- 1Department of Physical Therapy, Juntendo University Graduate School of Health Science, Tokyo, Japan
- 2Department of Physical Therapy, Human Health Sciences, Graduate School of Medicine, Kyoto University, Kyoto, Japan
- 3Department of Physical Therapy, Juntendo University Faculty of Health Science, Tokyo, Japan
- 4Department of Rehabilitation Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
Among the noninvasive electrical stimulation methods, transcutaneous auricular vagus nerve stimulation (taVNS) regulates the activity of various neural networks in the brain and autonomic nervous system and is expected to be applied clinically in many areas, including in patients with central nervous system, psychiatric, and cardiac diseases. Although systematic reviews and meta-analyses have been conducted on safety and efficacy, the variability of stimulation parameters and the lack of consistency in their effects remain significant issues. Therefore, the present study aimed to provide a comprehensive view of the safety, parameters, and efficacy of taVNS by focusing on studies in healthy participants, patients with stroke, and patients with Parkinson’s disease. A literature search was conducted from October 14 to 25 November 2024, using PubMed, Google Scholar, Web of Science, the Cochrane Library, and Scopus. The following search terms were used: “noninvasive VNS or nVNS or noninvasive vagus nerve stimulation,” “transcutaneous vagus nerve stimulation or tVNS,” and “transcutaneous auricular vagus nerve stimulation or taVNS.” In total, 154 papers were included, of which 139 were on healthy participants, nine on patients with stroke, and six on patients with Parkinson’s disease. The safety of taVNS was relatively high. Although minor side effects were reported, no serious adverse events were attributed to taVNS parameters used. taVNS could regulate brain activity, motor and mental functions, and autonomic nervous system activity in patients with stroke and Parkinson’s disease. Modulation of the autonomic nervous system and cortical excitability was also observed in healthy individuals. However, these effects may depend on the stimulation parameters. The lack of reports on safety and the stimulation parameters used was also highlighted. Further validation of parameters and accumulation of evidence regarding the efficacy of taVNS are necessary.
1 Introduction
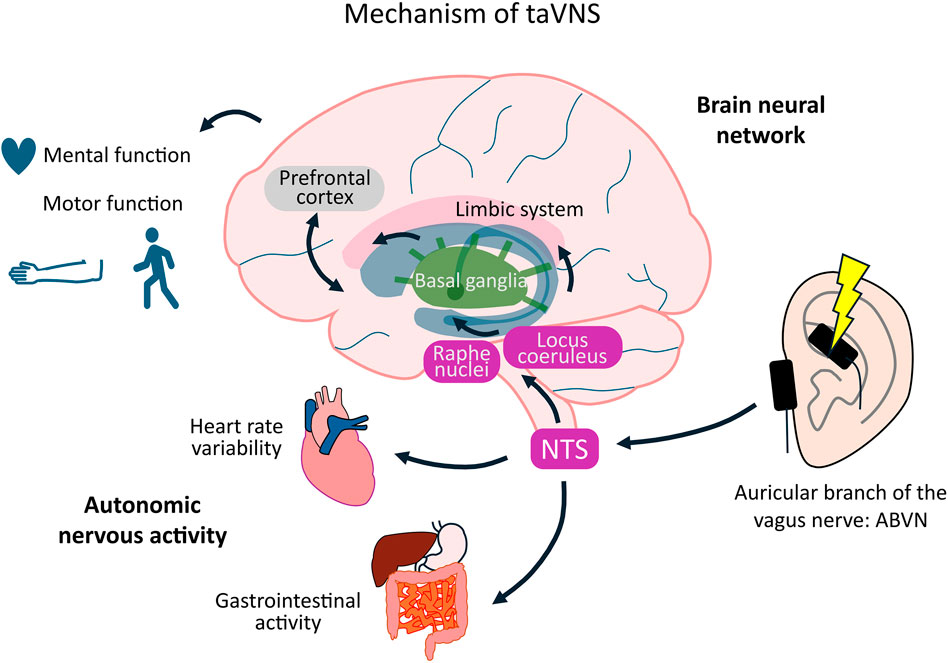
Transcutaneous auricular vagus nerve stimulation (taVNS) is a noninvasive neuromodulation technique that influences autonomic nervous system activity by stimulating the auricular branch of the vagus nerve (ABVN). The afferent fibers of the ABVN enter the vagal trunk and project to the nucleus tractus solitarius (NTS). Activation of the NTS alters brain activity, including that in the locus coeruleus (LC) and raphe nucleus, while affecting the functions of the prefrontal cortex, basal ganglia, and limbic system (Badran et al., 2018a). These effects improve consciousness, motor function, somatosensory function, and mental health in both healthy individuals and patients with neurological disorders (Gao et al., 2023; Dong et al., 2023; Hagen et al., 2014; Colzato et al., 2018a; Colzato et al., 2018b). Although these effects depend on stimulation parameters, such as current intensity, frequency, duration, and on–off time, the influence of these parameters on the outcome remains unclear. Therefore, optimizing taVNS parameters may offer therapeutic benefits to patients with neurological disorders during neurorehabilitation. Unlike earlier systematic reviews and meta-analyses that primarily assessed aggregate efficacy outcomes (Hua et al., 2023; Ramos-Castaneda et al., 2022; Redgrave et al., 2018), this review aimed to explore the potential clinical applications of taVNS in healthy participants, patients with stroke, and patients with Parkinson’s disease (PD), centering on three aspects: safety, stimulation parameters, and efficacy.
In this review, we focus on healthy individuals, stroke, and PD to align mechanistic insights with clinical translation. Healthy participants were included because most mechanistic studies of taVNS have been conducted in this group, providing essential information on stimulation parameters and physiological mechanisms. Healthy cohorts also offer a low-confounder setting to map taVNS stimulation parameters (frequency, duration, intensity, and on-off interval) onto neurophysiological effects (brainstem/cortical activity, electroencephalogram, and autonomic modulation). Stroke and PD then represent canonical motor network disorders in which these mechanisms are hypothesized to support recovery of motor control, gait, and learning (Mondal et al., 2023; Engineer et al., 2019). In addition, taVNS has recently gained attention as a promising adjunct in neurorehabilitation for these conditions, where novel interventions to improve motor and cognitive outcomes are particularly needed (Dolphin et al., 2022; Galvez-Garcia et al., 2024; Maraver et al., 2020; Shi et al., 2023). Additionally, Zhang et al. (2025) provided a comprehensive review of both invasive and non-invasive VNS mechanisms in stroke rehabilitation. They reported that VNS promotes synaptic plasticity, inhibits inflammatory responses, facilitates vascular regeneration, and protects the integrity of the blood–brain barrier, collectively contributing to functional remodeling after ischemic injury. Furthermore, clinical evidence suggests that invasive VNS and taVNS yield significant improvements in upper-limb motor function post-stroke. Previous research on other neuropsychiatric conditions such as depression, epilepsy, and migraine has already been synthesized in systematic reviews and meta-analyses (Lim et al., 2022; Reuter et al., 2019; Wu et al., 2018), whereas the evidence base for stroke and PD remains comparatively limited and heterogeneous. Focusing on these three groups enables harmonized outcome frameworks [e.g., functional magnetic resonance imaging (fMRI), electroencephalogram (EEG), heart rate variability (HRV), task performance, the Fugl–Meyer Assessment of the upper extremity (FMA-UE), gait metrics, Unified Parkinson’s Disease Rating Scale (UPDRS)] and avoids cross-indication heterogeneity that would obscure parameter–effect relationships central to this review. Optimizing taVNS parameters may offer therapeutic benefits to patients with neurological disorders during neurorehabilitation. Given the novelty of taVNS, the current evidence base is still heterogeneous, with relatively few controlled clinical trials available. Therefore, instead of a systematic review, we adopted a narrative review format to provide an overview of existing studies and to outline key challenges for future standardized research.
Regarding safety, the most commonly reported side effects are ear pain, headache, and numbness (Kim et al., 2022). Researchers have reported a low rate of adverse events and no serious events when applying taVNS to healthy individuals as well as patients with stroke, depression, epilepsy, cognitive impairment, and migraine (Redgrave et al., 2018; Tan et al., 2023; Wang et al., 2022; Wu et al., 2020). No differences in the risk of adverse or serious events were observed between the active and sham stimulation conditions. However, the incidence and impacts of adverse events vary despite the application of the same parameters (Gerges et al., 2024b). Moreover, the relationship between taVNS parameters and the incidence or severity of adverse events remains unknown owing to inconsistent reporting of adverse events. Therefore, the association between these parameters and adverse events should be investigated.
The taVNS parameters vary according to individual stimulation sensitivity and disease specificity. To effectively apply taVNS, establishing stimulation parameters that reflect participant characteristics is essential.
Furthermore, the outcomes used to evaluate the effect of taVNS differ across studies, complicating comparisons and potentially hindering clinical applications (Badran et al., 2018b). This review serves as an initial step in exploring the variability and efficacy of taVNS parameters by focusing on the parameters used for each disease and the associated evaluation indices.
Previous studies have reported the effect of taVNS on neural brain networks and motor performance in healthy individuals and those with stroke and PD (Li et al., 2022; Sigurdsson et al., 2021; Wang et al., 2021). However, a functional magnetic resonance imaging (fMRI) study indicated that varying the taVNS settings, such as frequency and stimulus duration, led to different brain network activities. In addition, HRV, which reflects the function of the autonomic nervous system, changes depending on the stimulus intensity and frequency of taVNS (Badran et al., 2018b; Clancy et al., 2014). Thus, it remains unclear which parameters are most effective in specific settings and populations, including healthy individuals and those with neurological disorders (Thompson et al., 2021). Further studies are required to explore the differences and individual variabilities in the effect of taVNS according to different parameters to optimize and maximize its effect (Yokota et al., 2022; Ng et al., 2024).
2 Methods
A literature search was conducted from October 14 to 25 November 2024, using PubMed, Google Scholar, Web of Science, the Cochrane Library, and Scopus. The search terms and Boolean combinations used were: (“Non-invasive VNS” OR “nVNS” OR “Non-invasive vagus nerve stimulation”), (“Transcutaneous vagus nerve stimulation” OR “tVNS”), (“Transcutaneous auricular vagus nerve stimulation” OR “taVNS”).
The target population was defined to include healthy adults, patients with stroke, and patients with PD. This selection was guided by (i) original articles published in peer-reviewed journals and written in English; (ii) the density of parameterized taVNS studies in healthy cohorts; (iii) the neurorehabilitation relevance of stroke and PD, where motor and cognitive endpoints allow cross-study comparability; and (iv) the need to minimize heterogeneity from conditions with disparate pathophysiology and outcomes (e.g., mood or cardiovascular indications), which would compromise our parameter-focused synthesis. For patient populations, all studies involving stroke or PD were eligible, irrespective of study design. Studies were included if they applied taVNS with reported stimulation parameters and outcome measures. Exclusion criteria were: (i) unspecified taVNS parameters, (ii) absence of assessment items, and (iii) preprints and study protocols.
The search terms “tVNS” and “nVNS” may also retrieve studies using percutaneous cervical vagus nerve stimulation (tcVNS, e.g., gammaCore), because using “tVNS” alone could exclude papers that mentioned the auricular approach but were labeled differently. Therefore, all abstracts and full texts identified by our search terms were screened, and non-auricular approaches were excluded. This review includes only studies using auricular stimulation (taVNS). Although transcutaneous cervical vagus nerve stimulation (tcVNS) is also a promising approach, it differs in stimulation site and mechanisms; thus, our scope was limited to taVNS.
Studies involving healthy adults of any age were eligible. No specific age restrictions were applied; however, most included studies were conducted in young to middle-aged adults, with only a few explicitly targeting older adults.
This review was conducted in a narrative format. A systematic literature search was performed, and findings were synthesized qualitatively. No statistical pooling or meta-analysis was undertaken because of the heterogeneity of study designs, stimulation protocols, and outcome measures. Outcomes were extracted and narratively organized into three domains: safety, stimulation parameters, and efficacy. This narrative review was not pre-registered on OSF or any other public repository. Screening and data extraction were performed by a single reviewer.
3 Results
3.1 Overview
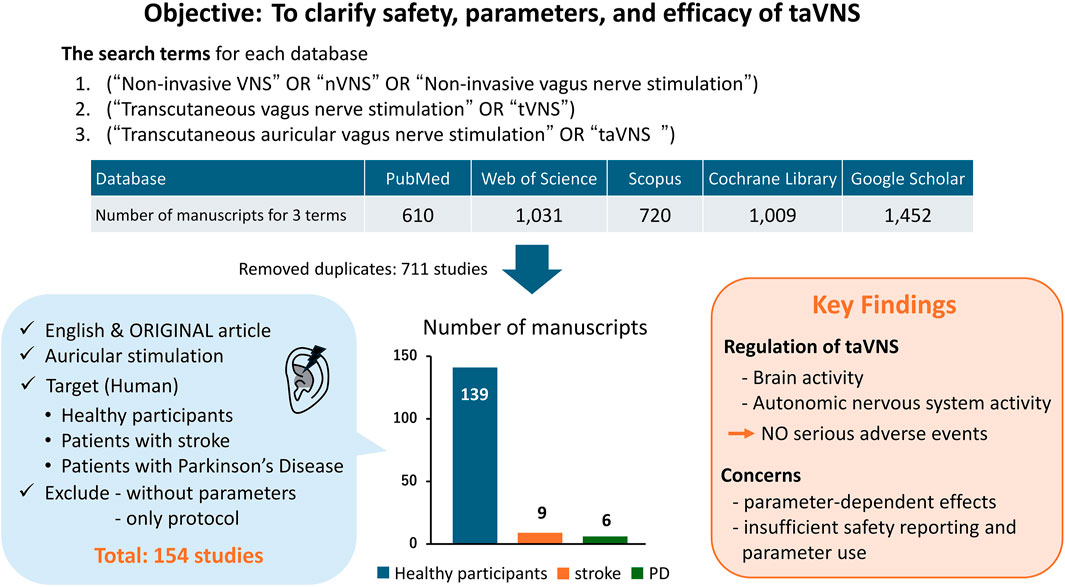
A total of 4,822 records were retrieved from PubMed, Web of Science, Scopus, Google Scholar, and the Cochrane Library. After removing duplicates, 711 unique records remained. Following title/abstract and full-text screening based on the inclusion and exclusion criteria, 154 studies were included in this review (139 healthy, nine stroke, six PD). A simplified flow summary is presented in Figure 1.
Regarding the safety of taVNS, most studies found that the participants did not experience any adverse events. Common side effects included warmth, vibration, and numbness; however, these were not serious. Some studies lacked descriptions of side effects or adverse events.
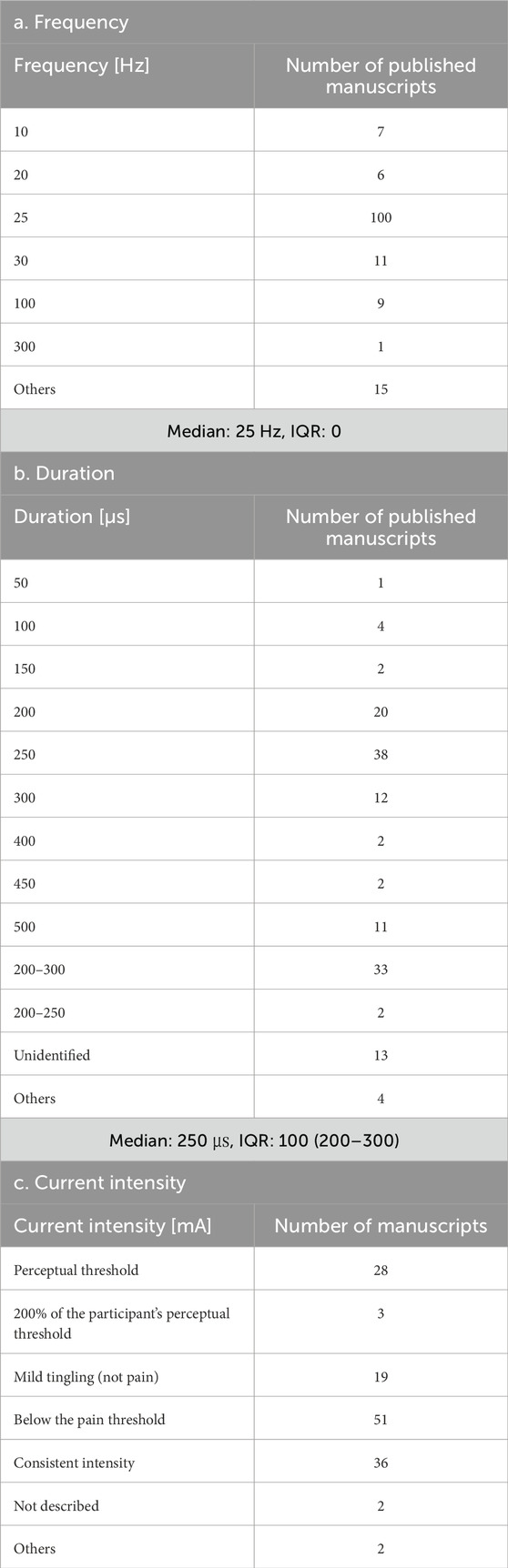
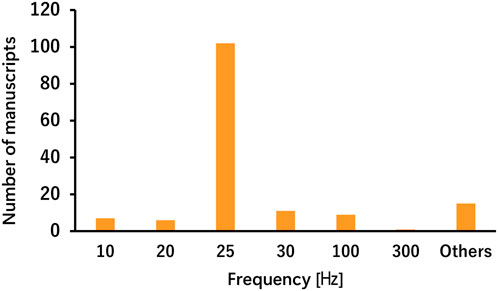
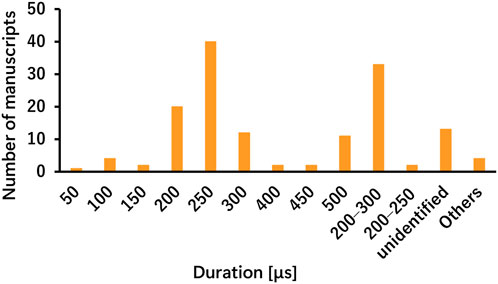
The parameters varied according to disease and stimulation target, demonstrating less consistency across studies involving healthy participants, patients with stroke, and patients with PD (Tables 1, 2). For each parameter, a frequency of 25 Hz and a pulse duration of 200–300 μs were the most commonly used in both healthy participants and patient populations in the included studies. The most common stimulation intensity was below the pain threshold, followed by the sensory threshold, mild tingling, uniform intensity, and 200% sensory threshold. Methods for determining intensity varied across studies, and there were also nuances in the interpretations of terms such as “tolerable” and “below the pain threshold.” For the on-off interval, a 30-s “on” and 30-s “off” pattern was applied in both healthy participants and patients with stroke; however, the duty cycle in patients with PD varied across studies, including patterns such as 60-s “on” and 10-s “off” or 60-s “on” and 30-s “off”. Some studies applied continuous stimulation without on-off cycle. taVNS was applied for ≤60 min in most studies. However, in healthy individuals, large variations were observed, such as >60 min, only a few minutes, and occasionally during task performance. The outcome measures primarily included disease-specific rating scales and taVNS efficacy measures.
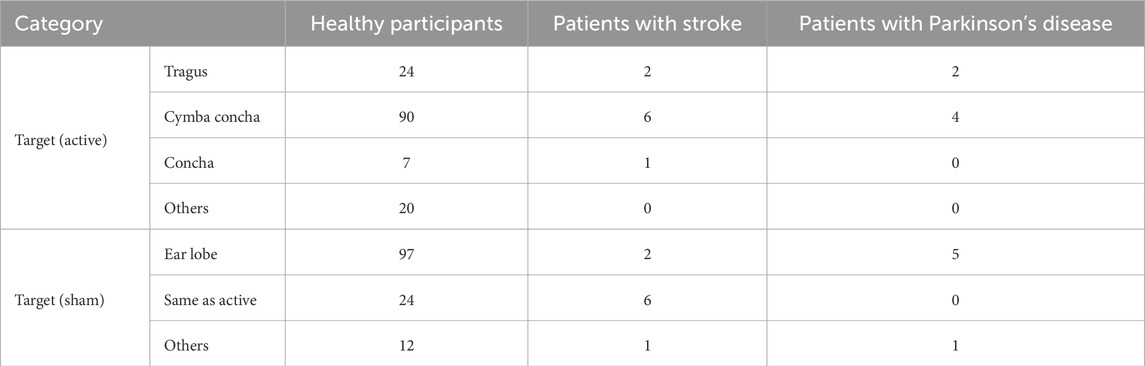
Table 2. Electrode attachment sites used in taVNS studies (number of manuscripts by participant type).
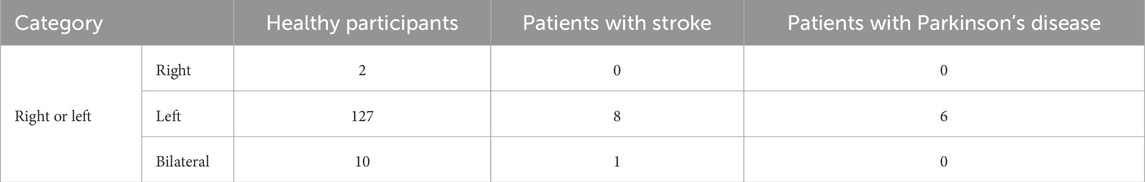
Regarding electrode lateralization, both right-sided and left-sided taVNS were found to equally enhance cortical excitability and induce neurophysiological changes in the frontal area (Konakoglu et al., 2023; Camargo et al., 2024). Bilateral stimulation also showed a modulating effect on heart rate variability (Percin et al., 2024; De Couck et al., 2017). Hatik et al. (2023) and Laqua et al. (2014) reported that bilateral stimulation resulted in significantly less stimulation-related pain. Peng et al. (2023) examined the effects of taVNS delivered to different ear targets relative to the lesion (ipsilesional vs. contralesional vs. bilateral vs. sham) in patients with stroke and found that ipsilesional stimulation produced the largest direct brain activation. However, in healthy individuals, activity increased in the wrist extensor muscles on the side opposite to the stimulated side (Konakoglu et al., 2023). Overall, this review found no studies reporting negative results for bilateral stimulation; however, effects may differ between healthy individuals and patient populations, necessitating further investigation. Only 2 studies performed right-sided taVNS (Gauthey et al., 2020; Sinkovec et al., 2021).
Across all included studies (including overlaps), 52 assessed autonomic outcomes (e.g., HR, HRV, pupil size, salivary markers), 60 investigated cortical/neurophysiological indices (e.g., fMRI, EEG, TMS), 42 reported motor outcomes, and 25 assessed cognitive or affective measures. Representative findings included HRV increases and improved pupil size in autonomic measures, modulation of limbic and prefrontal activity in neurophysiological studies, improvements in gait and upper-limb function in motor outcomes, and enhanced attention and reduced anxiety in cognitive/affective measures. Studies involving patients with stroke and those with PD more often evaluated motor function than autonomic nervous system activity. In contrast, studies in healthy participants primarily investigated the neurological and physiological mechanisms of taVNS, employing fMRI, HRV, and electroencephalography (EEG) (Badran et al., 2018a; Gianlorenco et al., 2024; Keute et al., 2021; Rufener et al., 2018; Rufener et al., 2023; Sclocco et al., 2019). Other studies have demonstrated improved performance in tasks involving rewards, fatigue, and memory. In studies on patients with stroke, taVNS contributed to reducing inflammation and vasospasm and enhancing motor and sensory functions, walking speed, gait cycle, balance, and emotional responses (Capone et al., 2017; Chang et al., 2021; Li et al., 2022; Sellaro et al., 2015b; Wang M. H. et al., 2024). Modulation of the default mode network using taVNS underlies these effects (Peng et al., 2023). Regarding the effect of taVNS in patients with PD, the improvement in walking velocity or stride length, anxiety, and modulation of brain areas, such as the superior parietal lobule, anterior central gyrus, posterior central gyrus, middle occipital gyrus, and cuneus, has been reported (van Midden et al., 2024b; Fu et al., 2024; Zhang et al., 2024).
3.2 Healthy participants
3.2.1 Safety
Of the 139 studies in healthy participants, 76 (55%) did not report adverse events, 41 (29%) explicitly stated that no adverse events occurred, and 13 (9%) described minor adverse events (e.g., headache, ear pain, tingling, transient fatigue), and nine (6.5%) provided insufficient detail for classification. All reported events were mild and self-limiting, with no serious adverse events documented. Nine studies included sham stimulation, and none observed significant differences in adverse event rates between active and sham groups. 13 studies reported various side effects, including headache; pain; discomfort; nausea; muscle contractions, tingling, or burning sensations; and fatigue. These studies also noted that such side effects were not serious (D’Agostini et al., 2021; Jacobs et al., 2015; Tona et al., 2020). One study concluded that the physiological and potential side effects of taVNS were unknown when used bilaterally because the parasympathetic nervous system of the heart is innervated by the right vagus nerve (Kraus et al., 2007). However, Hatik et al. (2023) subsequently reported that taVNS did not cause bradycardia or hypotension even with bilateral stimulation. Overall, this high rate of non-reporting underscores the potential for underreporting bias; therefore, it was unclear whether any adverse events had occurred. These findings suggest that taVNS is generally safe, although underreporting remains a limitation.
3.2.2 Parameters
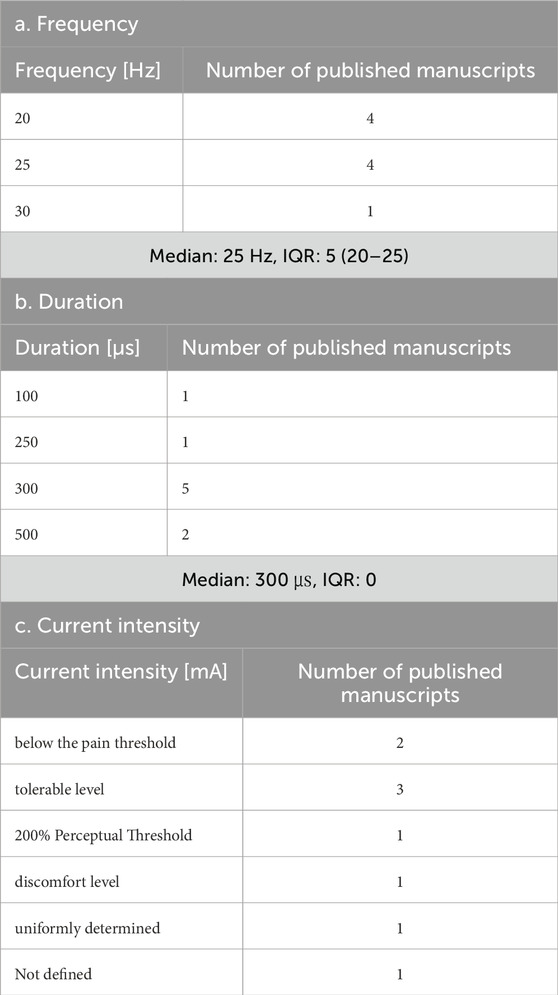
The taVNS parameters are summarized in Tables 3a–c as well as illustrated in Figures 2, 3. Table 3 presents the total number of studies that examined multiple frequencies, durations, and intensities. The most commonly used parameters were a frequency of 25 Hz, stimulus interval of 250 μs, and intensity below the pain threshold. However, the stimulus parameters varied among studies. Several studies examined the effects of these parameters. For instance, a study that applied taVNS at different intensities and examined its effects reported that pupillary dilation was most effectively induced at 2 mA (Capone et al., 2021). Regarding other parameters, 500 μs and 10 Hz had the most substantial effect on heart rate (HR) (Badran et al., 2018b), and taVNS at 250 μs, 100 Hz, and 3.0 mA was effective in suppressing pain (St Pierre and Shinohara, 2023; Yokota et al., 2024). Additionally, taVNS at 100 Hz increased cerebellar brain inhibition to a greater extent than that at 25 Hz (van Midden et al., 2024a).
Regarding the location of the electrodes, stimulation of the cymba conchae elicited a stronger and more significant activation of the NTS and LC, key brainstem targets of vagal afferents, compared with stimulation of other auricular sites (Forte et al., 2022; Yakunina et al., 2017). However, the fossa triangularis was also an effective stimulation site (Machetanz et al., 2021), and some studies applied bilateral stimulation (Camargo et al., 2024; Ferstl et al., 2022; Gianlorenco et al., 2024; Hatik et al., 2023; Konakoglu et al., 2023; Laqua et al., 2014; Machetanz et al., 2021; Müller et al., 2021; Ng et al., 2024; Percin et al., 2024). On the other hand, Borges et al. (2020) have reported the cardiac vagal activity may be similarly influenced by afferent vagal stimuli triggered by active and sham stimulation with different stimulation intensities.
Overall, the taVNS parameters are an important consideration; however, the optimal settings remain uncertain because of the variability in parameter combinations and assessment methods.
3.2.3 Effects
The effects of taVNS were predominantly categorized into three domains: modulation of activity in the brainstem and cortical areas; regulation of the autonomic nervous system, including the cardiovascular and gastrointestinal systems; and enhancement of motor and cognitive functions.
3.2.3.1 Modulation of activity in the brainstem and cortical areas
fMRI studies investigating the neuromodulatory effects of taVNS consistently demonstrated increased BOLD signals in key regions, such as the insular cortex, thalamus, prefrontal cortex, and cingulate cortex (Borgmann et al., 2021; Kraus et al., 2007). Furthermore, brainstem regions, including the NTS and LC, were activated following taVNS (Sclocco et al., 2019). These areas constitute the core components of vagal afferent pathways and are critically involved in interoception, autonomic regulation, and emotional processing (Alicart et al., 2021; Dietrich et al., 2008; Johnson and Steenbergen, 2022; Mao et al., 2022). Several studies examining the effects of taVNS on the resting-state brain network have reported increased activation in limbic system regions, including the putamen, caudate, posterior cingulate cortex, amygdala, and parahippocampal gyrus (Keatch et al., 2022; Peng et al., 2018). Teckentrup et al. (2021) reported that taVNS increased network activation in the NTS. However, Kraus et al. (2007) reported that taVNS led to decreased network activation in the limbic system. This apparent discrepancy is likely attributable to variations in stimulation parameters and electrode placement. Specifically, studies reporting increased limbic system activation used electrodes affixed to the cymba concha, whereas those observing reduced activity used electrodes placed on the tragus. Several EEG-based studies demonstrated that taVNS enhanced attention, motor learning, and emotion regulation, indicating its potential as a tool for cognitive and affective modulation (Muller et al., 2022; Wang et al., 2023). Specifically, taVNS modulated alpha and beta brainwave activities, particularly within regions associated with attention, working memory, and cognitive processing (Konjusha et al., 2023; Sun et al., 2021; Vertanen, 2023). Notably, reductions in high-frequency (HF) alpha activity correlated with increased reaction times (RTs), suggesting enhanced cortical alertness and attention (Chen, et al., 2023a; Konakoglu et al., 2023; Konjusha et al., 2022). Changes in motor-related cortical potentials and RT following taVNS suggested enhanced neural efficiency during motor execution (Chen et al., 2024a; Chen et al., 2022; Chen, et al., 2023b; Gadeyne et al., 2022; Gurtubay et al., 2023). Functional connectivity analyses further highlighted alterations in the parietal lobe, reinforcing its role in sensorimotor integration (Poppa et al., 2022; Warren et al., 2020). Emotion-related event-related potentials were also affected by taVNS, with evidence suggesting that taVNS modulated neurophysiological activity in the frontal lobe. Specifically, taVNS enhanced the lateralization of alpha waves toward the right frontal hemisphere during Go/No-Go tasks, potentially supporting improved executive control (Keute et al., 2018; Kuhnel et al., 2020). However, although taVNS consistently produces neurophysiological effects, certain anticipated outcomes, such as alpha suppression, emotional memory formation, emotional responses, and autonomic modulation—have not been reliably replicated. These inconsistencies suggest that the precise mechanisms underlying the effects of taVNS remain poorly understood and warrant further investigation (Camargo et al., 2024). Moreover, motor-evoked potentials (MEPs) and TMS-evoked EEG potentials (TEPs) were used to evaluate the effects of taVNS, particularly on motor-related brain activities. MEPs and TEPs were not significantly influenced at the group level; taVNS with a higher intensity (>2.5 mA) decreased cortical excitability, as evidenced by increased resting motor thresholds, reduced MEP amplitudes, and EEG analysis (D’Agostini, 2023; Pihlaja et al., 2020; Sharon et al., 2021; Ventura-Bort et al., 2021; Warren et al., 2019). taVNS selectively influenced motor performance through the modulation of the GABAergic system in the motor cortex without cholinergic circuits. These were evaluated using short-interval intracortical inhibition and short-latency afferent inhibition. These outcome measures are integral to cognitive processing and motor function (Capone et al., 2015; Horinouchi et al., 2024; Mertens et al., 2022; van Midden et al., 2024a; Wang et al., 2022).
Overall, taVNS modulates cortical activity related to motor function, and these effects can be evaluated using noninvasive brain imaging techniques.
3.2.3.2 Regulation of the autonomic nervous system
Autonomic nervous system effects were assessed using HR, blood pressure, HRV, pupil size, and salivary biomarkers as key outcome measures. Sinkovec et al. (2021) reported significant reductions in HR, left ventricular contractility, and left ventricular output with right taVNS, resulting in a beneficial reduction in left ventricular workload. HRV serves as a robust indicator of parasympathetic activity and sympathetic suppression, with increases in the root mean square of successive differences and HF components of HRV, along with decreases in the low-frequency (LF) to HF (LF/HF) ratio, representing significant markers of autonomic modulation (Geng D. et al., 2022; Geng D. Y. et al., 2022; Petersen et al., 2024; Sclocco et al., 2020; Tobaldini et al., 2019; Toschi et al., 2023). Changes in HRV have been associated with elevated nociceptive withdrawal reflex thresholds and attenuated stress responses, highlighting their potential for treating chronic pain and psychiatric disorders (Yokota et al., 2024). Furthermore, HRV enhancement has been implicated in the restoration of autonomic balance through sympathetic inhibition and parasympathetic activation, particularly in conditions characterized by sympathetic overactivity, such as heart failure (Clancy et al., 2014). Additionally, taVNS improves cardiac baroreflex sensitivity and autonomic modulation (Antonino et al., 2017). HRV modulation is also associated with the mitigation of initial stress responses, nociceptive processing, and the enhancement of cognitive function, highlighting its broader physiological relevance (Austelle et al., 2024; Le Roy et al., 2023). taVNS influences pupil diameter under specific conditions, with significantly greater dilation observed during scotopic illumination and phasic stimulation compared with sham conditions (Skora et al., 2024). The pupillary response appears to scale with the intensity of the taVNS, suggesting possible engagement of the LC–noradrenergic (LC–NA) system (D’Agostini et al., 2023). However, interindividual variability and dependence on prestimulation pupil diameter, a proxy for tonic LC–NA activity, pose challenges to consistency. Further investigations are warranted to determine whether the evoked pupillary changes reflect orienting responses or are attributable to somatosensory perception. Hence, although pupillometry shows promise as a noninvasive biomarker of taVNS effect, its reliability and specificity require rigorous validation (Keute et al., 2019b; Villani et al., 2022; Wienke et al., 2023).
Salivary alpha-amylase (sAA) has been used as a biomarker to evaluate the effects of taVNS, potentially reflecting its NA activity. Although some studies have reported significant increases in sAA following taVNS, others have found no difference compared with sham conditions, resulting in inconsistent findings. Although sAA elevation has been observed under both sustained and brief stimulations, it remains unclear whether these changes directly correspond to NA activation. However, further studies are required to validate the reliability of sAA as a biomarker. Overall, although sAA has potential as an indicator of the effects of taVNS, further validation studies are required (Bömmer et al., 2024; D’Agostini et al., 2022; Fischer et al., 2018; Zhu et al., 2022).
Furthermore, in a study that simultaneously evaluated neurological indices, such as the autonomic nervous index and EEG, pupil dilation after taVNS suggested temporary noradrenaline activation and was associated with autonomic nervous regulation; however, no evident effect was observed on EEG (Lloyd et al., 2023). Thus, although effects have been observed for a single endpoint, inconsistent results have been reported across endpoints. In terms of gastrointestinal effects, HF taVNS enhances gastric motility by increasing the amplitude of peristaltic waves (Frokjaer et al., 2016; Steidel et al., 2021; Teckentrup et al., 2020). Furthermore, taVNS strengthens gastric-to-brain connectivity in the NTS and midbrain while simultaneously enhancing connectivity across broader brain regions (Muller et al., 2022). However, Gancheva et al. (2018) reported that taVNS did not acutely modulate the autonomic tone to the visceral organs, indicating discrepancies in findings between studies.
3.2.3.3 Enhancement of motor and cognitive functions
To investigate the effects of taVNS on the performance, Hatik et al. (2023) examined the effects of taVNS on exercise-induced pain and fatigue. Although taVNS significantly reduced pain and fatigue, it did not improve exercise performance. The observed fatigue recovery effect was attributed to attenuation of exercise-induced sympathetic overactivity by taVNS. Performance outcomes appear to be strongly influenced by individual motivation during experimental tasks (St Pierre and Shinohara, 2023; Wang et al., 2022).
Combining taVNS with interference control training enhances both cognitive function and task performance (Borges et al., 2019). In contrast, failure to apply taVNS at a certain time may impair motor learning (St Pierre and Shinohara, 2023). Studies investigating the effects of taVNS on memory have reported improved memory task scores (Cibulcova et al., 2024; Giraudier et al., 2020; Muller et al., 2022; Yakunina et al., 2017), although no significant effects have been observed on delayed recall (Keatch et al., 2022). Taking advantage of the overlapping brain modulation regions of taVNS and transcranial direct current stimulation (tDCS), a combined stimulation protocol yielded greater enhancement in working memory performance than either intervention alone (Zhao et al., 2022). Furthermore, taVNS may modulate selective aspects of memory processing.
In a multiday fear conditioning and extinction paradigm, Szeska et al. (2020) demonstrated that long-term use of taVNS enhanced the amygdala modulation of the fear-enhancing startle and its cognitive effects. Administering taVNS during the memory extinction phase facilitates the suppression of fear-enhancing startle responses and cognitive risk assessment, thereby reducing the likelihood of fear reinstatement. Collectively, these findings suggest that taVNS improves cognitive and memory functions and may influence memory processing mechanisms, potentially contributing to enhanced learning.
Based on the modulatory effects of taVNS on emotion and mood, studies have suggested that taVNS reduces emotional reactions or anxiety (Burger et al., 2016; Burger et al., 2017 Burger et al., 2018; Burger, 2019; Burger et al., 2019; Ferreira et al., 2024; Finisguerra et al., 2019; Jongkees et al., 2018; Keatch et al., 2023; Sellaro et al., 2018; Steenbergen et al., 2021; Szeska et al., 2021) and improves positive mood during effort tasks (Ferstl et al., 2022). Specifically, the lower the baseline positive mood, the more immediate was the improvement in motivation with taVNS. Regarding its effects on the reward system, some studies have reported the activation of reward functions, indicating increased motivation for reward acquisition rather than effort maintenance (Ferstl et al., 2022; Neuser et al., 2020). Conversely, taVNS does not result in reward activation, regardless of the reward type, scale, or trial difficulty in effort tasks (Lucchi et al., 2024). Neurological investigations have shown that taVNS may enhance the inhibitory control of emotions by acting within the inhibitory control network of the prefrontal cortex (Zhu et al., 2023).
Several studies have demonstrated the effectiveness of taVNS in addressing obesity, sleep disorders, pain, motion sickness, and language skills (Alicart et al., 2021; Altinkaya et al., 2023; Anzolin et al., 2023; Crupper, 2023; Kozorosky et al., 2022; Honda et al., 2024; Jackowska et al., 2022; Llanos et al., 2020; Maharjan, 2018; Mertens et al., 2020; Molefi et al., 2023; Müller et al., 2021; Obst et al., 2020; Phillips et al., 2021; Thakkar et al., 2020; Vosseler et al., 2020; Vishal et al., 2025).
3.3 Stroke
3.3.1 Safety
No serious adverse events were observed during the experiments in six of the nine studies. Li et al. (2022) noted that 2 of the 60 participants experienced skin redness, which promptly and completely subsided after adjusting the current intensity. Capone et al. (2017) reported no adverse events or discomfort. Two studies did not describe adverse events (Baig et al., 2019; Peng et al., 2023).
3.3.2 Parameters
All nine studies provided detailed information on the stimulation parameters (Baig et al., 2019; Capone et al., 2017; Chang et al., 2021; Huguenard et al., 2024; Li et al., 2022; Liu et al., 2024; Peng et al., 2023; Wang L. et al., 2024; Wang M. H. et al., 2024). The parameters are summarized in Tables 4a–c. The predominant frequencies used were 25 Hz, with a duration of 300 µs and a duty cycle of 30 s “on” and 30 s “off”. However, there were inconsistencies in determining the intensity across studies. Most studies implemented session schedules lasting ≥10 days, indicating medium-to long-term intervention strategies (Capone et al., 2017; Chang et al., 2021; Li et al., 2022; Liu et al., 2024; Wang M. H. et al., 2024).
3.3.3 Efficacy
The clinical efficacy of taVNS in conjunction with rehabilitation interventions and other neuromodulatory devices has been demonstrated. Of the nine stroke studies, three applied taVNS alone and reported improvements in upper-limb motor recovery, sensory function, or depressive symptoms. The remaining six studies combined taVNS with rehabilitation training, robotic therapy, or tDCS, reported enhanced functional outcomes such as gait, balance, and activities of daily living (Baig et al., 2019; Capone et al., 2017; Chang et al., 2021; Li et al., 2022; Wang L. et al., 2024; Wang M. H. et al., 2024). In the robotic therapy trials, participants received identical robotic training with either taVNS or sham taVNS; both studies reported larger gains in upper-limb function in the taVNS + robotic arm relative to robotic + sham, indicating an added benefit of taVNS under matched co-intervention (Capone et al., 2017; Chang et al., 2021). In trials combining taVNS and tDCS, arm structures varied (e.g., taVNS + tDCS vs. tDCS alone vs. taVNS alone vs. control). The combination arm generally showed the largest improvements; however, formal taVNS × tDCS interaction tests were not reported, so synergy cannot be established. Across studies, taVNS-alone arms improved motor outcomes versus their control/sham comparators, albeit with small samples and heterogeneous dosing (Wang M. H. et al., 2024). Futhermore, combining taVNS with rehabilitation has demonstrated notable improvements in motor and sensory functions and in managing post-stroke depression (Baig et al., 2019; Li et al., 2022). Peng et al. (2023) indicated that all active taVNS conditions (ipsilesional, contralesional, and bilateral) significantly attenuated activity in the contralesional default mode network compared with the sham condition. However, only ipsilesional taVNS led to a significant increase in activation in the ipsilesional visuomotor and secondary visual cortices, while simultaneously reducing visuomotor activity in the contralesional hemisphere. These results highlighted the strong influence of laterality on the effectiveness of taVNS, with ipsilesional stimulation having the most direct effect on brain activation. Huguenard et al. (2024) demonstrated the immunomodulatory effects of taVNS in patients with subarachnoid hemorrhage. This study excluded patients with SAH of traumatic etiology, patients with negative vascular imaging for aneurysm, and those receiving ongoing cancer therapy or immunosuppressive medication. taVNS significantly reduced pro-inflammatory cytokine levels in the plasma, attenuated moderate-to-severe vasospasm, and increased vessel caliber, highlighting its potential therapeutic role.
For the clinical evaluation to verify the effects of taVNS, FMA-UE was frequently used, with some studies also including assessments of the FMA lower extremity and the FMA sensory function. Motor performance outcomes, such as the Wolf Motor Function Test, timed up and go test (TUGT), Modified Barthel Index, and Berg Balance Scale scores, were measured. Only one study has used MEPs to assess cortical excitability (Wang M. H. et al., 2024). The evaluation of mental function included scales for depressive symptoms, such as the Hamilton Rating Scale for Depression and Hospital Anxiety and Depression Scale. fMRI was used to assess the effectiveness of taVNS.
3.4 Parkinson’s disease
3.4.1 Safety
Four studies reported no adverse reactions during the trials (Fu et al., 2024; Van Midden et al., 2024b; Zhang et al., 2023; Zhang et al., 2024). Lench et al. (2023) reported that some participants who received either taVNS or sham reported adverse events. The most frequently reported adverse event in the active taVNS group was difficulty sleeping, followed by lightheadedness, fatigue, nausea, tinnitus, tooth grinding, ear fluid, anxiety, and dizziness. The most frequently reported adverse event in the sham group was lightheadedness, followed by difficulty sleeping, headache, fatigue, difficulty concentrating, and neck pain. Only a few patients present with each of these symptoms. There were no reports of pain at the stimulation site, and no serious adverse events occurred in either group (Lench et al., 2023). One study reported no adverse events (Marano et al., 2024).
3.4.2 Parameters
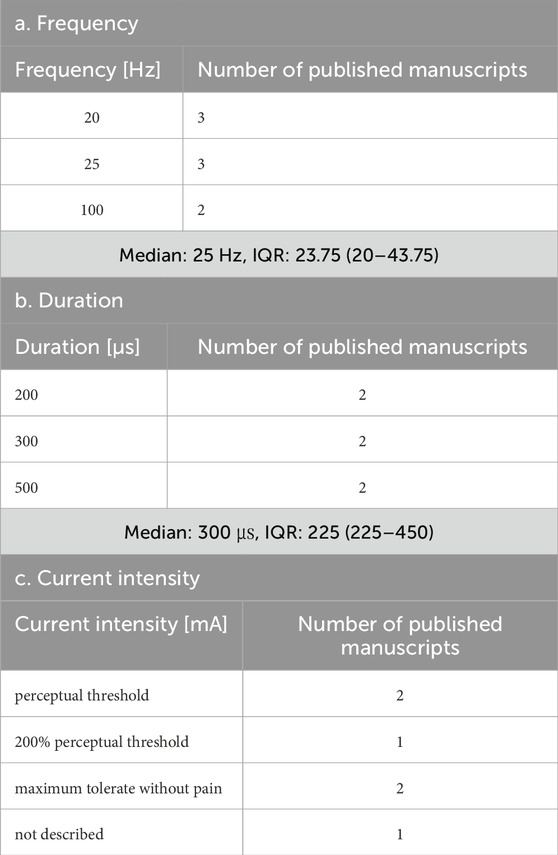
In all six studies, the electrodes were placed in the left ear. The specific parameters varied across each study. The parameters are summarized in Tables 5a–c. van Midden et al. (2024b) proposed that 25 Hz taVNS enhanced physical function and gait in patients with PD.
3.4.3 Efficacy
In PD, all six included studies investigated taVNS as a standalone intervention. Reported benefits included improvements in gait (speed, stride length, turning) and reductions in anxiety, with no combination interventions reported. Several studies have reported improvements in gait parameters, including gait speed, stride length, and 360° turn duration (van Midden et al., 2024b; Marano et al., 2024; Zhang et al., 2024). However, Marano et al. (2024) and Lench et al. (2023) found no significant clinical changes in the UPDRS scores in their respective reports. In terms of the mental function, Zhang et al. (2023) used the Hamilton Anxiety Rating Scale to assess anxiety symptoms and concluded that taVNS could alleviate anxiety in patients with PD. Regarding neurological background, MRI studies have shown that taVNS is associated with a widespread decrease in the amplitude of LF fluctuations (ALFF) in the right hemisphere, including the superior parietal lobule, precentral gyrus, postcentral gyrus, middle occipital gyrus, and cuneus. The ALFF in the right superior parietal lobule during taVNS was negatively correlated with the UPDRS Part III (Fu et al., 2024).
To confirm the efficacy of taVNS in patients with PD, the brain neural activity and autonomic nervous system were assessed using functional near-infrared spectroscopy, fMRI, HR monitoring, and blood pressure measurements. The UPDRS and TUGT scores were used as outcome measures to assess PD symptoms.
4 Discussion
This review investigated taVNS, a noninvasive neuromodulation technique, focusing on three aspects, safety, efficacy, and stimulation parameters, in healthy participants, patients with stroke, and patients with PD. A total of 154 articles, including 6,485 participants, were examined. The majority of studies in healthy participants involved young to middle-aged adults, with relatively few focused on older populations. Age-related differences in neuroplasticity and autonomic regulation may influence the response to taVNS and warrant further investigation, particularly when comparing healthy adults with patients with stroke or PD. taVNS was demonstrated to be safe, with no serious adverse events reported in healthy individuals or in patients with stroke or PD. In terms of efficacy, taVNS modulates cortical excitability and autonomic nervous system activity, leading to improvements in motor and cognitive functions, as well as learning. The results were evaluated using neurophysiological indicators and task performance. However, outcome measures are insufficiently uniform, leading to differences in results and perspectives. Although commonly used parameters have been identified, they vary across studies, and many studies have emphasized their importance. Although some studies have focused on these parameters, the number of studies remains limited. It was also suggested that the effect may be influenced by the stimulus condition.
The overall quality of the evidence base is limited. Most studies had small sample sizes, many lacked blinding or sham controls, and outcome measures and stimulation parameters were heterogeneous. Reporting of adverse events was frequently incomplete. Consequently, confidence in efficacy remains low-to-moderate, whereas conclusions on safety are more consistent but constrained by potential under-reporting. In addition, a limitation of this review is that its scope excludes other indications (e.g., depression, epilepsy, gastrointestinal or cardiac disorders), which may limit generalizability. Future reviews should extend this parameter–outcome framework to additional conditions once sufficient methodologically comparable evidence becomes available. Finally, a methodological limitation of this review is that screening and data extraction were performed by a single reviewer. Although this ensured consistency in study selection and data handling, it may have increased the risk of bias compared to a double-review process. Future reviews in this field should ideally incorporate independent screening and extraction by at least two reviewers to enhance methodological rigor.
4.1 Safety of taVNS
taVNS activates the ipsilateral NTS to stimulate and send impulses to the heart via the efferent cervical vagus nerve, suggesting the possibility of avoiding direct and asymmetric stimulation of the vagus nerve, unlike implanted VNS (Kim et al., 2022). Previous systematic reviews and meta-analyses have noted a low incidence of arrhythmia and bradycardia, which are concerning side effects of implantable VNS. Furthermore, cardiac side effects are rare, because taVNS is currently used to treat heart failure and atrial fibrillation (Dalgleish et al., 2021). Based on these results, taVNS is considered safe.
In this review, the adverse events associated with taVNS mainly included transient ear pain, redness, itching, and other forms of discomfort. No severe adverse effects were observed. However, safety reports may be inadequate, which can be considered a limitation of the study. Most clinical studies, including those involving patients with stroke and PD, have reported the presence or absence of adverse events and their safety. Consistent with our findings, a recent systematic review of tVNS in patients with PD reported a favorable safety profile, with no serious adverse events and only mild, transient side effects such as headache, fatigue, or local discomfort (Shan et al., 2024). This further supports the view that tVNS can be safely applied in PD when appropriate stimulation parameters are used. However, 54% of the studies included in this review on healthy participants did not report the adverse events, leaving it unclear whether any occurred.
Previous reviews have stated that taVNS is well-tolerated and safe in humans at the doses tested, with no adverse events directly attributable to it. However, concerns have been raised regarding underreporting, mandatory reporting of safety and tolerability results, and the need for standardization of adverse event measurement methods. Moreover, the different taVNS parameters used in studies reporting adverse events may not allow accurate comparisons (Redgrave et al., 2018). Based on these insights, although this review partially supports previous findings on the safety of taVNS, the evidence remains insufficient for generalization. Future studies should thoroughly report side effects and adverse events as well as focus on the conditions of use, including stimulation parameters, to ensure safety.
4.2 Parameters of taVNS
This review found that although parameters are important for safety and efficacy, more consistent parameters and measures are required to assess them. The most frequently used frequency was 25 Hz, and the duration was 200–300 μs, which was consistently adopted. However, there are various ways to determine the current intensity, such as below the pain threshold, at the sensory threshold, twice the sensory threshold, and mild tingling. This result highlights the importance of not only maintaining consistency in the intensity value itself but also clearly defining how the intensity is set. Future studies focusing on the current intensity are necessary. Similarly, the stimulation time and duty cycle significantly vary among studies and should be considered in the context of subject burden and safety. Although most included studies applied taVNS to the left ear, partly owing to theoretical concerns regarding cardiac parasympathetic innervation of the right vagus, right-sided and bilateral approaches were also reported as safe and effective. Laterality is a crucial parameter, and although the majority of studies used left-ear stimulation for safety reasons, studies employing right-sided or bilateral stimulation similarly demonstrated safety and effects. Future research should continue to explore laterality to determine the conditions for optimal taVNS effectiveness. Parameter considerations have received attention in studies of healthy participants, and further studies aimed at clarifying stimulus parameters are expected in the future. The outcome measures were relatively consistent in basic studies including healthy individuals but varied owing to the multiple measures of autonomic assessment, such as HR, HRV, pupil size, and sAA. However, another limitation is the variability across studies, largely because of the small number of available clinical investigations. Therefore, in the present review, the variability in the assessment measures identified as challenges could be due to the selection of multiple autonomic activity measures and the small number of clinical studies. A relatively large number of assessments using the same indicators in healthy participants may facilitate the establishment of evidence in the field of taVNS research by allowing for comparisons of stimulus settings and treatment outcomes.
Future studies should report the detailed and standardized parameters and metrics used. The clinical effectiveness must be tested in consistent stimulus settings, which would allow for comparisons between taVNS studies and contribute to evidence building.
4.2.1 Standardized reporting and parameter recommendations
To facilitate clinical translation, future studies should adopt standardized reporting guidelines, including complete disclosure of stimulation parameters (frequency, duration, intensity relative to pain/sensory thresholds, electrode placement, and session duration) and participant characteristics (Badran et al., 2018b; Dumoulin et al., 2021; Wang M. H. et al., 2024; Zhang et al., 2024). Based on currently available evidence, commonly applied settings, such as 25 Hz frequency, 200–300 μs duration, and sub-pain threshold intensities, may serve as starting points but should be validated and optimized across conditions. Core outcome measures, including HRV for autonomic modulation, the Fugl–Meyer Assessment or UPDRS for motor outcomes, and validated cognitive and mood scales, should be prioritized to enable comparability and evidence accumulation. Inconsistencies among studies may stem not only from methodological heterogeneity but also from biological and clinical variability. Factors such as age, sex, disease severity, and concomitant treatments may modulate vagal sensitivity and responsiveness. Study design differences—including unilateral versus bilateral stimulation, task-related versus resting-state application, and acute versus multi-session protocols—also likely contribute to divergent findings (Camargo et al., 2024; Gerges et al., 2024a; Janner et al., 2018; Sellaro et al., 2015a; Yokota et al., 2022; Szulczewski et al., 2023). Addressing these dimensions in future research will be critical to clarify mechanistic underpinnings and maximize the therapeutic potential of taVNS.
4.3 Efficacy of taVNS
The mechanism of action of taVNS is projection from the ABVN, which is an afferent fiber innervating mainly the ear, to the LC and raphe nuclei via the NTS, and modulatory effects on the cortex, limbic system, and other areas. Consequently, taVNS has shown potential for the treatment of epilepsy, depression, stroke, and PD. The vagus nerve is an important component of the parasympathetic nervous system, and taVNS increases parasympathetic activity (Kania et al., 2021). Modulation of parasympathetic activity is associated with clinical conditions, such as heart failure, inflammatory bowel disease, and chronic pain syndromes (Butt et al., 2020; Veiz et al., 2022). Mechanistically, taVNS activates afferent projections from the auricular branch of the vagus nerve to the NTS. The NTS, in turn, engages the locus coeruleus–noradrenergic and raphe nuclei–serotonergic systems, influencing widespread cortical and subcortical regions, including the prefrontal cortex, cingulate cortex, basal ganglia, and limbic areas (Ludwig et al., 2021; Huang et al., 2023). In addition, taVNS has been reported to modulate GABAergic activity in the motor cortex (Van Midden et al., 2023; Chen et al., 2024b; Keute et al., 2019a; Beste et al., 2016). Collectively, these pathways provide a neurophysiological basis for the observed effects of taVNS on cognition, motor control, and emotional regulation (Johnson and Steenbergen, 2022). This review focuses on the applications of taVNS in central nervous system diseases. These findings indicate that taVNS enhances activity in brainstem regions, such as the NTS, LC, and raphe nuclei, and in the insular cortex, thalamus, prefrontal cortex, motor cortex, cingulate cortex, amygdala, and striatum, as observed in experiments utilizing fMRI and EEG. This stimulation increases the cortical excitability and affects motor function, attention, and emotion regulation. Modulatory effects on autonomic nervous system activity have also been reported using biomarkers, such as HR, blood pressure, HRV, pupil diameter, and salivary amylase. These markers are promising in determining the effect of taVNS. These effects have also been observed in previous patient studies. Modulation of these brain regions and their functions have also been observed in healthy participants, although some reports have found no effects on HRV, pupil diameter, sAA, or memory performance. These differences indicate that consistent results have not been achieved. Thus, the present review found that the effects of taVNS were typically consistent with the mechanism of action described in previous studies; however, further studies are needed to confirm whether consistent results can be achieved (Figure 4).
In clinical reserches, in stroke patients, taVNS has been used in combination with rehabilitation and tDCS. However, because most combination studies did not implement full factorial designs or report interaction terms, current evidence supports an added benefit of taVNS when layered onto rehabilitation or tDCS, rather than demonstrating synergy per se. Differences in session number, stimulus dosing, and small samples further limit causal attribution. Future trials should adopt adequately powered factorial designs with matched dosing and pre-specified interaction analyses to formally adjudicate additivity vs. synergy. In patients with PD, after our search window closed, a recent meta-analysis reported no significant overall improvement in global motor symptoms with non-invasive VNS in PD, while suggesting a possible benefit for freezing of gait; these findings reinforce the need for larger, well-controlled trials with harmonized endpoints (Abouelmagd et al., 2025). Further research and discussion are required to examine the consistent efficacy of taVNS in clinical studies.
5 Conclusion
taVNS appears to be safe and shows promise for modulating motor, cognitive, and autonomic functions in healthy individuals, as well as in patients with stroke and PD. However, the current evidence is limited by small sample sizes, heterogeneous protocols, and short-term follow-up. Future research should prioritize long-term safety monitoring, standardization of stimulation parameters, harmonization of outcome measures, and large-scale multicenter clinical trials to generate definitive evidence for clinical application.
Author contributions
MM: Writing – original draft, Writing – review and editing, Conceptualization. TY: Writing – original draft, Writing – review and editing, Conceptualization, Funding acquisition. TF: Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the JSPS KAKENHI (grant no. 23K27943 to TY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1693907/full#supplementary-material
Abbreviations
ABVN, auricular branch of the vagus nerve; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; HR, heart rate; HRV, heart rate variability; LC, locus coeruleus; NTS, nucleus tractus solitarius; PD, Parkinson’s disease; sAA, salivary alpha-amylase; taVNS, transcutaneous auricular vagus nerve stimulation.
References
Abouelmagd M. E., Yousef O., Ibrahim I. A., Elshahat A. (2025). Effectiveness of non-invasive vagal nerve stimulation in Parkinson's disease: a comprehensive systematic review and meta-analysis. J. Clin. Neurosci- 133, 111016. doi:10.1016/j.jocn.2024.111016
Alicart H., Heldmann M., Gottlich M., Obst M. A., Tittgemeyer M., Munte T. F. (2021). Modulation of visual processing of food by transcutaneous vagus nerve stimulation (tVNS). Brain Imaging Behav. 15, 1886–1897. doi:10.1007/s11682-020-00382-8
Altinkaya Z., Ozturk L., Buyukguduk I., Yanik H., Yilmaz D. D., Yar B., et al. (2023). Non-invasive vagus nerve stimulation in a hungry state decreases heart rate variability. Physiol. Behav. 258, 114016. doi:10.1016/j.physbeh.2022.114016
Antonino D., Teixeira A. L., Maia-Lopes P. M., Souza M. C., Sabino-Carvalho J. L., Murray A. R., et al. (2017). Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul. 10, 875–881. doi:10.1016/j.brs.2017.05.006
Anzolin A., Das P., Garcia R. G., Chen A., Grahl A., Ellis S., et al. (2023). Delta power during sleep is modulated by EEG-gated auricular vagal afferent nerve stimulation (EAVANS). Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2023, 1–4. doi:10.1109/embc40787.2023.10340971
Austelle C. W., Sege C. T., Kahn A. T., Gregoski M. J., Taylor D. L., Mcteague L. M., et al. (2024). Transcutaneous auricular vagus nerve stimulation attenuates early increases in heart rate associated with the cold pressor test. Neuromodulation 27, 1227–1233. doi:10.1016/j.neurom.2023.07.012
Badran B. W., Dowdle L. T., Mithoefer O. J., Labate N. T., Coatsworth J., Brown J. C., et al. (2018a). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 11, 492–500. doi:10.1016/j.brs.2017.12.009
Badran B. W., Mithoefer O. J., Summer C. E., Labate N. T., Glusman C. E., Badran A. W., et al. (2018b). Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 11, 699–708. doi:10.1016/j.brs.2018.04.004
Baig S. S., Falidas K., Laud P. J., Snowdon N., Farooq M. U., Ali A., et al. (2019). Transcutaneous auricular vagus nerve stimulation with upper limb repetitive task practice may improve sensory recovery in chronic stroke. J. Stroke Cerebrovasc. Dis. 28, 104348. doi:10.1016/j.jstrokecerebrovasdis.2019.104348
Beste C., Steenbergen L., Sellaro R., Grigoriadou S., Zhang R., Chmielewski W., et al. (2016). Effects of concomitant stimulation of the GABAergic and norepinephrine system on inhibitory control - a study using transcutaneous vagus nerve stimulation. Brain Stimul. 9, 811–818. doi:10.1016/j.brs.2016.07.004
Bömmer T., Schmidt L. M., Meier K., Kricheldorff J., Stecher H., Herrmann C. S., et al. (2024). Impact of stimulation duration in taVNS-exploring multiple physiological and cognitive outcomes. Brain Sci. 14, 875. doi:10.3390/brainsci14090875
Borges U., Laborde S., Raab M. (2019). Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: not different from sham stimulation and no effect of stimulation intensity. PLoS One 14, e0223848. doi:10.1371/journal.pone.0223848
Borges U., Knops L., Laborde S., Klatt S., Raab M. (2020). Transcutaneous vagus nerve stimulation may enhance only specific aspects of the core executive functions. A randomized crossover trial. Front. Neurosci. 14, 523. doi:10.3389/fnins.2020.00523
Borgmann D., Rigoux L., Kuzmanovic B., Thanarajah S. E., Munte T. F., Fenselau H., et al. (2021). Technical note: modulation of fMRI brainstem responses by transcutaneous vagus nerve stimulation. Neuroimage 244, 118566. doi:10.1016/j.neuroimage.2021.118566
Burger A. M. (2019). “Hitting the right nerve: effects of transcutaneous vagus nerve stimulation on symptoms of anxiety,”. Dissertation/master’s thesis (Leiden, Netherlands: Leiden University).
Burger A. M., Verkuil B., Van Diest I., Van der Does W., Thayer J. F., Brosschot J. F. (2016). The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol. Learn Mem. 132, 49–56. doi:10.1016/j.nlm.2016.05.007
Burger A. M., Verkuil B., Fenlon H., Thijs L., Cools L., Miller H., et al. (2017). Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behav. Res. Ther. 97, 64–74. doi:10.1016/j.brat.2017.07.005
Burger A. M., Van Diest I., Van der Does W., Hysaj M., Thayer J. F., Brosschot J. F., et al. (2018). Transcutaneous vagus nerve stimulation and extinction of prepared fear: a conceptual non-replication. Sci. Rep. 8, 11471. doi:10.1038/s41598-018-29561-w
Burger A. M., Van Diest I., Van der Does W., Korbee J. N., Waziri N., Brosschot J. F., et al. (2019). The effect of transcutaneous vagus nerve stimulation on fear generalization and subsequent fear extinction. Neurobiol. Learn Mem. 161, 192–201. doi:10.1016/j.nlm.2019.04.006
Butt M. F., Albusoda A., Farmer A. D., Aziz Q. (2020). The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 236, 588–611. doi:10.1111/joa.13122
Camargo L., Pacheco-Barrios K., Gianlorenco A. C., Menacho M., Choi H., Song J. J., et al. (2024). Evidence of bottom-up homeostatic modulation induced taVNS during emotional and Go/No-Go tasks. Exp. Brain Res. 242, 2069–2081. doi:10.1007/s00221-024-06876-x
Capone F., Assenza G., Di Pino G., Musumeci G., Ranieri F., Florio L., et al. (2015). The effect of transcutaneous vagus nerve stimulation on cortical excitability. J. Neural. Transm. (Vienna) 122, 679–685. doi:10.1007/s00702-014-1299-7
Capone F., Miccinilli S., Pellegrino G., Zollo L., Simonetti D., Bressi F., et al. (2017). Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural. Plast. 2017, 7876507. doi:10.1155/2017/7876507
Capone F., Motolese F., Di Zazzo A., Antonini M., Magliozzi A., Rossi M., et al. (2021). The effects of transcutaneous auricular vagal nerve stimulation on pupil size. Clin. Neurophysiol. 132, 1859–1865. doi:10.1016/j.clinph.2021.05.014
Chang J. L., Coggins A. N., Saul M., Paget-Blanc A., Straka M., Wright J., et al. (2021). Transcutaneous auricular vagus nerve stimulation (tAVNS) delivered during upper limb interactive robotic training demonstrates novel antagonist control for reaching movements following stroke. Front. Neurosci. 15, 767302. doi:10.3389/fnins.2021.767302
Chen L., Zhang J., Wang Z., Zhang X., Zhang L., Xu M., et al. (2022). Effects of transcutaneous vagus nerve stimulation (tVNS) on action planning: a behavioural and EEG study. IEEE Trans. Neural. Syst. Rehabil. Eng. 30, 1675–1683. doi:10.1109/TNSRE.2021.3131497
Chen Y., Lu X., Hu L. (2023a). Transcutaneous auricular vagus nerve stimulation facilitates cortical arousal and alertness. Int. J. Environ. Res. Public Health 20, 1402. doi:10.3390/ijerph20021402
Chen Y., Yang H., Wang F., Lu X., Hu L. (2023b). Modulatory effects of transcutaneous auricular vagus nerve stimulation (taVNS) on attentional processes. Gen. Psychiatr. 36, e101176. doi:10.1136/gpsych-2023-101176
Chen L., He J., Zhang J., Wang Z., Zhang L., Gu B., et al. (2024a). Influence of transcutaneous vagus nerve stimulation on motor planning: a resting-state and task-state EEG study. IEEE J. Biomed. Health Inf. 28, 1374–1385. doi:10.1109/JBHI.2023.3324085
Chen L., Tang C., Wang Z., Zhang L., Gu B., Liu X., et al. (2024b). Enhancing motor sequence learning via transcutaneous auricular vagus nerve stimulation (taVNS): an EEG study. IEEE J. Biomed. Health Inf. 28, 1285–1296. doi:10.1109/jbhi.2023.3344176
Cibulcova V., Koenig J., Jackowska M., Jandackova V. K. (2024). Influence of a 2-week transcutaneous auricular vagus nerve stimulation on memory: findings from a randomized placebo controlled trial in non-clinical adults. Clin. Auton. Res. 34, 447–462. doi:10.1007/s10286-024-01053-0
Clancy J. A., Mary D. A., Witte K. K., Greenwood J. P., Deuchars S. A., Deuchars J. (2014). Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 7, 871–877. doi:10.1016/j.brs.2014.07.031
Colzato L. S., Ritter S. M., Steenbergen L. (2018a). Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia 111, 72–76. doi:10.1016/j.neuropsychologia.2018.01.003
Colzato L. S., Wolters G., Peifer C. (2018b). Transcutaneous vagus nerve stimulation (tVNS) modulates flow experience. Exp. Brain Res. 236, 253–257. doi:10.1007/s00221-017-5123-0
Crupper J. (2023). The effects of transcutaneous auricular vagus nerve stimulation on language retention in college-aged students. Boller Rev. 7, 1–22. doi:10.18776/tcu/br/7/168
Dalgleish A. S., Kania A. M., Stauss H. M., Jelen A. Z. (2021). Occipitoatlantal decompression and noninvasive vagus nerve stimulation slow conduction velocity through the atrioventricular node in healthy participants. J. Osteopath. Med. 121, 349–359. doi:10.1515/jom-2020-0213
De Couck M., Cserjesi R., Caers R., Zijlstra W. P., Widjaja D., Wolf N., et al. (2017). Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton. Neurosci. 203, 88–96. doi:10.1016/j.autneu.2016.11.003
Dietrich S., Smith J., Scherzinger C., Hofmann-Preiss K., Freitag T., Eisenkolb A., et al. (2008). A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed. Tech. Berl. 53, 104–111. doi:10.1515/BMT.2008.022
Dolphin H., Dukelow T., Finucane C., Commins S., McElwaine P., Kennelly S. P. (2022). The wandering nerve linking heart and mind - the complementary role of transcutaneous Vagus nerve stimulation in modulating neuro-cardiovascular and cognitive performance. Front. Neurosci. 16, 897303. doi:10.3389/fnins.2022.897303
Dong X., Tang Y., Zhou Y., Feng Z. (2023). Stimulation of vagus nerve for patients with disorders of consciousness: a systematic review. Front. Neuroscience- 17, 1257378. doi:10.3389/fnins.2023.1257378
Dumoulin M., Liberati G., Mouraux A., Santos S. F., El Tahry R. (2021). Transcutaneous auricular VNS applied to experimental pain: a paired behavioral and EEG study using thermonociceptive CO2 laser. PLoS One 16, e0254480. doi:10.1371/journal.pone.0254480
D’agostini M., Burger A. M., Franssen M., Claes N., Weymar M., Von Leupoldt A., et al. (2021). Effects of transcutaneous auricular vagus nerve stimulation on reversal learning, tonic pupil size, salivary alpha-amylase, and cortisol. Psychophysiology 58, e13885. doi:10.1111/psyp.13885
D’agostini M., Burger A. M., Villca Ponce G., Claes S., Von Leupoldt A., Van Diest I. (2022). No evidence for a modulating effect of continuous transcutaneous auricular vagus nerve stimulation on markers of noradrenergic activity. Psychophysiology 59, e13984. doi:10.1111/psyp.13984
D’Agostini M., Burger A. M., Jelinčić V., von Leupoldt A., Van Diest I. (2023). Effects of transcutaneous auricular vagus nerve stimulation on P300 magnitudes and salivary alpha-amylase during an auditory oddball task. Biol. Psychol. 182, 108646. doi:10.1016/j.biopsycho.2023.108646
D’Agostini M., Burger A. M., Franssen M., Perkovic A., Claes S., von Leupoldt A., et al. (2023). Short bursts of transcutaneous auricular vagus nerve stimulation enhance evoked pupil dilation as a function of stimulation parameters. Cortex 159, 233–253. doi:10.1016/j.cortex.2022.11.012
Engineer N. D., Kimberley T. J., Prudente C. N., Dawson J., Tarver W. B., Hays S. A. (2019). Targeted Vagus nerve stimulation for rehabilitation after stroke. Front. Neurosci- 13, 280. doi:10.3389/fnins.2019.00280
Ferreira L. M. A., Brites R., Fraião G., Pereira G., Fernandes H., De Brito J. A. A., et al. (2024). Transcutaneous auricular vagus nerve stimulation modulates masseter muscle activity, pain perception, and anxiety levels in university students: a double-blind, randomized, controlled clinical trial. Neurosci 18, 1422312. doi:10.3389/fnint.2024.1422312
Ferstl M., Teckentrup V., Lin W. M., Krautlein F., Kuhnel A., Klaus J., et al. (2022). Non-invasive vagus nerve stimulation boosts mood recovery after effort exertion. Psychol. Med. 52, 3029–3039. doi:10.1017/S0033291720005073
Finisguerra A., Crescentini C., Urgesi C. (2019). Transcutaneous vagus nerve stimulation affects implicit spiritual self-representations. Neuroscience 412, 144–159. doi:10.1016/j.neuroscience.2019.05.059
Fischer R., Ventura-Bort C., Hamm A., Weymar M. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cogn. Affect. Behav. Neurosci. 18, 680–693. doi:10.3758/s13415-018-0596-2
Forte G., Favieri F., Leemhuis E., De Martino M. L., Giannini A. M., De Gennaro L., et al. (2022). Ear your heart: transcutaneous auricular vagus nerve stimulation on heart rate variability in healthy young participants. PeerJ 10, e14447. doi:10.7717/peerj.14447
Frokjaer J. B., Bergmann S., Brock C., Madzak A., Farmer A. D., Ellrich J., et al. (2016). Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol. Motil. 28, 592–598. doi:10.1111/nmo.12760
Fu C., Hou X., Zheng C., Zhang Y., Gao Z., Yan Z., et al. (2024). Immediate modulatory effects of transcutaneous vagus nerve stimulation on patients with Parkinson's disease: a crossover self-controlled fMRI study. Front. Aging Neurosci. 16, 1444703. doi:10.3389/fnagi.2024.1444703
Gadeyne S., Mertens A., Carrette E., Van Den Bossche F., Boon P., Raedt R., et al. (2022). Transcutaneous auricular vagus nerve stimulation cannot modulate the P3b event-related potential in healthy volunteers. Clin. Neurophysiol. 135, 22–29. doi:10.1016/j.clinph.2021.11.079
Galvez-Garcia G., Mena-Chamorro P., Espinoza-Palavicino T., Romero-Arias T., Barramuno-Medina M., Bascour-Sandoval C. (2024). Mixing transcutaneous vagal nerve stimulation and galvanic cutaneous stimulation to decrease simulator adaptation syndrome. Front. Psychol. 15, 1476021. doi:10.3389/fpsyg.2024.1476021
Gancheva S., Bierwagen A., Markgraf D. F., Bonhof G. J., Murphy K. G., Hatziagelaki E., et al. (2018). Constant hepatic ATP concentrations during prolonged fasting and absence of effects of Cerbomed nemos® on parasympathetic tone and hepatic energy metabolism. Mol. Metab. 7, 71–79. doi:10.1016/j.molmet.2017.10.002
Gao Y., Zhu Y., Lu X., Wang N., Zhu S., Gong J., et al. (2023). Vagus nerve stimulation paired with rehabilitation for motor function, mental health and activities of daily living after stroke: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 94, 257–266. doi:10.1136/jnnp-2022-329275
Gauthey A., Morra S., Van De Borne P., Deriaz D., Maes N., Le Polain De Waroux J. B. (2020). Sympathetic effect of auricular transcutaneous vagus nerve stimulation on healthy subjects: a crossover controlled clinical trial comparing vagally mediated and active control stimulation using microneurography. Front. Physiol. 11, 599896. doi:10.3389/fphys.2020.599896
Geng D., Liu X., Wang Y., Wang J. (2022a). The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS One 17, e0263833. doi:10.1371/journal.pone.0263833
Geng D. Y., Yang K., Fu Z. G., Zhang Y., Wang C., An H. X. (2022b). Circadian stage-dependent and stimulation duration effects of transcutaneous auricular vagus nerve stimulation on heart rate variability. Plos One 17, e0277090. doi:10.1371/journal.pone.0277090
Gerges A. N. H., Graetz L., Hillier S., Uy J., Hamilton T., Opie G., et al. (2024a). Transcutaneous auricular vagus nerve stimulation modifies cortical excitability in middle-aged and older adults. Psychophysiology 62, e14584. doi:10.1111/psyp.14584
Gerges A. N. H., Williams E. E. R., Hillier S., Uy J., Hamilton T., Chamberlain S., et al. (2024b). Clinical application of transcutaneous auricular vagus nerve stimulation: a scoping review. Disabil. Rehabil. 46, 5730–5760. doi:10.1080/09638288.2024.2313123
Gianlorenco A. C., Pacheco-Barrios K., Camargo L., Pichardo E., Choi H., Song J. J., et al. (2024). Understanding the effects of non-invasive transauricular vagus nerve stimulation on EEG and HRV. J. Vis. Exp. 203. doi:10.3791/66309
Giraudier M., Ventura-Bort C., Weymar M. (2020). Transcutaneous vagus nerve stimulation (tVNS) improves high-confidence recognition memory but not emotional word processing. Front. Psychol. 11, 1276. doi:10.3389/fpsyg.2020.01276
Gurtubay I. G., Perez-Rodriguez D. R., Fernandez E., Librero-Lopez J., Calvo D., Bermejo P., et al. (2023). Immediate effects and duration of a short and single application of transcutaneous auricular vagus nerve stimulation on P300 event related potential. Front. Neurosci. 17, 1096865. doi:10.3389/fnins.2023.1096865
Hagen K., Ehlis A. C., Schneider S., Haeussinger F. B., Fallgatter A. J., Metzger F. G. (2014). Influence of different stimulation parameters on the somatosensory evoked potentials of the nervus vagus--how varied stimulation parameters affect VSEP. J. Clin. Neurophysiol. 31, 143–148. doi:10.1097/WNP.0000000000000038
Hatik S. H., Asrlan M., Demirbilek O., Ozden A. V. (2023). The effect of transcutaneous auricular vagus nerve stimulation on cycling ergometry and recovery in healthy young individuals. Brain Behav. 13, e3332. doi:10.1002/brb3.3332
Honda C. T., Bhutani N., Clayards M., Baum S. (2024). No clear benefit of transcutaneous auricular vagus nerve stimulation for non-native speech sound learning. Front. Lang. Sci. 3, 1403080. doi:10.3389/flang.2024.1403080
Horinouchi T., Nezu T., Saita K., Date S., Kurumadani H., Maruyama H., et al. (2024). Transcutaneous auricular vagus nerve stimulation enhances short-latency afferent inhibition via central cholinergic system activation. Sci. Rep. 14, 11224. doi:10.1038/s41598-024-61958-8
Hua K., Cummings M., Bernatik M., Brinkhaus B., Usichenko T., Dietzel J. (2023). Cardiovascular effects of auricular stimulation -a systematic review and meta-analysis of randomized controlled clinical trials. Front. Neurosci. 17, 1227858. doi:10.3389/fnins.2023.1227858
Huang Y., Zhang Y., Hodges S., Li H., Yan Z., Liu X., et al. (2023). The modulation effects of repeated transcutaneous auricular vagus nerve stimulation on the functional connectivity of key brainstem regions along the vagus nerve pathway in migraine patients. Front. Mol. Neurosci. 16, 1160006. doi:10.3389/fnmol.2023.1160006
Huguenard A., Tan G., Johnson G., Adamek M., Coxon A., Kummer T., et al. (2024). Non-invasive auricular vagus nerve stimulation for subarachnoid hemorrhage (NAVSaH): protocol for a prospective, triple-blinded, randomized controlled trial. PLoS One 19, e0301154. doi:10.1371/journal.pone.0301154
Jackowska M., Koenig J., Vasendova V., Jandackova V. K. (2022). A two-week course of transcutaneous vagal nerve stimulation improves global sleep: findings from a randomised trial in community-dwelling adults. Auton. Neurosci. 240, 102972. doi:10.1016/j.autneu.2022.102972
Jacobs H. I., Riphagen J. M., Razat C. M., Wiese S., Sack A. T. (2015). Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging 36, 1860–1867. doi:10.1016/j.neurobiolaging.2015.02.023
Janner H., Klausenitz C., Gurtler N., Hahnenkamp K., Usichenko T. I. (2018). Effects of electrical transcutaneous vagus nerve stimulation on the perceived intensity of repetitive painful heat stimuli: a blinded placebo- and sham-controlled randomized crossover investigation. Anesth. Analg. 126, 2085–2092. doi:10.1213/ANE.0000000000002820
Johnson K. V., Steenbergen L. (2022). Gut feelings: vagal stimulation reduces emotional biases. Neuroscience 494, 119–131. doi:10.1016/j.neuroscience.2022.04.026
Jongkees B. J., Immink M. A., Finisguerra A., Colzato L. S. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during sequential action. Front. Psychol. 9, 1159. doi:10.3389/fpsyg.2018.01159
Kania A. M., Weiler K. N., Kurian A. P., Opena M. L., Orellana J. N., Stauss H. M. (2021). Activation of the cholinergic antiinflammatory reflex by occipitoatlantal decompression and transcutaneous auricular vagus nerve stimulation. J. Osteopath. Med. 121, 401–415. doi:10.1515/jom-2020-0071
Keatch C., Lambert E., Woods W., Kameneva T. (2022). Measuring brain response to transcutaneous vagus nerve stimulation (tVNS) using simultaneous magnetoencephalography (MEG). J. Neural. Eng. 19, 026038. doi:10.1088/1741-2552/ac620c
Keatch C., Lambert E., Kameneva T., Woods W. (2023). Functional connectivity analysis of transcutaneous vagus nerve stimulation (tVNS) using magnetoencephalography (MEG). IEEE Trans. Neural. Syst. Rehabil. Eng. 31, 3630–3640. doi:10.1109/TNSRE.2023.3297736
Keute M., Ruhnau P., Heinze H. J., Zaehle T. (2018). Behavioral and electrophysiological evidence for GABAergic modulation through transcutaneous vagus nerve stimulation. Clin. Neurophysiol. 129, 1789–1795. doi:10.1016/j.clinph.2018.05.026
Keute M., Boehrer L., Ruhnau P., Heinze H. J., Zaehle T. (2019a). Transcutaneous vagus nerve stimulation (tVNS) and the dynamics of visual bistable perception. Front. Neurosci. 13, 227. doi:10.3389/fnins.2019.00227
Keute M., Demirezen M., Graf A., Mueller N. G., Zaehle T. (2019b). No modulation of pupil size and event-related pupil response by transcutaneous auricular vagus nerve stimulation (taVNS). Sci. Rep. 9, 11452. doi:10.1038/s41598-019-47961-4
Keute M., Wienke C., Ruhnau P., Zaehle T. (2021). Effects of transcutaneous vagus nerve stimulation (tVNS) on beta and gamma brain oscillations. Cortex 140, 222–231. doi:10.1016/j.cortex.2021.04.004
Kim A. Y., Marduy A., De Melo P. S., Gianlorenco A. C., Kim C. K., Choi H., et al. (2022). Safety of transcutaneous auricular vagus nerve stimulation (taVNS): a systematic review and meta-analysis. Sci. Rep. 12, 22055. doi:10.1038/s41598-022-25864-1
Konakoglu G., Özden A. V., Solmaz H., Bildik C. (2023). The effect of auricular vagus nerve stimulation on electroencephalography and electromyography measurements in healthy persons. Front. Physiol. 14, 1–15. doi:10.3389/fphys.2023.1215757
Konjusha A., Colzato L., Muckschel M., Beste C. (2022). Auricular transcutaneous vagus nerve stimulation diminishes alpha-band-related inhibitory gating processes during conflict monitoring in frontal cortices. Int. J. Neuropsychopharmacol. 25, 457–467. doi:10.1093/ijnp/pyac013
Konjusha A., Yu S., Muckschel M., Colzato L., Ziemssen T., Beste C. (2023). Auricular transcutaneous vagus nerve stimulation specifically enhances working memory gate closing mechanism: a system neurophysiological study. J. Neurosci. 4, 4709–4724. doi:10.1523/JNEUROSCI.2004-22.2023
Kozorosky E. M., Lee C. H., Lee J. G., Nunez Martinez V., Padayachee L. E., Stauss H. M. (2022). Transcutaneous auricular vagus nerve stimulation augments postprandial inhibition of ghrelin. Physiol. Rep. 10, e15253. doi:10.14814/phy2.15253
Kraus T., Hosl K., Kiess O., Schanze A., Kornhuber J., Forster C. (2007). BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural. Transm. (Vienna) 114, 1485–1493. doi:10.1007/s00702-007-0755-z
Kuhnel A., Teckentrup V., Neuser M. P., Huys Q. J. M., Burrasch C., Walter M., et al. (2020). Stimulation of the vagus nerve reduces learning in a go/no-go reinforcement learning task. Eur. Neuropsychopharmacol. 35, 17–29. doi:10.1016/j.euroneuro.2020.03.023
Laqua R., Leutzow B., Wendt M., Usichenko T. (2014). Transcutaneous vagal nerve stimulation may elicit anti- and pro-nociceptive effects under experimentally-induced pain - a crossover placebo-controlled investigation. Auton. Neurosci. 185, 120–122. doi:10.1016/j.autneu.2014.07.008
Le Roy B., Martin-Krumm C., Gille A., Jacob S., Vigier C., Laborde S., et al. (2023). Evaluation of taVNS for extreme environments: an exploration study of health benefits and stress operationality. Front. Neurol. 14, 1286919. doi:10.3389/fneur.2023.1286919
Lench D. H., Turner T. H., Mcleod C., Boger H. A., Lovera L., Heidelberg L., et al. (2023). Multi-session transcutaneous auricular vagus nerve stimulation for Parkinson's disease: evaluating feasibility, safety, and preliminary efficacy. Front. Neurol. 14, 1210103. doi:10.3389/fneur.2023.1210103
Li J. N., Xie C. C., Li C. Q., Zhang G. F., Tang H., Jin C. N., et al. (2022). Efficacy and safety of transcutaneous auricular vagus nerve stimulation combined with conventional rehabilitation training in acute stroke patients: a randomized controlled trial conducted for 1 year involving 60 patients. Neural. Regen. Res. 17, 1809–1813. doi:10.4103/1673-5374.332155
Lim M. J. R., Fong K. Y., Zheng Y., Chua C. Y. K., Miny S., Lin J. B., et al. (2022). Vagus nerve stimulation for treatment of drug-resistant epilepsy: a systematic review and meta-analysis. Neurosurg. Rev. 45, 2361–2373. doi:10.1007/s10143-022-01757-9
Liu C., Tang H., Liu C., Ma J., Liu G., Niu L., et al. (2024). Transcutaneous auricular vagus nerve stimulation for post-stroke depression: a double-blind, randomized, placebo-controlled trial. J. Affect. Disord. 354, 82–88. doi:10.1016/j.jad.2024.03.005
Llanos F., Mchaney J. R., Schuerman W. L., Yi H. G., Leonard M. K., Chandrasekaran B. (2020). Non-invasive peripheral nerve stimulation selectively enhances speech category learning in adults. NPJ Sci. Learn. 5, 12. doi:10.1038/s41539-020-0070-0
Lloyd B., Wurm F., De Kleijn R., Nieuwenhuis S. (2023). Short-term transcutaneous vagus nerve stimulation increases pupil size but does not affect EEG alpha power: a replication of Sharon et al. (2021, Journal of Neuroscience). Brain Stimul. 16, 1001–1008. doi:10.1016/j.brs.2023.06.010
Lucchi F., Lloyd B., Nieuwenhuis S. (2024). Non-invasive vagus nerve stimulation and the motivation to work for rewards: a replication of Neuser et al. (2020, Nature Communications). Psychophysiology 61, e14484. doi:10.1111/psyp.14484
Ludwig M., Wienke C., Betts M. J., Zaehle T., Hammerer D. (2021). Current challenges in reliably targeting the noradrenergic locus coeruleus using transcutaneous auricular vagus nerve stimulation (taVNS). Auton. Neurosci. 236, 102900. doi:10.1016/j.autneu.2021.102900
Machetanz K., Berelidze L., Guggenberger R., Gharabaghi A. (2021). Transcutaneous auricular vagus nerve stimulation and heart rate variability: analysis of parameters and targets. Auton. Neurosci. 236, 102894. doi:10.1016/j.autneu.2021.102894
Maharjan A. (2018). “An investigation in the neuromodulation ofolfactory function using non-invasive vagus nerve stimulation,”. Dissertation/master’s thesis (Dunedin, New Zealand: University of Otago).
Mao Y., Chen C., Falahpour M., Macniven K. H., Heit G., Sharma V., et al. (2022). Effects of sub-threshold transcutaneous auricular vagus nerve stimulation on cingulate cortex and insula resting-state functional connectivity. Front. Hum. Neurosci. 16, 862443. doi:10.3389/fnhum.2022.862443
Marano M., Anzini G., Saltarocchi L., Ricciuti R., Capone F., Tan H., et al. (2024). Left vagus stimulation modulates contralateral subthalamic beta power improving the gait in Parkinson's disease. Mov. Disord. 39, 424–428. doi:10.1002/mds.29690
Maraver M. J., Steenbergen L., Hossein R., Actis-Grosso R., Ricciardelli P., Hommel B., et al. (2020). Transcutaneous vagus nerve stimulation modulates attentional resource deployment towards social cues. Neuropsychologia 143, 107465. doi:10.1016/j.neuropsychologia.2020.107465
Mertens A., Naert L., Miatton M., Poppa T., Carrette E., Gadeyne S., et al. (2020). Transcutaneous vagus nerve stimulation does not affect verbal memory performance in healthy volunteers. Front. Psychol. 11, 551. doi:10.3389/fpsyg.2020.00551
Mertens A., Carrette S., Klooster D., Lescrauwaet E., Delbeke J., Wadman W. J., et al. (2022). Investigating the effect of transcutaneous auricular vagus nerve stimulation on cortical excitability in healthy males. Neuromodulation 25, 395–406. doi:10.1111/ner.13488
Molefi E., Mcloughlin I., Palaniappan R. (2023). On the potential of transauricular electrical stimulation to reduce visually induced motion sickness. Sci. Rep. 13, 3272. doi:10.1038/s41598-023-29765-9
Mondal B., Choudhury S., Banerjee R., Roy A., Chatterjee K., Basu P., et al. (2023). Effects of non-invasive vagus nerve stimulation on clinical symptoms and molecular biomarkers in Parkinson's disease. Front. Aging Neurosci. 15, 1331575. doi:10.3389/fnagi.2023.1331575
Müller F. K., Teckentrup V., Kühnel A., Ferstl M., Kroemer N. B. (2021). Acute vagus nerve stimulation does not affect liking or wanting ratings of food in healthy participants. Appetite 169, 105813. doi:10.1016/j.appet.2021.105813
Muller S. J., Teckentrup V., Rebollo I., Hallschmid M., Kroemer N. B. (2022). Vagus nerve stimulation increases stomach-brain coupling via a vagal afferent pathway. Brain Stimul. 15, 1279–1289. doi:10.1016/j.brs.2022.08.019
Neuser M. P., Teckentrup V., Kuhnel A., Hallschmid M., Walter M., Kroemer N. B. (2020). Vagus nerve stimulation boosts the drive to work for rewards. Nat. Commun. 11, 3555. doi:10.1038/s41467-020-17344-9
Ng K. B., Guiu Hernandez E., Haszard J., Macrae P., Huckabee M. L., Cakmak Y. O. (2024). Transcutaneous auricular vagus nerve stimulation alters cough sensitivity depending on stimulation parameters: potential implications for aspiration risk. Front. Neurosci. 18, 1265894. doi:10.3389/fnins.2024.1265894
Obst M. A., Heldmann M., Alicart H., Tittgemeyer M., Munte T. F. (2020). Effect of short-term transcutaneous vagus nerve stimulation (tVNS) on brain processing of food cues: an electrophysiological study. Front. Hum. Neurosci. 14, 206. doi:10.3389/fnhum.2020.00206
Peng L., Mu K., Liu A., Zhou L., Gao Y., Shenoy I. T., et al. (2018). Transauricular vagus nerve stimulation at auricular acupoints kindey (CO10), Yidan (CO11), Liver (CO12) and Shenmen (TF4) can induce auditory and limbic cortices activation measured by fMRI. Hear Res. 359, 1–12. doi:10.1016/j.heares.2017.12.003
Peng X., Baker-Vogel B., Sarhan M., Short E. B., Zhu W., Liu H., et al. (2023). Left or right ear? A neuroimaging study using combined taVNS/fMRI to understand the interaction between ear stimulation target and lesion location in chronic stroke. Brain Stimul. 16, 1144–1153. doi:10.1016/j.brs.2023.07.050
Percin A., Ozden A. V., Yenisehir S., Pehlivanoglu B. E., Yilmaz R. C. (2024). The effect of in-ear and behind-ear transcutaneous auricular vagus nerve stimulation on autonomic function: a randomized, single-blind, sham-controlled study. J. Clin. Med. 13, 4385. doi:10.3390/jcm13154385
Petersen J. C. G., Becker R., Petersen L. G. (2024). Transcutaneous vagal nerve stimulation during lower body negative pressure. Auton. Neurosci. 254, 103192. doi:10.1016/j.autneu.2024.103192
Phillips I., Calloway R. C., Karuzis V. P., Pandza N. B., O'rourke P., Kuchinsky S. E. (2021). Transcutaneous auricular vagus nerve stimulation strengthens semantic representations of foreign language tone words during initial stages of learning. J. Cogn. Neurosci. 34, 127–152. doi:10.1162/jocn_a_01783
Pihlaja M., Failla L., Perakyla J., Hartikainen K. M. (2020). Reduced frontal Nogo-N2 with uncompromised response inhibition during transcutaneous vagus nerve stimulation-more efficient cognitive control? Front. Hum. Neurosci. 14, 561780. doi:10.3389/fnhum.2020.561780
Poppa T., Benschop L., Horczak P., Vanderhasselt M. A., Carrette E., Bechara A., et al. (2022). Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. 15, 260–269. doi:10.1016/j.brs.2021.12.004
Ramos-Castaneda J. A., Barreto-Cortes C. F., Losada-Floriano D., Sanabria-Barrera S. M., Silva-Sieger F. A., Garcia R. G. (2022). Efficacy and safety of vagus nerve stimulation on upper limb motor recovery after stroke. A systematic review and meta-analysis. Front. Neurol. 13, 889953. doi:10.3389/fneur.2022.889953
Redgrave J., Day D., Leung H., Laud P. J., Ali A., Lindert R., et al. (2018). Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. 11, 1225–1238. doi:10.1016/j.brs.2018.08.010
Reuter U., McClure C., Liebler E., Pozo-Rosich P. (2019). Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. J. Neurol. Neurosurg. Psychiatry 90, 796–804. doi:10.1136/jnnp-2018-320113
Rufener K. S., Geyer U., Janitzky K., Heinze H. J., Zaehle T. (2018). Modulating auditory selective attention by non-invasive brain stimulation: differential effects of transcutaneous vagal nerve stimulation and transcranial random noise stimulation. Eur. J. Neurosci. 48, 2301–2309. doi:10.1111/ejn.14128
Rufener K. S., Wienke C., Salanje A., Haghikia A., Zaehle T. (2023). Effects of transcutaneous auricular vagus nerve stimulation paired with tones on electrophysiological markers of auditory perception. Brain Stimul. 16, 982–989. doi:10.1016/j.brs.2023.06.006
Sclocco R., Garcia R. G., Kettner N. W., Isenburg K., Fisher H. P., Hubbard C. S., et al. (2019). The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: a multimodal ultrahigh-field (7T) fMRI study. Brain Stimul. 12, 911–921. doi:10.1016/j.brs.2019.02.003
Sclocco R., Garcia R. G., Kettner N. W., Fisher H. P., Isenburg K., Makarovsky M., et al. (2020). Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimul. 13, 970–978. doi:10.1016/j.brs.2020.03.011
Sellaro R., Steenbergen L., Verkuil B., Van I. M. H., Colzato L. S. (2015a). Transcutaneous vagus nerve stimulation (tVNS) does not increase prosocial behavior in Cyberball. Front. Psychol. 6, 499. doi:10.3389/fpsyg.2015.00499
Sellaro R., Van Leusden J. W., Tona K. D., Verkuil B., Nieuwenhuis S., Colzato L. S. (2015b). Transcutaneous vagus nerve stimulation enhances post-error slowing. J. Cogn. Neurosci. 27, 2126–2132. doi:10.1162/jocn_a_00851
Sellaro R., De Gelder B., Finisguerra A., Colzato L. S. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex 99, 213–223. doi:10.1016/j.cortex.2017.11.007
Shan J., Li Z., Ji M., Zhang M., Zhang C., Zhu Y., et al. (2024). Transcutaneous vagus nerve stimulation for Parkinson's disease: a systematic review and meta-analysis. Front. Aging Neurosci. 16, 1498176. doi:10.3389/fnagi.2024.1498176
Sharon O., Fahoum F., Nir Y. (2021). Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J. Neurosci. 41, 320–330. doi:10.1523/JNEUROSCI.1361-20.2020
Shi X., Zhao J., Xu S., Ren M., Wu Y., Chen X., et al. (2023). Clinical research progress of the post-stroke upper limb motor function improvement via transcutaneous Auricular vagus nerve stimulation. Neural Plast. 2023, 9532713. doi:10.1155/2023/9532713
Sigurdsson H. P., Raw R., Hunter H., Baker M. R., Taylor J. P., Rochester L., et al. (2021). Noninvasive vagus nerve stimulation in Parkinson's disease: current status and future prospects. Expert Rev. Med. Devices 18, 971–984. doi:10.1080/17434440.2021.1969913
Sinkovec M., Trobec R., Meglic B. (2021). Cardiovascular responses to low-level transcutaneous vagus nerve stimulation. Auton. Neurosci. 236, 102851. doi:10.1016/j.autneu.2021.102851
Skora L., Marzecova A., Jocham G. (2024). Tonic and phasic transcutaneous auricular vagus nerve stimulation (taVNS) both evoke rapid and transient pupil dilation. Brain Stimul. 17, 233–244. doi:10.1016/j.brs.2024.02.013
St Pierre M. A., Shinohara M. (2023). Transcutaneous vagus nerve stimulation at nonspecific timings during training can compromise motor adaptation in healthy humans. J. Neurophysiol. 130, 212–223. doi:10.1152/jn.00447.2022
Steenbergen L., Maraver M. J., Actis-Grosso R., Ricciardelli P., Colzato L. S. (2021). Recognizing emotions in bodies: vagus nerve stimulation enhances recognition of anger while impairing sadness. Cogn. Affect. Behav. Neurosci. 21, 1246–1261. doi:10.3758/s13415-021-00928-3
Steidel K., Krause K., Menzler K., Strzelczyk A., Immisch I., Fuest S., et al. (2021). Transcutaneous auricular vagus nerve stimulation influences gastric motility: a randomized, double-blind trial in healthy individuals. Brain Stimul. 14, 1126–1132. doi:10.1016/j.brs.2021.06.006
Sun J. B., Cheng C., Tian Q. Q., Yuan H., Yang X. J., Deng H., et al. (2021). Transcutaneous auricular vagus nerve stimulation improves spatial working memory in healthy young adults. Front. Neurosci. 15, 790793. doi:10.3389/fnins.2021.790793
Szeska C., Richter J., Wendt J., Weymar M., Hamm A. O. (2020). Promoting long-term inhibition of human fear responses by non-invasive transcutaneous vagus nerve stimulation during extinction training. Sci. Rep. 10, 1529. doi:10.1038/s41598-020-58412-w
Szeska C., Richter J., Wendt J., Weymar M., Hamm A. O. (2021). Attentive immobility in the face of inevitable distal threat-startle potentiation and fear bradycardia as an index of emotion and attention. Psychophysiology 58, e13812. doi:10.1111/psyp.13812
Szulczewski M. T., D'agostini M., Van Diest I. (2023). Expiratory-gated transcutaneous auricular vagus nerve stimulation (taVNS) does not further augment heart rate variability during slow breathing at 0.1 Hz. Appl. Psychophysiol. Biofeedback 48, 323–333. doi:10.1007/s10484-023-09584-4
Tan C., Qiao M., Ma Y., Luo Y., Fang J., Yang Y. (2023). The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: a systematic review and meta-analysis of randomized controlled trials. J. Affect. Disord. 337, 37–49. doi:10.1016/j.jad.2023.05.048
Teckentrup V., Neubert S., Santiago J. C. P., Hallschmid M., Walter M., Kroemer N. B. (2020). Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimul. 13, 470–473. doi:10.1016/j.brs.2019.12.018
Teckentrup V., Krylova M., Jamalabadi H., Neubert S., Neuser M. P., Hartig R., et al. (2021). Brain signaling dynamics after vagus nerve stimulation. NeuroImage 245, 118679. doi:10.1016/j.neuroimage.2021.118679
Thakkar V. J., Engelhart A. S., Khodaparast N., Abadzi H., Centanni T. M. (2020). Transcutaneous auricular vagus nerve stimulation enhances learning of novel letter-sound relationships in adults. Brain Stimul. 13, 1813–1820. doi:10.1016/j.brs.2020.10.012
Thompson S. L., O'Leary G. H., Austelle C. W., Gruber E., Kahn A. T., Manett A. J., et al. (2021). A review of parameter settings for invasive and non-invasive vagus nerve stimulation (VNS) applied in neurological and psychiatric disorders. Front. Neurosci. 15, 709436. doi:10.3389/fnins.2021.709436
Tobaldini E., Toschi-Dias E., Appratto De Souza L., Rabello Casali K., Vicenzi M., Sandrone G., et al. (2019). Cardiac and peripheral autonomic responses to orthostatic stress during transcutaneous vagus nerve stimulation in healthy subjects. J. Clin. Med. 8, 496. doi:10.3390/jcm8040496
Tona K. D., Revers H., Verkuil B., Nieuwenhuis S. (2020). Noradrenergic regulation of cognitive flexibility: no effects of stress, transcutaneous vagus nerve stimulation, and atomoxetine on task-switching in humans. J. Cogn. Neurosci. 32, 1881–1895. doi:10.1162/jocn_a_01603
Toschi N., Duggento A., Barbieri R., Garcia R. G., Fisher H. P., Kettner N. W., et al. (2023). Causal influence of brainstem response to transcutaneous vagus nerve stimulation on cardiovagal outflow. Brain Stimul. 16, 1557–1565. doi:10.1016/j.brs.2023.10.007
van Midden V., Demsar J., Pirtosek Z., Kojovic M. (2023). The effects of transcutaneous auricular vagal nerve stimulation on cortical GABAergic and cholinergic circuits: a transcranial magnetic stimulation study. Eur. J. Neurosci. 57, 2160–2173. doi:10.1111/ejn.16004
van Midden V., Pirtosek Z., Kojovic M. (2024a). The effect of taVNS on the cerebello-thalamo-cortical pathway: a TMS study. Cerebellum 23, 1013–1019. doi:10.1007/s12311-023-01595-5
van Midden V., Simoncic U., Pirtosek Z., Kojovic M. (2024b). The effect of taVNS at 25 Hz and 100 Hz on Parkinson's disease gait-a randomized motion sensor study. Mov. Disord. 39, 1375–1385. doi:10.1002/mds.29826
Veiz E., Kieslich S. K., Czesnik D., Herrmann-Lingen C., Meyer T., Staab J. (2022). Increased concentrations of circulating interleukins following non-invasive vagus nerve stimulation: results from a randomized, sham-controlled, crossover study in healthy subjects. Neuroimmunomodulation 29, 450–459. doi:10.1159/000524646
Ventura-Bort C., Wirkner J., Wendt J., Hamm A. O., Weymar M. (2021). Establishment of emotional memories is mediated by vagal nerve activation: evidence from noninvasive taVNS. J. Neurosci. 41, 7636–7648. doi:10.1523/JNEUROSCI.2329-20.2021
Vertanen E. T. M. (2023). “The impact of invasive and noninvasive vagus nerve stimulation on cognitive functions in humans,”. Dissertation/master’s thesis (Tampere, Finland: Tampere University).
Villani V., Finotti G., Di Lernia D., Tsakiris M., Azevedo R. T. (2022). Event-related transcutaneous vagus nerve stimulation modulates behaviour and pupillary responses during an auditory oddball task. Psychoneuroendocrinology 140, 105719. doi:10.1016/j.psyneuen.2022.105719
Vishal J., Thakkar J. E. C., Abby S. E., Centanni T. M. (2025). Parameter optimization of non-invasive vagus nerve stimulation for second language learning in typically developing young adults. J. Neurolinguistics 73, 101225. doi:10.1016/j.jneuroling.2024.101225
Vosseler A., Zhao D., Fritsche L., Lehmann R., Kantartzis K., Small D. M., et al. (2020). No modulation of postprandial metabolism by transcutaneous auricular vagus nerve stimulation: a cross-over study in 15 healthy men. Sci. Rep. 10, 20466. doi:10.1038/s41598-020-77430-2
Wang Y., Li S. Y., Wang D., Wu M. Z., He J. K., Zhang J. L., et al. (2021). Transcutaneous auricular vagus nerve stimulation: from concept to application. Neurosci. Bull. 37, 853–862. doi:10.1007/s12264-020-00619-y
Wang C., Cao X., Gao Z., Liu Y., Wen Z. (2022). Training and transfer effects of combining inhibitory control training with transcutaneous vagus nerve stimulation in healthy adults. Front. Psychol. 13, 858938. doi:10.3389/fpsyg.2022.858938
Wang C., Zeng L., Cao X., Dai J., Liu Y., Gao Z., et al. (2023). Synergistic effects of transcutaneous vagus nerve stimulation and inhibitory control training on electrophysiological performance in healthy adults. Front. Neurosci. 17, 1123860. doi:10.3389/fnins.2023.1123860
Wang L., Wang L., Wang Z., Zhao H., Wu J., Gao F., et al. (2024a). Efficacy observation of combined transcutaneous vagus nerve stimulation and transcranial direct current stimulation on gait in 169 subacute stroke patients. J. Rehabil. Med. 56, jrm40348. doi:10.2340/jrm.v56.40348
Wang M. H., Wang Y. X., Xie M., Chen L. Y., He M. F., Lin F., et al. (2024b). Transcutaneous auricular vagus nerve stimulation with task-oriented training improves upper extremity function in patients with subacute stroke: a randomized clinical trial. Front. Neurosci. 18, 1346634. doi:10.3389/fnins.2024.1346634
Warren C. M., Tona K. D., Ouwerkerk L., Van Paridon J., Poletiek F., Van Steenbergen H., et al. (2019). The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 12, 635–642. doi:10.1016/j.brs.2018.12.224
Warren C. V., Maraver M. J., De Luca A., Kopp B. (2020). The effect of transcutaneous auricular vagal nerve stimulation (taVNS) on P3 event-related potentials during a Bayesian oddball task. Brain Sci. 10, 404. doi:10.3390/brainsci10060404
Wienke C., Grueschow M., Haghikia A., Zaehle T. (2023). Phasic, event-related transcutaneous auricular vagus nerve stimulation modifies behavioral, pupillary, and low-frequency oscillatory power responses. J. Neurosci. 43, 6306–6319. doi:10.1523/JNEUROSCI.0452-23.2023
Wu C., Liu P., Fu H., Chen W., Cui S., Lu L., et al. (2018). Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: a systematic review and meta-analysis. Med. (Baltimore) 97, e13845. doi:10.1097/MD.0000000000013845
Wu K., Wang Z., Zhang Y., Yao J., Zhang Z. (2020). Transcutaneous vagus nerve stimulation for the treatment of drug-resistant epilepsy: a meta-analysis and systematic review. ANZ J. Surg. 90, 467–471. doi:10.1111/ans.15681
Yakunina N., Kim S. S., Nam E. C. (2017). Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 20, 290–300. doi:10.1111/ner.12541
Yokota H., Edama M., Hirabayashi R., Sekine C., Otsuru N., Saito K., et al. (2022). Effects of stimulus frequency, intensity, and sex on the autonomic response to transcutaneous vagus nerve stimulation. Brain Sci. 12, 1038. doi:10.3390/brainsci12081038
Yokota H., Edama M., Kawanabe Y., Hirabayashi R., Sekikne C., Akuzawa H., et al. (2024). Effects of transcutaneous auricular vagus nerve stimulation at left cymba concha on experimental pain as assessed with the nociceptive withdrawal reflex, and correlation with parasympathetic activity. Eur. J. Neurosci. 59, 2826–2835. doi:10.1111/ejn.16305
Zhang H., Cao X. Y., Wang L. N., Tong Q., Sun H. M., Gan C. T., et al. (2023). Transcutaneous auricular vagus nerve stimulation improves gait and cortical activity in Parkinson's disease: a pilot randomized study. CNS Neurosci. Ther. 29, 3889–3900. doi:10.1111/cns.14309
Zhang H., Shan A. D., Wan C. H., Cao X. Y., Yuan Y. S., Ye S. Y., et al. (2024). Transcutaneous auricular vagus nerve stimulation improves anxiety symptoms and cortical activity during verbal fluency task in Parkinson's disease with anxiety. J. Affect. Disord. 361, 556–563. doi:10.1016/j.jad.2024.06.083
Zhang J., Zhou F., Roberts N., Yuan Q., Wang M., Wu G., et al. (2025). Invasive or non-invasive vagus nerve stimulation modulation of brain function and remodeling after stroke: a review. Int. J. Surg. 111, 2148–2161. doi:10.1097/JS9.0000000000002173
Zhao R., He Z. Y., Cheng C., Tian Q. Q., Cui Y. P., Chang M. Y., et al. (2022). Assessing the effect of simultaneous combining of transcranial direct current stimulation and transcutaneous auricular vagus nerve stimulation on the improvement of working memory performance in healthy individuals. Front. Neurosci. 16, 947236. doi:10.3389/fnins.2022.947236
Zhu S., Qing Y., Zhang Y., Zhang X., Ding F., Zhang R., et al. (2022). Transcutaneous auricular vagus nerve stimulation increases eye-gaze on salient facial features and oxytocin release. Psychophysiology 59, e14107. doi:10.1111/psyp.14107
Keywords: noninvasive brain stimulation, taVNS, central nervous system disorder, neuromodulation, rehabilitation
Citation: Matsuoka M, Yamaguchi T and Fujiwara T (2025) Transcutaneous auricular vagus nerve stimulation in healthy individuals, stroke, and Parkinson’s disease: a narrative review of safety, parameters, and efficacy. Front. Physiol. 16:1693907. doi: 10.3389/fphys.2025.1693907
Received: 27 August 2025; Accepted: 10 October 2025;
Published: 24 October 2025.
Edited by:
Edmund O. Acevedo, Virginia Commonwealth University, United StatesReviewed by:
Hikari Kirimoto, Hiroshima University, JapanChen Xin, China Academy of Chinese Medical Sciences, China
Patrik Simko, Central European Institute of Technology (CEITEC), Czechia
Jiatong Shan, National University of Singapore, Singapore
Thorsten Herr, Universitätsklinikum Greifswald, Germany
Mohammad Yusuf Hasan, Aligarh Muslim University, India
Moaz Abouelmagd, Cairo University, Egypt
Copyright © 2025 Matsuoka, Yamaguchi and Fujiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomofumi Yamaguchi, eWFtYWd1Y2hpLnRvbW9mdW1pLjNpQGt5b3RvLXUuYWMuanA=
 Machiko Matsuoka
Machiko Matsuoka Tomofumi Yamaguchi
Tomofumi Yamaguchi Toshiyuki Fujiwara
Toshiyuki Fujiwara