Department of Physiology, Kühne Minerva Center for Studies of Visual Transduction, Faculty of Medicine, The Hebrew University, Jerusalem, Israel
Fly eyes have been a useful biological system in which fundamental principles of sensory signaling have been elucidated. The physiological optics of the fly compound eye, which was discovered in the Musca, Calliphora and Drosophila flies, has been widely exploited in pioneering genetic and developmental studies. The detailed photochemical cycle of bistable photopigments has been elucidated in Drosophila using the genetic approach. Studies of Drosophila phototransduction using the genetic approach have led to the discovery of novel proteins crucial to many biological processes. A notable example is the discovery of the inactivation no afterpotential D scaffold protein, which binds the light-activated channel, its activator the phospholipase C and it regulator protein kinase C. An additional protein discovered in the Drosophila eye is the light-activated channel transient receptor potential (TRP), the founding member of the diverse and widely spread TRP channel superfamily. The fly eye has thus played a major role in the molecular identification of processes and proteins with prime importance.
Vision of invertebrate species has been one of the first senses to be thoroughly studied, and many fundamental principles relevant to all senses have been first discovered in invertebrate eyes. A notable example is the discovery of lateral inhibition in the compound eye of the Limulus by the Nobel Prize Laurie, Haldan Keffer Hartline (Ratliff, 1990
). Surprisingly, invertebrate phototransduction, the process by which light quanta are translated into electrical signal is still not entirely understood in terms of its underlying molecular mechanism. The pioneering experiments, which exploited the size of giant photoreceptor cells in some invertebrate species like the Limulus (reviewed in Dorlochter and Stieve, 1997
), were followed by studies on Drosophila melanogaster, exploiting its great molecular genetics power (reviewed in Minke and Hardie, 2000
; Montell, 1989
; Pak, 1995
; Ranganathan et al., 1995
). In the present review, we focus on processes and molecules that have been discovered in invertebrate eyes in general and in the Drosophila eye in particular, which shed light on crucial functions of other cells and tissues. These landmark discoveries include: (i) Structural and optical properties of Diptera compound eyes. (ii) Bistable photopigments in which both the rhodopsin (R) and metarhodopsin (M) states of the photopigment are dark stable and photoconvertible. (iii) The photochemical cycle in which phosphorylated arrestin (ARR) and ARR translocation play a major role. (iv) Light-induced translocation of Gqα and the excess of Gqβ over Gqα, which prevents spontaneous activation of the Gq-protein in the dark. (v) The dual role of light-activated phospholipase Cβ (PLCβ) in vivo as a G-protein-mediated activator and negative regulator of phototransduction via its action as a GTPase activating protein (GAP). (vi) Unitary signaling events (e.g. single photon responses, quantum bumps). (vii) The light-activated channels, TRP and TRPL, the founding members of the TRP superfamily channel proteins. (viii) Light-induced translocation of the TRPL channel. (ix) The inactivation no afterpotential D (INAD) multimolecular signaling complex, which binds the TRP channel, its activator, the PLC and its regulator, eye-specific protein kinase C (ePKC).
General Anatomy
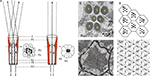
Two distinct types of eyes have evolved through evolution; the lens eye (or camera eye) typically encountered in vertebrates, and the compound eye typically encountered in arthropods. Many insects encompass both types of eyes. While, the compound eye is the primary image forming organ, the ocelli lens eye is small and primitive (Kirschfeld and Franceschini, 1968
, 1969
). The compound eyes are composed of many repeat and well-organized units termed ommatidia (Figure 1
B) embedded in a sphere (Figure 1
A). The number of ommatidia in insects vary from just a handful in the primitive Archaeognatha (jumping bristletails) and Thysanura (silverfish or bristletails) to several hundred up to thousands in Diptera (which includes the house fly Musca and the fruit fly Drosophila). In Drosophila, the ommatidium consists of about 20 cells, in which 8 (6–21 in different insect species) are the photoreceptor cells (RZ, Figure 1
A). Each ommatidium contains a dioptric apparatus composed of transparent chitinous cuticle, which forms the cornea (C, Figure 1
A) and an extracellular fluid-filled cavity, called the pseudocone (PC, Figure 1
A). The floor of the cavity is formed by four Semper cells (SZ, Figure 1
A) and the walls by primary pigment cells (PZ, Figure 1
A, red), which together circle the pseudocone, shielding the photoreceptor from stray light coming from adjacent ommatidia. The photoreceptor cells are highly polarized epithelial cells, with a specialized compartment known as the rhabdomere (Rh, Figure 1
A), consisting of a stack of ∼30,000–50,000 microvilli each ∼2 μm long and ∼60 nm in diameter. The transduction machinery is located in these tightly dense structures, while the nucleus and cellular organelles (N, Figure 1
C), such as submicrovillar cisternae (SMC, Figure 1
C) reside in the cell body. Pioneering studies conducted by Franceschini and Kirschfeld in Diptera (mainly in Musca) have elucidated the remarkable optics of the compound eye. In their studies, they showed that the highly ordered rhabdomeres form light guides (Kirschfeld and Snyder, 1976
) that have been widely exploited experimentally (Figure 3
). For example, the screening for retinal degeneration mutants of Drosophila has used the optical phenomenon designated deep pseudopupil (dpp), by Franceschini and Kirschfeld (1971)
, that is associated with their light guide property (see Figure 3
). The dpp, which disappears in retinal degeneration mutant flies such as in R defective mutants, has been used as an efficient tool for a fast screen of large populations of putative mutant flies. Other examples are spectral measurement of the compound eye such as the eye shine, resulting from tapetal reflection, transmittance spectra of photopigments and fluorescent measurements of M.
Figure 1. The morphology and optics of the compound eye. (A) The compound eye of Musca and the visual ganglionic layers: a schematic representation of a horizontal section. Inset – Schematic representation of the distal area of a single ommatidium. C – corneal lens, PC – pseudocone, RZ – retinula cells (photoreceptor), PZ – pigment cells, K – rhabdomere cap, SZ – Semper cells, Rh – rhabdomere, La – lamina, Me – medulla (modified from Kirschfeld, 1967
). (B,C) Electron microscopic (EM) cross-section of Drosophila ommatidia and a rhabdomere at the upper region of the photoreceptors respectively. M – microvilli, SMC – submicrovillar cisternae, N – nucleus (modified from Minke and Selinger, 1996
). (D) Optical properties of a single ommatidium demonstrated by “antidromic” illumination in Musca when a 30 μm diaphragm is placed over a single ommatidium seen when focused at the cornea (0 μm). Inverted images of the rhabdomere tips are seen when focusing above the cornea (1000 μm and 500 μm) and upright images below the cornea (−500 μm and −1000 μm). The optical path is shown on the right, F – focal plane, H – main plane, K – junction, a – outer, i – inner (modified from Kirschfeld and Franceschini, 1968
).
Open and Closed Rhabdom
Two kinds of rhabdomere architecture exist: closed rhabdom, in which all rhabdomeres are fused at the center of the ommatidium (Figures 2
A,C) and open rhabdom, in which the rhabdomeres are separated (Figures 2
A,B), forming a polygon pattern depending on the number of photoreceptors (hexagonal in Drosophila). Each ommatidium is connected by axons to the ganglionic layers providing a single or several image elements of space, depending on the rhabdomere architecture (Figure 1
A). In open rhabdomere, the repeated elements are arranged in a specific geometrical patterning and spacing, ensuring visual connectivity between adjacent ommatidia. Accordingly, the angles between the individual rhabdomeres in one ommatidium are identical to those between adjacent ommatidia. As a result, each of the seven rhabdomeres in one ommatidium portrays the same field of view as a rhabdomere in a neighboring ommatidium (Figures 2
D,E; Kirschfeld, 1967
). In addition, all six rhabdomeres that share a common field of view send their axons to the same place in the first ganglionic layer – the lamina (La, Figures 1
A and 2
E). The central rhabdomeres send their axons to the second ganglionic layer – the medulla (Me, Figure 1
A). In Drosophila, the seven rhabdomeres of each ommatidium are separated from each other and function as independent light guides (Figure 1
D) forming open rhabdomere architecture (Figures 2
A,B). In contrast, bees, beetles and various mosquitoes have a closed rhabdom architecture, in which rhabdomeres within each ommatidium are fused to each other, thus sharing the same visual axis (Figures 2
A,C). Recently, the power of Drosophila genetics was exploited to elucidate the molecular factors participating in the transition between open and closed rhabdom architecture by screening, isolating and characterizing Drosophila genes involved in this process. The study identified two genes, spacemaker (spam) and prominin (prom) which when mutated cause the collapse of the intra-rhabdomere space (IRS; Figure 2
B) resulting in the conversion of an open rhabdom system into a closed rhabdom architecture. Further analysis showed that SPAM is a secreted protein expressed in the IRS, which acts together with PROM, which is an evolutionary conserved transmembrane (TM) protein often associated with microvilli. Secretion of SPAM into the IRS forces the separation of the stalk membrane, pushing the rhabdomere apart, and the recruitment of SPAM to the microvilli surface by the binding to PROM prevents inter-rhabdomere adhesion. Furthermore, targeted expression of spam to photoreceptors of a closed system markedly reorganizes the architecture of the compound eyes to resemble an open system (Zelhof et al., 2006
).
Figure 2. Compound eyes with closed and open rhabdoms. (A) Schematic representation of an ommatidium with open rhabdom (left) and closed rhabdom architecture (right) (modified from Kirschfeld, 1971
). (B) EM cross-section of Drosophila ommatidium with open rhabdom architecture. (C) EM cross-section of Ephestia ommatidium in the region of distal tracheole ends with closed rhabdom architecture (from Fischer and Horstmann, 1971
). (D) Diagram of seven facets of the compound eye. The encircle rhabdomeres receive light from one and the same point in space (modified from Kirschfeld, 1967
). (E) Diagram of axonal connections between ommatidia. Axons of photoreceptor cells one to six receiving light from the same point in space are drawn converging on one and the same cartridge of the lamina (modified from Kirschfeld, 1967
).
The unusual stiffness of SPAM has been exploited in mechanoreceptors of Drosophila. Accordingly, a recent study has demonstrated the involvement of SPAM in maintaining cell shape and tone, crucial for integrity of the mechanosensory neurons. The authors argued that for poikilothermic organisms, like insects, changes in temperature may impact the function of mechanoreceptor neurons. SPAM role was found as protective of mechanosensory organ from massive cellular deformation caused by heat-induced osmotic imbalance, by forming an extracellular shield that guards mechanosensory neurons from environmental insult (Cook et al., 2008
).
Functional Retinal Organization
Drosophila ommatidia consist of eight photoreceptors that can be divided into two functional groups according to their position, functional involvement, spectral specificity and axonal projection. The R1–R6 cells (marked 1–6 in Figure 1
B) represent the major class of photoreceptors in the retina and are involved in image formation and motion detection. These cells have peripherally located rhabdomeres extending from the basal to the apical side of the retina. They express a single opsin called Rh1, which when combined with 11-cis 3-hydroxy retinal, forms a blue-absorbing R and orange-absorbing M. The R1–R6 cells (Figure 1
B) project their axons to the first optic lobe, the lamina (La, Figure 1
A green). The second group consists of two cells in the center of each ommatidium termed, R7 (marked 7 in Figure 1
B) and R8 (located below R7) each spanning only half of the retina in length. The central cells R7 and R8 are involved in color vision and detection of polarized light and project their axons to the second optic lobe, the medulla (Me, Figure 1
A, pink; Wernet et al., 2006
).
Color vision requires comparison between the electrical signals of photoreceptors that are sensitive to different ranges of wavelengths of light. In Drosophila, this is achieved by the inner photoreceptors (R7 and R8) that contain different Rs. The R7 rhabdomere is located distally in the retina and expresses one of two opsins, Rh3 or Rh4, characterized by a UV-absorbing R and blue-absorbing M. The R8 rhabdomere is located proximally in the retina, beneath the R7 rhabdomere (not shown) and expresses one of three opsins, Rh3, Rh5 or Rh6, characterized by a UV-, blue- or green-absorbing R, respectively. On the basis of opsin expression in the R7 and R8 cells, three ommatidia subtypes can be distinguished. The R7 and R8 cells in ommatidia, residing in the dorsal rim area of the eye, which functions as a polarized light detector, both express Rh3 opsin. The “pale” ommatidia subtype express Rh3 in R7 cells and Rh5 in R8 cells and constitute ∼30% of the total ommatidia, while the “yellow” ommatidia subtype express Rh4 in R7 cells and Rh6 in R8 cells and constitute ∼70% of the total ommatidia. Two types of comparisons, required for color vision, can thus occur in the fly: between the R7 (UV sensitive) and R8 (blue or green sensitive) photoreceptor cells within one ommatidium or between different ommatidia that contain spectrally distinct inner photoreceptors (Wernet et al., 2006
).
The intriguing repeated structure of fly compound eye has been a major scientific preparation for research of various aspects of cell differentiation and development. For example, in Drosophila, the hexagonal chiral orientation of the six rhabdomeres in the ommatidia is identical at the upper hemisphere of the compound eye and is reverted by 180° at the equator (Figure 1
D).This phenomenon is generally referred to as the planar cell polarity (PCP) of a tissue, a unique polarization within the plane of epithelium. Genetic screens in Drosophila pioneered the discovery of core PCP factors, which subsequently were found to be evolutionarily conserved. In vertebrate, the PCP factors participate in several developmental processes such as convergence extension, neural tube closure, eyelid closure, hair bundle orientation in inner ear sensory cells, and hair follicle orientation in the skin (for review see Wang and Nathans, 2007
).
Pupil Mechanism
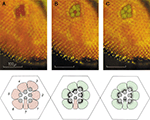
The pupil-like mechanism of the compound eye was first discovered and studied by Franceschini and Kirschfeld. Upon bright light illumination, tiny pigment granules about 0.2 μm in diameter migrate from dispersed areas of the cell body to the cytoplasmic face of the rhabdomere (Figure 3
; Kirschfeld and Franceschini, 1969
). The accumulation of pigment granules attenuates propagation of light along the rhabdomere by reducing the refractive index of the interface between the rhabdomere and the adjacent cell body region, thereby changing the waveguide property of the rhabdomere. As a consequence, the amount of light traveling through the rhabdomere is attenuated, activating less photopigment, much like a pupil. This mechanism can attenuate the light flux in the rhabdomere by up to one order of magnitude and operates in a time scale of seconds, making it an elegant adaptation mechanism. The pigment granule migration is Ca2+-dependent, as evidenced by injecting Ca2+ chelators into flies eye, resulting in the inhibition of pupil closure (Kirschfeld and Vogt, 1980
). The pupil mechanism was later found to occur transiently in the trp mutant fly (Lo and Pak, 1981
; Zuidervaart et al., 1979
) and was used by Minke as supporting evidence for his hypothesis that TRP is a major route for Ca2+ entry into the photoreceptor cell (see below, Minke and Selinger, 1991
). Recently, the molecular mechanism of pigment granules migration has been elaborated. It was shown that pigment migration is myosin V (MyoV), lightoid, calmodulin (CaM) and cytoplasmic myosin light chain dependent. A model of pigment migration has been put forward by Ready, in which MyoV pulls the pigment granules to the base of the rhabdomere upon Ca2+ elevation. Accordingly, lightoid, a Rab-related protein, links MyoV to pigment granules while both CaM and myosin light chain bind the long, multi-IQ domain of MyoV lever arms. Together, this Ca2+-dependent protein complex migrates to the plus ends (+) of the actin microfilaments, designated the rhabdomere terminal web, at the base of the rhabdomere upon Ca2+ influx induced by illumination (Satoh et al., 2008
).
Figure 3. Deep pseudopupil (dpp) observed in the eye of a living Drosophila under white orthodromic illumination. The dpp is the superposition of virtual images of adjacent ommatidia observed when a low power microscope is focused at the center of the curvature of the compound eye of Diptera. (A) Dark adapted. (B) After 60 s of medium intense illumination. Note that the central image corresponding to R7/R8 still appears red while the six peripheral images corresponding to R1–R6 reflect green light. (C) After 60 s of intense illumination (twofold higher). Note that all images reflect green light. The disparity between (B) and (C) arises from the difference in the absorption spectra between rhodopsin expressed in R1–R6 compared to R7 (upper panels). Schematic representation of the positioning of the pigment granules at each of the above states (lower panels) (modified from Franceschini and Kirschfeld, 1971
).
The signaling proteins of the phototransduction cascade are tightly assembled in the microvillar structure and linked to the actin cytoskeleton (F-actin) via two proteins: Dmoesin, which binds the TRP and TRPL channels, at the base of the microvilli to F-actin (Chorna-Ornan et al., 2005
), and no inactivation no afterpotential C (NINAC), that associates INAD to F-actin (Li et al., 1998
). The only protein that diffuses during the phototransduction cascade is Gqα (Figure 4
).
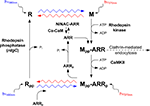
Figure 4. Schematic representation of the molecular components of the signal transduction cascade of Drosophila. Upon absorption of a photon, rhodopsin (R) is converted into metarhodopsin (M). This photoconversion leads to the activation of heterotrimeric G-protein (Gqα) by promoting the GDP to GTP exchange. In turn, this leads to activation of phospholipase Cβ (PLCβ), which hydrolyzes PIP2 into the soluble InsP3 and the membrane-bound DAG. Subsequently, two classes of light-sensitive channels, the TRP and TRPL open by a still unknown mechanism. PLC also promotes hydrolysis of the bound GTP, resulting in Gqα bound to GDP and this ensures the termination of Gqα activity. The TRP and TRPL channel openings lead to elevation of cellular Ca2+. Elevation of DAG and Ca2+ promote eye-specific protein kinas C activity, which regulates channel activity. PLC, PKC and the TRP ion channel form a supramolecular complex with the scaffolding protein INAD.
Upon absorption of a photon, R is converted into the active state of the photopigment, M (Figure 4
). This leads to the activation of heterotrimeric G-protein (DGq) by promoting the guanosine diphosphate (GDP) to guanosine triphosphate (GTP) exchange. In turn, this leads to activation of PLCβ, which hydrolyzes the minor phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2) into the soluble inositol 1,4,5-trisphosphate (InsP3) and the membrane-bound diacylglycerol (DAG). Subsequently, two classes of light-sensitive channels, TRP that is highly permeable to Ca2+ and TRPL that is a non-selective cation channel, open by a still unknown mechanism. PLC also promotes hydrolysis of the bound GTP, resulting in Gqα bound to GDP and this ensures the termination of Gqα activity. The TRP and TRPL channel openings lead to elevation of calcium ions extruded by the Na+/Ca2+ exchanger CALX. Elevation of DAG and Ca2+ promote ePKC activity, which regulates channel activity. PLC, ePKC and the TRP ion channel form a supramolecular complex with the scaffolding protein INAD (for reviews on the phototransduction cascade see Hardie and Raghu, 2001
; Minke and Cook, 2002
; Montell, 1989
).
Dim light stimulation induces discrete voltage (or current) fluctuations in most invertebrate species, which are called quantum bumps (Yeandle and Spiegler, 1973
; see Figure 5
A). Each bump is assumed to be evoked by the absorption of a single photon. The discrete nature of the unitary events of the photoreceptor cells is not due to the quantized nature of light. This has been demonstrated by the application of a non-quantized stimulus such as GTPγS, which elicit quantum bump-like events (Fein and Corson, 1981
). The bumps vary in latency, time course and amplitude for identical stimulation and are the consequence of synchronized activation of many light-sensitive channels. The number of channels, which are activated to produce a bump vary greatly in different species: few tens in Drosophila and up to several thousands in Limulus ventral photoreceptors (Nasi et al., 2000
). Bump generation is a stochastic process described by Poisson statistics where each effective absorbed photon elicits only one bump (Yeandle and Spiegler, 1973
). However in at least two Drosophila mutants (ninaC and arr, see Figure 5
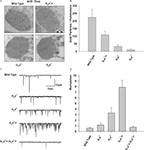
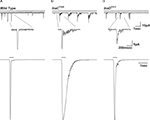
), absorption of a single photon elicits a train of bumps which do not overlap but are separated by intervals (Figures 5
B,C). This train of bumps is thought to be caused by a failed R inactivation process and a refractory period of the microvilli (Scott et al., 1997
).
Figure 5. Slow response termination in arr2 and ninaC null mutants. (A–C) Upper panels: Whole-cell voltage clamp recordings of quantum bumps in response to brief (1 ms) dim flashes of light with intensity sufficient to activate only a single rhodopsin molecule upon photon absorption in wild-type (WT), arr23 and ninaCP235 null Drosophila mutant flies. In WT, only a single bump is induced by a single flash and some flashes do not elicit any bump (middle trace). In contrary, a single flash in arr23 and ninaCP235 mutant flies elicits a train of bumps. (A–C) Lower panels: Whole-cell voltage clamp recordings of normalized macroscopic responses of WT and the corresponding mutants in response to 500-ms light pulses. A slow termination of macroscopic response is observed in arr23 and ninaCP235 mutant flies relative to WT.
A detailed study in Limulus photoreceptors has indicated that the latency of the bump is not correlated with the bump waveform, thus strongly suggesting that the triggering mechanism of the bump arises from different molecular processes than those determining the bump waveform (Dorlochter and Stieve, 1997
). These findings are partly explained by models in which the amplification process is preceded by a series of non-amplifying latency producing steps. To produce realistic bumps by such a model means that no step in the transduction cascade could have a life time greater than the duration of a bump generating mechanism which includes the latency, bump duration and bump refractory period. The single photon-single bump relationship requires that each step in the cascade must have not only an efficient “turn-on” mechanism, but also an equally effective “turn-off” mechanism (see below). The functional advantage of such a transduction mechanism is obvious; it produces a sensitive photon counter, very well suited for both the sensitivity and the temporal resolution required by the visual system.
A recent study has presented a quantitative model explaining how bumps emerge from stochastic non-linear dynamics of the signaling cascade. Three essential “modules” govern the production of bumps in this model: (i) an “activation module” downstream of PLC but upstream of the channels, (ii) a “bump-generation module” including channels and Ca2+-mediated positive feedback and (iii) a Ca2+-dependent “negative-feedback module”. The model shows that the cascade acts as an “integrate and fire” device conjectured formerly by Henderson et al. (2000)
much like the generation of spikes. The model explains both the reliability of bump formation and low background noise in the dark and is able to capture mutant bump behavior and explains the dependence on external calcium, which controls feedback regulation (Pumir et al., 2008
).
Bistable pigments
The G-protein-coupled receptor (GPCR), R, is composed of a 7-TM protein, opsin and the chromophore, 11-cis 3 hydroxy retinal (in Diptera; Vogt and Kirschfeld, 1984
). Isomerization of the chromophore by photon absorption induces conformational change in the opsin, which is photoconverted into the dark stable physiologically active photoproduct, M. The action spectrum of this reaction depends on the R type (see above) and spans a wavelength range between UV and green lights. To ensure high sensitivity, high temporal resolution and low dark noise of the photoresponse, the active M has to be quickly inactivated and recycled (Figure 6
). The latter requirement is achieved, in invertebrates, by two means: the absorption of an additional photon by the dark stable M, which photoconverts M back to R (Hillman et al., 1972
, 1983
), or by a multistep photochemical cycle (Figure 6
). The action spectrum of M to R conversion in the R1–R6 cells of Drosophila is in the orange range. The red screening pigment of the Drosophila eye prevents massive conversion of R to M, by formation of a red filter, which is preferential for M to R conversion. Genetic removal of the red screening pigment and application of blue light (which is preferentially absorbed by the R state) enables a large net photoconversion of R to its dark stable photoproduct M with a minimal conversion of M back to R (Figure 6
). A large net photoconversion of R to M, prevents phototransduction termination at the photopigment level when light is turned off (Minke et al., 1975a
). This is because the net photoconversion of R to M exceeds the amount of ARR (see below) and thereby its ability to inactivate M, resulting in a large amount of dark stable M, which does not undergo inactivation and thus remains physiologically active in the dark (Byk et al., 1993
; Dolph et al., 1993
). This brings the capacity of the phototransduction process to its upper limit and results in a phenomenon called prolonged depolarizing afterpotential (PDA; Hillman et al., 1972
, 1983
). Illumination with red light photoconverts M back to R and terminates the PDA after the light is turned off. The PDA protocol has been used efficiently to screen for phototransduction defective Drosophila mutants (Pak, 1995
) and has been widely exploited in studies of Drosophila phototransduction.
Figure 6. The photochemical cycle: the “turn-on” and “turn-off” of the photopigment. Upon photoconversion of rhodopsin (R) to metarhodopsin (M), by illuminating with blue light (wavy blue arrow), M is phosphorylated at multiple sites by rhodopsin kinase and the fly ARR2 binds to phosphorylated M. ARR2 is then phosphorylated by Ca2+ calmodulin-dependent kinase (CaMKII). Photoconversion of phosphorylated M (Mpp) back to phosphorylated R (Rpp) is achieved by illuminating with orange light (wavy red arrow). Upon photoregeneration of Mpp to Rpp, phosphorylated ARR2 is released and the phosphorylated rhodopsin (Rpp) is exposed to phosphatase activity by rhodopsin phosphatase (encoded by the rdgC gene). Unphosphorylated ARR2 also binds to myosin III (NINAC) in a Ca2+ calmodulin (Ca-CaM)-dependent manner (modified from Liu et al., 2008
; Selinger et al., 1993
).
The Role of Arrestin in Photoinactivation
The ARR family of proteins plays a key role in regulating the activity of GPCRs (Violin and Lefkowitz, 2007
). In Drosophila, two homologues of vertebrate ARR exist, which participate through binding, in M inactivation. Both ARRs undergo light-dependent phosphorylation by Ca2+ calmodulin-dependent kinase II (CaMKII) originally discovered by Matsumoto (Kahn and Matsumoto, 1997
). This phosphorylation is unique to the invertebrate visual ARRs and crucial for ARR dissociation from M (Alloway and Dolph, 1999
; Kiselev et al., 2000
; Yamada et al., 1990
).
The study, which clarified the regulatory role of ARR2, used in vitro assays of ARR2 and M, in Drosophila and Musca eyes. Upon photoconversion of R to M, by illumination with blue light (wavy blue arrow, Figure 6
), the fly ARR2 is found predominantly in the membrane fraction, while photoconversion of phosphorylated M (Mpp) back to phosphorylated R (Rpp), by illumination with orange light (wavy red arrow), result in the detection of ARR2 in the supernatant fraction (cytosol). ARR1 on the other hand, always remains membrane bound. The in vitro studies indicated that the functional role of ARR2 binding to M is to terminate its activity (Byk et al., 1993
). The isolation of Drosophila mutant fly arrestin2 (arr2), enabled demonstrating the physiological effect, in vivo, of ARR2 on the light response (Dolph et al., 1993
). Accordingly, these flies showed a slow response termination at the macroscopic level (Figure 5
B). Further investigations have shown that single photon absorption in these flies results in a train of quantum bumps while in wild-type flies it elicits a single bump (Figure 5
B).The train of bumps is a manifestation of the M’s incapability to inactivate, and explains the slow response termination seen at the macroscopic level (Scott et al., 1997
). Moreover, under the assumption that each bump is produced in a single microvillus, the train of bumps separated by intervals suggests a possible inactivation process of the microvilli (Hardie and Raghu, 2001
).
The binding of ARR2 also protects the Mpp from phosphatase activity (Figure 6
). Only upon photoregeneration of Mpp to Rpp, is ARR2 released and the Rpp is exposed to phosphatase activity by rhodopsin phosphatase, encoded by the rdgC gene (Steele et al., 1992
). These combined actions are crucial for preventing reinitiating of phototransduction in the dark, as the dissociation of ARR2 is coupled to conversion of Mpp to Rpp, thereby directing the protein phosphatase only towards the inactive Rpp (Byk et al., 1993
). Subsequent studies have revealed that both CaMKII-dependent phosphorylation of ARR2 at Ser366 and photoconversion of Mpp are required to release phosphorylated ARR2. They furthermore showed that the phosphorylation of ARR2 is required for its dissociation from Mpp upon photoconversion and that ARR2 phosphorylation prevents endocytotic internalization of the ARR2-Mpp complex by a clathrin-mediated mechanism (Alloway and Dolph, 1999
; Alloway et al., 2000
; Kiselev et al., 2000
).
Upon illumination, ARR2 translocates from the cell body to the rhabdomere, thereby elevating its concentration in the signaling compartment (Byk et al., 1993
). This process enables the ARR2-dependent inactivation of M, operating in massive photoconversion of R to M in bright daylight, thus preventing response saturation and ensures sufficient time resolution of the light response. A further study has shown that ARR2 translocation requires a phosphoinositide-mediated interaction with myosin III (NINAC; Lee and Montell, 2004
). Interestingly, the electrophysiological phenotype of the ninaC mutant is similar to that of arr2 mutant (Figures 5
B,C) and may be the consequence of reduced ARR2 concentration in the rhabdomere caused by the ninaC mutation. A recent study suggests that under low Ca2+ conditions, ARR2 binding to M is slowed down by its sequestration to NINAC. Accordingly, in physiological conditions, light-induced Ca2+ influx acting via CaM (Ca-CaM), rapidly releases ARR2 from NINAC and allows its binding to M and consequently, M inactivation (Liu et al., 2008
; Figure 6
).
It has been well established in photoreceptors of several invertebrate species that photoexcited R activates a heterotrimeric G-protein (Fein, 1986
). The first experiments, conducted on fly photoreceptors, showed that when pharmacological agents, known to activate G-proteins, are applied to Musca photoreceptors in the dark, they mimic the light-dependent activation of the photoreceptor cells (Minke and Stephenson, 1985
). Later studies using genetic screens isolated two genes encoding visual specific G-protein subunits. These genes, dgq (Lee et al., 1990
) and gβe (Dolph et al., 1994
), encode a Gqα and Gqβ subunit, respectively. The isolated eye-specific DGqα, shows ∼75% identity to mouse Gqα, which is known to activate PLC (Lee et al., 1990
). The most direct demonstration that DGqα participates in the phototransduction cascade came from studies of mutants defective in Gqα which showed highly reduced sensitivity to light. In the isolated Gαq1 mutant, DGqα protein levels are reduced to ∼1%, while Gqβ, PLC and R protein levels are virtually normal. The Gαq1 mutant exhibits a ∼1000-fold reduced sensitivity to light and slow response termination (Scott et al., 1995
), strongly suggesting that there is no parallel pathway mediated by the G-protein, as proposed for the Limulus eye (Dorlochter and Stieve, 1997
). Manipulations of the DGqα protein levels by the inducible heat-shock promoter made it possible to show a strong correlation between the sensitivity to light and DGqα protein levels, further establishing its major role in Drosophila phototransduction (Scott et al., 1995
).
The Drosophila fly has an eye-specific Gqβ (Gqβe) which shares 50% amino acid identity with other Gβ homologue proteins. Two defective Gqβe (Gβe1 and Gβe2) mutants with highly reduced Gqβ levels were isolated and showed a greatly (∼100-fold) decreased sensitivity to light and slow response termination (Dolph et al., 1994
). Studies conducted on these mutants revealed that Gqα is dependent on Gqβγ for both membrane attachment and targeting to the rhabdomere, suggesting that the decreased light sensitivity of these mutants may result from the mislocalization of the Gqα subunit (Elia et al., 2005
). Attachment of Gqα to Gqβγ prevents spontaneous GDP-GTP exchange and anchors Gqα to the plasma membrane. Therefore, in Gβe mutants Gqα concentration is highly reduced in the rhabdomere (Figures 7
A,B). Analysis of the stoichiometry between the Gqα and Gqβ subunits revealed a twofold excess of Gqβ over Gqα. Genetic elimination of the Gqβ excess leads to spontaneous activation of the visual cascade in the dark, demonstrating that Gqβ excess is essential for the suppression of dark electrical activity produced by spontaneous GDP-GTP exchange of Gqα. Reestablishing the excess of Gqβ over Gqα, by a double hetrozygote mutant fly, suppresses the dark electrical activity (Elia et al., 2005
; Figures 7
C bottom trace and D). These studies show a dual role for Gqβ: retention of Gqα in the signaling membrane and prevention of spontaneous activation of Gqα in the dark.
Figure 7. Excess of Gqβ over Gqα is required to prevent production of spontaneous bumps in the dark. (A) Immunogold EM analysis of a cross-section of a single rhabdomere, using a Gqα antibody that was applied to dark adapted wild-type flies and Gβ mutants (bar 500 nm). (B) Number of mean gold particles in cross-sections of 20 different single rhabdomeres. Error bars are SEM. (C) Whole-cell voltage clamp recordings of spontaneous bumps observed in complete darkness of various mutants as indicated. (D) Histogram plotting the mean bump frequency of the various mutants. Error bars are SEM. Note the high spontaneous bump frequency of Gβ hetrozygote compared to the reduced bump frequency of the Gqα/Gβ double hetrozygote mutant (modified from Elia et al., 2005
).
Heterotrimeric G-proteins relay signals between membrane-bound receptors and downstream effectors. Little is known, however, about the regulation of Gα subunit localization within the natural endogenous environment of a specialized signaling cell. Studies using Drosophila flies showed that prolonged lights cause massive and reversible translocation of Gqα to the cytosol (Kosloff et al., 2003
), in similar manner to light-induced translocation of the vertebrate Gtα transducin (Arshavsky, 2003
; Trojan et al., 2008
). A long exposure to light followed by minutes of darkness resulted in reduction in the efficiency with which each absorbed photon elicited single photon responses, while the size and shape of each single photon response did not change. To dissect the physiological significance of Gqα translocation by light, a series of Drosophila mutants were used. Genetic dissection showed a pivotal role for light-induced translocation of Gqα from the signaling membrane and the cytosol. Biochemical studies revealed that the sensitivity to light depends on the membrane Gqα concentration, which can be modulated either by light or by mutations that impair its membrane targeting. Thus, long-term adaptation is mediated by the movement of Gqα from the signaling membrane to the cytosol, thereby reducing the probability of each photon to elicit a bump (Frechter et al., 2007
).
PLC role in light excitation
Evidence for a light-dependent Gqα-mediated PLC activity in fly photoreceptors came from combined biochemical and electrophysiological experiments. These experiments, conducted in membrane preparations and intact Musca and Drosophila eyes, showed illumination and Gqα-dependent accumulation of InsP3 and InsP2, derived from PIP2 hydrolysis by PLC (Devary et al., 1987
; Figure 4
).
The key evidence for the participation of PLC in visual excitation of the fly was achieved by the isolation and analysis of Drosophila PLC gene, designated no receptor potential A (norpA). The norpA gene encodes a β-class PLC, predominately expressed in the rhabdomeres. Mutant flies in the norpA gene show a drastically reduced receptor potential. Transgenic Drosophila, carrying the norpA gene on a null norpA background, rescued the transformant flies from all the physiological, biochemical and morphological defects, which are associated with the norpA mutants (Bloomquist et al., 1988
). The norpA mutant thus provides essential evidence for the critical role of inositol-lipid signaling in phototransduction, by showing that no excitation takes place in the absence of functional PLC (Bloomquist et al., 1988
; Minke and Selinger, 1992
). However, the events required for light excitation downstream of PLC activation remain unresolved.
PLC Role in Response Termination
In general, the cytoplasmic GTP concentration in cells is much higher than GDP, making the inactivation process of Gα by hydrolysis of Gα-GTP to Gα-GDP unfavorable. In order to accelerate the GTPase reaction and terminate Gα activity, a specific GAP exists (Mukhopadhyay and Ross, 1999
). In vitro studies of mammalian PLC-β1 reconstituted into phospholipid vesicles with recombinant M1 muscarinic receptor and Gq/11 (Berstein et al., 1992
) have shown that, upon receptor stimulation, the addition of PLC-β1 increases the rate at which Gq hydrolyses GTP by three orders of magnitude, suggesting its action as GAP. A reduction in the levels of PLC in mutant flies affects the amplitude and activation kinetics of the light response (Pearn et al., 1996
), but also mysteriously slows response termination (compare Figure 8
A to Figure 8
B, lower panels). Biochemical and physiological studies conducted in Drosophila have revealed the requirement for PLC in the induction of GAP activity in vivo. Using several Drosophila norpA mutant flies, a high correlation between PLC protein level, GAP activity and response termination was observed (Cook et al., 2000
). The virtually complete dependence of GAP activity on PLC provides an efficient mechanism for ensuring the one photon, one bump relationship (Yeandle and Spiegler, 1973
), which is critical for the fidelity of phototransduction in dim light. The apparent inability to hydrolyze GTP without PLC ensures that every activated G-protein eventually encounters a PLC molecule and thereby produces a response by the downstream mechanisms. The instantaneous inactivation of the G-protein by its target, the PLC, guarantees that every G-protein produces no more than one bump (Cook et al., 2000
). This apparently complete dependence of GTPase activity on its activator PLC, in flies, differs from the partial dependence of GTPase activity on additional GAP factors in vertebrate phototransduction (Chen et al., 2000
). Vertebrate phototransduction depends on specific GAPs (Arshavsky and Pugh-EN, 1998
; Makino et al., 1999
). Accordingly, genetic elimination of regulators of G-protein signaling (RGS) proteins reduces and slows down GAP activity and leads to slow response termination to light (Chen et al., 2000
).
Figure 8. Slow response termination composed of bumps characterizes norpA mutants. (A,B) Upper panels: Whole-cell voltage clamp recordings of quantum bumps in response to continues dim light in wild-type and the weak allele of norpA, norpAP57 mutant flies. (A,B) Lower panels: Whole-cell voltage clamp recordings of normalized macroscopic responses of wild-type and the corresponding mutants in response to 200-ms light pulses. In contrast to the fast response termination of wild-type, slow termination of the light response of norpAP57 mutant flies is revealed. This slow response termination can be resolved into continuous production of bumps in the dark at a later time (inset, at higher magnification). (C) Electroretinogram (ERG) responses showing superimposed traces recorded from wild-type and norpAP76 (a weak norpA allele) to a brief flash (red arrow) and continuous light. The graph plots the relative steady state amplitude of the ERG to prolonged lights as a function of relative light intensity. The ERG responses of norpAP76 to a brief flash and to continuous light are indistinguishable.
The dual action of PLC as an activator and a negative regulator nicely accounts for all features of the PLC-deficient mutants. A striking demonstration of the poor temporal resolution of mutants with reduced PLC levels relative to wild-type flies is shown in Figure 8
C, which compares the ability of wild type and the PLC-deficient mutant norpAP76 to discriminate between intense lights of different durations (flash, red arrow, pulse, blue line). In contrast to the wild-type fly, where there is a pronounced difference between the responses to a flash compared with a long stimulus, no such difference is observed in the norpA mutants, where the two responses overlap (Figure 8
C). This result indicates that the PLC-deficient mutants cannot discriminate between long and short light stimuli. When PLC levels in the signaling membranes are low relative to the amount of the active G-protein, light induces production of Gqα-GTP at a higher rate than it is inactivated by PLC. The Gqα-GTP that has accumulated during illumination continues to produce bumps in the dark until all active Gqα-GTP molecules are hydrolyzed via GAP activity of the scarce PLC (Figure 8
B, lower panel, inset). Hence, the flies’ temporal resolution is reduced and become virtually blind at low levels of PLC (Cook et al., 2000
).
The Phosphoinositide (PI) Cycle
In the phototransduction cascade of Drosophila, light triggers the activation of PLCβ. This catalyzes hydrolysis of the membrane phospholipid PIP2 into water soluble InsP3 and membrane-bound DAG (Berridge, 1993
). The continuous functionality of the photoreceptors during illumination is maintained by rapid regeneration of PIP2 in a cyclic enzymatic pathway (the PI pathway, Figure 9
). Moreover, the PI pathway has emerged to be most important for activation of the TRP and TRPL channels (Hardie, 2003
; Raghu and Hardie, 2009
).
Figure 9. The phosphoinositide cycle. In the phototransduction cascade, light triggers the activation of phospholipase Cβ (PLCβ). This catalyzes hydrolysis of the membrane phospholipid PIP2 into InsP3 and DAG. DAG is transported by endocytosis to the endoplasmic reticulum and inactivated by phosphorylation converting it into phosphatidic acid (PA) via DAG kinase (DGK) and to CDP-DAG via CDP-DAG syntase. Subsequently, CDP-DAG is converted into phosphatidylinositol (PI), which is transferred back to the microvillar membrane, by the PI transfer protein. PIP and PIP2 are produced at the microvillar membrane by PI kinase and PIP kinase, respectively. There are probably two PIP kinases (PIPK I, PIPK II, which are unified in the scheme). PA can also be converted back to DAG by lipid phosphate phosphohydrolase. PA is also produced from phosphatidylcholine (PC) by phospholipase D (PLD). DAG is also hydrolyzes by DAG lipase into poly unsaturated fatty acids (PUFA).
The phospholipid branch of the PI cycle, following PLC activation, begins by DAG transport through endocytosis to the endoplasmic reticulum (SMC) and subsequently, inactivation by phosphorylation and conversion into phosphatidic acid (PA), via DAG kinase (DGK), encoded by the retinal degeneration A (rdgA) gene (Masai et al., 1993
, 1997
). Then, CDP-DAG syntase encoded by the cds gene (Wu et al., 1995
) produces DAG-CDP from PA. Both RDGA and CDS are located in the SMC (Figure 1
C). Subsequently, DAG-CDP is converted into phosphatidylinositol (PI), which is transferred back to the microvillar membrane, by the PI transfer protein (PITP), encoded by the rdgB gene (Vihtelic et al., 1991
) located in the SMC. PIP and PIP2 are produced at the microvillar membrane by PI kinase and PIP kinase, respectively. PA can be reconverted back to DAG by lipid phosphate phosphohydrolase, LPP, also designated phosphatidic acid phosphatase, PAP, encoded by the laza gene (Garcia-Murillas et al., 2006
; Kwon and Montell, 2006
) or produced from phosphatidylcholine (PC) by phospholipase D, PLD, encoded by the Pld gene (LaLonde et al., 2005
). DAG is also hydrolyzed by DAG lipase encoded by the inaE gene (Leung et al., 2008
) predominantly localized outside the rhabdomeres, into polyunsaturated fatty acid (PUFA, Figure 9
).
Mutations in most proteins of the PI pathway result in retinal degeneration. For example, rdgA mutant flies show light-independent retinal degeneration, thought to occur due to a sustained Ca2+ influx through the light-activated TRP and TRPL channels, making the PI pathway crucial for understanding phototransduction and TRP channels activation. Although it is possible to partially rescue the degeneration phenotypes by reducing the level of TRP (Raghu et al., 2000b
), it is still unclear whether this mutation promotes channel opening directly or through an indirect change in the photoreceptor, leading to channel opening.
The trp Mutant and the Discovery of the TRP Channel
A spontaneously occurring Drosophila mutant, showing a decline in the receptor potential to baseline during prolonged illumination (Cosens and Manning, 1969
), was designated transient receptor potential (trp) by Minke et al. (1975b)
(Figure 10
B, right). Minke and Selinger suggested in a review article, that the trp gene encodes a Ca2+ channel/transporter, mainly because application of the Ca2+ channel blocker La3+ to wild-type photoreceptors mimicked the trp phenotype (Minke and Selinger, 1991
). The cloning of the trp locus by Montell and Rubin (1989)
revealed a novel membrane protein. The available sequence of the trp gene led, several years later, to the discovery of mammalian TRPs and the TRP superfamily (Wes et al., 1995
; Zhu et al., 1995
). However, the significance of the trp sequence, as a gene encoding a putative channel protein, was only first appreciated after a trp homolog, the trp-like (trpl) gene was cloned. This was done by a screen for calmodulin-binding proteins which identified a TM protein. A comparison of its TM domain to that of voltage gated Ca2+ channels and the TRP protein led to the conclusion that this protein is a putative channel protein with high identity to TRP (Phillips et al., 1992
). The first direct physiological evidence for the notion that TRP is the major light-activated channel came from a comparative patch clamp study of isolated ommatidia of wild type and the trp mutant (Hardie and Minke, 1992
). The use of Ca2+ indicator dyes and Ca2+-selective microelectrodes, directly demonstrated that the TRP channel is the major route for Ca2+ entry into the photoreceptor cell (Peretz et al., 1994a
,b
). The final evidence showing that TRP and TRPL are the light-activated channels came from the isolation of a null mutant of the trpl gene and the construction of the double mutant, trpl;trp, which is blind (Niemeyer et al., 1996
). A third TRP homolog channel designated TRPγ has been cloned and sequenced (Xu et al., 2000
). Heterologous expression in HEK293 cells has revealed a functional channel (Jors et al., 2006
; Xu et al., 2000
). However, in Drosophila photoreceptors this channel cannot generate any light-activated conductance in isolation as revealed in the trpl;trp double null mutant and therefore its role in phototransduction, if any, is not clear.
Figure 10. The electrophysiological properties of WT, trp and trpl mutants. (A) Whole-cell voltage clamp recordings of quantum bumps in response to continuous dim light in wild-type, trpl302 and trpP343 null mutant flies. Highly reduced amplitude of trpP343 bumps is observed. (B) Whole-cell voltage clamp recordings in response to a 3-s light pulse of WT and the corresponding mutants. The transient response of the trpP343 mutant is observed. (C) A family of light-induced currents to 20-ms light pulse at voltage steps of 3 mV measured around Erev. (D) Histogram plotting the mean Erev of WT and the various mutants, error bars are SEM. Erev of wild-type is between the positive Erev of trpl302, which expresses only TRP and the Erev of trpP343 mutant, which expresses only TRPL.
Biophysical Properties of the TRP and TRPL Channels
The Drosophila light-sensitive channels, TRP and TRPL, can be studied separately by utilizing the trpl302 and trpP343 null mutants, respectively (Scott et al., 1997
; Figure 10
). The channels are permeable to a variety of monovalent and divalent ions including Na+, K+, Ca2+ and Mg2+ and even to large organic cations such as TRIS and TEA (Ranganathan et al., 1991
). The reversal potential of the light-induced current (LIC) shows a marked dependence on extracellular Ca2+ indicating a high permeability for this ion. Permeability ratio measurement for a variety of divalent and monovalent ions, determined under bi-ionic conditions, confirmed a high Ca2+ permeability of ∼57:1 = Ca2+:Cs+ in the trpl mutant and ∼4.3:1 = Ca2+:Cs+ for the trp mutant (Reuss et al., 1997
). The large Ca2+ permeability of TRP is reflected in its positive reversal potential (Erev; Figures 10
C,D).
The TRP and TRPL channels show voltage-dependent conductance during illumination. An early study revealed that the light response can be blocked by physiological concentrations of Mg2+ ions (Hardie and Mojet, 1995
). The block mainly influenced the TRP channel and affected its voltage dependence. Later, detailed analyses described the voltage dependence of heterologously expressed TRPL channels in S2 cells and of the native TRPL channels, using the Drosophila trp null mutant. These studies indicated that the voltage dependence of the TRPL channel is not an intrinsic property, as is thought for some other members of the TRP family, but arises from divalent cations open channel block that can be removed by depolarization. The open channel block by divalent cations is thought to play a role in improving the signal to noise ratio of the response to intense light and may function in light adaptation and response termination (Parnas et al., 2007
).
A comparison with voltage-gated K+ channels and cyclic nucleotide gated (CNG) channels, postulates that both TRP and TRPL are assembled as tetrameric channels, thus raising the question whether they assemble as homomultimers or as heteromultimers. Since null trp and trpl mutants both respond to light, each can clearly function without the other. However, heterologous co-expression studies and co-immunoprecipitation, led to the suggestion that the TRP and TRPL channels can assemble into heteromultimers (Xu et al., 1997
). Detailed measurements of biophysical properties, questioned this conclusion since they found that the wild-type conductance could be quantitatively accounted for by the sum of the conductances determined in the trp and trpl mutants (Reuss et al., 1997
). In addition, a study demonstrated that the TRPL, but not the TRP channel reversibly translocates from the rhabdomere to the cell body upon illumination (Bahner et al., 2002
) further imply that TRPL assemble as homomers.
Light-Regulated Subcellular Translocation of Drosophila TRPL Channels
In neurons the expression pattern of ion channels determines the physiological properties of the cell. Besides regulation at the level of gene expression that determines which channels are present in a given neuron, trafficking of ion channels into and out of the plasma membrane is an important mechanism for manipulating the number of channels at a specific cellular site (for reviews see Lai and Jan, 2006
; Sheng and Lee, 2001
). In Drosophila photoreceptors activation of the phototransduction cascade and the influx of Ca2+ through the TRP channels initiate the translocation of the TRPL but not the TRP channels from the signaling compartment, the rhabdomere, to the cell body (Bahner et al., 2002
; Meyer et al., 2006
). The TRPL translocation process occurs in two stages, a fast translocation (5 min) to the neighboring stalk membrane and a slow translocation (over 6 h) to the basolateral membrane (Cronin et al., 2006
). Thus, the TRPL translocation timescale conforms to day night cycle and act in light adaptation (Bahner et al., 2002
). While, Ca2+ influx has been shown to be necessary for TRPL translocation the molecular mechanism and structural determinants of the TRPL involved in translocation, are still unknown. Signal dependent translocation of mammalian TRP channels was found to be a widespread phenomenon (Bezzerides et al., 2004
; Kanzaki et al., 1999
; Zhang et al., 2005
). Nevertheless, many of these researches are conducted on TRP channels expressed in tissue culture cells. This makes the Drosophila photoreceptors a unique system in which TRPL channels translocation can be studied in vivo.
Activation Mechanisms of TRP and TRPL Channels
It has been well established that hydrolysis of PIP2 by PLC, encoded by the norpA gene, activates the light-sensitive channels TRP and TRPL in Drosophila photoreceptors. However, the mechanism by which PLC activity results in channels opening is still under debate. Several hypotheses have been presented through the years. (i) The InsP3 hypothesis, suggested that the elevation of InsP3, following PIP2 hydrolysis, activates the InsP3R (InsP3 receptor) resulting in Ca2+ store depletion and activation of the channels in a store-operated mechanism (Hardie and Minke, 1993
). This mechanism of activation has also been suggested for a number of mammalian TRPC channels (Putney, 2007
; Yuan et al., 2007
). In addition, direct activation of the channels as in the Limulus ventral photoreceptors (Payne et al., 1986
) using caged Ca2+ or InsP3 to elevate Ca2+ did not activate the channels (Hardie, 1995
; Hardie and Raghu, 1998
). Rather, direct application of Ca2+ in excised inside-out patches inhibits expressed TRPL channels in S2 cells by an open channel block mechanism (Parnas et al., 2007
), suggesting an inhibition rather than activation effect of Ca2+. Furthermore, genetic elimination of the only InsP3R in Drosophila had no effect on the light response (Acharya et al., 1997
; Raghu et al., 2000a
). Therefore, the InsP3 hypothesis was abundant. It therefore became evident that the alternative branch of PLC, DAG production should be investigated. The most familiar action of DAG is to activate the classical protein kinase C (PKC) synergistically with Ca2+. However, mutations in the ePKC, encoded by the inaC gene lead to defects in response termination with no apparent effects on activation (Hardie et al., 1993
; compare Figure 11
A to Figure 11
B). (ii) The PUFA or DAG hypothesis argues that the elevation of DAG and consequently of PUFA acting as second messengers results in channel opening. This hypothesis emerged from a detailed pharmacological study which tested the effect of various fatty acids (including PUFAs) on TRP and TRPL channels activation in vivo and TRPL expressed in Drosophila S2 cells (Chyb et al., 1999
). In addition, a detailed analysis of the rdgA mutant encoding DAG kinase has established the importance of the DAG branch in channel activation. This mutant shows light-independent retinal degeneration and constitutive activity of the light-activated channels, while a partial rescue of the degeneration is achieved by eliminating the TRP channel in the double mutant rdgA;trpP343 (Raghu et al., 2000b
). Furthermore, it has been shown that the double mutant norpAP24, rdgA partially rescues the light response in the almost null norpAP24 mutant. This finding further supports the hypothesis that DAG or its surrogate PUFA are involved in channel activation (Hardie et al., 2003
). Several lines of evidence challenge this hypothesis: first, application of DAG to intact ommatidia does not activate the channels (unpublished data), while application of DAG analogs 1-oleoyl-2-acetyl-sn-glycerol (OAG) at low concentration (2 μM) in inside-out patches excised from the microvilli of dissociated ommatidia result in activation of the TRP and TRPL channels in kinetics slower by three orders of magnitude (∼60 s after application) compared to the light stimuli (Delgado and Bacigalupo, 2009
). Second, the localization of RDGA in the SMC, a relatively distant cellular compartment from the transduction machinery (Masai et al., 1997
) makes it unlikely that DAG could act as a second messenger without considerably slowing response termination kinetics, which does not fit to the fast termination of the response to light. Further establishment of this hypothesis requires identification of a functional-binding domain for DAG or PUFA on the TRP and TRPL channel and further elucidating the complex enzymatic machinery of PUFA production by DAG lipases. Recently, the inaE gene was identified as encoding a homologue of mammalian sn-1 type DAG lipase and was shown to be expressed predominantly in the cell body of Drosophila photoreceptors (Figure 9
). Mutant flies, expressing low levels of the inaE gene product, have an abnormal light response, while the activation of the light-sensitive channels was not prevented (Leung et al., 2008
). The discovery of the inaE gene is a first step in an endeavor to elucidate lipids regulation of the channels (see review, Raghu and Hardie, 2009
). Thus, the participation of DAG or PUFAs in TRP and TRPL activation in vivo needs further exploration. (iii) The PIP2 depletion and DAG accumulation hypothesis argues that PIP2 acts as a negative modulator, while DAG or its surrogates acts as positive modulators of the TRPL channel. Schilling and colleagues demonstrated in Sf9 cells expressing TRPL that application of DAG or PUFA activates the channels, while application of PIP2 in inside-out patches inhibit their activity (Estacion et al., 2001
). However, Hardie et al. (2001)
showed that the trpP343 mutant phenotype (in which the light response decays to baseline) is a result of PIP2 depletion which is not compatible with a PIP2 inhibitory action. In addition, a PIP2-binding domain has not been functionally identified in the TRP or TRPL channels. Together, the above arguments put the PIP2 depletion and DAG accumulation hypothesis in question. (iv) A recently new hypothesis was formulated, suggesting that plasma membrane lipid–channel interaction controls channel gating. Accordingly, disruption of this interaction by membrane lipid modification through PLC activation causes the opening of the channels (Parnas et al., 2009
). It is important to realize that PLC activation, which converts PIP2, a charged molecule, containing a large hydrophilic head-group, into DAG, devoid of the hydrophilic head-group, is known to cause major changes in lipid packing and lipid–channel interactions (Janmey and Kinnunen, 2006
). It is therefore possible that neither PIP2 hydrolysis nor DAG production affect the TRP and TRPL channel as second messengers, but rather act as modifiers of membrane lipid–channel interactions. This may in turn act as a possible mechanism of channel activation (Parnas et al., 2009
). This hypothesis evades the two main problems of the DAG or PUFA hypothesis: the need of RDGA at closed proximity to the channels and a channel-binding domain for DAG and/or PUFA. This hypothesis suffers from insufficient direct demonstration both in cell expression systems and in vivo.
Figure 11. The inaCP209 and inaDP215 mutants reveal slow response termination of the macroscopic response to light and of the single bumps. (A–C) Upper panels: Whole-cell voltage clamp quantum bump responses to continues dim light in wild-type, inaCP209 and inaDP215 mutant flies. A slow termination of the bumps is observed in inaCP209 and inaDP215 mutant flies. (A–C) Lower panels: Whole-cell voltage clamp recordings of normalized responses to a 500-ms light pulse of the above mutants. A slow termination of macroscopic response is observed in inaCP209 null mutant and in the inaDP215 mutant in which the binding of INAD to TRP is disrupted (Chevesich et al., 1997
; Shieh and Zhu, 1996
).
An important step towards understanding Drosophila phototransduction has been achieved by the finding that some of the key elements of the phototransduction cascade are incorporated into supramolecular signaling complexes via a scaffold protein, INAD (Figure 4
). The INAD protein was discovered using a PDA screen which isolated a defective Drosophila mutant (inaD). The first discovered inaD mutant, the inaDP215, was isolated by Pak (1995) and was subsequently cloned and sequenced by Shieh and Niemeyer (1995)
. Later studies in Calliphora have shown that INAD binds not only TRP but also PLC (NORPA) and ePKC (INAC) (Huber et al., 1996
). The interaction of INAD with TRP, NORPA and INAC was later confirmed in Drosophila (Tsunoda et al., 1997
). It was further found that inaD is a scaffold protein, which consists of five ∼90 amino acid (aa) protein interaction motifs called PDZ (PSD95, DLG, ZO1) domains. These domains are recognized as protein modules which bind to a diversity of signaling, cell adhesion and cytoskeletal proteins (Dimitratos et al., 1999
; Schillace and Scott, 1999
) by specific binding to target sequences typically, though not always, in the final three residues of the C-terminal. The PDZ domains of INAD bind to the signaling molecules as follows: PDZ1 and PDZ5 bind PLC (Shieh et al., 1997
; van Huizen et al., 1998
), PDZ2 or PDZ4 bind ePKC (Adamski et al., 1998
) and PDZ3 binds TRP (Chevesich et al., 1997
; Shieh and Zhu, 1996
). This binding pattern is still under debate due to several contradictory reports. Contrary to TRP, TRPL appears not to be a member of the complex, since unlike INAC, NORPA and TRP it remains strictly localized to the microvilli in the inaD1 null mutant (Tsunoda et al., 1997
). Several studies have suggested that, in addition to PLC, PKC and TRP, other signaling molecules such as CaM, R, TRPL and NINAC bind to the INAD signaling complex. Such binding, however, must be dynamic. Biochemical studies conducted in Calliphora have revealed that both INAD and TRP are targets for phosphorylation by the nearby ePKC (Huber et al., 1998
). Accordingly, the association of TRP into transduction complexes may be related to increasing speed and efficiency of transduction events as reflected by the immediate vicinity of TRP to its upstream activator, PLC, and its possible regulator, ePKC (Huber et al., 1998
). Indeed, genetic elimination of INAC affected the shape of the quantum bump of the inaC null mutant, by inducing slow termination of the bump, composed of dumped oscillating current noise of an unclear underlying mechanism (Hardie et al., 1993
; Henderson et al., 2000
; Figure 11
B). Interestingly, a similar phenotype was observed in the inaDP215 mutant, whereby the INAD complex and TRP channel are dissociated (Henderson et al., 2000
; Figure 11
C), also with a still unclear underlying mechanism.
TRP plays a major role in localizing the entire INAD multimolecular complex. Association between TRP and INAD is essential for correct localization of the complex in the rhabdomeres, as found in other signaling systems (Arnold and Clapham, 1999
). This conclusion was derived from the use of Drosophila mutants in which the signaling proteins, which constitute the INAD complex, were removed genetically, and also by deletions of the specific binding domains, which bind TRP to INAD. These experiments showed that INAD is correctly localized to the rhabdomeres in inaC mutants (where ePKC is missing) and in norpA mutants (where PLC is missing), but severely mislocalized in null trp mutants (Li and Montell, 2000
; Tsunoda et al., 2001
), thus indicating that TRP but not PLC or PKC is essential for localization of the signaling complex to the rhabdomere. To demonstrate that a specific interaction of INAD with TRP is required for rhabdomeric localization of the complex, the binding site at the C-terminal of TRP was removed or three conserved residues in PDZ3, which are expected to disrupt the interaction between PDZ domains and their targets were modified. As predicted, both TRP and INAD were mislocalized in these mutants. The study of the above mutants was also used to show that TRP and INAD do not depend on each other for targeting to the rhabdomeres. Thus, INAD–TRP interaction is not required for targeting but for anchoring of the signaling complex (Li and Montell, 2000
; Tsunoda et al., 2001
). Additional experiments on TRP and INAD further showed that INAD has other functions in addition to anchoring the signaling complex. One important function is to preassemble the proteins of the signaling complex. Another important function, at least in the case of PLC, is to prevent degradation of the unbound signaling protein.
A recent study by Ranganathan and colleagues has suggested that the binding of signaling proteins to INAD may be a dynamic process that allows an additional level of phototransduction regulation. Their study showed two crystal structural states of isolated INAD PDZ5 domain, differing mainly by the formation of a disulfide bond. This conformational change has light-dependent dynamics that was demonstrated by the use of transgenic Drosophila flies expressing INAD with a point mutation disrupting the formation of the disulfide bond. They proposed a model in which, ePKC phosphorylation at a still unknown site promotes the light-dependent conformational change of PDZ5, distorting its ligand-binding groove to PLC and thus regulating phototransduction (Mishra et al., 2007
).
The study of fly photoreceptors has opened new avenues in biological research, mainly through the exploitation of the power of Drosophila molecular genetics. Processes and proteins that were discovered in Drosophila have been found to be highly conserved through evolution and thus paved the way for the discovery of important proteins and mechanisms in development and cell signaling in mammals. A striking example is the discovery of the TRP channel protein in the Drosophila photoreceptors, which led to the discovery of the widespread TRP superfamily, which plays crucial roles in sensory signaling of insects and mammals. The activation and regulation of Drosophila TRPs by the inositol-lipid signaling pathway and the major role of PLC in the activation of these channels has wide implications for understanding the activation and regulations of mammalian TRPs. Even today Drosophila photoreceptors are one of the few systems in which TRP channels are studied in vivo. Another novel molecule that was discovered in Drosophila photoreceptors is the INAD scaffold protein which forms a supramolecular signaling complex. This protein has introduced new concepts in cell signaling dynamics which are still under investigations. An additional advantage of using the fly for research on cellular signaling is that frequently the fly system is less evolutionary evolved relative to mammals, making it simpler to study, while maintaining its core function. It is therefore anticipated that research using the Drosophila sensory and motor systems will continue to identify new proteins and mechanisms of high biological importance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Shaya Lev, Moshe Parnas and Daniela Dadon for critical reading of the manuscript. The research part of this review was supported by grants from the National Institute of Health (EY 03529), the Israel Science Foundation (ISF), the US-Israel Binational Science Foundation (BSF), the Moscona Foundation and the Minerva Foundation.
Huber, A., Sander, P., Gobert, A., Bahner, M., Hermann, R., and Paulsen, R. (1996). The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 15, 7036–7045.