1
Section on Behavioral Science and Genetics, Laboratory for Integrative Neuroscience, National Institute on Alcoholism and Alcohol Abuse, NIH, Rockville, MD, USA
2
Department of Experimental Psychology, University of Cambridge, Cambridge, UK
3
Medical Research Council and Wellcome Trust Behavioral and Clinical Neuroscience Institute, Cambridge, UK
Subchronic treatment with the psychotomimetic phencyclidine (PCP) has been proposed as a rodent model of the negative and cognitive/executive symptoms of schizophrenia. There has, however, been a paucity of studies on this model in mice, despite the growing use of the mouse as a subject in genetic and molecular studies of schizophrenia. In the present study, we evaluated the effects of subchronic PCP treatment (5àmg/kg twice dailyà×à7àdays, followed by 7àdays withdrawal) in C57BL/6J mice on (1) social behaviors using a sociability/social novelty-preference paradigm, and (2) pairwise visual discrimination and reversal learning using a touchscreen-based operant system. Results showed that mice subchronically treated with PCP made more visits to (but did not spend more time with) a social stimulus relative to an inanimate one, and made more visits and spent more time investigating a novel social stimulus over a familiar one. Subchronic PCP treatment did not significantly affect behavior in either the discrimination or reversal learning tasks. These data encourage further analysis of the potential utility of mouse subchronic PCP treatment for modeling the social withdrawal component of schizophrenia. They also indicate that the treatment regimen employed was insufficient to impair our measures of discrimination and reversal learning in the C57BL/6J strain. Further work will be needed to identify alternative methods (e.g., repeated cycles of subchronic PCP treatment, use of different mouse strains) that reliably produce discrimination and/or reversal impairment, as well as other cognitive/executive measures that are sensitive to chronic PCP treatment in mice.
Schizophrenia is a heterogeneous disease characterized by positive, negative and cognitive/executive symptoms (DSM-IV, 1994
). Positive symptoms (so-called because they add to the normal behavioral repertoire) include hallucinations, psychomotor agitation and hypersensitivity to psychotomimetics. Amongst the negative symptoms (so-called because they subtract from the normal behavioral repertoire) are blunted affect, anhedonia and low motivation, and social withdrawal. The cognitive/executive symptom category encompasses a range of deficits including impaired discrimination learning and reversal learning (Murray et al., 2008
) and attentional set-shifting problem (Braff et al., 1991
).
Drugs with N-methyl-D-aspartate receptor (NMDAR) antagonist properties, such as ketamine and phencyclidine (PCP), mimic the symptoms of schizophrenia in healthy human subjects and cause relapse in schizophrenics (Adler et al., 1999
; Krystal et al., 1994
; Malhotra et al., 1997
; Newcomer et al., 1999
). In rodents, treatment with NMDAR antagonists also produce various behavioral abnormalities posited to be relevant to positive symptoms of schizophrenia (Arguello and Gogos, 2006
; Powell and Miyakawa, 2006
). For example, acute administration of the NMDAR antagonists PCP or MK-801 (dizocilpine) produces a profound locomotor hyperactivity that is reversible by antipsychotics. This drug response has been proposed as a measure relevant to positive symptoms in schizophrenia, and has been extensively studied as such (Arguello and Gogos, 2006
; Powell and Miyakawa, 2006
).
There is increasing recognition that the negative and cognitive/executive symptoms of schizophrenia are inadequately treated and underrepresented in rodent models of the disease (Carter et al., 2008
). In this context, repeated (‘subchronic’) treatment with PCP has been found to produce a range of persistent behavioral disturbances in non-human primates, rats and mice, that may model some of these symptoms (Jentsch and Roth, 1999
). For example, previous studies have demonstrated that following subchronic PCP treatment, rats display impairments in T-maze spatial working memory (Jentsch and Taylor, 2001
; Jentsch et al., 1997b
) (but see Stefani and Moghaddam, 2002
), T-maze spatial reversal learning (Jentsch and Taylor, 2001
), extra-dimensional set-shifting (EDS; Egerton et al., 2008
; Rodefer et al., 2005
, 2008
), and reversal learning in maze- and operant-based tasks (Abdul-Monim et al., 2006
, 2007
; Jentsch and Taylor, 2001
). Although there have been fewer reports on subchronic PCP effects in mice, one recent study observed deficits in reversal learning and EDS in the C57BL/6J inbred mouse strain (Laurent and Podhorna, 2004
), while another found no impairment in radial arm maze working memory in the same strain (Li et al., 2003
).
Previous studies have shown that subchronic PCP treatment also models social withdrawal component of schizophrenia, in that rats having undergone this treatment show reduced social interaction with a conspecific in a dyadic encounter (Lee et al., 2005
; Sams-Dodd, 1995
; Schwabe et al., 2006
; Snigdha and Neill, 2008a
,b
; Tanaka et al., 2003
) (but see Egerton et al., 2008
). However, there have been fewer published data showing similar deficits in social behavior of mice (Qiao et al., 2001
; Wang et al., 2007
).
The general paucity of literature on subchronic PCP effects on behavioral measures in mice related to both the social and cognitive/executive symptoms of schizophrenia, contrasts with the growing use of the mouse as the subject of choice in genetic and molecular studies of the role of NMDARs and other components of the glutamate system in the pathophysiology of schizophrenia (e.g., Karlsson et al., 2008
, 2009
; Labrie et al., 2008
; Miyamoto et al., 2001
; Mohn et al., 1999
; Wiedholz et al., 2008
) and frequently comorbid disorders such as anxiety and alcoholism (e.g., Boyce-Rustay and Holmes, 2005
, 2006
; Crabbe et al., 2006
; Cryan and Dev, 2007
; Palachick et al., 2008
). A better understanding of the potentialÃÂ effects of subchronic PCP on these behaviors would facilitate the comparison and convergence of mouse NMDAR-related pharmacological and gene mutant models of schizophrenia.
In the present study we tested for persistent effects of subchronic PCP treatment in C57BL/6J mice on social behaviors, using a task recently shown to be valuable in the study of social abnormalities in mouse models of neurodevelopmental disorders such as autism (Crawley, 2004
). In addition, we tested for subchronic PCP effects on pairwise visual discrimination and reversal learning using a mouse touchscreen-based operant system (Brigman et al., 2005
; Izquierdo et al., 2006
; Morton et al., 2006
).
Subjects
Subjects were male C57BL/6J obtained from The Jackson Laboratory (Bar Harbor, ME, USA) at 2àmonths of age and tested/treated beginning at 2–5àmonths of age. The C57BL/6J strain was selected on the basis of its frequent use in behavioral neuroscience and as a genetic background for mutant models of schizophrenia, as well as its inclusion as a “group A” priority strain in the Mouse Phenome Project, an international effort to provide the biomedical research community with phenotypic data on the most commonly used mouse strains (www.jax.org/phenome). Mice were housed two per cage in a temperature- (72à±Ã 5°C) and humidity- (45à±Ã 15%) controlled vivarium under a 12-h light/dark cycle (lights on 0600àh). The number of mice used is given in the figure legends. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research’ and the local Animal Care and Use Committees.
Subchronic PCP Treatment
Mice were intraperitoneally injected with 5ÃÂ mg/kg PCP (Sigma-Aldrich, St Louis, MO, USA) or saline vehicle (in a volume of 10ÃÂ mL/kg body weight) twice daily (morning and evening, separated by 12ÃÂ h) for 7 consecutive days. Fresh solutions were prepared daily. To facilitate the comparison of drug effects on the social and reversal tasks, mice used in these experiments were treated at the same time using the same drug solutions. Testing was conducted (for social) or began (for operant) 7ÃÂ days after the final PCP treatment. One cohort of mice was tested for sociability and social novelty preference. A separate cohort of was trainedÃÂ through pre-training criterion then treated with PCP and tested for discrimination 7ÃÂ days later. A third cohort of mice was trained through discrimination criterion then treated with PCP and tested for reversal 7ÃÂ days later. The dose and treatment/withdrawal procedure employed was based on a previous study demonstrating that the same procedure impaired reversal learning in rats (Jentsch and Taylor, 2001
).
Sociability and Social Novelty Preference
The effects of subchronic PCP treatment on social behavior were assessed based on methods previously described to test for autism- and schizophrenia-related phenotypes in mice (Crawley, 2007
; McFarlane et al., 2008
). The apparatus was a white Plexiglas square arena (40àcmà×à40àcmà×à35àcm, 25àlux) containing two wire-bar cups (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH, USA) placed on either side of the arena. Plastic cups were placed over the wire-bar cups to prevent the subject from climbing on top. The mouse was initially habituated to the arena for 20àmin, during which time two black Plexiglas (38àcmà×à13.5àcmà×à14àcm) boxes completely covered the sides of the arena containing the cups.
The mouse was initially habituated to the arena for 20ÃÂ min, during which time it was prevented from entering the sides of the arena containing the cups by the insertion of black Plexiglas partitions.
Sociability
The Plexiglas partitions were removed and an unfamiliar male C57BL/6J mouse was placed in one wire-bar cup (social stimulus). The other wire-bar cup remained empty (inanimate stimulus). The test mouse was manually observed for the total duration of investigation (defined as snout in physical contact with the wire-bar cup) and the cumulative number of investigations (a discrete investigation was scored when the snout was continuously in contact with the cup and until the snout was turned away from the cup) of the social and inanimate stimuli, and transitions between stimuli, over a 10-min test session using the Hindsight software program (Scientific Programming Services, Wokingham, UK).
Social novelty preference
Immediately after the sociability test, the inanimate stimulus was replaced with another unfamiliar male C57BL/6J mouse such that the test mouse now had a choice between investigating the now-familiar social stimulus vs. the novel social stimulus. Stimuli investigation and inter-stimuli transitions were measured as above over a 10-min test session.
Analysis of sociability and social novelty preference data
Consistent with earlier reports (e.g., McFarlane et al., 2008
) we did not consider drug treatment and stimulus-type as independent factors and analyze by use of analysis of variance. Instead, the effects of stimulus-type on dependent measures in the sociability and social novelty preference tests were analyzed within each treatment group by use of paired t-tests with Bonferroni correction for multiple comparisons. Drug treatment groups were compared for transitions between stimuli by unpaired t-test.
Pairwise Discrimination Learning and Reversal
The effects of subchronic PCP treatment on pairwise visual discrimination learning and reversal using a murine touchscreen-based operant system, as previously described (Brigman et al., 2008
; Izquierdo et al., 2006
).
Apparatus
An operant chamber measuring 21.6àcmà×à17.8àcmà×à12.7àcm (model # ENV-307W, Med Associates, St Albans, VT, USA) was housed within a sound and light attenuating box (Med Associates). The grid floor of the chamber was covered with solid Plexiglas to facilitate ambulation. A pellet dispenser delivering 14àmg dustless pellets (#F05684, BioServ, Frenchtown, NJ, USA) into a magazine located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Light Industrial Metal Cased TFT LCD Monitor, Craft Data Limited, Chesham, UK), a house-light, and a tone generator. The touchscreen was covered by a black Plexiglas panel that had 2àcmà×à5àcm windows separated by 0.5àcm and located at a height of 6.5àcm from the floor of the chamber. Stimuli presented on the screen were controlled by custom software (‘MouseCat’, L.M. Saksida) and were visible through the windows (one stimulus/window). Nosepokes at the stimuli were detected by the touchscreen and recorded by the software.
Autoshaping and instrumental pre-training
Mice were slowly reduced and then maintained at 85% free-feeding body weight. Prior to testing, mice were acclimated to the 14ÃÂ mg pellet food reward by provision of ∼10 pellets/mouse in the home cage for 1–3ÃÂ days. Mice were then acclimated to the operant chamber and to eating out of the pellet magazine by being placed in the chamber for 30ÃÂ min with pellets available in the magazine. Mice eating 10 pellets within 30ÃÂ min were moved onto autoshaping. During autoshaping variously shaped stimuli were presented in the touchscreen windows (one per window) for 10ÃÂ s [inter-trial interval (ITI) 15ÃÂ s]. The disappearance of the stimuli coincided with provision of a single pellet food reward, concomitant with presentation of stimuli (2-s 65ÃÂ dB auditory tone and illumination of pellet magazine) that served to support instrumental learning. Retrieval of the pellet from the pellet magazine (detected as a head entry) triggered the next trial. To encourage screen approaches and touches at this stage, nosepokes at the touchscreen delivered three pellets in the magazine.
Mice retrieving 30 pellets within 30ÃÂ min were moved onto pre-training. During pre-training mice first obtained rewards by responding to a (variously-shaped) stimulus that appeared in one of the two windows (spatially pseudorandomized) and remained on the screen until a response was made (‘respond’ phase). Mice retrieving 30 pellets within 30ÃÂ min were next required to initiate each new trial with a head entry into the pellet magazine (‘respond’ phase). In addition, responses at a blank window during stimulus presentation produced a 5ÃÂ s timeout (signaled by extinction of the house light) to discourage indiscriminate screen responding (‘punish’ phase). Incorrect responses were followed by correction trials in which the same stimulus and spatial configuration was presented until a correct response was made. Mice making ≥75% (excluding correction trials) of their responses at a stimulus-containing window over a 30-trial session were moved onto discrimination.
Discrimination
Two novel approximately equiluminescent stimuli were presented in spatially pseudorandomized manner over 30-trial sessions (15ÃÂ s ITI). Responses at one stimulus (correct) resulted in reward; responses at the other stimulus (incorrect) resulted in a 5ÃÂ s timeout (signaled by extinction of the house light) and were followed by a correction trial. Stimuli remained on screen until a response was made. Designation of the correct and incorrect stimulus was counterbalanced across drug treatment. Performance criterion was performing at an average of 85% correct (excluding correction trials) over two consecutive sessions.
Reversal
The session after attaining discrimination criterion, the designation of stimuli as correct vs. incorrect was reversed for each mouse and performance tested over 30-trial daily sessions to a criterion of 85% correct (excluding correction trials) on each of 2 consecutive days.
Operant data analysis
The dependent variable measured during autoshaping and pre-training was trials to criterion on each phase. The dependent variables measured during discrimination and reversal were trials, errors and correction errors to criterion, and average reaction time and reward retrieval latency. To further examine perseverative responding during reversal, we calculated a perseveration index (=average number of correction errors committed per error committed) (Brigman et al., 2008
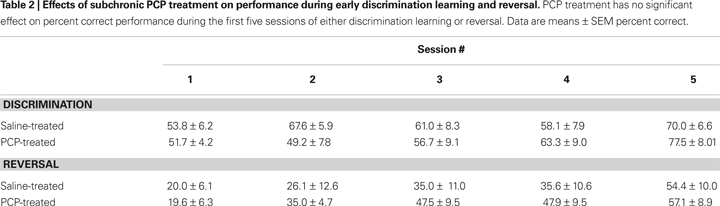
). The effect of drug treatment on these measures was analyzed using Student’s t-test. Finally, to test whether drug treatment effects were restricted to the early phases of the discrimination and reversal tasks (and potentially diluted in the to-criterion measures), we examined percent correct performance during the first five sessions on each task using two-factor drug treatmentà×àsession ANOVAs.
Sociability and Social Novelty Preference
Sociability
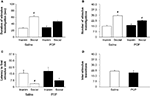
Saline-treated mice spent significantly more time with the social than inanimate stimulus (tÃÂ =ÃÂ 6.04, dfÃÂ =ÃÂ 7, pÃÂ <ÃÂ 0.001) whereas PCP-treated mice showed trend in the same direction that was not statistically significant (tÃÂ =ÃÂ 2.71, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.035, non-significant after Bonferroni correction) (Figure 1
A). Both saline-treated (tÃÂ =ÃÂ 8.56, dfÃÂ =ÃÂ 7, pÃÂ <ÃÂ 0.001) and PCP-treated (tÃÂ =ÃÂ 4.00, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.007) mice made significantly more visits to the social than the inanimate stimulus (Figure 1
B). Saline-treated mice were significantly quicker to first investigate the social than inanimate stimulus (tÃÂ =ÃÂ 3.15, dfÃÂ =ÃÂ 7, pÃÂ =ÃÂ 0.016) whereas PCP-treated mice (tÃÂ =ÃÂ 1.79, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.124) were not (Figure 1
C). The number of transitions between the social and inanimate stimuli did not differ between saline- and PCP-treated mice (tÃÂ =ÃÂ 0.80, dfÃÂ =ÃÂ 13, pÃÂ =ÃÂ 0.439) (Figure 1
D).
Figureà1. Effects of subchronic PCP treatment on sociability in C57BL/6J mice. In contrast to saline-treated controls, mice subchronically treated with PCP did not spend significantly more time investigating the social stimulus than the inanimate stimulus (A). Mice subchronically treated with either PCP or saline made significantly more investigations of the social stimulus than the inanimate stimulus (B). Saline but not PCP-treated mice were significantly faster to first investigate the social stimulus than the inanimate stimulus (C). Treatment did not affect the number of transitions between stimuli (D). Inaminà=àinanimate stimulus. Data are meanà±Ã SEM. n =à7–8 per treatment. #pà<à0.05 vs. inanimate/same treatment group.
Social novelty preference
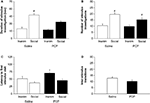
Saline-treated (tÃÂ =ÃÂ 5.83, dfÃÂ =ÃÂ 7, pÃÂ <ÃÂ 0.001) and PCP-treated (tÃÂ =ÃÂ 5.59, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.001) mice spent significantly more time with the novel than the familiar social stimulus (Figure 2
A). Both saline-treated (tÃÂ =ÃÂ 4.89, dfÃÂ =ÃÂ 7, pÃÂ =ÃÂ 0.002) and PCP-treated (tÃÂ =ÃÂ 4.24, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.006) mice also made significantly more visits to the novel than the familiar social stimulus (Figure 2
B). Neither saline-treated (tÃÂ =ÃÂ 1.53, dfÃÂ =ÃÂ 7, pÃÂ =ÃÂ 0.169) nor PCP-treated (tÃÂ =ÃÂ 1.80, dfÃÂ =ÃÂ 6, pÃÂ =ÃÂ 0.122) mice were significantly quicker to first investigate the novel than the familiar social stimulus (Figure 2
C). The number of transitions between the social and inanimate stimuli did not differ between saline- and PCP-treated mice (tÃÂ =ÃÂ 2.07, dfÃÂ =ÃÂ 13, pÃÂ =ÃÂ 0.059) (Figure 2
D).
Figureà2. Effects of subchronic PCP treatment on social novelty preference in C57BL/6J mice. Mice subchronically treated with either PCP or saline spent significantly more time investigating (A) and made significantly more investigations of (B) the novel social stimulus than the familiar social stimulus. Neither treatment group showed a significant difference in latency to first visit the novel social stimulus than the familiar social stimulus (C). Treatment did not affect the number of transitions between stimuli (D). nà=à7–8 per treatment. Inaminà=àinanimate stimulus. Data are meanà±Ã SEM. #pà<à0.05 vs. familiar/same treatment group.
Pairwise Discrimination Learning and Reversal
Discrimination learning
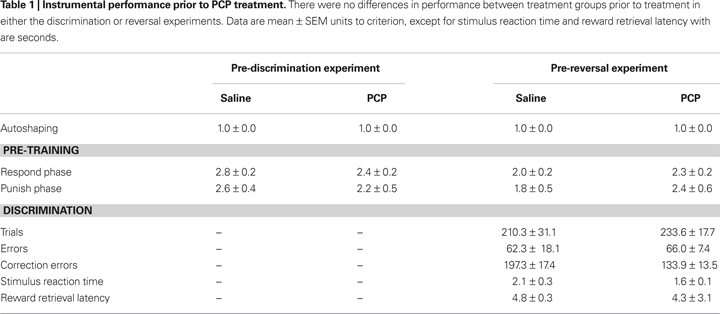
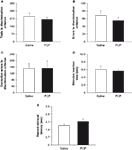
Prior to drug treatment, saline and PCP groups did not significantly differ in the number of sessions taken to attain autoshaping or pre-training criteria (Table 1
). Following treatment, there was no significant effect of PCP treatment on the number of trials, errors or correction errors committed to attain discrimination criterion (Figures 3
A–C). Stimulus reaction time and reward retrieval latency were also no different between treatment groups (Figures 3
D,E). Examination of the first five discrimination sessions found a significant effect of session (F4,48à=à8.17, pà<à0.01) but not drug treatment and no sessionà×àtreatment interaction (Table 2
).
Figureà3. Effects of subchronic PCP treatment on visual discrimination learning in C57BL/6J mice. Mice subchronically treated with either PCP or saline did not differ in the number of trials (A), errors (B) or correction errors (C) taken to attain discrimination criterion. Neither stimulus reaction time (D) nor reward retrieval latency (E) differed between treatment groups. Data are meanà±Ã SEM. nà=à8 per treatment. #pà<à0.05 vs. inanimate/same treatment group, *pà<à0.05 vs. saline.
Reversal
Prior to drug treatment, saline and PCP groups did not significantly differ in the number of sessions taken to attain autoshaping or pre-training criteria, or in the number of trials, errors or correction errors committed in reaching discrimination criterion (Table 1
). Following treatment, there was no significant effect of PCP treatment on the number of trials, errors or correction errors committed to attain reversal criterion (Figures 4
A–C). Perseveration index, stimulus reaction time and reward retrieval latency were also no different between treatment groups (Figures 4
D–F). Examination of the first five reversal sessions found a significant effect of session (F4,48à=à16.05, pà<à0.01) but not drug treatment and no sessionà×àtreatment interaction (Table 2
). (Note: one saline-treated mouse failed to reach criterion after being offered 900 trials was excluded from the analysis).
Figureà4. Effects of subchronic PCP treatment on reversal in C57BL/6J mice. Mice subchronically treated with either PCP or saline did not differ in the number of trials (A), errors (B) or correction errors (C) taken to attain reversal criterion. Perseveration index (correction errors committed per error) did not differ between treatment groups (D). Neither stimulus reaction time (E) nor reward retrieval latency (F) differed between treatment groups. nà=à7–8 per treatment. Data are meanà±Ã SEM. #pà<à0.05 vs. familiar/same treatment group, *pà<à0.05 vs. saline.
The results of the current study showed that subchronic PCP treatment did not affect either pairwise discrimination or reversal learning in C57BL/6J mice. The same treatment did, however, produce preliminary evidence of a partial decrease in social behavior.
The sociability/social novelty preference paradigm we employed to test for PCP-induced social withdrawal has been proposed (Crawley, 2004
) and proven valuable (Jamain et al., 2008
; McFarlane et al., 2008
; Moy et al., 2009
) as a test for social deficits in mouse models of autism spectrum disorders. Extending these findings, we examined social behaviors in the task following subchronic PCP treatment in C57BL/6J mice. PCP-treated mice showed more visits to a social stimulus than an inanimate stimulus, but only showed a trend for spending more time with the social stimulus, suggesting a partial attenuation of social preference in the drug treated mice. Underscoring the modest nature of the social deficit, PCP-treated mice retained a preference for a novel social stimulus over a familiar social stimulus.
Importantly, given the known locomotor hyperactivity-inducing effects of PCP, assessment of social behaviors in PCP-treated mice was not associated with a non-specific change exploratory locomotion, as measured by the number of transitions between stimuli (which were no different than saline controls). We have recently found that knockout mice lacking the glutamate transporter (GLAST), which display an antipsychotic-reversible ‘schizophrenia-like phenotype’ on measures related to the positive symptoms of the disease (i.e., novelty- and psychotomimetic-induced locomotor hyperactivity), also exhibit reduced social behaviors in this task (Karlsson et al., 2009
). Taken together with current findings, these data suggest that the sociability/social novelty preference paradigm has some utility as a measure for social withdrawal in both pharmacological and gene mutant models of schizophrenia.
Previous work has show that various regimens of subchronic PCP treatment significantly reduces social interaction during a dyadic encounter with an unfamiliar conspecific in rats (Lee et al., 2005
; Sams-Dodd, 1995
; Schwabe et al., 2006
; Snigdha and Neill, 2008a
,b
; Tanaka et al., 2003
) (but see Egerton et al., 2008
) and (ICR or ddY strains of) mice (Qiao et al., 2001
; Wang et al., 2007
). One distinguishing feature of the sociability/social novelty preference paradigm is that social interactions must be initiated and maintained by the test subject (Crawley, 2004
). By contrast, in a ‘free’ dyadic social encounter, social behavior can be supported by both the subject and the stimulus. This difference could render the sociability/social novelty preference paradigm more sensitive to true-positive social deficits in the subject mouse. Indeed, this has been borne out in recent examples in which putative mouse mutant models of autism (Jamain et al., 2008
; McFarlane et al., 2008
) and schizophrenia (Karlsson et al., 2009
) exhibited reduced social behavior in the sociability/social novelty preference test but not in a free dyadic social interaction test. It would be of interest in future studies to directly compare the effects of subchronic PCP treatment on the sociability/social novelty preference and a free interaction test in C57BL/6J mice to determine whether the approaches differ in their sensitivity to this treatment. Testing under free social interaction would also allow for the measurement of alterations in aggression as a result of subchronic PCP treatment; which would be of interest given known effects of acute PCP on this behavior in mice (Miczek and Haney, 1994
; Tyler and Miczek, 1982
; Wilmot et al., 1987
).
Subchronic PCP treatment had no observable effects on either pairwise discrimination learning or reversal in a murine touchscreen-based operant task previously shown to be sensitive to dopamine D1 agonist treatment in C57BL/6J mice, as well as knockout of the NR2A NMDAR subunit (Brigman et al., 2008
; Izquierdo et al., 2006
). The negative effect of PCP was evidenced on multiple measures of performance that included trials and errors to discrimination criterion, and trials, errors, and correction errors to reversal criterion, as well as stimulus reaction time and reward retrieval latency in both tasks. Moreover, additional analysis of the first five sessions of the discrimination or reversal tasks also failed to uncover a drug effect. Thus, it appears unlikely that there was a subtle effect of treatment that our analysis was not able to detect.
While there are no directly comparable data from mouse or rat versions of this task, prior studies have examined subchronic PCP effects on discrimination and reversal in other experimental settings. For example, studies have tested PCP effects on discrimination and reversal as components within the intra-dimensional set-shifting (IDS)/Extra-dimentional set-shifting (EDS) digging-based task developed by Birrell and Brown (Birrell and Brown, 2000
) as an analogue of the Wisconsin Card Sorting Task in humans (Braff et al., 1991
). Using the same subchronic PCP treatment regimen we used (albeit with a 10, rather than 7, day withdrawal period) Rodefer et al. (2005
, 2008)
found impaired EDS in rats, but no deficits on either discrimination, reversal or IDS. A similar pattern of impairment restricted to EDS was recently observed by Egerton et al. (2008)
in rats exposed to a somewhat milder PCP treatment regimen (5ÃÂ days of once daily 2.6ÃÂ mg/kg PCP and a 3-day withdrawal period). The lack of effects on discrimination or reversal in these studies would be in line with our data.
There are, however, a number of examples of impairments in discrimination and/or reversal following subchronic PCP treatment. One study found that C57BL/6J mice administered (1.3ÃÂ mg/kg) PCP once daily for 5ÃÂ days showed a general deficit across components of the Birrell and Brown task, including discrimination and reversal (Laurent and Podhorna, 2004
). However, this generalized profile of impairment may have resulted from PCP treatment 2ÃÂ h prior to testing, as acute PCP treatment is known to produce deficits, including impaired reversal, per se (Abdul-Monim et al., 2003
; Idris et al., 2005
).
Perhaps more difficult to reconcile with current negative data is a report of reversal (but not discrimination) impairment in rats given the same subchronic treatment as we used (Jentsch and Taylor, 2001
). The fact that their study differed from ours in a number of important methodological factors, such as the use of a T-maze- rather than an operant-based procedure, could account for the differences in findings. However, there is also recent work by Neill and colleagues showing that the same regimen of treatment and withdrawal as we used (albeit with a lower, 2ÃÂ mg/kg, dose) produced reversal (antipsychotic-reversible) deficits on a rat operant task (Abdul-Monim et al., 2006
, 2007
). Again, variations in methodology could account for the difference. Of particular note, these authors employed a task similar in certain respects to a go/no-go procedure (i.e., subjects learned to respond for reward signaled by the presence or absence of a cue). This procedure likely placed a greater demand on inhibitory control than our task, which did not have the additional requirement that subjects inhibit responding on no-go. Thus, given evidence from Jentsch and colleagues that subchronic PCP treatment significantly impairs inhibitory control in rats and non-human primates (Jentsch and Taylor, 2001
; Jentsch et al., 1997a
, 2000
), this might have rendered their task more sensitive to the treatment than ours. Additional studies could test this hypothesis; e.g., by testing whether subchronic PCP impairs C57BL/6J mice on tasks in our touchscreen task that strongly tax inhibitory control, such as operant extinction (Hefner et al., 2008
) and go/no-go. An alternative approach would be to modify our discrimination and reversal procedure to increase task difficulty; e.g., by increasing the perceptual similarity of the visual stimuli, presenting distracters or making reinforcement probabilistic.
Another interesting avenue for future work would be to determine regimens of PCP treatment that are sufficient to impair the forms of discrimination and/or reversal tested in the current study; e.g., by increasing dose, treatment chronicity or the number of cycles of subchronic PCP treatment. Of note in this regard, chronic hospitalized schizophrenic patients with frontal lobe damage have a profound impairment profile that includes poor discrimination and reversal (Pantelis et al., 1999
), while chronic stabilized patients are impaired on reversal and extra-dimensional shifts (Elliott et al., 1995
; Waltz and Gold, 2007
), and first-episode schizophrenics show milder profile restricted to extra-dimensional impairment (Braw et al., 2008
; Hutton et al., 1998
; Joyce et al., 2002
). These data could suggest that the severity of cognitive impairment on discrimination/reversal/EDS in schizophrenia may be positively correlated with the chronicity of the disease (Joyce et al., 2002
). Although arguing against this, however, are reports of reversal and extra-dimensional deficits in first-episode schizophrenics (Barnett et al., 2005
; Murray et al., 2008
). Notwithstanding, these clinical data do raise the interesting question of whether discrimination and/or reversal deficits in our task would become evident after more extensive PCP treatment.
While the main aim of the current study was to describe effects of subchronic PCP on measures of social behavior and cognitive/executive function in mice, rather than elucidate the mechanisms underlying these effects, the observed dissociation between effects on these measures provided some initial clues into these mechanisms. PFC dysfunction in schizophrenia is thought to be a principle locus of the negative and cognitive/executive symptoms of the disease (Tamminga, 2006
; Tan et al., 2007
). Subchronic PCP treatment has been shown to produce various long-lasting abnormalities in the rat PFC, including evidence of reduced dopamine utilization up to 3ÃÂ weeks after the end of treatment (Jentsch et al., 1997b
, 1998
) and loss of GABAergic (parvalbumin-positive) interneurons in the anterior cingulate cortex (ACC) 6ÃÂ weeks post-treatment (Abdul-Monim et al., 2007
). These intervals are well within the time window in which we tested cognitive and social behavior. Interestingly in this context, a recent study in rats demonstrated that excitotoxic lesions of the ACC but not orbitofrontal cortex (OFC) reduced social interaction (and social memory) (Rudebeck et al., 2007
)ÃÂ – mimicking the PCP-induced deficit in our study. By contrast, there is compelling evidence that reversal learning is mediated by the OFC and not ACC (Schoenbaum et al., 2006
), and preliminary evidence suggests that this subregion dissociation also holds for our mouse reversal task (while discrimination is not PFC-mediated) (Brigman etÃÂ al. unpublished). On the basis of these various findings, an heuristic model for further research isÃÂ that the profile of behavioral deficits produced by subchronic PCP treatment in our study were produced by selective dysfunction of the mouse ACC.
In summary, current data investigated the effects of a regimen of subchronic PCP treatment – previously proposed as a pharmacological model of the negative and cognitive/executive symptoms of schizophrenia (Jentsch and Roth, 1999
; Jentsch and Taylor, 2001
). Results suggested a partial deficit in social behavior after subchronic PCP treatment and encourage further analysis of the potential utility of this pharmacological model for studying the social withdrawal component of schizophrenia. It will also be of interest to test whether other negative symptoms of the disease (e.g.,ÃÂ abnormal affect and anhedonia) are also modeled by this treatment. The current study did not observe deficits in either discrimination or reversal learning in C57BL/6J mice after subchronic PCP treatment, despite evidence that similar cognitive/executive processes are impaired in schizophrenics and PCP-treated rats. Further work will be needed to identify cognitive/executive measures that are sensitive to chronic PCP treatment in C57BL/6J, as well as alternative methods that produce discrimination and/or reversal impairment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism (Z01-AA000411).
Carter, C. S., Barch, D. M., Buchanan,ÃÂ R.ÃÂ W., Bullmore, E., Krystal,ÃÂ J. H., Cohen, J., Geyer, M., Green, M., Nuechterlein,ÃÂ K.ÃÂ H., Robbins, T., Silverstein, S., Smith, E. E., Strauss,ÃÂ M., Wykes, T., and Heinssen, R. (2008). Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the cognitive neuroscience treatment research to improve cognition in schizophrenia initiative. Biol. Psychiatry 64, 4–10.
Jamain, S., Radyushkin, K., Hammerschmidt, K., Granon,ÃÂ S., Boretius, S., Varoqueaux, F., Ramanantsoa, N., Gallego, J., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., Bourgeron,ÃÂ T., Ehrenreich,ÃÂ H., and Brose, N. (2008). Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 105, 1710–1715.
Karlsson, R. M., Tanaka, K., Heilig, M., and Holmes, A. (2008). Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol. Psychiatry 64, 810–814.
Krystal, J. H., Karper, L. P., Seibyl,ÃÂ J.ÃÂ P., Freeman, G. K., Delaney, R., Bremner,ÃÂ J.ÃÂ D., Heninger, G. R., Bowers,ÃÂ M. B. Jr., and Charney, D. S. (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214.
Wiedholz, L. M., Owens, W. A., Horton,ÃÂ R.ÃÂ E., Feyder, M., Karlsson,ÃÂ R.ÃÂ M., Hefner, K., Sprengel,ÃÂ R., Celikel, T., Daws, L. C., and Holmes, A. (2008). Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol. Psychiatry 13, 631–640.