- 1Engineering Research Center of Sports Health Intelligent Equipment of Hubei Province, Wuhan Sports University, Wuhan, Hubei, China
- 2Department of Rehabilitation Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 3Beijing University of Chinese Medicine Shenzhen Hospital (Long gang), Shenzhen, Guangdong, China
Objectives: To evaluate whether transcranial direct current stimulation (tDCS) combined with exercise therapy enhances balance ability in chronic ankle instability (CAI) individuals.
Methods: Systematic searches were conducted in PubMed, Embase, Web of Science, Cochrane Library, and Scopus databases up to July 10, 2025. The Cochrane Risk of Bias tool version 2. Was used to assess the methodological quality of studies meeting the inclusion criteria. Meta-analyses were performed using random-effects models, with results expressed as standardized mean differences (SMD) and 95% confidence intervals (95% CI). Evidence quality was evaluated using the GRADE methodology.
Results: Eight studies involving 216 participants were included in the meta-analysis. Overall analysis revealed that tDCS combined with exercise therapy did not significantly improve dynamic balance (SMD: 0.08, 95% CI: −0.36 to 0.52, P = 0.72) or static balance (SMD: −0.53, 95% CI: −1.08 to 0.02, P = 0.06) in individuals with CAI. Subgroup analysis by exercise type showed that tDCS combined with non-balance training significantly enhanced dynamic balance ability (SMD: 0.52, 95% CI: 0.07 to 0.97, P = 0.02), while tDCS combined with balance training showed no significant improvements in either composite dynamic balance measures (SMD: −0.26, 95% CI: −0.82 to 0.30, P = 0.36) or Y-balance reach distances in any direction (P > 0.05 for all directions).
Conclusion: tDCS provides therapeutic benefits for dynamic balance only when combined with non-balance exercises. Current evidence is insufficient to demonstrate improvements in static balance with tDCS adjunctive therapy.
1 Introduction
Lateral ankle sprains are among the most common types of sports injuries, with approximately 70% of first-time sprain patients eventually developing chronic ankle instability (CAI) (Herzog et al., 2019). Individuals with CAI frequently experience symptoms such as recurrent ankle “giving way,” repeated sprains, functional limitations, and diminished balance ability, which significantly impact daily activities and athletic performance (Gribble et al., 2016; Thompson et al., 2016). Extensive research has confirmed that exercise therapy can improve balance control and reduce re-injury risk in individuals with CAI by enhancing ankle proprioception and ankle muscle strength, making it the preferred conservative treatment approach for CAI (Yang et al., 2025; Zhang et al., 2025a). Recent studies have revealed that CAI development is not solely related to local ligament and proprioceptive damage, but also involves adaptive changes within the central nervous system (Maricot et al., 2023; Li et al., 2025). These changes are specifically characterized by decreased neural excitability at the spinal and/or cortical levels, which subsequently leads to altered muscle activation patterns in the lower extremity and compensatory biomechanical adaptations (Suttmiller and McCann, 2020; Li et al., 2025). However, traditional exercise therapy has certain limitations in promoting central nervous system remodeling and improving cortical excitability.
As a non-invasive neuromodulation technique, transcranial direct current stimulation (tDCS) employs weak direct electrical currents delivered between electrodes placed on the cerebral cortex. This technique modulates cortical excitability by altering neuronal resting membrane potentials, thereby promoting neuroplasticity and enhancing motor learning capacity (Auvichayapat and Auvichayapat, 2011; Chase et al., 2020). In rehabilitation of neurological conditions such as Parkinson’s disease, tDCS has been proven effective as an adjunct to conventional rehabilitation, further enhancing patients’ balance and postural stability (Nguyen et al., 2024). For individuals with CAI, researchers have attempted to combine exercise therapy with tDCS to explore the comprehensive therapeutic effects. However, current research findings remain inconsistent, Ma et al. found that combining tDCS with short-foot exercises improved dynamic balance ability in CAI individuals compared to short-foot exercises alone (Ma et al., 2020), while Kim et al. revealed that tDCS combined with active joint mobilization training did not provide significant additional clinical benefits (Kim et al., 2025).
Given the inconsistent research results regarding tDCS combined with exercise therapy for CAI treatment and the lack of systematic synthesis, this study aims to comprehensively evaluate the therapeutic efficacy of tDCS combined with exercise therapy versus exercise therapy alone in improving balance ability in CAI individuals using systematic review and meta-analysis methodology. By integrating existing research evidence, we seek to determine whether tDCS can serve as an effective adjunctive treatment to further enhance patients’ balance ability, while analyzing key factors that may influence treatment outcomes to provide reliable evidence-based guidance for clinical practice.
2 Materials and methods
This study adhered rigorously to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook (Cumpston et al., 2019; Page et al., 2021). The study protocol has been registered on the PROSPERO platform (CRD420251110630).
2.1 Search strategy
Two researchers (ZJW and CRX) independently conducted searches in PubMed, Embase, Web of Science, Cochrane Library, and Scopus databases. The search was conducted up to July 10th. The specific search strategies for each database are provided in Supplementary Table S1.
2.2 Eligibility criteria
Inclusion criteria: 1) Participants were individuals with CAI as defined by the Intentional Ankle Consortium statement; 2) The intervention group received tDCS combined with any form of exercise therapy, while the control group underwent exercise therapy alone or exercise therapy supplemented with sham tDCS intervention; 3) Outcome measures included balance ability assessments; 4) Studies were randomized controlled trials. Exclusion criteria: 1) Publications in languages other than English; 2) Animal studies and conference abstracts.
2.3 Study selection and data extraction
Screening of the retrieved studies was conducted according to predetermined inclusion and exclusion criteria through a systematic examination of study titles, abstracts, and complete manuscripts. The following basic characteristics were extracted from studies that satisfied the final criteria: 1. First author, country, and publication year; 2. Number of participants, gender, and age; 3. Intervention methods for experimental and control groups, including intervention duration and frequency; 4. Outcome measures and measurement timepoints; 5. tDCS electrode placement, stimulation intensity, stimulation duration, sham tDCS protocol, and adverse events; 6. Changes in balance assessment parameters following intervention were measured using multiple indicators: the dynamic postural stability indices (DPSI) and Y balance test (YBT) for evaluating dynamic balance capabilities, and center of pressure (COP) displacement and balance error scoring system (BESS) for assessing static balance performance. Two researchers (ZJW, CRX) performed all operations independently, with conflicts resolved through consensus discussion involving a third reviewer (WSF).
When studies did not provide complete data, emails were sent to corresponding authors to request the missing information. For studies presenting data in graphical format, data extraction was performed using GetData software (V2.26).
2.4 Bias risk assessment
Two researchers (ZJW, CRX) independently assessed the risk of bias for each included study using the Cochrane Risk of Bias tool version 2 (RoB 2) (Sterne et al., 2019). This assessment tool evaluates five critical domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Each domain was rated as low risk, some concerns, or high risk of bias. Any disagreements between the two assessors were resolved through consultation with a third researcher (WSF).
2.5 Certainty of evidence
Two independent researchers (ZJW, CRX) conducted evidence quality assessment of the meta-analysis results using GRADEpro software. Five domains were evaluated: bias risk, inconsistency, indirectness, imprecision, and publication bias, leading to evidence quality ratings of high, moderate, low, or very low (Guyatt et al., 2011). A third researcher (WSF) resolved any assessment disagreements.
2.6 Statistical analysis
Review Manager V.5.3 software was utilized to perform the meta-analysis, analyzing the mean and standard deviation (SD) of baseline-to-post-intervention changes for both the intervention and control groups in each study. When required data were unavailable in the original publications, calculations were undertaken using methods derived from previous research approaches (Ye et al., 2023). Due to variations in intervention protocols and measurement units among the included studies, all outcome measures were synthesized using a random-effects model and expressed as standardized mean differences (SMD) with 95% confidence intervals (CI) (Borenstein et al., 2010; Andrade, 2020). Specifically, Hedges’ g was used to calculate the SMD, with effect sizes interpreted as follows: >0.8 indicating a large effect, 0.5–0.8 a medium effect, 0.2–0.5 a small effect, and <0.2 a trivial effect (Wu et al., 2021). The I2 statistic assessed between-study heterogeneity: I2 < 30% (no heterogeneity), 30%–50% (moderate heterogeneity), 50%–75% (substantial heterogeneity), and >75% (considerable heterogeneity) (Cumpston et al., 2019). To examine result stability, sensitivity analysis was conducted by excluding studies individually and assessing how these removals affected the calculated effect sizes and their statistical significance (Viechtbauer and Cheung, 2010). Statistical significance was defined as P < 0.05 for the overall effect.
Given that balance training has been proven to significantly improve balance function in individuals with CAI (Guo et al., 2024), subgroup analyses were further conducted based on the type of exercise intervention to explore the differential effects of tDCS combined with different exercise modalities on balance ability in CAI, specifically categorized into tDCS combined with balance training group and tDCS combined with non-balance training group. Additionally, given that the YBT involves three testing directions, subgroup analyses were also performed based on YBT directions (anterior, posteromedial, and posterolateral) to further evaluate the direction-specific effects of tDCS on dynamic balance ability in individuals with CAI.
3 Results
3.1 Study selection
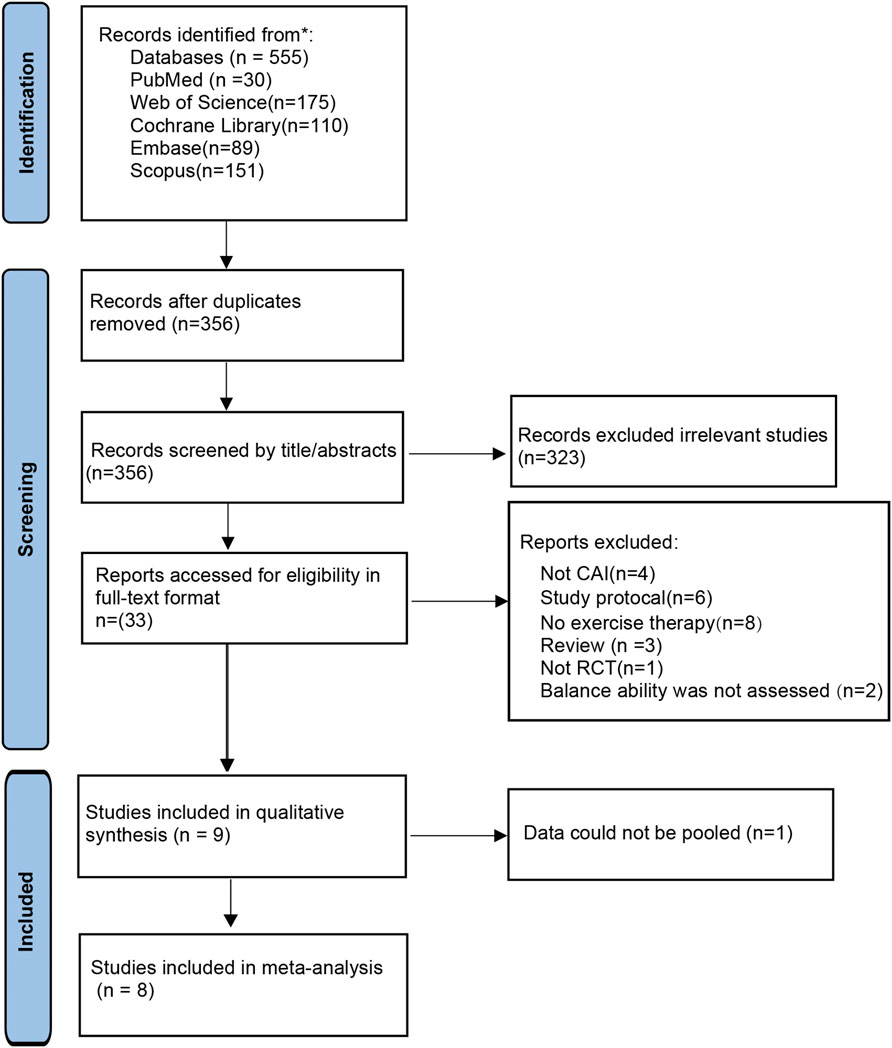
The initial search across five databases generated 555 studies, which was reduced to 356 studies after removing duplicates. Following the application of inclusion and exclusion criteria through title and abstract screening, followed by full-text review, 9 studies were included for qualitative analysis (Bruce et al., 2020; Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b). One study was excluded from quantitative synthesis due to incompatible data (Beyraghi et al., 2025), resulting in 8 studies being included in the meta-analysis (Bruce et al., 2020; Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Ge et al., 2025; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b). The detailed selection process is illustrated in Figure 1.
3.2 Characteristics of the included studies
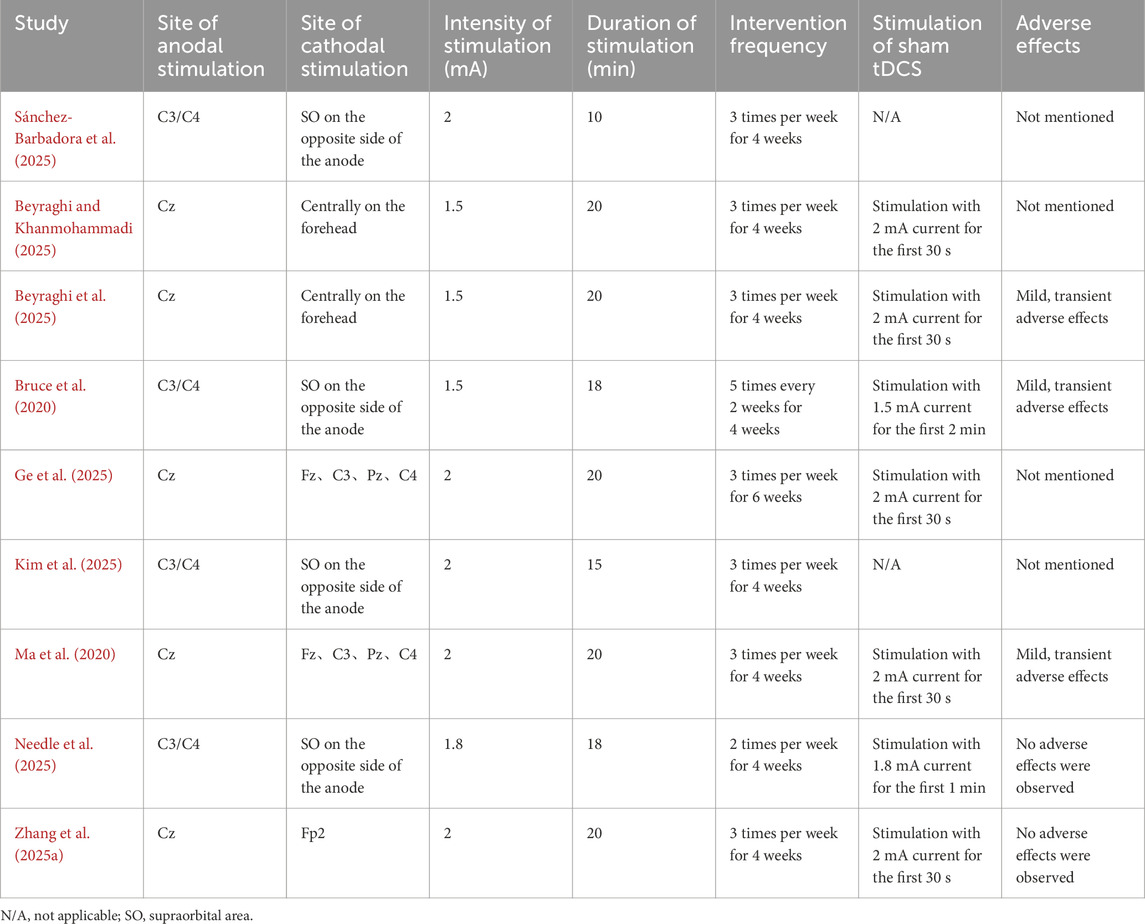
Among the included studies, a total of 248 individuals with CAI were included. The exercise therapy varied across studies: six studies implemented balance training (Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b), one focused on short foot exercises (Ma et al., 2020), one utilized active joint mobilization techniques (Kim et al., 2025), and one employed ankle strengthening exercises (Bruce et al., 2020). Regarding control group interventions, two studies used exercise therapy alone (Kim et al., 2025; Sánchez-Barbadora et al., 2025), while the remaining seven studies employed exercise therapy combined with sham tDCS (Bruce et al., 2020; Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025; Needle et al., 2025; Zhang et al., 2025b). For outcome measures, seven studies assessed dynamic balance ability (Bruce et al., 2020; Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b), with five utilizing the YBT (Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Kim et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b) and two employing the DPSI (Bruce et al., 2020; Needle et al., 2025). Two studies evaluated static balance using COP sway measures (Ge et al., 2025; Kim et al., 2025), while one study evaluated static balance ability using the BESS (Zhang et al., 2025b), and one study assessed postural control during gait using COP sway amplitude (Beyraghi et al., 2025) (Table 1).
Concerning tDCS parameters, two studies utilized transcranial direct current stimulation (HD-tDCS) (Ma et al., 2020; Ge et al., 2025), five studies positioned the anode at Cz (Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025; Zhang et al., 2025b), while others placed it at C3 or C4 (Bruce et al., 2020; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025). Stimulation intensity ranged from 1.5 to 2 mA, with session durations of 10–20 min. One study conducted interventions for 6 weeks (Ge et al., 2025), while all others implemented 4-week intervention periods (Bruce et al., 2020; Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b). For sham stimulation protocols, five studies applied current only during the initial 30 s (Ma et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025; Zhang et al., 2025b), one study used 2 min of initial stimulation (Bruce et al., 2020), and one employed 1 min of initial current application (Needle et al., 2025). Regarding adverse effects, two studies explicitly reported no adverse events during interventions (Needle et al., 2025; Zhang et al., 2025b), three studies indicated only mild and transient side effects (Bruce et al., 2020; Ma et al., 2020; Beyraghi et al., 2025), while four studies did not mention adverse effects (Beyraghi and Khanmohammadi, 2025; Ge et al., 2025; Kim et al., 2025; Sánchez-Barbadora et al., 2025) (Table 2).
3.3 Risk of bias assessment
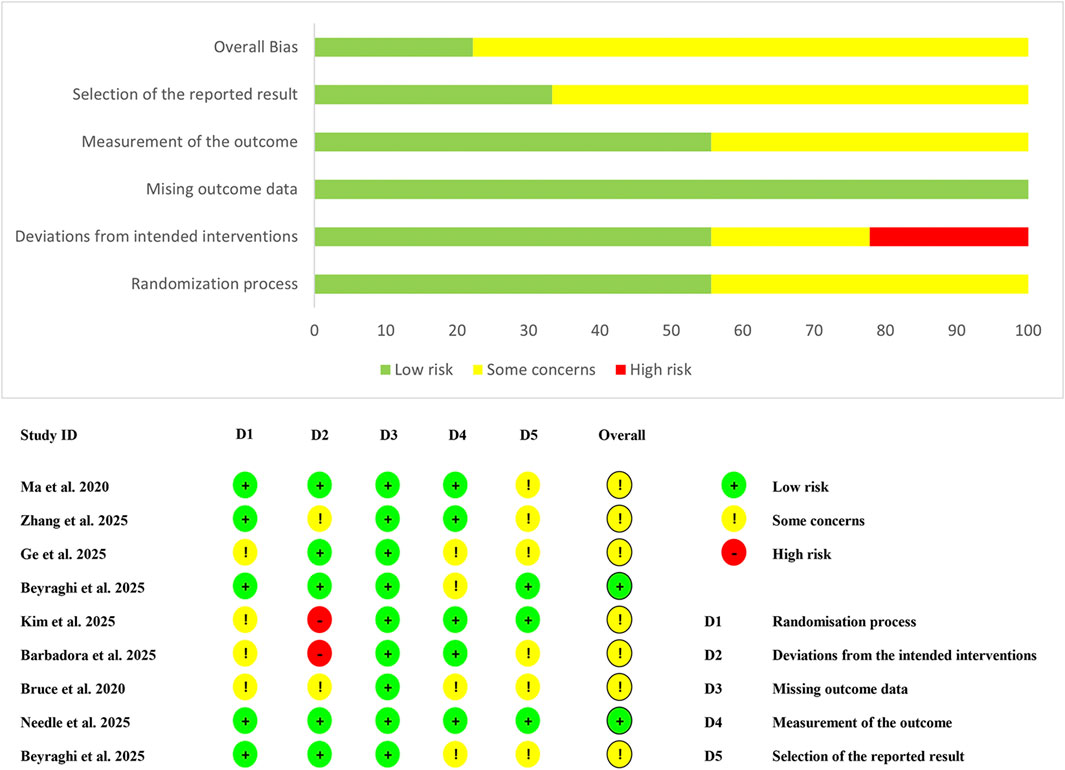
Figure 2 shows the results of the risk of bias assessment for the included studies. For the randomization process domain, four studies failed to adequately describe their random allocation procedures and were rated as “some concerns” (Bruce et al., 2020; Ge et al., 2025; Kim et al., 2025; Sánchez-Barbadora et al., 2025), while the remaining studies demonstrated low risk. Regarding deviations from intended interventions, two studies were classified as high risk due to lack of blinding for both participants and intervention providers (Kim et al., 2025; Sánchez-Barbadora et al., 2025), and two additional studies received “some concerns” ratings for inadequate blinding of intervention providers (Bruce et al., 2020; Zhang et al., 2025b). All studies demonstrated low risk for missing outcome data. In the measurement of outcomes domain, four studies did not specify whether outcome assessors were blinded; however, considering the minimal influence of assessor knowledge on outcome measurement, these were rated as “some concerns” (Bruce et al., 2020; Beyraghi and Khanmohammadi, 2025; Beyraghi et al., 2025; Ge et al., 2025). For selective reporting, only three studies provided trial registration numbers and protocols (Beyraghi and Khanmohammadi, 2025; Kim et al., 2025; Needle et al., 2025), earning low risk ratings, while the remaining six studies were classified as “some concerns” due to insufficient reporting transparency (Bruce et al., 2020; Ma et al., 2020; Beyraghi et al., 2025; Ge et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b).
Overall, merely two studies achieved an overall low risk of bias rating (Beyraghi and Khanmohammadi, 2025; Needle et al., 2025), with all other studies demonstrating “some concerns” across multiple domains (Bruce et al., 2020; Ma et al., 2020; Beyraghi et al., 2025; Ge et al., 2025; Kim et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b), indicating moderate methodological limitations that may potentially influence the reliability of findings.
3.4 Meta-analysis results
Due to the limited number of studies and the diversity of balance assessment methods, dynamic balance ability was accessed by YBT composite score and the DPSI. In addition, subgroup analyses of the three YBT directional reach distances were conducted to assess the direction-specific characteristics of dynamic balance. For static balance, COP displacement and the BESS total score were pooled for analysis.
3.4.1 Effects of combined exercise therapy and tDCS on dynamic balance
Six studies assessed the impact of combined tDCS and exercise therapy on dynamic balance performance in CAI individuals (Bruce et al., 2020; Ma et al., 2020; Kim et al., 2025; Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b), utilizing YBT composite scores and DPSI measurements across 146 CAI participants. Meta-analysis results showed that compared with exercise therapy alone, the addition of tDCS did not significantly improve dynamic balance ability in CAI individuals (SMD: 0.08, 95% CI: −0.36 to 0.52; P = 0.72), representing a trivial effect size, with substantial heterogeneity observed between studies (I2 = 53%) (Figure 3).
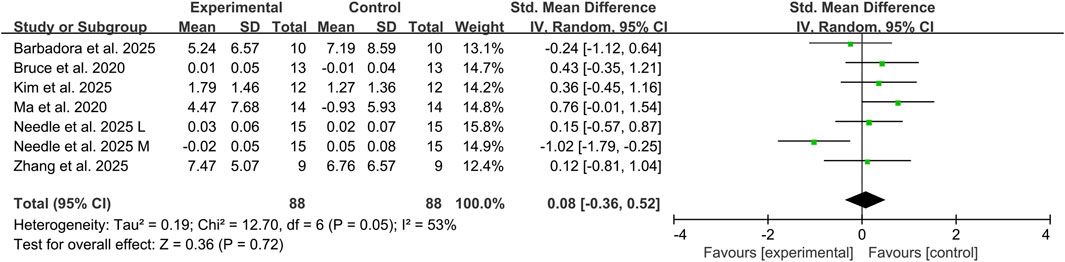
Figure 3. Effects of combined exercise therapy and tDCS on dynamic balance. Note: L, lateral; M, medial.
3.4.2 Subgroup analysis of combined exercise therapy and tDCS on dynamic balance
3.4.2.1 Different type of exercise therapy
Three studies assessed the effects of tDCS combined with balance training on dynamic balance ability in CAI individuals using YBT composite scores and DPSI, involving 68 participants (Needle et al., 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b). The addition of tDCS did not significantly improve dynamic balance ability, showing a small effect size (SMD: −0.26, 95% CI: −0.82 to 0.30, P = 0.36), with moderate heterogeneity between studies (I2 = 47%). Another three studies evaluated the effects of combined non-balance training on dynamic balance ability, involving 58 participants (Bruce et al., 2020; Ma et al., 2020; Kim et al., 2025). The results demonstrated a medium effect size in favor of combined tDCS and non-balance training for improving dynamic balance ability in CAI individuals, (SMD: 0.52, 95% CI: 0.07 to 0.97, P = 0.02) and no heterogeneity observed between studies (I2 = 0%) (Figure 4).However, these findings should be interpreted with caution given the limited number of studies in this subgroup analysis.
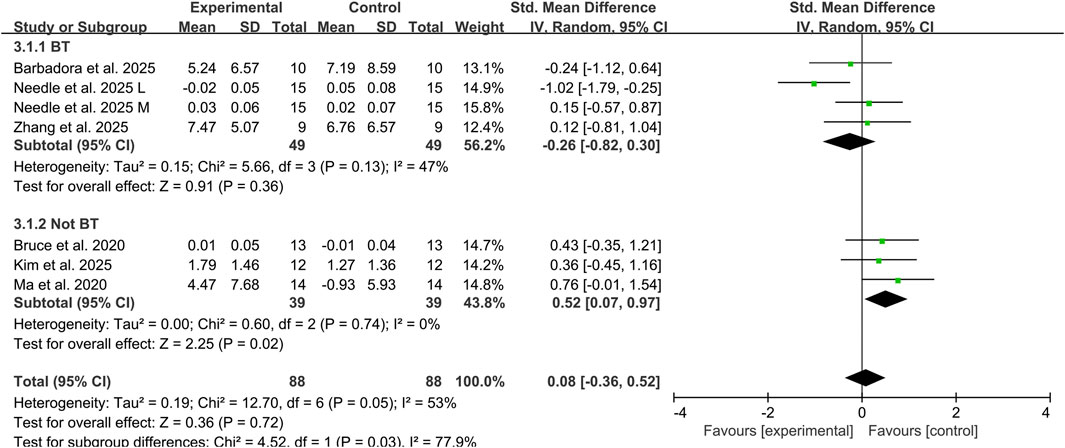
Figure 4. Subgroup analysis of the effects of tDCS combined with different types of exercise therapy on dynamic balance ability. Note: BT, balance training; L, lateral; M, medial.
3.4.2.2 Different directions of YBT
Three studies assessed the impact of combined tDCS and exercise therapy on YBT reach distances in three directions among 68 CAI participants (Beyraghi and Khanmohammadi, 2025; Sánchez-Barbadora et al., 2025; Zhang et al., 2025b). The results revealed trivial to small effect sizes with no significant improvements in any direction: ANT (SMD: 0.04, 95% CI: −0.44 to 0.51, P = 0.88), PM (SMD: 0.01, 95% CI: −0.47 to 0.49, P = 0.96), PL (SMD: 0.24, 95% CI: −0.24 to 0.73, P = 0.32). No heterogeneity was observed between studies across all three directions (I2 = 0%) (Figure 5). These directional analyses should be interpreted cautiously due to the limited evidence base.
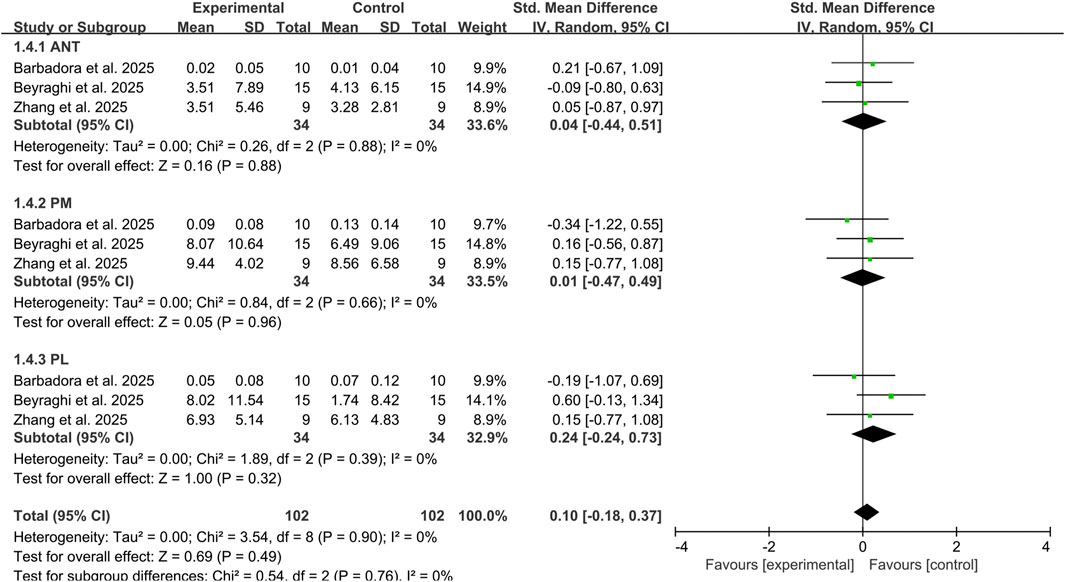
Figure 5. Subgroup analysis of the effects of tDCS combined with exercise therapy on each direction of the YBT. Note: ANT, anterior reach; PL, posterolateral reach; PM, posteromedial reach.
3.4.3 Effects of combined exercise therapy and tDCS on static balance
Three studies examined the effects of combined tDCS and exercise therapy on static balance in CAI, with two studies using COP (Ge et al., 2025; Kim et al., 2025) and one study using BESS for assessment (Zhang et al., 2025b), involving a total of 82 participants. Figure 6 showed that combined tDCS and exercise therapy failed to show significant enhancement in static balance performance among CAI, demonstrating a medium effect size (SMD: −0.53, 95% CI: −1.08 to 0.02, P = 0.06), with substantial heterogeneity observed between studies (I2 = 53%) (Figure 6). Given the limited number of studies, this finding require cautious interpretation.
![Forest plot showing a meta-analysis of four studies comparing experimental and control groups. Ge et al. 2025 AP and ML, Kim et al. 2025, and Zhang et al. 2025 are analyzed. Standardized mean differences with 95% confidence intervals are presented. The overall effect shows a standardized mean difference of -0.53 with a 95% confidence interval of [-1.08, 0.02]. Heterogeneity indicates I² = 53%. The plot suggests no significant difference, favoring neither experimental nor control groups.](https://www.frontiersin.org/files/Articles/1681272/fphys-16-1681272-HTML/image_m/fphys-16-1681272-g006.jpg)
Figure 6. Effects of combined exercise therapy and tDCS on static balance. Note: AP, anteroposterior; ML, mediolateral.
3.5 Sensitivity analysis results
To identify potential sources of high heterogeneity, sensitivity analyses were performed. Exclusion of the study by Needle et al. reduced heterogeneity for dynamic balance ability from I2 = 53%–0%, and in the tDCS combined with balance training subgroup from I2 = 47%–0%. For static balance outcomes, exclusion of the medial-lateral COP values reported by Ge et al. reduced heterogeneity from I2 = 53%–0%. To assess the stability of results, removal of the study by Ma et al. rendered the subgroup analysis of tDCS combined with non-balance training on dynamic balance ability non-significant (P = 0.17). Conversely, exclusion of the study by Kim et al. resulted in a significant pooled effect for static balance (P = 0.04). The other analyses were unaffected by individual study. Details are presented in Supplementary Table S2.
These results indicate that Needle et al. and Ge et al. were the primary sources of heterogeneity for dynamic and static balance outcomes, respectively. Importantly, the pooled effects for static balance and for tDCS combined with non-balance training on dynamic balance demonstrated instability, being significantly influenced by individual studies.
3.6 Certainty of evidence
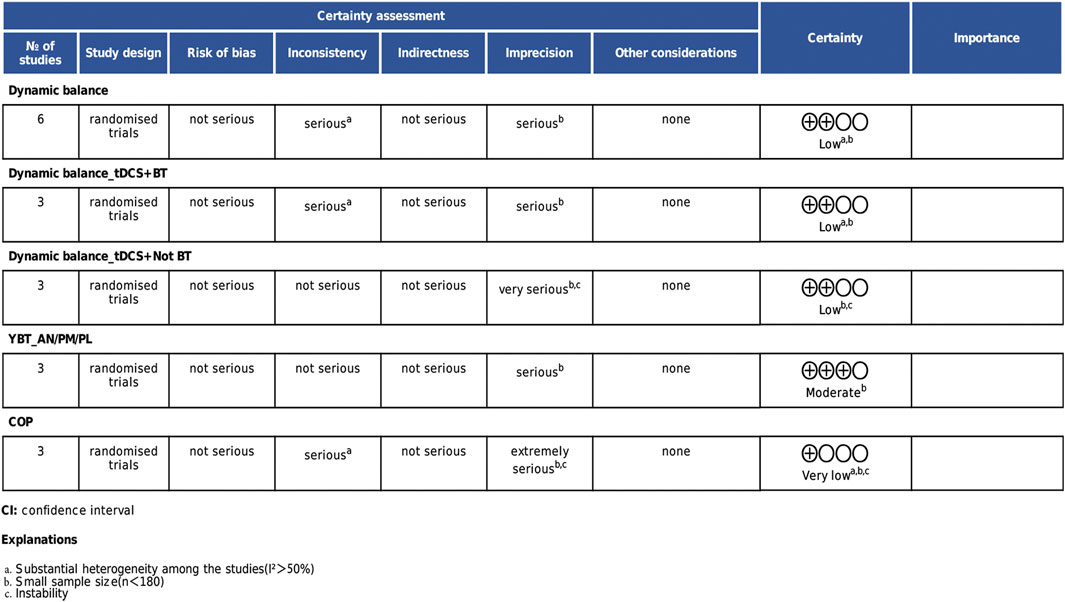
According to the GRADE assessment, the quality of evidence varied across outcomes. The overall dynamic balance results and the subgroup analysis of dynamic balance combined with balance training were rated as low quality due to high heterogeneity and insufficient sample sizes. Static balance results were rated as very low quality due to result instability. Within the dynamic balance subgroup analyses, the YBT composite score analysis was rated as moderate quality due to limited sample size, while the subgroup combined with non-balance training was downgraded to low quality due to result instability. Details are presented in Figure 7.
4 Discussion
This systematic review and meta-analysis provides the first comprehensive evaluation of combined tDCS and exercise therapy effects on balance performance in CAI individuals. The results show that when tDCS is combined with non-balance training, it provides additional improvements in dynamic balance ability. However, when combined with balance training, no significant additional benefits are observed. Regarding static balance, the combination of tDCS with exercise therapy showed no significant improvement compared to exercise therapy alone.
4.1 Dynamic balance
Balance dysfunction is common in CAI individuals, potentially resulting from central nervous system reorganization following ligament injury (Thompson et al., 2018; Ghislieri et al., 2023). CAI individuals exhibit reduced cortical excitability in the primary motor cortex (M1) and supplementary motor area (SMA), which disrupts motor control and leads to balance deficits (Solis-Escalante et al., 2019; Lepley and Lepley, 2022; Egger et al., 2023). Since tDCS can enhance M1 cortical excitability through anodal stimulation and improve motor learning and balance control (Devanathan and Madhavan, 2016; Kaminski et al., 2016), combining tDCS with exercise therapy should theoretically provide synergistic benefits for addressing dynamic balance deficits in CAI individuals. However, our meta-analysis shows that tDCS provides additional clinical benefits only when combined with non-balance training (strength training, SFE, and AJM). When tDCS combined with balance training, neither the comprehensive dynamic balance assessment indicators (YBT composite scores and DPSI) nor the specific directional results of YBT testing showed significant differences compared to balance training alone. This finding may have important clinical and scientific implications. It suggests that the effectiveness of tDCS as an adjunctive treatment for improving dynamic balance in CAI may have conditional limitations, potentially related to differences in neural networks and plasticity mechanisms activated by different training modalities.
From a neurophysiological perspective, a possible hypothesis may explain why the combination of tDCS and balance training failed to yield additional benefits. Balance training primarily enhances the efficacy of GABAergic inhibitory pathways in the motor cortex, significantly increasing short-interval intracortical inhibition (SICI), thereby suppressing co-contraction of irrelevant muscles and achieving refined motor control regulation (Mouthon and Taube, 2019; Taube et al., 2020). In contrast, strength training reduces SICI to release M1 output potential and achieve increased strength (Kidgell et al., 2017). However, existing meta-analyses have confirmed that anodal tDCS suppresses GABA synthesis and weakens SICI function (Biabani et al., 2018). This contradiction in neuroplasticity mechanisms may be one of reasons why tDCS combined with balance training fails to further improve balance capacity in CAI individuals. However, we must cautiously note that this explanation remains speculative at present. First, no relevant studies have validated the baseline characteristics of SICI in CAI populations, which makes it unclear whether interventions aimed at modulating cortical inhibition are equally applicable in individuals with CAI. Second, none of the studies included in this meta-analysis directly measured neurophysiological indicators such as SICI through techniques like TMS to confirm that tDCS and balance training indeed induced the aforementioned mutually antagonistic neuromodulatory effects in participants’ brains. Therefore, this hypothesis still requires further validation through future research. Furthermore, balance training alone has been extensively demonstrated to significantly improve dynamic balance function in CAI individuals, and the resulting ceiling effect may also limit the potential for additional benefits from tDCS (Mollà-Casanova et al., 2021; Guo et al., 2024).
It should also be noted that although our findings indicate that tDCS combined with different types of exercise therapy may exert differential effects on dynamic balance with CAI, the limited number of studies included in the subgroup analyses and the instability of the pooled results for tDCS combined with balance training in the sensitivity analysis suggest that these conclusions should be interpreted with caution. Further high-quality randomized controlled trials are warranted to substantiate these findings.
4.2 Static balance
This meta-analysis shows that adding tDCS to exercise therapy did not significantly enhance static balance ability in CAI individuals compared to exercise therapy alone. Neuroimaging studies reveal that dynamic balance tasks elicit higher activation in motor control networks, requiring elevated cortical excitability to coordinate complex muscle activation patterns and postural adjustments (Taube et al., 2015). In contrast, static balance control relies on automated postural mechanisms with lower neural resource demands (Jahn et al., 2004). Consequently, the neuromodulatory effects of tDCS through enhanced cortical excitability may have limited impact on improving static balance control (Xiao et al., 2020; Xiao et al., 2022). However, several limitations should be acknowledged. Subgroup analyses examining different exercise therapy types could not be conducted for static balance outcomes due to the limited study sample, which prevented assessment of whether specific training modalities demonstrate superior synergistic effects with tDCS. Additionally, the included studies exhibited heterogeneity in static balance assessment methods, which may have affected the accuracy of the meta-analysis results.
Although the overall results did not reach statistical significance, the effect size and confidence intervals suggest that tDCS may have potential clinical benefits in improving static balance. Furthermore, sensitivity analysis revealed that the results became statistically significant after excluding the Kim et al. Combined with findings from Ge et al. which demonstrated that tDCS combined with exercise therapy significantly improved mediolateral sway amplitude of the COP in individuals with CAI, these findings indicate that tDCS combined with exercise therapy may indeed improve static balance in CAI. However, this effect is currently obscured by between-study heterogeneity and insufficient sample sizes. Future research with larger sample sizes and more rigorous designs is needed to validate and elucidate this potential therapeutic effect.
4.3 Clinical implications
Our research findings provide certain guidance for the clinical application of CAI rehabilitation. In clinical practice, individualized treatment protocols should be developed based on specific patient conditions. For CAI individuals who have not received balance training, tDCS can serve as an effective adjunctive therapy to significantly improve their dynamic balance ability. However, for individuals already undergoing systematic balance training, the additional use of tDCS may not provide extra benefits for balance function improvement. Regarding stimulation parameters, based on current evidence, we recommend adopting a treatment protocol of 1.5–2 mA anodal stimulation over the M1 area for 15–20 min. Nevertheless, due to the limited number of related studies, future high-quality research is still needed to explore the effects of different stimulation intensities, durations, and target sites on balance function in CAI individuals, thereby establishing optimal stimulation parameters to provide more precise evidence for clinical decision-making.
4.4 Limitations
This study has several limitations: 1. The relatively small sample sizes of included studies and heterogeneity observed in some meta-analyses resulted in low to moderate certainty of evidence; 2. Variations in balance assessment methods across studies, while not identified as a major source of heterogeneity in sensitivity analysis, may still affect the precision of results; 3. Limited by the insufficient number of studies on static balance, subgroup analyses by different exercise therapy types were not feasible, preventing clear determination of differential effects of tDCS combined with various exercise interventions on static balance; 4. Analyses stratified by tDCS stimulation parameters and intervention duration were not conducted, leaving unclear whether different stimulation parameters and treatment durations produce varying effects.
5 Conclusion
Our findings demonstrate that the effectiveness of tDCS combined with different types of exercise therapy on dynamic balance improvement in individuals with CAI may vary. Preliminary evidence indicates that tDCS combined with non-balance training may offer potential benefits for dynamic balance ability, though this finding requires further validation due to its demonstrated instability. Conversely, tDCS combined with balance training showed no significant additional benefits. Regarding static balance ability, current evidence is insufficient to demonstrate that tDCS combined with exercise therapy provides further improvement effects. Future research should conduct more high-quality randomized controlled trials to validate this conclusion and systematically explore optimal combination protocols between different exercise therapy modalities and tDCS, while establishing ideal stimulation parameter configurations, thereby providing higher-quality evidence-based medicine for clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. RC: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – review and editing. SW: Methodology, Validation, Writing – review and editing. JC: Supervision, Validation, Writing – review and editing. XL: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Longgang District Medical Key Discipline Construction Project (2024–2027).
Acknowledgments
The authors would like to express their gratitude to Professor Song Qipeng and his research team for their generous provision of original data that supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1681272/full#supplementary-material
References
Andrade C. (2020). Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J. Clin. Psychiatry 81, 20f13681. doi:10.4088/JCP.20f13681
Auvichayapat P., Auvichayapat N. (2011). Basic knowledge of transcranial direct current stimulation. J. Med. Assoc. Thai 94, 518–527.
Beyraghi Z., Khanmohammadi R. (2025). No additive effect of transcranial direct current stimulation on balance exercises for brain activity and clinical outcomes in patients with chronic ankle instability: a randomised controlled trial. BMJ Open Sport Exerc Med. 11, e002401. doi:10.1136/bmjsem-2024-002401
Beyraghi Z., Khanmohammadi R., Hadian M. R. (2025). Effects of combining transcranial direct current stimulation with balance training on anticipatory postural adjustments in persons with chronic ankle instability. Sports Health 17, 383–393. doi:10.1177/19417381241247746
Biabani M., Aminitehrani M., Zoghi M., Farrell M., Egan G., Jaberzadeh S. (2018). The effects of transcranial direct current stimulation on short-interval intracortical inhibition and intracortical facilitation: a systematic review and meta-analysis. Rev. Neurosci. 29, 99–114. doi:10.1515/revneuro-2017-0023
Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111. doi:10.1002/jrsm.12
Bruce A. S., Howard J. S., VAN Werkhoven H., McBride J. M., Needle A. R. (2020). The effects of transcranial direct current stimulation on chronic ankle instability. Med. Sci. Sports Exerc 52, 335–344. doi:10.1249/MSS.0000000000002129
Chase H. W., Boudewyn M. A., Carter C. S., Phillips M. L. (2020). Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol. Psychiatry 25, 397–407. doi:10.1038/s41380-019-0499-9
Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.ED000142
Devanathan D., Madhavan S. (2016). Effects of anodal tDCS of the lower limb M1 on ankle reaction time in young adults. Exp. Brain Res. 234, 377–385. doi:10.1007/s00221-015-4470-y
Egger S., Wälchli M., Rüeger E., Taube W. (2023). Short-term balance consolidation relies on the primary motor cortex: a rTMS study. Sci. Rep. 13, 5169. doi:10.1038/s41598-023-32065-x
Ge Y., Gao H., Huang X., Luo X., Liu Y., Wang D., et al. (2025). Effects of HD-tDCS combined with bosu ball training on static and dynamic postural stability among individuals with chronic ankle instability. Front. Sports Act. Living 7, 1618683. doi:10.3389/fspor.2025.1618683
Ghislieri M., Labanca L., Mosca M., Bragonzoni L., Knaflitz M., Benedetti M. G., et al. (2023). Balance and muscle synergies during a single-limb stance task in individuals with chronic ankle instability. IEEE Trans. Neural Syst. Rehabilitation Eng. 31, 4367–4375. doi:10.1109/TNSRE.2023.3328933
Gribble P. A., Bleakley C. M., Caulfield B. M., Docherty C. L., Fourchet F., Fong D. T.-P., et al. (2016). Evidence review for the 2016 international ankle consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br. J. Sports Med. 50, 1496–1505. doi:10.1136/bjsports-2016-096189
Guo Y., Cheng T., Yang Z., Huang Y., Li M., Wang T. (2024). A systematic review and meta-analysis of balance training in patients with chronic ankle instability. Syst. Rev. 13, 64. doi:10.1186/s13643-024-02455-x
Guyatt G., Oxman A. D., Akl E. A., Kunz R., Vist G., Brozek J., et al. (2011). GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, 383–394. doi:10.1016/j.jclinepi.2010.04.026
Herzog M. M., Kerr Z. Y., Marshall S. W., Wikstrom E. A. (2019). Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 54, 603–610. doi:10.4085/1062-6050-447-17
Jahn K., Deutschländer A., Stephan T., Strupp M., Wiesmann M., Brandt T. (2004). Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 22, 1722–1731. doi:10.1016/j.neuroimage.2004.05.017
Kaminski E., Steele C. J., Hoff M., Gundlach C., Rjosk V., Sehm B., et al. (2016). Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin. Neurophysiol. 127, 2455–2462. doi:10.1016/j.clinph.2016.03.018
Kidgell D. J., Bonanno D. R., Frazer A. K., Howatson G., Pearce A. J. (2017). Corticospinal responses following strength training: a systematic review and meta-analysis. Eur. J. Neurosci. 46, 2648–2661. doi:10.1111/ejn.13710
Kim Y., Kim H., Jung J., Lee S. (2025). Synergistic effects of joint-biased rehabilitation and combined transcranial direct current stimulation (tDCS) in chronic ankle instability: a single-blind, three-armed randomized controlled trial. Brain Sci. 15, 458. doi:10.3390/brainsci15050458
Lepley A. S., Lepley L. K. (2022). Mechanisms of arthrogenic muscle inhibition. J. Sport Rehabil. 31, 707–716. doi:10.1123/jsr.2020-0479
Li Y., Wang Z., Yang Y., Deng Y., Shen Y., Wang X., et al. (2025). Exploring low- and high-order functional connectivity in chronic ankle instability through resting-state fMRI. Med. Phys. 52, 565–575. doi:10.1002/mp.17474
Ma Y., Yin K., Zhuang W., Zhang C., Jiang Y., Huang J., et al. (2020). Effects of combining high-definition transcranial direct current stimulation with short-foot exercise on chronic ankle instability: a pilot randomized and double-blinded study. Brain Sci. 10, 749. doi:10.3390/brainsci10100749
Maricot A., Dick E., Walravens A., Pluym B., Lathouwers E., De Pauw K., et al. (2023). Brain neuroplasticity related to lateral ankle ligamentous injuries: a systematic review. Sports Med. 53, 1423–1443. doi:10.1007/s40279-023-01834-z
Mollà-Casanova S., Inglés M., Serra-Añó P. (2021). Effects of balance training on functionality, ankle instability, and dynamic balance outcomes in people with chronic ankle instability: systematic review and meta-analysis. Clin. Rehabil. 35, 1694–1709. doi:10.1177/02692155211022009
Mouthon A., Taube W. (2019). Intracortical inhibition increases during postural task execution in response to balance training. Neuroscience 401, 35–42. doi:10.1016/j.neuroscience.2019.01.007
Needle A. R., Skinner J. W., Watson M. G., Roberts S. E., Grattan S. A., Howard J. S. (2025). Transcranial direct current stimulation over the motor or frontal cortex in patients with chronic ankle instability. J. Athl. Train. 60, 664–674. doi:10.4085/1062-6050-0728.24
Nguyen T. X. D., Mai P. T., Chang Y.-J., Hsieh T.-H. (2024). Effects of transcranial direct current stimulation alone and in combination with rehabilitation therapies on gait and balance among individuals with Parkinson’s disease: a systematic review and meta-analysis. J. Neuroeng Rehabil. 21, 27. doi:10.1186/s12984-024-01311-2
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Sánchez-Barbadora M., Moreno-Segura N., Medina-Mirapeix F., Martín-San Agustín R. (2025). The effects of a balance training program with and without transcranial direct current stimulation on dynamic balance in recreationally active young adults: a randomized controlled trial. Appl. Sci. 15, 1807. doi:10.3390/app15041807
Solis-Escalante T., van der Cruijsen J., de Kam D., van Kordelaar J., Weerdesteyn V., Schouten A. C. (2019). Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. Neuroimage 188, 557–571. doi:10.1016/j.neuroimage.2018.12.045
Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Suttmiller A. M. B., McCann R. S. (2020). Neural excitability of lower extremity musculature in individuals with and without chronic ankle instability: a systematic review and meta-analysis. J. Electromyogr. Kinesiol 53, 102436. doi:10.1016/j.jelekin.2020.102436
Taube W., Mouthon M., Leukel C., Hoogewoud H.-M., Annoni J.-M., Keller M. (2015). Brain activity during observation and motor imagery of different balance tasks: an fMRI study. Cortex 64, 102–114. doi:10.1016/j.cortex.2014.09.022
Taube W., Gollhofer A., Lauber B. (2020). Training-muscle- and task-specific up- and downregulation of cortical inhibitory processes. Eur. J. Neurosci. 51, 1428–1440. doi:10.1111/ejn.14538
Thompson C., Schabrun S., Romero R., Bialocerkowski A., Marshall P. (2016). Factors contributing to chronic ankle instability: a protocol for a systematic review of systematic reviews. Syst. Rev. 5, 94. doi:10.1186/s13643-016-0275-8
Thompson C., Schabrun S., Romero R., Bialocerkowski A., van Dieen J., Marshall P. (2018). Factors contributing to chronic ankle instability: a systematic review and meta-analysis of systematic reviews. Sports Med. 48, 189–205. doi:10.1007/s40279-017-0781-4
Viechtbauer W., Cheung M. W.-L. (2010). Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1, 112–125. doi:10.1002/jrsm.11
Wu J., Zeng A., Chen Z., Wei Y., Huang K., Chen J., et al. (2021). Effects of virtual reality training on upper limb function and balance in stroke patients: systematic review and meta-meta-analysis. J. Med. Internet Res. 23, e31051. doi:10.2196/31051
Xiao S., Wang B., Zhang X., Zhou J., Fu W. (2020). Acute effects of high-definition transcranial direct current stimulation on foot muscle strength, passive ankle kinesthesia, and static balance: a pilot study. Brain Sci. 10, 246. doi:10.3390/brainsci10040246
Xiao S., Wang B., Yu C., Shen B., Zhang X., Ye D., et al. (2022). Effects of intervention combining transcranial direct current stimulation and foot core exercise on sensorimotor function in foot and static balance. J. Neuroeng Rehabil. 19, 98. doi:10.1186/s12984-022-01077-5
Yang Y.-S., Lai P.-C., Liu Z.-W., Fang C.-J., Tu Y.-K., Chang C.-H., et al. (2025). What will deliver the best bang-for-your-treatment-buck? Treatment effects of physical therapy approaches to managing chronic ankle instability: a network meta-analysis of randomized controlled trials. J. Orthop. Sports Phys. Ther. 55, 26–44. doi:10.2519/jospt.2024.12601
Ye H., Yang J.-M., Luo Y., Long Y., Zhang J.-H., Zhong Y.-B., et al. (2023). Do dietary supplements prevent loss of muscle mass and strength during muscle disuse? A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 10, 1093988. doi:10.3389/fnut.2023.1093988
Zhang C., Luo Z., Wu D., Fei J., Xie T., Su M. (2025a). Effectiveness of exercise therapy on chronic ankle instability: a meta-analysis. Sci. Rep. 15, 11709. doi:10.1038/s41598-025-95896-w
Keywords: transcranial direct current stimulation, chronic ankle instability, sports therapy, dynamic balance ability, static balance ability
Citation: Zheng J, Chen R, Wang S, Chen J and Lin X (2025) Can exercise therapy combined with transcranial direct current stimulation further improve balance ability in individuals with chronic ankle instability? A systematic review and meta-analysis. Front. Physiol. 16:1681272. doi: 10.3389/fphys.2025.1681272
Received: 07 August 2025; Accepted: 07 October 2025;
Published: 15 October 2025.
Edited by:
Daniel Gomes Da Silva Machado, Federal University of Rio Grande do Norte, BrazilReviewed by:
Baofeng Wang, Shandong First Medical University, ChinaRoya Khanmohammadi, Iran University of Medical Sciences, Iran
Copyright © 2025 Zheng, Chen, Wang, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xikai Lin, MTAwNjg2NTYzOEBxcS5jb20=
 Jiawei Zheng
Jiawei Zheng Ruixiong Chen2
Ruixiong Chen2 Xikai Lin
Xikai Lin