Abstract
Pulmonary hypertension (PH) is a known and life limiting complication of preterm born young adults with bronchopulmonary dysplasia (BPD), ultimately leading to progressive right ventricular (RV) failure. Prognosis remains poor, especially in patients unresponsive to modern vasoactive pharmacotherapy. Therefore, lung transplantation presents the treatment of choice to avert cardiac failure. With limited donor organ availability and long waiting times, the implantation of a paracorporeal lung assist device (PLAD) is a way to bridge the patient as an alternative to veno-arterial ECMO. Herein, we present the case of a prematurely born 23-year-old female, who developed severe PH due to BPD and consequently experienced therapy refractory RV failure. Urgent PLAD implantation was performed and the patient successfully underwent double-lung transplantation after 215 days of PLAD support. No major PLAD-associated complications occurred and full recovery of RV function could be observed after double-lung transplantation.
1. Introduction
Bronchopulmonary dysplasia (BPD) is a consequence of abnormal bronchopulmonary development in preterm born infants, especially if born prior to a gestational age of 28 weeks (1, 2). Its association with pulmonary vascular disease in terms of dysmorphic capillary configuration and abnormal vascular remodeling poses an increased risk for pulmonary hypertension (PH) and approximately 20%–44% of preterm born infants with BPD are affected (1, 3–6). Whereas advances in ventilation and vasoactive treatment strategies for infants with BPD led to improved outcome, survivors remain at risk for developing PH and consequently right ventricular (RV) failure, even in adulthood (5–7). Therefore, further screening of patients with BPD for PH has been recommended in recently published guidelines (8). The initial treatment of PH consists of vasoactive agent therapy, and lung transplantation is preferred in selected patients unresponsive to optimal medical treatment. However, with limited donor organ availability, waiting times often exceed survival. Paracorporeal lung assist devices (PLAD) have been introduced as a possible bridge-to-transplantation, however evidence regarding outcome is still insufficient (9, 10). We report on a 23-year-old female patient, who had been listed for lung transplantation due to severe PH. She deteriorated and went into RV failure, so emergency PLAD implantation was performed as a bridging therapy. She successfully underwent lung transplantation after 215 days of PLAD support.
2. Case description
We describe the clinical course of a 23-year-old female, who was prematurely born at gestational week 27. A ventriculoperitoneal shunt was placed for hydrocephalus relief. She suffered from bronchopulmonary dysplasia and ultimately developed severe PH.
While cognitive development was normal, physical growth remained impaired (height 142 cm and weight 34 kg). At age 23, she presented with a progressive decline in exertional capacity and significant dyspnea (NYHA III-IV). Clinical examination showed central cyanosis, severe peripheral edema, clubbing of nails, signs of hepatomegaly and mild hypoxemia with continuous oxygen demand (2 L/min) to maintain saturations above 90%. Laboratory findings included an increased NT-proBNP (3,546 pg/ml) but normal levels of kidney and liver parameters. ECG depicted right axis deviation and signs of RV-hypertrophy. Echocardiography revealed a dilated right atrium and reduced RV function (tricuspid annular plane excursion: 12 mm), severe tricuspid valve regurgitation, congestion of the hepatic veins and signs of severe pulmonary hypertension (D-sign and a systolic pulmonary pressure: 83 mmHg + central venous pressure at systemic arterial pressures of 119/65 mmHg). Left ventricular ejection fraction was within normal range (Figure 2A). After recompensation with forced diuresis, right heart catheterization showed the following results: mean pulmonary pressure 60 mmHg, left atrial pressure 6 mmHg, transpulmonary pressure gradient 54 mmHg, pulmonary vascular resistance 24 Wood units, right atrial pressure 10 mmHg and a cardiac index of 1.9 L/min/m2. Arterial oxygen saturation at room air was 87%. According to the Sitbon criteria (11), pulmonary hypertension was not reversible with vasoactive agents (oxygen, nitric oxide and ilomedin were applied). The patient deteriorated despite treatment with Riociguat, Macitentan, oral prostacyclin and intravenous administration of Treprostinil via a subcutaneously implanted pump. In the following months, the patient suffered from frequent pulmonary infections with intermittent oxygen demand and a second cardiac decompensation occurred. In the mean time, the patient had been listed for double lung transplantation, however, her clinical status quickly deteriorated in terms of increased oxygen demand and right ventricular failure (NT-proBNP = 11,485 pg/ml) due to now suprasystemic pulmonary pressures (systolic pulmonary pressure 120 mmHg, central venous pressure 26 mmHg). As the waiting time for lung transplantation in Germany remains long for patients smaller in size, the interdisciplinary decision for urgent PLAD implantation as a bridge-to-transplantation was made. Preoperatively, a loading dose of Levosimendan was administered.
After careful induction of general anesthesia, rapid median sternotomy was performed, and cardiopulmonary bypass was established through bicaval and aortic cannulation. An atrial Berlin Heart EXCOR® cannula (9 mm) was inserted into the left atrium with 8 interrupted sutures (Prolene 4-0) enhanced with felt pledgets. A Berlin Heart EXCOR® graft adapter cannula (9 mm), extended by 12 mm Gelweave Vascutec Terumo straight graft prosthesis was sewn to the pulmonary artery with a continuous Prolene suture (Figure 1). Both cannulae were channeled subcutaneously to the subxiphoid area, de-aired and connected to a Medtronic Nautilus™ oxygenator with an incorporated heat exchanger. This ultimately resulted in a passive shunt from the pulmonary artery (PA) to the left atrium (LA) with a flow of 2.5 L/min, and prompt hemodynamic stabilization could be achieved. Weaning from cardiopulmonary bypass was uneventful, and the chest was closed primarily. The pressure gradient across the oxygenator was 12 mmHg. Postoperatively, the patient required renal replacement therapy for 3 weeks due to acute renal failure and a tracheostomy was performed two weeks post PLAD implantation. As pulmonary pressures decreased (systolic pulmonary pressure approximately 70 mmHg; 2/3 of systemic systolic pressure), Treprostinil was discontinued, right ventricular function progressively improved and inotropic support could be discontinued (Figure 2B). The anticoagulation regime included Aspirine, Clopidogrel and Vitamin K antagonists (target INR 3–3.5). Nurses and perfusionists checked the whole system 3 times a day. When clot formation >2 mm was visible, the system was changed at the bedside with the patient being awake. The patient required more than 20 exchanges of the tubing system or solely of the oxygenator due to frequent thrombus formation. Systemic embolization could be successfully averted with frequent PLAD inspections and liberal system exchanges. No major complications occurred during PLAD support, and the patient recovered to the point of being able to walk on the hospital ward (Figure 3). The patient underwent lung transplantation after 215 days of PLAD support. No thrombotic material was found in the Berlin Heart EXCOR® cannulae or the left atrium. After lung transplant, the patient was put on central veno-arterial ECLS support for prolonged weaning, which is a standard procedure for patients with severe PH at our institution and the chest remained open. A single rethoracotomy was performed on the first day post-transplant due to bleeding and the patient underwent ECLS-explantation and final sternal closure on the 5th postoperative day. She required three weeks of renal replacement therapy. The tracheostomy was closed six weeks after lung transplant. The patient was discharged in good clinical condition on the 54th day after lung transplant without supplementary oxygen and only slightly impaired renal function. Echocardiography revealed a remodeled right ventricle with adequate size as well as function and only minor tricuspid valve insufficiency.
Figure 1
Figure 2
Figure 3
2.1. Timeline
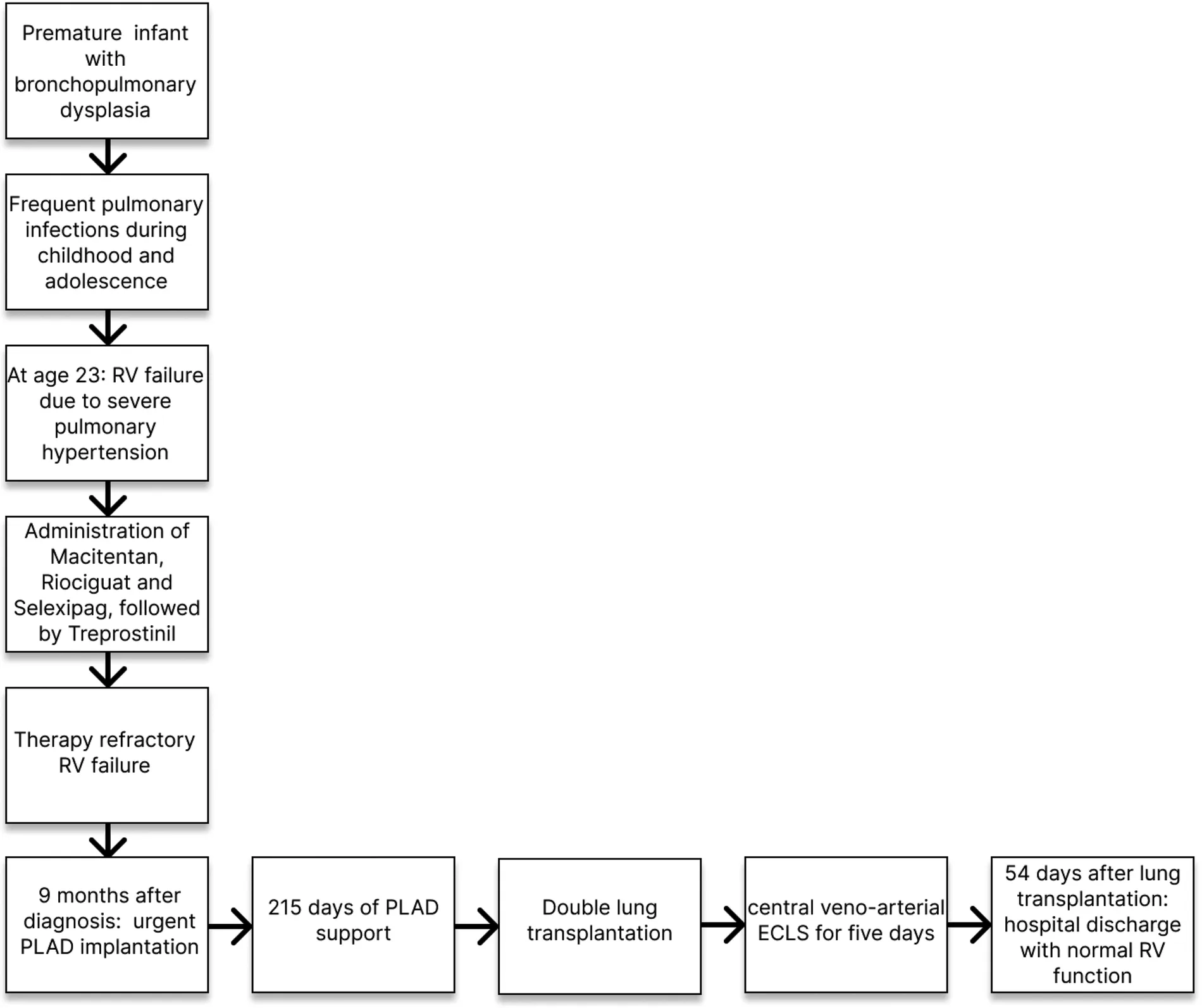
RV, right ventricle, PLAD, paracorporeal lung assist device, ECLS, extracorporeal life support.
3. Discussion
In patients with systemic pulmonary pressures, the creation of a PA-LA shunt results in a passive blood flow through the paracorporeal system containing a low resistance oxygenator, which is crucial to avoid the need of a pump as a driving force (12, 13). Avoiding pump-associated complications (e.g., hemolysis), this concept enables longer support times in comparison to veno-arterial extracorporeal membrane oxygenation. As our patient suffered from RV failure and faced an additionally extended waiting time for transplantation due to the low body surface area (1.18 m2), a long-term solution for cardiopulmonary support was required. Therefore, we decided to proceed with PLAD implantation instead of veno-arterial extracorporeal membrane oxygenation. Furthermore, PLAD leads to an increase in left ventricular preload, which preconditions the left ventricle and avoids LV failure after lung transplant (13). One requirement for successful PLAD implantation is preserved LV function (10). The option of intensified physical therapy after extubation and thus improving pre-transplant physical condition presents an additional advantage of PLAD over veno-arterial ECMO which always has peripheral cannulae in the bridge-to-transplant setting. Peripheral cannulation has a particularly high morbidity in pediatric and small patients (10).
The Medtronic Nautilus™ Smart ECMO Module oxygenator was chosen for three reasons: first, the design with a circular oxygenator profile and the transverse blood flow minimizes pressure drop, resulting in a low transoxygenator pressure gradient. In our patient, the pressure gradient remained <15 mmHg at flow rates of 2–2.5 L/min. Second, it contains a heat exchanger, which was necessary to counteract heat loss along the paracorporeal circuit. Third, continuous monitoring of pressure and oxygenation data via the oxygenator screen provides live information in terms of pulmonary pressures and RV unloading, which was particularly helpful in the early postoperative phase.
To our knowledge, this is the longest time of PLAD-support (>7 months) ever reported without PLAD-associated systemic embolic complications. Nevertheless, with a passive and consequently low blood flow along the paracorporeal circuit, patients remain at substantial risk for thromboembolic events. Therefore, we opted for an anticoagulant strategy including a target INR of 3–3.5 as well as dual antiplatelet therapy, mimicking the strategy after Berlin Heart ventricular assist device implantation for heart failure at our center. Nevertheless, we saw recurrent thrombus depositions, mostly at the connectors and to a lesser extent in the oxygenator without clinical relevance for the patient. We think the passive flow in this system (without a pump) is mainly responsible for the thrombogenicity. This underlines that technical improvement is necessary to reduce the risk of thromboembolic complications.
PLAD as a therapeutic approach has been introduced in 2009 and, to this day, reports are limited to a small number of patients with significantly shorter support times compared to our case (10, 12–16). De Perrot et al. described 4 cases of PA-LA Novalung implantations due to PH and RV failure (support time 9–69 days, cannulation of LA with right-angle cannula and PA with straight cannula) using unfractionated heparin (target activated clotting time 160–200 s) for anticoagulation and reported that none of the patients suffered from embolic complications (14). All of the patients underwent double lung transplantation and one patient died due to primary graft dysfunction. The authors concluded that an aggressive approach using circulatory support in patients with PH (VA ECMO and PA-LA Novalung) could effectively reduce waitlist mortality without increasing the risk for severe primary graft dysfunction (14). Strueber et al. presented the clinical course of four patients (support time 8–30 days) receiving the Novalung (PA-LA through median sternotomy or left thoracotomy) due to severe PH and RV failure (12). All patients survived transplantation, one patient underwent heart-lung transplantation and three patients received double lung transplantation. Since recovery of RV function and improved LV-filling and -function was always seen after PLAD, the authors concluded that this technique can avoid combined heart lung transplantation for the diagnosis of pulmonary hypertension.
While these results seem promising, pediatric patients tend to have worse outcome. Hoganson et al. reported on 4 pediatric patients (one neonate) who underwent PLAD implantation using the Novalung or Maquet Quadrox-iD oxygenator (support time 5–74 days) with a mortality rate of 50% and a stroke rate of 75%—possibly related to thrombus buildup in the left atrium and the left atrial metal tip cannula that was used in the first three cases (10). Thrombus formation (however without clinical relevance) was also seen at the right angle LA cannulae in de Perrot’s study (14). The Hoganson group therefore developed a novel technique of LA cannulation by creation of an ASD and suturing a Goretex graft (as an extension attached to the Berlin Heart cannula) into the rim of the ASD. The right atrium was closed around the Goretex tube. That way, there was no material in the LA that could cause thrombus formation.
In our patient, we implanted a Berlin Heart atrial cannula into the LA with single interrupted sutures. This cannula is designed for long-term use in VAD patients and not a regular atrial cannula for cardiopulmonary bypass. We think that this is a way to effectively avoid thrombus formation in the LA.
Alternative bridge-to-lung transplant strategies in patients with PH are balloon atrioseptostomy (at our center preferably combined with the implantation of an atrial flow regulator) or the creation of a reverse Potts-shunt (either surgically or interventionally). Both procedures increase cardiac output and decrease RV afterload but carry the risk of cyanosis by right-to-left-shunting. Therefore, these procedures can only be applied in PH patients who are not oxygen dependent because the increase in cardiac output goes along with a decrease in oxygen saturation (17, 18).
In conclusion, long-term PLAD support presents a feasible therapeutic option for patients suffering from severe PH and RV failure as a bridge-to-lung transplantation. The patient can be fully awake and perform physical therapy much easier than with venoarterial ECMO, which puts him in a better condition for the following lung transplant. Nevertheless, daily inspections of the paracorporeal circuit for thrombotic depositions and liberal system exchanges are necessary to avoid thromboembolic complications even when cannulae are implanted that are designed for long-term use. Further studies are necessary to confirm our findings.
Statements
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM, MH, JP, JPa, CS, TK, NK, JB, KM, JB, TV, CK, CM, RD, LC, MI, RT, JA, PS, MF, RD, MF, AJ, MH, NH, CH, JH contributed to conception and design of the study. SM and MH wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
SM and TV declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
HansmannGSallmonHRoehrCCKourembanasSAustinEDKoestenbergerMet alPulmonary hypertension in bronchopulmonary dysplasia. Pediatr Res. (2021) 89(3):446–55. 10.1038/s41390-020-0993-4
2.
GossKNBeshishAGBartonGPHaraldsdottirKLevinTSTetriLHet alEarly pulmonary vascular disease in young adults born preterm. Am J Respir Crit Care Med. (2018) 198(12):1549–58. 10.1164/rccm.201710-2016OC
3.
CoalsonJJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. (2003) 8(1):73–81. 10.1016/s1084-2756(02)00193-8
4.
BerkelhamerSKMestanKKSteinhornRH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. (2013) 37(2):124–31. 10.1053/j.semperi.2013.01.009
5.
ArjaansSHaarmanMGRoofthooftMTRFriesMWFKooiEMWBosAFet alFate of pulmonary hypertension associated with bronchopulmonary dysplasia beyond 36 weeks postmenstrual age. Arch Dis Child Fetal Neonatal Ed. (2021) 106(1):45–50. 10.1136/archdischild-2019-318531
6.
ArjaansSZwartEAHPloegstraMJBosAFKooiEMWHillegeHLet alIdentification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. (2018) 32(3):258–67. 10.1111/ppe.12444
7.
VargheseNRiosD. Pulmonary hypertension associated with bronchopulmonary dysplasia: a review. Pediatr Allergy Immunol Pulmonol. (2019) 32(4):140–8. 10.1089/ped.2018.0984
8.
HumbertMKovacsGHoeperMMBadagliaccaRBergerRMFBridaMet al2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 43(38):3618–731. 10.1093/eurheartj/ehac237
9.
MaulTMNelsonJSWeardenPD. Paracorporeal lung devices: thinking outside the box. Front Pediatr. (2018) 6:243. 10.3389/fped.2018.00243
10.
HogansonDMGazitAZBostonUSSweetSCGradyRMHuddlestonCBet alParacorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J Thorac Cardiovasc Surg. (2014) 147(1):420–6. 10.1016/j.jtcvs.2013.08.078
11.
SitbonOHumbertMJaisXIoosVHamidAMProvencherSet alLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. (2005) 111:3105–11. 10.1161/CIRCULATIONAHA.104.488486
12.
StrueberMHoeperMMFischerSCypelMWarneckeGGottliebJet alBridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. (2009) 9(4):853–7. 10.1111/j.1600-6143.2009.02549.x
13.
HoeperMMBenzaRLCorrisPde PerrotMFadelEKeoghAMet alIntensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J. (2019) 53(1):1801906. 10.1183/13993003.01906-2018
14.
de PerrotMGrantonJTMcRaeKCypelMPierreAWaddellTKet alImpact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. (2011) 30(9):997–1002. 10.1016/j.healun.2011.03.002
15.
HoetzeneckerKDonahoeLYeungJCAzadSFanEFergusonNDet alExtracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg. (2018) 155(3):1316–1328.e1. 10.1016/j.jtcvs.2017.09.161
16.
TaylorKHoltbyH. Emergency interventional lung assist for pulmonary hypertension. Anesth Analg. (2009) 109(2):382–5. 10.1213/ane.0b013e3181ac5461
17.
HansmannGApitzC. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European paediatric pulmonary vascular disease network, endorsed by ISHLT and DGPK. Heart. (2016) 102(Suppl 2):ii67–85. 10.1136/heartjnl-2015-309103
18.
LehnerASchulze-NeickIFischerMFernandez-RodriguezSUlrichSHaasNAet alThe creation of an interatrial right-to-left shunt in patients with severe, irreversible pulmonary hypertension: rationale, devices, outcomes. Curr Cardiol Rep. (2019) 21(5):31. 10.1007/s11886-019-1118-8
Summary
Keywords
lung assist device, lung transplantation, pulmonary hypertension, bridge-to-lung transplant, ECMOextracorporeal membrane oxygenation
Citation
Michel SG, Hanuna M, Pattathu J, Pabst von Ohain J, Schneider C, Kauke T, Kneidinger N, Behr J, Milger K, Barton J, Veit T, Kamla C, Mueller C, Dzieciol R, Christen L, Irlbeck M, Tomasi R, Abicht J, Scheiermann P, Feuerecker M, Dalla-Pozza R, Fischer M, Jakob A, Hermann M, Haas N, Hagl C and Hörer J (2023) Case report: Paracorporeal lung assist device for 215 days as a bridge-to-lung transplantation in a patient with bronchopulmonary dysplasia and severe pulmonary hypertension. Front. Transplant. 2:1197906. doi: 10.3389/frtra.2023.1197906
Received
31 March 2023
Accepted
19 June 2023
Published
06 July 2023
Volume
2 - 2023
Edited by
Varun Puri, Washington University in St. Louis, United States
Reviewed by
Lucian Durham, Medical College of Wisconsin, United States Stephanie Chang, New York University, United States
Updates
Copyright
© 2023 Michel, Hanuna, Pattathu, Pabst von Ohain, Schneider, Kauke, Kneidinger, Behr, Milger, Barton, Veit, Kamla, Mueller, Dzieciol, Christen, Irlbeck, Tomasi, Abicht, Scheiermann, Feuerecker, Dalla-Pozza, Fischer, Jakob, Hermann, Haas, Hagl and Hörer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian G. Michel sebastian.michel@med.uni-muenchen.de
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.