Abstract
Calcium influx through plasma membrane ion channels is crucial for many events in cellular physiology. Cell surface stimuli lead to the production of inositol 1,4,5-trisphosphate (IP3), which binds to IP3 receptors (IP3R) in the endoplasmic reticulum (ER) to release calcium pools from the ER lumen. This leads to the depletion of ER calcium pools, which has been termed store depletion. Store depletion leads to the dissociation of calcium ions from the EF-hand motif of the ER calcium sensor Stromal Interaction Molecule 1 (STIM1). This leads to a conformational change in STIM1, which helps it to interact with the plasma membrane (PM) at ER:PM junctions. At these ER:PM junctions, STIM1 binds to and activates a calcium channel known as Orai1 to form calcium release-activated calcium (CRAC) channels. Activation of Orai1 leads to calcium influx, known as store-operated calcium entry (SOCE). In addition to Orai1 and STIM1, the homologs of Orai1 and STIM1, such as Orai2/3 and STIM2, also play a crucial role in calcium homeostasis. The influx of calcium through the Orai channel activates a calcium current that has been termed the CRAC current. CRAC channels form multimers and cluster together in large macromolecular assemblies termed “puncta”. How CRAC channels form puncta has been contentious since their discovery. In this review, we will outline the history of SOCE, the molecular players involved in this process, as well as the models that have been proposed to explain this critical mechanism in cellular physiology.
Introduction
Calcium is a crucial secondary messenger that serves a multitude of functions ranging from subcellular signaling to organ system-level changes. It serves many roles in biological processes, including growth, disease, and death (Berridge et al., 2000; Carafoli, 2002; Clapham, 2007). Calcium levels in the cytosol are maintained at ∼100 nM in many ways, including calcium pumps and exchangers on the plasma membrane and various organelles. The extracellular calcium concentration is 1–1.5 mM (Clapham, 2007). The calcium ion gradient across the plasma membrane (PM) is maintained by calcium efflux pumps and ion channels, in addition to calcium reuptake mechanisms in ER. Sarco-endoplasmic reticulum calcium ATPase (SERCA) pumps use an active transport mechanism to move calcium across the concentration gradient from the cytosol into the ER lumen (Hasselbach and Makinose, 1961; Ebashi and Lipmann, 1962). Upon cellular stimulation by agonist actions at plasma membrane receptors, a multitude of signaling cascades starts that lead to the production of inositol 1,4,5-trisphosphate (IP3) due to phospholipase-C-mediated cleavage of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) (Rhee, 2001). Once produced, IP3 binds to and activates the IP3 receptors (IP3Rs) on the ER membrane to promote calcium release from the ER lumen into the cytosol. This decrease in calcium levels in the ER leads to intracellular calcium store depletion (Parekh and Penner, 1997). Store depletion leads to the activation of an ER transmembrane protein known as stromal interaction molecule 1 (STIM1), which then binds to Orai1 channels on the plasma membrane to promote store-operated calcium entry (SOCE) (Parekh and Penner, 1997; Parekh and Putney, 2005). The channel formed by Orai1 and STIM1 is known as the calcium release-activated calcium (CRAC) channel. A general overview of SOCE is presented in Figure 1.
FIGURE 1
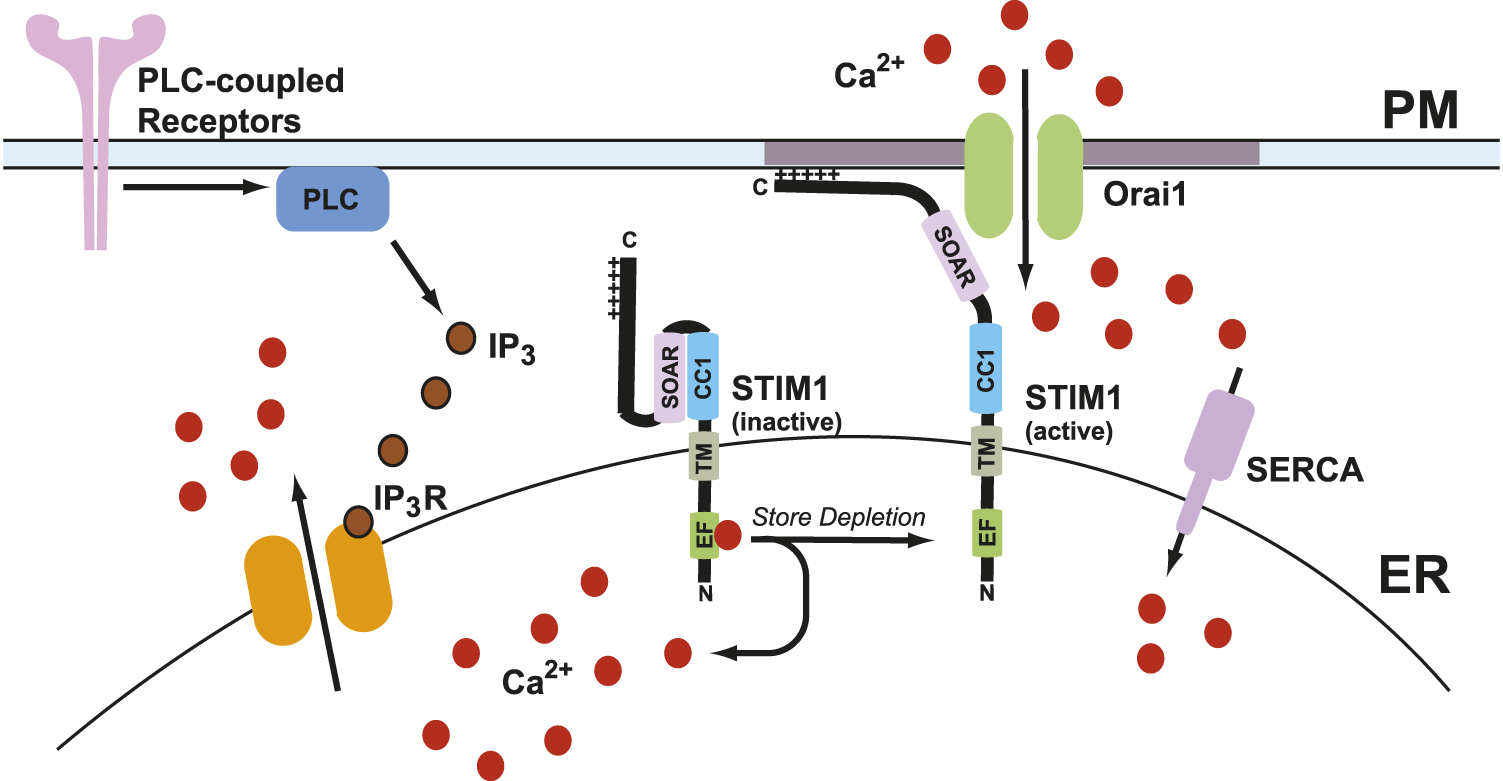
General overview of store-operated calcium entry (SOCE). Agonist stimulation through the PLC-coupled receptors leads to IP3-mediated calcium release which leads to ER calcium store depletion. As a result, calcium dissociates from the EF-hand of STIM1 and leads to its activation. This leads to a conformation change in STIM1 which extends its C-terminus toward plasma membrane where it binds to Orai1 channels and form calcium release-activated calcium (CRAC) channels. CRAC channels promote calcium entry from extracellular milieu into the cells. One subunit of STIM1 dimer is shown here for simplicity.
Store-operated calcium entry is a predominant mechanism for calcium entry from the extracellular milieu to increase cytosolic calcium after store depletion. It also serves to refill the ER calcium stores after IP3-mediated calcium release (Negulescu and Machen, 1988; Jousset et al., 2007). The most important role SOCE plays is a sustained calcium entry that can last from several seconds to up to an hour in some cellular systems (Cheng et al., 2011; Quintana et al., 2011). One specific case where this differential role of calcium entry plays a crucial role is the activation of T cells. The activation of nuclear factor of activated T cells (NFAT), which regulates IL-2 production, a sustained calcium entry is needed, which lasts for several minutes (Hogan et al., 2003). While for activation of NF-ΚB, calcium oscillations of lower frequency from Orai1 channels are required. Finally, CaMKII, a calcium sensitive kinase, needs calcium oscillations of higher frequency for its activation. Thus, cellular functions regulated by calcium are mediated by changes in both frequency and amplitude (Smedler and Uhlen, 2014). Calcium entry from the plasma membrane is essential for shaping the spatiotemporal aspects of calcium transients, including the amplitude and duration of calcium release events. Calcium entry contributes to many cellular functions such as secretion, gene transcription, and enzyme activation. Efficient SOCE requires the formation of multiple Orai1-STIM1 complexes at defined ER:PM junctions known as “puncta” (Wu et al., 2006; Liou et al., 2007; Barr et al., 2008; Penna et al., 2008). A key difference between CRAC channels and other calcium channels such as voltage or ligand gated ion channels is their ability to conduct calcium currents at resting membrane potentials without ligand binding (Lewis and Cahalan, 1989). This property allows CRAC channels to be gated in non-excitable cells by exclusively by binding to STIM1. This facilitates precise spatiotemporal regulation of cellular calcium signaling by store depletion.
In this review, we look at the historical origins of SOCE and summarize a few landmark studies that established the characteristics of SOCE. We will delve into the proteins that form the CRAC channel complex, such as Orai1 and STIM1, and how these proteins are regulated. In addition to STIM1, STIM2 is another member of ER resident calcium sensor that promotes SOCE. Orai2 and Orai3 are genes in the Orai family with Orai1. Proteins outside these two families such as TRP channels also play a role in SOCE. Finally, we will also review the effect of SOCE-associated regulatory factor (SARAF) and role it plays in SOCE. SARAF promotes calcium dependent inactivation, but how it functions as an inactivator of SOCE is not well known. Our review outlines the key modulators of SOCE. We will conclude with a discussion of very recent work indicating the protein S-acylation is a key mediator of CRAC channel assembly and function.
History of SOCE
The earliest known proposal for SOCE was introduced by the Putney group in 1985 as outlined by their model for receptor-regulated calcium entry, in which they proposed the mechanism for sustained calcium entry from extracellular matrix upon agonist binding to G-protein coupled receptors (Putney, 1986). Termed as the capacitive calcium entry model, they carefully analyzed the calcium release from the ER and entry from extracellular milieu, and the coupling between these two processes. A few years later, Hoth and Penner identified a similar inward rectifying calcium current in mast cells upon depletion of intracellular calcium stores using IP3 (Hoth and Penner, 1992). Zweifach and Lewis then showed store depletion in T cells leads to a similar calcium current using thapsigargin, a SERCA pump inhibitor (Zweifach and Lewis, 1993). Later, studies published by Liou et al., and Roos J et al., identified STIM proteins as sensors of ER luminal calcium (Liou et al., 2005; Zhang et al., 2005). A year later, studies by Feske et al. showed how a mutation in Orai1 protein causes immune deficiency by affecting SOCE (Feske et al., 1996). Other studies during this same period also showed Orai1 is crucial for SOCE (Vig et al., 2006b). A genome wide RNAi screen in drosophila S2 cells showed the role of both Orai1 and STIM1 in SOCE. They demonstrated that RNAi of olf186-f, the drosophila Orai1 homologue, reduced thapsigargin-evoked SOCE, which was improved by 3-fold upon its overexpression. In addition, co-expression of STIM with olf186-f increased SOCE by approximately 8-fold (Gwack et al., 2006; Yeromin et al., 2006). These seminal studies identified the key regulatory proteins involved in SOCE. What followed is an expansion of studies involving Orai and STIM proteins and their role in CRAC channel formation to promote SOCE.
IP3-mediated calcium release
Cleavage of membrane phospholipids by phospholipase C (PLC) leads to the production of IP3 and diacylglycerol (Rhee, 2001). Liberated IP3 binds to IP3 receptor calcium channels on ER membranes and leads to calcium efflux from the ER lumen (Streb et al., 1983). Depending on the strength and duration of agonist stimulation, IP3R activation leads to a decrease inf calcium levels in the ER lumen which is termed store depletion (Streb et al., 1983; Parekh and Penner, 1997). The role of IP3 in activating calcium release from the endoplasmic reticulum was initially discovered by experiments by the Berridge group in blowfly salivary glands based on the ability of those tissues to respond to hydrolysis of PIP2 (Berridge and Irvine, 1984). Subsequently, the role of IP3 as a secondary messenger was to mobilize internal calcium stores in mammalian cells was demonstrated in saponin-permeabilized hepatocytes (Joseph et al., 1984). Application of IP3 led to a brief rise in intracellular calcium levels followed by a sustained calcium plateau (Joseph et al., 1984). Capacitive calcium entry was the first model proposed to explain the calcium entry observed in cells upon emptying intracellular calcium pools (Putney, 1986). This idea originated from the observation that calcium entry from the extracellular milieu lasted for a long duration after the levels of IP3 returned to the baseline. These observations were made in rat parotid gland upon carbachol (Aub et al., 1982) application, and rabbit ear artery upon application of noradrenaline (Casteels and Droogmans, 1981). Based on the biphasic nature of agonist-induced increase in the cytosolic calcium, it was proposed that the initial increase is a result of calcium release from the ER due to the action of IP3 followed by influx of calcium through channels in the plasma membrane until the levels of calcium in the ER reaches to a significant level that stops the entry (Putney, 1986).
CRAC channel characterization and models of activation
An effort to accurately define the mechanism of SOCE and to distinguish it from other calcium currents led to further research into the channels underlying these currents. CRAC channels conduct calcium currents at a negative membrane potential. The voltage independence of CRAC channel gating was first observed in patch recordings conducted on Jurkat T cells treated with various cell mitogens such as phytohaemagglutinin (PHA), a substance known to activate T-cell signaling pathway (Lewis and Cahalan, 1989). A similar current was also observed in these cells recorded in low extracellular calcium. In a separate set of experiments conducted in mast cells, intracellular dialysis with IP3 and extracellular application of substance P generated a similar low-noise current (1–2 pA) in patch-clamp recordings (Penner et al., 1988; Matthews et al., 1989). This current developed in these cells with a concomitant increase of intracellular calcium. The currents observed by both groups showed similar features such as inward rectifying current-voltage relationship, voltage-independent gating, very high calcium selectivity, an extremely low unitary conductance, and extracellular calcium-dependent feedback inhibition.
The discovery of thapsigargin as a potent, selective, and irreversible SERCA pump inhibitor helped in delineating the difference between CRAC currents and other calcium currents (Thastrup et al., 1989). Thapsigargin promotes a slow calcium leak from the ER lumen into the cytosol, and thereby passively depletes ER calcium stores, possibly through the Sec61 translocon on the ER membranes (Van Coppenolle et al., 2004). In addition, the development of calcium sensitive fluorescent dyes made live-calcium imaging feasible without the need for electrophysiological recordings (Grynkiewicz et al., 1985). One established model of isolating SOCE currents using calcium-sensitive dyes involves depletion of ER calcium stores by treatment with thapsigargin in calcium-free buffer. Subsequent treatment of these cells with calcium replete buffer results in calcium entry from extracellular milieu which can easily be monitored using microscopy in live cells. Using this methodology, along with other imaging paradigms, many groups have reported SOCE in both excitable and non-excitable cells. It was discovered that PLC-mediated IP3 production was dispensable for SOCE (Gouy et al., 1990; Mason et al., 1991; Sarkadi et al., 1991). A seminal observation made in parotid acinar cells using thapsigargin to deplete stores garnered evidence that the ER calcium store levels and activate SOCE independently of IP3 production (Takemura et al., 1989). A subsequent set of experiments done on T cells using thapsigargin established the role of ER calcium store depletion and T-cell activation. The term ICRAC was coined by Hoth and Penner after identifying calcium currents in whole cell currents elicited by a number of agents such as ionomycin, IP3, and EGTA (Hoth and Penner, 1992). The crucial experiments conducted by Lewis and others using perforated-patch clamp and thapsigargin in T cells showed the similarity between TCR-mediated calcium currents and CRAC currents (Zweifach and Lewis, 1993; Fanger et al., 1995; Serafini et al., 1995). These studies led to the definitive conclusion that CRAC currents are controlled by intracellular ER calcium stores.
Hypotheses for CRAC channel activation
The research into how ER calcium depletion leads to CRAC channel activation led to three main hypotheses: 1) diffusion of an activating factor released from the ER to the plasma membrane, 2) targeting of active CRAC channels to the PM by membrane fusion, and 3) conformational coupling between a putative ER calcium sensor and a PM calcium channel. Here we will discuss these proposals in detail.
Calcium influx factor (CIF): The earliest proposal for a diffusible mediator that is released from the ER into the cytosol and/or the extracellular milieu to activate calcium influx was presented in early 1990s. Application of phytohaemagglutinin (PHA)-treated Jurkat T cell extracts to P388D1 macrophage cells showed a sustained but fluctuating calcium increase. A diluted version of the extract decreased the amplitude and increased the latency of the calcium flux (Randriamampita and Tsien, 1993). Interestingly, NG115-401L cells did not show CIF-induced calcium entry (Thomas and Hanley, 1995). In later studies, it was shown that NG115-401L cells do not express endogenous STIM1 (Zhang and Thomas, 2016). The treatment of putative CIF-containing extracts with alkaline phosphatase neutralized the effect of these extracts. These observations led to a proposal where cellular stimulation leads to the production of a diffusible factor that activates extracellular influx of calcium (Parekh and Penner, 1997; Parekh and Putney, 2005).
Membrane fusion of active CRAC channels: It was found that acid extracts from thapsigargin-treated Jurkat cells leads to a chloride current in Xenopus oocytes (Thomas and Hanley, 1995). Microinjection of Xenopus oocytes with the Rho GTPase inhibitor clostridium C3 transferase potentiated a calcium entry current termed ISOC. In addition, the expression of wild-type or constitutively active Rho inhibited ISOC. Interestingly, botulinum neurotoxin A and dominant negative SNAP-25 mutants activated ISOC. Treatment of these cells with brefeldin A, an agent that blocks exocytosis by inhibiting protein maturation and exit from Golgi apparatus, has no effect on ISOC. Based on these results, Tsien and others proposed the model where SOCE is mediated by exocytosis, leading to CRAC channels being inserted into the plasma membrane (Yao et al., 1999).
Conformational coupling of an ER calcium sensor and a PM calcium channel: The IP3 receptor has calcium binding sites in its luminal domain. This calcium binding site on the IP3 receptor was proposed to regulate calcium efflux from the ER into the cytosol (Supattapone et al., 1988; Gill, 1989). Based on these observations, it was proposed that the IP3R was a sensor for ER calcium levels. Upon depletion of ER calcium stores, a cytosolic domain of the receptor binds to the plasma membrane to promote calcium influx. In this hypothesis, the IP3 receptor is the regulator of calcium homeostasis in the cells, resulting in release from the ER lumen, as well as influx from the extracellular milieu (Irvine, 1990). The role the IP3R plays in this conformational coupling model is analogous to the role of ryanodine receptors and dihydropyridine receptors in muscle cells (Lee et al., 2004).
Identifying the mechanism(s) of CRAC channel activation ultimately required the cloning and characterization of the relevant calcium channel(s) activated by store depletion. In the next section, we will discuss the putative role of transient receptor potential (TRP) channels in mediating SOCE.
TRP channels as potential CRAC channels
Transient receptor potential (TRP) channels are ion channels that show diverse ion selectivity, activation mechanisms, and physiological functions. All TRP channels share some common features such as six transmembrane domains, tetrameric structure, cation selectivity, and sequence homology. The first TRP channels were characterized in Drosophila visual transduction mutants (Montell, 2001; Montell, 2005). The Drosophila trp and trpl mutants have a significant decrease in light-induced calcium influx (Cosens and Manning, 1969; Hardie and Minke, 1992). Combined with other mutants in the PLC pathway which also affected vision, it was hypothesized that trp/trpl channels are putative SOCE channels. Cloning and characterization of these channels showed that the protein encoded by the trp gene localizes to the eye, contains four N-terminal ankyrin repeats, and a transmembrane topology similar to voltage-gated ion channels. (Montell et al., 1985; Montell and Rubin, 1989). Whole cells currents recorded from Sf9 insect cells showed the channels encoded by trp gene are activated upon ER calcium store depletion and are moderately selective for calcium over monovalent cations such as sodium (PCa:PNa ∼ 10:1) (Vaca et al., 1994).
Drosophila trp channels: In Drosophila, three TRPC members are expressed in the eye that play an important role in phototransduction. TRP, TRPL, and TRPγ are the three proteins work in concert for successful operation of fly vision (Phillips et al., 1992; Xu et al., 2000). TRPL and TRPγ have ∼50% sequence identity with TRP in the six TM domains, and only differ in the TRP domain, which contains highly conserved regions known as TRP boxes 1 and 2. Loss-of-function and dominant-negative mutations in Drosophila TRPCs have demonstrated the importance of these channels in calcium influx in photoreceptors of Drosophila upon light stimulus. The trpl mutant flies affect the specificity of different cation influx and a decreased response to light stimulus of long duration. In addition, trp/trpl double mutant flies cannot respond to light, showing the importance of these channels. Finally, TRPγ heteromultimerizes with TRPL to form light-regulated channels, and a dominant-negative form of TRPγ suppresses TRPL currents (Niemeyer et al., 1996; Reuss et al., 1997; Leung et al., 2000; Xu et al., 2000).
The Drosophila TRP can be activated in vitro by blocking the SERCA (sarcoplasmic endoplasmic reticulum calcium ATPase) pumps that maintain the calcium gradient between ER lumen and cytosol (Phillips et al., 1992; Vaca et al., 1994). Thapsigargin irreversibly blocks SERCA pumps and causes a passive ER calcium leak, which will then activate store-operated calcium channels (Thastrup et al., 1989; Thastrup et al., 1990). However, in vivo observations do not support the in vitro analyses. Thapsigargin-mediated SERCA blockade or IP3 receptor activation does not promote calcium influx or affect phototransduction (Ranganathan et al., 1994; Hardie, 1996; Hardie and Raghu, 1998). Some studies also evaluated the potential effect of diacylglycerol, another product of PLC hydrolysis of PIP2, on calcium influx and phototransduction. Isolated photoreceptor cells from drosophila wild type and rdgA (DAG kinase) mutants show constitutive TRP activity. Recently, endocannabinoids produced in Drosophila photoreceptor cells in response to light have been shown to activate TRP channels (Sokabe et al., 2022). In addition, using flies engineered to express genetically encoded ER calcium indicator, the sodium/calcium exchanger has been shown to result in rapid calcium release from the ER upon light exposure (Liu et al., 2020).
Mammalian TRP channels: The seven TRPC proteins in mammals are divided into four groups based on sequence homology (Wes et al., 1995; Zhu et al., 1995). Like Drosophila TRPC proteins, the mammalian counterparts also include three to four ankyrin repeats, 6 TM domains, and high sequence homology in the TRP box domain in the N-terminus (Philipp et al., 1996; Okada et al., 1998; Vannier et al., 1998; Wissenbach et al., 1998; Okada et al., 1999). Like Drosophila TRPCs, the activation of mammalian TRP channels can be activated in cultured cells through PLC activation (Philipp et al., 1996; Zhu et al., 1996). The specific mediator in the PLC pathway that ultimately activates these channels is still unknown, and hypotheses include activation by IP3, DAG, and calcium. TRPC1-4 proteins have been shown to be activated upon store depletion, either by IP3 or thapsigargin treatment in cultured cells expressing these proteins (Philipp et al., 1996; Zhu et al., 1996; Zitt et al., 1997). Calcium influx factor was proposed to be a factor to activate these channels to promote calcium influx (Randriamampita and Tsien, 1993; Thomas and Hanley, 1995; Smani et al., 2004). Additional hypotheses also included conformational coupling between IP3R and the TRP channels (Irvine, 1990). Some models also suggested internalization of TRPC1/3/4 channels upon store depletion (Lockwich et al., 2001; Itagaki et al., 2004). Other TRPC proteins, such as TRP6/7, are activated by DAG and show similarities to Drosophila TRP channels (Hofmann et al., 1999; Itagaki et al., 2004).
Another complexity in mammalian TRPC channel activation comes from context and cell type-specific activation mechanisms. For example, mouse TRPC2 in vomeronasal neurons has been shown to be activated by DAG, whereas the same protein in mouse sperm has been shown to be activated by calcium release (Jungnickel et al., 2001; Lucas et al., 2003). Whether this differential activation is due to alternate splicing or different multimerization is unknown (Vannier et al., 1999; Hofmann et al., 2000). TRPC3 also shows similar differential activation between DAG and store depletion. Some reports also suggest direct binding between TRPC3 and IP3R as a mode of activation of these channels (Zhu et al., 1996; Kiselyov et al., 1998; Hofmann et al., 1999).
TRP Channels and their role in SOCE: Of the many roles TRP channels play in cellular physiology, a crucial one is store-operated calcium entry (Brough et al., 2001). TRP channels have low calcium selectivity, which distinguishes them from conventional CRAC channels or voltage-gated calcium channels. As such, TRP channels are high conductance and non-selective cation channels with varying permeability ratios between calcium, potassium, and sodium (Petersen et al., 1995; Xu et al., 1997). The mechanism of TRP channel activation and gating ranges from changes in cytosolic calcium concentration, to membrane depolarization, and external cellular stimuli (Hoth and Penner, 1992). The loop between transmembrane domains 5 and 6 form the channel pore of these TRP channels. Four individual TRP subunits form an active channel complex to promote calcium entry (Venkatachalam and Montell, 2007; Hellmich and Gaudet, 2014). The domain architecture of different TRP family proteins regulates the functions of these proteins. For example, TRPC, TRPV, and TRPA channels have repeats of ankyrin regions in their N-terminus (Hellmich and Gaudet, 2014) and TRPC and TRPM channels have a conserved TRP domain adjacent to the last transmembrane domain (Montell, 2005). TRPM subfamily of TRP channels have a catalytic kinase domain in their C-terminus. These proteins also have a highly conserved TRP box sequence (Glu-Trp-Lys-Phe-Ala-Arg) and proline-rich sequence that regulates signal transduction and gating (Gregorio-Teruel et al., 2014). In addition, coiled-coil domains in C- and N- terminal domains aid in the assembly of some TRP channel subfamilies (Baez-Nieto et al., 2011). These coiled-coil domains also regulate the channel binding to STIM1, the ER calcium sensor, to regulate SOCE (Lee et al., 2014). A C-terminal calmodulin and IP3R binding region in TRPC channels is known to regulate both store-independent, and store-dependent calcium entry (Wedel et al., 2003; Dionisio et al., 2011a).
Support for TRP channels acting as store-operated channels came from the studies done using TRPC1 expression in vitro that showed increased calcium entry after store depletion (Zhu et al., 1996; Zitt et al., 1996; Zitt et al., 1997). One key difference in these experiments was even though these cells showed calcium entry upon store depletion, this current was different from the biophysical properties observed in CRAC currents (ICRAC) (Zitt et al., 1997; Trebak et al., 2003; Desai et al., 2015). Experiments later determined that TRPC1 is assembled with the Orai1-STIM1 complex (Ong et al., 2007; Jardin et al., 2008a). Immunofluorescence and confocal microscopy in human salivary gland cells showed colocalization of Orai1, STIM1, and TRPC1 at the plasma membrane upon store depletion (Ong et al., 2007). Activation and gating of TRPC1 is mediated by negatively charged aspartate residues which bind STIM1 at both the SOAR domain as the polybasic domain (Zeng et al., 2008). Examination of how calcium entry observed from Orai1:STIM1 complex upon store depletion differs from TRPC1:STIM1 complex led to many interesting hypotheses. One hypothesis is that the local increase of cytosolic calcium near ER:PM junctions upon store depletion activates cytosolic TRPC1 channels, which then gets embedded into the plasma membrane to be activated by STIM1 (Cheng et al., 2008; Cheng et al., 2011). This model can explain the spatiotemporal differences observed in calcium oscillations in Orai1:STIM1 complexes versus TRPC1:STIM1 complexes. These differences also affect the physiological and pathological outcomes of cytosolic calcium transients. One specific example is the activation of nuclear factor of activated T-cells (NFAT) versus activation of NF-kB. Calcium entry mediated by Orai1 after T-cell stimulation leads to activation of NFAT (Feske et al., 2005; McCarl et al., 2009; Akimzhanov et al., 2010), while TRPC1-dependent calcium entry leads to activation of NFkB (Ong et al., 2012). Interestingly, knockdown of Orai1 in Jurkat cells inhibits both SOC and CRAC currents, with no effect upon knockdown of TRPC1 or TRPC3 (Kim et al., 2009). In addition, calcium entry observed from the Orai1:STIM1:TRPC1 complex leads to insulin release from pancreatic beta cells, platelet activation during blood clotting, and SNARE complex formation for adipocyte differentiation and adiponectin secretion (Galán et al., 2009; Sabourin et al., 2015; Schaar et al., 2019). Furthermore, calcium entry from this complex contributes to prostate and colon cancer cell migration (Guéguinou et al., 2016; Perrouin-Verbe et al., 2019). Finally, calcium entry observed in STIM1-Orai1-TRPC1-TRPC4 complexes plays a pivotal role in right ventricular hypertrophy (Sabourin et al., 2018).
Decades of research has now shown the pivotal role of TRP channels modulating the spatiotemporal aspects of calcium release in many systems. This includes activation by store depletion and other IP3-mediated signaling pathways. However, TRP channels do not re-capitulate the “classic” biophysical properties of store-operated calcium currents originally described T cells, including small unitary conductance and very high calcium selectivity. We will next describe the cloning of Orai1 and STIM1 as mediators of this specific type of SOCE.
Discovery and cloning of Orai1 and STIM1
Feske and others reported abnormalities in T-cell activation in infants born to consanguineous parents that manifested as severe combined immunodeficiency (SCID) (Feske et al., 1996). They found increased CD4+ T cell counts and inability of these T cells to produce IL-2 upon stimulation with phorbol 12-myristate 13-acetate (PMA), concanavalin A, and anti-CD3 stimulation. Exogenous application of IL-2 restored the proliferative deficiencies of these cells. Further analyses into the DNA binding activity of transcription factors showed a lack of NFAT binding to DNA with no difference in AP-1, NF-kB, and Octamer binding proteins. They also found no differences in the expression of NFAT, but the dephosphorylation and nuclear translocation was affected in these T cells (Feske et al., 2000a). In subsequent experiments using PMA + ionomycin and increasing concentrations of extracellular calcium, they found that higher levels of extracellular calcium rescued IL-2 production in these T cells (Feske et al., 2000b). They concluded that dysregulation in upstream signaling events play a role in NFAT binding to DNA elements that ultimately results in SCID (Feske et al., 1996).
During this time, it was discovered that there exist two different calcium mobilization patterns in T cells after stimulation. An immediate transient calcium spike (Berridge, 1993) followed by a sustained calcium influx from extracellular milieu (Putney, 1986; Clapham, 1995). The transient calcium release activates NF-kB and JNK, but NFAT activation needs sustained elevations to promote dephosphorylation by the calcium-dependent phosphatase calcineurin (Timmerman et al., 1996; Dolmetsch et al., 1997; Crabtree, 1999; Kiani et al., 2000). Calcium imaging of T cells obtained from the patients with SCID showed dysregulated calcium entry upon stimulation with anti-CD3, ionomycin, and thapsigargin. More importantly, membrane hyperpolarization with valinomycin did not alter calcium entry abnormalities found in these cells, suggesting the lack of calcium entry is not a result of depolarization (Feske et al., 2001). These intriguing results warranted further research into the calcium entry in T cells upon their activation and the ion channels that promote sustained calcium entry.
Development of RNAi screening as a research tool in early 2000s opened avenues for high throughput identification of proteins that mediate specific signaling pathways (Downward, 2004; Dykxhoorn and Lieberman, 2005). Two important siRNA screens performed at this time identified the proteins involved in mediating ICRAC and pinpointed the possible mechanism of activation (Gwack et al., 2006; Zhang et al., 2006). These two screens were performed in the Drosophila S2 cells, which were known to have a store-operated channel with low conductance and high calcium selectivity similar to ICRAC in mammalian cells (Yeromin et al., 2004). The siRNA screen performed by Feske and others identified known proteins that affect calcium influx in addition to NFAT regulatory proteins (Gwack et al., 2006). When they used this RNAi screen to identify potential regulators of Ca+2/calcineurin mediated NFAT activation, they found a kinase that negatively regulated exogenously expressed NFAT. In addition, they also found other candidates in this screen that alter calcium levels in the cytosol such as SERCA, Homer, and STIM. Concurrently, Zhang and others independently performed a similar genome-wide RNAi screen (Zhang et al., 2006). Probing for hits that resulted in an inhibition of calcium influx after store depletion by thapsigargin, they identified 11 transmembrane proteins including STIM. Of note was another four transmembrane protein olf186-F, which showed a reduction in SOCE and CRAC currents. They followed up this experiment with an overexpression paradigm, which showed a three-fold increase in CRAC currents, which was further increased to eight-fold upon co-expression with STIM (Zhang et al., 2006). These two pivotal experiments identified Orai1 and STIM1 as the core proteins that form the CRAC channel.
At the same time these RNAi experiments were being conducted, Feske and others continued their research into understand the T-cell activation abnormalities in SCID infants. Using whole-cell patch-clamp electrophysiology performed on T cells from control and SCID patients, they showed that T cells obtained from some SCID patients lacked SOCE. Furthermore, they also found that exogenous expression of STIM1 in these SCID cells did not rescue SOCE in these cells (Feske et al., 2005). Next, they used a combined approach of Drosophila RNAi screening and linkage analysis with a single-nucleotide polymorphism (SNP) array to screen for regulators of SOCE and nuclear import of NFAT. T cells obtained from SCID infants and relatives of these infants were analyzed for SOCE deficits and SNP mapping arrays using their genomic DNA. This, in conjunction with Drosophila RNAi screen for NFAT regulators, pointed to a gene olf186-f in Drosophila and to the TMEM142A gene on chromosome 12 in humans which they named Orai1 (Feske et al., 2006). Genomic DNA sequencing showed a mutation of an arginine to tryptophan at residue 91 (R91W) on Orai1, identifying the molecular defect in these patients leading to reduced SOCE. Finally, exogenous expression of wild-type Orai1 in T cells from SCID patients restored CRAC channel activity.
STIM1 is mammalian homolog of Drosophila STIM, which is essential for CRAC channel activation in human T cells. Roos and others showed in S2, HEK293, and Jurkat cell lines that silencing of STIM1 abrogates SOCE (Roos et al., 2005). Zhang et al. determined the role of the luminal EF hand motif by expressing calcium binding mutants in Jurkat T cells and analyzing SOCE. The STIM1 EF hand calcium binding mutants 1A3A and 12Q were found to be constitutively active STIM1 mutants (Zhang et al., 2005). At the time, the pore forming Orai1 subunit had not been cloned and it was hypothesized that STIM1 may traffic to the PM upon store depletion to activate a channel or form a channel itself. The critical role of the EF hand in SOCE and puncta formation was independently confirmed by another group in HeLa cells using a D76A mutant (Liou et al., 2005). This discovery, along with the discovery of Orai1 as CRAC channel pore forming subunit, potentiated a barrage of research into these proteins in SOCE.
The discovery of Orai1 as pore-forming subunit of CRAC channels was a direct result of the RNAi approaches in Drosophila S2 cells by several groups as outline above. Following the research into calcium deficits observed in SCID human patients, two different groups pursued RNAi screens focused toward decreases in NFAT translocation into nucleus, and they individually found the same four transmembrane plasma membrane protein with intracellular C and N termini olf186-F (Feske et al., 2001; Vig et al., 2006b), and named it CRACM1 (Vig et al., 2006b). The human homolog of this protein was eventually named Orai1. There are three homologs of Orai proteins with high sequence identity, with up to 90% identity in the transmembrane regions. Overexpression of Orai1 and STIM1 in HEK293 cells helped identify the pore forming subunit of CRAC channels. The definitive proof for Orai1 as the pore forming subunit came from experiments that showed the importance of acidic residues in the transmembrane domains of Orai1. Residues E106 and E190 are two important glutamic acid residues that line the pore of Orai1 channel. Mutation of these residues to aspartate and glutamine respectively decreased the calcium influx, increased monovalent cation current, and made the channel cesium permeable (Prakriya et al., 2006). These experiments strongly supported Orai1 as the pore forming unit of CRAC channel.
Molecular players in SOCE
The discovery of STIM1 as ER calcium sensor that activates SOCE upon store depletion was a pivotal moment in furthering the field of store-operated calcium entry (Liou et al., 2005). Soon after the discovery of STIM1, multiple research groups discovered a four transmembrane cell surface protein in Drosophila and its homologs in mammalian cells which would ultimately be known as Orai (Vig et al., 2006b; Feske et al., 2006; Zhang et al., 2006). Overexpression of Orai1 in T cells isolated from patients with SCID restored the calcium entry deficits in those cells. In addition, co-expression of Orai1 with STIM1 in HEK293 cells showed calcium currents in these cells that matched the biophysical properties of CRAC currents (Mercer et al., 2006). CRAC channel formation is interesting in that is requires the assembly of proteins from two different membranous subcellular compartments to form the active channel complex (Zhang et al., 2005; Ong et al., 2007; Lioudyno et al., 2008; Zhou et al., 2010a; Kim and Muallem, 2011). In this next section, we will discuss how this mechanism of activation was discovered.
STIM1
Depletion of ER calcium stores initiates a cascade of events starting with the activation of STIM1 and culminates with calcium entry from the extracellular milieu. STIM1 is a dimer of two single pass type I ER transmembrane proteins. In the N-terminus, there is a canonical and a noncanonical EF hand motifs that play a role in calcium sensing in the ER (Stathopulos et al., 2006; Stathopulos et al., 2009). Immediately adjacent to the EF hand motifs, there is a sterile alpha motif (SAM) domain. On the cytosolic side, there is a calcium-activating domain (CAD) which is also known as STIM-Orai activation region (SOAR), which binds to the Orai1 channel (Yuan et al., 2009). The cytosolic C-terminus also contains three coiled-coil domains (CC1, CC2, CC3) that help maintaining STIM1 in its inactive conformation when ER calcium stores are full. Near the C-terminus, there is a polybasic domain which binds to the PIP2 in the plasma membrane (Yuan et al., 2009). In addition to these domains, STIM1 also has and inhibitory domain (ID), a proline-serine rich domain (P/S domain), EB1 binding domain (EB) in its cytosolic side. These domains act in conjunction with each other upon store depletion to colocalize with Orai1 to form CRAC channels.
The calcium binding EF hand motifs are required for STIM1 to sense ER calcium store levels. This was confirmed using experiments with mutations in the EF hand motif. The mutations D76A, D78A, and E87A reduce the affinity of this motif to bind to calcium and show constitutive puncta formation as well as CRAC currents in resting cells (Liou et al., 2005; Zhang et al., 2005; Mercer et al., 2006). The EF-SAM domains of STIM1 and STIM2 show similar affinities to calcium in vitro, however, structural differences in the two proteins results in dramatically different abilities to oligomerize in response to store depletion (Stathopulos et al., 2006; Zheng et al., 2008).
The first step in the CRAC channel activation after ER calcium store depletion is the conformational change in STIM1. This was elucidated in experiments conducted using the cytosolic fragment composed of amino acids 233–685, which is capable of activating SOCE regardless of store depletion (Huang et al., 2006). Further experiments involving truncation mutants of STIM1 led to identification of STIM1-Orai1 activating region (SOAR/CAD), the stretch of amino acids that can activate SOCE without store depletion. It is, however, interesting to note the difference in the amino acid sequences identified by individual groups ranged from 342 to 448 (Park et al., 2009), 344–442 (Yuan et al., 2009), and 339–444 (Kawasaki et al., 2009). Despite these differences, the consensus is that the SOAR/CAD region can activate CRAC currents independently of store depletion. Further research into CAD domain also showed that CAD binds both the N- and C-termini of Orai1, but the strength of binding is higher at the C-terminus (Park et al., 2009). This binding between Orai1 and CAD is mediated by a coiled-coil interaction between the CC2 region of STIM1-CAD and the polar residues in the C terminus of Orai1 (Stathopulos et al., 2013).
In resting conditions, STIM1 is diffusely localized throughout the ER, but upon store depletion redistributes into clusters that are termed “puncta” (Park et al., 2009). In resting conditions, STIM1 is in a compact conformation that keeps it from interacting with Orai1. Upon store depletion, a conformational change in STIM1 leads to an extended conformation with its N-terminus reaching toward the plasma membrane where it binds to the C-terminus of Orai1 (Zhang et al., 2005; Wu et al., 2006; Xu et al., 2006). Following store depletion, a complex choreography of intramolecular events happens between several domains of STIM1 that stabilizes STIM1 in its extended conformation to promote binding with Orai1. The ER:PM sites of cells are held in close apposition to promote STIM1 and Orai1 binding to promote CRAC channel formation by a multitude of accessory proteins such a septins, synptogamins, junctate, and others as described above (Wu et al., 2006). The extended conformation of STIM1 was proposed to trap Orai1 into puncta, leading to channel gating to promote SOCE (Hoover and Lewis, 2011; Wu et al., 2014). This is known as the “diffusion-trap” model.
STIM1 exists as a dimer in cells with calcium replete stores in the resting state. This was confirmed using the C-terminal cytosolic fractions in vitro which formed dimers in solution. The specific domains involved in dimerization of STIM1 were further resolved using co-immunoprecipitation and fluorescence photobleaching of individual fragments (Covington et al., 2010). The coiled-coiled domain fragments and CAD domain independently can form dimers in vitro. The CAD domain dimerization is interesting because STIM1 binding to Orai1 is also mediated by this domain. The CC1 domains alone can form dimers, but they are weak and unstable (Muik et al., 2009; Covington et al., 2010). This supports the conclusion that STIM1 dimerization is a complex process requiring the interaction of multiple domains in the protein.
The CAD domain of STIM1 can independently and constitutively activate Orai1 and promote CRAC currents. Based on a crystal structure of CAD domain, hydrophobic residues form hydrogen bonds between the two monomers to stabilize the dimer (Kawasaki et al., 2009). In addition, interactions between the CC2 and CC3 regions of the STIM1 are purported to stabilize the extended conformation of STIM1. One interesting finding in the structure of CAD domain is the role of the CC2 and CC3 helices in binding to and activating Orai1 channels. In resting inactive state, the two CC2 helices (in a dimer) are in a parallel configuration in a tight hairpin structure with CC3 helices. Upon store depletion, these CC2 helices pivot and twist to an antiparallel orientation, allowing CC3 to extend out and allow for binding with Orai1 (Stathopulos et al., 2008; Yang et al., 2012; Stathopulos et al., 2013; Fahrner et al., 2014). These are highly complex and precise molecular movements in a restricted space between ER and PM within seconds of store depletion.
The EF hand domains of STIM1 residing in the ER lumen also undergo dimerization upon release of calcium from their binding pockets. In resting state, these domains exist as compact monomers bound to calcium (Stathopulos et al., 2006). NMR-resolved calcium-bound STIM1/2 EF-SAM fragments show an α-helical structure with a canonical (cEF) and non-canonical (nEF) EF hand domains. The cEF domain contains a helix-loop-helix structure that binds to calcium and nEF domain does not bind to calcium but stabilizes the cEF through hydrogen bonding (Zheng et al., 2011). Upon calcium release, the EF-SAM domains unfold leading to conformational changes in luminal and cytosolic domains of STIM1. Mutations in the glutamate (E87A) or leucine residues (L195R) individually, and phenylalanine and glycine (F108D and G110D) together, in the EF-hand domain led to puncta formation irrespective of calcium depletion (Stathopulos et al., 2008). This led to a proposal that calcium release from EF-hands leads to STIM1 oligomerization upon store depletion. Live-cell imaging and FRET studies using STIM1 mutants added support to this hypothesis, as they show increased FRET between STIM1 fused with YFP and CFP in RBL cells, which was reverse upon calcium addback (Liou et al., 2007). Furthermore, FRET experiments conducted using Orai1-CFP and STIM1-YFP also show a spatiotemporal correlation between STIM1 oligomerization and Orai1-STIM1 binding (Muik et al., 2008).
Orai1
There are three homologs of Orai proteins (Orai1, Orai2, Orai3) in humans, and all three proteins promote calcium entry upon store depletion with varying biophysical properties. They can also form both homo- and hetero-multimers. For example, Orai1 and Orai3 can assemble as heteromultimers to promote store-independent calcium channels that are regulated by arachidonic acid or leukotriene C4 (Mignen et al., 2008a; Mignen et al., 2009; Gonzalez-Cobos et al., 2013; Thompson and Shuttleworth, 2013). These are called ARC channels and LRC channels, respectively.
Orai protein subunits are composed of approximately 300 amino acids. Early evidence from co-immunoprecipitation and FRET experiments suggested that these proteins are oligomers in functional CRAC channels (Vig et al., 2006a; Gwack et al., 2007; Muik et al., 2008; Navarro-Borelly et al., 2008). Experiments using preassembled tandem Orai1 multimers and co-expression with dominant-negative Orai1 mutants showed that Orai1 may form homotetramers in CRAC channels similar to other ion channels (Mignen et al., 2008b). Using photo-bleaching of individual fluorophores in tandem Orai1-STIM1 multimers suggested a similar result of four Orai1 molecules with two STIM1 dimers, which was confirmed using FRET (Ji et al., 2008). These results were replicated in live mammalian cells as well as cellular lysates obtained from lymphocytes (Penna et al., 2008; Madl et al., 2010). However, the X-ray crystallographic structure of Drosophila Orai revealed a hexameric stoichiometry challenging these observations (Hou et al., 2012).
The X-ray and CryoEM structures of the Drosophila Orai channel helped resolve the oligomeric status of Orai and revealed the mechanisms for gating and ion permeation (Hou et al., 2012; Hou et al., 2018; Hou et al., 2020). This structure shows Orai1 as a four transmembrane protein with cytosolic C and N termini. The TM1 forms the channel pore with the Orai1 N terminus. TM2 and TM3 shield the pore from the membrane and TM4 and C terminus extend away from the channel pore. The channel pore has a ring of glutamate residues on TM1 (E178 is Drosophila, E106 in humans) that form a highly negative electrostatic region and serves as a selectivity filter. The side chains of these glutamate residues extend into the central pore, where the oxygen atoms of the carboxylic groups are in close proximity (∼6 Å). Below the selectivity filter is a region of hydrophobic amino acids followed by positively charged residues that extend into the cytosolic lumen. One interesting observation from the crystal structure is that the C terminal tails of Orai1 helices form anti-parallel helices with each other in the hexamer. These interactions are held together by hydrophobic interactions between leucine residues at 316 and 219 (273 and 276 in humans). Mutations at these residues, such as L273S/D and L276D, lower the coiled-coil probability of the C terminus as well as inhibit STIM1 binding and channel activity (Li et al., 2007; Navarro-Borelly et al., 2008; Li et al., 2011). However, an NMR structure between Orai1 272–292 fragment and 312–387 STIM1 fragment shows that the anti-parrel orientation does not change upon binding, but only leads to a small change in the angle or Orai1 helices (Stathopulos et al., 2013). The information obtained from these multitude of experimental approaches revealed key details regarding the gating of CRAC channels, especially how the allosteric modulators regulate Orai1 channel function. Four seminal studies helped us elucidate the structural features regulating gating and ion permeation in the Orai channel (Hou et al., 2012; Hou et al., 2018; Hou et al., 2020). Glutamate residues in the extracellular side of TM1 (E106, E178 in Drosophila), as explained earlier in the review, were hypothesized to form a selectivity filter by selectively binding calcium over other divalent cations. Mutation of a valine at position 102 (V174 in Drosophila) in the TM1 domain to alanine or serine made the channels constitutively active (McNally et al., 2012). The Drosophila Orai structure also shows the hydrophobic valine residues making extensive connections protruding into the ion permeation pathway that presents a de-solvation barrier for ions in closed configuration of the channel (Hou et al., 2012; Dong et al., 2013). Using terbium luminescence and disulfide crosslinking, others have also shown that STIM1 binding to Orai1 leads to a conformational change in the extracellular side near E106 and V102, and that this short segment forms a STIM1-dependent barrier (Gudlur et al., 2014). Based on these observations, V102 was proposed as a hydrophobic gate, which was eventually refuted and F171 is now considered to be the hydrophobic gate (discussed in more detail below). Surprisingly, mutation of glycine and arginine residues in the intracellular side of TM1 region of Orai1, at 98 and 91 respectively, resulted in constitutive activation of Orai1 channels. This led to a speculation whether there is another gate in the in the cytosolic side of the channel (Zhang et al., 2011). Based on these observations, R91 was thought to be the physical gate at the intracellular side that leads to dilation of the helices upon STIM1 binding. G98 was suggested to serve as a hinge, upon which the N terminus can rotate to allow for calcium entry. In addition, a phenyl alanine at 99 is on the opposite side of the pore helix to G98. STIM1 binding has been proposed to evoke a conformational change that exposes G98 while concealing F99 away from the channel pore (Yamashita et al., 2017). The Drosophila Orai crystal structure revealed basic residues in the immediate inner cytosolic end of channel pore which led to another hypothesis where these residues stabilize the closed channel through either binding of anions or electrostatic repulsion of cations near this pore (Hou et al., 2012). With the knowledge obtained from structural and functional studies using Orai1 and STIM1 mutants, several hypotheses have been put forward to explain the mechanism of activation of Orai1 by STIM1. STIM1 has been proposed to activate Orai1 in stepwise manner, first by initial binding to the C terminus for docking and a subsequent weaker binding to the N terminus to initiate conformational changes in the TM pore leading to pore opening (Zheng et al., 2013).
A stretch of conserved acidic amino acids of Orai1 have been shown to confer calcium selectivity. These include residues at E106, E190, D110, D112, and D114 all of which are either in the TM1 or TM1-2 loop. Mutation of aspartate residues to alanine resulted in increased cesium permeability in these channels (Yamashita et al., 2007; Zhou et al., 2010b). In addition, mutation of E106 residue in the channel pore resulted in the same increase in cesium permeability. These mutants also increase the pore diameter as evidenced by increased permeability of methylammonium derivatives (Yamashita et al., 2007). Finally, E106D mutant also increases the fast calcium-dependent inactivation time latency compared to wild-type Orai1 (Yamashita et al., 2007).
The structure of the open conformation of Orai1 was recently resolved at 3.3 Å using cryo-electron microscopy (Hou et al., 2020). As mentioned earlier, the hydrophobic amino acids F99 and V102 (F171 and V174 in human) within the channel pore were thought to function as channel gate (Hou et al., 2012). This structure shows the channel pore lined by acidic residues facing the extracellular entrance by D184, D182, Q180 and E178 followed by hydrophobic residues V174, F171, and L167 lining the middle of the pore, and positively charged K163 and K159 lining the pore on the intracellular side. A comparison of the open and closed structures revealed that F171 is the primary gate, with the side chain rotating into the ion conduction pathway in the closed state (Hou et al., 2020). Amino acids Q180, D182, and D184 on the extracellular surface create a negative electrostatic surface to attract cations near the mouth of the pore with E178 acting as a selectivity filter at the entrance to the ion conduction pathway.
SARAF
SARAF (SOCE-associated regulatory factor) is an ER transmembrane protein known to be bind to STIM1, keeping it in an inactive state. It primarily localizes to the ER membrane and possibly to the plasma membrane (Palty et al., 2012; Albarran et al., 2017). SARAF was discovered in a functional expression screen to identify proteins that affect mitochondrial calcium homeostasis (Palty et al., 2012). HEK293 cells transfected with SARAF cDNA showed lower baseline cytosolic, ER, and mitochondrial calcium levels. In addition, siRNA-based knockdown of SARAF resulted in an increase of basal cytosolic and ER calcium levels. This suggested a role for SARAF in cellular calcium homeostasis. Further experiments conducted using SARAF in conjunction with Orai1 and STIM1 led to deciphering its role in calcium entry. Electrophysiology recordings of Jurkat T cells and HEK293 cells showed SARAF regulates SOCE, specifically the slow calcium-dependent inactivation of CRAC channels (Palty et al., 2012). Subsequent experiments conducted in SH-SY5Y and NG115-401L cell lines also showed that SARAF is expressed in the plasma membrane where it interacts with and negatively regulates ARC channels (Albarran et al., 2016c). In addition, SARAF can also inhibit calcium entry mediated by TRPC1 channels (Albarran et al., 2016b).
How SARAF affects STIM1 structure and function is still not completely understood. Several reports indicate that SARAF interacts with STIM1 in resting conditions to keep STIM1 in its inactive state (Jha et al., 2013; Albarran et al., 2016a). Co-immunoprecipitation studies have shown that SARAF binds to STIM1, and this binding is mediated by the C-terminus of SARAF. The TM and ER-luminal domains are not required for this interaction (Palty et al., 2012). Interestingly, constitutively active STIM1 which has four glutamates mutated to alanine in its SOAR domain does not bind to SARAF and has deficits in slow calcium-dependent inactivation (Jha et al., 2013). The physical interaction between STIM1 and SARAF has been confirmed by multiple reports (Jha et al., 2013; Albarran et al., 2016a; Albarran et al., 2017; Lopez et al., 2019). In addition, SARAF has been shown to bind Orai1 and EFHB (EF hand containing family member B) (Jha et al., 2013; Maleth et al., 2014; Albarran et al., 2016a; Albarran et al., 2018; Lopez et al., 2019).
The nature of SARAF binding to STIM1 and Orai1 is interesting, especially when put into the context of SOCE. SARAF binds to STIM1 in resting conditions to keep it from spontaneously activating. Upon store depletion, co-immunoprecipitation experiments have shown that SARAF immediately dissociates from STIM1 within the first minute, but then they re-associate rapidly (Maleth et al., 2014; Albarran et al., 2016a; Albarran et al., 2018). It is possible that SARAF binds to Orai1 after store depletion, not STIM1. The exact sequence of events by which SARAF binds/releases STIM1 and Orai1 during SOCE is difficult to determine using existing techniques. Experiments conducted using fragments of these proteins show that SARAF interacts with STIM1 at the CC1 and the C-terminal inhibitory domain (CTID) of STIM1 (Jha et al., 2013). TIRF microscopy using STIM1-mCherry and SARAF-GFP show that these two proteins co-localize at the ER:PM junctions upon store depletion using thapsigargin. Co-immunoprecipitation of the SARAF N- and C-termini with STIM1 shows that SARAF binds to the C-terminus of STIM1 (Palty et al., 2012).
Membrane phospholipids play a crucial role in the interaction between SARAF and STIM1/Orai1. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) is a membrane phospholipid that mediates many functions in cellular signaling. It has been shown that the CRAC complex translocates between PI(4,5)P2 poor and rich microdomains during SOCE, and this regulates SOCE (Calloway et al., 2011). STIM1 interactions with Orai1 and PI(4,5)P2 are both dependent on SARAF (Maleth et al., 2014). In addition, localization of Oria1/STIM1 in PI(4,5)P2 microdomains are maintained by stabilizing proteins such as extended synaptogamin 1 (E-Syt1) and septin4 (Maleth et al., 2014). The dynamic regulation of this complex in membrane microdomains during store depletion has yet to be determined with suitable kinetic resolution. More recently, the SOAR domain of STIM1 has been shown to bind interact with plasma membrane phospholipids. Interactions between lysine residues 382, 384, 385, and 386 in the SOAR domain with plasma membrane phospholipids PI(3,4)P2, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P2 are crucial for the stability of ER:PM junctions as well as SOCE (Jha et al., 2013). The Orai1/STIM1 complex localized to the PI(4,5)P2 rich subdomains binds to SARAF and this localization and binding is crucial for SARAF to regulate slow calcium dependent inactivation (Maleth et al., 2014). These complex binding interactions between the SOAR and CTID domains of STIM1 and membrane phospholipids are crucial for the activity of SARAF.
How SARAF regulates slow calcium-dependent inactivation is still unknown. Multiple hypotheses have been proposed to explain this phenomenon. One of them is that an additional calcium-binding protein mediates SARAF activation in slow calcium-dependent inactivation (Zhang et al., 2020). Calcium binding proteins have been known to modulate SOCE, and some of them have been discussed earlier in the review such as EFHB and calmodulin (Mullins et al., 2009). Other hypotheses include modulation by the calcium sensing abilities of STIM1 and Orai1.
Selectivity and permeation of CRAC channels
One of the most distinguishing properties of CRAC channels is their very high selectivity toward calcium over other cations. CRAC channels show 1,000 times higher selectivity over sodium. Mutagenesis of the residues lining the channel pore have provided insights into the mechanisms mediating the remarkable selectivity of Orai1 channels toward calcium. Alanine substitutions of E106 in TM1 region, D110, D112, and D114 residues in the TM1-TM2 loop, and E190 in the TM2-TM3 loop all result in a loss of calcium selectivity and increased cesium permeating. In addition, these mutations also diminish calcium-dependent inactivation of these channels. Cesium permeability reveals that the loss of selectivity is likely due to an increase in pore diameter (Yamashita et al., 2007). The E190Q mutation in the TM2-TM3 loop also resulted in loss of calcium selectivity (Vig et al., 2006a; Prakriya et al., 2006). Also, alanine substitution of the D180 residue changed these channels from calcium selective and inward rectifying channels to sodium/cesium selective and outward rectifying channels (Yeromin et al., 2006). These observations highlight the selectivity filter in the Orai1 channels is formed by the conserved acidic residues lining the outer pore of the channel.
Cysteine scanning experiments showed that the channel pore diameter is largely responsible for the low conductance and high calcium selectivity. Lack of cysteine reactivity suggests that the E190 residue does not form the channel pore. Molecular dynamics simulation suggested that mutating this residue to glutamate (E190Q) decreases the pore hydration of the channel (Alavizargar et al., 2018). In addition, mutating this residue to a cysteine (E190C) decreased the calcium selectivity of Orai1 channel (Yeung et al., 2018). It is possible this residue helps in maintaining the pore size by regulating hydration of the ion conduction pathway. Finally, cysteine substitution of aspartate residues at 110, 112, 114 in the TM1-TM2 loop did not change calcium selectivity of these channels (McNally et al., 2009; Zhou et al., 2010a). These results show that TM1 lines the channel pore and a E106 residue (E178 dOrai1) from each monomer forms the selectivity filter in the extracellular side of Orai1 channel.
The combination of electrophysiological analysis and high-resolution structures have resolved many questions regarding Orai structure/function. Orai is unique with regards to subunit composition, ion permeation properties, and the kinetics of gating compared to other calcium channels. Indeed, they have very little sequence homology to other calcium channel families such as voltage-gated calcium channels and ligand-gated channels. Orai channels may have evolved from the very large and ubiquitous Cation Diffusion Facilitator (CDF) carrier family (Matias et al., 2010).
It has been shown that store depletion leads to the relatively slow activation of Orai1 channels (seconds to tens of seconds) (Prakriya and Lewis, 2006). A non-linear gating mechanism has been proposed to regulate CRAC channel gating, where these channels exhibit a ‘modal gating’ mechanism where the channels alternate between silent and high open probability (Yamashita and Prakriya, 2014). Modal gating mechanism was based on an observation that 2-APB, a widely used as ICRAC inhibitor, elicits strong SOCE at low concentrations and inhibition at high concentrations. This hypothesis proposed that STIM1 binding to the Orai1 channel increases the time spent by these channels in the high open probability state. And because the frequency of these transitions is lower, calcium entry is enhanced (Yamashita and Prakriya, 2014).
Taken together, how calcium depletion in the ER leads to activation of STIM1 shows a complicated picture of multiple events happening in a precise sequential order mediated by several domains of STIM1. First, the binding of calcium ions to the EF-hand keeps the EF-SAM in a compact state which helps interactions between CC1 and CAD regions of STIM1 in the cytosolic side. An inhibitory clamp formed by intramolecular interactions between CC1/2/3 regions of STIM1 helps keep it in inactive state (Xu et al., 2006; Yang et al., 2012; Yu et al., 2013). Store depletion and calcium dissociation from EF-hands leads to a conformational change that releases this clamp and formation of a coiled-coil dimer between CC1 domains. Experiments done using isolated cytosolic STIM1 fragments show that in resting state, these cytosolic fragments are tightly bound, which are extended upon binding to Orai1 (Muik et al., 2011). Mutations in the CC1 regions that cause spontaneous activation of STIM1 also show a similar phenotype (Fahrner et al., 2014). Finally, several leucine residues (L251, L261, L419, and L416) in the CAD region play a crucial role in extension of STIM1 toward plasma membrane for STIM1 binding to Orai1 (Zhou et al., 2013; Fahrner et al., 2014; Ma et al., 2015). This intra-and-intermolecular choreography of STIM1 proteins upon store depletion controls SOCE. An overview of important binding events between STIM1 and plasma membrane lipids is shown in Figure 2. These amino acid residues of STIM1 that interact with plasma membrane are also highlighted using an AlphaFold model of the compact and extended conformations of STIM1 (Jumper et al., 2021).
FIGURE 2
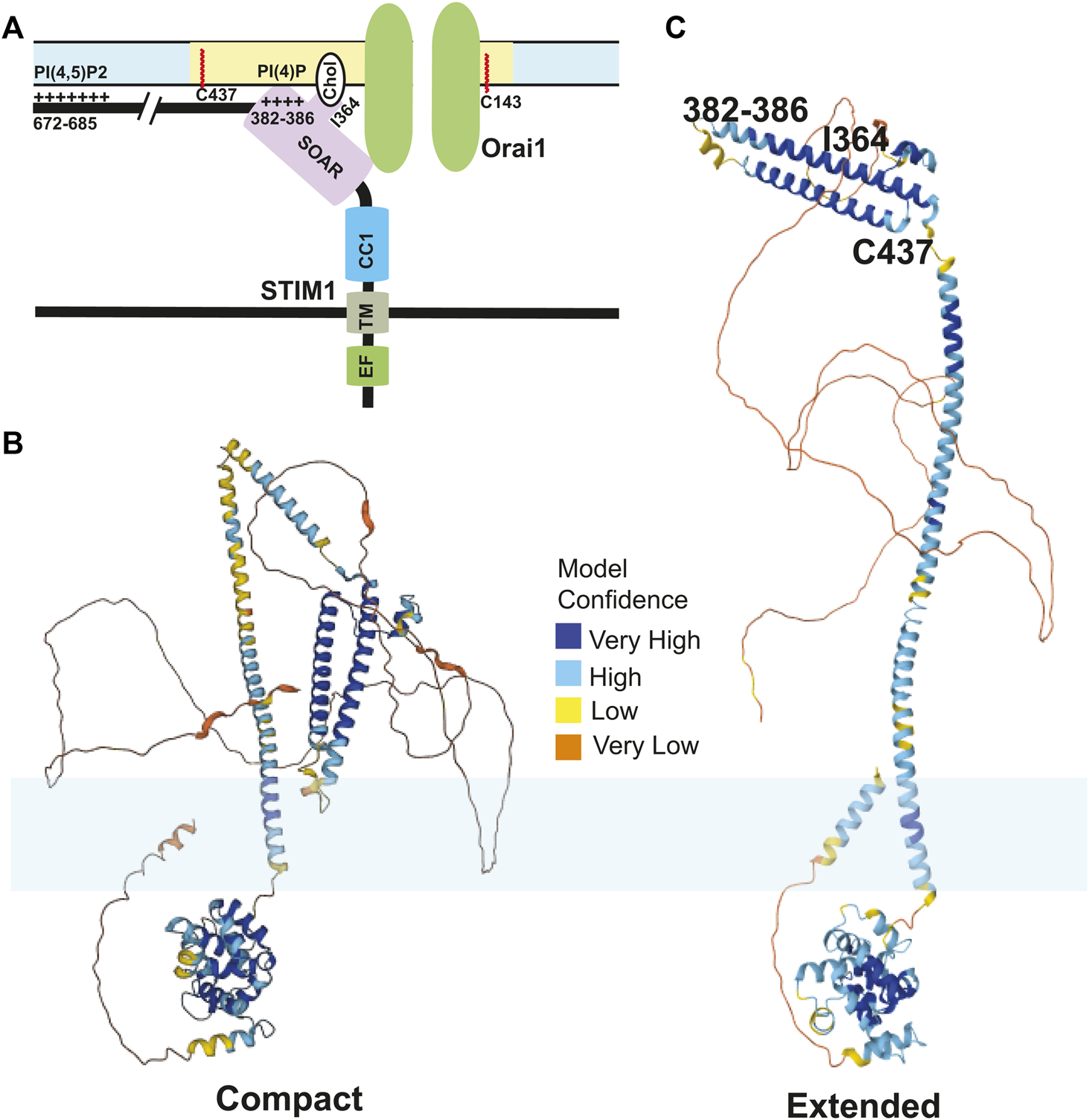
STIM1-lipid binding at the plasma membrane: (A) The CRAC channels are stabilized at the ER:PM junctions by a multitude of bindings between different domains of Orai1 and STIM1 with plasma membrane lipids upon store depletion. Orai1 undergoes S-acylation at its C143 residue which shuttles the channels to lipid rafts. The SOAR domain of STIM1 binds to the N-terminus region of Orai1 and leads to activation of Orai1 channels. The cholesterol binding domain (CBD) in the SOAR domain also binds to the cholesterol rich phospholipids in the plasma membrane, which is mediated by I364 residue. The polybasic domain of STIM1 binds to the PI(4,5)P2 phospholipids in the plasma membrane which is mediated by the positively charged amino acids in the C-terminal tail of STIM1. Finally, STIM1 undergoes S-acylation at its C437 residue which is crucial for SOCE. One subunit of STIM1 dimer is shown here for simplicity. (B,C) The AlphaFold structure predictions of the compact (presumably inactive) and extended (presumably active) conformations of STIM1 are shown. The residues on STIM1 that interact with the plasma membrane as well as C437 that undergoes S-acylation are highlighted in (C). In (B,C), the lipid bilayer is represented in light blue. The compact model is the AlphaFold prediction of Uniprot #V5J3L2. The extended model is the AlphaFold prediction of Uniprot #Q13586).
Orai1/STIM1 clustering/puncta
Orai1 in the plasma membrane and STIM1 in the ER membrane are diffusely distributed in cells with replete ER calcium stores. Upon store depletion, Orai1-STIM1 complexes are recruited to ER:PM junctions where they form CRAC channel clusters to promote efficient calcium entry upon store depletion (Liou et al., 2007). These clusters were denoted as “puncta” upon observation under fluorescence microscopy of tagged-STIM1 (Liou et al., 2005; Zhang et al., 2005; Baba et al., 2006; Luik et al., 2006; Wu et al., 2006; Xu et al., 2006). These ER:PM junctions are regions within the cell where PM and ER are held in close apposition (∼10–20 nm) (Wu et al., 2006; Orci et al., 2009). Upon store depletion, STIM1 accumulates near the thin cortical tubules of the ER (Orci et al., 2009). An interesting observation in CRAC puncta formation is the longevity of these puncta. Depending on the downstream effect of calcium influx, the puncta can be active for a few minutes or up to an hour. How these puncta can stay active to promote calcium entry for this duration is still not completely understood. Even more compelling is the ability of STIM1 to translocate to the sites of already formed puncta repeatedly upon subsequent store depletion (Smyth et al., 2008). A multitude of factors regulate this behavior to increase the number of cortical ER tubules near the plasma membrane. One mechanism is a secretion-like coupling model which includes redistribution of F-actin into cortical layers (Patterson et al., 1999). STIM1 has been known to interact with microtube attachment protein EB1 and maintain ER tubule length (Grigoriev et al., 2008). STIM1 also binds to plasma membrane using its polybasic domain, which might strengthen the STIM1 localization in the puncta (Liou et al., 2007). The SOAR region of STIM1 also has a cholesterol binding domain which has been shown to bind to cholesterol rich regions in the plasma membrane (Pacheco et al., 2016). Several additional proteins like synaptogamins and septins maintain the integrity of these ER:PM junctions (Chang et al., 2013; Sharma et al., 2013). Cytosolic calcium elevation leads to increased translocation of E-Syt1 to ER:PM junctions, which subsequently recruits the phosphatidylinositol transfer protein (PITP) Nir2 to these junctions to strengthen ER:PM junction stability (Chang et al., 2013). E-Syt2 and E-Syt3 also regulate the ER:PM junctions, in a calcium-independent manner (Giordano et al., 2013). Interestingly, siRNA-mediated knockdown of E-Syt proteins decreases the number of ER:PM contact sites but does not affect SOCE (Giordano et al., 2013). This explains the possibility that E-Syt proteins maintain the stability of ER:PM junctions with no specific effect on Orai1 or STIM1. Another protein that has shown a role in regulating SOCE as well as long-term maintenance of ER:PM junctions is an ER transmembrane protein TMEM110, also known as junctate (Quintana et al., 2015). Junctate is a calcium binding protein in the ER membrane that forms supramolecular complexes with IP3 receptors and TRPC3 calcium channels. Junctate has an EF-hand domain in its ER luminal side, which is required for CRAC channel activation independently of store depletion (Treves et al., 2004; Srikanth et al., 2012). In addition to these proteins mentioned above, many other proteins play a crucial role in maintenance of stable ER:PM junctions that help CRAC channel formation and function (Ivanova and Atakpa-Adaji, 2023).
Models of CRAC channel assembly
ATP-dependent puncta formation
Based on the observation in live cells that STIM1 translocates to the plasma membrane from the ER membrane and an earlier hypothesis that STIM1 is transported to PM via a secretory pathway (Hauser and Tsien, 2007), puncta formation was hypothesized to be ATP-dependent. Mitochondria are known to accumulate near the ER:PM junctions and regulate calcium homeostasis (Park et al., 2001; Quintana et al., 2011). In addition, depletion of intracellular ATP leads to decreased calcium entry in rat lymphocytes (Marriott and Mason, 1995). ATP depletion can also lead to translocation of STIM1 to puncta. However, this required depletion of PI(4,5)P2 in addition to ATP depletion (Chvanov et al., 2008).
Microtube-associated STIM1 translocation
As discussed earlier in the review, STIM1 binds to the EB1 protein that is known to modulate microtubule growth. In B cells, treatment with the anti-mitotic agent nocodazole, which inhibits polymerization of microtubules, did not show the puncta formation observed in untreated cells (Baba et al., 2006). Pull down assay with EB1, EB2, and EB3 proteins showed STIM1 binds to EB1. This was also confirmed using immunocytochemistry and co-immunoprecipitation experiments (Grigoriev et al., 2008). In addition, treatment of cells with ML-9, a myosin light chain kinase inhibitor, led to reversal of CRAC puncta as well inhibition of SOCE (Smyth et al., 2008). These observations led to a hypothesis that STIM1 traffics to the ER:PM junctions by microtubule-assisted transport mediated by its binding to EB1. However, this model shows that STIM1 and Orai1 proteins are confined at the junctions after store depletion but does not offer any explanation to why this happens.
Phosphatidylinositol-mediated membrane sorting
Early experiments conducted on STIM proteins led to discovery of a C-terminal polybasic domain that interact with PM phospholipids, such as PI(4,5)P2 (Ercan et al., 2009; Calloway et al., 2011). This polybasic domain was also found to mediate the inward rectification of SOCE currents (Yuan et al., 2009). HeLa cells treated with wortmannin and LY294002, inhibitors of phosphatidyl inositol 3-kinase (PI3K) and PI4K respectively, led to inhibition of STIM1 puncta formation as well as SOCE (Walsh et al., 2009). In addition, the binding between STIM1 and Orai1 was differentially modulated by the levels of PI(4,5)P2 (Calloway et al., 2011). Decreased PI(4,5)P2 concentrations resulted in reduced thapsigargin-mediated Orai1-STIM1 binding in the PM liquid-ordered phase/rafts, and this affinity was reversed in membrane disordered regions (Walsh et al., 2009). This led a hypothesis that membrane phospholipids play a role in CRAC channel formation and SOCE. However, depletion of phosphoinositides in cells overexpressing Orai1 did not affect either STIM1 puncta formation or SOCE (Walsh et al., 2009). In addition, the experiments showing a role for phosphoinositides was shown using inhibitors which do not discriminate between direct effects on Orai1/STIM versus general effects due to severely perturbing PM phospholipid composition.
IP3-mediated calcium depletion in ER lumen
Before the discovery of Orai1 and STIM1 as proteins forming CRAC channels, experiments performed on TRP3 transfected HEK293 cells using plasma membrane patches by treatment with IP3. Based on this observation, they proposed a hypothesis where the released IP3 binds to the IP3Rs and activates them, which in turn regulate SOCE and CRAC channels. However, they do not show physical binding between IP3Rs and TRP3 proteins (Kiselyov et al., 1998). This physical binding between the IP3Rs and TRP3 was shown later using coimmunoprecipitation of tagged constructs of IP3R and TRP3 (Boulay et al., 1999). They also show the role of this binding in the regulation of SOCE. In subsequent studies, it was also shown that TRP3 forms store-operated cation channels dependent and independently with IP3Rs using DT40 WT and IP3R knockout cells with rescue expression of TRP3 (Vazquez et al., 2001). However, the mechanism behind this modulation of SOCE by IP3Rs remained unresolved. Recently, it was shown that STIM1 proteins interact with IP3Rs in the ER membrane (Beliveau et al., 2014). Independently, others have shown that overexpression of IP3Rs leads to increased ER calcium depletion, larger CRAC puncta, and higher SOCE (Sampieri et al., 2018). In addition, using confocal microscopy, they also show IP3Rs are recruited to CRAC puncta upon activation of IP3Rs using bradykinin. Based on these observations, they proposed a mechanism for SOCE where recruitment of IP3Rs to CRAC puncta leads to the generation of localized calcium-free microenvironment in the luminal side of ER, which helps in activation of STIM1 (Sampieri et al., 2018).
Diffusion-trap model
The diffusion-trap model postulates that STIM1 undergoes a conformational change that exposes its C-terminal domains toward plasma membrane, where it binds to membrane phospholipids and stochastically binds to Orai1 channels laterally diffusing in the PM (Hoover and Lewis, 2011; Wu et al., 2014). In agreement with this hypothesis, super-resolution microscopy experiments conducted using tagged Orai1 and STIM1 constructs showed that these proteins diffuse randomly in resting conditions. Upon store depletion, these proteins slow down at distinct ER:PM junctions allowing them to accumulate and form puncta (Wu et al., 2014). Additionally, single particle tracking analysis shows single STIM1 and Orai1 particles diffusing freely before getting trapped at the junctions. Deletion of the polybasic domain in the C-terminus of STIM1 showed altered puncta formation after store depletion, suggesting a direct role for this domain. These experiments also demonstrated that STIM1 and Orai1 particles have a long half-life in puncta once trapped (Wu et al., 2014; Qin et al., 2020).
The diffusion-trap model fails to explain some aspects of puncta formation and SOCE. The polybasic domain was hypothesized to act as a trap to attract Orai1 to STIM1. However, a mutant STIM1 devoid of this polybasic domain is capable of binding with Orai1. Orai1 binding by itself can also trap STIM1 within ER:PM junctions which raises the possibility that Orai1 may be trapping STIM1 (Walsh et al., 2009). Interestingly, we have found that STIM1 can form puncta in the absence of Orai1, but Orai1 cannot form puncta in the absence of STIM1 (West et al., 2022b; Kodakandla et al., 2022). The strong binding between Orai1 and STIM1 in the puncta could also mean there is an equivalent amount of protein particles enter and leave the puncta, thereby maintaining a dynamic equilibrium at these ER:PM junctions (Wu et al., 2014). Finally, single particle tracking and polydispersity analyses conducted on Orai1 and STIM1 proteins show that the mobility of these proteins decreases after store depletion, but these proteins are also confined in compartmentalized membrane regions before and after store depletion (Qin et al., 2020). Based on these observations, the binding of Orai1 and STIM1 upon store depletion appears to be more complicated than a simple diffusion-trap model.
S-acylation as a regulator of Orai1/STIM1 assembly
S-acylation is a reversible addition of lipid moieties to cysteine residues of target proteins that affects protein stability, function, conformation, and trafficking between compartments within a cell (Chamberlain and Shipston, 2015; Chen et al., 2021). S-acylation is a post translational modification that is mediated by specific group of enzymes known as protein acyltransferases (PATs). PATs are also known as DHHC enzymes owing to the conserved aspartate-histidine-histidine-cysteine motif in their active site that carries out this reaction (Chamberlain and Shipston, 2015). The different classes of DHHC enzymes distinguish among themselves by their selectivity toward substrates, lipid preferences, and mechanisms that activate and inactivate them (Chen et al., 2021). Deficiencies in DHHC enzyme functions leads to a range of diseases ranging from cancers such as adenocarcinomas, lung cancer, bladder cancer, and breast cancer, to diseases that affect neurological functions such as epilepsy and schizophrenia, glioblastoma, and X-linked intellectual disability (Ladygina et al., 2011; Resh, 2016; Essandoh et al., 2020; Fraser et al., 2020; Chen et al., 2021). The subsequent process of removal of lipid moieties from protein substrates, known as deacylation, is mediated by another set of enzymes known as acyl protein thioesterases (APTs) (Duncan and Gilman, 1998; Lin and Conibear, 2015). These enzymes act in concert to dynamically regulate protein function in a stimulus-dependent manner. How most DHHC and APT enzymes are activated and inactivated is still unclear.
DHHC enzymes catalyze the addition of lipid moieties to proteins through a two-step mechanism, where the cysteine residue in the active site of the enzyme receives the lipid group and then transfers it to the cysteine residues of target proteins (Jennings and Linder, 2012). This first step, known as auto-acylation, is followed by the transfer of acyl group to target proteins. The DHHC enzymatic reaction is sometimes referred to as a “ping-pong” reaction. However, this may be a misnomer. Ping-pong mechanisms (also known as double-displacement reactions) always involve two substrates and two products, with the binding of the second substrate dependent upon the successful completion of the first reaction (Kullmann, 1984; Ulusu, 2015). The reaction mechanism for DHHC enzymes is highlighted in Figure 3. For the DHHC reaction to be considered ping-pong, we would have to accept that CoA is product #1, and that substrate #2 binding (the protein be S-acylated) depends upon autoacylation of the DHHC enzyme. We would argue that the autoacylated DHHC enzyme is an intermediate, not an enzyme newly capable of binding substrate #2. Regardless, the ultimate result is the S-acylation of the target protein. Motivated by the finding that the protein kinase Lck is dynamically S-acylated in T cells during Fas signaling, we investigated the role(s) of this modification in SOCE pathways (Akimzhanov and Boehning, 2015).
FIGURE 3
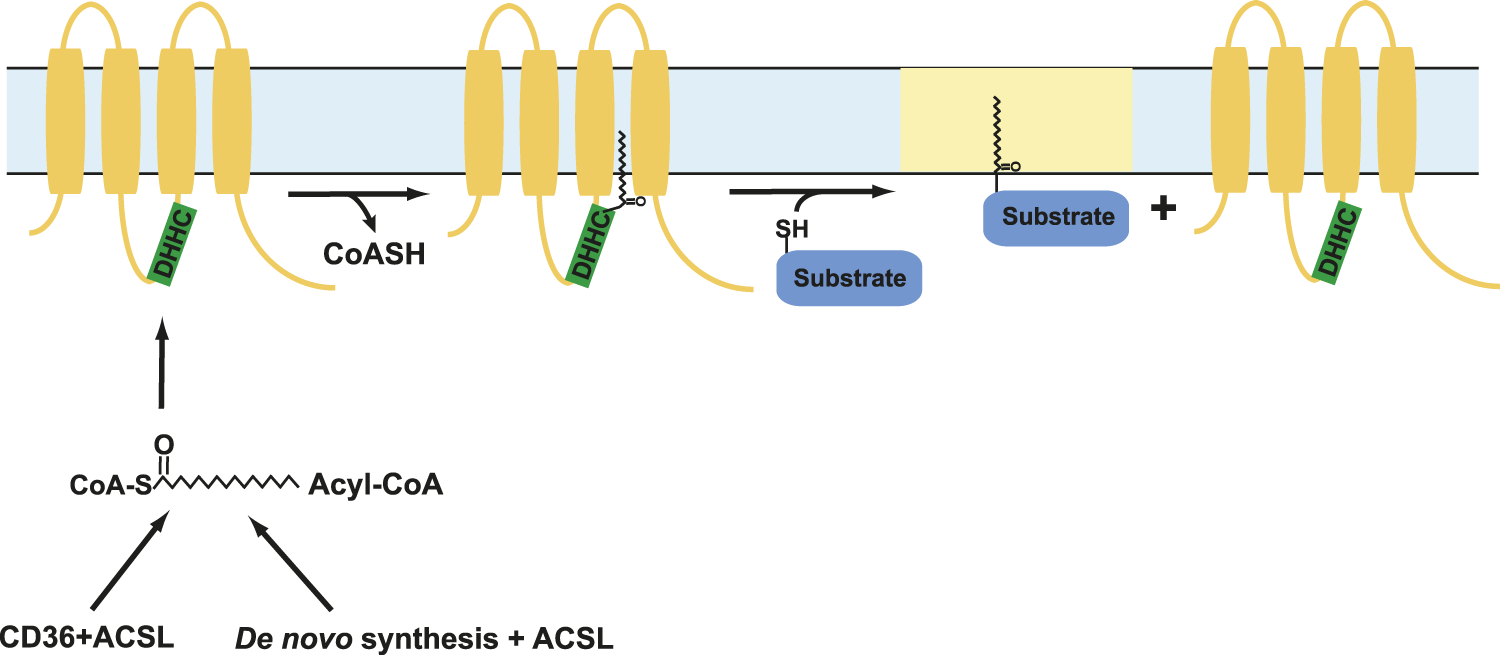
Mechanism of DHHC enzyme catalysis. DHHC enzymes S-acylate their substrates by a two-step mechanism which involves autoacylation followed by transfer of the acyl group to target protein cysteine residues (substrates). In this cartoon, lipid rafts are indicated in yellow. ACSL = Acyl-CoA synthetase long-chain family members.
Orai1 and STIM1 accumulate in lipid raft domains upon depletion of intracellular calcium stores. Depletion of PM cholesterol with methyl-beta-cyclodextrin impairs SOCE, implying a role for lipid rafts in SOCE (Jardin et al., 2008b; Dionisio et al., 2011b). As mentioned above, our group found that signaling through the Fas death receptor in T cells requires dynamic S-acylation of the kinase Lck, leading to translocation to rafts where it activates PLC-y1-mediated IP3 production (Wozniak et al., 2006). Subsequently, we found that many TCR components such as Lck, Fyn, ZAP70, and PLCy1 undergo dynamic S-acylation upon T cell activation (Akimzhanov et al., 2010; Akimzhanov and Boehning, 2015; West et al., 2022a). This S-acylation was required the calcium/calmodulin-dependent activation of the protein acyltransferase DHHC21. We next investigated T cell function in the mutant mouse depilated, which carry a functionally deficient DHHC21 with an in-frame deletion of phenylalanine 233 (F233) eliminated calmodulin binding. Depilated mice have severe deficits in T cell differentiation, including differentiation into Th1, Th2, and Th17 lineages (Bieerkehazhi et al., 2022). TCR signaling in depilated mice is severely disrupted due to reduced activation of ZAP70, Lck, PLCγ1, JNK, ERK1/2, and p38 in response to TCR stimulation (Fan et al., 2020; Bieerkehazhi et al., 2022). As Orai1 and STIM1 are essential for TCR signaling, we hypothesized that Orai1 and STIM1 undergo S-acylation to regulate CRAC channel formation and function in T cells.
We found that treating Jurkat T cells with anti-CD3 or HEK293 cells with thapsigargin leads to stimulus-dependent S-acylation of Orai1 and STIM1. The kinetics of S-acylation Orai1 and STIM1 are very rapid consistent with the assembly of puncta and channel activation. The cysteine-mutant versions of Orai1 and STIM1 that are incapable of undergoing S-acylation have significantly reduced puncta formation and SOCE, indicating a direct role for S-acylation in CRAC channel assembly (West et al., 2022b; Kodakandla et al., 2022).
One interesting observation between the cysteine mutants of Orai1 and STIM1 is that the Orai1 cysteine mutant (C143S) was an almost complete loss of function phenotype, whereas mutant STIM1 (C437S) retained some residual activity. The residue where STIM1 undergoes S-acylation is at the proximal end of its SOAR domain. As explained earlier in the review, the SOAR domain of STIM1 is crucial in the tethering of STIM1 to plasma membrane. The C-terminus of STIM1 also has the polybasic membrane binding domain. We hypothesize the S-acylation of STIM1 stabilizes the C-terminus in the plasma membrane. Due to redundant membrane-binding domains in the C-terminus, we postulate that the loss of S-acylation would only partially reduce the affinity of the C-terminus for the membrane, thus possibly explaining our partial loss-of-function phenotype (Figure 2) (Pacheco et al., 2016). Our model is that a plasma membrane resident DHHC enzyme, probably DHHC21, S-acylates STIM1 and anchors its C-terminus in the plasma membrane, thereby stabilizing the CRAC complex at these ER:PM junctions to facilitate SOCE (West et al., 2022b; Kodakandla et al., 2022). An overview of the models suggested in this section is presented in Figure 4.
FIGURE 4
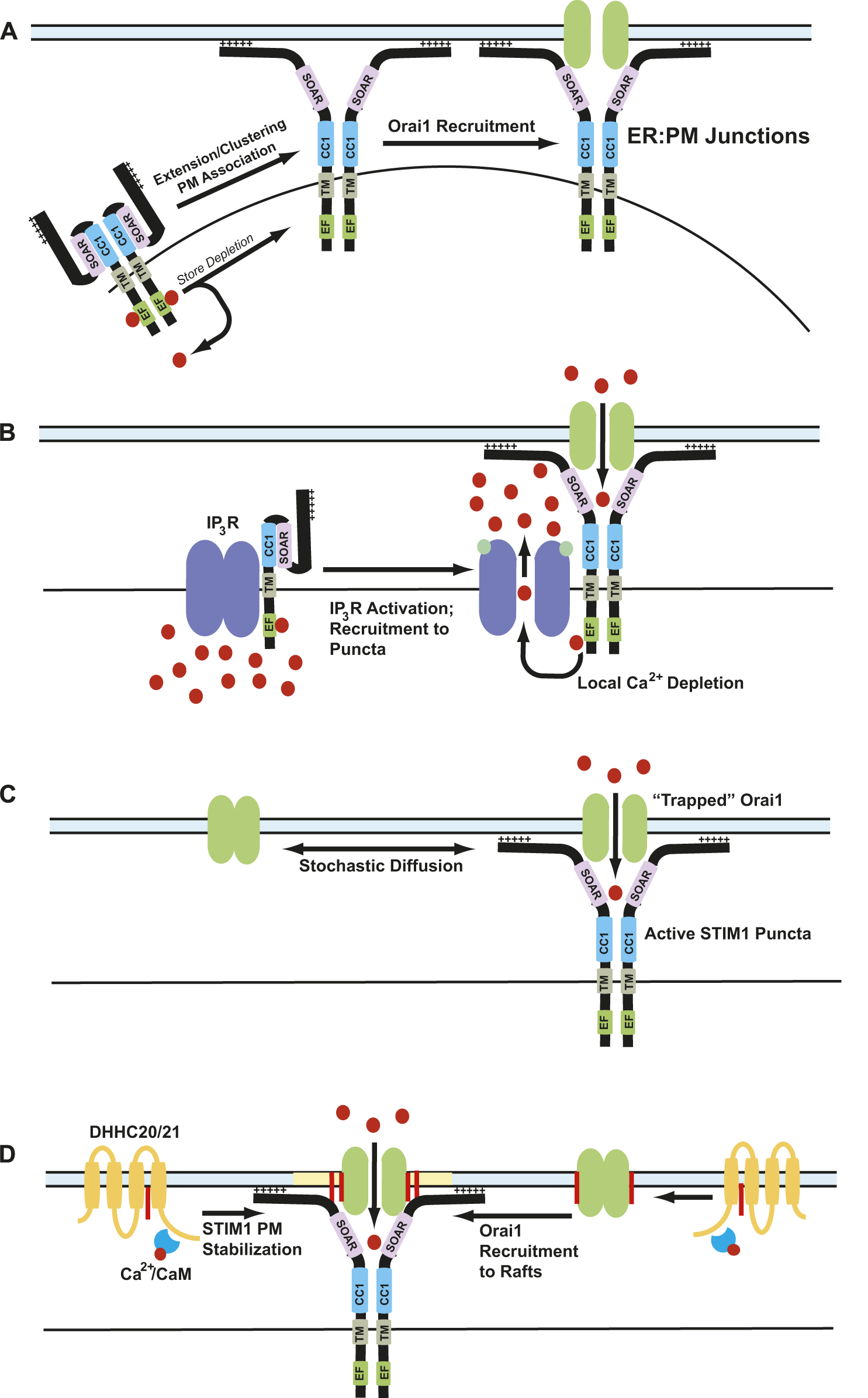
Models proposed to explain CRAC channel assembly in SOCE. (A) Overview of CRAC puncta formation after ER calcium store-depletion. (B) IP3R recruitment to puncta with STIM1 and IP3-mediated local calcium depletion (C) The Orai1 diffusion trap model, and (D) S-acylation of Orai1 and STIM1 and recruitment to lipid rafts. The three models are not necessarily mutually exclusive. See the text for details.
At the same time as our research into S-acylation of Orai1 and STIM1, the Demaurex group also showed that Orai1 undergoes S-acylation (Carreras-Sureda et al., 2021). It was found that S-acylation of Orai1 is critical for its recruitment to the immunological synapse as well as TCR-mediated calcium entry in Jurkat T cells. Using TIRF and confocal imaging techniques, they showed that S-acylation of Orai1 is critical for channel clustering as well as their trafficking to the lipid rafts. Finally, using overexpression analysis, they show that DHHC20 is the enzyme that mediates S-acylation of Orai1 in the plasma membrane, at least under resting conditions. We hypothesize that DHHC20 may mediate Orai1 S-acylation under resting conditions, whereas store depletion leads to calcium/calmodulin-dependent activation of DHHC21 leading to increased Orai1/STIM1 S-acylation and active partitioning to ER:PM junctions. This would be consistent with our data in depilated DHHC21 mutant mice showing altered T cell calcium signaling in response TCR activation (Fan et al., 2020; Tewari et al., 2021; Bieerkehazhi et al., 2022).
Unanswered questions and further directions
Our hypothesis that S-acylation plays a role in store-operated calcium entry was derived from the inability of current models to explain the specific characteristics that make this process unique. As explained earlier in the review, Orai1 and STIM1 proteins show distinct features upon store-depletion, such as decreased mobility in the membrane, targeting to membrane subdomains, and dynamic nature of CRAC puncta, among other observations. We based our hypothesis that S-acylation, being a process that is controlled by a set of enzymes, gives a dynamic control to Orai1 and STIM1 function.
Of the many topics not answered in our studies, we want to highlight a few that are more intriguing and necessitate scrutiny. We have discussed the role of DHHC enzymes in S-acylating CRAC channel components, including DHHC20 and DHHC21. But the enzymes that deacylated these proteins have not been studied yet. As explained earlier, S-acylation is reversible. Acyl protein thioesterases (APT) are responsible for removing the lipid moiety from S-acylated proteins. APT enzymes have been thought to be constitutively active. For example, radiolabeling studies using tritiated palmitate have shown than palmitate incorporation has a half-life of 20 min whereas depalmitoylation is 10–20 times faster (Magee et al., 1987; Rocks et al., 2005). Another difference between S-acylation and deacylation is the localization of proteins that undergo this process. Some reports suggest S-acylation is restricted to compartments where the specific DHHC enzyme resides, such the Golgi apparatus, while deacylation can happen throughout the cell (Rocks et al., 2010). However, the specific mechanisms how DHHC and APT enzymes coordinate these steps has not been explored. Interestingly, APT enzymes themselves are targets for S-acylation, indicating the DHHC enzymes regulate APT enzyme localization and function (Abrami et al., 2021). Nothing is known about the regulation of Orai1/STIM1 by APT enzymes other than the S-acylation of both proteins is transient after store depletion, suggesting a direct for APT enzymes in puncta disassembly (West et al., 2022b; Kodakandla et al., 2022).
How S-acylation drives the movement of these proteins to subdomains in the plasma membrane is also interesting. Addition of lipid moieties (long chain lipids) changes the hydrophobicity of the proteins. The result of this addition is an increased affinity toward phospholipids, cholesterol, or other moieties in membrane subdomains (Greaves and Chamberlain, 2007). For example, addition of saturated lipids can result in translocation to cholesterol and sphingolipid-enriched membrane subdomains such as lipid rafts (Pani and Singh, 2009; Resh, 2016). Indeed, previous work by our group has shown that S-acylation is critical for the assembly of the TCR complex in lipid rafts (Akimzhanov et al., 2010; Akimzhanov and Boehning, 2015; Fan et al., 2020; Tewari et al., 2021). This might also help us understand the differences observed between cysteine mutants of Orai1 and STIM1, which show different calcium entry patters upon store-depletion.
Another interesting point of emphasis which we failed to make is the specific lipid moiety that is added to target proteins, and how the lipid moiety affects the protein behavior. The lipid moiety could range from saturated palmitate (C16:0, 74%) to monounsaturated oleate (C18:1, 4%) or saturated stearate (C16:0, 22%) among others (Towler and Glaser, 1986). The specific type of lipid moiety attached to Orai1 and STIM1 will help answer some questions regarding the function of the S-acylated residue. For example, we hypothesized S-acylation of Orai1 helps it recruit to cholesterol-rich lipid rafts, whereas STIM1 S-acylation anchors the SOAR domain of STIM1 in the plasma membrane to facilitate its interaction with Orai1 in lipid rafts. Palmitic acid has long known to have higher affinity toward cholesterol compared to other phospholipids (Melkonian et al., 1999; Levental et al., 2010). So, we can test if Orai1 S-acylation leads to addition of a palmitate group using metabolic labeling. On the other hand, stearic acid is known to recruit to plasma membrane domains that have low levels of phosphatidylinositol-4,5-bisphosphate (PIP2) (Laquel et al., 2022). Some reports have suggested the CRAC channels translocate to these domains upon store-depletion for propagating SOCE currents (Maleth et al., 2014). Stearic acid conjugation to STIM1 may lead to differential association with plasma membrane domains depleted of PIP2. Delineating these hypotheses is crucial for understanding the role of S-acylation in SOCE.
The S-acylation of STIM1 may stabilize the C-terminus of the active conformation at the plasma membrane. In addition to S-acylation, two other critical binding events happen between the C-terminus of STIM1 and the plasma membrane. In particular, STIM1 binds to membrane lipids on the inner leaflet via the polybasic domain and the cholesterol-binding domain (Figure 2; Yuan et al., 2009; Pacheco et al., 2016). This functional redundancy of membrane-associated features in the C-terminus of STIM1 may explain the partial loss of function in the cysteine-mutant STIM1. Future work can test this hypothesis using different STIM1 mutants that lack the polybasic or CBD domains alone and in combination with the S-acylation mutant.
Finally, how the DHHC enzymes that S-acylate Orai1 and STIM1 are regulated is a question that remains unanswered. There is surprisingly little information about how these enzymes are regulated. Previously, we have shown that DHHC5, an enzyme the S-acylates many signaling proteins involved in beta adrenergic receptor function, is itself regulated by S-acylation of several cysteines in the C-terminal tail. S-acylation of DHHC5 in its C-terminal tail upon receptor stimulation increases its stability in the plasma membrane where it can S-acylate its target proteins (Chen et al., 2020). In addition, the DHHC enzymes regulate each other, where one DHHC enzyme can S-acylate another DHHC enzyme, which in turn affects its localization, function, or stability. For example, DHHC16 S-acylated DHHC6, which is an ER membrane protein that S-acylates many ER proteins such as calnexin, E3 ligase gp78, and IP3 receptor (Abrami et al., 2017). We previously showed that DHHC21 is a calcium-calmodulin regulated enzyme S-acylates many T cell proteins in vitro and in vivo. Future studies will determine if DHHC21 is also the key regulator of Orai1/STIM1 S-acylation. Ultimately, the regulation of CRAC channel formation and SOCE by S-acylation adds a regulatory step for channel assembly and disassembly during store depletion. This allows for the fine tuning of the spatiotemporal aspects of calcium signaling in cells expressing Orai1/STIM1.
Statements
Author contributions
GK: Conceptualization, Data curation, Investigation, Software, Visualization, Writing–original draft, Writing–review and editing. AA: Funding acquisition, Methodology, Writing–review and editing. DB: Conceptualization, Data curation, Funding acquisition, Methodology, Software, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Institute of General Medicine and Sciences (NIGMS) of the National Institutes of Health (NIH) under award numbers R01GM130840 (to DB and AA), R01GM115446 (to AA), and R01GM081685 (to DB) and startup funds provided by Cooper Medical School of Rowan University (DB).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Acyl-RAC, Acyl resin-assisted capture; BAPTA, l,2-bis(0-aminophenoxy)ethane-A;A^A′,A″-tetraacetic acid; BSA, bovine serum albumin; C-terminal, carboxy terminal; CaM, calmodulin; CRAC, calcium release-activated calcium; DHHC, aspartate-histidine-histidine-cysteine; DTT, dithiothreitol; EDTA, ethylene diamine-tetraacetic acid; EGTA, ethylene glycol-bis(P-aminoethyl ether)N,N,N′,N′-tetraacetic acid; ER, endoplasmic reticulum; HA, hydroxylamine; HEK293, human embryonic kidney 293 cell line; IP3, inositol 1,4,5 trisphosphate; IP3R, inositol 1,4,5 trisphosphate receptor; mRFP, monomeric red fluorescence protein; PAT, protein acyltransferase; PBS, phosphate buffered saline; PIP2, phosphatidylinositol 4,5 bisphosphate; PLC, phospholipase C; PM, plasma membrane; PMSF, phenyl methyl sulfonyl fluoride; SDS-PAGE, sodium dodecyl sulfide polyacrylamide gel electrophoresis; SERCA, sarco-endoplasmic reticulum calcium ATPase; SOCE, store-operated calcium entry; STIM1, stromal interaction molecule 1; TCR, T-cell receptor; TG, thapsigargin; TIRF, total internal reflection fluorescence.
References
1
AbramiL.AudagnottoM.HoS.MarcaidaM. J.MesquitaF. S.AnwarM. U.et al (2021). Palmitoylated acyl protein thioesterase APT2 deforms membranes to extract substrate acyl chains. Nat. Chem. Biol.17, 438–447. 10.1038/s41589-021-00753-2
2
AbramiL.DallavillaT.SandozP. A.DemirM.KunzB.SavoglidisG.et al (2017). Identification and dynamics of the human ZDHHC16-ZDHHC6 palmitoylation cascade. Elife6, e27826. 10.7554/eLife.27826
3
AkimzhanovA. M.BoehningD. (2015). Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc. Natl. Acad. Sci. U. S. A.112, 11876–11880. 10.1073/pnas.1509929112
4
AkimzhanovA. M.WangX.SunJ.BoehningD. (2010). T-cell receptor complex is essential for Fas signal transduction. Proc. Natl. Acad. Sci. U. S. A.107, 15105–15110. 10.1073/pnas.1005419107
5
AlavizargarA.BertiC.EjtehadiM. R.FuriniS. (2018). Molecular dynamics simulations of orai reveal how the third transmembrane segment contributes to hydration and Ca(2+) selectivity in calcium release-activated calcium channels. J. Phys. Chem. B122, 4407–4417. 10.1021/acs.jpcb.7b12453
6
AlbarranL.LopezJ. J.AmorN. B.Martin-CanoF. E.Berna-ErroA.SmaniT.et al (2016a). Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry. Sci. Rep.6, 24452. 10.1038/srep24452
7
AlbarranL.LopezJ. J.GomezL. J.SalidoG. M.RosadoJ. A. (2016b). SARAF modulates TRPC1, but not TRPC6, channel function in a STIM1-independent manner. Biochem. J.473, 3581–3595. 10.1042/BCJ20160348
8
AlbarranL.LopezJ. J.JardinI.Sanchez-ColladoJ.Berna-ErroA.SmaniT.et al (2018). EFHB is a novel cytosolic Ca2+ sensor that modulates STIM1-SARAF interaction. Cell Physiol. Biochem.51, 1164–1178. 10.1159/000495494
9
AlbarranL.LopezJ. J.WoodardG. E.SalidoG. M.RosadoJ. A. (2016c). Store-operated Ca2+ entry-associated regulatory factor (SARAF) plays an important role in the regulation of arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem.291, 6982–6988. 10.1074/jbc.M115.704940
10
AlbarranL.RegodonS.SalidoG. M.LopezJ. J.RosadoJ. A. (2017). Role of STIM1 in the surface expression of SARAF. Channels (Austin)11, 84–88. 10.1080/19336950.2016.1212141
11
AubD. L.MckinneyJ. S.PutneyJ. W.JR. (1982). Nature of the receptor-regulated calcium pool in the rat parotid gland. J. Physiol.331, 557–565. 10.1113/jphysiol.1982.sp014391
12
BabaY.HayashiK.FujiiY.MizushimaA.WataraiH.WakamoriM.et al (2006). Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A.103, 16704–16709. 10.1073/pnas.0608358103
13
Baez-NietoD.CastilloJ. P.DragicevicC.AlvarezO.LatorreR. (2011). Thermo-TRP channels: biophysics of polymodal receptors. Adv. Exp. Med. Biol.704, 469–490. 10.1007/978-94-007-0265-3_26
14
BarrV. A.BernotK. M.SrikanthS.GwackY.BalagopalanL.ReganC. K.et al (2008). Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol. Biol. Cell19, 2802–2817. 10.1091/mbc.e08-02-0146
15
BeliveauE.LessardV.GuillemetteG. (2014). STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells. PLoS One9, e114718. 10.1371/journal.pone.0114718
16
BerridgeM. J. (1993). Inositol trisphosphate and calcium signalling. Nature361, 315–325. 10.1038/361315a0
17
BerridgeM. J.IrvineR. F. (1984). Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature312, 315–321. 10.1038/312315a0
18
BerridgeM. J.LippP.BootmanM. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol.1, 11–21. 10.1038/35036035
19
BieerkehazhiS.FanY.WestS. J.TewariR.KoJ.MillsT.et al (2022). Ca2+-dependent protein acyltransferase DHHC21 controls activation of CD4+ T cells. J. Cell Sci.135, jcs258186. 10.1242/jcs.258186
20
BoulayG.BrownD. M.QinN.JiangM.DietrichA.ZhuM. X.et al (1999). Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc. Natl. Acad. Sci. U. S. A.96, 14955–14960. 10.1073/pnas.96.26.14955
21
BroughG. H.WuS.CioffiD.MooreT. M.LiM.DeanN.et al (2001). Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J.15, 1704–1710. 10.1096/fj.010085rev
22
CallowayN.OwensT.CorwithK.RodgersW.HolowkaD.BairdB. (2011). Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P₂ between distinct membrane pools. J. Cell Sci.124, 2602–2610. 10.1242/jcs.084178
23
CarafoliE. (2002). Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. U. S. A.99, 1115–1122. 10.1073/pnas.032427999
24
Carreras-SuredaA.AbramiL.Ji-HeeK.WangW. A.HenryC.FriedenM.et al (2021). S-acylation by ZDHHC20 targets ORAI1 channels to lipid rafts for efficient Ca2+ signaling by Jurkat T cell receptors at the immune synapse. Elife10, e72051. 10.7554/eLife.72051
25
CasteelsR.DroogmansG. (1981). Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J. Physiol.317, 263–279. 10.1113/jphysiol.1981.sp013824
26
ChamberlainL. H.ShipstonM. J. (2015). The physiology of protein S-acylation. Physiol. Rev.95, 341–376. 10.1152/physrev.00032.2014
27
ChangC. L.HsiehT. S.YangT. T.RothbergK. G.AzizogluD. B.VolkE.et al (2013). Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep.5, 813–825. 10.1016/j.celrep.2013.09.038
28
ChenJ. J.FanY.BoehningD. (2021). Regulation of dynamic protein S-acylation. Front. Mol. Biosci.8, 656440. 10.3389/fmolb.2021.656440
29
ChenJ. J.MarsdenA. N.ScottC. A.AkimzhanovA. M.BoehningD. (2020). DHHC5 mediates β-adrenergic signaling in cardiomyocytes by targeting gα proteins. Biophys. J.118, 826–835. 10.1016/j.bpj.2019.08.018
30
ChengK. T.LiuX.OngH. L.AmbudkarI. S. (2008). Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J. Biol. Chem.283, 12935–12940. 10.1074/jbc.C800008200
31
ChengK. T.LiuX.OngH. L.SwaimW.AmbudkarI. S. (2011). Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol.9, e1001025. 10.1371/journal.pbio.1001025
32
ChvanovM.WalshC. M.HaynesL. P.VoroninaS. G.LurG.GerasimenkoO. V.et al (2008). ATP depletion induces translocation of STIM1 to puncta and formation of STIM1-ORAI1 clusters: translocation and re-translocation of STIM1 does not require ATP. Pflugers Arch.457, 505–517. 10.1007/s00424-008-0529-y
33
ClaphamD. E. (1995). Intracellular calcium. Replenishing the stores. Nature375, 634–635. 10.1038/375634a0
34
ClaphamD. E. (2007). Calcium signaling. Cell131, 1047–1058. 10.1016/j.cell.2007.11.028
35
CosensD. J.ManningA. (1969). Abnormal electroretinogram from a Drosophila mutant. Nature224, 285–287. 10.1038/224285a0
36
CovingtonE. D.WuM. M.LewisR. S. (2010). Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol. Biol. Cell21, 1897–1907. 10.1091/mbc.e10-02-0145
37
CrabtreeG. R. (1999). Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell96, 611–614. 10.1016/s0092-8674(00)80571-1
38
DesaiP. N.ZhangX.WuS.JanoshaziA.BolimunthaS.PutneyJ. W.et al (2015). Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci. Signal8, ra74. 10.1126/scisignal.aaa8323
39
DionisioN.AlbarranL.Berna-ErroA.Hernandez-CruzJ. M.SalidoG. M.RosadoJ. A. (2011a). Functional role of the calmodulin- and inositol 1,4,5-trisphosphate receptor-binding (CIRB) site of TRPC6 in human platelet activation. Cell Signal23, 1850–1856. 10.1016/j.cellsig.2011.06.022
40
DionisioN.GalanC.JardinI.SalidoG. M.RosadoJ. A. (2011b). Lipid rafts are essential for the regulation of SOCE by plasma membrane resident STIM1 in human platelets. Biochim. Biophys. Acta1813, 431–437. 10.1016/j.bbamcr.2011.01.010
41
DolmetschR. E.LewisR. S.GoodnowC. C.HealyJ. I. (1997). Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature386, 855–858. 10.1038/386855a0
42
DongH.FiorinG.CarnevaleV.TreptowW.KleinM. L. (2013). Pore waters regulate ion permeation in a calcium release-activated calcium channel. Proc. Natl. Acad. Sci. U. S. A.110, 17332–17337. 10.1073/pnas.1316969110
43
DownwardJ. (2004). RNA interference. BMJ328, 1245–1248. 10.1136/bmj.328.7450.1245
44
DuncanJ. A.GilmanA. G. (1998). A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem.273, 15830–15837. 10.1074/jbc.273.25.15830
45
DykxhoornD. M.LiebermanJ. (2005). The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annu. Rev. Med.56, 401–423. 10.1146/annurev.med.56.082103.104606
46
EbashiS.LipmannF. (1962). Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J. Cell Biol.14, 389–400. 10.1083/jcb.14.3.389
47
ErcanE.MomburgF.EngelU.TemmermanK.NickelW.SeedorfM. (2009). A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic10, 1802–1818. 10.1111/j.1600-0854.2009.00995.x
48
EssandohK.PhilippeJ. M.JenkinsP. M.BrodyM. J. (2020). Palmitoylation: a fatty regulator of myocardial electrophysiology. Front. Physiol.11, 108. 10.3389/fphys.2020.00108
49
FahrnerM.MuikM.SchindlR.ButoracC.StathopulosP.ZhengL.et al (2014). A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1). J. Biol. Chem.289, 33231–33244. 10.1074/jbc.M114.610022
50
FangerC. M.HothM.CrabtreeG. R.LewisR. S. (1995). Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J. Cell Biol.131, 655–667. 10.1083/jcb.131.3.655
51
FanY.ShayahatiB.TewariR.BoehningD.AkimzhanovA. M. (2020). Regulation of T cell receptor signaling by protein acyltransferase DHHC21. Mol. Biol. Rep.47, 6471–6478. 10.1007/s11033-020-05691-1
52
FeskeS.DraegerR.PeterH. H.EichmannK.RaoA. (2000a). The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J. Immunol.165, 297–305. 10.4049/jimmunol.165.1.297
53
FeskeS.DraegerR.PeterH. H.RaoA. (2000b). Impaired NFAT regulation and its role in a severe combined immunodeficiency. Immunobiology202, 134–150. 10.1016/S0171-2985(00)80060-1
54
FeskeS.GiltnaneJ.DolmetschR.StaudtL. M.RaoA. (2001). Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol.2, 316–324. 10.1038/86318
55
FeskeS.GwackY.PrakriyaM.SrikanthS.PuppelS. H.TanasaB.et al (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature441, 179–185. 10.1038/nature04702
56
FeskeS.MullerJ. M.GrafD.KroczekR. A.DragerR.NiemeyerC.et al (1996). Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur. J. Immunol.26, 2119–2126. 10.1002/eji.1830260924
57
FeskeS.PrakriyaM.RaoA.LewisR. S. (2005). A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J. Exp. Med.202, 651–662. 10.1084/jem.20050687
58
FraserN. J.HowieJ.WypijewskiK. J.FullerW. (2020). Therapeutic targeting of protein S-acylation for the treatment of disease. Biochem. Soc. Trans.48, 281–290. 10.1042/BST20190707
59
GalánC.ZbidiH.BartegiA.SalidoG. M.RosadoJ. A. (2009). STIM1, Orai1 and hTRPC1 are important for thrombin- and ADP-induced aggregation in human platelets. Arch. Biochem. Biophys.490, 137–144. 10.1016/j.abb.2009.08.007
60
GillD. L. (1989). Calcium signalling: receptor kinships revealed. Nature342, 16–18. 10.1038/342016a0
61
GiordanoF.SahekiY.Idevall-HagrenO.ColomboS. F.PirruccelloM.MilosevicI.et al (2013). PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell153, 1494–1509. 10.1016/j.cell.2013.05.026
62
Gonzalez-CobosJ. C.ZhangX.ZhangW.RuhleB.MotianiR. K.SchindlR.et al (2013). Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia. Circ. Res.112, 1013–1025. 10.1161/CIRCRESAHA.111.300220
63
GouyH.CefaiD.ChristensenS. B.DebreP.BismuthG. (1990). Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur. J. Immunol.20, 2269–2275. 10.1002/eji.1830201016
64
GreavesJ.ChamberlainL. H. (2007). Palmitoylation-dependent protein sorting. J. Cell Biol.176, 249–254. 10.1083/jcb.200610151
65
Gregorio-TeruelL.ValenteP.González-RosJ. M.Fernández-BallesterG.Ferrer-MontielA. (2014). Mutation of I696 and W697 in the TRP box of vanilloid receptor subtype I modulates allosteric channel activation. J. Gen. Physiol.143, 361–375. 10.1085/jgp.201311070
66
GrigorievI.GouveiaS. M.Van Der VaartB.DemmersJ.SmythJ. T.HonnappaS.et al (2008). STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol.18, 177–182. 10.1016/j.cub.2007.12.050
67
GrynkiewiczG.PoenieM.TsienR. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem.260, 3440–3450. 10.1016/s0021-9258(19)83641-4
68
GudlurA.QuintanaA.ZhouY.HirveN.MahapatraS.HoganP. G. (2014). STIM1 triggers a gating rearrangement at the extracellular mouth of the ORAI1 channel. Nat. Commun.5, 5164. 10.1038/ncomms6164
69
GuéguinouM.HarnoisT.CrottesD.UguenA.DeliotN.GambadeA.et al (2016). SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget7, 36168–36184. 10.18632/oncotarget.8786
70
GwackY.SharmaS.NardoneJ.TanasaB.IugaA.SrikanthS.et al (2006). A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature441, 646–650. 10.1038/nature04631
71
GwackY.SrikanthS.FeskeS.Cruz-GuillotyF.Oh-HoraM.NeemsD. S.et al (2007). Biochemical and functional characterization of Orai proteins. J. Biol. Chem.282, 16232–16243. 10.1074/jbc.M609630200
72
HardieR. C. (1996). Excitation of Drosophila photoreceptors by BAPTA and ionomycin: evidence for capacitative Ca2+ entry?Cell Calcium20, 315–327. 10.1016/s0143-4160(96)90037-8
73
HardieR. C.MinkeB. (1992). The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron8, 643–651. 10.1016/0896-6273(92)90086-s
74
HardieR. C.RaghuP. (1998). Activation of heterologously expressed Drosophila TRPL channels: Ca2+ is not required and InsP3 is not sufficient. Cell Calcium24, 153–163. 10.1016/s0143-4160(98)90125-7
75
HasselbachW.MakinoseM. (1961). The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting. Biochem. Z333, 518–528.
76
HauserC. T.TsienR. Y. (2007). A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc. Natl. Acad. Sci. U. S. A.104, 3693–3697. 10.1073/pnas.0611713104
77
HellmichU. A.GaudetR. (2014). Structural biology of TRP channels. Handb. Exp. Pharmacol.223, 963–990. 10.1007/978-3-319-05161-1_10
78
HofmannT.ObukhovA. G.SchaeferM.HarteneckC.GudermannT.SchultzG. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature397, 259–263. 10.1038/16711
79
HofmannT.SchaeferM.SchultzG.GudermannT. (2000). Cloning, expression and subcellular localization of two novel splice variants of mouse transient receptor potential channel 2. Biochem. J.351, 115–122. 10.1042/0264-6021:3510115
80
HoganP. G.ChenL.NardoneJ.RaoA. (2003). Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev.17, 2205–2232. 10.1101/gad.1102703
81
HooverP. J.LewisR. S. (2011). Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. U. S. A.108, 13299–13304. 10.1073/pnas.1101664108
82
HothM.PennerR. (1992). Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature355, 353–356. 10.1038/355353a0
83
HouX.BursteinS. R.LongS. B. (2018). Structures reveal opening of the store-operated calcium channel Orai. Elife7, e36758. 10.7554/eLife.36758
84
HouX.OuthwaiteI. R.PediL.LongS. B. (2020). Cryo-EM structure of the calcium release-activated calcium channel Orai in an open conformation. Elife9, e62772. 10.7554/eLife.62772
85
HouX.PediL.DiverM. M.LongS. B. (2012). Crystal structure of the calcium release-activated calcium channel Orai. Science338, 1308–1313. 10.1126/science.1228757
86
HuangG. N.ZengW.KimJ. Y.YuanJ. P.HanL.MuallemS.et al (2006). STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat. Cell Biol.8, 1003–1010. 10.1038/ncb1454
87
IrvineR. F. (1990). 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett.263, 5–9. 10.1016/0014-5793(90)80692-c
88
ItagakiK.KannanK. B.SinghB. B.HauserC. J. (2004). Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J. Immunol.172, 601–607. 10.4049/jimmunol.172.1.601
89
IvanovaA.Atakpa-AdajiP. (2023). Phosphatidylinositol 4,5-bisphosphate and calcium at ER-PM junctions - complex interplay of simple messengers. Biochim. Biophys. Acta Mol. Cell Res.1870, 119475. 10.1016/j.bbamcr.2023.119475
90
JardinI.LopezJ. J.SalidoG. M.RosadoJ. A. (2008a). Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J. Biol. Chem.283, 25296–25304. 10.1074/jbc.M802904200
91
JardinI.SalidoG. M.RosadoJ. A. (2008b). Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels (Austin)2, 401–403. 10.4161/chan.2.6.7055
92
JenningsB. C.LinderM. E. (2012). DHHC protein S-acyltransferases use similar ping-pong kinetic mechanisms but display different acyl-CoA specificities. J. Biol. Chem.287, 7236–7245. 10.1074/jbc.M111.337246
93
JhaA.AhujaM.MaléthJ.MorenoC. M.YuanJ. P.KimM. S.et al (2013). The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J. Cell Biol.202, 71–79. 10.1083/jcb.201301148
94
JiW.XuP.LiZ.LuJ.LiuL.ZhanY.et al (2008). Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc. Natl. Acad. Sci. U. S. A.105, 13668–13673. 10.1073/pnas.0806499105
95
JosephS. K.ThomasA. P.WilliamsR. J.IrvineR. F.WilliamsonJ. R. (1984). myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J. Biol. Chem.259, 3077–3081. 10.1016/s0021-9258(17)43262-5
96
JoussetH.FriedenM.DemaurexN. (2007). STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J. Biol. Chem.282, 11456–11464. 10.1074/jbc.M609551200
97
JumperJ.EvansR.PritzelA.GreenT.FigurnovM.RonnebergerO.et al (2021). Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589. 10.1038/s41586-021-03819-2
98
JungnickelM. K.MarreroH.BirnbaumerL.LémosJ. R.FlormanH. M. (2001). Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat. Cell Biol.3, 499–502. 10.1038/35074570
99
KawasakiT.LangeI.FeskeS. (2009). A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem. Biophys. Res. Commun.385, 49–54. 10.1016/j.bbrc.2009.05.020
100
KianiA.RaoA.AramburuJ. (2000). Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity12, 359–372. 10.1016/s1074-7613(00)80188-0
101
KimJ. Y.MuallemS. (2011). Unlocking SOAR releases STIM. EMBO J.30, 1673–1675. 10.1038/emboj.2011.107
102
KimM. S.ZengW.YuanJ. P.ShinD. M.WorleyP. F.MuallemS. (2009). Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J. Biol. Chem.284, 9733–9741. 10.1074/jbc.M808097200
103
KiselyovK.XuX.MozhayevaG.KuoT.PessahI.MigneryG.et al (1998). Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature396, 478–482. 10.1038/24890
104
KodakandlaG.WestS. J.WangQ.TewariR.ZhuM. X.AkimzhanovA. M.et al (2022). Dynamic S-acylation of the ER-resident protein stromal interaction molecule 1 (STIM1) is required for store-operated Ca(2+) entry. J. Biol. Chem.298, 102303. 10.1016/j.jbc.2022.102303
105
KullmannW. (1984). Kinetics of chymotrypsin- and papain-catalysed synthesis of [leucine]enkephalin and [methionine]enkephalin. Biochem. J.220, 405–416. 10.1042/bj2200405
106
LadyginaN.MartinB. R.AltmanA. (2011). Dynamic palmitoylation and the role of DHHC proteins in T cell activation and anergy. Adv. Immunol.109, 1–44. 10.1016/B978-0-12-387664-5.00001-7
107
LaquelP.TestetE.TuphileK.CullinC.FouillenL.BessouleJ. J.et al (2022). Phosphoinositides containing stearic acid are required for interaction between Rho GTPases and the exocyst to control the late steps of polarized exocytosis. Traffic23, 120–136. 10.1111/tra.12829
108
LeeE. H.LopezJ. R.LiJ.ProtasiF.PessahI. N.KimD. H.et al (2004). Conformational coupling of DHPR and RyR1 in skeletal myotubes is influenced by long-range allosterism: evidence for a negative regulatory module. Am. J. Physiol. Cell Physiol.286, C179–C189. 10.1152/ajpcell.00176.2003
109
LeeK. P.ChoiS.HongJ. H.AhujaM.GrahamS.MaR.et al (2014). Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J. Biol. Chem.289, 6372–6382. 10.1074/jbc.M113.546556
110
LeungH. T.GengC.PakW. L. (2000). Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J. Neurosci.20, 6797–6803. 10.1523/JNEUROSCI.20-18-06797.2000
111
LeventalI.GrzybekM.SimonsK. (2010). Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry49, 6305–6316. 10.1021/bi100882y
112
LewisR. S.CahalanM. D. (1989). Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul.1, 99–112. 10.1091/mbc.1.1.99
113
LinD. T.ConibearE. (2015). ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife4, e11306. 10.7554/eLife.11306
114
LioudynoM. I.KozakJ. A.PennaA.SafrinaO.ZhangS. L.SenD.et al (2008). Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Natl. Acad. Sci. U. S. A.105, 2011–2016. 10.1073/pnas.0706122105
115
LiouJ.FivazM.InoueT.MeyerT. (2007). Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. U. S. A.104, 9301–9306. 10.1073/pnas.0702866104
116
LiouJ.KimM. L.HeoW. D.JonesJ. T.MyersJ. W.FerrellJ. E.JR.et al (2005). STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol.15, 1235–1241. 10.1016/j.cub.2005.05.055
117
LiuC. H.ChenZ.OlivaM. K.LuoJ.CollierS.MontellC.et al (2020). Rapid release of Ca2+ from endoplasmic reticulum mediated by Na+/Ca2+ exchange. J. Neurosci.40, 3152–3164. 10.1523/JNEUROSCI.2675-19.2020
118
LiZ.LiuL.DengY.JiW.DuW.XuP.et al (2011). Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res.21, 305–315. 10.1038/cr.2010.131
119
LiZ.LuJ.XuP.XieX.ChenL.XuT. (2007). Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem.282, 29448–29456. 10.1074/jbc.M703573200
120
LockwichT.SinghB. B.LiuX.AmbudkarI. S. (2001). Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. J. Biol. Chem.276, 42401–42408. 10.1074/jbc.M106956200
121
LopezE.FrischaufI.JardinI.DerlerI.MuikM.CantoneroC.et al (2019). STIM1 phosphorylation at Y(316) modulates its interaction with SARAF and the activation of SOCE and I(CRAC). J. Cell Sci.132, jcs226019. 10.1242/jcs.226019
122
LucasP.UkhanovK.Leinders-ZufallT.ZufallF. (2003). A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron40, 551–561. 10.1016/s0896-6273(03)00675-5
123
LuikR. M.WuM. M.BuchananJ.LewisR. S. (2006). The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol.174, 815–825. 10.1083/jcb.200604015
124
MadlJ.WeghuberJ.FritschR.DerlerI.FahrnerM.FrischaufI.et al (2010). Resting state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells. J. Biol. Chem.285, 41135–41142. 10.1074/jbc.M110.177881
125
MaG.WeiM.HeL.LiuC.WuB.ZhangS. L.et al (2015). Inside-out Ca(2+) signalling prompted by STIM1 conformational switch. Nat. Commun.6, 7826. 10.1038/ncomms8826
126
MageeA. I.GutierrezL.MckayI. A.MarshallC. J.HallA. (1987). Dynamic fatty acylation of p21N-ras. EMBO J.6, 3353–3357. 10.1002/j.1460-2075.1987.tb02656.x
127
MalethJ.ChoiS.MuallemS.AhujaM. (2014). Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat. Commun.5, 5843. 10.1038/ncomms6843
128
MarriottI.MasonM. J. (1995). ATP depletion inhibits capacitative Ca2+ entry in rat thymic lymphocytes. Am. J. Physiol.269, C766–C774. 10.1152/ajpcell.1995.269.3.C766
129
MasonM. J.Mahaut-SmithM. P.GrinsteinS. (1991). The role of intracellular Ca2+ in the regulation of the plasma membrane Ca2+ permeability of unstimulated rat lymphocytes. J. Biol. Chem.266, 10872–10879. 10.1016/s0021-9258(18)99100-3
130
MatiasM. G.GomolplitinantK. M.TamangD. G.SaierM. H.JR. (2010). Animal Ca2+ release-activated Ca2+ (CRAC) channels appear to be homologous to and derived from the ubiquitous cation diffusion facilitators. BMC Res. Notes3, 158. 10.1186/1756-0500-3-158
131
MatthewsG.NeherE.PennerR. (1989). Second messenger-activated calcium influx in rat peritoneal mast cells. J. Physiol.418, 105–130. 10.1113/jphysiol.1989.sp017830
132
MccarlC. A.PicardC.KhalilS.KawasakiT.RötherJ.PapolosA.et al (2009). ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J. Allergy Clin. Immunol.124, 1311–1318. 10.1016/j.jaci.2009.10.007
133
McnallyB. A.SomasundaramA.YamashitaM.PrakriyaM. (2012). Gated regulation of CRAC channel ion selectivity by STIM1. Nature482, 241–245. 10.1038/nature10752
134
McnallyB. A.YamashitaM.EnghA.PrakriyaM. (2009). Structural determinants of ion permeation in CRAC channels. Proc. Natl. Acad. Sci. U. S. A.106, 22516–22521. 10.1073/pnas.0909574106
135
MelkonianK. A.OstermeyerA. G.ChenJ. Z.RothM. G.BrownD. A. (1999). Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem.274, 3910–3917. 10.1074/jbc.274.6.3910
136
MercerJ. C.DehavenW. I.SmythJ. T.WedelB.BoylesR. R.BirdG. S.et al (2006). Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem.281, 24979–24990. 10.1074/jbc.M604589200
137
MignenO.ThompsonJ. L.ShuttleworthT. J. (2008a). Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J. Physiol.586, 185–195. 10.1113/jphysiol.2007.146258
138
MignenO.ThompsonJ. L.ShuttleworthT. J. (2008b). Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J. Physiol.586, 419–425. 10.1113/jphysiol.2007.147249
139
MignenO.ThompsonJ. L.ShuttleworthT. J. (2009). The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J. Physiol.587, 4181–4197. 10.1113/jphysiol.2009.174193
140
MontellC. (2001). Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci. STKE2001, re1. 10.1126/stke.2001.90.re1
141
MontellC. (2005). The TRP superfamily of cation channels. Sci. STKE2005, re3. 10.1126/stke.2722005re3
142
MontellC.JonesK.HafenE.RubinG. (1985). Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science230, 1040–1043. 10.1126/science.3933112
143
MontellC.RubinG. M. (1989). Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron2, 1313–1323. 10.1016/0896-6273(89)90069-x
144
MuikM.FahrnerM.DerlerI.SchindlR.BergsmannJ.FrischaufI.et al (2009). A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J. Biol. Chem.284, 8421–8426. 10.1074/jbc.C800229200
145
MuikM.FahrnerM.SchindlR.StathopulosP.FrischaufI.DerlerI.et al (2011). STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J.30, 1678–1689. 10.1038/emboj.2011.79
146
MuikM.FrischaufI.DerlerI.FahrnerM.BergsmannJ.EderP.et al (2008). Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem.283, 8014–8022. 10.1074/jbc.M708898200
147
MullinsF. M.ParkC. Y.DolmetschR. E.LewisR. S. (2009). STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc. Natl. Acad. Sci. U. S. A.106, 15495–15500. 10.1073/pnas.0906781106
148
Navarro-BorellyL.SomasundaramA.YamashitaM.RenD.MillerR. J.PrakriyaM. (2008). STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol.586, 5383–5401. 10.1113/jphysiol.2008.162503
149
NegulescuP. A.MachenT. E. (1988). Release and reloading of intracellular Ca stores after cholinergic stimulation of the parietal cell. Am. J. Physiol.254, C498–C504. 10.1152/ajpcell.1988.254.4.C498
150
NiemeyerB. A.SuzukiE.ScottK.JalinkK.ZukerC. S. (1996). The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell85, 651–659. 10.1016/s0092-8674(00)81232-5
151
OkadaT.InoueR.YamazakiK.MaedaA.KurosakiT.YamakuniT.et al (1999). Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem.274, 27359–27370. 10.1074/jbc.274.39.27359
152
OkadaT.ShimizuS.WakamoriM.MaedaA.KurosakiT.TakadaN.et al (1998). Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem.273, 10279–10287. 10.1074/jbc.273.17.10279
153
OngH. L.ChengK. T.LiuX.BandyopadhyayB. C.PariaB. C.SoboloffJ.et al (2007). Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem.282, 9105–9116. 10.1074/jbc.M608942200
154
OngH. L.JangS. I.AmbudkarI. S. (2012). Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca(2+)](i) signals determine specificity of Ca(2+)-dependent gene expression. PLoS One7, e47146. 10.1371/journal.pone.0047146
155
OrciL.RavazzolaM.Le CoadicM.ShenW. W.DemaurexN.CossonP. (2009). From the Cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A.106, 19358–19362. 10.1073/pnas.0911280106
156
PachecoJ.DominguezL.Bohórquez-HernándezA.AsanovA.VacaL. (2016). A cholesterol-binding domain in STIM1 modulates STIM1-Orai1 physical and functional interactions. Sci. Rep.6, 29634. 10.1038/srep29634
157
PaltyR.RavehA.KaminskyI.MellerR.ReuvenyE. (2012). SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell149, 425–438. 10.1016/j.cell.2012.01.055
158
PaniB.SinghB. B. (2009). Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium45, 625–633. 10.1016/j.ceca.2009.02.009
159
ParekhA. B.PennerR. (1997). Store depletion and calcium influx. Physiol. Rev.77, 901–930. 10.1152/physrev.1997.77.4.901
160
ParekhA. B.PutneyJ. W. (2005). Store-operated calcium channels. Physiol. Rev.85, 757–810. 10.1152/physrev.00057.2003
161
ParkC. Y.HooverP. J.MullinsF. M.BachhawatP.CovingtonE. D.RaunserS.et al (2009). STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell136, 876–890. 10.1016/j.cell.2009.02.014
162
ParkM. K.AshbyM. C.ErdemliG.PetersenO. H.TepikinA. V. (2001). Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J.20, 1863–1874. 10.1093/emboj/20.8.1863
163
PattersonR. L.Van RossumD. B.GillD. L. (1999). Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell98, 487–499. 10.1016/s0092-8674(00)81977-7
164
PennaA.DemuroA.YerominA. V.ZhangS. L.SafrinaO.ParkerI.et al (2008). The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature456, 116–120. 10.1038/nature07338
165
PennerR.MatthewsG.NeherE. (1988). Regulation of calcium influx by second messengers in rat mast cells. Nature334, 499–504. 10.1038/334499a0
166
Perrouin-VerbeM. A.SchoentgenN.TalagasM.GarlantezecR.UguenA.DoucetL.et al (2019). Overexpression of certain transient receptor potential and Orai channels in prostate cancer is associated with decreased risk of systemic recurrence after radical prostatectomy. Prostate79, 1793–1804. 10.1002/pros.23904
167
PetersenC. C.BerridgeM. J.BorgeseM. F.BennettD. L. (1995). Putative capacitative calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem. J.311 (1), 41–44. 10.1042/bj3110041
168
PhilippS.CavaliéA.FreichelM.WissenbachU.ZimmerS.TrostC.et al (1996). A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J.15, 6166–6171. 10.1002/j.1460-2075.1996.tb01004.x
169
PhillipsA. M.BullA.KellyL. E. (1992). Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron8, 631–642. 10.1016/0896-6273(92)90085-r
170
PrakriyaM.FeskeS.GwackY.SrikanthS.RaoA.HoganP. G. (2006). Orai1 is an essential pore subunit of the CRAC channel. Nature443, 230–233. 10.1038/nature05122
171
PrakriyaM.LewisR. S. (2006). Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J. Gen. Physiol.128, 373–386. 10.1085/jgp.200609588
172
PutneyJ. W.JR. (1986). A model for receptor-regulated calcium entry. Cell Calcium7, 1–12. 10.1016/0143-4160(86)90026-6
173
QinX.LiuL.LeeS. K.AlsinaA.LiuT.WuC.et al (2020). Increased confinement and polydispersity of STIM1 and Orai1 after Ca(2+) store depletion. Biophys. J.118, 70–84. 10.1016/j.bpj.2019.11.019
174
QuintanaA.PascheM.JunkerC.Al-AnsaryD.RiegerH.KummerowC.et al (2011). Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J.30, 3895–3912. 10.1038/emboj.2011.289
175
QuintanaA.RajanikanthV.Farber-KatzS.GudlurA.ZhangC.JingJ.et al (2015). TMEM110 regulates the maintenance and remodeling of mammalian ER-plasma membrane junctions competent for STIM-ORAI signaling. Proc. Natl. Acad. Sci. U. S. A.112, E7083–E7092. 10.1073/pnas.1521924112
176
RandriamampitaC.TsienR. Y. (1993). Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature364, 809–814. 10.1038/364809a0
177
RanganathanR.BacskaiB. J.TsienR. Y.ZukerC. S. (1994). Cytosolic calcium transients: spatial localization and role in Drosophila photoreceptor cell function. Neuron13, 837–848. 10.1016/0896-6273(94)90250-x
178
ReshM. D. (2016). Fatty acylation of proteins: the long and the short of it. Prog. Lipid Res.63, 120–131. 10.1016/j.plipres.2016.05.002
179
ReussH.MojetM. H.ChybS.HardieR. C. (1997). In vivo analysis of the drosophila light-sensitive channels, TRP and TRPL. Neuron19, 1249–1259. 10.1016/s0896-6273(00)80416-x
180
RheeS. G. (2001). Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem.70, 281–312. 10.1146/annurev.biochem.70.1.281
181
RocksO.GerauerM.VartakN.KochS.HuangZ. P.PechlivanisM.et al (2010). The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell141, 458–471. 10.1016/j.cell.2010.04.007
182
RocksO.PeykerA.KahmsM.VerveerP. J.KoernerC.LumbierresM.et al (2005). An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science307, 1746–1752. 10.1126/science.1105654
183
RoosJ.DigregorioP. J.YerominA. V.OhlsenK.LioudynoM.ZhangS.et al (2005). STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol.169, 435–445. 10.1083/jcb.200502019
184
SabourinJ.BoetA.Rucker-MartinC.LambertM.GomezA. M.BenitahJ. P.et al (2018). Ca2+ handling remodeling and STIM1L/Orai1/TRPC1/TRPC4 upregulation in monocrotaline-induced right ventricular hypertrophy. J. Mol. Cell Cardiol.118, 208–224. 10.1016/j.yjmcc.2018.04.003
185
SabourinJ.Le GalL.SaurweinL.HaefligerJ. A.RaddatzE.AllagnatF. (2015). Store-operated Ca2+ entry mediated by Orai1 and TRPC1 participates to insulin secretion in rat β-cells. J. Biol. Chem.290, 30530–30539. 10.1074/jbc.M115.682583
186
SampieriA.SantoyoK.AsanovA.VacaL. (2018). Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep.8, 13252. 10.1038/s41598-018-31621-0
187
SarkadiB.TordaiA.HomolyaL.ScharffO.GardosG. (1991). Calcium influx and intracellular calcium release in anti-CD3 antibody-stimulated and thapsigargin-treated human T lymphoblasts. J. Membr. Biol.123, 9–21. 10.1007/BF01993958
188
SchaarA.SunY.SukumaranP.RosenbergerT. A.KroutD.RoemmichJ. N.et al (2019). Ca2+ entry via TRPC1 is essential for cellular differentiation and modulates secretion via the SNARE complex. J. Cell Sci.132, jcs231878. 10.1242/jcs.231878
189
SerafiniA. T.LewisR. S.ClipstoneN. A.BramR. J.FangerC.FieringS.et al (1995). Isolation of mutant T lymphocytes with defects in capacitative calcium entry. Immunity3, 239–250. 10.1016/1074-7613(95)90093-4
190
SharmaS.QuintanaA.FindlayG. M.MettlenM.BaustB.JainM.et al (2013). An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature499, 238–242. 10.1038/nature12229
191
SmaniT.ZakharovS. I.CsutoraP.LenoE.TrepakovaE. S.BolotinaV. M. (2004). A novel mechanism for the store-operated calcium influx pathway. Nat. Cell Biol.6, 113–120. 10.1038/ncb1089
192
SmedlerE.UhlenP. (2014). Frequency decoding of calcium oscillations. Biochim. Biophys. Acta1840, 964–969. 10.1016/j.bbagen.2013.11.015
193
SmythJ. T.DehavenW. I.BirdG. S.PutneyJ. W.JR. (2008). Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J. Cell Sci.121, 762–772. 10.1242/jcs.023903
194
SokabeT.BradshawH. B.TominagaM.LeishmanE.ChandelA.MontellC. (2022). Endocannabinoids produced in photoreceptor cells in response to light activate Drosophila TRP channels. Sci. Signal15, eabl6179. 10.1126/scisignal.abl6179
195
SrikanthS.JewM.KimK. D.YeeM. K.AbramsonJ.GwackY. (2012). Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. U. S. A.109, 8682–8687. 10.1073/pnas.1200667109
196
StathopulosP. B.LiG. Y.PlevinM. J.AmesJ. B.IkuraM. (2006). Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem.281, 35855–35862. 10.1074/jbc.M608247200
197
StathopulosP. B.SchindlR.FahrnerM.ZhengL.Gasmi-SeabrookG. M.MuikM.et al (2013). STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat. Commun.4, 2963. 10.1038/ncomms3963
198
StathopulosP. B.ZhengL.IkuraM. (2009). Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J. Biol. Chem.284, 728–732. 10.1074/jbc.C800178200
199
StathopulosP. B.ZhengL.LiG. Y.PlevinM. J.IkuraM. (2008). Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell135, 110–122. 10.1016/j.cell.2008.08.006
200
StrebH.IrvineR. F.BerridgeM. J.SchulzI. (1983). Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature306, 67–69. 10.1038/306067a0
201
SupattaponeS.WorleyP. F.BarabanJ. M.SnyderS. H. (1988). Solubilization, purification, and characterization of an inositol trisphosphate receptor. J. Biol. Chem.263, 1530–1534. 10.1016/s0021-9258(19)57336-7
202
TakemuraH.HughesA. R.ThastrupO.PutneyJ. W.JR. (1989). Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. J. Biol. Chem.264, 12266–12271. 10.1016/s0021-9258(18)63852-9
203
TewariR.ShayahatiB.FanY.AkimzhanovA. M. (2021). T cell receptor-dependent S-acylation of ZAP-70 controls activation of T cells. J. Biol. Chem.296, 100311. 10.1016/j.jbc.2021.100311
204
ThastrupO.CullenP. J.DrøbakB. K.HanleyM. R.DawsonA. P. (1990). Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U. S. A.87, 2466–2470. 10.1073/pnas.87.7.2466
205
ThastrupO.DawsonA. P.ScharffO.FoderB.CullenP. J.DrøbakB. K.et al (1989). Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions27, 17–23. 10.1007/BF02222186
206
ThomasD.HanleyM. R. (1995). Evaluation of calcium influx factors from stimulated Jurkat T-lymphocytes by microinjection into Xenopus oocytes. J. Biol. Chem.270, 6429–6432. 10.1074/jbc.270.12.6429
207
ThompsonJ. L.ShuttleworthT. J. (2013). Molecular basis of activation of the arachidonate-regulated Ca2+ (ARC) channel, a store-independent Orai channel, by plasma membrane STIM1. J. Physiol.591, 3507–3523. 10.1113/jphysiol.2013.256784
208
TimmermanL. A.ClipstoneN. A.HoS. N.NorthropJ. P.CrabtreeG. R. (1996). Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature383, 837–840. 10.1038/383837a0
209
TowlerD.GlaserL. (1986). Acylation of cellular proteins with endogenously synthesized fatty acids. Biochemistry25, 878–884. 10.1021/bi00352a021
210
TrebakM.St J BirdG.MckayR. R.BirnbaumerL.PutneyJ. W. (2003). Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J. Biol. Chem.278, 16244–16252. 10.1074/jbc.M300544200
211
TrevesS.Franzini-ArmstrongC.MoccagattaL.ArnoultC.GrassoC.SchrumA.et al (2004). Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J. Cell Biol.166, 537–548. 10.1083/jcb.200404079
212
UlusuN. N. (2015). Evolution of enzyme kinetic mechanisms. J. Mol. Evol.80, 251–257. 10.1007/s00239-015-9681-0
213
VacaL.SinkinsW. G.HuY.KunzeD. L.SchillingW. P. (1994). Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am. J. Physiol.267, C1501–C1505. 10.1152/ajpcell.1994.267.5.C1501
214
Van CoppenolleF.Vanden AbeeleF.SlomiannyC.FlourakisM.HeskethJ.DewaillyE.et al (2004). Ribosome-translocon complex mediates calcium leakage from endoplasmic reticulum stores. J. Cell Sci.117, 4135–4142. 10.1242/jcs.01274
215
VannierB.PeytonM.BoulayG.BrownD.QinN.JiangM.et al (1999). Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc. Natl. Acad. Sci. U. S. A.96, 2060–2064. 10.1073/pnas.96.5.2060
216
VannierB.ZhuX.BrownD.BirnbaumerL. (1998). The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem.273, 8675–8679. 10.1074/jbc.273.15.8675
217
VazquezG.LievremontJ. P.StJ. B. G.PutneyJ. W.JR. (2001). Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc. Natl. Acad. Sci. U. S. A.98, 11777–11782. 10.1073/pnas.201238198
218
VenkatachalamK.MontellC. (2007). TRP channels. Annu. Rev. Biochem.76, 387–417. 10.1146/annurev.biochem.75.103004.142819
219
VigM.BeckA.BillingsleyJ. M.LisA.ParvezS.PeineltC.et al (2006a). CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol.16, 2073–2079. 10.1016/j.cub.2006.08.085
220
VigM.PeineltC.BeckA.KoomoaD. L.RabahD.Koblan-HubersonM.et al (2006b). CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science312, 1220–1223. 10.1126/science.1127883
221
WalshC. M.ChvanovM.HaynesL. P.PetersenO. H.TepikinA. V.BurgoyneR. D. (2009). Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem. J.425, 159–168. 10.1042/BJ20090884
222
WedelB. J.VazquezG.MckayR. R.St J BirdG.PutneyJ. W. (2003). A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J. Biol. Chem.278, 25758–25765. 10.1074/jbc.M303890200
223
WesP. D.ChevesichJ.JerominA.RosenbergC.StettenG.MontellC. (1995). TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U. S. A.92, 9652–9656. 10.1073/pnas.92.21.9652
224
WestS. J.BoehningD.AkimzhanovA. M. (2022a). Regulation of T cell function by protein S-acylation. Front. Physiol.13, 1040968. 10.3389/fphys.2022.1040968
225
WestS. J.KodakandlaG.WangQ.TewariR.ZhuM. X.BoehningD.et al (2022b). S-acylation of Orai1 regulates store-operated Ca2+ entry. J. Cell Sci.135, jcs258579. 10.1242/jcs.258579
226
WissenbachU.SchrothG.PhilippS.FlockerziV. (1998). Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett.429, 61–66. 10.1016/s0014-5793(98)00561-4
227
WozniakA. L.WangX.StierenE. S.ScarbroughS. G.ElferinkC. J.BoehningD. (2006). Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J. Cell Biol.175, 709–714. 10.1083/jcb.200608035
228
WuM. M.BuchananJ.LuikR. M.LewisR. S. (2006). Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol.174, 803–813. 10.1083/jcb.200604014
229
WuM. M.CovingtonE. D.LewisR. S. (2014). Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum-plasma membrane junctions. Mol. Biol. Cell25, 3672–3685. 10.1091/mbc.E14-06-1107
230
XuX. Z.ChienF.ButlerA.SalkoffL.MontellC. (2000). TRPgamma, a drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron26, 647–657. 10.1016/s0896-6273(00)81201-5
231
XuX. Z.LiH. S.GugginoW. B.MontellC. (1997). Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell89, 1155–1164. 10.1016/s0092-8674(00)80302-5
232
XuP.LuJ.LiZ.YuX.ChenL.XuT. (2006). Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun.350, 969–976. 10.1016/j.bbrc.2006.09.134
233
YamashitaM.Navarro-BorellyL.McnallyB. A.PrakriyaM. (2007). Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J. Gen. Physiol.130, 525–540. 10.1085/jgp.200709872
234
YamashitaM.PrakriyaM. (2014). Divergence of Ca(2+) selectivity and equilibrium Ca(2+) blockade in a Ca(2+) release-activated Ca(2+) channel. J. Gen. Physiol.143, 325–343. 10.1085/jgp.201311108
235
YamashitaM.YeungP. S.IngC. E.McnallyB. A.PomesR.PrakriyaM. (2017). STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate. Nat. Commun.8, 14512. 10.1038/ncomms14512
236
YangX.JinH.CaiX.LiS.ShenY. (2012). Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. U. S. A.109, 5657–5662. 10.1073/pnas.1118947109
237
YaoY.Ferrer-MontielA. V.MontalM.TsienR. Y. (1999). Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell98, 475–485. 10.1016/s0092-8674(00)81976-5
238
YerominA. V.RoosJ.StaudermanK. A.CahalanM. D. (2004). A store-operated calcium channel in Drosophila S2 cells. J. Gen. Physiol.123, 167–182. 10.1085/jgp.200308982
239
YerominA. V.ZhangS. L.JiangW.YuY.SafrinaO.CahalanM. D. (2006). Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature443, 226–229. 10.1038/nature05108
240
YeungP. S.YamashitaM.IngC. E.PomesR.FreymannD. M.PrakriyaM. (2018). Mapping the functional anatomy of Orai1 transmembrane domains for CRAC channel gating. Proc. Natl. Acad. Sci. U. S. A.115, E5193–E5202. 10.1073/pnas.1718373115
241
YuanJ. P.ZengW.DorwartM. R.ChoiY. J.WorleyP. F.MuallemS. (2009). SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol.11, 337–343. 10.1038/ncb1842
242
YuJ.ZhangH.ZhangM.DengY.WangH.LuJ.et al (2013). An aromatic amino acid in the coiled-coil 1 domain plays a crucial role in the auto-inhibitory mechanism of STIM1. Biochem. J.454, 401–409. 10.1042/BJ20130292
243
ZengW.YuanJ. P.KimM. S.ChoiY. J.HuangG. N.WorleyP. F.et al (2008). STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell32, 439–448. 10.1016/j.molcel.2008.09.020
244
ZhangS. L.YerominA. V.HuJ.AmcheslavskyA.ZhengH.CahalanM. D. (2011). Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc. Natl. Acad. Sci. U. S. A.108, 17838–17843. 10.1073/pnas.1114821108
245
ZhangS. L.YerominA. V.ZhangX. H.YuY.SafrinaO.PennaA.et al (2006). Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. U. S. A.103, 9357–9362. 10.1073/pnas.0603161103
246
ZhangS. L.YuY.RoosJ.KozakJ. A.DeerinckT. J.EllismanM. H.et al (2005). STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature437, 902–905. 10.1038/nature04147
247
ZhangC.ThomasD. W. (2016). Stromal Interaction Molecule 1 rescues store-operated calcium entry and protects NG115-401L cells against cell death induced by endoplasmic reticulum and mitochondrial oxidative stress. Neurochem. Int.97, 137–145. 10.1016/j.neuint.2016.04.002
248
ZhangW.MuramatsuA.MatsuoR.TeranishiN.KaharaY.TakaharaT.et al (2020). The penta-EF-hand ALG-2 protein interacts with the cytosolic domain of the SOCE regulator SARAF and interferes with ubiquitination. Int. J. Mol. Sci.21, 6315. 10.3390/ijms21176315
249
ZhengH.ZhouM. H.HuC.KuoE.PengX.HuJ.et al (2013). Differential roles of the C and N termini of Orai1 protein in interacting with stromal interaction molecule 1 (STIM1) for Ca2+ release-activated Ca2+ (CRAC) channel activation. J. Biol. Chem.288, 11263–11272. 10.1074/jbc.M113.450254
250
ZhengL.StathopulosP. B.LiG. Y.IkuraM. (2008). Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun.369, 240–246. 10.1016/j.bbrc.2007.12.129
251
ZhengL.StathopulosP. B.SchindlR.LiG. Y.RomaninC.IkuraM. (2011). Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc. Natl. Acad. Sci. U. S. A.108, 1337–1342. 10.1073/pnas.1015125108
252
ZhouY.MeranerP.KwonH. T.MachnesD.Oh-HoraM.ZimmerJ.et al (2010a). STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol.17, 112–116. 10.1038/nsmb.1724
253
ZhouY.RamachandranS.Oh-HoraM.RaoA.HoganP. G. (2010b). Pore architecture of the ORAI1 store-operated calcium channel. Proc. Natl. Acad. Sci. U. S. A.107, 4896–4901. 10.1073/pnas.1001169107
254
ZhouY.SrinivasanP.RazaviS.SeymourS.MeranerP.GudlurA.et al (2013). Initial activation of STIM1, the regulator of store-operated calcium entry. Nat. Struct. Mol. Biol.20, 973–981. 10.1038/nsmb.2625
255
ZhuX.ChuP. B.PeytonM.BirnbaumerL. (1995). Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett.373, 193–198. 10.1016/0014-5793(95)01038-g
256
ZhuX.JiangM.PeytonM.BoulayG.HurstR.StefaniE.et al (1996). trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell85, 661–671. 10.1016/s0092-8674(00)81233-7
257
ZittC.ObukhovA. G.StrübingC.ZobelA.KalkbrennerF.LückhoffA.et al (1997). Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J. Cell Biol.138, 1333–1341. 10.1083/jcb.138.6.1333
258
ZittC.ZobelA.ObukhovA. G.HarteneckC.KalkbrennerF.LückhoffA.et al (1996). Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron16, 1189–1196. 10.1016/s0896-6273(00)80145-2
259
ZweifachA.LewisR. S. (1993). Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. U. S. A.90, 6295–6299. 10.1073/pnas.90.13.6295
Summary
Keywords
calcium, Orai1, STIM1, DHHC21, S-acylation, store-operated calcium entry, immune diseases
Citation
Kodakandla G, Akimzhanov AM and Boehning D (2023) Regulatory mechanisms controlling store-operated calcium entry. Front. Physiol. 14:1330259. doi: 10.3389/fphys.2023.1330259
Received
30 October 2023
Accepted
08 December 2023
Published
19 December 2023
Volume
14 - 2023
Edited by
Manuel F. Navedo, University of California, Davis, United States
Reviewed by
Luis Vaca, National Autonomous University of Mexico, Mexico
Oscar Cerda, University of Chile, Chile
Updates
Copyright
© 2023 Kodakandla, Akimzhanov and Boehning.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Goutham Kodakandla, kodaka78@rowan.edu; Darren Boehning, boehning@rowan.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.