- 1Functional Gastrointestinal Disorders Research Group, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
- 2Laboratory of Movement and Wellness, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
- 3Rheumatology Outpatient Clinic, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
- 4Outpatient Pain Management Unit, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
- 5Core Facility Biobank, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
- 6Laboratory of Clinical Pathology, National Institute of Gastroenterology IRCCS “Saverio de Bellis”, Castellana Grotte, Italy
Background: Intestinal barrier dysfunction is increasingly implicated in the pathophysiology of fibromyalgia (FM) and irritable bowel syndrome (IBS), particularly in their comorbid form. Physical capacity (PC) influences systemic inflammation and metabolic resilience, but its relationship with gut barrier integrity in these conditions remains poorly defined.
Methods: We conducted a cross-sectional study of 56 patients with FM (n = 14), IBS (n = 23), or both (FM + IBS, n = 19). Intestinal barrier function was assessed using serum and fecal zonulin, intestinal fatty acid-binding protein (I-FABP), and the lactulose/mannitol (Lac/Man) urinary excretion test. PC was quantified using the Global Physical Capacity Score (GPCS). Clinical symptoms were evaluated using the Fibromyalgia Impact Questionnaire–Revised (FIQ-R) and IBS Severity Scoring System (IBS-SSS).
Results: FM + IBS patients exhibited the greatest barrier dysfunction, with elevated fecal zonulin and Lac/Man ratio. IBS patients showed increased I-FABP, consistent with epithelial injury, whereas FM patients had milder gastrointestinal symptoms and less pronounced biomarker alterations. Although overall PC scores did not significantly differ across groups, serum zonulin levels showed a strong inverse correlation with GPCS. When stratified by GPCS (cut-off ≥6), patients in the high PC group exhibited significantly lower (p = 0.01) serum zonulin concentrations (48.23 ± 12.44 ng/mL) compared to those in the low PC group (57.63 ± 9.69 ng/mL). Multiple regression analysis confirmed GPCS as an independent predictor of serum zonulin (β = −9.67, p = 0.01), while BMI was not a significant contributor (p = 0.79). Furthermore, urinary indole levels correlated positively with both lactulose excretion and the Lac/Man ratio, supporting the existence of a dysbiosis-permeability feedback loop in these disorders.
Conclusion: Intestinal barrier dysfunction in FM and IBS displays phenotype-specific patterns and is significantly modulated by PC. These findings support the integration of PC assessment into clinical phenotyping and highlight potential targets for personalized management of chronic overlapping pain syndromes.
1 Introduction
Fibromyalgia (FM) and irritable bowel syndrome (IBS) are two common chronic functional disorders that often occur together, with epidemiological studies showing comorbidity rates between 30% and 70% (Drossman, 2016; Lichtenstein et al., 2018; Erdrich et al., 2020b; Masood et al., 2023). Although these conditions share key clinical features such as chronic pain, fatigue, and gastrointestinal (GI) issues, their underlying mechanisms are not fully understood. While central sensitization and gut-brain axis dysregulation have been well studied (Labanski et al., 2020), recent evidence indicates that intestinal barrier dysfunction might play a crucial role in linking GI and musculoskeletal symptoms in these overlapping disorders (Erdrich et al., 2020a). The integrity of the intestinal barrier, maintained by the coordinated function of tight junction (TJ) proteins, the mucus layer, and mucosal immune surveillance (Okumura and Takeda, 2025), seems compromised in both FM and IBS. Elevated serum zonulin levels, a marker of increased intestinal permeability, have been consistently observed in IBS patients (Linsalata et al., 2018; Seethaler et al., 2021) and, to a lesser extent, in FM populations (Erdrich et al., 2020a). This barrier dysfunction allows luminal components, such as lipopolysaccharides (LPS), to translocate, activating Toll-like receptor 4 -dependent inflammatory cascades that may increase systemic pain and contribute to symptom persistence (Ohira et al., 2017). Multiple factors contribute to intestinal barrier disruption in these conditions. Psychological stress, a well-known exacerbating factor in IBS, has been shown to increase intestinal permeability by modulating TJ proteins via cortisol (Martel et al., 2022). At the same time, intestinal dysbiosis, characterized by a decreased abundance of short-chain fatty acid-producing bacteria, reduces butyrate availability (Canani et al., 2011). This metabolic change impairs histone deacetylase (HDAC) inhibition, leading to upregulation of myosin light chain kinase and phosphorylation of TJ proteins (Peng et al., 2009).
Physical deconditioning is another significant factor, particularly in FM patients, who often exhibit reduced aerobic capacity, decreased muscle strength, and unfavorable body composition compared to healthy controls (Johannesson et al., 2011; Ursini et al., 2011; Góes et al., 2012). This reduced physical capacity (PC) results from pain- and fatigue-driven activity avoidance (Góes et al., 2012), creating a vicious cycle that may further harm gut barrier function by decreasing splanchnic perfusion and altering microbial ecology (Aya et al., 2021; Majnarić et al., 2021). The Global Physical Capacity Score (GPCS), a combined index based on validated performance tests, has become a sensitive tool to measure these functional impairments (Bianco et al., 2023). Notably, higher GPCS scores are associated with better clinical outcomes in IBS (Bianco et al., 2023), potentially indicating improved stress resilience and better control of inflammation (Johannesson et al., 2011; Riezzo et al., 2023). Despite these advances, significant knowledge gaps remain regarding the interactions between the gut and muscle. The comorbid FM + IBS presentation, although clinically common, remains poorly understood at the mechanistic level. Current research approaches typically focus on PC as a treatment outcome rather than exploring its potential as a modulator of intestinal barrier function. Additionally, existing biomarker studies often fail to differentiate between distinct patterns of barrier disruption, such as epithelial injury, reflected by intestinal fatty acid-binding protein (I-FABP), and paracellular permeability, assessed by zonulin or the lactulose/mannitol (Lac/Man) ratio, across different clinical phenotypes (Grootjans et al., 2016; Sturgeon and Fasano, 2016).
The present study was designed to address these limitations by providing a detailed analysis of intestinal barrier dysfunction in FM, IBS, and their overlap. Using multiple biomarkers, including serum and fecal zonulin, I-FABP, and the Lac/Man ratio, in combination with GPCS assessments, we sought to identify specific patterns of gut barrier disruption associated with different phenotypes and their relationships to physical performance measures. Our approach offers new insights into how objective measures of PC may affect intestinal health in functional disorders.
We hypothesized that: (1) FM + IBS comorbidity would show the most significant TJ disruption (increased zonulin and Lac/Man ratio), (2) IBS patients would mainly display epithelial injury markers (higher I-FABP levels), and (3) higher GPCS scores would be associated with better barrier integrity across all groups. These findings could help develop personalized treatments targeting dysfunction of the gut-muscle axis in functional GI and pain disorders.
2 Methods
2.1 Patient recruitment
Adult patients (aged 18–65 years) with FM and/or IBS were recruited from the medical staff of the “Functional Gastrointestinal Disorders” Outpatients Clinic, the “Rheumatology” Outpatient Clinic, and the “Outpatient Pain Management” Unit of the IRCCS ″S. de Bellis” in Castellana Grotte, Italy.
Regarding the inclusion criteria, FM diagnosis was established according to the 2021 clinical practice guidelines of the Italian Society for Rheumatology (SIR) (Ariani et al., 2021). These national guidelines endorse the American College of Rheumatology (ACR) 2010/2011 diagnostic criteria (Wolfe et al., 2011; Wolfe and Häuser, 2011) as a validated, symptom-based framework for both clinical and research use. Accordingly, all enrolled FM patients fulfilled the ACR 2010/2011 criteria as defined by a Widespread Pain Index (WPI) ≥ 7 and Symptom Severity Scale (SSS) score ≥5, or WPI 4–6 and SSS ≥9, with symptoms persisting for at least 3 months and not better explained by another disorder. Besides, they had to present a clinical phenotype consistent with the SIR diagnostic framework. For IBS patients, diagnosis was based on Rome IV criteria, with a total score ≥125 on the IBS-Severity Scoring System (IBS-SSS), indicating a moderate to severe degree of symptoms (Farrukh, 2022).
Exclusion criteria were: severe cardiac, hepatic, neurological, or psychiatric diseases, as well as GI disorders other than IBS, such as inflammatory bowel disease or diverticular disease. Patients receiving ongoing symptom-targeted therapies, including probiotics, specialized diets, educational interventions, or complementary medicine, were excluded. Any FM or IBS management medications were to be discontinued at least 15 days before enrollment.
The present study is part of a broader research project investigating the effects of a physical activity program on symptomatology (GI and psychological) and biochemical parameters in patients with FM and IBS, with or without comorbid conditions. Specifically, the present study focuses on baseline differences in biochemical markers related to intestinal permeability and membrane integrity among patients with FM, IBS, and FM + IBS at study enrollment. Patient recruitment and assessment followed a standardized protocol as outlined in Figure 1.
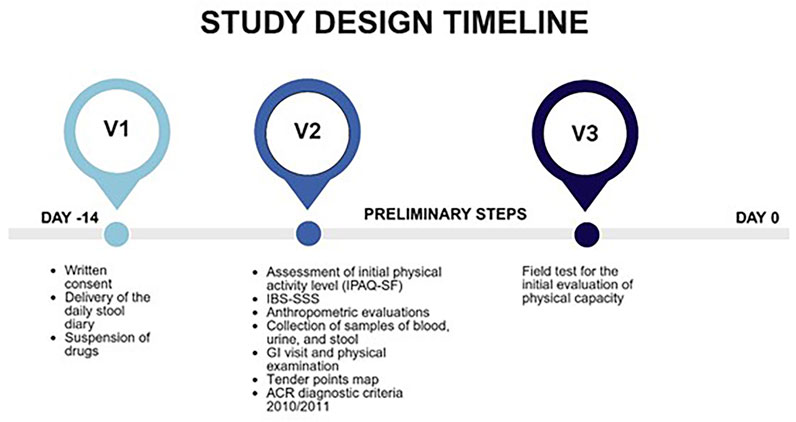
Figure 1. Overview of the study assessment protocol. Timeline and battery of clinical, functional, and laboratory evaluations administered to all participants upon enrollment. IPAQ-SF, International Physical Activity Questionnaire short-form; IBS-SSS, Irritable Bowel Syndrome Severity Scoring System; GI, Gastrointestinal; ACR, American College of Rheumatology.
The research project received approval from both the local Scientific Committee and the Institutional Ethics Committee of the IRCCS Ospedale Oncologico di Bari “Istituto Oncologico Giovanni Paolo II” (Protocol No. 117; Ethics Committee approval granted on March 3, 2023). The trial was conducted following the ethical standards outlined in the 1964 Declaration of Helsinki and its subsequent amendments, and it was prospectively registered at http://www.clinicaltrials.gov under the identifier NCT06166563. The final data access occurred on April 30, 2025.
2.2 Assessment of fibromyalgia and gastrointestinal symptoms
The severity of FM symptoms was evaluated using validated questionnaires: a) Fibromyalgia Impact Questionnaire, Revised Version (FIQ-R) (Sarzi-Puttini et al., 2003); b) Visual analog scale pain score (Crawford et al., 2011); c) Fatigue severity scale (Learmonth et al., 2013); d) Digital tender point examination (Cott et al., 1992). Before evaluating GI symptoms, a detailed clinical history was recorded.
The IBS-SSS was administered to assess the GI-symptom profile. The severity of IBS was determined using the generally accepted IBS-SSS cut-off scores: >75–175 for mild IBS, 175–300 for moderate IBS, and >300 for severe IBS (Francis et al., 1997). Additionally, stool characteristics were evaluated using the Bristol Stool Scale (Riegler and Esposito, 2001).
2.3 Sugar absorption test for small intestinal permeability
All study participants underwent evaluation of small intestinal permeability using an in vivo permeability test, which involved a sugar absorption test following an overnight fast. Before test administration, baseline urine samples were collected in the laboratory to screen for endogenous presence of the target sugars. Subsequently, subjects ingested a test solution containing 10 g of lactulose, 5 g of mannitol, and 40 g of sucrose dissolved in 100 mL of water. The inclusion of sucrose in the test solution was based on established protocols from our previous research (Linsalata et al., 2018) and is intended to provide an additional measure of gastric and proximal small intestinal permeability, as sucrose is not absorbed in healthy individuals and its urinary excretion can indicate upper GI barrier dysfunction. The dose of 40 g was selected to ensure detectable levels while minimizing potential osmotic effects, consistent with prior methodological studies (Linsalata et al., 2018).
Urine was collected over 5 h post-ingestion. Total urine volume for each participant was measured and documented. From each sample, a 2 mL aliquot was taken, centrifuged, and stored at −80 °C until further analysis.
Quantification of the three sugar markers (lactulose, mannitol, and sucrose) in urine was performed by high-performance liquid chromatography using an analytical method previously established by our research team (Linsalata et al., 2018). The percentage excretion of each ingested sugar (%Lac, %Man, and %Suc) was calculated. Additionally, the Lac/Man ratio was determined for each sample. A ratio exceeding 0.03 was considered indicative of altered intestinal permeability (Linsalata et al., 2018).
2.4 Biomarkers of intestinal barrier function, integrity, and dysbiosis
Blood and stool samples collected from participants were promptly frozen and stored at −80 °C within 12 h of collection. Zonulin levels in both serum and fecal samples were quantified using ELISA kits provided by Immunodiagnostik AG (Bensheim, Germany), following the manufacturer’s instructions. Serum zonulin concentrations below 48 ng/mL and fecal zonulin levels under 107 ng/mL were classified as within the normal reference range (Russo et al., 2018).
Serum concentrations of I-FABP were measured using commercially available ELISA kits from Thermo Fisher Scientific (Waltham, MA, USA).
Urinary indole levels were quantified as a potential indicator of altered microbial metabolism, using a colorimetric assay (Indican Assay Kit, ABNOVA Corporation, Taipei, Taiwan). Concentrations exceeding 20 mg/L were deemed to indicate increased bacterial activity (Linsalata et al., 2023).
2.5 Physical activity and physical capacity evaluation
Physical activity levels were evaluated using the short-form International Physical Activity Questionnaire (IPAQ-SF) (Lee et al., 2011), which categorizes activity into low, moderate, or high based on self-reported data.
Field-based fitness assessments were conducted to evaluate various components of PC. The 2-kilometer walking test (Laukkanen et al., 1992) was used to assess cardiorespiratory capacity, serving as a reliable indicator of cardiovascular health, overall fitness, and metabolic efficiency. The Handgrip Test was used to measure strength. A dynamometer was used to evaluate the maximum isometric strength of the forearm muscles (Pearn and Bullock, 1979). Flexibility was assessed using the sit-and-reach test (Hoeger and Hopkins, 1992), which measures the ability to reach forward while seated and reflects flexibility of the lower back and hamstrings.
Specific precautions were taken during field testing with participants diagnosed with FM, as recommended by the American College of Sports Medicine (Han et al., 2024). Before testing, symptom assessments evaluated pain severity, fatigue levels, and exercise tolerance. Participants were verbally encouraged to perform to the best of their ability while ensuring safety throughout the procedures. Adequate rest periods were incorporated between tests to prevent symptom exacerbation. Continuous monitoring of pain and fatigue was maintained during all assessments. In addition, participants were instructed on how to distinguish post-exercise muscle soreness from fatigue and pain related to FM to avoid misinterpreting symptoms.
Several standardization procedures were established to ensure the reliability and reproducibility of test results. All assessments were conducted under consistent conditions, including location, time of day, personnel, and equipment. Clear instructions and demonstrations were provided before each test, and participants were encouraged to wear comfortable clothing and suitable footwear.
2.6 Global Physical Capacity Score
PC was evaluated using motor tests of varying difficulty, previously validated in adult populations, to estimate cardiorespiratory fitness, muscular strength, and flexibility, as detailed in the preceding section. PC scores were determined from each test’s results, using age- and sex-specific normative reference charts. Each physical test was scored 1 to 5, and the scores from the three tests were combined to obtain an overall GPCS, with a possible total score of 3–15. In the absence of a standardized cut-off, we used the previously proposed value of GPCS ≥6 (Verrelli et al., 2025). Thus, to facilitate clinical interpretation, participants were dichotomized based on this cut-off value: ≥6 (high PC group) and <6 (low PC group). This composite scoring approach offers a more comprehensive PC assessment by integrating multiple functional domains relevant to daily life, rather than interpreting each test in isolation.
2.7 Statistical analysis
Continuous variables are reported as means ± standard error of the mean (SEM), unless otherwise specified. Categorical data are represented as numbers and percentages. Group comparisons were carried out using non-parametric tests. Correlations between variables were calculated using the Spearman correlation test.
Given the exploratory nature of this descriptive study on the Italian population and the lack of prior data on the expected magnitude of between-group differences, a formal a priori sample size calculation was not feasible. This approach aligns with observational studies that aim to generate hypotheses and inform the design of future trials, thereby enhancing the statistical power of those trials.
A multiple linear regression analysis was conducted on the raw data to identify predictors of mucosal integrity, incorporating clinical and biochemical variables as independent predictors through a stepwise selection method. To include categorical variables in the model, dummy variables were created by assigning binary codes (typically 0 and 1). The proportion of variance explained by the model was quantified using the adjusted R-squared, and its overall significance was tested via an F-statistic. The individual contributions of each predictor were assessed using t-statistics and corresponding p-values to determine whether the regression coefficients differed significantly from zero.
All statistical analyses were performed using SigmaStat version 11.0 (Systat Software, Inc., San Jose, CA, USA) and GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA). A p-value below 0.05 was considered indicative of statistical significance.
3 Results
3.1 Participant characteristics
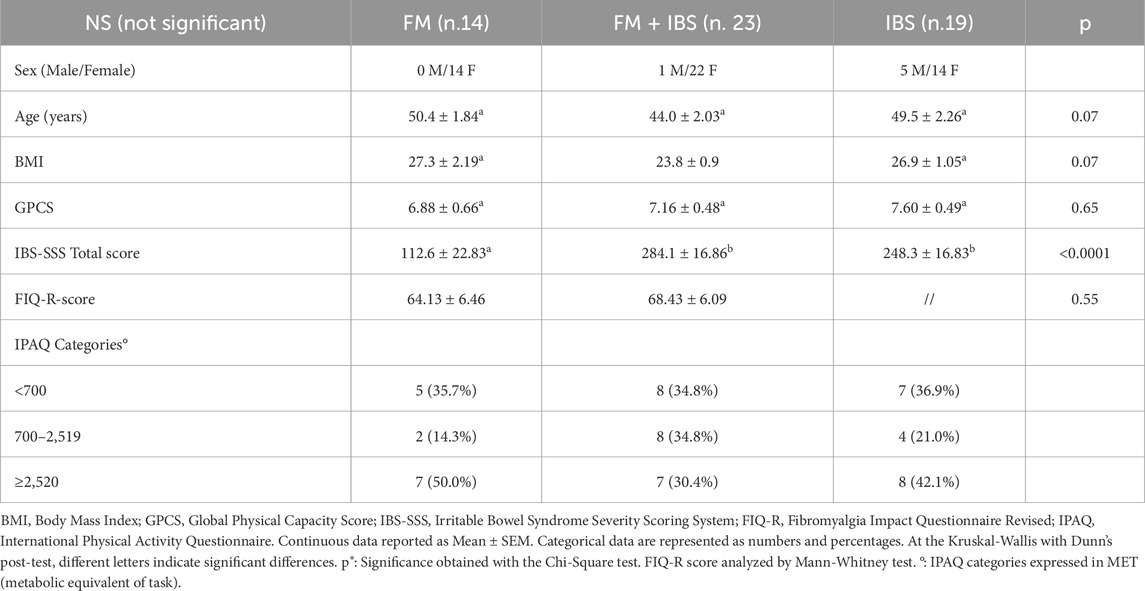
The study included 56 participants classified into three groups: FM (n = 14), FM + IBS (n = 23), and IBS (n = 19).
A flowchart summarizing participant recruitment and group allocation is shown in Figure 2.
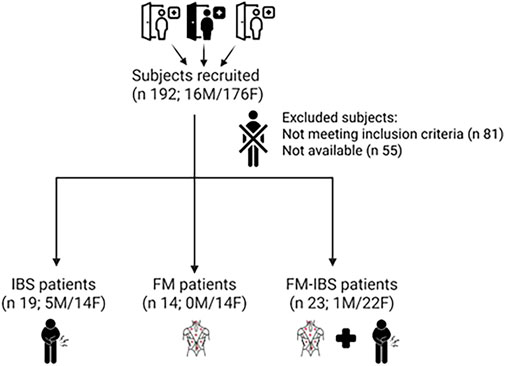
Figure 2. Participant flow through the study. The diagram details the number of individuals screened, eligible, enrolled, and included in the final analysis across the three diagnostic groups: fibromyalgia (FM), irritable bowel syndrome (IBS), and comorbid FM and IBS (FM + IBS).
Table 1 collects the baseline characteristics of the patients entered in the study. Age was not different among the 3 groups. The mean body mass index (BMI) of participants was within the normal-to-overweight range across all study groups, with no statistically significant intergroup differences detected, consistent with the high prevalence of excess weight reported in populations with chronic conditions, particularly FM. PC evaluation (performed by the GPCS) did not show a statistical difference among groups. The baseline IBS-SSS scores indicated moderate symptom severity in both the IBS (248.3 ± 16.83) and FM + IBS (284.1 ± 16.86) groups, while the FM group reported significantly lower scores (112.6 ± 22.83). Lastly, symptom severity, as measured by the FIQ-R, did not differ between FM and FM + IBS (Table 1). Analysis of physical activity levels using the IPAQ-SF showed a similar distribution among the three groups (FM, FM + IBS, and IBS), with no statistically significant differences.
All biochemical data for the patients included in the study remained within normal reference ranges, indicating no clinically relevant alterations between the groups (see Supplementary Table S1).
The assessments of the specific biomarkers of intestinal barrier function (Lac/Man ratio, zonulin, I-FABP) and urinary indole are reported in the following paragraphs.
3.2 Intestinal permeability
Intestinal permeability was assessed using the percentages of urinary excretion of the sugar probes (%Lac, %Man, %Suc) and the Lac/Man ratio. Significant differences were observed between groups for %Lac (p = 0.01) and the Lac/Man ratio (p = 0.02). Post-hoc analysis revealed that the FM + IBS group exhibited significantly higher %Lac values (0.46 ± 0.06) compared to both the FM group (0.274 ± 0.03, p = 0.003) and the IBS group (0.31 ± 0.04, p = 0.048). Similarly, the Lac/Man ratio was significantly higher in the FM + IBS group (0.03 ± 0.003) compared to the IBS group (0.02 ± 0.003, p = 0.04) (Figures 3A–D). Finally, no significant differences were observed between the groups in terms of %Suc.
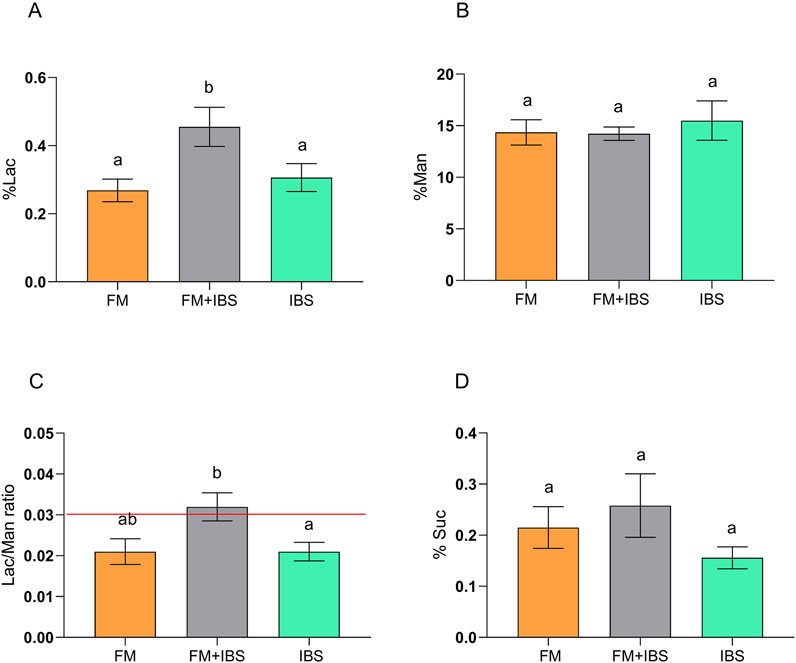
Figure 3. Gastrointestinal permeability assessed by the sugar absorption test in patients with fibromyalgia (FM; n = 14), irritable bowel syndrome (IBS; n = 19), and comorbid FM and IBS (FM + IBS; n = 23). Panel (A) Percentage of ingested lactulose recovered in urine (%Lac); Panel (B) Percentage of ingested mannitol recovered in urine (%Man); Panel (C) Lactulose-to-Mannitol urinary excretion ratio (Lac/Man); Panel (D) Percentage of ingested sucrose recovered in urine (%Suc). Data are presented as mean ± standard error of the mean (SEM). Groups were compared using the Kruskal–Wallis test followed by Dunn’s post hoc multiple comparisons test; bars labeled with different letters indicate statistically significant differences (p < 0.05). The red line in Panel (C) represents the upper limit of the normal reference range for the Lac/Man ratio, as established in healthy control populations.
3.3 Zonulin levels
Both serum and fecal zonulin levels were analyzed as markers of intestinal barrier integrity (Figure 4). No differences were found between the groups concerning serum zonulin levels (Figure 4A). In contrast, significant differences in fecal zonulin levels were observed between groups (p = 0.001). The FM + IBS group exhibited the highest fecal zonulin levels (243.5 ± 16.33 ng/mL), showing a 35.9% increase compared to the FM group (172.8 ± 21.87 ng/mL, p = 0.04) and a 46.0% increase compared to the IBS group (160.80 ± 14.2 ng/mL, p = 0.01) (Figure 4B).
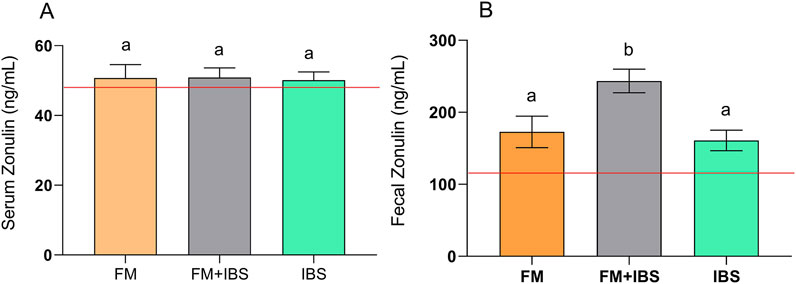
Figure 4. Serum Zonulin (Panel (A)) and Fecal Zonulin (Panel (B)) concentrations in patients with fibromyalgia (FM; n = 14), irritable bowel syndrome (IBS; n = 19), and comorbid FM and IBS (FM + IBS; n = 23). Data are presented as mean ± SEM. Groups were compared using the Kruskal–Wallis test followed by Dunn’s post hoc multiple comparisons test; bars labeled with different letters indicate statistically significant differences (p < 0.05). The red line represents the upper limit of the normal reference ranges for the serum and fecal zonulin levels, as established in healthy control populations.
3.4 Enterocyte damage and gut dysbiosis
Figure 5A, reports the serum levels of I-FABP, a marker of enterocyte damage. I-FABP levels were significantly elevated in the FM + IBS group (6.29 ± 1.14 ng/mL), showing a 127.5% increase compared to the FM group (2.76 ± 0.68 ng/mL, p = 0.049). No significant difference was observed between the FM and IBS groups (p > 0.05).
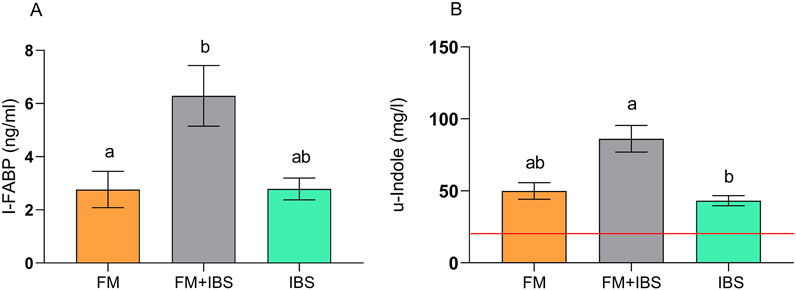
Figure 5. Intestinal fatty acid-binding protein (I-FABP; Panel (A)) and urinary indole (u-indole; Panel (B)) concentrations in patients with fibromyalgia (FM; n = 14), irritable bowel syndrome (IBS; n = 19), and comorbid FM and IBS (FM + IBS; n = 23). Data are presented as mean ± SEM. Groups were compared using the Kruskal–Wallis test followed by Dunn’s post hoc multiple comparisons test; bars labeled with different letters indicate statistically significant differences (p < 0.05). The red line in Panel (B) represents the upper limit of the normal reference range for the urinary indole, as established in healthy control populations.
Urinary indole levels, an indicator of gut dysbiosis, were significantly higher in the FM + IBS group (86.19 ± 9.22 μg/mL), with a not significant 58.9% increase compared to the FM group (49.96 ± 5.74 μg/mL, p = 0.03) and a significant 99.6% increase compared to the IBS group (43.18 ± 3.52 μg/mL, p < 0.001) (Figure 5B). Lastly, the examination of barrier integrity and dysbiosis parameters revealed a significant positive correlation between the Lac/Man ratio and urinary indole (rho = 0.36, p = 0.01).
3.5 Relationship among zonulin, global physical capacity score, body mass index, lactulose/mannitol ratio, and indole
The correlation study on the raw data revealed that BMI was positively correlated with CRP (rho = 0.55, p < 0.001) and inversely correlated with GPCS (rho = −0.37, p = 0.01). Intestinal permeability, expressed as the Lac/Man ratio, correlated with urinary indole concentration, a marker of intestinal dysbiosis (rho = 0.32, p = 0.02). In particular, %Lac, but not %Man, correlated with urinary indole (rho = 0.35, p = 0.01), confirming that dysbiosis alters the paracellular pathway and compromises TJ.
Regression analysis of the raw data revealed that serum zonulin levels could be linearly predicted from both clinical and biochemical variables. Specifically, the GPCS group showed a positive effect on serum zonulin levels, confirming the relationship between PC and mucosal barrier integrity. BMI did not significantly contribute to the equation’s ability to predict serum zonulin (F = 3.29; df = 2; p = 0.045; adjusted R2 = 0.08).
In the multivariable regression model, the association between each predictor and the outcome was assessed. BMI showed no statistically significant association with the outcome (β = −0.08, SE = 0.29, p = 0.79; 95% CI: −0.64 to 0.49). In contrast, membership in the GPCS group was significantly associated with a lower outcome score (β = −9.67, SE = 3.82, p = 0.02; 95% CI: −17.25 to −2.28).
Stratifying for GPCS, serum zonulin levels in the whole group were significantly lower in the high PC group than in the low PC group (48.231 ± 1.920 vs. 57.63 ± 2.59, p = 0.01), with an increase of 15.41% in serum zonulin concentration. No significant differences were found among other intestinal permeability markers (fecal zonulin, I-FABP, %Lac, and Lac/Man ratio), urinary indole levels or symptom profile in the other groups (p > 0.05). Data analyzed by Mann-Whitney test. These results demonstrate the importance of assessing multiple mucosal barrier integrity indices to evaluate GPCS in patients.
4 Discussion
Our findings establish intestinal barrier dysfunction as a key feature of FM, exacerbated in FM + IBS comorbidity. Notably, serum zonulin levels inversely correlated with PC across all groups (FM, FM + IBS, IBS), supporting the protective role of PC in gut health.
Comparative analysis revealed distinct phenotypic patterns of barrier disruption. FM + IBS patients demonstrated the most severe paracellular permeability, as reflected in elevated fecal zonulin and Lac/Man ratios. No significant differences were observed between the groups in %Suc, suggesting preserved gastric and proximal small intestinal barrier integrity. In contrast, IBS patients exhibited predominantly epithelial injury, as evidenced by increased I-FABP levels.
Additionally, patients with FM reported mild GI symptoms, which were significantly lower than those in the IBS and FM + IBS groups, both of which showed moderate IBS-SSS total scores.
These phenotypic differences imply distinct pathogenic mechanisms: FM-related barrier dysfunction likely reflects dysregulation of TJ proteins (stress-mediated and inflammatory pathways). At the same time, IBS appears driven by epithelial injury (mast cell-mediated hypersensitivity) (Hasler et al., 2022). Such mechanistic divergence underscores the necessity for phenotype-stratified therapies targeting specific barrier defects.
The microbial metabolite indole emerged as a key microbial metabolite, with FM + IBS patients showing significantly higher levels than those with FM. It is important to note that urinary indole levels reflect a complex interplay between gut microbial tryptophan metabolism and intestinal barrier integrity. Furthermore, their concentration is strongly influenced by dietary tryptophan intake (Kim et al., 2025). In the absence of detailed dietary records or metagenomic profiling, elevated indole excretion should be interpreted cautiously as a standalone marker of dysbiosis. Future studies should integrate dietary monitoring and microbiome analysis to better contextualize these metabolic signals.
Notably, the positive correlation between urinary indole and the Lac/Man ratio supports the concept of a dysbiosis-permeability loop, in which microbial imbalances exacerbate barrier dysfunction, thereby fueling systemic inflammation and nociceptive sensitization (Singh et al., 2021). This vicious cycle could be critical in FM + IBS patients, in whom central sensitization and visceral hypersensitivity may intensify the effects of dysbiosis and barrier disruption (Ho et al., 2025). In this framework, the dual role of indole, as both a marker of dysbiosis and a modulator of intestinal permeability, makes this finding particularly compelling (Roager and Licht, 2018).
Our findings identify distinct therapeutic opportunities that align with specific phenotypic mechanisms. In FM + IBS, targeted barrier restoration, potentially through glutamine supplementation to modulate zonulin-dependent permeability, may be prioritized, consistent with its efficacy in other permeability disorders (Arribas-López et al., 2021). For IBS cases, mast cell stabilizers such as ketotifen are rational candidates given their demonstrated epithelial-protective effects in similar cohorts (Fortea et al., 2021). Conversely, FM patients may benefit most from combined stress-axis modulation and TJ stabilization, addressing cortisol-mediated barrier dysregulation (Ohira et al., 2017). This mechanistic stratification underscores the potential for precision management of gut-muscle axis dysfunction. In this perspective, our data may also serve as a preliminary foundation for the future development of a translational decision-making algorithm to guide clinicians in a phenotype-oriented management of FM and IBS. At the current stage, however, these findings should be regarded as exploratory, and further validation in larger cohorts, incorporating additional clinical and molecular parameters, will be required before this approach can be translated into routine clinical practice.
Furthermore, our study reveals a novel association between physical deconditioning (lower GPCS) and elevated serum zonulin, suggesting that reduced PC contributes to intestinal barrier dysfunction. Notably, this association was specific to serum zonulin, which may reflect systemic barrier disruption more sensitively than fecal zonulin, I-FABP, or %Lac. Unlike BMI, a crude metabolic surrogate, GPCS provides a functional assessment of physical status and correlates more strongly with zonulin-mediated barrier impairment. Mechanistically, PC may influence barrier integrity through splanchnic hypoperfusion, oxidative stress, and Hypotalamic-Pituitary-Adrenal (HPA) axis dysregulation (de Oliveira and Burini, 2009; Bilski et al., 2018), which collectively promote cortisol-driven zonulin release, downregulation of TJ proteins (e.g., occludin, claudin), and microbial dysbiosis (Panwar et al., 2021; Riezzo et al., 2023).
The observed trends support the hypothesis that greater PC may promote improved gut health. In this context, it has been demonstrated that enhanced cardiovascular fitness may improve splanchnic perfusion, thereby reducing intestinal hypoxia and oxidative stress, which are known to compromise the TJ integrity (Keirns et al., 2020). Additionally, physical exercise promotes microbial diversity, particularly enriching butyrate-producing species such as Faecalibacterium prausnitzii, which strengthens the epithelial barrier through HDAC Inhibition (Zhang et al., 2021) and upregulation of occludin (O'Riordan et al., 2022). Finally, exercise modulates the HPA axis, potentially reducing cortisol-mediated zonulin release (Clark and Mach, 2016).
Physical exercise can be viewed as a pathogenically targeted intervention, akin to pharmacological agents, with dose-dependent and phenotype-specific effects. Its ability to modulate intestinal, inflammatory, and functional biomarkers positions it as both a preventive strategy and a primary therapeutic approach, particularly when tailored to individual patient characteristics. This aligns with the principles of precision medicine, which aim to tailor interventions based on individual biological and functional profiles. Integrating non-invasive biomarkers (e.g., zonulin, I-FABP) with physical performance indices, such as the GPCS, could facilitate a shift from symptom-based management to mechanism-driven stratification and care. Phenotypic stratification guided by such biomarkers may enable a precision medicine approach. For example, a patient with FM + IBS, high zonulin, and low GPCS might receive a combined regimen of graded exercise, probiotics targeting zonulin production (e.g., Lactobacillus rhamnosus GG, which has shown efficacy in modulating gut permeability (Chen et al., 2019), and gut-directed nutrients. In contrast, an IBS patient with elevated I-FABP may initially benefit from mast cell modulation and epithelial repair agents, such as zinc carnosine (Mahmood et al., 2007). This paradigm shift from symptom management to mechanistic targeting aligns with emerging frameworks for complex chronic disorders (Clauw, 2014).
While we advocate for the integration of PC assessment and exercise-based interventions, it is imperative to acknowledge that fatigue is a core and often debilitating symptom of FM. Any exercise program must be carefully graded and individualized, starting at a very low intensity and gradually increasing, to avoid exacerbating fatigue and pain. Patient education on pacing and energy conservation strategies is equally crucial to ensure adherence and prevent symptom flare-ups.
Although our study provides critical new insights into the gut-muscle axis in FM, several limitations must be acknowledged. Most notably, the cross-sectional design precludes causal inference regarding the relationships among PC, intestinal barrier function, and clinical symptom severity. As such, while our results reveal significant associations, they do not clarify whether reduced PC promotes barrier dysfunction or if mucosal alterations contribute to fatigue and deconditioning. Future longitudinal or interventional studies are needed to establish causality and determine whether targeted improvements in physical fitness lead to measurable benefits for gut barrier function.
Second, the absence of a healthy control group limits the generalizability of our findings. However, the sample included individuals from diverse geographic and socioeconomic backgrounds in Southern Italy, thereby enhancing external validity despite being recruited from a single center. Stratification by clinical phenotype, along with comparisons to published reference values for zonulin and I-FABP, allowed us to identify meaningful intergroup differences. Future studies should include healthy controls to establish baseline levels for intestinal barrier biomarkers and strengthen comparative analyses. A significant limitation of this study is the lack of formal classification of IBS subtypes (e.g., diarrhea-predominant, constipation-predominant). As different IBS subtypes may exhibit distinct patterns of barrier dysfunction and dysbiosis (Linsalata et al., 2018), this represents a crucial area for future investigation to refine phenotype-specific therapeutic approaches.
The acknowledged limitation of uncontrolled dietary intake must temper the interpretation of fecal zonulin levels. As dietary components, particularly gluten, have been shown to modulate fecal zonulin (Fasano, 2020), the observed elevations in the FM + IBS group may reflect dietary habits rather than, or in addition to, intrinsic barrier dysfunction. This highlights the importance of dietary standardization in future research utilizing this biomarker.
Third, dietary intake, a known influence on gut permeability and microbiota composition, was not systematically assessed. This omission may confound the interpretation of indole and zonulin levels. Future studies should include validated dietary questionnaires to address this limitation. Additionally, while anthropometric assessment (BMI) confirmed the link between FM and overweight/obesity (Ursini et al., 2011), our regression model demonstrated that BMI does not affect intestinal permeability in these patients.
Another important limitation is the potential confounding effect of sex. Given the known influence of sex hormones on intestinal epithelial permeability (Larauche et al., 2025) and the higher female prevalence of FM (Daher et al., 2024) and IBS (Kim and Kim, 2018), the observed differences in biomarkers might be partially attributable to sex distribution rather than disease phenotype alone. Future studies should aim for balanced sex representation or perform sex-stratified analyses.
Finally, some limitations affect the interpretation of zonulin: current ELISA assays lack specificity due to cross-reactivity with properdin and may detect non-haptoglobin-2 isoforms (Scheffler et al., 2018). To mitigate this, we contextualized zonulin data with I-FABP and sugar absorption tests. Similarly, while urinary indole shows promise as a non-invasive marker of dysbiosis, its pathophysiological relevance requires validation through targeted metabolomics and microbiome-based analyses.
In conclusion, this study demonstrates that intestinal barrier dysfunction is a shared yet phenotype-specific hallmark of FM and IBS, with the most severe impairment in comorbid cases. Distinct mechanisms (i.e., TJ dysfunction in FM and epithelial injury in IBS) underscore the need for personalized approaches. The observed inverse relationship between PC and barrier dysfunction underscores the potential to integrate exercise-based interventions and biomarker-guided stratification in the management of these complex functional disorders.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data presented in this study are openly available at 10.6084/m9.figshare.29656031.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of the IRCCS Ospedale Oncologico di Bari “Istituto Oncologico Giovanni Paolo II” (Protocol No. 117; Ethics Committee approval granted on March 3, 2023). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FR: Conceptualization, Formal Analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review and editing. AB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review and editing. LP: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. GL: Investigation, Methodology, Writing – review and editing. MG: Investigation, Writing – review and editing. BD’A: Investigation, Writing – review and editing. NV: Data curation, Investigation, Methodology, Writing – review and editing. AI: Investigation, Writing – review and editing. IF: Data curation, Investigation, Methodology, Writing – review and editing. FG: Data curation, Investigation, Writing – review and editing. CB: Data curation, Investigation, Methodology, Writing – review and editing. AA: Investigation, Writing – review and editing. MN: Investigation, Writing – review and editing. ML: Investigation, Writing – review and editing. GR: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Italian Ministry of Health, Ricerca Corrente 2025.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors used Grammarly.com to enhance the quality of the manuscript’s English language.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1695163/full#supplementary-material
References
Ariani A., Bazzichi L., Sarzi-Puttini P., Salaffi F., Manara M., Prevete I., et al. (2021). The Italian society for rheumatology clinical practice guidelines for the diagnosis and management of fibromyalgia best practices based on current scientific evidence. Reumatismo 73 (2), 89–105. doi:10.4081/reumatismo.2021.1362
Arribas-López E., Zand N., Ojo O., Snowden M. J., Kochhar T. (2021). The effect of amino acids on wound healing: a systematic review and meta-analysis on arginine and glutamine. Nutrients 13 (8), 2498. doi:10.3390/nu13082498
Aya V., Flórez A., Perez L., Ramírez J. D. (2021). Association between physical activity and changes in intestinal microbiota composition: a systematic review. PloS one 16 (2), e0247039. doi:10.1371/journal.pone.0247039
Bianco A., Russo F., Franco I., Riezzo G., Donghia R., Curci R., et al. (2023). Enhanced physical capacity and gastrointestinal symptom improvement in Southern Italian IBS patients following three months of moderate aerobic exercise. J. Clin. Med. 12 (21), 6786. doi:10.3390/jcm12216786
Bilski J., Mazur-Bialy A., Magierowski M., Kwiecien S., Wojcik D., Ptak-Belowska A., et al. (2018). Exploiting significance of physical exercise in prevention of gastrointestinal disorders. Curr. Pharm. Des. 24 (18), 1916–1925. doi:10.2174/1381612824666180522103759
Canani R. B., Di Costanzo M., Leone L., Pedata M., Meli R., Calignano A. (2011). Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. gastroenterology WJG 17 (12), 1519–1528. doi:10.3748/wjg.v17.i12.1519
Chen L., Li H., Li J., Chen Y., Yang Y. (2019). Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int. J. Mol. Med. 43 (3), 1139–1148. doi:10.3892/ijmm.2019.4050
Clark A., Mach N. (2016). Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J. Int. Soc. Sports Nutr. 13, 43. doi:10.1186/s12970-016-0155-6
Clauw D. J. (2014). Fibromyalgia: a clinical review. Jama 311 (15), 1547–1555. doi:10.1001/jama.2014.3266
Cott A., Parkinson W., Bell M., Adachi J., Bedard M., Cividino A., et al. (1992). Interrater reliability of the tender point criterion for fibromyalgia. J. rheumatology 19 (12), 1955–1959.
Crawford B., Piault E., Lai C., Bennett R. (2011). Assessing fibromyalgia-related fatigue: content validity and psychometric performance of the Fatigue Visual analog scale in adult patients with fibromyalgia. Clin. Exp. Rheumatology-Incl Suppl. 29 (6), S34–S43.
Daher M., Abbas S., Asaad Z., Khalil K., Jadid G. (2024). Prevalence of fibromyalgia and irritable bowel syndrome and its association with studying medicine, a cross-sectional study in Al-Baath University, Syria. Brain Behav. 14 (3), e3445. doi:10.1002/brb3.3445
de Oliveira E. P., Burini R. C. (2009). The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 12 (5), 533–538. doi:10.1097/MCO.0b013e32832e6776
Drossman D. A. (2016). Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 150 (6), 1262–1279.e2. doi:10.1053/j.gastro.2016.02.032
Erdrich S., Hawrelak J. A., Myers S. P., Harnett J. E. (2020a). Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet. Disord. 21 (1), 181. doi:10.1186/s12891-020-03201-9
Erdrich S., Hawrelak J. A., Myers S. P., Harnett J. E. (2020b). A systematic review of the association between fibromyalgia and functional gastrointestinal disorders. Ther. Adv. Gastroenterology 13, 1756284820977402. doi:10.1177/1756284820977402
Farrukh A. (2022). Measurement of pain and related symptoms in irritable bowel syndrome: the use of validated pain measurement tools. Gastrointest. Disord. 4 (1), 22–29. doi:10.3390/gidisord4010004
Fasano A. (2020). All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 9, F1000 Faculty Rev-69. doi:10.12688/f1000research.20510.1
Fortea M., Albert-Bayo M., Abril-Gil M., Ganda Mall J. P., Serra-Ruiz X., Henao-Paez A., et al. (2021). Present and future therapeutic approaches to barrier dysfunction. Front. Nutr. 8, 718093. doi:10.3389/fnut.2021.718093
Francis C. Y., Morris J., Whorwell P. J. (1997). The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacol. and Ther. 11 (2), 395–402. doi:10.1046/j.1365-2036.1997.142318000.x
Góes S. M., Leite N., Shay B. L., Homann D., Stefanello J. M., Rodacki A. L. (2012). Functional capacity, muscle strength and falls in women with fibromyalgia. Clin. Biomech. 27 (6), 578–583. doi:10.1016/j.clinbiomech.2011.12.009
Grootjans J., Lenaerts K., Buurman W. A., Dejong C. H., Derikx J. P. (2016). Life and death at the mucosal-luminal interface: new perspectives on human intestinal ischemia-reperfusion. World J. gastroenterology 22 (9), 2760–2770. doi:10.3748/wjg.v22.i9.2760
Han T., Xi R., Wang J., Yan H., Li L. (2024). Adherence to ACSM exercise guidelines and its influence on fibromyalgia treatment outcomes: a meta-analysis of randomized controlled trials. Front. Physiol. 15, 1413038. doi:10.3389/fphys.2024.1413038
Hasler W. L., Grabauskas G., Singh P., Owyang C. (2022). Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol. Motil. 34 (7), e14339. doi:10.1111/nmo.14339
Ho T., Elma Ö., Kocanda L., Brain K., Lam T., Kanhere T., et al. (2025). The brain-gut axis and chronic pain: mechanisms and therapeutic opportunities. Front. Neurosci. 19, 1545997. doi:10.3389/fnins.2025.1545997
Hoeger W. W., Hopkins D. R. (1992). A comparison of the sit and reach and the modified sit and reach in the measurement of flexibility in women. Res. Q Exerc. Sport. 63 (2), 191–195. doi:10.1080/02701367.1992.10607580
Johannesson E., Simrén M., Strid H., Bajor A., Sadik R. (2011). Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Official J. Am. Coll. Gastroenterology| ACG 106 (5), 915–922. doi:10.1038/ajg.2010.480
Keirns B. H., Koemel N. A., Sciarrillo C. M., Anderson K. L., Emerson S. R. (2020). Exercise and intestinal permeability: another form of exercise-induced hormesis? Am. J. Physiol. Gastrointest. Liver Physiol. 319 (4), G512–g518. doi:10.1152/ajpgi.00232.2020
Kim J. Y., Cartwright I. M., Colgan S. P. (2025). Indole dysbiosis and mucosal inflammation. Microbiota Host. 3 (1), e250004. doi:10.1530/mah-25-0004
Kim Y. S., Kim N. (2018). Sex-gender differences in irritable bowel syndrome. J. Neurogastroenterol. Motil. 24 (4), 544–558. doi:10.5056/jnm18082
Labanski A., Langhorst J., Engler H., Elsenbruch S. (2020). Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: a transdisciplinary challenge. Psychoneuroendocrinology 111, 104501. doi:10.1016/j.psyneuen.2019.104501
Larauche M., Mahurkar-Joshi S., Biraud M., Ju T., Mayer E. A., Chang L. (2025). Sex-dependent alterations of colonic epithelial permeability: relevance to irritable bowel syndrome. Front. Physiology 16, 1509935. doi:10.3389/fphys.2025.1509935
Laukkanen R., Oja P., Pasanen M., Vuori I. (1992). Validity of a two kilometre walking test for estimating maximal aerobic power in overweight adults. Int. J. Obes. Relat. Metab. Disord. 16 (4), 263–268.
Learmonth Y., Dlugonski D., Pilutti L., Sandroff B., Klaren R., Motl R. (2013). Psychometric properties of the fatigue severity scale and the modified fatigue impact scale. J. neurological Sci. 331 (1-2), 102–107. doi:10.1016/j.jns.2013.05.023
Lee P. H., Macfarlane D. J., Lam T. H., Stewart S. M. (2011). Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Act. 8, 115. doi:10.1186/1479-5868-8-115
Lichtenstein A., Tiosano S., Amital H. (2018). The complexities of fibromyalgia and its comorbidities. Curr. Opin. rheumatology 30 (1), 94–100. doi:10.1097/BOR.0000000000000464
Linsalata M., Riezzo G., D’Attoma B., Clemente C., Orlando A., Russo F. (2018). Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC Gastroenterol. 18 (1), 167. doi:10.1186/s12876-018-0888-6
Linsalata M., Riezzo G., Orlando A, D’Attoma B., Prospero L., Ignazzi A., et al. (2023). The role of intestinal barrier function in overweight patients with IBS with diarrhea undergoing a long-term low fermentable Oligo-, Di-, and monosaccharide and polyol diet. Nutrients 15 (21), 4683. doi:10.3390/nu15214683
Mahmood A., FitzGerald A. J., Marchbank T., Ntatsaki E., Murray D., Ghosh S., et al. (2007). Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 56 (2), 168–175. doi:10.1136/gut.2006.099929
Majnarić L. T., Bosnić Z., Guljaš S., Vučić D., Kurevija T., Volarić M., et al. (2021). Low psychological resilience in older individuals: an association with increased inflammation, oxidative stress and the presence of chronic medical conditions. Int. J. Mol. Sci. 22 (16), 8970. doi:10.3390/ijms22168970
Martel J., Chang S.-H., Ko Y.-F., Hwang T.-L., Young J. D., Ojcius D. M. (2022). Gut barrier disruption and chronic disease. Trends Endocrinol. and Metabolism 33 (4), 247–265. doi:10.1016/j.tem.2022.01.002
Masood R., Mandalia K., Moverman M. A., Puzzitiello R. N., Pagani N. R., Menendez M. E., et al. (2023). Patients with functional somatic syndromes—Fibromyalgia, irritable bowel syndrome, chronic headaches, and chronic low back pain—have lower outcomes and higher opioid usage and cost after shoulder and elbow surgery. Arthrosc. J. Arthrosc. and Relat. Surg. 39 (6), 1529–1538. doi:10.1016/j.arthro.2022.12.028
O'Riordan K. J., Collins M. K., Moloney G. M., Knox E. G., Aburto M. R., Fülling C., et al. (2022). Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 546, 111572. doi:10.1016/j.mce.2022.111572
Ohira H., Tsutsui W., Fujioka Y. (2017). Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. thrombosis 24 (7), 660–672. doi:10.5551/jat.RV17006
Okumura R., Takeda K. (2025) “The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation,” in Seminars in immunopathology, 2. Springer.
Panwar S., Sharma S., Tripathi P. (2021). Role of barrier integrity and dysfunctions in maintaining the healthy gut and their health outcomes. Front. Physiol. 12, 715611. doi:10.3389/fphys.2021.715611
Pearn J., Bullock K. (1979). A portable hand-grip dynamometer. Aust. Paediatr. J. 15 (2), 107–109. doi:10.1111/j.1440-1754.1979.tb01200.x
Peng L., Li Z.-R., Green R. S., Holzmanr I. R., Lin J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139 (9), 1619–1625. doi:10.3945/jn.109.104638
Riegler G., Esposito I. (2001). Bristol scale stool form. A still valid help in medical practice and clinical research. Tech. coloproctology 5 (3), 163–164. doi:10.1007/s101510100019
Riezzo G., Prospero L., D’Attoma B., Ignazzi A., Bianco A., Franco I., et al. (2023). The impact of a twelve-week moderate aerobic exercise program on gastrointestinal symptom profile and psychological well-being of irritable bowel syndrome patients: Preliminary data from a southern Italy cohort. J. Clin. Med. 12 (16), 5359. doi:10.3390/jcm12165359
Roager H. M., Licht T. R. (2018). Microbial tryptophan catabolites in health and disease. Nat. Commun. 9 (1), 3294. doi:10.1038/s41467-018-05470-4
Russo F., Chimienti G., Riezzo G., Linsalata M., D’Attoma B., Clemente C., et al. (2018). Adipose tissue-derived biomarkers of intestinal barrier functions for the characterization of diarrhoea-predominant IBS. Dis. Markers 2018 (1), 1827937. doi:10.1155/2018/1827937
Sarzi-Puttini P., Atzeni F., Fiorini T., Panni B., Randisi G., Turiel M., et al. (2003). Validation of an Italian version of the fibromyalgia impact questionnaire (FIQ-I). Clin. Exp. Rheumatol. 21 (4), 459–464.
Scheffler L., Crane A., Heyne H., Tönjes A., Schleinitz D., Ihling C. H., et al. (2018). Widely used commercial ELISA does not detect precursor of Haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front. Endocrinol. (Lausanne) 9, 22. doi:10.3389/fendo.2018.00022
Seethaler B., Basrai M., Neyrinck A. M., Nazare J.-A., Walter J., Delzenne N. M., et al. (2021). Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 321 (1), G11–G17.
Singh R., Zogg H., Wei L., Bartlett A., Ghoshal U. C., Rajender S., et al. (2021). Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J. Neurogastroenterol. Motil. 27 (1), 19–34. doi:10.5056/jnm20149
Sturgeon C., Fasano A. (2016). Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue barriers. 4 (4), e1251384. doi:10.1080/21688370.2016.1251384
Ursini F., Naty S., Grembiale R. D. (2011). Fibromyalgia and obesity: the hidden link. Rheumatol. Int. 31 (11), 1403–1408. doi:10.1007/s00296-011-1885-z
Verrelli N., Bonfiglio C., Franco I., Bagnato C. B., Stabile D., Shahini E., et al. (2025). The role of global physical capacity score in key parameters of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Clin. Med. 14 (11), 3821. doi:10.3390/jcm14113821
Wolfe F., Clauw D. J., Fitzcharles M. A., Goldenberg D. L., Häuser W., Katz R. S. (2011). Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 38 (6), 1113–1122. doi:10.3899/jrheum.100594
Wolfe F., Häuser W. (2011). Fibromyalgia diagnosis and diagnostic criteria. Ann. Med. 43 (7), 495–502. doi:10.3109/07853890.2011.595734
Keywords: fibromyalgia, irritable bowel syndrome, physical capacity, gut barrier, dysbiosis
Citation: Russo F, Bianco A, Prospero L, Laselva G, Grasso M, D’Attoma B, Verrelli N, Ignazzi A, Franco I, Goscilo F, Bagnato CB, Ancona A, Notarnicola M, Linsalata M and Riezzo G (2025) Physical capacity modulates intestinal barrier dysfunction in functional disorders: phenotype-specific patterns in fibromyalgia and irritable bowel syndrome. Front. Physiol. 16:1695163. doi: 10.3389/fphys.2025.1695163
Received: 29 August 2025; Accepted: 17 October 2025;
Published: 29 October 2025.
Edited by:
Stephen J. Pandol, Cedars Sinai Medical Center, United StatesReviewed by:
Sharon Erdrich, The University of Sydney, AustraliaAnastasiia Badaeva, Sechenov First Moscow State Medical University, Russia
Copyright © 2025 Russo, Bianco, Prospero, Laselva, Grasso, D’Attoma, Verrelli, Ignazzi, Franco, Goscilo, Bagnato, Ancona, Notarnicola, Linsalata and Riezzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Russo, ZnJhbmNlc2NvLnJ1c3NvQGlyY2NzZGViZWxsaXMuaXQ=; Antonella Bianco, YW50b25lbGxhLmJpYW5jb0BpcmNjc2RlYmVsbGlzLml0
†These authors have contributed equally to this work
 Francesco Russo
Francesco Russo Antonella Bianco
Antonella Bianco Laura Prospero1
Laura Prospero1 Benedetta D’Attoma
Benedetta D’Attoma Francesco Goscilo
Francesco Goscilo Maria Notarnicola
Maria Notarnicola Michele Linsalata
Michele Linsalata