- 1Department of Bio-Therapeutic, the First Medical Centre, Chinese People's Liberation Army General Hospital, Beijing, China
- 2Department of Bio-Therapeutic, the Fifth Medical Centre, Chinese People's Liberation Army General Hospital, Beijing, China
- 3Department of Myeloma and Lymphoma, Beijing GoBroad Boren Hospital, Beijing, China
- 4Changping Laboratory, Beijing, China
Background: CD19-targeted chimeric antigen receptor T (CAR T) cell therapy has revolutionized the treatment of refractory/relapsed B-cell malignancies. However, this therapy introduces significant safety concerns, including cytokine release syndrome (CRS) and infections, both of which can lead to life-threatening complications. These two complications often require conflicting treatment approaches, making it challenging to balance patient safety and therapeutic effectiveness. The optimal approach to managing infections complicated by CRS remains unclear.
Case presentation: A 54-year-old man with primary refractory high-grade B-cell lymphoma, who had failed multiple prior therapies, received CD19 CAR T-cell therapy after bridging therapy and intensive lymphodepletion. He developed a severe diffuse alveolar hemorrhage induced by CRS complicated with virus infection following CAR T-cell infusion. Despite aggressive therapeutic approaches including anti-infection measures, immune modulation, and anticytokine agents, no significant clinical improvement was initially observed. The patient’s toxicity was effectively managed, ultimately leading to a complete response (CR), only after the introduction of glucocorticoids following the median time to peak CAR T-cell expansion. The patient sustained this CR for over 36 months, until January 2025.
Conclusion: This case highlights the importance of early diagnosis and management of CRS and infection after CAR T-cell therapy, offering critical insights into managing adverse reactions and optimizing patient outcomes.
1 Introduction
CD19-targeted chimeric antigen receptor T (CAR-T) cell therapy has demonstrated exceptional efficacy for refractory/relapsed B-cell malignancies (1) and received the US Food and Drug Administration (FDA) approval in 2017; however, this therapy has also shown increased risk of cytokine release syndrome (CRS) infection. The incidence of CRS ranges from 50% to 100% (2) and is mediated by elevated levels of proinflammatory cytokines, which result from the extensive expansion and activation of infused CAR T cells during the first 3 to 4 weeks post-infusion (3, 4). In addition to CRS, approximately 70% of patients develop infections caused by identifiable pathogens within the first month following CAR T-cell therapy (5). The malignancy itself, multiple lines of anticancer treatments, and lymphodepleting chemotherapy administered before CAR T-cell infusion result in immunodeficiency, thereby increasing the risk of infections (6, 7). Of note, nearly 20% of patients may encounter overlapping CRS and infection, with viral infection accounting for 8% (8). The convergence of CRS and infections may lead to severe systemic inflammation, posing life-threatening risks. Therefore, balancing the management of infection and CRS during CAR T-cell therapy is crucial for optimizing patient outcomes. To date, only a few studies have reported the concurrence of CRS and infections following CAR T-cell therapy (1, 6, 7, 9, 10), providing limited guidance for treatment strategies. Currently, how to balance and manage this complex scenario remains unclear.
Here, we present a case of a patient successfully treated for severe viral pneumonia complicated by grade 3 CRS and even severe diffuse alveolar hemorrhage (DAH) during CAR T-cell therapy. The patient was diagnosed with high-grade B-cell lymphoma (HGBL) with primary refractory disease, high tumor burden, and failure of fourth-line treatment before CAR T-cell treatment. The patient achieved and sustained a complete response (CR) for over 36 months after CAR T-cell therapy. This report indicates that introducing glucocorticoids after the median time to peak CAR T-cell expansion may be a viable option for balancing and managing CRS and infections, and enhances awareness of severe DAH as a critical complication following CAR T-cell therapy.
2 Case presentation
A 54-year-old man was diagnosed with stage IV HGBL, characterized by extranodal involvement, positivity for CD19, Ki-67 expression of over 90%, and features indicative of double expression and double-hit lymphoma (DHL). Genetic analysis revealed a mutation in MYD88/CD79B and TP53. Despite undergoing four lines of therapy, including rituximab-based and anthracycline-based chemotherapies and autologous hematopoietic stem cell transplantation (auto-HSCT), the patient never achieved CR (Figure 1A). In late 2021, the patient presented to our department with weakness and B symptoms and elevated levels of aminotransferases, bilirubin, and lactate dehydrogenase (LDH) (Supplementary Figure S1). Positron emission tomography-computed tomography (PET-CT) revealed hypermetabolism in multiple lymph nodes, lungs, liver, spleen, and bones (Supplementary Figure S2A).
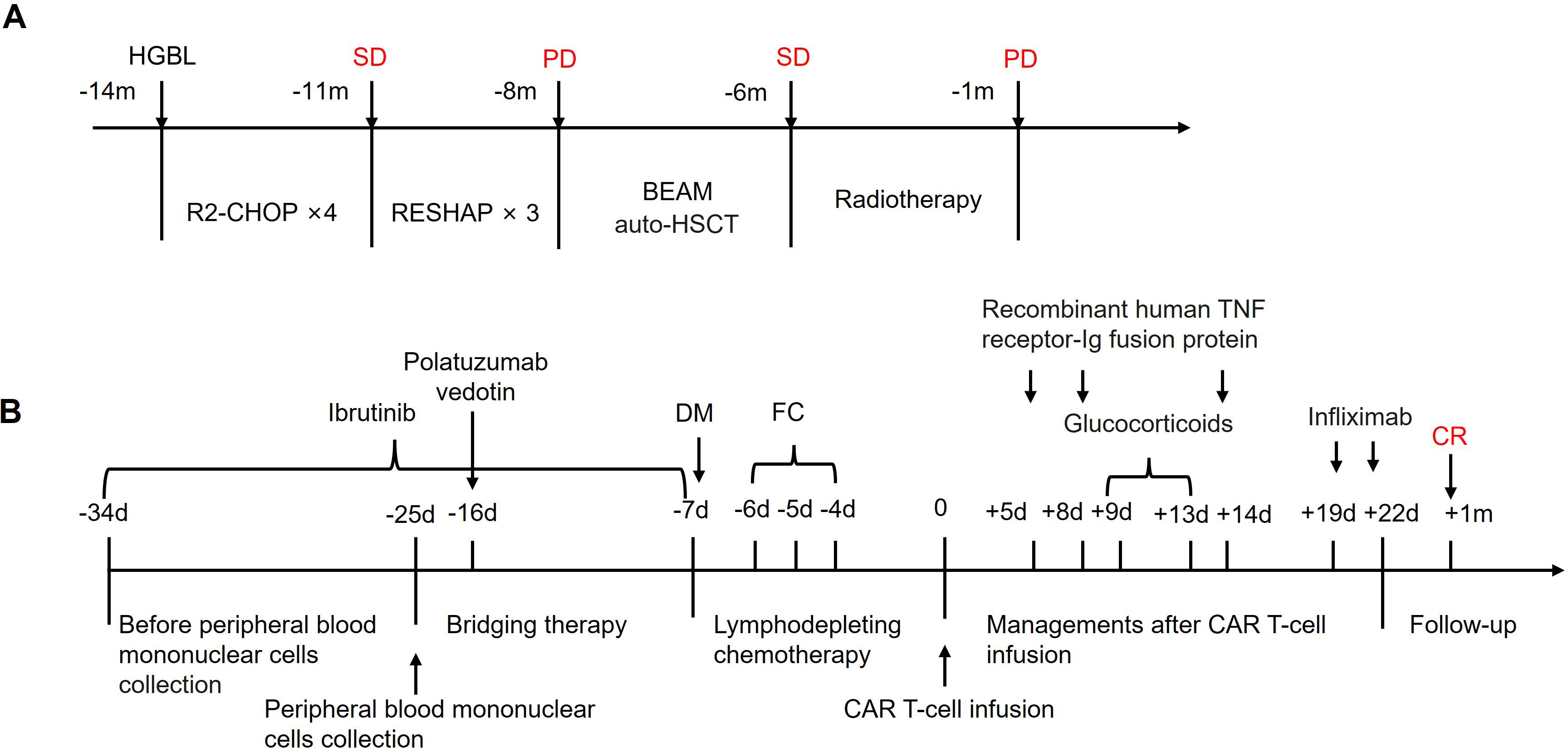
Figure 1. Treatment of the patient. (A) The history of treatment before CAR T-cell therapy. R2-CHOP included rituximab 700 mg on day 1, cyclophosphamide 1.4 g on day 2, doxorubicin 130 mg on day 2, vincristine 2 mg on day 2, prednisone 50 mg daily from day 2 to day 6, and lenalidomide 25 mg daily from day 2 to day 11; R-ESHAP included rituximab 700 mg on day 1, etoposide 74 mg daily from day 1 to day 4, cisplatin 46 mg daily from day 1 to day 4, cytarabine 3.7 g on day 5, and prednisone 50 mg daily from day 1 to day 5; BEAM included carmustine 450 mg on day −7, etoposide 360 mg daily from day −6 to day −3, cytarabine 360 mg on day −3, and melphalan 250 mg on day −2. (B) The clinical application scheme of the CAR T-cell therapy. CAR, chimeric antigen receptor; auto-HSCT, autologous hematopoietic stem cell transplantation; SD, stable disease; PD, progressive disease; m, month; DM, doxorubicin hydrochloride liposome and chlormethine hydrochloride; FC, fludarabine and cyclophosphamide; d, day.
Considering the patient’s refractory condition, CAR T-cell therapy was deemed a rational course of action. To optimize the efficacy of CAR T-cell treatment, the patient received ibrutinib (560 mg once daily from day −34 to −7) and polatuzumab vedotin (140 mg once daily on day −16) as bridging therapy. This was followed by an intensified lymphodepleting chemotherapy regimen consisting of doxorubicin hydrochloride liposome (20 mg once daily on day −7), chlormethine hydrochloride (15 mg once daily on day −7), fludarabine (20 mg once daily on days −6, −5, −4), and cyclophosphamide (0.4 g once daily on days −6, −5, −4) (Figure 1B). On day −5, the patient developed herpes zoster, presenting with a maculopapular rash and vesicles. Antiviral treatments were initiated alongside human immunoglobulin. On the next day, the patient developed a fever with a maximum temperature of 39.0°C. The leukocyte count dropped to 0.05 × 109/L, and procalcitonin (PCT) levels were elevated at 25.74 ng/mL (reference range < 0.5 ng/mL). Broad-spectrum antibiotics and antifungal agents were administered as a precaution.
On 7 January 2022, the patient received a CAR T-cell infusion, with a total of 100 × 106 CD19 CAR T cells (relmacabtagene autoleucel). Between days 3 and 5 post-infusion, the patient experienced hemoptysis, dyspnea, and fever, with auscultation revealing sporadic wet rales. Arterial blood gas analysis indicated respiratory failure (PaO2: 53 mmHg), and hemoglobin levels fell to 66 g/L. Chest radiography revealed bilateral diffuse exudation, and chest CT showed extensive ground-glass opacification (GGO) and consolidation with interlobular septal thickening (Figure 2), leading to a diagnosis of DAH. PMseq-DNA Pro high-throughput gene detection revealed the presence of human beta-herpesvirus 5 (13 gene sequences) in the phlegm and human beta-herpesvirus 5 (7 gene sequences) and human alpha-herpesvirus 3 (780 gene sequences) in the peripheral blood (Supplementary Table S1), suggesting reactivation of the herpesvirus. Simultaneously, cytokine levels increased and CAR T cells massively proliferated in the peripheral blood (Figure 3), leading to a diagnosis of grade 3 CRS. In addition to symptomatic, supportive therapies, antiviral treatments, and plasma were administered to support immunologic function, along with recombinant human TNF receptor-Ig fusion protein that was initiated to manage CRS. However, on day 8, pneumonia and respiratory failure worsened. Methylprednisolone sodium succinate (80 mg once daily from day 9 to 11, 60 mg once daily on day 12, 40 mg once daily on day 13) was administered from day 9 to 13 for its anti-inflammatory and immunosuppressive properties. Subsequently, the patient’s vital signs stabilized, and the clinical manifestations and pneumonia gradually improved (Figure 2). The patient achieved CR from the first month after CAR T-cell infusion and lasted for 36 months until the cutoff date in January 2025 (Supplementary Figure S2).
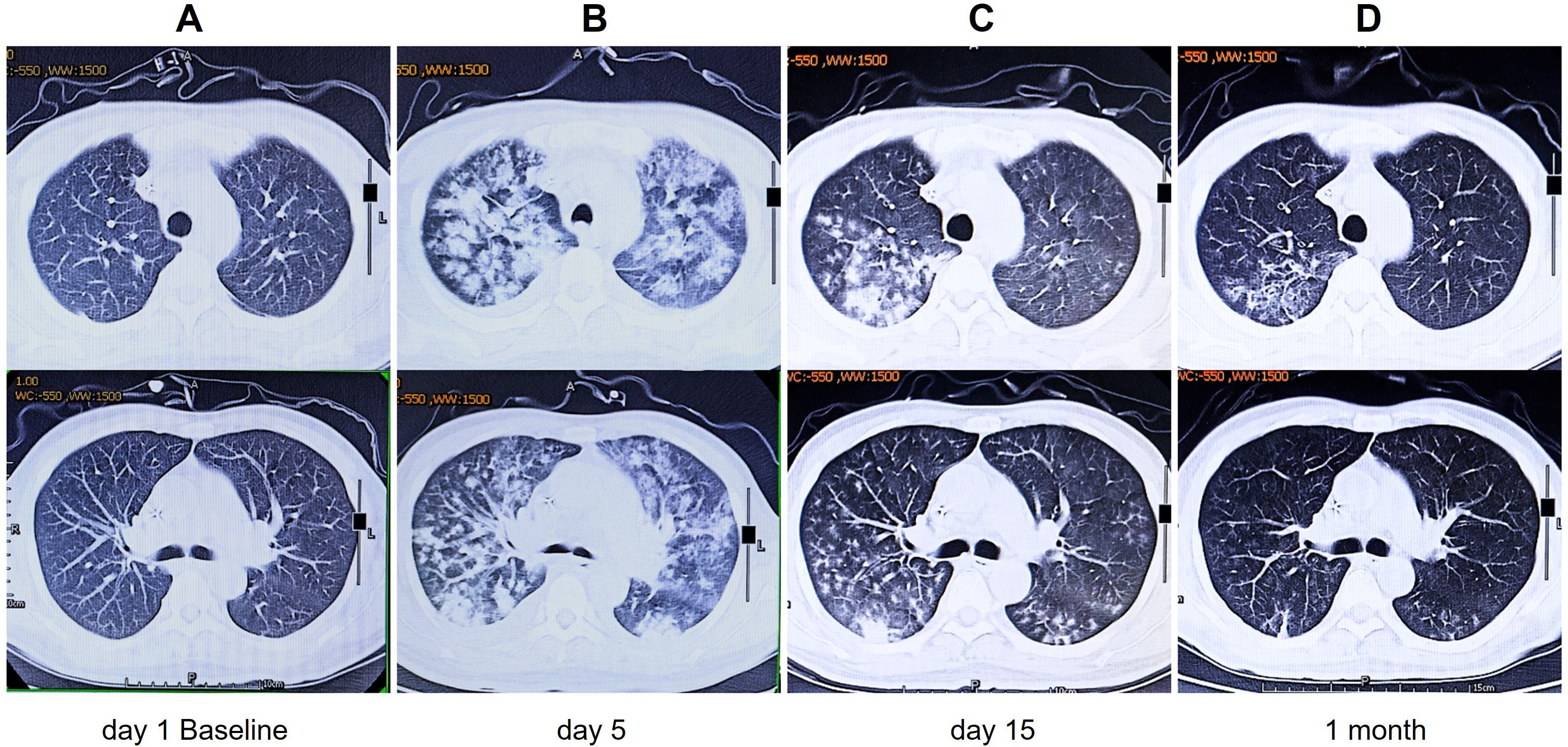
Figure 2. Changes in pulmonary inflammation at different time points after CAR T-cell infusion. (A) Before CAR T-cell infusion. (B) Five days after CAR T-cell infusion: extensive GGOs and consolidation with interlobular septal thickening. (C) Fifteen days after CAR T-cell infusion: notably reduced GGOs and consolidations. (D) One month after CAR T-cell infusion: there was almost complete resolution of pulmonary inflammation. CAR, chimeric antigen receptor; GGOs, ground-glass opacities.
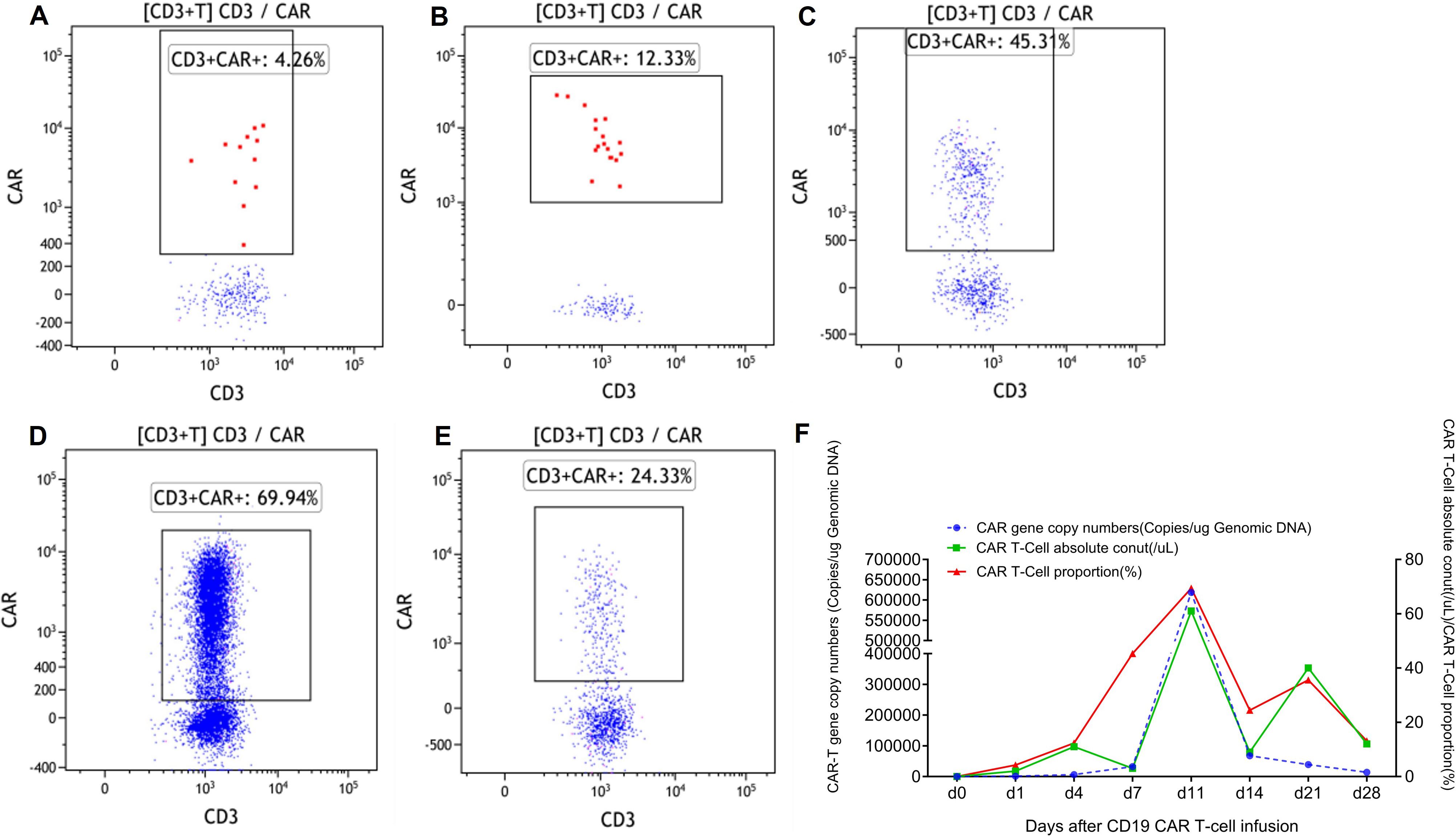
Figure 3. The proliferation of CAR T cells in peripheral blood. (A) One day after CAR T-cell infusion. (B) Four days after CAR T-cell infusion. (C) Seven days after CAR T-cell infusion. (D) Twelve days after CAR T-cell infusion. (E) Fourteen days after CAR T-cell infusion. (F) CAR gene copy number, CAR T-cell absolute number, and CAR T-cell proportions in peripheral blood. CAR, chimeric antigen receptor.
3 Discussion
The prevention and management of infection and CRS during CAR T-cell therapy is the focus of ongoing work. While CAR T-cell therapy has manifested remarkable efficacy in treating hematological malignancies, it is associated with several adverse effects. A meta-analysis indicated that infections are the primary driver of non-relapse mortality, regardless of the type of CAR T-cell product or disease entity, accounting for more than half (50.9%) of all reported non-relapse deaths (11). CRS, as a CAR T-cell side effect, is associated with 4.7% of non-relapse deaths (11). Thus, infection complicated by CRS may pose a potentially life-threatening risk to the patient. However, specific treatment guidelines are still lacking, and balancing these two conditions remains challenging. To our knowledge, this represents the first reported case of severe viral pneumonia complicated by grade 3 CRS-induced DAH during CAR T-cell therapy that was successfully managed by introducing glucocorticoids following the median peak expansion of CAR T cells.
In our study, the patient was diagnosed with a highly aggressive, relapsed/refractory stage IV HGBL and adverse genetic mutations, which may indicate a poor prognosis. He had previously undergone multiple lines of chemotherapy and an auto-HSCT. Prior to receiving CAR T-cell therapy, he also received bridging therapy followed by intensive lymphodepletion conditioning. These cumulative treatments collectively compromised the patient’s immune function and increased susceptibility to infections (6, 7, 12). The patient developed herpes zoster 5 days before CAR T-cell infusion. In response, antiviral medication and human immunoglobulin were administered to manage the viral infection. Additionally, broad-spectrum antibiotics and antifungal drugs were provided as prophylactic treatment. Unfortunately, within 3 to 5 days following CAR T-cell infusion, the patient developed symptoms including cough, hemoptysis, and dyspnea. Further diagnosis through DNA polymerase chain reaction sequencing and imaging studies indicated the presence of viral pneumonia. Additionally, the patient exhibited pulmonary lymphoma infiltration, respiratory failure, and grade 3 CRS. CT scans reveal bilateral consolidative changes, diffuse ill-defined patchy GGO, and interlobular septal thickening (Figure 2). Notably, these findings suggest that this viral infection and severe CRS have induced the occurrence of DAH, a condition that has not been previously reported in the literature.
There is no consensus on the optimal management of viral infections and CRS occurring after CAR T-cell therapy. Corticosteroid treatment might be a strategy to balance the impact of both conditions. Corticosteroids, recommended by multiple guidelines for the treatment of severe infections (13, 14), can effectively reduce inflammatory responses and minimize organ damage. Furthermore, it can mitigate CRS by inhibiting the proliferation of CAR T cells and other immune cells, as well as reducing the production of inflammatory cytokines (15–18). Nevertheless, the use of corticosteroids during the early phase of CAR T-cell therapy can influence the expansion of CAR T cells, thereby affecting clinical efficacy (19). Therefore, the timing of corticosteroid administration is critical and requires a careful balance between effectiveness and safety to ensure optimal treatment outcomes. Currently, the optimal timing of corticosteroid administration to balance CRS and infections remains unclear. In this case, given the potential for early administration of corticosteroids to hinder antitumor activity and CAR T-cell expansion, thereby affecting long-term prognosis (10), glucocorticoids were not immediately introduced to treat early severe infections and CRS. Aggressive therapeutic approaches involving anti-infection measures, immune modulation, and anti-cytokine agents were undertaken. However, no significant clinical improvement was observed initially. Until the introduction of glucocorticoids after the median time to peak CAR T-cell expansion (Figures 1, 3), the patient’s toxicity was effectively managed, ultimately resulting in the achievement of a long-term CR (Supplementary Figure S2). This case offers clinicians early diagnosis and treatment recommendations for CRS and infection following CAR T-cell therapy, providing references for treatment strategies and medication time points.
Although a study reported a case of a patient with relapsed/refractory B-cell acute lymphoblastic leukemia who successfully underwent treatment for severe CRS and infection via hemofiltration after CD19 CAR T-cell infusion, that patient did not develop DAH as observed in this study (9). This is the first documented case of a patient with primary refractory HGBL developing CRS and reactivation of herpesvirus infection, inducing a severe DAH, following CAR T-cell therapy. The FDA has issued black box warnings for CAR T-cell therapies, emphasizing the importance of reporting novel safety concerns. Therefore, our study is also crucial for identifying potential toxicity signals associated with CAR T-cell products.
4 Conclusion
In summary, we report the first case of severe viral infection complicated by grade 3 CRS-induced DAH during CAR T-cell therapy that was successfully managed by introducing glucocorticoids following the median peak expansion of CAR T cells. A comprehensive approach, incorporating anti-infective measures, immunosuppression, and strategic use of steroids following the peak of CAR T-cell amplification, offers the best potential for optimizing patient outcomes and provides valuable insights for managing similar cases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The patient provided written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NL: Data curation, Writing – original draft. YZ: Writing – review & editing, Project administration, Data curation. CW: Data curation, Writing – review & editing. QY: Supervision, Writing – review & editing, Project administration, Data curation. GR: Supervision, Formal analysis, Writing – review & editing. YL: Data curation, Supervision, Writing – review & editing, Project administration. WH: Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by funds from the National Natural Science Foundation of China (82341208 and 82430012).
Acknowledgments
The authors are grateful for the nurses and staff involved in the treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2025.1616504/full#supplementary-material
References
1. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
2. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. Fda approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. (2018) 23:943–7. doi: 10.1634/theoncologist.2018-0028
3. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z car T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. (2014) 6:224ra25. doi: 10.1126/scitranslmed.3008226
4. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
5. Wudhikarn K, Pennisi M, Garcia-Recio M, Flynn JR, Afuye A, Silverberg ML, et al. Dlbcl patients treated with cd19 car T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. (2020) 4:3024–33. doi: 10.1182/bloodadvances.2020001972
6. Gao Z, Lian Y, Ti J, Ren R, and Ma L. Therapeutic efficacy and infectious complications of cd19-targeted chimeric antigen receptor-modified T cell immunotherapy. Anticancer Drugs. (2023) 34:551–7. doi: 10.1097/cad.0000000000001485
7. Korell F, Schubert ML, Sauer T, Schmitt A, Derigs P, Weber TF, et al. Infection complications after lymphodepletion and dosing of chimeric antigen receptor T (Car-T) cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia or B cell non-hodgkin lymphoma. Cancers (Basel). (2021) 13:1684. doi: 10.3390/cancers13071684
8. Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of cd19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. (2018) 131:121–30. doi: 10.1182/blood-2017-07-793760
9. Liu Y, Chen X, Wang D, Li H, Huang J, Zhang Z, et al. Hemofiltration successfully eliminates severe cytokine release syndrome following cd19 car-T-cell therapy. J Immunother. (2018) 41:406–10. doi: 10.1097/cji.0000000000000243
10. Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. (2018) 67:533–40. doi: 10.1093/cid/ciy152
11. Cordas Dos Santos DM, Tix T, Shouval R, Gafter-Gvili A, Alberge JB, Cliff ERS, et al. A systematic review and meta-analysis of nonrelapse mortality after car T cell therapy. Nat Med. (2024) 30:2667–78. doi: 10.1038/s41591-024-03084-6
12. McKay SL, Guo A, Pergam SA, and Dooling K. Herpes zoster risk in immunocompromised adults in the United States: A systematic review. Clin Infect Dis. (2020) 71:e125–e34. doi: 10.1093/cid/ciz1090
13. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
14. Chaudhuri D, Nei AM, Rochwerg B, Balk RA, Asehnoune K, Cadena R, et al. Focused update: guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-Acquired pneumonia. Crit Care Med. (2024) 52:e219–e33. doi: 10.1097/ccm.0000000000006172
15. Thompson JA, Schneider BJ, Brahmer J, Zaid MA, Achufusi A, Armand P, et al. Nccn guidelines® Insights: management of immunotherapy-related toxicities, version 2.2024. J Natl Compr Canc Netw. (2024) 22:582–92. doi: 10.6004/jnccn.2024.0057
16. Paliogianni F, Ahuja SS, Balow JP, Balow JE, and Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through il-2 receptor. J Immunol. (1993) 151:4081–9. doi: 10.4049/jimmunol.151.8.4081
17. Lanza L, Scudeletti M, Puppo F, Bosco O, Peirano L, Filaci G, et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol. (1996) 103:482–90. doi: 10.1111/j.1365-2249.1996.tb08306.x
18. Franchimont D, Louis E, Dewe W, Martens H, Vrindts-Gevaert Y, De Groote D, et al. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul Pept. (1998) 73:59–65. doi: 10.1016/s0167-0115(97)01063-x
Keywords: B-cell lymphoma, CAR T-cell therapy, cytokine release syndrome, herpesvirus infection, corticosteroids
Citation: Lu N, Zhang Y, Wang C, Yang Q, Rong G, Liu Y and Han W (2025) Balance and management of CRS and infection following CD19-targeted CAR T-cell therapy in primary refractory high-grade B-cell lymphoma: a case report. Front. Hematol. 4:1616504. doi: 10.3389/frhem.2025.1616504
Received: 22 April 2025; Accepted: 30 June 2025;
Published: 24 July 2025.
Edited by:
Andrés Gómez-De León, Autonomous University of Nuevo León, MexicoReviewed by:
Senthilnathan Palaniyandi, University of Missouri, United StatesLukasz Chlewicki, Eli Lilly, United States
Copyright © 2025 Lu, Zhang, Wang, Yang, Rong, Liu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, bGl1eWFuZzMwMWJsb29kQDE2My5jb20=; Weidong Han, aGFud2Ryc3dAMTYzLmNvbQ==
 Nannan Lu
Nannan Lu Yajing Zhang3
Yajing Zhang3 Chunmeng Wang
Chunmeng Wang Weidong Han
Weidong Han