Abstract
Introduction:
Trigeminal neuralgia (TN) is a chronic neuropathic pain disorder characterized by spontaneous or triggered electric shock-like facial pain. Microvascular decompression (MVD) is the most effective surgical intervention for classical TN that is refractory to medication. Recent advances in neuroimaging have enhanced visualization of the trigeminal nerve’s vascular anatomy, deepening insights into TN pathophysiology and paving the way for improved diagnostics and therapies. Resting-state functional magnetic resonance imaging (rs-fMRI) has been extensively applied in studies of TN, uncovering alterations in brain activity, functional connectivity, cortical thickness and neural networks.
Methods:
Independent component analysis (ICA) presents a powerful alternative for analyzing fMRI data, offering several advantages over traditional region of interests (ROIs) approaches. The sensorimotor network playing a key role in pain modulation, identifying neuroimaging differences in the sensorimotor network is crucial for detecting and intervening in TN, Forty TN patients underwent MVD surgery, with follow-up assessments conducted 6 months postoperatively and twenty-five healthy controls (HC) were recruited and scanned with resting state fMRI (rs-fMRI). Group ICA was used to identify ROIs and assessed inter-group differences in neural activity using false discovery rate (FDR) correction.
Results:
Compared to the HC, increased activity was observed in the right frontal operculum cortex, right insular cortex, right inferior frontal gyrus (pars opercularis), and right frontal pole in TN patients. Conversely, decreased activity was found in the right cerebellum (lobule IX) and left cerebellum (lobules VIII and IX). Compared to the pre-surgery, increased activity was found in the right precentral gyrus in the post-surgery group. Compared to the HC, long-term increased activity was still present in the right frontal operculum cortex, right insular cortex, right inferior frontal gyrus (pars opercularis), and right frontal pole despite the effectiveness of MVD surgery. In contrast, MVD significantly reduced the area of aberrant activation regions, particularly in the operculo-insular cortex, and also normalized cerebellar abnormalities.
Discussion:
Our study demonstrates that ICA can effectively identify distinct patterns of functional connectivity in the sensorimotor network associated with TN and MVD surgery. These regions are involved in altered pain processing, including nociceptive stimulus integration, subjective pain perception, pain chronification, and pain-related empathy. Our findings suggest promising biomarkers for TN and provide insights for developing targeted treatments.
1 Introduction
Trigeminal neuralgia (TN) is a chronic neuropathic disorder characterized by sudden, electric shock-like facial pain - either spontaneous or triggered - that typically affects a specific facial region and may persist between paroxysms. This disease can significantly impair quality of life, and in refractory cases, may be associated with suicidal ideation (Zakrzewska et al., 2017). The diagnosis of TN is based on three clinical criteria: (1) pain localized to one or more trigeminal nerve branches, (2) paroxysmal, electric-shock-like pain episodes, and (3) pain triggered by innocuous stimuli in the affected facial or intraoral regions. Triggered paroxysmal pain is pathognomonic of TN, occurring in 91%–99% of reported cases (Di Stefano et al., 2018; Cruccu et al., 2016; Maarbjerg et al., 2014). TN is currently classified into three distinct subtypes: classical (the most common), secondary, and idiopathic. The classical form arises from neurovascular compression (NVC) at the trigeminal nerve root entry zone (REZ), typically causing focal demyelination. NVC can be visualized using functional magnetic resonance imaging (fMRI) and 3D reconstruction. Anticonvulsants such as carbamazepine (typically 200–1,200 mg daily) and oxcarbazepine (300–1800 mg daily) are considered first-line treatments for managing paroxysmal pain in TN. Although these drugs are effective, they are associated with significant side effects and often have limited efficacy in treating persistent pain (Bendtsen et al., 2019). Microvascular decompression (MVD) has emerged as the treatment of choice for medication-refractory TN (Cruccu et al., 2016). MVD represents the most effective surgical treatment for classical TN. Postoperative outcomes demonstrate 68%–88% initial success rates (1–2 years), with 61%–80% of patients sustaining pain relief at 4–5 years follow-up (Tuleasca et al., 2014; Zakrzewska and Akram, 2011). Resting fMRI (rs-fMRI) has become an invaluable tool for examining central mechanisms, particularly in structure and mapping functional connectivity within the human brain (Biswal et al., 2010). Patients with TN show significant cortical thinning in the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and midcingulate cortex (MCC), accompanied by extensive subcortical volume reductions (Mo et al., 2021). In addition, TN patients exhibited significantly greater cortical thickness in the contralateral primary somatosensory cortex and frontal pole compared to controls (DeSouza et al., 2013). Compared with healthy controls (HC), TN patients showed significantly altered regional homogeneity (ReHo) and fractional amplitude of low-frequency fluctuations (fALFF) in multiple brain regions, including the cerebellum, temporal lobe, cingulate cortex, putamen, limbic lobe, occipital lobe, precuneus, insula, and medial/superior frontal gyri (Yuan et al., 2018). TN patients displayed increased functional connectivity between the mPFC and left planum temporale, alongside decreased connectivity between the mPFC and left superior frontal gyrus (Wu et al., 2025). Another study revealed that patients with TN exhibited increased functional connectivity between the right insula/secondary somatosensory cortex (S2) and several key regions, including the ACC, PCC, medial prefrontal cortex (mPFC), and bilateral dorsolateral prefrontal cortex (dlPFC) (Wang et al., 2017). The sensorimotor network plays an important role in the pain modulation, as it integrates sensory input and motor output, which is crucial for bodily movements and responses to sensory stimuli. Xu et al. found that distinct patterns of functional connectivity are found in default mode network, somatosensory network, and salience network in TN (Xu et al., 2022). Disruptions in this network can be indicative of neurological disorders or conditions like TN. Costs et al. found that The TN group performed significantly worse than controls on both sensorimotor (tracking and aiming) and cognitive tasks (Coats et al., 2020). Recent fMRI studies indicate that sensorimotor impairments in low back pain (LBP) may be linked to alterations in brain function and structure (Goossens et al., 2018). Voxel-based morphometry (VBM) analyses demonstrated widespread gray matter volume (GMV) reductions throughout the brain in classical TN patients, particularly affecting the insula/secondary somatosensory cortex (S2), cingulate cortex, and multiple temporal regions (Wang et al., 2017). Zhou et al. reported that TN patients exhibited enhanced functional connectivity between the somatosensory-motor network and the dorsal attention network (Zhou et al., 2024). Tsai et al. found the activity in the sensorimotor network and default mode network was reduced in TN patients but increased following surgery (Tsai et al., 2019). Applying transcranial direct current stimulation (tDCS) over motor cortex reduces somatosensory cortex activity, which may lead to pain relief (Khodashenas et al., 2019). Currently, there remains a paucity of research investigating TN-related alterations in sensorimotor networks, as well as the impact of MVD surgery on these networks. Previous studies have analyzed blood oxygen level-dependent (BOLD) signal time series using general linear modeling (GLM), which are well-established correlates of task-evoked brain activity. However, GLM has two key limitations: it relies on a predefined experimental design matrix, and it cannot detect functional connectivity between brain regions (Kim et al., 2009; Specht and Laeng, 2011). To mitigate these limitations, we used independent component analysis (ICA), a blind-source separation method, to identify distinct clusters of brain regions with coherent hemodynamic temporal patterns without relying on prior temporal response information (e.g., an experimental design matrix). Unlike previous research that examined isolated regions, we used ICA to assess functional connectivity changes within sensorimotor networks in patients undergoing microvascular decompression surgery for classic TN, yielding potential insights for future interventions.
2 Materials and methods
2.1 Participants
This study was conducted in compliance with the Declaration of Helsinki and received ethical approval from the Federal Center of Neurosurgery’s Ethics Committee (Protocol #7, 25.05.21). This study includes raw neuroimaging data from the primary TN, collected at the Federal Neurosurgical Center, in Novosibirsk, Russia. Data were collected between 2022 and 2024. Participants were adults diagnosed with primary TN based on the International Classification of Headache Disorders (ICHD) criteria. All participants, including both patients and HC, provided written informed consent prior to study participation. Twenty-five age- and sex-matched HC were recruited via hospital advertisement systems and 40 TN patients who required MVD surgery due to pharmacological treatment failure or intolerable side effects were enrolled. Inclusion criteria were as follows:
(1) The TN group received MVD surgery and had a follow-up fMRI examination 6 months after the surgery. (2) Patients experience constant or episodic unilateral/bilateral pain in the V1, V2, and/or V3 trigeminal nerve distributions. (3) Neurovascular conflict was graded according to the M. Sindo classification system as follows: Grade 1—slight contact between the nerve and offending vessel; Grade 2—nerve distortion or displacement; and Grade 3—marked nerve indentation (Sindou et al., 2002). (4) Neuroimaging findings revealed no intracranial structural abnormalities or maxillofacial region anomalies (e.g., tumors, vascular malformations, aneurysms). (5) Right-handedness was determined based on manual asymmetry profiling. The exclusion criteria comprised: (1) History of mental disorders or neurological conditions; (2) Clinically significant cognitive impairment (Mini-Mental State Examination (MMSE) total score below the standard cutoff of 24 points) (Lecrubier et al., 1997); (3) Secondary TN (e.g., due to multiple sclerosis, brain tumors, or other intracranial lesions); (4) participants with claustrophobia; or (5) individuals with mental implants or tattoos on their neck or head.
2.2 Image acquisition
MRI data were acquired at the Federal Neurosurgical Center (Novosibirsk, Russia) using a 3T Philips Ingenia system (Philips Healthcare, Netherlands) with a 16-channel head-neck coil. The protocol included: 1. Functional imaging: T2*-weighted interleaved sequence (TR = 3,000 ms, TE = 30 ms, FOV = 256 × 256 mm2, flip angle = 90°, matrix = 80 × 80, slice thickness = 3 mm). 2. Structural imaging: High-resolution 3D sagittal T1-weighted Turbo Field Echo (TFE) sequence (TR = 6.60 ms, TE = 2.96 ms, FOV = 256 × 256 mm2, flip angle = 8°, matrix = 235 × 235, slice thickness = 1 mm). The total scan time was about 30 min.
2.3 fMRI data preprocessing and analysis
Functional connectivity analysis was conducted using the CONN-fMRI toolbox (version 22a) implemented in SPM12 (Wellcome Centre for Human Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The CONN processing pipeline incorporated comprehensive preprocessing of both functional and structural MRI data, including: realignment, slice-timing correction, ART-based outlier detection, segmentation and normalization of anatomical images, functional normalization, outlier detection and smoothing (Whitfield-Gabrieli and Nieto-Castanon, 2012). All neuroimaging data underwent standard volume-based preprocessing using CONN’s default pipeline. To account for magnetic field stabilization, we discarded the first five volumes of each functional run prior to analysis. Structural images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) components and spatially normalized to the Montreal Neurological Institute (MNI) template space. Functional images were then smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. For denoising, CONN implemented component-based noise correction (CompCor) to regress out nuisance signals derived from white matter and CSF regions, along with motion parameters and other confounding variables (Behzadi et al., 2007). To minimize residual motion artifacts and physiological noise, we applied bandpass filtering (0.008–0.09 Hz), linear detrending, and scrubbing using the Artifact Detection Tool (ART). Following standard neuroimaging preprocessing protocols, we applied strict head motion exclusion criteria (translational >2.5 mm or rotational >2.5° in any direction) to minimize movement-related artifacts.
2.4 fMRI data processing and connectivity analysis
Group ICA was performed using CONN toolbox v22a’s default settings, identifying several robust intrinsic connectivity networks. This implementation utilized Calhoun’s group information-guided ICA (GIG-ICA) approach for group-level component extraction (Calhoun et al., 2001). Group ICA was implemented to assess connectivity changes between region-of-interest (ROI) pairs. First-level analysis conducted voxel-wise generated comprehensive functional connectivity profiles across all voxels. Local correlation analysis quantified functional segregation at each observation point through voxel-by-voxel measurements. The dimensionality was reduced to 20 independent components (ICs) based on the minimum description length criteria and previous studies (Xiang et al., 2025; Dai et al., 2023; Moser et al., 2023). Each IC represented a spatially and temporally distinct functional brain network, characterized by synchronous co-fluctuation patterns throughout the scan duration (Li et al., 2007). At the subject level, z-score normalized BOLD signals underwent dimensionality reduction via singular value decomposition (SVD), extracting 64 components per subject. These were further reduced to 20 components through group-level SVD. Group-independent networks were then identified via FastICA algorithm (fixed-point iteration with G1 hyperbolic tangent contrast function). Individual subject maps were reconstructed using GICA3 back-projection. Group-level analyses were performed within a GLM framework. For each IC, we constructed separate GLMs treating voxel-wise first-level connectivity measures as dependent variables (one per subject) and group membership as the independent variable. Voxel-wise statistical inference used multivariate parametric statistics accounting for between-subject random effects and measurement covariance. Cluster-level inference was implemented via Gaussian random field theory (GRFT), identifying contiguous voxel clusters while controlling family-wise error (FWE) rates (Whitfield-Gabrieli and Nieto-Castanon, 2012; Worsley et al., 1996). The neural relevance of these components was quantified using the Dice Similarity Coefficient (DSC), which measured spatial overlap between suprathreshold activation areas and visually identified, best-matched ICs for each functional network (Cocchi et al., 2012).
2.5 Statistical analyses
All statistical analyses were performed using GraphPad Prism 10.1 (GraphPad Software, USA). Demographic data normality was first verified with Shapiro-Wilk tests, parametric (Student's t-test) or non-parametric (Chi-square test) analyses was performed with appropriate post hoc corrections. For neuroimaging second-level analyses, between-subject contrasts were computed using a [1, −1] covariate design, with statistical significance determined by a voxel-level threshold of p < 0.001 (uncorrected), cluster-level FDR correction at p < 0.05 and a minimum cluster extent of 30 contiguous voxels. For the comparison of TN patients before and after surgery, a paired t-test was used.
3 Results
3.1 Demographic information of participants
The demographic of the study population is shown in Table 1. The data in the analysis consisted of 25 HC aged from 43 to 74 years (Mean ± SD: 56.8 ± 7.07), 40 TN patients aged from 45 to 74 years (Mean ± SD: 59.45 ± 10.38), participant details are shown in Supplementary Figure S1. The HC group consist of 10 male and 15 female, while the TN group has 18 female and 22 males. The Sindo grade in the TN patients varied from 0 to 3 (Mean ± SD: 1.68 ± 1.14). The disease duration for the TN patients was 1–30 years (Mean ± SD: 8.8 ± 6.48).
TABLE 1
| Items | HC n = 25 | TN n = 40 | P |
|---|---|---|---|
| Age (Mean ± SD) | 56.8 ± 7.07 | 59.45 ± 10.38 | 0.2661a |
| Sex (Male/Female) | 10/15 | 18/22 | 0.7988b |
| Sindo grade (Mean ± SD) | 0 | 1.67 ± 1.14 | <0.0001c |
| Disease Duration | 0 | 8.8 ± 6.48 | <0.0001c |
Demographic information of participants.
HC, healthy controls; TN, trigeminal neuralgia; a: Student’s t-test, b: Chi-square test, c: One sample t and Wilcoxon test analysis.
3.2 Independent components identification
Among the 20 components, the Sensorimotor (IC-9) (r = 0.375) was selected (Figure 1). In the between subjects contrast. Enhanced activity was found in the right frontal operculum cortex, right insular cortex, right inferior frontal gyrus (pars opercularis) and right frontal pole, compared to the HC. However, decreased activity was observed in the right cerebelum (lobule IX) and the left cerebelum (lobules VIII and IX) (Figure 2; Table 2) in the TN pretreatment patients. After the MVD surgery, compared to the HC group, increased activity was observed in the right frontal pole, right insular cortex, right inferior frontal gyrus (pars opercularis), and right frontal right operculum cortex. The surgery significantly decreased the right insular cortex and operculum cortex activation area postoperatively (Figure 3; Table 3). We then assessed changes in brain activity in TN patients before and after surgery. The post-surgery group exhibited increased activity in the right precentral gyrus compared to the pre-surgery group (Figure 4). Since pretreatment TN patients’ brain activity may correlate with disease duration, we performed additional correlation analyses. However, no significant association was found between altered pretreatment brain regions and disease duration (Supplementary Figure S2).
FIGURE 1
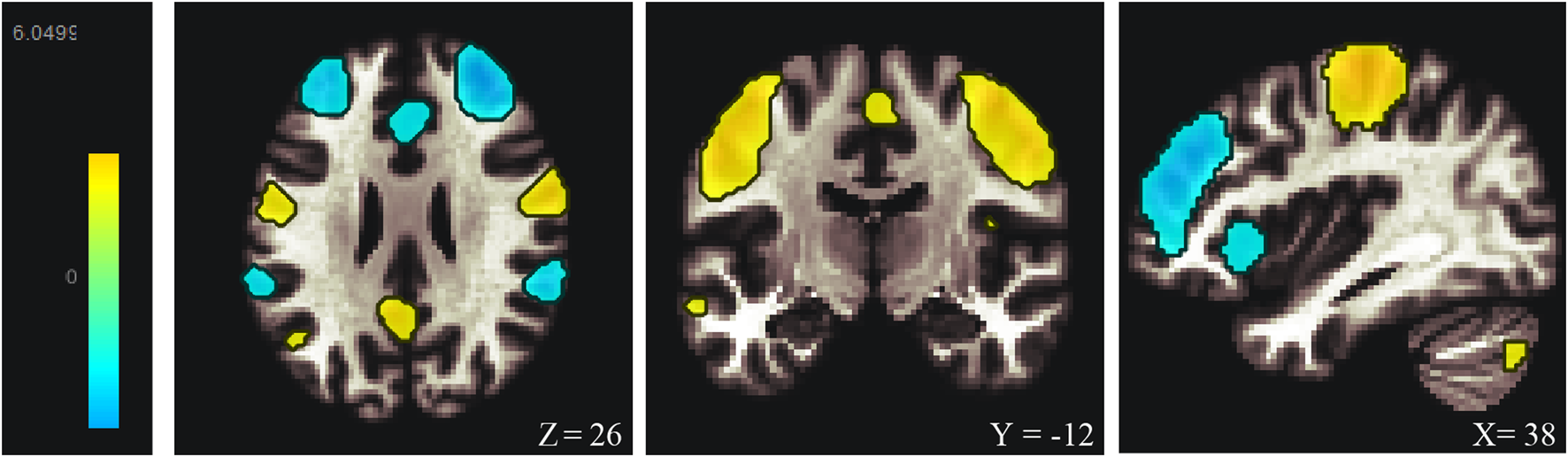
Resting-state sensorimortor networks (IC-9) generated by ICA. ICA, independent component analysis.
FIGURE 2
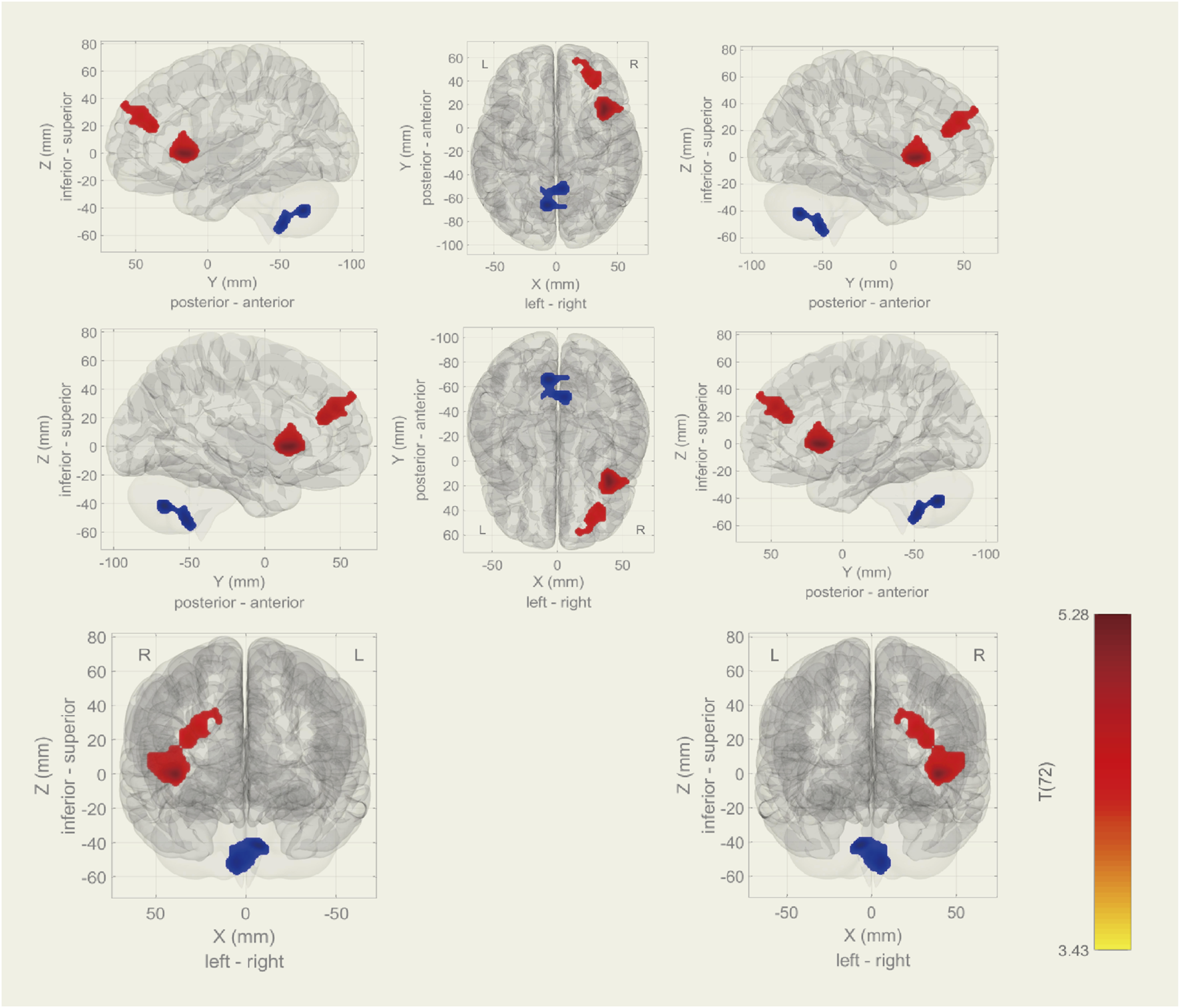
During rs-fMRI, the TN group exhibited increased activity (TN > HC) in the right frontal operculum cortex, insular cortex, inferior frontal gyrus (pars opercularis), and frontal pole. Conversely, decreased activity was found in the right cerebellum (lobule IX) and left cerebellum (lobules VIII and IX).
TABLE 2
| MNI coordinates (x,y,z) | Total voxel size | Size P-FDR | Network | Area | ROIs (%) | Voxel size |
|---|---|---|---|---|---|---|
| +40 + 16 +00 | 488 | 0.000306 | IC-9 | Right frontal operculum cortex | 63 | 196 |
| Right insular cortex | 13 | 181 | ||||
| Right inferior frontal gyrus, pars opercularis | 10 | 67 | ||||
| +32 + 38 +20 | 324 | 0.004028 | Right frontal pole | 4 | 292 | |
| −6 -66–42 | 308 | 0.005288 | Right cerebelum (lobule IX) | 14 | 113 | |
| Left cerebelum (lobules VIII) | 4 | 71 | ||||
| Left cerebelum (lobules IX) | 8 | 67 |
Rs-fMRI results (TN > HC).
Note: Analyses were performed using CONN, which aligns the Oxford-Harvard ROI, atlas with MNI, coordinates. Abbreviations: FDR, false discovery rate; IC, independent component; MNI, montreal neurological institute; ROI, region of interest.
FIGURE 3
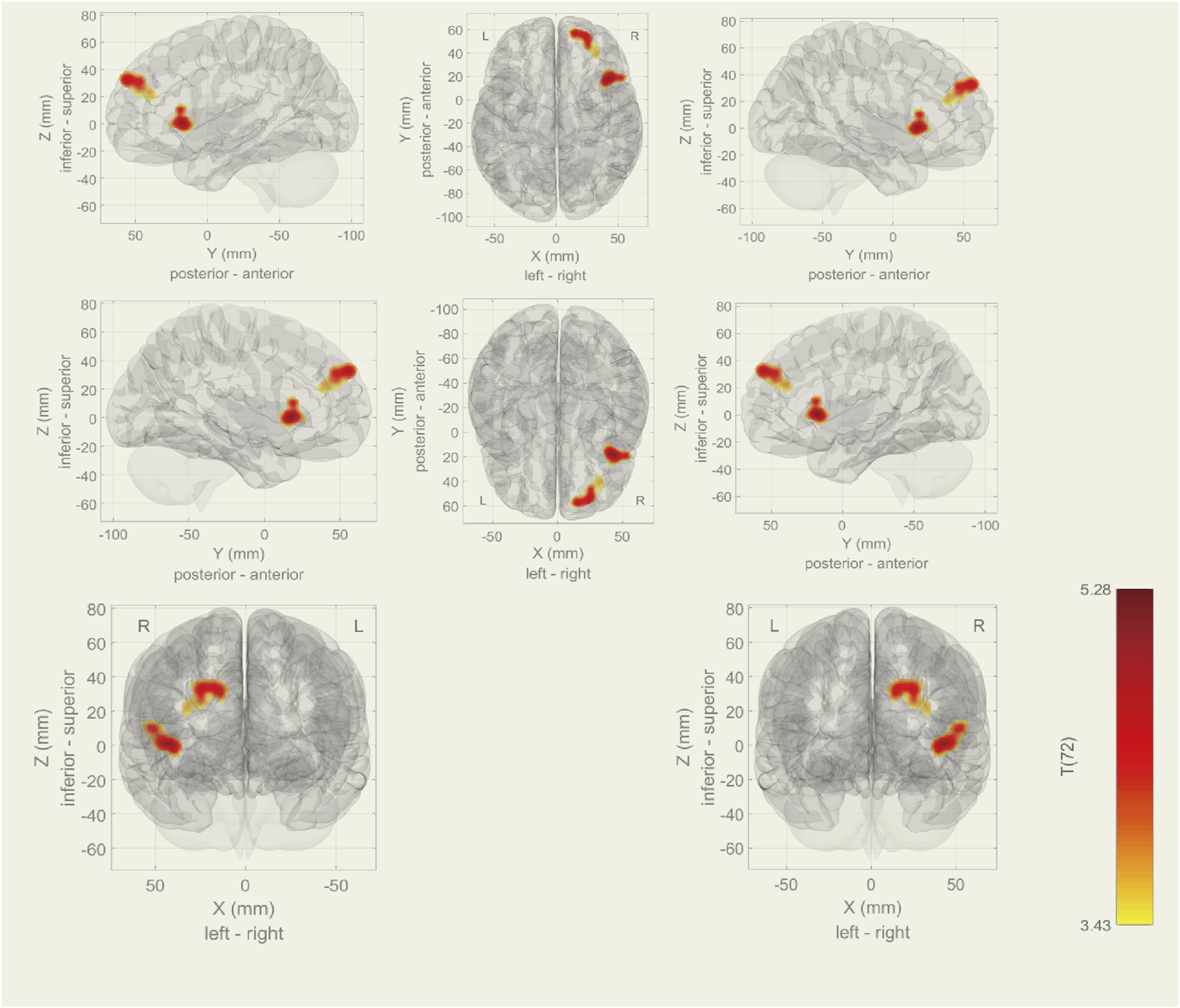
During rs-fMRI, post-surgery group exhibited increased activity (TN (Post MVD) > HC) in the right frontal pole, frontal operculum cortex, insular cortex and right inferior frontal gyrus (pars opercularis).
TABLE 3
| MNI coordinates (x,y,z) | Total voxel size | Size P-FDR | Network | Area | ROIs (%) | Voxel size |
|---|---|---|---|---|---|---|
| +24 + 54 +34 | 347 | 0.003678 | IC-9 | Right frontal pole | 4 | 330 |
| +44 + 18 +02 | 276 | 0.011771 | Right frontal operculum cortex | 34 | 107 | |
| Right insular cortex | 6 | 76 | ||||
| Right inferior frontal gyrus, pars opercularis | 10 | 69 |
Rs-fMRI results (TN (Post-surgery) > HC).
Note: Analyses were performed using CONN, which aligns the Oxford-Harvard ROI, atlas with MNI, coordinates. Abbreviations: FDR, false discovery rate; IC, independent component; MNI, montreal neurological institute; ROI, region of interest.
FIGURE 4

During rs-fMRI, post-surgery group exhibited increased activity in the right precentral gyrus than the pre-surgery group.
4 Discussion
This study employed ICA to examine functional connectivity changes after MVD surgery for TN. Unlike hypothesis-driven approaches, ICA is data-driven and can reveal patterns of brain dysfunction beyond the constraints of predefined experimental models. Our findings successfully revealed changes in individual functional connectivity associated with MVD surgery.
Previous studies have used ICA to identify consistent neural activity patterns across groups of individuals (Eyler et al., 2019). Compared to HC group, we found increased activity in the right frontal pole, right frontal operculum cortex, right insular cortex and right inferior frontal gyrus, pars opercularis pre-surgery of the TN group and decreased activity in the right cerebellum (lobule IX) and the left cerebellum (lobules VIII and lobules IX). The frontal pole is associated with various functions, including motor control and sensory processing (Koechlin, 2011) Changes in connectivity in this region may indicate altered neural processing related to pain. Recent neuroimaging studies have reported that pain induces both structural and functional plasticity in pain-processing brain regions, including the PFC, insula, ACC, and somatosensory cortex (Coppieters et al., 2016; Niddam et al., 2017), these changes affect sensory, emotional, and cognitive dimensions of pains. Our results are consistent with the previous study.
The insula plays a critical role in both physiological and possibly pathological pain (Garcia-Larrea, 2012; Brooks and Tracey, 2007). The insular cortex is one of the earliest brain regions activated by nociceptive stimuli. Its activation level strongly correlates with both the intensity of noxious stimuli and the subjective perception of pain (Coghill et al., 1999; Oshiro et al., 2009). The relationship between altered insular activity (Hsieh et al., 1995), structural changes (Gustin et al., 2011), and pain chronification has been increasingly recognized. In chronic LBP patients, studies have demonstrated disrupted functional connectivity of the insular with the somatosensory (Kim et al., 2019) and the medial prefrontal cortices (Baliki et al., 2011). On the other hand, pharmacological interventions for chronic pain have been shown to partially reverse these aberrant insular connectivity (Čeko et al., 2015). The observed increase in activity in the right insular cortex may indicate a neural adaptation or maladaptive changes due to chronic pain in our study. This change could reflect heightened processing of pain. In our study, we found the MVD surgery can effectively reduce the active areas in the insular cortex. However, the persistent insular activation postoperatively may contribute to the recurrence of TN in the future. Recent clinical studies have proposed the insular cortex as a potential therapeutic target for deep brain stimulation in chronic pain management. In healthy subjects, transcranial magnetic stimulation (TMS) of the posterior opercular insular cortex was found to elevate heat pain thresholds mediated by Aδ fibers (Lenoir et al., 2018). Similarly, repetitive TMS targeting the posterior insula produced significant increases in thermal pain thresholds among central neuropathic pain patients (Galhardoni et al., 2019). Notably, a study demonstrated TMS in posterior superior insula has a significant analgesic effects when applied to patients with peripheral neuropathic pain (Dongyang et al., 2021). These consistent findings across multiple pain models suggest the insular cortex may serve as a promising target for non-pharmacological pain interventions. Although our results confirm that MVD surgery is effective in reducing insular activation area, further interventions target insular cortex may be needed in the future to enhance efficacy.
The right inferior frontal gyrus (rIFG) comprises part of Broca’s area homolog, a region traditionally implicated in speech production and language processing. However, it is also involved in emotional processing, executive function, and integration of sensory information, making it relevant to pain procession and responses (Symonds et al., 2006). Recent tDCS and repetitive TMS studies suggest that the rIFG may be involved in pain-related empathy, yielding novel insights into the neurobiological substrates underlying empathy for others’ suffering (Wu et al., 2018; Li et al., 2021). Our results demonstrate that increased rIFG activity persists both pre- and post-operatively, potentially reflecting enhanced pain empathy resulting from the long-lasting pain experience in TN.
The operculum, defined as the cortical region adjacent to the insula, comprises three major subdivisions and plays well-established roles in various neurological and psychiatric disorders. This structurally complex area mediates multiple functions including sensory processing, motor control, autonomic regulation, and cognitive operations (Garcia-Larrea, 2012). The dorsal posterior insula and adjacent medial operculum are considered nociception-specific regions. Lesions in this area may predispose to neuropathic pain development, while the adjoining medial operculum mediates non-noxious thermal processing (Garcia-Larrea, 2012). Patients with chronic traumatic neck pain exhibited enhanced left pallidum-left frontal operculum connectivity compared to HC (Coppieters et al., 2021). The operculo-insular cortex plays a crucial role in pain processing. Building on this understanding, Ulf Baumgärtner and colleagues investigated somatotopic organization within this region across 11 healthy subjects using noxious heat and pinprick stimulation paradigms. Their study revealed multiple discrete pain representations within this region, likely reflecting its critical role as a multisensory integration hub that generates body site-specific emotional and behavioral responses to injury (Baumgärtner et al., 2010). Our results indicate increased activity in this brain area related neuropathic pain development in TN. MVD surgery is effective in reducing operculum activation area.
The cerebellum plays a modulatory role in pain through its communication with sensorimotor, executive, reward, and limbic functional regions. Although substantial evidence suggests that the cerebellum may be critically involved in trigeminal nociceptive pain, few studies have investigated this function. Even less is known about which specific cerebellar regions participate in trigeminal surgery. In a recent meta-analysis, Moulton and colleagues demonstrated that multiple cerebellar regions are typically activated during nociceptive processing in human, beyond this, the cerebellum contributes to pathological nociceptive sensitivity and processing in chronic pain (Moulton et al., 2010). In addition to evidence of altered activation patterns during stimulation in chronic pain, PET studies have also investigated baseline activity levels in individuals experiencing chronic pain, with some reporting sustained cerebellar blood flow changes (Hsieh et al., 1995). Beyond altered blood flow, the cerebellum in patients with chronic orofacial neuropathic pain also exhibits modified patterns of ongoing activity (Alshelh et al., 2016). In addition to observed cerebellar activation in chronic pain patients, recent neuroimaging studies have also identified abnormal functional connectivity between the cerebellum and other cortical/subcortical areas in these individuals (Simons et al., 2014). We are the first found abnormal connectivity in the right cerebellum (lobules IX) and the left cerebellum (VIII and IX) in TN. Our results indicated MVD can effectively treat this abnormality, this could be considered an important criterion for postoperative evaluation. Investigating connectivity changes pre- and post-peratively in TN is very important. They could lead to the identification of biomarkers for TN severity or potential treatment evaluation.
The precentral gyrus, also known as the primary motor cortex or Brodmann area 4, is located in the frontal lobe of the brain and is responsible for initiating and controlling voluntary movements (Banker and Tadi, 2019). The precentral gyrus also play a modulatory role in pain processing. Previous studies have identified significant group differences in functional connectivity between the right precentral gyrus and regions such as the cingulate gyrus, precuneus, and left paracentral lobule in patients with chronic shoulder pain (Wei et al., 2022). Our findings suggest that the motor cortex may serve as a key brain region in chronic pain, and that MVD can effectively enhance its activity, thereby mitigating chronic pain progression. This aligns with prior moderate-quality evidence demonstrating that high-frequency rTMS of the primary motor cortex induces significant analgesic effects in neuropathic pain (NP) and fibromyalgia (Xiong et al., 2022).
5 Limitations
While our findings demonstrate potential clinical relevance, this study has limitations. Most notably, the modest sample size may reduce statistical power and limit the generalizability of the results. Future research with larger samples is needed to validate these results. Second, our fMRI preprocessing used head motion thresholds of 2.5 mm translation and 2.5° rotation, which may have been insufficiently conservative. This leniency could have introduced motion-related artifacts, potentially compromising the validity of the functional connectivity results. In addition, longer-term data tracking would be beneficial to further investigate the correlation between TN recurrence post-surgery and activation in these brain regions. Notwithstanding these limitations, our findings demonstrate the potential utility of ICA for evaluating post-operative pain outcomes following MVD surgery, while providing a neurobiological foundation for developing targeted interventions.
6 Conclusion
Our study demonstrates that ICA can effectively identifies distinct patterns of functional connectivity associated with TN and MVD surgery. In the sensorimotor network, TN was found to increase neural activity in broad brain regions, including the right frontal operculum cortex, right insular cortex, right inferior frontal gyrus (pars opercularis), and right frontal pole. Conversely, decreased activity was found in the right cerebellum (lobule IX) and left cerebellum (lobules VIII and IX). These regions are critically involved in altered pain processing, including nociceptive stimuli integration, subjective pain perception, and pain chronification. Additionally, TN enhanced activity in areas linked to pain-related empathy while disrupting cerebellar function. Compared to the pre-surgey, increased activity was found in the right precentral gyrus in the post-surgery group. Although MVD surgery is an effective treatment, long-term hyperactivity persisted in the right frontal operculum cortex, right insular cortex, right inferior frontal gyrus (pars opercularis), and right frontal pole. However, MVD significantly reduced aberrant activation in the operculo-insular cortex and normalized cerebellar abnormalities. Our findings identify promising biomarkers for TN and provide insights for developing targeted treatments.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by this study was conducted in compliance with the Declaration of Helsinki and received ethical approval from the Federal Center of Neurosurgery’s Ethics Committee (Protocol #7, 25.05.21). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Formal Analysis, Funding acquisition, Investigation, Visualization, Writing – original draft. XjW: Methodology, Software, Validation, Writing – review and editing. XfW: Writing – review and editing. GdH: Conceptualization, Funding acquisition, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by Shenzhen Science and Technology Program (Grant No. RCBS20221008093252091), the Fund of the Science and Technology Planning Project of Shenzhen Municipality (20210617155253001) and China Postdoctoral Science Foundation (2024M750240).
Acknowledgments
We acknowledge Elena Filimonova, Anton Pashkov, Azniv Martirosyan, Galina Moisak and Jamil Rzaev share their data on the website of Open Neuro: A large-scale dataset of pre- and post-surgical MRI data in patients with chronic trigeminal neuralgia. OpenNeuro. [Dataset] doi: doi:10.18112/openneuro.ds005713.v1.0.1. License:CC0.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1633028/full#supplementary-material
Glossary
- TN
trigeminal neuralgia
- MVD
microvascular decompression
- HC
healthy controls
- NVC
neurovascular compression
- fMRI
functional magnetic resonance imaging
- rs-fMRI
resting-state functional magnetic resonance imaging
- FDR
False discovery rate
- REZ
root entry zone
- ACC
anterior cingulate cortex
- MCC
midcingulate cortex
- PCC
posterior cingulate cortex
- ReHo
regional homogeneity
- fALFF
fractional amplitude of low-frequency fluctuations
- mPFC
medial prefrontal cortex
- dlPFC
dorsolateral prefrontal cortex
- LBP
low back pain
- VBM
voxel-based morphometry
- GMV
gray matter volume
- DMN
default mode networks
- SN
salience network
- SSN
somatosensory networks
- GLM
general linear modeling
- BOLD
blood oxygen level-dependent
- ICA
independent component analysis
- MMSE
mini-mental state examination
- TFE
turbo field echo
- CSF
cerebrospinal fluid
- MNI
montreal neurological institute
- FWHM
full-width at half-maximum
- CompCor
component-based noise correction
- ART
artifact detection tool
- GIG-ICA
group information-guided ICA
- ROIs
regions of interest
- ICs
independent components
- SVD
singular value decomposition
- GRFT
gaussian random field theory
- FWE
family-wise error
- TMS
transcranial magnetic stimulation
- rIFG
right inferior frontal gyrus
- tDCS
transcranial direct current stimulation
References
1
AlshelhZ.Di PietroF.YoussefA. M.ReevesJ. M.MaceyP. M.VickersE. R.et al (2016). Chronic neuropathic pain: it's about the rhythm. J. Neurosci.36, 1008–1018. 10.1523/JNEUROSCI.2768-15.2016
2
BalikiM. N.BariaA. T.ApkarianA. V. (2011). The cortical rhythms of chronic back pain. J. Neurosci.31, 13981–13990. 10.1523/JNEUROSCI.1984-11.2011
3
BankerL.TadiP. (2019). Neuroanatomy, precentral gyrus.
4
BaumgärtnerU.IannettiG. D.ZambreanuL.StoeterP.TreedeR. D.TraceyI. (2010). Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study. J. Neurophysiol.104 (5), 2863 –2872.
5
BehzadiY.RestomK.LiauJ.LiuT. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage37, 90–101. 10.1016/j.neuroimage.2007.04.042
6
BendtsenL.ZakrzewskaJ. M.AbbottJ. A.BraschinskyM.Di StefanoG.DonnetA.et al (2019). European academy of neurology guideline on trigeminal neuralgia. Eur. J. neurology26, 831–849. 10.1111/ene.13950
7
BiswalB. B.MennesM.ZuoX.-N.GohelS.KellyC.SmithS. M.et al (2010). Toward discovery science of human brain function, Proc. Natl. Acad. Sci. U. S. A.107 (10), 4734–4739. 10.1073/pnas.0911855107
8
BrooksJ. C. W.TraceyI. (2007). The insula: a multidimensional integration site for pain. Pain128 (1), 1–2.
9
CalhounV. D.AdaliT.PearlsonG. D.PekarJ. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Hum. brain Mapp.14, 140–151. 10.1002/hbm.1048
10
ČekoM.ShirY.OuelletJ. A.WareM. A.StoneL. S.SeminowiczD. A. (2015). Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum. brain Mapp.36, 2075–2092. 10.1002/hbm.22757
11
CoatsR. O.CrossleyK. L.ConlinN.WuJ.ZakrzewskaJ. M.PavittS. H.et al (2020). Cognitive and sensorimotor function in participants being treated for trigeminal neuralgia pain. J. headache pain21, 91. 10.1186/s10194-020-01156-9
12
CocchiL.BramatiI. E.ZaleskyA.FurukawaE.FontenelleL. F.MollJ.et al (2012). Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J. Neurosci.32, 17753–17761. 10.1523/JNEUROSCI.3272-12.2012
13
CoghillR. C.SangC. N.MaisogJ. M.IadarolaM. J. (1999). Pain intensity processing within the human brain: a bilateral, distributed mechanism. J. neurophysiology82, 1934–1943. 10.1152/jn.1999.82.4.1934
14
CoppietersI.CagnieB.De PauwR.MeeusM.TimmersI. (2021). Enhanced amygdala-frontal operculum functional connectivity during rest in women with chronic neck pain: associations with impaired conditioned pain modulation. NeuroImage Clin.30, 102638. 10.1016/j.nicl.2021.102638
15
CoppietersI.MeeusM.KregelJ.CaeyenberghsK.De PauwR.GoubertD.et al (2016). Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J. Pain17, 949–962. 10.1016/j.jpain.2016.04.005
16
CruccuG.FinnerupN. B.JensenT. S.ScholzJ.SindouM.SvenssonP.et al (2016). Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology87, 220–228. 10.1212/WNL.0000000000002840
17
DaiT.SeewooB. J.HennessyL. A.BollandS. J.RosenowT.RodgerJ. (2023). Identifying reproducible resting state networks and functional connectivity alterations following chronic restraint stress in anaesthetized rats. Front. Neurosci.17, 1151525. 10.3389/fnins.2023.1151525
18
DesouzaD. D.MoayediM.ChenD. Q.DavisK. D.HodaieM. (2013). Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: a paroxysmal, sensory-triggered neuropathic pain. PloS one8, e66340. 10.1371/journal.pone.0066340
19
Di StefanoG.MaarbjergS.NurmikkoT.TruiniA.CruccuG. (2018). Triggering trigeminal neuralgia. Cephalalgia38, 1049–1056. 10.1177/0333102417721677
20
DongyangL.FernandesA. M.Da CunhaP. H. M.TibesR.SatoJ.ListikC.et al (2021). Posterior-superior Insular deep transcranial magnetic stimulation alleviates peripheral neuropathic pain—A pilot double-blind, randomized cross-over study. Neurophysiol. Clin.51, 291–302. 10.1016/j.neucli.2021.06.003
21
EylerL. T.ElmanJ. A.HattonS. N.GoughS.MischelA. K.HaglerD. J.et al (2019). Resting state abnormalities of the default mode network in mild cognitive impairment: a systematic review and meta-analysis. J. Alzheimer's Dis.70, 107–120. 10.3233/JAD-180847
22
GalhardoniR.Aparecida Da SilvaV.GarcíA-LarreaL.DaleC.BaptistaA. F.BarbosaL. M.et al (2019). Insular and anterior cingulate cortex deep stimulation for central neuropathic pain: disassembling the percept of pain. Neurology92, e2165–e2175. 10.1212/WNL.0000000000007396
23
Garcia-LarreaL. (2012). The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol. Clinique/Clinical Neurophysiol.42, 299–313. 10.1016/j.neucli.2012.06.001
24
GoossensN.RummensS.JanssensL.CaeyenberghsK.BrumagneS. (2018). Association between sensorimotor impairments and functional brain changes in patients with low back pain: a critical review. Am. J. Phys. Med. and rehabilitation97, 200–211. 10.1097/PHM.0000000000000859
25
GustinS. M.PeckC. C.WilcoxS. L.NashP. G.MurrayG. M.HendersonL. A. (2011). Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J. Neurosci.31, 5956–5964. 10.1523/JNEUROSCI.5980-10.2011
26
HsiehJ.-C.BelfrageM.Stone-ElanderS.HanssonP.IngvarM. (1995). Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain63, 225–236. 10.1016/0304-3959(95)00048-w
27
KhodashenasM.BaghdadiG.TowhidkhahF. (2019). A modified hodgkin–huxley model to show the effect of motor cortex stimulation on the trigeminal neuralgia network. J. Math. Neurosci.9, 4. 10.1186/s13408-019-0072-5
28
KimD. I.ManoachD. S.MathalonD. H.TurnerJ. A.MannellM.BrownG. G.et al (2009). Dysregulation of working memory and default‐mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum. brain Mapp.30, 3795–3811. 10.1002/hbm.20807
29
KimJ.MawlaI.KongJ.LeeJ.GerberJ.OrtizA.et al (2019). Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain160, 1594–1605. 10.1097/j.pain.0000000000001541
30
KoechlinE. (2011). Frontal pole function: what is specifically human?Trends cognitive Sci.15, 241; author reply 243. 10.1016/j.tics.2011.04.005
31
LecrubierY.SheehanD. V.WeillerE.AmorimP.BonoraI.SheehanK. H.et al (1997). The mini international neuropsychiatric interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. psychiatry12, 224–231. 10.1016/s0924-9338(97)83296-8
32
LenoirC.AlgoetM.MourauxA. (2018). Deep continuous theta burst stimulation of the operculo‐insular cortex selectively affects Aδ‐fibre heat pain. J. physiology596, 4767–4787. 10.1113/JP276359
33
LiY.LiW.ZhangT.ZhangJ.JinZ.LiL. (2021). Probing the role of the right inferior frontal gyrus during pain‐related empathy processing: evidence from fMRI and TMS. Hum. brain Mapp.42, 1518–1531. 10.1002/hbm.25310
34
LiY. O.AdalıT.CalhounV. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Hum. brain Mapp.28, 1251–1266. 10.1002/hbm.20359
35
MaarbjergS.GozalovA.OlesenJ.BendtsenL. (2014). Trigeminal Neuralgia–A prospective systematic study of clinical characteristics in 158 patients. Headache J. Head Face Pain54, 1574–1582. 10.1111/head.12441
36
MoJ.ZhangJ.HuW.LuoF.ZhangK. (2021). Whole-brain morphological alterations associated with trigeminal neuralgia. J. headache pain22, 95–10. 10.1186/s10194-021-01308-5
37
MoserD. A.DricuM.KotikalapudiR.DoucetG. E.AueT. (2023). Within‐network brain connectivity during a social optimism task is related to personal optimism and optimism for in‐group members. Hum. brain Mapp.44, 4561–4571. 10.1002/hbm.26400
38
MoultonE. A.SchmahmannJ. D.BecerraL.BorsookD. (2010). The cerebellum and pain: passive integrator or active participator?Brain Res. Rev.65, 14–27. 10.1016/j.brainresrev.2010.05.005
39
NiddamD.LeeS. H.SuY. T.ChanR. C. (2017). Brain structural changes in patients with chronic myofascial pain. Eur. J. Pain21, 148–158. 10.1002/ejp.911
40
OshiroY.QuevedoA. S.MchaffieJ. G.KraftR. A.CoghillR. C. (2009). Brain mechanisms supporting discrimination of sensory features of pain: a new model. J. Neurosci.29, 14924–14931. 10.1523/JNEUROSCI.5538-08.2009
41
SimonsL.PielechM.ErpeldingN.LinnmanC.MoultonE.SavaS.et al (2014). The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. PAIN®155, 1727–1742. 10.1016/j.pain.2014.05.023
42
SindouM.HoweidyT.AcevedoG. (2002). Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir.144, 1–12. 10.1007/s701-002-8269-4
43
SpechtK.LaengB. (2011). An independent component analysis of fMRI data of graphene–colour synaesthesia. J. neuropsychology5, 203–213. 10.1111/j.1748-6653.2011.02008.x
44
SymondsL. L.GordonN. S.BixbyJ. C.MandeM. M. (2006). Right-lateralized pain processing in the human cortex: an FMRI study. J. neurophysiology95, 3823–3830. 10.1152/jn.01162.2005
45
TsaiY.-H.LiangX.YangJ.-T.HsuL.-M. (2019). Modular organization of brain resting state networks in patients with classical trigeminal neuralgia. NeuroImage Clin.24, 102027. 10.1016/j.nicl.2019.102027
46
TuleascaC.CarronR.ResseguierN.DonnetA.RousselP.GaudartJ.et al (2014). Repeat gamma knife surgery for recurrent trigeminal neuralgia: long-term outcomes and systematic review. J. Neurosurg.121, 210–221. 10.3171/2014.8.GKS141487
47
WangY.CaoD.-Y.RemeniukB.KrimmelS.SeminowiczD. A.ZhangM. (2017). Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain158, 1561–1570. 10.1097/j.pain.0000000000000951
48
WeiX.ShiG.TuJ.ZhouH.DuanY.LeeC. K.et al (2022). Structural and functional asymmetry in precentral and postcentral gyrus in patients with unilateral chronic shoulder pain. Front. Neurology13, 792695. 10.3389/fneur.2022.792695
49
Whitfield-GabrieliS.Nieto-CastanonA. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connect.2, 125–141. 10.1089/brain.2012.0073
50
WorsleyK. J.MarrettS.NeelinP.VandalA. C.FristonK. J.EvansA. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Hum. brain Mapp.4, 58–73. 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O
51
WuM.QiuJ.ChenY.JiangX. (2025). Stratifying trigeminal neuralgia and characterizing an abnormal property of brain functional organization: a resting-state fMRI and machine learning study. J. Neurosurg.1, 74–82. 10.3171/2024.11.JNS241935
52
WuX.XuF.ChenX.WangL.HuangW.WanK.et al (2018). The effect of high-definition transcranial direct current stimulation of the right inferior frontal gyrus on empathy in healthy individuals. Front. Hum. Neurosci.12, 446. 10.3389/fnhum.2018.00446
53
XiangY.XingX.HuaX.ZhangY.XueX.WuJ.et al (2025). Resting-state brain network remodeling after different nerve reconstruction surgeries: a functional magnetic resonance imaging study in brachial plexus injury rats. Neural Regen. Res.20, 1495–1504. 10.4103/NRR.NRR-D-23-00493
54
XiongH.-Y.ZhengJ.-J.WangX.-Q. (2022). Non-invasive brain stimulation for chronic pain: state of the art and future directions. Front. Mol. Neurosci.15, 888716. 10.3389/fnmol.2022.888716
55
XuH.SeminowiczD. A.KrimmelS. R.ZhangM.GaoL.WangY. (2022). Altered structural and functional connectivity of salience network in patients with classic trigeminal neuralgia. J. pain23, 1389–1399. 10.1016/j.jpain.2022.02.012
56
YuanJ.CaoS.HuangY.ZhangY.XieP.ZhangY.et al (2018). Altered spontaneous brain activity in patients with idiopathic trigeminal neuralgia: a resting-state functional MRI study. Clin. J. Pain34, 600–609. 10.1097/AJP.0000000000000578
57
ZakrzewskaJ. M.AkramH. (2011). Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst. Rev.2011, CD007312. 10.1002/14651858.CD007312.pub2
58
ZakrzewskaJ. M.WuJ.Mon-WilliamsM.PhillipsN.PavittS. H. (2017). Evaluating the impact of trigeminal neuralgia. Pain158, 1166–1174. 10.1097/j.pain.0000000000000853
59
ZhouQ.ZhaoR.QinZ.QiY.TangW.LiuL.et al (2024). Altered intra-and inter-network functional activity among migraine, chronic migraine, and trigeminal neuralgia. Mol. Pain20, 17448069241300939. 10.1177/17448069241300939
Summary
Keywords
trigeminal neuralgia, microvascular decompression, functional connectivity, independent component analysis, sensorimotor network
Citation
Zhang Y, Wang X, Wang X and Huang G (2025) The alteration of the sensorimotor network in trigeminal neuralgia after microvascular decompression surgery: a follow-up study using independent component analysis. Front. Physiol. 16:1633028. doi: 10.3389/fphys.2025.1633028
Received
22 May 2025
Accepted
21 July 2025
Published
05 August 2025
Volume
16 - 2025
Edited by
Valeria Sacca, Massachusetts General Hospital and Harvard Medical School, United States
Reviewed by
Liang Dong, First Affiliated Hospital of Chongqing Medical University, China
Qianling Zhou, Guangdong Provincial Hospital of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Zhang, Wang, Wang and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gengdi Huang, gengdi_huang@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.