Abstract
Objective:
To systematically evaluate the regulatory effects of exercise intervention on telomere length (TL) and telomerase activity (TA), and to provide evidence for formulating precise exercise prescriptions based on telomere protection.
Methods:
Databases including China National Knowledge Infrastructure, Wanfang, VIP, PubMed, Web of Science, Cochrane Library, and Embase were searched to collect randomized controlled trials (RCTs) regarding the regulation of TL and TA by exercise intervention up to February 2025. The Cochrane risk assessment tool was used to evaluate the quality of the included literature. Meta-analysis, heterogeneity test, subgroup analysis, sensitivity analysis, univariate meta-regression analysis, and publication bias test were conducted using Review Manager 5.3 and Stata 18.0 software.
Results:
Exercise intervention significantly maintained TL (SMD = 0.59, 95% CI: 0.14–1.06, P = 0.01) and enhanced TA (SMD = 0.35, 95% CI: 0.20–0.51, P < 0.00001). A single study suggests high-intensity interval training (HIIT) may maintain TL (SMD = 0.66, P = 0.01), but this requires further validation due to limited evidence. Aerobic exercise (AE) consistently increased TA (SMD = 0.33, P = 0.0001), while resistance exercise (RE) showed non-significant trends (SMD = 0.16, P = 0.43). Subgroup analysis by sex showed a trend toward greater TL maintenance in females (SMD = 0.48, P = 0.06) compared to males (SMD = 0.38, P = 0.40). An exercise duration of ≥16 weeks was necessary for significant effects. High heterogeneity (I2 = 92% for TL) was partially explained by measurement methods, age, and baseline health.
Conclusion:
Exercise maintains TL and enhances TA, potentially contributing to delayed aging. AE shows robust effects on TA, while HIIT and RE require further research due to limited studies. Future studies should standardize measurement methods and explore confounders like diet and genetics.
Systematic Review Registration:
PROSPERO, identifier CRD420251006569.
1 Introduction
Research indicates that the proportion of the world’s population aged 60 and above is increasing rapidly. It is projected that by 2050, this proportion will rise by 20%, surpassing the number of children globally. This phenomenon suggests that the population structure of most countries is tending towards aging (Stambler, 2017). Therefore, developing interventions that can slow down the aging process or reduce the incidence of aging-related diseases has become an urgent task, which also holds significant application value in improving the quality of life and reducing medical costs (Chakrabarti and Mohanakumar, 2016; Konar et al., 2016). Studies on human and animal models have shown that various genetic, dietary, exercise, and drug interventions can extend lifespan. Meanwhile, these lifespan - extending methods also contribute to delaying the onset of age - related diseases (Kenyon, 2010; Tacutu et al., 2013). In recent years, research has revealed the importance of telomere length (TL) and its integrity in the aging process, as well as potential interventions to delay aging, such as physical exercise and a healthy diet (Mercken et al., 2012). Since TL plays a crucial role in cellular aging and telomere shortening is associated with a decrease in life expectancy and an increased risk of chronic diseases, telomere attrition has been described as one of the important biological features of aging (López-Otín et al., 2013).
Telomeres are special structures at the ends of linear chromosomes, composed of repetitive G - and C - rich DNA sequences (5’ - TTAGGG - 3’/3’ - CCCTAA - 5′) and bound to a protein complex (shelterin), including telomeric repeat binding factor 1 (TRF1), telomeric repeat binding factor 2 (TRF2), protection of telomeres 1 protein (POT1), TRF1 - and TRF2 - interacting nuclear protein 2 (TIN2), TIN2 and POT1 interacting protein 1 (TPP1), and repressor activator protein 1 (RAP1). These proteins directly recognize telomere sequences and assist in forming T - loop and D - loop structures, thus hiding the telomere ends and suppressing the DNA damage response, preventing the activation of ataxia - telangiectasia mutation (ATM) and RAD3 - related (ATR) kinases (Balan et al., 2018; Blackburn et al., 2015; de Lange, 2005). Telomeres play a key role in stabilizing chromosomes, preventing DNA degradation and end - to - end fusion, and regulating cell growth. Simultaneously, as a mitotic clock, their length gradually shortens with cell division, serving as an indicator of cellular replication potential (Arnoult and Karlseder, 2015; Blackburn, 2010). With aging, telomere shortening leads to functional impairment, triggering genomic instability, cell senescence, and apoptosis (Blackburn et al., 2015). Biological aging is a process independent of chronological aging, which reduces the organism’s viability and increases vulnerability. TL, as a biomarker of biological aging, records both chronological and biological age (Brown et al., 2017). When TL shortens below a threshold, it can trigger chromosome fusion, genomic instability, and DNA damage, resulting in the production of non - functional proteins (Cleal et al., 2018; Hemann et al., 2001). These proteins may induce apoptosis or promote cancer development. Although telomere shortening can suppress tumors, its functional loss accelerates cell aging and tissue degeneration, driving organismal aging (Vakonaki et al., 2018). Therefore, maintaining TL is crucial for delaying aging.
Telomerase is an RNA - dependent DNA polymerase composed of telomerase reverse transcriptase (TERT) and telomerase RNA template (TERC), which can provide cells with unlimited proliferation potential by lengthening telomeric DNA (Blackburn, 2001; Cong et al., 2002). Due to the “end - replication problem”, the telomeres of somatic cells gradually shorten with age, while telomerase can slow down this process (Harley et al., 1990; Beyne-Rauzy et al., 2005). The polymorphism of TERT is associated with a reduced risk of breast cancer (Helbig et al., 2017), and telomerase plays a key role in maintaining genomic stability by synthesizing telomeres and counteracting telomere erosion (Zhang F. et al., 2016). In addition, the regulation of telomerase activity (TA) has potential value in anti - aging and cancer treatment (Cong et al., 2002; Aviv, 2002).
With the change of lifestyle, the lifespan and quality of life of the elderly have improved, especially with regular physical exercise. However, the underlying mechanisms remain unclear, which has, to some extent, promoted research on the relationship between exercise and telomere biology, such as whether exercise can delay aging and improve diseases. This systematic review and meta - analysis aim to integrate existing clinical studies and systematically evaluate the regulatory effects of exercise intervention on TL and TA, providing evidence - based support for formulating precise exercise prescriptions based on telomere protection.
2 Methods
This study was preregistered at PROSPERO (CRD420251006569) and adheres to PRISMA guidelines.
2.1 Literature inclusion and exclusion criteria
Inclusion criteria: Randomized controlled trials (RCTs) from database inception to February 2025, with no baseline differences between experimental and control groups. The control group maintained a regular lifestyle without exercise, while the experimental group received exercise intervention (minimum 16 weeks, ≥60 min/week). Outcome indicators: TL and TA.
Exclusion criteria: Non-RCTs, studies with ineligible outcomes (e.g., animal studies), exercise combined with diet or other interventions, no control group, non-continuous exercise, duplicated publications, or exercise perception training.
2.2 Literature search strategy
Databases (PubMed, Web of Science, Cochrane Library, Embase, CNKI, Wanfang, VIP) were searched using terms “telomeres, telomerase, exercise, senescence” up to February 2025. The PubMed search strategy is shown in Figure 1.
FIGURE 1

PubMed database search strategy.
2.3 Data extraction
Data were extracted on author, publication year, participant characteristics, sample size, intervention details (time, frequency, method), cell/tissue types, measurement methods, and outcomes. Ineligible studies were excluded after title/abstract or full-text review.
2.4 Quality evaluation
The Cochrane risk assessment tool evaluated selection, implementation, detection, followup, reporting, and other biases, with studies classified as high (5+ points), medium (3–4 points), or low quality (2 or fewer points) (Higgins et al., 2011).
2.5 Statistical analysis
Meta-analysis used Review Manager 5.3 and Stata 18.0. Standardized mean difference (SMD) and 95% confidence intervals (CI) were calculated. Significance was set at P < 0.05. Heterogeneity was assessed via Q-test (α = 0.1) (Hatala et al., 2005). A fixed-effects model was used if I2 ≤ 50%; otherwise, a random-effects model was applied, with subgroup, sensitivity, and meta-regression analyses to explore heterogeneity. Egger’s test assessed publication bias (Aviv, 2002).
3 Results
3.1 Literature search results
A total of 1,566 papers were initially obtained by searching various databases, including Chinese databases (CNKI, Wanfang, VIP) and English databases (PubMed, Web of Science, Cochrane Library, Embase). After importing them into EndNote X9 literature management software to remove duplicate papers, 741 papers remained. Preliminary screening by reading the titles and abstracts led to the exclusion of 689 irrelevant papers, leaving 52 papers. Following further full-text review, 41 papers were excluded due to intervention methods not complying (n = 5) or being non-randomized controlled trials (n = 36). Additionally, 5 manually searched literature pieces were added. Ultimately, 16 randomized controlled trial (RCT) papers were included in the qualitative and meta-analyses (Figure 2).
FIGURE 2
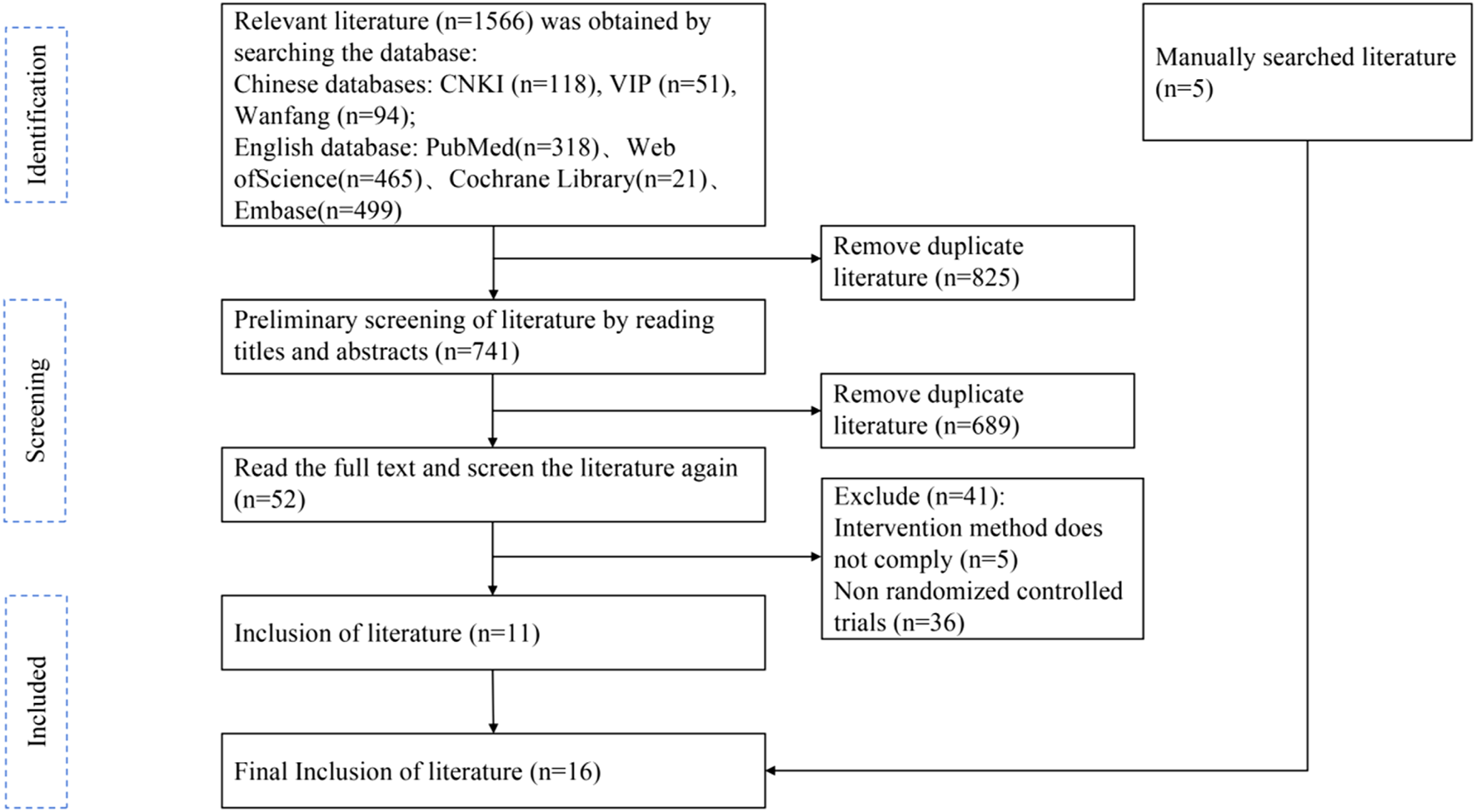
Flow Diagram of literature selection.
3.2 Basic characteristics and quality evaluation of the included papers
The basic characteristics of the 16 papers included in the Meta-analysis of this study are shown in Table 1. A total of 1,908 subjects were included in the Meta-analysis, with 1,005 in the experimental group and 903 in the control group. Among them, 11 papers adopted aerobic exercise (AE) intervention, 1 paper used high intensity interval training (HIIT) intervention, 3 papers applied resistance exercise (RE) intervention, and 3 paper used a combination of aerobic and resistance exercise intervention. The control groups in all included papers did not undergo any exercise intervention. The participants varied in type, including patients with breast cancer, women suffering from intimate partner violence, healthy women, people with high stress and lack of exercise, healthy populations, menopausal women, healthy elderly people, overweight and obese women, chronic disease patients, obese middle-aged females, postmenopausal women, PCOS women, myocardial infarction patients, and healthy older women. Gender distribution varied across studies, with some focusing on females, males, or mixed populations. Exercise intervention durations ranged from 8 to 52 weeks, with frequencies from 2 to 7 times per week.
TABLE 1
| Study | Country | Participants | Intervention | Outcome | Research quality/ score |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Age/y | N | Gender (M/F) | Method | Intensity | Time / min·times-1 |
Frequency / Times/ week |
Time/ weeks |
||||
| (Brown et al., 2023) | U.S.A | Patients with breast cancer | T 58.9 ± 8.4 C 59.2 ± 8.1 |
86 88 |
0/154 | AE + RE | 10RM/Moderate intensity | 2–3 sets /30 | 2/3-6 | 52 | ① | 4 |
| (Cheung et al., 2019) | China | Women who suffer from intimate partner violence | T42.0±8.7 C 41.5 ± 9.3 |
136 135 |
0/271 | AE | Baduanjin | 30 | 7 | 22 | ② | 6 |
| (Eigendorf et al., 2019) | Germany | Healthy women | T 53.0±4.9 C 52.8±4.7 |
146 145 |
0/291 | AE | — | 20 | 3 | 24 | ① | 4 |
| (Puterman et al., 2018) | U.S.A | People with high stress and lack of exercise | T 59.3 ± 5.7 C63.3±6.4 |
34 34 |
13/55 | AE | Low intensity - medium high intensity | 20–30 | 3–5 | 16 | ①② | 6 |
| (Werner et al., 2019) | Germany | Healthy population | T 50.2±7.4 49.5±7.0 48.4±6.5 C 48.1±7.5 |
26 29 34 35 |
45/79 | AE HIIT RE |
60%HRR 4 × 4 20RM |
45 | 3 | 26 | ①② | 4 |
| (Friedenreich et al., 2018) | Canada | Lack of exercise and healthy menopausal women | T 60.4 C 60.0 |
99 113 |
0/212 | AE | 70–80%HRR | 45 | 5 | 48 | ① | 6 |
| (Duan et al., 2016) | China | Healthy elderly people | T 59.6±5.6 C 59.9±5.7 |
43 37 |
32/48 | AE | Yang’s Tai Chi | 60 | 5 | 24 | ② | 6 |
| (Dimauro et al., 2016) | Italy | Healthy elderly people | T 72± 1 C 72± 1 |
10 10 |
10/10 | RE | 10-12 times | 3–4 sets | 2 | 12 | ① | 3 |
| (Mason et al., 2014) | U.S.A | Overweight and obese women | T 58.1±5.0 C 57.4±4.4 |
106 79 |
0/185 | AE | 70–85%HRR | 45 | 5 | 48 | ① | 4 |
| (Ho et al., 2012) | China | Chronic disease patients | T 42.1±7.3 C 42.5±5.5 |
33 31 |
13/51 | AE | Five element balance skill | 30 | 7 | 16 | ② | 4 |
| (Shin et al., 2008) | Korea | Obese middle-aged female | 46.8±6.4 | 8 8 |
0/16 | AE | 60% VO2R | 45 | 3 | 24 | ① | 3 |
| (Hagstrom and Denham, 2018) | Australia | Postmenopausal women | T 60.4 C 60.0 |
99 113 |
0/212 | AE | 70%–80% HRR | 45 | 5 | 48 | ① | 6 |
| (Ribeiro et al., 2021) | Brazil | PCOS women | T 28.5±5.8 C 29.0±4.3 |
58 29 |
0/87 | AE | 50%–60% HRR | 30–60 | 3 | 16 | ① | 4 |
| (Saks et al., 2016) | Iran | Myocardial infarction patients | T 57.3 ± 5.6 C 58.4 ± 5.4 |
10 10 |
20/0 | AE + RE | 8-15RM/50%–60% HRR | 1-3sets/30 | 3 | 8 | ①② | 4 |
| (Sanchez-Gonzalez et al., 2021) | Spain | Healthy older women | T 71.2± 4.3 C 72.7±4.1 |
33 41 |
0/74 | AE + RE | — | — | 3 | 24 | ① | 3 |
| (Hoodenand-Moghadam et al., 2020) | Iran | Healthy elderly men | T 66.3± 3.4 C 66.1± 3 |
15 15 |
30/0 | RE | 60% 1RM | 4 sets of the 6 exercise circuits | 3 | 12 | ② | 5 |
Characteristics of the studies included in the Meta⁃analysis.
T: experimental group C: control group; AE: aerobic exercise; RE: resistance exercise; HIIT: High-intensity interval exercise; HR: Heart rate.
HRR: heart rate reserve; VO2R: The difference between maximum VO2 and resting VO2; ①: TL; ②: TA; PCOS: polycystic ovary syndrome.
Table 2 outlines the cell/tissue types used for analysis and the methods for measuring TL and TA. Leukocytes were commonly used for TL measurement via qPCR, while PBMCs were frequently used for TA measurement through methods like PCR ELISA PLUS or TRAP ELISA. DNA extraction methods also varied, including kits such as QIAamp DNA Mini kit, PAXgeneTM Blood DNA Tube, or Macherey-Nagel NucleoMag Blood 200 μL kit.
TABLE 2
| Study | Cell/tissue type | TL | TA | DNA |
|---|---|---|---|---|
| (Brown et al., 2023) | PBMCs, Lymphocyte | qPCR | — | PAXgeneTM Blood DNA Tube, BD Sciences |
| (Cheung et al., 2019) | PBMCs | — | PCR ELISAPLUS | ELISA |
| (Eigendorf et al., 2019) | PBMCs | qPCR | — | QIAamp DNA Mini kit |
| (Puterman et al., 2018) | PBMCs, Leukocytes | qPCR | ddPCR | QIAamp® DNA Blood Midi kit |
| (Werner et al., 2019) | PBMCs, Leukocytes | Flow cytometry, FISH, PCR | Lightcycler | QIAamp DNA Blood Mini Kit(Column extraction) |
| (Friedenreich et al., 2018) | PBMCs, Leukocytes | qPCR | — | Macherey-Nagel NucleoMag Blood 200 μL kit |
| (Duan et al., 2016) | PBMCs | — | TE ELISA | Sodium citrate tube |
| (Dimauro et al., 2016) | PBMCs | RT-PCR | — | ChargeSwitch gDNA 50–100 μL blood Kit |
| (Mason et al., 2014) | PBMCs, Leukocytes | qPCR | — | Qiagen Midi Kit Kit(Column extraction) |
| (Ho et al., 2012) | PBMCs | — | TRAP ELISA | Ficoll-Paque PLUS |
| (Shin et al., 2008) | PBMCs | qPCR | — | Wizard Genomic DNA Purification Kit |
| (Hagstrom and Denham, 2018) | PBMCs, Leukocytes | qPCR | — | Macherey-Nagel NucleoMag Blood 200 μL kit |
| (Ribeiro et al., 2021) | PBMCs, Leukocytes | qPCR | — | MasterPure Complete DNA and RNA Purification Kit |
| (Saks et al., 2016) | PBMCs | qPCR | qPCR | — |
| (Sanchez-Gonzalez et al., 2021) | Saliva | qPCR | — | NanoDropTM 2000/2001 spectrophotometer |
| (Hoodenand-Moghadam et al., 2020) | PBMCs | — | ELISA human kit | ELISA human kit |
Extraction methods of the Studies Included in the Meta⁃analysis.
PBMCs:Peripheral blood mononuclear cells; qPCR: Quantitative Polymerase Chain Reaction; TRAP: Telomeric Repeat Amplification Protocol; ddPCR: Droplet Digital PCR; TE-ELISA: human telomerase–enzyme linked immunosorbent assay.
The Cochrane risk of bias assessment tool was used to evaluate the quality of the above papers. Six papers were of high quality, and nine were of medium quality. The evaluation results are shown in Figures 3, 4.
FIGURE 3
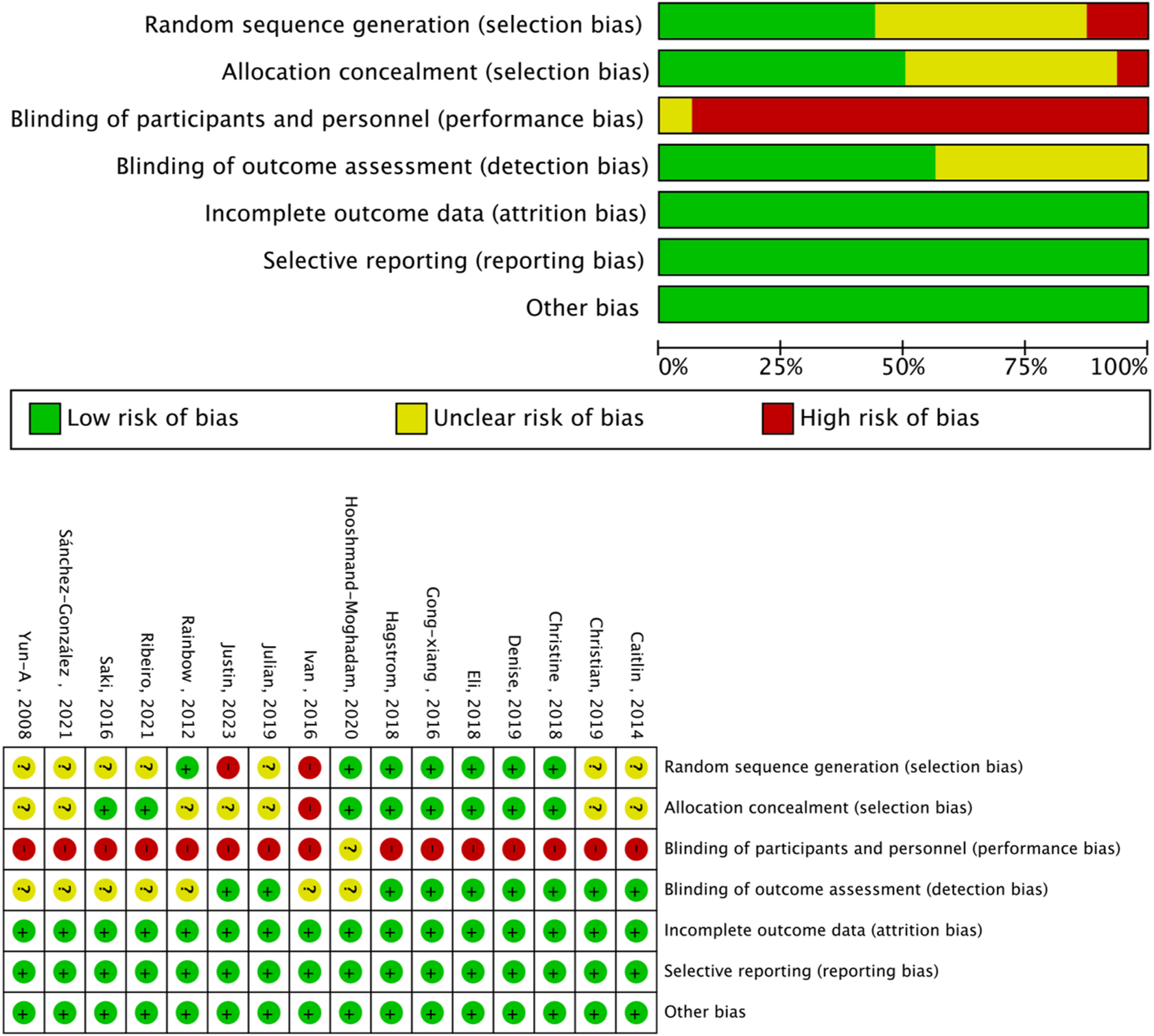
Analysis of the risk of bias in Accordance with the Cochrane Collaboration Guidelines.
FIGURE 4
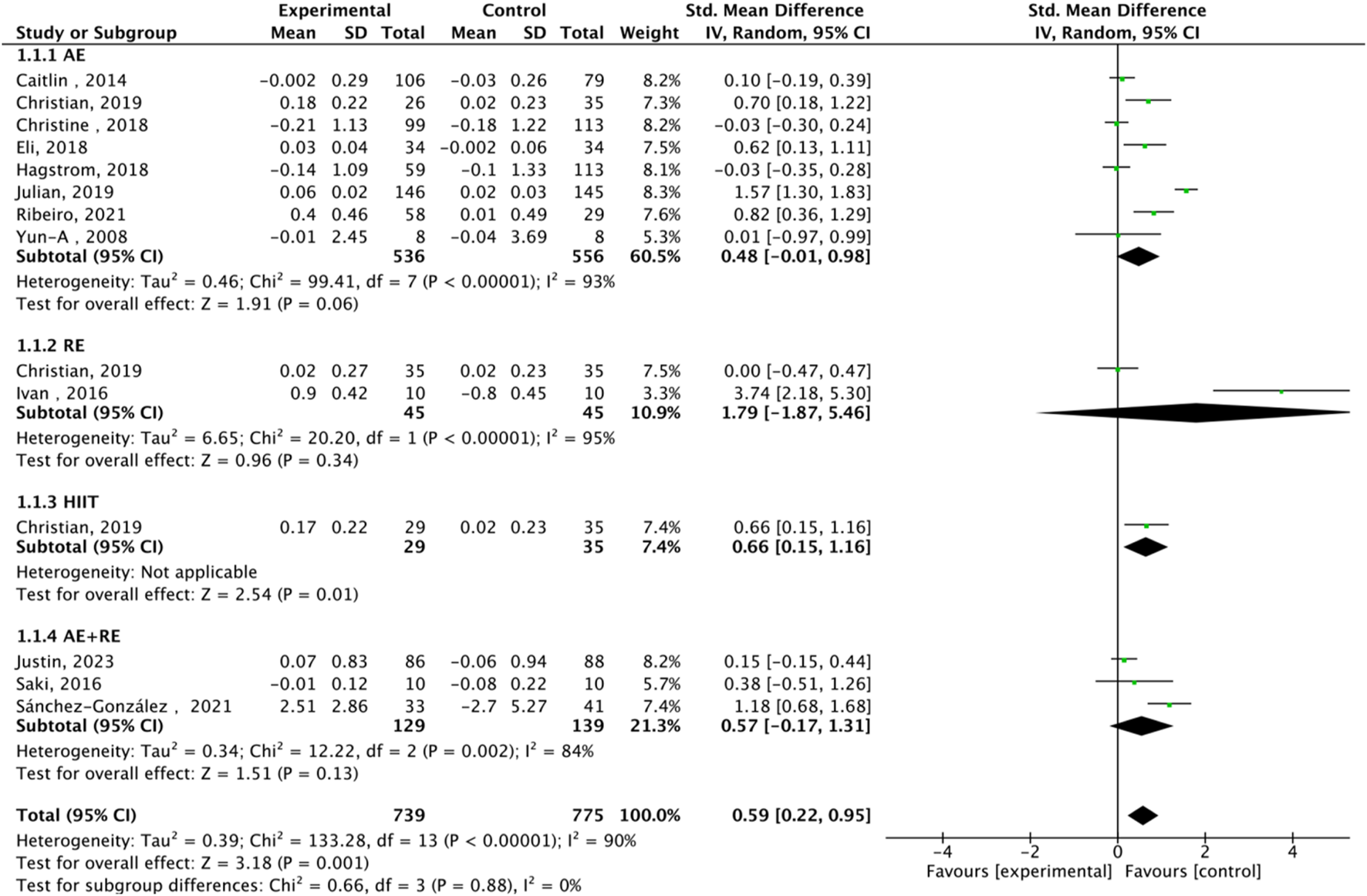
Subgroup analysis of TL effect size under Different Modes of exercise.
3.3 Meta-analysis results
3.3.1 Meta-analysis of the effect size of TL
Fourteen studies assessed TL. Exercise maintained TL (SMD = 0.59, 95% CI: 0.22–0.95, P = 0.001, I2 = 92%, random-effects model) (Figure 4). Subgroup analysis by exercise type showed trends for AE (SMD = 0.48, P = 0.06, I2 = 93%), RE (SMD = 1.79, P = 0.34, I2 = 95%), HIIT (SMD = 0.66, P = 0.01, single study), and AE + RE (SMD = 0.57, P = 0.13). The HIIT result is preliminary due to reliance on a single study. Subgroup analysis by sex showed a trend for females (SMD = 0.48, P = 0.06) over males (SMD = 0.38, P = 0.40) (Figure 5). Sensitivity analysis indicated stable results (Figure 6). Meta-regression identified publication year (2016–2018) as a heterogeneity source (β = −1.256, P = 0.026) (Table 3)
FIGURE 5
![Forest plot showing standard mean differences for experimental and control groups across different studies categorized by gender: male, female, and mixed. Each study's data includes sample size, mean, standard deviation, weight, and confidence intervals. Subtotals and overall totals reflect heterogeneity and test effects, with a total sample size of 775. Significant overall effect favors the experimental group with a standardized mean difference of 0.57, 95% CI [0.16, 0.97].](https://www.frontiersin.org/files/Articles/1627292/xml-images/fphys-16-1627292-g005.webp)
Subgroup analysis of TL effect size under Different gender of exercise.
FIGURE 6
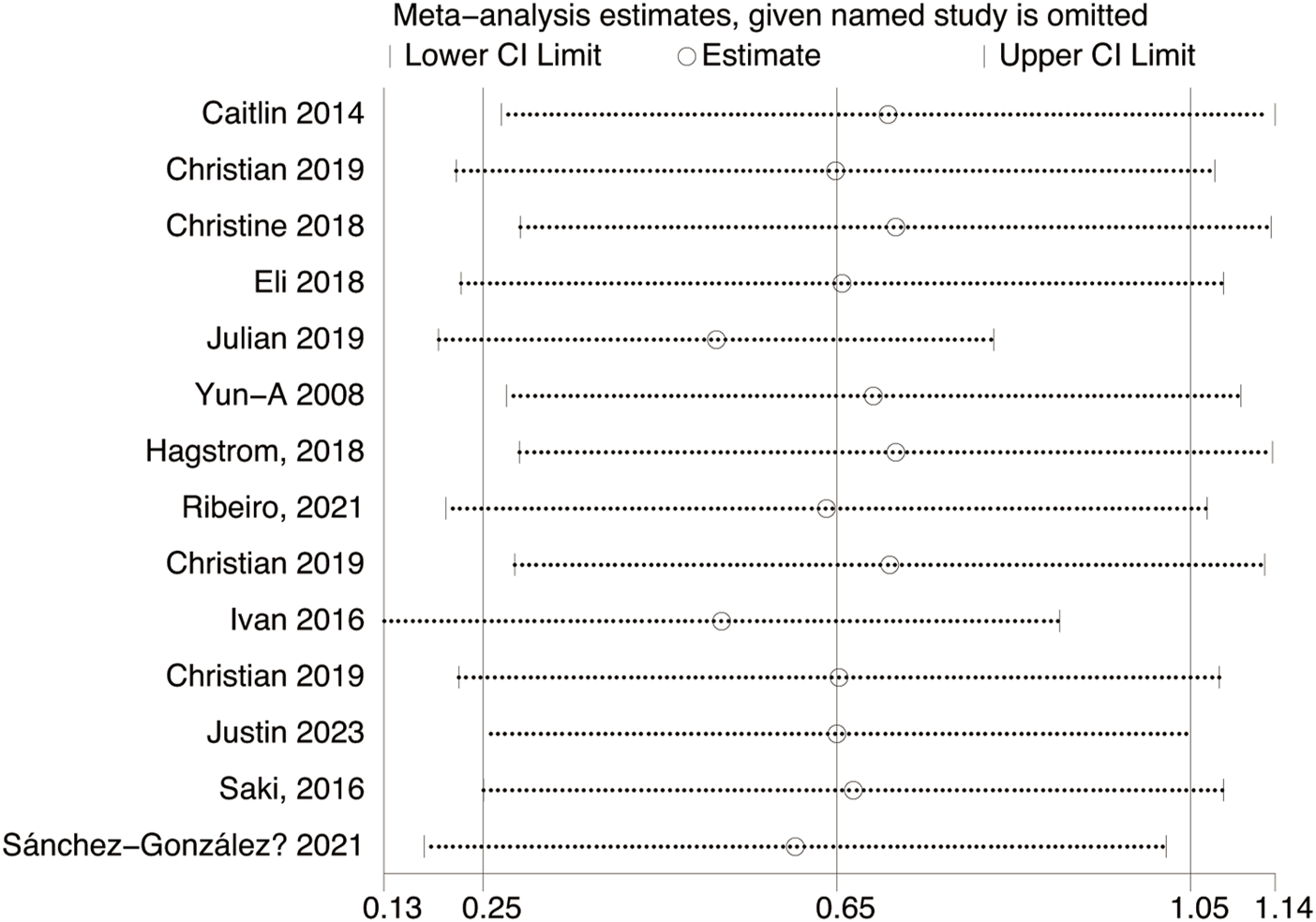
Sensitivity analysis of TL effect size under Different Modes of exercise.
TABLE 3
| Research features | Regression coefficient(β) | 95%CI | t | p |
|---|---|---|---|---|
| Intervention time | −0.04 | −0.08∼0.008 | −1.91 | 0.09 |
| Sample size | −0.002 | −0.01∼0.01 | −0.39 | 0.71 |
| health | 0.69 | −0.79∼2.16 | 1.07 | 0.32 |
| country | 0.40 | −0.21∼1.00 | 1.51 | 0.17 |
| Gender | 0.56 | −0.95∼2.07 | 0.85 | 0.42 |
| Article quality | −0.45 | −2.34∼1.44 | −0.55 | 0.60 |
| Publication Year 2016–2018 | −1.25628 | −2.32∼−0.20 | −2.73 | 0.026 |
Meta-regression analysis results of heterogeneity factors Affecting TL effect size.
3.3.2 Meta - analysis of the effect size of TA
Nine studies assessed TA. Exercise enhanced TA (SMD = 0.36, 95% CI: 0.22–0.51, P < 0.00001, I2 = 39%, fixed-effects model) (Figure 7). Subgroup analysis showed significant effects for AE (SMD = 0.33, P = 0.0001, I2 = 44%) and HIIT (SMD = 0.78, P = 0.003, single study), but not RE (SMD = 0.16, P = 0.43). Mixed-gender groups showed significant TA increases (SMD = 1.12, P = 0.02) (Figure 8).
FIGURE 7
![Forest plot showing a meta-analysis of studies comparing experimental and control groups. It includes subgroups AE, RE, HIIT, and AE+RE with their standard mean differences and confidence intervals. Diamonds represent the pooled effect size for each subgroup. The overall total (95% CI) favors the experimental group at 0.36 [0.22, 0.51]. Heterogeneity measures are provided for each subgroup, with varying degrees of heterogeneity reported.](https://www.frontiersin.org/files/Articles/1627292/xml-images/fphys-16-1627292-g007.webp)
Subgroup analysis of TA effect size under different modes of exercise.
FIGURE 8
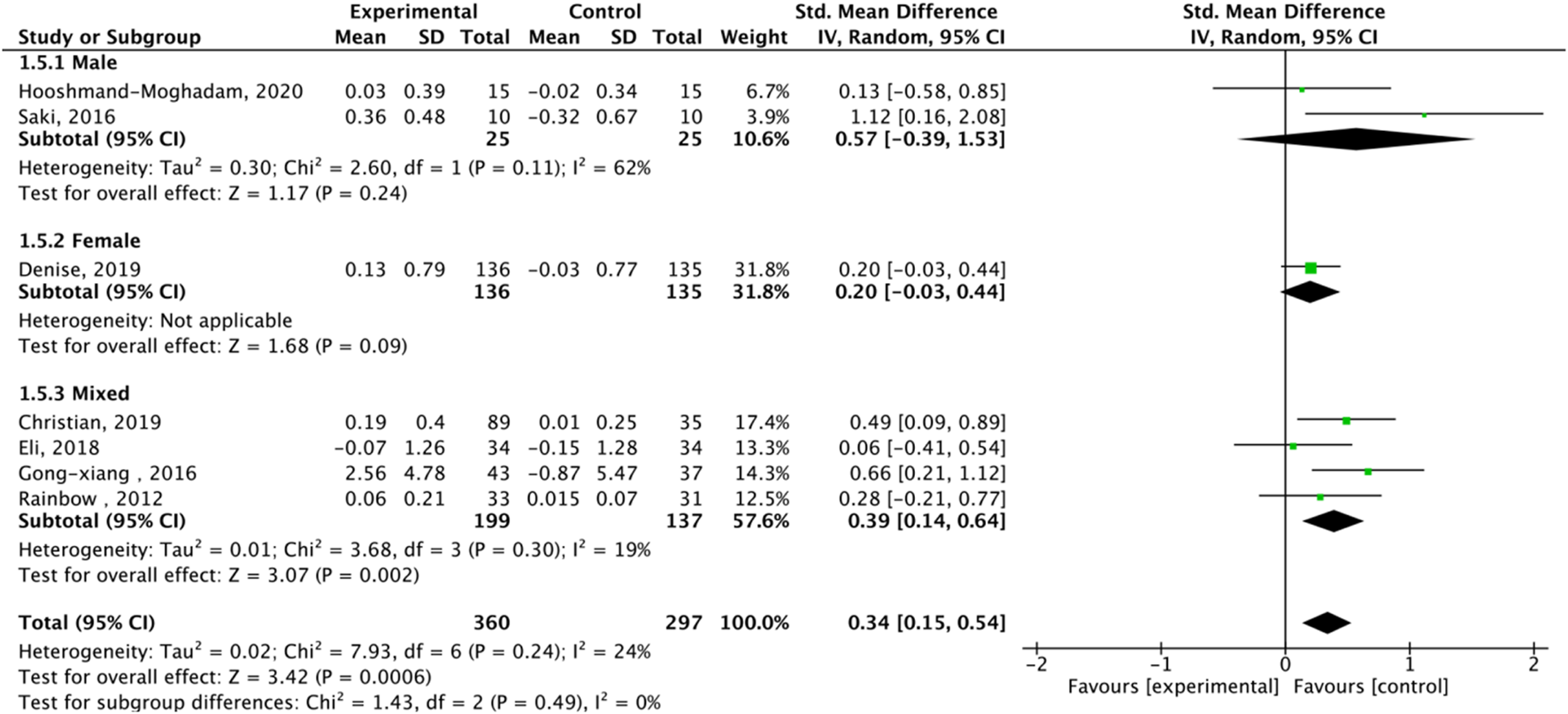
Subgroup analysis of TA effect size under different gender of exercise.
3.3.3 Publication bias analysis
Egger’s test was used to study the publication bias of the literature from two aspects: the intervention effect of exercise on TL and TA. When the intercept segment crossed the zero point, the publication bias was low. For the intervention effect of exercise on TL, the test result was t = 0.46, P = 0.66, 95% CI: (−5.42–8.15), which included 0, indicating that there was no obvious publication bias in the intervention effect of exercise on TL, and the results of the Meta - analysis were relatively stable. For the intervention effect of exercise on TA, the test result was t = 1.35, P = 0.24, 95% CI: (−1.91–6.11), which included 0, indicating that there was no obvious publication bias in the intervention effect of exercise on TA, and the results of the Meta - analysis were relatively stable (Figures 9, 10).
FIGURE 9
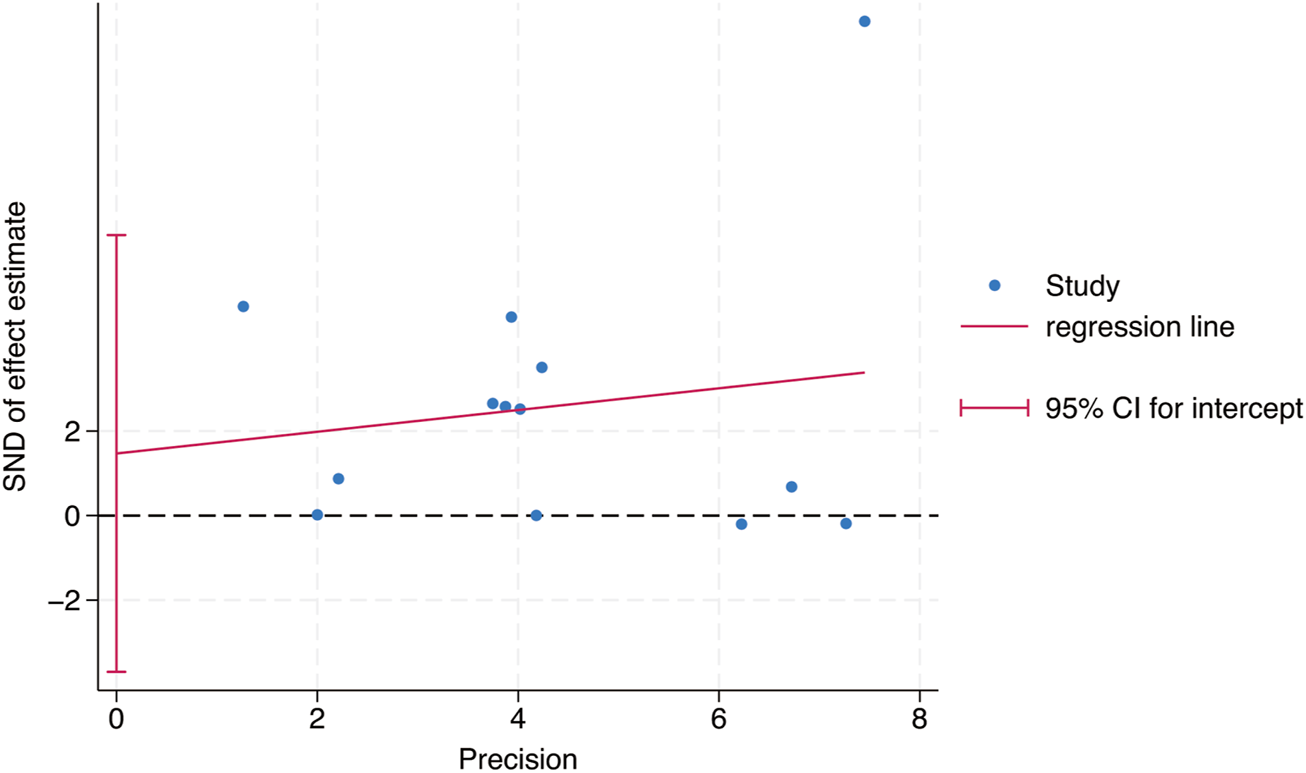
Bias analysis of the impact of exercise intervention on TL
FIGURE 10
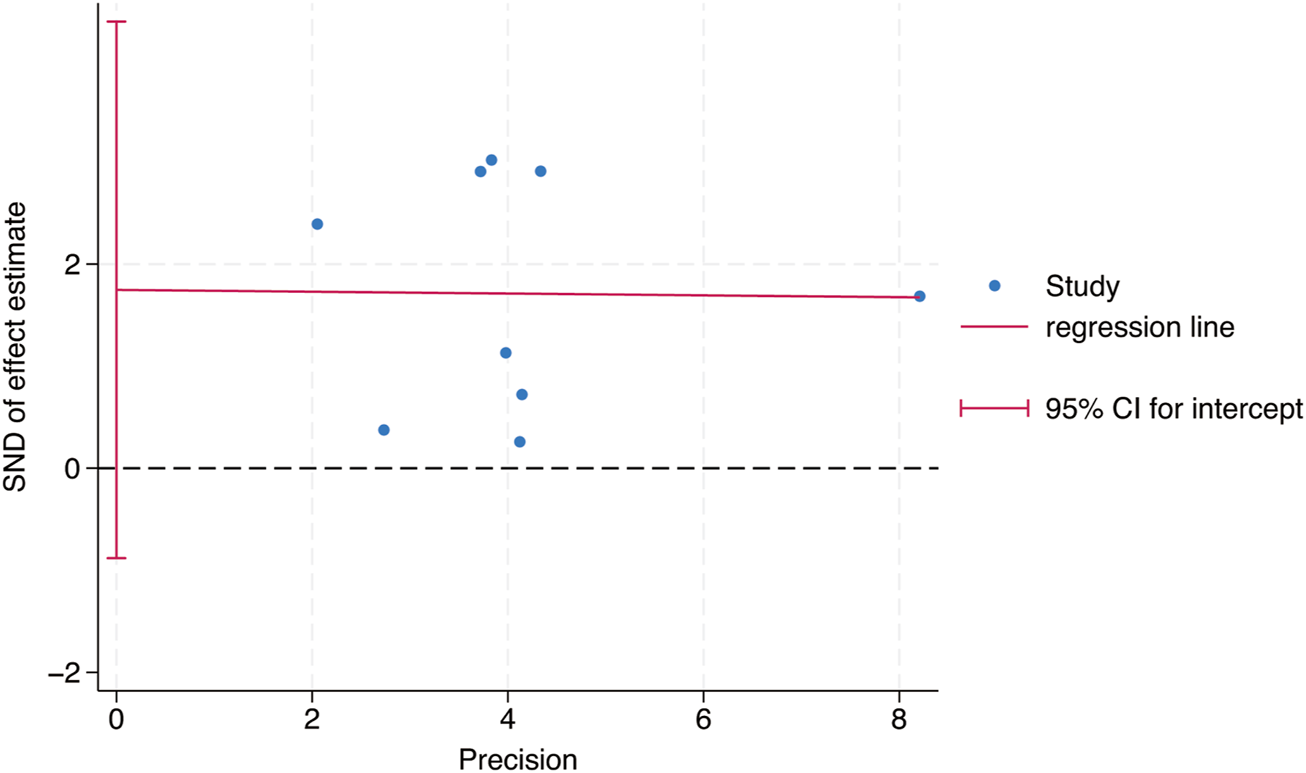
Bias analysis of the impact of exercise intervention on TA
4 Discussion
Exercise maintains TL and enhances TA, potentially contributing to delayed aging. This meta-analysis of 16 RCTs provides evidence for exercise prescriptions targeting telomere protection, aligning with prior meta-analyses like Schellnegger et al. (2022), which found exercise associated with longer TL in leukocytes (SMD = 0.41, P < 0.05) but noted similar heterogeneity challenges (Schellnegger et al., 2022). TL and TA are robust biomarkers of cellular aging, reflecting replication potential more directly than oxidative stress or inflammatory markers (Tacutu et al., 2013). Exercise maintained TL (SMD = 0.60, P = 0.01) and enhanced TA (SMD = 0.35, P < 0.00001). The claim of telomere lengthening is tempered by mechanisms such as selective apoptosis of cells with short telomeres, which may increase the proportion of cells with longer telomeres without actual elongation (Beyne-Rauzy et al., 2005). Thus, exercise primarily maintains TL relative to sedentary controls. TA increases may result from telomerase recruitment to short telomeres (Zou et al., 2004), immune cell proliferation (Simpson et al., 2010), or upregulation of TERT expression (Zhang J. et al., 2016). Mechanistically, exercise reduces oxidative stress via enhanced antioxidant enzyme activity (e.g., superoxide dismutase) (Shin et al., 2008) and suppresses inflammation through reduced pro-inflammatory cytokines (e.g., IL-6, TNF-α) (Werner et al., 2019; von Zglinicki, 2002), both of which protect telomeres from damage (von Zglinicki, 2002).
Subgroup analysis by sex showed a stronger TL maintenance trend in females (SMD = 0.48, P = 0.06) than males (SMD = 0.38, P = 0.40), possibly due to estrogen’s role in telomerase regulation (Konar et al., 2016). AE consistently enhanced TA (SMD = 0.33, P = 0.0001), while HIIT showed promise for TL maintenance (SMD = 0.66, P = 0.01), though this finding is limited by a single study (Werner et al., 2019). RE showed non-significant trends (SMD = 0.16, P = 0.43), likely due to only three studies and high variability in protocols (e.g., intensity, volume) (Zhang F. et al., 2016). Merging AE and RE categories was considered but not implemented, as their distinct physiological mechanisms (e.g., oxidative stress reduction in AE vs muscle hypertrophy in RE) justify separate analyses (Vakonaki et al., 2018).
High heterogeneity (I2 = 92% for TL) was partially explained by measurement methods (e.g., qPCR, Flow-FISH, Southern blot), participant age, and baseline health (Table 2). For example, qPCR is less precise than Southern blot for TL measurement, potentially inflating variability (Chakrabarti and Mohanakumar, 2016). TRAP ELISA for TA is less reliable than gel-based TRAP or droplet digital PCR (Friedenreich et al., 2018). Participant diversity (healthy, cancer, obese, stressed) and age (20–80 years) likely amplify heterogeneity, as disease states or older age may enhance exercise effects (Puterman et al., 2018). Metaregression identified publication year as a significant heterogeneity source, but only 25.2% of variance was explained, suggesting unexamined confounders like diet or genetics (von Zglinicki, 2002). The forest plots (Figures 4,6, ) correctly represent effect sizes favoring exercise, with positive SMD indicating TL/TA increases.
Causal claims about exercise delaying aging are tempered by potential confounders. Diet (e.g., antioxidant intake) and genetic factors (e.g., TERT polymorphisms) may influence TL and TA independently or interact with exercise effects (von Zglinicki, 2002). For instance, high antioxidant diets may synergize with exercise to reduce oxidative stress, while genetic predispositions may modulate telomerase response (de Lange, 2005). These factors were not controlled in most included studies, limiting causal inferences.
Exercise prescriptions include:
• TL maintenance: HIIT, ≥16 weeks, ≥60 min/week, 80%–90% max heart rate, pending further validation.
• TA enhancement: AE (e.g., running, swimming), ≥150 min/week, 60%–75% heart rate reserve, ≥6 months.
• Comprehensive strategy: Combine AE and RE (e.g., Taijiquan) for synergistic effects (Blackburn, 2001).
Limitations include reliance on English literature, limited HIIT/RE studies, measurement variability, and uncontrolled confounders like diet and genetics. Compared to Schellnegger et al. (2022), our study includes more recent RCTs and TA outcomes but faces similar heterogeneity challenges (Schellnegger et al., 2022). Future research should standardize TL/TA measurement methods (e.g., adopt Southern blot or droplet digital PCR), control for confounders, and explore sex- and cell-specific effects.
5 Conclusion
Exercise maintains TL and enhances TA, potentially contributing to delayed aging. AE shows robust effects on TA, while HIIT and RE require further research due to limited studies and non-significant results for RE. Standardized measurement methods and control for confounders like diet and genetics are needed to strengthen causal inferences.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LS: Writing – original draft. TZ: Writing – original draft. LL: Writing – original draft. YY: Writing – original draft. CW: Writing – original draft. JL: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Social Science Foundation of China (Grant No: 19ZDA352).
Acknowledgments
We thank the reviewers for their insightful feedback, which significantly improved this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- RCTs
randomized controlled trials
- TRF1
telomeric repeat binding factor 1
- TRF2
telomeric repeat binding factor 2
- POT1
protection of telomeres 1 protein
- TIN2
TRF1 - and TRF2 - interacting nuclear protein 2
- TPP1
TIN2 and POT1 interacting protein 1
- RAP1
repressor activator protein 1
- TERT
telomerase reverse transcriptase
- TERC
telomerase RNA template
- AE
Aerobic exercise
- RE
Resistance exercise
- HIIT
High-intensity interval exercise
- HR
Heart rate
References
1
Arnoult N. Karlseder J. (2015). Complex interactions between the DNA-Damage response and Mammalian telomeres. Nat. Struct. Mol. Biol.22 (11), 859–866. 10.1038/nsmb.3092
2
Aviv A. (2002). Telomeres, sex reactive oxygen species, and human cardiovascular aging. J. Mol. Med. Berl.80 (11), 689–695. 10.1007/s00109-002-0377-8
3
Balan E. Decottignies A. Deldicque L. (2018). Physical activity and nutrition: two promising strategies for telomere maintenance?Nutrients10 (12), 1942. 10.3390/nu10121942
4
Beyne-Rauzy O. Prade-Houdellier N. Demur C. Recher C. Ayel J. Laurent G. et al (2005). Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood106 (9), 3200–3205. 10.1182/blood-2005-04-1386
5
Blackburn E. H. (2001). Switching and signaling at the telomere. Cell106 (6), 661–673. 10.1016/s0092-8674(01)00492-5
6
Blackburn E. H. (2010). Telomeres and telomerase: the means to the end (nobel lecture). Angew. Chem. Int. Ed. Engl.49 (41), 7405–7421. 10.1002/anie.201002387
7
Blackburn E. H. Epel E. S. Lin J. (2015). Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science350 (6265), 1193–1198. 10.1126/science.aab3389
8
Brown J. C. Sturgeon K. Sarwer D. B. Troxel A. B. DeMichele A. M. Denlinger C. S. et al (2023). The effects of exercise and diet on oxidative stress and telomere length in breast cancer survivors. Breast Cancer Res. Treat.199 (1), 109–117. 10.1007/s10549-023-06868-5
9
Brown L. Needham B. Ailshire J. (2017). Telomere length among older U.S. adults: differences by race/ethnicity, gender, and age. J. Aging Health29 (8), 1350–1366. 10.1177/0898264316661390
10
Chakrabarti S. Mohanakumar K. P. (2016). Aging and neurodegeneration: a tangle of models and mechanisms. Aging Dis.7 (2), 111–113. 10.14336/AD.2016.0312
11
Cheung D. S. T. Deng W. Tsao S. W. Ho R. T. H. Chan C. L. W. Fong D. Y. T. et al (2019). Effect of a qigong intervention on telomerase activity and mental health in Chinese women survivors of intimate partner violence: a randomized clinical trial. JAMA Netw. Open2 (1), e186967. 10.1001/jamanetworkopen.2018.6967
12
Cleal K. Norris K. Baird D. (2018). Telomere length dynamics and the evolution of cancer genome architecture. Int. J. Mol. Sci.19 (2), 482. 10.3390/ijms19020482
13
Cong Y. S. Wright W. E. Shay J. W. (2002). Human telomerase and its regulation. Microbiol. Mol. Biol. Rev.66 (3), 407–425. table of contents. 10.1128/MMBR.66.3.407-425.2002
14
de Lange T. (2005). Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev.19 (18), 2100–2110. 10.1101/gad.1346005
15
Dimauro I. Scalabrin M. Fantini C. Grazioli E. Beltran Valls M. R. Mercatelli N. et al (2016). Resistance training and redox homeostasis: correlation with age-associated genomic changes. Redox Biol.10, 34–44. 10.1016/j.redox.2016.09.008
16
Duan G.-x. Wang K. Su Y.-h. Tang S.-y. Jia H.-l. Chen X.-m. et al (2016). Effects of Tai chi on telomerase activity and gerotranscendence in middle aged and elderly adults in Chinese society. Int. J. Nurs. Sci.3, 235–241. 10.1016/j.ijnss.2016.07.005
17
Eigendorf J. Melk A. Haufe S. Boethig D. Berliner D. Kerling A. et al (2019). Effects of personalized endurance training on cellular age and vascular function in middle-aged sedentary women. Eur. J. Prev. Cardiol.26 (17), 1903–1906. 10.1177/2047487319849505
18
Friedenreich C. M. Wang Q. Ting N. S. Brenner D. R. Conroy S. M. McIntyre J. B. et al (2018). Effect of a 12-month exercise intervention on leukocyte telomere length: results from the ALPHA trial. Cancer Epidemiol.56, 67–74. 10.1016/j.canep.2018.07.012
19
Hagstrom A. D. Denham J. (2018). The effect of resistance training on telomere length in women recovering from breast cancer. J. Strength Cond. Res.32, 1589–1596. 10.3390/jfmk3010009
20
Harley C. B. Futcher A. B. Greider C. W. (1990). Telomeres shorten during ageing of human fibroblasts. Nature345 (6274), 458–460. 10.1038/345458a0
21
Hatala R. Keitz S. Wyer P. Guyatt G. Evidence-Based Medicine Teaching Tips Working Group (2005). Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. Cmaj172 (5), 661–665. 10.1503/cmaj.1031920
22
Helbig S. Wockner L. Bouendeu A. Hille-Betz U. McCue K. French J. D. et al (2017). Functional dissection of breast cancer risk-associated TERT promoter variants. Oncotarget8 (40), 67203–67217. 10.18632/oncotarget.18226
23
Hemann M. T. Strong M. A. Hao L. Y. Greider C. W. (2001). The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell107 (1), 67–77. 10.1016/s0092-8674(01)00504-9
24
Higgins J. P. Altman D. G. Gøtzsche P. C. Jüni P. Moher D. Oxman A. D. et al (2011). The cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj343, d5928. 10.1136/bmj.d5928
25
Ho R. T. Chan J. S. Wang C. W. Lau B. W. So K. F. Yuen L. P. et al (2012). A randomized controlled trial of qigong exercise on fatigue symptoms, functioning, and telomerase activity in persons with chronic fatigue or chronic fatigue syndrome. Ann. Behav. Med.44 (2), 160–170. 10.1007/s12160-012-9381-6
26
Hoodenand-Moghadam M. Najafi A. Mohammadi M. Malekzadeh J. M. (2020). Effect of aerobic training on telomerase activity in peripheral blood mononuclear cells of middleaged men. J. Biol. Regul. Homeost. Agents34, 1895–1901. 10.23812/20-123-L
27
Kenyon C. J. (2010). The genetics of ageing. Nature464 (7288), 504–512. 10.1038/nature08980
28
Konar A. Singh P. Thakur M. K. (2016). Age-associated cognitive decline: insights into molecular switches and recovery avenues. Aging Dis.7 (2), 121–129. 10.14336/AD.2015.1004
29
López-Otín C. Blasco M. A. Partridge L. Serrano M. Kroemer G. (2013). The hallmarks of aging. Cell153 (6), 1194–1217. 10.1016/j.cell.2013.05.039
30
Mason C. Risques R. A. Xiao L. Duggan C. R. Imayama I. Campbell K. L. et al (2014). Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obes. (Silver Spring)21 (12), E549–E554. 10.1002/oby.20509
31
Mercken E. M. Carboneau B. A. Krzysik-Walker S. M. de Cabo R. (2012). Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev.11 (3), 390–398. 10.1016/j.arr.2011.11.005
32
Puterman E. Weiss J. Lin J. Schilf S. Slusher A. L. Johansen K. L. et al (2018). Aerobic exercise lengthens telomeres and reduces stress in family caregivers: a randomized controlled trial - curt richter award paper 2018. Psychoneuroendocrinology98, 245–252. 10.1016/j.psyneuen.2018.08.002
33
Ribeiro J. Oliveira A. Silva I. Santos C. Duarte J. A. (2021). Effects of exercise on leukocyte telomere length in healthy adults: a randomized controlled trial. Eur. J. Sport Sci.21, 567–575. 10.1080/17461391.2020.1754467
34
Saks E. Tiit M. Veldre G. Riso E. M. Märtson A. Jürimäe J. (2016). Exercise and telomere length in peripheral blood mononuclear cells in older adults. Clin. Sci.130, 1357–1366. 10.1042/CS20160058
35
Sanchez-Gonzalez J. L. Sanchez-Rodriguez J. L. Martin-Vallejo J. Martel-Martel A. Gonzalez-Sarmiento R. (2021). Effects of an exercise program on salivary telomere length and stress biomarkers in schoolchildren. Oral Dis.27, 629–636. 10.1111/odi.13594
36
Schellnegger M. Lin A. C. Hammer N. Kamolz L. P. (2022). Physical activity on telomere length as a biomarker for aging: a systematic review. Sports Med. Open8, 111. 10.1186/s40798-022-00503-1
37
Shin Y. A. Lee J. H. Song W. Jun T. W. (2008). Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing Dev.129 (5), 254–260. 10.1016/j.mad.2008.01.001
38
Simpson R. J. Cosgrove C. Ingram L. A. Florida-James G. D. Whyte G. P. Pircher H. et al (2010). Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Exerc Immunol. Rev.16, 96–116.
39
Stambler I. (2017). Recognizing degenerative aging as a treatable medical condition: methodology and policy. Aging Dis.8 (5), 583–589. 10.14336/AD.2017.0130
40
Tacutu R. Craig T. Budovsky A. Wuttke D. Lehmann G. Taranukha D. et al (2013). Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res.41 (Database issue), D1027–D1033. 10.1093/nar/gks1155
41
Vakonaki E. Tsiminikaki K. Plaitis S. Fragkiadaki P. Tsoukalas D. Katsikantami I. et al (2018). Common mental disorders and association with telomere length. Biomed. Rep.8 (2), 111–116. 10.3892/br.2018.1040
42
von Zglinicki T. (2002). Oxidative stress shortens telomeres. Trends Biochem. Sci.27, 339–344. 10.1016/S0968-0004(02)02110-2
43
Werner C. M. Hecksteden A. Morsch A. Zundler J. Wegmann M. Kratzsch J. et al (2019). Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J.40 (1), 34–46. 10.1093/eurheartj/ehy585
44
Zhang F. Cheng D. Wang S. Zhu J. (2016a). Human specific regulation of the telomerase reverse transcriptase gene. Genes (Basel)7 (7), 30. 10.3390/genes7070030
45
Zhang J. Rane G. Dai X. Shanmugam M. K. Arfuso F. Samy R. P. et al (2016b). Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res. Rev.25, 55–69. 10.1016/j.arr.2015.11.006
46
Zou Y. Sfeir A. Gryaznov S. M. Shay J. W. Wright W. E. (2004). Does a sentinel or a subset of short telomeres determine replicative senescence?Genes Dev.18, 3074–3085. 10.1101/gad.1252104
Summary
Keywords
exercise, aging, telomeres, telomerase, meta analysis
Citation
Sun L, Zhang T, Luo L, Yang Y, Wang C and Luo J (2025) Exercise delays aging: evidence from telomeres and telomerase —a systematic review and meta-analysis of randomized controlled trials. Front. Physiol. 16:1627292. doi: 10.3389/fphys.2025.1627292
Received
12 May 2025
Accepted
18 June 2025
Published
26 June 2025
Volume
16 - 2025
Edited by
Jose A. Parraca, Universidade de Évora, Portugal
Reviewed by
Enrico Tam, University of Verona, Italy
Andrew T. Ludlow, University of Michigan, United States
Updates
Copyright
© 2025 Sun, Zhang, Luo, Yang, Wang and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Luo, 784682301@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.