- 1Key Laboratory of Neuromolecular Biology, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
- 2Department of Neurology, The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
Mesenchymal stem cell-derived extracellular vesicles, as carriers for intercellular communication, are rich in bioactive substances such as proteins and nucleic acids, and show unique potential in the treatment of neurodegenerative diseases. Their vesicular structure, with a diameter of 30–150 nm, can penetrate the blood–brain barrier and modulate the activity of microglia and astrocytes by delivering functional molecules. This process inhibits the release of pro-inflammatory factors and enhances the expression of anti-inflammatory mediators, thereby alleviating neuroinflammation in the pathological process of neurodegenerative diseases. As natural drug carriers, extracellular vesicles can improve the targeted delivery efficiency of therapeutic molecules. However, their specific anti-inflammatory mechanisms remain not fully understood and require further exploration. This article discusses the anti-inflammatory effects in the context of neurodegenerative diseases and provides a summary and outlook on the anti-inflammatory actions associated with extracellular vesicles from past research.
1 Introduction
Stem cells possess unique properties that enable them to differentiate into various cell types and self-renew in vivo (Zakrzewski et al., 2019). Extracellular vesicles (EVs) facilitate intercellular communication by carrying various biologically active molecules, including proteins, lipids, and RNA. Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have been shown to regulate nerve-related cell functions, reduce neuroinflammation, and thus contribute to neuroprotection. EVs can affect the expression of genes related to inflammation and apoptosis, thereby promoting neuronal survival and reducing inflammatory response (Zhang et al., 2023). MSC-EVs enhance angiogenesis, essential for neurodegenerative disease (NDD) recovery, through upregulation of intercellular cell adhesion molecule-1 (ICAM-1) and activation of associated signaling pathways (Xue et al., 2021). Improving blood flow helps reduce inflammation and supports neuronal health. On the other hand, MSC secrete or package neurotrophic factors into EVs or exosomes, which can promote angiogenesis and have a protective effect on dopaminergic neurons (d’Angelo et al., 2020; Hofer and Tuan, 2016). More importantly, EVs are rich in miRNA, which can regulate gene expression in recipient cells. The miR-100-5p found in MSC-EVs derived from the trophoblast stage has been shown to target NADPH oxidase 4 (NOX4). By inhibiting NOX4 in a Parkinson’s disease (PD) model, these EVs can reduce the production of reactive oxygen species, thereby mitigating oxidative stress and its inflammatory consequences in dopaminergic neurons (He et al., 2023). The ability of EVs to cross the blood–brain barrier (BBB) enhances their therapeutic potential by allowing them to exert anti-inflammatory effects directly within the central nervous system (CNS) (Chen et al., 2020). The use of EVs as a treatment strategy has many advantages over traditional stem cell therapy. Compared with whole-cell therapy, EVs can be produced in large quantities and have a lower risk of tumorigenicity and immune rejection. This makes them a more feasible choice for clinical application in the treatment of NDDs.
Neurodegenerative diseases are a group of diseases caused by the gradual loss of specific neuronal populations, affecting the lives of millions of people around the world (Heemels, 2016). Neurodegeneration is the gradual loss of neuronal structure and function, including neuronal death and glial cell imbalance, which can lead to cognitive impairments such as dementia. Currently, common NDDs include Alzheimer’s disease (AD), PD, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). As the global population ages, the incidence of NDDs is increasing annually, placing a substantial burden on patients’ families and society. The pathogenesis of these diseases is complex, often involving multiple pathological processes such as neuroinflammation, oxidative stress, and apoptosis. Recent studies have demonstrated that inflammation plays a crucial role in disease progression, suggesting that inhibiting neuroinflammation may offer new therapeutic strategies. The inflammatory response is mainly driven by glial cells such as microglia and astrocytes, rather than by infiltration of peripheral white blood cells (Minghetti, 2005). The regulation of inflammation relies on the ability of microglia and macrophages to exhibit two opposing phenotypes: the M1 phenotype, which can produce cytotoxic effects, and the M2 phenotype, which can produce neuroprotective effects (Amici et al., 2017). The dynamic changes in M1/M2 phenotypes are closely associated with NDDs, including AD, PD, ALS, and MS (Tang and Le, 2016). The phenotype of inflammatory cells changes depending on the stage and severity of the disease. Furthermore, effectively managing the phase transition of the M1/M2 phenotypes within an appropriate time window may enhance therapeutic outcomes (Carata et al., 2023). In some cases, the inflammatory response can promote the recovery of damaged neurons, but it may also lead to or aggravate NDDs. Studies have shown that although acute inflammation may help repair damaged neurons, persistent chronic inflammation often leads to neuronal dysfunction and death (Wyss-Coray and Mucke, 2002). Inflammatory factors can regulate neuronal excitability and synaptic function by interacting with specific receptors on the surface of neurons. In NDDs, inflammation is typically triggered by the accumulation of abnormal proteins, such as amyloid-beta and tau, or signals released by damaged cells. These abnormal proteins and cell signals activate microglia, leading to the inflammatory response (Ising et al., 2019; Kwon and Koh, 2020; Leng and Edison, 2021; Peterson and Fujinami, 2007; Wang et al., 2015). It is particularly important to emphasize that the traditional microglia M1/M2 dichotomy has been proven insufficient to fully reflect their highly dynamic and heterogeneous functional states. Recent single-cell transcriptomics studies have revealed that microglia undergo continuous functional state transitions in NDDs, involving multi-dimensional features such as metabolic reprogramming, phagocytic functional plasticity, and microenvironment-dependent epigenetic remodeling (Paolicelli et al., 2022). This will provide new theoretical foundations for the dynamic impact of precise therapeutic strategies using stem cell-derived EVs, while simultaneously emphasizing the heterogeneity of microenvironment-specific regulatory targets at different stages of disease progression.
2 Characteristics of stem cell extracellular vesicles
2.1 Classification and function of stem cells
Mesenchymal stem cells are a type of stem cell with multidirectional differentiation potential, naturally found in tissues such as bone marrow, adipose tissue, and umbilical cord (Galipeau and Sensébé, 2018). They can be isolated and expanded using adherent culture methods. Their ability to differentiate into CNS cells depends on specific induction conditions, such as chemical factors like β-mercaptoethanol and retinoic acid or regulation by growth factors like brain-derived neurotrophic factor (BDNF) (Liu et al., 2022a). iPSCs are pluripotent stem cells reprogrammed from mature somatic cells (Malik and Rao, 2013). This reprogramming is achieved by introducing key transcription factors such as octamer-binding transcription factor (OCT4), sex determining region Y-box 2 (SOX2), Krüppel-like factor 4 (KLF4), and c-Myc into mature somatic cells, like skin fibroblasts. Subsequently, these cells can be directed to differentiate into neural precursor cells by inhibiting the SMAD signaling pathway. Further combination with developmental signals such as FGF8 can induce differentiation into specific CNS cell types, such as dopaminergic neurons (Huang et al., 2024). Furthermore, three-dimensional organoids or microglial cells (iMGLs) derived from iPSCs can be used to study NDDs by simulating the microenvironment of brain development (Bhargava et al., 2022; Karagiannis et al., 2019). The advantages of MSCs lie in their inherent immunomodulatory functions and ease of acquisition, while iPSCs can construct patient-specific disease models. Both have complementary potential in exploring neural regeneration mechanisms and clinical translation (Joo et al., 2020; Shi et al., 2024).
2.2 Structure and biological function of extracellular vesicles
2.2.1 The formation and secretion process of extracellular vesicles
Extracellular vesicles were first discovered in 1981, initially known as exfoliative vesicles (Trams et al., 1981). EVs, are lipid-delimited biological nanoparticles that appear to be released by all cell types. EVs are usually between 30 and 150 nm in diameter. The biogenesis of exosomes begins with the endocytosis of the cell membrane forming early endosomes (Wang et al., 2024; Xie et al., 2022) and subsequently matures into multivesicular bodies (MVBs), within which intraluminal vesicles (ILVs) are formed through membrane invagination (Hessvik and Llorente, 2018; Yue et al., 2020). After MVBs fuse with the plasma membrane, ILVs are released as exosomes, a process dependent on the Rab protein family and membrane fusion mechanisms (Kim et al., 2018; Xu et al., 2023) (Figure 1). In addition to exosomes that originate from the endocytic pathway, cells can also secrete small extracellular vesicles (sEVs) directly through budding from the plasma membrane. Their formation involves the ESCRT complex or lipid raft microdomains (Gurung et al., 2021; Ishii et al., 2023; Moyano et al., 2019). Recent studies emphasize the high heterogeneity of EVs, including subtypes such as exosomes, microvesicles, and apoptotic bodies, which exhibit significant differences in biogenetic pathways and molecular characteristics (Rincón-Riveros et al., 2021; Yuan et al., 2023). Their efficient delivery capability may be related to their natural or acquired tropism, which allows them to cross cellular barriers and result in fewer off-target effects compared to synthetic nanoparticles (Elsharkasy et al., 2020; Witwer and Wolfram, 2021). EVs derived from MSCs can modulate signaling pathways in recipient cells, playing important roles in tissue repair and immune regulation (Gurung et al., 2021; Yu et al., 2020; Yuan et al., 2023). Recent advancements demonstrate that engineering modifications of EV surface proteins can enhance their therapeutic specificity, such as the simultaneous display of two decoy receptors significantly improving anti-inflammatory effects. These findings highlight the unique advantages of EVs as natural delivery vectors (Cheng and Hill, 2022; Gupta et al., 2021; Herrmann et al., 2021; Kalluri and LeBleu, 2020; Möller and Lobb, 2020; van Dommelen et al., 2012).
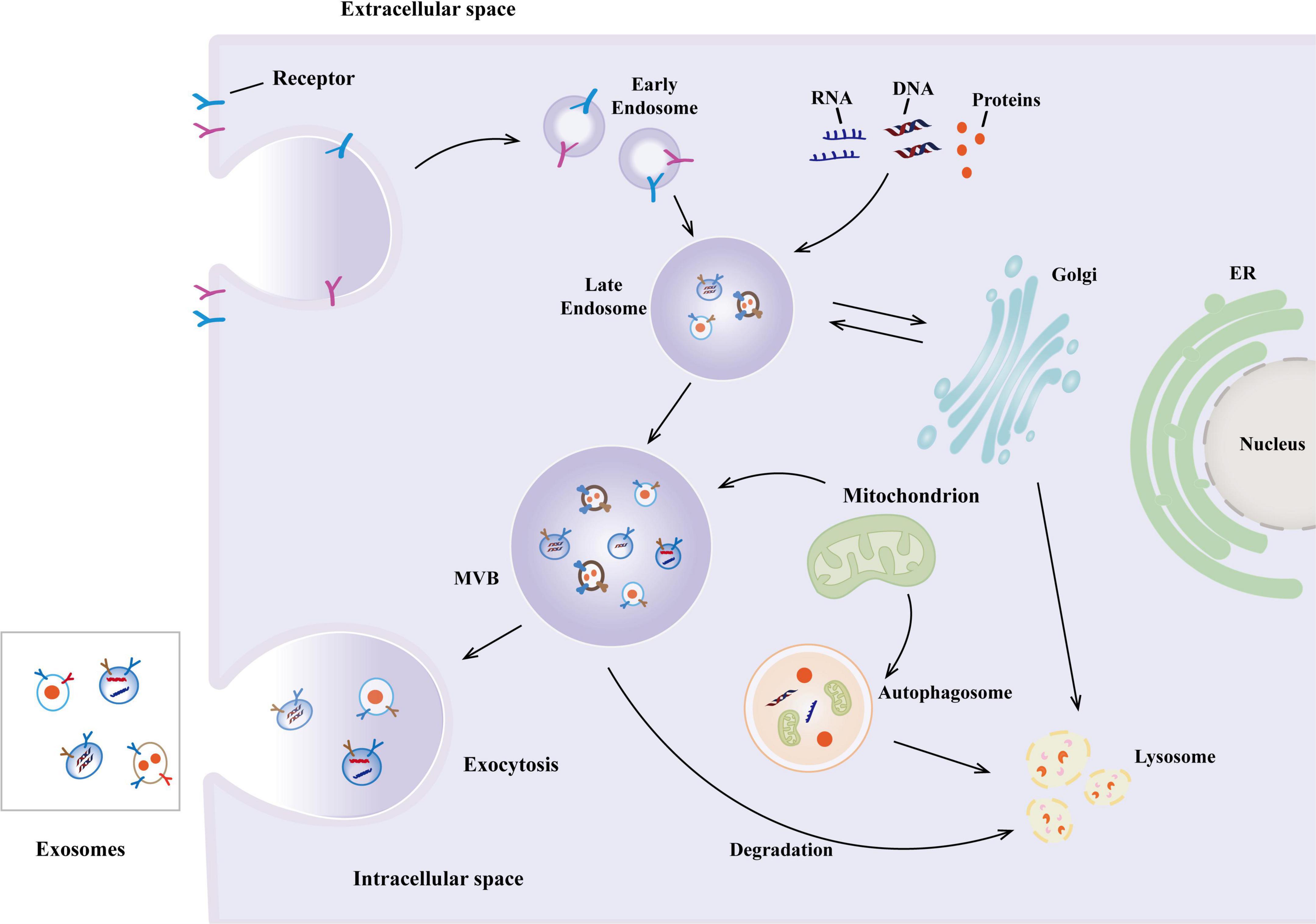
Figure 1. The biogenesis of exosomes. The biogenesis of exosomes begins with the formation of early endosomes in the endocytosis of the cell membrane and subsequently matures into multivesicular bodies (MVBs), in which intraluminal vesicles (ILVs) are formed through membrane invagination. After MVBs fuse with the plasma membrane, ILVs are released as exosomes.
2.2.2 Bioactive components of extracellular vesicles
Extracellular vesicles contain a range of proteins, including membrane and cytoplasmic proteins, which are crucial for cell signal transduction, intercellular interactions, and immune responses (Yuan et al., 2023). The quantity of bioactive molecules transported by EVs varies depending on the type of cell (Jeppesen et al., 2019) and the stage of cell differentiation (Romancino et al., 2013). MSC-EVs are typically rich in growth factors and cytokines related to cell survival, proliferation, and differentiation, such as transforming growth factor (TGF) and platelet-derived growth factor (PDGF) (Tan et al., 2024). The lipid bilayer membrane of EVs not only maintains structural integrity but also carries various bioactive substances that are essential for regulating their biological functions (Sun et al., 2021). EVs contain various effector proteins, messenger RNA (mRNA), microRNA (miRNA), and mitochondrial RNA (mtRNA), mitochondrial DNA (mtDNA), which can potentially affect the fate of target cells by regulating gene expression through interaction with receiving cells (Fakouri et al., 2024).
3 The anti-inflammatory mechanism of stem cell extracellular vesicles
3.1 The role of extracellular vesicles in immune regulation
Immune cell-derived exosomes (IEXs) are key mediators of intercellular communication that play a central, bidirectional regulatory role in inflammatory responses, with their functions being highly dependent on the type and state of their parent cells (Glass et al., 2010; Hickman et al., 2018). When activated, microglia exhibit heterogeneous responses toward surrounding cells, manifesting either pro- or anti-inflammatory effects (Ajami et al., 2018; Ransohoff, 2016). Microglia-driven inflammation may further compromise astrocytic function, impairing their capacity to support neuronal survival and growth while promoting neurotoxic phenotypes (Jha et al., 2019; Liddelow et al., 2017). Under pathological conditions such as hypertension, activated macrophages release EVs enriched with pro-inflammatory factors. Concurrently, reduced levels of miR-17—a negative regulator of ICAM-1—in these EVs establish a pro-inflammatory feedback loop that activates the downstream NLRP3 inflammasome pathway, thereby amplifying the inflammatory cascade (Hazrati et al., 2022; Osada-Oka et al., 2017). Conversely, MSC-EVs demonstrate potent anti-inflammatory and immunomodulatory properties. These EVs induce regulatory T cell (Treg) differentiation, suppress pro-inflammatory Th17 cell activity (Heidari et al., 2022; Yang et al., 2021), and modulate dendritic cell (DC) signaling pathways through targeted molecular delivery. MSC-EVs engineered to overexpress miR-540-3p inhibit the CD74/NF-κB axis in DCs, reducing pro-inflammatory cytokines IL-1β, IFN-γ while enhancing anti-inflammatory mediators IL-10, TGF-β1, ultimately attenuating inflammation and tissue damage (He et al., 2024). Similarly, Treg-derived EVs deliver bioactive molecules (dimethyl fumarate, DMF) directly to local tissue cells such as keratinocytes, suppressing inflammation and promoting immune tolerance (Ou et al., 2025). Collectively, stem cell EVs function as versatile carriers of specific biomolecular “cargo,” possessing inherent cell-targeting capabilities, engineerable plasticity for functional reprogramming, and context-dependent duality in disease pathogenesis.
3.2 Inhibition of proinflammatory cytokines by extracellular vesicles
Mesenchymal stem cell-derived extracellular vesicles exert immunomodulatory effects by regulating the function of immune cells. In a lipopolysaccharide-induced neuroinflammation mouse model, MSC-EVs can inhibit microglial activation and significantly reduce the levels of pro-inflammatory factors TNF-a and IL-6 in the hippocampal region (Domenis et al., 2018). This anti-inflammatory effect is particularly prominent in models of chronic NDDs. For instance, studies on transgenic mice with AD have shown that EVs treatment can reduce inflammatory infiltration around amyloid plaques (Wang et al., 2021). Notably, specific cytokines can optimize the therapeutic potential of EVs. Croitoru-Lamoury et al. (2011) discovered that IFN-γ-stimulated bone marrow MSCs enhance immunosuppressive capabilities by upregulating indoleamine-2,3-dioxygenase (IDO) expression, a mechanism that has shown efficacy in experimental autoimmune encephalomyelitis (EAE) mouse models. MSC-EVs may exert their effects by carrying specific microRNAs. In the serum EVs model of cynoglossus semilaevis, Sun et al. (2022) found that miR-133-3p can reduce the production of IL-1β and IL-6 by inhibiting the PP2A/NF-κB pathway, a mechanism that has also been validated in mammalian macrophage lines. MSC-EVs treated with proinflammatory cytokines have been found to enhance immunosuppressive and anti-inflammatory potential (Domenis et al., 2018). Wozniak et al. (2020) demonstrated, using a hepatitis virus infection model, that inflammatory signals can promote the selective loading of exosomal miRNA mediated by the RNA-binding protein FMR1. Additionally, EVs can reduce pro-inflammatory responses by inhibiting the formation of inflammasome complexes. The systematic review by Noonin and Thongboonkerd (2021) indicates that MSC-EVs can inhibit caspase-1 activation and IL-1β maturation in a sepsis model by blocking the assembly of the NLRP3 inflammasome. These studies suggest that EVs have different regulatory mechanisms in various stages of inflammation and disease models.
3.3 Extracellular vesicles and intercellular communication
This communication is crucial for coordinating cellular responses and maintaining tissue homeostasis (Hade et al., 2021; Tan et al., 2024). EVs can transfer bioactive molecules from one cell to another. This process can be carried out through direct cell contact or through intercellular space. When EVs bind to target cells, the molecules in EVs can be released and affect the function of target cells. MiRNAs in stem cell EVs can regulate gene expression in target cells, thereby affecting cell proliferation and differentiation (Liu et al., 2022b; Singh G. et al., 2024). MiRNAs play a crucial role in regulating the development and function of DCs. Studies have shown that miRNAs can influence the maturation, migration, and antigen presentation of DCs, thereby affecting the strength and nature of the immune response (Zhou and Wu, 2017). MiRNAs not only regulate gene expression independently, but also regulate the function of peripheral cells by interacting with signal transduction pathways. In response to peripheral nerve injury, the expression pattern of miRNAs changes and regulates signaling pathways related to nerve protection and regeneration, suggesting that miRNAs play a crucial role in the repair and regeneration of peripheral nerves (Borger et al., 2022).
4 The core pathogenic role of inflammation in neurodegenerative diseases
4.1 Alzheimer’s disease
Alzheimer’s disease is a chronic, progressive neurodegenerative disorder, accounting for approximately 60%–80% of all dementia cases (De-Paula et al., 2012; Scheltens et al., 2021). It is characterized by cognitive and behavioral impairments that significantly affect patients’ social and occupational abilities (Zhang et al., 2024). Currently, it is an incurable disease with a lengthy preclinical course. The pathogenesis of AD is not yet fully understood. Its main characteristics involve the synergistic pathological interaction between amyloid beta (Aβ) accumulation and excessive phosphorylation of tau protein, both of which are key drivers of neurodegenerative lesions (Burns and Wang, 2017; Ising et al., 2019; Madar et al., 2024). The study found that microglia are activated in response to amyloid plaques, leading to the release of pro-inflammatory cytokines that contribute to neuroinflammation and neuronal damage (Balducci and Forloni, 2018; Leng and Edison, 2021; von Bernhardi et al., 2015).
In summary, Aβ plaques induce synaptic dysfunction through amyloid-mediated interference and further trigger cascade events that directly promote tau pathology (Abdelnour et al., 2022; Hyman et al., 2012; Serrano-Pozo et al., 2011). Research indicates that Aβ oligomers activate the NLRP3 inflammasome in microglia, promoting the release of interleukin-1β (IL-1β), thereby enhancing tau kinase activities (such as GSK-3β) while inhibiting phosphatases. This neuroinflammatory environment leads to excessive tau phosphorylation, tangle formation, and subsequent neuronal death (Cummings, 2021; Wang C. et al., 2022; Zhao et al., 2024). In mouse models, intracerebral injection of Aβ induces tau pathology, while NLRP3 gene knockout alleviates tau aggregation and cognitive deficits (Wang C. et al., 2022; Zhao et al., 2024). This creates a self-amplifying cycle in which Aβ-induced neuroinflammation exacerbates tau toxicity, ultimately accelerating synaptic loss and hippocampal atrophy (Pini et al., 2016; Singh M. et al., 2024; Tangaro et al., 2014). It has been found that the release of TNF-a, IL-1β, and nitric oxide (NO) regulates the transformation of microglia into pro-inflammatory phenotype (Huang R. et al., 2023; Li et al., 2023). A recent study demonstrated that C-X-C motif chemokine ligand 10 (CXCL10) and its receptor, CXCR3, are pivotal in regulating the infiltration of CD8+ T cells into the brain. These infiltrating CD8+ T cells interact with microglia, collectively promoting neurodegenerative changes associated with AD (Jorfi et al., 2023). TREM2 is a potential therapeutic target due to its neuroprotective role in early AD, mitigating neuroinflammation, cognitive impairment, and M1 microglia polarization via the PI3K/AKT/FoxO3a pathway in AD models (Wang Y. et al., 2020).
Tau pathology exacerbates Aβ accumulation by impairing microglial clearance functions and promoting astrocyte dysfunction. Reactive astrocytes lose their ability to degrade Aβ (Han et al., 2019; Lana et al., 2023; Rather et al., 2021) and release pro-inflammatory mediators TNF-a, IL-6, further polarizing microglia toward a neurotoxic phenotype (Kitazawa et al., 2005; Novoa et al., 2022). This interaction between glial cells underscores the interdependence of Aβ and tau, as both pathologies jointly sustain chronic neuroinflammation and neuronal death (Arranz and De Strooper, 2019; Gopinath et al., 2023; Rossi and Volterra, 2009). Emerging therapeutic strategies reflect attention to this dual pathology, with MSCs modulating microglial polarization toward an anti-inflammatory phenotype (M2) through the secretion of TGF-β1, resulting in better cognitive recovery compared to single-target approaches (Ju and Tam, 2020; Wang Y. et al., 2020) (Figure 2).
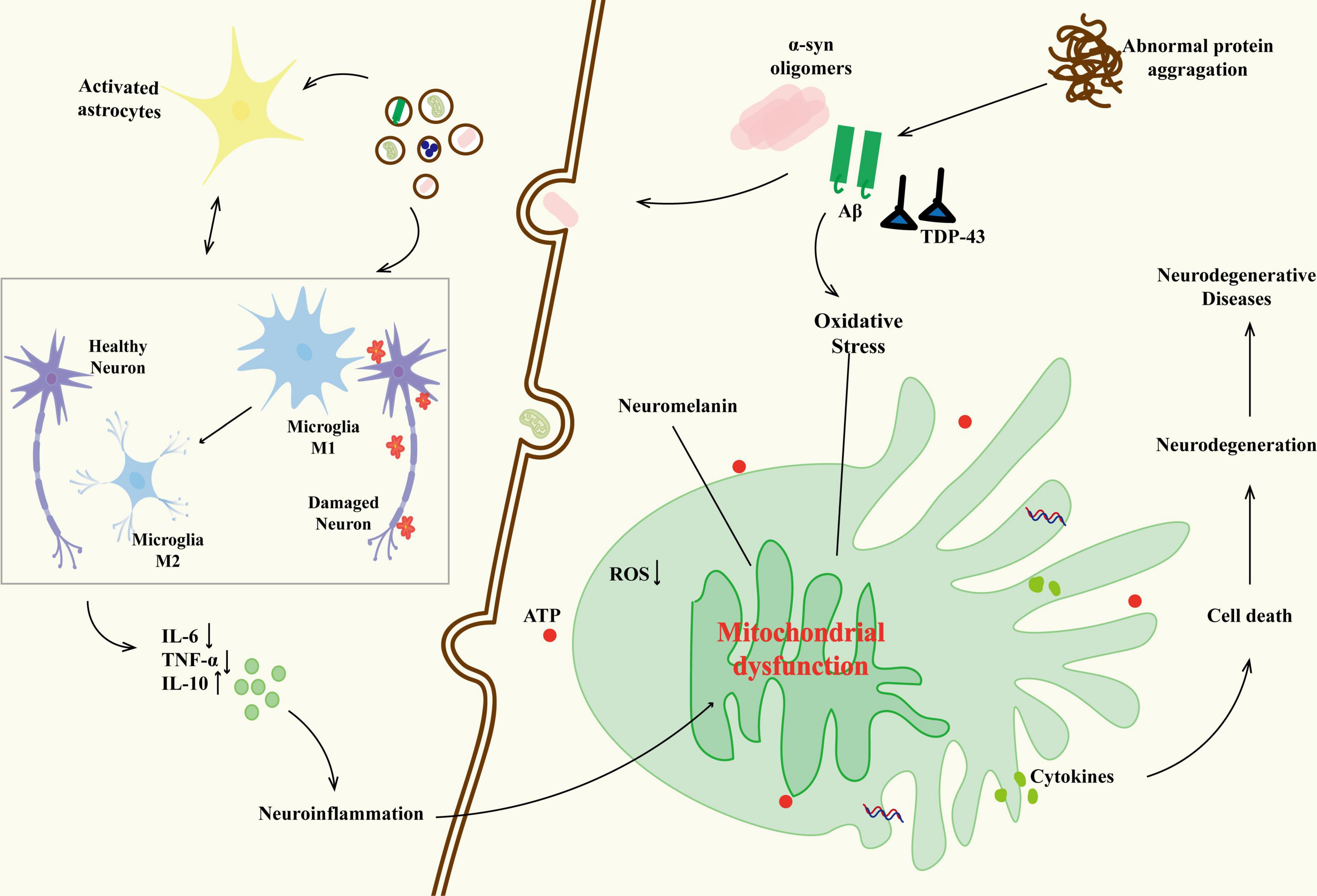
Figure 2. The relationship between neurodegenerative diseases and glial cells. The abnormal protein aggregation activates microglia (M1 phenotype releases IL-6 and TNF-a) and triggers their interaction with astrocytes (IL-6), forming a positive feedback loop of neuroinflammation. As the disease progresses, some M1 microglia transform into the M2 phenotype, releasing IL-10 in an attempt to repair; however, their function is inhibited by the oxidative stress microenvironment. The persistent activation of glial cells results in neuronal mitochondrial dysfunction (reduced ATP production, oxidative stress imbalance), abnormal deposition of neuromelanin, and loss of synaptic plasticity, ultimately driving the malignant progression of neurodegenerative diseases through blood–brain barrier leakage and inflammatory factor infiltration.
4.2 Parkinson’s disease
Parkinson’s disease is the second most common NDDs after AD. Globally, 10 million patients are affected, with a higher incidence among people over 60 years old (Dorsey et al., 2018; GBD 2016 Neurology Collaborators, 2019). The core pathological features are the progressive loss of dopaminergic neurons in the substantia nigra and the formation of Lewy bodies (LBs) (Tolosa et al., 2021; Ye et al., 2023). The death of dopaminergic neurons leads to motor symptoms, tremor, rigidity, and non-motor symptoms, cognitive impairment, mood disorders, directly related to the dysfunction of the nigrostriatal pathway (Chaudhuri et al., 2006; Pfeiffer, 2016; Poewe, 2008). Neuroinflammation is a key driving factor in the progression of PD (Grayson, 2016; MacMahon Copas et al., 2021; Samii et al., 2004). The degree of microglial activation is associated with the dopaminergic terminal loss in early-stage PD (Ouchi et al., 2005). Abnormal aggregation of a-synuclein is not only a major component of LBs but also triggers a neuroinflammatory cascade by activating the TLR4/NLRP3 inflammasome pathway in microglia, leading to the release of pro-inflammatory factors such as IL-1β and IL-18 (Fu et al., 2023). This inflammatory microenvironment exacerbates neuronal damage and motor/cognitive dysfunction by regulating synaptic plasticity and neurotransmitter metabolism (Erta et al., 2012; Santello and Volterra, 2012). Promoting the activation of M2-type microglia may have potential benefits in slowing the progression of PD (Badanjak et al., 2021). The NLRP3 inflammasome is activated by ROS, upregulating IL-18 levels, which is positively correlated with the severity of motor symptoms and can serve as an inflammatory biomarker for PD (Cabrera Ranaldi et al., 2023). There is a bidirectional regulation between the metabolic reprogramming of microglia and the activation of the NLRP3 inflammasome: mitochondrial dysfunction can enhance inflammasome assembly, whereas blocking NLRP3 can reverse the polarization of microglia toward the pro-inflammatory M1 type (Huang R. et al., 2023). Additionally, peripheral inflammation promotes the spread of central inflammation through BBB leakage, and elevated serum CRP in elderly patients is associated with increased risk of PD (Jin et al., 2020). Imaging studies show a positive correlation between the degree of microglial activation in the substantia nigra of PD patients and the loss of dopaminergic terminals, providing a dynamic assessment indicator for inflammation-targeted therapies (Tansey et al., 2022). EVs can act as central conduits for the transmission of a-synuclein, amplifying neuroinflammation and shuttling peripheral inflammatory signals, making them key diagnostic/therapeutic targets.
4.3 Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is a NDD that primarily affects motor neurons in the brain and spinal cord (Feldman et al., 2022). Known for its progressive and lethal nature, the disease gradually causes patients to lose muscle control, ultimately affecting breathing and other basic physical functions (Hardiman et al., 2017). ALS is pathologically characterized by degeneration of upper motor neurons in the cerebral cortex and lower motor neurons in the spinal cord/brainstem (Rizea et al., 2024; van den Bos et al., 2019). With a global incidence rate of 1–2 per 100,000, this neurodegenerative disorder demonstrates epidemiological patterns of slight male predominance and higher prevalence in middle-aged and elderly populations. Despite being classified as a rare disease, its progressive and debilitating nature imposes substantial physical and psychological burdens on both patients and their families (Arthur et al., 2016; Xu et al., 2020). Its core pathological features include abnormal aggregation of TDP-43 protein within motor neurons and a glial cell-mediated neuroinflammatory cascade (de Boer et al., 2020; Lee et al., 2011). Recent studies emphasize that neuroinflammation driven by non-neuronal cells (Appel et al., 2021; Clarke and Patani, 2020) is a key mechanism accelerating disease progression: the polarization of microglia from an early protective M2 phenotype to a late toxic M1 phenotype (Liao et al., 2012), together with astrocytes forming a pro-inflammatory microenvironment through Cx30-mediated inflammatory signaling (Hashimoto et al., 2022). The degeneration of oligodendrocytes leads to dysfunction, causing axonal lesions in the motor neurons of ALS patients (Kang et al., 2013; Philips et al., 2013). Activation of the NLRP3 inflammasome results in the release of cytokines such as IL-1β and IL-18 (Beers and Appel, 2019), forming an inflammatory amplification loop along with the NF-κB pathway abnormalities induced by optineurin (OPTN) gene mutations (Akizuki et al., 2013; Markovinovic et al., 2018; Toth and Atkin, 2018). Additionally, some ALS cases have a familial hereditary nature. Known genetic mutations, such as superoxide dismutase 1 (SOD1) and C9orf72, are closely related to the pathogenesis of this disease (Marino et al., 2015; Ross and Poirier, 2005). Furthermore, these mutations may affect protein folding and function, leading to neuronal dysfunction and death (Aguzzi and Rajendran, 2009; Polymenidou and Cleveland, 2011). Notably, specific interventions targeting astrocytes in SOD1 mutant mice have confirmed that targeting glial cell inflammation can significantly delay disease progression (Ferraiuolo et al., 2016; Wang et al., 2011). Thus, EVs act as central performers to direct the transport of mutant cargo that drives neuronal protein toxicity, while providing actionable targets for intervention through EV modulation or engineering.
4.4 Multiple sclerosis
Multiple sclerosis is a complex, chronic autoimmune disease affecting the CNS (Bjornevik et al., 2022; Olsson et al., 2017), where the immune system mistakenly attacks the myelin sheath of the CNS, leading to neuronal damage and dysfunction. As the myelin is progressively damaged, patients may experience a range of symptoms, including motor dysfunction, sensory disturbances, and visual problems (Correale et al., 2017; Doshi and Chataway, 2016). This immune response may be driven by genetic susceptibility involving variations in multiple immune-related genes (Ghasemi et al., 2017; Goodin, 2024), which synergistically interact with environmental factors [such as Epstein-Barr virus (EBV) infection and vitamin D deficiency] through epigenetic regulation to produce a pathogenic effect (Fujinami et al., 2006; O’Gorman et al., 2014; Speer, 2013; Zhang et al., 2000). The core pathological hallmark manifests as autoimmune-mediated destruction of CNS myelin sheaths, with disease progression being persistently accompanied by continuously evolving neuroinflammatory processes (Compston and Coles, 2008; Lassmann, 2018). Investigations reveal that microglia and astrocytes exhibit spatiotemporal-specific activation patterns in neuroinflammatory cascades: During acute phases, microglia facilitate tissue repair through myelin debris clearance and anti-inflammatory factor secretion (Dendrou et al., 2015; Dobson and Giovannoni, 2019; Lassmann, 2018), whereas chronic activation exacerbates axonal damage via pro-inflammatory mediators, such as TNF-a (Arnett et al., 2001; Liu et al., 1998). Notably, this cytokine demonstrates biphasic regulatory capacity by concurrently promoting oligodendrocyte precursor cell proliferation (Compston and Coles, 2008; Marcus, 2022). Aberrant activation of the receptor-interacting protein kinase 1 (RIPK1) signaling pathway has been identified as a critical molecular mechanism driving neuroinflammation, demonstrating specific upregulation in MS patients’ brain tissues and mediating the formation of astrocyte-microglia inflammatory networks (Zelic et al., 2021). At the immunoregulatory level, anti-inflammatory cytokines IL-4 and IL-10 exert direct neuroprotective functions through modulation of Th cell differentiation (Hulshof et al., 2002; Wang et al., 2018), with their receptors exhibiting specific distribution patterns in MS lesions. While astrocyte-derived glial scars effectively confine inflammatory spread, their physical barrier properties may concurrently impair regenerative processes, this dual functionality shows close association with cellular activation states and microenvironmental factors (Ponath et al., 2018; Sen et al., 2022; Sofroniew and Vinters, 2010; Voss et al., 2012). Furthermore, BDNF has demonstrated pivotal neuroprotective effects in EAE models by sustaining oligodendrocyte survival and promoting myelin regeneration (Linker et al., 2010), whereas IFN-κ’s immunomodulatory functions influence disease progression through Th1/Th2 balance regulation (Billiau et al., 1988).
5 The application of stem cell extracellular vesicles in neurodegenerative diseases
5.1 In vitro studies
Stem cell extracellular vesicles show multidimensional therapeutic potential in in vitro models of NDDs. Its core mechanism relies on the inherent biologically active “cargo” delivery capacity of EVs. Neuroprotection, human amniotic fluid stem cell exosomes (hAFSC-Exos) play a crucial role by inhibiting microglial activation. After exosomal intervention in LPS-activated microglia, the expression of pro-inflammatory markers is significantly downregulated, and Aβ-induced oxidative stress and neuronal apoptosis are effectively alleviated, confirming their neuroprotective effects through the regulation of neuroinflammatory pathways (Zavatti et al., 2022). Bone marrow mesenchymal stem cell-derived extracellular vesicles (BMSCs-EVs) carry miR-29c-3p, which reduces Aβ production by inhibiting BACE1 expression (Sha et al., 2021). The therapeutic mechanisms of EVs include inhibiting the overactivation of microglial cells and blocking the inflammatory damage to dopaminergic neurons. However, catalase enriched in neural stem cell-derived extracellular vesicles (NSC-EVs) in PD attenuates oxidative stress to protect dopaminergic neurons (Díaz Reyes et al., 2025). In terms of anti-inflammatory regulation, BMSCs-EVs-derived TNF-stimulated gene-6 (TSG-6) maintains dopaminergic neuronal survival by blocking the ubiquitinated degradation of LRRK2 by inhibiting the STAT3-miR-7-NEDD4 pathway, while engineered miR-7-overexpressing EVs attenuate MPP Induced neurotoxicity (Huang et al., 2022). Li et al. (2019) confirmed that BMSCs-EVs alleviate central nervous inflammation effectively by modulating immune polarization, elevating IL-10 and TGF-B, and inhibiting TNF-a and IL-12. These findings systematically uncover the core mechanisms by which stem cell EVs intervene in NDDs through multi-target, multi-pathway interactions.
5.2 Preclinical studies
Preclinical studies of stem cell EVs in NDDs have demonstrated therapeutic potential across a spectrum of diseases, with their mechanisms of action achieved through delivery system optimization and multi-target regulation. In the field of AD, breakthroughs in CNS-targeted delivery technology have significantly enhanced efficacy. Its molecular mechanisms involve the reduction of inflammatory factors such as IL-1β and TNF-a, the clearance of Aβ deposits, and the promotion of neuron survival and regeneration. Cui et al. (2019) developed RVG-modified MSC-EVs, which, upon intravenous injection, significantly improved cognitive function in APP/PS1 model mice and reduced levels of inflammatory factors TNF-a, IL-1β, and IL-6. Cone et al. (2021) demonstrated that intranasal administration of human bone marrow mesenchymal stem cells-derived extracellular vesicles (hBMSCs-EVs) in A5XFAD mice, which carry five familial AD mutations, significantly enhanced the clearance of Aβ plaques in the hippocampus and effectively decelerated disease progression compared to saline-treated controls. PD research reveals core pathways through multi-model validation: in the 6-OHDA injury model, BMSCs-EVs significantly repair the dopaminergic pathway through striatal-targeted delivery (Bouchez et al., 2008). Ahmed et al. (2016) found that the rotenone model further reveals that BMSCs-EVs reverse the neuroinflammatory microenvironment by regulating the TGF-β1/IL-17 axis. Nasally administered umbilical cord MSC-EVs cross the BBB and improve motor coordination in PD mice (Huang et al., 2024). Chan et al. (2023) confirmed that human adipose-derived stem cell exosomes (hADSCs-Exos) exert neuroprotective effects in PD mouse models through anti-inflammatory properties. Mechanistic studies confirm their dual role, suppressing abnormal aggregation of a-syn (Gouda et al., 2022) while upregulating tyrosine hydroxylase (TH) expression (Ahmed et al., 2016). MSC-EVs of different origins exert their effects through characteristic pathways, hADSCs-Exos target the inhibition of the IL-17 signaling axis (Ghasemi et al., 2014; Shalaby et al., 2016), and Bai et al. (2012) found that BMSCs-EVs mediate myelin repair through HGF. A systematic review by Barabadi et al. (2024) confirmed that MSC-EVs achieve improvement in neurological function scores in 80% of preclinical models. Zhang et al. (2022) reported that mouse MSC-EVs can alleviate demyelinate ion-induced functional impairment and promote neurological function recovery. In the field of ALS, ASC EVs have demonstrated neuroprotective ubiquity through dual administration routes: intravenous injection primarily improves the survival of lumbar motor neurons, while nasal administration achieves targeted enrichment in brainstem lesion areas (Kuzma-Kozakiewicz et al., 2018). Their mechanism of action involves the repair of neuromuscular junction structures and the inhibition of glial cell activation, providing new evidence for research on trans-synaptic transmission. These studies collectively build a therapeutic network of stem cell EVs from local intervention to systemic regulation, laying a theoretical foundation for clinical translation.
5.3 Clinical trials
The clinical translational research of stem cell EVs in NDDs has shown multidimensional breakthroughs. In the field of AD, innovative therapy through nasal delivery of MSC-EVs has achieved key progress: In a clinical trial involving patients with mild to moderate AD, treatment with MSC-EVs via nasal administration significantly improved patients’ cognitive functions. Evaluations using the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) showed that scores in the treatment group improved significantly, indicating enhanced cognitive function. The daily living abilities of patients in the treatment group also improved, with enhanced capabilities in activities such as dressing and eating, suggesting that stem cell EVs therapy helps improve cognitive and self-care abilities in AD patients. Although the sample size was small and the follow-up time was short, these preliminary results provide a reference for the clinical application of stem cell EVs therapy in AD and support further large-scale trials (Xie et al., 2023; Table 1). Clinical research on PD reveals the optimization direction of EVs drug delivery systems: hypoxic preconditioning of olfactory mucosa-derived exosome (hOM-Exos) treatment significantly reduces the Unified Parkinson’s Disease Rating Scale (UPDRS) scores in PD patients, indicating improved motor function (Zhuo et al., 2024; Table 2). Research on ALS focuses on mechanism innovation and efficacy validation: although mesenchymal stem cell-derived neurotrophic factor (MSC-NTF) treatment shows changes in patients’ ALSFRS-R scores, its efficacy requires further validation (Fisher-Shoval et al., 2012; Table 3). Research on MS has achieved a leap from scale assessment to functional recovery: BMSCs-EVs treatment significantly improved scores in the 25-foot walk test (T25FW) on the EDSS scale in MS patients (Kråkenes et al., 2024). Several clinical trials have confirmed that stem cell EVs therapy can enhance patients’ motor function and quality of life, marking a paradigm shift in EVs treatment from symptom control to functional remodeling (Table 4).
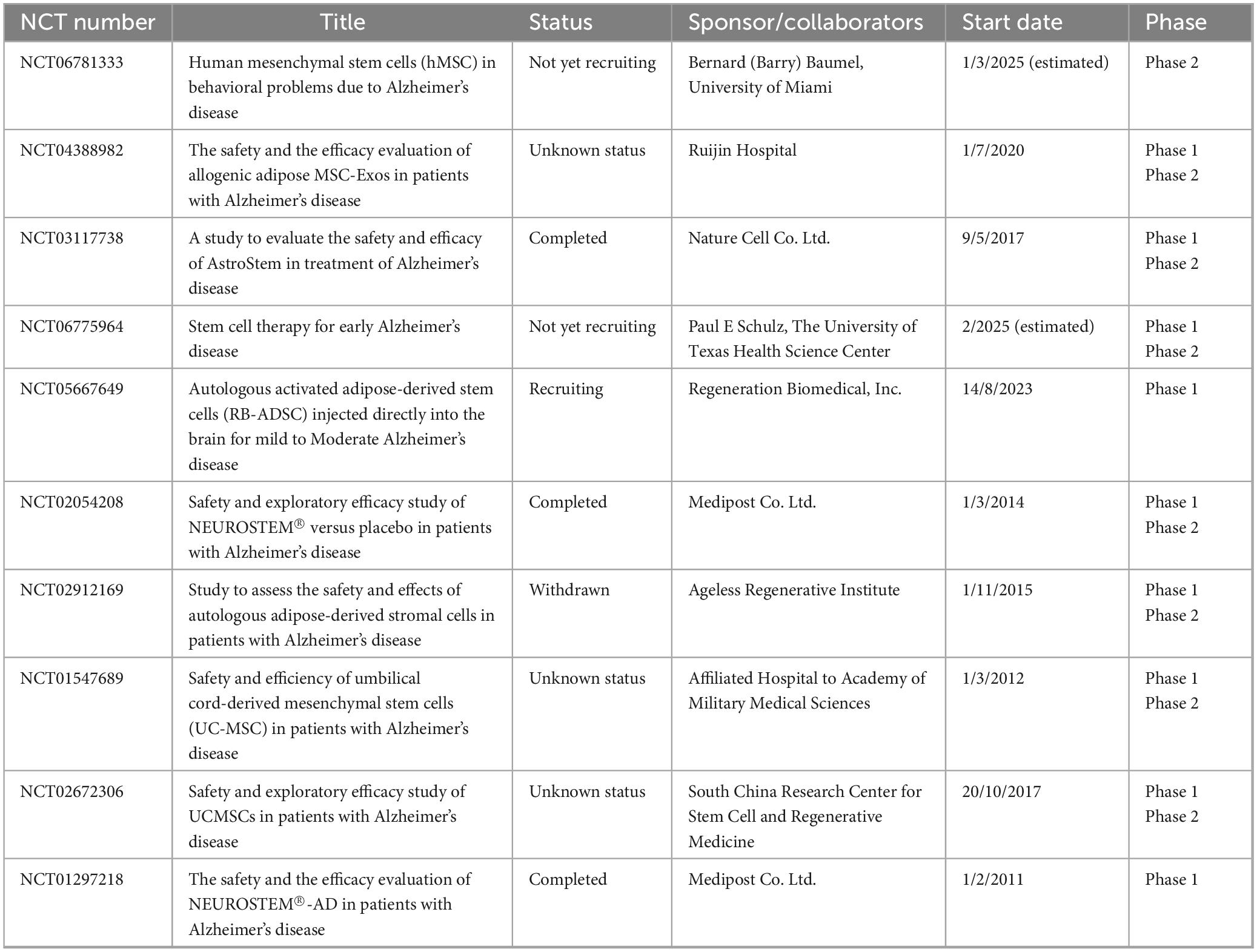
Table 1. The summary of clinical trial projects on stem cell-derived exosome therapy for Alzheimer’s disease.
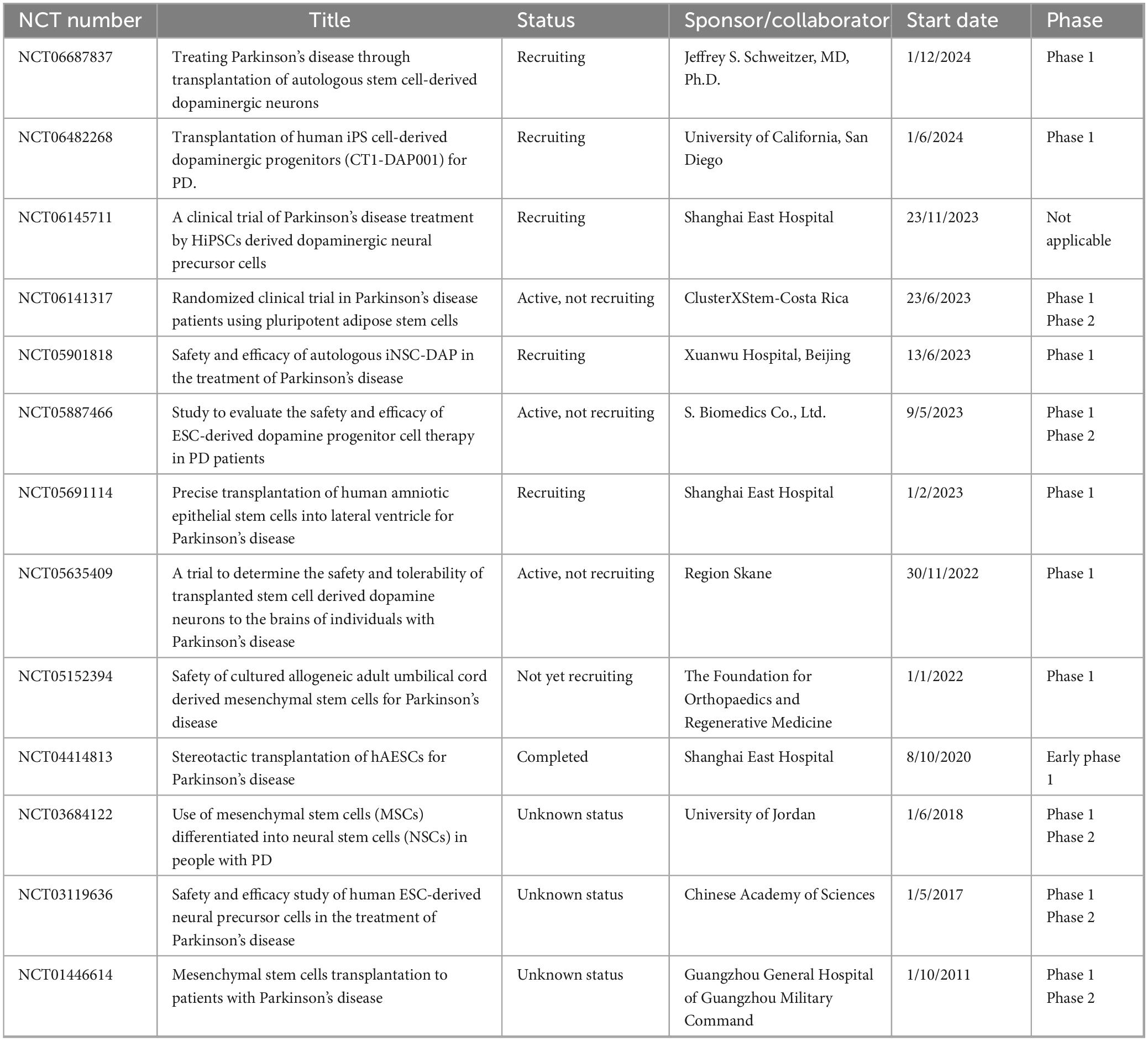
Table 2. The summary of clinical trial projects on stem cell-derived exosome therapy for Parkinson’s disease.
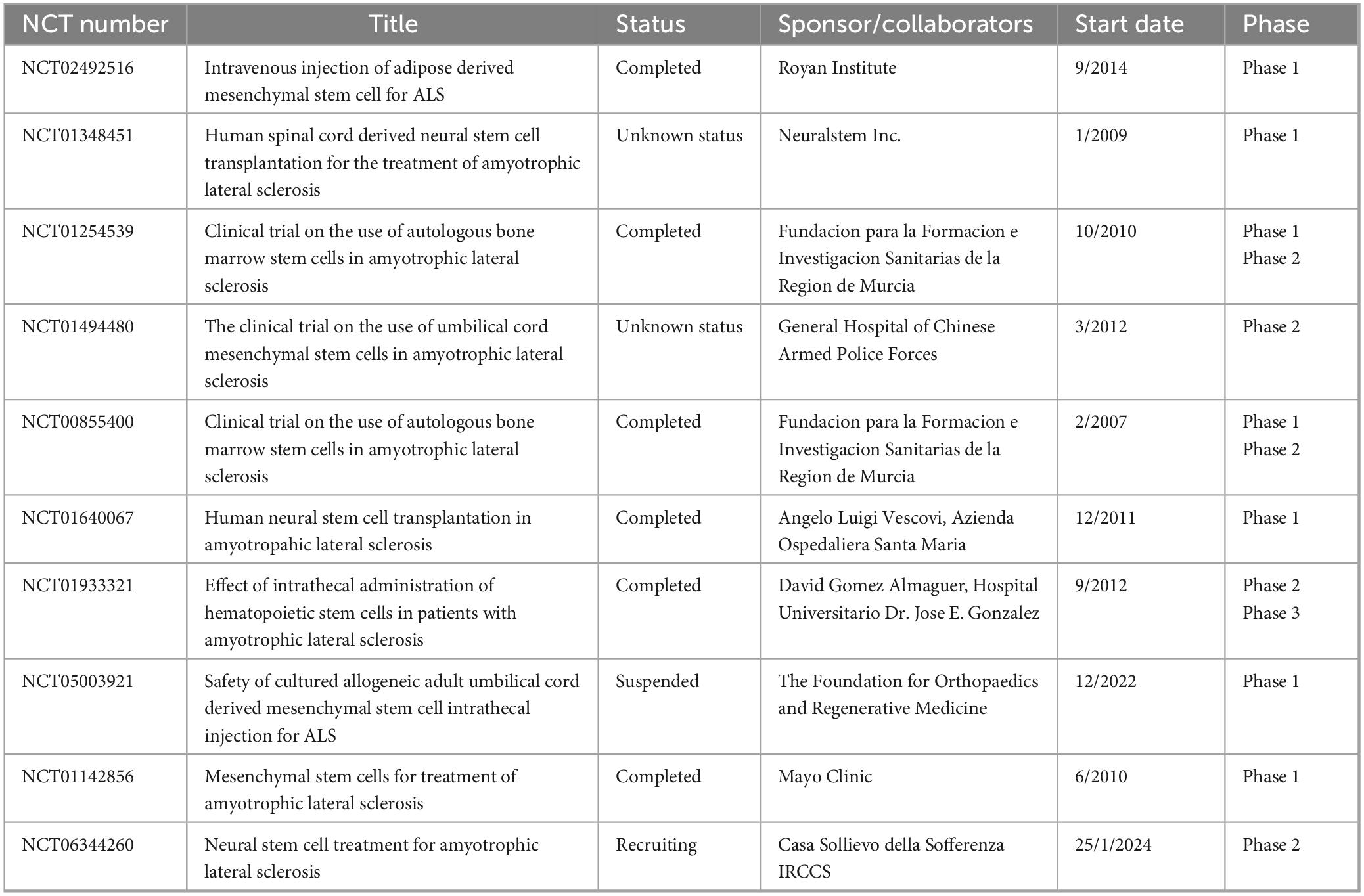
Table 3. The summary of clinical trial projects on stem cell-derived exosome therapy for amyotrophic lateral sclerosis.
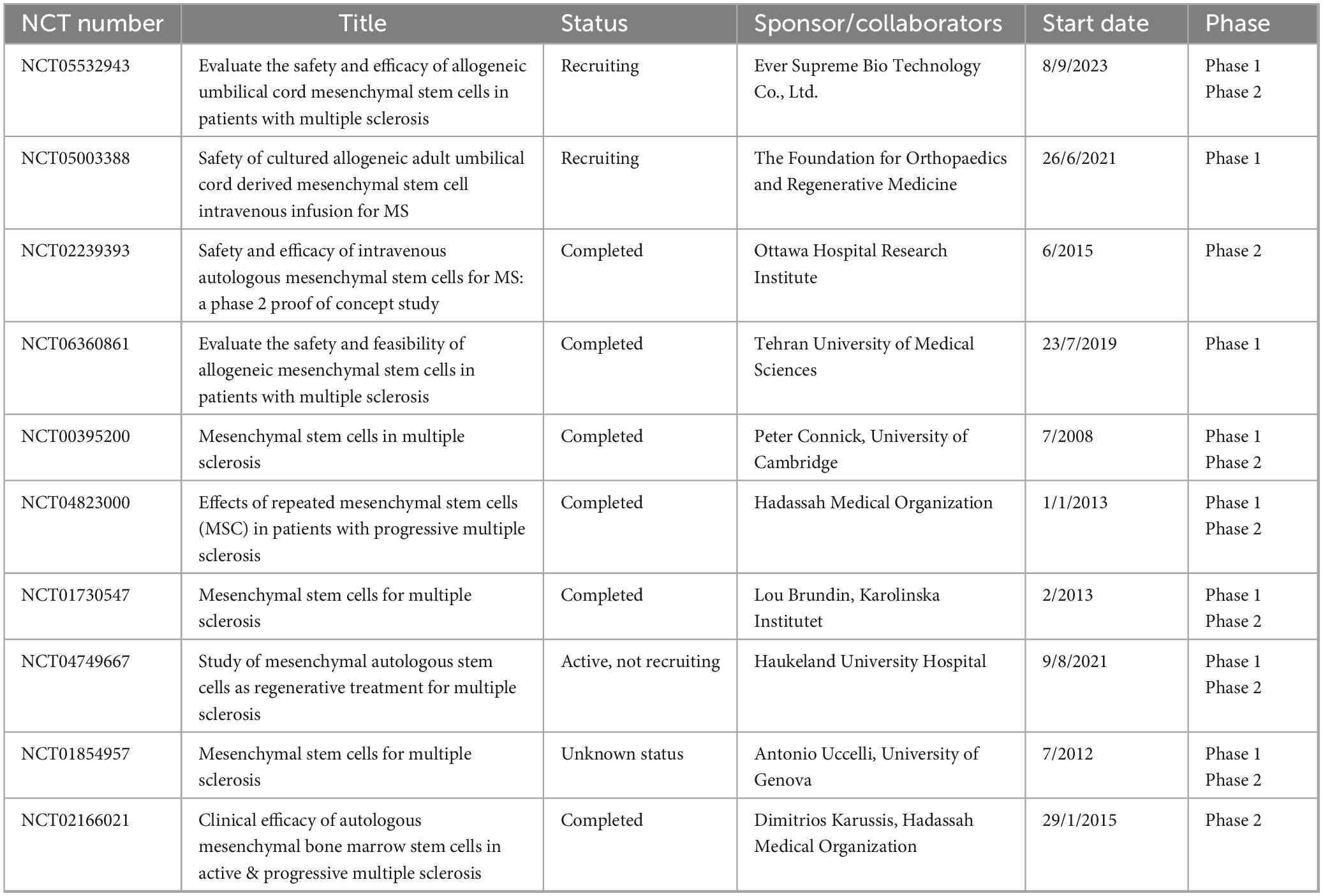
Table 4. The summary of clinical trial projects on stem cell-derived exosome therapy for multiple sclerosis.
6 Advantages and challenges
Stem cell EVs have significant advantages in the treatment of NDDs (Ranganath et al., 2012; Sajeesh et al., 2020; Santamaria et al., 2021). The lipid bilayer membrane of EVs effectively shields cargo components (miRNAs, enzymes) from degradation by blood-borne nucleases and proteases, thereby prolonging their circulatory half-life. In contrast, free anti-inflammatory factors like IL-10 undergo rapid clearance in systemic circulation. EVs demonstrate BBB penetration capability through receptor-mediated transcytosis (Saint-Pol et al., 2020; van Niel et al., 2018), whereas most anti-inflammatory proteins (including antibodies) lack direct access to the CNS. Notably, EVs can detect inflammatory signals in pathological tissues via surface molecules such as integrins, and exhibit tropism toward injury sites through biological recognition mechanisms (Shimaoka et al., 2019). This active targeting capacity (Liu et al., 2022b) remains unattainable for free molecular therapeutics. In terms of immune regulation, MSC-EVs can function through multiple mechanisms: they achieve bidirectional immune modulation by regulating the TLR/NF-κB signaling pathway and the Th17/Treg cell balance (de Godoy et al., 2018; Wang J. et al., 2020), while simultaneously delivering neurotrophic factors to promote neuron survival and reduce apoptosis rates (Fakouri et al., 2024; Namini et al., 2023; Tambe et al., 2024). Studies have also found that EVs can activate neural regeneration-related transcription factors such as Sox2/Pax6. Engineered EVs modified via the Notch signaling pathway can significantly enhance the differentiation efficiency of oligodendrocytes (Fričová et al., 2020). Thanks to their low immunogenicity (Heris et al., 2022) and potential for large-scale production (Chan et al., 2023), these characteristics make them a promising vehicle for clinical translation in neural repair.
However, the clinical application of this technology still faces significant challenges. Firstly, cross-contamination often occurs during EVs isolation and purification, and existing techniques are unable to completely remove other cellular components, which directly affects the purity and functional stability of the preparation (Moon et al., 2023). Secondly, although research indicates that EVs can target brain lesion areas via the BBB, their non-specific distribution in systemic circulation may lead to off-target effects (Wan et al., 2020). Intravenous injection acts quickly but is prone to off-target effects and is suitable for systemic diseases (Wiklander et al., 2015). Intraperitoneal targeting affects the metabolic system (Zhou et al., 2020), oral administration targets the digestive system (Umezu et al., 2021), and localized injections are precise and efficient but require caution regarding abnormal accumulation (Wang D. et al., 2022). Therefore, the choice should be based on therapeutic goals and targeting the specific organs needed (Song et al., 2022). Although the surface protein CD47 sends a “don’t eat me” signal by binding to SIRPa on macrophages, thus reducing the binding of EVs to macrophages and maximizing circulation time (Koh et al., 2017), optimizing targeting remains a challenge. Furthermore, the complex composition of EVs contents and their interaction mechanisms with host cells are not yet fully elucidated, which poses obstacles to the design and standardization of treatment protocols (Fričová et al., 2020). In order to solve the current obstacles based on stem cell EVs treatment, there is still a lack of uniform standards and guidelines for the preparation, storage and application of EVs. This lack of standardization may lead to inconsistent results between different studies, thus affecting the clinical application of EVs, and its potential mechanism needs further study.
7 Conclusion
Stem cell EVs demonstrate immense potential in the treatment of NDDs due to their ability to cross the BBB and multifunctional regulatory properties. Current research confirms that EVs alleviate neuroinflammation through mechanisms including immune response modulation, oxidative stress reduction, neuroprotection enhancement, and cellular metabolism optimization. These findings not only provide novel insights into the complexity of neuroinflammation but also establish a foundation for innovative therapeutic strategies. Longitudinal monitoring of inflammatory factor profiles within EVs isolated from patient serum or cerebrospinal fluid could enable the development of novel therapeutic biomarkers, facilitating early efficacy prediction and dynamic intervention. Emerging studies propose synergistic integration of EVs-based therapies with existing pharmacological agents to amplify therapeutic outcomes. Crucially, standardization of EVs isolation and characterization methodologies is essential for ensuring treatment consistency. Researchers are currently engineering MSCs via gene editing to optimize exosomal cargo composition, while developing advanced biomaterials to enhance targeting specificity, innovations poised to overcome current technical limitations. The anti-inflammatory properties of MSC-EVs hold significant promise for NDD management. Future investigations should prioritize mechanistic elucidation of EVs-mediated neuroinflammatory regulation, neuronal protection, and regeneration, with emphasis on identifying precise molecular targets and signaling pathways. Exploratory integration with emerging technologies such as brain-computer interfaces and cerebral organoid transplantation may enable neural network remodeling. Multidimensional innovations in this field are anticipated to transcend conventional therapeutic boundaries, ultimately driving a paradigm shift from delaying disease progression to achieving functional cure in neurodegenerative disorders.
Author contributions
MY: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation. HM: Conceptualization, Formal Analysis, Investigation, Writing – review & editing. XL: Conceptualization, Investigation, Writing – review & editing, Data curation. JW: Data curation, Methodology, Writing – review & editing, Formal Analysis. MS: Data curation, Methodology, Writing – review & editing. JY: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all the authors involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NDDs, neurodegenerative diseases; PD, Parkinson’s disease; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis; MSCs, mesenchymal stem cells; BBB, blood–brain barrier; iMGLs, iPSC-derived microglia; MVBs, multivesicular bodies; ILVs, intraluminal vesicles; BM-MSCs, bone marrow mesenchymal stem cells; iPSCs, induced pluripotent stem cells; TGF, transforming growth factor; PDGF, platelet-derived growth factor; mRNA, messenger RNA; miRNA, microRNA; mtRNA, mitochondrial RNA; PLEVs, plant-derived extracellular vesicles; TNF-a, tumor necrosis factor-alpha; IL-1 β, interleukin-1 β; IL-6, interleukin-6; IFN-γ, interferon gamma; IDO, indoleamine 2,3-dioxygenase; PGE2, prostaglandin E2; PRRs, pattern recognition receptors; IL-1, interleukin-1; A β, amyloid beta; NO, nitric oxide; DCs, dendritic cells; NF-κ B, nuclear factor kappa B; CXCL10, C-X-C motif chemokine ligand 10; SNpc, substantia nigra pars compacta; NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; UMN, upper motor neurons; LMN, lower motor neurons; SOD1, superoxide dismutase 1; Cx30, connexin 30; RIPK1, receptor-interacting protein kinase 1; BDNF, brain-derived neurotrophic factor; EAE, experimental autoimmune encephalomyelitis; RVG, rabies virus glycoprotein; hAFSC, human amniotic fluid stem cells; LPS, lipopolysaccharide; hADSC, human adipocyte-derived stem cells; 6-OHDA, 6-hydroxydopamine; TH, tyrosine hydroxylase; hOM, hypoxic preconditioning olfactory mucosa; UPDRS, Unified Parkinson’s Disease Rating Scale; ASC-EXOs, exosomes derived from adipose-derived stem cells; MSC-NTF, mesenchymal stem cell-induced neurotrophic factor; ALSFRS-R, ALS function rating scale; hWJ-MSC-derived OPCs, human umbilical cord-derived stem cell-derived oligodendrocyte precursor cells; hES-MSCs, human embryonic stem cell-derived mesenchymal stem cells; PL-MSCs, human placental MSCs; TSG-6, TNF-a-stimulated gene/protein 6; hPDLSCs, human periodontal ligament stem cells; NPCs, neural precursor cells; HGF, hepatocyte growth factor; ADSCs, adipose-derived stem cells; CNS, central nervous system; T25FW, timed 25-foot walk; IL-18, interleukin-18; CRP, C-reactive protein; LBs, Lewy bodies; IEXs, exosomes derived from immune cells; DEXs, dendritic cell-derived exosomes; Tregs, regulatory T cells; DMF, dimethyl fumarate; EVs, extracellular vesicles; sEVs, small extracellular vesicles; mtDNA, mitochondrial DNA; ALR-ELNs, exosome-like nanoparticles from atractylodes lancea roots.
References
Abdelnour, C., Agosta, F., Bozzali, M., Fougère, B., Iwata, A., Nilforooshan, R., et al. (2022). Perspectives and challenges in patient stratification in Alzheimer’s disease. Alzheimers Res. Ther. 14:112. doi: 10.1186/s13195-022-01055-y
Aguzzi, A., and Rajendran, L. (2009). The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64, 783–790. doi: 10.1016/j.neuron.2009.12.016
Ahmed, H., Salem, A., Atta, H., Eskandar, E., Farrag, A., Ghazy, M., et al. (2016). Updates in the pathophysiological mechanisms of Parkinson’s disease: Emerging role of bone marrow mesenchymal stem cells. World J. Stem. Cells 8, 106–117. doi: 10.4252/wjsc.v8.i3.106
Ajami, B., Samusik, N., Wieghofer, P., Ho, P., Crotti, A., Bjornson, Z., et al. (2018). Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551. doi: 10.1038/s41593-018-0100-x
Akizuki, M., Yamashita, H., Uemura, K., Maruyama, H., Kawakami, H., Ito, H., et al. (2013). Optineurin suppression causes neuronal cell death via NF-κB pathway. J. Neurochem. 126, 699–704. doi: 10.1111/jnc.12326
Amici, S., Dong, J., and Guerau-de-Arellano, M. (2017). Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 8:1520. doi: 10.3389/fimmu.2017.01520
Appel, S., Beers, D., and Zhao, W. (2021). Amyotrophic lateral sclerosis is a systemic disease: Peripheral contributions to inflammation-mediated neurodegeneration. Curr. Opin. Neurol. 34, 765–772. doi: 10.1097/WCO.0000000000000983
Arnett, H., Mason, J., Marino, M., Suzuki, K., Matsushima, G., and Ting, J. P. (2001). TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4, 1116–1122. doi: 10.1038/nn738
Arranz, A., and De Strooper, B. (2019). The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 18, 406–414. doi: 10.1016/S1474-4422(18)30490-3
Arthur, K., Calvo, A., Price, T., Geiger, J., Chiò, A., and Traynor, B. (2016). Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 7:12408. doi: 10.1038/ncomms12408
Badanjak, K., Fixemer, S., Smajić, S., Skupin, A., and Grünewald, A. (2021). The contribution of microglia to neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 22:4676. doi: 10.3390/ijms22094676
Bai, L., Lennon, D., Caplan, A., DeChant, A., Hecker, J., Kranso, J., et al. (2012). Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 15, 862–870. doi: 10.1038/nn.3109
Balducci, C., and Forloni, G. (2018). Novel targets in Alzheimer’s disease: A special focus on microglia. Pharmacol. Res. 130, 402–413. doi: 10.1016/j.phrs.2018.01.017
Barabadi, M., Paton, M., Kumar, N., Lim, R., and Payne, N. (2024). Stem cell derived extracellular vesicle therapy for multiple sclerosis, a systematic review and meta-analysis of preclinical studies. Stem. Cells Transl. Med. 13, 436–447. doi: 10.1093/stcltm/szae011
Beers, D., and Appel, S. (2019). Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 18, 211–220. doi: 10.1016/S1474-4422(18)30394-6
Bhargava, A., Sandoval Castellanos, A., Shah, S., and Ning, K. (2022). An insight into the iPSCs-derived two-dimensional culture and three-dimensional organoid models for neurodegenerative disorders. Interface Focus 12, 20220040. doi: 10.1098/rsfs.2022.0040
Billiau, A., Heremans, H., Vandekerckhove, F., Dijkmans, R., Sobis, H., Meulepas, E., et al. (1988). Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J. Immunol. 140, 1506–1510.
Bjornevik, K., Cortese, M., Healy, B., Kuhle, J., Mina, M., Leng, Y., et al. (2022). Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301. doi: 10.1126/science.abj8222
Borger, A., Stadlmayr, S., Haertinger, M., Semmler, L., Supper, P., Millesi, F., et al. (2022). How miRNAs regulate schwann cells during peripheral nerve regeneration-a systemic review. Int. J. Mol. Sci. 23:3440. doi: 10.3390/ijms23073440
Bouchez, G., Sensebé, L., Vourc’h, P., Garreau, L., Bodard, S., Rico, A., et al. (2008). Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem. Int. 52, 1332–1342. doi: 10.1016/j.neuint.2008.02.003
Burns, L., and Wang, H. (2017). Altered filamin A enables amyloid beta-induced tau hyperphosphorylation and neuroinflammation in Alzheimer’s disease. Neuroimmunol. Neuroinflamm. 4, 263–271. doi: 10.20517/2347-8659.2017.50
Cabrera Ranaldi, E., Nuytemans, K., Martinez, A., Luca, C., Keane, R., and de Rivero Vaccari, J. (2023). Proof-of-principle study of inflammasome signaling proteins as diagnostic biomarkers of the inflammatory response in Parkinson’s disease. Pharmaceuticals 16:883. doi: 10.3390/ph16060883
Carata, E., Muci, M., Di Giulio, S., Mariano, S., and Panzarini, E. (2023). Looking to the future of the role of macrophages and extracellular vesicles in neuroinflammation in ALS. Int. J. Mol. Sci. 24:11251. doi: 10.3390/ijms241411251
Chan, L., Hsu, W., Chen, K., Wang, W., Hung, Y., and Hong, C. (2023). Therapeutic effect of human adipocyte-derived stem cell-derived exosomes on a transgenic mouse model of Parkinson’s disease. Vivo 37, 2028–2038. doi: 10.21873/invivo.13300
Chaudhuri, K., Healy, D., and Schapira, A. (2006). National institute for clinical excellence. non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
Chen, H., Liang, F., Gu, P., Xu, B., Xu, H., Wang, W., et al. (2020). Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 11:288. doi: 10.1038/s41419-020-2473-5
Cheng, L., and Hill, A. (2022). Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399. doi: 10.1038/s41573-022-00410-w
Clarke, B., and Patani, R. (2020). The microglial component of amyotrophic lateral sclerosis. Brain 143, 3526–3539. doi: 10.1093/brain/awaa309
Compston, A., and Coles, A. (2008). Multiple sclerosis. Lancet 372, 1502–1517. doi: 10.1016/S0140-6736(08)61620-7
Cone, A., Yuan, X., Sun, L., Duke, L., Vreones, M., Carrier, A., et al. (2021). Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 11, 8129–8142. doi: 10.7150/thno.62069
Correale, J., Gaitán, M., Ysrraelit, M., and Fiol, M. (2017). Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 140, 527–546. doi: 10.1093/brain/aww258
Croitoru-Lamoury, J., Lamoury, F., Caristo, M., Suzuki, K., Walker, D., Takikawa, O., et al. (2011). Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One 6:e14698. doi: 10.1371/journal.pone.0014698
Cui, G., Guo, H., Li, H., Zhai, Y., Gong, Z., Wu, J., et al. (2019). modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 16:10. doi: 10.1186/s12979-019-0150-2
Cummings, J. (2021). New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 16:2. doi: 10.1186/s13024-021-00424-9
d’Angelo, M., Cimini, A., and Castelli, V. (2020). Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson’s disease. Int. J. Mol. Sci. 21:5241. doi: 10.3390/ijms21155241
de Boer, E., Orie, V., Williams, T., Baker, M., De Oliveira, H., Polvikoski, T., et al. (2020). TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 92, 86–95. doi: 10.1136/jnnp-2020-322983
de Godoy, M., Saraiva, L., de Carvalho, L., Vasconcelos-Dos-Santos, A., Beiral, H., Ramos, A., et al. (2018). Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem. 293, 1957–1975. doi: 10.1074/jbc.M117.807180
Dendrou, C., Fugger, L., and Friese, M. (2015). Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558. doi: 10.1038/nri3871
De-Paula, V., Radanovic, M., Diniz, B., and Forlenza, O. (2012). Alzheimer’s disease. Subcell. Biochem. 65, 329–352. doi: 10.1007/978-94-007-5416-4_14
Díaz Reyes, M., Gatti, S., Delgado Ocaña, S., Ortega, H., and Banchio, C. (2025). Neuroprotective effect of NSCs-derived extracellular vesicles in Parkinson’s disease models. Sci. Rep. 15:6092. doi: 10.1038/s41598-025-87238-7
Dobson, R., and Giovannoni, G. (2019). Multiple sclerosis - A review. Eur. J. Neurol. 26, 27–40. doi: 10.1111/ene.13819
Domenis, R., Cifù, A., Quaglia, S., Pistis, C., Moretti, M., Vicario, A., et al. (2018). Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 8:13325. doi: 10.1038/s41598-018-31707-9
Dorsey, E., Sherer, T., Okun, M., and Bloem, B. (2018). The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 8, S3–S8. doi: 10.3233/JPD-181474
Doshi, A., and Chataway, J. (2016). Multiple sclerosis, a treatable disease. Clin. Med. 16, S53–S59. doi: 10.7861/clinmedicine.16-6-s53
Elsharkasy, O., Nordin, J., Hagey, D., de Jong, O., Schiffelers, R., Andaloussi, S., et al. (2020). Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 159, 332–343. doi: 10.1016/j.addr.2020.04.004
Erta, M., Quintana, A., and Hidalgo, J. (2012). Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 8, 1254–1266. doi: 10.7150/ijbs.4679
Fakouri, A., Razavi, Z., Mohammed, A., Hussein, A., Afkhami, H., and Hooshiar, M. (2024). Applications of mesenchymal stem cell-exosome components in wound infection healing: New insights. Burns Trauma 12:tkae021. doi: 10.1093/burnst/tkae021
Feldman, E., Goutman, S., Petri, S., Mazzini, L., Savelieff, M., Shaw, P., et al. (2022). Amyotrophic lateral sclerosis. Lancet 400, 1363–1380. doi: 10.1016/S0140-6736(22)01272-7
Ferraiuolo, L., Meyer, K., Sherwood, T., Vick, J., Likhite, S., Frakes, A., et al. (2016). Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc. Natl. Acad. Sci. U S A. 113, E6496–E6505. doi: 10.1073/pnas.1607496113
Fisher-Shoval, Y., Barhum, Y., Sadan, O., Yust-Katz, S., Ben-Zur, T., Lev, N., et al. (2012). Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J. Mol. Neurosci. 48, 176–184. doi: 10.1007/s12031-012-9805-6
Fričová, D., Korchak, J., and Zubair, A. (2020). Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 5:20. doi: 10.1038/s41536-020-00106-y
Fu, J., Chen, S., Liu, J., Yang, J., Ou, R., Zhang, L., et al. (2023). Serum inflammatory cytokines levels and the correlation analyses in Parkinson’s disease. Front. Cell. Dev. Biol. 11:1104393. doi: 10.3389/fcell.2023.1104393
Fujinami, R., von Herrath, M., Christen, U., and Whitton, J. (2006). Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin. Microbiol. Rev. 19, 80–94. doi: 10.1128/CMR.19.1.80-94.2006
Galipeau, J., and Sensébé, L. (2018). Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell. Stem. Cell. 22, 824–833. doi: 10.1016/j.stem.2018.05.004
GBD 2016 Neurology Collaborators. (2019). Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 18, 459–480. doi: 10.1016/S1474-4422(18)30499-X
Ghasemi, N., Razavi, S., and Nikzad, E. (2017). Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 19, 1–10. doi: 10.22074/cellj.2016.4867
Ghasemi, N., Razavi, S., Mardani, M., Esfandiari, E., Salehi, H., and Zarkesh Esfahani, S. (2014). Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Mol. Biotechnol. 56, 470–478. doi: 10.1007/s12033-014-9744-2
Glass, C., Saijo, K., Winner, B., Marchetto, M., and Gage, F. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. doi: 10.1016/j.cell.2010.02.016
Goodin, D. (2024). Pathogenesis of multiple sclerosis: Genetic, environmental and random mechanisms. J. Neurol. Neurosurg. Psychiatry 95, 1002–1011. doi: 10.1136/jnnp-2023-333296
Gopinath, A., Mackie, P., Phan, L., Tansey, M., and Khoshbouei, H. (2023). The complex role of inflammation and gliotransmitters in Parkinson’s disease. Neurobiol. Dis. 176:105940. doi: 10.1016/j.nbd.2022.105940
Gouda, N., Elkamhawy, A., and Cho, J. (2022). Emerging therapeutic strategies for Parkinson’s disease and future prospects: A 2021 update. Biomedicines 10:371. doi: 10.3390/biomedicines10020371
Gupta, D., Wiklander, O., Görgens, A., Conceição, M., Corso, G., Liang, X., et al. (2021). Amelioration of systemic inflammation via the display of two different decoy protein receptors on extracellular vesicles. Nat. Biomed. Eng. 5, 1084–1098. doi: 10.1038/s41551-021-00792-z
Gurung, S., Perocheau, D., Touramanidou, L., and Baruteau, J. (2021). The exosome journey: From biogenesis to uptake and intracellular signalling. Cell. Commun. Signal. 19:47. doi: 10.1186/s12964-021-00730-1
Hade, M., Suire, C., and Suo, Z. (2021). Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 10:1959. doi: 10.3390/cells10081959
Han, F., Perrin, R., Wang, Q., Wang, Y., Perlmutter, J., Morris, J., et al. (2019). Neuroinflammation and myelin status in Alzheimer’s disease, Parkinson’s disease, and normal aging brains: A small sample study. Parkinsons Dis. 2019:7975407. doi: 10.1155/2019/7975407
Hardiman, O., Al-Chalabi, A., Chio, A., Corr, E., Logroscino, G., Robberecht, W., et al. (2017). Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3:17071. doi: 10.1038/nrdp.2017.71
Hashimoto, Y., Yamasaki, R., Ko, S., Matsuo, E., Kobayakawa, Y., Masaki, K., et al. (2022). Connexin 30 deficiency ameliorates disease progression at the early phase in a mouse model of amyotrophic lateral sclerosis by suppressing glial inflammation. Int. J. Mol. Sci. 23:16046. doi: 10.3390/ijms232416046
Hazrati, A., Soudi, S., Malekpour, K., Mahmoudi, M., Rahimi, A., Hashemi, S., et al. (2022). Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 10:30. doi: 10.1186/s40364-022-00374-4
He, J., Wu, X., Li, S., Yan, D., Xiao, G., and Mao, F. (2024). Exosomes derived from microRNA-540-3p overexpressing mesenchymal stem cells promote immune tolerance via the CD74/nuclear factor-kappaB pathway in cardiac allograft. World J. Stem. Cells 16, 1022–1046. doi: 10.4252/wjsc.v16.i12.1022
He, S., Wang, Q., Chen, L., He, Y., Wang, X., and Qu, S. (2023). miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson’s disease model via regulation of Nox4/ROS/Nrf2 signaling. J. Transl. Med. 21:747. doi: 10.1186/s12967-023-04638-x
Heidari, N., Abbasi-Kenarsari, H., Namaki, S., Baghaei, K., Zali, M., Mirsanei, Z., et al. (2022). Regulation of the Th17/Treg balance by human umbilical cord mesenchymal stem cell-derived exosomes protects against acute experimental colitis. Exp. Cell. Res. 419:113296. doi: 10.1016/j.yexcr.2022.113296
Heris, R., Shirvaliloo, M., Abbaspour-Aghdam, S., Hazrati, A., Shariati, A., Youshanlouei, H., et al. (2022). The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem Cell Res. Therapy 13:371. doi: 10.1186/s13287-022-03050-4
Herrmann, I., Wood, M., and Fuhrmann, G. (2021). Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759. doi: 10.1038/s41565-021-00931-2
Hessvik, N., and Llorente, A. (2018). Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75, 193–208. doi: 10.1007/s00018-017-2595-9
Hickman, S., Izzy, S., Sen, P., Morsett, L., and El Khoury, J. (2018). Microglia in neurodegeneration. Nat. Neurosci. 21, 1359–1369. doi: 10.1038/s41593-018-0242-x
Hofer, H., and Tuan, R. (2016). Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem. Cell. Res. Ther. 7:131. doi: 10.1186/s13287-016-0394-0
Huang, D., Zhang, M., and Tan, Z. (2022). Bone marrow stem cell-exo-derived TSG-6 attenuates 1-Methyl-4-Phenylpyridinium+-Induced neurotoxicity via the STAT3/miR-7/NEDD4/LRRK2 axis. J. Neuropathol. Exp. Neurol. 81, 621–634. doi: 10.1093/jnen/nlac049
Huang, R., Zhu, Z., Wu, Q., Bekhit, A., Wu, S., Chen, M., et al. (2023). Whole-plant foods and their macromolecules: Untapped approaches to modulate neuroinflammation in Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 63, 2388–2406. doi: 10.1080/10408398.2021.1975093
Huang, W., Zhang, T., Li, X., Gong, L., Zhang, Y., Luan, C., et al. (2024). Intranasal administration of umbilical cord mesenchymal stem cell exosomes alleviates Parkinson’s disease. Neuroscience 549, 1–12. doi: 10.1016/j.neuroscience.2024.04.010
Huang, Z., Liu, S., Zhuang, J., Li, L., Li, M., Huang, Y., et al. (2023). Role of microglial metabolic reprogramming in Parkinson’s disease. Biochem. Pharmacol. 213:115619. doi: 10.1016/j.bcp.2023.115619
Hulshof, S., Montagne, L., De Groot, C., and Van Der Valk, P. (2002). Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia 38, 24–35. doi: 10.1002/glia.10050
Hyman, B., Phelps, C., Beach, T., Bigio, E., Cairns, N., Carrillo, M., et al. (2012). National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13. doi: 10.1016/j.jalz.2011.10.007
Ishii, N., Noguchi, K., Ikemoto, M., Yohda, M., and Odahara, T. (2023). Optimizing exosome preparation based on size and morphology: Insights from electron microscopy. Microsc. Microanal. 29, 2068–2079. doi: 10.1093/micmic/ozad103
Ising, C., Venegas, C., Zhang, S., Scheiblich, H., Schmidt, S., Vieira-Saecker, A., et al. (2019). NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673. doi: 10.1038/s41586-019-1769-z
Jeppesen, D., Fenix, A., Franklin, J., Higginbotham, J., Zhang, Q., Zimmerman, L., et al. (2019). Reassessment of exosome composition. Cell 177, 428–445.e18. doi: 10.1016/j.cell.2019.02.029
Jha, M., Jo, M., Kim, J., and Suk, K. (2019). Microglia-astrocyte crosstalk: An intimate molecular conversation. Neuroscientist 25, 227–240. doi: 10.1177/1073858418783959
Jin, H., Gu, H., Mao, C., Chen, J., and Liu, C. (2020). Association of inflammatory factors and aging in Parkinson’s disease. Neurosci. Lett. 736:135259. doi: 10.1016/j.neulet.2020.135259
Joo, H., Suh, J., Lee, H., Bang, E., and Lee, J. (2020). Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int. J. Mol. Sci. 21:727. doi: 10.3390/ijms21030727
Jorfi, M., Park, J., Hall, C., Lin, C., Chen, M., von Maydell, D., et al. (2023). Infiltrating CD8+ T cells exacerbate Alzheimer’s disease pathology in a 3D human neuroimmune axis model. Nat. Neurosci. 26, 1489–1504. doi: 10.1038/s41593-023-01415-3
Ju, Y., and Tam, K. (2020). 9R, the cholinesterase and amyloid beta aggregation dual inhibitor, as a multifunctional agent to improve cognitive deficit and neuropathology in the triple-transgenic Alzheimer’s disease mouse model. Neuropharmacology 181:108354. doi: 10.1016/j.neuropharm.2020.108354
Kalluri, R., and LeBleu, V. (2020). The biology, function, and biomedical applications of exosomes. Science 367:eaau6977. doi: 10.1126/science.aau6977
Kang, S., Li, Y., Fukaya, M., Lorenzini, I., Cleveland, D., Ostrow, L., et al. (2013). Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 16, 571–579. doi: 10.1038/nn.3357
Karagiannis, P., Takahashi, K., Saito, M., Yoshida, Y., Okita, K., Watanabe, A., et al. (2019). Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 99, 79–114. doi: 10.1152/physrev.00039.2017
Kim, S., Cho, S., and Kim, S. (2018). Exosomal secretion of truncated cytosolic lysyl-tRNA synthetase induces inflammation during cell starvation. Cell Stress 2, 119–121. doi: 10.15698/cst2018.05.137
Kitazawa, M., Oddo, S., Yamasaki, T., Green, K., and LaFerla, F. (2005). Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J. Neurosci. 25, 8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005
Koh, E., Lee, E., Nam, G., Hong, Y., Cho, E., Yang, Y., et al. (2017). Exosome-SIRPa, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 121, 121–129. doi: 10.1016/j.biomaterials.2017.01.004
Kråkenes, T., Sandvik, C., Ytterdal, M., Gavasso, S., Evjenth, E., Bø, L., et al. (2024). The therapeutic potential of exosomes from mesenchymal stem cells in multiple sclerosis. Int. J. Mol. Sci. 25:10292. doi: 10.3390/ijms251910292
Kuzma-Kozakiewicz, M., Marchel, A., Kaminska, A., Gawel, M., Sznajder, J., Figiel-Dabrowska, A., et al. (2018). Intraspinal Transplantation of the adipose tissue-derived regenerative cells in amyotrophic lateral sclerosis in accordance with the current experts’ recommendations: Choosing optimal monitoring tools. Stem Cells Int. 2018:4392017. doi: 10.1155/2018/4392017
Kwon, H., and Koh, S. (2020). Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 9:42. doi: 10.1186/s40035-020-00221-2
Lana, D., Branca, J., Delfino, G., Giovannini, M., Casamenti, F., Nardiello, P., et al. (2023). Morphofunctional investigation in a transgenic mouse model of Alzheimer’s disease: Non-reactive astrocytes are involved in Aβ load and reactive astrocytes in plaque build-up. Cells 12:2258. doi: 10.3390/cells12182258
Lassmann, H. (2018). Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 8:a028936. doi: 10.1101/cshperspect.a028936
Lee, E., Lee, V., and Trojanowski, J. (2011). Gains or losses: Molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 13, 38–50. doi: 10.1038/nrn3121
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Li, Z., Liu, F., He, X., Yang, X., Shan, F., and Feng, J. (2019). Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 67, 268–280. doi: 10.1016/j.intimp.2018.12.001
Li, Z., Yin, B., Zhang, S., Lan, Z., and Zhang, L. (2023). Targeting protein kinases for the treatment of Alzheimer’s disease: Recent progress and future perspectives. Eur. J. Med. Chem. 261:115817. doi: 10.1016/j.ejmech.2023.115817
Liao, B., Zhao, W., Beers, D., Henkel, J., and Appel, S. (2012). Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 237, 147–152. doi: 10.1016/j.expneurol.2012.06.011
Liddelow, S., Guttenplan, K., Clarke, L., Bennett, F., Bohlen, C., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Linker, R., Lee, D., Demir, S., Wiese, S., Kruse, N., Siglienti, I., et al. (2010). Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: Therapeutic implications in a model of multiple sclerosis. Brain 133, 2248–2263. doi: 10.1093/brain/awq179
Liu, J., Marino, M., Wong, G., Grail, D., Dunn, A., Bettadapura, J., et al. (1998). TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat. Med. 4, 78–83. doi: 10.1038/nm0198-078
Liu, S., Fan, M., Xu, J., Yang, L., Qi, C., Xia, Q., et al. (2022a). Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflammation 19:35. doi: 10.1186/s12974-022-02393-2
Liu, S., Li, L., Zhuang, J., Li, M., Ye, L., Chen, X., et al. (2022b). Update on the application of mesenchymal stem cell-derived exosomes in the treatment of Parkinson’s disease: A systematic review. Front. Neurol. 13:950715. doi: 10.3389/fneur.2022.950715
MacMahon Copas, A., McComish, S., Fletcher, J., and Caldwell, M. (2021). The pathogenesis of Parkinson’s disease: A complex interplay between astrocytes, microglia, and T lymphocytes? Front. Neurol. 12:666737. doi: 10.3389/fneur.2021.666737
Madar, P., Nagalapur, P., Chaudhari, S., Sharma, D., Koparde, A., Buchade, R., et al. (2024). The unveiling of therapeutic targets for Alzheimer’s disease: An integrative review. Curr. Top. Med. Chem. 24, 850–868. doi: 10.2174/0115680266282492240220101049
Malik, N., and Rao, M. S. (2013). A review of the methods for human iPSC derivation. Methods Mol. Biol. 997, 23–33. doi: 10.1007/978-1-62703-348-0_3
Marino, M., Papa, S., Crippa, V., Nardo, G., Peviani, M., Cheroni, C., et al. (2015). Differences in protein quality control correlate with phenotype variability in 2 mouse models of familial amyotrophic lateral sclerosis. Neurobiol. Aging 36, 492–504. doi: 10.1016/j.neurobiolaging.2014.06.026
Markovinovic, A., Ljutic, T., Béland, L., and Munitic, I. (2018). Optineurin insufficiency disbalances proinflammatory and anti-inflammatory factors by reducing microglial IFN-β responses. Neuroscience 388, 139–151. doi: 10.1016/j.neuroscience.2018.07.007
Minghetti, L. (2005). Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 18, 315–321. doi: 10.1097/01.wco.0000169752.54191.97
Möller, A., and Lobb, R. (2020). The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 20, 697–709. doi: 10.1038/s41568-020-00299-w
Moon, H., Kim, B., Kwon, I., and Oh, Y. (2023). Challenges involved in cell therapy for Parkinson’s disease using human pluripotent stem cells. Front. Cell Dev. Biol. 11:1288168. doi: 10.3389/fcell.2023.1288168
Moyano, S., Musso, J., Feliziani, C., Zamponi, N., Frontera, L., Ropolo, A., et al. (2019). Exosome biogenesis in the protozoa parasite Giardia lamblia: A model of reduced interorganellar crosstalk. Cells 8:1600. doi: 10.3390/cells8121600
Namini, M., Daneshimehr, F., Beheshtizadeh, N., Mansouri, V., Ai, J., Jahromi, H., et al. (2023). Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem. Cell. Res. Ther. 14:254. doi: 10.1186/s13287-023-03467-5
Noonin, C., and Thongboonkerd, V. (2021). Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 11, 4436–4451. doi: 10.7150/thno.54004
Novoa, C., Salazar, P., Cisternas, P., Gherardelli, C., Vera-Salazar, R., Zolezzi, J., et al. (2022). Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 55:39. doi: 10.1186/s40659-022-00404-3
O’Gorman, C., Bukhari, W., Todd, A., Freeman, S., and Broadley, S. (2014). Smoking increases the risk of multiple sclerosis in Queensland, Australia. J. Clin. Neurosci. 21, 1730–1733. doi: 10.1016/j.jocn.2014.01.009
Olsson, T., Barcellos, L., and Alfredsson, L. (2017). Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36. doi: 10.1038/nrneurol.2016.187
Osada-Oka, M., Shiota, M., Izumi, Y., Nishiyama, M., Tanaka, M., Yamaguchi, T., et al. (2017). Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res. 40, 353–360. doi: 10.1038/hr.2016.163
Ou, M., Cao, J., Luo, R., Zhu, B., Miao, R., Yu, L., et al. (2025). Drug-loaded microneedle patches containing regulatory T cell-derived exosomes for psoriasis treatment. Acta Biomater. S1742-7061:00256–9. doi: 10.1016/j.actbio.2025.04.015
Ouchi, Y., Yoshikawa, E., Sekine, Y., Futatsubashi, M., Kanno, T., Ogusu, T., et al. (2005). Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 57, 168–175. doi: 10.1002/ana.20338
Paolicelli, R., Sierra, A., Stevens, B., Tremblay, M., Aguzzi, A., Ajami, B., et al. (2022). Microglia states and nomenclature: A field at its crossroads. Neuron 110, 3458–3483. doi: 10.1016/j.neuron.2022.10.020
Peterson, L., and Fujinami, R. (2007). Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 184, 37–44. doi: 10.1016/j.jneuroim.2006.11.015
Pfeiffer, R. (2016). Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 22, S119–S122. doi: 10.1016/j.parkreldis.2015.09.004
Philips, T., Bento-Abreu, A., Nonneman, A., Haeck, W., Staats, K., Geelen, V., et al. (2013). Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 136, 471–482. doi: 10.1093/brain/aws339
Pini, L., Pievani, M., Bocchetta, M., Altomare, D., Bosco, P., Cavedo, E., et al. (2016). Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev. 30, 25–48. doi: 10.1016/j.arr.2016.01.002
Poewe, W. (2008). Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 15, 14–20. doi: 10.1111/j.1468-1331.2008.02056.x
Polymenidou, M., and Cleveland, D. (2011). The seeds of neurodegeneration: Prion-like spreading in ALS. Cell 147, 498–508. doi: 10.1016/j.cell.2011.10.011
Ponath, G., Park, C., and Pitt, D. (2018). The Role of astrocytes in multiple sclerosis. Front. Immunol. 9:217. doi: 10.3389/fimmu.2018.00217
Ranganath, S., Levy, O., Inamdar, M., and Karp, J. (2012). Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell. Stem. Cell. 10, 244–258. doi: 10.1016/j.stem.2012.02.005
Ransohoff, R. M. (2016). A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991. doi: 10.1038/nn.4338
Rather, M., Khan, A., Alshahrani, S., Rashid, H., Qadri, M., Rashid, S., et al. (2021). Inflammation and Alzheimer’s disease: Mechanisms and therapeutic implications by natural products. Mediators Inflamm. 2021:9982954. doi: 10.1155/2021/9982954
Rincón-Riveros, A., Lopez, L., Villegas, E., and Antonia Rodriguez, J. (2021). Regulation of antitumor immune responses by exosomes derived from tumor and immune cells. Cancers 13:847. doi: 10.3390/cancers13040847
Rizea, R., Corlatescu, A., Costin, H., Dumitru, A., and Ciurea, A. (2024). Understanding amyotrophic lateral sclerosis: pathophysiology, diagnosis, and therapeutic advances. Int. J. Mol. Sci. 25:9966. doi: 10.3390/ijms25189966
Romancino, D., Paterniti, G., Campos, Y., De Luca, A., Di Felice, V., d’Azzo, A., et al. (2013). Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 587, 1379–1384. doi: 10.1016/j.febslet.2013.03.012
Ross, C., and Poirier, M. (2005). Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol 6, 891–898. doi: 10.1038/nrm1742
Rossi, D., and Volterra, A. (2009). Astrocytic dysfunction: Insights on the role in neurodegeneration. Brain Res. Bull. 80, 224–232. doi: 10.1016/j.brainresbull.2009.07.012
Saint-Pol, J., Gosselet, F., Duban-Deweer, S., Pottiez, G., and Karamanos, Y. (2020). Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells 9:851. doi: 10.3390/cells9040851
Sajeesh, S., Broekelman, T., Mecham, R., and Ramamurthi, A. (2020). Stem cell derived extracellular vesicles for vascular elastic matrix regenerative repair. Acta Biomater. 113, 267–278. doi: 10.1016/j.actbio.2020.07.002
Samii, A., Nutt, J., and Ransom, B. (2004). Parkinson’s disease. Lancet 363, 1783–1793. doi: 10.1016/S0140-6736(04)16305-8
Santamaria, G., Brandi, E., Vitola, P., Grandi, F., Ferrara, G., Pischiutta, F., et al. (2021). Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 28, 203–218. doi: 10.1038/s41418-020-0592-2
Santello, M., and Volterra, A. (2012). TNFa in synaptic function: Switching gears. Trends Neurosci. 35, 638–647. doi: 10.1016/j.tins.2012.06.001
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C., et al. (2021). Alzheimer’s disease. Lancet 397, 1577–1590. doi: 10.1016/S0140-6736(20)32205-4
Sen, M., Mahns, D., Coorssen, J., and Shortland, P. (2022). The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia 70, 1215–1250. doi: 10.1002/glia.24148
Serrano-Pozo, A., Frosch, M., Masliah, E., and Hyman, B. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1:a006189. doi: 10.1101/cshperspect.a006189
Sha, S., Shen, X., Cao, Y., and Qu, L. (2021). Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging 13, 15285–15306. doi: 10.18632/aging.203088
Shalaby, S., Sabbah, N., Saber, T., and Abdel Hamid, R. (2016). Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model. IUBMB Life 68, 106–115. doi: 10.1002/iub.1469
Shi, X., Zhang, K., Yu, F., Qi, Q., Cai, X., and Zhang, Y. (2024). Advancements and innovative strategies in induced pluripotent stem cell-derived mesenchymal stem cell therapy: A comprehensive review. Stem. Cells Int. 2024, 4073485. doi: 10.1155/2024/4073485
Shimaoka, M., Kawamoto, E., Gaowa, A., Okamoto, T., and Park, E. (2019). Connexins and integrins in exosomes. Cancers 11:106. doi: 10.3390/cancers11010106
Singh, G., Mehra, A., Arora, S., Gugulothu, D., Vora, L., Prasad, R., et al. (2024). Exosome-mediated delivery and regulation in neurological disease progression. Int. J. Biol. Macromol. 264:130728. doi: 10.1016/j.ijbiomac.2024.130728
Singh, M., Shin, Y., Ju, S., Han, S., Kim, S., and Kang, I. (2024). Comprehensive overview of Alzheimer’s disease: Etiological insights and degradation strategies. Int. J. Mol. Sci. 25:6901. doi: 10.3390/ijms25136901
Sofroniew, M., and Vinters, H. (2010). Astrocytes: Biology and pathology. Acta Neuropathol. 119, 7–35. doi: 10.1007/s00401-009-0619-8
Song, H., Chen, X., Hao, Y., Wang, J., Xie, Q., and Wang, X. (2022). Nanoengineering facilitating the target mission: Targeted extracellular vesicles delivery systems design. J. Nanobiotechnol. 20:431. doi: 10.1186/s12951-022-01638-9
Speer, G. (2013). [Impact of vitamin D in neurological diseases and neurorehabilitation: From dementia to multiple sclerosis. Part I: The role of vitamin D in the prevention and treatment of multiple sclerosis]. Ideggyogy Sz 66, 293–303.
Sun, Y., Liu, G., Zhang, K., Cao, Q., Liu, T., and Li, J. (2021). Mesenchymal stem cells-derived exosomes for drug delivery. Stem. Cell. Res. Ther. 12:561. doi: 10.1186/s13287-021-02629-7
Sun, Z., Liu, X., Lu, M., Zhang, X., and Sun, J. (2022). Serum-derived exosomes induce proinflammatory cytokines production in Cynoglossus semilaevis via miR-133-3p. Developmental Comp. Immunol. 136: 104497. doi: 10.1016/j.dci.2022.104497
Tambe, P., Undale, V., Sanap, A., Bhonde, R., and Mante, N. (2024). The prospective role of mesenchymal stem cells in Parkinson’s disease. Parkin. Relat. Disord. 127:107087. doi: 10.1016/j.parkreldis.2024.107087
Tan, F., Li, X., Wang, Z., Li, J., Shahzad, K., and Zheng, J. (2024). Clinical applications of stem cell-derived exosomes. Signal. Transduct. Target Ther. 9:17. doi: 10.1038/s41392-023-01704-0
Tang, Y., and Le, W. (2016). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. doi: 10.1007/s12035-014-9070-5
Tangaro, S., Amoroso, N., Boccardi, M., Bruno, S., Chincarini, A., Ferraro, G., et al. (2014). Automated voxel-by-voxel tissue classification for hippocampal segmentation: Methods and validation. Phys. Med. 30, 878–887. doi: 10.1016/j.ejmp.2014.06.044
Tansey, M., Wallings, R., Houser, M., Herrick, M., Keating, C., and Joers, V. (2022). Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673. doi: 10.1038/s41577-022-00684-6
Tolosa, E., Garrido, A., Scholz, S., and Poewe, W. (2021). Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397.
Toth, R., and Atkin, J. (2018). Dysfunction of optineurin in amyotrophic lateral sclerosis and glaucoma. Front. Immunol. 9:1017. doi: 10.3389/fimmu.2018.01017
Trams, E., Lauter, C., Salem, N., and Heine, U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645, 63–70. doi: 10.1016/0005-2736(81)90512-5
Umezu, T., Takanashi, M., Murakami, Y., Ohno, S., Kanekura, K., Sudo, K., et al. (2021). Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 21, 199–208. doi: 10.1016/j.omtm.2021.03.006
van den Bos, M., Geevasinga, N., Higashihara, M., Menon, P., and Vucic, S. (2019). Pathophysiology and diagnosis of ALS: Insights from advances in neurophysiological techniques. Int. J. Mol. Sci. 20:2818. doi: 10.3390/ijms20112818
van Dommelen, S., Vader, P., Lakhal, S., Kooijmans, S., van Solinge, W. W., Wood, M. J. A., et al. (2012). Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control Release 161, 635–644. doi: 10.1016/j.jconrel.2011.11.021
van Niel, G., D’Angelo, G., and Rasurname>Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19, 213–228.
von Bernhardi, R., Eugenín-von Bernhardi, L., and Eugenín, J. (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 7:124. doi: 10.3389/fnagi.2015.00124
Voss, E., Škuljec, J., Gudi, V., Skripuletz, T., Pul, R., Trebst, C., et al. (2012). Characterisation of microglia during de- and remyelination: Can they create a repair promoting environment? Neurobiol. Dis. 45, 519–528. doi: 10.1016/j.nbd.2011.09.008
Wan, Z., Zhao, L., Lu, F., Gao, X., Dong, Y., Zhao, Y., et al. (2020). Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 10, 218–230. doi: 10.7150/thno.38198
Wang, C., Fan, L., Khawaja, R., Liu, B., Zhan, L., Kodama, L., et al. (2022). Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat. Commun. 13:1969. doi: 10.1038/s41467-022-29552-6
Wang, D., Wan, Z., Yang, Q., Chen, J., Liu, Y., Lu, F., et al. (2022). Sonodynamical reversion of immunosuppressive microenvironment in prostate cancer via engineered exosomes. Drug Deliv. 29, 702–713. doi: 10.1080/10717544.2022.2044937
Wang, H., Liu, Y., Li, J., Wang, T., Hei, Y., Li, H., et al. (2021). Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP / PS1 mice. Cell. Death Discov. 7:230. doi: 10.1038/s41420-021-00620-y
Wang, J., Liu, X., Sun, J., Yang, J., Xu, D., and Yan, S. (2020). Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: A systematic review. World J. Stem. Cells 12, 879–896. doi: 10.4252/wjsc.v12.i8.879
Wang, K., Song, F., Fernandez-Escobar, A., Luo, G., Wang, J., and Sun, Y. (2018). The properties of cytokines in multiple sclerosis: Pros and cons. Am. J. Med. Sci. 356, 552–560. doi: 10.1016/j.amjms.2018.08.018
Wang, L., Gutmann, D., and Roos, R. (2011). Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum. Mol. Genet. 20, 286–293. doi: 10.1093/hmg/ddq463
Wang, Q., Liu, Y., and Zhou, J. (2015). Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 4:19. doi: 10.1186/s40035-015-0042-0
Wang, Y., Lin, Y., Wang, L., Zhan, H., Luo, X., Zeng, Y., et al. (2020). TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging 12, 20862–20879. doi: 10.18632/aging.104104
Wang, Y., Xiao, T., Zhao, C., and Li, G. (2024). The regulation of exosome generation and function in physiological and pathological processes. Int. J. Mol. Sci. 25:255. doi: 10.3390/ijms25010255
Wiklander, O., Nordin, J., O’Loughlin, A., Gustafsson, Y., Corso, G., Mäger, I., et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4:26316. doi: 10.3402/jev.v4.26316
Witwer, K., and Wolfram, J. (2021). Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 6, 103–106. doi: 10.1038/s41578-020-00277-6
Wozniak, A., Adams, A., King, K., Dunn, W., Christenson, L., Hung, W., et al. (2020). The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 219:e201912074. doi: 10.1083/jcb.201912074
Wyss-Coray, T., and Mucke, L. (2002). Inflammation in neurodegenerative disease—A double-edged sword. Neuron 35, 419–432. doi: 10.1016/S0896-6273(02)00794-8
Xie, S., Zhang, Q., and Jiang, L. (2022). Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes 12:498. doi: 10.3390/membranes12050498
Xie, X., Song, Q., Dai, C., Cui, S., Tang, R., Li, S., et al. (2023). Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: A phase I/II clinical trial. Gen. Psychiatr. 36:e101143. doi: 10.1136/gpsych-2023-101143
Xu, L., Liu, T., Liu, L., Yao, X., Chen, L., Fan, D., et al. (2020). Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 267, 944–953. doi: 10.1007/s00415-019-09652-y
Xu, M., Ji, J., Jin, D., Wu, Y., Wu, T., Lin, R., et al. (2023). The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 10, 1894–1907.
Xue, C., Li, X., Ba, L., Zhang, M., Yang, Y., Gao, Y., et al. (2021). Derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson’s disease. Aging Dis. 12, 1211–1222. doi: 10.14336/AD.2020.1221
Yang, S., Liang, X., Song, J., Li, C., Liu, A., Luo, Y., et al. (2021). novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem. Cell. Res. Ther. 12:315. doi: 10.1186/s13287-021-02404-8
Ye, H., Robak, L., Yu, M., Cykowski, M., and Shulman, J. (2023). Genetics and pathogenesis of Parkinson’s syndrome. Annu. Rev. Pathol. 18, 95–121. doi: 10.1146/annurev-pathmechdis-031521-034145
Yu, H., Sun, T., An, J., Wen, L., Liu, F., Bu, Z., et al. (2020). Potential Roles of exosomes in Parkinson’s disease: From pathogenesis, diagnosis, and treatment to prognosis. Front. Cell. Dev. Biol. 8:86. doi: 10.3389/fcell.2020.00086
Yuan, Y., Wang, J., Zhang, Y., Li, L., Reza, A., and Gurunathan, S. (2023). Biogenesis, composition and potential therapeutic applications of mesenchymal stem cells derived exosomes in various diseases. IJN 18, 3177–3210. doi: 10.2147/IJN.S407029
Yue, B., Yang, H., Wang, J., Ru, W., Wu, J., Huang, Y., et al. (2020). Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Proliferation 53:e12857. doi: 10.1111/cpr.12857
Zakrzewski, W., Dobrzynski, M., Szymonowicz, M., and Rybak, Z. (2019). Stem cells: Past, present, and future. Stem. Cell. Res. Ther. 10:68. doi: 10.1186/s13287-019-1165-5
Zavatti, M., Gatti, M., Beretti, F., Palumbo, C., and Maraldi, T. (2022). Exosomes derived from human amniotic fluid mesenchymal stem cells preserve microglia and neuron cells from Aβ. Int. J. Mol. Sci. 23:4967. doi: 10.3390/ijms23094967
Zelic, M., Pontarelli, F., Woodworth, L., Zhu, C., Mahan, A., Ren, Y., et al. (2021). RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 35:109112. doi: 10.1016/j.celrep.2021.109112
Zhang, J., Buller, B., Zhang, Z., Zhang, Y., Lu, M., Rosene, D., et al. (2022). Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 347:113895. doi: 10.1016/j.expneurol.2021.113895
Zhang, J., Zhang, Y., Wang, J., Xia, Y., Zhang, J., and Chen, L. (2024). Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal. Transduct. Target Ther. 9:211. doi: 10.1038/s41392-024-01911-3
Zhang, S., Willett, W., Hernán, M., Olek, M., and Ascherio, A. (2000). Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am. J. Epidemiol. 152, 1056–1064. doi: 10.1093/aje/152.11.1056
Zhang, Z., Zhou, Y., Gu, P., Zhao, W., Chen, H., Wu, R., et al. (2023). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson’s disease and neuronal damage through inhibition of microglia. Neural Regen. Res. 18, 2291–2300. doi: 10.4103/1673-5374.368300
Zhao, K., Liu, J., Sun, T., Zeng, L., Cai, Z., Li, Z., et al. (2024). The miR-25802/KLF4/NF-κB signaling axis regulates microglia-mediated neuroinflammation in Alzheimer’s disease. Brain Behav. Immun. 118, 31–48. doi: 10.1016/j.bbi.2024.02.016
Zhou, H., and Wu, L. (2017). The development and function of dendritic cell populations and their regulation by miRNAs. Protein Cell. 8, 501–513. doi: 10.1007/s13238-017-0398-2
Zhou, X., Li, Z., Sun, W., Yang, G., Xing, C., and Yuan, L. (2020). Delivery efficacy differences of intravenous and intraperitoneal injection of exosomes: Perspectives from tracking dye labeled and MiRNA encapsulated exosomes. Curr. Drug Deliv. 17, 186–194. doi: 10.2174/1567201817666200122163251
Keywords: stem cell therapy, extracellular vesicles, neuroinflammation, neurodegenerative diseases, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis
Citation: Yu M, Ma H, Lai X, Wu J, Shen M and Yan J (2025) Stem cell extracellular vesicles: a new dawn for anti-inflammatory treatment of neurodegenerative diseases. Front. Aging Neurosci. 17:1592578. doi: 10.3389/fnagi.2025.1592578
Received: 12 March 2025; Accepted: 27 June 2025;
Published: 11 July 2025.
Edited by:
R. M. Damian Holsinger, The University of Sydney, AustraliaReviewed by:
Aurélie Ledreux, University of Colorado Anschutz Medical Campus, United StatesMehmet Ozansoy, Istanbul Medipol University, Türkiye
Copyright © 2025 Yu, Ma, Lai, Wu, Shen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junqiang Yan, eWFuanFAaGF1c3QuZWR1LmNu
 Miao Yu
Miao Yu Hongxia Ma1
Hongxia Ma1 Junqiang Yan
Junqiang Yan