- 1Department of Intensive Care Unit, The Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, China
- 2Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing, China
- 3Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, Beijing, China
- 4Department of Anesthesia, Zhangjiagang Hospital of Traditional Medicine, Suzhou, China
- 5Department of Child Healthcare, Shenzhen Baoan Women’s and Children’s Hospital, Shenzhen, China
Interpretation: Hb and respiratory infection showed a nonlinear U-shaped association; such a relation is modified by the chronotype sleep behavior.
Objective: To examine the association between Hb and the incidence of hospitalized respiratory infection, and to explore potential modification effects of sleep behaviors.
Methods: Included were 292,568 individuals without respiratory disease, cancer, or anemia diagnosis in the United Kingdom Biobank . Hb (g/dL) was measured at baseline. The interaction between Hb and sleep behaviors, including sleep duration, insomnia, chronotype, and daytime sleepiness with respiratory infection, was tested.
Results: The cohort was followed up at a median 12.6 years, and 16,669 incident respiratory infections (9,334 in men, 7,335 in women) were identified. There was a nonlinear U-shaped association between Hb and respiratory infection in both men and women, where the risk increased markedly with Hb above 15.0 g/dL for men and 13.5 g/dL for women. In men, compared with the third quintile group, the hazard ratio (HR; 95% confidence interval [CI]) of respiratory infection in the Q1, Q2, Q4, and Q5 quintile groups was 1.28 (1.21–1.37), 1.07 (1.00–1.14), 1.06 (0.99–1.13), and 1.09 (1.02–1.17), respectively. In women, the HR (95% CI) was 1.20 (1.12–1.29), 1.09 (1.01–1.17), 1.01 (0.94–1.09), and 1.05 (0.98–1.13) in the Q1, Q2, Q4, and Q5 quintile groups of Hb, respectively, compared with the third quintile group. There was a significant interaction between Hb concentration and chronotype on the risk of respiratory infection (P for interaction = 0.005). The elevated risk of respiratory infection associated with Hb was more pronounced among participants with late chronotype.
Conclusion: The study suggests that Hb and respiratory infection have a nonlinear U-shaped association and that such a relation is modified by chronotype.
Introduction
Hemoglobin (Hb) is an iron-containing protein responsible for oxygen transport in red blood cells (Ciaccio et al., 2022). Lower Hb concentrations have been recognized as an indicator of anemia (Stevens et al., 2013). Symptoms including fatigue, weakness, and cognitive decline may originate from anemia, in which tissue oxygen delivery is limited (Schumann and Solomons, 2017). Globally, approximately 1.93 billion people had anemia (Kassebaum and Collaborators, 2016). Previous studies have shown that Hb concentration is associated with adverse health outcomes including infections, chronic heart failure, and mortality (Harrison et al., 2021; K et al., 2020; Gelaw et al., 2021). Of note, respiratory infections are one of the most common causes of mortality and disability-adjusted life-years worldwide, especially during the coronavirus disease 2019 (COVID-19) pandemic (Diseases and Injuries, 2020; Williamson et al., 2020). Lower respiratory infections have high morbidity and mortality rates and contribute to a substantial burden on individuals, families, and the society (GBD 2016 Lower Respiratory Infections Collaborators, 2018). Recent meta-analyses have evaluated the association between Hb and respiratory infection (Taneri et al., 2020; Kowsar et al., 2023), but conflicting results were reported. For example, a meta-analysis found that lower concentrations of Hb were associated with higher mortality in COVID-19 patients (Kowsar et al., 2023). However, Taneri et al. (2020) observed a non-significant difference in Hb concentrations between survivors and non-survivors among COVID-19 patients. An observational study also showed that Hb did not differ significantly between critically ill and moderate COVID-19 patients (Zeng et al., 2020). In addition, the sex-specific dose response association between Hb concentration and respiratory infection risk remains poorly explored in large-scale cohorts, despite sex differences in anemia prevalence and respiratory infection risk being documented (Levi et al., 2019; Zhang et al., 2022). Considering the physiological differences in Hb concentration thresholds, immune function, and respiratory disease susceptibility between men and women (Levi et al., 2019; Zhang et al., 2022), the current study specifically addresses this gap using a large, longitudinal cohort.
Previous studies have shown that the modification effects of various lifestyle factors might partly explain inconsistent associations between biomarkers and health outcomes (Wang et al., 2020; Wang et al., 2022). Notably, sleep behaviors are emerging lifestyle factors that are closely related to both Hb and respiratory infection (Jackowska et al., 2013; Chen et al., 2020; Shafiee et al., 2023; Chiner et al., 2016; Chen et al., 2021). Population-based investigations have shown that sleep duration and disturbance were related to Hb concentration (Jackowska et al., 2013; Chen et al., 2020). Furthermore, sleep disturbance was related to elevated risk of respiratory infection (Shafiee et al., 2023; Chiner et al., 2016; Chen et al., 2021). A recently published study showed that obstructive sleep apnea was associated with severe COVID-19 and longer hospitalization (Arish et al., 2023). Therefore, sleep behaviors might be potential modifiers in the association between Hb and the risk of respiratory infection. However, there has been limited investigation into the role of sleep behaviors in the relationship between Hb and respiratory infection.
Using data from the United Kingdom Biobank (UKB), we tested the sex-specific dose–response association between Hb and the risk of any hospitalized respiratory infection incidence and explored the potential modification effects of several sleep behaviors, including excessive daytime sleepiness, insomnia, sleep duration, and chronotype.
Methods
Study design and population
The detailed study design and participants of UKB have been described in Sudlow et al. (2015). In brief, more than 500,000 community-based volunteers aged 37–73 years across the United Kingdom were enrolled between 2006 and 2010. All participants completed touchscreen questionnaires and physical measurements during a baseline survey. They also provided blood samples for hematology and biomarker testing. The follow-up information for all participants was mainly obtained through record linkage to national electronic health data, including death and cancer registers, primary care, and hospital in-patient. All participants gave written consent, and ethical approval was obtained from the North-West Multi-Centre Research Ethics Committee (Ref 11/NW/0382).
For the current analysis, participants with any respiratory disease, cancer, or anemia diagnosis were excluded (n = 27 258). Those without Hb measurements (n = 51 634) or sleep variables (n = 74 303) at baseline were also excluded, leaving a total of 292 568 individuals in the analysis.
Assessment of hemoglobin concentration
Blood samples were collected from participants for hematology analysis during baseline visit. Four Beckman Coulter LH750 instruments were used to analyze samples for hematology data. The detailed information on this data collection is provided at the UKB website (https://biobank.ndph.ox.ac.uk/showcase/refer.cgi?id=1453).
Assessment of sleep behaviors
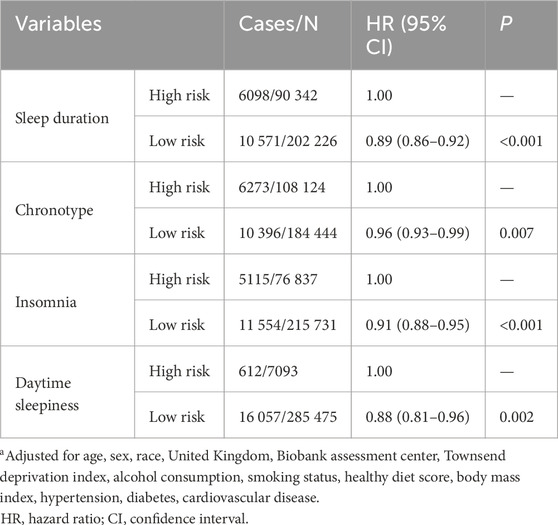
Sleep behaviors data were collected through a self-reported touchscreen questionnaire in the UKB. Details about questions of sleep behaviors were described by Fan et al. (2019). In the current study, only four sleep behaviors (excessive daytime sleepiness, sleep duration, chronotype, insomnia) were included since snoring was closely related to respiratory diseases. Each sleep behavior was classified as low- or high-risk and coded as “1” or “0”, respectively. Low-risk sleep behaviors were defined according to the following criteria: sleep 7–8 h per day; early chronotype (“morning” or “morning than evening”); never or rarely reported insomnia symptoms; no excessive daytime sleepiness (“never/rarely” or “sometimes”). High risk sleep behaviors referred to sleep less than 7 h/day or more than 8 h/day, late chronotype (“evening than morning” or “evening”), sometimes or usually reported insomnia symptoms, and excessive daytime sleepiness (“often” or “all of the time”).
Assessment of outcomes
Health outcomes in the UKB were defined based on multiple resources of records including death register, cancer register, hospital admissions data, and self-report information. Hospital admission data were obtained through Scottish Morbidity Record data in Scotland, Hospital Episode Statistics in England, and the Patient Episode Database in Wales. Any incident hospitalized respiratory infection was ascertained with the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes of J00, J01, J02, J03, J04, J05, J06, J09, J10, J11, J12, J13, J14, J15, J16, J17, J18, J20, J21, and J22. Specifically, J00, J01, J02, J03, J04, J05, J06, J09, J10, and J11 were identified as upper respiratory infections, while J12, J13, J14, J15, J16, J17, J18, J20, J21, and J22 were lower respiratory infections.
Statistical analysis
Male and female participants were separately classified into five groups according to quintiles of Hb concentrations. For the baseline characteristics of participants, continuous and categorical variables were described as means or percentages, respectively, according to the quintile groups. The period between the baseline date and the first occurrence of any respiratory infection diagnosis, death, or the census date (30 September 2021) was estimated as the follow-up time. The Cox proportional hazards model was adopted to calculate the sex-specific hazard ratio (HR) and 95% confidence interval (CI) of respiratory infection, with the third quintile as the reference group. In the first model, adjustments were made for demographics including age, race (white European, mixed, South Asian, black, others), Townsend deprivation index, and UKB assessment center. Model 2 was further adjusted for lifestyle factors such as alcohol consumption (never, former, current), smoking status (never, former, current), healthy diet score (0–5), iron supplement intake (yes/no), and body mass index (BMI, kg/m2). In model 3, we further adjusted for hypertension (yes/no), diabetes (yes/no), and cardiovascular disease (yes/no) at baseline. We tested the proportional hazards assumption using the Schoenfeld residuals method, and the models were satisfied for Hb. The healthy diet score was calculated by using five dietary factors: fruits, vegetables, unprocessed red meat, processed meat, and fish. Each was classified as a low risk factor and scored one point according to the favorable corresponding median values: vegetables at least four tablespoons/day; fruits at least three pieces/day; fish at least twice/week; unprocessed red meat no more than twice/week; processed meat no more than twice/week. The total healthy diet score ranged from 0 to 5 (Pazoki et al., 2018). Restricted cubic splines were constructed to assess the association of Hb with respiratory infection based on model 3. Notably, quintile-based categorization of Hb concentration was adopted for maintaining relatively sufficient statistical power and capturing the nonlinear association between Hb and respiratory infection risk. For missing continuous variables, mean values were imputed. For missing categorical variables, a missing indicator approach was used. The missing rate of race, alcohol consumption, and smoking status was 0.3%, 0.9%, and 0.3%, respectively. The likelihood ratio test comparing models with and without a cross-product term of Hb and each sleep factor was conducted to test the interaction between Hb and sleep behaviors. All sleep behaviors were included in models simultaneously.
Sensitivity analyses with restricting incident respiratory infection cases to more than 2 years from the baseline survey time or excluding participants with extreme hemoglobin values (physiologically plausible range: 12–16 g/dL for men and 11–15 g/dL for women) were conducted to confirm the robustness of the results.
SAS software (version 9.4; SAS Institute Inc., Cary, NC, United States) and R (version 4.5.1) were used to conduct the analyses. A two-sided P-value less than 0.05 was considered statistical significant.
Results
Baseline characteristics
Baseline characteristics of the study populations according to quintiles of Hb concentrations are shown in Table 1. Among all participants included in the current analysis, 46.3% (135 351 individuals) were male. The mean (standard deviation [SD]) Hb concentrations were 15.0 (1.0) and 13.5 (0.9) g/dL for men and women, respectively. Participants with higher Hb concentrations were older, had higher BMI levels, tended to be current smokers, but were less likely to have a healthy diet. Furthermore, participants with higher Hb concentrations also tended to have hypertension but had a lower prevalence of iron supplement intake than those with lower Hb concentrations.
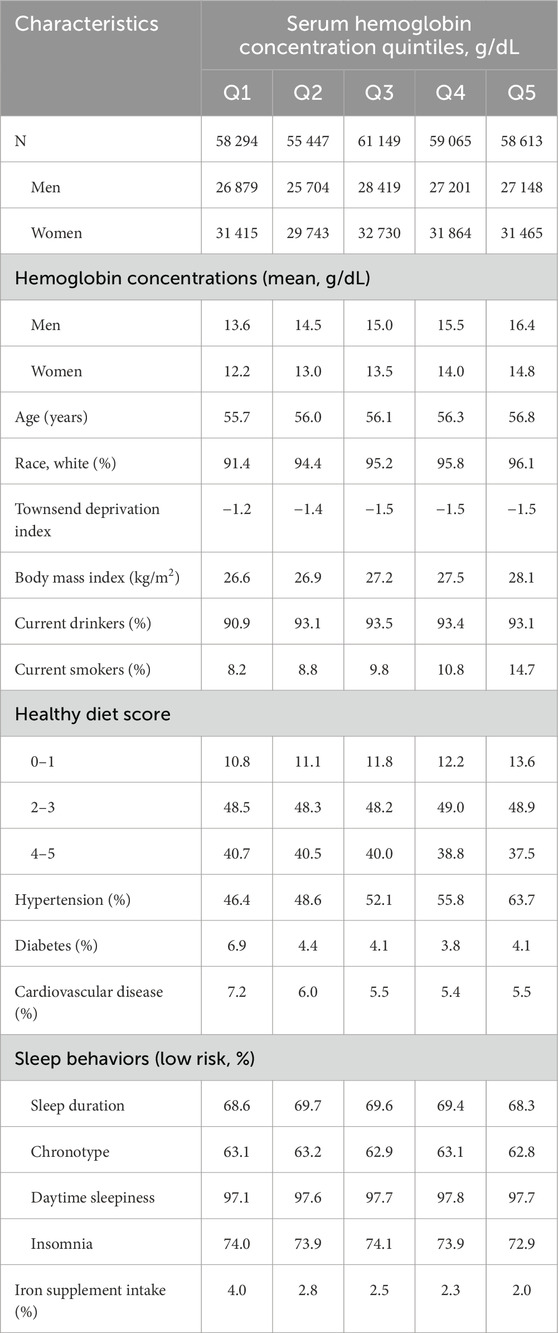
Table 1. Baseline characteristics of participants according to quintiles of serum hemoglobin concentration.
Sex-specific associations between Hb concentrations and respiratory infections
The median follow-up time of the cohort was 12.6 years, during which 16,669 incident respiratory infections (9,334 in males, 7,335 in females) were identified. The incidence rate of any respiratory infection was 5.7/1,000 person-years for men and 3.8/1,000 person-years for women. Of note, the corresponding cases of upper and lower respiratory infections were 2067 (12.4%) and 14,602 (87.6%), respectively. A U-shaped association was detected between Hb concentration and respiratory infection for all participants as well as for men and women separately (Figures 1, 2). Statistically significant nonlinear associations between Hb concentration and respiratory infection risk were observed in both men (P for nonlinear <0.01) and women (P for nonlinear <0.01). The C-index for the restricted cubic spline was 0.70 in males and 0.68 in females, respectively. The risk increased markedly with Hb concentration above 15.0 g/dL in males and 13.5 g/dL in females.
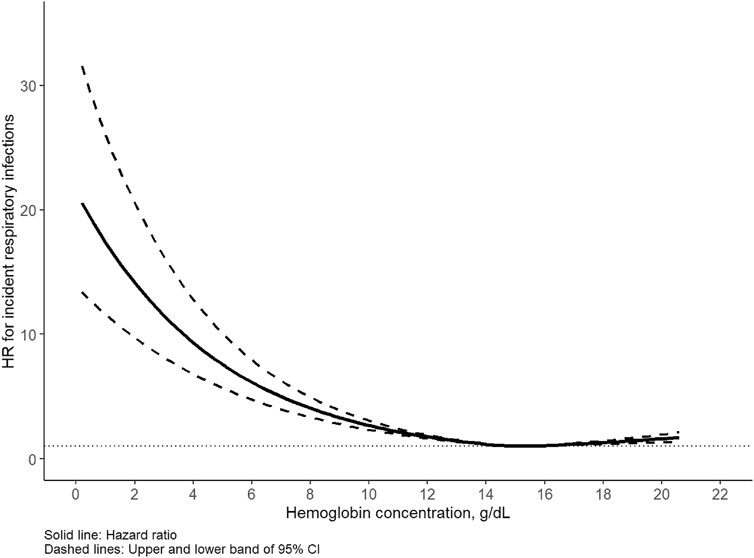
Figure 1. Association between hemoglobin and respiratory infection in males. Hazard ratio (HR) adjusted for age, race, United Kingdom Biobank assessment center, Townsend deprivation index, alcohol consumption, smoking status, healthy diet score, body mass index, iron supplement intake, hypertension, diabetes, cardiovascular disease.
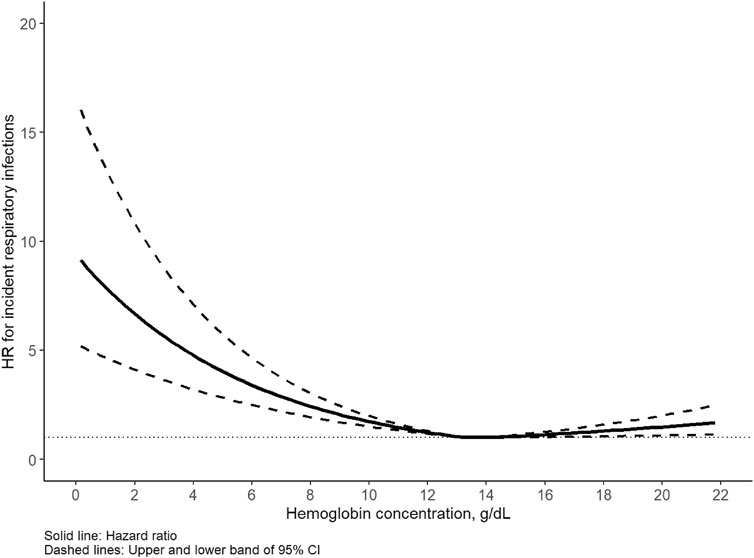
Figure 2. Association between hemoglobin and respiratory infection in females. Hazard ratio (HR) adjusted for age, race, United Kingdom Biobank assessment center, Townsend deprivation index, alcohol consumption, smoking status, healthy diet score, body mass index, iron supplement intake, hypertension, diabetes, cardiovascular disease.
In all multivariable-adjusted models, compared with the third quintile group, other quintile groups were associated with higher risks of respiratory infection incidents. In model 3 with adjustment for sex, age, race, Townsend deprivation index, UKB assessment center, alcohol intake, smoking, healthy diet score, BMI, iron supplement intake, hypertension, diabetes, and cardiovascular disease, the HR (95% CI) was 1.25 (1.20–1.32), 1.08 (1.02–1.13), 1.04 (0.99–1.09), and 1.07 (1.02–1.12), respectively, in the first, second, fourth, and fifth quintile groups compared with the third quintile group of Hb concentration. In men, the HR (95% CI) was 1.28 (1.21–1.37), 1.07 (1.00–1.14), 1.06 (0.99–1.13), and 1.09 (1.02–1.17), respectively, in the first, second, fourth, and fifth quintile groups compared with the third quintile group of Hb concentration. In females, the corresponding HR (95% CI) was 1.20 (1.12–1.29), 1.09 (1.01–1.17), 1.01 (0.94–1.09), and 1.05 (0.98–1.13) (Table 2). However, no statistically significant interaction between Hb concentration and sex on respiratory infection was observed in the current analysis (P for interaction >0.05).
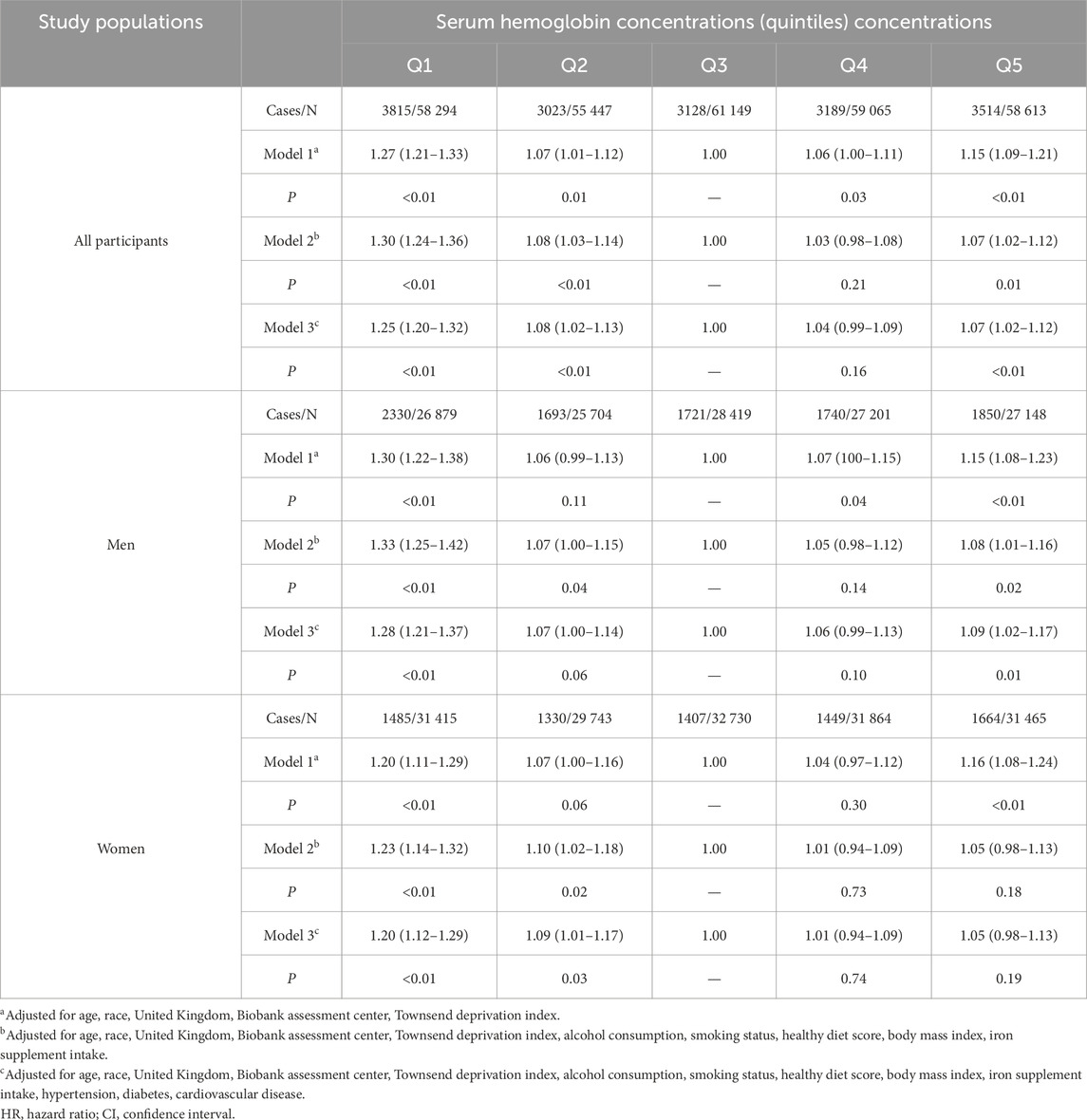
Table 2. Sex-specific adjusted HRs and 95% CI for hemoglobin concentrations with respiratory infections.
Associations between sleep behaviors and respiratory infections
Associations between sleep behaviors and respiratory infections are shown in Table 3. We found that all healthy sleep behaviors were associated with lower risks of respiratory infections. In detail, the HR (95% CI) of respiratory infection was 0.89 (0.86–0.92) in the low-risk group of sleep duration compared with the high-risk group. In addition, the low-risk groups for chronotype, insomnia, and daytime sleepiness were associated with 4%, 9%, and 12% lower risk of respiratory infection, respectively.
Interactions between Hb and sleep behaviors with respiratory infections
Interaction and subgroup analyses according to sleep factors were conducted to evaluate whether sleep behaviors modified the relationship between Hb and the risk of respiratory infection. There was a significant interaction between Hb concentration and chronotype for the risk of incident respiratory infection (P for interaction = 0.005); the HR (95% CI) was 1.32 (1.22, 1.42), 1.09 (1.00, 1.18), 1.07 (0.99, 1.16), and 1.08 (1.00, 1.17), respectively, in the first, second, fourth, and fifth quintile groups compared with the third quintile group of Hb concentration when further adjusted for the cross-product term of Hb and chronotype in the model. Subgroup analysis showed that the elevated HR of respiratory infection associated with Hb was more evident among participants in the high-risk chronotype group than in the low-risk group. The HR (95% CI) of respiratory infection incidents in first, second, fourth, and fifth quintile groups of Hb concentration was 1.33 (1.23–1.43), 1.09 (1.01–1.19), 1.06 (0.98–1.15), and 1.08 (0.99–1.17), respectively, among participants with late chronotype, and 1.21 (1.14–1.29), 1.07 (1.00–1.14), 1.02 (0.96–1.09), and 1.07 (1.00–1.13), respectively, among participants with early chronotype (Figure 3). The interactions between Hb concentration and other sleep behaviors did not show statistical significance.
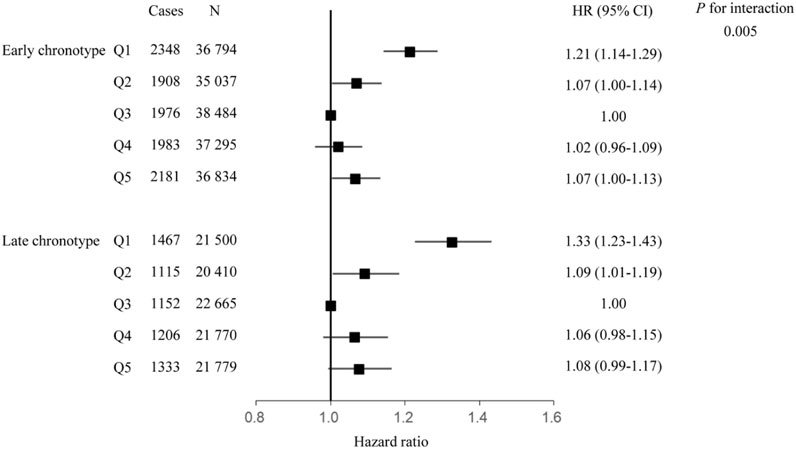
Figure 3. Association between hemoglobin and respiratory infection, stratified by chronotype. Hazard ratio adjusted for age, race, United Kingdom Biobank assessment center, Townsend deprivation index, alcohol consumption, smoking status, healthy diet score, body mass index, iron supplement intake, hypertension, diabetes, cardiovascular disease, sleep duration, insomnia, daytime sleepiness.
Sensitivity analysis
Sensitivity analysis showed that the results were generally stable when excluding participants with a follow-up time ≤2 years or those with extreme hemoglobin values. The results are shown in Supplementary Tables S1, S2.
Discussion
In UKB, we observed a U-shaped association between Hb concentration and any incidence of hospitalized respiratory infection in which the risk of respiratory infection increased markedly with Hb above 15.0 g/dL for men and 13.5 g/dL for women. Significant associations of four sleep behaviors—excessive daytime sleepiness, sleep duration, chronotype, and insomnia—with respiratory infections were also observed. We also found that the association between Hb and respiratory infection was significantly modified by chronotype, and the higher risk of respiratory infection associated with either lower or higher Hb concentration appeared to be stronger among participants with late chronotype.
The present study showed that the association between Hb and respiratory infection was nonlinear and U-shaped, with either low or high Hb concentration being associated with a higher risk of respiratory infection. Patino-Aldana et al. (2022) found a nonlinear, U-shaped association between Hb concentration and the risk of COVID-19 mortality similar to our results. Furthermore, a U-shaped association was also observed between serum iron and severe respiratory failure in COVID-19 patients (Tojo et al., 2021). The U-shaped association between Hb concentration and the risk of respiratory infection is biologically explainable. Hb concentration reflects the oxygen-carrying capacity of the blood (Hayden et al., 2012), and hypoxia may exert an adverse effect on immune responses through the NF-κB-driven inflammation pathway (Taylor et al., 2014) and programmed death-ligand 1 (PD-L1)/PD-1 crosstalk (Cubillos-Zapata et al., 2017). At the same time, higher Hb concentration might cause excess iron. Animal studies have shown that iron overload might cause lung injury due to apoptosis of the lung tissue, which consequently increases the risk of respiratory infection (Zhang et al., 2020; Liu et al., 2022). Additionally, the release of free iron from Hb might enhance oxidant damage in isolated rat lungs caused by t-buOOH (Seibert et al., 1991). The results illustrate the importance of achieving a delicate balance in iron intake in which neither too-low nor too-high Hb concentration has a negative health effect on respiratory tract. The proportion of lower respiratory infections is particularly higher than upper respiratory infections in the current analysis. Lower respiratory infections including pneumonia and bronchitis typically present with more severe symptoms, which may increase the likelihood of medical consultation and diagnosis. In contrast, milder upper respiratory infections may often remain self-managed and undiagnosed. This pattern is consistent with the case definition based on ICD codes in the UKB, which primarily captures clinically attended cases. Therefore, our findings may predominantly reflect the association between Hb and severe respiratory infections requiring medical attention rather than total community infections. The interpretation of our results should be addressed in future studies with more upper respiratory infection cases. Notably, no statistically significant interaction between sex and Hb concentration on respiratory infection was observed in the current study. However, biological differences in Hb concentration by sex is well-documented in clinical guidelines. Additionally, the current analysis also showed significantly different hemoglobin concentrations between sexes, with men having higher concentrations than women. Therefore, the results of the association between Hb concentration and respiratory infection were still presented by sex.
We also assessed the association between sleep behaviors and respiratory infection in the current analysis. The results were consistent with previous studies, which showed that unhealthy sleep behaviors might be related to a higher risk of respiratory infection (Cohen et al., 2009; Prather et al., 2015; Quan et al., 2023; Nieters et al., 2019). Like Hb, sleep also plays a vital role in maintaining optimal immune function (Besedovsky et al., 2012) which is strongly associated with respiratory infection (Garbarino et al., 2021). Several studies have demonstrated that sleep duration and sleep disturbances are related to the continuous production of inflammation markers, including pro-inflammatory cytokines, which might result in increased risks of inflammatory diseases (Garbarino et al., 2021; Irwin et al., 2016). Furthermore, insomnia is related to increased stress and the activation of the sympathetic nervous system, as well as elevated cortisol (Bonnet and Arand, 2010), which can inhibit the transcription of antiviral interferon genes through increased release of norepinephrine, thus inducing a state of chronic inflammation (Irwin and Opp, 2017; Furman et al., 2019). As emerging lifestyle risk factors, sleep behaviors—including no frequent excessive daytime sleepiness, sleep 7–8 h per day, no or rare insomnia, and early chronotype—have been reported to reduce the risks of cardiovascular diseases and type 2 diabetes in recent studies (Fan et al., 2019; Zhuang et al., 2023). The current study contributes to a more comprehensive picture of sleep behaviors and health outcomes.
Interestingly, in the interaction analysis, we observed that chronotype significantly modified the association between Hb concentration and respiratory infection risk. The finding of the interaction between chronotype and Hb seemed biologically plausible. It has been shown that Hb production might be disrupted by inflammation such as IL-6 (Mehta et al., 2020). Notably, inflammatory responses are gated by the circadian clock (Gibbs et al., 2012). An evening chronotype is associated with higher levels of circulating inflammatory markers (Kim et al., 2018). Therefore, the inflammatory pathway could partly explain the interaction between chronotype and Hb concentration for respiratory infection. The interaction analysis findings suggest that sleep behaviors are potential modifiers in the Hb-respiratory infection association and that the heterogeneity in associations between Hb and respiratory infection across different studies might be partly caused by sleep behaviors.
Smoking status should be an important consideration in interpreting results of the current study. Previous studies have shown that smoking is associated with both Hb concentration and susceptibility to respiratory infection (Pedersen et al., 2019; Jiang et al., 2020). Additionally, smoking may also contribute to a higher risk of sleep disturbance (Amiri and Behnezhad, 2020). In the current analysis, despite its adjustment for self-reported smoking status in the multivariable models, information bias and residual confounding may persist. Furthermore, smoking may act as an effect modifier in the hemoglobin–respiratory infection association, although no significant interaction was detected in the current analysis. Further studies are needed to clarify these complex pathways.
Strengths and limitations
This study used UKB data to assess the sex-specific association between Hb and respiratory infection, and particularly to explore the interaction between Hb concentration and sleep behaviors with incident respiratory infection risk. The cohort study has a large sample size, longitudinal design, and valid measurements of Hb concentration. Additionally, a number of confounders including lifestyles, physical measurements, and medical records were adjusted to reduce potential bias. However, there were still limitations to be addressed. First, causality relationships could not be inferred since this was an observational study. Therefore, intervention studies and mechanistic experiments are needed to confirm and explain the findings. Second, although several important confounding variables were taken into account, some unmeasurable confounders might affect the association. Third, self-reported sleep data were adopted in the study, so misclassification of exposures might exist. Fourthly, only a single measurement of Hb concentration is available at baseline. Repeated measurements of Hb concentration are needed to confirm the association. Fifthly, ICD-10 codes were used to identify respiratory infections in the UKB, which may capture more severe infections recorded in clinical settings. Thus, the respiratory incidence rates should not be interpreted as estimates of total community infection incidence, and the results should be interpreted with caution. Additionally, in order to increase statistical efficiency, especially in stratified analyses, different subtypes of respiratory infection were not considered. Finally, more caution is needed when generalizing and interpreting the results to other populations since the participants were all enrolled in the United Kingdom.
Conclusion
The study indicates that either lower or higher Hb concentration is associated with an elevated risk of hospitalized respiratory infection, indicating a U-shaped relation in both males and females. In addition, individual sleep behaviors including excessive daytime sleepiness, sleep duration, chronotype, and insomnia are related to the risk of hospitalized respiratory infection. More importantly, the association between Hb concentration and hospitalized respiratory infection is modified by chronotype. It is important to consider sleep behaviors when investigating the association between Hb and hospitalized respiratory infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by North-West Multi-Centre Research Ethics Committee (Ref 11/NW/0382). The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Formal Analysis, Writing – original draft, Writing – review and editing. QC: Writing – review and editing. MW: Formal Analysis, Writing – original draft. HQ: Writing – review and editing. QS: Conceptualization, Writing – review and editing. BL: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This research was conducted using the United Kingdom Biobank resources under application 87134.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1638819/full#supplementary-material
References
Amiri S., Behnezhad S. (2020). Smoking and risk of sleep-related issues: a systematic review and meta-analysis of prospective studies. Can. J. Public Health 111 (5), 775–786. doi:10.17269/s41997-020-00308-3
Arish N., Izbicki G., Rokach A., Jarjou'i A., Kalak G., Goldberg S. (2023). Association of the risk of obstructive sleep apnoea with the severity of COVID-19. PLoS One 18 (7), e0284063. doi:10.1371/journal.pone.0284063
Besedovsky L., Lange T., Born J. (2012). Sleep and immune function. Pflugers Arch. 463 (1), 121–137. doi:10.1007/s00424-011-1044-0
Bonnet M. H., Arand D. L. (2010). Hyperarousal and insomnia: state of the science. Sleep. Med. Rev. 14 (1), 9–15. doi:10.1016/j.smrv.2009.05.002
Chen X., Wang S. B., Li X. L., Huang Z. H., Tan W. Y., Lin H. C., et al. (2020). Relationship between sleep duration and sociodemographic characteristics, mental health and chronic diseases in individuals aged from 18 to 85 years old in Guangdong province in China: a population-based cross-sectional study. BMC Psychiatry 20 (1), 455. doi:10.1186/s12888-020-02866-9
Chen T. Y., Chang R., Chiu L. T., Hung Y. M., Wei J. C. C. (2021). Obstructive sleep apnea and influenza infection: a nationwide population-based cohort study. Sleep. Med. 81, 202–209. doi:10.1016/j.sleep.2021.02.034
Chiner E., Llombart M., Valls J., Pastor E., Sancho-Chust J. N., Andreu A. L., et al. (2016). Association between obstructive sleep apnea and community-acquired pneumonia. Plos One 11 (4), e0152749. doi:10.1371/journal.pone.0152749
Ciaccio C., Coletta A., Coletta M. (2022). Role of hemoglobin structural-functional relationships in oxygen transport. Mol. Asp. Med. 84, 101022. doi:10.1016/j.mam.2021.101022
Cohen S., Doyle W. J., Alper C. M., Janicki-Deverts D., Turner R. B. (2009). Sleep habits and susceptibility to the common cold. Arch. Intern Med. 169 (1), 62–67. doi:10.1001/archinternmed.2008.505
GBD 2016 Lower Respiratory Infections Collaborators (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18 (11), 1191–1210. doi:10.1016/S1473-3099(18)30310-4
Cubillos-Zapata C., Avendaño-Ortiz J., Hernandez-Jimenez E., Toledano V., Casas-Martin J., Varela-Serrano A., et al. (2017). Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 50 (4), 1700833. doi:10.1183/13993003.00833-2017
Diseases G. B. D., Injuries C. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 (10258), 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Fan M., Sun D., Zhou T., Heianza Y., Lv J., Li L., et al. (2019). Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 41, 1182–1189. doi:10.1093/eurheartj/ehz849
Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25 (12), 1822–1832. doi:10.1038/s41591-019-0675-0
Garbarino S., Lanteri P., Bragazzi N. L., Magnavita N., Scoditti E. (2021). Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 4 (1), 1304. doi:10.1038/s42003-021-02825-4
Gelaw Y., Getaneh Z., Melku M. (2021). Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ. Health Prev. Med. 26 (1), 13. doi:10.1186/s12199-020-00931-z
Gibbs J. E., Blaikley J., Beesley S., Matthews L., Simpson K. D., Boyce S. H., et al. (2012). The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 109 (2), 582–587. doi:10.1073/pnas.1106750109
Harrison R. K., Lauhon S. R., Colvin Z. A., McIntosh J. J. (2021). Maternal anemia and severe maternal morbidity in a US cohort. Am. J. Obstet. Gynecol. MFM 3 (5), 100395. doi:10.1016/j.ajogmf.2021.100395
Hayden S. J., Albert T. J., Watkins T. R., Swenson E. R. (2012). Anemia in critical illness: insights into etiology, consequences, and management. Am. J. Respir. Crit. Care Med. 185 (10), 1049–1057. doi:10.1164/rccm.201110-1915CI
Irwin M. R., Opp M. R. (2017). Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42 (1), 129–155. doi:10.1038/npp.2016.148
Irwin M. R., Olmstead R., Carroll J. E. (2016). Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80 (1), 40–52. doi:10.1016/j.biopsych.2015.05.014
Jackowska M., Kumari M., Steptoe A. (2013). Sleep and biomarkers in the English Longitudinal Study of Ageing: associations with C-reactive protein, fibrinogen, dehydroepiandrosterone sulfate and hemoglobin. Psychoneuroendocrinology 38 (9), 1484–1493. doi:10.1016/j.psyneuen.2012.12.015
Jiang C., Chen Q., Xie M. (2020). Smoking increases the risk of infectious diseases: a narrative review. Tob. Induc. Dis. 18, 60. doi:10.18332/tid/123845
Kurz K., Lanser L., Seifert M., Kocher F., Pölzl G., Weiss G. (2020). Anaemia, iron status, and gender predict the outcome in patients with chronic heart failure. Esc. Heart Fail 7 (4), 1880–1890. doi:10.1002/ehf2.12755
Kassebaum N. J., Collaborators G. B. D. A. (2016). The global burden of anemia. Hematol. Oncol. Clin. North Am. 30 (2), 247–308. doi:10.1016/j.hoc.2015.11.002
Kim S. M., Neuendorff N., Alaniz R. C., Sun Y., Chapkin R. S., Earnest D. J. (2018). Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J. 32 (6), 3085–3095. doi:10.1096/fj.201700784R
Kowsar R., Rahimi A. M., Sroka M., Mansouri A., Sadeghi K., Bonakdar E., et al. (2023). Risk of mortality in COVID-19 patients: a meta- and network analysis. Sci. Rep. 13 (1), 2138. doi:10.1038/s41598-023-29364-8
Levi M., Simonetti M., Marconi E., Brignoli O., Cancian M., Masotti A., et al. (2019). Gender differences in determinants of iron-deficiency anemia: a population-based study conducted in four European countries. Ann. Hematol. 98 (7), 1573–1582. doi:10.1007/s00277-019-03707-w
Liu X., Zhang J., Xie W. (2022). The role of ferroptosis in acute lung injury. Mol. Cell Biochem. 477 (5), 1453–1461. doi:10.1007/s11010-021-04327-7
Mehta P., McAuley D. F., Brown M., Sanchez E., Tattersall R. S., Manson J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395 (10229), 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Nieters A., Blagitko-Dorfs N., Peter H. H., Weber S. (2019). Psychophysiological insomnia and respiratory tract infections: results of an infection-diary-based cohort study. Sleep 42 (8), zsz098. doi:10.1093/sleep/zsz098
Patino-Aldana A. F., Ruíz Sternberg Á. M., Pinzón Rondón Á. M., Molano-Gonzalez N., Rodriguez Lima D. R. (2022). Interaction effect between hemoglobin and hypoxemia on COVID-19 mortality: an observational study from bogotá, Colombia. Int. J. Gen. Med. 15, 6965–6976. doi:10.2147/IJGM.S371067
Pazoki R., Dehghan A., Evangelou E., Warren H., Gao H., Caulfield M., et al. (2018). Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood Pressure levels and cardiovascular events. Circulation 137 (7), 653–661. doi:10.1161/CIRCULATIONAHA.117.030898
Pedersen K. M., Çolak Y., Ellervik C., Hasselbalch H. C., Bojesen S. E., Nordestgaard B. G. (2019). Smoking and increased white and red blood cells. Arterioscler. Thromb. Vasc. Biol. 39 (5), 965–977. doi:10.1161/ATVBAHA.118.312338
Prather A. A., Janicki-Deverts D., Hall M. H., Cohen S. (2015). Behaviorally assessed sleep and susceptibility to the common cold. Sleep 38 (9), 1353–1359. doi:10.5665/sleep.4968
Quan S. F., Weaver M. D., Czeisler M. É., Barger L. K., Booker L. A., Howard M. E., et al. (2023). Insomnia, poor sleep quality and sleep duration, and risk for COVID-19 infection and hospitalization. Am. J. Med. 136 (8), 780–788 e5. doi:10.1016/j.amjmed.2023.04.002
Schumann K., Solomons N. W. (2017). Perspective: what makes it So difficult to mitigate worldwide anemia prevalence? Adv. Nutr. 8 (3), 401–408. doi:10.3945/an.116.013847
Seibert A. F., Taylor A. E., Bass J. B., Haynes J. (1991). Hemoglobin potentiates oxidant injury in isolated rat lungs. Am. J. Physiol. 260 (6 Pt 2), H1980–H1984. doi:10.1152/ajpheart.1991.260.6.H1980
Shafiee A., Jafarabady K., Rajai S., Mohammadi I., Mozhgani S. H. (2023). Sleep disturbance increases the risk of severity and acquisition of COVID-19: a systematic review and meta-analysis. Eur. J. Med. Res. 28 (1), 442. doi:10.1186/s40001-023-01415-w
Stevens G. A., Finucane M. M., De-Regil L. M., Paciorek C. J., Flaxman S. R., Branca F., et al. (2013). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob. Health 1 (1), e16–e25. doi:10.1016/S2214-109X(13)70001-9
Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 (3), e1001779. doi:10.1371/journal.pmed.1001779
Taneri P. E., Gómez-Ochoa S. A., Llanaj E., Raguindin P. F., Rojas L. Z., Roa-Díaz Z. M., et al. (2020). Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur. J. Epidemiol. 35 (8), 763–773. doi:10.1007/s10654-020-00678-5
Taylor C. T., Kent B. D., Crinion S. J., McNicholas W. T., Ryan S. (2014). Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem. Biophys. Res. Commun. 447 (4), 660–665. doi:10.1016/j.bbrc.2014.04.062
Tojo K., Sugawara Y., Oi Y., Ogawa F., Higurashi T., Yoshimura Y., et al. (2021). The U-shaped association of serum iron level with disease severity in adult hospitalized patients with COVID-19. Sci. Rep. 11 (1), 13431. doi:10.1038/s41598-021-92921-6
Wang M., Zhou T., Li X., Ma H., Liang Z., Fonseca V. A., et al. (2020). Baseline Vitamin D status, sleep patterns, and the risk of incident type 2 diabetes in data from the UK biobank study. Diabetes Care 43 (11), 2776–2784. doi:10.2337/dc20-1109
Wang X., Cheng S., Lv J., Yu C., Guo Y., Pei P., et al. (2022). Liver biomarkers, genetic and lifestyle risk factors in relation to risk of cardiovascular disease in Chinese. Front. Cardiovasc Med. 9, 938902. doi:10.3389/fcvm.2022.938902
Williamson E. J., Walker A. J., Bhaskaran K., Bacon S., Bates C., Morton C. E., et al. (2020). Factors associated with COVID-19-related death using OpenSAFELY. Nature 584 (7821), 430–436. doi:10.1038/s41586-020-2521-4
Zeng Z., Yu H., Chen H., Qi W., Chen L., Chen G., et al. (2020). Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit. Care 24 (1), 525. doi:10.1186/s13054-020-03255-0
Zhang V., Ganz T., Nemeth E., Kim A. (2020). Iron overload causes a mild and transient increase in acute lung injury. Physiol. Rep. 8 (12), e14470. doi:10.14814/phy2.14470
Zhang T., Abdelrahman Z., Liu Q., Wang X., Chen Z. (2022). Comparisons of the immunological landscape of COVID-19 patients based on sex and disease severity by multi-omics analysis. Chem. Biol. Interact. 352, 109777. doi:10.1016/j.cbi.2021.109777
Keywords: sleep behaviors, hemoglobin concentration, respiratory infection, cohort, interaction
Citation: Zhu Y, Chen Q, Wang M, Qian H, Song Q and Liu B (2025) Sleep behaviors modify the association between hemoglobin concentration and respiratory infection: a prospective cohort analysis. Front. Physiol. 16:1638819. doi: 10.3389/fphys.2025.1638819
Received: 04 June 2025; Accepted: 08 September 2025;
Published: 30 September 2025.
Edited by:
Dongsheng Yan, Wenzhou Medical University, ChinaReviewed by:
Zachary Harvanek, Yale University, United StatesEmrah Güler, Lefke Avrupa Universitesi, Cyprus
Copyright © 2025 Zhu, Chen, Wang, Qian, Song and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiying Song, c29uZ3FpeWluZ0Biam11LmVkdS5jbg==; Bofei Liu, bGl1Ym9mZWkuempnLmpzQDE2My5jb20=
 Yongkui Zhu1
Yongkui Zhu1 Mengying Wang
Mengying Wang Qiying Song
Qiying Song