- 1Division of Hematology/Oncology, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
- 2Bone Marrow Transplantation Program, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
- 3Department of Pathology and Laboratory Medicine, American University of Beirut, Beirut, Lebanon
This letter describes the experience of the American University of Beirut Medical Center in Lebanon with haploidentical stem cell transplant (haplo-SCT) for hematological malignancies in adult patients. Haplo-SCT made it possible through universal and rapid donor availability for most of the adult patients with leukemia or lymphoma not only in the Middle East but also globally. Moreover, the use of post-transplant cyclophosphamide (PTCy) and reduced intensity conditioning (RIC) regimens when indicated improved the outcome and decreased the toxicity of haploidentical stem cell transplant.RIC regimens also allowed its use in the elderly population. Patients from throughout the Middle East come to our center, the American university of Beirut Medical Center, to receive this transformative type of stem cell transplant. In this paper, we discuss the results of haplo-SCT with PTCy done on adult patients with hematological malignancies in our center from 2015 to 2021. The results are encouraging and show that haplo-SCT should be considered more often in the Middle Eastern countries. The subgroup analysis showed the importance of achieving complete remission of the disease prior to transplant to improve outcomes in our center. There is a paucity of literature on the outcomes of haplo-SCT in the Middle East which may contribute to the limited number of centers that offer this type of SCT. Herein, we aim to fill this gap in the hopes of encouraging the implementation of this potentially curative modality of treatment to a larger extent in the Middle East.
Background and Introduction
Haploidentical stem cell transplant (Haplo) has made it possible that almost every allotransplant candidate has a donor (1). The outcome of Haplo has improved dramatically by using T-cell replete grafts with administration of post-transplantation cyclophosphamide (PTCy). The results of such transplants have been comparable to other historical transplant modalities (1). Locally, it has been offered at our center, the American University of Beirut Medical Center (AUBMC) in Beirut, Lebanon, to patients from throughout the Middle East and the Gulf area since 2013 (2, 3).
Autologous SCT activity in Lebanon has remained mostly stable since 2012 compared to allogeneic activity, which increased drastically (2). This increase in allogeneic stem cell transplantation (allo-SCT) can be mainly attributed to increased availability. Allo-SCT in Lebanon is limited by many factors related to the cost, third party coverage, referral and availability of donors (2). Allo-SCT with matched-related donor (MRD) is considered the best source for allo-SCT among patients with hematologic malignancies but is available for only 25% of patients (1). For the rest of the patients, matched unrelated donor (MUD) transplant is an alternative option worldwide (1). The odds of finding a MUD in international registries can range from 20% to 80% depending on patient’s ethnic background (1). However, in Lebanon and the Middle East area, the probability is less than 5% (2, 3). The low representation of the Middle East population in international registries and the lack of a public registry in the region make it impractical for almost 70% of patients with leukemia and lymphoma (2, 3). For all these reasons, only six patients received allo-SCT from MUD during those last years at our center (2, 3).
In contrast to MUD, almost 95% of the patients have at least one haploidentical donor with an average of 2.7 donors per patient (2). This universal availability of a haploidentical donor, the lower cost, and the short time needed to find the appropriate donor makes the possibility of a Haplo attainable for most patients in Lebanon (2, 3). Moreover, the use of PTCy, anti-thymocyte globulin, graft manipulation (T-cell repletion), and reduced intensity or toxicity conditioning regimen (RIC/RTC) decreased the rate of non-relapse mortality (NRM) associated with Haplo (4–10).
Haplo at AUBMC is predominantly performed for adult patients with hematological malignancies such as acute leukemia and lymphoproliferative disorders, namely Hodgkin’s and Non-Hodgkin’s lymphoma (2, 3). Haplo use in Lebanon started in 2013 (5% of allo-SCT) and reached around 40 to 50% of the allogeneic transplant activity since 2016 (2, 3). Until now, 130 (adults and pediatric) patients received Haplo at AUBMC with 117 of them from 2015 to this date (2, 3).
Haploidentical stem cell transplant in adult patients at AUBMC from 2015 to 2021
In this letter, we describe the outcomes of Haplo with post-transplant cyclophosphamide in 99 consecutive adult patients with leukemia and lymphoma at AUBMC from January 2015 to December 2021. On average, 17 adult patients received Haplo every year. The age of the patients ranged from 17 to 79 years with a median of 41 years. One patient included in our cohort was 17-year-old at the time of transplant. The patient was referred to our center to be treated as an adult patient for the following reasons: (1) the patient’s disease and characteristics (2) the patient was close to turning 18 at the time of the transplant. The rest of our cohort was at 18-year-old at the time of the transplant. Hence, this paper is reporting the experience of adult patients. As for patients older than 60, Haplo was made possible by using RTC/RIC and updated supportive care. This procedure was performed mainly for acute leukemia, predominantly myeloid (64%), and lymphoma (24%). Almost all (98%) of these transplants were performed using peripheral blood stem cell sources (PBSC) and 63% of patients were in complete remission (CR) at the time of transplantation. Different conditioning regimens were used depending on disease type, disease status, and patient characteristics. The conditioning regimens include Thiotepa-busulfan-fludarabine with anti-thymocyte globulin (TBF- ATG) (11–13) (57%) or fludarabine cyclophosphamide with total body irradiation 2 to 4 Gy (TBI) (Flu + Cyclo + TBI) (14) (14%). Patients with relapsed/refractory (R/R) malignancies received a sequential regimen consisting of Thiotepa-etoposide-cyclophosphamide (TEC) and RIC (15) in (17%) if younger than 65 and fit for the regimen. Patients with R/R malignancies but older than 65-year-old or unfit for intensive regimens received flu-ATG-TBI (12, 13, 16) (2%), or either Clofarabine (Clo) or flu with 4 to 8 Gy total body irradiation (TBI) (17) (9%) if older than 65 years or deemed unfit for intensive regimens. All of our patients received cyclophosphamide 50 mg/kg/day at day 3 and day 5 post-transplant (18). The patients and transplant characteristics are listed in Table 1.
Statistics and methods
This retrospective study was approved by the IRB ethics committee in our center, AUBMC. Patients who underwent haplo with post-transplant cyclophosphamide between 01, January, 2015 and 31, December, 2021 in our center were considered for this study. We collected all data retrospectively from our center’s database and used standard descriptive statistical methods to summarize it. Data collected were related to patient characteristics, such as gender and age, transplant and donor characteristics disease characteristics, such as stem cell source, disease status at transplant and conditioning regimens, and clinical events such as treatment-related complications and survival data at last follow-up. Categorical variables were compared using the X2 test, while continuous variables were compared using Student’s t-test. Progression-free survival (PFS) was defined as survival without relapse or progression of hematological disease. For patients without disease or progression, we censored their data at the last follow-up. Overall survival (OS) and NRM were defined as death from any cause and without evidence of relapse, respectively. We utilized the Kaplan-Meier method to calculate the probabilities of graft-vs.-host disease (GVHD), PFS, and OS. The cumulative incidence functions were employed to estimate the relapse incidence (RI) and NRM in a competing risk setting. A p-value <0.05 was considered to indicate a significant difference. All analyses were conducted using SPSS version 26.0 and R-Studio version 1.2.5019.
Results and discussion
GVHD and NRM
After a median follow-up of 710 days, nearly 2 years, the incidence of acute and chronic GvHD was found to be low compared to that reported in the literature in regard to Haplo with post-transplant cyclophosphamide (19, 20). Thirty four patients (34%) developed acute GvHD at the time of the last follow-up and 9 patients (9%) developed chronic GvHD at the time of the last follow-up. Seventeen (17%) of our patients developed grade III-IV acute GvHD while 9% developed grade II acute GvHD. As for the patients who developed chronic GvHD, 5 patients (5%) developed limited chronic GvHD while 4 patients (4%) developed extensive chronic GvHD. The relatively low incidence of GvHD reported here could be related to the small sample size or to the systematic concomitant use of PTCy and ATG. Infectious complications that happened in our cohort include hemorrhagic cystitis (28%), CMV reactivation (56%), clostridium difficile (17%), EBV reactivation (16%), human herpes virus 6 (7%), and central line-associated bloodstream infection (3%). As for engraftment, platelet and white blood cell engraftment failure happened in 17% and 3% of patients, respectively. NRM in our sample was 9% on day 100 after transplantation, 21% at 1 year after transplantation, and 24% at 2 years after transplantation (Table 2).
Relapse
As for relapse, the RI was 24% and 30% at 1-year and 2-years post-transplant respectively. This cumulative incidence of relapse is comparable to the cumulative incidence of relapse published by EBMT: 44% in ALL patients and 32% in AML patients after three years of transplantation (19, 20). Relapse or refractory disease related mortality turned out to be the main mortality cause in our cohort and accounted for 31% of deaths at the median follow-up time of 2-years post-transplant.
OS and PFS
PFS was 53% and 44% at 1 year and 2 years after transplantation with a median PFS 17 months. The 1-year and 2-year posttransplant probability of OS was 57% and 47%, respectively, with median OS of 21 months. Both OS and PFS curves reached a plateau around two years after transplantation (Figure 1). These results are satisfactory since 33% of the patients in our sample had active disease at the time of the transplant, which is associated with worse outcomes (21).
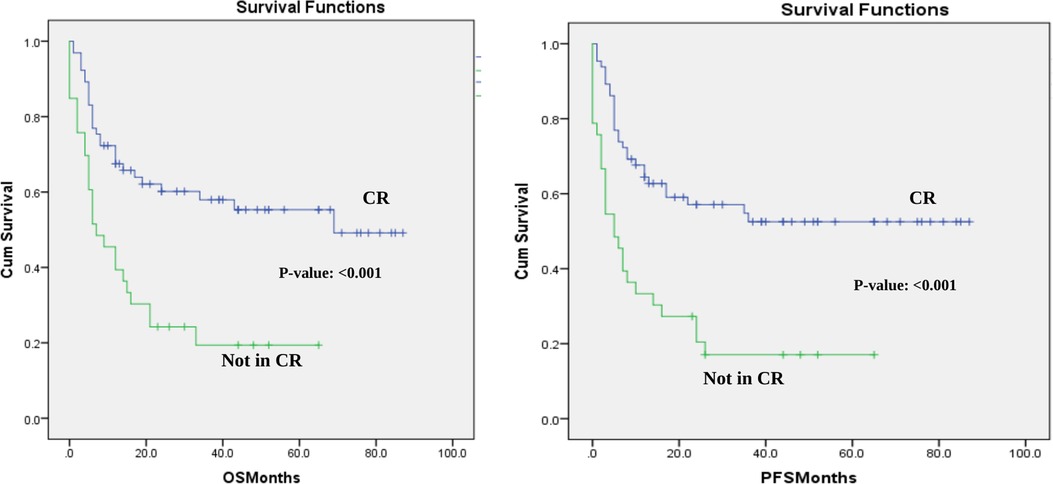
Figure 1. Overall survival and progression-free survival in the cohort-CR vs. not in CR at the time of transplant. OS and PFS for the whole cohort based in disease status at transplant- CR vs. not in CR at the time of transplant.
Transplant outcomes according to disease status at transplant
The analysis for the whole cohort showed that complete remission (CR) at transplant significantly affected the median OS, median PFS, and NRM in our cohort. The median OS for patients who had CR at the time of transplantation not reached compared to only 7 months in patients who did not have CR at transplant.(p-value: <0.001) (Figure 1). The median PFS in the CR subgroup was not reached while in the other group it was only 5 months (p-value: <0.001) (Figure 1). Finally, patients who were in CR had a 4% less NRM rate compared to the other group (17% vs. 21%; p-value: 0.002). These results show that the survival outcomes in the whole cohort can be partly explained with the worse prognosis in patients who had the transplant with residual or active disease.
This effect was still significant for all outcomes when the analysis was performed in patients with AML. In patients with AML, patients who were in complete remission at the time of transplant did not even reach the median OS or median PFS. Patients with AML who were not in CR had only a median OS of 5 months (P-value: <0.001) and a median PFS of 3 months (p-value: <0.001) (Figure 2). Patients with AML who had the transplant at CR had 13% less NRM rate than patients who had the transplant with residual disease (16% vs. 29% p-value: <0.001). On the other hand, the subgroup analysis did not show a significant effect of disease status at transplantation on any of the outcomes in the cohort of patients with lymphoma, although a positive trend was observed. In our center, an allogeneic transplant is administered for patients with lymphoma only after other therapy has failed, which could have affected the results of the lymphoma subgroup analysis.
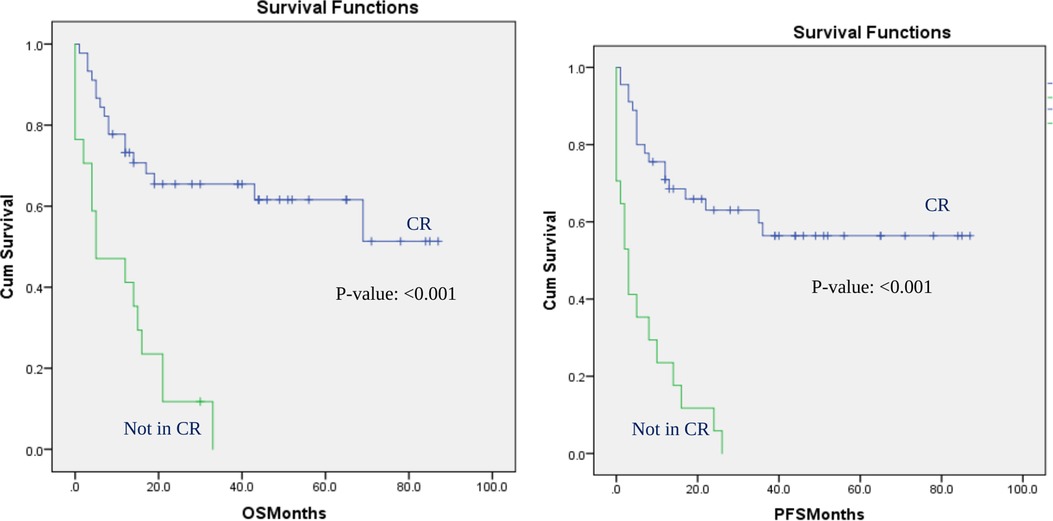
Figure 2. Overall survival and progression-free survival in patients with AML: CR vs. not in CR at the time of transplant. OS and PFS for AML patients based on disease status at transplant: CR vs. not in CR at the time of transplant.
Finally, a subgroup analysis was performed for patients who received TBF, the most utilized myeloablative regimen in our cohort. CR at the time of transplantation was significantly associated with a higher median PFS in this subgroup (36 months vs. 10 months; p-value: 0.044). The median OS also showed a positive trend but did not reach significance.
Conclusion
In conclusion, Haplo has been and is still used successfully at AUBMC to fill the gap in adult patients with acute leukemia and lymphoma, owing to the universal and rapid donor availability, cost, and outcomes. Our analysis showed that CR should be achieved before transplant to improve transplantation in the center. Since almost all of our patients have at least one haploidentical donor, it should now be possible to and offer this potentially curative option for patients with high-risk hematologic disease (1).
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author contributions
All the authors contributed to the manuscript. JC, the corresponding and first author, came up with the idea given the gap in the literature regarding haploidentical stem cell transplantation with post-transplant cyclophosphamide specially in the middle east countries. JC also supervised the study and reviewed the manuscript extensively. GB, the second author, synthesized the manuscript writing, contributed in data collection, and reviewed the paper extensively. LS, the third author, contributed with GB on the data collection and cleaning. RM contributed to the statistics including overall and subgroup analysis. AZ contributed to patient and case database organization and management. The final draft was reviewed and edited by JC, NM, ID, and AB recruited patients and contributed on the writing of the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors JE-C and RM declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khan MA, Bashir Q, Chaudhry QUN, Ahmed P, Satti TM, Mahmood SK. Review of haploidentical hematopoietic cell transplantation. J Glob Oncol. (2018) 4:1–13. doi: 10.1200/jgo.18.00130
2. Ali B, Ammar Z, Radwan M, Jean EC, Colette H, Fadi N, et al. Trends in hematopoietic stem cell transplant activity in Lebanon. Trends Hematopoietic Stem Cell Transplant Activ Lebanon. (2017) 10:315–20. doi: 10.1016/j.hemonc.2017.05.003
3. Bazarbachi A, Hatoum H, Mugharbel A, Otrock Z, Yassine N, Muwakkit S, et al. Hematopoietic stem cell transplantation in Lebanon: first comprehensive report. Bone Marrow Transplant. (2008) 42(Suppl 1):S96–102. doi: 10.1038/bmt.2008.128
4. Im A, Rashidi A, Wang T, Hemmer M, MacMillan ML, Pidala J, et al. Risk factors for graft-versus-host disease in haploidentical hematopoietic cell transplantation using post-transplant cyclophosphamide. Biol Blood Marrow Transplant. (2020) 26(8):1459–68. doi: 10.1016/j.bbmt.2020.05.001
5. Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. (2013) 31(10):1310–6. doi: 10.1200/JCO.2012.44.3523
6. Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. (2015) 126(8):1033–40. doi: 10.1182/blood-2015-04-639831
7. Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs. HLA-matched unrelated donors. Blood. (2016) 127(7):938–47. doi: 10.1182/blood-2015-09-671834
8. Kasamon YL, Bolaños-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. (2015) 33(28):3152–61. doi: 10.1200/JCO.2014.60.4777
9. Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. (2019) 12(1):88. doi: 10.1186/s13045-019-0781-y
10. Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. (1998) 339(17):1186–93. doi: 10.1056/NEJM199810223391702
11. Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, busulfan, and fludarabine conditioning regimen in T cell-replete HLA-haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25(7):1407–15. doi: 10.1016/j.bbmt.2019.02.025
12. Salas MQ, Atenafu EG, Law AD, Lam W, Pasic I, Chen C, et al. Lower dose of ATG combined with post-transplant cyclophosphamide for HLA matched RIC alloHCT is associated with effective control of GVHD and less viral infections. Leuk Lymphoma. (2021) 62(14):3373–83. doi: 10.1080/10428194.2021.1966781
13. Xu X, Yang J, Cai Y, Li S, Niu J, Zhou K, et al. Low dose anti-thymocyte globulin with low dose posttransplant cyclophosphamide (low dose ATG/PTCy) can reduce the risk of graft-versus-host disease as compared with standard-dose anti-thymocyte globulin in haploidentical peripheral hematopoietic stem cell transplantation combined with unrelated cord blood. Bone Marrow Transplant. (2021) 56(3):705–8. doi: 10.1038/s41409-020-01047-2
14. Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. (2008) 14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005
15. Duléry R, Ménard AL, Chantepie S, El-Cheikh J, François S, Delage J, et al. Sequential conditioning with thiotepa in T cell- replete hematopoietic stem cell transplantation for the treatment of refractory hematologic malignancies: comparison with matched related, haplo-mismatched, and unrelated donors. Biol Blood Marrow Transplant. (2018) 24(5):1013–21. doi: 10.1016/j.bbmt.2018.01.005
16. Cao J, Pei R, Lu Y, Zheng Z, Yuan Z, Li D, et al. Fludarabine and antithymocyte globulin-based conditioning regimen combined with post-transplantation cyclophosphamide for haploidentical allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia and myelodysplastic syndrome. Curr Res Transl Med. (2023) 71(1):103360. doi: 10.1016/j.retram.2022.103360
17. Cheikh J E, Bidaoui G, Atoui A, Terro K, Sharrouf L, Zahreddine A, et al. Clofarabine and total body irradiation (TBI) as conditioning regimen for allogeneic stem cell transplantation in high-risk acute leukemia patients: a two-center retrospective cohort study. Bone Marrow Transplant. (2023). doi: 10.1038/s41409-023-01947-z
18. Ruggeri A, Labopin M, Battipaglia G, Chiusolo P, Tischer J, Diez-Martin JL, et al. Timing of post-transplantation cyclophosphamide administration in haploidentical transplantation: a comparative study on behalf of the acute leukemia working party of the European society for blood and marrow transplantation. Biol Blood Marrow Transplant. (2020) 26(10):1915–22. doi: 10.1016/j.bbmt.2020.06.026
19. Piemontese S, Boumendil A, Labopin M, Schmid C, Ciceri F, Arcese W, et al. Leukemia relapse following unmanipulated haploidentical transplantation: a risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol. (2019) 12(1):68. doi: 10.1186/s13045-019-0751-4
20. Adhikari J, Gyawali B, Sharma P, Bhatt VR. Outcomes of haploidentical transplant compared with matched donor allogeneic stem cell transplant. Future Oncol. (2017) 13(10):935–44. doi: 10.2217/fon-2016-0443
Keywords: haploidentical hematopoietic stem cell transplantation, low- and lower-middle-income countries, hematologic malignancies, post transplant cyclophosphamide, tertiaiy care
Citation: El Cheikh J, Bidaoui G, Sharrouf L, Zahreddine A, Massoud R, Nehme R, Kreidieh N, Moukalled N, Abou Dalle I, Mahfouz R and Bazarbachi A (2023) Haploidentical stem cell transplantation with post-transplant cyclophosphamide challenges and outcome from a tertiary care center in Lebanon. Front. Transplant. 2:1149393. doi: 10.3389/frtra.2023.1149393
Received: 21 January 2023; Accepted: 26 May 2023;
Published: 12 June 2023.
Edited by:
Maria Teresa Lupo Stanghellini, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Meltem Kurt Yuksel, Ankara University, TürkiyeKris Michael Mahadeo, Duke University, United States
© 2023 El Cheikh, Bidaoui, Sharrouf, Zahreddine, Massoud, Nehme, Kreidieh, Moukalled, Abou Dalle, Mahfouz and Bazarbachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean El Cheikh amU0NkBhdWIuZWR1Lmxi
 Jean El Cheikh
Jean El Cheikh Ghassan Bidaoui
Ghassan Bidaoui Layal Sharrouf
Layal Sharrouf Ammar Zahreddine2
Ammar Zahreddine2 Rita Nehme
Rita Nehme Nour Moukalled
Nour Moukalled Iman Abou Dalle
Iman Abou Dalle Rami Mahfouz
Rami Mahfouz Ali Bazarbachi
Ali Bazarbachi