Abstract
Pain management in chronic pancreatitis (CP) patients remains a major challenge, largely due to complex and refractory pain. Such pain detrimentally impacts patients by reducing quality of life, limiting daily activities, increasing psychological distress, necessitating frequent hospitalizations, and contributing to opioid dependence and socioeconomic burden. This review delineates the multifaceted nature of CP-related pain, highlighting the roles of neurogenic inflammation, maladaptive neuroplasticity, and disrupted pain modulation pathways. Current management strategies are multidisciplinary, encompassing lifestyle modification, pharmacologic therapies, endoscopic and surgical interventions, and nerve-targeted procedures (e.g., celiac plexus blocks and neurolysis). Advances in genetics, bioinformatics and biomarker research have further enhanced our understanding of CP-related pain pathogenesis, paving the way for precision medicine approaches. This review highlights current evidence and emerging innovations in the evolving landscape of CP-related pain management, emphasizing the importance of tailored and interdisciplinary care to address the intricate mechanism of CP-related pain and improve patient outcomes.
1 Introduction
Chronic pancreatitis (CP) is a progressive inflammatory disorder of the pancreas characterized by a fibro-inflammatory process leading to irreversible destruction of the pancreas, ultimately resulting in fibrosis, calcification and exocrine and endocrine insufficiencies. One clinical hallmark of CP is persistent abdominal pain, commonly described as a dull, sharp or nagging sensation in the upper abdomen or radiating to the back (Yadav et al., 2023). Severe and constant pain in CP not only reduces quality of life but also impairs physical functioning, and contributes to considerable psychological distress, manifesting as anxiety, insomnia and depression (Yadav et al., 2023) (Figure 1).
FIGURE 1
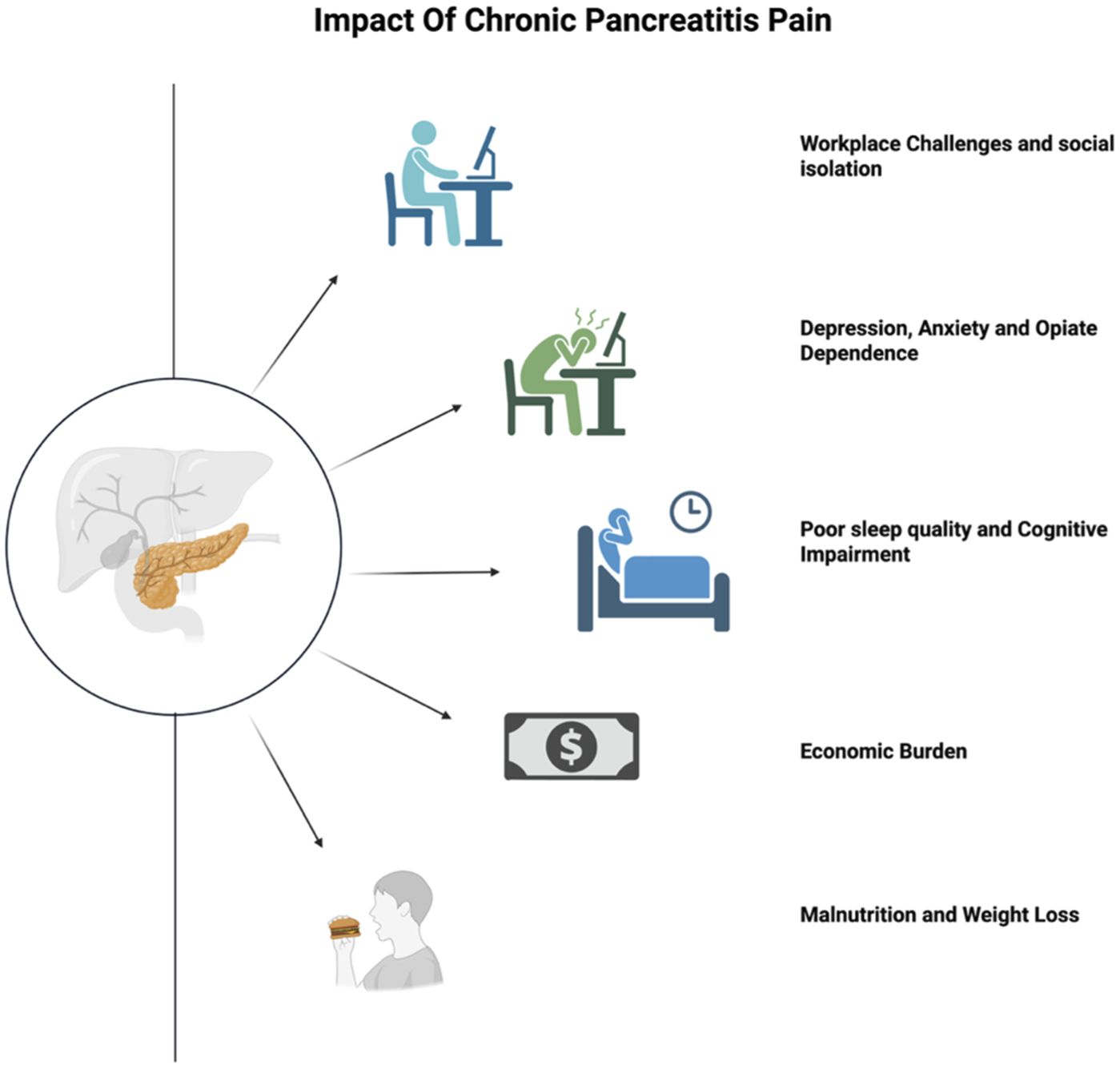
Burden of Pain from Chronic Pancreatitis. Figure created with www.biorender.com.
CP affects an estimated 50 individuals per 100,000 in the general population (Mann et al., 2021), with risk shaped by factors such as alcohol consumption, smoking genetic predispositions, and socioeconomic status. About 85% patients report persistent pain within four to 5 years of disease onset (Ammann et al., 1984). Even after more than a decade of follow-up, up to 60% of CP patients continue experiencing painful attacks (Lankisch et al., 1995). The North American Pancreatitis Study 2 (NAPS2), the largest multi-center study of prospectively enrolled CP patients in the United States, has further revealed race-specific disparities in pain severity. Compared to white patients, Black individuals with CP exhibit more severe disease manifestations, including pronounced morphological pancreatic abnormalities, higher rates of persistent pain, increased prevalence of endocrine dysfunction, and greater disease-related disability (Wilcox et al., 2016).
CP-related pain imposes a substantial economic burden, with repeated emergency department visits, opioid prescriptions, and surgical or endoscopic interventions escalating expenses for both the healthcare system and individual patients (Wen et al., 2024).
Despite advancements in understanding CP pathophysiology, effective pain management remains a persistent challenge. Conventional treatments, including analgesics, neuromodulators, surgery, endoscopic procedures, and nerve-targeted procedures, often yield only partial relief and carry notable side effects. Additionally, the increasing reliance on opioids raises concerns about dependence and misuse. This issue is further complicated by limited data on opioid utilization in CP and the lack of standardized prescribing guidelines, leaving providers uncertain about the most effective treatment strategies (Singh et al., 2019).
2 Pathophysiology of pain in chronic pancreatitis
Pain in CP is multifactorial, involving a combination of nociceptive and neuropathic components. Nociceptive pain originates from tissue inflammation and damage within the pancreas, whereas neuropathic pain is caused by injury to pancreatic or extra-pancreatic nerves, leading to disrupted nerve signaling. Prolonged inflammation also fosters structural and functional changes in nociceptive pathways, driving pain amplification and chronicity (van Zeggeren et al., 2025; Thierens et al., 2025). A foundational understanding of the normal innervation (Figure 2) of the pancreas is essential for contextualizing the mechanisms underlying pain in CP (Lkhagvasuren et al., 2021).
FIGURE 2
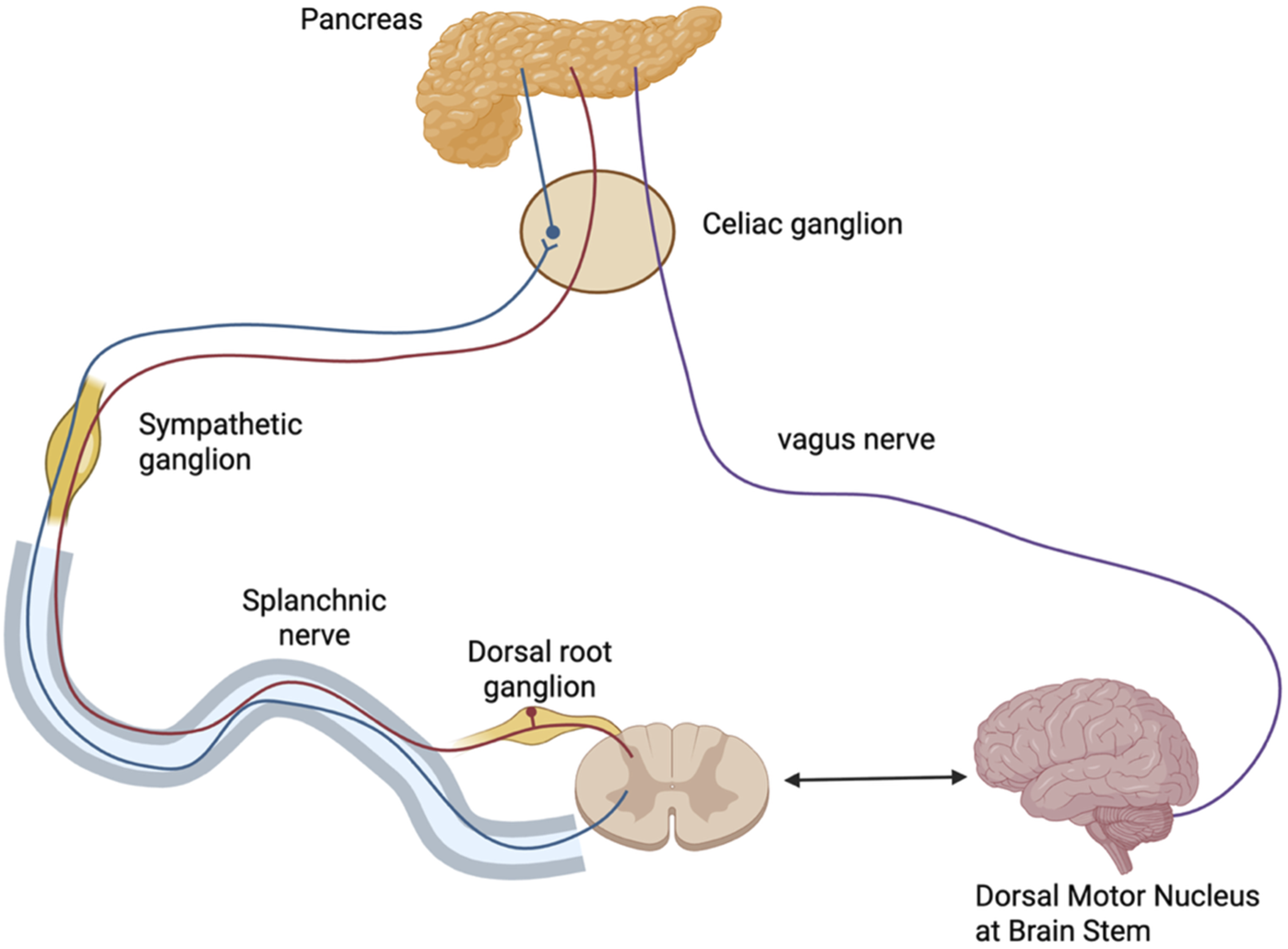
Innervation of the Pancreas. Parasympathetic fibers (purple) originate from the dorsal motor nucleus of the vagus in the brainstem and travel via the vagus nerve through the celiac region to reach intrapancreatic ganglia. Sympathetic fibers (blue) arise from the thoracic spinal cord, with preganglionic fibers projecting via the splanchnic nerve to synapse in the celiac ganglion; postganglionic fibers continue to the pancreas. Sensory afferents (red) from the pancreas travel back through the splanchnic nerve to the dorsal root ganglion and spinal cord, forming a bidirectional communication loop with the brain. Figure created with www.biorender.com.
2.1 Peripheral nociception and sensitization
2.1.1 Neurogenic inflammation as a key driver of peripheral sensitization
Peripheral sensitization in CP results from the heightened responsiveness of pancreatic nociceptors due to sustained exposure to inflammatory mediators. This process is driven in part by neurogenic inflammation, which is initiated by the release of neuropeptides—such as substance P, calcitonin gene-related peptide (CGRP), and brain-derived neurotrophic factor (BDNF), from sensory nerves themselves, but also other cells such as immune cells and glial cells (Figure 3).
FIGURE 3
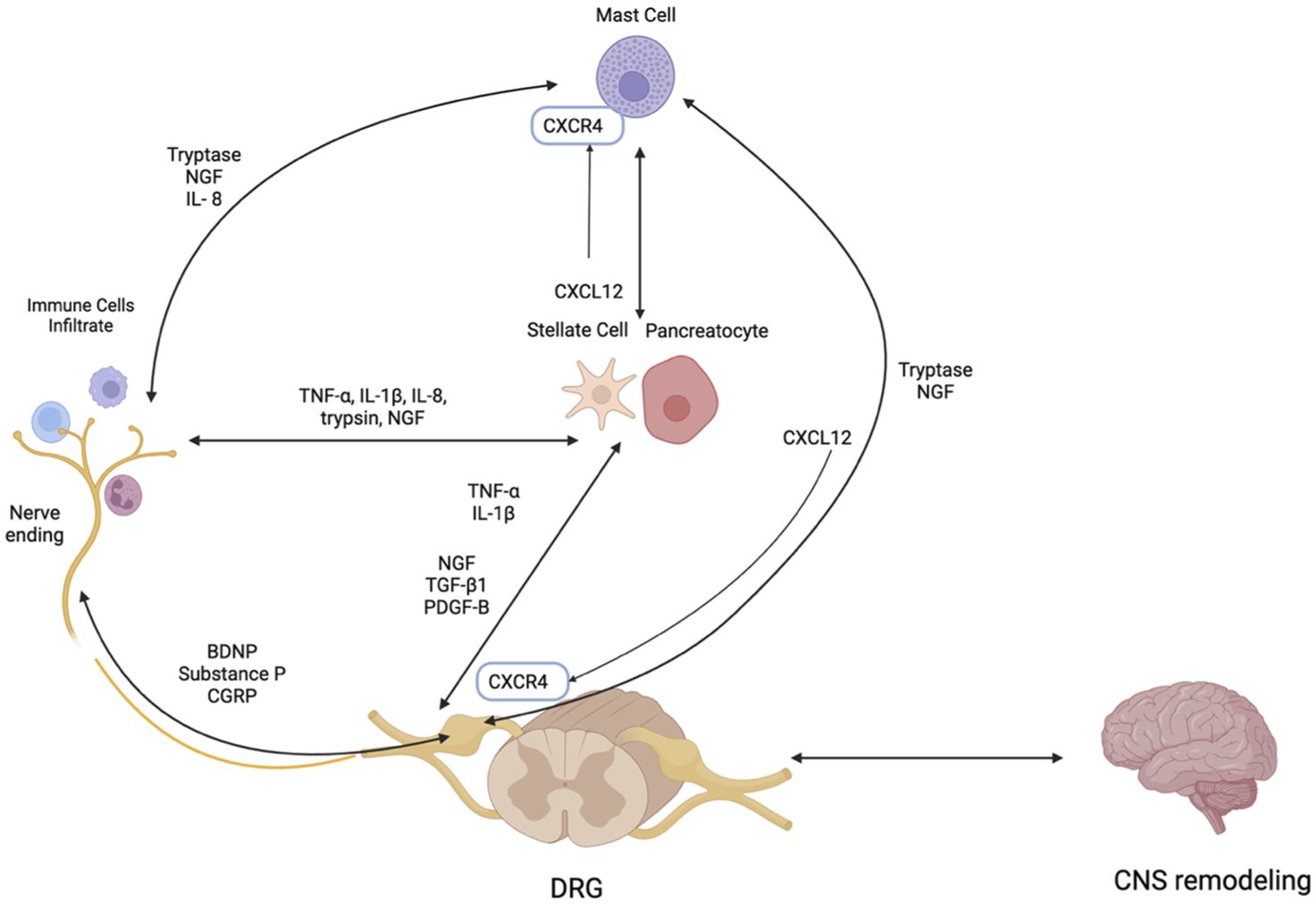
Neuroimmune Interactions in Chronic Pancreatitis: Key Mediators and Pathways. PDGF-B - Platelet-derived growth factor subunit B, CXCL 12- C-X-C motif ligand 12, CNS–Central nervous system, CXCR4 – C-X-C chemokine receptor 4, IL-1β–Interleukin-1 beta, NGF–Nerve growth factor, TGF-β1 – Transforming growth factor-beta, TNF-α–Tumor necrosis factor-alpha, NGF- Nerve Growth Factor, DRG- Dorsal Root Ganglion, BDNF–Brain-Derived Neurotrophic Factor. Figure created with www.biorender.com.
In CP, while acinar cell injury is often the initial trigger, neurogenic inflammation can occur early and contribute to the amplification and perpetuation of the inflammatory response. Unlike inflammation initiated by immune cell infiltration, neurogenic inflammation originates from the peripheral nerve terminals themselves, through the release of neuropeptides. Experimental models of CP demonstrate that pharmacologic blockade of substance P, CGRP, and BDNF can attenuate pain (Winston et al., 2005; Fregni et al., 2007), underscoring their critical role in this process. The resulting inflammatory milieu lowers the activation threshold of peripheral sensory fibers, perpetuating pain even in the absence of ongoing pancreatic damage (Winston et al., 2005). In addition, neurogenic inflammation promotes vascular permeability, edema, and further nerve sensitization, highlighting its potential as a strategic target for therapeutic intervention (De et al., 2011).
2.1.2 Critical signaling in peripheral sensitization
Peripheral sensitization is further amplified by a diverse set of mediators. Key contributors include pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), chemokines like C-X-C motif ligand 12 (CXCL12), and neurotrophic factors like nerve growth factor (NGF). These mediators alter the function of ion channels on nociceptive neurons, especially transient receptor potential vanilloid (TRPV)1 and 4 and reduce voltage-gated A-type (IA) potassium currents, lowering the threshold for pain perception. Pharmacologic antagonism of TRPV1 or TRPV4 has been shown to significantly reduce pain behaviors and hypersensitivity in CP models (Xu et al., 2007).
Pancreatic proteases such as trypsin further exacerbate sensitization by activating protease-activated receptor-2 (PAR-2) on sensory neurons, which in turn sensitizes TRPV1 (Olesen et al., 2017) and promotes the release of pro-nociceptive neuropeptides like substance P and CGRP (Olesen et al., 2017). These interactions fuel neurogenic inflammation and intensify pain signaling, especially during episodes of acute exacerbation.
NGF, normally confined to pancreatic islets, becomes widespread in CP, upregulating the TRPV1 expression and contributing to hyperalgesia (Olesen et al., 2017). In CP models, anti-NGF therapy reduces pancreatic hyperalgesia by decreasing TRPV1 current density, suppressing its expression (Olesen et al., 2017), and restoring IA potassium currents. This results in reduced nociceptor excitability and highlights NGF’s pivotal role in modulating pain pathways in CP (Zhu et al., 2011; Zhu et al., 2012a).
Additional factors such as transforming growth factor-beta1 (TGF-β1), platelet-derived growth factor B (PDGF-B), and glycoprotein 130 (GP130) are also upregulated in CP and contribute to persistent pain. TGF-β1 increases sensory neuron excitability by prolonging action potential duration, and downregulating IA currents, and its blockade has been shown to alleviate pain in CP models (Zhu et al., 2012b). Elevated levels of PDGF-B and GP130 in patients with opioid-refractory pain phenotypes in CP further implicate these molecules in sensitization and suggest a broader role for immune signaling in chronic pain maintenance (Saloman et al., 2023).
2.2 Peripheral neuropathy
2.2.1 Pancreatic neuroplasticity and neural remodeling
In addition to neurogenic inflammation and a wide array of inflammatory and neurotrophic mediators, pancreatic neuroplasticity–characterized by increased nerve diameter and density, plays central roles in amplifying and maintaining pain in CP. This phenomenon, distinguished by intrapancreatic neural hypertrophy and hyperinnervation, is uniquely observed in CP and pancreatic cancer among pancreatic disorders (Ceyhan et al., 2009). Two key mediators driving these changes are growth-associated protein 43 (GAP-43) and neuturin. GAP-43, a critical molecule for neuronal development and axonal regeneration, is elevated in CP tissues and correlates with enhanced neural plasticity. Neurturin supports neuronal survival and differentiation. Its overexpression not only accelerates neuroplasticity but also enhances the invasiveness of pancreatic cancer cells, indicating a dual role in disease progression and pain manifestation (Wang et al., 2013; Demir et al., 2012).
Neural remodeling in CP refers to qualitative changes in pancreatic nerves, including shifts in the relative proportions of autonomic and sensory fibers (Demir et al., 2015). This process involves not only neurons but also glial cells. Notably, remodeled nerves in CP show increased expression of the neuroepithelial stem cell marker nestin and reduced expression of the glial transcription factor SOX10, suggesting enhanced activity of sprouting neural progenitor cells and potential dedifferentiation of peripheral glia. These findings point to a complex structural and cellular reorganization that serves as the qualitative basis for sustained pain and neuropathy in CP (Olesen et al., 2017; Ceyhan et al., 2010).
2.2.2 Pancreatic neuritis and neuro-immune interactions
Pancreatic neuritis, the infiltration of immune cells into intrapancreatic nerves, is a hallmark feature of CP and plays a significant role in chronic pain (Demir et al., 2015). A growing body of evidence supports the contribution of neuro-immune interactions to pain mechanisms in CP, particularly through the presence of CD8+ cytotoxic T cells, macrophages, and mast cells (Demir et al., 2013). Among these, mast cells are especially notable for their bidirectional communication with nerve endings, a mechanism well-established in other gastrointestinal disorders such as irritable bowel syndrome (Barbara et al., 2004). This crosstalk may promote sustained neuropathic pain in CP (Buhner and Schemann, 2012).
A key chemokine signaling pathway mediating this neuro-immune interplay is the CXCL12/C-X-C chemokine receptor type 4 (CXCR4) axis. This axis is upregulated in CP and facilitates the recruitment of immune cells to perineural regions. In animal models, CXCL12 and its receptor CXCR4 are significantly elevated in the dorsal root ganglion (DRG) of CP rats, linking local immune activation to persistent pain (Zhu et al., 2017). As a result, the CXCL12/CXCR4 axis represents a potential therapeutic target, and strategies such as cytokine inhibitors, mast cell stabilizers, and anti-inflammatory agents are currently under investigation to reduce neuritis-associated pain (Cheng et al., 2019).
2.2.3 Role of dorsal root ganglion in neuropathic pain
The DRG serves as a critical interface in the pathophysiology of CP-related pain, particularly in peripheral neuropathy. In CP, DRG neurons exhibit stromal hypertrophy, spontaneous firing, and axonal hyperbranching (Takamido et al., 2006), along with upregulation of pain-related ion channels and growth-associated proteins such as TRPV1 and GAP-43 (Di Sebastiano et al., 2003). These structural and molecular changes enhance DRG excitability, contributing to persistent pain signaling.
Furthermore, immune-driven inflammation, including mast cell infiltration and the release of inflammatory mediators such as IL-8 and histamine, further sensitizes DRG neurons (Demir et al., 2013). As described above, chemokine signaling via CXCL12/CXCR4 axis also plays a dual role, both in immune cell recruitment and in directly modulating DRG excitability, further bridging immune activation with peripheral neuropathic pain (Zhu et al., 2017). Altogether, the DRG acts as a dynamic modulator of neuroinflammation, making it a compelling site for therapeutic intervention in CP-associated neuropathy.
2.3 Central sensitization
Central sensitization is a fundamental mechanism underlying chronic pain, characterized by heightened excitability of neurons in the central nervous system (CNS) following prolonged peripheral nociceptive input (Kuhlmann et al., 2025), marked by increased synaptic efficacy, loss of inhibitory control and glial cell activation. These changes result in the amplification of pain perception (hyperalgesia) and the emergence of pain in response to non-painful stimuli (allodynia), even in the absence of ongoing tissue damage (Woolf, 2011).
2.3.1 Spinal contributions to central sensitization
At the spinal level, the dorsal horn of the spinal cord plays a central role in amplifying pancreatic pain through mechanisms of central sensitization. CP induces increased spontaneous activity and excitability in DRG neurons, which represent peripheral sensitization and contribute to enhanced nociceptive input to the spinal cord. This amplified input triggers central sensitization in the dorsal horn, characterized by increased responsiveness and synaptic efficacy of second-order neurons, leading to prolonged hyperalgesia and pain independent of initial peripheral triggers (Olesen et al., 2017). Specifically, recent animal models have shown that CP induces significant upregulation of P2X7 receptor expression in spinal microglia, which mediates their activation and contributes to visceral hyperalgesia in a rat model of CP (Liu et al., 2012).
Glial cells, particularly microglia and astrocytes, play essential roles in the development and maintenance of central sensitization in CP-related pain. At the spinal cord level, microglial activation has been strongly implicated in the maintenance of visceral hyperalgesia. Liu et al. observed a marked transition of microglia from a resting to an activated state in the thoracic spinal cord 3 weeks after CP induction in rats. This prolonged activation is likely driven by persistent visceral inflammation and contributes to sustained pain states. Pharmacological inhibition of microglial activity using minocycline effectively attenuated pain behaviors, highlighting microglia as a central mediator of pain persistence in CP (Liu et al., 2012). Subsequent studies further showed that activated microglia colocalize with upregulated P2X7 receptor expression in the spinal cord, and that inhibition of P2X7 receptor significantly reduced nociceptive behaviors in CP models (Liu et al., 2012; Liu et al., 2015).
In parallel, astrocytes have also been shown to contribute to spinal central sensitization. Feng et al. demonstrated increased expression of glial fibrillary acidic protein (GFAP) in the thoracic spinal cord of CP rats, indicating astrocytic activation. Inhibition of astrocytic activation could attenuate pain of CP, suggesting that spinal astrocytes are integral to central sensitization in CP-related pain (Feng et al., 2010).
2.3.2 Supraspinal structures involved in central sensitization
Supraspinally, several CNS regions have been implicated in the modulation of CP-related pain. These include the anterior cingulate cortex (ACC), anterior insular cortex (aIC), paraventricular hypothalamic nucleus (PVH), periaqueductal gray (PAG), nucleus tractus solitarius (NTS), and lateral parabrachial nucleus (LPB) (Ren et al., 2022; Bai et al., 2019a; Liu et al., 2020).
Ren et al. identified the ACC as a key cortical hub mediating both hyperalgesia and anxiety in a rat model of CP. Tract tracing and immunostaining demonstrated a direct nociceptive projection from the NTS to the ACC. CP induced glutamatergic hyperactivity in the ACC, marked by increased vesicular glutamate transporter (VGluT) 1 expression and enhanced membrane trafficking and phosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor glutamate receptor subunit 1(GluR1) and N-methyl-D-aspartate (NMDA) receptor NR2B subunits. Inhibition of ACC glutamatergic transmission through receptor antagonists or chemogenic suppression of pyramidal neurons significantly reduced pain and anxiety behaviors, highlighting the ACC as a neuromodulatory target in CP pain (Ren et al., 2022). Similarly, Bai et al. demonstrated that the aIC is critically involved in mediating both hyperalgesia and pain-related anxiety in a rat model of CP. In CP rats, aIC neurons exhibited upregulated VGluT1 expression, along with increased membrane trafficking and phosphorylation of NR2B and GluR1 subunits (Bai et al., 2019a).
The PAG has also been implicated in CP-associated central sensitization. Liu et al. found that dysfunction of the brain-gut axis in the midbrain ventrolateral PAG contributes to visceral pain, with reduced presynaptic glutamate release and diminished postsynaptic AMPA receptor-mediated responses. These neuroplastic changes weaken excitatory synaptic strength and impair descending pain inhibition (Liu et al., 2020). In a separate study, Yang et al. reported sustained activation of PAG neurons in CP models, as indicated by elevated c-Fos expression. Using a gene therapy approach to express enkephalin, they showed reduced PAG activation and visceral nociceptive behaviors, supporting a role for the PAG in mediating pain responses in CP (Yang et al., 2008).
The hypothalamus has also emerged as a relevant supraspinal region in CP pain. Recent animal studies have demonstrated that glutamatergic neurons in the PVH mediate visceral pain responses (Luo et al., 2024). Dysfunctional astrocyte-mediated glutamate clearance in this region contributes to both pain and anxiety-like behaviors in mice, implicating the PVH in affective-motivational components of CP pain (Luo et al., 2024; Ji et al., 2024).
Within the brainstem, the NTS functions as a key integration center for visceral afferents. In CP animal models, increased Fos expression, enhanced excitatory synaptic transmission, and upregulation of glutamatergic receptors (VGluT2, GluR1, NR2B) are observed within NTS (Bai et al., 2019b). Direct modulation of this region via glutamate receptor antagonists significantly reduces visceral pain, supporting the NTS as a contributor of CP-related central sensitization (Bai et al., 2019b).
Finally, the LPB has been implicated in mediating visceral hypersensitivity in CP. Wu et al. showed that CP induces glutamate release and selective NR2B upregulation in LPB neurons (Wu et al., 2025). Inhibition of NR2B receptors or LPB glutamatergic neurons significantly alleviated pain, confirming the LPB’s role in central sensitization (Wu et al., 2025).
At the supraspinal level, glial contributions have also been identified. Luo et al. reported that dysfunctional astrocytic glutamate uptake in the PVH contributes to visceral pain and anxiety in mice with CP, potentially due to deficiency of astroglial glutamate transporter-1 (GLT-1). Pharmacological activation of GLT-1 alleviated VGluT2 neuron hyperexcitability, as well as abdominal visceral pain and anxiety-like behaviors, suggesting GLT-1 as a potential therapeutic target for CP-related pain (Luo et al., 2024).
2.3.3 Emerging tools and knowledge gaps in central sensitization of CP
Recent studies have begun to systematically characterize central hyperalgesia, impaired descending inhibitory pain modulation, and disrupted brain resting activity in CP patients. Notably, electroencephalogram recordings have revealed increased delta and decreased alpha peak frequencies, indicating disrupted cortical dynamics (de Vries et al., 2013). Structural and functional brain alterations have also been reported, including microstructural changes in the amygdala, cingulate cortex, and insula, as well as cortical thinning in the prefrontal and secondary somatosensory cortices. These structural changes particularly in cingulate and prefrontal cortices, identified by diffusion tensor imaging and magnetic resonance imaging (MRI), correlate with patients’ reported pain intensity (Frøkjær et al., 2011).
Despite this progress, significant gaps remain in our understanding of central sensitization in CP-related pain. For instance, the degree to which genetic predisposition, environmental exposures, or individual variability influence the development and persistence of central sensitization remains unclear. Key questions include, but are not limited to, whether certain individuals are more genetically or epigenetically predisposed to central sensitization, which risk factors contribute to the chronification of pain hypersensitivity. Additionally, improving the diagnostic accuracy of central sensitization will be essential for patient phenotyping and for selecting treatments aimed at normalizing hyperexcitable central neural circuits (Woolf, 2011). There is a critical need for further research to elucidate the precise contribution of central neural circuits to CP-related pain, which may ultimately inform more targeted neuromodulatory treatments.
2.4 Structural Abnormality
Traditional theories have attributed pain in CP to structural abnormalities, such as ductal obstructions, pancreatic fibrosis, and associated complications like pseudocysts. Ductal blockages elevate intraductal pressure, disrupt exocrine flow, and may induce localized ischemia, all of which can intensify nociceptive signaling via visceral nerves. Interventions aimed at reducing these obstructions (e.g., endoscopic stone removal and surgical decompression) often yield varying success in pain relief. However, studies demonstrate a limited correlation between ductal obstruction relief and long-term improvement in pain scores, suggesting that structural changes alone do not fully account for the pain experienced in CP (Whitcomb et al., 2008; Wilcox et al., 2015).
Progressive pancreatic fibrosis, a hallmark of CP, often contributes to the formation of pseudocysts. These fluid-filled structures can compress surrounding tissues and provoke severe abdominal pain. As CP advances, fibrosis and inflammation can extend to adjacent organs, including the biliary system, duodenum, stomach, and spleen, leading to complications in bile duct or duodenal strictures. Fibrotic remodeling of the duodenum results in reduced duodenal flexibility and luminal narrowing, while pseudocysts impose external pressure. While endoscopic ultrasound-guided drainage is often effective for pseudocyst-induced obstruction, surgical solutions (e.g., bypass procedures) may be necessary for fibrotic obstructions to restore gastrointestinal continuity (Bansal et al., 2019).
Recent large-center studies indicate that the severity and patterns of CP-related abdominal pain do not consistently match the extent of structural anomalies on MRI, radiographs and endoscopic imaging. Although patients with obstructive changes or with inflammatory disease more frequently report severe pain, those with pseudocysts were more likely to report mild pain (Whitcomb et al., 2008; Wilcox et al., 2015).
2.5 Additional pathophysiologic drivers of CP-related pain
2.5.1 Genetic underpinnings of pain sensitization
Recent investigations on genetics of pain among CP patients underscore the involvement of several key pathways in peripheral sensitization, inflammation, and central sensitization. Three pancreas-related genes (CTRC, NEURL3, HSF2) have been associated with constant chronic pain, while the injury-response gene (REG3) correlates with pain severity (Dunbar et al., 2025). Further, four genes implicated in psychiatric stress disorders (TMEM65, RVFOX1, ZNF385D and LDLR) were linked to more severe pain, suggesting psychological factors exacerbate CP-related pain (Dunbar et al., 2021; Dunbar et al., 2020). Notably, four additional genes (SNYPR, NTF3, DOK6, RBFOX1) are implicated in BDNF-mediated neuropathic pain, with elevated BDNF levels observed in CP patients. These changes further lead to increased release of glutamate, CGRP, and substance P in both the periphery and CNS (Dunbar et al., 2025). Further exploration could guide the development of mechanism-based therapies, paving the way for individualized pain management in CP patients.
2.5.2 Role of pancreatic stellate cells in fibro-inflammatory pain pathways
Fibrosis and persistent inflammation are central to the pathogenesis of CP and contribute significantly to associated pain. Pancreatic stellate cells (PSCs) are key mediators of this fibro-inflammatory response. In their quiescent state, PSCs store vitamin A, but become activated upon pancreatic injury or inflammation, transitioning into myofibroblast-like cells that secrete extracellular matrix proteins such as type I collagen (Masamune et al., 2009; Kong et al., 2024). Activated PSCs also release pro-inflammatory cytokines (e.g., IL-1, IL-6, TNF-α, TGF-β1), chemokines, and matrix metalloproteinases, which perpetuate immune cell recruitment and tissue remodeling. This creates a pro-inflammatory microenvironment that supports neuronal sensitization and chronic pain (Hines and Pandol, 2024). Notably, PSCs secrete neuroectodermal markers such as GFAP, as well as neurotrophins such as NGF and artemin (Jiang et al., 2020; Haas et al., 2009; Ceyhan et al., 2007); the latter could play a role in the neural hypertrophy seen in CP. Interestingly, PSCs can also activate mast cells, which in turn secrete IL-13 and tryptase, both of which activate PSCs (Ma et al., 2013). PSCs also secrete CXCL12 (Lu et al., 2022), the ligand for the axis implicated in neuropathic pain (Luo et al., 2016). Thus, PSCs are likely to play an important role in pancreatic pain, a subject that is worthy of further research. Indeed, several intracellular signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinases (MAPKs), and reactive oxygen species (ROS) that regulate PSC activation are targets for emerging therapies (Masamune et al., 2009; Masamune and Shimosegawa, 2009).
3 Current pain management
3.1 Drug therapeutics or treatment
For symptomatic pain relief in CP, patients typically follow World Health Organization guidelines, starting with non-opioid analgesics [e.g., acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs)]. Co-analgesics or adjuvant analgesics, including antidepressants and neuromodulators, are also widely employed in managing chronic visceral and neuropathic pain (Figure 4) (Table 1).
FIGURE 4
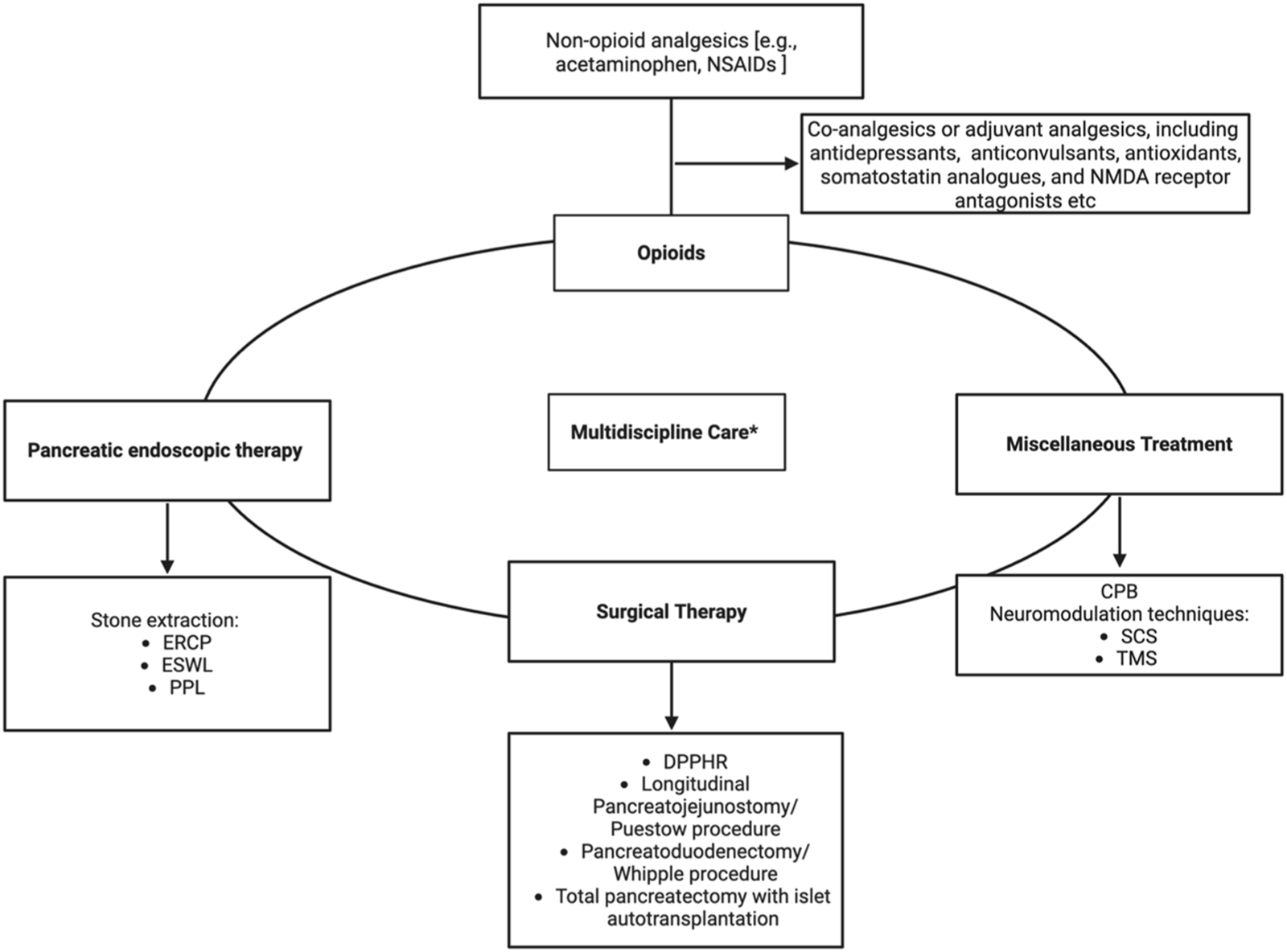
Algorithm for Multimodal Pain Management in Chronic Pancreatitis. NSAIDs–Nonsteroidal anti-inflammatory drugs, ERCP–Endoscopic Retrograde Cholangiopancreatography, ESWL–Extracorporeal Shock Wave Lithotripsy, PPL–Pancreatoscopy-guided lithotripsy, DPPHR–Duodenum-preserving pancreatic head resection, CPB–Celiac plexus block, SCS–Spinal cord stimulation, TMS–Transcranial magnetic stimulation. Figure created with www.biorender.com.
Gabapentinoids, such as pregabalin and gabapentin, have demonstrated efficacy in neuropathic pain conditions. Recent animal studies suggest that gabapentin may potentiate the analgesic effect of morphine in pancreatitis (Smiley et al., 2004). Antidepressants, including serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs), are frequently used as neuromodulators. While direct evidence in CP remains limited (Lunn et al., 2014), most authoritative guidelines acknowledge their potential role (Talukdar and Reddy, 2013). However, recommendations are largely based on lower-level evidence and extrapolation from other chronic neuropathic pain conditions.
Oxidative stress plays a significant role in the pancreatic injury and contributes to persistent inflammation. Both clinical and experimental studies have demonstrated increased production of ROS and depletion of endogenous antioxidants, particularly within pancreatic acinar and stellate cells. Accordingly, antioxidant therapy has been proposed as a disease-modifying intervention. A combination of micronutrients—commonly including methionine, selenium, vitamins C and E, and β-carotene—has been shown to restore systemic antioxidant capacity (Zhou et al., 2015). Clinical trials and meta-analyses suggest that such supplementation may reduce pain intensity and analgesic consumption in a subset of patients, presumably through suppression of ROS-mediated inflammatory signaling and modulation of neuroimmune interactions within the pancreas (Talukdar et al., 2015; Zhou et al., 2015). However, the precise mechanisms by which oxidative stress influences nociceptive processing in CP remain to be fully elucidated.
Often, managing pain in CP necessitates the use of opioid therapy. Tramadol, a weak opioid agonist that inhibits norepinephrine and serotonin reuptake, is frequently utilized for moderate pain management in CP due to its more favorable side-effect profile compared with stronger opioids like morphine (Edinoff et al., 2021). For more severe or persistent pain, stronger opioid, such as fentanyl patches, may be considered particularly for patients who have difficulty swallowing or adhering to oral medication regimens (Fu et al., 2024). However, achieving adequate analgesia often necessitates doses above the manufacturer’s recommendations. Thus, these agents should be reserved for individuals with established opioid tolerance or dysphasia (Niemann et al., 2000).
Less commonly used agents also show promise in treating refractory pain. Somatostatin analogues and ketamine have been explored for pain relief (Horváth et al., 2022), although their clinical benefits remain primarily anecdotal. Additionally, clonidine, benzodiazepines, antipsychotics and cannabinoids have been used off-label for refractory CP pain clinically.
In cases where analgesic therapy fails to adequately control pain, current practice emphasizes a multimodal approach tailored to the patient’s specific anatomy, disease etiology, and symptom severity, rather than strictly adhering to a linear progression of medical to surgical therapies. Previously, it was thought that in cases where pain persists despite prolonged use of analgesics (often opioids) and multiple endoscopic procedures, up to 75% of patients may ultimately require invasive surgical intervention (Bouwense et al., 2015).
3.2 Endoscopic intervention
Pancreatic endoscopic therapy is most frequently indicated for CP patients with proximal main pancreatic duct obstructions, commonly a single stone or a stricture in the head of the pancreas (Strand et al., 2022). Early intervention tends to yield better outcomes, before extensive fibrotic or inflammatory changes become entrenched.
Two primary techniques that address pancreatic duct obstruction are stenting and stone extraction. Stenting involves the placement of pancreatic duct stents to relieve obstruction and facilitate ductal drainage, while stone extraction involves endoscopic balloon retrieval and often combined with extracorporeal shockwave lithotripsy (ESWL) to fragment and remove intraductal stones (Tandan et al., 2019).
In cases of a dominant stricture in the main pancreatic duct that results in significant ductal dilation, ductal stenting is often employed alongside endoscopic therapy. Stents help to bypass the obstruction, restore ductal flow, and alleviate the buildup of intrapancreatic pressure that contributes to pain and tissue damage.
Most recent guidelines suggest that small (≤5 mm) stones should be treated with pancreatography and conventional stone extraction maneuvers under Endoscopic Retrograde Cholangiopancreatography (ERCP) (Strand et al., 2022). For patients with large obstructive stones (>5 mm) in the pancreatic head, endoscopic therapy is frequently combined with ESWL (Tandan et al., 2019). ESWL uses focused acoustic waves to fragment pancreatic duct stones into smaller pieces, allowing their subsequent removal through endoscopic techniques (Drewes et al., 2017). Evidence supports the efficacy of ESWL; short-term pain relief is reported in around 85% of patients receiving endotherapy, with long-term improvements observed in around two-thirds of patients (Talukdar et al., 2024). Pancreatoscopy-guided lithotripsy, a newer technology, is also commonly employed and has achieved 88%–100% stone clearance compared to ∼68% with ESWL (Gerges et al., 2023; van Huijgevoort et al., 2020).
The success and timing of pancreatic endoscopic therapy in CP are influenced by a complex interplay of factors, including patient demographics, disease progression, and socioeconomic status. For example, women and individuals with lower income are more likely to receive endoscopic treatment (Machicado et al., 2018). One study noted that endoscopic therapy typically occurred within 1 year after CP diagnosis, involved a median 2-month treatment span, and an average of two sessions per course (Issa et al., 2020; Clarke et al., 2012). Although ∼65% of patients experience improvement, recurrent ductal strictures or stones lead 30%–50% to need additional procedures within 2–3 years (Tringali et al., 2019; Han et al., 2024). Roughly one-third showed no response to their initial endoscopic therapy course (Issa et al., 2020), and only a small subset of patients proceeded to surgery, despite randomized controlled trial evidence suggesting that early surgical intervention may offer superior pain relief (Issa et al., 2020; Díte et al., 2003; Bordaçahar et al., 2018).
3.3 Surgical management
Surgical intervention for CP plays a critical role in disease management, with its timing and sequence relative to medical and endoscopic therapies remaining a subject of ongoing debate. While traditionally reserved for patients with severe, long-standing symptoms refractory to conservative treatments, emerging evidence suggests potential benefits of earlier surgical intervention in select cases.
Pancreatoduodenectomy (Whipple procedure) is typically indicated for disease predominantly affecting the pancreatic head, this involves the removal of the pancreatic head along with the duodenum and other adjacent structures. It effectively relieves pain but carries a substantial risk of loss of pancreatic function (Andersen and Frey, 2010). Duodenum-preserving pancreatic head resection (DPPHR) including procedures such as Frey and Beger/Berne techniques, targeting the inflamed pancreatic head while preserving the duodenum and maintaining pancreatic function. Comparative studies have shown that these procedures provide pain relief equivalent to pancreatoduodenectomy, with superior preservation of exocrine and endocrine functions. A meta-analysis comparing pancreatoduodenectomy and DPPHR suggests that both are equally effective in improving quality of life and pain relief; however, DPPHR is generally preferred due to its lower complication rates and better functional outcomes (Guo et al., 2023).
Longitudinal pancreatojejunostomy (Puestow procedure) involves creating a drainage channel by opening the pancreatic duct along its length and attaching it to the jejunum. This approach is particularly useful for patients with a diffusely dilated duct and widespread disease (Tchouta and Schrope, 2024). Distal pancreatectomy is indicated for disease localized to the body or tail of the pancreas. While this procedure can effectively provide pain relief, it may result in loss of pancreatic endocrine and exocrine functions (Hallac et al., 2020).
For patients with intractable pain who have exhausted other treatment modalities, total pancreatectomy with islet autotransplantation (TPIAT) is an advanced option (McEachron and Bellin, 2018; Bellin et al., 2017a). For children with genetic risk factors or greater disease burden, it may be utilized earlier (Bellin et al., 2017b; Heinzman et al., 2023). This procedure entails the complete removal of the pancreas, followed by transplantation of isolated islets into the liver to preserve insulin production. A meta-analysis conducted by the Dutch Pancreatitis Study Group has shown an increase in opioid-free rates from 0% to 15% pre-operatively to 63% postoperatively, with insulin dependence rate decreasing to 30% (Kempeneers et al., 2019). Observational data from Sutherland et al. showed durable pain relief in 85% of patients, with 60% achieving narcotic independence and approximately 30% attaining insulin independence (Sutherland et al., 2012). Additionally, a prospective study also reported reduced pain severity in young children with severe CP (Heinzman et al., 2023).
The optimal sequence of using endoscopic procedure or surgery to relieve pain in CP patients remains a topic of debate among clinicians. Cahen et al. demonstrated that early surgery provided better pain control over 24-month, whereas endoscopic treatment required more procedures (Cahen et al., 2007). Similarly, the ESCAPE trial showed a significant lower integrated pain score in the early surgery group compared with endoscopy-first group (37 vs. 49) at 18-month follow up (Issa et al., 2020). A subsequent follow-up publication confirmed that the early surgery group had superior outcomes compared to the endoscopy-first group, demonstrating better pain control (as measured by Izbicki and VAS pain scores), higher rates of complete pain relief, and greater patient satisfaction (van Veldhuisen et al., 2025). Despite concerns over strict inclusion criteria and limited generalizability, findings from ESCAPE trial and the follow-up data challenge the traditional paradigm of reserving surgery as a last resort. Instead, they suggest that earlier surgical interventions may provide significant benefits for select patients.
3.4 Miscellaneous treatment
Celiac plexus block (CPB) is a standard intervention for managing pain associated with CP and other upper abdominal conditions. Steroid injections, most commonly triamcinolone, provide temporary relief by reducing local inflammation and edema (Cornman-Homonoff et al., 2017; Sheth et al., 2024). CPB alleviates CP-related pain by targeting the celiac plexus, disrupting nociceptive pathways and reducing peripheral input to the CNS. This may mitigate central sensitization and, through autonomic modulation, relieve ischemia and inflammation-related discomfort (Shienbaum, 2024).
Traditionally performed via percutaneous techniques under fluoroscopic or computed tomography (CT) guidance, CPBs increasingly utilize endoscopic ultrasonography (EUS), which offers advanced imaging visualization of the celiac axis and surrounding structures through Doppler imaging. In one trial, CPB reduced pain scores in 70% of patients with CP (Santosh et al., 2009). This approach is particularly beneficial in managing CP-related pain, where pain is often driven by local inflammation secondary to the release of pancreatic enzymes (Wilcox et al., 2024; Wyse and Sahai, 2018).
For more enduring relief, neurolytic agents such as ethanol and phenol are employed to chemically ablate the nerve fibers of the celiac plexus (Hooten, 2024). Neurolytic interventions are primarily reserved for malignant conditions or refractory CP pain due to their irreversible effects (Noble and Gress, 2006). A currently ongoing clinical trial is evaluating the feasibility of EUS-guided CPB and neurolysis (Wilcox et al., 2024). Common side effects of CPB include transient hypotension, diarrhea, and localized back pain (Dos Santos Silva et al., 2023). Neurolytic procedures, due to their more destructive nature, may result in rare (<2%) (Kambadakone et al., 2011), but more serious complications such as orthostatic hypotension, retroperitoneal hemorrhage, or unintended motor and sensory deficits secondary to inadvertent neural injury (Shienbaum, 2024). Therefore, careful patient selection and procedural planning are essential.
Neuromodulation techniques, such as spinal cord stimulation (SCS) and transcranial magnetic stimulation (TMS), are emerging as potential adjuncts for managing refractory pain in CP. SCS delivers electrical stimulation to the dorsal columns, activating large-diameter Aβ fibers that suppress nociceptive input from smaller fibers. This reduces pain perception via mechanisms aligned with gate control theory and enhancement of descending inhibitory pathways (Deer et al., 2014). SCS involves the implantation of electrodes near the spinal cord, usually T6-T8, to deliver electrical impulses, achieving pain relief in approximately 50%–60% of patients, with a 6% infection rate as the primary complication (Ratnayake et al., 2020).
TMS, a non-invasive neuromodulation technique using magnetic fields to stimulate specific brain regions (typically the primary motor cortex or dorsolateral prefrontal cortex), has shown promise in neuropathic pain and depression, but remain under investigation for CP-related pain (Yang and Chang, 2020). Preliminary results suggest feasibility and safety of TMS, though larger, disease-specific trials are needed (Yang and Chang, 2020; O'Connell et al., 2018). Its analgesic effects are thought to involve modulation of cortical excitability and enhancement of descending pain inhibition, likely mediated by changes in thalamic and limbic activity as well as alterations in neurotransmitters (Lefaucheur et al., 2020).
4 Emerging diagnostics and therapeutic approaches
4.1 Pain phenotyping: the role of pancreatic-specific quantitative sensory testing in CP-related pain
Phenotyping of pain in CP is an emerging area of research aimed at improving diagnostic accuracy and personalizing treatment. Quantitative Sensory Testing (QST), particularly the pancreatic-specific QST (P-QST), has showed potential in differentiating pain subtypes and identifying central sensitization. By applying controlled stimuli, P-QST helps elucidate underlying pain mechanisms, with a specific emphasis on segmental and widespread hyperalgesia as indicators of central sensitization. This approach is especially valuable given that central sensitization, a key driver of pain chronicity in CP, is often challenging to detect using conventional imaging or routine clinical assessments (Buscaglia and Chang, 2022; Faghih et al., 2022).
An ongoing observational clinical trial is evaluating the predictive value of P-QST phenotyping for pain relief following endoscopic or surgery interventions in 150 CP patients recruited from three U.S. tertiary care centers. Participants are classified as having no central sensitization, segmental sensitization, or widespread hyperalgesia prior to treatment. Six-month pain reduction via a Numeric Rating Scale, serves as the primary outcome. These findings are expected to guide the development of individualized treatment strategies based on P-QST results (Phillips et al., 2024).
4.2 Emerging therapies and ongoing clinical trials for CP and associated pain
A growing body of research highlights the importance of novel interventions that target not only the pancreatic pathology but also the multifaceted nature of CP-related pain. These emerging therapies encompass novel pharmacologic agents, minimally invasive procedural innovations, and digital health solutions, all of which aim to alleviate pain while addressing key aspects of CP pathophysiology (Table 2).
TABLE 1
| Drug category | Example | Mechanism of action | Key evidence | Potential side effects |
|---|---|---|---|---|
| Gabapentinoids | Gabapentin, Pregabalin |
|
|
|
| SNRIs | Duloxetine, Venlafaxine |
|
|
|
| TCAs | Amitriptyline, Nortriptyline, Desipramine |
|
|
|
| Antioxidants | methionine, organic selenium, β-carotene, ascorbic acid, and α-tocoferol (Talukdar et al., 2016) |
|
|
|
| Somatostatin Analogues | Octreotide |
|
|
|
| NMDA Receptor Antagonists | Ketamine |
|
|
|
| Others | Clonidine |
|
|
|
| Cannabinoids |
|
|
|
Non-opioid pharmacologic interventions for chronic Pancreatitis pain: Mechanisms, evidence, and side effects.
SNRI, Serotonin-Norepinephrine Reuptake Inhibitor; CNS, Central Nervous System; RCT, Randomized Controlled Trial; TCA, Tricyclic Antidepressant, NMDA–N-Methyl-D-Aspartate, CB1 – Cannabinoid Receptor Type 1, CB2 – Cannabinoid Receptor Type 2, CP, Chronic Pancreatitis; MAOIs, Monoamine Oxidase Inhibitors.
TABLE 2
| NCT Number | Title | Enrollment | Study type | Intervention | Locations | Estimated completion |
|---|---|---|---|---|---|---|
| NCT06426160 | Tocilizumab for Painful Chronic Pancreatitis | 36 | A randomized, placebo-controlled trial evaluating tocilizumab for reducing CP pain by reducing inflammation and fibrosis | Drug: Tocilizumab, Sodium Chloride | Europe | 2026-6 |
| NCT04115826 | ESWL vs. PPL for Painful Chronic Calcific Pancreatitis | 150 | A randomized trial comparing ESWL and PPL for pancreatic duct stone clearance and pain relief | Procedure: ESWL, PPL | US | 2026-6 |
| NCT041572 | Role of Home-Based TEA for Treatment of Pain in Chronic Pancreatitis | 40 | A crossover trial assessing TEA, a non-invasive therapy stimulating acupuncture points via electrical currents to modulate pain pathways | Device: TEA | US | 2027-12 |
| NCT063262616 | QOLAPI: Quality of Life Assessment of Chronic Pancreatitis Endoscopic Interventions | 500 | A multicenter cohort study evaluating endoscopic approaches to relieve ductal obstruction and inflammation | Endoscopic Procedures | US | 2026-8 |
| NCT06362187 | VR Pilot for Pancreatitis | 20 | A pilot study exploring VR integrating cognitive-behavioral techniques to improve pain perception and emotional wellbeing | Device: Gut-Directed VR, Sham Control | US | 2026-5 |
| NCT06178315 | EUS-CPB vs. Sham in Chronic Pancreatitis | 94 | A randomized trial investigating EUS-CPB, using steroids and anesthetics to inhibit sympathetic nerve activity and relieve CP pain | Procedure: EUS-Guided Celiac Plexus Block | US | 2026-12 |
| NCT04043074 | EUS-CPN for Chronic Pancreatitis | 35 | A prospective trial examining EUS-CPN, where neurolytic agents disrupt sympathetic pathways for long-term pain relief | Procedure: Celiac Plexus Neurolysis | US | 2029-10 |
| NCT05925036 | Novel Cellular Therapy for the Treatment of Pain Associated with Chronic Pancreatitis | 40 | A phase 1 trial assessing Mesenchymal stromal cell for their anti-inflammatory and immunomodulatory properties in reducing CP-related fibrosis, inflammation, and pain | Drug: Mesenchymal Stem Cells, Placebo | US | 2027-12 |
| NCT05603702 | STTEPP: Safety, Tolerability, and Dose-Limiting Toxicity of Lacosamide in Patients with CP Pain | 24 | A dose-escalation trial testing lacosamide as an adjunct to opioids to reduce hyperalgesia and central sensitization | Drug: Lacosamide | US | 2025-3 |
| NCT05664880 | ALLIANCE: A Pilot Clinical Trial of Paricalcitol for Chronic Pancreatitis | 24 | A pilot study evaluating paricalcitol for its anti-inflammatory and antifibrotic effects in CP pain management | Drug: Paricalcitol | US | 2026-1 |
| NCT05771675 | Simvastatin Treatment to Improve Patient-Reported Outcomes in Patients with CP | 90 | A double-blind trial testing potential effect from simvastatin to enhance autophagic clearance and reduce fibro-inflammatory responses to improve CP pain outcomes | Drug: Simvastatin, Placebo | US | 2026-6 |
Ongoing clinical trials exploring emerging therapies for chronic pancreatitis pain management.
ESWL- extracorporeal shock wave lithotripsy, PPL, Pancreatoscopy-Guided Lithotripsy; TEA, Transcutaneous Electrical Acustimulation; VR, Virtual reality; EUS-CPB, endoscopic ultrasound-guided celiac plexus block; EUS-CPN, Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis.
Among pharmacologic therapies, tocilizumab, an anti-IL-6 monoclonal antibody, is under investigation in the TOPAC trial (Hagn-Meincke et al., 2025), for its potential to reduce systemic and pancreatic inflammation, thereby potentially normalizing pain processing and improving quality of life. IL-6 has emerged as a key mediator in the complex immune signaling networks of CP, based on evidence from both preclinical models and human studies using pancreatic cell lines and tissue samples (Komar et al., 2017). In peripheral sensitization and pancreatic neuroplasticity models, IL-6 appears to be upregulated in both the pancreas and thoracic DRG, sensitizing primary afferent nociceptors via autocrine or paracrine mechanisms, including enhanced TRPV1 activity (Xu et al., 2007). Clinically, a recent pilot study showed a progressive rise in plasma IL-6 levels correlating with the severity of CP, suggesting its utility as a biomarker across disease stages (Hagn-Meincke et al., 2024). By blocking both membrane-bound and soluble forms of IL-6 receptor (IL-6R), tocilizumab disrupts downstream JAK/STAT and MAPK signaling, attenuating inflammatory and fibrotic cascades as well as nociceptive transmission (Chen et al., 2023). While prior studies have shown its effectiveness in modulating systemic inflammation in acute pancreatitis (Chen et al., 2016), emerging evidence supports its potential in chronic, immune-driven pain phenotypes in CP (Chen et al., 2023). The novel small molecule IL-6R antagonist TB-2–081 has also demonstrated effectiveness in reversing CP-induced referred abdominal hypersensitivity in experimental models (Vardanyan et al., 2010).
Lacosamide, a sodium channel blocker, has garnered interest for its selective modulation of NaV1.7—a voltage-gated sodium channel critical in amplifying subthreshold depolarizations and increasing the excitability of nociceptive neurons, particularly within the DRG and peripheral sensory pathways. By enhancing the slow inactivation of NaV1.7, lacosamide reduces neuronal hyperexcitability associated with chronic pain (Dib-Hajj et al., 2013). This mechanism positions it as a potential therapeutic candidate for managing pain in CP (Fogel et al., 2024). In addition, it is currently under evaluation to determine its ability to mitigate opioid-induced hyperalgesia, a frequent complication in patients with CP who rely on long-term opioid therapy (Fogel et al., 2024).
Emerging evidence also supports the role of simvastatin, traditionally used as a lipid-lowering agent, in restoring acinar cell homeostasis and modulating fibro-inflammatory pathways, which are critical in CP pathogenesis. Mechanistically, simvastatin and other HMG-CoA reductase inhibitors suppress PSC activation by inhibiting the mevalonate pathway, which disrupts isoprenylation and membrane localization of Ras and Rho GTPases. This downregulates the Ras-Raf-ERK pathway, inhibits PDGF-induced RhoA activation, reduces α-smooth muscle actin expression, and promotes apoptosis in activated PSCs, thereby attenuating fibrogenic signaling (Jaster et al., 2003).
Paricalcitol, a vitamin D analog, is another agent under study for its dual anti-fibrotic and anti-inflammatory properties. It acts by activating the vitamin D receptor on PSCs, promoting transcriptional reprogramming that suppresses PSC activation and extracellular matrix production. Mechanistically, this includes inhibition of TGF-β/SMAD signaling, downregulation of pro-fibrotic genes, and a shift of PSCs toward a quiescent phenotype, resulting in reduced inflammation and stromal fibrosis (Sherman et al., 2014).
Early data also suggest that mesenchymal stem cell transplantation may exert regenerative properties on the inflamed pancreas, potentially reducing fibrosis and pain by suppressing inflammatory cytokines and PSCs (Kawakubo et al., 2018).
Procedural innovations remain a cornerstone of pain management in CP. Endoscopic approaches, such as EUS-CPB and EUS-guided celiac plexus neurolysis (EUS-CPN), represent minimally invasive methods to achieve localized nerve inhibition. Ongoing trials are assessing their safety and efficacy in CP patients with refractory pain. Lithotripsy Techniques such as ESWL and pancreatoscopy-guided lithotripsy are both under evaluation to determine the most effective approach for achieving pancreatic duct stone clearance. Successful duct clearance can significantly reduce pain and mitigate complications related to ductal hypertension.
Incorporating digital health tools and behavioral interventions is also a growing area of focus in managing CP-related pain. Among these, cognitive behavioral therapy reduces pain by modulating central sensitization (Wang et al., 2025). The Internet-Delivered Pain Self-Management for Persons With Acute Recurrent and Chronic Pancreatitis Pain (IMPACT-2) trial explores an internet-delivered pain self-management program that teaches essential skills like relaxation, goal-setting, and activity pacing. These skills empower patients to actively participate in their pain management, potentially improving both adherence and outcomes. Virtual reality therapy aims to reshape pain perception by leveraging immersive technology, engaging cognitive-behavioral techniques, and altering attitudes, emotions, and coping mechanisms related to pain.
Transcutaneous electrical acustimulation (TEA), provides a non-invasive, home-based option to modulate pain pathways without needles or medication, thus broadening the range of pain management strategies. TEA has demonstrated analgesic effects in patients with early-stage acute pancreatitis, primarily through autonomic modulation (decreasing sympathetic tone while enhancing vagal activity) to reduce visceral hypersensitivity and improve gastrointestinal motility (Xuan et al., 2022). Its role in CP-related pain, however, remains to be clarified.
5 Conclusion
Advancements in CP pain management will hinge on enhanced mechanistic understanding, precision diagnostics, and targeted therapies. Future research aims to integrate large cohort studies and big data to identify genetic markers and refine risk stratification, while artificial intelligence (AI) and advanced imaging techniques will enable more precise diagnostics and prediction of disease progression. Mechanistic studies focusing on inflammation, fibrosis, and neuropathy may uncover genetic pathways for tailored treatments. Novel and repurposed therapies, including paricalcitol, statins, lacosamide, and cystic fibrosis transmembrane conductance regulator (CFTR) modulators, etc., are generating optimism that they may address CP’s underlying pathophysiology. Additionally, digital health tools, such as AI-assisted cognitive behavioral therapy and wearable sensors, provide new non-pharmacologic and objective outcome measures. Optimizing clinical trial designs by incorporating genetic profiles, tailoring interventions, and quantifying placebo effects will improve therapeutic efficacy in this heterogeneous disease. These measures aim to refine treatment approaches, ultimately improving patient outcomes and quality of life.
Statements
Author contributions
ZL: Methodology, Visualization, Conceptualization, Writing – review and editing, Resources, Writing – original draft. SP: Conceptualization, Investigation, Supervision, Writing – review and editing. MA: Conceptualization, Writing – review and editing. YJ: Supervision, Writing – review and editing, Conceptualization, Writing – original draft, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was suppoerted by NIH U01 DK108314 DoD HT9425-23-1-0150.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript.This manuscript was revised with the assistance of ChatGPT (GPT-4 March 2024 version), developed by OpenAI (https://openai.com/chatgpt), which was used solely for language refinement and clarity improvements. No AI tools were used for content generation, data interpretation, or critical analysis. All revisions were reviewed and approved by the authors to ensure accuracy and integrity.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AmmannR. W.AkovbiantzA.LargiaderF.SchuelerG. (1984). Course and outcome of chronic pancreatitis. Gastroenterology86, 820–828. 10.1016/s0016-5085(24)05129-1
2
AmnaS.ØhlenschlaegerT.SaedderE. A.SigaardJ. V.BergmannT. K. (2024). Review of clinical pharmacokinetics and pharmacodynamics of clonidine as an adjunct to opioids in palliative care. Basic Clin. Pharmacol. Toxicol.134, 485–497. 10.1111/bcpt.13979
3
AndersenD. K.FreyC. F. (2010). The evolution of the surgical treatment of chronic pancreatitis. Ann. Surg.251, 18–32. 10.1097/SLA.0b013e3181ae3471
4
BaiY.ChenY. B.QiuX. T.ChenY. B.MaL. T.LiY. Q.et al (2019b). Nucleus tractus solitarius mediates hyperalgesia induced by chronic pancreatitis in rats. World J. Gastroenterol.25, 6077–6093. 10.3748/wjg.v25.i40.6077
5
BaiY.MaL. T.ChenY. B.RenD.ChenY. B.LiY. Q.et al (2019a). Anterior Insular cortex mediates hyperalgesia induced by chronic pancreatitis in rats. Mol. Brain12, 76. 10.1186/s13041-019-0497-5
6
BansalA.GuptaP.SinghH.SamantaJ.MandavdhareH.SharmaV.et al (2019). Gastrointestinal complications in acute and chronic pancreatitis. JGH Open3, 450–455. 10.1002/jgh3.12185
7
BarbaraG.StanghelliniV.De GiorgioR.CremonC.CottrellG. S.SantiniD.et al (2004). Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology126, 693–702. 10.1053/j.gastro.2003.11.055
8
BellinM. D.ForlenzaG. P.MajumderK.BergerM.FreemanM. L.BeilmanG. J.et al (2017a). Total pancreatectomy with islet autotransplantation resolves pain in young children with severe chronic pancreatitis. J. Pediatr. Gastroenterology Nutr.64, 440–445. 10.1097/MPG.0000000000001314
9
BellinM. D.ForlenzaG. P.MajumderK.BergerM.FreemanM. L.BeilmanG. J.et al (2017b). Total pancreatectomy with islet autotransplantation resolves pain in young children with severe chronic pancreatitis. J. Pediatr. Gastroenterol. Nutr.64, 440–445. 10.1097/mpg.0000000000001314
10
BordaçaharB.CouvelardA.VulliermeM. P.BucchiniL.SauvanetA.DokmakS.et al (2018). Predicting the efficacy of surgery for pain relief in patients with alcoholic chronic pancreatitis. Surgery164, 1064–1070. 10.1016/j.surg.2018.05.025
11
BouwenseS. A.de VriesM.SchreuderL. T. W.OlesenS. S.FrøkjærJ. B.DrewesA. M.et al (2015). Systematic mechanism-orientated approach to chronic pancreatitis pain. World J. Gastroenterol.21, 47–59. 10.3748/wjg.v21.i1.47
12
BouwenseS. A.OlesenS. S.DrewesA. M.PoleyJ. W.van GoorH.Wilder-SmithO. H. G. (2012). Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One7, e42096. 10.1371/journal.pone.0042096
13
BuhnerS.SchemannM. (2012). Mast cell-nerve axis with a focus on the human gut. Biochim. Biophys. Acta1822, 85–92. 10.1016/j.bbadis.2011.06.004
14
BuscagliaJ. M.ChangL. (2022). Pain phenotypes in chronic pancreatitis: beginning to fine-tune our approach to treatment. Clin. Gastroenterol. Hepatol.20, 28–30. 10.1016/j.cgh.2020.12.032
15
CahenD. L.GoumaD. J.NioY.RauwsE. A. J.BoermeesterM. A.BuschO. R.et al (2007). Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N. Engl. J. Med.356, 676–684. 10.1056/NEJMoa060610
16
CeyhanG. O.BergmannF.KadihasanogluM.AltintasB.DemirI. E.HinzU.et al (2009). Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology136, 177–186. 10.1053/j.gastro.2008.09.029
17
CeyhanG. O.BergmannF.KadihasanogluM.ErkanM.ParkW.HinzU.et al (2007). The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut56, 534–544. 10.1136/gut.2006.105528
18
CeyhanG. O.DemirI. E.MaakM.FriessH. (2010). Fate of nerves in chronic pancreatitis: neural remodeling and pancreatic neuropathy. Best Pract. and Res. Clin. Gastroenterology24, 311–322. 10.1016/j.bpg.2010.03.001
19
ChenK. L.LvZ. Y.YangH. W.LiuY.LongF. W.ZhouB.et al (2016). Effects of tocilizumab on experimental severe acute pancreatitis and associated acute lung injury. Crit. Care Med.44, e664–e677. 10.1097/ccm.0000000000001639
20
ChenY.ZhouJ.XuS.NieJ. (2023). Role of Interleukin-6 family cytokines in organ fibrosis. Kidney Dis. (Basel)9, 239–253. 10.1159/000530288
21
ChengY.MaX.-l.WeiY.-q.WeiX.-W. (2019). Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochimica Biophysica Acta (BBA) - Rev. Cancer1871, 289–312. 10.1016/j.bbcan.2019.01.005
22
ChincholkarM. (2020). Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br. J. Pain14, 104–114. 10.1177/2049463720912496
23
ClarkeB.SlivkaA.TomizawaY.SandersM.PapachristouG. I.WhitcombD. C.et al (2012). Endoscopic therapy is effective for patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol.10, 795–802. 10.1016/j.cgh.2011.12.040
24
Cornman-HomonoffJ.HolzwangerD. J.LeeK. S.MadoffD. C.LiD. (2017). Celiac plexus block and neurolysis in the management of chronic upper abdominal pain. Semin. Interv. Radiol.34, 376–386. 10.1055/s-0037-1608861
25
DeerT. R.MekhailN.ProvenzanoD.PopeJ.KramesE.LeongM.et al (2014). The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the neuromodulation appropriateness consensus committee. Neuromodulation17, 515–550. 10.1111/ner.12208
26
DemirI. E.FriessH.CeyhanG. O. (2015). Neural plasticity in pancreatitis and pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol.12, 649–659. 10.1038/nrgastro.2015.166
27
DemirI. E.SchornS.Schremmer-DanningerE.WangK.KehlT.GieseN. A.et al (2013). Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One8, e60529. 10.1371/journal.pone.0060529
28
DemirI. E.TieftrunkE.MaakM.FriessH.CeyhanG. O. (2011). Pain mechanisms in chronic pancreatitis: of a master and his fire. Langenbeck's Archives Surg.396, 151–160. 10.1007/s00423-010-0731-1
29
DemirI. E.WangK.TieftrunkE.GieseN. A.XingB.FriessH.et al (2012). Neuronal plasticity in chronic pancreatitis is mediated via the neurturin/GFRα2 axis. Am. J. Physiol. Gastrointest. Liver Physiol.303, G1017–G1028. 10.1152/ajpgi.00517.2011
30
de VriesM.Wilder-SmithO. H.JongsmaM. L.van den BroekeE. N.ArnsM.van GoorH.et al (2013). Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J. Pain Res.6, 815–824. 10.2147/jpr.S50919
31
Dib-HajjS. D.YangY.BlackJ. A.WaxmanS. G. (2013). The Na(V)1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci.14, 49–62. 10.1038/nrn3404
32
Di SebastianoP.di MolaF. F.BockmanD. E.FriessH.BüchlerM. W. (2003). Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut52, 907–911. 10.1136/gut.52.6.907
33
DíteP.RuzickaM.ZborilV.NovotnýI. (2003). A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy35, 553–558. 10.1055/s-2003-40237
34
Dos Santos SilvaR. P.LopesA. J. M.BezerraR. B.AndradeR. A.AndradeR. G.da CostaL. M. F.et al (2023). Persistent hypotension and other complications of celiac plexus neurolysis: a case report and literature review. Clin. Case Rep.11, e7505. 10.1002/ccr3.7505
35
DrewesA. M.BouwenseS. A. W.CampbellC. M.CeyhanG. O.DelhayeM.DemirI. E.et al (2017). Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology17, 720–731. 10.1016/j.pan.2017.07.006
36
DunbarE.GreerP. J.MelhemN.AlkaadeS.AmannS. T.BrandR.et al (2020). Constant-severe pain in chronic pancreatitis is associated with genetic loci for major depression in the NAPS2 cohort. J. Gastroenterol.55, 1000–1009. 10.1007/s00535-020-01703-w
37
DunbarE. K.GreerP. J.SalomanJ. L.AlbersK. M.YadavD.WhitcombD. C. (2025). Genetics of constant and severe pain in the NAPS2 cohort of recurrent acute and chronic pancreatitis patients. J. Pain27, 104754. 10.1016/j.jpain.2024.104754
38
DunbarE. K.GreerP. J.AmannS. T.AlkaadeS.BanksP.BrandR.et al (2021). Pain experience in pancreatitis: strong association of genetic risk loci for anxiety and PTSD in patients with severe, constant, and constant-severe pain. Am. J. Gastroenterol.116, 2128–2136. 10.14309/ajg.0000000000001366
39
EdinoffA. N.KaplanL. A.KhanS.PetersenM.SauceE.CauseyC. D.et al (2021). Full opioid agonists and tramadol: pharmacological and clinical considerations. Anesth. Pain Med.11, e119156. 10.5812/aapm.119156
40
FaghihM.PhillipsA. E.KuhlmannL.AfghaniE.DrewesA. M.YadavD.et al (2022). Pancreatic QST differentiates chronic pancreatitis patients into distinct pain phenotypes independent of psychiatric comorbidities. Clin. Gastroenterol. Hepatol.20, 153–161.e2. 10.1016/j.cgh.2020.10.036
41
FengQ. X.WangW.FengX. Y.MeiX. P.ZhuC.LiuZ. C.et al (2010). Astrocytic activation in thoracic spinal cord contributes to persistent pain in rat model of chronic pancreatitis. Neuroscience167, 501–509. 10.1016/j.neuroscience.2010.02.005
42
FinkK.DooleyD. J.MederW. P.Suman-ChauhanN.DuffyS.ClusmannH.et al (2002). Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology42, 229–236. 10.1016/s0028-3908(01)00172-1
43
FogelE. L.EaslerJ. J.YuanY.YadavD.ConwellD. L.VegeS. S.et al (2024). Safety, tolerability, and dose-limiting toxicity of lacosamide in patients with painful chronic pancreatitis: protocol for a phase 1 clinical trial to determine safety and identify side effects. JMIR Res. Protoc.13, e50513. 10.2196/50513
44
FrancescangeliJ.KaramchandaniK.PowellM.BonaviaA. (2019). The serotonin syndrome: from molecular mechanisms to clinical practice. Int. J. Mol. Sci.20, 2288. 10.3390/ijms20092288
45
FregniF.Pascual-LeoneA.FreedmanS. D. (2007). Pain in chronic pancreatitis: a salutogenic mechanism or a maladaptive brain response?Pancreatology7, 411–422. 10.1159/000108958
46
FrøkjærJ. B.OlesenS. S.GramM.YavarianY.BouwenseS. A. W.Wilder-SmithO. H. G.et al (2011). Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut60, 1554–1562. 10.1136/gut.2010.236620
47
FuQ.HanN.LiN.GuiL.ShiC.RongP.et al (2024). Guidelines for rational clinical use of fentanyl transdermal patch. Drug Des. Devel Ther.18, 233–255. 10.2147/dddt.S414318
48
GergesC.AlbersD.SchmitzL.GoniE.CappelloA.SchirraJ.et al (2023). Digital single-operator pancreatoscopy for the treatment of symptomatic pancreatic duct stones: a prospective multicenter cohort trial. Endoscopy55, 150–157. 10.1055/a-1870-3403
49
GoodmanM. T.LombardiC.TorrensA.BreseeC.SalomanJ. L.LiL.et al (2025). Association of serum endocannabinoid levels with pancreatitis and pancreatitis-related pain. Cannabis Cannabinoid Res.10, 60–70. 10.1089/can.2024.0079
50
GuoS.ZhouQ.YangJ.TaoJ.ZhangJ.WangH. (2023). Duodenum-preserving pancreatic head resection compared to pancreaticoduodenectomy: a systematic review and network meta-analysis of surgical outcomes. Front. Surg.10, 1107613. 10.3389/fsurg.2023.1107613
51
HaasS. L.FitznerB.JasterR.WiercinskaE.GaitantziH.JesnowskiR.et al (2009). Transforming growth factor-beta induces nerve growth factor expression in pancreatic stellate cells by activation of the ALK-5 pathway. Growth factors.27, 289–299. 10.1080/08977190903132273
52
Hagn-MeinckeR.FrøkjærJ. B.DrewesA. M.HolmboeC. H.KroghK.NedergaardR. B.et al (2025). Tocilizumab for painful chronic pancreatitis (TOPAC trial): protocol for a phase 2 randomized, placebo-controlled, double-blind, investigator-initiated trial. Pancreatology25, 343–352. 10.1016/j.pan.2025.03.001
53
Hagn-MeinckeR.HartP. A.AndersenD. K.VegeS. S.FogelE. L.SerranoJ.et al (2024). Circulating immune signatures across clinical stages of chronic pancreatitis: a pilot study. Eur. J. Gastroenterol. Hepatol.36, 177–183. 10.1097/meg.0000000000002691
54
HallacA.AleassaE. M.RogersM.FalkG. A.Morris-StiffG. (2020). Exocrine pancreatic insufficiency in distal pancreatectomy: incidence and risk factors. HPB22, 275–281. 10.1016/j.hpb.2019.06.017
55
HanS.ConwellD. L.EaslerJ. J.YangY.AndersenD. K.FisherW. E.et al (2024). Use of pancreatic endotherapy in patients with chronic pancreatitis: results from a multicenter cohort study in the United States. Gastrointest. Endosc.100, 262–272.e1. 10.1016/j.gie.2024.04.002
56
HeinzmanC.HornungL.LinT. K.LoweC. M. O.VitaleD. S.Abu-El-HaijaM.et al (2023). Total pancreatectomy with islet autotransplantation reduces opioid use and improves nutritional support in children with debilitating pancreatitis. PLoS One18, e0289620. 10.1371/journal.pone.0289620
57
HinesO. J.PandolS. J. (2024). Management of chronic pancreatitis. Bmj384, e070920. 10.1136/bmj-2023-070920
58
HootenR. S. D. S. W. M. (2024). “Neurolytic blocks. In: Statpearls,”. Treasure Island (FL): StatPearls.
59
HorváthI. L.BunducS.FehérváriP.VáncsaS.NagyR.GarmaaG.et al (2022). The combination of ulinastatin and somatostatin reduces complication rates in acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Sci. Rep.12, 17979. 10.1038/s41598-022-22341-7
60
IssaY.KempeneersM. A.BrunoM. J.FockensP.PoleyJ. W.Ahmed AliU.et al (2020). Effect of early surgery vs endoscopy-first approach on pain in patients with chronic pancreatitis: the ESCAPE randomized clinical trial. Jama323, 237–247. 10.1001/jama.2019.20967
61
JasterR.BrockP.SparmannG.EmmrichJ.LiebeS. (2003). Inhibition of pancreatic stellate cell activation by the hydroxymethylglutaryl coenzyme A reductase inhibitor lovastatin. Biochem. Pharmacol.65, 1295–1303. 10.1016/s0006-2952(03)00075-3
62
JiN. N.CaoS.SongX. L.PeiB.JinC. Y.FanB. F.et al (2024). Glutamatergic neurons in the paraventricular nucleus of the hypothalamus participate in the regulation of visceral pain induced by pancreatic cancer in mice. Hepatobiliary Surg. Nutr.13, 258–272. 10.21037/hbsn-23-442
63
JiangJ.BaiJ.QinT.WangZ.HanL. (2020). NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. J. Cell Mol. Med.24, 5901–5910. 10.1111/jcmm.15265
64
JohnsonB. W.StrandN. H.RaynakJ. C.JaraC.HabtegiorgisK.HandB. A.et al (2025). Cannabinoids in chronic pain management: a review of the history, efficacy, applications, and risks. Biomedicines13, 530. 10.3390/biomedicines13030530
65
KambadakoneA.ThabetA.GervaisD. A.MuellerP. R.ArellanoR. S. (2011). CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics31, 1599–1621. 10.1148/rg.316115526
66
KawakuboK.OhnishiS.KuwataniM.SakamotoN. (2018). Mesenchymal stem cell therapy for acute and chronic pancreatitis. J. Gastroenterology53, 1–5. 10.1007/s00535-017-1363-9
67
KempeneersM. A.ScholtenL.VerkadeC. R.van HooftJ. E.van SantvoortH. C.BuschO. R.et al (2019). Efficacy of total pancreatectomy with islet autotransplantation on opioid and insulin requirement in painful chronic pancreatitis: a systematic review and meta-analysis. Surgery166, 263–270. 10.1016/j.surg.2019.03.014
68
KetamineV. J. O. J. S. R. N. L. S. P. (2023). in Acute and chronic pain management (StatPearls).Cohen.
69
KomarH. M.HartP. A.Cruz-MonserrateZ.ConwellD. L.LesinskiG. B. (2017). Local and systemic expression of immunomodulatory factors in chronic pancreatitis. Pancreas46, 986–993. 10.1097/mpa.0000000000000896
70
KongF.PanY.WuD. (2024). Activation and regulation of pancreatic stellate cells in chronic pancreatic fibrosis: a potential therapeutic approach for chronic pancreatitis. Biomedicines12, 108. 10.3390/biomedicines12010108
71
KuhlmannL.OlesenS. S.DrewesA. M. (2025). Pathophysiology, assessment, and management of pain associated with chronic pancreatitis. Gastroenterol. Clin. North Am.54, 129–142. 10.1016/j.gtc.2024.09.005
72
LankischP. G.SeidenstickerF.Löhr-HappeA.OttoJ.CreutzfeldtW. (1995). The course of pain is the same in alcohol- and nonalcohol-induced chronic pancreatitis. Pancreas10, 338–341. 10.1097/00006676-199505000-00003
73
LefaucheurJ. P.AlemanA.BaekenC.BenningerD. H.BrunelinJ.Di LazzaroV.et al (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin. Neurophysiol.131, 474–528. 10.1016/j.clinph.2019.11.002
74
LiuP. Y.LeeI. H.TanP. H.WangY. P.TsaiC. F.LinH. C.et al (2015). P2X7 receptor mediates spinal microglia activation of visceral hyperalgesia in a rat model of chronic pancreatitis. Cell Mol. Gastroenterol. Hepatol.1, 710–720. 10.1016/j.jcmgh.2015.07.008
75
LiuP. Y.LuC. L.WangC. C.LeeI. H.HsiehJ. C.ChenC. C.et al (2012). Spinal microglia initiate and maintain hyperalgesia in a rat model of chronic pancreatitis. Gastroenterology142, 165–173. 10.1053/j.gastro.2011.09.041
76
LiuQ.KoC. Y.ZhengC.YeL.LiuB.GaoH.et al (2020). Decreased glutamatergic synaptic strength in the periaqueductal gray contributes to maintenance of visceral pain in Male rats with experimental pancreatitis. Neuroscience428, 60–69. 10.1016/j.neuroscience.2019.12.004
77
LkhagvasurenB.Mee-IntaO.ZhaoZ. W.HiramotoT.BoldbaatarD.KuoY. M. (2021). Pancreas-brain crosstalk. Front. Neuroanat.15, 691777. 10.3389/fnana.2021.691777
78
LuX.WuY.CaoR.YuX.GongJ. (2022). CXCL12 secreted by pancreatic stellate cells accelerates gemcitabine resistance of pancreatic cancer by enhancing glycolytic reprogramming. Anim. Cells Syst. Seoul.26, 148–157. 10.1080/19768354.2022.2091019
79
LunnM. P.HughesR. A.WiffenP. J. (2014). Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev.2014, Cd007115. 10.1002/14651858.CD007115.pub3
80
LuoR.HuX.LiX.LeiF.LiaoP.YiL.et al (2024). Dysfunctional astrocyte glutamate uptake in the hypothalamic paraventricular nucleus contributes to visceral pain and anxiety-like behavior in mice with chronic pancreatitis. Glia72, 2022–2037. 10.1002/glia.24595
81
LuoX.WangX.XiaZ.ChungS. K.CheungC. W. (2016). CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev. Neurosci.27, 83–92. 10.1515/revneuro-2015-0016
82
MaY.HwangR. F.LogsdonC. D.UllrichS. E. (2013). Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res.73, 3927–3937. 10.1158/0008-5472.Can-12-4479
83
MachicadoJ. D.ChariS. T.TimmonsL.TangG.YadavD. (2018). A population-based evaluation of the natural history of chronic pancreatitis. Pancreatology18, 39–45. 10.1016/j.pan.2017.11.012
84
MannR.BoregowdaU.VyasN.GajendranM.UmapathyC. P.SayanaH.et al (2021). Current advances in the management of chronic pancreatitis. Disease-a-Month67, 101225. 10.1016/j.disamonth.2021.101225
85
MasamuneA.ShimosegawaT. (2009). Signal transduction in pancreatic stellate cells. J. Gastroenterol.44, 249–260. 10.1007/s00535-009-0013-2
86
MasamuneA.WatanabeT.KikutaK.ShimosegawaT. (2009). Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin. Gastroenterol. Hepatol.7, S48–S54. 10.1016/j.cgh.2009.07.038
87
MayoralV.GalvezR.FerrándizM.Miguéns VázquezX.Cordero-GarcíaC.Alcántara MonteroA.et al (2024). Pregabalin vs. gabapentin in the treatment of neuropathic pain: a comprehensive systematic review and meta-analysis of effectiveness and safety. Front. Pain Res. (Lausanne)5, 1513597. 10.3389/fpain.2024.1513597
88
McEachronK. R.BellinM. D. (2018). Total pancreatectomy and islet autotransplantion for chronic and recurrent acute pancreatitis. Curr. Opin. Gastroenterol.34, 367–373. 10.1097/mog.0000000000000458
89
MoraczewskiJ. A. A.AedmaK. K. (2023). Tricyclic antidepressants. StatPearls. Treasure Island (FL).
90
NiemannT.MadsenL. G.LarsenS.ThorsgaardN. (2000). Opioid treatment of painful chronic pancreatitis. Int. J. Pancreatol.27, 235–240. 10.1385/ijgc:27:3:235
91
NobleM.GressF. G. (2006). Techniques and results of neurolysis for chronic pancreatitis and pancreatic cancer pain. Curr. Gastroenterol. Rep.8, 99–103. 10.1007/s11894-006-0004-x
92
O'ConnellN. E.MarstonL.SpencerS.DeSouzaL. H.WandB. M. (2018). Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev.4, Cd008208. 10.1002/14651858.CD008208.pub5
93
OlesenS. S.BouwenseS. A.Wilder-SmithO. H.van GoorH.DrewesA. M. (2011). Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology141, 536–543. 10.1053/j.gastro.2011.04.003
94
OlesenS. S.KraussT.DemirI. E.Wilder-SmithO. H.CeyhanG. O.PasrichaP. J.et al (2017). Towards a neurobiological understanding of pain in chronic pancreatitis: mechanisms and implications for treatment. Pain Rep.2, e625. 10.1097/PR9.0000000000000625
95
PertovaaraA. (2006). Noradrenergic pain modulation. Prog. Neurobiol.80, 53–83. 10.1016/j.pneurobio.2006.08.001
96
PhillipsA. E.AfghaniE.AkshintalaV. S.BenosP. Y.DasR.DrewesA. M.et al (2024). Pancreatic quantitative sensory testing to predict treatment response of endoscopic therapy or surgery for painful chronic pancreatitis with pancreatic duct obstruction: study protocol for an observational clinical trial. BMJ Open14, e081505. 10.1136/bmjopen-2023-081505
97
RatnayakeC. B.BunnA.PandanaboyanaS.WindsorJ. A. (2020). Spinal cord stimulation for management of pain in chronic pancreatitis: a systematic review of efficacy and complications. Neuromodulation23, 19–25. 10.1111/ner.13051
98
RenD.LiJ. N.QiuX. T.WanF. P.WuZ. Y.FanB. Y.et al (2022). Anterior cingulate cortex mediates hyperalgesia and anxiety induced by chronic pancreatitis in rats. Neurosci. Bull.38, 342–358. 10.1007/s12264-021-00800-x
99
RuscianoD. (2024). Molecular mechanisms and therapeutic potential of Gabapentin with a focus on topical formulations to treat ocular surface diseases. Pharmaceuticals17, 623. 10.3390/ph17050623
100
SalomanJ. L.LiY.StelloK.LiW.LiS.PhillipsA. E.et al (2023). Serum biomarkers of nociceptive and neuropathic pain in chronic pancreatitis. J. Pain24, 2199–2210. 10.1016/j.jpain.2023.07.006
101
SantoshD.LakhtakiaS.GuptaR.ReddyD. N.RaoG. V.TandanM.et al (2009). Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment. Pharmacol. Ther.29, 979–984. 10.1111/j.1365-2036.2009.03963.x
102
ShermanM. H.YuR. T.EngleD. D.DingN.AtkinsA. R.TiriacH.et al (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell159, 80–93. 10.1016/j.cell.2014.08.007
103
ShethS. G.MachicadoJ. D.ChalhoubJ. M.ForsmarkC.ZyromskiN.ThosaniN. C.et al (2024). American society for gastrointestinal endoscopy guideline on the role of endoscopy in the management of chronic pancreatitis: summary and recommendations. Gastrointest. Endosc.100, 584–594. 10.1016/j.gie.2024.05.016
104
ShienbaumR. S. J. B. D. J. M. H. R.Celiac plexus block. (2024).
105
SinghV. K.YadavD.GargP. K. (2019). Diagnosis and management of chronic pancreatitis: a review. Jama322, 2422–2434. 10.1001/jama.2019.19411
106
SmileyM. M.LuY.Vera-PortocarreroL. P.ZidanA.WestlundK. N. (2004). Intrathecal gabapentin enhances the analgesic effects of subtherapeutic dose morphine in a rat experimental pancreatitis model. Anesthesiology101, 759–765. 10.1097/00000542-200409000-00026
107
StrandD. S.LawR. J.YangD.ElmunzerB. J. (2022). AGA clinical practice update on the endoscopic approach to recurrent acute and chronic pancreatitis: expert review. Gastroenterology163, 1107–1114. 10.1053/j.gastro.2022.07.079
108
SutherlandD. E.RadosevichD. M.BellinM. D.HeringB. J.BeilmanG. J.DunnT. B.et al (2012). Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J. Am. Coll. Surg.214, 409–424. 10.1016/j.jamcollsurg.2011.12.040
109
SwentekL.ChungD.IchiiH. (2021). Antioxidant therapy in pancreatitis. Antioxidants (Basel)10, 657. 10.3390/antiox10050657
110
TakamidoS.KataokaY.TananoA.CuiY.IkeuraT.ShimataniM.et al (2006). Intrapancreatic axonal hyperbranching of dorsal root ganglia neurons in chronic pancreatitis model rats and its relation to pancreatic pain. Pancreas33, 268–279. 10.1097/01.mpa.0000240600.72946.23
111
TalukdarR.LakhtakiaS.Nageshwar ReddyD.RaoG. V.PradeepR.BanerjeeR.et al (2016). Ameliorating effect of antioxidants and pregabalin combination in pain recurrence after ductal clearance in chronic pancreatitis: results of a randomized, double blind, placebo-controlled trial. J. Gastroenterol. Hepatol.31, 1654–1662. 10.1111/jgh.13332
112
TalukdarR.MurthyH. V.ReddyD. N. (2015). Role of methionine containing antioxidant combination in the management of pain in chronic pancreatitis: a systematic review and meta-analysis. Pancreatology15, 136–144. 10.1016/j.pan.2015.01.003
113
TalukdarR.OlesenS. S.UnnisaM.BedarkarA.SarkarS.TandanM.et al (2024). Extracorporeal shock-wave lithotripsy and endoscopy for the treatment of pain in chronic pancreatitis: a sham-controlled, randomized trial. Ann. Intern. Med.177, 749–758. 10.7326/m24-0210/m38801774
114
TalukdarR.ReddyD. N. (2013). Pain in chronic pancreatitis: managing beyond the pancreatic duct. World J. Gastroenterol.19, 6319–6328. 10.3748/wjg.v19.i38.6319
115
TandanM.Nageshwar ReddyD.TalukdarR.VinodK.KiranS. V. V. S.SantoshD.et al (2019). ESWL for large pancreatic calculi: report of over 5000 patients. Pancreatology19, 916–921. 10.1016/j.pan.2019.08.001
116
TchoutaL. N.SchropeB. A. (2024). Evolving technique for Puestow-Type procedure for chronic pancreatitis: the combined Roux-en-Y proximal end-to-side and distal longitudinal pancreatojejunostomy. Am. J. Case Rep.25, e942066. 10.12659/ajcr.942066
117
TemmermandR.BarrettJ. E.FontanaA. C. K. (2022). Glutamatergic systems in neuropathic pain and emerging non-opioid therapies. Pharmacol. Res.185, 106492. 10.1016/j.phrs.2022.106492
118
ThierensN.VerdonkR. C.LöhrJ. M.van SantvoortH. C.BouwenseS. A.van HooftJ. E. (2025). Chronic pancreatitis. Lancet404, 2605–2618. 10.1016/s0140-6736(24)02187-1
119
TringaliA.BoveV.Vadalà di PramperoS. F.BoškoskiI.FamiliariP.PerriV.et al (2019). Long-term follow-up after multiple plastic stenting for refractory pancreatic duct strictures in chronic pancreatitis. Endoscopy51, 930–935. 10.1055/a-0959-6163
120
van HuijgevoortN. C. M.VeldJ. V.FockensP.BesselinkM. G.BoermeesterM. A.ArvanitakisM.et al (2020). Success of extracorporeal shock wave lithotripsy and ERCP in symptomatic pancreatic duct stones: a systematic review and meta-analysis. Endosc. Int. Open8, E1070-E1085–e1085. 10.1055/a-1171-1322
121
van VeldhuisenC. L.KempeneersM. A.de RijkF. E. M.BouwenseS. A.BrunoM. J.FockensP.et al (2025). Long-term outcomes of early surgery vs endoscopy first in chronic pancreatitis: follow-up analysis of the ESCAPE randomized clinical trial. JAMA Surg.160, 126–133. 10.1001/jamasurg.2024.5182
122
van ZeggerenL.Boelens NabbiR.KallewaardJ. W.SteegersM.CohenS. P.KapuralL.et al (2025). 16. Pain in chronic pancreatitis. Pain Pract.25, e70030. 10.1111/papr.70030
123
VardanyanM.MelemedjianO. K.PriceT. J.OssipovM. H.LaiJ.RobertsE.et al (2010). Reversal of pancreatitis-induced pain by an orally available, small molecule interleukin-6 receptor antagonist. Pain151, 257–265. 10.1016/j.pain.2010.05.022
124
WangK.DemirI. E.D'HaeseJ. G.TieftrunkE.KujundzicK.SchornS.et al (2013). The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis35, 103–113. 10.1093/carcin/bgt312
125
WangY.AaronR.AttalN.CollocaL. (2025). An update on non-pharmacological interventions for pain relief. Cell Rep. Med.6, 101940. 10.1016/j.xcrm.2025.101940
126
WenY.LuoY.HuangY.ZhangZ.XiongL.WangY. (2024). Global, regional, and national pancreatitis burden and health inequality of pancreatitis from 1990 to 2019 with a prediction from 2020 to 2034. BMC Public Health24, 3329. 10.1186/s12889-024-20796-z
127
WhitcombD. C.YadavD.AdamS.HawesR. H.BrandR. E.AndersonM. A.et al (2008). Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the north American pancreatitis study 2 (NAPS2). Pancreatology8, 520–531. 10.1159/000152001
128
WilcoxC. M.BangJ. Y.BuxbaumJ.GardnerT. B.HawesR.KediaP.et al (2024). Effect of endoscopic ultrasound guided celiac plexus block on the palliation of pain in chronic pancreatitis (EPOCH Trial): study protocol for a randomized multicenter sham-controlled trial {1}. Trials25, 676. 10.1186/s13063-024-08478-y
129
WilcoxC. M.YadavD.YeT.GardnerT. B.GelrudA.SandhuB. S.et al (2015). Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin. Gastroenterol. Hepatol.13, 552–560. 10.1016/j.cgh.2014.10.015
130
WilcoxM. C.SandhuB. S.SinghV.GelrudA.AbberbockJ. N.ShermanS.et al (2016). Racial differences in the clinical profile, causes, and outcome of chronic pancreatitis. Official J. Am. Coll. Gastroenterology | ACG111, 1488–1496. 10.1038/ajg.2016.316
131
WinstonJ. H.HeZ. J.ShenoyM.XiaoS. Y.PasrichaP. J. (2005). Molecular and behavioral changes in nociception in a novel rat model of chronic pancreatitis for the study of pain. Pain117, 214–222. 10.1016/j.pain.2005.06.013
132
WoolfC. J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain152, S2–s15. 10.1016/j.pain.2010.09.030
133
WuJ. L.KuangW. Q.ZhuZ. Y.DouJ. H.YaoJ. H.CaoJ.et al (2025). Upregulation of NR2B subunits of NMDA receptors in the lateral parabrachial nucleus contributes to chronic pancreatitis pain. CNS Neurosci. Ther.31, e70313. 10.1111/cns.70313
134
WyseJ. M.SahaiA. V. (2018). Endoscopic ultrasound-guided management of pain in chronic pancreatitis and pancreatic cancer: an update. Curr. Treat. Options Gastroenterol.16, 417–427. 10.1007/s11938-018-0193-z
135
XuG. Y.WinstonJ. H.ShenoyM.YinH.PendyalaS.PasrichaP. J. (2007). Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology133, 1282–1292. 10.1053/j.gastro.2007.06.015
136
XuanJ. L.ZhuY. W.XuW. H.ZhaoH.ChenJ. D. Z.WuG. J.et al (2022). Integrative effects of transcutaneous electrical acustimulation on abdominal pain, gastrointestinal motility, and inflammation in patients with early-stage acute pancreatitis. Neurogastroenterol. Motil.34, e14249. 10.1111/nmo.14249
137
YadavD.AskewR. L.PalermoT.LiL.AndersenD. K.ChenM.et al (2023). Association of chronic pancreatitis pain features with physical, mental, and social health. Clin. Gastroenterol. Hepatol.21, 1781–1791.e4. 10.1016/j.cgh.2022.09.026
138
YangH.McNearneyT. A.ChuR.LuY.RenY.YeomansD. C.et al (2008). Enkephalin-encoding Herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol. Pain4, 8. 10.1186/1744-8069-4-8
139
YangS.ChangM. C. (2020). Effect of repetitive transcranial magnetic stimulation on pain management: a systematic narrative review. Front. Neurol.11, 114. 10.3389/fneur.2020.00114
140
ZhouD.WangW.ChengX.WeiJ.ZhengS. (2015). Antioxidant therapy for patients with chronic pancreatitis: a systematic review and meta-analysis. Clin. Nutr.34, 627–634. 10.1016/j.clnu.2014.07.003
141
ZhuH. Y.LiuX.MiaoX.LiD.WangS.XuG. Y. (2017). Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol. Pain13, 1744806917697979. 10.1177/1744806917697979
142
ZhuY.ColakT.ShenoyM.LiuL.MehtaK.PaiR.et al (2012b). Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol. Pain8, 65. 10.1186/1744-8069-8-65
143
ZhuY.ColakT.ShenoyM.LiuL.PaiR.LiC.et al (2011). Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology141, 370–377. 10.1053/j.gastro.2011.03.046
144
ZhuY.MehtaK.LiC.XuG. Y.LiuL.ColakT.et al (2012a). Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol.302, G176–G181. 10.1152/ajpgi.00053.2011
Summary
Keywords
central sensitisation, chronic pancreatitis, quality of life, pain managemant, neurogenic inflammation
Citation
Lin Z, Pandol S, Apte M and Jiang Y (2025) Navigating chronic pancreatitis pain: a pathophysiological and therapeutic overview. Front. Physiol. 16:1622845. doi: 10.3389/fphys.2025.1622845
Received
04 May 2025
Accepted
26 June 2025
Published
10 July 2025
Volume
16 - 2025
Edited by
Oleg Gerasimenko, Cardiff University, United Kingdom
Reviewed by
Kang Chen, Sun Yat-sen University Cancer Center (SYSUCC), China
Menghan Mao, Shanghai Jiao Tong University, China
Updates
Copyright
© 2025 Lin, Pandol, Apte and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Jiang, yijiangmd@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.