- Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, Jinan, China
Objectives: This study aims to investigate whether the ratios of cell types in peripheral blood could be used as reliable predictors of off-pump coronary artery bypass grafting (CABG)-associated acute kidney injury (AKI).
Materials and methods: We retrospectively reviewed patients (n = 420) undergoing off-pump CABG from January 1, 2021 to January 1, 2022 in Qilu Hospital of Shandong University. We used logistic regression analysis to identify the potential predictors of off-pump CABG-associated AKI and construct a predictive model. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive ability of predictors and prediction models.
Results: The prevalence of AKI associated with off-pump CABG was 20.95%. Patients in the AKI group had significantly higher ratios of peripheral blood cells on postoperative day (POD)1 than patients in the non-AKI group (P < 0.01). The area under the ROC curve (AUC) of the neutrophil:lymphocyte ratio (NLR) on POD1 for predicting off-pump CABG-associated AKI was 0.780 and the cutoff value was 20.07. Patients with high NLR on POD1 had a poor short-term prognosis. The AUC of the predictive model constructed by logistic regression analysis was 0.882. The sensitivity was 68.2% and the specificity was 93.1%.
Conclusion: The NLR on POD1 was a reliable predictive biomarker of off-pump CABG-associated AKI. And we successfully construct a prediction model, which contribute to the early recognition and management of off-pump CABG-associated AKI.
Introduction
Acute kidney injury (AKI) involves a sudden decline in renal function. Approximately 20% of adult patients develop AKI during hospitalization, 10% of whom require dialysis (1). Studies have indicated that even mild AKI is associated with a significantly increased risk of death, and the mortality in patients requiring renal replacement therapy (RRT) is 50%, which poses a huge challenge for medical professionals (1–4).
AKI development is heterogenous, and several mechanisms may be involved (5). Patients undergoing cardiac surgery are more likely to develop AKI due to hemodynamic changes, an increased inflammatory response, and use of nephrotoxic medications (6). Moreover, cardiac surgery-associated AKI (CSA-AKI) is associated independently with short-term and long-term mortality (7–9). Considering the high prevalence (≤42%) and severe effects of CSA-AKI, early recognition and intervention are very important (10).
The inflammatory cascade is considered to be a major event that aggravates injury to tubular epithelial cells and reduces the glomerular filtration rate (GFR) during the extension phase: this represents the most promising phase for successful treatment and intervention of AKI (11). Therefore, the predictive role of inflammatory response-related biomarkers in CSA-AKI has been studied extensively.
The relevant ratios of different cell types in peripheral blood are able to reflect the inflammation and have been found to be potential predictors of AKI after acute type-A aortic dissection, on-pump coronary artery bypass grafting (CABG), and transcatheter implantation of aortic valves (12–17). However, studies on the relationship between off-pump CABG-associated AKI and the inflammatory response are lacking.
Off-pump CABG-associated AKI also significantly increases the risk of renal replacement therapy and death in patients (18–20). The risk factors for the development of AKI behind off-pump CABG are not well understood. We investigated whether the ratios of cell types in peripheral blood could be predictors of off-pump CABG-associated AKI. We look forward to providing guidance for the early recognition and treatment of off-pump CABG-associated AKI.
Materials and methods
Ethical approval of the study protocol
This study protocol was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (Jinan, China). Written informed consent was waived and all patient information was stored anonymously.
Study design
We retrospectively reviewed patients undergoing off-pump CABG from January 2021 to January 2022 at the Department of Cardiovascular Surgery within Qilu Hospital of Shandong University.
Exclusion criteria
Patients were excluded if: (i) they needed intraoperative CPB; (ii) they had preoperative severe chronic kidney disease necessitating RRT; (iii) their postoperative serum creatinine (SCr) data were incomplete.
Surgical procedures
Off-pump CABG was undertaken in patients with severe coronary artery disease [left main disease, three-vessel disease, combined with diabetes mellitus (DM)] or failed stenting. After the induction of general anesthesia with endotracheal intubation, a median sternal incision was made. The left internal mammary artery and great saphenous vein were freed as bridge vessels simultaneously. After heparinization, the anastomotic site was secured using a stabilizer. The anastomosis was undertaken with an intra-coronary shunt and deep pericardial suture. The operating surgeon measured the flow of vein grafts after the anastomosis to ensure the patency of grafted vascular bridges.
Definition
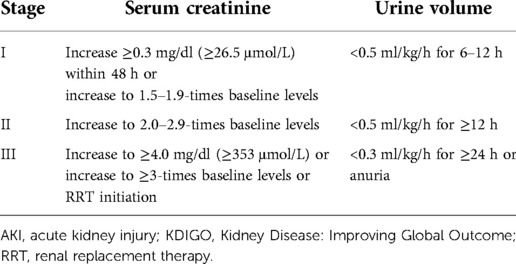
We selected the most recent SCr value before the surgical procedure as the baseline level. We applied the Chronic Kidney Disease Epidemiology Collaboration equation to obtain the estimated glomerular filtration rate (eGFR) (21). The diagnosis and staging of AKI followed the criteria of the Kidney Disease: Improving Global Outcome (KDIGO) guideline (22) (Table 1).
We calculated the neutrophil:lymphocyte ratio (NLR), monocyte:lymphocyte ratio (MLR), and platelet:lymphocyte ratio (PLR) as biomarkers associated with the inflammatory response.
Data collection
We documented the perioperative variables of patients. These were: age, gender, body mass index (BMI), tobacco smoking, hypertension, DM, hyperlipemia, history of cerebral diseases, kidney disease without RRT, chronic obstructive pulmonary disease (COPD), percutaneous coronary intervention (PCI), New York Heart Association (NYHA) functional classification, preoperative left ventricular ejection fraction (LVEF), preoperative peripheral blood counts, preoperative blood biochemistry, preoperative renal function, intraoperative erythrocyte transfusion, intraoperative urine volume, intraoperative fluid replacement, central venous pressure (CVP) and mean arterial pressure (MAP) at intensive care unit (ICU) admission, use of an intra-aortic balloon pump (IABP), low cardiac output syndrome (LCOS), RRT, application of vasoactive agents, duration of mechanical ventilation, peripheral blood counts on postoperative day (POD)1, postoperative renal function, postoperative erythrocyte transfusion, complications, duration of hospital stay, duration of ICU stay, death.
Statistical analyses
We used SPSS 25.0 (IBM, Armonk, NY, United States) for statistical analyses. Measurement data were tested to see if they had a normal distribution. Variables with a normal distribution are expressed as the mean ± SD and were analyzed by the Student's t-test. Variables with a non-normal distribution are expressed as medians and quartiles and were analyzed by the Mann–Whitney U-test. Categorical data are expressed as frequencies and percentages and were compared by the chi-square test or Fisher's exact test. P < 0.05 (two-sided) was considered significant. Multivariate analysis incorporated variables with significant differences in univariate analysis. The results of multivariate logistic regression analysis are expressed as odds ratio (OR) and 95% confidence interval (CI). A receiver operating characteristic (ROC) curve and Hosmer–Lemeshow goodness of fit test were applied to evaluate the ability of predictive models. The maximum value of the Youden index was used to determine the cutoff value.
Results
Characteristics of the study cohort
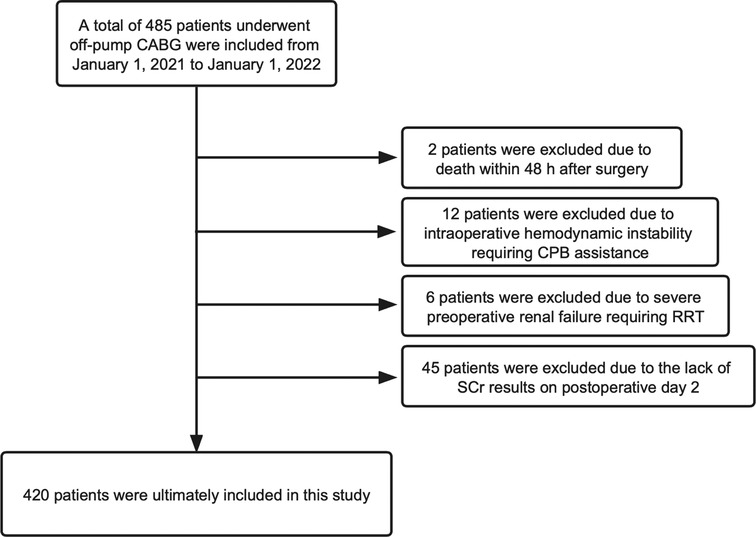
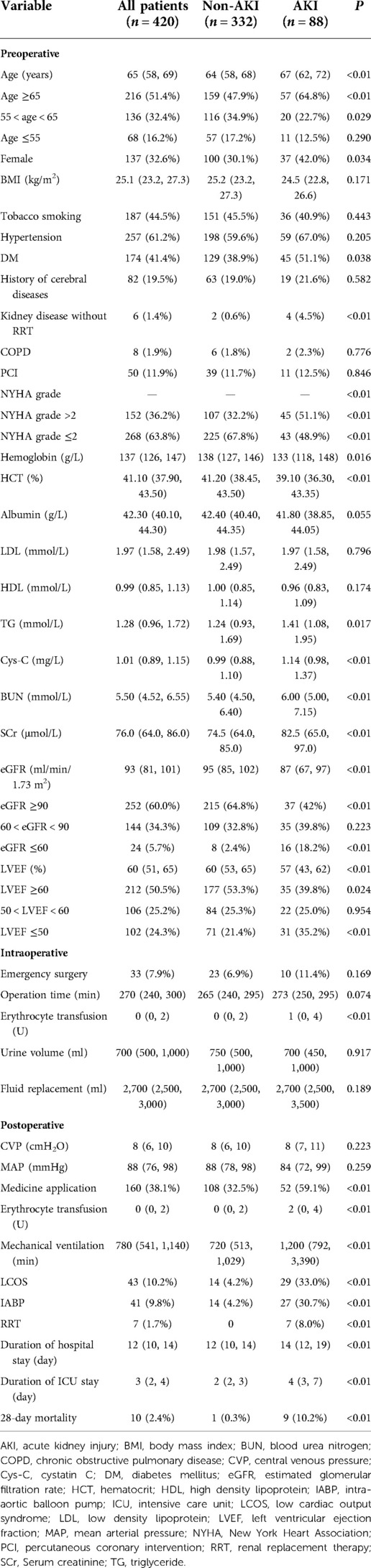
From January 1, 2021 to January 1, 2022, 485 patients underwent off-pump CABG in the Department of Cardiovascular Surgery within Qilu Hospital of Shandong University. We excluded 65 patients according to our exclusion criteria (Figure 1). Finally, the data of 420 patients were analyzed and their baseline characteristics are shown in Table 2.
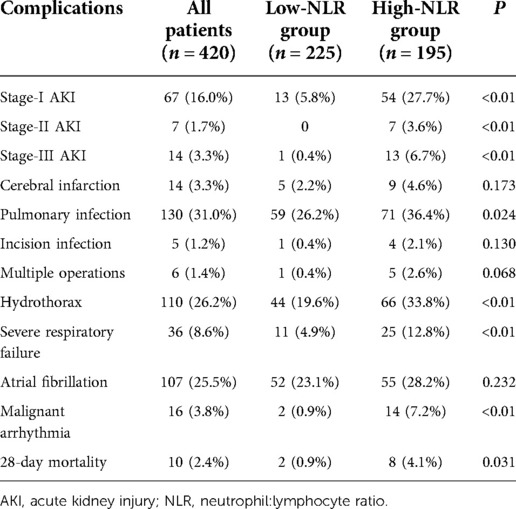
Eighty-eight patients (20.95%) developed AKI after off-pump CABG (67 patients with stage-I, 7 patients with stage-II, and 14 patients with stage-III AKI). Sixteen patients were diagnosed with AKI on POD1. The peak value of SCr occurred on POD2. Patients in the AKI group had a longer stay in the ICU and hospital. Seven patients received RRT after off-pump CABG, and 10 patients died within 28 days after CABG.
Inflammation-related biomarkers and off-pump CABG-associated AKI
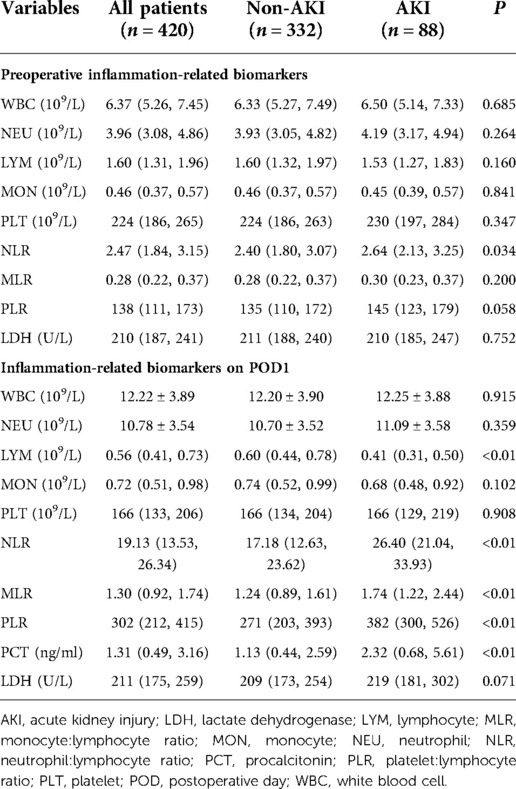
We measured the levels of inflammation-related biomarkers before and on POD1 (Table 3). The counts for leukocytes, neutrophils, monocytes, and the levels of procalcitonin (PCT), which are correlated positively with inflammation, increased significantly on POD1. The lymphocyte count and platelet count, which are correlated negatively with the inflammatory response, decreased significantly on POD1. These results demonstrated that patients undergoing off-pump CABG experienced a dramatic inflammatory response. Moreover, there were significant differences in the ratios of cell types in peripheral blood on POD1 between patients in the AKI group and non-AKI group (P < 0.01), which implied a more pronounced inflammatory response in the AKI group. Patients in the AKI group had significantly higher levels of interleukin (IL)-6 than patients in the non-AKI group on POD1 (Supplementary Table S1 and Supplementary Figure S1).
In addition, we found that ten of the patients who were diagnosed with AKI on POD1 developed more severe AKI in the following days. We divided them into the deteriorate and stable group based on their development of AKI. Patients in the deteriorate group had higher NLR on POD1 than those in the stable group, which indicated that NLR on POD1 may be used as predictors for more severe AKI in these patients (Supplementary Table S2 and Supplementary Figure S2).

Figure 2. The results of ROC-curve analysis: (A) the ROC curve of the NLR for predicting off-pump CABG-associated AKI. (B) The ROC curve of the predictive model.
Independent risk factors of off-pump CABG-associated AKI
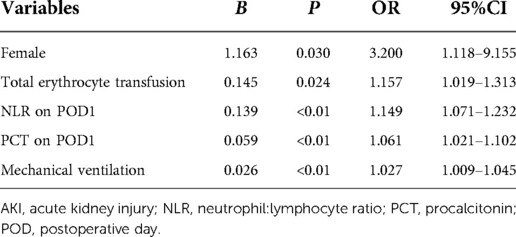
The results of univariate analysis exhibited that there were statistical differences between AKI and no-AKI groups regarding older than 65, female gender, DM, kidney disease without RRT, NYHA score greater than 2, hemoglobin, hematocrit, triglyceride, preoperative cystatin C, preoperative blood urea nitrogen, preoperative SCr, eGFR below 60%, LVEF below 50%, application of vasoactive agents, erythrocyte transfusion, duration of mechanical ventilation, LCOS, use of an IABP, preoperative NLR, NLR on POD1, MLR on POD1, PLR on POD1, PCT on POD1 (Table 2 and Table 3).
We included variables described above into a multivariate logistics regression model. Being female (OR = 3.200, 95%CI = 1.118–9.115), total erythrocyte transfusion (1.157, 1.019–1.31), NLR on POD1 (1.149, 1.071–1.232), PCT level on POD1 (1.061, 1.021–1.102), and duration of mechanical ventilation (1.027, 1.009–1.045) were independent risk factors of off-pump CABG-associated AKI (Table 4).
Predictive model of off-pump CABG-associated AKI
We used ROC-curve analysis to calculate the predictive ability of the NLR on POD1. The area under the ROC curve (AUC) of the NLR for predicting off-pump CABG-associated AKI was 0.780 (Figure 2A). The sensitivity was 84.1% and the specificity was 63.6%. When the Youden index reached a maximum, the cutoff value of the NLR was 20.07. Next, we included all the independent risk factors obtained by multivariate analysis into a predictive model for ROC-curve analysis. The AUC of the new predictive model was 0.882 (Figure 2B), which exhibited a better predictive ability. The sensitivity was 68.2% and the specificity was 93.1%. And the P value of Hosmer–Lemeshow goodness of fit test equals 0.074 (P > 0.05).
Correlation between the NLR and postoperative complications
We divided patients into a high-NLR group and low-NLR group according to the cutoff value (20.07) of the NLR on POD1. A high NLR on POD1 was closely associated with more severe AKI, pulmonary infection, hydrothorax, severe respiratory failure, and malignant arrhythmia (Table 5). Moreover, postoperative 28-day mortality was significantly higher in patients with a high NLR than in those with a low NLR (P < 0.05). These results demonstrated that patients with a high NLR on POD1 had poor short-term outcomes.
Discussion
Ischemic AKI is the most prevalent type of CSA-AKI. According to the change in the GFR, the development of ischemic AKI can be divided into four phases: initiation, extension, maintenance, and recovery (23). In the initiation phase, ischemia-induced damage to tubular epithelial cells and endothelial cells leads to the release of chemokines and cytokines that activate inflammatory cascades (24). The inflammatory response aggravates tubular-cell injury in the extension phase, which leads to a continued reduction in the GFR. Significant infiltration of inflammatory cells in the renal outer medulla occurs as early as 24 h after ischemia (25), and leukocytes may appear as early as 2 h after ischemia (26). Therefore, early identification and interrupting the amplification of the inflammatory response in the extension phase is very important.
The SCr level peaked on POD2 and the infiltration of inflammatory cells would appear early, so we chose the cell ratios in peripheral blood on POD1 as biomarkers. Our study found that the NLR on POD1 was a reliable biomarker for the early prediction of off-pump CABG-associated AKI (AUC = 0.780, cutoff = 20.07). The NLR was derived from the hematological observation that the neutrophil count is associated positively with cardiovascular events and the lymphocyte count is associated negatively with cardiovascular events (27, 28). Neutrophil activation is an important sign of acute inflammatory response, and lymphopenia is a marker of poor health condition and physiological stress. Compared with C-reactive protein, IL-6, and other inflammation-specific biomarkers, the NLR can be obtained more readily and in an inexpensive manner, so it has attracted the attention of researchers. A meta-analysis involving five randomized clinical trials with large cohorts showed that the NLR at baseline independently predicted the risk of cardiovascular events and all-cause mortality in patients (29). Kim and colleagues revealed a high NLR on POD1 to be closely associated with an increased risk of CSA-AKI and 1-year mortality (30).
The high NLR level on POD1 may indicate the early inflammatory response after off-pump CABG, which is one of the most important mechanisms of AKI. Many animal experiments have proved the important role of inflammatory response in the development of AKI. Kelly and colleagues found that anti-intercellular adhesion molecule-1 therapy prevented ischemic AKI in mice via neutrophil-dependent pathway (31, 32). Rabb and coworkers confirmed that the adhesion molecules CD11 and CD18 on the surface of leukocytes play an important role in ischemic AKI in rats (33). In addition, selectin ligand inhibitors also successfully attenuated renal ischemia-reperfusion injury in rat and pig models (34, 35). Besides the adhesion molecules described above, chemokines, proinflammatory cytokines, reactive oxygen species, C-reactive protein and danger-associated molecular patterns also participate in the inflammatory response leading to unacceptable AKI.
CPB during cardiac surgery activates the immune system significantly: a large number of cytokines and chemokines are released, which increases the risk of AKI (36). Parlar and coworkers found that the postoperative NLR was an independent predictor of AKI after on-pump CABG (37). However, we found that the leukocyte count, PCT level, and IL-6 level were also significantly higher in patients after off-pump CABG, which reflected a marked inflammatory response. Studies correlating inflammation with off-pump CABG-associated AKI are lacking. Our findings demonstrated this association and revealed the predictive value of the NLR on POD1.
We also discovered four other independent risk factors of off-pump CABG-associated AKI according to multivariate analysis: gender, total erythrocyte transfusion, PCT level on POD1, and duration of mechanical ventilation. In our study, female patients accounted for 32.6% of the study cohort, but the prevalence of AKI was much higher than that in male patients (P < 0.05). After adjustment for other variables, being female remained an independent risk factor of off-pump CABG-associated AKI. This observation is consistent with findings in some studies (38, 39) but not in other studies (40, 41).
The free hemoglobin and free iron released from an erythrocyte transfusion would cause oxidative stress, which aggravates inflammation and ischemia–reperfusion injury in the kidney (42, 43). Therefore, many researchers expect to reduce the occurrence of CSA-AKI by restricting erythrocyte transfusion (44, 45). The association between mechanical ventilation and AKI was documented first by Drury and coworkers in 1947. They found that continuous-pressure ventilation caused a decline in renal function (46). Afterwards, Kuiperet and colleagues proposed that mechanical ventilation may affect renal function due to hemodynamic alterations and ventilator-induced lung injury which activates a systemic inflammatory response (47). We also found a correlation between higher levels of inflammatory biomarkers on POD1 and pulmonary complications.
PCT is a commonly used indicator for the diagnosis of sepsis in the ICU. The PCT level can be used to assess bacterial infection in the body. Studies of PCT and AKI have focused mainly on patients with sepsis, with fewer studies concentrating on patients undergoing cardiac surgery. Heredia-Rodríguez and colleagues enrolled patients with a systemic inflammatory response or sepsis after cardiac surgery. They showed that the PCT level was significantly higher in patients with CSA-AKI (48). Our results also confirmed this correlation, and we found that a high PCT level on POD1 was an independent risk factor of off-pump CABG-associated AKI. We hypothesize that patients on POD1 did not develop a bacterial infection, but the high PCT level may suggest an inflammatory reaction occurring in vivo and a higher risk of infection.
We included the independent risk factors stated above into a prediction model and obtained a high predictive ability (AUC = 0.882). Our study provides a predictive biomarker and predictive model for the early recognition and timely intervention of off-pump CABG-associated AKI. We aim to improve the prognosis of patients undergoing off-pump CABG.
Our study had three main limitations. First, this was a single-center retrospective study. The predictors obtained and prediction model created must undergo external validation. Second, we assessed only the short-term outcomes of patients after off-pump CABG. Third, the predictive value of NLR on POD1 was attenuated in patients who have had AKI on POD1. In the future, we will dedicate ourselves to improving the intraoperative management of off-pump CABG and carrying out a prospective study that documents the prevalence of postoperative AKI in patients undergoing different treatments.
Conclusion
We demonstrated the NLR on POD1 to be a reliable biomarker for predicting off-pump CABG-associated AKI. Also, we constructed a prediction model that may contribute to the early recognition and management of off-pump CABG-associated AKI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Qilu Hospital of Shandong University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RY performed data analysis, statistics, and draft writing. HS performed data collection. XM and YB designed the study and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Dr. Tingyi Liang for her assistance with the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1047050/full#supplementary-material.
References
1. Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of aki: a meta-analysis. Clin J Am Soc Nephrol. (2013) 8(9):1482–93. doi: 10.2215/cjn.00710113
2. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of Serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. (2004) 15(6):1597–605. doi: 10.1097/01.asn.0000130340.93930.dd
3. Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, et al. Impact of minimal increases in Serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. (2008) 36(4):1129–37. doi: 10.1097/CCM.0b013e318169181a
4. Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet. (2013) 382(9887):170–9. doi: 10.1016/s0140-6736(13)60647-9
5. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394(10212):1949–64. doi: 10.1016/s0140-6736(19)32563-2
6. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the adqi (acute disease quality initiative) group. J Am Heart Assoc. (2018) 7(11):e008834. doi: 10.1161/jaha.118.008834
7. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119(18):2444–53. doi: 10.1161/circulationaha.108.800011
8. Lopez-Delgado JC, Esteve F, Torrado H, Rodríguez-Castro D, Carrio ML, Farrero E, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified rifle classification. Crit Care. (2013) 17(6):R293. doi: 10.1186/cc13159
9. Machado MN, Nakazone MA, Maia LN. Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (kdigo) criteria. PLoS One. (2014) 9(5):e98028. doi: 10.1371/journal.pone.0098028
10. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. (2017) 13(11):697–711. doi: 10.1038/nrneph.2017.119
11. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. (2012) 2(2):1303–53. doi: 10.1002/cphy.c110041
12. Ma X, Chen S, Yun Y, Zhao D, Li J, Wu Z, et al. The predictive role of lymphocyte-to-monocyte ratio in acute kidney injury in acute debakey type I aortic dissection. Front Surg. (2021) 8:704345. doi: 10.3389/fsurg.2021.704345
13. Chen W, Song X, Hong L, Xu H, Qian Y, Zhang W, et al. The association between lymphocyte-monocyte ratio and postoperative acute kidney injury in patients with acute type a aortic dissection. J Cardiothorac Surg. (2022) 17(1):60. doi: 10.1186/s13019-022-01813-x
14. Fisher LA, Stephenson S, Reid MT, Anderson SG. Acute kidney injury following cardiopulmonary bypass in Jamaica. JTCVS Open. (2022) 36(11):161–75. doi: 10.1016/j.xjon.2022.05.012
15. Olasińska-Wiśniewska A, Perek B, Grygier M, Urbanowicz T, Misterski M, Puślecki M, et al. Increased neutrophil-to-lymphocyte ratio is associated with higher incidence of acute kidney injury and worse survival after transcatheter aortic valve implantation. Cardiol J. (2021). doi: 10.5603/CJ.a2021.0149. [Epub ahead of print]
16. Koo CH, Eun Jung D, Park YS, Bae J, Cho YJ, Kim WH, et al. Neutrophil, lymphocyte, and platelet counts and acute kidney injury after cardiovascular surgery. J Cardiothorac Vasc Anesth. (2018) 32(1):212–22. doi: 10.1053/j.jvca.2017.08.033
17. Parlar H, Şaşkın H. Are Pre and postoperative platelet to lymphocyte ratio and neutrophil to lymphocyte ratio associated with early postoperative aki following cabg? Braz J Cardiovasc Surg. (2018) 33(3):233–41. doi: 10.21470/1678-9741-2017-0164
18. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. (2012) 366(16):1489–97. doi: 10.1056/NEJMoa1200388
19. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. (2013) 368(13):1179–88. doi: 10.1056/NEJMoa1301228
20. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, O'Corragain OA, Edmonds PJ, et al. Comparison of renal outcomes in off-pump versus on-pump coronary artery bypass grafting: a systematic review and meta-analysis of randomized controlled trials. Nephrology (Carlton). (2015) 20(10):727–35. doi: 10.1111/nep.12506
21. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate gfr without race. N Engl J Med. (2021) 385(19):1737–49. doi: 10.1056/NEJMoa2102953
22. Khwaja A. Kdigo clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120(4):c179–84. doi: 10.1159/000339789
23. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. (2002) 62(5):1539–49. doi: 10.1046/j.1523-1755.2002.00631.x
24. Rabb H, O'Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. (1997) 51(5):1463–8. doi: 10.1038/ki.1997.200
25. Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. (2000) 15(10):1562–74. doi: 10.1093/ndt/15.10.1562
26. Willinger CC, Schramek H, Pfaller K, Pfaller W. Tissue distribution of neutrophils in postischemic acute renal failure. Virchows Arch B Cell Pathol Incl Mol Pathol. (1992) 62(4):237–43. doi: 10.1007/bf02899687
27. Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. (1974) 290(23):1275–8. doi: 10.1056/nejm197406062902302
28. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. (2005) 45(10):1638–43. doi: 10.1016/j.jacc.2005.02.054
29. Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. (2021) 42(9):896–903. doi: 10.1093/eurheartj/ehaa1034
30. Kim WH, Park JY, Ok SH, Shin IW, Sohn JT. Association between the neutrophil/lymphocyte ratio and acute kidney injury after cardiovascular surgery: a retrospective observational study. Medicine (Baltimore). (2015) 94(43):e1867. doi: 10.1097/md.0000000000001867
31. Kelly KJ, Williams WW Jr., Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A. (1994) 91(2):812–6. doi: 10.1073/pnas.91.2.812
32. Kelly KJ, Williams WW Jr., Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. (1996) 97(4):1056–63. doi: 10.1172/jci118498
33. Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, et al. Role of Cd11a and Cd11b in ischemic acute renal failure in rats. Am J Physiol. (1994) 267(6 Pt 2):F1052–8. doi: 10.1152/ajprenal.1994.267.6.F1052
34. Nemoto T, Burne MJ, Daniels F, O'Donnell MP, Crosson J, Berens K, et al. Small molecule selectin ligand inhibition improves outcome in ischemic acute renal failure. Kidney Int. (2001) 60(6):2205–14. doi: 10.1046/j.1523-1755.2001.00054.x
35. Jayle C, Milinkevitch S, Favreau F, Doucet C, Richer JP, Deretz S, et al. Protective role of selectin ligand inhibition in a large animal model of kidney ischemia-reperfusion injury. Kidney Int. (2006) 69(10):1749–55. doi: 10.1038/sj.ki.5000335
36. Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al. Plasma il-6 and il-10 concentrations predict aki and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol. (2015) 26(12):3123–32. doi: 10.1681/asn.2014080764
37. Parlar H, Arıkan AA, Önmez A. Dynamic changes in perioperative cellular inflammation and acute kidney injury after coronary artery bypass Grafting. Braz J Cardiovasc Surg. (2021) 36(3):354–64. doi: 10.21470/1678-9741-2020-0163
38. Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, et al. Arf after open-heart surgery: influence of gender and race. Am J Kidney Dis. (2003) 41(4):742–51. doi: 10.1016/s0272-6386(03)00021-0
39. Birnie K, Verheyden V, Pagano D, Bhabra M, Tilling K, Sterne JA, et al. Predictive models for kidney disease: improving global outcomes (kdigo) defined acute kidney injury in UK cardiac surgery. Crit Care. (2014) 18(6):606. doi: 10.1186/s13054-014-0606-x
40. Fang Y, Teng J, Ding X. Acute kidney injury in China. Hemodial Int. (2015) 19(1):2–10. doi: 10.1111/hdi.12193
41. Jiang W, Teng J, Xu J, Shen B, Wang Y, Fang Y, et al. Dynamic predictive scores for cardiac surgery-associated acute kidney injury. J Am Heart Assoc. (2016) 5(8):e003754. doi: 10.1161/jaha.116.003754
42. Billings FTT, Ball SK, Roberts LJ 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. (2011) 50(11):1480–7. doi: 10.1016/j.freeradbiomed.2011.02.011
43. Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. (2010) 55(19):2024–33. doi: 10.1016/j.jacc.2009.12.046
44. Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. (2017) 377(22):2133–44. doi: 10.1056/NEJMoa1711818
45. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. (2015) 372(11):997–1008. doi: 10.1056/NEJMoa1403612
46. Drury DR, Henry JP, Goodman J. The effects of continuous pressure breathing on kidney function. J Clin Invest. (1947) 26(5):945–51. doi: 10.1172/jci101889
47. Kuiper JW, Groeneveld AB, Slutsky AS, Plötz FB. Mechanical ventilation and acute renal failure. Crit Care Med. (2005) 33(6):1408–15. doi: 10.1097/01.ccm.0000165808.30416.ef
Keywords: coronary artery bypass grafting, acute kidney injury, risk factors, predictive model, cardiopulmonary bypass
Citation: Yu R, Song H, Bi Y and Meng X (2022) Predictive role of the neutrophil: lymphocyte ratio in acute kidney injury associated with off-pump coronary artery bypass grafting. Front. Surg. 9:1047050. doi: 10.3389/fsurg.2022.1047050
Received: 17 September 2022; Accepted: 18 October 2022;
Published: 8 November 2022.
Edited by:
Shahzad Raja, Harefield Hospital, United KingdomReviewed by:
Birkan Bozkurt, Başakşehir Çam & Sakura City Hospital Organ Transplant Center, TurkeyYang Yu, Capital Medical University, China
© 2022 Yu, Song, Bi and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangbin Meng henry2008meng@hotmail.com
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Ruiming Yu
Ruiming Yu Han Song
Han Song Yanwen Bi
Yanwen Bi Xiangbin Meng
Xiangbin Meng