Abstract
During pregnancy, marked changes in vasculature occur. The placenta is developed, and uteroplacental and fetoplacental circulations are established. These processes may be negatively affected by genetic anomalies, maternal environment (i.e., obesity or diabetes), and environmental conditions such as pollutants and hypoxia. Chronic hypoxia has detrimental effects on the vascular adaptations to pregnancy and fetal growth. The typical pregnancy-dependent rise in uterine blood flow by vascular remodeling and vasodilation of maternal uterine arteries is reduced, leading to increases in vascular tone. These maladaptations may lead to complications such as fetal growth restriction (FGR) and preeclampsia. In this review, the effect of hypoxia on uteroplacental and fetoplacental circulation and its impact on pregnancy outcomes in humans and animal models are discussed. Evidence is provided for several mechanisms that affect pregnancy through hypoxia-induced alterations. Future directions to fill gaps in knowledge and develop therapeutic strategies to prevent or alleviate hypoxia-related pregnancy complications, such as FGR and preeclampsia, are suggested.
1 Introduction
1.1 Uteroplacental and fetoplacental circulation during healthy pregnancy
Mammalian pregnancy causes profound and progressive adaptations in the maternal cardiovascular system intended to sustain the developing fetus. Maternal cardiac output is increased, arterial blood pressure is decreased, and peripheral vascular resistance is reduced, among other changes (Mahendru et al., 2014). Importantly, decreased vascular resistance leads to increased blood flow directed to the uterine circulation (Ford, 1982; Palmer et al., 1992; Konje et al., 2001), which is responsible for nutrient and gas exchange between the mother and the fetus through the placenta. Any interruption to these adaptations can result in suboptimal pregnancy outcomes or complications.
The circulation between the mother and the fetus can be divided into maternal, placental, and fetal compartments (Figure 1). In the human maternal vascular component, the main uterine arteries (UtAs) bifurcate from the bilateral internal iliac arteries. UtAs run along the serosal surface of the uterus and provide most of its blood supply, although there is also contribution from the ovarian arteries. Branching off the main UtAs are the arcuate arteries, which are parallel to, and embedded in the uterine smooth muscle layer, called the myometrium. Radial arteries (also known as myometrial arteries) branch off from the arcuate arteries towards the endometrium (or decidua in the pregnant state) – the inner layer of the uterus – where some end in a specialized coiled shape and are called spiral arteries (Degner et al., 2017; James et al., 2017). In non-pregnant individuals, the spiral arteries remain coiled and relatively constricted, as the demand for blood to the endometrium is small. However, during pregnancy, the spiral arteries near the embryo implantation site are invaded by placental trophoblast cells and remodeled to become wider and lower resistance to meet the blood flow demanded by the developing fetus (Pijnenborg et al., 2006; Zhang et al., 2023). Once uteroplacental circulation is established, the placental vascular component comes in contact with the maternal blood, which bathes the intervillous space of the placenta, where the syncytiotrophoblast cells allow the transport of gases and nutrients to and from the fetal circulation. In the fetal vascular component, the fetal internal iliac arteries connect to two umbilical arteries which run through the umbilical cord into the chorionic plate of the placenta. These arteries bifurcate into smaller branches (the chorionic plate arteries) and deeper into the chorionic villi to generate the fetal capillaries that will, in turn, become venules leaving the villous tree and converging into the chorionic plate veins and the umbilical vein (Chappell et al., 2023). The umbilical vein carries oxygenated blood and nutrients to the fetal circulation via the ductus venosus and small portal sinus (Basta and Lipsett, 2024; Remien and Majmundar, 2023) (Figure 1).
FIGURE 1
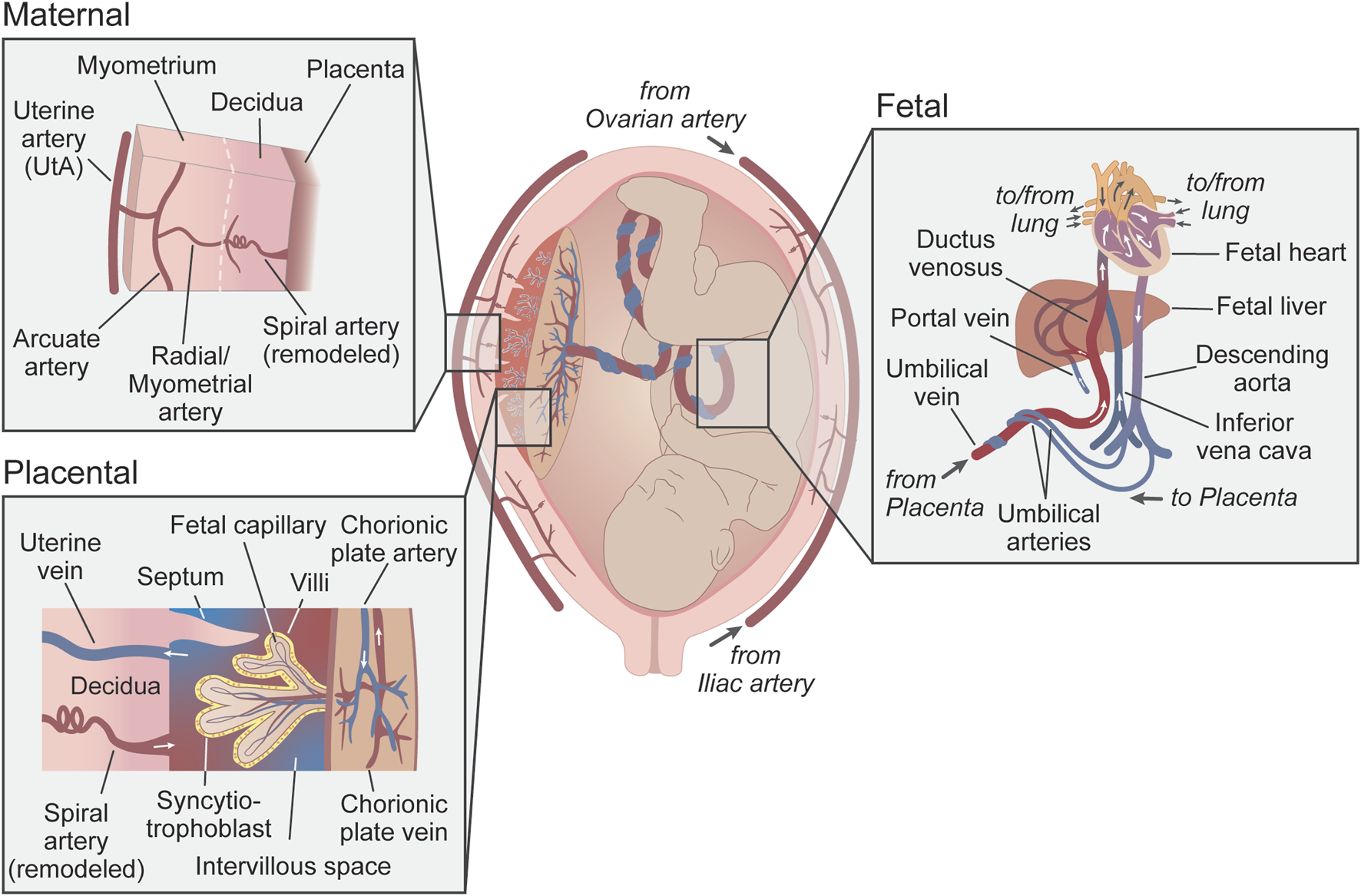
Uteroplacental and fetoplacental circulations. Schematic representation of the uteroplacental and fetoplacental blood vessels during pregnancy. Insets show a more detailed anatomical organization of the circulatory systems (maternal, placental, and fetal). Red vessels represent oxygenated blood, blue is non-oxygenated blood, and purple is mixed blood. Arrows show the direction of blood flow. Uterine veins and venules have been omitted from the maternal inset for simplification.
1.2 Maternal vascular changes during pregnancy
Early in human pregnancy, the embryo implants into the endometrium, which is then named decidua basalis, but uteroplacental circulation is not established until the end of the first trimester. Around this time, placental extravillous trophoblast cells migrate into the maternal spiral arteries, through somatic tissue and vessel lumen, and enlarge these blood vessels by replacing the endothelial cells (ECs), dedifferentiating smooth muscle cells (SMCs), and increasing vasodilation (Burton and Jauniaux, 2018; Ma et al., 2021).
The major changes to the maternal vasculature are both structural and functional. Structurally, the UtA undergoes remodeling, evidenced as an increase in diameter with species-dependent changes in wall thickness (Hilgers et al., 2003; Osol and Moore, 2014). There are several mechanisms for this remodeling, including cell hypertrophy, hyperplasia, and extracellular matrix remodeling (Osol and Moore, 2014). Assuming blood flow to be laminar, this increase in UtA diameter largely leads to increased blood flow as determined by Poiseuille’s law for laminar flow (Equation 1), in which the volumetric flow rate (Q) increases with the fourth power of the radius (r). Increased length (l) of UtA and uterine veins is inversely linearly related to Q, which is particularly important in multiparous animals. Both viscosity of the fluid (η) and pressure gradient (ΔP) are also linearly associated with Q.
Functionally, UtAs exhibit decreased vasoconstriction and increased vasodilatory responses, which may contribute to the pregnancy-dependent rise in blood flow, as vessel diameter increases and resistance decreases. Several endothelial and vascular smooth muscle factors contribute to this regulation of vasoreactivity. Increased production of nitric oxide (NO), via endothelial NO synthase (eNOS), is elicited by estrogen, shear stress, vascular endothelial growth factor, or other mechanisms in ECs (Bird et al., 2003; Luksha et al., 2010). Whereas, in SMCs, increased K+ channel-dependent hyperpolarization is an important contributor to the reduced UtA vascular tone and increased diameter observed during pregnancy (Bresnitz and Lorca, 2022).
1.3 Impairments in vascular adaptations during pregnancy
Appropriately timed pregnancy-dependent changes in vasculature are critical for healthy pregnancy outcomes. Thus, impaired vascular adaptations are associated with several pregnancy complications, such as fetal growth restriction (FGR) and preeclampsia, which exhibit a blunted rise in UtA blood flow (Konje et al., 2003; Julian et al., 2008; Browne et al., 2011). For instance, human myometrial arteries from FGR pregnancies have less vasodilatory response than appropriate for gestational age (AGA) controls (Ong et al., 2003; Lorca et al., 2020), which is consistent with UtAs in animal models of FGR (Aljunaidy et al., 2016). Moreover, pregnant eNOS knockout mice develop FGR and are associated with impaired UtA function, showing higher vasoconstriction and impaired vasodilation compared to wild-type mice (Kusinski et al., 2012). The same mouse model also showed increased UtA resistance associated with structural and cellular changes contributing to dysregulated uteroplacental blood flow during pregnancy (van der Heijden et al., 2005; Rennie et al., 2015). Similarly, preeclampsia reduces the vasodilatory responses in the UtAs and myometrial arteries (Cockell and Poston, 1997; Kublickiene et al., 2000; Luksha et al., 2010). Although the etiology of preeclampsia is likely multifactorial, a common contributor to this pregnancy complication is a shallow invasion of the maternal spiral arteries by the extravillous trophoblast. This prevents the proper remodeling of the spiral arteries and impairs the normal function of the placenta (Burton et al., 2009). Maternal endothelial progenitor cells may also contribute to the etiology of preeclampsia insofar as endothelial progenitor cells from preeclamptic pregnancies promote a transition of spiral artery SMCs into a synthetic phenotype that accumulates extracellular matrix components before trophoblast invasion/remodeling, ultimately contributing to reduced uteroplacental perfusion (Tan et al., 2024). Other insults that reduce UtA blood flow, such as surgical ligations of the uterine vessels or exposure to hypoxic conditions, are often utilized in animal models of these pregnancy complications (Alexander et al., 2001; Vuguin, 2007; Janot et al., 2014; Aljunaidy et al., 2016; Lane et al., 2020c).
1.4 Effect of hypoxia on systemic vascular function
Hypoxia is a strong driver of vascular reactivity, inducing constriction in pulmonary arteries and vasodilation in systemic arteries. These diverse physiological effects of hypoxia respond to the need to preserve gas exchange in the lungs and promote blood delivery to systemic tissues under low oxygen conditions. Systemic arteries dilate in response to hypoxia via multiple mechanisms. Specifically, hypoxia produces a decrease in ATP levels, activation of ATP-sensitive K+ (KATP) channels, and reduction in intracellular Ca2+ levels in smooth muscle, leading to dilation (Taggart and Wray, 1998). Chronic hypoxia also elicits vascular remodeling. For instance, in pulmonary arteries, high-altitude residence and animals exposed to artificial ambient hypoxic conditions thicken the arterial wall and increase the production of extracellular matrix proteins (Arias-Stella and Saldana, 1963; Rabinovitch et al., 1979; Heath et al., 1981; Wang and Chesler, 2012).
2 Maternal vascular dysfunction induced by hypoxia
In uterine vasculature, hypoxia decreases vasodilation and increases vascular tone by multiple mechanisms (Figure 2). Hypoxia is a necessary trait during early mammalian development to prevent excess reactive oxygen species (ROS) during the highly proliferative early pregnancy stages. However, prolonged exposure to low oxygen levels during pregnancy can lead to FGR (Jensen and Moore, 1997; Julian et al., 2008) and preeclampsia (Palmer et al., 1999; Moore, 2021). Hypoxia-induced uterine vascular dysfunction is characterized by alterations in hemodynamic parameters. Several reports have shown increased UtA resistance and decreased UtA diameter reduce blood flow in women living at high altitudes (defined as elevations above 2500 m) compared to low altitudes (Zamudio et al., 1995; Krampl et al., 2001; Julian et al., 2008). Reduced UtA blood flow in high-altitude pregnancies is attributed, in part, to a high endothelin-1/NO metabolites ratio (Julian et al., 2008). Similarly, chronic hypoxia-exposed pregnant guinea pigs show increased UtA pulsatility and resistance indexes (Turan et al., 2017) and decreased UtA capacity for growth during pregnancy. These responses to hypoxia are due to modifications in the proliferative response of vascular SMCs in vitro (Rockwell et al., 2006) and compromised biomechanical properties of the UtA, such as increased blood vessel distensibility and an altered stress-strain relationship (Mateev et al., 2006). The similar effects of hypoxia on maternal UtA blood flow in humans and guinea pigs could be a result of the similarities in their pregnancies, as both species develop haemomonochorial placentas, deliver precocial neonates, present similar uterine blood flow distribution throughout pregnancy, and fetal growth and development (Morrison et al., 2018; Carter, 2020; Candia et al., 2023).
FIGURE 2
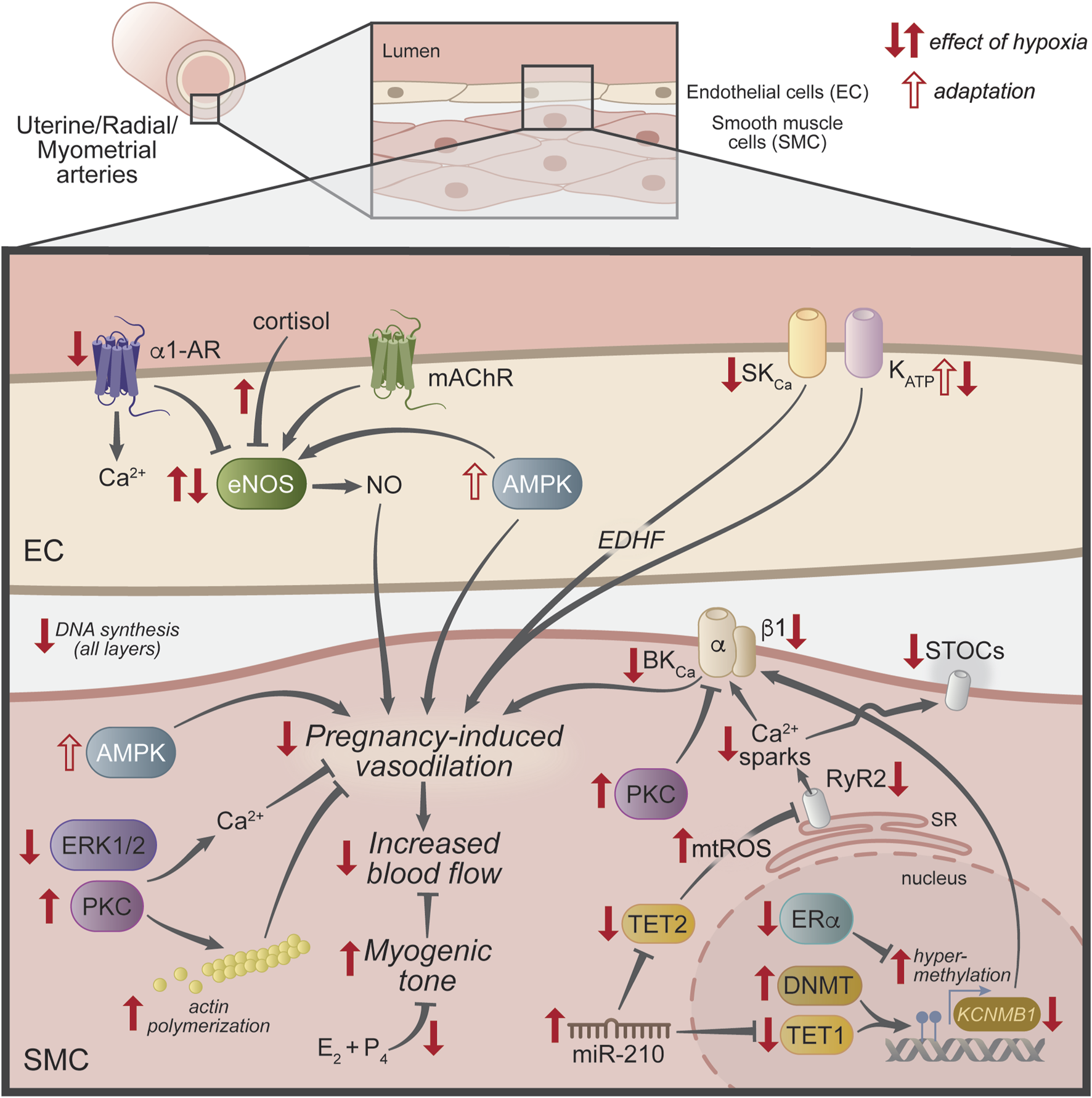
Mechanisms underlying the effect of hypoxia in uterine vasculature. Schematic representation of uterine vascular regulation mechanisms that are modified by hypoxia (filled red arrows) during pregnancy to reduce the rise in uterine blood flow. The open red arrows show mechanisms thought to be compensatory or adaptions to the lack of oxygen. Abbreviations: α1-AR, alpha-1 adrenergic receptor; AMPK, AMP-activated protein kinase; BKCa, large-conductance Ca2+- activated K+ channel; DNMT, DNA methyltransferase; E2, 17β-estradiol; EC, endothelial cell; EDHF, endothelial-derived hyperpolarizing factor; eNOS, endothelial nitric oxide synthase; ERα, estrogen receptor-α; ERK1/2, extracellular signal-regulated kinase 1/2; KATP, ATP-sensitive K+ channel; KCNMB1, large-conductance Ca2+-activated K+ channel β1 subunit gene; mAChR, muscarinic acetylcholine receptor; mir-210, micro RNA 210; mtROS, mitochondrial reactive oxygen species; NO, nitric oxide; P4, progesterone; PKC, protein kinase C; RyR1/2, ryanodine receptors 1 and 2; SKCa, small-conductance Ca2+-activated K+ channel; SMC, smooth muscle cell; SR, sarcoplasmic reticulum; STOCs, spontaneous transient outward currents; TET1 and 2, ten-eleven translocation methylcytosine dioxygenase 1 and 2.
Rats exposed to chronic hypoxia during pregnancy also exhibit reduced endothelium-dependent vasodilatory responses, however, their UtA pulsatility and resistance indexes are decreased (Aljunaidy et al., 2016). Likewise, mice exposed to chronic hypoxia during pregnancy show an increase or no change in UtA blood flow compared to normoxic mice (Lane et al., 2020b; Lane et al., 2020c). This hypoxia-induced increase in UtA blood flow in mice and rats, opposite humans and guinea pigs, may be a compensatory mechanism, although it is insufficient to prevent hypoxia-induced FGR (Aljunaidy et al., 2016; Lane et al., 2020b). This compensatory mechanism could be associated with the higher tolerance to hypoxia observed in rodents compared to humans. Mice and rats typically have the ability to develop higher tissue capillary density, can decrease their body temperature, and increase their whole-body oxygen consumption as adaptive mechanisms to resist hypoxia (Rising and D'Alecy, 1989; Dzhalilova and Makarova, 2020; Arias-Reyes et al., 2021). Hence, rodent models must be exposed to more severe hypoxic conditions than humans to create comparable stressors. Another explanation for these differences between mice and rats with humans and guinea pigs could be due to the distinct placental structures among these species: mice and rats develop a haemotrichorial placenta (two syncytiotrophoblast layers and one cytotrophoblast layer) as opposed to the haemomonochorial structure observed in humans and guinea pigs (Georgiades et al., 2002).
Specific cellular and molecular mechanisms known to underlie the hypoxia-induced reduction of uteroplacental perfusion are discussed below.
2.1 Estrogen and other steroid hormones
Estrogen, acting on estrogen receptor-α (ERα) and estrogen receptor-β (ERβ), is a regulator of vascular function in different vascular beds (Rubanyi et al., 2002). ERα and ERβ modulate the transcription of several genes due to the direct interaction of the ER complex with estrogen response elements (Albrecht and Pepe, 1990; Mahendru et al., 2014). In human UtAs, increased expression of ERα correlates inversely with collagen levels and distensibility of these arteries (Lydrup and Ferno, 2003). During pregnancy, the dramatic increase in circulating estrogen levels contributes to the increase in UtA blood flow (Li et al., 2022). Increased ERα expression leads to greater binding of circulating estrogen, which induces the transcription of eNOS, with a consequent increase in NO production in the UtA endothelium (Magness et al., 2001). Estrogen also binds to membrane receptors, which activate UtA vasodilator pathways mediated mainly by acute activation of eNOS (Chen et al., 2004).
Chronic hypoxia prevents the normal pregnancy- and sex steroid-induced increase in ERα expression in the pregnant ovine UtA (Xiao et al., 2009; Chang et al., 2010). Mechanistically, hypoxia induces epigenetic modifications (i.e., DNA methylation and histone modification) that repress ERα gene expression in the UtA (Dasgupta et al., 2012; Chen et al., 2015). In DNA methylation, a methyl group binds to cytosine residues in CpG sequences, which can suppress gene transcription by rendering the DNA unrecognizable in response to the binding of some transcription factors (Handy et al., 2011; Fuentes and Silveyra, 2019). Hypoxia has been reported to induce CpG methylation at a wide range, including repression of ERα (Chen et al., 2015). In ovine UtA, ERα gene repression is mediated through increased promoter methylation at critical transcription factor binding sites, such as specific protein 1 and upstream stimulatory factor, reducing ERα promoter activity (Dasgupta et al., 2012). Thus, the hypoxia-induced repression of ERα gene expression impairs normal vascular adaptations to pregnancy, largely by epigenetic modulation.
ERβ also contributes to UtA vasodilation during pregnancy. ERβ is upregulated during pregnancy in endothelial and vascular smooth muscle cells in the UtA of pregnant ewes (Byers et al., 2005; Liao et al., 2005). In UtA endothelial cells, specific activation of ERβ alone induces a decrease in the inhibitory site of eNOS (Thr495), leading to elevated levels of NO, similarly to the effect of ERα expression (Pastore et al., 2016). In addition, increased ERβ during pregnancy mediates upregulation of the angiotensin II type 2 receptor (AT2R) expression in UtA endothelium via transactivation of the AT2R promoter (Mishra et al., 2019), and AT2R activation increases UtA blood flow in rats (Mishra et al., 2018). In addition, pharmacological activation of ERβ by diarylpropionitrile reduces protein kinase C (PKC)-dependent maximal vasoconstriction in UtA isolated from pregnant ewes (Chang et al., 2010). In the same study, the authors showed that ERβ abundance in the UtA does not change in hypoxic pregnant ewes compared to low-altitude controls (Chang et al., 2010), suggesting that ERβ is not modulated by gestational chronic hypoxia.
Progesterone (P4) treatment of sheep UtA upregulates eNOS expression in non-pregnant ewes, and this effect is enhanced in the presence of 17β-estradiol (E2) (Rupnow et al., 2001). Treatment with both steroid hormones (P4+E2), at levels similar to those observed during pregnancy, decreases myogenic tone in non-pregnant UtA. In contrast, selective blockade of their receptors induces an increase in UtA myogenic tone (Xiao et al., 2009). Notably, this sex steroid hormone-dependent reduction of UtA myogenic tone is blunted by chronic exposure to hypoxia during gestation (Chang et al., 2010).
Cortisol may also play a role in the regulation of UtA vascular tone. Although maternal cortisol has been shown to be elevated during pregnancy (Nolten and Rueckert, 1981), its effects on the uterine vasculature remain unclear. Studies performed in UtA from non-pregnant ewes treated with cortisol showed a potentiation in the constrictor response to norepinephrine (NE) and a decrease in eNOS expression, contributing to a pro-constrictor state (Xiao et al., 2002). However, reducing endogenous cortisol levels via adrenalectomy in non-pregnant ewes also reduces eNOS expression in the UtA (Li et al., 2007). Pregnancy decreases the cortisol-induced increase in UtA vasoconstriction and reduces by half the cortisol-dependent downregulation in eNOS expression (Xiao et al., 2002). Notably, pregnant ewes exposed to acute cortisol treatment show no difference in UtA flow compared to untreated controls (Vaughan et al., 2016). A prospective cohort study demonstrated an association between high maternal cortisol levels and low birth weight (Shriyan et al., 2023), suggesting a relationship between cortisol-dependent UtA constriction and birth weight. Further studies are required to determine the precise role of cortisol in regulating UtA vasoreactivity during pregnancy. In relation to hypoxia, one study has shown that chronic hypoxic exposure during pregnancy increases cortisol sensitivity in the UtA, which contributes to a pro-constrictor state (Xiao et al., 2004).
2.2 Nitric oxide (NO) signaling
Healthy pregnancy typically involves an increase in NO-dependent vasodilation of UtA (White et al., 2000). In one study, pregnant women exposed to chronic hypoxia due to high-altitude residency show decreased vasodilatory responses to acetylcholine in myometrial arteries compared to low-altitude residents. The reduction was caused by decreased NO signaling, but eNOS expression was unchanged (Lorca et al., 2019), suggesting that the reduced effect of NO could be upstream of eNOS (i.e., at the cholinergic receptor or its coupling to Ca2+ signaling) or downstream of eNOS (i.e., cyclic GMP signaling, nitrosylation of target proteins, etc.). In pregnant animal models, chronic hypoxia impairs UtA flow-dependent vasodilator responses (Mateev et al., 2003) and decreases NO-dependent vasodilation (White et al., 2000). Intermittent hypoxia in pregnant mice also impairs NO-mediated UtA vasodilation (Badran et al., 2019). In contrast, chronic hypoxia has been found to lead to enhanced NO-mediated vasodilation and increased eNOS expression in UtA from pregnant ewes (Xiao et al., 2001), which contributes to increased endothelium-dependent vasodilator signaling. Interestingly, pregnant ewes exposed to high-altitude chronic hypoxia from these studies do not develop FGR (Kamitomo et al., 1992), unlike pregnant sheep studied at other high-altitude locations (Herrera et al., 2007) or other animals such as mice, rats, or guinea pigs (Aljunaidy et al., 2016; Turan et al., 2017; Lane et al., 2020b). This difference among species may be due to the hypoxia-evoked increase in NO response observed in UtAs from the pregnant ewes used in these studies (Xiao et al., 2001). In sheep studies that observed FGR development (Herrera et al., 2007), differences in fetal growth induced by hypoxia could be due to specific responses by different breeds of sheep or environmental and/or nutritional differences.
2.3 Adrenergic signaling
Vasoconstrictor responses to adrenergic stimulation in pregnancy are species-specific. Studies conducted in pregnant ovine UtA showed increased sensitivity to α1-adrenergic receptor stimulation with NE compared to non-pregnant sheep (Xiao et al., 2002). In humans, pregnant UtA also showed increased sensitivity to NE when compared to UtA from non-pregnant women (Rosenfeld et al., 2012). UtA from late-pregnant rats showed three-fold increases in vasoconstrictor responses to the α-1 agonist phenylephrine (PE) compared to non-pregnant rats (Osol and Cipolla, 1993). However, in pregnant guinea pig UtA, no differences in constriction induced by NE were observed (Jovanovic et al., 1995). However, in another study, pregnancy decreased PE-mediated vasoconstrictor responses in guinea pig UtA (White et al., 1998). Chronic hypoxia does not alter blunted pregnancy-associated contractile response to adrenergic stimulation in UtA from guinea pigs (White et al., 1998) nor in human myometrial vessels (Lorca et al., 2019). Conversely, long-term hypoxia exposure decreases α-1 adrenergic receptor-mediated vasoconstrictor activity in pregnant sheep UtA by reducing adrenergic receptor densities (Hu et al., 1996) and diminishing the sensitivity of α-1 adrenergic receptor to inositol 1,4,5-trisphosphate (IP3)-mediated signaling (Hu et al., 1999). Complementary studies in the same animal model demonstrated that exposure to chronic hypoxia during pregnancy increases Ca2+ mobilization in response to α-1 adrenergic receptor agonist stimulus (i.e., NE), but reduces Ca2+ sensitivity in UtA myofilaments (Xiao and Zhang, 2004). This mechanism of diminished Ca2+ sensitivity in myofilaments may be associated, in part, with increased eNOS expression and subsequent cyclic GMP formation as shown in other vascular beds (McDaniel et al., 1992; Soloviev et al., 2004; Van Hove et al., 2009).
2.4 Protein kinase C (PKC)
Another mechanism that contributes to the regulation of vascular tone during pregnancy is signaling via PKC. Research in this pathway has been performed almost exclusively in ovine models, which are described here unless otherwise noted. In healthy, non-pregnant sheep, PKC activation induces sustained vasoconstriction in UtAs (Xiao and Zhang, 2002). During pregnancy, this vasoconstriction is attenuated by a reduction in PKC signaling and a consequent decrease in Ca2+ sensitivity in vascular SMCs (Xiao et al., 2006). Furthermore, PKC activation inhibits PE-dependent contractions by reducing the adrenergic-dependent [Ca2+]i mobilization in normoxic pregnancies (Zhang et al., 2006). Actin polymerization, mediated by the PKC/ERK1/2 pathway, is responsible for the regulation of myogenic tone in UtA and is also decreased in UtAs during normal pregnancy, further decreasing vasoconstriction (Xiao et al., 2010b). In chronic hypoxia, the normal pregnancy-induced suppression of PKC signaling pathways is inhibited (Chang et al., 2009), increasing vascular tone and pro-constrictor response in the UtA via increases in basal Ca2+ sensitivity and actin polymerization (Xiao et al., 2010a; Xiao et al., 2012). This process has also been described in Sprague-Dawley rat resistance vessels exposed to prolonged stimulation with vasoconstrictors (Staiculescu et al., 2013). This maladaptation may be linked to the downregulation of ERα expression (Chang et al., 2010), since steroid hormones decrease PKC activity (Xiao et al., 2009).
2.5 K+ channels
Gestational hypoxia additionally induces PKC-mediated inhibition of UtA K+ channel activity, contributing to vascular dysfunction (Xiao et al., 2014). Large-conductance Ca2+-activated K+ (BKCa) channels are responsible for the regulation of membrane potential in many cell types (Sancho and Kyle, 2021). BKCa channels are composed of pore-forming α subunits and their activity is regulated by several auxiliary subunits (β1-β4 and γ1-γ4) (Gonzalez-Perez and Lingle, 2019). In the vasculature, BKCa channels are mainly expressed in vascular SMCs and associated with β1 and γ1 subunits, which increase channel activity (Tanaka et al., 1997; Brenner et al., 2000; Evanson et al., 2014). In SMCs, BKCa channels hyperpolarize the plasma membrane in response to increases in [Ca2+]i, promoting vasodilation and opposing myogenic tone (Nelson et al., 1995). In the uterine circulation, pregnancy increases the activity of BKCa channels through regulation of its auxiliary subunits, leading to an increase in the diameter of the UtA, a decrease in myogenic tone, and increased UtA blood flow [reviewed by (Bresnitz and Lorca, 2022)]. Gestational hypoxia inhibits the increase in BKCa channel activity caused by estrogen in UtA during pregnancy (Chen et al., 2015). Several studies propose epigenetic modifications as a key mechanism in the hypoxia-dependent regulation of BKCa channel activity. Chronic hypoxia enhances the expression and activity of DNA methyltransferase (DNMT), resulting in excessive methylation of the promoter region of the BKCa channel β1 subunit (KCNMB1) and consequent suppression of its expression (Hu et al., 2017a). In one study, DNMT inhibitors effectively reversed the hypermethylation of the BKCa β1 promoter region caused by hypoxia, restored KCNMB1 expression, and normalized channel activity, leading to enhanced UtA function (Hu et al., 2017a). Thus, hypoxia-induced epigenetic silencing is associated with reduced function of BKCa channels and impaired UtA adaptation to pregnancy.
MicroRNA-210 (miR-210) has emerged as a critical regulator in the hypoxia-induced repression of BKCa channels. miR-210 is a highly conserved small non-coding RNA that is involved in processes such as cell cycle and angiogenesis, and is upregulated in the UtA during gestational hypoxia (Huang et al., 2010; Ivan and Huang, 2014). miR-210 downregulates ten-eleven translocation methylcytosine dioxygenase 1 (TET1) expression (Hu et al., 2017b), a key enzyme involved in DNA demethylation (Guo et al., 2011b). This suppression of TET1 by miR-210 results in methylation of the KCNMB1 promoter, impairing BKCa channel β1 subunit expression and function (Hu et al., 2017a; Hu et al., 2017b). Moreover, miR-210 also targets and downregulates the ryanodine type 2 receptor (RyR2) (Hu et al., 2021), a major regulator of Ca2+ release from the sarcoplasmic reticulum and modulator of vascular tone (Kassmann et al., 2019). Reduced RyR2 and BKCa channel β1 subunit expression lead to a decrease in Ca2+ sparks. In normal function, Ca2+ sparks that result from local Ca2+ release into the cytosol of the SMC activate the BKCa channel which, in turn, generate spontaneous transient outward currents (STOCs) in UtA (Zhuge et al., 2002; Song et al., 2021), contributing to membrane hyperpolarization and opposing vasoconstriction (Nelson et al., 1995). Thus, the hypoxia-elicited reduction in Ca2+ sparks increases uterine vascular myogenic tone. Studies in humans residing at high altitudes also have shown a SMC-specific reduction in BKCa channel activity, evidenced by a diminished sensitivity of myometrial arteries to the blocker tetraethylammonium (Fallahi et al., 2022). However, the mechanism(s) underlying the hypoxia-dependent regulation of BKCa in human uterine vasculature remain unknown.
Another Ca2+-activated K+ channel involved in regulating vascular tone and UtA adaptation during pregnancy is the small-conductance Ca2+-activated K+ channel (SKCa). Specifically, SKCa types 2 and 3 are upregulated in the UtA during pregnancy (Zhu et al., 2013), facilitating vascular relaxation and adaptation. However, chronic hypoxia impedes this upregulation, leading to diminished SKCa channel activity and impaired myogenic reactivity in pregnant animals (Zhu et al., 2013), thus contributing to maladaptation of the uteroplacental circulation.
Hypoxia also leads to activation of KATP channels via a reduction in ATP levels. In human myometrial arteries from women with AGA pregnancies residing at high altitudes, there is an increased endothelium-dependent sensitivity of KATP channels to the blocker glibenclamide (Fallahi et al., 2022). This suggests that KATP channels are more active (or available) under chronic hypoxic conditions and could act as a compensatory mechanism in these uncomplicated human pregnancies at high altitudes. Interestingly, although these high-altitude pregnancies are AGA, they still show a non-pathological reduction in birth weight compared to lower altitudes (Lorca et al., 2019; Fallahi et al., 2022). However, in ovine models of high-altitude pregnancy, KATP channel activity is reduced in the UtA (Xiao et al., 2010c). These dissimilar observations between humans and sheep further highlight species-specific responses to hypoxia in the uterine vasculature. Moreover, the apparent redundancy of K+ channel activity promoting uterine vasodilation and increased blood flow may be an adaptive mechanism to preserve the uterine vascular adaptation to pregnancy under adverse conditions, such as chronic hypoxia.
2.6 Oxidative stress
Hypoxia elevates endoplasmic reticulum stress and oxidative stress in UtA, which also suppresses Ca2+ sparks/STOCs in the ovine pregnancy, increasing vascular tone (Hu et al., 2020). Additionally, mitochondrial dysfunction plays a crucial role in this process. Hypoxia and miR-210 enhance mitochondrial ROS (mtROS) production, inhibiting STOCs and contributing to increased myogenic tone (Hu et al., 2022). Notably, another target of miR-210, ten-eleven translocation methylcytosine dioxygenase 2 (TET2), which promotes DNA demethylation (Guo et al., 2011a), has been identified as a key regulator in this pathway. Downregulation of TET2 results in mitochondrial dysfunction and increased mtROS, thus decreasing STOCs and increasing myogenic contractions in the UtA, whereas overexpression of TET2 can mitigate these effects (Hu et al., 2023).
Furthermore, rats exposed to hypoxia during gestation and treated with a mitochondria-targeted antioxidant (MitoQ) showed an increase in maternal placental blood space and restoration of placental efficiency compared to untreated hypoxic animals (Nuzzo et al., 2018). Another study found that treatment with nanoparticle-encapsulated MitoQ reverses hypoxia-induced FGR and alleviates placental oxidative stress in a sex-dependent manner (Ganguly et al., 2021). Moreover, a recent study has shown a protective effect of MitoQ against UtA dysfunction and remodeling induced by chronic hypoxia during pregnancy in rats (Wang et al., 2024). Nevertheless, it should be considered that early gestation treatment with MitoQ could exacerbate the pre-eclamptic phenotype in mice by interfering with proper placentation (Yang et al., 2021).
Peroxisome proliferator-activated receptor gamma (PPARγ) is a hypoxia-sensitive ligand-inducible transcription factor with diverse functions, including the modulation of redox signaling in the vasculature (Kim and Yang, 2013). Inhibition of PPARγ during the second half of pregnancy decreased the vasodilator responses of the rat UtA, resulting in FGR (Gokina et al., 2013). In a mouse model of hypoxia-induced FGR, the pharmacological activation of PPARγ rescued fetal weight and prevented placental insufficiency (Lane et al., 2019). In the same rodent model, exposure to hypoxia during pregnancy increased endothelin-1-mediated UtA vasoconstriction, which was decreased by applying the selective PPAR-γ agonist troglitazone (TGZ) ex vivo (Lane et al., 2020a). In addition, UtA from hypoxic mice were more sensitive to TGZ-dependent vasodilation than UtA from normoxic animals (Lane et al., 2020a). These studies highlight PPARγ as a potential target to reverse the detrimental effects of oxidative stress and hypoxia during late gestation. Future studies should address the specific mechanism of PPARγ agonists in pregnant human uteroplacental circulation.
Resveratrol, an antioxidant drug, has also been widely studied in animal models of pregnancy. Resveratrol treatment improves fetal weight in a diabetic embryopathy model (Singh et al., 2011), induces UtA vasodilation in non-pregnant guinea pigs (Naderali et al., 2000), and reverses fetal death, but not the reduction in fetal weight, in a rat model exposed to hypoxia during pregnancy (Bourque et al., 2012). Moreover, resveratrol administered in the diet increases fetal weight and maternal UtA blood flow without changes in UtA vasoconstrictor or vasodilatory responses in a catechol-O-methyltransferase knockout model, which recapitulates characteristics of FGR and preeclampsia (Poudel et al., 2013). Sustained subcutaneous treatment with resveratrol in pregnant ewes increased UtA blood flow velocity and fetal growth (Darby et al., 2019). Subsequent studies demonstrated that acute administration of resveratrol in pregnant ewes has no significant effect on fetal hemodynamic improvements (Darby et al., 2023), indicating that successful treatment would require long-term intervention. Despite these benefits during pregnancy, resveratrol has not yet been sufficiently tested as a treatment for hypoxic human pregnancies and warrants further clinical study.
Melatonin, a neurohormone that upregulates antioxidant enzymes (Antolin et al., 1996), has been studied in several sheep models of FGR. Antenatal melatonin treatment has been shown to decrease brain injury in ovine ischemic-induced FGR offspring (Miller et al., 2014) and improve cerebrovascular function in ovine high-altitude-evoked FGR offspring (Candia et al., 2022). However, potential adverse effects have been reported in sheep exposed to chronic high-altitude hypoxia where antenatal treatment worsened FGR (Gonzalez-Candia et al., 2016). Moreover, antenatal melatonin treatment increased offspring mortality in rats (Singh et al., 2012) and failed to improve fetal weight or restore UtA vasodilatory function in an eNOS knockout mouse model of FGR (Renshall et al., 2018). A clinical trial in which melatonin was supplemented in women with early onset preeclampsia found no differences in the UtA pulsatility index but reduced the need for increasing antihypertensive drugs compared to the untreated women (Hobson et al., 2018).
N-acetylcysteine (NAC), an antioxidant likely acting as a precursor for glutathione synthesis (Samuni et al., 2013), partially reversed FGR in a guinea pig model and prevented fetal endothelial dysfunction (Herrera et al., 2017). Furthermore, NAC also restored vasodilatory responses in fetal arteries from chicken embryos exposed to hypoxia during development and in human chorionic arteries from FGR pregnancies (Krause et al., 2024). In addition, antenatal NAC treatment in FGR rats increased fetal brain weight at term without augmenting fetal weight (Chang et al., 2005).
Clinical studies have found limited or no effects of other antioxidants, such as vitamins C and E (Conde-Agudelo et al., 2011) and selenium (McDougall et al., 2023), in improving pregnancy outcomes (i.e., fetal weight). One clinical trial determined that using vitamins C and E in high concentrations exacerbates low birth weight (Poston et al., 2006).
Future efforts may focus on finding one or a combination of antioxidant treatment(s) for the improvement of maternal and fetal outcomes.
2.7 High-altitude ancestry
In human populations, high-altitude hypoxia has divergent effects on fetal growth and pregnancy outcomes depending on the ancestry of the individuals. Studies conducted in La Paz, Bolivia (elevation 3,600–4,100 m) revealed that women of Andean origin exhibit greater UtA diameter, cross-sectional area, and blood flow during pregnancy than those of European origin, resulting in improved uteroplacental oxygen delivery (Wilson et al., 2007). These physiological adaptations contribute to higher birth weights in newborns from Andean ancestry compared to their European counterparts at high altitudes (Julian et al., 2007). Interestingly, this is independent of maternal arterial oxygen content between the groups (Wilson et al., 2007; Julian et al., 2009). Women from lowland ancestry raised at high altitudes are not protected against the effects of high altitude on uteroplacental O2 delivery or reductions in birth weight, indicating this adaptation involves genetic, rather than developmental, factors (Julian et al., 2011). This may be due to a single nucleotide polymorphism (SNP) located near PRKAA1, the gene that encodes for the AMP-activated protein kinase (AMPK) α1 catalytic subunit, which is associated with higher UtA diameter and birthweight in altitude-adapted Andean populations (Bigham et al., 2014). Similar to Andeans, Tibetan populations living at high altitudes are protected from reduced birth weight compared to newcomers of Han ancestry (Moore et al., 2001). In addition, a high-altitude population in Ladakh, India (elevation 3,540 m), with mostly Tibetan ancestry, shows higher birth weights and larger UtA diameters than a low-altitude Indian population (Dolma et al., 2022). Furthermore, although no genome-wide significance of SNP was observed in Ladakhi populations, seven variants showed nominal associations in genes associated with birth weight (Bhandari et al., 2022). Overall, these associations underscore the critical role of UtA blood flow in fetal growth and highlight genetic factors that may enable high-altitude populations to better adapt to high-altitude hypoxia, reducing the incidence of adverse pregnancy outcomes.
2.8 AMP-activated protein kinase (AMPK)
Recent studies have highlighted the significant role of AMPK in modulating UtA blood flow and protecting against FGR under hypoxic conditions, including high altitude. AMPK acts as a metabolic sensor and is activated by ATP depletion, nutrient starvation, and hypoxia, among other stressors (Kim et al., 2016). AMPK induces vasodilatory responses by increasing NO bioavailability in ECs (Chen et al., 1999) and by decreasing [Ca2+]i through sarcoplasmic/endoplasmic Ca2+-ATPase and BKCa channel activation in vascular SMC (Schneider et al., 2015). Following the identification of the PRKAA1 SNP in a high-altitude human population (Bigham et al., 2014), a study in mice exposed to chronic hypoxia showed increased expression and activation of AMPK in UtAs (Skeffington et al., 2016). Accordingly, mice exposed to hypoxia during late pregnancy and treated in vivo with the AMPK agonist AICAR showed further increased UtA blood flow and partially attenuated reduction in fetal weight (Lane et al., 2020c). Furthermore, human myometrial arteries from women with AGA pregnancies residing at high altitudes showed increased AMPK-dependent vasodilation compared to low-altitude counterparts (Lorca et al., 2020). However, AMPK-dependent vasodilation was blunted in FGR pregnancies at high altitudes (Lorca et al., 2020). Thus, this vasodilatory response seems crucial for maintaining uteroplacental perfusion and supporting fetal growth in hypoxic environments. The limited research on AMPK activation in the human placenta at high altitude is mixed. In one study, high-altitude environments increased the activation of AMPK, measured as the ratio of total AMPK and its phosphorylated (Thr172) form (Lorca et al., 2021), whereas another study showed no change in AMPK activation (Yung et al., 2012). This discrepancy could be due to different sample sizes and/or altitude gradients between these studies. In mouse placenta, hypoxia during late pregnancy induces a reduction in phosphorylated AMPK (Lane et al., 2020b). Although the significance of the hypoxia-dependent regulation of placental AMPK for the regulation of uteroplacental blood flow remains unclear, taken together, these observations suggest an adaptive mechanism by which uterine vascular AMPK helps sustain the pregnancy-dependent rise in UtA blood flow under hypoxic conditions. There are potential therapeutic opportunities that may arise from these findings, and drugs that activate AMPK, such as metformin, are approved for certain pregnancy complications, such as gestational diabetes mellitus and polycystic ovary syndrome (Lautatzis et al., 2013). However, clinical randomized controlled trials have indicated that in utero exposure to metformin may lead to metabolic issues, increasing the risk of obesity in children (Hanem et al., 2018; Rowan et al., 2018). In addition, metformin can cross the placenta and affect fetal tissue (Charles et al., 2006), raising the likelihood of off-target effects. Thus, the wide-ranging effects of metformin discourage its use for specifically increasing uterine vasodilation in cases of reduced uteroplacental perfusion. Specific regulators of vascular AMPK targets could also be further studied to treat hypoxia-related vascular complications of pregnancy.
3 Hypoxia-induced regulation in fetoplacental circulation
The fetoplacental vascular bed is represented by umbilical cord arteries, umbilical vein, and the chorionic plate and villous blood vessels which include arteries, capillaries, and veins. Early studies aimed at addressing the acute effects of oxygen tension were performed in human placental cotyledons, in which acute hypoxia induces vasoconstriction, mainly in small caliber arteries (Howard et al., 1987; Hampl et al., 2002). This hypoxia-elicited vasoconstriction is partially mediated by a decrease in basal NO release by the endothelium (Byrne et al., 1997) and by an inhibition of voltage-gated K+ channels in the arteries of the chorionic plate (Hampl et al., 2002) with consequent activation of L-type Ca2+ channels (Jakoubek et al., 2006). Vasoconstrictor responses of the fetoplacental blood vessels to acute hypoxia appear to be very similar to that of the human pulmonary circulation (reviewed by (Ward and McMurtry, 2009)). Lowering O2 in pressurized chorionic plate veins induces vasoconstriction, whereas it evokes a moderate vasodilation in chorionic plate arteries (Wareing, 2012). Furthermore, nitrite-dependent vasodilation of chorionic plate arteries and veins is increased by acute hypoxia (Tropea et al., 2018). Chronic hypoxia due to residence at high altitudes also induces changes in the placental vasculature by increasing placental capillary density with decreased remodeling (Tissot van Patot et al., 2003). Future studies should investigate the effect of long-term hypoxia on the functional regulation of human fetoplacental circulation.
Studies using various animal models have been conducted to understand the impact of chronic hypoxia on the fetoplacental vascular bed. In rats, chronic hypoxia during pregnancy induces an increased vasoconstrictor response to angiotensin II and acute hypoxic challenges in fetoplacental vessels (Jakoubek et al., 2008). Furthermore, rat fetoplacental arteries exposed to chronic hypoxia during pregnancy showed increased collagen fiber accumulation, indicative of remodeling towards a pro-constrictor phenotype (Hvizdosova-Klescova et al., 2013). Similar to the observations in human placentas (Tissot van Patot et al., 2003), mouse and ewe models of gestational hypoxia showed an increase in the capillary network in the placenta (Parraguez et al., 2006; Cahill et al., 2018). This angiogenic response to hypoxia is also evident in the chorioallantoic membrane (Strick et al., 1991), the avian equivalent of the fetoplacental arterial circulation (Lindgren et al., 2010). Notably, the hypoxic chick embryo has been used as an animal model to study FGR independent of maternal hypoxic influences (Itani et al., 2018).
These studies in fetoplacental vessels showed that acute hypoxia-induced vasoconstriction of fetoplacental arteries reduces placental perfusion. However, capillary network expansion in the chronically hypoxic placenta acts as a local compensatory drive that potentially ensures the correct blood flow distribution and, therefore, oxygen supply to the fetus. Understanding this physiological adaptation may facilitate the identification of mechanisms that are compromised in pathological pregnancy conditions associated with impaired fetoplacental vasculature.
4 Conclusion
According to the developmental origins of adult diseases hypothesis (Barker’s hypothesis), pregnancy complications leading to decreased uteroplacental perfusion, placental dysfunction, and subsequent low birth weight affect early life and increase cardiometabolic risk in adulthood (Barker, 1990; de Boo and Harding, 2006). Since chronic hypoxia is a contributor to many vascular-associated pregnancy complications, such as FGR and preeclampsia, the mechanisms involved in the maternal and fetal vascular responses to hypoxic environments could highlight possible therapies to prevent or alleviate these complications. Particular attention should be given to the protective mechanisms observed in human populations residing at high altitudes (Julian et al., 2009; Bigham et al., 2014; Bhandari et al., 2022), as they could reveal novel targets for improving uteroplacental and fetoplacental perfusion. The development of preclinical models of chronic hypoxia have also been important for the testing of new drugs and therapies. For example, antioxidants and other metabolism-modifying drugs have been described in animal models to attenuate the effects of hypoxia on maternal vasculature, alleviating the development of pregnancy complications (Lane et al., 2019; Lane et al., 2020c; Wang et al., 2024). Future studies aiming to develop treatments targeted at uteroplacental and/or fetoplacental vasculature may prevent the non-specific adverse vascular effects observed in previous clinical trials using broad vasodilators (Pels et al., 2020). Additionally, the impact of hypoxia on placental physiology and its link to pregnancy complications has been extensively studied, reviewed by (Colson et al., 2021), and this information should also be taken into account when developing therapies. Thus, using animal models of impaired placental function can enhance our understanding of pregnancy complications associated with vascular issues.
Statements
Author contributions
GA: Writing–original draft, Writing–review and editing, Conceptualization. RL: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Institutes of Health HD109564, HD111908, and HD113796 Grants (RL), The Lorna Grindlay Moore Faculty Launch Award from the University of Colorado Anschutz Medical Campus (RL), and the Society for Reproductive Investigation International Training Grant 2023 (GA).
Acknowledgments
The authors thank Dr. Hannah Dimmick for the critical reading and editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AlbrechtE. D.PepeG. J. (1990). Placental steroid hormone biosynthesis in primate pregnancy. Endocr. Rev.11, 124–150. 10.1210/edrv-11-1-124
2
AlexanderB. T.KassabS. E.MillerM. T.AbramS. R.ReckelhoffJ. F.BennettW. A.et al (2001). Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension37, 1191–1195. 10.1161/01.hyp.37.4.1191
3
AljunaidyM. M.MortonJ. S.CookeC. L.DavidgeS. T. (2016). Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol.311, R1068-R1075–r1075. 10.1152/ajpregu.00119.2016
4
AntolinI.RodriguezC.SainzR. M.MayoJ. C.UriaH.KotlerM. L.et al (1996). Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J.10, 882–890. 10.1096/fasebj.10.8.8666165
5
Arias-ReyesC.SolizJ.JosephV. (2021). Mice and rats display different ventilatory, hematological, and metabolic features of acclimatization to hypoxia. Front. Physiol.12, 647822. 10.3389/fphys.2021.647822
6
Arias-StellaJ.SaldanaM. (1963). The terminal portion of the pulmonary arterial tree in people native to high altitudes. Circulation28, 915–925. 10.1161/01.cir.28.5.915
7
BadranM.AbuyassinB.AyasN.LaherI. (2019). Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol.597, 2639–2650. 10.1113/JP277775
8
BarkerD. J. (1990). The fetal and infant origins of adult disease. BMJ301, 1111. 10.1136/bmj.301.6761.1111
9
BastaM.LipsettB. J. (2024). “Anatomy, abdomen and pelvis: umbilical cord,” inStatPearls [Internet]. Treasure Island, FL: StatPearls Publishing.
10
BhandariS.DolmaP.MukerjiM.PrasherB.MontgomeryH.KularD.et al (2022). Population history and genome wide association studies of birth weight in a native high altitude Ladakhi population. PLoS One17, e0269671. 10.1371/journal.pone.0269671
11
BighamA. W.JulianC. G.WilsonM. J.VargasE.BrowneV. A.ShriverM. D.et al (2014). Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol. Genomics46, 687–697. 10.1152/physiolgenomics.00063.2014
12
BirdI. M.ZhangL.MagnessR. R. (2003). Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol.284, R245–R258. 10.1152/ajpregu.00108.2002
13
BourqueS. L.DolinskyV. W.DyckJ. R.DavidgeS. T. (2012). Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta33, 449–452. 10.1016/j.placenta.2012.01.012
14
BrennerR.PerezG. J.BonevA. D.EckmanD. M.KosekJ. C.WilerS. W.et al (2000). Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature407, 870–876. 10.1038/35038011
15
BresnitzW.LorcaR. A. (2022). Potassium channels in the uterine vasculature: role in healthy and complicated pregnancies. Int. J. Mol. Sci.23, 9446. 10.3390/ijms23169446
16
BrowneV. A.Toledo-JaldinL.DavilaR. D.LopezL. P.YamashiroH.Cioffi-RaganD.et al (2011). High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol.300, R1221–R1229. 10.1152/ajpregu.91046.2008
17
BurtonG. J.JauniauxE. (2018). Development of the human placenta and fetal heart: synergic or independent?Front. Physiol.9, 373. 10.3389/fphys.2018.00373
18
BurtonG. J.WoodsA. W.JauniauxE.KingdomJ. C. (2009). Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta30, 473–482. 10.1016/j.placenta.2009.02.009
19
ByersM. J.ZanglA.PhernettonT. M.LopezG.ChenD. B.MagnessR. R. (2005). Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J. Physiol.565, 85–99. 10.1113/jphysiol.2005.085753
20
ByrneB. M.HowardR. B.MorrowR. J.WhiteleyK. J.AdamsonS. L. (1997). Role of the L-arginine nitric oxide pathway in hypoxic fetoplacental vasoconstriction. Placenta18, 627–634. 10.1016/s0143-4004(97)90003-5
21
CahillL. S.RennieM. Y.HoggarthJ.YuL. X.RahmanA.KingdomJ. C.et al (2018). Feto- and utero-placental vascular adaptations to chronic maternal hypoxia in the mouse. J. Physiol.596, 3285–3297. 10.1113/JP274845
22
CandiaA. A.AriasP. V.Gonzalez-CandiaC.NavarreteA.EbenspergerG.ReyesR. V.et al (2022). Melatonin treatment during chronic hypoxic gestation improves neonatal cerebrovascular function. Vasc. Pharmacol.144, 106971. 10.1016/j.vph.2022.106971
23
CandiaA. A.JimenezT.NavarreteA.BenaldoF.SilvaP.Garcia-HerreraC.et al (2023). Developmental ultrasound characteristics in Guinea pigs: similarities with human pregnancy. Vet. Sci.10, 144. 10.3390/vetsci10020144
24
CarterA. M. (2020). Animal models of human pregnancy and placentation: alternatives to the mouse. Reproduction160, R129-R143–R143. 10.1530/REP-20-0354
25
ChangE. Y.BarbosaE.PaintliaM. K.SinghA.SinghI. (2005). The use of N-acetylcysteine for the prevention of hypertension in the reduced uterine perfusion pressure model for preeclampsia in Sprague-Dawley rats. Am. J. Obstet. Gynecol.193, 952–956. 10.1016/j.ajog.2005.05.083
26
ChangK.XiaoD.HuangX.LongoL. D.ZhangL. (2009). Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am. J. Physiol. Heart Circ. Physiol.296, H1840–H1849. 10.1152/ajpheart.00090.2009
27
ChangK.XiaoD.HuangX.XueZ.YangS.LongoL. D.et al (2010). Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension56, 750–757. 10.1161/HYPERTENSIONAHA.110.155812
28
ChappellJ.AughwaneR.ClarkA. R.OurselinS.DavidA. L.MelbourneA. (2023). A review of feto-placental vasculature flow modelling. Placenta142, 56–63. 10.1016/j.placenta.2023.08.068
29
CharlesB.NorrisR.XiaoX.HagueW. (2006). Population pharmacokinetics of metformin in late pregnancy. Ther. Drug Monit.28, 67–72. 10.1097/01.ftd.0000184161.52573.0e
30
ChenD. B.BirdI. M.ZhengJ.MagnessR. R. (2004). Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology145, 113–125. 10.1210/en.2003-0547
31
ChenM.XiaoD.HuX. Q.DasguptaC.YangS.ZhangL. (2015). Hypoxia represses ER-α expression and inhibits estrogen-induced regulation of Ca2+-activated K+ channel activity and myogenic tone in ovine uterine arteries: causal role of DNA methylation. Hypertension66, 44–51. 10.1161/HYPERTENSIONAHA.115.05299
32
ChenZ. P.MitchelhillK. I.MichellB. J.StapletonD.Rodriguez-CrespoI.WittersL. A.et al (1999). AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett.443, 285–289. 10.1016/s0014-5793(98)01705-0
33
CockellA. P.PostonL. (1997). Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension30, 247–251. 10.1161/01.hyp.30.2.247
34
ColsonA.SonveauxP.DebieveF.Sferruzzi-PerriA. N. (2021). Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction. Hum. Reprod. Update27, 531–569. 10.1093/humupd/dmaa053
35
Conde-AgudeloA.RomeroR.KusanovicJ. P.HassanS. S. (2011). Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am. J. Obstet. Gynecol.204, 503 e501–e512. 10.1016/j.ajog.2011.02.020
36
DarbyJ. R. T.SainiB. S.SooJ. Y.LockM. C.HolmanS. L.BradshawE. L.et al (2019). Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant Ewe and increases fetal but not cardiac growth. J. Physiol.597, 5063–5077. 10.1113/JP278110
37
DarbyJ. R. T.WilliamsG. K.ChoS. K. S.MeakinA. S.HolmanS. L.QuinnM.et al (2023). Acute resveratrol exposure does not impact hemodynamics of the fetal sheep. Physiol. Rep.11, e15749. 10.14814/phy2.15749
38
DasguptaC.ChenM.ZhangH.YangS.ZhangL. (2012). Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-α gene in ovine uterine arteries via heightened promoter methylation. Hypertension60, 697–704. 10.1161/HYPERTENSIONAHA.112.198242
39
De BooH. A.HardingJ. E. (2006). The developmental origins of adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol.46, 4–14. 10.1111/j.1479-828X.2006.00506.x
40
DegnerK.MagnessR. R.ShahD. M. (2017). Establishment of the human uteroplacental circulation: a historical perspective. Reprod. Sci.24, 753–761. 10.1177/1933719116669056
41
DolmaP.AngchukP. T.JainV.DadhwalV.KularD.WilliamsD. J.et al (2022). High-altitude population neonatal and maternal phenotypes associated with birthweight protection. Pediatr. Res.91, 137–142. 10.1038/s41390-021-01593-5
42
DzhalilovaD.MakarovaO. (2020). Differences in tolerance to hypoxia: physiological, biochemical, and molecular-biological characteristics. Biomedicines8, 428. 10.3390/biomedicines8100428
43
EvansonK. W.BannisterJ. P.LeoM. D.JaggarJ. H. (2014). LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circ. Res.115, 423–431. 10.1161/CIRCRESAHA.115.303407
44
FallahiS.HouckJ. A.EuserA. G.JulianC. G.MooreL. G.LorcaR. A. (2022). High altitude differentially modulates potassium channel-evoked vasodilatation in pregnant human myometrial arteries. J. Physiol.600, 5353–5364. 10.1113/JP283741
45
FordS. P. (1982). Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J. Anim. Sci.55 (Suppl. 2), 32–42.
46
FuentesN.SilveyraP. (2019). Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol.116, 135–170. 10.1016/bs.apcsb.2019.01.001
47
GangulyE.KirschenmanR.SpaansF.HolodyC. D.PhillipsT. E. J.CaseC. P.et al (2021). Nanoparticle-encapsulated antioxidant improves placental mitochondrial function in a sexually dimorphic manner in a rat model of prenatal hypoxia. FASEB J.35, e21338. 10.1096/fj.202002193R
48
GeorgiadesP.Ferguson-SmithA. C.BurtonG. J. (2002). Comparative developmental anatomy of the murine and human definitive placentae. Placenta23, 3–19. 10.1053/plac.2001.0738
49
GokinaN. I.ChanS. L.ChapmanA. C.OppenheimerK.JettonT. L.CipollaM. J. (2013). Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Front. Physiol.4, 184. 10.3389/fphys.2013.00184
50
Gonzalez-CandiaA.VelizM.ArayaC.QuezadaS.EbenspergerG.Seron-FerreM.et al (2016). Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: findings in pregnant sheep. Am. J. Obstet. Gynecol.215, 245 e241–e7. 10.1016/j.ajog.2016.02.040
51
Gonzalez-PerezV.LingleC. J. (2019). Regulation of BK channels by beta and gamma subunits. Annu. Rev. Physiol.81, 113–137. 10.1146/annurev-physiol-022516-034038
52
GuoJ. U.SuY.ZhongC.MingG. L.SongH. (2011a). Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell. Cycle10, 2662–2668. 10.4161/cc.10.16.17093
53
GuoJ. U.SuY.ZhongC.MingG. L.SongH. (2011b). Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell.145, 423–434. 10.1016/j.cell.2011.03.022
54
HamplV.BibovaJ.StranakZ.WuX.MichelakisE. D.HashimotoK.et al (2002). Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am. J. Physiol. Heart Circ. Physiol.283, H2440–H2449. 10.1152/ajpheart.01033.2001
55
HandyD. E.CastroR.LoscalzoJ. (2011). Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation123, 2145–2156. 10.1161/CIRCULATIONAHA.110.956839
56
HanemL. G. E.StridsklevS.JuliussonP. B.SalvesenO.RoelantsM.CarlsenS. M.et al (2018). Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 Years of age: follow-up of two RCTs. J. Clin. Endocrinol. Metab.103, 1612–1621. 10.1210/jc.2017-02419
57
HeathD.SmithP.Rios DalenzJ.WilliamsD.HarrisP. (1981). Small pulmonary arteries in some natives of La Paz, Bolivia. Thorax36, 599–604. 10.1136/thx.36.8.599
58
HerreraE. A.Cifuentes-ZunigaF.FigueroaE.VillanuevaC.HernandezC.AlegriaR.et al (2017). N-Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted Guinea pigs. J. Physiol.595, 1077–1092. 10.1113/JP273396
59
HerreraE. A.PulgarV. M.RiquelmeR. A.SanhuezaE. M.ReyesR. V.EbenspergerG.et al (2007). High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol.292, R2234–R2240. 10.1152/ajpregu.00909.2006
60
HilgersR. H.BergayaS.SchiffersP. M.MenetonP.BoulangerC. M.HenrionD.et al (2003). Uterine artery structural and functional changes during pregnancy in tissue kallikrein-deficient mice. Arterioscler. Thromb. Vasc. Biol.23, 1826–1832. 10.1161/01.ATV.0000090672.07568.60
61
HobsonS. R.GurusingheS.LimR.AlersN. O.MillerS. L.KingdomJ. C.et al (2018). Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res.65, e12508. 10.1111/jpi.12508
62
HowardR. B.HosokawaT.MaguireM. H. (1987). Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am. J. Obstet. Gynecol.157, 1261–1266. 10.1016/s0002-9378(87)80307-1
63
HuX. Q.ChenM.DasguptaC.XiaoD.HuangX.YangS.et al (2017a). Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol. Reprod.96, 424–434. 10.1095/biolreprod.116.145946
64
HuX. -Q.DasguptaC.SongR.RomeroM.WilsonS. M.ZhangL. (2021). MicroRNA-210 mediates hypoxia-induced repression of spontaneous transient outward currents in sheep uterine arteries during gestation. Hypertension77, 1412–1427. 10.1161/HYPERTENSIONAHA.120.16831
65
HuX. -Q.DasguptaC.XiaoD.HuangX.YangS.ZhangL. (2017b). MicroRNA-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance Ca2+-activated K+ channel expression and function in ovine uterine arteries. Hypertension70, 601–612. 10.1161/hypertensionaha.117.09864
66
HuX. Q.LongoL. D.GilbertR. D.ZhangL. (1996). Effects of long-term high-altitude hypoxemia on alpha 1-adrenergic receptors in the ovine uterine artery. Am. J. Physiol.270, H1001–H1007. 10.1152/ajpheart.1996.270.3.H1001
67
HuX. Q.SongR.DasguptaC.BloodA. B.ZhangL. (2023). TET2 confers a mechanistic link of microRNA-210 and mtROS in hypoxia-suppressed spontaneous transient outward currents in uterine arteries of pregnant sheep. J. Physiol.601, 1501–1514. 10.1113/JP284336
68
HuX. Q.SongR.DasguptaC.RomeroM.JuarezR.HansonJ.et al (2022). MicroRNA-210-mediated mtROS confer hypoxia-induced suppression of STOCs in ovine uterine arteries. Br. J. Pharmacol.179, 4640–4654. 10.1111/bph.15914
69
HuX. Q.SongR.RomeroM.DasguptaC.MinJ.HatcherD.et al (2020). Gestational hypoxia inhibits pregnancy-induced upregulation of Ca(2+) sparks and spontaneous transient outward currents in uterine arteries via heightened endoplasmic reticulum/oxidative stress. Hypertension76, 930–942. 10.1161/HYPERTENSIONAHA.120.15235
70
HuX. Q.YangS.PearceW. J.LongoL. D.ZhangL. (1999). Effect of chronic hypoxia on alpha-1 adrenoceptors-mediated inositol 1,4,5-trisphosphate signaling in ovine uterine artery. J. Pharmacol. Exp. Ther.288, 977–983.
71
HuangX.LeQ. T.GiacciaA. J. (2010). MiR-210-micromanager of the hypoxia pathway. Trends Mol. Med.16, 230–237. 10.1016/j.molmed.2010.03.004
72
Hvizdosova-KlescovaA.UhlikJ.MalinaM.VulterinovaH.NovotnyT.VajnerL. (2013). Remodeling of fetoplacental arteries in rats due to chronic hypoxia. Exp. Toxicol. Pathol.65, 97–103. 10.1016/j.etp.2011.06.006
73
ItaniN.SalinasC. E.VillenaM.SkeffingtonK. L.BeckC.VillamorE.et al (2018). The highs and lows of programmed cardiovascular disease by developmental hypoxia: studies in the chicken embryo. J. Physiol.596, 2991–3006. 10.1113/JP274111
74
IvanM.HuangX. (2014). miR-210: fine-tuning the hypoxic response. Adv. Exp. Med. Biol.772, 205–227. 10.1007/978-1-4614-5915-6_10
75
JakoubekV.BibovaJ.HamplV. (2006). Voltage-gated calcium channels mediate hypoxic vasoconstriction in the human placenta. Placenta27, 1030–1033. 10.1016/j.placenta.2005.10.006
76
JakoubekV.BibovaJ.HergetJ.HamplV. (2008). Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am. J. Physiol. Heart Circ. Physiol.294, H1638–H1644. 10.1152/ajpheart.01120.2007
77
JamesJ. L.ChamleyL. W.ClarkA. R. (2017). Feeding your baby in utero: how the uteroplacental circulation impacts pregnancy. Physiol. (Bethesda)32, 234–245. 10.1152/physiol.00033.2016
78
JanotM.Cortes-DublyM. L.RodriguezS.Huynh-DoU. (2014). Bilateral uterine vessel ligation as a model of intrauterine growth restriction in mice. Reprod. Biol. Endocrinol.12, 62. 10.1186/1477-7827-12-62
79
JensenG. M.MooreL. G. (1997). The effect of high altitude and other risk factors on birthweight: independent or interactive effects?Am. J. Public Health87, 1003–1007. 10.2105/ajph.87.6.1003
80
JovanovicA.GrbovicL.JovanovicS. (1995). Effect of the vascular endothelium on noradrenaline-induced contractions in non-pregnant and pregnant Guinea-pig uterine arteries. Br. J. Pharmacol.114, 805–815. 10.1111/j.1476-5381.1995.tb13276.x
81
JulianC. G.GalanH. L.WilsonM. J.DesilvaW.Cioffi-RaganD.SchwartzJ.et al (2008). Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol.295, R906–R915. 10.1152/ajpregu.00164.2008
82
JulianC. G.HagemanJ. L.WilsonM. J.VargasE.MooreL. G. (2011). Lowland origin women raised at high altitude are not protected against lower uteroplacental O2 delivery during pregnancy or reduced birth weight. Am. J. Hum. Biol.23, 509–516. 10.1002/ajhb.21167
83
JulianC. G.VargasE.ArmazaJ. F.WilsonM. J.NiermeyerS.MooreL. G. (2007). High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch. Dis. Child. Fetal Neonatal Ed.92, F372–F377. 10.1136/adc.2006.109579
84
JulianC. G.WilsonM. J.LopezM.YamashiroH.TellezW.RodriguezA.et al (2009). Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am. J. Physiol. Regul. Integr. Comp. Physiol.296, R1564–R1575. 10.1152/ajpregu.90945.2008
85
KamitomoM.LongoL. D.GilbertR. D. (1992). Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am. J. Physiol.262, H399–H405. 10.1152/ajpheart.1992.262.2.H399
86
KassmannM.SzijartoI. A.Garcia-PrietoC. F.FanG.SchleifenbaumJ.AnistanY. M.et al (2019). Role of ryanodine type 2 receptors in elementary Ca(2+) signaling in arteries and vascular adaptive responses. J. Am. Heart Assoc.8, e010090. 10.1161/JAHA.118.010090
87
KimJ.YangG.KimY.KimJ.HaJ. (2016). AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med.48, e224. 10.1038/emm.2016.16
88
KimT.YangQ. (2013). Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J. Cardiol.5, 164–174. 10.4330/wjc.v5.i6.164
89
KonjeJ. C.HowarthE. S.KaufmannP.TaylorD. J. (2003). Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG110, 301–305. 10.1046/j.1471-0528.2003.t01-1-02163.x
90
KonjeJ. C.KaufmannP.BellS. C.TaylorD. J. (2001). A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol.185, 608–613. 10.1067/mob.2001.117187
91
KramplE. R.Espinoza-DoradoJ.LeesC. C.MoscosoG.BlandJ. M.CampbellS. (2001). Maternal uterine artery Doppler studies at high altitude and sea level. Ultrasound Obstet. Gynecol.18, 578–582. 10.1046/j.0960-7692.2001.00579.x
92
KrauseB. J.PazA. A.GarrudT. a.C.PenalozaE.Vega-TapiaF.FordS. G.et al (2024). Epigenetic regulation by hypoxia, N-acetylcysteine and hydrogen sulphide of the fetal vasculature in growth restricted offspring: a study in humans and chicken embryos. J. Physiol.602, 3833–3852. 10.1113/JP286266
93
KublickieneK. R.LindblomB.KrugerK.NisellH. (2000). Preeclampsia: evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am. J. Obstet. Gynecol.183, 160–166. 10.1067/mob.2000.105820
94
KusinskiL. C.StanleyJ. L.DilworthM. R.HirtC. J.AnderssonI. J.RenshallL. J.et al (2012). eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am. J. Physiol. Regul. Integr. Comp. Physiol.303, R86–R93. 10.1152/ajpregu.00600.2011
95
LaneS. L.DodsonR. B.DoyleA. S.ParkH.RathiH.MatarrazoC. J.et al (2019). Pharmacological activation of peroxisome proliferator-activated receptor gamma (PPAR-gamma) protects against hypoxia-associated fetal growth restriction. FASEB J.33, 8999–9007. 10.1096/fj.201900214R
96
LaneS. L.DoyleA. S.BalesE. S.HouckJ. A.LorcaR. A.MooreL. G.et al (2020a). Peroxisome proliferator-activated receptor gamma blunts endothelin-1-mediated contraction of the uterine artery in a murine model of high-altitude pregnancy. FASEB J.34, 4283–4292. 10.1096/fj.201902264RR
97
LaneS. L.DoyleA. S.BalesE. S.LorcaR. A.JulianC. G.MooreL. G. (2020b). Increased uterine artery blood flow in hypoxic murine pregnancy is not sufficient to prevent fetal growth restriction†. Biol. Reprod.102, 660–670. 10.1093/biolre/ioz208
98
LaneS. L.HouckJ. A.DoyleA. S.BalesE. S.LorcaR. A.JulianC. G.et al (2020c). AMP-activated protein kinase activator AICAR attenuates hypoxia-induced murine fetal growth restriction in part by improving uterine artery blood flow. J. Physiol.598, 4093–4105. 10.1113/JP279341
99
LautatzisM. E.GoulisD. G.VrontakisM. (2013). Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: a systematic review. Metabolism62, 1522–1534. 10.1016/j.metabol.2013.06.006
100
LiF.WoodC. E.Keller-WoodM. (2007). Adrenalectomy alters regulation of blood pressure and endothelial nitric oxide synthase in sheep: modulation by estradiol. Am. J. Physiol. Regul. Integr. Comp. Physiol.293, R257–R266. 10.1152/ajpregu.00082.2007
101
LiY.HanB.SalmeronA. G.BaiJ.ChenD. B. (2022). Estrogen-induced uterine vasodilation in pregnancy and preeclampsia. Matern. Fetal Med.4, 52–60. 10.1097/fm9.0000000000000132
102
LiaoW. X.MagnessR. R.ChenD. B. (2005). Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol. Reprod.72, 530–537. 10.1095/biolreprod.104.035949
103
LindgrenI.ZoerB.AltimirasJ.VillamorE. (2010). Reactivity of chicken chorioallantoic arteries, avian homologue of human fetoplacental arteries. J. Physiol. Pharmacol.61, 619–628.
104
LorcaR. A.HouckJ. A.LaurentL. C.MatarazzoC. J.BakerK.HoriiM.et al (2021). High altitude regulates the expression of AMPK pathways in human placenta. Placenta104, 267–276. 10.1016/j.placenta.2021.01.010
105
LorcaR. A.LaneS. L.BalesE. S.NsierH.YiH.DonnellyM. A.et al (2019). High altitude reduces NO-dependent myometrial artery vasodilator response during pregnancy. Hypertension73, 1319–1326. 10.1161/HYPERTENSIONAHA.119.12641
106
LorcaR. A.MatarazzoC. J.BalesE. S.HouckJ. A.OrlickyD. J.EuserA. G.et al (2020). AMPK activation in pregnant human myometrial arteries from high-altitude and intrauterine growth-restricted pregnancies. Am. J. Physiol. Heart Circ. Physiol.319, H203-H212–h212. 10.1152/ajpheart.00644.2019
107
LukshaL.LukshaN.KublickasM.NisellH.KublickieneK. (2010). Diverse mechanisms of endothelium-derived hyperpolarizing factor-mediated dilatation in small myometrial arteries in normal human pregnancy and preeclampsia. Biol. Reprod.83, 728–735. 10.1095/biolreprod.110.084426
108
LydrupM. L.FernoM. (2003). Correlation between estrogen receptor alpha expression, collagen content and stiffness in human uterine arteries. Acta Obstet. Gynecol. Scand.82, 610–615. 10.1080/j.1600-0412.2003.00209.x
109
MaY.YuX.ZhangL.LiuJ.ShaoX.LiY. X.et al (2021). Uterine decidual niche modulates the progressive dedifferentiation of spiral artery vascular smooth muscle cells during human pregnancy†. Biol. Reprod.104, 624–637. 10.1093/biolre/ioaa208
110
MagnessR. R.SullivanJ. A.LiY.PhernettonT. M.BirdI. M. (2001). Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am. J. Physiol. Heart Circ. Physiol.280, H1692–H1698. 10.1152/ajpheart.2001.280.4.H1692
111
MahendruA. A.EverettT. R.WilkinsonI. B.LeesC. C.McenieryC. M. (2014). A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J. Hypertens.32, 849–856. 10.1097/HJH.0000000000000090
112
MateevS.SillauA. H.MouserR.McculloughR. E.WhiteM. M.YoungD. A.et al (2003). Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am. J. Physiol. Heart Circ. Physiol.284, H820–H829. 10.1152/ajpheart.00701.2002
113
MateevS. N.MouserR.YoungD. A.MechamR. P.MooreL. G. (2006). Chronic hypoxia augments uterine artery distensibility and alters the circumferential wall stress-strain relationship during pregnancy. J. Appl. Physiol.100, 1842–1850. 10.1152/japplphysiol.00618.2005
114
McdanielN. L.ChenX. L.SingerH. A.MurphyR. A.RemboldC. M. (1992). Nitrovasodilators relax arterial smooth muscle by decreasing [Ca2+]i and uncoupling stress from myosin phosphorylation. Am. J. Physiol.263, C461–C467. 10.1152/ajpcell.1992.263.2.C461
115
McdougallA. R.DoreG.AboudL.MakamaM.NguyenP. Y.MillsK.et al (2023). The effect of selenium supplementation in pregnant women on maternal, fetal, and newborn outcomes: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM5, 101160. 10.1016/j.ajogmf.2023.101160
116
MillerS. L.YawnoT.AlersN. O.Castillo-MelendezM.SupramaniamV. G.VanzylN.et al (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res.56, 283–294. 10.1111/jpi.12121
117
MishraJ. S.GopalakrishnanK.KumarS. (2018). Pregnancy upregulates angiotensin type 2 receptor expression and increases blood flow in uterine arteries of rats. Biol. Reprod.99, 1091–1099. 10.1093/biolre/ioy130
118
MishraJ. S.Te RieleG. M.QiQ. R.LechugaT. J.GopalakrishnanK.ChenD. B.et al (2019). Estrogen receptor-β mediates estradiol-induced pregnancy-specific uterine artery endothelial cell angiotensin type-2 receptor expression. Hypertension74, 967–974. 10.1161/HYPERTENSIONAHA.119.13429
119
MooreL. G. (2021). Hypoxia and reproductive health: reproductive challenges at high altitude: fertility, pregnancy and neonatal well-being. Reproduction161, F81–F90. 10.1530/REP-20-0349
120
MooreL. G.YoungD.McculloughR. E.DromaT.ZamudioS. (2001). Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am. J. Hum. Biol.13, 635–644. 10.1002/ajhb.1102
121
MorrisonJ. L.BottingK. J.DarbyJ. R. T.DavidA. L.DysonR. M.GatfordK. L.et al (2018). Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol.596, 5535–5569. 10.1113/JP274948
122
NaderaliE. K.DoyleP. J.WilliamsG. (2000). Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female Guinea-pigs. Clin. Sci. (Lond)98, 537–543. 10.1042/cs19990303
123
NelsonM. T.ChengH.RubartM.SantanaL. F.BonevA. D.KnotH. J.et al (1995). Relaxation of arterial smooth muscle by calcium sparks. Science270, 633–637. 10.1126/science.270.5236.633
124
NoltenW. E.RueckertP. A. (1981). Elevated free cortisol index in pregnancy: possible regulatory mechanisms. Am. J. Obstet. Gynecol.139, 492–498. 10.1016/0002-9378(81)90331-8
125
NuzzoA. M.CammE. J.Sferruzzi-PerriA. N.AshmoreT. J.YungH. W.Cindrova-DaviesT.et al (2018). Placental adaptation to early-onset hypoxic pregnancy and mitochondria-targeted antioxidant therapy in a rodent model. Am. J. Pathol.188, 2704–2716. 10.1016/j.ajpath.2018.07.027
126
OngS. S.MooreR. J.WarrenA. Y.CrockerI. P.FulfordJ.TylerD. J.et al (2003). Myometrial and placental artery reactivity alone cannot explain reduced placental perfusion in pre-eclampsia and intrauterine growth restriction. BJOG110, 909–915. 10.1111/j.1471-0528.2003.02368.x
127
OsolG.CipollaM. (1993). Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am. J. Obstet. Gynecol.168, 697–705. 10.1016/0002-9378(93)90519-o
128
OsolG.MooreL. G. (2014). Maternal uterine vascular remodeling during pregnancy. Microcirculation21, 38–47. 10.1111/micc.12080
129
PalmerS. K.MooreL. G.YoungD.CreggerB.BermanJ. C.ZamudioS. (1999). Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am. J. Obstet. Gynecol.180, 1161–1168. 10.1016/s0002-9378(99)70611-3
130
PalmerS. K.ZamudioS.CoffinC.ParkerS.StammE.MooreL. G. (1992). Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol.80, 1000–1006.
131
ParraguezV. H.AtlagichM.DiazR.CepedaR.GonzalezC.De Los ReyesM.et al (2006). Ovine placenta at high altitudes: comparison of animals with different times of adaptation to hypoxic environment. Anim. Reprod. Sci.95, 151–157. 10.1016/j.anireprosci.2005.11.003
132
PastoreM. B.TalwarS.ConleyM. R.MagnessR. R. (2016). Identification of differential ER-alpha versus ER-beta mediated activation of eNOS in ovine uterine artery endothelial cells. Biol. Reprod.94, 139. 10.1095/biolreprod.115.137554
133
PelsA.DerksJ.Elvan-TaspinarA.Van DrongelenJ.De BoerM.DuvekotH.et al (2020). Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction: a randomized clinical trial. JAMA Netw. Open3, e205323. 10.1001/jamanetworkopen.2020.5323
134
PijnenborgR.VercruysseL.HanssensM. (2006). The uterine spiral arteries in human pregnancy: facts and controversies. Placenta27, 939–958. 10.1016/j.placenta.2005.12.006
135
PostonL.BrileyA. L.SeedP. T.KellyF. J.ShennanA. H.Vitamins in Pre-Eclampsia TrialC. (2006). Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet367, 1145–1154. 10.1016/S0140-6736(06)68433-X
136
PoudelR.StanleyJ. L.Rueda-ClausenC. F.AnderssonI. J.SibleyC. P.DavidgeS. T.et al (2013). Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One8, e64401. 10.1371/journal.pone.0064401
137
RabinovitchM.GambleW.NadasA. S.MiettinenO. S.ReidL. (1979). Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol.236, H818–H827. 10.1152/ajpheart.1979.236.6.H818
138
RemienK.MajmundarS. H. (2023). –Physiology, fetal circulation,– in StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing.
139
RennieM. Y.RahmanA.WhiteleyK. J.SledJ. G.AdamsonS. L. (2015). Site-specific increases in utero- and fetoplacental arterial vascular resistance in eNOS-deficient mice due to impaired arterial enlargement. Biol. Reprod.92, 48. 10.1095/biolreprod.114.123968
140
RenshallL. J.MorganH. L.MoensH.CansfieldD.Finn-SellS. L.TropeaT.et al (2018). Melatonin increases fetal weight in wild-type mice but not in mouse models of fetal growth restriction. Front. Physiol.9, 1141. 10.3389/fphys.2018.01141
141
RisingC. L.D'alecyL. G. (1989). Hypoxia-induced increases in hypoxic tolerance augmented by beta-hydroxybutyrate in mice. Stroke20, 1219–1225. 10.1161/01.str.20.9.1219
142
RockwellL. C.DempseyE. C.MooreL. G. (2006). Chronic hypoxia diminishes the proliferative response of Guinea pig uterine artery vascular smooth muscle cells in vitro. High. Alt. Med. Biol.7, 237–244. 10.1089/ham.2006.7.237
143
RosenfeldC. R.DespainK.WordR. A.LiuX. T. (2012). Differential sensitivity to angiotensin II and norepinephrine in human uterine arteries. J. Clin. Endocrinol. Metab.97, 138–147. 10.1210/jc.2011-1818
144
RowanJ. A.RushE. C.PlankL. D.LuJ.ObolonkinV.CoatS.et al (2018). Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res. Care6, e000456. 10.1136/bmjdrc-2017-000456
145
RubanyiG. M.KauserK.JohnsA. (2002). Role of estrogen receptors in the vascular system. Vasc. Pharmacol.38, 81–88. 10.1016/s0306-3623(02)00130-1
146
RupnowH. L.PhernettonT. M.ShawC. E.ModrickM. L.BirdI. M.MagnessR. R. (2001). Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am. J. Physiol. Heart Circ. Physiol.280, H1699–H1705. 10.1152/ajpheart.2001.280.4.H1699
147
SamuniY.GoldsteinS.DeanO. M.BerkM. (2013). The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta1830, 4117–4129. 10.1016/j.bbagen.2013.04.016
148
SanchoM.KyleB. D. (2021). The large-conductance, calcium-activated potassium channel: a big key regulator of cell physiology. Front. Physiol.12, 750615. 10.3389/fphys.2021.750615
149
SchneiderH.SchubertK. M.BlodowS.KreutzC. P.ErdogmusS.WiedenmannM.et al (2015). AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension66, 108–116. 10.1161/HYPERTENSIONAHA.115.05514
150
ShriyanP.SudhirP.Van SchayckO. C. P.BabuG. R. (2023). Association of high cortisol levels in pregnancy and altered fetal growth. Results from the MAASTHI, a prospective cohort study, Bengaluru. Lancet Reg. Health Southeast Asia14, 100196. 10.1016/j.lansea.2023.100196
151
SinghC. K.KumarA.HitchcockD. B.FanD.GoodwinR.LavoieH. A.et al (2011). Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol. Nutr. Food Res.55, 1186–1196. 10.1002/mnfr.201000457
152
SinghH. J.KeahL. S.KumarA.SirajudeenK. N. (2012). Adverse effects of melatonin on rat pups of Wistar-Kyoto dams receiving melatonin supplementation during pregnancy. Exp. Toxicol. Pathol.64, 751–752. 10.1016/j.etp.2011.01.011
153
SkeffingtonK. L.HigginsJ. S.MahmoudA. D.EvansA. M.Sferruzzi-PerriA. N.FowdenA. L.et al (2016). Hypoxia, AMPK activation and uterine artery vasoreactivity. J. Physiol.594, 1357–1369. 10.1113/JP270995
154
SolovievA.Lehen'kyiV.ZelenskyS.HellstrandP. (2004). Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell. Calcium36, 165–173. 10.1016/j.ceca.2004.02.002
155
SongR.HuX. Q.RomeroM.HolguinM. A.KagaboW.XiaoD.et al (2021). Ryanodine receptor subtypes regulate Ca2+ sparks/spontaneous transient outward currents and myogenic tone of uterine arteries in pregnancy. Cardiovasc Res.117, 792–804. 10.1093/cvr/cvaa089
156
StaiculescuM. C.GalinanesE. L.ZhaoG.UlloaU.JinM.BeigM. I.et al (2013). Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res.98, 428–436. 10.1093/cvr/cvt034
157
StrickD. M.WaycasterR. L.MontaniJ. P.GayW. J.AdairT. H. (1991). Morphometric measurements of chorioallantoic membrane vascularity: effects of hypoxia and hyperoxia. Am. J. Physiol.260, H1385–H1389. 10.1152/ajpheart.1991.260.4.H1385
158
TaggartM. J.WrayS. (1998). Hypoxia and smooth muscle function: key regulatory events during metabolic stress. J. Physiol.509 (Pt 2), 315–325. 10.1111/j.1469-7793.1998.315bn.x
159
TanB.LinL.YuanY.LongY.KangY.HuangB.et al (2024). Endothelial progenitor cells control remodeling of uterine spiral arteries for the establishment of utero-placental circulation. Dev. Cell.59, 1842–1859.e12. 10.1016/j.devcel.2024.04.009
160
TanakaY.MeeraP.SongM.KnausH. G.ToroL. (1997). Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J. Physiol.502 (Pt 3), 545–557. 10.1111/j.1469-7793.1997.545bj.x
161
Tissot Van PatotM.GrilliA.ChapmanP.BroadE.TysonW.HellerD. S.et al (2003). Remodelling of uteroplacental arteries is decreased in high altitude placentae. Placenta24, 326–335. 10.1053/plac.2002.0899
162
TropeaT.WareingM.GreenwoodS. L.FeelischM.SibleyC. P.CottrellE. C. (2018). Nitrite mediated vasorelaxation in human chorionic plate vessels is enhanced by hypoxia and dependent on the NO-sGC-cGMP pathway. Nitric Oxide80, 82–88. 10.1016/j.niox.2018.08.009
163
TuranS.AberdeenG. W.ThompsonL. P. (2017). Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant Guinea pig. Am. J. Physiol. Regul. Integr. Comp. Physiol.313, R330-R339–R339. 10.1152/ajpregu.00056.2017
164
Van Der HeijdenO. W.EssersY. P.FazziG.PeetersL. L.De MeyJ. G.Van EysG. J. (2005). Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol. Reprod.72, 1161–1168. 10.1095/biolreprod.104.033985
165
Van HoveC. E.Van Der DoncktC.HermanA. G.BultH.FransenP. (2009). Vasodilator efficacy of nitric oxide depends on mechanisms of intracellular calcium mobilization in mouse aortic smooth muscle cells. Br. J. Pharmacol.158, 920–930. 10.1111/j.1476-5381.2009.00396.x
166
VaughanO. R.DaviesK. L.WardJ. W.De BlasioM. J.FowdenA. L. (2016). A physiological increase in maternal cortisol alters uteroplacental metabolism in the pregnant Ewe. J. Physiol.594, 6407–6418. 10.1113/JP272301
167
VuguinP. M. (2007). Animal models for small for gestational age and fetal programming of adult disease. Horm. Res.68, 113–123. 10.1159/000100545
168
WangZ.CammE. J.NuzzoA. M.SpiroskiA. M.SkeffingtonK. L.AshmoreT. J.et al (2024). In vivo mitochondria-targeted protection against uterine artery vascular dysfunction and remodelling in rodent hypoxic pregnancy. J. Physiol.602, 1211–1225. 10.1113/JP286178
169
WangZ.CheslerN. C. (2012). Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech. Model. Mechanobiol.11, 279–289. 10.1007/s10237-011-0309-z
170
WardJ. P.McmurtryI. F. (2009). Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr. Opin. Pharmacol.9, 287–296. 10.1016/j.coph.2009.02.006
171
WareingM. (2012). Effects of oxygenation and luminal flow on human placenta chorionic plate blood vessel function. J. Obstet. Gynaecol. Res.38, 185–191. 10.1111/j.1447-0756.2011.01666.x
172
WhiteM. M.McculloughR. E.DyckesR.RobertsonA. D.MooreL. G. (1998). Effects of pregnancy and chronic hypoxia on contractile responsiveness to alpha1-adrenergic stimulation. J. Appl. Physiol.85, 2322–2329. 10.1152/jappl.1998.85.6.2322
173
WhiteM. M.McculloughR. E.DyckesR.RobertsonA. D.MooreL. G. (2000). Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in Guinea pig uterine and thoracic arteries. Am. J. Physiol. Heart Circ. Physiol.278, H2069–H2075. 10.1152/ajpheart.2000.278.6.H2069
174
WilsonM. J.LopezM.VargasM.JulianC.TellezW.RodriguezA.et al (2007). Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am. J. Physiol. Regul. Integr. Comp. Physiol.293, R1313–R1324. 10.1152/ajpregu.00806.2006
175
XiaoD.BirdI. M.MagnessR. R.LongoL. D.ZhangL. (2001). Upregulation of eNOS in pregnant ovine uterine arteries by chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol.280, H812–H820. 10.1152/ajpheart.2001.280.2.H812
176
XiaoD.BuchholzJ. N.ZhangL. (2006). Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am. J. Physiol. Heart Circ. Physiol.290, H2337–H2343. 10.1152/ajpheart.01238.2005
177
XiaoD.HuangX.BaeS.DucsayC. A.LongoL. D.ZhangL. (2004). Cortisol-mediated regulation of uterine artery contractility: effect of chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol.286, H716–H722. 10.1152/ajpheart.00805.2003
178
XiaoD.HuangX.BaeS.DucsayC. A.ZhangL. (2002). Cortisol-mediated potentiation of uterine artery contractility: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol.283, H238–H246. 10.1152/ajpheart.00842.2001
179
XiaoD.HuangX.LongoL. D.ZhangL. (2010a). PKC regulates alpha(1)-adrenoceptors-mediated contractions and baseline Ca(2+) sensitivity in the uterine arteries of nonpregnant and pregnant sheep acclimatized to high altitude hypoxia. High. Alt. Med. Biol.11, 153–161. 10.1089/ham.2009.1076
180
XiaoD.HuangX.YangS.LongoL. D.ZhangL. (2010b). Pregnancy downregulates actin polymerization and pressure-dependent myogenic tone in ovine uterine arteries. Hypertension56, 1009–1015. 10.1161/HYPERTENSIONAHA.110.159137
181
XiaoD.HuangX.YangS.ZhangL. (2009). Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension54, 352–358. 10.1161/HYPERTENSIONAHA.109.130781
182
XiaoD.HuangX.ZhangL. (2012). Chronic hypoxia differentially up-regulates protein kinase C-mediated ovine uterine arterial contraction via actin polymerization signaling in pregnancy. Biol. Reprod.87, 142. 10.1095/biolreprod.112.104448
183
XiaoD.LongoL. D.ZhangL. (2010c). Role of KATP and L-type Ca2+ channel activities in regulation of ovine uterine vascular contractility: effect of pregnancy and chronic hypoxia. Am. J. Obstet. Gynecol.203, 596.e6–596.e12. 10.1016/j.ajog.2010.07.038
184
XiaoD.ZhangL. (2002). ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol.282, H292–H300. 10.1152/ajpheart.2002.282.1.H292
185
XiaoD.ZhangL. (2004). Calcium homeostasis and contraction of the uterine artery: effect of pregnancy and chronic hypoxia. Biol. Reprod.70, 1171–1177. 10.1095/biolreprod.103.024943
186
XiaoD.ZhuR.ZhangL. (2014). Gestational hypoxia up-regulates protein kinase C and inhibits calcium-activated potassium channels in ovine uterine arteries. Int. J. Med. Sci.11, 886–892. 10.7150/ijms.9338
187
YangY.XuP.ZhuF.LiaoJ.WuY.HuM.et al (2021). The potent antioxidant MitoQ protects against preeclampsia during late gestation but increases the risk of preeclampsia when administered in early pregnancy. Antioxid. Redox Signal34, 118–136. 10.1089/ars.2019.7891
188
YungH. W.CoxM.Tissot Van PatotM.BurtonG. J. (2012). Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J.26, 1970–1981. 10.1096/fj.11-190082
189
ZamudioS.PalmerS. K.DromaT.StammE.CoffinC.MooreL. G. (1995). Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol.79, 7–14. 10.1152/jappl.1995.79.1.7
190
ZhangH.XiaoD.LongoL. D.ZhangL. (2006). Regulation of alpha1-adrenoceptors-mediated contractions of uterine arteries by PKC: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol.291, H2282–H2289. 10.1152/ajpheart.00321.2006
191
ZhangL.LiuJ.FengX.LashG. E. (2023). Unraveling the mysteries of spiral artery remodeling. Placenta141, 51–56. 10.1016/j.placenta.2023.05.013
192
ZhuR.HuX. Q.XiaoD.YangS.WilsonS. M.LongoL. D.et al (2013). Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension62, 367–374. 10.1161/HYPERTENSIONAHA.113.01236
193
ZhugeR.FogartyK. E.TuftR. A.WalshJ. V.Jr. (2002). Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca(2+) concentration on the order of 10 microM during a Ca(2+) spark. J. Gen. Physiol.120, 15–27. 10.1085/jgp.20028571
Summary
Keywords
hypoxia, pregnancy, uterine artery, placenta, vascular reactivity
Citation
Arenas GA and Lorca RA (2024) Effects of hypoxia on uteroplacental and fetoplacental vascular function during pregnancy. Front. Physiol. 15:1490154. doi: 10.3389/fphys.2024.1490154
Received
02 September 2024
Accepted
03 December 2024
Published
18 December 2024
Volume
15 - 2024
Edited by
Mitchell C. Lock, The University of Manchester, United Kingdom
Reviewed by
Josele Flores-Santin, Universidad Autónoma del Estado de México, Mexico
Ashley Meakin, University of South Australia, Australia
Updates
Copyright
© 2024 Arenas and Lorca.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramón A. Lorca, ramon.lorca@cuanschutz.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.