Abstract
Plastic pollution poses a major threat to both human health and ecosystems. This study investigates the formation and effects of nanoplastics (NPs) derived from the fragmentation and degradation of plastic waste. The study highlights adsorption as a promising method for the removal of NPs and emphasizes the development of adsorption materials such as biochar, sponges and aerogels. After a detailed overview of adsorption mechanisms, including hydrogen bonding, electrostatic attraction and pore filling, the study identifies key factors that influence adsorption performance, such as pH, pore size and adsorbent composition. It also discusses the challenges associated with adsorbent design, regeneration and environmental hazards, and encourages the development of biocompatible adsorbents and cost-effective solutions. The conclusions emphasize the urgent need to address the problem of NP pollution and provide a roadmap for future research and technological innovation in water treatment and environmental protection.
1 Introduction
The extent of the threat posed by pollution of the oceans and landfill sites with various plastic items has been the subject of much discussion recently. According to one report, 400.3 million tons of plastic waste will be produced in 2022, which is 180 times the amount of plastic waste produced in 1950 (Pilapitiya and Ratnayake, 2024). Between 26 and 36% of the plastic produced is intended for single use only (De, 2020). From processing natural rubber to make ribbons, figurines and balls in 1600 BC (Andrady and Neal, 2009) to meeting the need for food packaging for the military in the 1800s, people have used polymers in various areas of life, but the plastics revolution took place in the late 1800s and led to an expansion into our everyday household products (Gündoğdu et al., 2024). Plastics have different chemical compositions such as polystyrene (PS), polycarbonate (PC), polyethylene (PE), polypropylene (PP) and polyvinyl chloride (PVC) (Forest and Pourchez, 2023). Properties of plastics such as thermal and electrical insulation, durability, easy mouldability, high strength and easy repairability (Forest and Pourchez, 2023) are the reasons for their extensive use in various areas of life such as PP&PVC in medical devices, PP in the automotive and transportation industries as well as in packaging materials, PVC in the construction industry and acrylic (polymethyl methacrylate: PMMA) in sports protective equipment (Gilbert, 2016). So many applications lead to excessive consumption and ultimately inadequate disposal, which has resulted in a considerable amount of plastic waste polluting the environment today (Lee et al., 2023). Without proper disposal, plastic waste released into the environment is transformed over time through fragmentation, biodegradation, weathering and decomposition of plastics (Forest and Pourchez, 2023) into microplastics (<5 mm) and nanoplastics (<1 μm–100 nm) that pollute the environment (Thaiba et al., 2023). The sources of nanoplastics (NPs) production can be divided into two areas: primary (biomedical, cosmetic and color 3D printing processes and plastic cutting applications) and secondary sources (due to physical wear, mechanical and chemical UV exposure, processes in water and wind, including fragmentation of microplastics (MP) into smaller particles) (Gonçalves and Bebianno, 2021). Masks are also a constant source of NPs, as cases of Covid-19 have skyrocketed (Hu et al., 2024). From all these sources, NPs have formed and entered our environment, including oceans, rivers and lakes (Gonçalves and Bebianno, 2021). Figure 1 shows an overview of the formation of nanoplastics in the environment.
Figure 1

An overview of the formation of nanoplastics in the environment.
The characterization techniques used for microplastics can also be used in part or in combination for the characterization of nanoplastics, as the latter only has a smaller size and mass. The constantly changing charges, shapes, sizes and densities complicate the assessment of potential hazards (Ivleva, 2021). In recent decades, studies on NPs have focused on functional groups for measurement and identification to remove NPs (Vohl et al., 2024). Various techniques such as microscopy (e.g., SEM, TEM) (Huang et al., 2005; Lombardo et al., 2020) dynamic light scattering (DLS) (Venâncio et al., 2019; Lombardo et al., 2020) field flux fractionation (FFF) (Schwaferts et al., 2020; Bocca et al., 2021; Cai et al., 2021), Fourier transform infrared spectroscopy (FTIR) (Dong et al., 2021; Devi et al., 2022), μ-Raman spectroscopy (Fang et al., 2020; Vélez-Escamilla and Contreras-Torres, 2022), X-ray photoelectron spectroscopy (XPS) (Mendes, 2022; Aynard et al., 2023), capillary electrophoresis (CE) (Adelantado et al., 2024) mass spectrometry (e.g., GC–MS pyrolysis) (Devi et al., 2022; Li et al., 2024) small-angle X-ray scattering (SAXS) (Lombardo et al., 2020; Ompala et al., 2024) have already been used to characterize nanoplastics.
These NPs have different chemical and physical properties due to their smaller size, larger surface area and functional group modifications, which are ultimately of great concern due to their exceptional ability to bind hazardous pollutants, including heavy metal ions (Mitrano et al., 2021; Forest and Pourchez, 2023; Pelegrini et al., 2023). This can result in these harmful particles being present in the air, water and soil and eventually accumulating in living organisms through ingestion, inhalation and skin contact (Forest and Pourchez, 2023; Ramsperger et al., 2022; Sun L. et al., 2024). As it becomes increasingly likely that NPs are toxic to humans and other living organisms, the presence of NPs in the environment is a growing concern. Various types of polymers in different sizes and shapes have been found in muscle tissue (Ramsperger et al., 2022). One study showed that the largest number of plastic particles was found in mussels with a size of more than 2.2 μm and in the range of 20–200 nm and that humans in Europe could ingest more than 2 mg of nanoplastics annually by eating mussels alone (Fraissinet et al., 2024). Another study conducted on water samples from around the world found that 81% of tap water samples contained microplastics that were also significantly contaminated with nanoplastics (Wibuloutai et al., 2023). These NPs can migrate through the pleura and be taken up by epithelial cells after inhalation or removed by the mucociliary clearance mechanism (Banerjee and Shelver, 2021). NPs have been found to cause inflammation and congestion in organisms (Rahman et al., 2021). By serving as transporters for viruses, bacteria or pollutants (such as hazardous organic compounds and heavy metals), NPs can influence the bioavailability and toxicity of pollutants. NPs have reportedly been found in human faeces (Jiménez-Arroyo et al., 2023). Serious health risks such as physical damage to living cells (Chen T. et al., 2024), oxidative stress (Kaluç et al., 2024), immunological responses (Yang et al., 2024), neurotoxic reactions (Liu et al., 2024), genotoxicity and metabolic problems (He et al., 2024; Shi et al., 2024) have been associated with the accumulation of NPs in tissues (Summer et al., 2024). Therefore, there is an urgent need for the removal of NPs already present in water systems. Figure 2 shows the toxicological effects of nanoplastics on living cells.
Figure 2
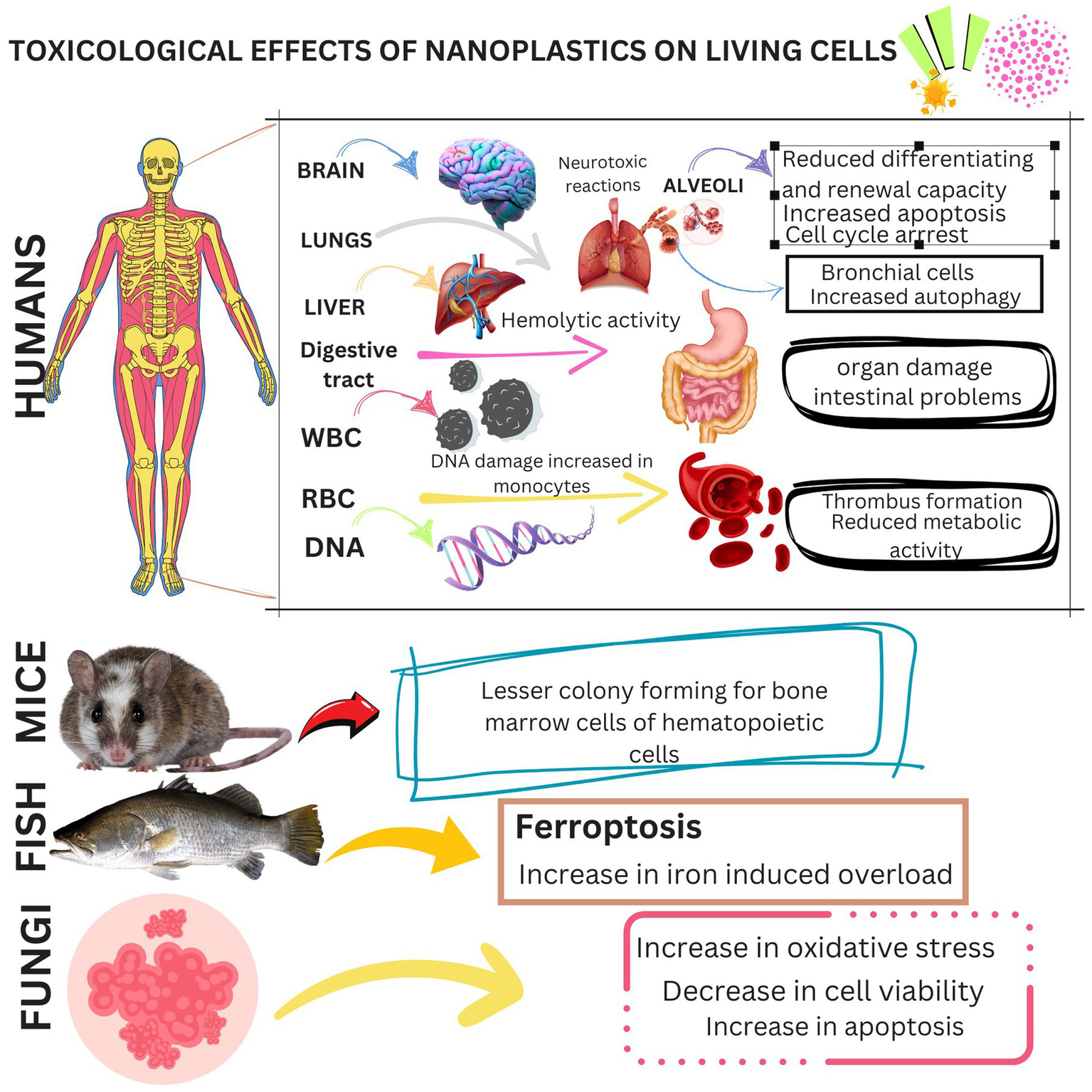
Summary of the toxicological effects of nanoplastics on living cells.
Water treatment is carried out using a variety of techniques such as photocatalysis, filtration, flotation, magnetic separation, advanced oxidation processes, bioremediation, photolysis, coagulation/flocculation and adsorption, etc. (Devi et al., 2022). Among these processes, adsorption is an important water treatment technique. Chemical and physical interactions are two important keys for adsorption, which makes it beneficial for the removal of toxic metals and organic metals and other pollutants from contaminated water (Lujanienė et al., 2017; Lujanienė et al., 2022; Novikau and Lujaniene, 2022; Novikau et al., 2022; Lujanienė et al., 2023; Lujanienė et al., 2024). Due to the small size, significant hydrophobicity and numerous functional groups of NPs, numerous adsorbents have been developed to remove them from contaminated water. The main mechanisms of adsorption are hydrogen bonding, pore filling, π–π stacking, electrostatic attraction, hydrophobic contact and surface complexation (Huang et al., 2024). In this article, we have provided a comprehensive overview of the current developments in adsorbents for the extraction of NPs from contaminated water. The adsorption capacity, a key performance metric for adsorption, is significantly influenced by the physicochemical properties of the adsorbents, including their chemical composition, electronic properties and nanostructure (Sable et al., 2024). The efficiency of adsorbents can be influenced by experimental parameters (such as pH, interfering chemical ions, etc.) (Salahshoori et al., 2024). However, the experimental results cannot be fully generalized. After analyzing the chemical structure of NPs, removal methods and recent developments in adsorption technology for the removal of NPs from contaminated water, recommendations are given on the opportunities and challenges in this field that could guide the development of the next generation of highly efficient adsorbents.
2 Challenges in the research of nanoplastics
Since the beginning of the polymer industry, the disposal of plastics has never been properly regulated, resulting in environmental pollution due to various degradation processes of plastics. Various physicochemical characteristics such as polymer type, size, surface area and properties are almost identical in nanoplastics and microplastics, but microplastics consist of particles with greater structural diversity due to less fragmentation than nanoplastics. The main challenges in removing and analyzing these plastic particles of different sizes are listed below:
-
Low concentration in real samples in terms of mass fraction and the possibility of hetero-agglomeration with the decrease in nanoplastics size (Ivleva, 2021).
-
Small size (up to 100 nm), irregular shapes and combination of different types of degraded fragments (can agglomerate with other plastic particles or metals and organic components) (Magrì et al., 2018; Mitrano et al., 2021).
-
Difficult characterization in complex matrices (Blancho et al., 2021) and problems with reference materials (e.g., limited number of materials resembling real nanoplastics) (Ivleva, 2021).
-
Nanoplastics have different surface charge, hydrophobicity and hydrophilicity (Chen C. et al., 2024; Ko et al., 2024).
All these unresolved issues have led to significant methodological gaps in the characterization and removal of nanoplastics from freshwater.
3 Chemical structures
Ideally, plastics could break down into monomers, but in the environment, these can decompose under various thermal and mechanical stresses at different binding sites. These fragmented plastics further degrade into nanoplastics whose structure cannot be fully formed due to their smaller size and reactivity. To investigate the formation of fragmented plastics, various polymer samples were pyrolyzed to mark signature peaks for degraded particles of the most commonly used plastics. For example, polyethylene has methylene repeating units, but in the formation of nanoplastics, different processes can lead to the formation various new products such as carbon–oxygen bonds, carbonyl groups (Sorasan et al., 2021) and 1,14-pentadeca-diene, 81, 1-pentadecen (m/z 83, 97, 111, 140) (Dierkes et al., 2019).
For polypropylene, propylene group is the monomer, but for NPs particle there can be 2,4-dimethyl-hept-1-ene (C9), indicator ion (m/z 70, 83), meso-2,4,6-trimethyl-1-nonene, syndiotactic (C12s), m/z 1120, 2,4,6,8-tetramethyl-1-undecene (C15s), m/z 1244 (Blancho et al., 2021) and 7-methyl-decene,3-dodecene (m/z 709), 5-methyl-undecene (m/z 745), 7-methyl-1-undecene (m/z 788), 7-methyl-2-decene (m/z 864, 706), 1-Tridecene (m/z 924), 2,6,8-Trimethyl-octene (m/z 1101) (Sullivan et al., 2020) can occur due to UV radiation or thermal stress. Figure 3 shows the fragmentation of propylene into its microplastics.
Figure 3

Fragmentation of propylene into its microplastics.
In the case of polyvinylchloride, microplastics may contain benzene (m/z 78, 74, 52), toluene (m/z 437), ethylbenzene (m/z 534), styrene (m/z 565), indane (m/z 714) and indene (m/z 722), 1,4-dihydronaphthalene (m/z 834), naphthalene (m/z 852), 1-methyl-naphthalene (m/z 945–960), biphenyl (m/z 1009) can also be formed by external processes (Okoffo et al., 2020). These microplastics could be degraded into further smaller particles that form nanoplastics.
For polystyrene, Niu et al. (2024) found C8H8+ ions as tracer for the detection of nanoplastics. However, due to environmental factors (i.e., photodegradation or oxidation) these can also include styrene (m/z 104) and styrene-d5 (m/z 561) (Dierkes et al., 2019), toluene (m/z 441), α-methylstyrene, 2-phenyl naphthalene (Blancho et al., 2021), 1-ethyl-2-methylbenzene (m/z 621), 1,2,3-trimethyl-benzene (m/z 656), 2-propenyl-benzene (m/z 697), 3-butenyl-benzene (m/z 716), 3-phenyl-1-propyne (m/z 722), 1-methylenepropyl-benzene (m/z 729), bibenzyl (m/z 1112), 1,1′-(1-methyl-1,2-ethanediyl)bis-benzene (m/z 1138), stilbene (m/z 1239), 1,2-dihydro-3-phenylnaphthalene (m/z 1311), 2,5-diphenyl-1,5-hexadiene (m/z 1351) (Sullivan et al., 2020), 5-hexene-1,3,5-triyltribenzene (styrene trimer (m/z 91, 117, 194, 312)) (Okoffo et al., 2020).
In the case of polyethylene terephthalate (PET) may have aromatic rings of benzene, vinyl benzoate (m/z values of 105, 77, 148, 51) (Okoffo et al., 2020). However, PET may contain aromatic rings after oxidative degradation or cleavage of the polymer chains.
Similarly, methyl methacrylate, ester group (-COO-), methyl group (-CH3) and hydrocarbon backbone are part of polymer, pyrolysis product results peaks for methyl methacrylate at m/z value of 69, 100, 89 (Okoffo et al., 2020). For Polycarbonate polymer Bisphenol A and Phosgene (-COCl2) are basic monomers but NPs may contain Bisphenol A at m/z value of 213, 119, 91, 165, 288 (Okoffo et al., 2020). These fragmented parts of plastics can react with chemicals in the medium, in which these end up producing further pollutants and may carry harmful metals or organic materials with them.
4 Adsorption
Several historical records, including medical traditions from Sanskrit (from 2000 BC) to the reports of the Greek historian Herodotus on water treatment with silver bottles by the Persian Great King Cyrus (590–529 BC) and the writings of Hippocrates (460–377 BC), deal with the treatment of water for better quality and the damage that poor quality water causes to living organisms (Faust and Aly, 2018). Traditionally used water treatment techniques, already mentioned in the introduction, have their disadvantages. Adsorption is a cost-effective and efficient process that is suitable for both very small doses and continuous processes and offers the possibility of reuse and regeneration (Gkika et al., 2022). In this article, we have provided a thorough overview of current developments in adsorbents for the extraction of NPs from contaminated water. Most adsorbents have been developed for the extraction of polystyrene nanoplastics. Table 1 summarizes the adsorbents that have been developed for the removal of nanoplastics.
Table 1
| Adsorbent | Types of nanoplastics | Best fit model | Best fit kinetics | Adsorption capacity and removal efficiency | Mechanism | References |
|---|---|---|---|---|---|---|
| Biochar with Fe3O4 nanoparticles, MBC300, 500, 700 | PS NPs,100 nm | Langmuir | Pseudo-second-order | 107.7181–229.5772 mg/g, 43.67, 82.73, 57.02% | Hydrophobicity, electrostatic attraction, H-bonding formation and π–π conjugation | Shi Q. et al. (2023) |
| Rice straw biochar modified with cetyltrimethylammonium bromide (CTAB) | PS NPs, <100 nm | Langmuir | Pseudo-second-order kinetic model | 54.07 mg/g, 99.56% | Electrostatic interaction, hyrdophobic interactions, pore filling, and surface complexation | Xing et al. (2024) |
| Mesoporous biochar | PS NPs, 100 nm | Langmuir | Pseudo-second-order | 56.02 mg/g | Transportation, diffusion into the pores/cracks | Zhu et al. (2022) |
| FeMg-LDO@BC | PS NPs, 50–100 nm | Sips | PFO kinetic model | 57.09 mg/g,99.8% | π–π interactions, surface complexation, and hydrogen bonding | Xing et al. (2025) |
| Fe BC | PSNPs, 100 nm | Langmuir | 1626.3 mg/g | Electrostatic interaction, Fe-O bonding, and heterogeneous aggregation | Huang et al. (2024) | |
| Cetyltrimethylammonium bromide (CTAB) modified magnetic biochar | polystyrene (PS), carboxylate-modified polystyrene (CPS), 600–1,000 nm | 95.3, 97.8% | Aggregation | Shi Y. et al. (2023) | ||
| Graphene oxide/chitosan/genipin sponges | PS NPs, 0.026 μ m |
Freundlich model | Pseudo-second-order models | 7.42 mg/g | Pore filling interaction, hydrophobic interaction, π–π interaction |
Ko et al. (2024) |
| Corn starch and gelatin sponge | PS NPs, 100 nm | Elovich model | Pseudo-first-order | 40%–55% | Heterogeneous diffusion | Fu et al. (2023) |
| SA/GO/CS composite membrane | PS NPs,500 nm and 50 nm | 99.87 and 97.10% | Polar interaction, electrostatic force | Li et al. (2023) | ||
| NiFe2O4@CNF | PSNPs, 20 nm | Langmuir | Pseudo-second order | 147.842 mg/g | Electrostatic interactions, metal complexation interaction | Teng et al. (2024) |
| Electro spun polyurethane nanofiber | ABS, PMMA, and PS NPs, 825, 190, and 90 nm | Langmuir | Pseudo-first-order | 417 mg/g | Electrostatic attraction and π−π stacking interactions | Juraij et al. (2023) |
| CuNi carbon material | PS NPs, 100 nm | Langmuir | Pseudo-first order kinetic | 99.18% | Electrostatic attraction | Zhou et al. (2022) |
| Cellulose/MgAl layered double hydroxides composite beads | PS NPs ~ 100 nm | Langmuir | Pseudo-second-order | 6.08 mg/g, 90% | Intra-particle diffusion, hydrogen bonding, electrostatic interaction | Sun et al. (2022) |
| Zn-Al layered double hydroxide | Hydrophilic PS NPs, 55 nm | Freundlich | General–order kinetic | 164.49 mg/g (Deionized water), 162.62 mg/g (synthetic freshwater) 0.100% | Electrostatic interaction | Tiwari et al. (2020) |
| Mg/Al-layered double hydroxides | PS NPs, 0.05–0.1 μm | 90.0% | Electrostatic adsorption, molecular force | Chen et al. (2021) | ||
| Mesoporous metal organic frameworks (MOFs) | PS NPs,26 nm | up to 100% | Electrostatic interaction | Pedrero et al. (2024) | ||
| Untreated coffee grounds | PS NPs, 100 nm | Dubinin-Radushkevich model | Pseudo-second-order | 74% | π–π interaction, electrostatic interactions, hydrogen bonding | Yen et al. (2022) |
| Triclosan | 50 nm, 100 nm, 200 nm, 500 nm, 900 nm PSNPs-COOH, 500 nm, PSNPs-NH2, 500 nm |
Temkin and D-R models | Weber-Morris model | Hydrophobicity, hydrogen bonding, π–π interaction, surface functionalization | Chen C. et al. (2024) | |
| Iron oxide (Fe3O4) nanoparticles | PS NPs, 0.08, 0.43, 0.7, and 1 μm | Langmuir isotherm model | Pseudo-first order | 3151.0 mg/g, 93.9 ± 2.0% | Hydrophobic interactions | Heo et al. (2022) |
| Fe-modified FA, NMA | PS NPs, 80 nm | Sips | Pseudo-first-order | 82.8–89.9 mg/g | Electrostatic attraction, complexation and π–π interactions | Zhao et al. (2022) |
Recent developments in the production of adsorbents, the types of adsorbed nanoplastics, isothermal models, kinetic models, adsorption capacity, removal efficiency, and the mechanisms involved in adsorption.
4.1 Biochar
Biochar (BC) produced from inexpensive biomass is often used for the adsorption of various environmental pollutants due to its physicochemical properties such as large specific surface area, high porosity, environmental friendliness and abundance of functional groups (Panahi et al., 2020; Chen et al., 2022b). The nanostructure and surface chemistry of biochar have a great influence on its adsorption capacity (Feng et al., 2021). It has been hypothesized that biochar produced at a comparatively higher temperature (approx. ~750°C) has a larger surface area and greater pore fullness, fewer carbonyl functional groups and also a higher removal efficiency (i.e., >99% during the first 4 min) compared to PS nanoparticles (Ganie et al., 2021). The adsorption of polystyrene nanoplastics by mesoporous biochar was investigated (Zhu et al., 2022). Mesoporous biochar with larger surface area, high porosity and high structural capacity showed reusability up to 5 cycles with an adsorption capacity of up to 45 mg·g−1. Rice straw biochar modified with cetyltrimethylammonium bromide (CTAB) was also used to separate polystyrene nanoplastics. Modifying the surface of the adsorbent with alkyl chains improved the adsorption capacity and surface activity, resulting in a removal efficiency of 99.56% in this experiment (Xing et al., 2024). The removal of nanoplastics was investigated by adsorption on biochar (Magid et al., 2021). It was found that surface functionalization controls the adsorption performance of biochar in addition to the pyrolysis temperature. The HNO3/H2SO4 oxidation step, which occurs after pyrolysis at higher temperatures (900°C), can increase the number of oxygen-carrying groups and specific surface area (SSA) in corncob biochar. In the case of oxidized biochar, the oxygen-containing groups promote hydrogen bonding and significantly support the adsorption of PS NPs (Magid et al., 2021). Zhu and co-authors formulated a hydrothermal method to degrade PS NPs in addition to adsorption (Zhu et al., 2022). The hydrothermal approach effectively degrades the PS nanoparticles on the charcoal, with the predominant degradation products being lower molecular weight oligomers (Zhu et al., 2022). The properties of the biochar are well preserved and show high reusability throughout the degradation process. Composite Fe BC has proven to be useful for the separation of polystyrene nanoplastics, where it can be reused up to three times (Huang et al., 2024). Recently, various magnetic biochar modifications have also been used for the separation of nanoplastics. In another study, biochar with Fe3O4 nanoparticles was used for the removal of nanoplastics (i.e., polystyrene, amine-modified polystyrene, carboxylate-modified polystyrene nanoplastics) was used. The biochar produced at different temperatures (i.e., 300, 500, 700°C) showed different adsorption capacities for the different adsorbents. Due to the largest surface area and the high number of oxygen-containing functional groups, Magnetic biochar (MBC) 500 had the highest removal efficiency of about 95.2% (Shi Q. et al., 2023) Magnetic biochar modified with cetyltrimethylammonium bromide (CTAB) was used to remove polystyrene (PS) and carboxylate-modified polystyrene (CPS). The adsorption of PS and CPS was improved by the increased hydrophobicity of CTAB with 5 reuse cycles (Shi Y. et al., 2023). Briefly, the adsorption performance of biochar for nanoplastics is not solely dictated by surface area or porosity, but by a complex interplay between pyrolysis temperature, functional group composition, surface hydrophobicity, and the presence of metal or surfactant modifications. While high-temperature biochars excel in structural features, surface functionalization via acid oxidation, metal doping, or surfactant grafting fine-tunes the interaction mechanisms, such as π–π bonding, hydrogen bonding, electrostatic attraction, or aggregation. As emerging pollutants like nanoplastics become more chemically diverse, tailoring biochar structure–function relationships will be essential for broad spectrum, reusable, and sustainable remediation technologies.
4.2 Sponge/aerogel/fiber materials
In addition to carbon materials from biochar, graphene oxide (Ko et al., 2024), activated carbon (Arenas et al., 2021), coffee grounds (Yen et al., 2022), Cu-Ni carbon materials (Özteki̇n and Sponza, 2024) and magnetic carbon nanotubes (Teng et al., 2024) have also been used for the adsorption of NPs. It should be noted that these carbon compounds have different primary NPs adsorption processes. In contrast, the adsorption of NPs by graphene oxide is primarily caused by the strong π–π interaction between the carbon ring of graphene oxides and the benzene ring of PS molecules. The composite membrane Sodium alginate along with Graphene oxide and chitosan (SA/GO/CS) of was prepared for the extraction of polystyrene nanoplastics (50 nm and 5,000 nm) in aqueous media (Li et al., 2023). In addition to the removal of polystyrene nanoplastics, this membrane has also proven to be efficient in the removal of other contaminants (e.g., six types of oil contaminants in water emulsions, Congo red and methylene blue from water). It is reusable and resistant to acidic and alkaline environments. Graphene oxide/chitosan/genipin sponges have been developed for the removal of nanoplastics from hospital waste and seawater. In addition to an adsorption capacity of 73%, graphene oxide/chitosan/genipin also have up to 5 adsorption and desorption cycles (Ko et al., 2024). In addition to graphene-based adsorbents, carbon-based adsorbents have also been shown to be effective for the removal of nanoplastics. Recently, a Ni-Cu-C nanocomposite was developed for the removal of polyethylene terephthalate (PET) NPs (Özteki̇n and Sponza, 2024). The adsorption mechanisms followed hydrogen bonding, electrostatic and π–π interactions. When adsorbed under optimal conditions (i.e., pH = 7, optimal temperature of 25o C, concentrations of PET NPs—10 mg/L and Ni-Cu-C—300 mg/L), an adsorption capacity of 99.20 and 99.42 was achieved. In another study, CuNi carbon material produced by a hydrothermal process was used to extract polystyrene nanoplastics. After a spontaneous and endothermic reaction, the CuNi carbon material developed an electrostatic interaction in an acidic environment and maintained a removal efficiency of 75% after 4 cycles. In addition, this material only needs to be washed and dried for reuse (Zhou et al., 2022). Coffee ground biowaste has also been used to remove amino-modified polystyrene nanoplastics. The main interactions that occur during adsorption include electrostatic interactions and hydrogen bonding between the functional groups on the surface of the coffee grounds and the charges on the surface of the nanoplastics. The porous surface of the coffee grounds ensures physical entrapment of the nanoplastics (Yen et al., 2022). Teng et al. (2024) experimented with NiFe2O4@CNF as an adsorbent for the removal of polystyrene nanoplastics. Both X-ray electron spectroscopy (XPS) and computational analyses were used to analyse the adsorption. The data (XPS) and density functional theory (DFT) analysis provided a solid basis for the existence of an electrostatic interaction between the positive electrostatic potential (ESP) and the negative ESP of the carboxyl groups of the nanoplastics. Decreasing binding energies between Fe+3 and Ni+2 also show the metal complexation of Fe and the carboxyl groups of the nanoplastics (Teng et al., 2024). Nanostructures made of sponge and fibre materials as well as aerogel are remarkable. Materials with a three-dimensional porous network include sponges (Sun et al., 2021), which are essential for the adsorption of nanoparticles from water. In general, porosity, mechanical properties and functional groups are considered in the development of sponge-based adsorbents. A chemical cross-linking process has been developed for the production of sponge materials from low-cost plant protein (Wang Z. et al., 2021). The remarkable removal efficiency of PS NPs (81.2%) is the result of the excellent fatigue resistance, water permeability and high porosity of the sponge. Hydrophobic contact and diffusion within the particles are the main adsorption mechanisms. It is proposed to add more functional nanomaterials to the sponge to improve its performance (Wang Z. et al., 2021).
The chitin-graphene oxide sponge has significant adsorption capabilities and can remove three different types of NPs from water, including PS, NH2-modified PS and COOH-modified PS. The three main adsorption mechanisms: π–π stacking, hydrogen bonding, electrostatic interaction and diffusion within the particles are involved in the adsorption process. Similarly, the double cross-linked and freeze-dried chitin-O-C3N4 (ChCN) sponge can bind various types of PS-NPs via hydrogen bonding and electrostatic adsorption (Sun C. et al., 2024). One type of very porous network with a large SSA and high porosity (i.e., 90%) is an aerogel (Ahankari et al., 2021). An electrospun polyurethane nanofibrous membrane was synthesized for the adsorption of PMMA, PS, ABS (acrylonitrile butadiene styrene) and MB (methylene blue). This membrane has an adsorption capacity of 417 mg/g, which has led to a new spectrum for the investigation of materials for the removal of different types of nanoplastics from complex matrices. A developed polydopamine-modified magnetic chitosan material (PDA-MCS) showed that the physical and electrostatic adhesion of NPs to aerogels drives the adsorption process (Chen et al., 2022a). In a more recent study, a directed cellulose nanofiber aerogel doped with quaternary ammonium salts showed a high adsorption capacity for PS-NPs (146.38 mg·g−1). On the other hand, work still needs to be done on durability and recycling, as the adsorption performance after four days was only 59.4% (Zhuang et al., 2022). Compared to pure cellulose fibres, PEI@CE (the one-dimensional cellulose fibres coated with cross-linked polyethyleneimine (PEI)) show a better adsorption capacity compared to polymethyl methacrylate (PMMA), PVC NPs and polyvinyl acetate (PVAc) from spiked water samples (Batool and Valiyaveettil, 2021). It should be noted that PEI adds amino groups to the CE fibre surface that facilitates the electrostatic contact that controls the adsorption of negatively charged NPs. To develop a sponge that can extract nanoplastics from actual environmental samples such as seawater, takeaway soup and surfactants in soil, a sponge was made from corn starch and gelatin (Fu et al., 2023). Water can be treated efficiently with controlled pore size, sustainability and degradability of the sponge. In addition to these advantages, there is a third: cost-efficient water treatment, as 0.6 RMB is required for 1 liter of water. In short, Carbonaceous adsorbents such as graphene oxide (GO), activated carbon (AC), coffee grounds, Cu-Ni-doped carbon materials, and magnetic carbon nanotubes (MCNTs) exhibit unique structural and chemical features that influence their adsorption mechanisms for nanoplastics (NPs). While all are carbon-based, their structural morphologies and surface chemistries lead to both overlapping and distinct adsorption behaviors.
4.3 Metal hydroxides
Recently, Mg/Al flakes have been prepared for the removal of PSNPs from aqueous media (Chen et al., 2021). It has been shown that the Mg/Al flakes turn into Mg/Al double hydroxide layers when polystyrene nanoplastics is removed as the pH changes. In another study, Mg-Al LDHs were modified with cellulose to produce composite beads for the extraction of polystyrene nanoplastics. During the adsorption process, various impurities such as humic acid and other ions (e.g., Na+, K+, Ca+, SO4−2) showed effects on the removal efficiency. Otherwise, these modified beads have shown a removal efficiency of 90% (Sun et al., 2022). Iron magnesium layered double hydroxides biochar FeMg-LDO@BC were investigated for the adsorption of polystyrene nanoplastics with a removal efficiency of up to 80% and proved to be reusable for 5 adsorption and desorption cycles (Xing et al., 2025). Zn-Al LDHs prepared by the co-precipitation technique have a high maximum sorption capacity of about 160 mg g−1 and can remove PS NPs from a variety of waters (deionized water, synthetic freshwater). These LDHs interact with nanoplastics by weak electrostatic attraction (Tiwari et al., 2020). Fly ash (FA)-derived porous materials for the adsorption of PS NPs have been developed to reduce the cost of adsorbent production (Zhao et al., 2022). High adsorption values of 82.8–89.9 mg g−1 were achieved at pH 5–7. Moreover, the developed new magnetic material (NMA) is extremely reusable and after 4 adsorption cycles the adsorption efficiency gradually decreased from 94.1 to 89.8%. Further studies confirm that the PS NPs are fixed on the surface and move into the pores of the NMA adsorbent by complexation and electrostatic interaction (Zhao et al., 2022).
In addition to metal hydroxides, magnetic oxides have also been used to remove nanoplastics from water systems. Nanoparticles of iron oxide (Fe3O4) were synthesized and applied to polystyrene nanoplastics. It was found that the adhesion of these particles to the nanoplastics follows a two-step oxidation process. These nanoplastics were then removed using a magnetic field (Heo et al., 2022). In another study, Fe-modified FA, called NMA, was synthesized to remove nanoplastics. Recent studies have shown that naturally occurring chlorophyll-added sodium and calcium montmorillonite clays (SMCH and CMCH) can be used to remove mixed micro- and NPs (Wang et al., 2024). After adsorption, the other important task is to completely remove the Zn-Al LDHs, fly ash-derived NMAs and clay-derived adsorbents from the solution, which needs to be further investigated (Chen et al., 2022a). These materials share a common reliance on surface charge properties and functional group interactions, yet differ significantly in structural properties like porosity, crystallinity, and composite integration. Materials like LDH-cellulose beads and FeMg-LDO@BC present a synergy of green synthesis, high efficiency, and recyclability. However, challenges remain in removing adsorbents from treated water, especially with fine particles (e.g., Zn-Al LDH, FA-based NMA). Future research should target: Hybrid structures that combine magnetic retrieval with porous surfaces, enhancing selectivity, durability under environmental conditions (e.g., high ionic strength, mixed contaminants) and scaling low-cost bio-based adsorbents like modified fly ash or agricultural waste composites.
4.4 Zeolites/MOFs/COFs
Metal organic frameworks (MOFs), zeolites and covalent organic frameworks (COFs) consist of porous crystalline substances that can be prepared using a variety of techniques, including solvothermal, hydrothermal, ion thermal solution precipitation, microwaves, sonication and more (Qian et al., 2021). Due to their large specific surface areas, periodic and clear topologies, flexible pore geometries and abundant adsorption sites, MOFs, zeolites and COFs have been used computationally and experimentally for the adsorption of NPs (Du et al., 2021; Wang Q. et al., 2021). It has been shown that the low-cost zeolite imidazolate Framework-67 can efficiently adsorb and remove PS NPs (<10 μm and 5 mg mL−1) from water by p–p stacking, hydrogen bonding and electrostatic interactions (adsorption efficiency—92.1%) (Wan et al., 2022). Similar studies have shown that a co-precipitated magnetic zeolite adsorbent can remove PS NPs with an equilibrium adsorption capacity of up to 34.2 mg g−1 (Zhao et al., 2023). A key factor to improve the adsorption performance is the Fe-O functional groups on the magnetic zeolite. Prepared mesoporous Metal Organic Frameworks (MOFs) and then studied their application for adsorption of nanoplastics and found to be effective in removal of nanoplastics with up to 100% removal efficiency per 1 g/L−1 (Pedrero et al., 2024). A recent computational analysis suggests that the functional groups on the COF TpPa-H can significantly affect the adsorption of three NPs (PE, PET and Nylon-6 (PA 6)), although COFs have not been experimentally used for the adsorption of NPs. Due to its favorable electrostatic potential distribution, TpPa-OH exhibits higher adsorption capacity compared to other analogues. These results give atomic-level insights for the interfacial adsorption of NPs onto COFs, which will help for the development of functional adsorbents (Shang et al., 2022; Townsend, 2024). MOFs, zeolites, and COFs differ significantly in structure, yet share key performance traits when applied to nanoplastics (NPs) adsorption. Collectively, these crystalline porous adsorbents exhibit similar performance through high affinity for nanoplastics via π–π interactions, hydrogen bonding, and tunable surface polarity, though their structural compositions metal–ligand for MOFs, silicate-based for zeolites, and covalently-bonded light elements for COFs distinguish their specific interaction profiles and operational environments.
5 Mechanisms involved in adsorption of nanoplastics
There are various mechanisms that play a role in the removal of nanoplastics by adsorption. These adsorption mechanisms are illustrated in Figure 4. Details of each of these mechanisms can be found below:
-
Nanoplastics have various functional groups such as -NH2, -OH, -COOH and various other metals or organic substances that they can carry. All these structures create a bond between the nanoplastics and the surface of the adsorbent, which already have different functional groups for chemical interaction, leading to the development of hydrogen bonds (Shi Q. et al., 2023). Varying pH leads to two types of interactions, namely competition between the binding sites and changes in the functional group. Both interactions influence the association between adsorbent and adsorbate (Tiwari et al., 2020).
-
Pore filling: On the one hand, nanoplastics have a smaller size, which makes them difficult to remove. On the other hand, adsorbents have a porous structure in which the particles are physically trapped, which leads to pore filling and ultimately to the removal of nanoplastics during adsorption (Zhu et al., 2022).
-
π–π stacking: A higher degree of aromatization, the interaction between aromatic rings nanoplastics and acidic functional groups (e.g., C–O, O–C=O) can serve as an exchange point for electrons of nanoplastics and adsorbents, leading to π–π stacking (Xing et al., 2025; Yen et al., 2022).
-
Electrostatic attraction: Adsorbents have electrostatic properties, which are the reason for a nucleation center that eventually leads to crystal growth for the removal of pollutants. In the case of nanoplastics, the overall surface charge of the nanoplastics is negative, so adsorbents with positive surface charge are better for better adsorption efficiency (Tiwari et al., 2020; Chen et al., 2021; Zhao et al., 2022).
-
Hydrophobic contact: Nanoplastics can have hydrophobic properties that make it easy for adsorbents with hydrophobic functional groups to bundle and capture the plastic nanoparticles (Heo et al., 2022; Shi Q. et al., 2023; Chen C. et al., 2024).
-
Surface complexation: The surface of the adsorbent can have various oxygen-containing functional groups, which can lead to the formation of complexes with plastic nanoparticles. The addition of metals to adsorbents or nanoplastics can also increase the probability of the formation of metal complexes (Chen C. et al., 2024).
Figure 4
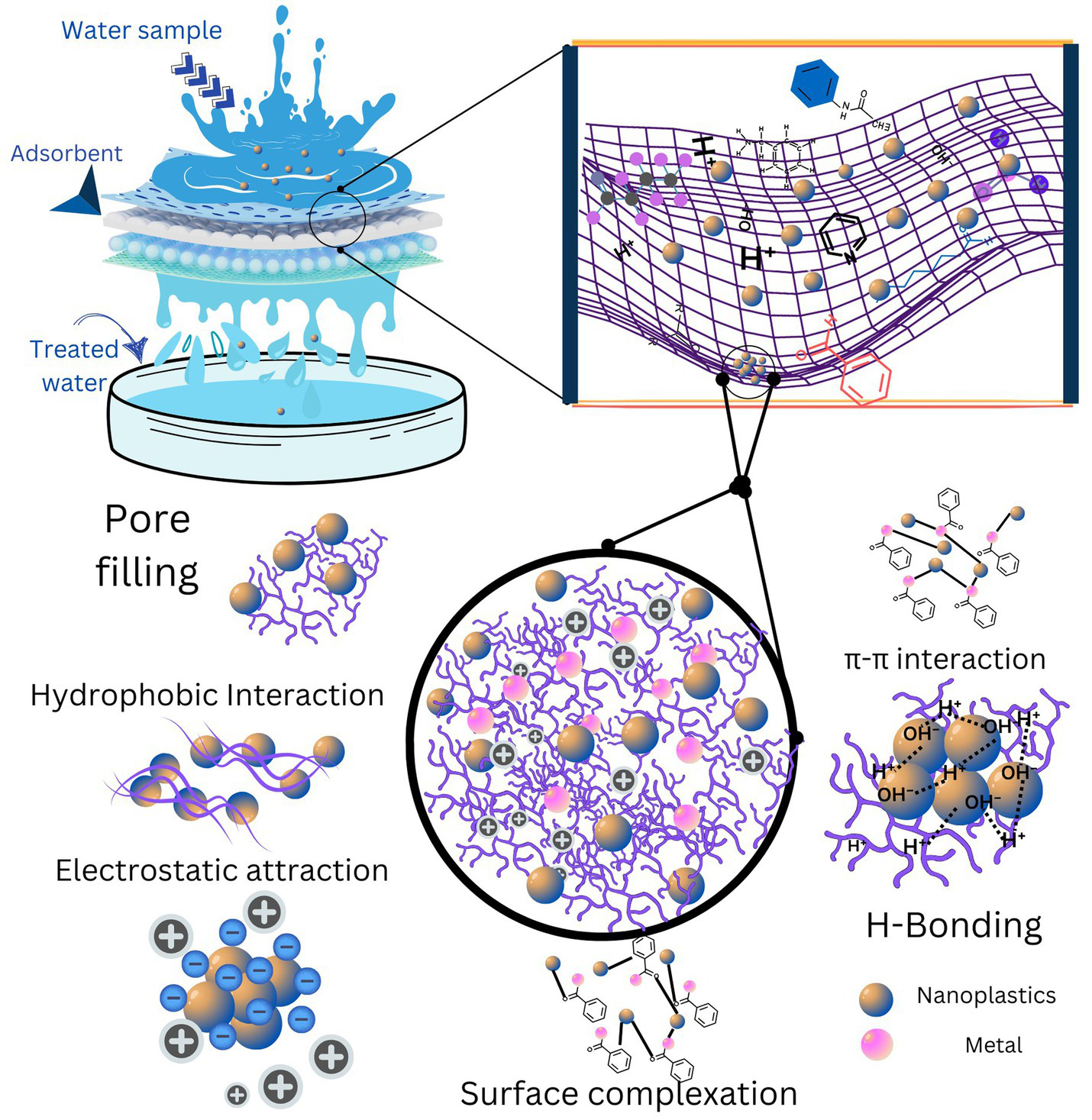
Mechanisms involved in nanoplastic adsorption.
6 Factors affecting adsorption capacity for adsorbents
Factors influencing the adsorption capacity of adsorbents: Adsorption capacity is the removal of mass in grammes of adsorbate per unit (mass in grammes) of adsorbent. Each adsorbent has a different adsorption capacity for nanoplastics and reusability, while the number of cycles an adsorbent can be used to clean nanoplastics from a water system is called reusability. It is considered the most important factor related to adsorption capacity. The adsorption capacity can be influenced by various factors, such as:
6.1 pH
Adsorbents have a unique surface charge due to the protonation state of the functional groups, which leads to different chemical properties such as charge density and distribution as well as charge strength. These surface charges are the cause of the interaction between adsorbent and adsorbate. Just as adsorbents have a surface charge, the nanoplastics also have a charge. The adsorbents therefore react with the nanoplastics according to the surface charge present, whereby the pH value plays a major role. For example, Mg/Al double layer hydroxides remove nanoplastics at a pH value of 9–10 (Chen et al., 2021). Zn-Al layered double hydroxide (Tiwari et al., 2020) and cellulose/Mg-Al layered double hydroxides (Sun et al., 2022) work well in acidic medium for adsorption. Triclosan works at a soil pH = 3 (Chen C. et al., 2024). Mesoporous biochar (Zhu et al., 2022), rice straw biochar modified with cetyltrimethylammonium bromide (CTAB) (Xing et al., 2024), magnetic material (Zhao et al., 2022), NiFe2O4@CNF (Teng et al., 2024) work at pH 3–7. Corn starch and gelatin sponge have adsorption at neutral pH (Fu et al., 2023) which can be explained by the different zero charge point for each adsorbent depending on the nanoplastics Therefore, the pH of the surface of the nanoplastics, the adsorbent and the medium in which the adsorption takes place have a great influence on the separation of the nanoplastics.
6.2 Adsorbent size
Adsorbents are the main components, the most important factor in influencing the removal of nanoplastics. Since both the chemical and physical interaction depend on the available active sites and the amount of adsorbent, the size of the adsorbent has a direct influence on the efficiency of nanoplastics removal (Zhu et al., 2022; Shi Y. et al., 2023).
6.3 Pore size
The size of nanoplastics can vary between 1 μm and 100 nm, which is very small to physically grasp these particles. However, there are various materials such as sponges or aerogels that have been produced for the extraction of nanoplastics. The pore size on the surface of these adsorbents confirms the permeability to water and the binding of nanoplastics to them. Large pores could be a reason for the leakage of nanoplastics or for the release of metals or other elements that could be released during the adsorption process. Therefore, the pore size of these adsorbents plays a crucial role in the removal efficiency of nanoplastics (Xing et al., 2024).
6.4 Concentration of nanoplastics
The existence of nanoplastics in water systems has not yet been fully recognized, so that samples from water systems may contain different amounts of nanoplastics. In the separation of nanoplastics by adsorbents, the concentration of nanoplastics may be an important factor affecting the adsorption capacity of adsorbents. As more nanoplastics can occupy all the active sites available for adsorption, some of them cannot be captured by physical and chemical interaction and leave the system, which ultimately affects the adsorption capacity of the material (Zhu et al., 2022; Xing et al., 2024).
6.5 Contact time
The contact time plays a decisive role in the interaction between adsorbate and adsorbent. The nanoplastics require a certain amount of time to interact with all active sites on the surface of the adsorbent. It is therefore important to achieve an optimal contact time, as the reusability of the adsorbent and the adsorption capacity are directly influenced by the optimal contact time (Shi Y. et al., 2023; Xing et al., 2024).
6.6 Temperature
All adsorbents have a different optimum temperature for the adsorption of nanoplastics depending on their composition, which is an important factor influencing the adsorption capacity of nanoplastics. Most adsorbents have been used at room temperature (triclosan 25°C; Chen C. et al., 2024) but some have other temperatures (corn starch and gelatin sponge 20°C; Fu et al., 2023) at which they function.
7 Challenges and perspectives in the adsorptive removal of NPs from water
Despite encouraging developments, the adsorption of NPs is still in its infancy and current studies are not yet sufficient to solve the NPs problem. In this section, difficulties and possible solutions for the adsorption of NPs in water systems are presented.
7.1 Adsorbent design
Most studies indicate a long adsorption time and a specific removal efficiency. Therefore, highly effective and affordable adsorbents are required for the wide application of adsorption techniques. Porous materials such as sponges and aerogels could provide additional adsorption sites for NPs, both on the surface and within the pores. Reducing particle size is another method of increasing the surface area of an adsorbent. In general, the chemistry of the surfaces can be easily controlled by synthetic parameters. In addition, it is advisable to perform computational studies such as density functional theory calculations and molecular dynamics simulations to predict how adsorbents and NPs would interact and subsequently determine the design of effective adsorbents. It is possible to develop adsorbents from materials that are abundant in the earth, as well as from troublesome wastes (such as clay, biowaste and fly ash), which improves the cost-effectiveness of adsorbents and promotes the industrial utilization of adsorption processes. The “waste-to-value” method generally benefits the environment, as the proven biochar from biowaste is a highly effective adsorbent for the circular economy and NPs wastewater treatment.
7.2 Regeneration of adsorbents
The practical use of adsorbents is determined by their desorption capacity and their regeneration, which are essential prerequisites. The regeneration of adsorbents has recently been investigated by chemical regeneration and thermal treatment (Tang et al., 2021; Wang J. et al., 2021). There are more questions to be answered, e.g., how to effectively desorb these NPs or recycle old adsorbents and also restore the adsorption efficiency. To preserve the structure and surface activity of the known carbon-based adsorbents and to increase their economic and environmental sustainability, regeneration by thermal treatment at relatively low temperatures is recommended. Of particular interest is the possible formation of gaseous pollutants during thermal regeneration and their impact on air quality. After the regeneration treatment, the performance of the adsorbents usually decreases. A new functionalization of these recycled adsorbents could improve the adsorption performance. In summary, improving the utilization efficiency of adsorbents requires the development of environmentally friendly regeneration and modification techniques. Regeneration methods like thermal treatment, while effective, can also generate volatile emissions unless adequately controlled.
7.3 Environmental risks of adsorbents and biocompatibility
It is important to address the structural stability and environmental hazards of adsorbents. The structural integrity of the adsorbents and their potential hazards to the environment must be considered as the leaching of metals (e.g., Fe, Cu, Ni) or surfactants from these nanostructured or porous materials and the formation of nanoparticles would be the result of the degradation of adsorbents. These materials from adsorbents that enter the water can cause secondary contamination. In addition, adsorbents can interact with other organisms and create new contaminants in the water. Advanced adsorbents such as MOFs, carbon nanotubes, and chemically modified biochars may involve the use of toxic solvents, high energy inputs, or hazardous reagents, which can pose environmental and occupational risks if not properly managed. These disadvantages are decisive for the actual use of adsorbents. In this context, the formation and stability of adsorbents in actual water systems must be measured. Biocompatibility is an important concern as aquatic organisms must not be harmed by the use of adsorbents. It is also a challenge to completely and successfully remove the adsorbents from the water after use.
7.4 Degradation after adsorption
Adsorption only removes the NPs from the solution. Additional processing of the collected NPs could further reduce the negative impact of NPs on the environment. Methods for the degradation of NPs include thermal degradation, photocatalysis, electrocatalysis, enhanced oxidation techniques, biodegradation and photocatalysis (Chen J. et al., 2022; Pivato et al., 2022). The method of adsorption degradation is extremely important for the assessment of plastic waste and the production of important chemicals. Plastic polymers can either be degraded to water, carbon dioxide and short-chain monomers or converted into carbon nanomaterials. Uogintė et al. (2023) conducted degradation experiments under UV light to optimize the initial pH, contact time and amount of photocatalyst and achieved a mass loss of 35.66–50.46% in 480 min. Adsorbed plastics can be degraded to styrene and various alkenes and other aromatic hydrocarbons by thermal regeneration (Xing et al., 2025). The growing scientific interest in the electrochemical recycling of plastics is remarkable. At the cathode and anode, plastic waste can be selectively converted into hydrogen gas and value-adding chemical raw materials. The electrolysis of plastics can produce green hydrogen and simultaneously degrade pollutants (Shi et al., 2021; Ma et al., 2022). In addition, the electrolysis of plastics produces hydrogen with less energy than the splitting of water, which could contribute to the development of the hydrogen industry (Pichler et al., 2021).
8 Key knowledge gaps and future research directions
Despite significant progress in understanding nanoplastics and developing adsorption-based removal strategies, several critical knowledge gaps and practical challenges remain unresolved and hindering the translation of current research into scalable and effective environmental solutions. The major key knowledge gaps and future research directions related to nanoplastic adsorption identified are listed below:
-
Surface Property Influence: The role of nanoplastics’ surface properties (e.g., charge, roughness, and functional groups) on adsorption mechanisms is not fully understood. This hampers the accurate prediction of their interaction with adsorbents.
-
Interaction Mechanisms: There is limited understanding of the specific mechanisms (e.g., hydrogen bonding, hydrophobic interactions and π–π interactions) driving adsorption between nanoplastics and various adsorbents. These interactions are complex and not yet quantitatively described in many cases.
-
Environmental Condition Effects: The influence of varying environmental conditions—such as pH, ionic strength, temperature, and presence of natural organic matter—on nanoplastics adsorption efficiency remains underexplored.
-
Lack of Standardization: A lack of standardized methodologies and experimental conditions makes it difficult to compare results across studies or develop universal design criteria for effective adsorbents.
-
Real-World Application Challenges: Most studies are based on laboratory conditions using polystyrene nanoplastics, whereas real environmental samples contain aged, hetero-aggregated, and coated nanoplastics. This limits practical applicability.
-
Desorption and Reusability: There is inadequate research on desorption behavior and regeneration performance of nanoplastics-loaded adsorbents, which is essential for evaluating long-term applicability and economic feasibility.
-
Material Safety and Cost: Concerns about the safety and toxicity of some advanced adsorbents (e.g., MOFs, CNTs) and the high cost of synthesis remain barriers for their real-world deployment.
-
Modeling and Predictive Tools: Current studies lack predictive models to simulate nanoplastics’ fate and transport in complex matrices post-adsorption. Machine learning and computational methods are underutilized.
9 Conclusion
The extent of misuse of plastics in the environment has raised global concern about plastic pollution and the possibility that NPs are ubiquitous. Even though NPs are present in enormous quantities in water, there is much research specifically addressing their presence in the atmosphere. Techniques are being developed to sample and characterize these NPs, so it is difficult to draw many parallels between the various studies in this area. This may be due to the different factors considered in the study, such as size, concentration, shape and air conditions during the experimental process. Future studies should focus on methods to successfully characterize the NPs present in water, which could provide an understanding of dispersion. Studies are needed to fully understand the potential long-term effects and mechanisms of action of the complex toxicological paradigm of these particles. In addition, research should focus on the toxicity that waterborne NPs may have on the human respiratory system, as we still know very little about this. Based on the newly developed adsorbents discussed in this article, it can be concluded that among the developed adsorbents, iron oxide (Fe3O4) nanoparticles and Fe BC have the best adsorption capacities (i.e., 3151.0 mg/g and 1626.3 mg/g). Due to the best removal efficiency, Zn-Al bilayer hydroxide and mesoporous metal–organic frameworks (MOFs) proved to be highly efficient adsorbents with a removal efficiency of up to 100%. It is also important to develop effective adsorbents to remove NPs and improve their biocompatibility, biodegradation and reusability. In this article, we thoroughly examine the current developments of adsorbents for the removal of nanoparticles from water. Finally, we highlight the key issues and future directions in the following areas: regeneration of adsorbents, design of adsorbents, analysis of environmental risks and biocompatibility, unified approaches, valorization and degradation. Future research should also prioritize real-world validation of adsorbents under environmentally relevant conditions, develop standardized protocols for performance assessment, and evaluate the economic and environmental sustainability of adsorbent materials through comprehensive life cycle assessments.
Statements
Author contributions
MJ: Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. GL: Writing – review & editing, Project administration, Supervision, Validation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/frwa.2025.1641086.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adelantado C. Lapizco-Encinas B. H. Jordens J. Voorspoels S. Velimirovic M. Tirez K. (2024). Capillary electrophoresis as a complementary analytical tool for the separation and detection of nanoplastic particles. Anal. Chem.96, 7706–7713. doi: 10.1021/acs.analchem.4c00822
2
Ahankari S. Paliwal P. Subhedar A. Kargarzadeh H. (2021). Recent developments in nanocellulose-based aerogels in thermal applications: a review. ACS Nano15, 3849–3874. doi: 10.1021/acsnano.0c09678
3
Andrady A. L. Neal M. A. (2009). Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci.364, 1977–1984. doi: 10.1098/rstb.2008.0304
4
Arenas L. R. Gentile S. R. Zimmermann S. Stoll S. (2021). Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ.791:148175. doi: 10.1016/j.scitotenv.2021.148175
5
Aynard A. Courrèges C. Jiménez-Lamana J. Raad A. Miqueu C. Grassl B. et al . (2023). Trace metal sorption on nanoplastics: an innovative analytical approach combining surface analysis and mass spectrometry techniques. Environ. Pollut.323:121229. doi: 10.1016/j.envpol.2023.121229
6
Banerjee A. Shelver W. L. (2021). Micro-and nanoplastic induced cellular toxicity in mammals: a review. Sci. Total Environ.755:142518. doi: 10.1016/j.scitotenv.2020.142518
7
Batool A. Valiyaveettil S. (2021). Surface functionalized cellulose fibers–a renewable adsorbent for removal of plastic nanoparticles from water. J. Hazard. Mater.413:125301. doi: 10.1016/j.jhazmat.2021.125301
8
Blancho F. Davranche M. Hadri H. E. Grassl B. Gigault J. (2021). Nanoplastics identification in complex environmental matrices: strategies for polystyrene and polypropylene. Environ. Sci. Technol.55, 8753–8759. doi: 10.1021/acs.est.1c01351
9
Bocca B. Battistini B. Petrucci F. (2021). "A protocol for size-based measurements of nanoplastics across the range 20 nm-200 nm", in: AIP conference proceedings: AIP Publishing).
10
Cai H. Xu E. G. Du F. Li R. Liu J. Shi H. (2021). Analysis of environmental nanoplastics: progress and challenges. Chem. Eng. J.410:128208. doi: 10.1016/j.cej.2020.128208
11
Chen Z. Fang J. Wei W. Ngo H. H. Guo W. Ni B.-J. (2022a). Emerging adsorbents for micro/nanoplastics removal from contaminated water: advances and perspectives. J. Clean. Prod.371:133676. doi: 10.1016/j.jclepro.2022.133676
12
Chen Z. Huang Z. Liu J. Wu E. Zheng Q. Cui L. (2021). Phase transition of mg/Al-flocs to mg/Al-layered double hydroxides during flocculation and polystyrene nanoplastics removal. J. Hazard. Mater.406:124697. doi: 10.1016/j.jhazmat.2020.124697
13
Chen T. Jiang H. He Y. Shen Y. Huang Z. Gu Y. et al . (2024). Nanoplastics and chrysene pollution: potential new triggers for nonalcoholic fatty liver disease and hepatitis, insights from juvenile Siniperca chuatsi. Sci. Total Environ.922:171125. doi: 10.1016/j.scitotenv.2024.171125
14
Chen C. Sun C. Wang B. Zhang Z. Yu G. (2024). Adsorption behavior of triclosan on polystyrene nanoplastics: the roles of particle size, surface functionalization, and environmental factors. Sci. Total Environ.906:167430. doi: 10.1016/j.scitotenv.2023.167430
15
Chen J. Wu J. Sherrell P. C. Chen J. Wang H. Zhang W. X. et al . (2022). How to build a microplastics-free environment: strategies for microplastics degradation and plastics recycling. Adv. Sci.9:e2103764. doi: 10.1002/advs.202103764
16
Chen Z. Zheng R. Wei W. Wei W. Zou W. Li J. et al . (2022b). Recycling spent water treatment adsorbents for efficient electrocatalytic water oxidation reaction. Resour. Conserv. Recycl.178:106037. doi: 10.1016/j.resconrec.2021.106037
17
De L. (2020). Single use plastics-its impact and sustainability. Res. Today2, 428–431.
18
Devi M. K. Karmegam N. Manikandan S. Subbaiya R. Song H. Kwon E. E. et al . (2022). Removal of nanoplastics in water treatment processes: a review. Sci. Total Environ.845:157168. doi: 10.1016/j.scitotenv.2022.157168
19
Dierkes G. Lauschke T. Becher S. Schumacher H. Földi C. Ternes T. (2019). Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem.411, 6959–6968. doi: 10.1007/s00216-019-02066-9
20
Dong S. Cai W. Xia J. Sheng L. Wang W. Liu H. (2021). Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: complex roles of electrolytes, pH and humic acid. Environ. Pollut.268:115828. doi: 10.1016/j.envpol.2020.115828
21
Du C. Zhang Z. Yu G. Wu H. Chen H. Zhou L. et al . (2021). A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere272:129501. doi: 10.1016/j.chemosphere.2020.129501
22
Fang C. Sobhani Z. Zhang X. Gibson C. T. Tang Y. Naidu R. (2020). Identification and visualisation of microplastics/nanoplastics by Raman imaging (ii): smaller than the diffraction limit of laser?Water Res.183:116046. doi: 10.1016/j.watres.2020.116046
23
Faust S. D. Aly O. M. (2018). Chemistry of water treatment. Boca Raton, FL: CRC Press.
24
Feng D. Guo D. Zhang Y. Sun S. Zhao Y. Shang Q. et al . (2021). Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J.410:127707. doi: 10.1016/j.cej.2020.127707
25
Forest V. Pourchez J. (2023). Can the impact of micro-and nanoplastics on human health really be assessed using in vitro models? A review of methodological issues. Environ. Int.178:108115. doi: 10.1016/j.envint.2023.108115
26
Fraissinet S. De Benedetto G. E. Malitesta C. Holzinger R. Materić D. (2024). Microplastics and nanoplastics size distribution in farmed mussel tissues. Commun. Earth Environ.5:128. doi: 10.1038/s43247-024-01300-2
27
Fu J. Liu N. Peng Y. Wang G. Wang X. Wang Q. et al . (2023). An ultra-light sustainable sponge for elimination of microplastics and nanoplastics. J. Hazard. Mater.456:131685. doi: 10.1016/j.jhazmat.2023.131685
28
Ganie Z. A. Khandelwal N. Tiwari E. Singh N. Darbha G. K. (2021). Biochar-facilitated remediation of nanoplastic contaminated water: effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater.417:126096. doi: 10.1016/j.jhazmat.2021.126096
29
Gilbert M. (2016). Brydson's plastics materials. Norwich, NY: William Andrew.
30
Gkika D. A. Mitropoulos A. C. Kyzas G. Z. (2022). Why reuse spent adsorbents? The latest challenges and limitations. Sci. Total Environ.822:153612. doi: 10.1016/j.scitotenv.2022.153612
31
Gonçalves J. M. Bebianno M. J. (2021). Nanoplastics impact on marine biota: a review. Environ. Pollut.273:116426. doi: 10.1016/j.envpol.2021.116426
32
Gündoğdu S. Bour A. Köşker A. R. Walther B. A. Napierska D. Mihai F.-C. et al . (2024). Review of microplastics and chemical risk posed by plastic packaging on the marine environment to inform the global plastics treaty. Sci. Total Environ.946:174000. doi: 10.1016/j.scitotenv.2024.174000
33
He L. Lu Z. Zhang Y. Yan L. Ma L. Dong X. et al . (2024). The effect of polystyrene nanoplastics on arsenic-induced apoptosis in HepG2 cells. Ecotoxicol. Environ. Saf.269:115814. doi: 10.1016/j.ecoenv.2023.115814
34
Heo Y. Lee E.-H. Lee S.-W. (2022). Adsorptive removal of micron-sized polystyrene particles using magnetic iron oxide nanoparticles. Chemosphere307:135672. doi: 10.1016/j.chemosphere.2022.135672
35
Hu F. Zhao H. Ding J. Jing C. Zhang W. Chen X. (2024). Uptake and toxicity of micro−/nanoplastics derived from naturally weathered disposable face masks in developing zebrafish: impact of COVID-19 pandemic on aquatic life. Environ. Pollut.343:123129. doi: 10.1016/j.envpol.2023.123129
36
Huang J. Tan X. Ali I. Ok Y. S. Duan Z. Liang J. et al . (2024). Efficient removal of nanoplastics by iron-modified biochar: understanding the removal mechanisms. Environ. Pollut.363:125121. doi: 10.1016/j.envpol.2024.125121
37
Huang M.-F. Yu J.-G. Ma X.-F. Jin P. (2005). High performance biodegradable thermoplastic starch—EMMT nanoplastics. Polymer46, 3157–3162. doi: 10.1016/j.polymer.2005.01.090
38
Ivleva N. P. (2021). Chemical analysis of microplastics and nanoplastics: challenges, advanced methods, and perspectives. Chem. Rev.121, 11886–11936. doi: 10.1021/acs.chemrev.1c00178
39
Jiménez-Arroyo C. Tamargo A. Molinero N. Moreno-Arribas M. V. (2023). The gut microbiota, a key to understanding the health implications of micro (nano) plastics and their biodegradation. Microb. Biotechnol.16, 34–53. doi: 10.1111/1751-7915.14182
40
Juraij K. Ammed S. P. Chingakham C. Ramasubramanian B. Ramakrishna S. Vasudevan S. et al . (2023). Electrospun polyurethane nanofiber membranes for microplastic and nanoplastic separation. ACS Appl. Nano Mater.6, 4636–4650. doi: 10.1021/acsanm.3c00112
41
Kaluç N. Çötelli E. L. Tuncay S. Thomas P. B. (2024). Polyethylene terephthalate nanoplastics cause oxidative stress induced cell death in Saccharomyces cerevisiae. J. Environ. Sci. Health A59, 180–188. doi: 10.1080/10934529.2024.2345026
42
Ko M. Jang T. Yoon S. Lee J. Choi J.-H. Choi J.-W. et al . (2024). Synthesis of recyclable and light-weight graphene oxide/chitosan/genipin sponges for the adsorption of diclofenac, triclosan, and microplastics. Chemosphere356:141956. doi: 10.1016/j.chemosphere.2024.141956
43
Lee Y. Cho S. Park K. Kim T. Kim J. Ryu D.-Y. et al . (2023). Potential lifetime effects caused by cellular uptake of nanoplastics: a review. Environ. Pollut.329:121668. doi: 10.1016/j.envpol.2023.121668
44
Li H. Lee L. M. Yu D. Chan S. H. Li A. (2024). An optimized multi-technique based analytical platform for identification, characterization and quantification of nanoplastics in water. Talanta272:125800. doi: 10.1016/j.talanta.2024.125800
45
Li Z. Xie W. Zhang Z. Wei S. Chen J. Li Z. (2023). Multifunctional sodium alginate/chitosan-modified graphene oxide reinforced membrane for simultaneous removal of nanoplastics, emulsified oil, and dyes in water. Int. J. Biol. Macromol.245:125524. doi: 10.1016/j.ijbiomac.2023.125524
46
Liu S. He Y. Yin J. Zhu Q. Liao C. Jiang G. (2024). Neurotoxicities induced by micro/nanoplastics: a review focusing on the risks of neurological diseases. J. Hazard. Mater.469:134054. doi: 10.1016/j.jhazmat.2024.134054
47
Lombardo D. Calandra P. Kiselev M. A. (2020). Structural characterization of biomaterials by means of small angle X-rays and neutron scattering (SAXS and SANS), and light scattering experiments. Molecules25:5624. doi: 10.3390/molecules25235624
48
Lujanienė G. Novikau R. Joel E. F. Karalevičiūtė K. Šemčuk S. Mažeika K. et al . (2022). Preparation of graphene oxide-maghemite-chitosan composites for the adsorption of europium ions from aqueous solutions. Molecules27:8035. doi: 10.3390/molecules27228035
49
Lujanienė G. Novikau R. Karalevičiūtė K. Pakštas V. Talaikis M. Levinskaitė L. et al . (2024). Chitosan-minerals-based composites for adsorption of caesium, cobalt and europium. J. Hazard. Mater.462:132747. doi: 10.1016/j.jhazmat.2023.132747
50
Lujanienė G. Novikau R. Leščinskytė A. Mažeika K. Pakštas V. Tumėnas S. et al . (2023). Prussian blue composites for Cs adsorption–modification of the method and modelling of the adsorption processes. J. Radioanal. Nucl. Chem.332, 1033–1045. doi: 10.1007/s10967-022-08660-z
51
Lujanienė G. Šemčuk S. Lečinskytė A. Kulakauskaitė I. Mažeika K. S. Valiulis D. et al . (2017). Magnetic graphene oxide based nano-composites for removal of radionuclides and metals from contaminated solutions. J. Environ. Radioact.166, 166–174. doi: 10.1016/j.jenvrad.2016.02.014
52
Ma F. Wang S. Gong X. Liu X. Wang Z. Wang P. et al . (2022). Highly efficient electrocatalytic hydrogen evolution coupled with upcycling of microplastics in seawater enabled via Ni3N/W5N4 janus nanostructures. Appl. Catal. B Environ.307:121198. doi: 10.1016/j.apcatb.2022.121198
53
Magid A. S. I. A. Islam M. S. Chen Y. Weng L. Li J. Ma J. et al . (2021). Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Sci. Total Environ.784:147115. doi: 10.1016/j.scitotenv.2021.147115
54
Magrì D. Sánchez-Moreno P. Caputo G. Gatto F. Veronesi M. Bardi G. et al . (2018). Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: characterization and toxicology assessment. ACS Nano12, 7690–7700. doi: 10.1021/acsnano.8b01331
55
Mendes A. S. S. (2022). Analysis of micro and nanoplastics with x-ray photoelectron spectroscopy.
56
Mitrano D. M. Wick P. Nowack B. (2021). Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol.16, 491–500. doi: 10.1038/s41565-021-00888-2
57
Niu S. Liu R. Zhao Q. Gagan S. Dodero A. Ying Q. et al . (2024). Quantifying the chemical composition and real-time mass loading of nanoplastic particles in the atmosphere using aerosol mass spectrometry. Environ. Sci. Technol.58, 3363–3374. doi: 10.1021/acs.est.3c10286
58
Novikau R. Lujaniene G. (2022). Adsorption behaviour of pollutants: heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): a review. J. Environ. Manag.309:114685. doi: 10.1016/j.jenvman.2022.114685
59
Novikau R. Lujanienė G. Pakštas V. Talaikis M. Mažeika K. Drabavičius A. et al . (2022). Adsorption of caesium and cobalt ions on the muscovite mica clay-graphene oxide-γ-Fe2O3-Fe3O4 composite. Environ. Sci. Pollut. Res.29, 74933–74950. doi: 10.1007/s11356-022-21078-0
60
Okoffo E. D. Ribeiro F. O'brien J. W. O'brien S. Tscharke B. J. Gallen M. et al . (2020). Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography–mass spectrometry. Sci. Total Environ.715:136924. doi: 10.1016/j.scitotenv.2020.136924
61
Ompala C. Renault J.-P. Taché O. Cournède É. Devineau S. Chivas-Joly C. (2024). Stability and dispersibility of microplastics in experimental exposure medium and their dimensional characterization by SMLS, SAXS, Raman microscopy, and SEM. J. Hazard. Mater.469:134083. doi: 10.1016/j.jhazmat.2024.134083
62
Özteki̇n N. R. Y. Sponza D. A. T. (2024). Removals of polyethylene terephthalate (PET) nanoplastics from an activated sludge: improvement of yields by Ni-cu-C nanocomposite. Int. J. Chem. Eng. Mater.3, 101–131. doi: 10.37394/232031.2024.3.10
63
Panahi H. K. S. Dehhaghi M. Ok Y. S. Nizami A.-S. Khoshnevisan B. Mussatto S. I. et al . (2020). A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J. Clean. Prod.270:122462. doi: 10.1016/j.jclepro.2020.122462
64
Pedrero D. Edo C. Fernández-Piñas F. Rosal R. Aguado S. (2024). Efficient removal of nanoplastics from water using mesoporous metal organic frameworks. Sep. Purif. Technol.333:125816. doi: 10.1016/j.seppur.2023.125816
65
Pelegrini K. Pereira T. C. B. Maraschin T. G. Teodoro L. D. S. Basso N. R. D. S. De Galland G. L. B. et al . (2023). Micro-and nanoplastic toxicity: a review on size, type, source, and test-organism implications. Sci. Total Environ.878:162954. doi: 10.1016/j.scitotenv.2023.162954
66
Pichler C. M. Bhattacharjee S. Rahaman M. Uekert T. Reisner E. (2021). Conversion of polyethylene waste into gaseous hydrocarbons via integrated tandem chemical–photo/electrocatalytic processes. ACS Catal.11, 9159–9167. doi: 10.1021/acscatal.1c02133
67
Pilapitiya P. N. T. Ratnayake A. S. (2024). The world of plastic waste: a review. Cleaner Mater.100220. doi: 10.1016/j.clema.2024.100220
68
Pivato A. F. Miranda G. M. Prichula J. Lima J. E. Ligabue R. A. Seixas A. et al . (2022). Hydrocarbon-based plastics: progress and perspectives on consumption and biodegradation by insect larvae. Chemosphere293:133600. doi: 10.1016/j.chemosphere.2022.133600
69
Qian Y. Zhang F. Pang H. (2021). A review of MOFs and their composites-based photocatalysts: synthesis and applications. Adv. Funct. Mater.31:2104231. doi: 10.1002/adfm.202104231
70
Rahman A. Sarkar A. Yadav O. P. Achari G. Slobodnik J. (2021). Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci. Total Environ.757:143872. doi: 10.1016/j.scitotenv.2020.143872
71
Ramsperger A. F. Bergamaschi E. Panizzolo M. Fenoglio I. Barbero F. Peters R. et al . (2022). Nano- and microplastics: a comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact29:100441. doi: 10.1016/j.impact.2022.100441
72
Sable H. Kumar V. Singh V. Rustagi S. Chahal S. Chaudhary V. (2024). Strategically engineering advanced nanomaterials for heavy-metal remediation from wastewater. Coord. Chem. Rev.518:216079. doi: 10.1016/j.ccr.2024.216079
73
Salahshoori I. Wang Q. Nobre M. A. Mohammadi A. H. Dawi E. A. Khonakdar H. A. (2024). Molecular simulation-based insights into dye pollutant adsorption: a perspective review. Adv. Colloid Interf. Sci.333:103281. doi: 10.1016/j.cis.2024.103281
74
Schwaferts C. Sogne V. Welz R. Meier F. Klein T. Niessner R. et al . (2020). Nanoplastic analysis by online coupling of Raman microscopy and field-flow fractionation enabled by optical tweezers. Anal. Chem.92, 5813–5820. doi: 10.1021/acs.analchem.9b05336
75
Shang S. Liu Y. Liu M. Bai Y. Wang X. Wu B. et al . (2022). Studying the adsorption mechanisms of nanoplastics on covalent organic frameworks via molecular dynamics simulations. J. Hazard. Mater.421:126796. doi: 10.1016/j.jhazmat.2021.126796
76
Shi Y. Du J. Zhao T. Feng B. Bian H. Shan S. et al . (2023). Removal of nanoplastics from aqueous solution by aggregation using reusable magnetic biochar modified with cetyltrimethylammonium bromide. Environ. Pollut.318:120897. doi: 10.1016/j.envpol.2022.120897
77
Shi Q. Guo S. Tang J. Lyu H. Ri C. Sun H. (2023). Enhanced removal of aged and differently functionalized polystyrene nanoplastics using ball-milled magnetic pinewood biochars. Environ. Pollut.316:120696. doi: 10.1016/j.envpol.2022.120696
78
Shi R. Liu K.-S. Liu F. Yang X. Hou C.-C. Chen Y. (2021). Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel. Chem. Commun.57, 12595–12598. doi: 10.1039/D1CC05032J
79
Shi J. Yu X. Zhao J. Wang T. Li N. Yu J. et al . (2024). Integrated transcriptomics and metabolomics reveal the mechanism of polystyrene nanoplastics toxicity to mice. Ecotoxicol. Environ. Saf.284:116925. doi: 10.1016/j.ecoenv.2024.116925
80
Sorasan C. Edo C. González-Pleiter M. Fernández-Piñas F. Leganés F. Rodríguez A. et al . (2021). Generation of nanoplastics during the photoageing of low-density polyethylene. Environ. Pollut.289:117919. doi: 10.1016/j.envpol.2021.117919
81
Sullivan G. Gallardo J. D. Jones E. Hollliman P. Watson T. Sarp S. (2020). Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere249:126179. doi: 10.1016/j.chemosphere.2020.126179
82
Summer M. Ashraf R. Ali S. Bach H. Noor S. Noor Q. et al . (2024). Inflammatory response of nanoparticles: mechanisms, consequences, and strategies for mitigation. Chemosphere363:142826. doi: 10.1016/j.chemosphere.2024.142826
83
Sun L. Li Y. Lan J. Bao Y. Zhao Z. Shi R. et al . (2024). Enhanced sinks of polystyrene nanoplastics (PSNPs) in marine sediment compared to freshwater sediment: influencing factors and mechanisms. Sci. Total Environ.939:173586. doi: 10.1016/j.scitotenv.2024.173586
84
Sun J. Wang Y. He Y. Liu J. Xu L. Zeng Z. et al . (2022). Effective removal of nanoplastics from water by cellulose/MgAl layered double hydroxides composite beads. Carbohydr. Polym.298:120059. doi: 10.1016/j.carbpol.2022.120059
85
Sun C. Wang Z. Zheng H. Zhao S. Luo X. Li C. et al . (2024). Synthesis of compressible and reusable chitin/O-gCN sponges for efficient removal of phthalate esters in water environments. Sep. Purif. Technol.331:125586. doi: 10.1016/j.seppur.2023.125586
86
Sun Y. Yu F. Li L. Ma J. (2021). Adsorption-reduction synergistic effect for rapid removal of Cr (VI) ions on superelastic NH2-graphene sponge. Chem. Eng. J.421:129933. doi: 10.1016/j.cej.2021.129933
87
Tang Y. Zhang S. Su Y. Wu D. Zhao Y. Xie B. (2021). Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J.406:126804. doi: 10.1016/j.cej.2020.126804
88
Teng J. Yu H. Liu Z. Bai L. Liu Z. (2024). Fabrication of novel metal oxide nanosheets-decorated carbon nanofibers for highly efficient removal of ultra-small nanoplastics. J. Environ. Chem. Eng.12:114094. doi: 10.1016/j.jece.2024.114094
89
Thaiba B. M. Sedai T. Bastakoti S. Karki A. Anuradha K. Khadka G. et al . (2023). A review on analytical performance of micro- and nanoplastics analysis methods. Arab. J. Chem.16:104686. doi: 10.1016/j.arabjc.2023.104686
90
Tiwari E. Singh N. Khandelwal N. Monikh F. A. Darbha G. K. (2020). Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J. Hazard. Mater.397:122769. doi: 10.1016/j.jhazmat.2020.122769
91
Townsend P. A. (2024). Adsorption in action: molecular dynamics as a tool to study adsorption at the surface of fine plastic particles in aquatic environments. ACS Omega9, 5142–5156. doi: 10.1021/acsomega.3c07488
92
Uogintė I. Pleskytė S. Skapas M. Stanionytė S. Lujanienė G. (2023). Degradation and optimization of microplastic in aqueous solutions with graphene oxide-based nanomaterials. Int. J. Environ. Sci. Technol.20, 9693–9706. doi: 10.1007/s13762-022-04657-z
93
Vélez-Escamilla L. Y. Contreras-Torres F. F. (2022). Latest advances and developments to detection of micro-and nanoplastics using surface-enhanced Raman spectroscopy. Part. Part. Syst. Charact.39:2100217. doi: 10.1002/ppsc.202100217
94
Venâncio C. Ferreira I. Martins M. A. Soares A. M. Lopes I. Oliveira M. (2019). The effects of nanoplastics on marine plankton: a case study with polymethylmethacrylate. Ecotoxicol. Environ. Saf.184:109632. doi: 10.1016/j.ecoenv.2019.109632
95
Vohl S. Kristl M. Stergar J. (2024). Harnessing magnetic nanoparticles for the effective removal of Micro-and nanoplastics: a critical review. Nano14:1179. doi: 10.3390/nano14141179
96
Wan H. Wang J. Sheng X. Yan J. Zhang W. Xu Y. (2022). Removal of polystyrene microplastics from aqueous solution using the metal–organic framework material of ZIF-67. Toxics10:70. doi: 10.3390/toxics10020070
97
Wang M. Lilly K. Martin L. M. Xu W. Tamamis P. Phillips T. D. (2024). Adsorption and removal of polystyrene nanoplastics from water by green-engineered clays. Water Res. 249:120944.
98
Wang J. Sun C. Huang Q.-X. Chi Y. Yan J.-H. (2021). Adsorption and thermal degradation of microplastics from aqueous solutions by mg/Zn modified magnetic biochars. J. Hazard. Mater.419:126486. doi: 10.1016/j.jhazmat.2021.126486
99
Wang Z. Sun C. Li F. Chen L. (2021). Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chem. Eng. J.415:129006. doi: 10.1016/j.cej.2021.129006
100
Wang Q. Zhao Y. Shi Z. Sun X. Bu T. Zhang C. et al . (2021). Magnetic amino-functionalized-MOF (M= Fe, Ti, Zr)@ COFs with superior biocompatibility: performance and mechanism on adsorption of azo dyes in soft drinks. Chem. Eng. J.420:129955. doi: 10.1016/j.cej.2021.129955
101
Wibuloutai J. Thongkum W. Khiewkhern S. Thunyasirinon C. Prathumchai N. (2023). Microplastics and nanoplastics contamination in raw and treated water. Water Supply23, 2267–2282. doi: 10.2166/ws.2023.116
102
Xing Y. Shen X. Niu Q. Duan H. Tang C. Tao B. et al . (2025). Thermally and chemically stable Fe/mg-layered double oxides-biochar for enhanced polystyrene Nanoplastic adsorption and sustainable recycling. Chem. Eng. J.508:160918. doi: 10.1016/j.cej.2025.160918
103
Xing Y. Zhang B. Niu Q. Ji G. (2024). Enhanced adsorption of polystyrene nanoplastics by cetyltrimethylammonium bromide surface-modified magnetic rice straw biochar: efficient performance and adsorption mechanisms. Sep. Purif. Technol.344:127264. doi: 10.1016/j.seppur.2024.127264
104
Yang H. Ju J. Wang Y. Zhu Z. Lu W. Zhang Y. (2024). Micro- and nano-plastics induce kidney damage and suppression of innate immune function in zebrafish (Danio rerio) larvae. Sci. Total Environ.931:172952. doi: 10.1016/j.scitotenv.2024.172952
105
Yen P.-L. Hsu C.-H. Huang M.-L. Liao V. H.-C. (2022). Removal of nano-sized polystyrene plastic from aqueous solutions using untreated coffee grounds. Chemosphere286:131863. doi: 10.1016/j.chemosphere.2021.131863
106
Zhao H. Huang X. Wang L. Zhao X. Yan F. Yang Y. et al . (2022). Removal of polystyrene nanoplastics from aqueous solutions using a novel magnetic material: Adsorbability, mechanism, and reusability. Chem. Eng. J.430:133122. doi: 10.1016/j.cej.2021.133122
107
Zhao H. Wu J. Su F. He X. (2023). Removal of polystyrene nanoplastics from aqueous solutions by a novel magnetic zeolite adsorbent. Hum. Ecol. Risk Assess. Int. J.29, 327–346. doi: 10.1080/10807039.2022.2071209
108
Zhou G. Huang X. Xu H. Wang Q. Wang M. Wang Y. et al . (2022). Removal of polystyrene nanoplastics from water by CuNi carbon material: the role of adsorption. Sci. Total Environ.820:153190. doi: 10.1016/j.scitotenv.2022.153190
109
Zhu N. Yan Q. He Y. Wang X. Wei Z. Liang D. et al . (2022). Insights into the removal of polystyrene nanoplastics using the contaminated corncob-derived mesoporous biochar from mining area. J. Hazard. Mater.433:128756. doi: 10.1016/j.jhazmat.2022.128756
110
Zhuang J. Rong N. Wang X. Chen C. Xu Z. (2022). Adsorption of small size microplastics based on cellulose nanofiber aerogel modified by quaternary ammonium salt in water. Sep. Purif. Technol.293:121133. doi: 10.1016/j.seppur.2022.121133
Summary
Keywords
plastic pollution, nanoplastics, adsorption, water treatment, biocompatible adsorbents, environmental protection
Citation
Javed M and Lujanienė G (2025) Nanoplastics in aquatic systems: challenges and advances in adsorptive removal technologies. Front. Water 7:1611558. doi: 10.3389/frwa.2025.1611558
Received
14 April 2025
Accepted
19 May 2025
Published
04 June 2025
Corrected
18 June 2025
Volume
7 - 2025
Edited by
Venkatramanan Senapathi, National College, India
Reviewed by
Sivakumar Karthikeyan, Alagappa University, India
Zhijie Chen, University of New South Wales, Australia
Shilpa Susan Scaria, Christ University, India
Israa Murtadha Hameed Altameemi, Mustansiriyah University, Iraq
Updates
Copyright
© 2025 Javed and Lujanienė.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahrosh Javed, mahrosh.javed@ftmc.lt
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.