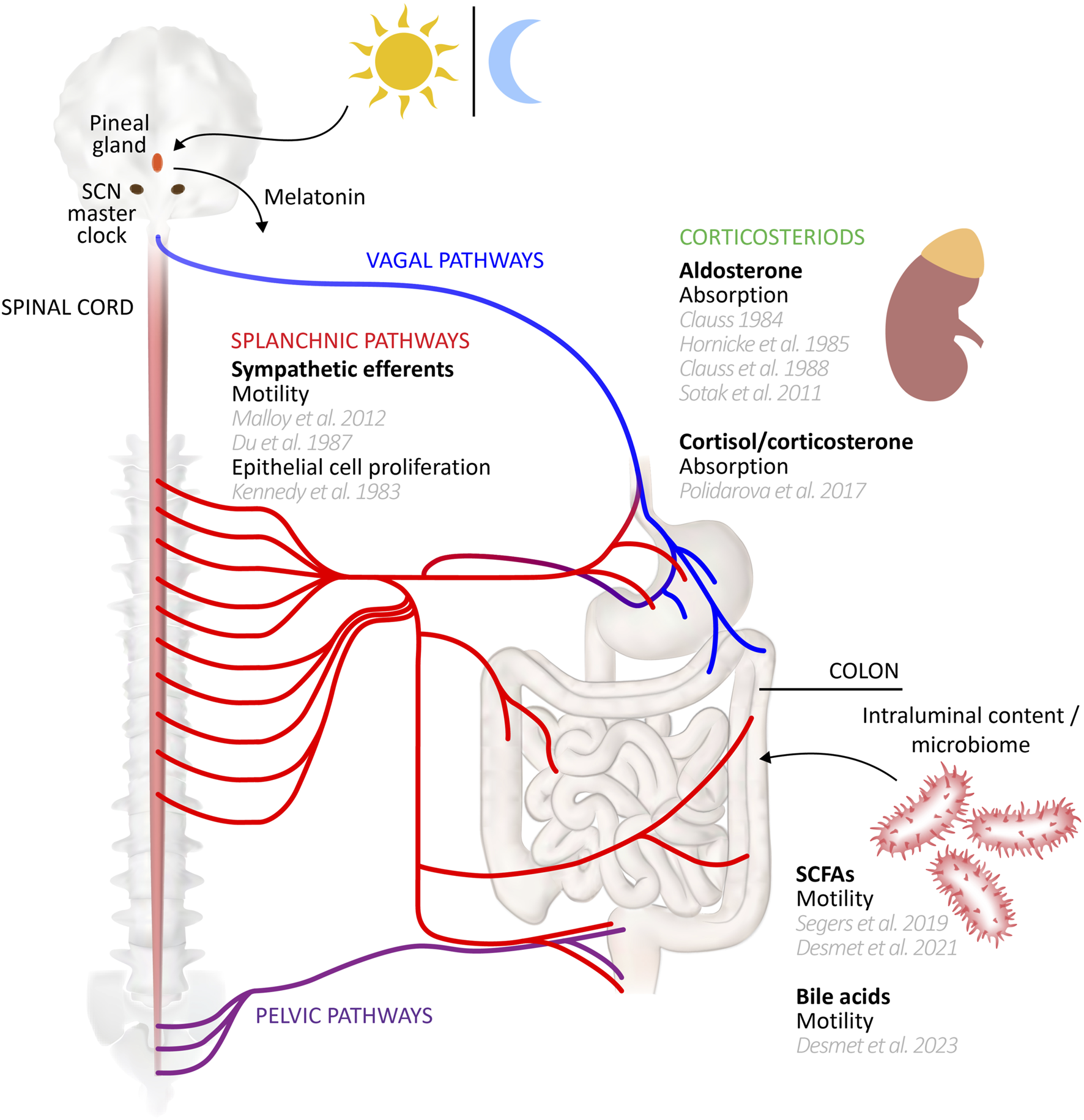
Circadian entrainers of colon function. A range of influences may entrain or modulate peripheral clocks underlying circadian rhythms of colonic functions. This schematic diagram summarizes those influences, citing supporting studies. Several influences, such as the vagal and pelvic efferent and afferent pathways remain to be studied in detail for their potential role in driving colonic function rhythmicity. Gut-CNS schematic based on Young (2012).
As seen in most mammals, including humans, both faecal defecation and urinary voiding exhibit a daily rhythm of increased occurrence during the “active period” (i.e., the daytime in diurnal animals, and night in nocturnal animals) and a decreased occurrence during the “inactive period,” or “rest period” (Kirkland et al., 1983; Herrera and Meredith, 2010; Noh et al., 2011; Negoro et al., 2012; Duboc et al., 2020). In humans, faecal defecation peaks early morning, usually shortly after waking, and following a meal (Heaton et al., 1992). Similarly, urinary voiding also peaks during the early morning, with a consistent pattern throughout the day and little to no occurrence at night (Noh et al., 2011). Chronic disruption to circadian rhythms can significantly impact health, sleep and quality of life (Xie et al., 2019; Vetter, 2020), with recent research turning towards the hormone melatonin as one of the potential treatments.
The SCN drives the activation of sympathetic nerves in the superior cervical ganglia that project to the pineal gland, evoking nocturnal melatonin synthesis and release into the circulation (Reiter, 1991; Claustrat et al., 2005). Melatonin could be partially responsible for synchronisation of the peripheral clocks by the central clock, but also serves as a feedback mechanism to the SCN (Prasai et al., 2011). Plasma levels of melatonin represent one of the most robust circadian rhythms with concentrations in the blood and urine peaking during the night, stabilising the sleep-wake cycle (Reiter et al., 2009). In the SCN, melatonin acts via G-protein coupled receptors; melatonin 1 (MT1) receptors reducing neuronal activity, and melatonin 2 (MT2) receptors causing a circadian phase shift (Dubocovich, 2007). MT1 and MT2 receptors have been identified in the neurons of the central nervous system (CNS) and peripheral organs such as blood vessels, heart, lung, kidney, bladder, liver, gut, and others (Dubocovich and Markowska, 2005; Pandi-Perumal et al., 2008). Exogenous melatonin can act peripherally on smooth muscle and enteric neurons influencing colonic motility, albeit in concentration ranges significantly higher than its physiological levels. Symptoms of functional dyspepsia, irritable bowel syndrome (IBS) and ulcerative colitis (UC) are significantly exacerbated by circadian disruptions (Kim et al., 2013; Fowler et al., 2022). Melatonin has been considered a potential treatment for gut and bladder disorders, such as functional dyspepsia, IBS (Lu et al., 2009; Chojnacki et al., 2013; Fowler et al., 2022), UC (Liu and Wang, 2019), and nocturia (Drake et al., 2004; Ramsay and Zagorodnyuk, 2023). This review summarises the circadian rhythmicity of the colon and the influence of melatonin on its function.
Circadian rhythms of colonic motility
The large intestine receives from the ileum undigested content as well as endogenous secretions, metabolites and dead epithelial cells. Undigested material may be fermented by microbiota in the caecum and proximal colon. In the more proximal regions, intraluminal content is an amorphous semi-liquid. Water, electrolytes, and microbial products are absorbed along the colon as the content forms a stool that is released on defecation (Costa et al., 2021). These processes, including the motor behaviours that propel content along the large intestine show distinct circadian profiles.
Defecation
Defection is an overt indication of colonic motility that shows daily rhythmicity, peaking in the active period. This has been reported in numerous species, including diurnal humans (Rendtorff and Kashgarian, 1967; Heaton et al., 1992; Aschoff, 1994; Shemerovskii, 2002) and non-human primates (Bernstein, 1964; Caton et al., 1996), birds (Clarke, 1979; Rodriguez-Sinovas et al., 1994; Malek et al., 2020), dogs (Hirabayashi et al., 2009), horses (Piccione et al., 2005), camels (Aubè et al., 2017), and sheep (Piccione et al., 2005); and nocturnal rodents (Gosling, 1979; Magot and Chevallier, 1983; Firpo et al., 2005; Hoogerwerf et al., 2010; de Azevedo et al., 2011; Platt et al., 2013; Allen and Johnson, 2018), foxes (Klenk, 1971), antechinus (Cowan et al., 1974), rabbit (Jilge, 1974; Jilge and Hudson, 2001), hare (Pehrson, 1983), and house musk (Kobayashi et al., 2022). Some species, such as degu and the Mongolian gerbil that can show either diurnal or nocturnal activity patterns (Refinetti, 2006) have a more constant defecation pattern (Kenagy et al., 1999). Animals showing activity and defecation peaks around the day-night transitions may be referred to as “crepuscular,” such as the predominantly nocturnal cat (Wienbeck and Kreuzpaintner, 1976) and diurnal guinea pig (Elfers et al., 2021).
Most observations of the daily rhythmicity in defecation patterns arise from subjects with typical, ongoing photoperiods and ad-libitum food access. However, the persistence of defecation patterns during the active period under constant lighting conditions has also been identified in mice (Hoogerwerf et al., 2010), rabbits (Jilge, 1982) and humans (Aschoff, 1994). This suggests daily rhythms in defecation is not acutely sensitive to lighting conditions and thus likely represents an endogenous circadian rhythm. Yet, daily feeding rhythms show circadian rhythmicity and food intake potently stimulates gut motility, including defecation (Dorfman et al., 2022). Thus it remains possible that defecation patterns are not intrinsically circadian but is triggered by processes that are, such as feeding. This is tricky since food ultimately supplies most colonic content so its restriction limits defecation capacity. Interestingly however, restricting food availability to a 4-h period in rabbits during the light (inactive) period fully shifted hard faeces defecation to this period, along with general activity patterns (Jilge and Stähle, 1993). This illustrates the potency of the FEO in this species and the importance of food intake and availability in determining defecation and activity patterns. Whilst these data point to the potential role of a different oscillator and/or zeitgeber in determining daily rhythmicity of defecation, it does not clarify whether defecation patterns reflect intrinsic circadian rhythmicity of the colon. In a more recent study, the food intake and fecal pellet output of guinea pigs was tracked hourly, under normal light/dark conditions and ad-libitum food access (Elfers et al., 2021). An interesting finding of this study was that although guinea pigs consumed less food during the dark (inactive) period, the difference was modest, and the animals continued to consume food at around 65% of the mean active period rate. At the same time, mean fecal pellet output fell to near zero for most of the inactive period, and overall was less than 20% of the active period rate (Elfers et al., 2021). This would suggest daily defecation patterns are governed by more factors than food intake alone, pointing to the possibility of true intrinsic circadian rhythmicity of colonic motor behaviours.
Colonic motor behaviours
The motor behaviours of the entire gastrointestinal tract are under circadian influence (for review, see Leembruggen et al., 2022). Here we principally focus on colonic motor behaviours and adjacent regions. Most studies that describe daily variability in colonic motor activity, in vivo, has been done in humans in 24-h manometry studies (Bassotti et al., 1999; Bharucha and Brookes, 2012). One of the most prominent motor activities of the human colon are referred to as high amplitude propagating contractions (HAPCs). HAPCs are strong propulsive contractions that typically initiate in the proximal colon and may mediate defecation (Corsetti et al., 2019). Compatible with circadian rhythmicity of human defecation, human colonic manometry studies report most (up to 90%) HAPCs occur in the daytime and are relatively rare at night (Narducci et al., 1987; Bassotti and Gaburri, 1988; Crowell et al., 1991; Bassotti et al., 1992; Furukawa et al., 1994; Hagger et al., 2002; Rao et al., 2010). Where studies report the hourly distribution of HAPCs, the peak occurrence has been detected at awakening ∼7a.m. (Bassotti and Gaburri, 1988; Bassotti et al., 1992), just after breakfast ∼9a.m. (∼7a.m. wake, 8a.m. breakfast) (Narducci et al., 1987) and following a 12p.m. lunch at ∼1p.m. (Crowell et al., 1991). The preponderance of HAPCs in the day (active) period was observed where subjects were confined to a supine or side-lying position for recordings, indicating ambulation cannot fully account for daily HAPC variability (Narducci et al., 1987; Bassotti and Gaburri, 1988; Bassotti et al., 1992; Furukawa et al., 1994). Food intake is a well-known stimulus of HAPCs and other colonic motor patterns, taking effect within minutes of eating and lasting up to 2 h postprandially (Dinning et al., 2014). The rate of HAPCs increases just prior to, or upon waking in the morning, before breakfast (Crowell et al., 1991; Bassotti et al., 1992; Furukawa et al., 1994). This suggests daily rhythmicity of HAPCs is not fully accountable by a simple response to feeding, and thus more likely to be circadian.
HAPCs may be important for colonic propulsion but represent a small proportion of the motor patterns present in the human colon. Several lower amplitude propagating motor patterns have been identified by high resolution manometry. The most prominent of these is the cyclic motor pattern. This motor pattern consists of rhythmic pressure waves, occurring between 2-6 cycles per minute, that can propagate in an antegrade or retrograde direction. Single propagating contractions of varying length, speed and polarity can also occur (Dinning et al., 2014; Dinning et al., 2016). Given the short duration of colonic high-resolution manometry studies (typically between 4-8hrs), the daily rhythmicity of motor patterns quantified with this technique has not been established. However, in low-resolution manometry studies the aggregate area under the curve and frequency of all ongoing contractility (not just HAPCs) along the human colon was significantly suppressed at night compared to the day (Narducci et al., 1987; Soffer et al., 1989; Furukawa et al., 1994; Hagger et al., 2002; Rao et al., 2004; Rao et al., 2010). Furthermore, low-resolution manometry studies had identified bouts of rhythmic contraction in the rectum with the same frequency as the cyclic motor pattern described above (see Figure 5 in Patton et al., 2013). In those studies, the motor pattern was labelled rectal motor complexes (RMCs), or period rectal motor activity (PRMA). Although negative or contradictory findings have been reported (Auwerda et al., 2001; Hagger et al., 2002), most 24 h studies have reported that this rectal activity was more frequent at night, compared to day (Kumar et al., 1989; Orkin et al., 1989; Ronholt et al., 1999; Rao et al., 2001a; Rao et al., 2001b; Rao et al., 2004). It was speculated that the increased nocturnal presence may help to prevent rectal filling while sleeping; a concept built upon with high-resolution manometry studies, which have now provided evidence for this rhythmic cyclic motor pattern acting as a rectosigmoid brake (Lin et al., 2017a; Lin et al., 2017b; Heitmann et al., 2022).
Compatible with the manometry data, an electromyographic (EMG) study of human colonic smooth muscle electrical behaviour distinguished long and short burst of spiking activity (Frexinos et al., 1985). However, short spike bursts were relatively constant, lacking daily rhythmicity, while long spike bursts were significantly more abundant during the day (Frexinos et al., 1985). In addition, total colonic pressure is reported to be lowest during the night, allowing accommodation of greater intraluminal volumes (Steadman et al., 1991). Indeed, colonic manometry combined with electroencephalography to monitor sleep stages revealed an inverse relationship between total colonic pressure and sleep depth (Furukawa et al., 1994).
Taken together, the available data suggest the human colon and rectum show complementary daily rhythmicity favouring increased diurnal motility in colon and nocturnal motility in the recto-sigmoid region. Food intake promptly enhances colonic motility but does not appear to fully account for daily rhythmicity, nor does ambulation. We speculate the daily rhythms in human colonic and rectal motor activity represent true circadian rhythms but this remains to be shown in temporally-isolated subjects.
In diurnal animals, available evidence shows similar daily rhythmicity to humans; total colonic contractility measured by pressure transducers in pigs was also significantly greater in the day compared to night time (Crowell et al., 1992). Colonic high amplitude propagating contractions in dogs, as measured by force transducers in vivo, were significantly more prominent in the early day period compared to other periods (Hirabayashi et al., 2009). In the chicken, EMG analysis of caecal and colonic smooth muscle firing activity revealed that periodic bursts of spikes that underlie contractility were relatively quiescent at night, compared to their frequency during the day (Rodriguez-Sinovas et al., 1994).
Colonic motor behaviour, in vivo, has also been assessed in nocturnal animals such as mice (Hoogerwerf et al., 2010), rats (Du et al., 1987; Gálvez-Robleño et al., 2022) and the house musk shrew, Suncus murinus (Kobayashi et al., 2022). In the house musk shrew, force transducers were used to detect ongoing contractility, including “giant migrating contractions” in the distal colon (GMCs) which were associated with defecation (Kobayashi et al., 2022). GMCs probably represent neurogenic peristalsis identified in more common experimental animals (Costa et al., 2013), and HAPCs in human colon (Spencer et al., 2016). The frequency of GMCs in the nocturnal house musk was almost 3 times higher in the night compared to the day period (Kobayashi et al., 2022). In mice, intracolonic pressure monitored in vivo showed a sustained elevation of basal pressure in the dark (active) period (Hoogerwerf et al., 2010), reminiscent of similar findings in humans (Steadman et al., 1991; Furukawa et al., 1994). Importantly, the daily oscillation in intracolonic pressure in mouse colon persisted under continuous dark conditions, consistent with circadian rhythmicity. In rats, colonic smooth muscle EMG recordings revealed periodic bursts of muscle action potentials. These spikes bursts were supressed during the day (inactive period), compared to the night (Du et al., 1987). Sympathetic preganglionic neurons to the prevertebral ganglia that in turn supply noradrenergic postganglionic neurons to the colon (Trudrung et al., 1994) are predominantly located in the intermediolateral column of the thoracolumbar spinal cord (Strack et al., 1988). Interestingly, thoracolumbar spinal cord ablation prevented the daily suppression of colonic spike burst activity (Du et al., 1987), suggesting thoracolumbar sympathetic drive may be required to suppress colonic motility during the inactive period. More recently, gastrointestinal transit was monitored by x-ray imaging after barium gavage in rats, revealing more rapid entry of content into the colon during the active period (Gálvez-Robleño et al., 2022). This effect was more pronounced in females than males (Gálvez-Robleño et al., 2022), similar to interactions between female sex and time of day in the rate of upper gastrointestinal transit in mice (Soni et al., 2019).
Recent data published in abstract form reports daily rhythmicity in the excitability of colonic myenteric neurons, ex vivo (Leembruggen et al., 2020); the enteric neural plexus underlying colonic neurogenic motility (Costa and Furness, 1976). Agonists to nicotinic, tachykinin, serotonin receptors and P2 purinoreceptors each evoked significantly greater intracellular calcium responses in the dark (active) period, compared to the light (inactive) period (Leembruggen et al., 2020), which may be consistent with observed differences in motility during these periods. The flat sheet ex vivo gut preparations used for this type of calcium imaging study are isolated from extrinsic neural, hormonal, and microbial inputs, thereby pointing to the role of intrinsic clock gene oscillations and their effectors in myenteric neurons as a potential mechanism for the observed differences in excitability between the active and inactive periods (Leembruggen et al., 2020).
Clock genes and colonic motility
Recent correlative analyses of genetic variation across multiple organs and cell types identify the colon as a major cross organ regulator of gene expression, showing more genes under rhythmic circadian control than any other organ analysed (Zhou et al., 2023). Most clock genes have been identified in the healthy colon and may be controlled by non-SCN peripheral influences. Clock and Bmal1 mRNA are expressed in colonic epithelial cells and myenteric plexus (Hoogerwerf et al., 2007; Sládek et al., 2007), which are key coordinators of colonic function (Furness, 2012). The expression of both Clock and Bmal1 peaks during the rest period and nadirs during the active period in humans, mice, and male rats (Hoogerwerf et al., 2007; Sládek et al., 2007; Sládek et al., 2012; Soták et al., 2013). Whilst males and females showed similar core clock gene phases, there were significantly more genes rhythmically expressed, with higher amplitudes, in female compared to male transverse colon (Talamanca et al., 2023). This suggests there are sex differences in the downstream output of the core circadian genes. Per1/2, Cry1/2, and Rev-erb are also expressed in the colon, showing an opposite phase to Clock and Bmal1 where they peak during the active period and nadir during the inactive period in rats and mice (Hoogerwerf et al., 2007; Sládek et al., 2007; Sládek et al., 2012; Soták et al., 2013; Polidarová et al., 2014). RORα has been identified in the colon, however, its research focus has been primarily on its involvement in colorectal cancers (Karasek et al., 2002; Winczyk et al., 2002). During constant darkness or light with ad libitum food access, rhythmic Clock expression in the male rat colon is lost whilst rhythms of Bmal1, Per1/2, and Cry1/2 are maintained (Hoogerwerf et al., 2007; Sládek et al., 2007), suggesting dependence on an entraining light stimulus for rhythmic Clock expression. The persistence of Bmal1, Per1/2, and Cry1/2 rhythmicity under constant light schedules is consistent with intrinsic circadian rhythmicity.
Feeding behaviour is rhythmic and under the influence of the SCN (Challet, 2019), thereby indirectly linking gut functions to light conditions. Bilateral SCN ablation in mice caused complete loss of faecal defecation rhythms, which may be attributed to loss of food intake rhythms (Malloy et al., 2012). Imposing rhythmicity of food intake by food restriction in SCN ablated mice restored defecation rhythms (Malloy et al., 2012), suggesting food intake is a strong influence. Indeed, reversed feeding times in rats results in reversal of colonic Bmal1, Per1/2, Cry1/2, and Reverb rhythmicity (Hoogerwerf et al., 2007; Sládek et al., 2007). However, the clock genes Per2 and Cry1 (but not Clock) in mouse distal colon continued to show daily rhythms following 24 h of constant darkness and fasting (Hoogerwerf et al., 2008). This shows that the rhythmicity of peripheral clocks in the colon withstands the removal of a more potent zeitgeber for the gut (food intake) than light, consistent with an intrinsic circadian rhythm.
Amongst core clock genes, only Per1 and Per2 have been investigated for a role in determining daily rhythms of colonic motility (Hoogerwerf et al., 2010). A Per1/Per2 double gene knockout in mice (but not Per1 or Per2 knockout alone) abolished their daily rhythm of fecal pellet output, total colonic pressure and cholinergic agonist sensitivity in continuous dark conditions (120 h), leading to the conclusion that daily colonic motility rhythms are regulated by Period genes (Hoogerwerf et al., 2010). Whilst this conclusion may be correct, it has since been shown that the feeding behaviour of Per1/Per2 double knockout mice becomes arrhythmic in constant darkness conditions (Adamovich et al., 2014), which provides an alternative explanation for the loss of colonic motor rhythms (Hoogerwerf et al., 2010). Indeed, only 48 h of an altered feeding schedule was required to alter colonic clock gene expression (Hoogerwerf et al., 2007). Imposed feeding rhythms or cell-specific knockouts may be able to rule out a role of arrhythmic feeding behaviour to bolster the conclusion that Period genes are responsible for circadian rhythms of colonic motility.
Beyond core clock genes, important neurotransmitters used by myenteric neurons have been reported to show daily rhythms. For example, a loss of daily colonic motor rhythms was observed in neuronal nitric oxide synthase (nNOS) knockout mice (Hoogerwerf, 2010) suggesting these rhythms are neuronally mediated. However, it is currently unknown how nNOS is linked to core circadian genes in the gut, if at all. Daily variation in mouse colonic Calcb gene expression has also been reported (Drokhlyansky et al., 2020; Leembruggen et al., 2020). This gene encodes the β-calcitonin gene-related peptide, which excites myenteric neurons (Palmer et al., 1986) and selectively expressed by mouse colonic intrinsic primary afferent neurons (Furness et al., 2004; Thompson et al., 2008; Hibberd et al., 2022c). This class of enteric neuron may be responsible for initiating excitation of enteric motor circuits to sensory stimuli (Kunze and Furness, 1999) and generating cyclic motor patterns (Hibberd et al., 2022b). Thus, variations in Calcb expression may contribute to daily rhythms in colonic motility.
Extrinsic neural control of motility
The colonic myenteric plexus is the principal coordinator of colonic motor behaviour (Costa and Furness, 1976), allowing the persistence of propulsive activities even in absence of central inputs (Bayliss and Starling, 1900). Nevertheless, the colon receives dense innervation from extrinsic noradrenergic sympathetic nerves (Tassicker et al., 1999; Olsson et al., 2006; Parker et al., 2022) which potently inhibits motility by supressing myenteric neurotransmission via action on presynaptic α2-receptors (Hirst and McKirdy, 1974; Stebbing et al., 2001) and actions on non-neural elements (Gillespie, 1962; Beani et al., 1969; Furness, 1969; Gulbransen et al., 2010; Kurahashi et al., 2020a; Kurahashi et al., 2020b; Zhang et al., 2022). Sympathetic outputs are under SCN control (Ueyama et al., 1999) and influence circadian rhythmicity of peripheral organs (Warren et al., 1994; Vujovic et al., 2008). Tyrosine hydroxylase activity, required for noradrenaline synthesis in sympathetic neurons, also shows circadian rhythmicity in the coeliac-superior mesenteric ganglia (Brusco et al., 1998); a major source of sympathetic innervation in the colon (Trudrung et al., 1994). Peripheral sympathetic nerve output may also be modulated by retinal light exposure (Niijima et al., 1992; Niijima et al., 1993; Mutoh et al., 2003; Ishida et al., 2005). Like other entraining factors, sympathetic influence on the colon may contribute to rhythmicity entrainment but is not essential, since rhythmic clock gene expression and fecal output patterns in mice persisted following sympathectomy but could be phase shifted by adrenergic receptor agonists (Malloy et al., 2012). On the other hand, an earlier study found sympathetic ablation abolished circadian fecal output patterns in rats, suggesting a more critical role (Du et al., 1987). In any case, the extrinsic sympathetic influence on colonic motility raises the possibility of circadian modulation of other colonic functions under sympathetic control, such as secretion and blood flow (Szurszewski and Linden, 2012). It is worth mentioning that gut epithelial cell proliferation shows circadian rhythmicity (Buchi et al., 1991; Marra et al., 1994; Scheving, 2000; Bjarnason and Jordan, 2002; Pácha and Sumová, 2013; Balounová et al., 2020) which is principally determined by feeding patterns (Yoshida et al., 2015) but are also modulated by sympathetic input (Tutton and Barkla, 1980; Kennedy et al., 1983; Tutton and Barkla, 1989). Parasympathetic vagal efferents are another potential source of extrinsic influence on the colon (Berthoud et al., 1991) that could impact circadian rhythmicity in motility, but few data are currently available. In mice, vagal pathways regulate clock gene expression in respiratory tissues (Bando et al., 2007), but were not required for the maintenance of clock gene rhythmicity in the stomach (Hoogerwerf et al., 2007).
Microbial products and circadian control of colonic function
Intraluminal products of microbial metabolism, particularly secondary bile acids and short chain fatty acids (SCFAs), have received attention as potential circadian entraining factors. Microbes and their metabolites are themselves subject to daily rhythms, highlighting a major potential source of variability in studies of the microbiome (Allaband et al., 2022). Partly driving these oscillations is rhythmic delivery of intraluminal content to the gut by feeding behaviour that is ultimately controlled by the SCN (Nagai et al., 1978) and clock gene oscillations (Turek et al., 2005). Gut microbial characteristics, including relative abundances, spatial organization and metabolism oscillate with feeding rhythmicity (Thaiss et al., 2014; Zarrinpar et al., 2014; Thaiss et al., 2016), modulating circadian profile of host peripheral gene transcription programs via direct microbe-epithelium interactions (Abreu, 2010; Wells et al., 2011; Mukherji et al., 2013; Clasen et al., 2023) and microbial metabolites such as polyamines, SCFAs and unconjugated bile acids (Leone et al., 2015; Govindarajan et al., 2016; Thaiss et al., 2016; Tahara et al., 2018). Specifically, the SCFAs evoked shifts in clock gene expression of multiple peripheral cell types (Leone et al., 2015; Tahara et al., 2018), including colonic epithelia (Desmet et al., 2021b). Yet, despite their coordinating influence, microbial entraining mechanisms may not be strictly necessary for peripheral core clock entrainment, since peripheral clock gene rhythmicity persisted following microbial ablation (Thaiss et al., 2016). Indeed, microbial circadian rhythmicity may depend on gut epithelial circadian clocks (Mukherji et al., 2013; Altaha et al., 2022; Heddes et al., 2022), although time-restricted feeding recapitulates features of normal microbial oscillation after core clock gene knockout (Thaiss et al., 2014; Segers et al., 2020).
Endogenous circadian rhythms have been present throughout evolution (Jabbur and Johnson, 2021), and the molecular clock used by Cyanobacteria is well characterised (Johnson et al., 2017). There is currently limited evidence for intrinsic circadian rhythms in non-photosynthetic bacteria (Eelderink-Chen et al., 2021) but the field of prokaryotic chronobiology has been described as young compared to the study of eukaryotic circadian systems (Johnson et al., 2017), largely leaving open the question whether gut microbes have their own oscillators. At least one bacterial species in the human gut microbiome has been identified that shows entrainable, temperature-compensating circadian oscillations, in vitro (Paulose and Cassone, 2016; Paulose et al., 2016; Paulose et al., 2019).
SCFAs arise from microbial metabolism of undigested carbohydrates; they have been identified in the gut of amphibians, birds, reptiles, fish, and mammals, including humans (McNeil, 1984; Pryor and Bjorndal, 2005; Blaak et al., 2020). In mammals, most SCFAs are produced in the caecum and colon (den Besten et al., 2013), with concentrations showing daily oscillation. In mice and rats fed ad libitum, most reports of caecal and blood SCFAs show peak concentrations around the early to mid-active period (Tahara et al., 2018; Segers et al., 2020; Han et al., 2021; Ding et al., 2022), preceding a colonic peak from the late active to mid inactive period (Henning and Hird, 1972; Yajima and Sakata, 1992; Segers et al., 2019; Desmet et al., 2021a; Desmet et al., 2021b). Core clock gene Bmal1 knockout in mice disrupted feeding patterns, microbial rhythmicity (Liang et al., 2015), and circadian SCFA fluctuations (Segers et al., 2019). Interestingly, sleep duration correlated with SCFA production in humans (Shimizu et al., 2023), who also show daily fluctuations in circulating SCFAs, peaking in the latter half of the day, after lunch and dinner (Wolever et al., 1997; Swanson et al., 2020; Brignardello et al., 2022). Peak colonic concentrations, particularly in the distal regions are presumed to be somewhat later.
Aside a potential role in entraining circadian signalling, the question arises whether cycling colonic SCFA levels may more directly exert regulatory effects on colonic functions, such as colonic motility. Reports of the acute colonic motor effects of single or multiple SCFAs range from predominantly inhibitory (Squires et al., 1992; Ono et al., 2004; Dass et al., 2007; West et al., 2017), mixed (Cherbut et al., 1998; Mitsui et al., 2005a; Hurst et al., 2014; Shaidullov et al., 2021), excitatory (Yajima, 1985; McManus et al., 2002; Fukumoto et al., 2003; Rondeau et al., 2003; Mitsui et al., 2005b; Grider and Piland, 2007; Tan et al., 2020), or without detectable effects (Flourie et al., 1989; Jouët et al., 2013; Vincent et al., 2018). Similarly, chronic SCFA elevation by various methods have shown inhibitory effects on colonic transit and contractility (Bardon and Fioramonti, 1983; Bajka et al., 2010; Patten et al., 2015; Yuan et al., 2020), or increased transit and contractility (Soret et al., 2010; Suply et al., 2012). Taking these and other considerations (Sakata, 2019) into account, it is difficult to determine how SCFA rhythmicity may affect the circadian cycle of colonic motility, if at all. To this end, Segers et al. (2019) quantified SCFA-mediated inhibition of nerve evoked contractility in proximal and distal colonic strips across the circadian cycle. Maximal and minimal inhibition occurred in the inactive and active periods, respectively, paralleling oscillation in expression of free fatty acid receptors 2 and 3 (Segers et al., 2019). This would suggest SCFA oscillation may indeed support inhibition of colonic motility in the inactive period. However, it will be important to show whether propulsion is also affected, as studies of acute SCFA application have occasionally identified inhibitory effects on contractility whilst facilitating colonic propulsive behaviour (Cherbut et al., 1998; Tan et al., 2020; Shaidullov et al., 2021).
Finally, it may be speculated that colonic SCFAs exert long range motility effects. Since the enteroendocrine cells and neural circuits underlying the ileal brake also exist in colon (Szurszewski and Linden, 2012; Hibberd T. et al., 2022; Holst et al., 2022; Zhang et al., 2022), an untested possibility is that SCFAs contribute to glucagon like peptide 1 (GLP-1) and peptide tyrosine tyrosine (PYY) release from colonic enteroendocrine cells (Freeland and Wolever, 2010; Psichas et al., 2015; Christiansen et al., 2018; Larraufie et al., 2018), supporting upper gastrointestinal inhibition at the endogenous SCFA daily peak via an ileal brake mechanism (Van Citters and Lin, 2006; Zhang et al., 2022). Compatible with this, intracolonic infusion of exogenous SCFAs suppressed gastric tone in humans, coinciding with elevated plasma PYY but not GLP-1 (Ropert et al., 1996).
Primary bile acids are delivered to the small intestine for nutrient digestion and can be transformed by intraluminal bacteria that express bile salt hydrolase to form secondary bile acids. These microbially-modified bile acids show daily rhythmicity in blood (Setchell et al., 1982; Steiner et al., 2011; Zhang et al., 2011; Eggink et al., 2017; Al-Khaifi et al., 2018) and faecal concentrations (Cui et al., 2022a; Altaha et al., 2022; Cui et al., 2022b), and may modify peripheral clock gene expression in the ileum, colon and liver (Govindarajan et al., 2016). Like SCFAs, secondary bile acids can exert direct effects on colonic motility (Alemi et al., 2013). Interestingly, circadian disruption evoked de novo circadian rhythmicity in bile acid receptor expression (Desmet et al., 2023).
Colonic motility and disruptions of colon rhythms in IBS and UC
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterised by recurrent abdominal pain and altered bowel habits: (constipation, diarrhea, or both; Moayyedi et al., 2017). More than 90% of patients experience abdominal pain; the symptoms that most severely disrupts quality of life (Cain et al., 2006; American Gastroenterological Association, 2015; Mearin et al., 2016). Gut symptoms of IBS and functional dyspepsia are significantly exacerbated by disruptions of circadian rhythms (Kim et al., 2013; Fowler et al., 2022). Circadian disruptions commonly occur through shift work, or work outside the normal 9a.m.-5p.m. hours. Shift work is strongly associated with an increased prevalence of IBS-related symptoms such as constipation or diarrhea, bloating, gas, and abdominal pain (Wells et al., 2012; Kim et al., 2013; Hyun et al., 2019; Rahimimoghadam et al., 2020; Roman et al., 2023), and alterations in the composition of the gut microbiome (Mortaş et al., 2020). In constipation-related IBS (IBS-C), the frequency of high-amplitude propagating colon contractions in patients are decreased over a 24-period (Bassotti et al., 2003). Conversely, in diarrhoea-related IBS (IBS-D) patients, the frequency of high-amplitude propagated contractions were higher during the active period compared to controls (Clemens et al., 2003). Simulated shift work in mice led to increased colon motility and permeability (Summa et al., 2013; Tran et al., 2021), and decreased apical junction complexes (Tran et al., 2021); factors which likely contribute to IBS-D.
Inflammatory bowel diseases, including UC, are chronic relapsing gastrointestinal disorders with increasing prevalence worldwide (Ng et al., 2017). Most patients with UC experience abdominal pain throughout their disease, profoundly impacting their quality of life (Zeitz et al., 2016). The severity of UC, characterised by inflammation and development of ulcers in the colon, is exacerbated by circadian disruptions. In humans, sleep disruptions worsened UC symptoms with increased colon permeability and pro-inflammatory cytokines (Sobolewska-Włodarczyk et al., 2020; Swanson et al., 2021). Animal studies suggest the increased severity of UC associated with circadian disturbances is likely due to impaired recovery. Clock controlled genes are implicated by observations that deletion of Bmal1 in dextran sulfate sodium (DSS)-induced colitis mice delayed colon epithelium regeneration via disruptions to rhythms of cell proliferation (Taleb et al., 2021) suggesting Bmal1 is necessary for UC recovery. Further, jetlag-induced circadian disruptions in DSS-induced colitis mice aggravated colitis, disrupted rhythms of Clock and Bmal1 expression, and reduced Per2 expression. Decreased Per2 expression was associated with decreased adenosine triphosphate and cell proliferation in the colonic epithelium via circadian modification of dynamin-related protein 1, which mediates mitochondrial fission (Chen et al., 2022).
Circadian rhythms of colonic absorption, permeability, and hormone secretion
Absorption
The human colon contributes to body water balance by reabsorbing 1.5–2 L of daily fluid inputs, which represents ∼20% of the total fluid intake of the gut (Barrett and Keely, 2022). One of the primary ways this is achieved is via electrogenic import of sodium ions through epithelial sodium channels (ENaC) located on the apical membrane of mucosal cells (Kunzelmann and Mall, 2002). Daily rhythmicity in electrical potential difference across colonic epithelium, reflecting changes in electrogenic absorption, was reported in rabbit colon and rectum with peak absorption in the dark period (Clauss, 1984; Clauss et al., 1988). Rabbits produce two types of faeces, hard and soft, which are excreted in the dark (active) and light (inactive) periods, respectively (Jilge, 1974). The latter are reingested during the light period (Jilge and Hudson, 2001), recovering nutrients made available by hindgut fermentation, including SCFAs (Henning and Hird, 1972; Vernay et al., 1984; Vernay, 1989). The least colonic reabsorption of sodium and water in the light period coincides with soft faeces production in rabbits. In contrast, mice and rats have more uniform faeces than rabbits but also show daily rhythms of colonic and rectal sodium absorption via amiloride-sensitive ENaC (Wang et al., 2000; Wang et al., 2004; Wang et al., 2010; Frateschi et al., 2012; Malsure et al., 2014). In mice and rats, the night (active) period is the peak period for both sodium reabsorption and defecation.
In addition to ENaC-mediated electrogenic transport, electroneutral absorption via Na+/H+ exchangers may have circadian rhythmicity as transcription of Na+/H+ hydrogen exchanger 3 (Nhe3) in rat colonic epithelium showed circadian rhythmicity under constant lighting conditions, peaking in the night (active) period (Sládek et al., 2007; Soták et al., 2011), paralleling the daily cycle of electrogenic transport via ENaC.
Corticosteroid influences on absorption
The early studies of colonic absorption identified the parallel rhythmic oscillations in corticosteroids as possible underlying mechanism for daily rhythms of absorption (Clauss, 1984; Clauss et al., 1988). Indeed, adrenalectomy blunted circadian rhythmicity in Nhe3 in intestinal epithelia (Vagnerová et al., 2019) and clock gene rhythmicity in colonic epithelia, which could be restored by exogenous gluococorticoids (Polidarová et al., 2017). Mineralocorticoids are also candidate entrainers of colonic absorption as aldosterone may entrain renal ENaC via regulation of Per1 (Gumz et al., 2009).
Permeability
Colonic permeability has been positively correlated with stool frequency in rats (Hou et al., 2019). Compatible with this, colonic permeability is reported to have a daily rhythm in mice, peaking in the night (active) phase: the period of greatest faecal pellet output (Oh-oka et al., 2014). Epithelial tight junctions are the main regulators of colonic permeability (Lee, 2015). Some evidence suggests tight junction proteins such as occludins and claudins, may be expressed with daily rhythmicity in the colon, putatively controlled by CLOCK-BMAL1 (Oh-oka et al., 2014). Colonic permeability is inversely associated with the expression of the occludin and claudin proteins. Colonic epithelial occludin mRNA expression peaked during the day (inactive) period and nadirs during the night (active) period in mice (Summa et al., 2013; Oh-oka et al., 2014; Desmet et al., 2021b). Evidence is currently mixed as to whether the same pattern occurs with colonic epithelial Claudin-1 mRNA expression (Oh-oka et al., 2014; Desmet et al., 2021b) and Bmal1 knockout did not affect colonic Claudin-1 mRNA expression in a recent study (Taleb et al., 2022).
GLP-1 secretion
Epithelial L-cells secrete the hormone glucagon-like peptide 1 (GLP-1) in response to luminal nutrients such as glucose, potentiating pancreatic glucose-evoked insulin secretion while inhibiting glucagon secretion (Drucker, 2018; Holst, 2022) and contribute to the so called “ileal-brake” (Read et al., 1984; Spiller et al., 1984) to acutely inhibit appetite (Flint et al., 1998; Giralt and Vergara, 1998; 1999; Zhang et al., 2022). L-cells occur in large numbers in the distal small intestine (Knudsen et al., 1975; Eissele et al., 1992) where their physiological effects are best characterised. Interestingly, their density increases along the colon and rectum where the role of GLP-1 is less understood (Holst et al., 2022) and are more likely activated by bile acids (Christiansen et al., 2019) rather than nutrients like glucose that are absorbed in the more proximal gut.
A daily rhythmicity of GLP-1 secretion was suggested by the observation that identical meals consumed at different times evoked significantly different plasma GLP-1 responses in humans, favouring higher GLP-1 secretion in the morning, compared to evening (Lindgren et al., 2009). A circadian rhythmicity of GLP-1 secretion was confirmed in rats (Gil-Lozano et al., 2014) and mice (Biancolin et al., 2020; Desmet et al., 2021b), depending on circadian rhythmicity of the BMAL1-controlled SNARE regulatory protein, secretagogin (Biancolin et al., 2020; Biancolin et al., 2022). Interestingly, GLP-1 secretion rhythmicity may not depend on entrainment by glucocorticoid rhythms (Gil-Lozano et al., 2016). However, GLP-1 secretion and L-cell core clock gene rhythms were deranged by high fat diets and microbial ablation, pointing to a critical role for the microbiome in maintaining GLP-1 secretion rhythmicity (Gil-Lozano et al., 2016; Martchenko et al., 2018; Martchenko et al., 2020).
Circadian rhythm of colonic afferents and pain
Daily rhythmicity in pain perception in humans is commonly reported, with peak and nadir timing varying across sensory modalities and pathophysiological conditions (Aviram et al., 2015; Daguet et al., 2022; Mun et al., 2022). The first order neurons involved in sensory signalling from the colon are vagal and spinal afferents. In other gastrointestinal organs such as the stomach, mucosal and tension receptors of the vagal nerve have a circadian rhythm in mechanosensitivity, inversely proportionate to food intake (Page, 2021). Their excitability is higher at the onset of the active-compared to inactive period (Kentish et al., 2013). Currently no studies have investigated the circadian rhythm modulation of sensory vagal fibres that innervate the proximal or distal colon. However, recent work has identified that vagal afferent signalling to second order neurons in the nucleus tractus solitarius (NTS) also shows circadian variability that favours throughput of afferent-driven signalling during the active period, and passive spontaneous firing during the inactive period (Ragozzino et al., 2023). It remains to be determined whether similar mechanisms govern circadian variation of signalling efficacy to the CNS in spinal afferent pathways.
Colonic spinal afferents and their function have been reviewed extensively elsewhere (Brierley et al., 2018). In brief, colonic afferents send mechanical and chemical signals about the colon (e.g., luminal contents and wall stretch) to the spinal cord via the lumbar splanchnic and sacral pelvic nerves. These afferents have been classified into five major types, muscular, mucosal, muscular-mucosal, vascular, and silent (Brierley et al., 2018). Surprisingly, circadian rhythms of colonic afferents have, to date, not been directly investigated. Interestingly, bladder afferents derive from lumbar splanchnic and sacral pelvic nerves like the afferent supply to the distal colon and show strong time-of-day regulation of sensitivity, raising the possibility similar variations occur in colon. At least 3 classes of bladder afferents (stretch-insensitive mucosal and stretch-sensitive low and high threshold muscular-mucosal afferents) demonstrated significantly increased sensitivity to mechanical stimuli like stroking and stretch during the active-, compared to the inactive period, suggesting strong circadian regulation of spinal sensory neuron excitability (Christie and Zagorodnyuk, 2021; Ramsay and Zagorodnyuk, 2023).
In the distal colon, potential circadian regulation of colonic afferents could be inferred through measurements of visceromotor responses (VMRs), that can be assessed by recording abdominal EMG activity, evoked by colonic distension. Distension of hollow visceral organs evoked VMRs that may serve as an indirect indication of visceral afferent activity, and, at noxious distensions (>40 mmHg) VMRs are used as surrogate measure of pain (Ness and Gebhart, 1988; Ness and Elhefni, 2004; Zagorodnyuk et al., 2011; Kyloh et al., 2022). An early study reported that VMRs evoked by colorectal distension in rats exhibits a daily rhythm with significant increase in the response seen in active period (at night) (Gschossmann et al., 2001). However, a more recent study reported that distension-evoked VMRs in rats do not exhibit a daily rhythm (Botschuijver et al., 2016). The reason for this conflicting information between studies is not clear but may involve different distension methods (volume versus isobaric), conscious freely moving versus restrained animals, and/or differences in the strains of rats used. Compatible with the idea that visceral afferent sensitivity and signalling efficacy to the CNS may be enhanced during the active-compared to inactive period, human data indicates perception thresholds to rectal distension stimuli for urge and pain was lower in the morning than evening (Enck et al., 2009). Interestingly, daily variations in sensory signalling may differ by region and sensory modality; peak visceral pain sensitivities in the active period differs to those for cutaneous thermal and mechanical pain and in conditions like neuropathic pain and cluster headache which peak during the inactive period (Mun et al., 2022).
Melatonin
Melatonin arises from multiple sources, of which the best known is nocturnally generated pineal melatonin. However, extra-pineal melatonin is a far greater source of melatonin in the body, much of which may be generated in mitochondria where it controls oxidative processes and which may represent its original site of synthesis in evolution (for review, see Tan et al., 2013; Zimmerman and Reiter, 2019; Tan et al., 2023). In the gut, melatonin is predominantly contained in the epithelial cells along the whole gastrointestinal tract (Bubenik et al., 1977; Bubenik, 1980; Holloway et al., 1980; Lee and Pang, 1993; Poon et al., 1996; Söderquist et al., 2015). Like serotonin (Gershon, 2022), more of the body’s melatonin is synthesized in the gut than in the brain (Huether, 1993). Both melatonin and serotonin released from mucosa give rise locally to micromolar concentrations in mouse ileum and colon (Bertrand et al., 2010; Diss et al., 2013).
Melatonin effects on gut smooth muscle
Melatonin is both water and lipid-soluble, so it can penetrate the cell membrane and act on intracellular receptors of the RORα family and/or directly on intracellular proteins including Ca2+ binding protein, calmodulin and Ca2+/calmodulin-dependent kinase II (CaMKII) (Landau and Zisapei, 2007; Hardeland et al., 2011; Han et al., 2012). Melatonin is capable of inhibiting smooth muscle of urogenital organs including myometrium and detrusor muscle: these direct effects likely due to its ability to inhibit Ca2+ channels and Ca/MKII system (Ouyang and Vogel, 1998; Ayar et al., 2001; Han et al., 2012).
Melatonin may have two different effects on the vascular smooth muscle, with vasoconstriction mediated via MT1 and vasodilation–via MT2 (Harlow and Weekley, 1986). In dispersed gastric smooth muscle cells, melatonin-evoked contraction was mediated by MT1 activation of Gq to stimulate phosphoinositide hydrolysis and increased cytosolic Ca2+ (Ahmed et al., 2013). In small gut segments, melatonin decreased rat small intestine and colon contractility, whereas it evoked contraction of guinea pig proximal colon (Harlow and Weekley, 1986; Lucchelli et al., 1997). Melatonin’s inhibitory effects on rat ileal smooth muscle may be mediated by Ca2+ activated K+ channels (Reyes-Vázquez et al., 1997). Smooth muscle responses to melatonin in the studies by Lucchelli et al. (1997) and Reyes-Vázquez et al. (1997) were not significantly affected by neuronal blockade, suggesting enteric neurons were not involved. Taken together, melatonin has potential to directly affect colonic smooth muscle function, but its importance under normal physiological conditions is not characterised.
Melatonin effects on the enteric neurons
In enteric neurons, MT1 receptor immunofluorescence was weak or undetectable in human colonic submucous and myenteric plexus, but MT2 receptor immunoreactivity was generally stronger, ranging from weak to strong in both plexuses (Söderquist et al., 2015). Mtnr1a mRNA was also reported in rat small intestine myenteric neurons (Soták et al., 2006). Electrophysiologically, exogenous melatonin did not affect membrane potential or input resistance, but inhibited nicotinic synaptic input in guinea pig ileum submucous neurons (Barajas-López et al., 1996). In mouse colon, an inhibitory action of melatonin on neuronal NOS was inferred by its reduction of the slow (nitric oxide-mediated) (Shuttleworth et al., 1997; Kuriyama et al., 1998) component of the inhibitory junction potential (Storr et al., 2002). Whether these actions of exogenous melatonin relate to any endogenous role, or the circadian regulation of colonic functions remains to be established.
Melatonin and gut motility
Melatonin is released into circulation by the pineal gland during the dark and is hormonal regulator of circadian rhythms. There is some evidence of pineal melatonin involvement in regulation of the interdigestive migrating motor complex (MMC; Szurszewski, 1969) in rats (Bonouali-Pellissier, 1994). Pineal or exogenous melatonin does not affect clock gene expression in rat or mouse colonic epithelial cells (Polidarová et al., 2017), suggesting melatonin plays no role in entraining these peripheral clocks. Melatonin is produced peripherally (Huether et al., 1992; Huether, 1993) in a non-circadian manner (Bubenik, 2002) by the gut enterochromaffin cells in response to food intake, with melatonin levels sharply rising after a meal (Bron and Furness, 2009; Duboc et al., 2020). Exogenous melatonin can modulate colonic transit, and this may be dose dependent. One study has demonstrated that 3 mg of melatonin daily increases colon transit time in healthy humans (Lu et al., 2009). Another study in rats reported that low doses of melatonin (10 μg/kg) increased colonic transit whilst high doses (1 mg/kg) decreased it (Drago et al., 2002), suggesting a potential biphasic effect, which is often seen for G-protein coupled receptors. The underlying mechanisms of melatonin action on colonic motility are not known. In in vivo studies of the small intestine, nonselective MT1 and MT2 melatonin receptor antagonist, S-22153 suppresses nocturnal variations in interdigestive MMC frequency in the rat small intestine (Merle et al., 2000). This may suggest an involvement of melatonin in physiological regulation in the pre- and postprandial changes of intestinal motility (Merle et al., 2000). Melatonin in pharmacological doses (1 mg/kg) increased frequency of MMC by reducing the duration of irregular spiking activity and of the quiescent period (Merle et al., 2000).
Melatonin in the treatment of IBS and UC
Melatonin has potential as a therapeutic for the treatment of IBS and UC symptoms, although reports are conflicting. It has been shown that melatonin (3 mg) improves abdominal pain associated with both IBS-C and IBS-D (Song et al., 2005). However, it is also reported that melatonin (3 mg) improves abdominal pain in only IBS-C and not IBS-D (Chojnacki et al., 2013). Other studies also indicated that melatonin (3 mg) improved abdominal pain, however, the type of IBS was not specified (Saha et al., 2007). Similarly, the effect of melatonin on stool frequency and colonic transit in IBS is conflicting. It has been shown that melatonin (3 mg) only improves stool frequency and colonic transit in IBS-C patients (Chojnacki et al., 2013; Mishchuk et al., 2019). However, it is also reported that melatonin has no effect on stool frequency and colonic transit in IBC-D and IBC-C patients compared with placebo (Lu et al., 2009). It should be noted that other, greater affinity, MT1 and MT2 agonists, such as agomelatine, have been studied for their potential in the treatment of IBS-D. Agomelatine (25 mg) significantly improved overall symptoms in IBS-D patients (Balakina et al., 2014). However, agomelatine is also a 5-HT2C and 5-HT2B receptor antagonist (Guardiola-Lemaitre et al., 2014) which suggests agomelatine may influence colonic motility acting on 5-HT receptors.
As previously mentioned, disruptions to circadian rhythms can exacerbate UC signs and pathology. In UC-circadian disrupted mice, treatment with melatonin reduced the signs and severity of inflammation in the colon (Park et al., 2015; Liu and Wang, 2019) which was abolished by the non-specific MT1 and MT2 antagonist luzindole (Liu and Wang, 2019). Similar effects of melatonin are also seen in UC mice without circadian disruptions (Trivedi and Jena, 2013). It has been speculated that patients with UC may have increased synthesis of melatonin in the colonic mucosa (Vaccaro et al., 2023). It is likely that in the treatment of UC, melatonin exhibits a protective, anti-oxidative effect on the colonic mucosa.
Conclusion
A wide array of colonic functions shows circadian rhythmicity optimized to the period of food intake. Disruptions of these rhythms can cause organ disorders or exacerbate pre-existing ones. Multiple neural, hormonal and intraluminal mechanisms may contribute to the entrainment of circadian variation in colonic functions, but their full details remain to be elucidated. Gut melatonin, in contrast with pineal melatonin, may be principally arrhythmic in function but nevertheless may have therapeutic potential in its exogenous application for treatment of gut disorders that are exacerbated by circadian disruption.
Statements
Author contributions
SR and TH drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
National Health and Medical Research Council (NHMRC) Project grant #1156416 and Australian Research Council (ARC) Discovery Project grant #DP190103628 to NS, and NHMRC grant #1184546 to VZ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AbreuM. T. (2010). Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol.10, 131–144. 10.1038/nri2707
2
AdamovichY.Rousso-NooriL.ZwighaftZ.Neufeld-CohenA.GolikM.Kraut-CohenJ.et al (2014). Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab.19, 319–330. 10.1016/j.cmet.2013.12.016
3
AhmedR.MahavadiS.Al-ShboulO.BhattacharyaS.GriderJ. R.MurthyK. S. (2013). Characterization of signaling pathways coupled to melatonin receptors in gastrointestinal smooth muscle. Regul. Pept.184, 96–103. 10.1016/j.regpep.2013.03.028
4
Al-KhaifiA.StranieroS.VoronovaV.ChernikovaD.SokolovV.KumarC.et al (2018). Asynchronous rhythms of circulating conjugated and unconjugated bile acids in the modulation of human metabolism. J. Intern Med.284, 546–559. 10.1111/joim.12811
5
AlemiF.PooleD. P.ChiuJ.SchoonjansK.CattaruzzaF.GriderJ. R.et al (2013). The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology144, 145–154. 10.1053/j.gastro.2012.09.055
6
AllabandC.LingarajuA.RamosS. F.KumarT.JavaheriH.TiuM. D.et al (2022) Time of sample collection critical for microbiome replicability. bioRxiv. 2022.2010.2026.513817. 10.1101/2022.10.26.513817
7
AllenM.JohnsonR. A. (2018). Evaluation of self-injurious behavior, thermal sensitivity, food intake, fecal output, and pica after injection of three buprenorphine formulations in rats (Rattus norvegicus). Am. J. Vet. Res.79, 697–703. 10.2460/ajvr.79.7.697
8
AltahaB.HeddesM.PilorzV.NiuY.GorbunovaE.GiglM.et al (2022). Genetic and environmental circadian disruption induce weight gain through changes in the gut microbiome. Mol. Metab.66, 101628. 10.1016/j.molmet.2022.101628
9
American Gastroenterological Association (2015). IBS in America: Survey summary findings. Retrieved November 5, 2016.
10
AschoffJ. (1981). “Freerunning and entrained circadian rhythms,” in Biological rhythms. Editors AschoffJ. (Boston, MA: Springer US), 81–93.
11
AschoffJ. (1994). The timing of defecation within the sleep-wake cycle of humans during temporal isolation. J. Biol. Rhythms9, 43–50. 10.1177/074873049400900104
12
AstizM.HeydeI.OsterH. (2019). Mechanisms of communication in the mammalian circadian timing system. Int. J. Mol. Sci.20, 343. 10.3390/ijms20020343
13
AubèL.FatnassiM.MonacoD.KhorchaniT.LacalandraG. M.HammadiM.et al (2017). Daily rhythms of behavioral and hormonal patterns in male dromedary camels housed in boxes. PeerJ5, e3074. 10.7717/peerj.3074
14
AuwerdaJ. J.BacD. J.SchoutenW. R. (2001). Circadian rhythm of rectal motor complexes. Dis. Colon Rectum44, 1328–1332. 10.1007/BF02234793
15
AviramJ.ShochatT.PudD. (2015). Pain perception in healthy young men is modified by time-of-day and is modality dependent. Pain Med.16, 1137–1144. 10.1111/pme.12665
16
AyarA.KutluS.YilmazB.KelestimurH. (2001). Melatonin inhibits spontaneous and oxytocin-induced contractions of rat myometrium in vitro. Neuro Endocrinol. Lett.22, 199–207. https://www.nel.edu/melatonin-inhibits-spontaneous-and-oxytocin-induced-contractions-of-rat-myometrium-in-vitro-2344/.
17
BajkaB. H.ClarkeJ. M.ToppingD. L.CobiacL.AbeywardenaM. Y.PattenG. S. (2010). Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats. Nutr. Res.30, 427–434. 10.1016/j.nutres.2010.06.003
18
BalakinaI. V.MironovaT.GolovanovaE. N. (2014). Efficacy and safety of valdoxan in patients with irritable bowel syndrome. Zh Nevrol. Psikhiatr Im. S S Korsakova114, 90–92.
19
BalounováK.SotákM.ErgangP.VodičkaM.VagnerováK.PáchaJ. (2020). Effects of aging and tumorigenesis on coupling between the circadian clock and cell cycle in colonic mucosa. Mech. Ageing Dev.190, 111317. 10.1016/j.mad.2020.111317
20
BandoH.NishioT.van der HorstG. T.MasubuchiS.HisaY.OkamuraH. (2007). Vagal regulation of respiratory clocks in mice. J. Neurosci.27, 4359–4365. 10.1523/JNEUROSCI.4131-06.2007
21
Barajas-LópezC.PeresA. L.Espinosa-LunaR.Reyes-VázquezC.Prieto-GómezB. (1996). Melatonin modulates cholinergic transmission by blocking nicotinic channels in the Guinea-pig submucous plexus. Eur. J. Pharmacol.312, 319–325. 10.1016/0014-2999(96)00481-5
22
BardonT.FioramontiJ. (1983). Nature of the effects of bran on digestive transit time in pigs. Br. J. Nutr.50, 685–690. 10.1079/bjn19830140
23
BarrettK. E.KeelyS. J. (2022). Electrolyte secretion and absorption in the small intestine and colon. Yamada's Textb. Gastroenterology, 283–312. 10.1002/9781119600206.ch16
24
BasinouV.ParkJ. S.CederrothC. R.CanlonB. (2017). Circadian regulation of auditory function. Hear. Res.347, 47–55. 10.1016/j.heares.2016.08.018
25
BassottiG.BettiC.FusaroC.MorelliA. (1992). Colonic high‐amplitude propagated contractions (mass movements): repeated 24‐h manometric studies in healthy volunteers. Neurogastroenterol. Motil.4, 187–191. 10.1111/j.1365-2982.1992.tb00160.x
26
BassottiG.ChistoliniF.MarinozziG.MorelliA. (2003). Abnormal colonic propagated activity in patients with slow transit constipation and constipation-predominant irritable bowel syndrome. Digestion68, 178–183. 10.1159/000075554
27
BassottiG.GaburriM. (1988). Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am. J. Physiology255, G660–G664. 10.1152/ajpgi.1988.255.5.G660
28
BassottiG.IantornoG.FiorellaS.Bustos-FernandezL.BilderC. R. (1999). Colonic motility in man: features in normal subjects and in patients with chronic idiopathic constipation. Am. J. Gastroenterology94, 1760–1770. 10.1111/j.1572-0241.1999.01203.x
29
BaylissW. M.StarlingE. H. (1900). The movements and the innervation of the large intestine. J. Physiology26, 107–118. 10.1113/jphysiol.1900.sp000825
30
BeaniL.BianchiC.CremaA. (1969). The effect of catecholamines and sympathetic stimulation on the release of acetylcholine from the Guinea-pig colon. Br. J. Pharmacol.36, 1–17. 10.1111/j.1476-5381.1969.tb08298.x
31
BernsteinI. S. (1964). A field study of the activities of howler monkeys. Anim. Behav.12, 92–97. 10.1016/0003-3472(64)90108-3
32
BerthoudH.CarlsonN.PowleyT. (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiology260, R200–R207. 10.1152/ajpregu.1991.260.1.R200
33
BertrandP. P.BertrandR. L.CamelloP. J.PozoM. J. (2010). Simultaneous measurement of serotonin and melatonin from the intestine of old mice: the effects of daily melatonin supplementation. J. Pineal Res.49, 23–34. 10.1111/j.1600-079X.2010.00760.x
34
BharuchaA. E.BrookesS. J. H. (2012). “Neurophysiologic mechanisms of human large intestinal motility,” in Physiology of the gastrointestinal tract. Editors GhishanF. K.Fifth Edition (Boston: Academic Press), 977–1022.
35
BiancolinA. D.MartchenkoA.MitovaE.GurgesP.MichalchyshynE.ChalmersJ. A.et al (2020). The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab.31, 124–137. 10.1016/j.molmet.2019.11.004
36
BiancolinA. D.SrikrishnarajA.JeongH.MartchenkoA.BrubakerP. L. (2022). The cytoskeletal transport protein, secretagogin, is essential for diurnal glucagon-like peptide-1 secretion in mice. Endocrinology163, bqac142. 10.1210/endocr/bqac142
37
BjarnasonG. A.JordanR. (2002). Rhythms in human gastrointestinal mucosa and skin. Chronobiol Int.19, 129–140. 10.1081/cbi-120002595
38
BlaakE. E.CanforaE. E.TheisS.FrostG.GroenA. K.MithieuxG.et al (2020). Short chain fatty acids in human gut and metabolic health. Benef. Microbes11, 411–455. 10.3920/BM2020.0057
39
Bonouali-PellissierS. (1994). Melatonin is involved in cholecystokinin-induced changes of ileal motility in rats. J. Pineal Res.17, 79–85. 10.1111/j.1600-079x.1994.tb00117.x
40
BotschuijverS.YuZ.WeltingO.CailottoC.KalsbeekA.van den WijngaardR. (2016). Absence of diurnal variation in visceromotor response to colorectal distention in normal Long Evans rats. F1000Res5, 98. 10.12688/f1000research.7238.1
41
BrierleyS. M.HibberdT. J.SpencerN. J. (2018). Spinal afferent innervation of the colon and rectum. Front. Cell Neurosci.12, 467. 10.3389/fncel.2018.00467
42
BrignardelloJ.FountanaS.PosmaJ. M.ChambersE. S.NicholsonJ. K.WistJ.et al (2022). Characterization of diet-dependent temporal changes in circulating short-chain fatty acid concentrations: A randomized crossover dietary trial. Am. J. Clin. Nutr.116, 1368–1378. 10.1093/ajcn/nqab211
43
BronR.FurnessJ. B. (2009). Rhythm of digestion: keeping time in the gastrointestinal tract. Clin. Exp. Pharmacol. Physiol.36, 1041–1048. 10.1111/j.1440-1681.2009.05254.x
44
BruscoL. I.García-BonachoM.EsquifinoA. I.CardinaliD. P. (1998). Diurnal rhythms in norepinephrine and acetylcholine synthesis of sympathetic ganglia, heart and adrenals of aging rats: effect of melatonin. J. Aut. Nerv. Syst.74, 49–61. 10.1016/s0165-1838(98)00134-9
45
BubenikG. A.BrownG. M.GrotaL. J. (1977). Immunohistological localization of melatonin in the rat digestive system. Experientia33, 662–663. 10.1007/BF01946561
46
BubenikG. A. (2002). Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci.47, 2336–2348. 10.1023/a:1020107915919
47
BubenikG. A. (1980). Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Horm. Res.12, 313–323. 10.1159/000179137
48
BuchiK. N.MooreJ. G.HrusheskyW. J.SothernR. B.RubinN. H. (1991). Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterology101, 410–415. 10.1016/0016-5085(91)90019-h
49
CainK. C.HeadstromP.JarrettM. E.MotzerS. A.ParkH.BurrR. L.et al (2006). Abdominal pain impacts quality of life in women with irritable bowel syndrome. Am. J. Gastroenterol.101, 124–132. 10.1111/j.1572-0241.2006.00404.x
50
CaoX.WangL.SelbyC. P.Lindsey-BoltzL. A.SancarA. (2023). Analysis of mammalian circadian clock protein complexes over a circadian cycle. J. Biol. Chem.299, 102929. 10.1016/j.jbc.2023.102929
51
CaoX.YangY.SelbyC. P.LiuZ.SancarA. (2021). Molecular mechanism of the repressive phase of the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A.118, e2021174118. 10.1073/pnas.2021174118
52
CatonJ. M.HillD. M.HumeI. D.CrookG. A. (1996). The digestive strategy of the common marmoset, Callithrix jacchus. Comp. Biochem. Physiol. A Physiol.114, 1–8. 10.1016/0300-9629(95)02013-6
53
ChalletE. (2019). The circadian regulation of food intake. Nat. Rev. Endocrinol.15, 393–405. 10.1038/s41574-019-0210-x
54
ChenY. D.ZhaoR. F.ZhengG.LingF. M.LiJ. R.XuM. Y.et al (2022). The association between disruption of the circadian rhythm and aggravation of colitis in mice. Gastroenterol. Rep. (Oxf)10, goac028. 10.1093/gastro/goac028
55
CherbutC.FerrierL.RozeC.AniniY.BlottiereH.LecannuG.et al (1998). Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiology275, G1415–G1422. 10.1152/ajpgi.1998.275.6.G1415
56
ChoH.ZhaoX.HatoriM.YuR. T.BarishG. D.LamM. T.et al (2012). Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature485, 123–127. 10.1038/nature11048
57
ChojnackiC.Walecka-KapicaE.LokiećK.PawłowiczM.WinczykK.ChojnackiJ.et al (2013). Influence of melatonin on symptoms of irritable bowel syndrome in postmenopausal women. Endokrynol. Pol.64, 114–120. 10.5603/ep.34290
58
ChristiansenC. B.GabeM. B. N.SvendsenB.DragstedL. O.RosenkildeM. M.HolstJ. J. (2018). The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiology - Gastrointest. Liver Physiology315, G53–g65. 10.1152/ajpgi.00346.2017
59
ChristiansenC. B.TrammellS. A. J.Wewer AlbrechtsenN. J.SchoonjansK.AlbrechtsenR.GillumM. P.et al (2019). Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiology - Gastrointest. Liver Physiology316, G574–g584. 10.1152/ajpgi.00010.2019
60
ChristieS.ZagorodnyukV. (2021). Time-of-day dependent changes in Guinea pig bladder afferent mechano-sensitivity. Sci. Rep.11, 19283. 10.1038/s41598-021-98831-x
61
ClarkeP. L. (1979). Coccidial infection with Eimeria tenella and caecal defaecation in chicks. Br. Poult. Sci.20, 317–322. 10.1080/00071667908416586
62
ClasenS. J.BellM. E. W.BorbónA.LeeD. H.HenselerZ. M.de la Cuesta-ZuluagaJ.et al (2023). Silent recognition of flagellins from human gut commensal bacteria by Toll-like receptor 5. Sci. Immunol.8, eabq7001. 10.1126/sciimmunol.abq7001
63
ClaussW. (1984). Circadian rhythms in Na transport. Intestinal Absorpt. Secret., 273–283.
64
ClaussW.DürrJ. E.KrattenmacherR.HörnickeH.Van DriesscheW. (1988). Circadian rhythm of apical Na-channels and Na-transport in rabbit distal colon. Experientia44, 608–610. 10.1007/BF01953312
65
ClaustratB.BrunJ.ChazotG. (2005). The basic physiology and pathophysiology of melatonin. Sleep. Med. Rev.9, 11–24. 10.1016/j.smrv.2004.08.001
66
ClemensC. H.SamsomM.Van Berge HenegouwenG. P.SmoutA. J. (2003). Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig. Dis. Sci.48, 74–82. 10.1023/a:1021734414976
67
CorsettiM.CostaM.BassottiG.BharuchaA. E.BorrelliO.DinningP.et al (2019). First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat. Rev. Gastroenterol. Hepatol.16, 559–579. 10.1038/s41575-019-0167-1
68
CostaM.DoddsK. N.WiklendtL.SpencerN. J.BrookesS. J.DinningP. G. (2013). Neurogenic and myogenic motor activity in the colon of the Guinea pig, mouse, rabbit, and rat. Am. J. Physiology-Gastrointestinal Liver Physiology305, G749–G759. 10.1152/ajpgi.00227.2013
69
CostaM.FurnessJ. B. (1976). The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn-Schmiedeberg's Archives Pharmacol.294, 47–60. 10.1007/BF00692784
70
CostaM.HibberdT. J.KeightleyL. J.WiklendtL.KylohM. A.DinningP. G.et al (2021). Novel intrinsic neurogenic and myogenic mechanisms underlying the formation of faecal pellets along the large intestine of Guinea-pigs. J. Physiol.599, 4561–4579. 10.1113/JP282069
71
CowanI. M.O'riordanA.CowanJ. M. (1974). Energy requirements of the dasyurid marsupial mouse Antechinus swainsonii (Waterhouse). Can. J. Zoology52, 269–275. 10.1139/z74-033
72
CrowellM. D.BassottiG.CheskinL. J.SchusterM. M.WhiteheadW. E. (1991). Method for prolonged ambulatory monitoring of high-amplitude propagated contractions from colon. Am. J. Physiology261, G263–G268. 10.1152/ajpgi.1991.261.2.G263
73
CrowellM. D.MusialF.FrenchW.KitturD.AndersonD.WhiteheadW. E. (1992). Prolonged ambulatory monitoring of colonic motor activity in the pig. Physiology Behav.52, 471–474. 10.1016/0031-9384(92)90332-v
74
CuiY.LiS.YinY.LiX.LiX. (2022a). Daytime restricted feeding promotes circadian desynchrony and metabolic disruption with changes in bile acids profiles and gut microbiota in C57BL/6 Male Mice. J. Nutr. Biochem.109, 109121. 10.1016/j.jnutbio.2022.109121
75
CuiY.YinY.LiS.WuZ.XieY.QianQ.et al (2022b). Apple polyphenol extract modulates bile acid metabolism and gut microbiota by regulating the circadian rhythms in daytime-restricted high fat diet feeding C57BL/6 male mice. Food Funct.13, 2805–2822. 10.1039/d1fo04116a
76
DaguetI.RaverotV.BouhassiraD.GronfierC. (2022). Circadian rhythmicity of pain sensitivity in humans. Brain145, 3225–3235. 10.1093/brain/awac147
77
DamiolaF.Le MinhN.PreitnerN.KornmannB.Fleury-OlelaF.SchiblerU. (2000). Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev.14, 2950–2961. 10.1101/gad.183500
78
DassN. B.JohnA. K.BassilA. K.CrumbleyC. W.SheheeW. R.MaurioF. P.et al (2007). The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol. Motil.19, 66–74. 10.1111/j.1365-2982.2006.00853.x
79
de AzevedoG. V.RodriguezR.PortoS. M.Graeff-TeixeiraC.FornariF. (2011). Elimination of angiostrongylus costaricensis larvae in feces from experimentally infected Swiss mice: circadian rhythm and correlation with survival. Parasitol. Res.108, 537–540. 10.1007/s00436-010-2094-5
80
den BestenG.van EunenK.GroenA. K.VenemaK.ReijngoudD. J.BakkerB. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res.54, 2325–2340. 10.1194/jlr.R036012
81
DesmetL.ThijsT.MasR.VerbekeK.DepoortereI. (2021a). Time-restricted feeding in mice prevents the disruption of the peripheral circadian clocks and its metabolic impact during chronic jetlag. Nutrients13, 3846. 10.3390/nu13113846
82
DesmetL.ThijsT.SegersA.DepoortereI. (2023). Chronic jetlag reprograms gene expression in the colonic smooth muscle layer inducing diurnal rhythmicity in the effect of bile acids on colonic contractility. Neurogastroenterol. Motil.35, e14487. 10.1111/nmo.14487
83
DesmetL.ThijsT.SegersA.VerbekeK.DepoortereI. (2021b). Chronodisruption by chronic jetlag impacts metabolic and gastrointestinal homeostasis in male mice. Acta Physiol. (Oxf)233, e13703. 10.1111/apha.13703
84
DibnerC.SchiblerU.AlbrechtU. (2010). The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol.72, 517–549. 10.1146/annurev-physiol-021909-135821
85
DickmeisT. (2009). Glucocorticoids and the circadian clock. J. Endocrinol.200, 3–22. 10.1677/JOE-08-0415
86
DingL.LiuJ.ZhouL.JiaX.LiS.ZhangQ.et al (2022). A high-fat diet disrupts the hepatic and adipose circadian rhythms and modulates the diurnal rhythm of gut microbiota-derived short-chain fatty acids in gestational mice. Front. Nutr.9, 925390. 10.3389/fnut.2022.925390
87
DinningP.WiklendtL.MaslenL.GibbinsI.PattonV.ArkwrightJ.et al (2014). Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol. Motil.26, 1443–1457. 10.1111/nmo.12408
88
DinningP. G.SiaT. C.KumarR.Mohd RosliR.KylohM.WattchowD. A.et al (2016). High-resolution colonic motility recordings in vivo compared with ex vivo recordings after colectomy, in patients with slow transit constipation. Neurogastroenterol. Motil.28, 1824–1835. 10.1111/nmo.12884
89
DissL. B.RobinsonS. D.WuY.FidalgoS.YeomanM. S.PatelB. A. (2013). Age-related changes in melatonin release in the murine distal colon. ACS Chem. Neurosci.4, 879–887. 10.1021/cn4000617
90
DorfmanL.El-ChammasK.MansiS.KaulA. (2022). Gastrocolonic response. Curr. Gastroenterol. Rep.24, 137–144. 10.1007/s11894-022-00849-2
91
DragoF.MacaudaS.SalehiS. (2002). Small doses of melatonin increase intestinal motility in rats. Dig. Dis. Sci.47, 1969–1974. 10.1023/a:1019696006677
92
DrakeM. J.MillsI. W.NobleJ. G. (2004). Melatonin pharmacotherapy for nocturia in men with benign prostatic enlargement. J. Urology171, 1199–1202. 10.1097/01.ju.0000110442.47593.ea
93
DrokhlyanskyE.SmillieC. S.Van WittenbergheN.EricssonM.GriffinG. K.EraslanG.et al (2020). The human and mouse enteric nervous system at single-cell resolution. Cell182, 1606–1622. 10.1016/j.cell.2020.08.003
94
DruckerD. J. (2018). Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab.27, 740–756. 10.1016/j.cmet.2018.03.001
95
DuC.FerreJ. P.RuckebuschY. (1987). Spinal cord influences on the colonic myoelectrical activity of fed and fasted rats. J. Physiol.383, 395–404. 10.1113/jphysiol.1987.sp016415
96
DubocH.CoffinB.SiproudhisL. (2020). Disruption of circadian rhythms and gut motility: an overview of underlying mechanisms and associated pathologies. J. Clin. Gastroenterology54, 405–414. 10.1097/MCG.0000000000001333
97
DubocovichM. L.MarkowskaM. (2005). Functional MT1 and MT2 melatonin receptors in mammals. Endocrine27, 101–110. 10.1385/ENDO:27:2:101
98
DubocovichM. L. (2007). Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep. Med.8 (3), 34–42. 10.1016/j.sleep.2007.10.007
99
DuffyJ. F.CzeislerC. A. (2009). Effect of light on human circadian physiology. Sleep. Med. Clin.4, 165–177. 10.1016/j.jsmc.2009.01.004
100
Eelderink-ChenZ.BosmanJ.SartorF.DoddA. N.Kovács ÁT.MerrowM. (2021). A circadian clock in a nonphotosynthetic prokaryote. Sci. Adv.7, eabe2086. 10.1126/sciadv.abe2086
101
EgginkH. M.OostermanJ. E.de GoedeP.de VriesE. M.FoppenE.KoehorstM.et al (2017). Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Chronobiol Int.34, 1339–1353. 10.1080/07420528.2017.1363226
102
EisseleR.GökeR.WillemerS.HarthusH. P.VermeerH.ArnoldR.et al (1992). Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest.22, 283–291. 10.1111/j.1365-2362.1992.tb01464.x
103
ElfersK.ArmbrechtY.Mazzuoli-WeberG. (2021). Good to know: baseline data on feed intake, fecal pellet output and intestinal transit time in Guinea pig as a frequently used model in gastrointestinal researc. Anim. (Basel)11, 1593. 10.3390/ani11061593
104
EnckP.KaiserC.FelberM.RieplR. L.KlauserA.KlosterhalfenS.et al (2009). Circadian variation of rectal sensitivity and gastrointestinal peptides in healthy volunteers. Neurogastroenterol. Motil.21, 52–58. 10.1111/j.1365-2982.2008.01182.x
105
FingerA. M.DibnerC.KramerA. (2020). Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett.594, 2734–2769. 10.1002/1873-3468.13898
106
FirpoM. A.RollinsM. D.SzaboA.GullJ. D.JacksonJ. D.ShaoY.et al (2005). A conscious mouse model of gastric ileus using clinically relevant endpoints. BMC Gastroenterol.5, 18. 10.1186/1471-230X-5-18
107
FlintA.RabenA.AstrupA.HolstJ. J. (1998). Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investigation101, 515–520. 10.1172/JCI990
108
FlourieB.PhillipsS.RichterH.3rdAzpirozF. (1989). Cyclic motility in canine colon: responses to feeding and perfusion. Dig. Dis. Sci.34, 1185–1192. 10.1007/BF01537266
109
FowlerS.HoedtE. C.TalleyN. J.KeelyS.BurnsG. L. (2022). Circadian rhythms and melatonin metabolism in patients with disorders of gut-brain interactions. Front. Neurosci.16, 825246. 10.3389/fnins.2022.825246
110
FrateschiS.KeppnerA.MalsureS.IwaszkiewiczJ.SergiC.MerillatA. M.et al (2012). Mutations of the serine protease CAP1/Prss8 lead to reduced embryonic viability, skin defects, and decreased ENaC activity. Am. J. Pathol.181, 605–615. 10.1016/j.ajpath.2012.05.007
111
FreelandK. R.WoleverT. M. (2010). Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr.103, 460–466. 10.1017/S0007114509991863
112
FrexinosJ.BuenoL.FioramontiJ. (1985). Diurnal changes in myoelectric spiking activity of the human colon. Gastroenterology88, 1104–1110. 10.1016/s0016-5085(85)80067-6
113
FukumotoS.TatewakiM.YamadaT.FujimiyaM.MantyhC.VossM.et al (2003). Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol.284, R1269–R1276. 10.1152/ajpregu.00442.2002
114
FurnessJ. (1969). An electrophysiological study of the innervation of the smooth muscle of the colon. J. Physiology205, 549–562. 10.1113/jphysiol.1969.sp008982
115
FurnessJ. B.RobbinsH. L.XiaoJ.StebbingM. J.NurgaliK. (2004). Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res.317, 1–12. 10.1007/s00441-004-0895-5
116
FurnessJ. B. (2012). The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterology Hepatology9, 286–294. 10.1038/nrgastro.2012.32
117
FurukawaY.CookI. J.PanagopoulosV.McEvoyR. D.SharpD. J.SimulaM. (1994). Relationship between sleep patterns and human colonic motor patterns. Gastroenterology107, 1372–1381. 10.1016/0016-5085(94)90539-8
118
Gálvez-RobleñoC.López-TofiñoY.López-GómezL.BagüésA.Soto-MontenegroM. L.AbaloR. (2022). Radiographic assessment of the impact of sex and the circadian rhythm-dependent behaviour on gastrointestinal transit in the rat. Lab. Anim.57, 270–282. 10.1177/00236772221124381
119
GershonM. D. (2022). The shaggy dog story of enteric signaling: serotonin, a molecular megillah. Adv. Exp. Med. Biol.1383, 307–318. 10.1007/978-3-031-05843-1_28
120
Gil-LozanoM.MingomatajE. L.WuW. K.RidoutS. A.BrubakerP. L. (2014). Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes63, 3674–3685. 10.2337/db13-1501
121
Gil-LozanoM.WuW. K.MartchenkoA.BrubakerP. L. (2016). High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent L-cell. Endocrinology157, 586–599. 10.1210/en.2015-1732
122
GillespieJ. S. (1962). Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J. Physiol.162, 54–75. 10.1113/jphysiol.1962.sp006914
123
GiraltM.VergaraP. (1999). Glucagonlike peptide-1 (GLP-1) participation in ileal brake induced by intraluminal peptones in rat. Dig. Dis. Sci.44, 322–329. 10.1023/a:1026654417697
124
GiraltM.VergaraP. (1998). Sympathetic pathways mediate GLP-1 actions in the gastrointestinal tract of the rat. Regul. Pept.74, 19–25. 10.1016/s0167-0115(98)00010-x
125
GoslingL. (1979). The twenty‐four hour activity cycle of captive coypus (Myocastor coypus). J. Zoology187, 341–367. 10.1111/j.1469-7998.1979.tb03374.x
126
GovindarajanK.MacSharryJ.CaseyP. G.ShanahanF.JoyceS. A.GahanC. G. (2016). Unconjugated bile acids influence expression of circadian genes: A potential mechanism for microbe-host crosstalk. Plos One11, e0167319. 10.1371/journal.pone.0167319
127
GriderJ. R.PilandB. E. (2007). The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am. J. Physiology - Gastrointest. Liver Physiology292, G429–G437. 10.1152/ajpgi.00376.2006
128
GschossmannJ. M.BuengerL.AdamB.LiebregtsT.SallerB.MannK.et al (2001). Diurnal variation of abdominal motor responses to colorectal distension and plasma cortisol levels in rats. Neurogastroenterol. Motil.13, 585–589. 10.1046/j.1365-2982.2001.00293.x
129
Guardiola-LemaitreB.De BodinatC.DelagrangeP.MillanM. J.MunozC.MocaërE. (2014). Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br. J. Pharmacol.171, 3604–3619. 10.1111/bph.12720
130
GulbransenB. D.BainsJ. S.SharkeyK. A. (2010). Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the Guinea pig distal colon. J. Neurosci.30, 6801–6809. 10.1523/JNEUROSCI.0603-10.2010
131
GumzM. L.StowL. R.LynchI. J.GreenleeM. M.RudinA.CainB. D.et al (2009). The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Investigation119, 2423–2434. 10.1172/JCI36908
132
HaggerR.KumarD.BensonM.GrundyA. (2002). Periodic colonic motor activity identified by 24-h pancolonic ambulatory manometry in humans. Neurogastroenterol. Motil.14, 271–278. 10.1046/j.1365-2982.2002.00331.x
133
HanJ. H.ChangI. H.MyungS. C.LeeM. Y.KimW. Y.LeeS. Y.et al (2012). A novel pathway underlying the inhibitory effects of melatonin on isolated rat urinary bladder contraction. Korean J. Physiol. Pharmacol.16, 37–42. 10.4196/kjpp.2012.16.1.37
134
HanS.GaoH.SongR.ZhangW.LiY.ZhangJ. (2021). Oat fiber modulates hepatic circadian clock via promoting gut microbiota-derived short chain fatty acids. J. Agric. Food Chem.69, 15624–15635. 10.1021/acs.jafc.1c06130
135
HardelandR.CardinaliD. P.SrinivasanV.SpenceD. W.BrownG. M.Pandi-PerumalS. R. (2011). Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol.93, 350–384. 10.1016/j.pneurobio.2010.12.004
136
HarlowH. J.WeekleyB. L. (1986). Effect of melatonin on the force of spontaneous contractions of in vitro rat small and large intestine. J. Pineal Res.3, 277–284. 10.1111/j.1600-079x.1986.tb00750.x
137
HastingsM. H.MaywoodE. S.BrancaccioM. (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci.19, 453–469. 10.1038/s41583-018-0026-z
138
HeatonK. W.RadvanJ.CrippsH.MountfordR. A.BraddonF. E.HughesA. O. (1992). Defecation frequency and timing, and stool form in the general population: A prospective study. Gut33, 818–824. 10.1136/gut.33.6.818
139
HeddesM.AltahaB.NiuY.ReitmeierS.KleigreweK.HallerD.et al (2022). The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Nat. Commun.13, 6068. 10.1038/s41467-022-33609-x
140
HeitmannP. T.Mohd RosliR.MaslenL.WiklendtL.KumarR.OmariT. I.et al (2022). High-resolution impedance manometry characterizes the functional role of distal colonic motility in gas transit. Neurogastroenterol. Motil.34, e14178. 10.1111/nmo.14178
141
HenningS. J.HirdF. J. (1972). Diurnal variations in the concentrations of volatile fatty acids in the alimentary tracts of wild rabbits. Br. J. Nutr.27, 57–64. 10.1079/bjn19720069
142
HerreraG. M.MeredithA. L. (2010). Diurnal variation in urodynamics of rat. Plos One5, e12298. 10.1371/journal.pone.0012298
143
HibberdT.SpencerN. J.BrookesS.CostaM.YewW. P. (2022a). Enteric control of the sympathetic nervous system. Adv. Exp. Med. Biol.1383, 89–103. 10.1007/978-3-031-05843-1_9
144
HibberdT. J.CostaM.SmoliloD. J.KeightleyL. J.BrookesS. J.DinningP. G.et al (2022b). Mechanisms underlying initiation of propulsion in Guinea pig distal colon. Am. J. Physiology - Gastrointest. Liver Physiology323, G71–g87. 10.1152/ajpgi.00055.2022
145
HibberdT. J.YewW. P.DoddsK. N.XieZ.TravisL.BrookesS. J.et al (2022c). Quantification of CGRP-immunoreactive myenteric neurons in mouse colon. J. Comp. Neurology530, 3209–3225. 10.1002/cne.25403
146
HirabayashiT.MorikawaY.MatsufujiH.HoshinoK.HaganeK.OzakiK. (2009). Stimulatory action of mitemcinal (GM-611), an acid-resistant non-peptide motilin receptor agonist, on colonic motor activity and defecation: spontaneous and mitemcinal-induced giant migrating contractions during defecation in dogs. Neurogastroenterol. Motil.21, 1085–1e91. 10.1111/j.1365-2982.2009.01341.x
147
HirstG. D. S.McKirdyH. C. (1974). Presynaptic inhibition at mammalian peripheral synapse?Nature250, 430–431. 10.1038/250430a0
148
HollowayW. R.GrotaL. J.BrownG. M. (1980). Determination of immunoreactive melatonin in the colon of the rat by immunocytochemistry. J. Histochem Cytochem28, 255–262. 10.1177/28.3.6444434
149
HolstJ. J.AndersenD. B.GrunddalK. V. (2022). Actions of glucagon-like peptide-1 receptor ligands in the gut. Br. J. Pharmacol.179, 727–742. 10.1111/bph.15611
150
HolstJ. J. (2022). Discovery of the GI effects of GLP-1: an historical perspective. Dig. Dis. Sci.67, 2716–2720. 10.1007/s10620-022-07519-3
151
HoogerwerfW. A.HellmichH. L.CornélissenG.HalbergF.ShahinianV. B.BostwickJ.et al (2007). Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology133, 1250–1260. 10.1053/j.gastro.2007.07.009
152
HoogerwerfW. A. (2010). Role of clock genes in gastrointestinal motility. Am. J. Physiology - Gastrointest. Liver Physiology299, G549–G555. 10.1152/ajpgi.00147.2010
153
HoogerwerfW. A.ShahinianV. B.CornélissenG.HalbergF.BostwickJ.TimmJ.et al (2010). Rhythmic changes in colonic motility are regulated by period genes. Am. J. Physiology-Gastrointestinal Liver Physiology298, G143–G150. 10.1152/ajpgi.00402.2009
154
HoogerwerfW. A.SinhaM.ConesaA.LuxonB. A.ShahinianV. B.CornélissenG.et al (2008). Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology135, 2019–2029. 10.1053/j.gastro.2008.08.048
155
HouQ.HuangY.ZhuZ.LiaoL.ChenX.HanQ.et al (2019). Tong-Xie-Yao-Fang improves intestinal permeability in diarrhoea-predominant irritable bowel syndrome rats by inhibiting the NF-κB and notch signalling pathways. BMC Complement. Altern. Med.19, 337. 10.1186/s12906-019-2749-4
156
HuetherG.PoeggelerB.ReimerA.GeorgeA. (1992). Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci.51, 945–953. 10.1016/0024-3205(92)90402-b
157
HuetherG. (1993). The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia49, 665–670. 10.1007/BF01923948
158
HurstN. R.KendigD. M.MurthyK. S.GriderJ. R. (2014). The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the Guinea pig colon. Neurogastroenterol. Motil.26, 1586–1596. 10.1111/nmo.12425
159
HyunM. K.BaekY.LeeS. (2019). Association between digestive symptoms and sleep disturbance: A cross-sectional community-based study. BMC Gastroenterol.19, 34. 10.1186/s12876-019-0945-9
160
IshidaA.MutohT.UeyamaT.BandoH.MasubuchiS.NakaharaD.et al (2005). Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab.2, 297–307. 10.1016/j.cmet.2005.09.009
161
JabburM. L.JohnsonC. H. (2021). Spectres of clock evolution: past, present, and yet to come. Front. Physiol.12, 815847. 10.3389/fphys.2021.815847
162
JilgeB.HudsonR. (2001). Diversity and development of circadian rhythms in the European rabbit. Chronobiol Int.18, 1–26. 10.1081/cbi-100001275
163
JilgeB. (1982). Monophasic and diphasic patterns of the circadian caecotrophy rhythm of rabbits. Lab. Anim.16, 1–6. 10.1258/002367782780908832
164
JilgeB. (1974). Soft faeces excretion and passage time in the laboratory rabbit. Lab. Anim.8, 337–346. 10.1258/002367774780943698
165
JilgeB.StähleH. (1993). Restricted food access and light-dark: impact of conflicting zeitgebers on circadian rhythms of the rabbit. Am. J. Physiology264, R708–R715. 10.1152/ajpregu.1993.264.4.R708
166
JohnsonC. H.ZhaoC.XuY.MoriT. (2017). Timing the day: what makes bacterial clocks tick?Nat. Rev. Microbiol.15, 232–242. 10.1038/nrmicro.2016.196
167
JouëtP.MoussataD.DubocH.BoschettiG.AttarA.GorbatchefC.et al (2013). Effect of short-chain fatty acids and acidification on the phasic and tonic motor activity of the human colon. Neurogastroenterol. Motil.25, 943–949. 10.1111/nmo.12212
168
KarasekM.Carrillo-VicoA.GuerreroJ. M.WinczykK.PawlikowskiM. (2002). Expression of melatonin MT(1) and MT(2) receptors, and ROR alpha(1) receptor in transplantable murine Colon 38 cancer. Neuro Endocrinol. Lett.23 (1), 55–60https://www.nel.edu/expression-of-melatonin-mt-1-and-mt-2-receptors-and-ror-alpha-1-receptor-in-transplantable-murine-colon-38-cancer-2269/.
169
KenagyG. J.VelosoC.BozinovicF. (1999). Daily rhythms of food intake and feces reingestion in the degu, an herbivorous Chilean rodent: optimizing digestion through coprophagy. Physiol. Biochem. Zool.72, 78–86. 10.1086/316644
170
KennedyM. F.TuttonP. J.BarklaD. H. (1983). Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Clin. Exp. Pharmacol. Physiol.10, 577–586. 10.1111/j.1440-1681.1983.tb00226.x
171
KentishS. J.FrisbyC. L.KennawayD. J.WittertG. A.PageA. J. (2013). Circadian variation in gastric vagal afferent mechanosensitivity. J. Neurosci.33, 19238–19242. 10.1523/JNEUROSCI.3846-13.2013
172
KimH. I.JungS. A.ChoiJ. Y.KimS. E.JungH. K.ShimK. N.et al (2013). Impact of shiftwork on irritable bowel syndrome and functional dyspepsia. J. Korean Med. Sci.28, 431–437. 10.3346/jkms.2013.28.3.431
173
KirklandJ. L.LyeM.LevyD. W.BanerjeeA. K. (1983). Patterns of urine flow and electrolyte excretion in healthy elderly people. Br. Med. J. Clin. Res.287, 1665–1667. 10.1136/bmj.287.6406.1665
174
KlenkK. (1971). Das Aktivitätsmuster des Rotfuchses Vulpus vulpes (L). einem Freilandgehege mit künstlichem Bau.
175
KnudsenJ. B.HolstJ. J.AsnaesS.JohansenA. (1975). Identification of cells with pancreatic-type and gut-type glucagon immunoreactivity in the human colon. Acta Pathol. Microbiol. Scand. A83, 741–743. 10.1111/j.1699-0463.1975.tb01407.x
176
KobayashiY.TakemiS.SakaiT.ShibataC.SakataI. (2022). Diurnal changes of colonic motility and regulatory factors for colonic motility in Suncus murinus. Neurogastroenterol. Motil.34, e14302. 10.1111/nmo.14302
177
KochA. A.BagnallJ. S.SmyllieN. J.BegleyN.AdamsonA. D.FribourghJ. L.et al (2022). Quantification of protein abundance and interaction defines a mechanism for operation of the circadian clock. Elife11, e73976. 10.7554/eLife.73976
178
KumarD.WilliamsN. S.WaldronD.WingateD. L. (1989). Prolonged manometric recording of anorectal motor activity in ambulant human subjects: evidence of periodic activity. Gut30, 1007–1011. 10.1136/gut.30.7.1007
179
KunzeW. A. A.FurnessJ. B. (1999). The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiology61, 117–142. 10.1146/annurev.physiol.61.1.117
180
KunzelmannK.MallM. (2002). Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev.82, 245–289. 10.1152/physrev.00026.2001
181
KurahashiM.KitoY.BakerS. A.JenningsL. K.DowersJ. G. R.KohS. D.et al (2020a). A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB J.34, 5563–5577. 10.1096/fj.201903134R
182
KurahashiM.KitoY.HaraM.TakeyamaH.SandersK. M.HashitaniH. (2020b). Norepinephrine has dual effects on human colonic contractions through distinct subtypes of alpha 1 adrenoceptors. Cell Mol. Gastroenterol. Hepatol.10, 658–671. 10.1016/j.jcmgh.2020.04.015
183
KuriyamaH.KitamuraK.ItohT.InoueR. (1998). Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev.78, 811–920. 10.1152/physrev.1998.78.3.811
184
KylohM. A.HibberdT. J.CastroJ.HarringtonA. M.TravisL.DoddsK. N.et al (2022). Disengaging spinal afferent nerve communication with the brain in live mice. Commun. Biol.5, 915. 10.1038/s42003-022-03876-x
185
LabrecqueN.CermakianN. (2015). Circadian clocks in the immune system. J. Biol. Rhythms30, 277–290. 10.1177/0748730415577723
186
LandauM.ZisapelN. (2007). “The low affinity binding of melatonin to calmodulin: use of computational methods to explain its physiological relevance,” in Melatonin: from molecules to therapy. Editors Pandi-PerumalS. R.CardinaliD. P. (New York, NY: Nova Science Publishers, Inc), 69–79.
187
LarraufieP.Martin-GallausiauxC.LapaqueN.DoreJ.GribbleF. M.ReimannF.et al (2018). SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep.8, 74. 10.1038/s41598-017-18259-0
188
LeeP. P.PangS. F. (1993). Melatonin and its receptors in the gastrointestinal tract. Biol. Signals2, 181–193. 10.1159/000109491
189
LeeS. H. (2015). Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res.13, 11–18. 10.5217/ir.2015.13.1.11
190
LeembruggenA. J.StampL. A.BornsteinJ. C.HaoM. M. (2020). The role of the circadian rhythm on enteric neural plasticity and gut motility. Ferderation Neurogastroenterol. Motil.
191
LeembruggenA. J. L.StampL. A.BornsteinJ. C.HaoM. M. (2022). Circadian control of gastrointestinal motility. Adv. Exp. Med. Biol.1383, 191–203. 10.1007/978-3-031-05843-1_18
192
LeoneV.GibbonsS. M.MartinezK.HutchisonA. L.HuangE. Y.ChamC. M.et al (2015). Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe17, 681–689. 10.1016/j.chom.2015.03.006
193
LiangX.BushmanF. D.FitzGeraldG. A. (2015). Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. U. S. A.112, 10479–10484. 10.1073/pnas.1501305112
194
LinA. Y.DinningP. G.MilneT.BissettI. P.O'GradyG. (2017a). The "rectosigmoid brake": review of an emerging neuromodulation target for colorectal functional disorders. Clin. Exp. Pharmacol. Physiol.44, 719–728. 10.1111/1440-1681.12760
195
LinA. Y.DuP.DinningP. G.ArkwrightJ. W.KampJ. P.ChengL. K.et al (2017b). High-resolution anatomic correlation of cyclic motor patterns in the human colon: evidence of a rectosigmoid brake. Am. J. Physiology - Gastrointest. Liver Physiology312, G508–g515. 10.1152/ajpgi.00021.2017
196
LindgrenO.MariA.DeaconC. F.CarrR. D.WinzellM. S.VikmanJ.et al (2009). Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metabolism94, 2887–2892. 10.1210/jc.2009-0366
197
LiuX. W.WangC. D. (2019). Melatonin alleviates circadian rhythm disruption exacerbating DSS-induced colitis by inhibiting the distribution of HMGB1 in intestinal tissues. Int. Immunopharmacol.73, 108–117. 10.1016/j.intimp.2019.05.005
198
LowreyP. L.TakahashiJ. S. (2011). Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet.74, 175–230. 10.1016/B978-0-12-387690-4.00006-4
199
LuW. Z.SongG. H.GweeK. A.HoK. Y. (2009). The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig. Dis. Sci.54, 1087–1093. 10.1007/s10620-008-0463-z
200
LucchelliA.Santagostino-BarboneM. G.ToniniM. (1997). Investigation into the contractile response of melatonin in the Guinea-pig isolated proximal colon: the role of 5-HT4 and melatonin receptors. Br. J. Pharmacol.121, 1775–1781. 10.1038/sj.bjp.0701287
201
MagotT.ChevallierF. (1983). Influence of daily feeding within a limited time on weight, digestive transit and cholesterol turnover in adult rats. Reprod. Nutr. Développement23, 1019–1027. 10.1051/rnd:19830708
202
MalekI.HaimA.IzhakiI. (2020). Melatonin mends adverse temporal effects of bright light at night partially independent of its effect on stress responses in captive birds. Chronobiol Int.37, 189–208. 10.1080/07420528.2019.1698590
203
MalloyJ. N.PauloseJ. K.LiY.CassoneV. M. (2012). Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Am. J. Physiology - Gastrointest. Liver Physiology303, G461–G473. 10.1152/ajpgi.00369.2011
204
MalsureS.WangQ.CharlesR. P.SergiC.PerrierR.ChristensenB. M.et al (2014). Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. J. Am. Soc. Nephrol.25, 1453–1464. 10.1681/ASN.2013090936
205
MarraG.AntiM.PercesepeA.ArmelaoF.FicarelliR.CocoC.et al (1994). Circadian variations of epithelial cell proliferation in human rectal crypts. Gastroenterology106, 982–987. 10.1016/0016-5085(94)90757-9
206
MartchenkoA.OhR. H.WheelerS. E.GurgesP.ChalmersJ. A.BrubakerP. L. (2018). Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol. (Oxf)222, e13007. 10.1111/apha.13007
207
MartchenkoS. E.MartchenkoA.CoxB. J.NaismithK.WallerA.GurgesP.et al (2020). Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes69, 2589–2602. 10.2337/db20-0262
208
MaywoodE. S.DrynanL.CheshamJ. E.EdwardsM. D.DardenteH.FustinJ. M.et al (2013). Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse. Proc. Natl. Acad. Sci. U. S. A.110, 9547–9552. 10.1073/pnas.1220894110
209
McManusC. M.MichelK. E.SimonD. M.WashabauR. J. (2002). Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am. J. Vet. Res.63, 295–300. 10.2460/ajvr.2002.63.295
210
McNeilN. I. (1984). The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr.39, 338–342. 10.1093/ajcn/39.2.338
211
MearinF.LacyB. E.ChangL.CheyW. D.LemboA. J.SimrenM.et al (2016). Bowel disorders. Gastroenterology.
212
MenakerM.MurphyZ. C.SellixM. T. (2013). Central control of peripheral circadian oscillators. Curr. Opin. Neurobiol.23, 741–746. 10.1016/j.conb.2013.03.003
213
MerleA.DelagrangeP.RenardP.LesieurD.CuberJ. C.RocheM.et al (2000). Effect of melatonin on motility pattern of small intestine in rats and its inhibition by melatonin receptor antagonist S 22153. J. Pineal Res.29, 116–124. 10.1034/j.1600-079x.2000.290208.x
214
MishchukV. H.GrygorukG. V.HubinaN. V.KozinchukH. V.StupnytskaH. Y. (2019). Efficiency of synthetic melatonin in comprehensive therapy of patients with acombination of irritable bowel syndrome withconstipation, arterial hypertension and obesity. Romanian J. Med. Pract.14, 155–161. 10.37897/rjmp.2019.2.12
215
MistlbergerR. E. (1994). Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav Rev.18, 171–195. 10.1016/0149-7634(94)90023-x
216
MistlbergerR. E. (2020). Food as circadian time cue for appetitive behavior. F1000Res9, F1000 Faculty Rev-61. 10.12688/f1000research.20829
217
MitsuiR.OnoS.KarakiS.KuwaharaA. (2005b). Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol. Motil.17, 585–594. 10.1111/j.1365-2982.2005.00669.x
218
MitsuiR.OnoS.KarakiS.KuwaharaA. (2005a). Propionate modulates spontaneous contractions via enteric nerves and prostaglandin release in the rat distal colon. Jpn. J. Physiol.55, 331–338. 10.2170/jjphysiol.RP000205
219
MoayyediP.MearinF.AzpirozF.AndresenV.BarbaraG.CorsettiM.et al (2017). Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J.5, 773–788. 10.1177/2050640617731968
220
MortaşH.BiliciS.KarakanT. (2020). The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chronobiol Int.37, 1067–1081. 10.1080/07420528.2020.1778717
221
MukherjiA.KobiitaA.YeT.ChambonP. (2013). Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell153, 812–827. 10.1016/j.cell.2013.04.020
222
MunC. J.BurgessH. J.SearsD. D.ParthasarathyS.JamesD.AltamiranoU.et al (2022). Circadian rhythm and pain: A review of current research and future implications. Curr. Sleep. Med. Rep.8, 114–123. 10.1007/s40675-022-00228-3
223
MutohT.ShibataS.KorfH. W.OkamuraH. (2003). Melatonin modulates the light-induced sympathoexcitation and vagal suppression with participation of the suprachiasmatic nucleus in mice. J. Physiol.547, 317–332. 10.1113/jphysiol.2002.028001
224
NagaiK.NishioT.NakagawaH.NakamuraS.FukudaY. (1978). Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res.142, 384–389. 10.1016/0006-8993(78)90648-0
225
NarducciF.BassottiG.GaburriM.MorelliA. (1987). Twenty four hour manometric recording of colonic motor activity in healthy man. Gut28, 17–25. 10.1136/gut.28.1.17
226
NegoroH.KanematsuA.DoiM.SuadicaniS. O.MatsuoM.ImamuraM.et al (2012). Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat. Commun.3, 809. 10.1038/ncomms1812
227
NessT. J.ElhefniH. (2004). Reliable visceromotor responses are evoked by noxious bladder distention in mice. J. Urology171, 1704–1708. 10.1097/01.ju.0000116430.67100.8f
228
NessT. J.GebhartG. F. (1988). Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res.450, 153–169. 10.1016/0006-8993(88)91555-7
229
NgS. C.ShiH. Y.HamidiN.UnderwoodF. E.TangW.BenchimolE. I.et al (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet390, 2769–2778. 10.1016/S0140-6736(17)32448-0
230
NiijimaA.NagaiK.NagaiN.AkagawaH. (1993). Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiol. Behav.54, 555–561. 10.1016/0031-9384(93)90249-f
231
NiijimaA.NagaiK.NagaiN.NakagawaH. (1992). Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. J. Aut. Nerv. Syst.40, 155–160. 10.1016/0165-1838(92)90026-d
232
NohJ. Y.HanD. H.YoonJ. A.KimM. H.KimS. E.KoI. G.et al (2011). Circadian rhythms in urinary functions: possible roles of circadian clocks?Int. Neurourol. J.15, 64–73. 10.5213/inj.2011.15.2.64
233
Oh-okaK.KonoH.IshimaruK.MiyakeK.KubotaT.OgawaH.et al (2014). Expressions of tight junction proteins occludin and claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. Plos One9, e98016. 10.1371/journal.pone.0098016
234
OhdoS. (2010). Chronotherapeutic strategy: rhythm monitoring, manipulation and disruption. Adv. Drug Deliv. Rev.62, 859–875. 10.1016/j.addr.2010.01.006
235
OlssonC.ChenB. N.JonesS.ChatawayT. K.CostaM.BrookesS. J. H. (2006). Comparison of extrinsic efferent innervation of Guinea pig distal colon and rectum. J. Comp. Neurology496, 787–801. 10.1002/cne.20965
236
OnoS.KarakiS.KuwaharaA. (2004). Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Jpn. J. Physiol.54, 483–493. 10.2170/jjphysiol.54.483
237
OrkinB. A.HansonR. B.KellyK. A. (1989). The rectal motor complex. Neurogastroenterol. Motil.1, 5–8. 10.1111/j.1365-2982.1989.tb00138.x
238
OuyangH.VogelH. J. (1998). Melatonin and serotonin interactions with calmodulin: NMR, spectroscopic and biochemical studies. Biochimica Biophysica Acta1383, 37–47. 10.1016/s0167-4838(97)00157-x
239
PáchaJ.SumováA. (2013). Circadian regulation of epithelial functions in the intestine. Acta Physiol. (Oxf)208, 11–24. 10.1111/apha.12090
240
PageA. J. (2021). Gastrointestinal vagal afferents and food intake: relevance of circadian rhythms. Nutrients13, 844. 10.3390/nu13030844
241
PalmerJ. M.SchemannM.TamuraK.WoodJ. D. (1986). Calcitonin gene-related peptide excites myenteric neurons. Eur. J. Pharmacol.132, 163–170. 10.1016/0014-2999(86)90601-1
242
Pandi-PerumalS. R.TrakhtI.SrinivasanV.SpenceD. W.MaestroniG. J.ZisapelN.et al (2008). Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol.85, 335–353. 10.1016/j.pneurobio.2008.04.001
243
ParkY. S.ChungS. H.LeeS. K.KimJ. H.KimJ. B.KimT. K.et al (2015). Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med.35, 979–986. 10.3892/ijmm.2015.2080
244
ParkerD. R.WiklendtL.HumenickA.ChenB. N.SiaT. C.WattchowD. A.et al (2022). Sympathetic pathways target cholinergic neurons in the human colonic myenteric plexus. Front. Neurosci.16, 863662. 10.3389/fnins.2022.863662
245
PartchC. L.GreenC. B.TakahashiJ. S. (2014). Molecular architecture of the mammalian circadian clock. Trends Cell Biol.24, 90–99. 10.1016/j.tcb.2013.07.002
246
PatkeA.YoungM. W.AxelrodS. (2020). Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol.21, 67–84. 10.1038/s41580-019-0179-2
247
PattenG. S.KerrC. A.DunneR. A.ShawJ. M.BirdA. R.ReginaA.et al (2015). Resistant starch alters colonic contractility and expression of related genes in rats fed a Western diet. Dig. Dis. Sci.60, 1624–1632. 10.1007/s10620-015-3537-8
248
PattonV.WiklendtL.ArkwrightJ. W.LubowskiD. Z.DinningP. G. (2013). The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br. J. Surg.100, 959–968. 10.1002/bjs.9114
249
PauloseJ. K.CassoneC. V.GraniczkowskaK. B.CassoneV. M. (2019). Entrainment of the circadian clock of the enteric bacterium Klebsiella aerogenes by temperature cycles. iScience19, 1202–1213. 10.1016/j.isci.2019.09.007
250
PauloseJ. K.CassoneV. M. (2016). The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes. Gut microbes7, 424–427. 10.1080/19490976.2016.1208892
251
PauloseJ. K.WrightJ. M.PatelA. G.CassoneV. M. (2016). Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. Plos One11, e0146643. 10.1371/journal.pone.0146643
252
PehrsonÅ. (1983). Caecotrophy in caged mountain hares (Lepus timidus). J. Zoology199, 563–574. 10.1111/j.1469-7998.1983.tb05107.x
253
PendergastJ. S.YamazakiS. (2018). The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J. Biol. Rhythms33, 458–474. 10.1177/0748730418789043
254
PiccioneG.CaolaG.RefinettiR. (2005). Temporal relationships of 21 physiological variables in horse and sheep. Comp. Biochem. Physiol. A Mol. Integr. Physiol.142, 389–396. 10.1016/j.cbpa.2005.07.019
255
PilorzV.AstizM.HeinenK. O.RawashdehO.OsterH. (2020). The concept of coupling in the mammalian circadian clock network. J. Mol. Biol.432, 3618–3638. 10.1016/j.jmb.2019.12.037
256
PlattT. R.HusseyG. L.ZelmerD. A. (2013). Circadian egg production by Echinostoma caproni (Digenea: echinostomatidae) in ICR mice. J. Parasitol.99, 179–182. 10.1645/GE-3227.1
257
PolidarováL.HoudekP.SládekM.NovosadováZ.PáchaJ.SumováA. (2017). Mechanisms of hormonal regulation of the peripheral circadian clock in the colon. Chronobiol Int.34, 1–16. 10.1080/07420528.2016.1231198
258
PolidarováL.OlejníkováL.PaušlyováL.SládekM.SotákM.PáchaJ.et al (2014). Development and entrainment of the colonic circadian clock during ontogenesis. Am. J. Physiology - Gastrointest. Liver Physiology306, G346–G356. 10.1152/ajpgi.00340.2013
259
PoonA. M.MakA. S.LukH. T. (1996). Melatonin and 2[125I]iodomelatonin binding sites in the human colon. Endocr. Res.22, 77–94. 10.3109/07435809609030499
260
PrasaiM. J.PernicovaI.GrantP. J.ScottE. M. (2011). An endocrinologist's guide to the clock. J. Clin. Endocrinol. Metabolism96, 913–922. 10.1210/jc.2010-2449
261
PryorG. S.BjorndalK. A. (2005). Symbiotic fermentation, digesta passage, and gastrointestinal morphology in bullfrog tadpoles (Rana catesbeiana). Physiol. Biochem. Zool.78, 201–215. 10.1086/427050
262
PsichasA.SleethM. L.MurphyK. G.BrooksL.BewickG. A.HanyalogluA. C.et al (2015). The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. (Lond)39, 424–429. 10.1038/ijo.2014.153
263
RagozzinoF. J.PetersonB. A.KaratsoreosI. N.PetersJ. H. (2023). Circadian regulation of glutamate release pathways shapes synaptic throughput in the brainstem nucleus of the solitary tract (NTS). J. Physiol.601, 1881–1896. 10.1113/JP284370
264
RahimimoghadamS.KhanjaniN.NaderiM.RasekhR. (2020). Comparing the prevalence of gastrointestinal disorders between day workers and shift workers at kerman university of medical Sciences. Asian Pac. J. Environ. Cancer3, 19–25. 10.31557/APJEC.2020.3.1.19-25
265
RalphM. R.FosterR. G.DavisF. C.MenakerM. (1990). Transplanted suprachiasmatic nucleus determines circadian period. Science247, 975–978. 10.1126/science.2305266
266
RamsayS.ZagorodnyukV. (2023). Role of circadian rhythms and melatonin in bladder function in heath and diseases. Auton. Neurosci.246, 103083. 10.1016/j.autneu.2023.103083
267
RaoS. S.SadeghiP.BattersonK.BeatyJ. (2001a). Altered periodic rectal motor activity: A mechanism for slow transit constipation. Neurogastroenterol. Motil.13, 591–598. 10.1046/j.1365-2982.2001.00292.x
268
RaoS. S.SadeghiP.BeatyJ.KavlockR.AckersonK. (2001b). Ambulatory 24-h colonic manometry in healthy humans. Am. J. Physiology - Gastrointest. Liver Physiology280, G629–G639. 10.1152/ajpgi.2001.280.4.G629
269
RaoS. S.SadeghiP.BeatyJ.KavlockR. (2004). Ambulatory 24-hour colonic manometry in slow-transit constipation. Am. J. Gastroenterol.99, 2405–2416. 10.1111/j.1572-0241.2004.40453.x
270
RaoS. S.SinghS.MudipalliR. (2010). Day-to-day reproducibility of prolonged ambulatory colonic manometry in healthy subjects. Neurogastroenterol. Motil.22, 640–e178. 10.1111/j.1365-2982.2010.01492.x
271
ReadN. W.McFarlaneA.KinsmanR. I.BatesT. E.BlackhallN. W.FarrarG. B.et al (1984). Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology86, 274–280. 10.1016/0016-5085(84)90411-6
272
RefinettiR. (2006). Variability of diurnality in laboratory rodents. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol.192, 701–714. 10.1007/s00359-006-0093-x
273
ReinkeH.AsherG. (2016). Circadian clock control of liver metabolic functions. Gastroenterology150, 574–580. 10.1053/j.gastro.2015.11.043
274
ReiterR. J. (1991). Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev.12, 151–180. 10.1210/edrv-12-2-151
275
ReiterR. J.TanD. X.KorkmazA. (2009). The circadian melatonin rhythm and its modulation: possible impact on hypertension. J. Hypertens. - Suppl.27, S17–S20. 10.1097/01.hjh.0000358832.41181.bf
276
RendtorffR. C.KashgarianM. (1967). Stool patterns of healthy adult males. Dis. Colon Rectum10, 222–228. 10.1007/BF02617184
277
ReppertS. M.WeaverD. R. (2002). Coordination of circadian timing in mammals. Nature418, 935–941. 10.1038/nature00965
278
Reyes-VázquezC.Naranjo-RodríguezE. B.García-SegovianoJ. A.Trujillo-SantanaJ. T.Prieto-GómezB. (1997). Apamin blocks the direct relaxant effect of melatonin on rat ileal smooth muscle. J. Pineal Res.22, 1–8. 10.1111/j.1600-079x.1997.tb00295.x
279
RichardsJ.GumzM. L. (2012). Advances in understanding the peripheral circadian clocks. FASEB J.26, 3602–3613. 10.1096/fj.12-203554
280
Rodriguez-SinovasA.MartinM. T.FernandezE.GonalonsE. (1994). Cecocolonic motility in the chicken. Effects of cholecystokinin. Life Sci.55, 1743–1755. 10.1016/0024-3205(94)00343-2
281
RomanP.Perez-CayuelaI.Gil-HernándezE.Rodriguez-ArrastiaM.Aparicio-MotaA.Ropero-PadillaC.et al (2023). Influence of shift work on the health of nursing professionals. J. Personalized Med.13, 627. 10.3390/jpm13040627
282
RondeauM. P.MeltzerK.MichelK. E.McManusC. M.WashabauR. J. (2003). Short chain fatty acids stimulate feline colonic smooth muscle contraction. J. Feline Med. Surg.5, 167–173. 10.1016/S1098-612X(03)00002-0
283
RonholtC.RasmussenO. O.ChristiansenJ. (1999). Ambulatory manometric recording of anorectal activity. Dis. Colon Rectum42, 1551–1559. 10.1007/BF02236205
284
RopertA.CherbutC.RozéC.Le QuellecA.HolstJ. J.Fu-ChengX.et al (1996). Colonic fermentation and proximal gastric tone in humans. Gastroenterology111, 289–296. 10.1053/gast.1996.v111.pm8690193
285
SahaL.MalhotraS.RanaS.BhasinD.PandhiP. (2007). A preliminary study of melatonin in irritable bowel syndrome. J. Clin. Gastroenterology41, 29–32. 10.1097/MCG.0b013e31802df84c
286
SakataT. (2019). Pitfalls in short-chain fatty acid research: A methodological review. Anim. Sci. J.90, 3–13. 10.1111/asj.13118
287
SatoT.Sassone-CorsiP. (2022). Nutrition, metabolism, and epigenetics: pathways of circadian reprogramming. EMBO Rep.23, e52412. 10.15252/embr.202152412
288
SchevingL. A. (2000). Biological clocks and the digestive system. Gastroenterology119, 536–549. 10.1053/gast.2000.9305
289
SchlangenL. J. M.PriceL. L. A. (2021). The lighting environment, its metrology, and non-visual responses. Front. Neurol.12, 624861. 10.3389/fneur.2021.624861
290
SegersA.DepoortereI. (2021). Circadian clocks in the digestive system. Nat. Rev. Gastroenterol. Hepatol.18, 239–251. 10.1038/s41575-020-00401-5
291
SegersA.DesmetL.SunS.VerbekeK.TackJ.DepoortereI. (2020). Night-time feeding of Bmal1-/- mice restores SCFA rhythms and their effect on ghrelin. J. Endocrinol.245, 155–164. 10.1530/JOE-20-0011
292
SegersA.DesmetL.ThijsT.VerbekeK.TackJ.DepoortereI. (2019). The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol. (Oxf)225, e13193. 10.1111/apha.13193
293
SetchellK. D.LawsonA. M.BlackstockE. J.MurphyG. M. (1982). Diurnal changes in serum unconjugated bile acids in normal man. Gut23, 637–642. 10.1136/gut.23.8.637
294
ShaidullovI. F.SorokinaD. M.SitdikovF. G.HermannA.AbdulkhakovS. R.SitdikovaG. F. (2021). Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol.21, 37. 10.1186/s12876-021-01613-y
295
ShemerovskiiK. A. (2002). Circadian rhythm of rectal reactivity in individuals with regular and irregular bowel evacuation function. Bull. Exp. Biol. Med.134, 565–567. 10.1023/a:1022965212971
296
ShimizuY.YamamuraR.YokoiY.AyabeT.UkawaS.NakamuraK.et al (2023). Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production. Gut microbes15, 2190306. 10.1080/19490976.2023.2190306
297
ShuttleworthC. W.ConlonS. B.SandersK. M. (1997). Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br. J. Pharmacol.120, 707–713. 10.1038/sj.bjp.0700949
298
SládekM.PolidarováL.NovákováM.ParkanováD.SumováA. (2012). Early chronotype and tissue-specific alterations of circadian clock function in spontaneously hypertensive rats. Plos One7, e46951. 10.1371/journal.pone.0046951
299
SládekM.RybováM.JindrákováZ.ZemanováZ.PolidarováL.MrnkaL.et al (2007). Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology133, 1240–1249. 10.1053/j.gastro.2007.05.053
300
Sobolewska-WłodarczykA.WłodarczykM.ZielińskaA.SiwińskiP.Wiśniewska-JarosińskaM.GąsiorowskaA.et al (2020). Circadian rhythm abnormalities in patients with inflammatory bowel disease - association with adipokine profile. Scand. J. Gastroenterol.55, 294–300. 10.1080/00365521.2020.1737727
301
SöderquistF.HellströmP. M.CunninghamJ. L. (2015). Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. Plos One10, e0120195. 10.1371/journal.pone.0120195
302
SofferE. E.ScalabriniP.WingateD. L. (1989). Prolonged ambulant monitoring of human colonic motility. Am. J. Physiology257, G601–G606. 10.1152/ajpgi.1989.257.4.G601
303
SongG. H.LengP. H.GweeK. A.MoochhalaS. M.HoK. Y. (2005). Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study. Gut54, 1402–1407. 10.1136/gut.2004.062034
304
SoniK. G.HalderT.ConnerM. E.PreidisG. A. (2019). Sexual dimorphism in upper gastrointestinal motility is dependent on duration of fast, time of day, age, and strain of mice. Neurogastroenterol. Motil.31, e13654. 10.1111/nmo.13654
305
SoretR.ChevalierJ.De CoppetP.PoupeauG.DerkinderenP.SegainJ. P.et al (2010). Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology138, 1772–1782. 10.1053/j.gastro.2010.01.053
306
SotákM.MrnkaL.PáchaJ. (2006). Heterogeneous expression of melatonin receptor MT1 mRNA in the rat intestine under control and fasting conditions. J. Pineal Res.41, 183–188. 10.1111/j.1600-079X.2006.00355.x
307
SotákM.PolidarováL.ErgangP.SumováA.PáchaJ. (2013). An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int. J. Cancer132, 1032–1041. 10.1002/ijc.27760
308
SotákM.PolidarováL.MusílkováJ.HockM.SumováA.PáchaJ. (2011). Circadian regulation of electrolyte absorption in the rat colon. Am. J. Physiology - Gastrointest. Liver Physiology301, G1066–G1074. 10.1152/ajpgi.00256.2011
309
SpencerN. J.DinningP. G.BrookesS. J.CostaM. (2016). Insights into the mechanisms underlying colonic motor patterns. J. Physiol.594, 4099–4116. 10.1113/JP271919
310
SpillerR. C.TrotmanI. F.HigginsB. E.GhateiM. A.GrimbleG. K.LeeY. C.et al (1984). The ileal brake-inhibition of jejunal motility after ileal fat perfusion in man. Gut25, 365–374. 10.1136/gut.25.4.365
311
SquiresP. E.RumseyR. D.EdwardsC. A.ReadN. W. (1992). Effect of short-chain fatty acids on contractile activity and fluid flow in rat colon in vitro. Am. J. Physiology262, G813–G817. 10.1152/ajpgi.1992.262.5.G813
312
SteadmanC. J.PhillipsS. F.CamilleriM.HaddadA. C.HansonR. B. (1991). Variation of muscle tone in the human colon. Gastroenterology101, 373–381. 10.1016/0016-5085(91)90014-c
313
StebbingM.JohnsonP.VremecM.BornsteinJ. (2001). Role of alpha(2)-adrenoceptors in the sympathetic inhibition of motility reflexes of Guinea-pig ileum. J. Physiology534, 465–478. 10.1111/j.1469-7793.2001.00465.x
314
SteinerC.OthmanA.SaelyC. H.ReinP.DrexelH.von EckardsteinA.et al (2011). Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. Plos One6, e25006. 10.1371/journal.pone.0025006
315
StephanF. K. (2001). “Food-entrainable oscillators in mammals,” in Circadian clocks (Springer), 223–246.
316
StephanF. K. (2002). The "other" circadian system: food as a zeitgeber. J. Biol. Rhythms17, 284–292. 10.1177/074873040201700402
317
StokkanK. A.YamazakiS.TeiH.SakakiY.MenakerM. (2001). Entrainment of the circadian clock in the liver by feeding. Science291, 490–493. 10.1126/science.291.5503.490
318
StorrM.KoppitzP.SibaevA.SaurD.KurjakM.FranckH.et al (2002). Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J. Pineal Res.33, 101–108. 10.1034/j.1600-079x.2002.02909.x
319
StrackA. M.SawyerW. B.MarubioL. M.LoewyA. D. (1988). Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res.455, 187–191. 10.1016/0006-8993(88)90132-1
320
SummaK. C.VoigtR. M.ForsythC. B.ShaikhM.CavanaughK.TangY.et al (2013). Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. Plos One8, e67102. 10.1371/journal.pone.0067102
321
SuplyE.de VriesP.SoretR.CossaisF.NeunlistM. (2012). Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am. J. Physiology - Gastrointest. Liver Physiology302, G1373–G1380. 10.1152/ajpgi.00338.2011
322
SwansonG. R.KochmanN.AminJ.ChouhanV.YimW.EngenP. A.et al (2021). Disrupted circadian rest-activity cycles in inflammatory bowel disease are associated with aggressive disease phenotype, subclinical inflammation, and dysbiosis. Front. Med. (Lausanne)8, 770491. 10.3389/fmed.2021.770491
323
SwansonG. R.SiskinJ.GorenzA.ShaikhM.RaeisiS.FoggL.et al (2020). Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl. Res.221, 97–109. 10.1016/j.trsl.2020.04.004
324
SzurszewskiJ. H. (1969). A migrating electric complex of canine small intestine. Am. J. Physiology217, 1757–1763. 10.1152/ajplegacy.1969.217.6.1757
325
SzurszewskiJ. H.LindenD. R. (2012). “Physiology of prevertebral sympathetic ganglia,” in Physiology of the gastrointestinal tract. Editors JohnsonL. R., (Academic Press) 1, 583–627.
326
TaharaY.YamazakiM.SukigaraH.MotohashiH.SasakiH.MiyakawaH.et al (2018). Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep.8, 1395. 10.1038/s41598-018-19836-7
327
TakahashiJ. S. (2017). Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet.18, 164–179. 10.1038/nrg.2016.150
328
TalamancaL.GobetC.NaefF. (2023). Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythms in humans. Science379, 478–483. 10.1126/science.add0846
329
TalebZ.Carmona-AlcocerV.StokesK.HaireekM.WangH.CollinsS. M.et al (2022). BMAL1 regulates the daily timing of colitis. Front. Cell Infect. Microbiol.12, 773413. 10.3389/fcimb.2022.773413
330
TalebZ.KarpowiczP. (2022). Circadian regulation of digestive and metabolic tissues. Am. J. Physiol. Cell Physiol.323, C306–c321. 10.1152/ajpcell.00166.2022
331
TalebZ.StokesK.WangH.CollinsS. M.KhanW. I.KarpowiczP. (2021). The circadian timing of inflammatory bowel disease. J. Can. Assoc. Gastroenterology4, 4–5. 10.1093/jcag/gwab002.003
332
TanD. X.ManchesterL. C.LiuX.Rosales-CorralS. A.Acuna-CastroviejoD.ReiterR. J. (2013). Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res.54, 127–138. 10.1111/jpi.12026
333
TanD. X.ReiterR. J.ZimmermanS.HardelandR. (2023). Melatonin: both a messenger of darkness and a participant in the cellular actions of non-visible solar radiation of near infrared light. Biol. (Basel)12, 89. 10.3390/biology12010089
334
TanW.LeeG.ChenJ. H.HuizingaJ. D. (2020). Relationships between distention-butyrate- and pellet-induced stimulation of peristalsis in the mouse colon. Front. Physiol.11, 109. 10.3389/fphys.2020.00109
335
TassickerB. C.HennigG. W.CostaM.BrookesS. J. (1999). Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the Guinea-pig small intestine in vitro. Cell Tissue Res.295, 437–452. 10.1007/s004410051250
336
TaufiqueS. K. T.EhichioyaD. E.PendergastJ. S.YamazakiS. (2022) Genetics and functional significance of the understudied methamphetamine sensitive circadian oscillator (MASCO). F1000Res.11, 1018. 10.12688/f1000research.125432.2
337
ThaissC. A.LevyM.KoremT.DohnalováL.ShapiroH.JaitinD. A.et al (2016). Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell167, 1495–1510. 10.1016/j.cell.2016.11.003
338
ThaissC. A.ZeeviD.LevyM.Zilberman-SchapiraG.SuezJ.TengelerA. C.et al (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell159, 514–529. 10.1016/j.cell.2014.09.048
339
ThompsonB. J.WashingtonM. K.KurreU.SinghM.RulaE. Y.EmesonR. B. (2008). Protective roles of alpha-calcitonin and beta-calcitonin gene-related peptide in spontaneous and experimentally induced colitis. Dig. Dis. Sci.53, 229–241. 10.1007/s10620-007-9848-7
340
TranL.JochumS. B.ShaikhM.WilberS.ZhangL.HaydenD. M.et al (2021). Circadian misalignment by environmental light/dark shifting causes circadian disruption in colon. Plos One16, e0251604. 10.1371/journal.pone.0251604
341
TrivediP. P.JenaG. B. (2013). Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig. Dis. Sci.58, 3460–3474. 10.1007/s10620-013-2831-6
342
TrudrungP.FurnessJ. B.PompoloS.MessengerJ. (1994). Locations and chemistries of sympathetic nerve cells that project to the gastrointestinal tract and spleen. Archives histology Cytol.57, 139–150. 10.1679/aohc.57.139
343
TurekF. W.JoshuC.KohsakaA.LinE.IvanovaG.McDearmonE.et al (2005). Obesity and metabolic syndrome in circadian Clock mutant mice. Science308, 1043–1045. 10.1126/science.1108750
344
TuttonP. J.BarklaD. H. (1989). Effect of an inhibitor of noradrenaline uptake, desipramine, on cell proliferation in the intestinal crypt epithelium. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol.57, 349–352. 10.1007/BF02899100
345
TuttonP. J.BarklaD. H. (1980). Neural control of colonic cell proliferation. Cancer45, 1172–1177. 10.1002/1097-0142(19800315)45:5+<1172:aid-cncr2820451322>3.0.co;2-b
346
UeyamaT.KroutK. E.NguyenX. V.KarpitskiyV.KollertA.MettenleiterT. C.et al (1999). Suprachiasmatic nucleus: A central autonomic clock. Nat. Neurosci.2, 1051–1053. 10.1038/15973
347
VaccaroR.CasiniA.SeveriC.LamazzaA.PronioA.PalmaR. (2023). Serotonin and melatonin in human lower gastrointestinal tract. Diagn. (Basel)13, 204. 10.3390/diagnostics13020204
348
VagnerováK.ErgangP.SotákM.BalounováK.KvapilováP.VodičkaM.et al (2019). Diurnal expression of ABC and SLC transporters in jejunum is modulated by adrenalectomy. Comp. Biochem. Physiol. C Toxicol. Pharmacol.226, 108607. 10.1016/j.cbpc.2019.108607
349
Van CittersG. W.LinH. C. (2006). Ileal brake: neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep.8, 367–373. 10.1007/s11894-006-0021-9
350
VernayM. (1989). Incidence of the circadian rhythm of the excretion pattern on acetate absorption and metabolism in the rabbit hind-gut. Reprod. Nutr. Dev.29, 185–196. 10.1051/rnd:19890206
351
VernayM.MartyJ.MoattiJ. P. (1984). Absorption of electrolytes and volatile fatty acids in the hind-gut of the rabbit. Circadian rhythm of hind-gut electrolytes and plasma aldosterone. Br. J. Nutr.52, 419–428. 10.1079/bjn19840107
352
VetterC. (2020). Circadian disruption: what do we actually mean?Eur. J. Neurosci.51, 531–550. 10.1111/ejn.14255
353
VincentA. D.WangX. Y.ParsonsS. P.KhanW. I.HuizingaJ. D. (2018). Abnormal absorptive colonic motor activity in germ free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am. J. Physiology - Gastrointest. Liver Physiology315, G896–G907. 10.1152/ajpgi.00237.2017
354
von GallC.NotonE.LeeC.WeaverD. R. (2003). Light does not degrade the constitutively expressed BMAL1 protein in the mouse suprachiasmatic nucleus. Eur. J. Neurosci.18, 125–133. 10.1046/j.1460-9568.2003.02735.x
355
VujovicN.DavidsonA. J.MenakerM. (2008). Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am. J. Physiol. Regul. Integr. Comp. Physiol.295, R355–R360. 10.1152/ajpregu.00498.2007
356
WangQ.ClementS.GabbianiG.HorisbergerJ. D.BurnierM.RossierB. C.et al (2004). Chronic hyperaldosteronism in a transgenic mouse model fails to induce cardiac remodeling and fibrosis under a normal-salt diet. Am. J. Physiology - Ren. Physiology286, F1178–F1184. 10.1152/ajprenal.00386.2003
357
WangQ.HorisbergerJ. D.MaillardM.BrunnerH. R.RossierB. C.BurnierM. (2000). Salt- and angiotensin II-dependent variations in amiloride-sensitive rectal potential difference in mice. Clin. Exp. Pharmacol. Physiol.27, 60–66. 10.1046/j.1440-1681.2000.03204.x
358
WangQ.MaillardM.SchiblerU.BurnierM.GachonF. (2010). Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am. J. Physiol. Regul. Integr. Comp. Physiol.299, R1013–R1019. 10.1152/ajpregu.00241.2010
359
WarrenW. S.ChampneyT. H.CassoneV. M. (1994). The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol. Behav.55, 1091–1099. 10.1016/0031-9384(94)90392-1
360
WellsJ. M.RossiO.MeijerinkM.van BaarlenP. (2011). Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. U. S. A.108 (1), 4607–4614. 10.1073/pnas.1000092107
361
WellsM. M.RothL.ChandeN. (2012). Sleep disruption secondary to overnight call shifts is associated with irritable bowel syndrome in residents: A cross-sectional study. Am. J. Gastroenterol.107, 1151–1156. 10.1038/ajg.2011.486
362
WestC.WuR. Y.WongA.StaniszA. M.YanR.MinK. K.et al (2017). Lactobacillus rhamnosus strain JB-1 reverses restraint stress-induced gut dysmotility. Neurogastroenterol. Motil.29, e12903. 10.1111/nmo.12903
363
WienbeckM.KreuzpaintnerG. (1976). Circadian rhythm of colonic motility in the cat. Res. Exp. Med. Berl.169, 83–91. 10.1007/BF01851169
364
WinczykK.PawlikowskiM.GuerreroJ. M.KarasekM. (2002). Possible involvement of the nuclear RZR/ROR-alpha receptor in the antitumor action of melatonin on murine Colon 38 cancer. Tumour Biol.23, 298–302. 10.1159/000068569
365
WoleverT. M.JosseR. G.LeiterL. A.ChiassonJ. L. (1997). Time of day and glucose tolerance status affect serum short-chain fatty acid concentrations in humans. Metabolism Clin. Exp.46, 805–811. 10.1016/s0026-0495(97)90127-x
366
XieY.TangQ.ChenG.XieM.YuS.ZhaoJ.et al (2019). New insights into the circadian rhythm and its related diseases. Front. Physiol.10, 682. 10.3389/fphys.2019.00682
367
YajimaT. (1985). Contractile effect of short-chain fatty acids on the isolated colon of the rat. J. Physiology368, 667–678. 10.1113/jphysiol.1985.sp015882
368
YajimaT.SakataT. (1992). Core and periphery concentrations of short-chain fatty acids in luminal contents of the rat colon. Comp. Biochem. Physiol. Comp. Physiol.103, 353–355. 10.1016/0300-9629(92)90593-f
369
YamaguchiS.IsejimaH.MatsuoT.OkuraR.YagitaK.KobayashiM.et al (2003). Synchronization of cellular clocks in the suprachiasmatic nucleus. Science302, 1408–1412. 10.1126/science.1089287
370
YanL.SmaleL.NunezA. A. (2020). Circadian and photic modulation of daily rhythms in diurnal mammals. Eur. J. Neurosci.51, 551–566. 10.1111/ejn.14172
371
YangN.SmyllieN. J.MorrisH.GonçalvesC. F.DudekM.PathiranageD. R. J.et al (2020). Quantitative live imaging of Venus:BMAL1 in a mouse model reveals complex dynamics of the master circadian clock regulator. PLoS Genet.16, e1008729. 10.1371/journal.pgen.1008729
372
YoshidaD.AokiN.TanakaM.AoyamaS.ShibataS. (2015). The circadian clock controls fluctuations of colonic cell proliferation during the light/dark cycle via feeding behavior in mice. Chronobiol Int.32, 1145–1155. 10.3109/07420528.2015.1065415
373
YoungE. (2012). Gut instincts: the secrets of your second brain. New Scientist216, 38–42. 10.1016/S0262-4079(12)63204-7
374
YuanF.TanW.RenH.YanL.WangY.LuoH. (2020). The effects of short-chain fatty acids on rat colonic hypermotility induced by water avoidance stress. Drug Des. Devel Ther.14, 4671–4684. 10.2147/DDDT.S246619
375
ZagorodnyukV. P.KylohM.GregoryS. J.PeirisH.BrookesS. J.Nan ChenB.et al (2011). Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung's disease. J. Physiology589, 1691–1706. 10.1113/jphysiol.2010.202820
376
ZarrinparA.ChaixA.YoosephS.PandaS. (2014). Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab.20, 1006–1017. 10.1016/j.cmet.2014.11.008
377
ZeitzJ.AkM.Müller-MottetS.ScharlS.BiedermannL.FournierN.et al (2016). Pain in IBD patients: very frequent and frequently insufficiently taken into account. Plos One11, e0156666. 10.1371/journal.pone.0156666
378
ZhangR.LahensN. F.BallanceH. I.HughesM. E.HogeneschJ. B. (2014). A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A.111, 16219–16224. 10.1073/pnas.1408886111
379
ZhangS.DaiM.WangX.JiangS. H.HuL. P.ZhangX. L.et al (2020). Signalling entrains the peripheral circadian clock. Cell Signal69, 109433. 10.1016/j.cellsig.2019.109433
380
ZhangT.PerkinsM. H.ChangH.HanW.de AraujoI. E. (2022). An inter-organ neural circuit for appetite suppression. Cell185, 2478–2494.e28. 10.1016/j.cell.2022.05.007
381
ZhangY. K.GuoG. L.KlaassenC. D. (2011). Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. Plos One6, e16683. 10.1371/journal.pone.0016683
382
ZhouM.VanC.MolendijkJ.ChangI. Y-Y.JohnsonC.VelezL. M.et al (2023) Leveraging genetic correlation structure to target discrete signaling mechanisms across metabolic tissues. Elife. 10.7554/elife.88863.1
383
ZimmermanS.ReiterR. J. (2019). Melatonin and the optics of the human body. Melat. Res.2, 138–160. 10.32794/mr11250016
Summary
Keywords
colon, circadian rhythms, colonic motility, enteric nervous system, time of day, pain signaling, colonic absorption, colonic manometry
Citation
Hibberd TJ, Ramsay S, Spencer-Merris P, Dinning PG, Zagorodnyuk VP and Spencer NJ (2023) Circadian rhythms in colonic function. Front. Physiol. 14:1239278. doi: 10.3389/fphys.2023.1239278
Received
13 June 2023
Accepted
17 August 2023
Published
30 August 2023
Volume
14 - 2023
Edited by
Sumei Liu, University of Wisconsin–La Crosse, United States
Reviewed by
Gemma Mazzuoli-Weber, University of Veterinary Medicine Hannover, Germany
Alder Yu, University of Wisconsin La Crosse, United States
Updates
Copyright
© 2023 Hibberd, Ramsay, Spencer-Merris, Dinning, Zagorodnyuk and Spencer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nick J. Spencer, nicholas.spencer@flinders.edu.au
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.