- 1Rehabilitation Medicine Center and Institute of Rehabilitation Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2School of Rehabilitation Sciences, West China School of Medicine, Sichuan University, Chengdu, China
- 3Key Laboratory of Rehabilitation Medicine in Sichuan Province, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Rehabilitation Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
Introduction: This study aimed to evaluate the efficacy of platelet-rich plasma (PRP) in treating knee osteoarthritis (KOA) and the effects of baseline characteristics and PRP intervention parameters on treatment outcomes.
Methods: Overall, 140 individuals diagnosed with KOA who received PRP injections and completed a 6-month follow-up period were enrolled in this retrospective analysis. Knee pain and functional outcomes were assessed using the Visual Analog Scale (VAS) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Based on the Minimal Clinically Important Difference (MCID) in outcomes, the participants were divided into effective and ineffective groups. Using multivariable logistic regression to explore factors influencing treatment outcomes, we compared the effective and ineffective groups to identify predictors of response to PRP therapy.
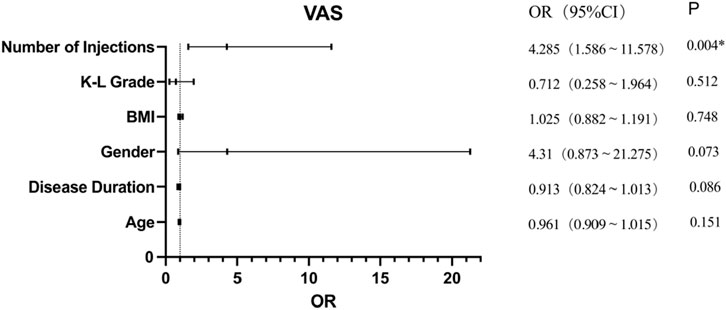
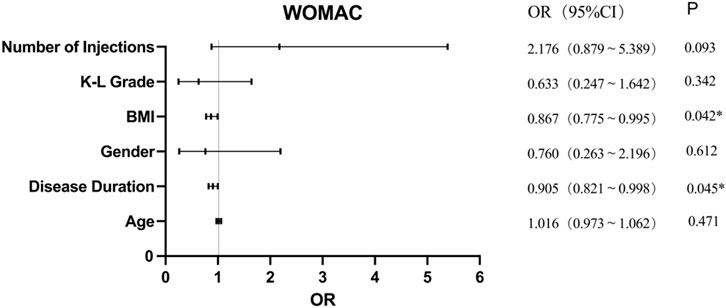
Results: At 6 months, the median (IQR) VAS score significantly decreased from 66.5 (27) to 24 (34) (95% CI = −38 to −30.5), p < 0.001), and WOMAC scores improved from 29 (22) to 12 (14) (95% CI = −16.5 to −12), p < 0.001). Five mild adverse events were reported. Multivariate analysis indicated that only the number of injections significantly influenced VAS outcomes (OR = 4.285, 95% CI: 1.586–11.578, p = 0.004). Regarding WOMAC, multivariate analysis revealed that body mass index (BMI) (OR = 0.867, 95% CI: 0.755–0.995, p = 0.042) and disease duration (OR = 0.905, 95% CI: 0.784–0.989, p = 0.045) significantly affected outcomes. Age, sex, Kellgren-Lawrence (KL) grade, number of PRP injections, and injection frequency did not significantly impact WOMAC scores.
Conclusion: PRP therapy is a safe and effective treatment option for KOA. In this 6-month follow-up investigation, we observed that the number of injections administered affected pain levels, while disease duration and BMI affected knee joint function. Insights from this study may facilitate patient selection and PRP treatment protocol optimization in clinical practice.
1 Introduction
Knee osteoarthritis (KOA) is a common musculoskeletal disease that affects approximately 364.58 million people globally and has become a major disabling condition. Notably, female individuals account for approximately two-thirds of the 225 million patients worldwide (Li et al., 2024). Therefore, effective and safe medical treatments for KOA are urgently required. Currently, no disease-modifying drugs have been approved, and existing non-operative therapies have demonstrated only limited benefits and may be associated with serious adverse effects (Arden et al., 2020; Kolasinski et al., 2020; McAlindon et al., 2014).
Platelet-rich plasma (PRP), a safe autologous blood product rich in various growth factors and cytokines, may influence the biological mechanisms underlying KOA progression and symptom manifestation (Fice et al., 2019; Gupta et al., 2020; Liu et al., 2023). Recently, PRP has gained popularity as a treatment option for individuals with KOA (Fice et al., 2019; Magruder et al., 2023; Werner et al., 2020), especially for pain management. For intra-articular injections, a meta-analysis indicated that for individuals with KOA, the combination therapy of PRP and hyaluronic acid is safe and also superior to PRP monotherapy in terms of pain relief and functional enhancement (Du and Liang, 2025). Additionally, several systematic reviews reported that PRP was associated with more favorable pain and function outcomes compared to intra-articular corticosteroids (Wang et al., 2024) or intra-articular hyaluronic acid (Hohmann et al., 2020), particularly in the long-term management of KOA (Patel et al., 2013; Bensa et al., 2025). However, a systematic review (Costa et al., 2022) indicated that there was limited evidence to support its clinical benefits. According to KOA clinical guidelines (Kolasinski et al., 2020; McAlindon et al., 2014), PRP is not recommended due to very low-certainty evidence and heterogeneity. Therefore, rigorous studies to demonstrate its effectiveness are required. Previous studies (Karaborklu et al., 2023; Saraf et al., 2023; Tao et al., 2023; Xiong et al., 2017) reported how individual characteristics such as age, sex, and PRP dosage affected the clinical outcomes of PRP treatment. These factors may potentially account for the heterogeneity observed in PRP treatment efficacy. Therefore, a large-scale retrospective study is needed to clarify these factors and their impact on treatment outcomes.
Within a 6-month follow-up period, this study aimed to evaluate the clinical effectiveness of PRP injections in relieving pain and enhancing joint function for individuals with KOA. Furthermore, we aimed to investigate whether individual characteristics (e.g., age, sex, disease duration, body mass index (BMI), and Kellgren–Lawrence (KL) grade) and PRP intervention parameters (e.g., number and frequency of injections) could influence the outcomes. The findings of this study will bridge this gap and provide more personalized recommendations for individuals with KOA of varying severities.
2 Methods
2.1 Study design and setting
This retrospective study was registered with the Chinese Clinical Trial Registry (Registration Number: ChiCTR2500103500). The data collection process adhered strictly to the ethical guidelines outlined by the World Medical Association’s Declaration of Helsinki. The study followed the STROBE guidelines (Supplementary Table S1) and was approved by the Biomedical Research Ethics Committee of West China Hospital, Sichuan University (Approval No. 691, reviewed in 2024). Given the retrospective nature of the study, the committee granted an exemption from obtaining informed consent. All data were kept strictly confidential and used exclusively for the purposes of this study.
2.2 Participants
Eligible participants were individuals aged 18–80 years who were diagnosed with KOA and received PRP therapy between January 2022 and November 2023 at a rehabilitation medical center. The inclusion criteria followed the national clinical guidelines (Zhu et al., 2023) and required recurrent knee pain in the previous month along with at least two of the following: (1) Radiographic findings (from standing or weight-bearing views) indicating joint space narrowing, subchondral sclerosis and/or cystic changes, and osteophyte formation at the joint margins; (2) age ≥50 years; (3) morning stiffness lasting ≤30 min; (4) audible joint crepitus during movement. Additional inclusion criteria included a VAS score of ≥40/100 and receiving at least one PRP injection. The exclusion criteria included individuals diagnosed with other lower limb disorders that affected daily activities or osteoarthritis of other joints (such as the hip or ankle), PRP injection duration of less than 8 weeks (Gobbi et al., 2015), or those receiving other biological treatments (such as stem cell therapy or systemic immunosuppressive medications) within the past year.
2.3 PRP preparation and injection
2.3.1 PRP preparation
PRP was prepared following a previously published protocol (Liu et al., 2023). A total of 46 mL blood was drawn from the median cubital vein, ensuring that 45 mL of them for preparing PRP for one knee, and 1 mL of blood was used for laboratory testing. The EasyPRP Centrifuge, model PRP520R (EasyPRP, China), was used to prepare PRP using a two-time centrifugation method. 45 mL of blood was anticoagulated with 5 mL of an anticoagulant agent and then centrifuged at low speed. After removing the supernatant, the sediment was centrifuged again. The final product was approximately 5 mL of leukocyte-poor PRP, and 1 mL of them was used for laboratory testing for comparing the number of platelet concentration to the baseline.
2.3.2 PRP injection
All injection procedures were performed by clinicians with over 5 years of experience in injection therapy. Standardization was ensured through uniform training in PRP preparation and application. All injections were administered under ultrasound guidance (LOGIQ e, GE Healthcare, Milwaukee, Wisconsin, United States), using a probe with frequencies between 7.5 and 14 MHz, to enhance the precision of all injections, minimize variability, and maximize the accuracy of the procedure. Trained operators administered 5 mL of PRP into the affected knee under ultrasound guidance within 30 min of preparation. Blood samples (1 mL of whole blood and 1 mL of PRP) were analyzed. In cases of joint effusion, aspiration via the suprapatellar pouch was performed before PRP injection. Participants were advised to avoid medications for 6 months post-treatment to ensure accurate assessment of PRP efficacy.
2.4 Outcomes
The primary outcomes were pain and functional assessments for 6 months after the first injection. Pain was assessed using the VAS, and function was evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), which is used for overall knee function assessment. The Minimal Clinically Important Difference (MCID) for both VAS (Lee et al., 2003) and WOMAC (Hmamouchi et al., 2012) is approximately 20%. The participants were classified into the effective and the ineffective groups based on the MCID of their VAS or WOMAC. Those with changes beyond the MCID were included in the effective group. The secondary outcome was safety, which was primarily evaluated through adverse event reports during the 6-month follow-up period.
2.5 Clinical data
Demographic data of the participants, laboratory results of PRP, and outcomes were obtained from the electronic medical system record (HIS) at a rehabilitation medical center. Data were extracted from the system at baseline and assessed manually at the 6-month follow-up. Specifically, we analyzed whether there were significant differences between the groups in terms of age, disease duration, sex, BMI, KL grade, injection frequency, and number of injections.
2.6 Statistical analysis
Statistical analysis was performed using SPSS Statistics, version 27.0 (IBM Corp., Armonk, NY, United States) and R version 4.1.0 (R Core Team, Vienna, Austria). A P-value <0.05 was considered statistically significant. Given the observational nature of our study, we followed the recommendation from a previous study (Ahmad and Halim, 2017) to set the sample size at 5–10 times the number of factors. For normally distributed continuous variables, estimated means and standard deviations were presented. Medians and interquartile ranges (IQR) were used for non-normally distributed variables. Normality was assessed through visual inspection of histograms or normal Q - Q plots and verified using the Kolmogorov–Smirnov test. Baseline between-group differences were determined using the Student’s t-test or Mann–Whitney U test, as appropriate. Categorical variables were analyzed via the chi-square or Fisher’s exact tests. Primary analyses were conducted on an available-case basis, including participants with both baseline and 6-month data; missing outcomes were not imputed. Additionally, a multivariate analysis was conducted with treatment effectiveness (categorized as effective or ineffective) as the dependent variable. Given that the dependent variable is categorical, logistic regression analysis was employed to assess the relationship between the predictors and the outcome of treatment effectiveness. Both continuous (age, BMI, disease duration) and categorical (sex, KL grade, number of injections) predictors were included in the model.
3 Results
3.1 Participants’ characteristics
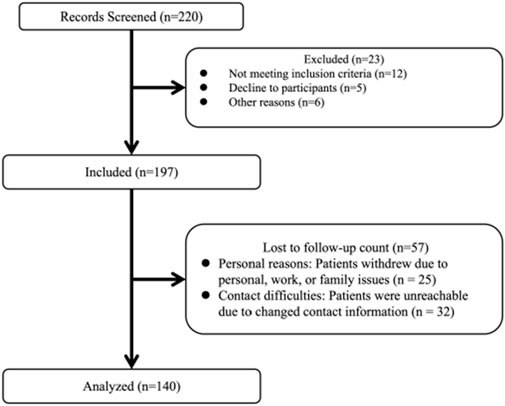
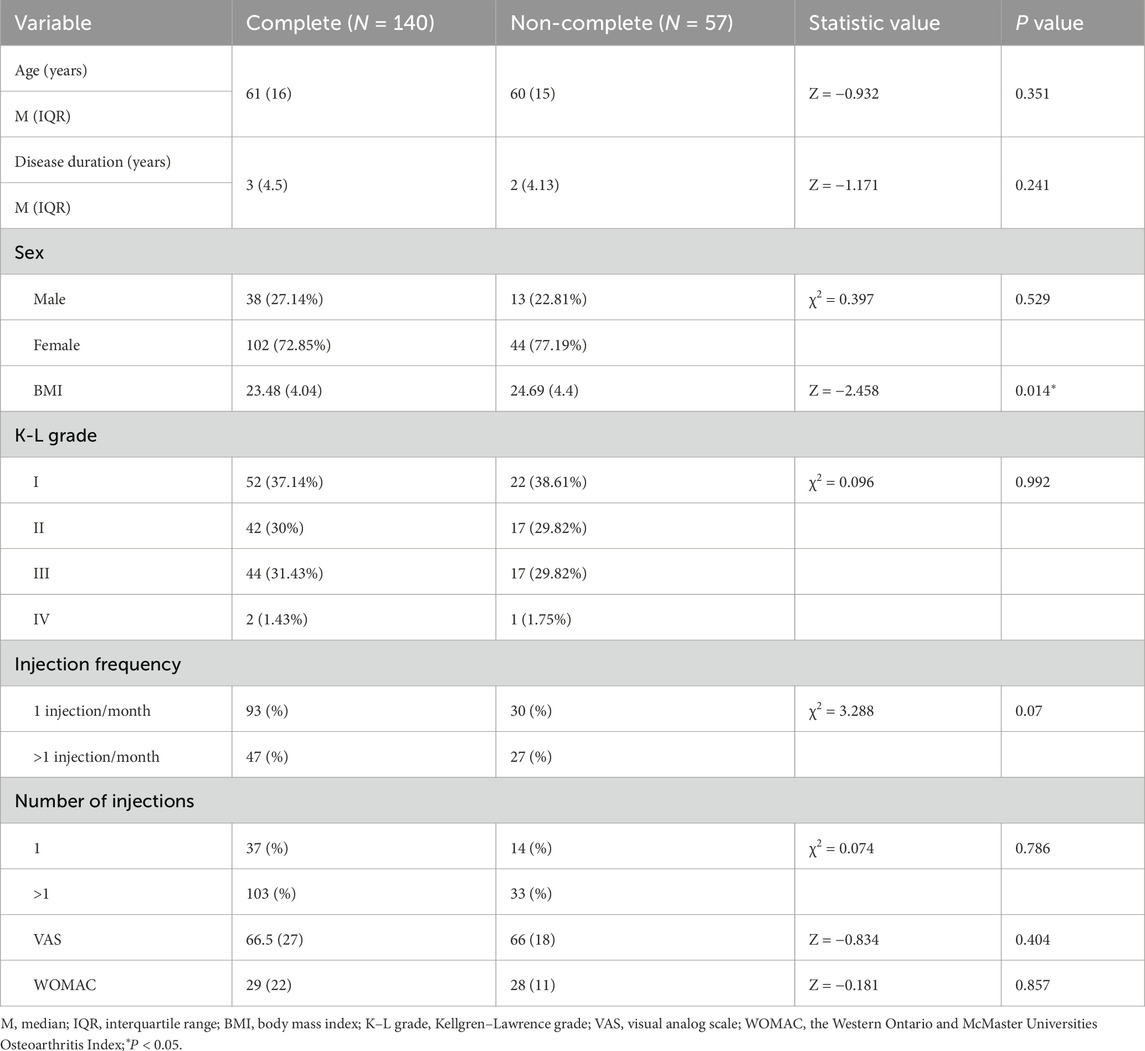
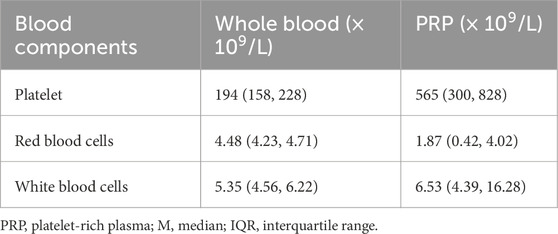
A total of 220 participants with KOA who received PRP injections participated in this trial. Overall, 23 participants were excluded at screening and 57 were lost to follow-up after 6 months. Finally, 140 individuals were included in this study (Figure 1). The baseline characteristics of those that completed the trial (completers) and those who did not (non-completers) are shown in Table 1. Among the participants, 102 (72.9%) were female and 38 (27.1%) were male. The mean age of the participants was 60.4 ± 10.7 years. The median disease duration was 3 years (IQR: 1.5–6.0 years). Based on the KL grade system, 52 participants (37.1%) were graded as level I, 42 individuals (30%) as level II, 44 individuals (34.1%) as level III, and 2 individuals (1.4%) as level IV. As shown in Table 2, the platelet concentration in the prepared PRP was approximately 2.91 times that of whole blood.
3.2 Within-group analysis
The median (IQR) VAS score decreased from 66.5 (Buendía-López et al., 2018) to 24 (Dhillon et al., 2017) at 6 months (Z = −10.159, r = −0.86, 95% CI = −38 to −30.5), p < 0.001). In terms of the WOMAC score, a significant improvement was observed as the score declined from 29 (Gobbi et al., 2015) to 12 (Bensa et al., 2025) after 6 months (Z = −9.790, r = −0.83, 95% CI = −16.5 to −12), p < 0.001).
3.3 PRP treatment effectiveness analysis
To investigate how baseline characteristics and PRP intervention parameters influenced treatment outcomes, the participants were divided into the effective and the ineffective groups based on the MCID criteria for VAS and WOMAC. Among the 140 individuals who received PRP treatment, 114 were classified as responders based on the VAS results, while 26 were classified as non-responders; regarding WOMAC, 109 were responders and 31 were non-responders.
3.3.1 Univariate analysis of PRP treatment effectiveness according to VAS
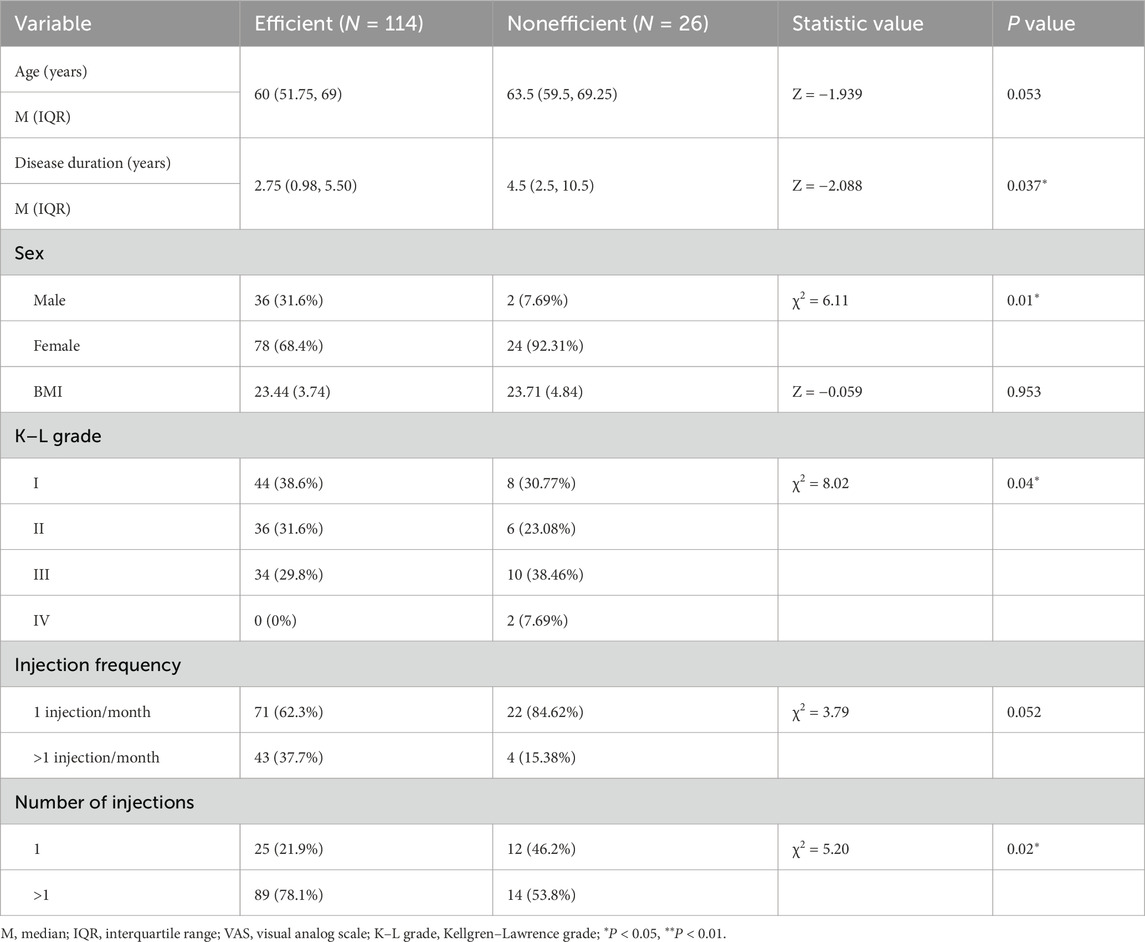
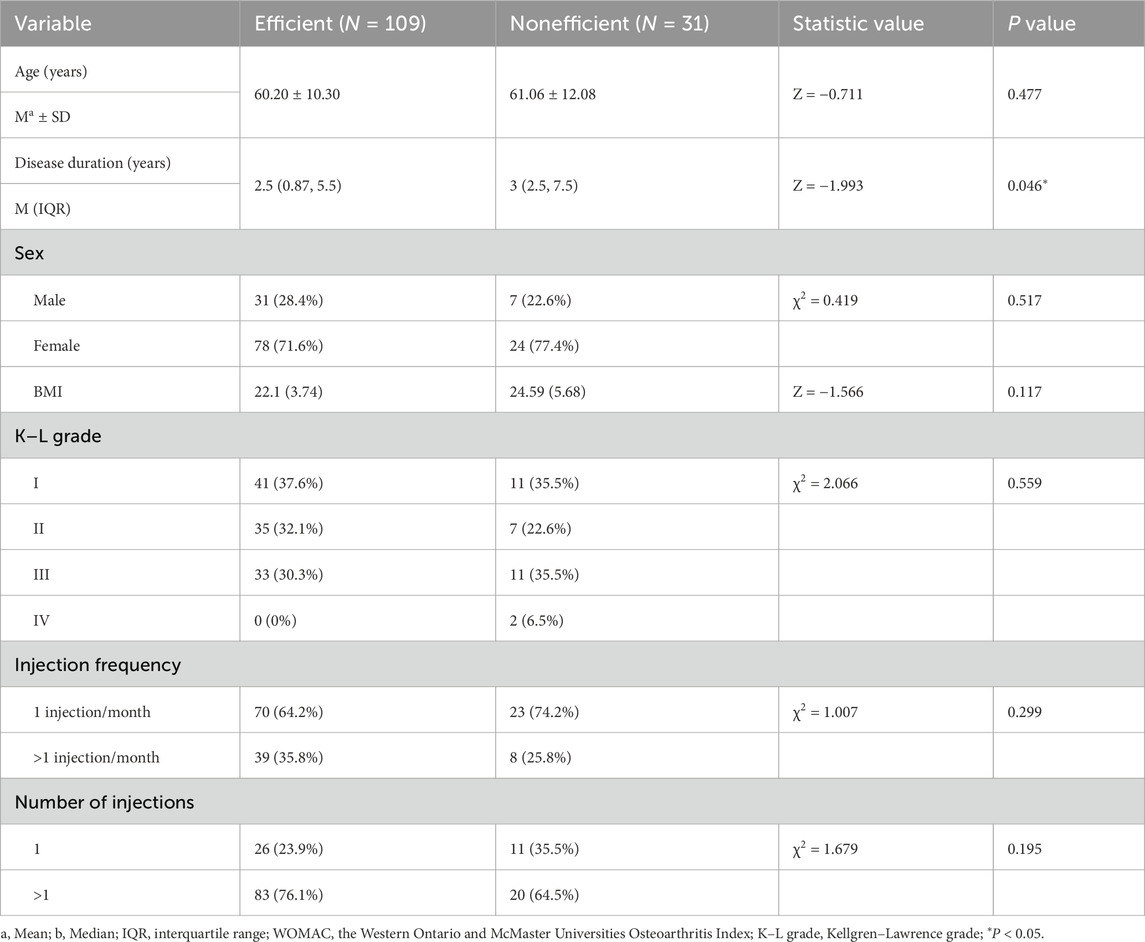
As shown in Table 3, significant differences were found between the effective and ineffective groups in terms of disease duration, sex distribution, KL grade, and number of injections. Participants in the effective group had a shorter disease duration (2.75 years, IQR: 0.98–5.50) compared to participants in the ineffective group (4.5 years, IQR: 2.5–10.5; Z = −2.088, P = 0.037). Regarding sex, within the effective group, 31.6% of the participants were male and 68.4% were female, while in the ineffective group, the corresponding proportions were 7.7% male and 92.3% female (χ2 = 6.11, P = 0.01). Specifically, 94.7% of male participants demonstrated a treatment response, compared to 76.5% of female participants. In terms of KL grade, a significant difference was found between the effective and ineffective groups (χ2 = 0.82, P = 0.04). In the effective group, 38.6% of the participants were classified as grade I, 31.6% as grade II, and 29.8% as grade III. In contrast, in the ineffective group, 30.8% were grade I, 23.1% were grade II, 38.5% were grade III, and 7.69% were grade IV. Additionally, the number of injections also appeared to exert a significant influence (χ2 = 5.2, P = 0.02). In the effective group, 21.9% of participants received a single injection, while 78.1% received repeated injections. Conversely, in the ineffective group, 46.2% received one injection and 53.8% received more than one injection. However, no significant differences were observed between the two groups in terms of age and injection frequency.
3.3.2 Multivariate analysis of PRP treatment effectiveness according to VAS
Univariate analysis showed that disease duration, sex, K-L grade, and number of injections influenced PRP treatment effectiveness according to VAS; however, further analysis of its effect using logistic regression analysis showed that only the number of injections (OR = 4.285, 95% CI: 1.586–11.578, p = 0.004) significantly affected PRP treatment effectiveness assessed using VAS scores (Figure 2). This suggests that repeated PRP injections are approximately four times more effective than a single injection in terms of VAS scores.
3.3.3 Univariate analysis of PRP treatment effectiveness according to WOMAC
As shown in Table 4, univariate analysis of WOMAC scores also indicated a significant difference in disease duration between the effective and ineffective groups. The effective group had a shorter disease duration (2.5 years, IQR: 0.87–5.5) compared to the ineffective group (3 years, IQR: 2.5–7.5; Z = −1.993, P = 0.046). No significant differences were found between the effective and ineffective groups in terms of age, sex, KL grade, injection frequency, and the number of injections.
3.3.4 Multivariate analysis of PRP treatment effectiveness according to WOMAC
Univariate analysis showed that only the disease duration influenced PRP treatment effectiveness according to WOAMC; however, further analysis of its effect using logistic regression analysis showed that BMI (OR = 0.867, 95% CI = 0.755–0.995, p = 0.042) and disease duration (OR = 0.905, 95% CI = 0.784–0.989, p = 0.045) significantly affected PRP treatment effectiveness on WOMAC scores (Figure 3). This revealed that for every 1 kg/m2 increase in BMI, the effectiveness of PRP treatment decreased by 13.3%. Similarly, in terms of disease duration, each 1-year increase was associated with a 9.5% reduction in the effectiveness of PRP injections on the WOMAC score. However, age, sex, K-L grade, number of injections of PRP, and injection frequency showed no significant difference.
3.4 Safety
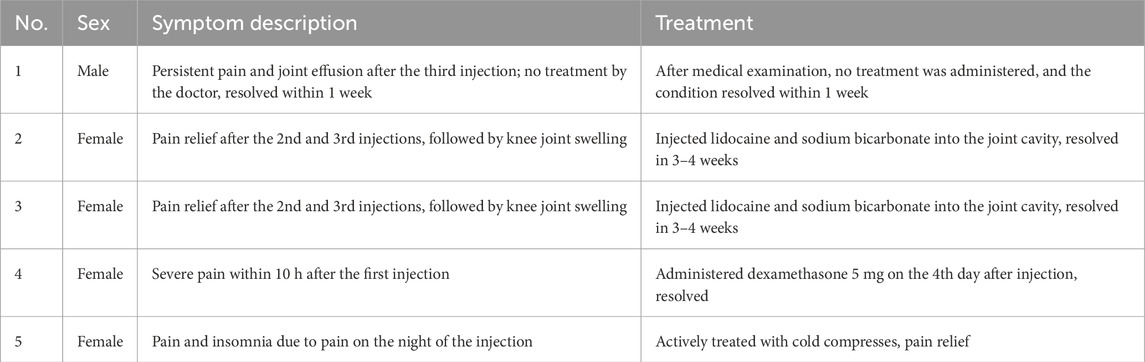
By the 6-month follow-up, four participants experienced a total of five adverse events associated with PRP intra-articular injections. The post-injection pain was effectively managed with lidocaine hydrochloride, sodium chloride solution, and diazepam, with the treatment durations ranging from immediate intervention to up to 4 weeks, depending on the characteristics and duration of the adverse reactions. The management of these adverse events is illustrated in Table 5.
3.5 Unchanged data before and after 6-month PRP injection
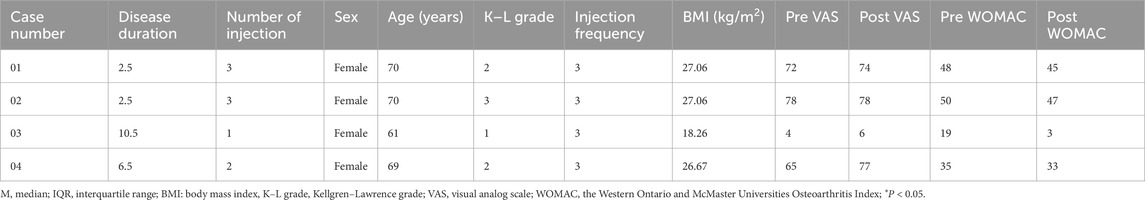
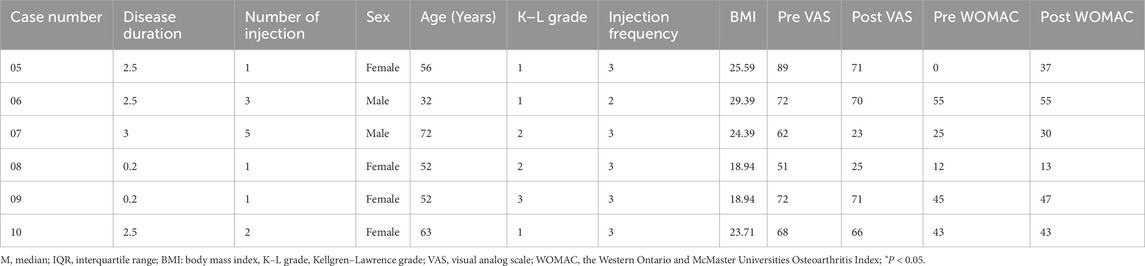
Among the 140 participants, four participants had no change in VAS after the intervention, and their information was listed in Table 6. Besides this, six participants had no change in WOMAC after PRP injection, and their information was listed in Table 7.
4 Discussion
This study evaluated the clinical effectiveness of PRP knee injections for KOA, observing significant pain reduction and joint function improvement within 6 months of follow-up, which is consistent with the findings of previous studies (Anil et al., 2021; Buendía-López et al., 2018; Elik et al., 2020; Riboh et al., 2015). Using the MCID, participants were divided into the “effective” and “noneffective” groups based on changes in their VAS and WOMAC scores. Both univariate and multivariate analyses were employed to investigate the factors that influence the effectiveness of PRP injection treatment. The results of our univariate analysis revealed that disease duration, sex, KL grade, and the number of injections administered significantly influenced the effectiveness of PRP treatment. However, multivariate analyses indicated that only the number of injections significantly affected pain levels, while disease duration and BMI influenced WOMAC outcomes. Specifically, PRP appeared to be more effective in pain management in individuals with shorter disease duration, lower BMI, and those who received repeated injections. This discrepancy between the univariate and multivariate analyses may be attributed to the interactions between variables and differences in model assumptions.
Khalilizad et al. (2025) and Vilchez-Cavazos et al. (2019) reported that repeated injections improved joint functionality more than a single injection at 6 months. Our findings are consistent with their observations. Our multivariate analysis, which accounted for potential confounding factors, showed that repeated PRP injections were about four times more effective than a single injection in reducing pain. This relief in pain may be due to the sustained release of growth factors from repeated injections, which promotes cartilage repair and reduces inflammation, thereby enhancing treatment efficacy. Moreover, BMI and disease duration significantly impacted WOMAC scores. These findings are consistent with those of previous studies (Dong et al., 2023; Bansal et al., 2021; Dhillon et al., 2017). In our study, for every 1 kg/m2 increase in BMI, the effectiveness of PRP decreased by 13.3%. Similarly, in terms of disease duration, each 1-year increase in disease duration was associated with a 9.5% reduction in the effectiveness of PRP injections on the WOMAC score. The influence of BMI on treatment outcomes may be related to the additional mechanical stress on the knee joint, which could exacerbate cartilage damage and diminish the effectiveness of PRP injections (Yu et al., 2025). Similarly, longer disease duration was associated with more severe cartilage degeneration, which may reduce the potential for repair and thus the effectiveness of PRP treatment (Kon et al., 2009; Kon et al., 2011).
However, in our multivariate analysis, age, sex, KL grade, and injection frequency did not significantly impact the efficacy of PRP treatment. Comparing our results with those previous studies revealed both consistencies and inconsistencies. Regarding age, it is widely recognized that cartilage repair capacity declines with age. However, the effectiveness of PRP may be more closely related to the local concentration of growth factors and the regulation of inflammation rather than to the patient’s age. This aligns with the findings of previous studies that suggest that PRP’s therapeutic potential can be maintained across different age groups due to its ability to enhance local healing factors (Chowdhary et al., 2022; Johnson et al., 2022). Our findings from our correlation analysis indicated an interaction effect with the number of injections. This suggests that the optimal therapeutic outcome may depend on a specific combination of these factors, which is consistent with the conclusions of other studies, highlighting the importance of tailored injection protocols (Tao et al., 2023; Khalilizad et al., 2025). Additionally, regarding KL grade, our analysis did not identify a significant impact on PRP efficacy. One possible reason is that KL grade reflects the structural severity of KOA; however, individuals with the same structural severity can exhibit varying levels of knee function due to individual differences (Sonobe et al., 2024). Therefore, the clinical response to PRP may not be directly correlated with radiographic findings. In contrast, our results for sex differed from those of previous studies. According to a previous research, sex may influence the efficacy of PRP; however, some studies indicate that the response of female subjects to PRP treatment when differ from that of male subjects (Li et al., 2025). Our analysis did not find a significant impact of sex on PRP efficacy. This discrepancy could be attributed to differences in study populations, sample sizes, or the specific methods used to assess PRP effectiveness. Further investigation is warranted to elucidate the role of sex in PRP treatment outcomes.
In terms of cost-effectiveness, PRP injections are relatively inexpensive (Alcerro and Lavernia, 2019; Carducci et al., 2019) compared to joint arthroplasty (Jones et al., 2018) or stem cell therapy (Bendich et al., 2020). We reviewed previous studies that reported on the costs of single and multiple injections, as well as the costs of PRP and hyaluronic acid. Wang et al. (2022) and Karabas and Tezcan (2025) found that single injections of PRP and hyaluronic acid had similar efficacy in improving outcomes. Conversely, Park et al. (2021) demonstrated that single-dose PRP injections were more effective in treating early-stage knee osteoarthritis than hyaluronic acid. Furthermore, Görmeli et al. (2015) reported that repeated PRP injections appeared to be more effective in the long-term management of pain and function compared to single injections of PRP or hyaluronic acid. Despite these findings, few studies have examined the cost-effectiveness of repeated PRP injections, particularly the improvement in pain and function relative to the financial investment when increasing from two to three injections. Further research is needed to determine the value of repeated PRP injection, weighing its added benefits against the increased cost.
4.1 Limitations
This study has several limitations. First, the absence of a control group limits our ability to compare the efficacy of PRP injections with other treatment methods, potentially introducing bias. Second, individuals’ compliance with the recommendation to avoid medications for up to 6 months could not be precisely controlled, which may have introduced co-intervention bias. Third, as a single-center trial, our study may be subject to center bias. Multicenter randomized controlled trials are needed to minimize selection bias and confounding factors, as well as to enhance the causal inference of the results.
5 Conclusion
This retrospective study analyzed the effectiveness of PRP treatment in individuals with KOA. Our findings showed that the effectiveness of PRP injections for treating KOA was influenced by multiple factors, including the number of injections, BMI, and disease duration. These findings highlight the importance of considering these variables when designing treatment protocols and suggested that personalized treatment strategies may be necessary to optimize outcomes. Future research should further explore the underlying mechanisms and interactions between these factors to refine PRP treatment protocols and improve clinical efficacy.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: In this study, strict restrictions have been implemented on the dataset to ensure the privacy and data security of the participants. All personally identifiable information has been anonymized, and access to the dataset is limited to authorized researchers who are bound by confidentiality agreements. The use of the dataset is strictly confined to the purposes of this study and has been approved by the relevant ethics review committee. To prevent data breaches, we have implemented advanced data security measures. Furthermore, sharing the dataset with third parties is strictly prohibited without the written consent of the data custodian and the ethics review committee. These measures are designed to safeguard the integrity of the research and respect the rights and privacy of the participants. Requests to access these datasets should be directed toaHhrZmhoY0AxMjYuY29t.
Ethics statement
The studies involving humans were approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
Y-TS: Writing – review and editing, Data curation, Formal Analysis, Writing – original draft. X-NX: Formal Analysis, Writing – review and editing, Writing – original draft, Data curation. JY: Data curation, Writing – review and editing, Formal Analysis. J-LP: Data curation, Writing – review and editing. H-CH: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Cheng-Qi He, PhD, and Quan Wei, PhD (all from West China Hospital, Sichuan University), for administrative assistance; Jing-Wen Jiang, PhD, (from West China Hospital, Sichuan University), for statistical method guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1678037/full#supplementary-material
Abbreviations
KOA, Knee osteoarthritis; PRP, Platelet-rich plasma; OA, Osteoarthritis; MCID, Minimal Clinically Important Difference; VAS, the Visual Analog Scale; WOMAC, the Western Ontario and McMaster Universities Osteoarthritis Index; BMI, Body Mass Index; KL, Kellgren-Lawrence; HIS, the electronic medical system record; IQR, Medians and interquartile ranges.
References
Ahmad H., Halim H. (2017). Determining sample size for research activities: the case of organizational research. Selangor Bus. Rev., 20–34.
Alcerro J. C., Lavernia C. J. (2019). Stem cells and platelet-rich plasma for knee osteoarthritis: prevalence and cost in south Florida. J. Am. Acad. Orthop. Surg. 27 (20), 779–783. doi:10.5435/JAAOS-D-18-00343
Anil U., Markus D. H., Hurley E. T., Manjunath A. K., Alaia M. J., Campbell K. A., et al. (2021). The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee 32, 173–182. doi:10.1016/j.knee.2021.08.008
Arden N. K., Perry T. A., Bannuru R. R., Bruyère O., Cooper C., Haugen I. K., et al. (2020). Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 17 (1), 59–66. doi:10.1038/s41584-020-00523-9
Bansal H., Leon J., Pont J. L., Wilson D. A., Bansal A., Agarwal D., et al. (2021). Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci. Rep. 11 (1), 3971. doi:10.1038/s41598-021-83025-2
Bendich I., Rubenstein W. J., Cole B. J., Ma C. B., Feeley B. T., Lansdown D. A. (2020). What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from level I randomized controlled trials. Arthroscopy 36 (7), 1983–1991.e1. doi:10.1016/j.arthro.2020.02.004
Bensa A., Previtali D., Sangiorgio A., Boffa A., Salerno M., Filardo G. (2025). PRP injections for the treatment of knee osteoarthritis: the improvement is clinically significant and influenced by platelet concentration: a meta-analysis of randomized controlled trials. Am. J. Sports Med. 53 (3), 745–754. doi:10.1177/03635465241246524
Buendía-López D., Medina-Quirós M.Fernández-Villacañas Marín MÁ (2018). Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J. Orthop. Traumatol. 19 (1), 3. doi:10.1186/s10195-018-0501-3
Carducci M. P., Gasbarro G., Menendez M. E., Mahendraraj K. A., Mattingly D. A., Talmo C., et al. (2019). Variation in the cost of care for different types of joint arthroplasty. J. Bone Jt. Surg. Am. 102 (5), 404–409. doi:10.2106/JBJS.19.00164
Chowdhary K., Sahu A., Iijima H., Shinde S., Borg-Stein J., Ambrosio F. (2022). Aging affects the efficacy of platelet-rich plasma treatment for osteoarthritis. Am. J. Phys. Med. Rehabil. 102 (7), 597–604. doi:10.1097/PHM.0000000000002161
Costa L. A. V., Lenza M., Irrgang J. J., Fu F. H., Ferretti M. (2022). How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am. J. Sports Med. 51 (4), 1074–1086. doi:10.1177/03635465211062243
Dhillon M. S., Patel S., John R. (2017). PRP in OA knee – update, current confusions and future options. SICOT J. 3, 27. doi:10.1051/sicotj/2017004
Dong Y., Yan Y., Zhou J., Zhou Q., Wei H. (2023). Evidence on risk factors for knee osteoarthritis in middle-older aged: a systematic review and meta analysis. J. Orthop. Surg. Res. 18 (1), 634. doi:10.1186/s13018-023-04089-6
Du D., Liang Y. (2025). A meta-analysis and systematic review of the clinical efficacy and safety of platelet-rich plasma combined with hyaluronic acid (PRP + HA) versus PRP monotherapy for knee osteoarthritis (KOA). J. Orthop. Surg. Res. 20 (1), 57. doi:10.1186/s13018-024-05429-w
Elik H., Doğu B., Yılmaz F., Begoğlu F. A., Kuran B. (2020). The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J. Back Musculoskelet. 33 (1), 127–138. doi:10.3233/BMR-181374
Fice M. P., Miller J. C., Christian R., Hannon C. P., Smyth N., Murawski C. D., et al. (2019). The role of platelet-rich plasma in cartilage pathology: an updated systematic review of the basic science evidence. Arthroscopy 35 (3), 961–976.e3. doi:10.1016/j.arthro.2018.10.125
Gobbi A., Lad D., Karnatzikos G. (2015). The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 23 (8), 2170–2177. doi:10.1007/s00167-014-2987-4
Görmeli G., Görmeli C. A., Ataoglu B., Çolak C., Aslantürk O., Ertem K. (2015). Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 25 (3), 958–965. doi:10.1007/s00167-015-3705-6
Gupta S., Paliczak A., Delgado D. (2020). Evidence-based indications of platelet-rich plasma therapy. Expert Rev. Hematol. 14 (1), 97–108. doi:10.1080/17474086.2021.1860002
Hmamouchi I., Allali F., Tahiri L., Khazzani H., Mansouri L. E., Ali Ou Alla S., et al. (2012). Clinically important improvement in the WOMAC and predictor factors for response to non-specific non-steroidal anti-inflammatory drugs in osteoarthritic patients: a prospective study. BMC Res. Notes 5 (1), 58. doi:10.1186/1756-0500-5-58
Hohmann E., Tetsworth K., Glatt V. (2020). Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 30 (6), 955–967. doi:10.1007/s00590-020-02623-4
Johnson D. S., Dhiman N., Badhal S., Wadhwa R. (2022). Effects of intra-articular platelet rich plasma on cartilage thickness, clinical and functional outcomes in knee osteoarthritis. Cureus 14 (12), e32256. doi:10.7759/cureus.32256
Jones I. A., Togashi R. C., Thomas Vangsness C. (2018). The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr. Rev. Musculoskelet. Med. 11 (4), 558–565. doi:10.1007/s12178-018-9514-z
Karabas C., Tezcan E. A. (2025). Comparative analysis of single-dose platelet-rich plasma and hyaluronic acid therapies in knee osteoarthritis: a 12-week follow-up study. North Clin. Istanb. 12 (2), 204–210. doi:10.14744/nci.2024.89587
Karaborklu A. S., Celik D., Naci Ergin O. N., Kilicoglu O. I. (2023). Factors affecting the features of platelet-rich plasma in patients with knee osteoarthritis. Acta Orthop. Traumato 57 (4), 148–153. doi:10.5152/j.aott.2023.22077
Khalilizad M., Emadian S. T., Marzban Abbas Abadi M. (2025). Comparative efficacy of different doses of platelet-rich plasma injection in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. J. Orthop. Surg. Res. 20 (1), 221. doi:10.1186/s13018-025-05650-1
Kolasinski S. L., Neogi T., Hochberg M. C., Oatis C., Guyatt G., Block J., et al. (2020). 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72 (2), 220–233. doi:10.1002/art.41142
Kon E., Buda R., Filardo G., Di Martino A., Timoncini A., Cenacchi A., et al. (2009). Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 18 (4), 472–479. doi:10.1007/s00167-009-0940-8
Kon E., Mandelbaum B., Buda R., Filardo G., Delcogliano M., Timoncini A., et al. (2011). Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27 (11), 1490–1501. doi:10.1016/j.arthro.2011.05.011
Lee J. S., Hobden E., Stiell I. G., Wells G. A. (2003). Clinically important change in the visual analog scale after adequate pain control. Acad. Emerg. Med. 10 (10), 1128–1130. doi:10.1111/j.1553-2712.2003.tb00586.x
Li E., Tan J., Xu K., Pan Y., Xu P. (2024). Global burden and socioeconomic impact of knee osteoarthritis: a comprehensive analysis. Front. Med. 11, 1323091. doi:10.3389/fmed.2024.1323091
Li X., Dong Y., Liu J., He W., Yan C., Zhang J. (2025). Efficacy of arthroscopic cartilage transplantation combined with platelet-rich plasma in the treatment of early knee osteoarthritis: a retrospective cohort study. Langenbeck Arch. Surg. 410 (1), 122. doi:10.1007/s00423-025-03690-z
Liu Y., Xiang X. N., Wang Q., He H. C. (2023). A comparison of different physical stimulation combined with platelet-rich plasma for the treatment of knee osteoarthritis: study protocol for a randomized controlled trial. Trials 24 (1), 200. doi:10.1186/s13063-023-07228-w
Magruder M. L., Caughey S., Gordon A. M., Capotosto B. S., Salvatore S., Rodeo S. A. (2023). Trends in utilization, demographics, and costs of platelet-rich plasma injections: a ten-year nationwide investigation. Physician Sportsmed. 52 (1), 89–97. doi:10.1080/00913847.2023.2178816
McAlindon T. E., Bannuru R. R., Sullivan M. C., Arden N. K., Berenbaum F., Bierma-Zeinstra S. M., et al. (2014). OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 22 (3), 363–388. doi:10.1016/j.joca.2014.01.003
Park Y. B., Kim J. H., Ha C. W., Lee D. H. (2021). Clinical efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: a randomized double-blind controlled clinical trial as compared with hyaluronic acid. Am. J. Sports Med. 49 (2), 487–496. doi:10.1177/0363546520986867
Patel S., Dhillon M. S., Aggarwal S., Marwaha N., Jain A. (2013). Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am. J. Sports Med. 41 (2), 356–364. doi:10.1177/0363546512471299
Riboh J. C., Saltzman B. M., Yanke A. B., Fortier L., Cole B. J. (2015). Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am. J. Sports Med. 44 (3), 792–800. doi:10.1177/0363546515580787
Saraf A., Hussain A., Singhal A., Arora V., Bishnoi S. (2023). Do age, gender, BMI and disease duration influence the clinical outcomes in patients of knee osteoarthritis treated with serial injections of autologous platelet rich plasma? J. Clin. Orthop. Trauma 43, 102226. doi:10.1016/j.jcot.2023.102226
Sonobe T., Otani K., Sekiguchi M., Otoshi K., Nikaido T., Konno S., et al. (2024). Influence of knee osteoarthritis severity, knee pain, and depression on physical function: a cross-sectional study. Clin. Interv. Aging 19, 1653–1662. doi:10.2147/CIA.S470473
Tao X., Aw A. A. L., Leeu J. J., Bin Abd Razak H. R. (2023). Three doses of platelet-rich plasma therapy are more effective than one dose of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthroscopy 39 (12), 2568–2576.e2. doi:10.1016/j.arthro.2023.05.018
Vilchez-Cavazos F., Millán-Alanís J. M., Blázquez-Saldaña J., Álvarez-Villalobos N., Peña-Martínez V. M., Acosta-Olivo C. A., et al. (2019). Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop. J. Sports Med. 7 (12), 2325967119887116. doi:10.1177/2325967119887116
Wang Y. C., Lee C. L., Chen Y. J., Tien Y. C., Lin S. Y., Chen C. H., et al. (2022). Comparing the efficacy of intra-articular single platelet-rich plasma (PRP) versus novel crosslinked hyaluronic acid for early-stage knee osteoarthritis: a prospective, double-blind, randomized controlled trial. Med. Lith. 58 (8), 1028. doi:10.3390/medicina58081028
Wang R., Xie Y., Xie L., Liu J., Jia J., Chen X., et al. (2024). Platelet-rich plasma versus corticosteroid in the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Georgian Med. News (349), 169–182.
Werner B. C., Cancienne J. M., Browning R., Verma N. N., Cole B. J. (2020). An analysis of current treatment trends in platelet-rich plasma therapy in the medicare database. Orthop. J. Sports Med. 8 (2), 2325967119900811. doi:10.1177/2325967119900811
Xiong G., Lingampalli N., Koltsov J. C. B., Leung L. L., Bhutani N., Robinson W. H., et al. (2017). Men and women differ in the biochemical composition of platelet-rich plasma. Am. J. Sports Med. 46 (2), 409–419. doi:10.1177/0363546517740845
Yu H., Wang J., Xu X., Li H., Guo J. (2025). Revealing the mediating mechanisms between BMI and osteoarthritis: a mendelian randomization and mediation analysis. Aging Clin. Exp. Res. 37 (1), 119. doi:10.1007/s40520-025-03035-2
Keywords: platelet-rich plasma, knee osteoarthritis, visual analog scale, WOMAC, treatment outcomes, predictors
Citation: Sun Y-T, Xiang X-N, Yang J, Peng J-L and He H-C (2025) Platelet-rich plasma improves pain and function in knee osteoarthritis: a retrospective study. Front. Physiol. 16:1678037. doi: 10.3389/fphys.2025.1678037
Received: 01 August 2025; Accepted: 14 October 2025;
Published: 29 October 2025.
Edited by:
Christina Maria Pabelick, Mayo Clinic, United StatesReviewed by:
Marc Henri De Longueville, UCB Pharma, BelgiumEzgi Akyildiz Tezcan, Selcuk University, Türkiye
Copyright © 2025 Sun, Xiang, Yang, Peng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Chen He, aHhrZmhoY0AxMjYuY29t
†These authors have contributed equally to this work
 Yi-Ting Sun
Yi-Ting Sun Xiao-Na Xiang
Xiao-Na Xiang Jie Yang4†
Jie Yang4† Hong-Chen He
Hong-Chen He