Anatomy/Neurobiology, Pediatrics and Neurology, University of California at Irvine, Irvine, CA, USA
Together with genetic factors, early-life experience governs the expression and function of stress-related genes throughout life. This, in turn, contributes to either resilience or vulnerability to depression and to aging-related cognitive decline. In humans and animal models, both the quality and quantity of early-life maternal care has been shown to be a predominant signal triggering bi-directional and enduring changes in expression profiles of genes including glucocorticoids and corticotropin releasing factor (CRH; hypothalamic and hippocampal), associated with the development of resilient or vulnerable phenotypes. However, many crucial questions remain unresolved. For examples, how is the maternal-derived signal transmitted to specific neuronal populations where enduring (likely epigenetic) regulation of gene expression takes place? What is the nature of this information? In other words, how do neurons know to ‘turn on’ epigenetic machinery? What are the direct functional consequences of altered gene expression? This review describes the voyage of recurrent bursts of sensory input from the mother (‘mother’s love’) to CRH-expressing hypothalamic neurons that govern the magnitude of the response to stress. In addition, the acute and enduring effects of both nurturing and fragmented maternal care on the structure, cellular signaling and function of specific hippocampal and hypothalamic neurons are discussed. The evolving understanding of the processes initiated by the early life experience of ‘mother’s love’ suggest novel molecular targets for prevention and therapy of stress-related affective and cognitive disorders.
Early Life Experience, and Especially Sensory Input from the Mother (Maternal Care) Leads to Enduring Neuroplasticity of Neuroendocrine Stress Response and Influences Cognitive Function
Why is this Topic Important? Human Perspective and History
Early postnatal life represents a period when the quality of the infant’s experience, and specifically of the interaction with the mother, is associated with emotional and cognitive development (Ammerman et al., 1991
; Fernald and Gunnar, 2009
). The important role of mother-infant interaction as a key determinant of the effects of early-postnatal experience on subsequent mental and cognitive health forms the basis of Bowlby’s social attachment theory stating that the mother is the primary social object mediating the effects of the external early environment on the infant (Bowlby, 1982
). The maternally mediated changes in the infant’s brain (programming) provide a mechanism for changes in the response of the infant to the environment, which last through adulthood. The enduring effects of early life programming may have adaptive and evolutionary advantages for an individual when the “programmed environment” reflects a real-life situation. The ‘programming’ effect has two facets: Human studies suggest that excellent early-life maternal care is associated with resilience to affective disorders (Gourion et al., 2008
). In contrast, adverse early life conditions in infancy, including poverty, substance abuse by the mother or maternal depression, have been associated with fragmented or reduced maternal care, and with vulnerability to psychopathology throughout life (Lupien et al., 2000
; Schore, 2000
; Agrawal et al., 2001
; Repetti et al., 2002
; Halligan et al., 2007
), as well as with cognitive decline later in life (Kaplan et al., 2001
; Wilson et al., 2007
). Among the most influential studies of these effects are those of institutionally reared children, where impoverished care was associated with cognitive and emotional deficits, and these were partially reversible by fostering (Gunnar et al., 2001
, 2009
; Nelson et al., 2007
).
These studies, and numerous additional clinical reports demonstrate a strong association between early life maternal interaction and cognitive function as well as resilience or vulnerability to mood disorders. Therefore, they provide strong impetus to understand the basis for the relationship between early-life experience and subsequent cognitive and emotional health. However, in human studies, causality between early-postnatal experience and long-term outcomes is difficult to infer: some reports are retrospective, introducing recall and other biases, and in prospective longitudinal and cohort studies, the influence of the genetic make-up of individuals on their early-life environment as well as on the predisposition to affective disorders such as depression (Caspi et al., 2003
) cannot be excluded.
Therefore, understanding the causal relationship between early experiences including interaction with the mother and life-long cognitive and emotional health benefits from animal models (e.g. Cottrell and Seckl, 2009
). These enable prospective longitudinal studies, as well as control of genetic background. In addition, parameters of interest can be manipulated and subsequent experiences can be controlled throughout the entire period of investigation. Finally, direct access to specific brain regions, coupled with neuroanatomical, biochemical and genetic approaches can tease out the regions, circuits, mediators and signaling cascades that might contribute to the profound effects of early-life maternal interaction on adult outcome (Joels and Baram, 2009
).
Historical Perspective of Work in Animal Models
Over the past half-century there has been a wealth of prospective experiments examining the short and long term consequences of physical and social elements of the early-postnatal environment on the development of stress responses and cognitive functions, with particular focus on the relevance of the mother-infant interaction (Heim et al., 2004
). Already the seminal work of Levine (1957) and Denenberg et al. (1967)
demonstrated that manipulations of the mother–pup relationship (in rat) have long-term consequences on neuroendocrine and behavioral stress responses of the pups later in life. Initial work on the ontogeny of the stress response suggested that that the first 2 weeks of life in the rodent, the hypothalamic–pituitary–adrenal (HPA) system was relatively unresponsive to stress (termed the stress hyporesponsive period or SHRP); (Schapiro et al., 1962
; Schoenfeld et al., 1980
; Rosenfeld et al., 1992
). However, this was found not to be the case, as immature rats elevated plasma glucocorticoids 300–400% in response to age-appropriate stressors, (Yi and Baram, 1994
; Dent et al., 2000
), and human neonatal cortisol increased with stress/pain (Gunnar, 1992
). Already in 1977, the observation that mother-pup interaction affected pups’ development, led Smothermann to propose the “maternal mediation” hypothesis stating that changes in maternal behavior underlie the effects of early manipulations on the pup (Smotherman et al., 1977
). Numerous other studies have been carried out in rodents (Levine and Lewis, 1959
; Hess, 1969
; Meaney et al., 1996
; Avishai-Eliner et al., 2001a
; Fenoglio et al., 2005
) and primates (Seay and Harlow, 1965
; Levine, 1993
; Heim et al., 1997
; Suomi, 1997
), building on these original hypotheses. Throughout these efforts, some of the most useful strategies used to study how altered quality and quantity of mother-pup interaction affects development of stress response and cognitive function long-term have included the ‘handling’, ‘maternal separation’ and ‘chronic early life stress’ paradigms. All of these manipulate specific parameters of maternal care, demonstrating the importance of the maternal factor in the ‘programming’ of the stress response and subsequent emotional and cognitive functions throughout life, and will be discussed in the next sections.
Quantity as Well as Quality of Interactions of Immature Individuals with their Mother Govern the Resulting Adult Phenotype
The interaction of the mother with her pups is a complex entity consisting of several aspects, each one impacting the pups’ brain in a different manner. In this context, it is helpful to consider this interaction from the perspective of the pup/infant, because the resulting persistent changes take place within specific regions of its brain. For example, a feeding pup receives both nutrition as well as sensory and motor elements derived from the pup’s suckling, and contact from the mother (Suchecki et al., 1995
; Eghbal-Ahmadi et al., 1999
). Studies aiming to dissect out these elements in the regulation of the development of the stress response indicated that different aspects of maternal inputs differentially regulate components of the HPA axis. Sensory stimulation [e.g., licking and grooming (LG) by the dam, as well as suckling motion of the pup] appeared to act directly on corticotropin releasing factor (CRH) – adrenocorticotropin releasing hormone (ACTH) expression and release (Suchecki et al., 1995
; Eghbal-Ahmadi et al., 1999
). Tactile stimulation is also important in maintaining basal levels of activity of enzymes and hormones necessary for normal growth (Kuhn et al., 1978
; Kuhn and Schanberg, 1998
). In contrast, nutrition from the mother regulates adrenal function (Suchecki et al., 1995
). These original studies have set the stage for experiments where the quantity and quality of specific elements of maternal care were manipulated, enabling analysis of the influence of this variation on infant outcome, as discussed below.
Maternal Care: Natural Variation and Strategies for Enhancement of this Care
An approach to the question of: ‘how does maternal care influence development of the stress response in pups?’ is to select mothers based on natural, individual variation in maternal care (e.g. amount of time spent LG and nurturing the pups) and compare the stress response of adults reared by dams exhibiting different level of maternal care. Individual differences in active maternal care in rats and mice were found to be directly associated with differences in stress response in the pups when they reached adulthood (Meaney, 2001
; Priebe et al., 2005
), such that hormonal stress responses of adults reared by dams exhibiting high levels of LG and arched back nursing were reduced. This was accompanied by reduced CRH expression in the hypothalamus and increased hippocampal glucocorticoid receptor (GR) expression when compared with pups from mothers with low levels of active maternal care (Plotsky and Meaney, 1993
; Francis and Meaney, 1999
; Meaney, 2001
). In addition the adult offspring of high LG mothers exhibited improved hippocampus-dependent cognitive function (Liu et al., 2000
) accompanied by longer dendritic branch length and increased spine density in CA1 neurons, alterations in electrophysiological properties at rest and enhanced LTP (Champagne et al., 2008
).
The quantity and quality of maternal care can be experimentally manipulated either to enhance or to impair it. Maternal care can be enhanced using the ‘handling’ procedure. This procedure involves brief (15 min) daily separation of rat pups from their mother followed by returning the pups to the home cage starting on postnatal day (P) 2 for a minimum of 1 week (Avishai-Eliner et al., 2001a
), or up to 3 weeks (Levine and Lewis, 1959
; Hess, 1969
; Plotsky and Meaney, 1993
; Bhatnagar and Meaney, 1995
) and has been shown to enhance mother pup interaction by provoking bursts of maternal sensory stimulation of pups immediately after their return to the home cage (Brown et al., 1977
; Fenoglio et al., 2006b
). Similarly to the adult rats reared by dams that naturally exhibit high levels of care, handling has consistently been found to suppress the stress response during adulthood (Sanchez et al., 2001
; Fenoglio et al., 2006b
) reduce CRH expression in the hypothalamus, and increase GR in the hippocampus (Plotsky and Meaney, 1993
; Avishai-Eliner et al., 2001a
). Levels of CRH and CRFR1 were reduced (Plotsky et al., 2005
), and of α1 and γ2 subunit of the GABAA receptor were increased in the locus coeruleus (LC). The GABA receptor was also increased in nucleus tractus solitarius, basolateral and central nucleus of the amygdala (ACe;) (Caldji et al., 2003
). Levels of vasopressin (AVP) were reduced in median eminence (Viau et al., 1993
) and found to be either decreased or increased in the hypothalamus (Viau et al., 1993
; Todeschin et al., 2009
) In addition, adult rats handled early in life were resilient to manipulations that lead to depressive-like behaviors (Meaney et al., 1991
) and had improved hippocampus-dependent cognitive function (Meaney et al., 1988
; Liu et al., 2000
; Tang, 2001
; Fenoglio et al., 2005
). Together, these studies indicate that either naturally occurring or experimentally enhanced sensory stimulation that pups receive from the dam programs the stress response, leading to blunted HPA axis tone, resilience to manipulations that lead to depressive-like behaviors and improved hippocampus-dependent learning and memory (Korosi and Baram, 2008
).
Recurrent Separation from the Mother: Recurrent Early-Life Stress
In the course of normal mother–pup interactions the dam is regularly away from the nest and pups for periods of 20–30 min (Rosenblatt, 1975
). Thus, the handling manipulation does not result in an abnormal period of separation or loss of maternal care. How do longer periods of separation influence the pups’ stress response acutely and long-term? Following a single prolonged (24 h) maternal deprivation, expression of CRH in the PVN was unchanged (Avishai-Eliner et al., 1995
; Dent et al., 2000
), or reduced (Smith et al., 1997
; Workel et al., 2001
; Schmidt et al., 2004
), and CRFR2 mRNA expression in the ventromedial hypothalamus was reduced (Eghbal-Ahmadi et al., 1997
). The response to stress, measured by ACTH and glucocorticoid levels, were augmented by single prolonged maternal separation (Avishai-Eliner et al., 1995
; Dent et al., 2000
).
Exposing the animals to recurrent, daily 3–6 h maternal separation during the first 2 weeks of life influenced variably maternal care upon returning the pups to the cage (Pryce et al., 2001
; Macri et al., 2004
). This recurrent separation augmented the stress response in adult graduates throughout life (Plotsky and Meaney, 1993
; Schmidt et al., 2004
), but see (Pryce et al., 2001
; Lehmann et al., 2002
; Giachino et al., 2007
), augmented fearfulness in tests of novelty and anxiety (Caldji et al., 1998
; Wigger and Neumann, 1999
; Ladd et al., 2000
) and lead to depressive-like behavior (Ladd et al., 2000
; Huot et al., 2002
; Aisa et al., 2007
). In addition, recurrent maternal separation led to an impaired hippocampus dependent learning and memory later in life (Oitzl et al., 2000
; Aisa et al., 2007
), as well as to abnormal synaptic plasticity and aberrant expression of the extracellular matrix protein NCAM (Aisa et al., 2009
; Bisaz et al., 2009
). Molecular changes reported in these recurrently separated rats have included decreased GR binding in both hippocampus and hypothalamus (Plotsky and Meaney, 1993
), increased CRH levels in the ACe, LC, and parabrachial nucleus, accompanied by increased CRFR1 mRNA expression levels in LC and the raphe nucleus (Ladd et al., 1996
). Levels of AVP were found either increased or decreased in the hypothalamus and BnST (Desbonnet et al., 2008
; Veenema and Neumann, 2009
) Together these findings indicate that recurrent prolonged maternal separation leads to increased stress responsiveness both at the molecular and at the behavioral levels during adulthood.
Erratic, Fragmented Maternal Care: Chronic Early-Life Stress
The studies described so far modulated the quantity of maternal care. In addition, they resulted in acute or recurrent, rather than chronic early-life stress. However, when infants and children are exposed to severe poverty, famine, war or drug-abusing mothers, the stress is typically chronic, and the mother is typically present, but her behavior is abnormal (Whipple and Webster-Stratton, 1991
; Koenen et al., 2003
; Kendall-Tackett, 2007
). A model of chronic early-life stress was developed to recapitulate these important elements of the human condition. Manipulation of the quality of care of a present mother in this model generates chronic (rather than recurrent) early-life ‘emotional stress’ (Gilles et al., 1996
; Avishai-Eliner et al., 2001b
; Ivy et al., 2008
). A hallmark of maternal behavior in neglect/abuse situations is its unpredictable and fragmented quality (Whipple and Webster-Stratton, 1991
; Gaudin et al., 1996
). The experimental paradigm, consisting of limiting the nesting material available to the dam recreates this pattern both in rat and mouse (Ivy et al., 2008
; Rice et al., 2008
). The limited nesting material environment results in stress of the mother, promoting fragmented care, i.e., shortened bouts of each nurturing behavior and frequent shifts between behaviors (Ivy et al., 2008
; Rice et al., 2008
). This aberrant maternal behavior induces chronic early-life stress in the pups, evident from elevated plasma glucocorticoids and increased adrenal weight, often associated with modest, transient reduction of weight gain. At the termination of the 1-week stress period, CRH mRNA is reduced in the PVN accompanied by reduced CRHR1 and GR expression in the hippocampus and unaltered hypothalamic AVP levels (Gilles et al., 1996
; Avishai-Eliner et al., 2001b
). Whereas these molecular changes are transient, lasting changes are evident in the hippocampus: elevated CRH mRNA levels in CA1 and CA3 (Fenoglio et al., 2006a
) and increased numbers of CRH-expressing neurons in both fields (Ivy et al., 2008
, Abstract at Society for Neuroscience), as well as dendritic atrophy of pyramidal cells. Importantly, these structural changes are accompanied by profound disruption of LTP in CA1 and CA3 and impaired hippocampus-mediated cognitive impairments, consisting of increased escape latency in the Morris Water maze test and inability to distinguish novel from familiar object in the Object recognition test (Brunson et al., 2005
). Thus a period of early-life chronic stress causes acute effects on the stress system and late onset and gradual deterioration of hippocampus mediated learning and memory.
Maternal Care Parameters Influence CRH mRNA Levels Acutely, and Modulate Stress Responses and Cognitive Function Long-Term: Potential Mechanisms
Reviewing the diverse manipulations of maternal care discussed above, it becomes evident that both the quality and quantity of maternal care program the stress response and specific cognitive functions in a bi-directional manner: Enhanced maternal care, either as a result of natural variation or via the ‘handling’ procedure, leads to diminished stress responsiveness and improved cognition. Thus, studies by several groups found augmented performance in the Morris water maze task, as discussed above. Conversely, absence or fragmentation of maternal care results in increased stress responsiveness and progressively impaired cognition commencing in adulthood (see Erratic, Fragmented Maternal Care: Chronic Early-Life Stress).
It is important to note that while varied maternal care leads to different phenotypes, handling and chronic early life stress both result in reduced CRH mRNA on P9. What are the potential mechanisms accounting for these differences? How can CRH mRNA reduction on P9 be associated with different phenotypes later in life?
The mechanisms accounting for the reduction of hypothalamic CRH mRNA expression after chronic early life stress differs from the early onset and permanent CRH reduction induced by handling, both in the associated neuroendocrine and physiological measures and in the associated phenotype. The reduced CRH levels induced by chronic-early life stress, while apparent immediately at the end of the stress period (P9) is transient in the rat (Avishai-Eliner et al., 2001b
) and still apparent in the adult mouse (Rice et al., 2008
). This reduction of CRH expression is associated with elevated basal CORT, increased adrenal weight, decreased body weight, decreased GR in the hypothalamus and frontal cortex and reduced CRH receptor binding in the pituitary, all indices of a chronic stress situation (Avishai-Eliner et al., 2001b
). The mechanisms underlying CRH mRNA reduction in this scenario may include altered glucocorticoid negative feedback, as suggested by the elevated glucocorticoids levels and the reduced GR mRNA expression of chronically stressed pups. Alternatively, or in concert, persistent increased CRH release, with limited compensatory enhanced synthesis, leads to depleted mRNA stores of hypothalamic CRH. In contrast to this early-life stress situation, CRH reduction after augmented maternal care (handling) is not associated with altered basal CORT, ACTH or GR in the hypothalamus, but is the first apparent change in a sequential reduction of hormonal stress response and of increased GR in the hippocampus (Figure 1
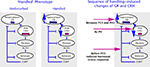
). This fact suggests that the CRH reduction in ‘handled’ pups might be independent of glucocorticoid feedback. In the absence of early alteration in other parameters of the stress system, the onset and persistence of reduced CRH expression might be governed by other mechanisms including epigenetic modulation of the Crh gene.
Figure 1. Schematic presentation of the adult handled phenotype (on the left panel) and the sequence of molecular changes in the infant’s brain after handling (on the right panel) (Avishai-Eliner et al., 2001a
).
An important step in understanding long-lasting phenotypes in graduates of varied maternal care is to define how each of these phenotypes arises, i.e., the sequential steps that are involved in its evolution. Therefore, the following sections will describe the brain regions involved in transmission of the maternal-derived ‘programming’ signals to brain areas involved in the stress response, and the molecules involved in initiating and maintaining the phenotype. We will focus on the phenotype induced by augmented maternal care (handling), which provides resilience to depression and enhanced cognitive function.
Sequence of Molecular Changes in the Infant’s Brain
Persistently altered mRNA expression levels of GR in hippocampus, and of the CRH in hypothalamic neurons have been established as key elements of the handling-induced programming (Plotsky and Meaney, 1993
; Avishai-Eliner et al., 2001a
). Specifically, GR expression levels in the hippocampus were elevated, whereas CRH mRNA levels in the hypothalamus were reduced. Discovering which of these fundamental changes occurred first should help in identifying the location and nature of the initial ‘programming’ steps triggered by the early life experience. The set-point and magnitude of the hormonal responses to stress are under tight and intricate regulation (Walker and Dallman, 1993
) and are influenced by both hippocampal GR and hypothalamic CRH function. What is the precise timing and sequence of the handling-induced alterations? Increased GR expression has been proposed as an early and critical effect of the enriched sensory input (Meaney et al., 1996
; Liu et al., 1997
; Francis et al., 1999
; Kaffman and Meaney, 2007
). The increased GR levels would then transmit more efficiently negative glucocorticoid feedback to the HPA axis, downregulating hypothalamic CRH and the subsequent responses to stress. However programming of Crh gene expression in PVN neurons to lower levels has been found to take place already by the end of the daily week-long handling period (P9), followed by attenuated hormonal responses to stress and enduring enhancement of hippocampal GR expression, which take place by P23 and between P23 and P45 respectively (Avishai-Eliner et al., 2001a
; Figure 1
). These findings establish that modulation of CRH expression precedes the increased GR expression, indicating that programming of the levels of Crh gene expression is an early and potentially key step in the mechanism of maternal-care induced stress-resilient phenotype. This supposition predicts that genetic variants or mutations of CRH or its receptors would confer similar phenotypes. In line with this supposition, it has been recently reported that specific polymorphism of CRHR1 moderates the effects of early-life stress on adult psychopathology. The presence of several single nucleotide polymorphisms (SNPs) in the Crhr1 gene interacted with childhood maltreatment to predict depressive symptoms (Bradley et al., 2008
). A haplotype including a specific combination of the SNPs was protective against depressive symptoms among maltreated subjects (Bradley et al., 2008
) and moderated the effect of childhood maltreatment on cortisol responses to the Dexhamethasone/CRH test (Tyrka et al., 2009
).
Neuronal Pathways Involved in Transmitting the Sensory Input (Maternal Care) from Mother to Pups, and within the Pup’s Brain to the ‘Programmed’ Regions
If the initial consequences of the augmented maternal-derived sensory input is to change CRH expression levels in the PVN, then we need to understand how the maternal signal reaches this brain region, and more specifically, the parvocellular CRH-expressing neurons in the PVN. Using the immediate-early gene Fos to visualize neurons activated by maternal-derived sensory signals, a pathway regulating the hypothalamic PVN emerged: Fos expression was induced in the bed nucleus of the stria terminalis (BnST) and the ACe after a single day of handling (Fenoglio et al., 2006b
), and both these regions generally augment CRH expression in PVN (Feldman et al., 1994
; Akana and Dallman, 1997
). In contrast, repeated handling induced Fos expression also in the thalamic paraventricular nucleus (PVT), a region with major inhibitory output onto ACe (Bhatnagar and Dallman, 1998
; Spencer et al., 2004
), and thus on the PVN. The activation of PVT neurons after recurrent handling likely altered their firing rate and/or neurotransmitter release, altering the combinatorial afferent information arriving at CRH-expressing neurons in PVN (Figure 2
). Indeed, PVT has been proposed previously as a region involved in processing the ‘memory’ of experiences related to the stress-response system (Bhatnagar and Dallman, 1998
; Bell et al., 2000
; Jaferi and Bhatnagar, 2006
). In addition recurrent daily handling was required for reduced hypothalamic CRH expression (Fenoglio et al., 2006b
), because a single day of increased maternal care did not result in altered expression of CRH in the PVN. Together with the patterns of fos-expression described above, these findings suggest that a key distinguishing feature of the signal that ‘programs’ CRH expression at lower levels is the recurrence of this signal (Fenoglio et al., 2006b
; Korosi and Baram, 2008
).
Figure 2. Brain regions activated by maternal-derived sensory input (in fuchsia), and their relationship to the expression of CRH in PVN. The red arrow indicates excitatory input to PVN after single handling, and the blunt black arrows show the inhibitory consequences of activation of PVT after recurrent handling.
What Type of Information Reaches the Hypothalamic CRH Neurons, ‘Commanding’ it to Repress CRH Expression Persistently?
What is the nature of the signal, derived from maternal-derived sensory experience, which reaches the CRH neuron and results in persistently reduced expression of the Crh gene? The hypothalamic CRH neuron is the target of a neuronal network, activated by barrages of maternal care induced by the brief daily separation of rat pups from the dam (Fenoglio et al., 2006b
), and upon recurrence of this activation, the PVT is recruited, providing an inhibitory signal to the PVN. We therefore examined directly the excitatory and inhibitory afferent input onto the CRH neuron, and found that augmented early-life experience reduced excitatory synaptic input onto the CRH neuron (A. Korosi et al., in review). This reduced excitation, evident as reduced synapse number and function, should influence intracellular signaling cascades resulting in suppressed CRH expression (see next section).
Molecular Cascades Involved in Programming the Infant Brain
Because the handling-evoked maternal sensory stimulation elicits a signal that provokes repression of CRH expression in PVN neurons, it is reasonable to query whether signalling cascade and transcription factors involved in the regulation of CRH expression were altered in these cells. Phosphorylation of cAMP response element-binding protein (CREB) and ERK influence the initial activation of the critical CRE domain on the Crh gene promoter (Seasholtz et al., 1988
). In addition, phosphorylation of the transcription factor ERK (pERK) is crucial for maintaining CREB phosphorylated beyond the first seconds after synaptic activation, contributing to plasticity at longer timescale (West et al., 2002
). CREB and ERK are ubiquitously phosphorylated in the PVN of undisturbed P9 rats and can therefore be candidates for deactivation by inhibitory inputs evoked by handling. In fact a strong decrease in the number of pERK immunoreactive cells in the PVN was found in rats handled recurrently (Fenoglio et al., 2006b
). Accordingly, transcription of Crh gene (measured by CRH hnRNA) in response to separation stress decreased in PVN of recurrently handled pups (Fenoglio et al., 2006b
). This reduced transcription of the Crh gene should result in reduced steady state CRH mRNA levels, as indeed observed in handled rats throughout their lives.
Translational and Therapeutic Implications of the Programming Effects of Maternal Care on the Stress System
Can we recreate the salubrious consequences of augmented maternal care for improving human health? Is there a critical time window for these effects? The sequence of changes described above suggests that reduced CRH levels might be a key initial target of augmented maternal care. Reduced expression of CRH, in turn, will promote less peptide release in response to stress and thus diminished activation of CRH receptors in the pituitary and a resulting reduction in plasma glucocorticoids levels. This reduction should promote up-regulation of GR expression. If reduced activation of CRF1 by CRH (and consequent lower stress-evoked plasma glucocorticoid levels) is required for long-lasting up-regulation of GR expression in the hippocampus and improved learning and memory functions, then partially blocking CRF1 receptors in undisturbed rats should recapitulate these changes. Indeed reducing CRH-CRH-receptor signalling by a systemic, post hoc administration of CRF1 antagonist in immature rats, that did not receive augmented maternal care, from P10–17 was sufficient to upregulate hippocampal GR persistently and to confer the behavioral ‘phenotype’ of improved cognitive functions induced by augmented maternal care (Fenoglio et al., 2005
). Thus, the enduring effects of enhanced early-life maternal care in handled rats on hippocampal GR gene expression, CRH mRNA in the PVN, and learning and memory are recapitulated by post hoc partial blockade of CRF1 receptor in undisturbed animals. These results support the idea that programming of the levels of Crh gene expression is a key and early step in the cellular and molecular cascades by which enhanced maternal care ‘programs’ stress responses and enhances cognitive function long-term, and position CRH-CRH-receptor intersection as molecular targets for influencing emotional and cognitive function after adverse early-life experience.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by NIH NS28912 and MH73136.
Avishai-Eliner, S., Eghbal-Ahmadi, M., Tabachnik, E., Brunson, K. L., and Baram, T. Z. (2001a). Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology 142, 89–97.
Bradley, R. G., Binder, E. B., Epstein, M. P., Tang, Y., Nair, H. P., Liu, W., Gillespie, C. F., Berg, T., Evces, M., Newport, D. J., Stowe, Z. N., Heim, C. M., Nemeroff, C. B., Schwartz, A., Cubells, J. F., and Ressler, K. J. (2008). Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry 65, 190–200.
Champagne, D. L., Bagot, R. C., van Hasselt, F., Ramakers, G., Meaney, M. J., de Kloet, E. R., Joels, M., and Krugers, H. (2008). Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045.
Fenoglio, K. A., Brunson, K. L., Avishai-Eliner, S., Stone, B. A., Kapadia, B. J., and Baram, T. Z. (2005). Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology 146, 4090–4096.
Giachino, C., Canalia, N., Capone, F., Fasolo, A., Alleva, E., Riva, M. A., Cirulli, F., and Peretto, P. (2007). Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience 145, 568–578.
Todeschin, A. S., Winkelmann-Duarte, E. C., Jacob, M. H., Aranda, B. C., Jacobs, S., Fernandes, M. C., Ribeiro, M. F., Sanvitto, G. L., and Lucion, A. B. (2009). Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm. Behav. 56, 93–100.
Viau, V., Sharma, S., Plotsky, P. M., and Meaney, M. J. (1993). Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J. Neurosci. 13, 1097–1105.