Abstract
Background: Flavin monooxygenases (FMOs) are enzymes responsible for the oxidation of a broad spectrum of exogenous and endogenous amines. There is increasing evidence that trimethylamine (TMA), a compound produced by gut bacteria and also recognized as an industrial pollutant, contributes to cardiovascular diseases. FMOs convert TMA into trimethylamine oxide (TMAO), which is an emerging marker of cardiovascular risk. This study hypothesized that blood pressure phenotypes in rats might be associated with variations in the expression of FMOs.
Methods: The expression of FMO1, FMO3, and FMO5 was evaluated in the kidneys, liver, lungs, small intestine, and large intestine of normotensive male Wistar-Kyoto rats (WKY) and two distinct hypertensive rat models: spontaneously hypertensive rats (SHRs) and WKY rats with angiotensin II-induced hypertension (WKY-ANG). Plasma concentrations of TMA and TMAO were measured at baseline and after intravenous administration of TMA using liquid chromatography-mass spectrometry (LC-MS).
Results: We found that the expression of FMOs in WKY, SHR, and WKY-ANG rats was in the descending order of FMO3 > FMO1 >> FMO5. The highest expression of FMOs was observed in the liver. Notably, SHRs exhibited a significantly elevated expression of FMO3 in the liver compared to WKY and WKY-ANG rats. Additionally, the plasma TMAO/TMA ratio was significantly higher in SHRs than in WKY rats.
Conclusion: SHRs demonstrate enhanced expression of FMO3 and a higher plasma TMAO/TMA ratio. The variability in the expression of FMOs and the metabolism of amines might contribute to the hypertensive phenotype observed in SHRs.

Introduction
NADPH-dependent flavin-containing monooxygenases are a family of enzymes that catalyze the oxidation of a wide range of nitrogen-containing compounds and metabolize drugs (Eswaramoorthy et al., 2006; Phillips and Shephard, 2020). Based on the cDNA sequence, FMOs were classified into five subfamilies (FMO1 to 5) (Lawton et al., 1994; Lattard et al., 2003). Species, age, sex and tissue-dependent variability in the expression of FMOs has been described (Nagata et al., 1990; Hvattum et al., 1991; Lawton et al., 1991; Hines et al., 1994; Shehin-Johnson et al., 1995; Dolphin et al., 1996; Kawaji et al., 1997; Lattard et al., 2002; Lattard et al., 2003; Zhang and Cashman, 2006; Shimizu et al., 2011). FMOs are expressed in the liver, lungs, kidney and, to a lesser extent, in the heart, intestine and brain (Lawton et al., 1991; Bhamre et al., 1993; Kawaji et al., 1995; Bhagwat et al., 1996; Kawaji et al., 1997; Lattard et al., 2002; Novick et al., 2009).
FMO mediates N-oxygenation of tertiary amines, including vasoactive amines such as phenethylamine and tyramine, (Gut and Conney, 1993; Cashman, 1995; Mitchell et al., 1997; Cashman et al., 2004; Krueger et al., 2006). Changes in FMOs gene expression have been detected in the following diseases: trimethylaminuria (Cashman et al., 1997; Dolphin et al., 1997; Treacy et al., 1998; Akerman et al., 1999; Cashman et al., 2003), atherosclerosis (Motika et al., 2007; Shih et al., 2015), diabetes mellitus (Rouer et al., 1987; Rouer et al., 1988; Takamura et al., 2004; Toda et al., 2005), primary hemochromatosis (Muckenthaler et al., 2003; Cashman and Zhang, 2006), atrial fibrillation (Kim et al., 2003); sideroblastic anaemia (Barber et al., 2000) and in neoplastic tissues (Krueger and Williams, 2005; Fialka et al., 2008).
Trimethylamine is a gut microbiota metabolite and air pollutant originating from chemically synthetized compound used in industrial production of and an air pollutant (Pospischil et al., 2017). In mammalian organism TMA is generated by bacterial metabolism of dietary choline, betaine, and carnitine, trimethyllysine and by reduction of dietary trimethylamine N-oxide to the parent amine (Lang et al., 1998; Craciun et al., 2014; Koeth et al., 2014; Zhu et al., 2014; Hsu et al., 2019; Sun et al., 2019; Muralitharan et al., 2020). TMA is oxidized to TMAO by first-pass metabolism in the liver (Al-Waiz et al., 1987; Lin and Cashman, 1997b; Lang et al., 1998; Karoly and Rose, 2001; Krueger and Williams, 2005).
Interestingly, high TMAO concentrations has been suggested to corelate with increased cardiovascular risk (Tang et al., 2013; Qi et al., 2018). The blood TMAO level has been reported to be positively correlated with long-term mortality risk in patients with atherosclerosis, heart failure, and chronic kidney disease (Koeth et al., 2013; Tang et al., 2014; Tang et al., 2015).
However, the role of TMAO as a causative factor in cardiovascular disease is debatable as contradictory data on TMAO effects are available (Yin et al., 2015; Collins et al., 2016; Meyer et al., 2016; Huc et al., 2018; Stubbs et al., 2019; Aldana-Hernandez et al., 2020; Gawrys-Kopczynska et al., 2020; Maksymiuk et al., 2022). Previously, we have found that TMA, but not TMAO, administered intravenously IV) produced a significant hypertensive effect in normotensive rats (Jaworska et al., 2019). Furthermore, TMA after the administration was rapidly oxidized to TMAO, which was associated with a decrease in the hypertensive response (Jaworska et al., 2019).
We hypothesized that the hypertensive rat phenotype might be linked to changes in the expression and activity of FMOs. Consequently, the main aim of our study was to compare the expression of FMOs in normotensive and hypertensive rats. We carried out this experiment using two different models of hypertension: the genetic SHR model and the pharmacologically induced model using Ang II.
Materials and methods
Animals
All animal procedures conformed to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. The study was approved by the II Local Ethical Committee in Warsaw (Certificate of approval No. WAW2/082/2018). Wistar Kyoto rats (WKY) and Spontaneously Hypertensive Rats (SHR) were obtained from the Central Laboratory for Experimental Animals, Medical University of Warsaw, Poland.
Rats were housed in groups of two to three animals, in polypropylene cages with environmental enrichment, 12 h light/12 h dark cycle, temperature 22–23°C, humidity 45%–55%, food and water ad libitum. 12-week-old, male.
The experiments were performed on rats (WKY, n = 48) (SHR, n = 48) and (WKY-ANG, n = 48) WKY-ANG group constituted of WKY rats implanted at the age of 10 weeks with subcutaneous osmotic minipump (ALZET 2ML; Durect, Cupertino, CA). The minipumps were releasing Ang II at the rate of (0.76 pmol s−1; 0.8 ngs−1) as previously described (Zera et al., 2015). All surgical procedure were performed using general anaesthesia with ketamine 100 mg/kg body weight intraperitoneally and xylazine 10 mg/kg body weight.
Blood pressure measurement
Before the experiment, blood pressure was recorded in rats anaesthetized with urethane (1.5 g/kg intraperitoneally, Sigma-Aldrich, Poland) via a polyurethane catheter inserted into the femoral artery. Haemodynamics were recorded using Biopac MP 160 system (Biopac Systems, Goleta, CA, United States). Blood pressure was assessed as a baseline prior to the intravenous infusion of TMA.
Gene and protein expression
12-week-old WKY, SHR and WKY-Ang II rats were killed, tissues samples were collected and frozen immediately. Real-time PCR was used to detect FMO1, FMO3 and FMO5 gene expression in the kidney medulla, kidney cortex, liver, lungs, small intestine and colon.
Real-time PCR
In short, about 20 mg of every tissue was homogenized on BeadBug™ microtube homogenizer (Benchmark Scientific, Inc.). Total RNA was isolated from samples according to TRI Reagent® protocol. cDNA was transcribed from RNA samples according to iScript™ Reverse Transcription Supermix protocol (Bio-Rad). The qPCR mixes were prepared according to the Bio-Rad SsoAdvanced™ universal SYBR® Green Supermix protocol. Amplifications were performed in a Bio-Rad CFX Connect Real-Time System under standardized conditions using commercial assays.
We used semi-quantitative analysis of PCR products to carry out with glyceraldehyde 3-phosphate dehydrogenase (PrimePCR™ SYBR® Green Assay: Gapdh, Rat, qRnoCID0057018, Bio-Rad), actin (PrimePCR™ SYBR, qRnoCID0056984, Bio-Rad), succinate dehydrogenase (PrimePCR™ SYBR, qRnoCID0057011, Bio-Rad) as internal references.
Genes investigated in this study were flavin containing monooxygenase 1 (PrimePCR™ SYBR, qRnoCID0008990, Bio-Rad), flavin containing monooxygenase 3 (PrimePCR™ SYBR qRnoCID0003196, Bio-Rad) and flavin containing monooxygenase 5 (PrimePCR™ SYBR, qRnoCID0053250, Bio-Rad).
Western blot
For the analysis of target proteins, total protein extracts were prepared from the, liver,. In short, frozen samples were suspended in a histidine-sucrose buffer (30 mM histidine, 250 mM sucrose, 2 mM EDTA, proteases inhibitors, PMSF, pH 7.4), homogenized, centrifuged (10,000 RCF, 10 min, 4°C). After removing the supernatant, 150 µL of lysis buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EDTA, 2% Triton-X, proteases inhibitors) was added to the pellet and resuspended by vortexing. The supernatant was separated for protein concentration analysis using a Bradford Protein Assay (Bio-Rad, Hercules, CA, United States). For all Western blot analyses, a 4× Laemmli sample buffer was added to samples. To determinate the levels of FMO1, FMO3 and FMO5 all samples were resolved by electrophoresis on 12% SDS/PAGE gels. Resolved proteins were transferred onto PVDF membranes (Bio-Rad, Hercules, CA, United States), blocked using skim milk and incubated with primary and secondary antibodies. For quantitative analysis of protein content, reactive bands were quantified relative to those of actin using a ChemiDoc MP Imaging System, Densitometric analysis was performed using Quantity One software version 4.6.8 (Bio-Rad, Hercules, CA, United States). Uncropped blots and list of antibodies are presented in Supplementary Figure S5 and Supplementary Table S1.
Pharmacokinetics of TMA, TMA/TMAO oxidation
Twelve-week-old WKY, SHR, WKY-ANG were anaesthetized with urethane (1.5 g/kg intraperitoneally, Sigma-Aldrich, Poland) and catheterized with polyurethane catheters in femoral artery and both femoral veins.
Blood samples from femoral vein, were collected at baseline, 10 min and 20 min after the intravenous infusion of TMA at a dose of 45 μmol/kg, 135 μmol/kg or 405 μmol/kg.
Plasma concentrations of TMA and TMAO was evaluated using Waters Acquity Ultra Performance Liquid Chromatograph coupled with Waters TQ-S triple-quadrupole mass spectrometer. Samples were prepared using the derivatization technique based on Johnson’s protocol with modification (Johnson, 2008). The mass spectrometer was operated in multiple-reaction monitoring (MRM)- positive electrospray ionization (ESI+) mode for all analytes. The concentrations of analytes were calculated using calibration standard mix derived from a series of calibrator samples by spiking standard stock solutions into water. Plasma samples were compared with an obtained calibration curve.
Statistics
The Kolmogorov-Smirnov test was used to test normality of the distribution.
To evaluate changes in pharmacokinetic data in response to treatment, baseline values were compared with post-treatment values using one-way analysis of variance (ANOVA) for repeated measures. This was followed by Tukey’s post hoc test for multiple comparisons to identify differences between baseline and post-dose time points. Differences between groups/series were assessed using multivariate ANOVA, followed by Tukey’s post hoc test or by a t-test, as appropriate. A two-sided p-value of less than 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism version 8.4.3 (GraphPad Software Inc., San Diego, CA, USA). Sample size calculation for Fmo’s analysis was conducted using G*Power software version 3.1.9.7, estimating a minimum required number of animals per group to be 6. Measurements was determined based on the following assumed parameters: difference between subjects (groups) 40% population mean 10 arbitrary unit (a.u) common standard deviation 0.9, for alpha error 0.05, test power 0.8. The post hoc power analysis was performed for significant differences by utilizing the online calculator: https://clincalc.com/stats/Power.aspx (Supplementary Table S2). The analysis of false discovery rate (FDR) for FMO3 mRNA and protein expression was conducted (Supplementary Tables S3, S4).
Results
Blood pressure at baseline
Anaesthetized SHR (n = 17) rats and WKY-ANG (n = 14), had significantly higher mean arterial blood pressure than WKY (n = 17) 118.4 ± 1.3; 110.2 ± 0.9; 76.5 ± 1.2, respectively. SHR and WKY-ANG rats showed higher heart rate than WKY 331 ± 4, 322 ± 5 and 308 ± 4. respectively. Post-hoc test revealed significant differences only between SHR vs. WKY rats (p < 001).
FMO’s mRNA expression in tissues
We have characterized gene and protein expressions of FMO1, FMO3 and FMO5 subfamilies, in kidney medulla, kidney cortex, liver, lungs, small intestine and colon in WKY (n = 7), SHR (n = 6), and WKY-ANG (n = 6) groups (Figure 1).
FIGURE 1
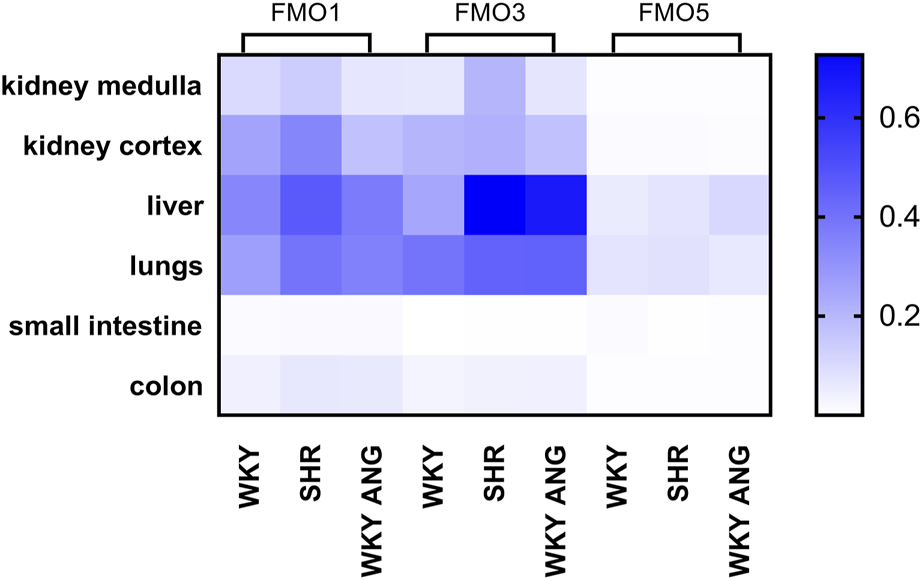
Heatmap of FMOs genes expression in WKY, SHR and WKY-ANG groups. Pattern expression peaks were found across tissues and FMOs. WKY - Wistar-Kyoto; SHR - Spontaneously Hypertensive Rats; WKY-ANG - Wistar-Kyoto with angiotensin II.
In general, all the groups, independently on tissue type, showed the gene expression of FMOs subfamilies in the following order of magnitude FMO3>FMO1>>FMO5 (Figure 1). With regard to tissue distribution of FMOs gene expression, high expression of FMOs was found in the liver, lungs and kidneys, whereas low FMOs expression was present in small intestine and colon. In relation to the liver’s most abundant mRNA FMO’s expression, we have conducted comprehensive investigations aimed at identifying the FMOs in this organ at the protein level.
Hepatic mRNA and protein expression of FMOs
In the liver, there was notably elevated mRNA expression of FMO3 in SHR compared to WKY (p < 0.01), while FMO1 and FMO5 exhibited no significant differences between the two strains. Interestingly, the WKY-ANG group showed significantly higher expression levels of FMO3(p < 0.01) and FMO5 (p < 0.05) than WKY strain (Figure 2 A).
FIGURE 2
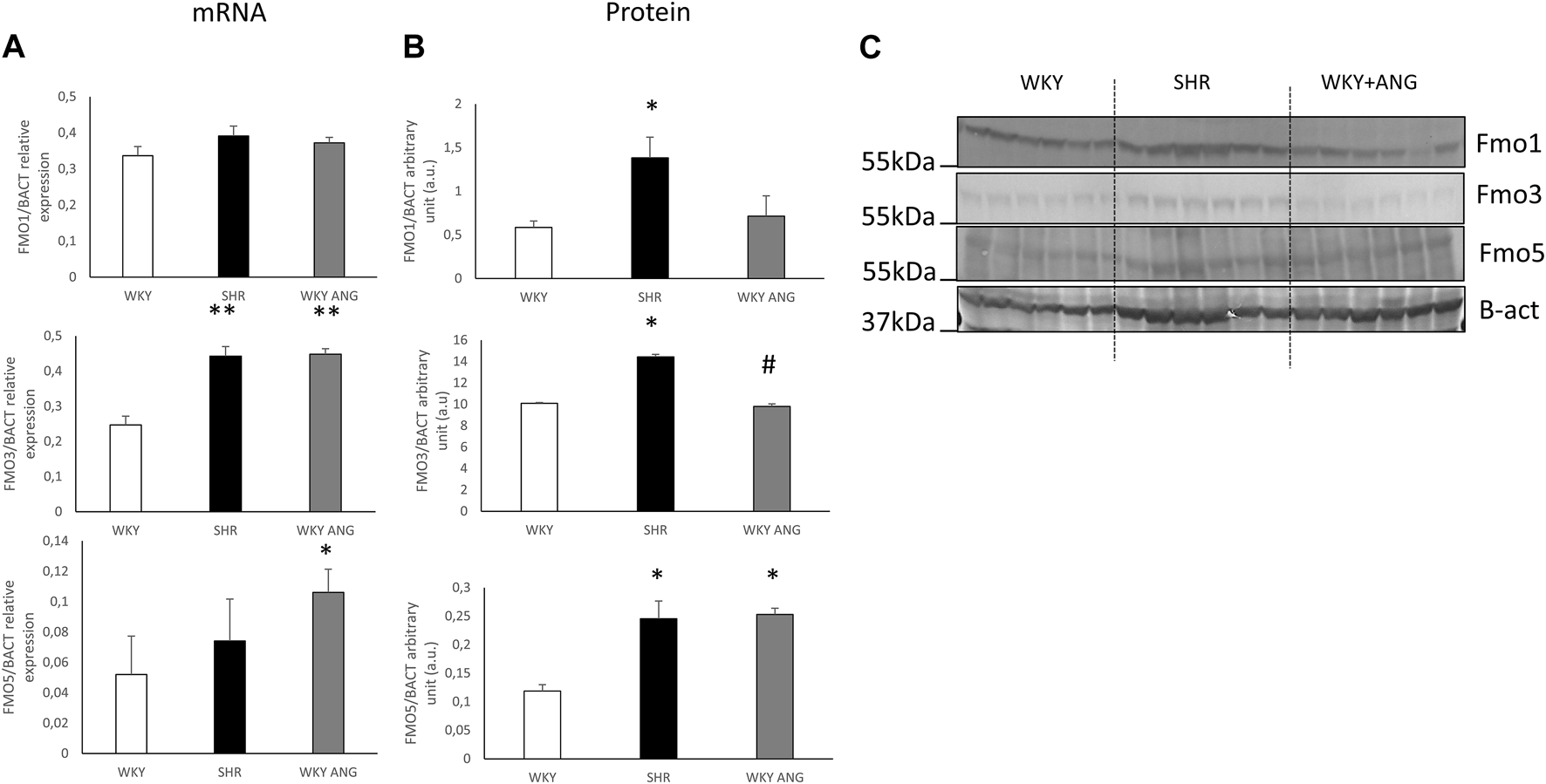
(A) RT-qPCR analysis of FMO1 FMO3 and FMO5 transcript levels in the liver of WKY, SHR and WKY-ANG rats (displays on histogram use arbitrary units). (B) FMO1, FMO3 and FMO5 protein levels in the liver examined by Western blot analysis. Beta-actin and the Ponceau-S staining were used as a reference for equal protein loading control. Quantification of the band intensity of protein expression was performed using Quantity One software The relative levels of the test proteins are plotted in arbitrary unit (means ± SD). (C) Representative blots of hepatic FMO’s protein of WKY, SHR and WKY-ANG rats. *p < 0.05 vs. WKY, **p < 0.01 vs. WKY #p < 0.05 SHR vs. WKY-ANG.
Moving to the protein level, SHR (n = 6) rats displayed significantly higher expression of all the mentioned FMOs (FMO1, FMO3, and FMO5) (p < 0.05) compared to WKY (n = 6). On the other hand, the WKY-ANG (n = 6) group demonstrated significantly higher expression of only FMO5 (p < 0.05) when compared to WKY (Figures 2B,C).
All statistical comparisons were made against WKY which was a control group in all gene and protein-based experiments.
Pharmacokinetics of TMA/TMAO oxidation
At baseline, SHR (n = 6) showed significantly higher TMAO plasma concentration than WKY (n = 6) and WKY-ANG (n = 6) 10.52 ± 0.97, 3.31 ± 0.57 and 6.11 ± 0.55 μmol/L, respectively. TMA plasma level was not significantly higher in SHR than in WKY and WKY-ANG 0.15 μmol/L ± 0.01, 0.14 μmol/L ± 0.02 and 0.09 ± 0.02 μmol/L, respectively) (Figures 3A,B).
FIGURE 3
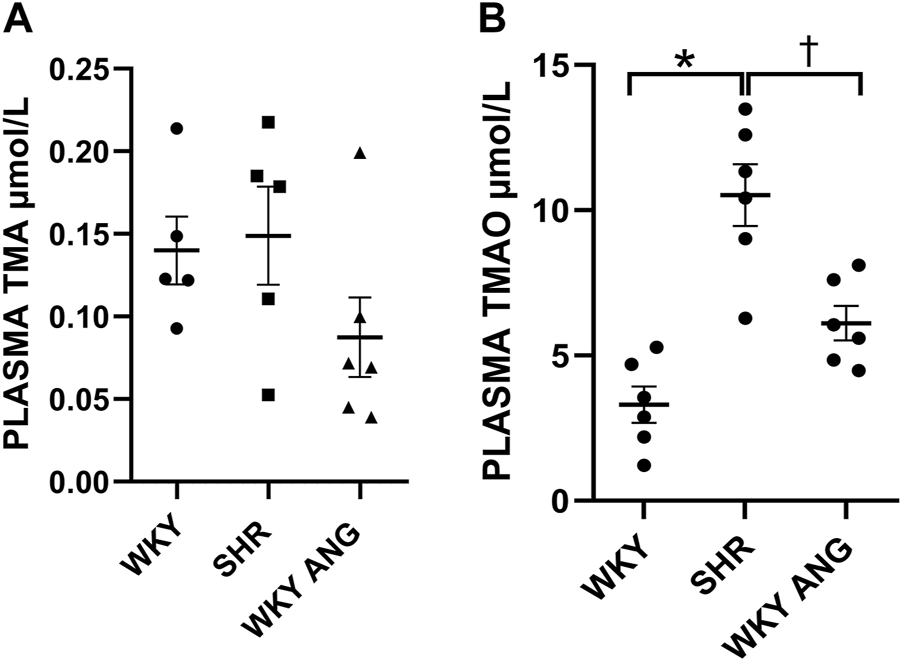
Plasma TMA (A) and TMAO (B) levels at baseline in WKY, SHR and WKY-ANG rats; *p < 0.05 SHR vs WKY, †p < 0.05 SHR vs WKY-ANG.
Infusion of TMA produced a significant, dose-dependent increase in plasma TMA and TMAO in all the groups. The increase in plasma TMAO was more rapid in SHR than in the other groups (Supplementary Figures S1-S4).
SHR group showed significantly higher plasma TMAO/TMA ratio than WKY and WKY-ANG 10 min after the infusion of TMA at a dose of 45 μmol/kg, whereas 20 min after the infusion, SHR showed significantly higher plasma TMAO/TMA ratio than WKY and WKY-ANG, for all TMA doses, i.e. 45, 135 and 405 μmol/kg (Figures 4A–C).
FIGURE 4
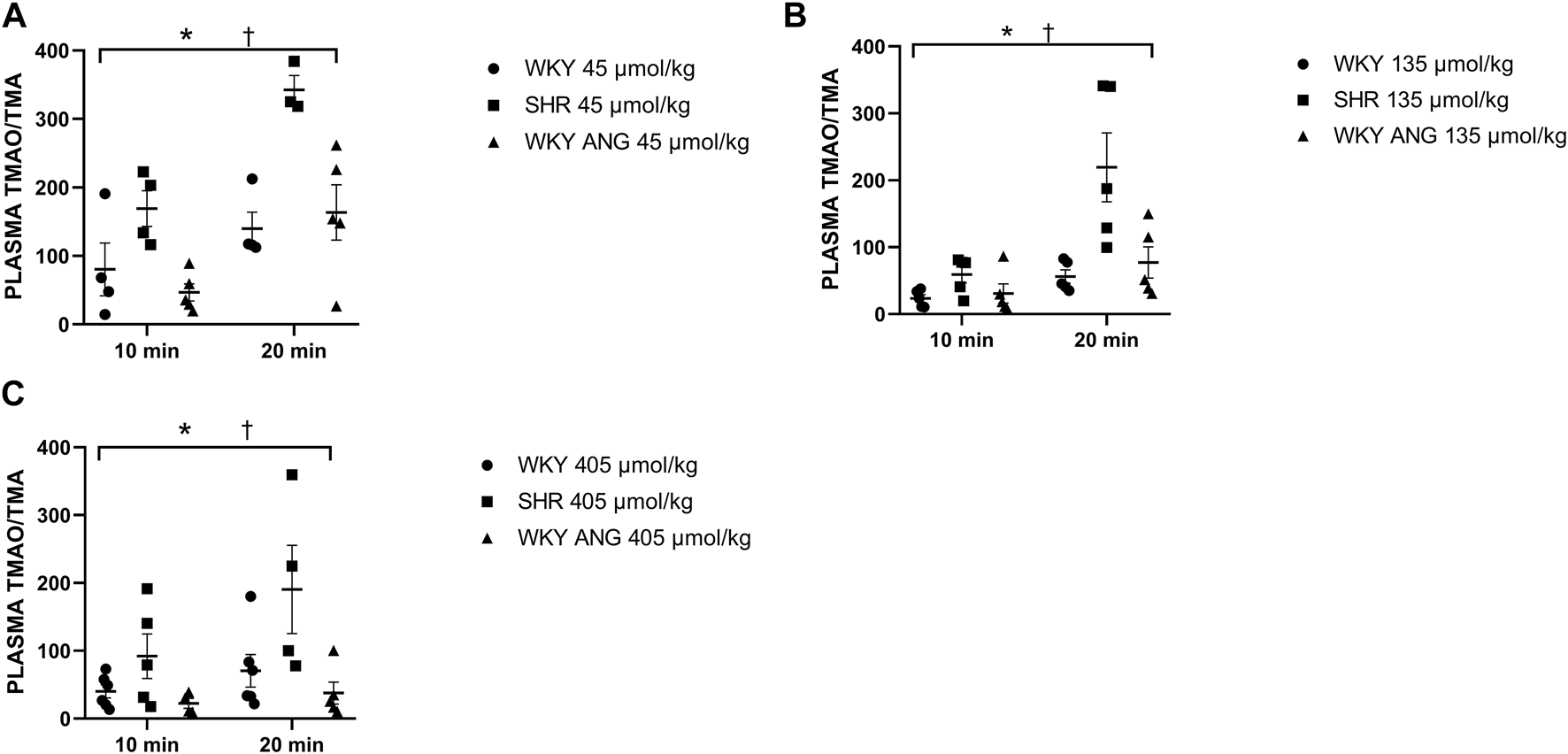
Plasma TMAO/TMA ratio in WKY, SHR and WKY-ANG rats after intravenous administration of TMA at a dose of 45 (A), 135 (B) and 405 (C) μmol/kg. Two way ANOVA *p < 0.05 SHR vs WKY, †p < 0.05 SHR vs WKY-ANG.
Discussion
The novel finding of our study is that SHRs show higher hepatic gene expression and protein levels of FMOs and more rapid oxidation of TMA to TMAO.
In the present study we evaluated two animal models of hypertension: SHRs and WKY-ANG. The SHR strain, derived from WKY rats, is the most commonly used animal model for essential hypertension in humans (Louis and Howes, 1990). SHRs begin to develop hypertension between the fourth and sixth weeks of age, and by the 10th week of life, their arterial blood pressure is 30% higher than that of WKY rats (Kokubo et al., 2005; Koga et al., 2008). Blood pressure measurements in anesthetized rats in this study revealed higher mean arterial blood pressure in both SHR and WKY-ANG rats, confirming their hypertensive phenotype.
Oxidation performed by FMOs is considered as one of important detoxifying mechanism (Sehlmeyer et al., 2010; Basaran and Can Eke, 2017). FMOs oxidize TMA as well as other amines including those exerting cardiovascular effect, for example, tyramine, phenethylamine, cys-teamine (Vrba et al., 1988), methionine and several cysteine-s-conjugates (Bull et al., 1964). Gut-bacteria derived TMA is oxidized to TMAO mostly by the FMO3 in the liver (Lang et al., 1998).
Here, we found that WKY, SHR and WKY-ANG show expression of the three subfamilies of FMO in the following order of magnitude FMO3>FMO1>>FMO5. Furthermore, we found that FMOs are expressed in the following tissues: liver, kidney, lungs, colon and intestines, with the greatest expression of FMOs was found in the liver.
In general, the most significant differences in gene and protein expression of FMOs and the pharmacokinetics of TMA were observed between the WKY and SHR, with WKY-ANG rats displaying characteristics that were a blend of both WKY and SHR strains. Specifically, compared to WKY, SHR exhibited significantly higher liver protein expression across all subfamilies of FMOs, whereas WKY-ANG rats showed an increase only in FMO5.
Importantly, the elevated expression of FMOs in SHR was linked to a more efficient and rapid oxidation of TMA to TMAO following the intravenous infusion of the amine. This was evidenced by SHRs demonstrating a significantly higher TMAO/TMA ratio after the administration of TMA in increasing doses. Lastly, SHRs also exhibited significantly higher baseline levels of TMAO, corroborating the findings of previous research (Huc et al., 2018). This study, suggests that greater oxidation of TMA to TMAO in SHRs may contribute to higher plasma TMAO levels in hypertensive rats, in addition to previously described factors such as increased gut-blood-barrier permeability to bacterial metabolites including TMA in hypertensive intestines (Jaworska et al., 2017; Drapala et al., 2020).
Some research suggest that alterations in FMOs expression are associated with several diseases including trimethylaminuria (TMAU) (Montoya Alvarez et al., 2009), diabetes mellitus (Rouer et al., 1988; Siddens et al., 2014), familial adenomatous polyposis (Cruz-Correa and Giardiello, 2003), breast (Krueger et al., 2006), prostate (Mondul et al., 2015) and colorectal cancer (Xie et al., 2012), peptic ulcer and gastro-oesophageal reflux (Chung et al., 2000) and hemochromatosis (Muckenthaler et al., 2003). Furthermore, some evidence suggests that patient with trimethylaminuria show higher blood pressure and exaggerated response to pressor amines like tyramine and phenethylamine (Forrest et al., 2001; Cashman et al., 2003), however, data are not consistent (Dolan et al., 2005; D'Angelo et al., 2013). There is also limited data on FMO3 polymorphisms and its effect on hypertension, but studies provide conflicting results (Akerman et al., 1999; Cashman et al., 2000; Cashman et al., 2003; Dolan et al., 2005; D'Angelo et al., 2013). Finally, some links between blood pressure and inactivation of biogenic amines by FMO3 (Cashman et al., 1997; Lin and Cashman, 1997; Treacy et al., 1998; Cashman et al., 2000) exist.
In the scientific literature, various models of hypertension are well-documented. For our research, we chose two models that are widely recognized and extensively used to represent human hypertension. This selection was influenced by the unique and differing etiologies of hypertension presented by these models, as well as their widespread acceptance as representative models for studying human hypertension (Jama et al., 2022). The presence of numerous underlying mechanisms driving hypertension underscores the critical need for future research to use alternative models for more comprehensive exploration.
The limitation of this study arises from its exclusive use of male rats, a decision aimed at minimizing biological variability due to hormonal fluctuations, which are known to significantly impact small experimental study outcomes. For future research, it is crucial to consider the inclusion of both sexes to ensure a more comprehensive understanding of TMA metabolism and FMOs activity in hypertensive rats. Additionally, measuring FMO expression in the heart, brain, and blood vessels would be beneficial, considering their potential impact on blood pressure and blood flow regulation within these tissues.
In conclusion, this study offers a comprehensive demonstration of the relationship between hepatic FMO expression and the oxidation of TMA to TMAO in the two animal models of hypertension. Our results indicate that hypertension in SHRs is linked to an increased expression and activity of liver FMOs. Further experimental research is necessary to clarify the role of FMOs in the pathogenesis of cardiovascular diseases. The findings from this study lay the groundwork for subsequent investigations into FMOs as a potential therapeutic target for hypertension treatment.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by II Local Ethical Committee in Warsaw (Certificate of approval No. WAW2/082/2018). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MG-K: Data curation, Formal Analysis, Writing–original draft. MS: Data curation, Formal Analysis, Investigation, Validation, Writing–review and editing. ES: Formal Analysis, Writing–review and editing. MK: Investigation, Writing–review and editing. DC: Writing–review and editing. MO: Formal Analysis, Writing–review and editing. MU: Conceptualization, Funding acquisition, Methodology, Supervision, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by the National Science Centre, Poland, grant no. 2020/37/B/NZ5/00366.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1340166/full#supplementary-material
References
1
AkermanB. R.ForrestS.ChowL.YouilR.KnightM.TreacyE. P. (1999a). Two novel mutations of the FMO3 gene in a proband with trimethylaminuria. Hum. Mutat.13, 376–379. 10.1002/(SICI1098-1004(1999)13:5<376::AID-HUMU5>3.0.CO;2-A
2
AkermanB. R.LemassH.ChowL. M.LambertD. M.GreenbergC.BibeauC.et al (1999b). Trimethylaminuria is caused by mutations of the FMO3 gene in a North American cohort. Mol. Genet. Metab.68, 24–31. 10.1006/mgme.1999.2885
3
Aldana-HernandezP.LeonardK. A.ZhaoY. Y.CurtisJ. M.FieldC. J.JacobsR. L. (2020). Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in ldlr-/- and apoe-/- male mice. J. Nutr.150 (2), 249–255. 10.1093/jn/nxz214
4
Al-WaizM.AyeshR.MitchellS. C.IdleJ. R.SmithR. L. (1987). Disclosure of the metabolic retroversion of trimethylamine N-oxide in humans: a pharmacogenetic approach. Clin. Pharmacol. Ther.42, 608–612. 10.1038/clpt.1987.207
5
BarberM.ConradM. E.UmbreitJ. N.BartonJ. C.MooreE. G. (2000). Abnormalities of flavin monooxygenase as an etiology for sideroblastic anemia. Am. J. Hematol.65 (2), 149–153. 10.1002/1096-8652(200010)65:2<149::aid-ajh10>3.0.co;2-b
6
BasaranR.Can EkeB. (2017). Flavin containing monooxygenases and metabolism of xenobiotics. Turk J. Pharm. Sci.14 (1), 90–94. 10.4274/tjps.30592
7
BhagwatS. V.BhamreS.BoydM. R.RavindranathV. (1996). Further characterization of rat brain flavin-containing monooxygenase. Metabolism of imipramine to its N-oxide. Biochem. Pharmacol.51 (11), 1469–1475. 10.1016/0006-2952(96)00088-3
8
BhamreS.BhagwatS. V.ShankarS. K.WilliamsD. E.RavindranathV. (1993). Cerebral flavin-containing monooxygenase-mediated metabolism of antidepressants in brain: immunochemical properties and immunocytochemical localization. J. Pharmacol. Exp. Ther.267 (1), 555–559.
9
BullC.ValverdeJ. M.BerletH. H.SpaideJ. K.TourlentesT. T.HimwichH. E. (1964). Hypertension with methionine in schizophrenic patients receiving tranylcypromine. Am. J. Psychiatry121, 381–382. 10.1176/ajp.121.4.381
10
CashmanJ. R. (1995). Structural and catalytic properties of the mammalian flavin-containing monooxygenase. Chem. Res. Toxicol.8 (2), 166–181. 10.1021/tx00044a001
11
CashmanJ. R.AkermanB. R.ForrestS. M.TreacyE. P. (2000). Population-specific polymorphisms of the human FMO3 gene: significance for detoxication. Drug Metab. Dispos.28 (2), 169–173.
12
CashmanJ. R.BiY. A.LinJ.YouilR.KnightM.ForrestS.et al (1997). Human flavin-containing monooxygenase form 3: cDNA expression of the enzymes containing amino acid substitutions observed in individuals with trimethylaminuria. Chem. Res. Toxicol.10 (8), 837–841. 10.1021/tx9700533
13
CashmanJ. R.CampK.FakharzadehS. S.FennesseyP. V.HinesR. N.MamerO. A.et al (2003). Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr. Drug Metab.4 (2), 151–170. 10.2174/1389200033489505
14
CashmanJ. R.LattardV.LinJ. (2004). Effect of total parenteral nutrition and choline on hepatic flavin-containing and cytochrome P-450 monooxygenase activity in rats. Drug Metab. Dispos.32 (2), 222–229. 10.1124/dmd.32.2.222
15
CashmanJ. R.ZhangJ. (2006). Human flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol.46, 65–100. 10.1146/annurev.pharmtox.46.120604.141043
16
ChungW. G.ParkC. S.RohH. K.LeeW. K.ChaY. N. (2000). Oxidation of ranitidine by isozymes of flavin-containing monooxygenase and cytochrome P450. Jpn. J. Pharmacol.84 (2), 213–220. 10.1254/jjp.84.213
17
CollinsH. L.Drazul-SchraderD.SulpizioA. C.KosterP. D.WilliamsonY.AdelmanS. J.et al (2016). L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis244, 29–37. 10.1016/j.atherosclerosis.2015.10.108
18
CraciunS.MarksJ. A.BalskusE. P. (2014). Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem. Biol.9 (7), 1408–1413. 10.1021/cb500113p
19
Cruz-CorreaM.GiardielloF. M. (2003). Familial adenomatous polyposis. Gastrointest. Endosc.58 (6), 885–894. 10.1016/s0016-5107(03)02336-8
20
D'AngeloR.EspositoT.CalabroM.RinaldiC.RobledoR.VarrialeB.et al (2013). FMO3 allelic variants in Sicilian and Sardinian populations: trimethylaminuria and absence of fish-like body odor. Gene515 (2), 410–415. 10.1016/j.gene.2012.12.047
21
DolanC.ShieldsD. C.StantonA.O'BrienE.LambertD. M.O'BrienJ. K.et al (2005). Polymorphisms of the Flavin containing monooxygenase 3 (FMO3) gene do not predispose to essential hypertension in Caucasians. BMC Med. Genet.6, 41. 10.1186/1471-2350-6-41
22
DolphinC. T.CullingfordT. E.ShephardE. A.SmithR. L.PhillipsI. R. (1996). Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FM04. Eur. J. Biochem.235 (3), 683–689. 10.1111/j.1432-1033.1996.00683.x
23
DolphinC. T.JanmohamedA.SmithR. L.ShephardE. A.PhillipsI. R. (1997). Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat. Genet.17 (4), 491–494. 10.1038/ng1297-491
24
DrapalaA.SzudzikM.ChabowskiD.MogilnickaI.JaworskaK.KraszewskaK.et al (2020). Heart failure disturbs gut-blood barrier and increases plasma trimethylamine, a toxic bacterial metabolite. Int. J. Mol. Sci.21 (17), 6161. 10.3390/ijms21176161
25
EswaramoorthyS.BonannoJ. B.BurleyS. K.SwaminathanS. (2006). Mechanism of action of a flavin-containing monooxygenase. Proc. Natl. Acad. Sci. U. S. A.103 (26), 9832–9837. 10.1073/pnas.0602398103
26
FialkaF.GruberR. M.HittR.OpitzL.BrunnerE.SchliephakeH.et al (2008). CPA6, FMO2, LGI1, SIAT1 and TNC are differentially expressed in early- and late-stage oral squamous cell carcinoma--a pilot study. Oral Oncol.44 (10), 941–948. 10.1016/j.oraloncology.2007.10.011
27
ForrestS. M.KnightM.AkermanB. R.CashmanJ. R.TreacyE. P. (2001). A novel deletion in the flavin-containing monooxygenase gene (FMO3) in a Greek patient with trimethylaminuria. Pharmacogenetics11 (2), 169–174. 10.1097/00008571-200103000-00007
28
Gawrys-KopczynskaM.KonopM.MaksymiukK.KraszewskaK.DerzsiL.SozanskiK.et al (2020). TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife9, e57028. 10.7554/eLife.57028
29
GutI.ConneyA. H. (1993). Trimethylamine N-oxygenation and N-demethylation in rat liver microsomes. Biochem. Pharmacol.46 (2), 239–244. 10.1016/0006-2952(93)90409-p
30
HinesR. N.CashmanJ. R.PhilpotR. M.WilliamsD. E.ZieglerD. M. (1994). The mammalian flavin-containing monooxygenases: molecular characterization and regulation of expression. Toxicol. Appl. Pharmacol.125 (1), 1–6. 10.1006/taap.1994.1042
31
HsuC. N.Chang-ChienG. P.LinS.HouC. Y.TainY. L. (2019). Targeting on gut microbial metabolite trimethylamine-N-oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res.63 (18), e1900073. 10.1002/mnfr.201900073
32
HucT.DrapalaA.GawrysM.KonopM.BielinskaK.ZaorskaE.et al (2018). Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol.315 (6), H1805–H1820. 10.1152/ajpheart.00536.2018
33
HvattumE.BergsethS.PedersenC. N.BremerJ.AarslandA.BergeR. K. (1991). Microsomal oxidation of dodecylthioacetic acid (a 3-thia fatty acid) in rat liver. Biochem. Pharmacol.41 (6-7), 945–953. 10.1016/0006-2952(91)90200-o
34
JamaH. A.MuralitharanR. R.XuC.O'DonnellJ. A.BertagnolliM.BroughtonB. R. S.et al (2022). Rodent models of hypertension. Br. J. Pharmacol.179 (5), 918–937. 10.1111/bph.15650
35
JaworskaK.BielinskaK.Gawrys-KopczynskaM.UfnalM. (2019). TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc Res.115 (14), 1948–1949. 10.1093/cvr/cvz231
36
JaworskaK.HucT.SamborowskaE.DobrowolskiL.BielinskaK.GawlakM.et al (2017). Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS One12 (12), e0189310. 10.1371/journal.pone.0189310
37
JohnsonD. W. (2008). A flow injection electrospray ionization tandem mass spectrometric method for the simultaneous measurement of trimethylamine and trimethylamine N-oxide in urine. J. Mass Spectrom.43 (4), 495–499. 10.1002/jms.1339
38
KarolyE. D.RoseR. L. (2001). Sequencing, expression, and characterization of cDNA expressed flavin-containing monooxygenase 2 from mouse. J. Biochem. Mol. Toxicol.15 (6), 300–308. 10.1002/jbt.10009
39
KawajiA.IsobeM.TakabatakeE. (1997). Differences in enzymatic properties of flavin-containing monooxygenase in brain microsomes of rat, mouse, hamster, Guinea pig and rabbit. Biol. Pharm. Bull.20 (8), 917–919. 10.1248/bpb.20.917
40
KawajiA.MikiT.TakabatakeE. (1995). Partial purification and substrate specificity of flavin-containing monooxygenase from rat brain microsomes. Biol. Pharm. Bull.18 (12), 1657–1659. 10.1248/bpb.18.1657
41
KimY. H.LimD. S.LeeJ. H.ShimW. J.RoY. M.ParkG. H.et al (2003). Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp. Mol. Med.35 (5), 336–349. 10.1038/emm.2003.45
42
KoethR. A.LevisonB. S.CulleyM. K.BuffaJ. A.WangZ.GregoryJ. C.et al (2014). γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab.20 (5), 799–812. 10.1016/j.cmet.2014.10.006
43
KoethR. A.WangZ.LevisonB. S.BuffaJ. A.OrgE.SheehyB. T.et al (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med.19 (5), 576–585. 10.1038/nm.3145
44
KogaY.HirookaY.ArakiS.NozoeM.KishiT.SunagawaK. (2008). High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens. Res.31 (11), 2075–2083. 10.1291/hypres.31.2075
45
KokuboM.UemuraA.MatsubaraT.MuroharaT. (2005). Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens. Res.28 (7), 601–609. 10.1291/hypres.28.601
46
KruegerS. K.VandykeJ. E.WilliamsD. E.HinesR. N. (2006). The role of flavin-containing monooxygenase (FMO) in the metabolism of tamoxifen and other tertiary amines. Drug Metab. Rev.38 (1-2), 139–147. 10.1080/03602530600569919
47
KruegerS. K.WilliamsD. E. (2005). Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther.106 (3), 357–387. 10.1016/j.pharmthera.2005.01.001
48
LangD. H.YeungC. K.PeterR. M.IbarraC.GasserR.ItagakiK.et al (1998). Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem. Pharmacol.56 (8), 1005–1012. 10.1016/s0006-2952(98)00218-4
49
LattardV.LachuerJ.BuronfosseT.GarnierF.BenoitE. (2002a). Physiological factors affecting the expression of FMO1 and FMO3 in the rat liver and kidney. Biochem. Pharmacol.63 (8), 1453–1464. 10.1016/s0006-2952(02)00886-9
50
LattardV.Longin-SauvageonC.BenoitE. (2003). Cloning, sequencing and tissue distribution of rat flavin-containing monooxygenase 4: two different forms are produced by tissue-specific alternative splicing. Mol. Pharmacol.63 (1), 253–261. 10.1124/mol.63.1.253
51
LattardV.Longin-SauvageonC.KruegerS. K.WilliamsD. E.BenoitE. (2002b). The FMO2 gene of laboratory rats, as in most humans, encodes a truncated protein. Biochem. Biophys. Res. Commun.292 (2), 558–563. 10.1006/bbrc.2002.6656
52
LawtonM. P.CashmanJ. R.CresteilT.DolphinC. T.ElfarraA. A.HinesR. N.et al (1994). A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch. Biochem. Biophys.308 (1), 254–257. 10.1006/abbi.1994.1035
53
LawtonM. P.KronbachT.JohnsonE. F.PhilpotR. M. (1991). Properties of expressed and native flavin-containing monooxygenases: evidence of multiple forms in rabbit liver and lung. Mol. Pharmacol.40 (5), 692–698.
54
LinJ.CashmanJ. R. (1997a). Detoxication of tyramine by the flavin-containing monooxygenase: stereoselective formation of the trans oxime. Chem. Res. Toxicol.10 (8), 842–852. 10.1021/tx970030o
55
LinJ.CashmanJ. R. (1997b). N-oxygenation of phenethylamine to the trans-oxime by adult human liver flavin-containing monooxygenase and retroreduction of phenethylamine hydroxylamine by human liver microsomes. J. Pharmacol. Exp. Ther.282 (3), 1269–1279.
56
LouisW. J.HowesL. G. (1990). Genealogy of the spontaneously hypertensive rat and Wistar-Kyoto rat strains: implications for studies of inherited hypertension. J. Cardiovasc Pharmacol.16 (7), S1–S5. 10.1097/00005344-199000167-00002
57
MaksymiukK. M.SzudzikM.Gawrys-KopczynskaM.OnyszkiewiczM.SamborowskaE.MogilnickaI.et al (2022). Trimethylamine, a gut bacteria metabolite and air pollutant, increases blood pressure and markers of kidney damage including proteinuria and KIM-1 in rats. J. Transl. Med.20 (1), 470. 10.1186/s12967-022-03687-y
58
MeyerK. A.BentonT. Z.BennettB. J.JacobsD. R.Jr.Lloyd-JonesD. M.GrossM. D.et al (2016). Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). J. Am. Heart Assoc.5 (10), e003970. 10.1161/JAHA.116.003970
59
MitchellS. C.ZhangA. Q.BarrettT.AyeshR.SmithR. L. (1997). Studies on the discontinuous N-oxidation of trimethylamine among Jordanian, Ecuadorian and New Guinean populations. Pharmacogenetics7 (1), 45–50. 10.1097/00008571-199702000-00006
60
MondulA. M.MooreS. C.WeinsteinS. J.KarolyE. D.SampsonJ. N.AlbanesD. (2015). Metabolomic analysis of prostate cancer risk in a prospective cohort: the alpha-tocolpherol, beta-carotene cancer prevention (ATBC) study. Int. J. Cancer137 (9), 2124–2132. 10.1002/ijc.29576
61
Montoya AlvarezT.GuardiolaP. D.RoldanJ. O.ElviroR.WeversR.GuijarroG. (2009). Primary trimethylaminuria: the fish odor syndrome. Endocrinol. Nutr.56 (6), 337–340. 10.1016/S1575-0922(09)71948-5
62
MotikaM. S.ZhangJ.CashmanJ. R. (2007). Flavin-containing monooxygenase 3 and human disease. Expert Opin. Drug Metab. Toxicol.3 (6), 831–845. 10.1517/17425255.3.6.831
63
MuckenthalerM.RoyC. N.CustodioA. O.MinanaB.deGraafJ.MontrossL. K.et al (2003). Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat. Genet.34 (1), 102–107. 10.1038/ng1152
64
MuralitharanR. R.JamaH. A.XieL.PehA.SnelsonM.MarquesF. Z. (2020). Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension76 (6), 1674–1687. 10.1161/HYPERTENSIONAHA.120.14473
65
NagataT.WilliamsD. E.ZieglerD. M. (1990). Substrate specificities of rabbit lung and porcine liver flavin-containing monooxygenases: differences due to substrate size. Chem. Res. Toxicol.3 (4), 372–376. 10.1021/tx00016a016
66
NovickR. M.MitzeyA. M.BrownfieldM. S.ElfarraA. A. (2009). Differential localization of flavin-containing monooxygenase (FMO) isoforms 1, 3, and 4 in rat liver and kidney and evidence for expression of FMO4 in mouse, rat, and human liver and kidney microsomes. J. Pharmacol. Exp. Ther.329 (3), 1148–1155. 10.1124/jpet.109.152058
67
PhillipsI. R.ShephardE. A. (2020). Flavin-containing monooxygenases: new structures from old proteins. Nat. Struct. Mol. Biol.27 (1), 3–4. 10.1038/s41594-019-0356-1
68
PospischilE. J. G.NielsenG. D.PapameletiouD.KleinC. L. (2017). SCOEL/REC/179 trimethylamine. Publ Sci Comm Occup Expo Lim Eur Union.
69
QiJ.YouT.LiJ.PanT.XiangL.HanY.et al (2018). Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med.22 (1), 185–194. 10.1111/jcmm.13307
70
RouerE.LemoineA.CresteilT.RouetP.LerouxJ. P. (1987). Effects of genetic or chemically induced diabetes on imipramine metabolism. Respective involvement of flavin monooxygenase and cytochrome P-450-dependent monooxygenases. Drug Metab. Dispos.15 (4), 524–528.
71
RouerE.RouetP.DelpechM.LerouxJ. P. (1988). Purification and comparison of liver microsomal flavin-containing monooxygenase from normal and streptozotocin-diabetic rats. Biochem. Pharmacol.37 (18), 3455–3459. 10.1016/0006-2952(88)90696-x
72
SehlmeyerS.WangL.LangelD.HeckelD. G.MohagheghiH.PetschenkaG.et al (2010). Flavin-dependent monooxygenases as a detoxification mechanism in insects: new insights from the arctiids (lepidoptera). PLoS One5 (5), e10435. 10.1371/journal.pone.0010435
73
Shehin-JohnsonS. E.WilliamsD. E.Larsen-SuS.StresserD. M.HinesR. N. (1995). Tissue-specific expression of flavin-containing monooxygenase (FMO) forms 1 and 2 in the rabbit. J. Pharmacol. Exp. Ther.272 (3), 1293–1299.
74
ShihD. M.WangZ.LeeR.MengY.CheN.CharugundlaS.et al (2015). Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res.56 (1), 22–37. 10.1194/jlr.M051680
75
ShimizuM.DentonT.KozonoM.CashmanJ. R.LeederJ. S.YamazakiH. (2011). Developmental variations in metabolic capacity of flavin-containing mono-oxygenase 3 in childhood. Br. J. Clin. Pharmacol.71 (4), 585–591. 10.1111/j.1365-2125.2010.03876.x
76
SiddensL. K.KruegerS. K.HendersonM. C.WilliamsD. E. (2014). Mammalian flavin-containing monooxygenase (FMO) as a source of hydrogen peroxide. Biochem. Pharmacol.89 (1), 141–147. 10.1016/j.bcp.2014.02.006
77
StubbsJ. R.StedmanM. R.LiuS.LongJ.FranchettiY.WestR. E.3rdet al (2019). Trimethylamine N-oxide and cardiovascular outcomes in patients with ESKD receiving maintenance hemodialysis. Clin. J. Am. Soc. Nephrol.14 (2), 261–267. 10.2215/CJN.06190518
78
SunJ.MauszM. A.ChenY.GiovannoniS. J. (2019). Microbial trimethylamine metabolism in marine environments. Environ. Microbiol.21 (2), 513–520. 10.1111/1462-2920.14461
79
TakamuraT.SakuraiM.OtaT.AndoH.HondaM.KanekoS. (2004). Genes for systemic vascular complications are differentially expressed in the livers of type 2 diabetic patients. Diabetologia47 (4), 638–647. 10.1007/s00125-004-1366-y
80
TangW. H.WangZ.FanY.LevisonB.HazenJ. E.DonahueL. M.et al (2014). Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol.64 (18), 1908–1914. 10.1016/j.jacc.2014.02.617
81
TangW. H.WangZ.KennedyD. J.WuY.BuffaJ. A.Agatisa-BoyleB.et al (2015). Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res.116 (3), 448–455. 10.1161/CIRCRESAHA.116.305360
82
TangW. H.WangZ.LevisonB. S.KoethR. A.BrittE. B.FuX.et al (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med.368 (17), 1575–1584. 10.1056/NEJMoa1109400
83
TodaA.EyanagiR.SaitoH.SoedaS.ShimenoH.MoriyamaM.et al (2005). Effects of experimental diabetes on hepatic drug metabolism in rats: the activities of flavin-containing monooxygenase, the phase II conjugation reactions and glutathione related enzymes. Res. Commun. Mol. Pathol. Pharmacol.117-118, 13–27.
84
TreacyE. P.AkermanB. R.ChowL. M.YouilR.BibeauC.LinJ.et al (1998). Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet.7 (5), 839–845. 10.1093/hmg/7.5.839
85
VrbaJ.LukicD.HaldarJ. (1988). Effects of cysteamine on blood pressure: possible mediation through vasopressin release. Proc. Soc. Exp. Biol. Med.188 (4), 485–488. 10.3181/00379727-188-42765
86
XieG.WongC. C.ChengK. W.HuangL.ConstantinidesP. P.RigasB. (2012). Regioselective oxidation of phospho-NSAIDs by human cytochrome P450 and flavin monooxygenase isoforms: implications for their pharmacokinetic properties and safety. Br. J. Pharmacol.167 (1), 222–232. 10.1111/j.1476-5381.2012.01982.x
87
YinJ.LiaoS. X.HeY.WangS.XiaG. H.LiuF. T.et al (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc.4 (11), e002699. 10.1161/JAHA.115.002699
88
ZeraT.UfnalM.Szczepanska-SadowskaE. (2015). TNF and angiotensin type 1 receptors interact in the brain control of blood pressure in heart failure. Cytokine71 (2), 272–277. 10.1016/j.cyto.2014.10.019
89
ZhangJ.CashmanJ. R. (2006). Quantitative analysis of FMO gene mRNA levels in human tissues. Drug Metab. Dispos.34 (1), 19–26. 10.1124/dmd.105.006171
90
ZhuY.JamesonE.CrosattiM.SchaferH.RajakumarK.BuggT. D.et al (2014). Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. U. S. A.111 (11), 4268–4273. 10.1073/pnas.1316569111
Summary
Keywords
bacterial metabolites, FMO, TMAO, TMA, cardiovascular disease
Citation
Gawryś-Kopczyńska M, Szudzik M, Samborowska E, Konop M, Chabowski D, Onyszkiewicz M and Ufnal M (2024) Spontaneously hypertensive rats exhibit increased liver flavin monooxygenase expression and elevated plasma TMAO levels compared to normotensive and Ang II-dependent hypertensive rats. Front. Physiol. 15:1340166. doi: 10.3389/fphys.2024.1340166
Received
17 November 2023
Accepted
28 February 2024
Published
12 April 2024
Volume
15 - 2024
Edited by
Leszek Dobrowolski, Mossakowski Medical Research Institute (PAS), Poland
Reviewed by
Karolina Skonieczna-Żydecka, Pomeranian Medical University, Poland
Michał Czapla, Wroclaw Medical University, Poland
Purnima Singh, University of Tennessee Health Science Center (UTHSC), United States
Updates
Copyright
© 2024 Gawryś-Kopczyńska, Szudzik, Samborowska, Konop, Chabowski, Onyszkiewicz and Ufnal.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mateusz Szudzik, mateusz.szudzik@wum.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.