Abstract
Alzheimer’s disease (AD) is the most common and costly chronic progressive neurodegenerative disorder, with the highest impact on public health worldwide. Pathological hallmarks of AD include progressive cognitive decline and memory impairment, dominantly mediated by oxidative neurodegeneration. Oxidative stress is commonly recognized as a key factor in the pathophysiological progression of AD. Despite significant advancements, a definitive and effective therapeutic intervention for AD remains elusive. In this study, we investigate the neuroprotective potential of ambroxol (Amb), known for its potent anti-inflammatory and antioxidant properties. Given ambroxol’s potential neuroprotective effects, we explore the underlying molecular mechanisms, explicitly examining its role in attenuating scopolamine-induced oxidative stress-mediated activation of the c-Jun N-terminal kinase (JNK) pathway, as well as its modulation of Akt and glycogen synthase kinase-3 beta (GSK-3β) signaling, which is a key contributor to neuroinflammation, synaptic dysfunction and neurodegeneration. AD pathology is induced by scopolamine administration, leading to excessive lipid peroxidation (LPO) and reactive oxygen species (ROS) generation, which leads to a decline in critical antioxidant proteins, including nuclear factor erythroid 2-related factor 2 (Nrf-2) and heme oxygenase-1 (HO-1). However, ambroxol treatment effectively attenuated oxidative stress by reducing the production of reactive oxidative species while restoring the expression of key antioxidant proteins. Similarly, ambroxol attenuated oxidative stress-induced JNK activation and modulated Akt and GSK-3β alterations. Immunofluorescence and western blot analyses revealed that ambroxol attenuated reactive gliosis by suppressing the expression of GFAP and Iba-1, alongside the downregulation of key pro-inflammatory mediators, such as IL-1β, TNF-α, and phosphorylated NF-κB (p-p65). Scopolamine also compromised synaptic integrity and induced deficits in memory formation and spatial learning. In contrast, ambroxol promoted synaptic integrity by upregulating the expression of SNAP-23 and PSD-95, thereby ameliorating scopolamine-induced impairments in spatial learning and memory.
1 Introduction
Progressive cognitive decline and memory impairment are defining characteristics of Alzheimer’s disease (AD), one of the most prevalent and complex neurodegenerative disorder. AD is a leading cause of disability and morbidity worldwide, significantly affecting the aging population, as advanced age is the primary risk factor for its onset and progression (Liu et al., 2017; Stephenson et al., 2018; Volkman and Offen, 2017). Pathological features of AD include degeneration of the brain’s cholinergic system, the formation of neurofibrillary tangles, and the accumulation of senile plaques (Zhang et al., 2023; Muhammad et al., 2019). The pathology of AD also involves neuronal loss, neuroinflammation, and oxidative stress, which contribute to progressive memory impairment and cognitive decline (Lee et al., 2012). Although each of these factors appears independent, they are intricately interconnected through the common oxidative stress pathway (Bai et al., 2022). Oxidative stress is a pivotal cellular response involved in AD and aging, resulting in excessive production of lipid peroxidation (LPO) products and reactive oxygen species (ROS). This imbalance surpasses the capacity of the antioxidant defense system, leading to cellular damage and accelerating neurodegeneration (Thingore et al., 2021). Oxidative stress plays a central role in multiple pathogenic pathways of AD, driving neuroinflammation, reducing the synthesis of antioxidant proteins, and contributing to synaptic dysfunction (Bai et al., 2022). Dysfunction of the cholinergic system results in a reduction of acetylcholine (ACh) levels, a key factor in the pathophysiology of dementia. Current therapeutic strategies focus on enhancing cholinergic neurotransmission by either increasing acetylcholine (ACh) levels or inhibiting its degradation through acetylcholinesterase (AChE) inhibition. Approved anti-alzheimer’s disease drugs, including acetylcholinesterase (AChE) inhibitors like tacrine, donepezil, and N-methyl-D-aspartate (NMDA) receptor antagonists, can alleviate symptoms, particularly in the early to moderate stages of the disease. However, their prolonged use may be linked to adverse effects (Hampel et al., 2018; Lahiri et al., 2004).
Scopolamine (Scop) is a well-known anticholinergic agent that acts as a non-selective muscarinic (M) receptor antagonist that efficiently crosses the blood-brain barrier. It competitively inhibits acetylcholine (ACh) binding to muscarinic receptors, resulting in learning and memory deficits, primarily by disrupting central cholinergic signaling (Muhammad et al., 2019; Drummond and Wisniewski, 2017; Rahimzadegan and Soodi, 2018; Zhao et al., 2024). Intraperitoneal injection of scopolamine induces cholinergic neuron degeneration and a reduction in acetylcholine (ACh) levels, primarily due to its antimuscarinic effect. This effect leads to diminished acetylcholine (ACh) activity and impaired cholinergic neurotransmission, which subsequently promotes the generation of reactive oxygen species (ROS) and lipid peroxidation (LPO). This further exacerbates oxidative stress, neuronal damage, neuroinflammation, and memory impairment, which are crucial factors in the progression of AD-like dementia (Wang et al., 2022; Abu Almaaty et al., 2021; Lu et al., 2018). Scopolamine administration induces behavioral and molecular features resembling those of Alzheimer’s disease and other neurocognitive disorders; therefore, scopolamine-treated animal models are widely used in neurocognitive research (Cheon et al., 2021). Numerous studies have demonstrated that scopolamine induces oxidative stress in the brains of rats and mice, leading to neuronal damage (Ju et al., 2021). Consequently, scopolamine-induced mouse models can partially replicate the cognitive deficits observed in AD patients, making them valuable tools for the rapid evaluation of potential therapeutic interventions targeting memory and learning. It has been demonstrated that c-Jun N-terminal kinase (JNK), Akt, and glycogen synthase kinase-3 beta (GSK-3β) play critical roles in the pathophysiology of AD. Phosphorylated JNK (the active form) and dysregulated Akt and GSK-3β have been implicated in the brains of individuals with AD, contributing to the progression of the disease (Mehan et al., 2011; Zhao et al., 2024). It is reported that scopolamine administration induces oxidative stress, which in turn activates JNK and disrupts Akt and GSK-3β signaling (Sandhu et al., 2022; SoukhakLari et al., 2018). Activated JNK and GSK-3β downregulation may contribute to the downregulation of antioxidant proteins, thereby exacerbating synaptic dysfunction, neuroinflammatory responses, and cognitive deficits associated with neurodegeneration. It demonstrates that JNK and GSK-3β could serve as promising therapeutic targets for the management and treatment of AD (Zhou et al., 2015; Yarza et al., 2016; Yang et al., 2016; Li et al., 2023).
Bioactive compounds are strategically utilized to modulate the pathophysiological mechanisms underlying neuronal dysfunction and degeneration, thereby mitigating the morbidity and mortality associated with neurodegenerative disorders (Franco et al., 2023). Plant-derived alkaloids have been shown to possess anti-inflammatory properties by inhibiting various pro-inflammatory protein complexes (Aryal et al., 2022). Ambroxol hydrochloride (2-amino-3,5-dibromo-N-methylbenzylamine hydrochloride), a synthetic derivative of the alkaloid vasicine, a natural alkaloid found in Justicia adhatoda (Rav Ali et al., 2024), is a pharmacological chaperone that has been FDA-approved as an expectorant. It is currently used as an antitussive and anti-asthmatic agent. Beyond its mucolytic effects, ambroxol exhibits antioxidant and anti-inflammatory effects that contribute to its neuroprotective properties. Its potential effects on the central nervous system (CNS) are currently under active investigation (Dhanve et al., 2023; Malerba and Ragnoli, 2008; Patzwaldt et al., 2023). It can cross the blood-brain barrier (BBB) and effectively penetrate the central nervous system (Istaiti et al., 2021). Ambroxol exerts its antioxidant and anti-inflammatory effects through multiple mechanisms (Ahmadi et al., 2024). It mitigates oxidative damage by scavenging free radicals, reducing mitochondrial reactive oxygen species (ROS) production, and enhancing the activity of endogenous antioxidant enzymes (Ullah et al., 2025). Additionally, ambroxol attenuates neuroinflammation and neuronal injury by downregulating pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), inhibiting the nuclear factor kappa B (NF-κB) signaling pathway, and suppressing microglial activation (Faldu and Shah, 2024). Ambroxol mitigates the elevation of phosphorylated c-Jun N-terminal kinase (p-JNK) in the liver and kidney (Bishr et al., 2019). Ambroxol has been reported to inhibit microglial activation and reduce the levels of proinflammatory cytokines in the brain following intracerebral hemorrhage (Jiang et al., 2020).
This study investigates the neuroprotective potential of ambroxol against scopolamine-induced oxidative stress, which triggers JNK activation, disrupts Akt and GSK-3β signaling pathways, and contributes to memory impairments, synaptic dysfunction, neuroinflammation, and neurodegeneration. Our findings demonstrate that ambroxol alleviates scopolamine-induced neuroinflammation, oxidative stress, synaptic dysfunction, and memory impairments in the brains of mice. These neuroprotective effects of ambroxol may be attributed to its inhibition of JNK and GSK-3β alteration, potential interactions with synaptic proteins, and its direct antioxidant and anti-inflammatory properties.
2 Materials and methods
2.1 Chemicals and antibodies
Scopolamine, ambroxol hydrochloride (CAS 23828-92-4), and 2,7-dichlorodihydrofluorescein diacetate (DCFHDA) were purchased from Sigma-Aldrich (St. Louis, MO, United States), and the primary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, United States). An overview of all antibodies utilized in the current study is provided in Table 1.
TABLE 1
| Antibody | Host | Catalog | Application | Dilution | Manufacturer |
| Nrf-2 | Rabbit | 12721S | WB/IF | 1:1000/1:100 | Cell Signaling, United States |
| Ho-1 | Mouse | Sc-136961 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| p-JNK | Mouse | Sc-6254 | WB/IF | 1:1000/1:100 | Santa Cruz Biotechnology, United States |
| p-GSK-3β | Mouse | Sc-373800 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| p-Akt | Rabbit | 9271S | WB | 1:1000 | Cell Signaling, United States |
| GFAP | Mouse | Sc-33673 | WB/IF | 1:1000/1:100 | Santa Cruz Biotechnology, United States |
| Iba-1 | Rabbit | 17,198 | WB/IF | 1:1000/1:100 | Cell Signaling, United States |
| p-NF-κB | Mouse | Sc-136548 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| TNF-α | Mouse | Sc-52746 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| IL-1 | Mouse | Sc-32294 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| PSD-95 | Mouse | Sc-71933 | WB/IF | 1:1000/100 | Santa Cruz Biotechnology, United States |
| SNAP-23 | Mouse | Sc-374215 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
| β-Actin | Mouse | Sc-47778 | WB | 1:1000 | Santa Cruz Biotechnology, United States |
List of primary antibodies used for western blot and immunofluorescence.
All the secondary antibodies were diluted in 1x TBST at a concentration of 1:10,000 μL.
2.2 Experimental animals
Male wild-type C57BL/6N mice (8 weeks old, with an average body weight of 25–30 g) were obtained from Samtako Bio (Osan, South Korea). Animals were housed and acclimatized for 1 week in the animal care facility under a 12 h light/dark cycle in a temperature-controlled environment (20 ± 2°C; 50 ± 10% humidity) with ad libitum access to food and water. All procedures and techniques were conducted following the guidelines established by the Animal Ethics Committee of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University, South Korea.
2.3 Animal grouping, drug administration, and preparation
Four groups, each consisting of eight mice, were randomly selected from the experimental cohort (n = 4 for western blot and n = 4 for immunohistochemistry).
-
1.
Control (Cont) group: Mice received a 14 days vehicle treatment with normal saline (0.9%, i.p.).
-
2.
Scopolamine (Scop) treated group: Mice were administered scopolamine (1 mg/kg/day, i.p.) dissolved in normal saline for 14 days (Istifo et al., 2024; Muhammad et al., 2019). Seven doses were administered over 2 weeks, on alternate days.
-
3.
Scopolamine + Ambroxol (Scop + Amb) co-treatment group: Mice were treated with scopolamine (1 mg/kg/day) and ambroxol (90 mg/kg/day in 0.9% normal saline) for 14 days. Prepared in different saline solutions and injected separately. Scopolamine was administered in seven doses over 2 weeks, on alternate days. Ambroxol was administered daily for 2 weeks.
-
4.
Ambroxol (Amb) treated group: Mice were treated with ambroxol (90 mg/kg/day in 0.9% normal saline) for 14 days (Su et al., 2004; Patzwaldt et al., 2023). Ambroxol was administered intraperitoneal injection (i.p.) for 14 doses for 2 weeks (Figure 1).
FIGURE 1

Diagrammatic representation of the treatment regimens, animal groupings, and experimental setup. Following a 7 days acclimatization period, mice were randomly allocated into four experimental groups. The animals received intraperitoneal (i.p.) injections of scopolamine and ambroxol (Amb) for 14 days. Subsequent to behavioral assessments, the animals were euthanized for subsequent biochemical and immunofluorescence analyses.
2.4 Behavioral study
After a 2 weeks regimen of scopolamine and ambroxol treatment, the mice’s behavioral performance was assessed using the Morris water maze (MWM) and Y-maze paradigms.
2.4.1 Morris water maze test
The Morris Water Maze (MWM) is a widely utilized paradigm for evaluating mice’s memory and learning capabilities. The MWM procedure was conducted with minor modifications, as previously described (Ali et al., 2015). The MWM apparatus consisted of a circular water tank with a diameter of 100 cm and a height of 35 cm. The tank was filled to a depth of 15.5 cm with water maintained at a temperature of 23 ± 1°C. The water was made opaque by applying non-toxic white-colored paint. A transparent escape platform, with a diameter of 4.5 cm and a height of 14.5 cm, was positioned at the center of one quadrant and submerged 1 cm beneath the water’s surface. Each mouse was placed in a designated quadrant and allowed a period of free exploration to locate the submerged platform. The hidden platform was employed for a 4 days consecutive training period for each mouse. During each trial, the escape latency, defined as the time taken to locate the hidden platform, was recorded for each mouse. On day 5, the probe test was conducted to assess memory consolidation. Following platform removal, each mouse was allotted a one-minute interval for free swimming. The probing trial evaluated the number of platform crossings, the duration spent in the target quadrant (the region where the platform was positioned during the hidden platform training), and the time spent in the remaining three quadrants (left, right, and opposite). The duration spent in the target quadrant following the learning phase served as an indicator of memory consolidation. Behavioral data were recorded using visual/video tracking software (SMART, Panlab Harvard Apparatus; Bioscience Company, Holliston, MA, Uited States).
2.4.2 Y-maze test
To evaluate spatial memory, a Y-maze apparatus (length = 50 cm, height = 20 cm, width = 10 cm) equipped with a camera sensor mounted above was employed (Rehman et al., 2017). Each mouse was placed at the center of the apparatus and allowed 8 min for free exploration. The attached sensor digitally recorded the sequence in which each arm was entered. The consecutive entries of each mouse into the three arms in overlapping triplet sets refer to spontaneous alternation. The formula used to determine the percentage of alteration behavior was (number of consecutive triplet sets/total number of arm entries) × 100. Superior cognitive performance is indicated by an increased percentage of spontaneous alternation behavior.
2.5 Brain tissue collection and sample preparation
Upon completion of pharmacological treatment and behavioral assessments, all experimental mice (n = 32) were euthanized using an intraperitoneal injection of 0.05 mL/100 g body weight of Rompun (Xylazine) and 0.1 mL/100 g body weight of Zoletil (Ketamine). Subsequently, the animals were promptly sacrificed for immunofluorescence and biochemical analyses. The experimental animals (n = 16 mice) underwent transcardial perfusion with ice-cold phosphate-buffered saline (PBS, 0.01 M), followed by neutral-buffered paraformaldehyde (NBP, 4%). The brains were then post-fixed in NBP (4%) for 48–72 h before immunofluorescence analysis. After removal of NBP, the post-fixed brains of each experimental mouse were rinsed with 1% PBS (0.01 M) and subsequently immersed in a 20% sucrose solution for 48 h, or until they sank to the bottom of the tube. Before sectioning the cortex and hippocampus (14 μm) using a CM-3050C Cryostat (Leica, Germany), the brains were carefully frozen in O.C.T. compound (A.O., United States), and the sections were then mounted onto microscopic slides. Thawed sections were mounted onto probe-on plus charged slides (Fisher, Rockford, IL, United States) for subsequent analysis. Similarly, for western blotting or biochemical analyses (n = 4 mice per group), the brains were swiftly excised, and the cortical and hippocampal tissues were meticulously dissected, snap-frozen on dry ice, and stored at −80°C. As directed by the manufacturer (iNtRON Biotechnology, Inc., Sungnam, South Korea), the tissues were homogenized in a protein extraction solution (PRO-PREP). The samples were centrifuged at 13,000 rpm for 25 min at 4°C, supernatants were carefully collected and stored at −80°C.
2.6 Western blot analysis
Western blotting was conducted as previously described, with slight modifications, to quantify the protein concentrations in the cortex and hippocampus (Sarubbo et al., 2018). Protein concentrations were determined using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, CA, United States). The protein samples were subsequently subjected to electrophoresis, with equal amounts (15–30 μg) loaded onto 10%/12% Bolt™ Mini Gels (Novex, Life Technologies, Kiryat Shmona, Israel). An anti-β-actin antibody (Santa Cruz Biotechnology, Dallas, TX, United States) was utilized as a loading control to confirm equal loading across the gel. After separation on the gel, the proteins were transferred to polyvinylidene difluoride (PVDF) membranes. To minimize non-specific binding, the membranes were blocked with 5% (w/v) skim milk and incubated with primary antibodies overnight at 4°C. Following incubation with primary antibodies (1:1000) in Tris-buffered saline with Tween (TBST), the membranes were exposed to secondary antibodies for 1–2 h. Subsequently, the membranes were washed three times for 10 min each with 1 × TBST. After incubation with a horseradish peroxidase-conjugated secondary antibody, protein visualization was achieved using the ECL detection reagent (ECL kit; Amersham, Japan) according to the manufacturer’s instructions, and the resulting signals were captured on X-ray films. Following the scanning of the developed X-ray films, histograms were generated by performing densitometric analysis of the bands using ImageJ and GraphPad Prism 6 software. The density values are presented as arbitrary units (A.U.) relative to the untreated control.
2.7 Immunofluorescence staining
Immunofluorescence staining was carried out with slight modifications to the previously described protocol (Ali et al., 2015). The 14 μm brain sections on the slides were washed twice with 0.01 M phosphate-buffered saline (PBS) for 10 min each. Subsequently, the slides were incubated for 1 h in a blocking solution composed of 0.3% Triton X-100 in PBS and 2% normal bovine serum, tailored to the specific antibody employed. Following the blocking, primary antibodies (diluted 1:100 in 1% PBS, i.e., 0.01 M) were applied to the slides and incubated overnight at 4°C. The slides were subsequently rinsed with 0.01 M PBS and incubated for 1.5–2 h with secondary antibodies (1:100) conjugated to fluorescein isothiocyanate (FITC, green) or tetramethylrhodamine isothiocyanate (TRITC, red). The slides were then counterstained with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) for 5–10 min, followed by mounting with glass coverslips using mounting media. The staining patterns were examined and assessed using a confocal laser scanning microscope (Fluoview FV 1000MPE, Olympus, Japan).
2.8 Reactive oxygen species (ROS) assay
The reactive oxygen species (ROS) assay was performed with minor modifications to the previously established protocols (Amin et al., 2017; Qian et al., 2008). The assay is primarily based on the oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to 2′,7′-dichlorofluorescein (DCF). Ice-cold Lock’s buffer was used to dilute the cortical and hippocampal brain homogenates at a 1:20 ratio, yielding a final tissue concentration of 2.5 mg per 500 μL. To generate fluorescent DCF from DCFH-DA, the reaction mixture was incubated for 15 min at room temperature, consisting of 1 mL of Lock’s buffer (pH 7.4), 0.2 mL of homogenate, and 10 μL of DCFH-DA (5 mM). The converted fluorescent product, DCF, was measured using a spectrofluorometer, with excitation and emission wavelengths set at 484 nm and 530 nm, respectively. Parallel measurements of blank samples were conducted to account for background fluorescence, which corresponds to the conversion of DCFH-DA in the absence of homogenate. The amount of reactive oxygen species (ROS) present was quantified in picomoles of DCF generated per minute per milligram of protein.
2.9 Lipid peroxidation (LPO) assay
As previously described, the lipid peroxidation (LPO) assay is essential for evaluating oxidative stress (Rehman et al., 2017). Following the manufacturer’s protocol, an MDA colorimetric/fluorometric assay kit (BioVision, United States, Cat # K739-100) was utilized to measure free malondialdehyde (MDA) in cortical and hippocampal protein homogenates. MDA was quantified as a marker of lipid peroxidation, serving as an indicator of oxidative lipid degradation. The mouse brains were homogenized in 300 μL of MDA lysis buffer containing 3 μL of butylated hydroxytoluene (BHT), followed by centrifugation at 13,000 rpm for 10 min. To each brain sample, 150 μL of distilled water, 3 μL of BHT, and 1 mL of 2 N perchloric acid were added to precipitate approximately 10 mg of protein. To isolate the precipitated protein, the mixture was vortexed and then centrifuged. The supernatant from each sample was transferred to a 96-well plate, and absorbance was measured at 532 nm using a microplate reader. The total MDA concentration was expressed as nmol of MDA per milligram of protein in each brain homogenate (cortex and hippocampus).
2.10 Statistical analysis
Western blot and immunofluorescence images were quantified using ImageJ software for densitometric analysis. The data, presented as the mean ± SEM from eight mice per group, were analyzed using Prism v8 software (GraphPad Software, Inc., San Diego, CA, United States). Group comparisons were performed using a one-way ANOVA, followed by Tukey’s post-hoc test for statistical analysis. A P-value less than 0.05 was regarded as statistically significant. The symbol “#” indicates a significant difference compared to the saline-injected group, while the symbol “*” denotes a significant difference relative to the scopolamine-injected group. Significance: #p ≤ 0.05, ##p ≤ 0.01, and ###p ≤ 0.001; *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
3 Results
3.1 Ambroxol rescues scopolamine-induced oxidative stress and enhances Nrf-2/HO-1 expression in the mouse brain
Scopolamine has been connected to neurodegeneration caused by oxidative stress (Gul et al., 2023; Muhammad et al., 2019; Skalicka-Wozniak et al., 2018; Ponne et al., 2020). While ambroxol exhibits antioxidant properties, mitigating oxidative stress-induced pathologies (Štětinová et al., 2004; Jiang et al., 2013; Cavalu et al., 2022). To assess the neuroprotective potential of ambroxol against scopolamine-induced oxidative brain damage in mice, we quantified reactive oxygen species (ROS) levels and lipid peroxidation (LPO) in hippocampal and cortical tissue homogenates from the experimental groups. Our findings revealed that scopolamine administration markedly increased LPO and ROS levels in cortical and hippocampal homogenates of scopolamine-treated mouse brains compared to saline-treated. Notably, ambroxol treatment significantly attenuated ROS and LPO levels in cortical and hippocampal homogenates of ambroxol + scopolamine co-treated mouse brains (Figures 2A, B). Furthermore, the antioxidant efficacy of ambroxol against scopolamine-induced oxidative stress in the mice brain was confirmed by assessing the expression levels of key antioxidant proteins, including nuclear factor erythroid 2-related factor 2 (Nrf-2) and heme oxygenase-1 (HO-1), via western blot analysis. The immunoblot analysis revealed a reduction in the expression levels of Nrf-2 and HO-1 in the cerebral tissues of scopolamine-administered mice. In contrast, treatment with ambroxol notably enhanced the expression of these antioxidant proteins in the brains of mice co-treated with ambroxol + scopolamine (Figures 2C–E). Furthermore, we performed an immunofluorescence analysis to validate the immunoblotting findings for Nrf-2, offering spatial insights into its subcellular localization within the nucleus. Immunofluorescence analysis demonstrated a reduction in Nrf-2 reactivity in both the cortex and hippocampus of scopolamine-treated mice, whereas a significant upregulation of Nrf-2 expression was observed in the ambroxol + scopolamine co-treated group (Figures 2F, G). Collectively, these findings suggest that ambroxol mitigates scopolamine-induced oxidative stress in the mouse brain, likely via its antioxidant properties.
FIGURE 2

Ambroxol reduced reactive oxygen species (ROS) and lipid peroxidation (LPO) levels and concurrently upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf-2) and heme oxygenase-1 (HO-1) in the brains of scopolamine-treated mice. (A,B) Representative histograms of ROS and LPO assays in the mice brain cortex and hippocampus. (C) Images of western blot analysis illustrating the protein expression levels of Nrf-2 and HO-1 in the cortex and hippocampus. (D,E) Histograms of Nrf-2 and HO-1 protein expression. (F) Immunofluorescence images depicting the immunoreactivity of Nrf-2 protein in the cortex and hippocampus. (G) Corresponding bar graphs of Nrf-2 immunofluorescence. β-actin was used as the loading control. Band intensities were cropped and quantified using ImageJ software, and the variations are illustrated in the corresponding histogram; Magnification 10×. Scale bar 50 μm. All data are presented as mean ± S.E.M, with corresponding bar graphs. An asterisk (*) denotes a significant difference from the normal saline-treated group; hash (#) indicates a significant difference from the scopolamine-treated group. Significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; #P ≤ 0.05, ##P ≤ 0.01; ###P ≤ 0.001.
3.2 Ambroxol exerts neuroprotective effects against scopolamine-induced neurotoxicity by modulating the JNK, Akt, and GSK-3β signaling in the mouse brain
An increasing amount of evidence revealed the involvement of c-Jun N-terminal kinase (JNK), also known as stress-activated protein kinases, in a range of pathophysiological processes underlying AD (Mehan et al., 2011; Zhou et al., 2015). The expression level of the phosphorylated JNK (p-JNK) protein was assessed via western blot to evaluate the effect of scopolamine-induced oxidative stress on the activation of stress kinases. The protein levels of JNK were significantly increased in the scopolamine-treated group, whereas ambroxol effectively attenuated the elevated production of phosphorylated JNK (p-JNK) (Figures 3A–D). Similarly, western blot analysis was employed to investigate whether ambroxol could restore the dysregulation of GSK-3β induced by scopolamine treatment. Glycogen synthase kinase-3β (GSK-3β) is a ubiquitously expressed, constitutively active serine/threonine kinase that plays a critical role in the regulation of numerous fundamental cellular pathways. It contributes to the pathophysiology of AD through multiple distinct mechanisms. Dysregulation of this kinase has been implicated in both in vitro and in vivo models of AD. It has been reported that scopolamine downregulates the expression of GSK-3β (Lauretti et al., 2020; Llorens-Marítin et al., 2014; SoukhakLari et al., 2018). GSK-3β is a key target of Akt, which induces its phosphorylation at the serine 9 position, leading to its inactivation. Previously, studies have reported that scopolamine-induced Akt deactivation and GSK-3β alteration (SoukhakLari et al., 2018; Muhammad et al., 2019). Our findings demonstrated a significant reduction in the protein levels of Akt and GSK-3β alteration in both the cortical and hippocampal regions of mice treated with scopolamine, compared to those treated with the control vehicle. However, ambroxol treatment significantly elevated the levels of Akt and GSK-3β in both the cortex and hippocampus (Figure 3A). Moreover, immunofluorescence analysis demonstrated a significant increase in p-JNK immunoreactivity in the cortex and hippocampus of scopolamine-treated mice, compared to the control saline-treated group. Interestingly, these elevated fluorescence levels were reduced in the brains of scopolamine + ambroxol co-treated mice, indicating that ambroxol effectively prevented scopolamine-induced JNK activation (Figures 3E, F).
FIGURE 3

Ambroxol modulates the expression of p-JNK, p-Akt, and p-GSK-3β in the mouse brain, thereby alleviating scopolamine-induced stress. (A) Images of western blot analysis illustrating the protein expression levels of p-JNK, p-Akt, and p-GSK-3β in the cortex and hippocampus. (B–D) Histograms of p-JNK, p-Akt, and p-GSK-3β protein expression. (E) Immunofluorescence images depicting the immunoreactivity of p-JNK protein in the cortex and hippocampus. (F) Corresponding bar graphs of p-JNK immunofluorescence. β-actin was used as the loading control. Band intensities were cropped and quantified using ImageJ software, and the variations are illustrated in the corresponding histogram; Magnification 10x. Scale bar 50 μm. All data are presented as mean ± S.E.M, with corresponding bar graphs. An asterisk (*) denotes a significant difference from the normal saline-treated group; hash (#) indicates a significant difference from the scopolamine-treated group. Significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; #P ≤ 0.05, ##P ≤ 0.01; ###P ≤ 0.001.
3.3 Ambroxol mitigates scopolamine-induced neuroinflammation and attenuates the activation of glial cells
Astrocytes and microglia play a pivotal role in both neuroinflammation and inflammatory neurodegeneration, serving as primary sources of various pro-inflammatory cytokines (Combs, 2009). Iba-1 and GFAP are well-established markers used to identify microglia and inflammatory astrocytes, respectively (Ahmad et al., 2024; Ali et al., 2024). We investigated the protective effect of ambroxol against ionized calcium-binding molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) in the cortex and hippocampus, which serve as key markers of reactive astrocytes and microglia. Western blot analysis revealed increased expression levels of Iba-1 and GFAP in the brains of scopolamine-treated mice. However, co-treatment with ambroxol effectively reduced the levels of these proteins (Figure 4A). Additionally, immunofluorescence staining corroborated the western blot results for GFAP and Iba-1, revealing an increased intensity of GFAP and Iba-1-positive cells in the brains of scopolamine-treated mice. Importantly, ambroxol treatment in combination with scopolamine significantly reduced the number of activated GFAP and Iba-1 positive cells in the cortical and hippocampal regions, compared to scopolamine treatment alone (Figures 4G, I). Previous studies have implicated an imbalance in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the pathogenesis of several neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s diseases. Furthermore, the expression of NF-κB has been shown to increase with aging (Sun et al., 2022; Hu et al., 2022; Calabrese et al., 2011; Ahmad et al., 2024). Consistent with previous reports, our results demonstrated that ambroxol co-treatment significantly attenuated the elevated expression of p-NF-κB (p-p65). Overexpression of NF-κB may trigger the activation of several pro-inflammatory markers, including tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), which are implicated in the progression of neurodegeneration (Ali et al., 2023). The levels of activated inflammatory markers, including TNF-α and IL-1β, were assessed in cortical and hippocampal samples from scopolamine-treated mice using western blot analysis. Our results demonstrated that ambroxol significantly mitigated scopolamine-induced neuroinflammation by reducing the expression levels of TNF-α and IL-1β (Figures 4A–F).
FIGURE 4

Ambroxol inhibited scopolamine-induced glial cell activation and suppressed the upregulation of inflammatory proteins in the mice brain. (A) Images of western blot analysis illustrating the protein expression levels of GFAP, Iba-1, p-NF-κB (p-p65), TNF-α, and IL-1β in the cortex and hippocampus. (B–F) Histograms of GFAP, Iba-1, p-NF-κB (p-p65), TNF-α, and IL-1β protein expression. (G) Immunofluorescence images depicting the immunoreactivity of GFAP and Iba-1 protein in the cortex and hippocampus. (H,I) Corresponding bar graphs of GFAP and Iba-1 immunofluorescence. β-actin was used as the loading control. Band intensities were cropped and quantified using ImageJ software, and the variations are illustrated in the corresponding histogram; Magnification 10x. Scale bar 50 μm. All data are presented as mean ± S.E.M, with corresponding bar graphs. An asterisk (*) denotes a significant difference from the normal saline-treated group; hash (#) indicates a significant difference from the scopolamine-treated group. Significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; #P ≤ 0.05, ##P ≤ 0.01; ###P ≤ 0.001.
3.4 Ambroxol ameliorated scopolamine-induced synaptic dysfunction and improved memory performance
Neuronal and synaptic dysfunction are key consequences of AD neuropathology and are strongly linked to cognitive decline and memory impairment (Oddo et al., 2003). To assess the protective effects of ambroxol against scopolamine-induced synaptic loss, we examined the expression of synaptic proteins in the cortical and hippocampal tissues of scopolamine-treated mice using western blot analysis and confocal microscopy. Our findings demonstrated that scopolamine significantly reduced the expression levels of synaptic proteins, such as postsynaptic density protein (PSD-95) and synaptosomal-associated protein 23 (SNAP-23), compared to the control group. However, ambroxol treatment significantly upregulated the expression of these proteins, thereby markedly enhancing synaptic protein levels in the cortical and hippocampal tissues of scopolamine-treated mice (Figures 5A–C). Furthermore, we employed an immunofluorescence assay for postsynaptic density protein (PSD-95) to corroborate the western blot data. Immunofluorescence results showed that scopolamine treatment significantly decreased postsynaptic density protein (PSD-95) levels compared to the control group. However, ambroxol co-treatment notably restored the levels of PSD-95 (Figures 5D, E).
FIGURE 5
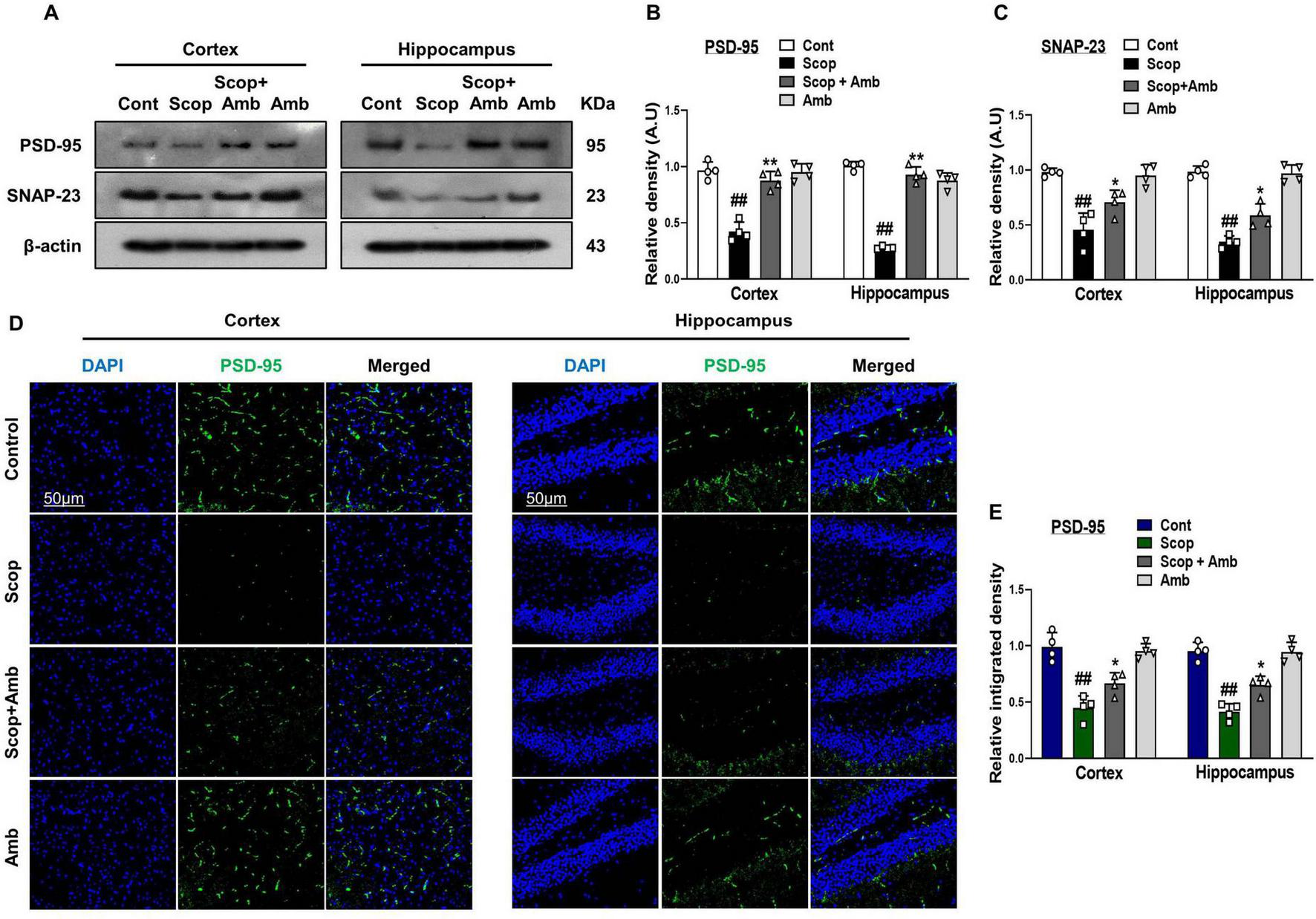
Effect of Ambroxol on scopolamine-induced synaptic dysfunction and memory impairment in mice brain. (A) Images of western blot analysis illustrating the protein expression levels of PSD-95 and SNAP-23 in the cortex and hippocampus. (B,C) Histograms of PSD-95 and SNAP-23 protein expression. (D) Immunofluorescence images depicting the immunoreactivity of PSD-95 protein in the cortex and hippocampus. (E) Corresponding bar graphs of PSD-95 immunofluorescence. β-actin was used as the loading control. Band intensities were cropped and quantified using ImageJ software, and the variations are illustrated in the corresponding histogram; Magnification 10×. Scale bar 50 μm. All data are presented as mean ± S.E.M, with corresponding bar graphs. An asterisk (*) denotes a significant difference from the normal saline-treated group; hash (#) indicates a significant difference from the scopolamine-treated group. Significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; #P ≤ 0.05, ##P ≤ 0.01; ###P ≤ 0.001.
3.5 Ambroxol attenuates scopolamine-induced behavioral and cognitive deficits in mice
The neuroprotective effects of ambroxol on spatial memory were evaluated in mice exhibiting cognitive and behavioral deficits induced by scopolamine. These effects were assessed using the Morris Water Maze (MWM) and Y-maze paradigms. In the MWM, mice from each experimental group were trained for 5 days to evaluate their learning abilities by practicing with a hidden platform. For the training period, the mean latency to locate the hidden platform steadily dropped. In contrast to the control group, the scopolamine-treated group showed a greater delay in locating the platform, suggesting impaired spatial learning and memory. Our findings demonstrated that administering ambroxol considerably reduced the elevated latency to find the platform during the training days brought on by scopolamine treatment. On day 6, we removed the platform and conducted a probe test to evaluate memory formation. Mice treated with scopolamine exhibited considerably fewer crossings and spent less time in the target quadrant than control mice. However, in the ambroxol co-treated group, both the number of platform crossings and the time spent in the target quadrant were significantly increased compared to the scopolamine-only treated group (Figures 6A–C). To further examine the cognitive abilities of mice, we assessed exploratory behavior and spatial working memory, a type of short-term memory, by determining the percentage (%) of spontaneous alternation behavior using the Y-maze test. An increase in the percentage of spontaneous alternation behavior was taken as an indication of enhanced memory function. Analysis of the spontaneous alternation rate from the center of the Y-maze is based on the percentage of spontaneous alternation behaviors, evaluated in the form of the total number of arm entries, a measure of exploratory activity, and successive triplets. Our results indicated that scopolamine-treated mice had a lower percentage of alternation than control mice, thus exhibiting reduced working memory. In contrast, the ambroxol + scopolamine-treated group significantly increased spontaneous alternation behavior (%) compared to the scopolamine-treated group, indicating that ambroxol mitigated memory deficits in the scopolamine-administered mice model. These findings provide evidence that ambroxol treatment not only increased exploratory behavior but also alleviated scopolamine-induced cognitive dysfunction (Figures 6A, D, E).
FIGURE 6

Ambroxol enhances learning memory and improves spontaneous alteration behavior in mice with scopolamine-induced memory impairment. (A) Trajectories of Y-maze and Morris water maze analysis. (B) Mean escape latency to reach the hidden platform during the training sessions. (C) Time spent in the platform quadrant, where the hidden platform was located, during the trial session. (D) The percentage of spontaneous alteration behavior during the Y-maze analysis. (E) The average number of target crossings at the hidden platform during the Morris water maze (MWM) test probe trial. For the behavioral study, each experimental group consisted of eight mice (n = 8). All data are presented as mean ± S.E.M, with corresponding bar graphs. An asterisk (*) denotes a significant difference from the normal saline-treated group; hash (#) indicates a significant difference from the scopolamine-treated group. Significance: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; #P ≤ 0.05, ##P ≤ 0.01; ###P ≤ 0.001.
4 Discussion
Alzheimer’s Disease (AD) stands as the most prevalent and predominant cause of dementia (Sequeira and Godad, 2024). Although the exact cause of AD is still unknown, oxidative stress and mitochondrial dysfunction are considered key factors in the disease’s progression (Tichon et al., 2023). It is characterized by neuronal degeneration, synaptic disruption, and behavioral anomalies, which subsequently lead to memory deficits and a progressive decline in cognitive functions (Rummel et al., 2022). The antimuscarinic agent scopolamine has been employed to antagonize muscarinic receptors, thereby inducing AD-like dementia in various animal models, which is known to induce oxidative stress, a key pathological feature in the development of AD.(Mostafa et al., 2021). Increased oxidative stress triggers a cascade of events that leads to synaptic dysfunction, neuroinflammation, and neurodegeneration, collectively contributing to the progression of neurodegenerative diseases (Tönnies and Trushina, 2017).
We investigated the neuroprotective effects of ambroxol against scopolamine-induced oxidative stress mouse model of AD. Nrf-2, a key regulator of endogenous antioxidant gene expression, acts as a stress-responsive transcription factor that mitigates reactive oxygen species (ROS)-induced oxidative stress across various pathological conditions.
Under normal physiological conditions, Nrf-2 is bound to its cytoplasmic inhibitor Keap1, facilitating its polyubiquitination and subsequent proteasomal degradation. However, under normal oxidative stress (moderate, acute), the Keap1-dependent ubiquitin ligase activity is inhibited, allowing Nrf-2 to escape degradation, translocate to the nucleus, which subsequently triggers the activation of other redox-regulated enzymes, such as heme oxygenase-1 (HO-1), to counteract the damaging effects of oxidative stress (Chen et al., 2012; Jana et al., 2018; Kim et al., 2020). However, previous studies have demonstrated that scopolamine-induced oxidative stress is more severe and sustained, which can impair the Nrf-2 signaling pathway and suppress the expression of Nrf-2 and HO-1, rather than activating it, thereby impairing the cellular defense mechanisms against oxidative damage and being involved in the pathogenesis of AD (Li et al., 2024; Ju et al., 2021; Yoo et al., 2024; Venkatesan et al., 2016; Subedi et al., 2019; Balakrishnan et al., 2023). According to reactive oxygen species (ROS) and lipid peroxidation (LPO) assays, ambroxol treatment significantly reduced ROS and LPO levels in the brains of mice subjected to scopolamine-induced oxidative stress. Furthermore, our findings demonstrate that ambroxol administration effectively reversed the scopolamine-induced effects, significantly increasing the expression levels of Nrf-2 and HO-1 proteins. Ambroxol alleviates scopolamine-induced oxidative stress and enhances the cellular antioxidant defense mechanism.
The c-Jun N-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK) family, is a highly conserved signal transduction pathway that can be activated by a diverse array of environmental stimuli. It plays a crucial role in regulating a variety of physiological processes (Cargnello and Roux, 2011; Gehi et al., 2022). Previous reports demonstrated that oxidative stress activates the JNK signaling pathway, resulting in prolonged phosphorylation of JNK, which disrupts cellular homeostasis (Mehan et al., 2011; Zhu et al., 2001; Quiroz-Baez et al., 2009). Glycogen synthase kinase-3β (GSK-3β) is a widely expressed and constitutively active serine/threonine kinase that plays a pivotal role in regulating numerous essential cellular pathways. It contributes to the pathogenesis of AD through multiple distinct mechanisms (Lauretti et al., 2020). It has been reported that oxidative stress plays a central role in disrupting GSK-3β, which in turn contributes to the key pathological features of AD (Dhapola et al., 2024; Lauretti et al., 2020). Accordingly, our findings indicated that scopolamine increased the expression levels of p-JNK and disrupted GSK-3β; however, ambroxol therapy significantly restores these proteins’ expression. Therefore, by inhibiting the JNK and restoring GSK-3β pathways, our results propose a novel and distinct neuroprotective mechanism of ambroxol in counteracting scopolamine-induced oxidative stress.
Phosphorylated JNK (p-JNK) and GSK-3β, in addition to their pivotal roles in regulating physiological processes and key cellular pathways, serve as crucial mediators of microglial activation and neuroinflammation (Wang et al., 2010; Biswas, 2023). Chronic neuroinflammation is a potential risk factor for various age-related diseases (Sanada et al., 2018). Glial cells, including microglia and astrocytes, constitute the frontline defense of the central nervous system and play a central role in amplifying the neuroinflammatory response in neurodegenerative disorders (Singh, 2022). Ionized calcium-binding adaptor molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) are specific markers of microglia and astrocyte activation (Norden et al., 2016). Previously, it has been reported that scopolamine administration induces activation of Iba-1 and GFAP (Muhammad et al., 2019). According to earlier research, activated glial cells overexpress the transcription factor NF-κB, leading to increased production of various proinflammatory mediators, such as TNF-α and IL-1β, which in turn contributes to neuroinflammation (Ahmad et al., 2024; Maqbool et al., 2013). Here, we found that scopolamine treatment induces the expression level of GFAP and Iba-1 in the cortex and hippocampus. In contrast, co-treatment of scopolamine and ambroxol markedly reduced the protein expression and immunofluorescence reactivity of GFAP and Iba-1 in the cortex and hippocampus. We also found that scopolamine enhances p-NF-κB (p-p65) and proinflammatory mediators such as TNF-α and IL-1β expression compared to saline-treated mice, which was significantly reversed by scopolamine + ambroxol treatment. These findings demonstrate that ambroxol treatment effectively inhibits the activation of glial cells and neuroinflammatory mediators, thereby preventing scopolamine-induced neuroinflammation.
Previously, it has been reported that oxidative stress, JNK, and GSK-3β signaling are involved in learning and memory deficits (Ullah et al., 2020; Takashima, 2006). Control of synaptic plasticity by synaptic proteins is essential for brain function (Lu et al., 2021). Scopolamine-induced synaptic and memory impairment by downregulating memory-associated synaptic protein levels in mice brains (Li et al., 2021; Zhang et al., 2022). Our results showed that scopolamine treatment reduced the expression levels of synaptic proteins, whereas cotreatment with ambroxol recovered the reduced synaptic protein levels. Furthermore, we found that scopolamine dramatically decreased memory function as measured by the Y-Maze and MWM tests. In the MWM test, ambroxol administration decreased the escape latency and increased the number of platform crossings and time spent by the mice in the target quadrant. An increase in the percentage (%) of spontaneous alteration behavior was detected in the Y-maze test. These results show that ambroxol treatment reduces scopolamine-induced synaptic protein loss and spatial learning/cognitive impairment, improving behavior and memory. We propose that ambroxol therapy is effective against scopolamine-induced cognitive and memory impairment.
Conclusion
Ambroxol alleviates scopolamine-induced oxidative stress, modulates neuroinflammatory pathways, and mitigates neurodegenerative changes. It reduces lipid peroxidation (LPO) and reactive oxygen species (ROS) production while upregulating the expression of key antioxidant defense proteins. Ambroxol inhibits c-Jun N-terminal kinase (JNK) activation and restores dysregulated Akt and GSK-3β signaling pathways. Additionally, it attenuates activated gliosis and downregulates inflammatory mediators. Furthermore, ambroxol promotes synaptic integrity and facilitates cognitive recovery (Figure 7).
FIGURE 7

Diagrammatic representation of Ambroxol’s suggested neuroprotective mechanism against oxidative stress, neuroinflammation, synaptic dysfunction, and memory impairment against scopolamine.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the animal ethics committee (IACUC) of the Division of Applied Life Science, Gyeongsang National University, Jinju, South Korea (Approval ID: 125, Code: GNU-200331-M0020). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WA: Writing – review and editing, Software, Conceptualization, Writing – original draft, Data curation, Formal Analysis, Methodology. KC: Writing – review and editing, Data curation, Conceptualization. RA: Writing – original draft, Visualization, Conceptualization. TP: Formal Analysis, Conceptualization, Writing – original draft. MOK: Project administration, Supervision, Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Government (MSIT) (RS-2024-00441331).
Conflict of interest
MOK was employed by the company Alz-Dementia Korea Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abu Almaaty A. H. Mosaad R. M. Hassan M. K. Ali E. H. Mahmoud G. A. Ahmed H. et al (2021). Urtica dioica extracts abolish scopolamine-induced neuropathies in rats.Environ. Sci. Pollut. Res. Int.2818134–18145. 10.1007/s11356-020-12025-y
2
Ahmad S. Choe K. Badshah H. Ahmad R. Ali W. Rehman I. U. et al (2024). Physcion mitigates LPS-induced neuroinflammation, oxidative stress, and memory impairments via TLR-4/NF-κB signaling in adult mice.Pharmaceuticals17:1199. 10.3390/ph17091199
3
Ahmadi E. Afrooghe A. Soltani Z. E. Elahi M. Shayan M. Ohadi M. A. D. et al (2024). Beyond the lungs: Exploring diverse applications of bromhexine and ambroxol.Life Sci.353:122909. 10.1016/j.lfs.2024.122909
4
Ali J. Khan A. Park J. S. Tahir M. Ahmad W. Choe K. et al (2023). Neuroprotective effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-induced Alzheimer’s disease mouse model.Nutrients15:4986. 10.3390/nu15234986
5
Ali T. Badshah H. Kim T. H. Kim M. (2015). Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model.J. Pineal Res.5871–85. 10.1111/jpi.12194
6
Ali W. Choe K. Park J. S. Ahmad R. Park H. Y. Kang M. H. et al (2024). Kojic acid reverses LPS-induced neuroinflammation and cognitive impairment by regulating the TLR4/NF-κB signaling pathway.Front. Pharmacol.15:1443552. 10.3389/fphar.2024.1443552
7
Amin F. U. Shah S. A. Kim M. O. (2017). Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice.Sci. Rep.7:40753. 10.1038/srep40753
8
Aryal B. Raut B. K. Bhattarai S. Bhandari S. Tandan P. Gyawali K. et al (2022). Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases.Evid. Based Complement. Alternat. Med.2022:7299778. 10.1155/2022/7299778
9
Bai R. Guo J. Ye X.-Y. Xie Y. Xie T. J. A. (2022). Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease.Ageing Res. Rev.77:101619. 10.1016/j.arr.2022.101619
10
Balakrishnan R. Park J.-Y. Cho D.-Y. Ahn J.-Y. Yoo D.-S. Seol S.-H. et al (2023). AD− 1 small molecule improves learning and memory function in scopolamine-induced amnesic mice model through regulation of CREB/BDNF and NF-κB/MAPK signaling pathway.Antioxidants (Basel)12:648. 10.3390/antiox12030648
11
Bishr A. Sallam N. Nour El-Din M. Awad A. S. Kenawy S. A. J. (2019). Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/p-ERK.Can. J. Physiol. Pharmacol.9755–64. 10.1139/cjpp-2018-0528
12
Biswas K. J. (2023). Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation.J. Neuroimmunol.383:578180. 10.1016/j.jneuroim.2023.578180
13
Calabrese V. Cornelius C. Cuzzocrea S. Iavicoli I. Rizzarelli E. Calabrese E. J. J. (2011). Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity.Mol. Aspects Med.32279–304. 10.1016/j.mam.2011.10.007
14
Cargnello M. Roux P. P. (2011). Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases.Microbiol. Mol. Biol. Rev.7550–83. 10.1128/MMBR.00031-10
15
Cavalu S. Sharaf H. Saber S. Youssef M. E. Abdelhamid A. M. Mourad A. A. et al (2022). Ambroxol, a mucolytic agent, boosts HO-1, suppresses NF-κB, and decreases the susceptibility of the inflamed rat colon to apoptosis: A new treatment option for treating ulcerative colitis.FASEB J.36:e22496. 10.1096/fj.202200749R
16
Chen X. Guo C. Kong J. J. (2012). Oxidative stress in neurodegenerative diseases⋆.Neural Regen Res.5376–385. 10.3969/j.issn.1673-5374.2012.05.009
17
Cheon S. Y. Koo B.-N. Kim S. Y. Kam E. H. Nam J. Kim E. J. (2021). Scopolamine promotes neuroinflammation and delirium-like neuropsychiatric disorder in mice.Sci. Rep.11:8376. 10.1038/s41598-021-87790-y
18
Combs C. K. (2009). Inflammation and microglia actions in Alzheimer’s disease.J. Neuroimmune Pharmacol.4380–388. 10.1007/s11481-009-9165-3
19
Dhanve P. Aggarwal P. Choure S. Dhaked D. K. Banerjee S. J. (2023). Ambroxol: A potential therapeutics against neurodegeneration.Amsterdam: Elsevier.
20
Dhapola R. Beura S. K. Sharma P. Singh S. K. Harikrishnareddy D. J. M. (2024). Oxidative stress in Alzheimer’s disease: Current knowledge of signaling pathways and therapeutics.Mol. Biol. Rep.51:48. 10.1007/s11033-023-09021-z
21
Drummond E. Wisniewski T. J. (2017). Alzheimer’s disease: Experimental models and reality.Acta Neuropathol.133155–175. 10.1007/s00401-016-1662-x
22
Faldu K. G. Shah J. S. J. (2024). Ambroxol improves amyloidogenic, NF-κB, and Nrf2 pathways in a scopolamine-induced cognitive impairment rat model of Alzheimer’s disease.Drug Dev. Res.85:e70017. 10.1002/ddr.70017
23
Franco G. A. Interdonato L. Cordaro M. Cuzzocrea S. Di Paola R. J. (2023). Bioactive compounds of the Mediterranean diet as nutritional support to fight neurodegenerative disease.Int. J. Mol. Sci.24:7318. 10.3390/ijms24087318
24
Gehi B. R. Gadhave K. Uversky V. N. Giri R. J. C. (2022). Intrinsic disorder in proteins associated with oxidative stress-induced JNK signaling.Cell Mol. Life Sci.79:202. 10.1007/s00018-022-04230-4
25
Gul S. Attaullah S. Alsugoor M. H. Bawazeer S. Shah S. A. Khan S. et al (2023). Folicitin abrogates scopolamine induced oxidative stress, hyperlipidemia mediated neuronal synapse and memory dysfunction in mice.Heliyon9:e16930. 10.1016/j.heliyon.2023.e16930
26
Hampel H. Mesulam M.-M. Cuello A. C. Farlow M. R. Giacobini E. Grossberg G. T. et al (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease.Brain1411917–1933. 10.1093/brain/awy132
27
Hu H. Guo L. Overholser J. Wang X. J. C. (2022). Mitochondrial VDAC1: A potential therapeutic target of inflammation-related diseases and clinical opportunities.Cells11:3174. 10.3390/cells11193174
28
Istaiti M. Revel-Vilk S. Becker-Cohen M. Dinur T. Ramaswami U. Castillo-Garcia D. et al (2021). Upgrading the evidence for the use of ambroxol in Gaucher disease and GBA related Parkinson: Investigator initiated registry based on real life data.Am. J. Hematol.96545–551. 10.1002/ajh.26131
29
Istifo N. N. Al-Zobaidy M. A. Abass K. S. J. D. (2024). Long-term effects of scopolamine on brain tissue of mice.J. Faculty Med. Baghdad1:3. 10.32007/jfacmedbaghdad.6632323
30
Jana S. Patra K. Jana J. Mandal D. P. Bhattacharjee S. J. (2018). Nrf-2 transcriptionally activates P21Cip/WAF1 and promotes A549 cell survival against oxidative stress induced by H2O2.Chem. Biol. Interact.28559–68. 10.1016/j.cbi.2018.02.030
31
Jiang K. Wang X. Mao X. Lao H. Zhang J. Wang G. et al (2013). “Ambroxol alleviates hepatic ischemia reperfusion injury by antioxidant and antiapoptotic pathways,” in Proceedings of the Transplantation, (Amsterdam: Elsevier), 2439–2445.
32
Jiang X. Zhang J. Kou B. Zhang C. Zhong J. Fang X. et al (2020). Ambroxol improves neuronal survival and reduces white matter damage through suppressing endoplasmic reticulum stress in microglia after intracerebral hemorrhage.Biomed. Res. Int.8131286. 10.1155/2020/8131286
33
Ju S. Seo J. Y. Lee S. K. Oh J. Kim J.-S. (2021). Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism.J. Ginseng Res.45108–118. 10.1016/j.jgr.2019.12.005
34
Kim H. J. Jang B. K. Park J.-H. Choi J. W. Park S. J. Byeon S. R. et al (2020). A novel chalcone derivative as Nrf2 activator attenuates learning and memory impairment in a scopolamine-induced mouse model.Eur. J. Med. Chem.185:111777. 10.1016/j.ejmech.2019.111777
35
Lahiri D. Rogers J. Greig N. Sambamurti K. J. (2004). Rationale for the development of cholinesterase inhibitors as anti-Alzheimer agents.Curr. Pharm. Des.103111–3119. 10.2174/1381612043383331
36
Lauretti E. Dincer O. Praticò D. J. B. (2020). Glycogen synthase kinase-3 signaling in Alzheimer’s disease.Biochim. Biophys. Acta Mol. Cell. Res.1867:118664. 10.1016/j.bbamcr.2020.118664
37
Lee M. Kwon B.-M. Suk K. Mcgeer E. Mcgeer P. L. J. (2012). Effects of obovatol on GSH depleted glia-mediated neurotoxicity and oxidative damage.J. Neuroimmune Pharmacol.7173–186. 10.1007/s11481-011-9300-9
38
Li D. Cai C. Liao Y. Wu Q. Ke H. Guo P. et al (2021). Systems pharmacology approach uncovers the therapeutic mechanism of medicarpin against scopolamine-induced memory loss.Phytomedicine91:153662. 10.1016/j.phymed.2021.153662
39
Li X. Zheng K. Chen H. Li W. J. C. (2024). Ginsenoside Re Regulates Oxidative Stress through the PI3K/Akt/Nrf2 Signaling Pathway in Mice with Scopolamine-Induced Memory Impairments.Curr. Issues Mol. Biol.4611359–11374. 10.3390/cimb46100677
40
Li Z. Yin B. Zhang S. Lan Z. Zhang L. J. (2023). Targeting protein kinases for the treatment of Alzheimer’s disease: Recent progress and future perspectives.Eur. J. Med. Chem.261:115817. 10.1016/j.ejmech.2023.115817
41
Liu Z. Zhou T. Ziegler A. C. Dimitrion P. Zuo L. J. O. M. Longevity C. (2017). Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications.Oxid. Med. Cell. Longev.2017:2525967. 10.1155/2017/2525967
42
Llorens-Marítin M. Jurado J. Hernández F. Ávila J. J. (2014). GSK-3β, a pivotal kinase in Alzheimer disease.Front. Mol. Neurosci.7:46. 10.3389/fnmol.2014.00046
43
Lu C. Wang Y. Xu T. Li Q. Wang D. Zhang L. et al (2018). Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling.Front. Pharmacol.9:1153. 10.3389/fphar.2018.01153
44
Lu H. Fang L. Wang J. Zhao F. Liu C. Gao Y. et al (2021). Pine nut antioxidant peptides ameliorate the memory impairment in a scopolamine-induced mouse model via SIRT3-induced synaptic plasticity.Food Funct.128026–8036.
45
Malerba M. Ragnoli B. J. E. (2008). Ambroxol in the 21st century: Pharmacological and clinical update.Expert. Opin. Drug Metab. Toxicol.41119–1129. 10.1517/17425255.4.8.1119
46
Maqbool A. Lattke M. Wirth T. Baumann B. J. M. N. (2013). Sustained, neuron-specific IKK/NF-κB activation generates a selective neuroinflammatory response promoting local neurodegeneration with aging.Mol. Neurodegener.81–18. 10.1186/1750-1326-8-40
47
Mehan S. Meena H. Sharma D. Sankhla R. J. J. (2011). JNK: A stress-activated protein kinase therapeutic strategies and involvement in Alzheimer’s and various neurodegenerative abnormalities.J. Mol. Neurosci.43376–390. 10.1007/s12031-010-9454-6
48
Mostafa N. M. Mostafa A. M. Ashour M. L. Elhady S. S. (2021). Neuroprotective effects of black pepper cold-pressed oil on scopolamine-induced oxidative stress and memory impairment in rats.Antioxidants10:1993. 10.3390/antiox10121993
49
Muhammad T. Ali T. Ikram M. Khan A. Alam S. I. Kim M. O. P. (2019). Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model.J. Neuroimmune Pharmacol.14278–294. 10.1007/s11481-018-9824-3
50
Norden D. M. Trojanowski P. J. Villanueva E. Navarro E. Godbout J. P. (2016). Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge.Glia64300–316. 10.1002/glia.22930
51
Oddo S. Caccamo A. Shepherd J. D. Murphy M. P. Golde T. E. Kayed R. et al (2003). Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction.Neuron39409–421. 10.1016/s0896-6273(03)00434-3
52
Patzwaldt K. Berezhnoy G. Ionescu T. Schramm L. Wang Y. Owczorz M. et al (2023). Repurposing the mucolytic agent ambroxol for treatment of sub-acute and chronic ischaemic stroke.Brain Commun.5:fcad099. 10.1093/braincomms/fcad099
53
Ponne S. Kumar C. R. Boopathy R. J. M. (2020). Verapamil attenuates scopolamine induced cognitive deficits by averting oxidative stress and mitochondrial injury–A potential therapeutic agent for Alzheimer’s Disease.Metab. Brain Dis.35503–515. 10.1007/s11011-019-00498-x
54
Qian Y. F. Wang H. Yao W. B. Gao X. D. J. (2008). Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade.Cell. Biol. Int.32304–311. 10.1016/j.cellbi.2007.10.004
55
Quiroz-Baez R. Rojas E. Arias C. J. (2009). Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of α-, β-and γ-secretase expression.Neurochem. Int.55662–670. 10.1016/j.neuint.2009.06.012
56
Rahimzadegan M. Soodi M. J. B. (2018). Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus.Basic Clin. Neurosci.95–14. 10.29252/NIRP.BCN.9.1.5
57
Ravali K. Kumary U. Sarathchandra G. Kannan T. Parthiban M. Kalaiselvi L. Vasicine a quinazoline alkaloid from Justicia adhatoda L.: Its antioxidant property. Int. J. Adv. Biochem. Res. 8 729–731. 10.33545/26174693.2024.v8.i1Sj.415
58
Rehman S. U. Shah S. A. Ali T. Chung J. I Kim M. O. (2017). Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats.Mol. Neurobiol.54255–271. 10.1007/s12035-015-9604-5
59
Rummel N. G. Butterfield D. A. Signaling R. (2022). Altered metabolism in Alzheimer disease brain: Role of oxidative stress.Antioxid. Redox Signal.361289–1305. 10.1089/ars.2021.0177
60
Sanada F. Taniyama Y. Muratsu J. Otsu R. Shimizu H. Rakugi H. et al (2018). Source of chronic inflammation in aging.Front. Cardiovasc. Med.22:12. 10.3389/fcvm.2018.00012
61
Sandhu M. Irfan H. M. Shah S. A. Ahmed M. Naz I. Akram M. et al (2022). Friedelin attenuates neuronal dysfunction and memory impairment by inhibition of the activated JNK/NF-κB signalling pathway in scopolamine-induced mice model of neurodegeneration.Molecules27:4513. 10.3390/molecules27144513
62
Sarubbo F. Ramis M. Kienzer C. Aparicio S. Esteban S. Miralles A. et al (2018). Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats.J. Neuroimmune Pharmacol.1324–38. 10.1007/s11481-017-9759-0
63
Sequeira R. C. Godad A. J. (2024). Understanding glycogen synthase kinase-3: A novel avenue for Alzheimer’s disease.Mol. Neurobiol.614203–4221. 10.1007/s12035-023-03839-1
64
Singh D. J. J. (2022). Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease.J. Neuroinflammation19:206. 10.1186/s12974-022-02565-0
65
Skalicka-Wozniak K. Budzynska B. Biala G. Boguszewska-Czubara A. J. (2018). Scopolamine-induced memory impairment is alleviated by xanthotoxin: Role of acetylcholinesterase and oxidative stress processes.ACS Chem. Neurosci.91184–1194. 10.1021/acschemneuro.8b00011
66
SoukhakLari R. Moezi L. Pirsalami F. Ashjazadeh N. Moosavi M. P. (2018). Curcumin ameliorates scopolamine-induced mice memory retrieval deficit and restores hippocampal p-Akt and p-GSK-3β.Eur. J. Pharmacol.84128–32. 10.1016/j.ejphar.2018.10.012
67
Stephenson J. Nutma E. van der Valk P. Amor S. J. I. (2018). Inflammation in CNS neurodegenerative diseases.Immunology154204–219. 10.1111/imm.12922
68
Štětinová V. Herout V. Květina J. J. (2004). In vitro and in vivo antioxidant activity of ambroxol.Clin. Exp. Med.4152–158. 10.1007/s10238-004-0050-3
69
Su X. Wang L. Song Y. Bai C. J. (2004). Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide.Intensive Care Med.30133–140. 10.1007/s00134-003-2001-y
70
Subedi L. Cho K. Park Y. U. Choi H. J. Kim S. Y. (2019). Sulforaphane-enriched broccoli sprouts pretreated by pulsed electric fields reduces neuroinflammation and ameliorates scopolamine-induced amnesia in mouse brain through its antioxidant ability via Nrf2-HO-1 activation.Oxid. Med. Cell. Longev.2019:3549274. 10.1155/2019/3549274
71
Sun E. Motolani A. Campos L. Lu T. J. (2022). The pivotal role of NF-kB in the pathogenesis and therapeutics of Alzheimer’s disease.Int. J. Mol. Sci.23:8972. 10.3390/ijms23168972
72
Takashima A. (2006). GSK-3 is essential in the pathogenesis of Alzheimer’s disease.J. Alzheimers Dis.9309–317. 10.3233/jad-2006-9s335
73
Thingore C. Kshirsagar V. Juvekar A. J. (2021). Amelioration of oxidative stress and neuroinflammation in lipopolysaccharide-induced memory impairment using Rosmarinic acid in mice.Metab. Brain Dis.36299–313. 10.1007/s11011-020-00629-9
74
Tichon A. Eitan E. Tsory S. Beit-Yanai E. Priel E. J. (2023). The Neuroprotective role of TERT influences the expression of SOD1 in motor neurons and mouse brain: Implications for fALS.J. Neurosci. Neurol. Disord.7113–125. 10.29328/journal.jnnd.1001085
75
Tönnies E. Trushina E. J. J. (2017). Oxidative stress, synaptic dysfunction, and Alzheimer’s disease.J. Alzheimer’s Dis.571105–1121. 10.3233/JAD-161088
76
Ullah R. Jo M. H. Riaz M. Alam S. I. Saeed K. Ali W. et al (2020). Glycine, the smallest amino acid, confers neuroprotection against D-galactose-induced neurodegeneration and memory impairment by regulating c-Jun N-terminal kinase in the mouse brain.J. Neuroinflammation17303. 10.1186/s12974-020-01989-w
77
Ullah S. Park T. J. Park J. S. Atiq A. Ali J. Kang M. H. et al (2025). Ambroxol attenuates detrimental effect of LPS-induced glia-mediated neuroinflammation, oxidative stress, and cognitive dysfunction in mice brain.Front. Immunol.16:1494114. 10.3389/fimmu.2025.1494114
78
Venkatesan R. Subedi L. Yeo E.-J. Kim S. Y. (2016). Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway.Neurochem. Int.99133–146. 10.1016/j.neuint.2016.06.010
79
Volkman R. Offen D. J. (2017). Concise review: Mesenchymal stem cells in neurodegenerative diseases.Stem Cells351867–1880. 10.1002/stem.2651
80
Wang M.-J. Huang H.-Y. Chen W.-F. Chang H.-F. Kuo J.-S. (2010). Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades.J. Neuroinflammation7:99. 10.1186/1742-2094-7-99
81
Wang Z.-X. Lian W.-W. He J. He X.-L. Wang Y.-M. Pan C.-H. et al (2022). Cornuside ameliorates cognitive impairments in scopolamine induced AD mice: Involvement of neurotransmitter and oxidative stress.J Ethnopharmacol.293:115252. 10.1016/j.jep.2022.115252
82
Yang L. Liu C.-C. Zheng H. Kanekiyo T. Atagi Y. Jia L. et al (2016). LRP1 modulates the microglial immune response via regulation of JNK and NF-κB signaling pathways.J. Neuroinflammation13:304. 10.1186/s12974-016-0772-7
83
Yarza R. Vela S. Solas M. Ramirez M. J. (2016). c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease.Front. Pharmacol.6:321. 10.3389/fphar.2015.00321
84
Yoo J. Nguyen C. D. Jeong S. J. Yang J. H. Lee G. Shin J. C. et al (2024). Prioritizing Nrf2/HO-1-mediated intrinsic antioxidant upregulation: The foremost neuroprotective mechanism of melittin in a scopolamine-induced animal model of neural stress, preceding anti-inflammatory effects.Durham, NC: Research Square Platform LLC, 10.21203/rs.3.rs-4002383/v1
85
Zhang G.-J. Zheng D. Yu H. Luo X.-P. Wu W. J. (2022). Ginkgo biloba extract ameliorates scopolamine-induced memory deficits via rescuing synaptic damage.Curr. Med. Sci.42474–482. 10.1007/s11596-022-2582-8
86
Zhang Y. Chen H. Li R. Sterling K. Song W. J. (2023). Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future.Signal. Transduct. Target. Ther.8:248. 10.1038/s41392-023-01484-7
87
Zhao Y. He C. Hu S. Ni H. Tan X. Zhi Y. et al (2024). Anti-oxidative stress and cognitive improvement of a semi-synthetic isoorientin-based GSK-3β inhibitor in rat pheochromocytoma cell PC12 and scopolamine-induced AD model mice via AKT/GSK-3β/Nrf2 pathway.Exp. Neurol.380:114881. 10.1016/j.expneurol.2024.114881
88
Zhou Q. Wang M. Du Y. Zhang W. Bai M. Zhang Z. et al (2015). Inhibition of c-J un N-terminal kinase activation reverses A lzheimer disease phenotypes in APP swe/PS 1dE 9 mice.Ann. Neurol.77637–654. 10.1002/ana.24361
89
Zhu X. Castellani R. Takeda A. Nunomura A. Atwood C. Perry G. et al (2021). Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: The ‘two hit’ hypothesis.Mech. Ageing Dev.12339–46. 10.1016/s0047-6374(01)00342-6
Summary
Keywords
Alzheimer’s disease, scopolamine, ambroxol, oxidative stress, neuroinflammation, synaptic dysfunction.
Citation
Ahmad W, Choe K, Ahmad R, Park TJ and Kim MO (2025) Ambroxol confers neuroprotection against scopolamine-induced Alzheimer’s-like pathology by modulating oxidative stress, neuroinflammation, and cognitive deficits via Nrf-2/JNK/GSK-3β signaling pathways. Front. Aging Neurosci. 17:1607289. doi: 10.3389/fnagi.2025.1607289
Received
07 April 2025
Accepted
04 July 2025
Published
23 July 2025
Volume
17 - 2025
Edited by
Chandra Prakash, Jawaharlal Nehru University, India
Reviewed by
Anne Kasus-Jacobi, University of Oklahoma Health Sciences Center, United States
Julie Ann Moreno, Colorado State University, United States
Updates
Copyright
© 2025 Ahmad, Choe, Ahmad, Park and Kim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae Ju Park, taeju.park@einsteinmed.eduMyeong Ok Kim, mokim@gnu.ac.kr
†These authors have equally contributed to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.