- 1Department of Medicine I, Division of Hematology and Hemostaseology, and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
- 2Austrian Group Medical Tumor Therapy, Salzburg, Austria
- 3Department of Pathology, Medical University of Vienna, Vienna, Austria
- 4Assign Data Management and Biostatistics GmbH, Innsbruck, Austria
- 5Department of Internal Medicine III with Hematology, Medical Oncology, Hemostaseology, Infectiology and Rheumatology, Oncologic Center, Salzburg Cancer Research Institute-Center for Clinical Cancer and Immunotherapy (SCRI-CCCIT), Paracelsus Medical University, Cancer Cluster Salzburg, Salzburg, Austria
- 6Department of Internal Medicine IV, Klinikum Wels-Grieskirchen GmbH, Wels, Austria
- 7Division of Hematology, Department of Internal Medicine, Medical University of Graz (MUG), Graz, Austria
- 8Department of Internal Medicine V, Hematology and Oncology, Medical University of Innsbruck, Innsbruck, Austria
- 9Department of Biomedical Imaging and Image-guided Therapy, Division of General and Pediatric Radiology, Medical University of Vienna, Vienna, Austria
- 10Department of Radiology, New York University (NYU) Grossman School of Medicine, New York University (NYU) Langone Health, New York, NY, United States
- 11Division of Nuclear Medicine, Department of Molecular Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria
- 12Department of Hematology and Oncology, Kepler University Hospital, Johannes Kepler University, Linz, Austria
Background: Patients with diffuse large B-cell lymphoma (DLBCL) relapsing early (within 12 months) or primary refractory to induction therapy with rituximab (R) and CHOP have a poor prognosis. We therefore initiated a study with obinutuzumab and venetoclax.
Study design and methods: Twenty-one patients with DLBCL (relapsed within 12 months or primary refractory), detectable Bcl-2 protein expression, and CD20 positivity were included in this prospective single-arm study between 2016 and 2021. Obinutuzumab was administered i.v. at a dose of 1,000 mg on days 1, 8, and 15 in cycle 1 and on day 1 of each of the following 21-day cycles. Venetoclax was given at 800 mg daily p.o. continuously. Treatment was repeated for up to three cycles. Eligible patients were planned to either proceed to cellular therapies or receive up to nine cycles of maintenance. The primary endpoint was objective response rate (ORR) after three cycles (Eudract Nr. 2016-001760-10 and NCT02987400).
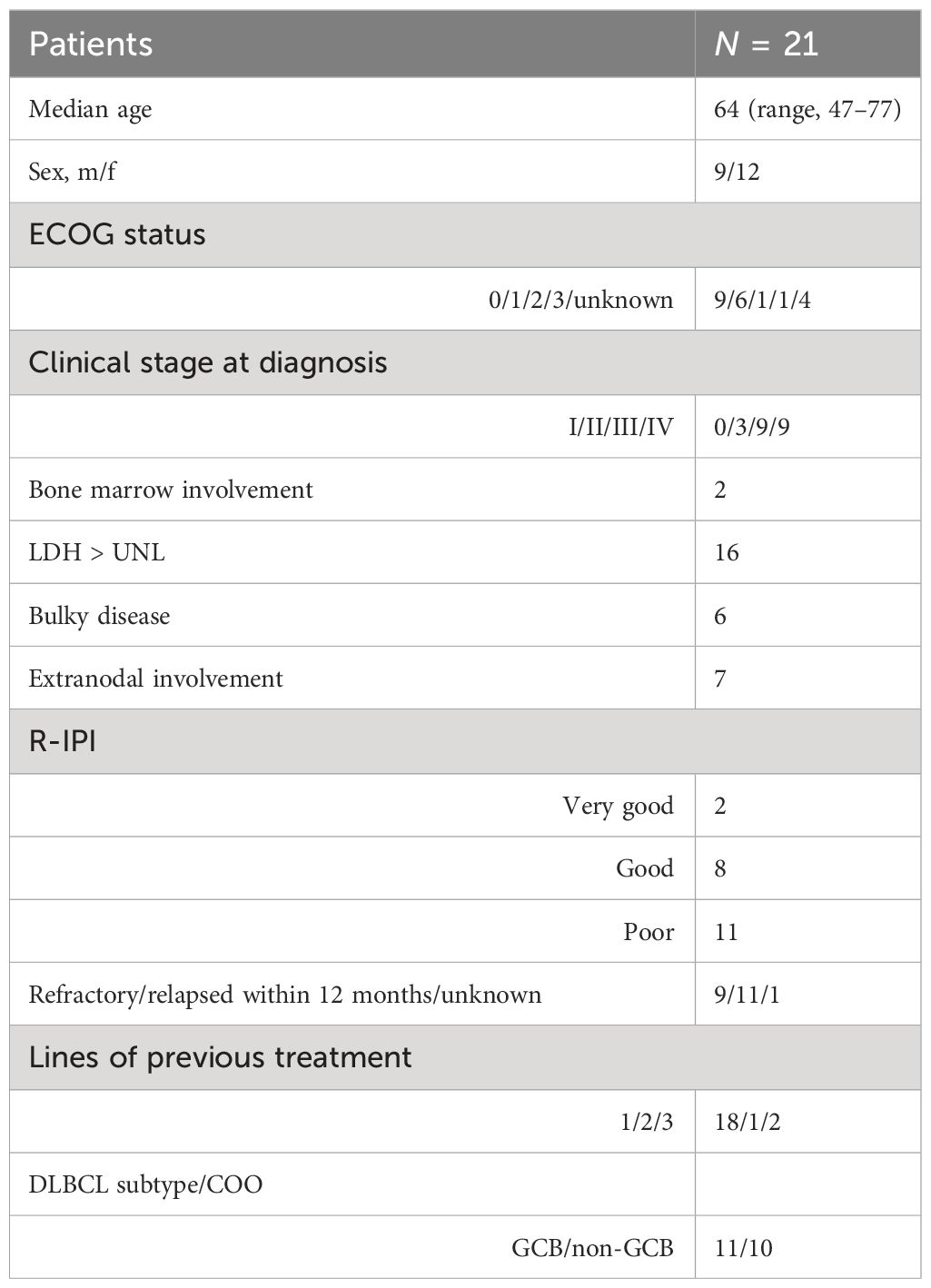
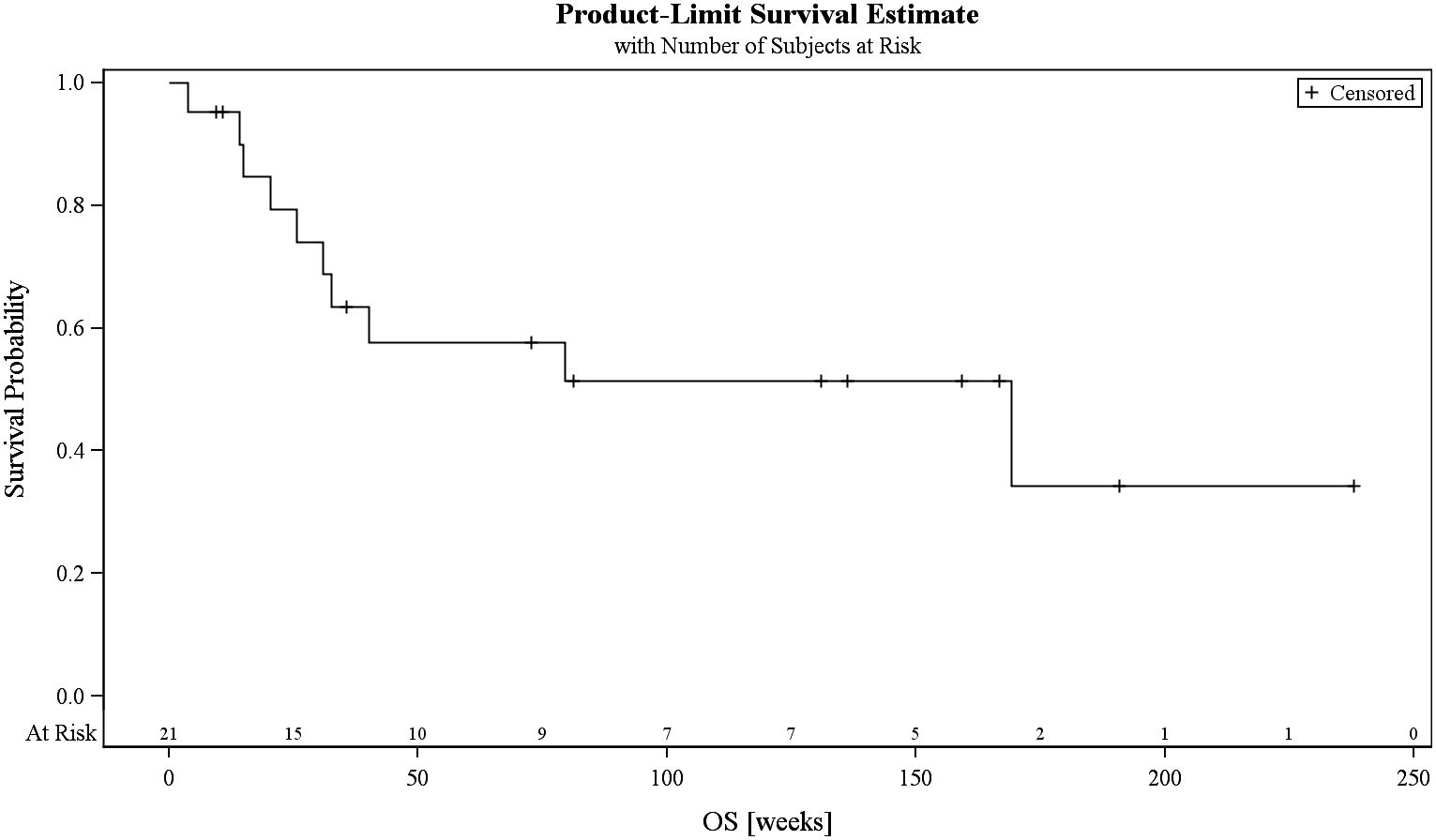
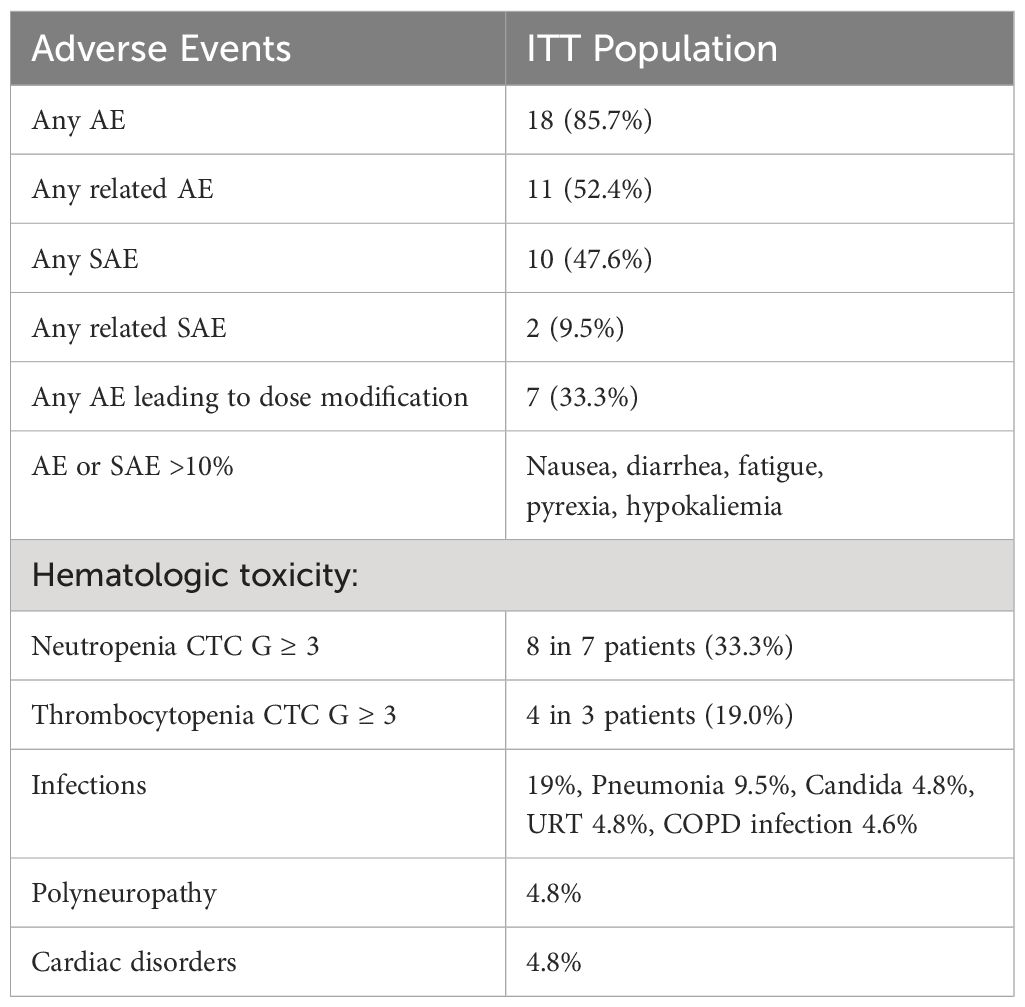
Results: Twenty-one patients (median age, 64 years) with refractory or early relapsed DLBCL after one (N = 11) to four previous lines of therapy were included. The majority of patients received three cycles of obinutuzumab/venetoclax (range, 1–8). The regimen was well tolerated with manageable cytopenias and infections. Severe adverse events related to treatment were observed in 9.5%. The ORR was 38.1% (8/21 patients) with a best response of five complete remissions (CRs; 23.8%) and three partial remissions (PRs; 14.2%). The primary endpoint (45% ORR) was not met. Response duration was 83.3% at 84 days, with a progression-free survival of 38.8% at 84 days and 25.9% at 168 days and a median overall survival of 169.1 weeks. All deaths were due to underlying disease. Seven patients became eligible for autologous transplant. Overall, nine patients (42.8%) received 11 cellular therapies (5 ASCT and 6 CAR-T). Three patients went directly from obinutuzumab/venetoclax to CAR-T therapy. All patients had successful peripheral stem cell or T-cell harvests. Characteristics of responders include relapsed disease (response rate, 6 of 11 = 54%), very good or good R-IPI (7 of 8), and low number of previous therapies (median = 1).
Conclusion: Obinutuzumab/venetoclax represents an effective chemo-free relapse regimen with low toxicity that can be followed by cellular therapies, particularly CAR-T cells.
Introduction
One-third of patients with diffuse large B-cell lymphoma (DLCBL) relapse after induction with rituximab (R) and anthracycline-containing therapy or have primary refractory disease (1, 2). Patients relapsing early (within 12 months) or primary refractory to induction therapy with rituximab (R) and CHOP or CHOP-like regimens have a particularly poor prognosis (3–5). Sequencing of treatment is of great importance, particularly with novel therapies emerging. Dose intensification of chemotherapy is ineffective in most cases of refractory DLBCL and many patients never achieve a remission, making them ineligible for stem cell transplantation. Patients with initially chemoresistant DLBCL have a 1-year survival of only 22% after treatment with high-dose therapy and autologous stem cell transplantation (6). Patients with DLBCL relapsing within 12 months and treated with conventional immunochemotherapy with R, followed by autologous stem cell transplantation, have a 3-year progression-free survival (PFS) of 23% (7–9). The new standard for refractory and early relapsing large B-cell lymphomas has been set by two seminal trials showing that therapy with chimeric antigen receptor T-cells (CAR-T) (axicabtagene ciloleucel and lisocabtagene maraleucel) is superior to autologous transplantation in eligible patients (10, 11). While bridging to CAR-T cell therapy was not allowed in one trial, 63% received bridging therapy before CAR-T infusion in the other. In the real world, the majority of patients are currently bridged (12–17).
Moreover, patients not eligible for transplantation may receive CAR-T cells or alternative therapies in the second or third line (12, 17).
This indicates the importance of sequencing therapies. Bridging to cellular therapies can be achieved by conventional immunochemotherapy in some patients, but lymphomas with molecular aberrations such as TP53 mutations or alterations of the MYC and BCL2 genes are frequently resistant to chemotherapy (18, 19). Therefore, agents like ibrutinib, lenalidomide, selinexor, or polatuzumab vedotin have been used (20–23). Importantly, the inclusion of agents like bendamustin in some of these therapies will hamper the quality of T-cell harvests for further treatment (24, 25).
The second-generation anti-CD20 antibody obinutuzumab (GA-101) and the BH3-mimetic/Bcl-2 inhibitor venetoclax (ABT-199, GDC-0199) have shown excellent activity in other B-cell lymphomas (26, 27). Obinutuzumab and venetoclax for initial treatment of DLBCL have been studied as singular additions to R-CHOP in the GOYA and CAVALLI trials (28, 29). Of note, venetoclax was able to overcome in part the poor prognostic effect of TP53 deletions in CLL (30). This is in line with the action of BH3-mimetics downstream of TP53 (31). In a recent study, a few complete remissions (CRs) with venetoclax monotherapy have been observed in r/r DLBCL (32).
Given the chemoresistance of refractory and early relapsing DLBCL, there was a strong rationale to use obinutuzumab and venetoclax in a “chemo-free” combination as novel targeted agents instead of classical relapse chemotherapies such as cisplatin-containing regimens. The immediate goal of such studies is to improve outcome in patients in whom intensification of chemotherapy is expected to fail.
The study presented here wanted to
● establish safety of the obinutuzumab/venetoclax combination therapy in r/r DLBCL including a 9-month consolidation phase
● prepare patients for cellular therapies without chemotherapy or to maintain remission in patients not eligible for transplant.
While novel antibodies and bispecific agents have shown superior activities since the study was conducted, the results of the NHL15B study serve as a proof of principle for chemo-free reinduction or bridging on the way to cellular therapies.
Patients and methods
Patients
Twenty-one patients with DLBCL (according to the WHO 2016 classification) were included in this prospective pilot trial between 09/2016 and 09/2021 (33). The last patient was treated in 06/2020. Major inclusion criteria were as follows: A histologically confirmed relapse within 12 months after having achieved a partial remission (PR) or CR after initial R-anthracycline containing therapy, or refractoriness to initial R-anthracycline containing therapy (not achieving at least a partial response), detectable Bcl-2 protein expression, and CD20 positivity (Supplementary 1 – Study Protocol). The study was approved by the EC of the Medical University of Vienna.
Treatment
Patients received obinutuzumab i.v. at a dose of 1,000 mg on days 1, 8, and 15 in cycle 1 and on day 1 of each of the following 21-day cycles (Supplementary 2.1). Venetoclax was given at 800 mg daily p.o. starting from day 1 of cycle 1. Treatment was repeated for up to three cycles. Eligible patients were initially planned to proceed to autologous stem cell transplantation or maintenance with up to nine cycles if ineligible for ASCT. During the conduct of the study, CAR-T cells became another option for these patients. While this is not formally amended in the Protocol, we refer to ASCT and CAR-T cell therapy as “cellular therapies” in this report.
The trial was registered under EudraCT Nr. 2016-001760-10 and NCT02987400.
Study design and statistical methods
The study was conducted as a non-comparative, Fleming-design Phase II single-arm study (Supplementary 1 – NHL15B protocol). A run-in phase for the first 6 patients and a futility analysis (after 10 patients) were done. The first response assessment (including PET-CT) was performed after the first cycle of obinutuzumab/venetoclax and patients with at least stable disease (SD) or better were planned to receive two additional cycles of therapy and then have assessment after a total of three cycles. Patients with CR or PR after three cycles of therapy could either go on to cellular therapy or receive nine further cycles of the combination therapy (if transplant ineligible). In this case, assessments were performed after 6, 9, and 12 cycles.
Patients with progressive disease at any time point or SD after three cycles were taken off study.
The recruitment period was 35 months after amendment #4. Data cutoff was conducted in July 2020, with a final overall survival follow-up in December 2022.
The primary endpoint was objective response rate (ORR) by the Lugano 2014 criteria after three cycles (investigator-assessed) (34). Responses were assessed based on local PET-CT results. There was no review adjudication committee (RAC) or centralized PET-CT review. Secondary objectives included dose-limiting toxicities, response duration, progression-free survival, overall survival, and ability to proceed to further stem cell therapy.
A Phase II Fleming design with α = 0.05 and β = 0.2 (power = 80%) was applied. The null hypothesis that the ORR is not greater than 20% was tested against the alternative hypothesis that the ORR is at least 45%. A total of 21 evaluable patients were analyzed at the end of the study, and the null hypothesis is rejected if at least eight responders are observed.
Biomarkers
A biomarker program investigated histopathologic, genomic, and biological factors associated with outcome. Genomic analysis from tumor samples was conducted by FoundationOne®Heme.
Results
Patient characteristics
Basic characteristics of the 21 patients with refractory or early relapsed DLBCL confirm the poor prognostic features of the intent-to-treat (ITT) study population (Table 1) (Supplementary 2.2. – CONSORT Flow Diagram). This includes advanced clinical stage and poor R-IPI at first diagnosis (35). Almost half of the patients were refractory to first-line treatment. Of note, 3 patients needed additional therapy between their first-line therapy and obinutuzumab and venetoclax, due to logistic delays. Patients received a median of three cycles of obinutuzumab/venetoclax (range, 1 to 8).
Response
The primary endpoint of the study was response rate (best overall response after three cycles of obinutuzumab and venetoclax at 45%). This endpoint was not met. An objective response was achieved in 8 of 21 patients (38.1%, 95% CI: 20.8, 59.1), namely, 5 complete responses (23.8%) and 3 partial responses (14.2%) (ITT population). Five patients had stable (25%) and eight had progressive disease (38%). Two complete responses were observed after cycle 1 and three complete responses were observed after cycle 3. After cycle 1, two CRs, five PRs, and six SDs were observed.
The PFS was 38.8% at 84 days and 25.9% at 168 days, while the median overall survival was 169.1 weeks (Figure 1; Supplementary 2.3.). At final visit, 20% were in complete response. Progressive disease was treated with various regimens including chemo-immunotherapy, bispecific agents, antibody–drug conjugates, immunomodulators, or small molecules. At the end of the study, 7 patients (33.3%) had died. All deaths were attributable to progressive disease.
Eligibility for cellular therapy and maintenance
One goal of this study was to enable patients to receive cellular therapies. Seven patients became eligible for autologous transplant due to their response status. Overall, nine patients (42.8%%) received 11 cellular therapies (5 ASCT and 6 CAR-T). Three patients went directly from obinutuzumab/venetoclax to cellular therapies (three CAR-T). All patients receiving ASCT had intermittent immunochemotherapies. Two patients had consecutive ASCT as well as CAR-T cell therapies. All patients had successful peripheral stem cell or T-cell harvests, indicating that obinutuzumab/venetoclax does not affect the ability to collect cells for further therapies.
Maintenance
Three patients went on to receive obinutuzumab/venetoclax maintenance after achieving a response. Two patients elected not to go on to cellular therapy at this stage.
Subgroup analysis
Characteristics of responding patients corresponded to good risk features of their DLBCL: 7 of 8 responding patients had an initial R-IPI of very good or good, while 10 of 13 non-responders had a poor R-IPI (p = 0.015) (Supplementary 2.4). Six of eight responding patients had relapsed as opposed to refractory disease. The response rate in relapsed patients was 6/11 (54%). Overall, responders were characterized by favorable R-IPI, only one line of pretreatment, and early relapse (not refractory). The cell of origin (GCB vs. non-GCB) did not seem to have an impact on response in this small patient group. There was also no impact of double-hit translocations.
Mutational analysis was available in 10 patients (Supplementary 2.5). There were no obvious findings associated with response. Of note, two TP53 aberrations were noted in non-responders. IGH-BCL2 rearrangements as well as BCL2 mutations were observed in responders and non-responders.
Safety
The study treatment was well tolerated (Table 2; Supplementary 2.6). Eleven patients had related adverse events, with only 9.5% related serious adverse events. Nausea, diarrhea, fatigue, pyrexia, and hypokalemia were frequent adverse events. Of note, hematologic toxicity (neutropenia and thrombocytopenia) was acceptable and was managed with G-CSF and antibiotics. None of the patients needed a transfusion of blood components.
Discussion
The NHL15B trial was intended to induce responses in refractory or early relapsing DLBCL with a chemo-free regimen with low toxicity. While it was not expected to produce durable responses, one major goal was to prepare these patients for cellular therapies. The rationale was that many of these patients are already refractory to conventional immune-chemotherapy and may not reach a partial or complete response to make them eligible for autologous transplantation, which was still the preferred second-line treatment at the start of the trial (36). This was also confirmed by the fact that in the ZUMA-7, TRANSFORM, and BELINDA trials, only 32% to 46% could be transplanted in the second line (10, 11, 37). On the other hand, more than 90% could receive CAR-T cells in all three trials investigating this cellular therapy as second-line therapy for DLBCL (10, 11, 37). Therefore, we have later also included patients who were able to receive CAR-T cells after obinutuzumab/venetoclax. Patients eligible for CAR-T cell treatment do not necessarily have to be in response; however, patients with (rapidly) progressive disease have a poorer outcome (38, 39). While the primary endpoint of the study (45% best ORR) was not met, obinutuzumab/venetoclax may be seen as a reasonable bridging therapy since 62% of patients in NHL15B had a CR, PR, or SD, making them good candidates for CAR-T cells. Indeed, in this trial, eight patients went on to cellular therapies including six CAR-T cell treatments.
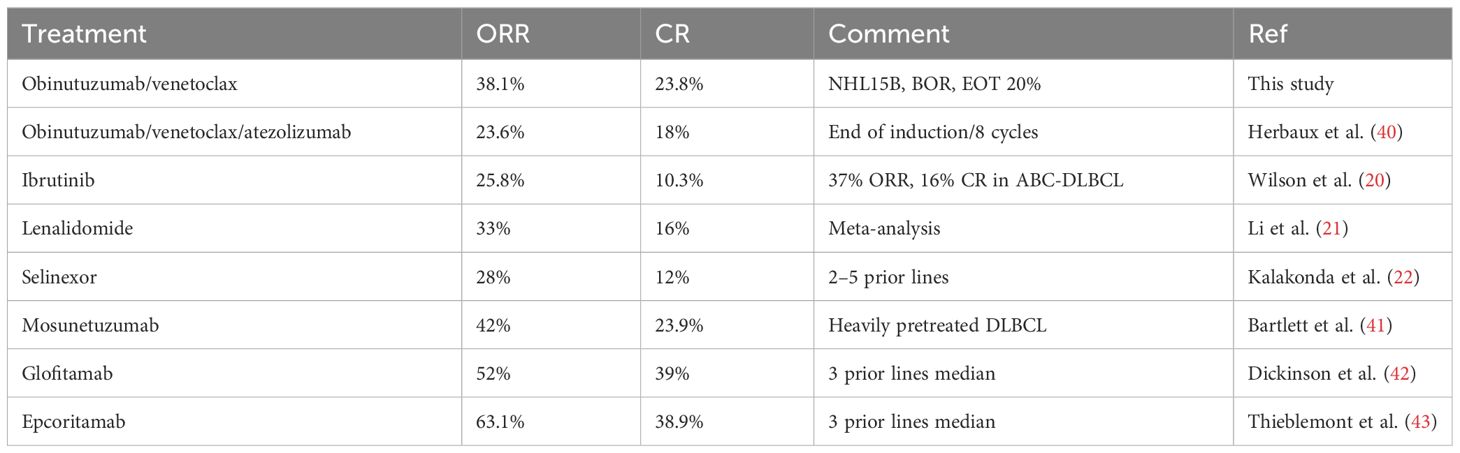
While this trial was recruiting, novel chemo-free therapies were studied and recently approved. Table 3 shows the response rates of these regimens in poor-risk relapsing or refractory patients (20, 21, 40–43). Only two of these studies (with bispecific antibodies) had ORRs above 45%. There are no data for most of these therapies in the second line. However, obinutuzumab/venetoclax in the second-line refractory or early relapsing patients was equally effective as other chemo-free regimens (albeit mostly administered in heavily pretreated patients) with the exception of the novel bispecific agents (42, 43). However, glofitamab or epcoritamab may currently not be accessible everywhere. The addition of a checkpoint inhibitor did not seem to add efficacy as compared to the study by Herbaux et al. (40).
Obinutuzumab/venetoclax was particularly effective in patients after first-line treatment with a relapse after initial response (not refractory) with a favorable IPI. The response rate in these patients makes this treatment an alternative bridging strategy to be considered. Obinutuzumab/venetoclax was less effective in refractory patients with a high R-IPI. Of note, three patients responded for longer than three cycles and went on to maintenance therapy.
While the obinutuzumab/venetoclax combination has not been studied in the second line except for this trial, both single substances have been used with (R)-CHOP therapy (28, 29). Obinutuzumab with CHOP showed benefit over R-CHOP in patients with GCB-DLBCL (28). This was not obvious in our study but we note that three patients with a GCB-type DLBCL were among the CR patients (Supplementary 2.2). Venetoclax showed a slightly improved long-term outcome compared with R-CHOP controls mainly in the Bcl-2 ICH-positive subgroup in the CAVALLI study (29). Since the NHL15B study required Bcl-2 positivity as entry criterion, this observation cannot be made. Nevertheless, inhibition of Bcl-2 may increase the rate of apoptosis in expressing cells in addition to obinutuzumab monotherapy (31, 44–47).
In this study, we also obtained genetic analysis of the initial tumor in half of the patients. With these low numbers, no definitive statements can be made. In a study investigating mutations in GOYA and CAVALLI, a trend towards improved survival outcomes for patients in the BCL2/EZH2 cluster upon treatment with the Bcl-2 inhibitor venetoclax was found (48).
We observed IGH-BCL2 or BCL2 as well as EZH2 mutations in both responding and non-responding patients. BTG1 and CDKN2A/B mutations that had been associated with resistance to venetoclax in CLL were also found in both subgroups (49). We note that the two TP53 mutations occurred in non-responders, pointing towards some resistance to the obinutuzumab/venetoclax combination as shown for CLL or immunotherapies in DLBCL (18, 19, 50, 51). We and others have shown that female patients respond better to CD20-antibody therapies (52). In this study, five of eight responders were female.
An increased myelotoxicity was observed with venetoclax in combination with immunochemotherapy (29). Based on the results of the CAVALLI 1b study, we applied 800 mg of venetoclax without ramp-up since tumor lysis was rarely observed in DLBCL (53, 54). In addition, venetoclax at a dose of 800 mg was given daily throughout the treatment, while in CAVALLI, an intermittent schedule was applied (29). We reasoned that hematologic toxicity should still be acceptable without concomitant chemotherapy.
Toxicity in NHL15 was manageable with acceptable rates of neutropenia and infections. This could represent argument for the use of obinutuzumab/venetoclax as bridging to cellular therapies. The therapy creates a window of approximately 3 months in responding patients. Another argument for its use for bridging before CAR-T cells would be that the seven patients responded and six patients had SD already after cycle 1 of obinutuzumab/venetoclax. Since late complications of CAR-T cell therapies may also be a function of preceding toxic treatments, a chemo-free regimen may help to avoid these events (55, 56). Finally, obinutuzumab/venetoclax did not impair the ability to harvest peripheral blood stem cells or T cells, even if followed by other treatments. Together with novel therapeutic possibilities, including CAR-T cells and bispecific antibodies is probably reflected in a reasonable overall survival.
Limitations
While this pilot study showed the feasibility and low toxicity of the therapy, and even though the study was designed to have enough power for the primary endpoint of the ORR, the ambitiously high assumption of 45% response rate contributed to the negative outcome of the study. Recruitment was slow due to the initiation of trials with other novel agents, particularly bispecific antibodies. Owing to logistic delays, three patients have received one (two patients) or two (one patient) additional cycles of R-DHAP while being prepared for the study. Two of these patients also progressed during obinutuzumab/venetoclax, while one patient responded with a CR. Given the 38% ORR, it is unlikely that this 2:1 distribution has influenced the study outcome.
The initial assumption that patients would go on to autologous stem cell transplant had to be revised for CAR-T cells/cellular therapies. Effective bispecific antibodies have now been approved after the second line and will probably be also used in the second line. Nevertheless, obinutuzumab/venetoclax may be a good alternative in some instances.
Conclusion
The NHL15B study showed that responses can be induced in 38% of patients with refractory or early relapsing DLBCL and can be followed by cellular therapies, particularly CAR-T cells. The low toxicity of this treatment may make it a useful alternative to other options in selected, low-risk DLBCL patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Medical University of Vienna. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
UJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. AE: Investigation, Writing – review & editing. IS-K: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PK: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. SH: Investigation, Writing – review & editing. PN: Investigation, Writing – review & editing. EW: Investigation, Writing – review & editing. FE: Data curation, Formal analysis, Software, Writing – review & editing. JL-S: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. PS: Investigation, Resources, Writing – review & editing. EP: Investigation, Writing – review & editing. CS: Investigation, Writing – review & editing. MM: Investigation, Writing – review & editing. MH: Investigation, Writing – review & editing. TM: Investigation, Writing – review & editing. MF: Conceptualization, Investigation, Writing – review & editing. RG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from Roche Austria and FoundationOne®Heme. Part of this work has been presented as a poster at the Annual Meeting of the American Society of Hematology 2020. UJ is currently supported by the Innovative Medicines Initiative (IMI) T2EVOLVE. The project receives funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 945393. This Joint Undertaking receives support from the European Union ís Horizon 2020 research and innovation program and EFPIA.
Acknowledgments
The authors are grateful to the dedicated study staff at individual centers and at the AGMT office.
Conflict of interest
Authors PK, FE, and JL-S was employed by the company Assign Data Management and Biostatistics GmbH. Author SH was employed by the company Klinikum Wels-Grieskirchen GmbH. UJ received honoraria from Roche, Abbvie, Amgen, Beigene, Astra Zeneca, Gilead, Janssen, Miltenyi, and Novartis. PN received honoraria from Roche, Abbvie, Amgen, Beigene, Astra Zeneca, Gilead, Janssen, Miltenyi, Novartis, BMS. MM received honoraria from GE, Siemens, and BMS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2024.1331008/full#supplementary-material
References
1. Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. (2005) 23:5027–33. doi: 10.1200/JCO.2005.09.137
2. Rovira J, Valera A, Colomo L, Setoain X, Rodríguez S, Martínez-Trillos A, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. (2015) 94:803–12. doi: 10.1007/s00277-014-2271-1
4. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. (2017) 130:1800–8. doi: 10.1182/blood-2017-03-769620
5. Maurer MJ, Habermann TM, Shi Q, Schmitz N, Cunningham D, Pfreundschuh M, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. (2018) 29:1822–7. doi: 10.1093/annonc/mdy203
6. Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. (1995) 333:1540–5. doi: 10.1056/NEJM199512073332305
7. Velasquez WS, Cabanillas F, Salvador P, Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. (1988) 71:117–22.
8. Gisselbrecht C. Is there any role for transplantation in the rituximab era for diffuse large B-cell lymphoma? Hematol Am Soc Hematol Educ Program. (2012) 2012:410–6.
9. Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. (2012) 30:4462–9. doi: 10.1200/JCO.2012.41.9416
10. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. All ZUMA-7 investigators and contributing kite members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. (2022) 386:640–54. doi: 10.1056/NEJMoa2116133
11. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. (2022) 399:2294–308. doi: 10.1016/S0140-6736(22)00662-6
12. Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. (2022) 28:2145–54. doi: 10.1038/s41591-022-01969-y
13. Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, et al. GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood. (2022) 140:349–58. doi: 10.1182/blood.2021015209
14. Kuhnl A, Roddie C, Kirkwood AA, Tholouli E, Menne T, Patel A, et al. A national service for delivering CD19 CAR-Tin large B-cell lymphoma - The UK real-world experience. Br J Haematol. (2022) 198:492–502. doi: 10.1111/bjh.18209
15. Tang K, Nastoupil LJ. Real-world experiences of CAR T-cell therapy for large B-cell lymphoma: how similar are they to the prospective studies? J Immunother Precis Oncol. (2021) 4:150–9. doi: 10.36401/JIPO-21-2
16. Vitale C, Strati P. CAR T-cell therapy for B-cell non-hodgkin lymphoma and chronic lymphocytic leukemia: clinical trials and real-world experiences. Front Oncol. (2020) 10:849. doi: 10.3389/fonc.2020.00849
17. Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transplant Cell Ther. (2022) 28:581.e1–8. doi: 10.1016/j.jtct.2022.05.026
18. Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. (2012) 120:3986–96. doi: 10.1182/blood-2012-05-433334
19. Schiefer AI, Kornauth C, Simonitsch-Klupp I, Skrabs C, Masel EK, Streubel B, et al. Impact of single or combined genomic alterations of TP53, MYC, and BCL2 on survival of patients with diffuse large B-cell lymphomas: A retrospective cohort study. Med (Baltimore). (2015) 94:e2388. doi: 10.1097/MD.0000000000002388
20. Wilson W, Young R, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. (2015) 21:922–6. doi: 10.1038/nm.3884
21. Li J, Zhou J, Guo W, Wang X, Zhao Y, Bai O. Efficacy and safety of lenalidomide monotherapy for relapsed/refractory diffuse large B cell lymphoma: systematic review and meta-analysis. Front Oncol. (2021) 11:756728. doi: 10.3389/fonc.2021.756728
22. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. (2020) 7:e511–22. doi: 10.1016/S2352-3026(20)30120-4
23. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. (2020) 38:155–65. doi: 10.1200/JCO.19.00172
24. Iacoboni G, Navarro V, Martín-López AÁ, Rejeski K, Kwon M, Jalowiec KA, et al. Recent bendamustine treatment before apheresis has a negative impact on outcomes in patients with large B-cell lymphoma receiving chimeric antigen receptor T-cell therapy. J Clin Oncol. (2024) 42(2):205–17. doi: 10.1200/JCO.23.01097
25. Worel N, Grabmeier-Pfistershammer K, Kratzer B, Schlager M, Tanzmann A, Rottal A, et al. The frequency of differentiated CD3+CD27-CD28- T cells predicts response to CART cell therapy in diffuse large B-cell lymphoma. Front Immunol. (2023) 13:1004703. doi: 10.3389/fimmu.2022.1004703
26. Davies A, Kater AP, Sharman JP, Stilgenbauer S, Vitolo U, Klein C, et al. Obinutuzumab in the treatment of B-cell Malignancies: a comprehensive review. Future Oncol. (2022) 18:2943–66. doi: 10.2217/fon-2022-0112
27. Rajendra A, Sengar M. Venetoclax: A narrative drug review. Cancer Res Stat Treat. (2022) 5:519–32. doi: 10.4103/crst.crst_179_22
28. Sehn LH, Martelli M, Trněný M, Liu W, Bolen CR, Knapp A, et al. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA. J Hematol Oncol. (2020) 13:71. doi: 10.1186/s13045-020-00900-7
29. Morschhauser F, Feugier P, Flinn IW, Gasiorowski R, Greil R, Illés Á, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. (2021) 137:600–9. doi: 10.1182/blood.2020006578
30. Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. (2019) 380:2225–36. doi: 10.1056/NEJMoa1815281
31. Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. (2003) 22:8590–607. doi: 10.1038/sj.onc.1207102
32. Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol. (2017) 35:826–33. doi: 10.1200/JCO.2016.70.4320
33. Swerdlow SH, Campo E, Stefano A, Pileri SA, Harris NL, Stein H, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
34. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. (2014) 32:3059–67. doi: 10.1200/JCO.2013.54.8800
35. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. (2007) 109:1857–61. doi: 10.1182/blood-2006-08-038257
36. Cuccuini W, Briere J, Mounier N, Voelker HU, Rosenwald A, Sundstrom C, et al. MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood. (2012) 119:4619–24. doi: 10.1182/blood-2012-01-406033
37. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. (2022) 386:629–39. doi: 10.1056/NEJMoa2116596
38. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
39. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
40. Herbaux C, Casasnovas O, Feugier P, Damaj G, Bouabdallah R, Guidez S, et al. Atezolizumab + obinutuzumab + venetoclax in patients with relapsed or refractory diffuse large B-cell Lymphomas (R/R DLBCL): Primary analysis of a phase II trial from LYSA. J Clin Oncol. (2020) 38:15_suppl:8053–3. doi: 10.1200/JCO.2020.38.15_suppl.8053
41. Bartlett NL, Assouline S, Giri P, Schuster SJ, Cheah CY, Matasar M, et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. (2023) 7:4926–35. doi: 10.1182/bloodadvances.2022009260
42. Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2022) 387:2220–31. doi: 10.1056/NEJMoa2206913
43. Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. (2023) 41:2238–47. doi: 10.1200/JCO.22.01725
44. Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, et al. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. (1988) 7:123–31. doi: 10.1002/j.1460-2075.1988.tb02791.x
45. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. (2002) 2:647–56. doi: 10.1038/nrc883
46. Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. (2016) 374(4):311–22. doi: 10.1056/NEJMoa1513257
47. Morschhauser FA, Cartron G, Thieblemont C, Solal-Céligny P, Haioun C, Bouabdallah R, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. (2013) 31:2912–9. doi: 10.1200/JCO.2012.46.9585
48. Kim E, Jiang Y, Xu T, Bazeos A, Knapp A, Bolen CR, et al. Prognostic mutational subtyping in de novo diffuse large B-cell lymphoma. BMC Cancer. (2022) 22:231. doi: 10.1186/s12885-022-09237-5
49. Herling CD, Abedpour N, Weiss J, Schmitt A, Jachimowicz RD, Merkel O, et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun. (2018) 9:727. doi: 10.1038/s41467-018-03170-7
50. Al-Sawaf O, Zhang C, Jin HY, Robrecht S, Choi Y, Balasubramanian S, et al. Transcriptomic profiles and 5-year results from the randomized CLL14 study of venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab in chronic lymphocytic leukemia. Nat Commun. (2023) 14:2147. doi: 10.1038/s41467-023-37648-w
51. Porpaczy E, Wohlfarth P, Königsbrügge O, Rabitsch W, Skrabs C, Staber P, et al. Influence of TP53 mutation on survival of diffuse large B-cell lymphoma in the CAR T-cell era. Cancers (Basel). (2021) 13:5592. doi: 10.3390/cancers13225592
52. Jäger U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, et al. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. (2012) 97:1431–8. doi: 10.3324/haematol.2011.059246
53. Zelenetz AD, Salles G, Mason KD, Casulo C, Le Gouill S, Sehn LH, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. (2019) 133:1964–76. doi: 10.1182/blood-2018-11-880526
54. Wei JX, Konopleva M. Bcl-2 inhibition in the treatment of hematologic Malignancies. Front Hematol. (2023) 2:1307661. doi: 10.3389/frhem.2023.1307661
55. Penack O, Peczynski C, Koenecke C, Polge E, Sanderson R, Yakoub-Agha I, et al. Organ complications after CD19 CAR T-cell therapy for large B cell lymphoma: a retrospective study from the EBMT transplant complications and lymphoma working party. Front Immunol. (2023) 14:1252811. doi: 10.3389/fimmu.2023.1252811
Keywords: diffuse large B-cell lymphoma, early relapsed or refractory, obinutuzumab, venetoclax, chemo-free
Citation: Jaeger U, Simonitsch-Klupp I, Klammer P, Egle A, Heibl S, Neumeister P, Willenbacher E, Erlsbacher F, Larcher-Senn J, Staber PB, Porpaczy E, Skrabs C, Mayerhoefer ME, Hacker M, Melchardt T, Fridrik MA and Greil R (2024) Phase II single-arm study of a combination of obinutuzumab and venetoclax in early relapsed or refractory diffuse large B-cell lymphoma—final results of the AGMT NHL15B study. Front. Hematol. 3:1331008. doi: 10.3389/frhem.2024.1331008
Received: 31 October 2023; Accepted: 15 February 2024;
Published: 28 March 2024.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Vincent Camus, Centre Henri Becquerel Rouen, FranceSolomon Graf, University of Washington, United States
Copyright © 2024 Jaeger, Simonitsch-Klupp, Klammer, Egle, Heibl, Neumeister, Willenbacher, Erlsbacher, Larcher-Senn, Staber, Porpaczy, Skrabs, Mayerhoefer, Hacker, Melchardt, Fridrik and Greil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrich Jaeger, dWxyaWNoLmphZWdlckBtZWR1bml3aWVuLmFjLmF0
†These authors share first authorship
 Ulrich Jaeger
Ulrich Jaeger Ingrid Simonitsch-Klupp3†
Ingrid Simonitsch-Klupp3† Alexander Egle
Alexander Egle Philipp B. Staber
Philipp B. Staber