- Department of Geriatrics Center, The Fourth People’s Hospital of Shenyang, Shenyang, China
Background: Brain-derived neurotrophic factor (BDNF) is essential for regulating neuronal proliferation and survival in neurodegenerative diseases, including Parkinson’s disease (PD). However, Studies on BDNF levels in peripheral blood and cerebrospinal fluid (CSF) have inconsistent results. Therefore, this study aimed to examine BDNF levels in patients with PD and to explore their correlation with non-motor symptoms.
Methods: Four databases (PubMed, Embase, Cochrane Library, and CNKI) were searched for eligible studies. The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS). Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated using Stata version 14.0, applying either a fixed-effect or a random-effects model based on heterogeneity. Furthermore, subgroup analysis, meta-regression, and sensitivity analysis were employed to identify and analyze sources of heterogeneity. Publication bias was assessed using funnel plots and Egger’s test.
Results: A comprehensive systematic review and meta-analysis was conducted, encompassing 48 articles. A total of 38 studies, covering 2,589 patients with PD and 2,422 healthy controls, were analyzed, revealing significantly lower peripheral blood BDNF levels in PD patients compared to healthy controls (SMD = −1.037; 95% CI [−1.412, −0.662]; P < 0.001), with substantial heterogeneity (I2 = 97.0%; P < 0.001). This result may be more applicable to serum samples and the Asian population according to subgroup analysis. PD patients with depression showed no significant difference in serum BDNF levels compared to those without depression (SMD = −0.511; 95% CI [−1.692, 0.671]; P = 0.397). A significant association was found between decreased serum BDNF concentrations and cognitive impairment in PD (SMD = −1.035; 95% CI [−1.340, −0.730]; P < 0.001). Moreover, negative correlations were observed between lower serum BDNF levels and autonomic dysfunction, rapid eye movement sleep behavior disorder (RBD), and restless legs syndrome (RLS), respectively. However, CSF BDNF levels showed no statistically significant difference between PD patients and controls (SMD = −0.398; 95% CI [−2.499, 1.703]; P = 0.711).
Conclusion: Reduced expression of BDNF is associated with both PD and its non-motor symptoms. Further research is needed to explore the potential of BDNF as a biomarker for non-motor symptoms of PD, particularly for cognitive impairment.
1 Introduction
Parkinson’s disease (PD), which is characterized by the deposition of toxic aggregated alpha-synuclein and the loss of dopaminergic neurons, is a complex and chronic neurodegenerative disease. The most frequently observed clinical manifestations among PD patients include bradykinesia and resting tremor, alongside a range of non-motor symptoms such as depression, cognitive impairment, and sleep disturbances. Non-motor symptoms can precede motor symptoms by 10–20 years, placing a significant burden on thousands of families (GBD 2016 Parkinson’s Disease Collaborators, 2018; Leite Silva et al., 2023; Schapira et al., 2017). Depression, affecting approximately 38% of patients with PD, typically emerges early in the disease and persists throughout its course, substantially reducing patients’ quality of life (Cong et al., 2022). The prevalence of cognitive impairment among patients diagnosed with PD is estimated to be as high as 40% (Baiano et al., 2020). However, the significance of non-motor symptoms for the preliminary diagnosis of PD has not been adequately reflected in international diagnostic criteria, highlighting the need for further research to investigate their potential as diagnostic markers for PD.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophic factor family, is widely distributed in the central nervous system, including astrocytes of the hippocampus and prefrontal cortex. It is also found in non-central nervous system tissues, such as platelets and cardiomyocytes (Palasz et al., 2020). BDNF has a high affinity for tropomyosin receptor kinase B (TrkB) and regulates neuronal maturation and survival–processes that are particularly important in the context of neurodegenerative diseases and psychiatric disorders (Colucci-D’Amato et al., 2020; Numakawa and Kajihara, 2025). Decreased BDNF expression in the striatum has been reported in a PD mouse model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Zhou et al., 2025). Low expression of BDNF in the medial prefrontal cortex has been observed in a depressive PD rat model, and activation of the BDNF/TrkB pathway can prevent depressive-like behavior in rats (Ma et al., 2024; Tekşen et al., 2024). Furthermore, the rs6265 BDNF allele and BDNF serum levels have been linked to various non-motor symptoms in PD (Nikitina et al., 2024a,b).
However, the association of BDNF levels in multiple body fluids with PD remains unclear, with conflicting results reported across studies. A consensus on the correlation between BDNF concentrations and the non-motor symptoms of PD has yet to be reached. Through a systematic review and meta-analysis, the current study aims to investigate BDNF expression in the blood and cerebrospinal fluid (CSF) of PD patients compared to healthy controls. Additionally, it will also explore potential differences between PD patients with and without non-motor symptoms.
2 Method
The present meta-analysis was conducted according to the guidelines that are recommended by the PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-Analysis).
2.1 Search strategy and study inclusion
Two investigators (Zhenzhen Zhao and Jiahui Sun) independently searched PubMed, Embase, the Cochrane Library, and CNKI for eligible studies up to March 1, 2025. The search terms were “cerebrospinal fluid,” “serum,” “plasma,” “brain-derived neurotrophic factor,” and “Parkinson’s disease.” The language was restricted to English and Chinese. The inclusion criteria were as follows: (1) case–control study, cross-sectional study, or cohort study that detected BDNF concentrations before experimental intervention; (2) original studies that recorded blood or CSF BDNF concentrations in at least two groups of subjects (PD without non-motor symptoms, PD with non-motor symptoms, and healthy controls); (3) BDNF levels that could be extracted or calculated as mean ± standard deviation from the included studies. The exclusion criteria were as follows: (1) review, report, or letter; (2) samples obtained post-mortem or from animals; (3) necessary data not available or duplicate data reported in different studies. Disagreements between the two investigators regarding study inclusion were resolved by Dr. Liu.
2.2 Quality evaluation
The quality of the eligible studies was independently assessed by two investigators using the Newcastle–Ottawa Scale (NOS). Disagreements were resolved by Dr. Liu. The scale evaluates the selection of the study population, the comparability between groups, and the measurement of exposure factors. Scores above 6 were considered high quality.
2.3 Data extraction
Data were extracted independently by two investigators, and any discrepancies were resolved through discussion. The extracted data from the eligible studies included the author’s name, year of publication, specimen type, sample size, participants’ age, gender proportion, country, measurement techniques, and BDNF levels. In addition, diagnostic criteria, disease duration, Hoehn–Yahr (H–Y) scores, years of education, and Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) scores of PD patients were also recorded.
2.4 Statistic analysis
Stata version 14.0 software was used to perform the meta-analysis. Standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated to compare BDNF levels between groups. The I2 statistic and Cochran’s Q test were used to assess heterogeneity between studies. I2 values of less than 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively. When significant heterogeneity was present, a random-effects model was applied to pool the data.
Three methods were used to explore the sources of heterogeneity. First, subgroup analysis was performed to assess the effects of specimen type, country of participants, and diagnostic criteria for PD on BDNF levels. Second, sensitivity analysis was conducted to assess the influence of excluding a single study on the overall results by systematically removing one study at a time and reanalyzing the remaining data. Finally, meta-regression was used to examine the effects of sample size, participants’ age, gender proportion, H–Y scores, disease duration, years of education, and UPDRS-III scores of PD patients on the results.
In addition, publication bias was evaluated using funnel plots and Egger’s test. It is worth noting that meta-regression and Egger’s test were not performed when fewer than 10 studies were available. In accordance with standard statistical practice, P-values < 0.05 were considered to indicate statistical significance.
3 Results
3.1 Systematic search and study selection
The search flowchart was shown in Figure 1. After a comprehensive retrieval and duplicate removal, a total of 3043 potential articles were found through PubMed, Cochrane Library, Embase and CNKI. Following an initial screening of titles and abstracts, 103 articles were deemed eligible for full-text review. Finally, 48 articles that met the inclusion criteria were included in the present systematic review and meta-analysis.
Among the included studies, 38 studies included in the 34 articles reported BDNF levels in the blood of PD patients (n = 2,589) and healthy controls (n = 2,422) (Alomari et al., 2018; Brockmann et al., 2016; Chen et al., 2015; Costa et al., 2019; Hernández-Vara et al., 2020; Hou et al., 2021; Huang et al., 2018, 2022; Jin et al., 2023; Ju et al., 2018; Khalil et al., 2017; Li and Zhong, n.d.; Li et al., 2019; Liu and Liu, 2023; Peng et al., 2019; Quan et al., 2020; Rocha et al., 2018; Scalzo et al., 2010; Schaeffer et al., 2022; Siuda et al., 2017; Sun, 2011; Szymura et al., 2020; Ventriglia et al., 2013; Wang et al., 2016; Wang and Liu, n.d.; Wu, 2018; Wu et al., 2022; Xie, 2017; Xu et al., 2024; Zhang et al., 2021; Zhao et al., 2021; Zhao and Xu, n.d.; Zhou et al., 2023). Seven studies included in the six articles reported BDNF levels in the blood of PD patients with depression (n = 358), PD patients without depression (n = 361), and healthy controls (n = 290) (Azevedo et al., 2022; Huang et al., 2021a; Ju et al., 2018; Wang et al., 2017, 2024; Wang and Liu, n.d.). Ten studies included in the nine articles examined BDNF levels in the blood of PD patients with cognitive impairment (n = 392), PD patients without cognitive impairment (n = 346), and healthy controls (n = 296) (Hu, 2021; Li and Zhong, n.d.; Li et al., 2019; Lin et al., 2021; Liu et al., 2015; Xiao, 2016; Xie, 2017; Ye et al., 2016; Zhao et al., 2023). Four additional articles explored the relationship between blood BDNF levels and fatigue, autonomic nerve dysfunction, rapid eye movement sleep behavior disorder (RBD), and restless legs syndrome (RLS), respectively (Azevedo et al., 2022; Huang et al., 2021b; Jin et al., 2023; Liu et al., 2024). Additionally, there were three articles noting the BDNF levels in CSF of PD patients (n = 78) and controls (n = 131) (Pålhagen et al., 2010; Salehi and Mashayekhi, 2009; Zhang et al., 2008). The characteristics of the eligible studies were shown in Supplementary Material.
3.2 Blood BDNF levels in PD
Thirty-eight qualified studies reported BDNF levels in blood samples of PD patients compared with healthy controls. Significant heterogeneity was observed among these studies, so a random-effects model was applied (I2 = 97.0%, P < 0.001). The forest plot showed that BDNF concentrations in blood were significantly lower in PD patients than in healthy controls (SMD = −1.037, 95% CI [−1.412, −0.662], P < 0.001). The forest plot was shown in Figure 2.

Figure 2. Meta-analysis of studies on blood BDNF in PD patients and controls. CI, confidence interval; SMD, standardized mean difference; BDNF, brain-derived neurotrophic factor; PD, Parkinson’s disease.
To explore potential sources of heterogeneity, subgroup analyses were conducted based on specimen type, country, and diagnostic criteria for PD. The results were shown in Table 1. Among the included studies, four used plasma samples, while thirty-four used serum samples. The results showed high heterogeneity in both serum and plasma (plasma: I2 = 79.8%, P = 0.002; serum: I2 = 97.3%, P < 0.001). However, the pooled SMD was statistically significant in serum studies (SMD = −1.099, 95% CI [−1.503, −0.694], P < 0.001), but not in plasma studies (SMD = −0.513, 95% CI [−1.136, 0.111], P = 0.107). These results suggested that the conclusion may only apply to serum.

Table 1. Subgroup analyses results of BDNF in peripheral blood of PD patients compared with healthy controls.
Of these studies, twenty-eight were in Asia, while ten were not in Asia. The results showed high heterogeneity in both Asian subgroup and non-Asian subgroup (Asian: I2 = 96.8%, P < 0.001; non-Asian: I2 = 86.0%, P < 0.001). A pooled SMD was found to be significant in the Asian subgroup (SMD = −1.436, 95% CI [−1.846, −1.026], P < 0.001), but not in the non-Asian subgroup (SMD = 0.105, 95% CI [−0.287, 0.496], P = 0.601). These results suggested that the conclusion may only apply to Asian population.
Regarding diagnostic criteria, fourteen studies used the United Kingdom PD Society Brain Bank Criteria (UKPDS), and twenty-four studies used other criteria. The results showed high heterogeneity in both subgroups (UKPDS: I2 = 97.5%, P < 0.001; other criteria: I2 = 96.1%, P < 0.001). However, the pooled SMD was significant in other criteria subgroup (SMD = −1.494, 95% CI [−1.932, −1.055], P < 0.001), but not in UKPDS subgroup (SMD = −0.259, 95% CI [−0.895, 0.377], P = 0.424). All results of subgroup analyses are summarized in Table 1.
In meta-regression analysis, sample sizes, age of participants, sex proportion of participants, H-Y scores, disease duration, year of education and UPDRS-III scores did not show any significant effects on the outcomes. A sensitivity analysis, in which one study was omitted at a time, demonstrated that no single study significantly altered the results.
The application of Egger’s test (P > 0.05) and a thorough examination of the funnel plot did not reveal any indication of publication bias.
3.3 Serum BDNF levels in PD with depression
Seven studies were included reporting serum BDNF levels in depressed PD patients. The pooled data indicated that BDNF levels were slightly lower in PD patients with depression compared to those without, although this difference was not statistically significant (SMD = −0.511; 95% CI [−1.692, 0.671]; P = 0.397), with high heterogeneity (I2 = 97.7%; P < 0.001). Moreover, there were significantly lower serum BDNF levels in PD with or without depression than healthy controls. The forest plot was shown in Figure 3. Sensitivity analysis indicated that removing one study significantly affected the results, suggesting the instability of the findings. No publication bias was found by visual inspection of the funnel plot.
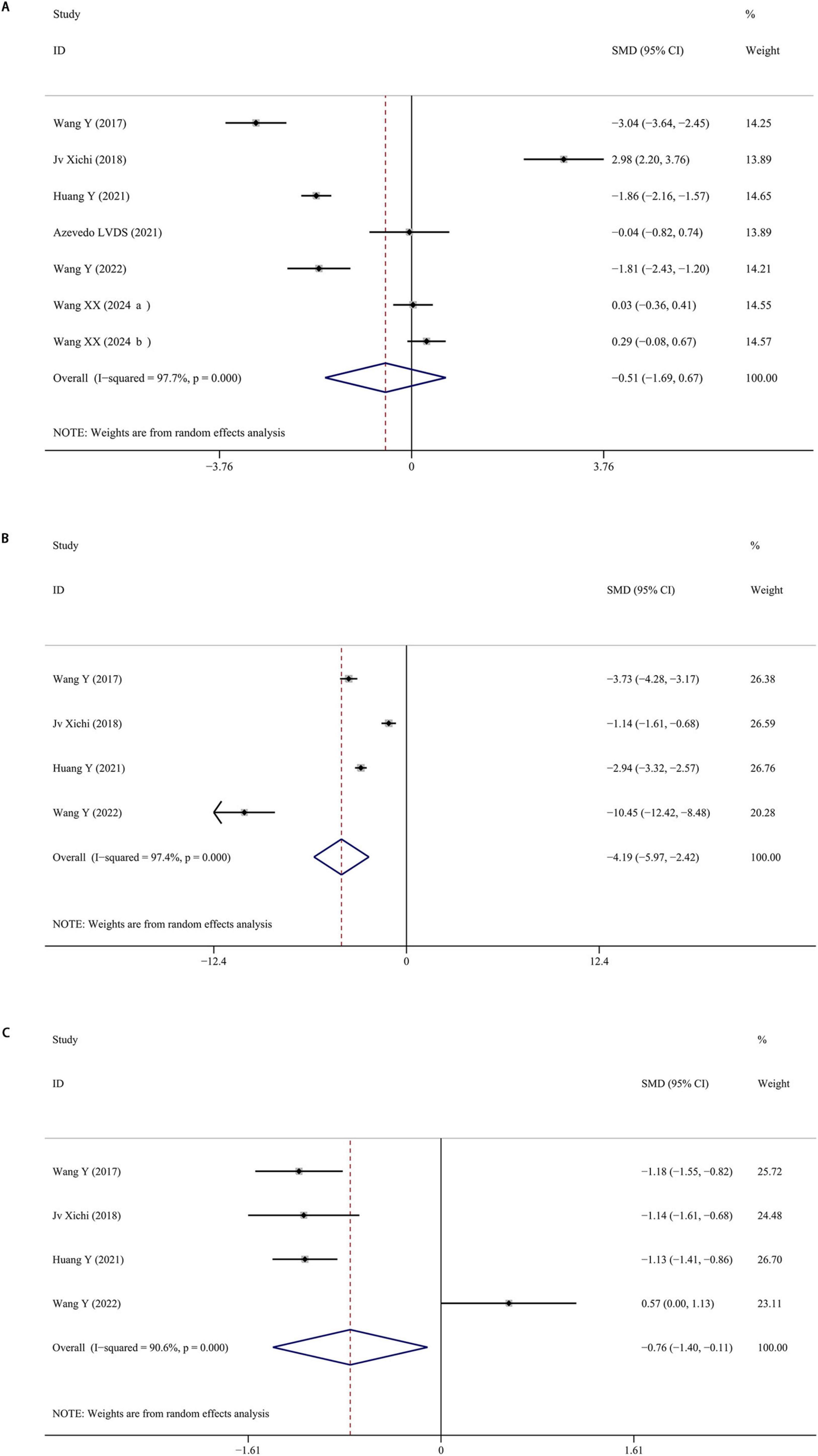
Figure 3. (A) Meta-analysis of studies on serum BDNF in PD patients with and without depression. (B) Meta-analysis of studies on serum BDNF in PD patients with depression and controls. (C) Meta-analysis of studies on serum BDNF in PD patients without depression and controls. CI, confidence interval; SMD, standardized mean difference; BDNF, brain-derived neurotrophic factor; PD, Parkinson’s disease.
3.4 Serum BDNF levels in PD with cognitive impairment
Ten studies were included reporting serum BDNF levels of PD patients with cognitive impairment. The pooled data showed that BDNF levels of PD patients with cognitive impairment was decreased than those without cognitive impairment (SMD = −1.035; 95% CI [−1.340, −0.730]; P < 0.001), with a high heterogeneity (I2 = 71.8%; P < 0.001). Furthermore, BDNF levels in patients diagnosed with PD, both with and without cognitive impairment, were notably lower compared to healthy control groups. The forest plot was shown in Figure 4. The results remained stable after omitting one of included studies, demonstrating the stability of the results. The application of Egger’s test (P > 0.05) and a thorough examination of the funnel plot did not reveal any indication of publication bias.

Figure 4. (A) Meta-analysis of studies on serum BDNF in PD patients with and without cognitive impairment. (B) Meta-analysis of studies on serum BDNF in PD patients with cognitive impairment and controls. (C) Meta-analysis of studies on serum BDNF in PD patients without cognitive impairment and controls. CI, confidence interval; SMD, standardized mean difference; BDNF, brain-derived neurotrophic factor; PD, Parkinson’s disease.
3.5 Serum BDNF levels in PD with other non-motor symptoms
A study performed in Brazil showed that serum concentrations of BDNF did not significantly differ between PD with fatigue and those without fatigue.
A study performed in China showed that BDNF concentrations of PD with and without autonomic nerve dysfunction were both lower than healthy controls. Furthermore, BDNF levels were significantly lower in PD patients with autonomic nerve dysfunction. This study also revealed a correlation between autonomic nerve dysfunction and H-Y scores through univariate analysis.
A study performed in China indicated that PD patients with RBD had lower BDNF levels than those without RBD. Additionally, BDNF levels were identified as an independent predictor of RBD in PD patients through logistic regression and P-trend analysis.
A study performed in China indicated that PD with RLS had lower BDNF levels than PD without RLS and healthy controls. The study also suggested a correlation between BDNF levels and the severity of RLS.
3.6 CSF BDNF levels in PD
Only three studies reported BDNF levels in the CSF of PD patients. The forest plots from these studies indicated that CSF BDNF levels were slightly lower in PD patients compared to healthy controls, but no significant difference was observed (SMD = −0.398; 95% CI [−2.499, 1.703]; P = 0.711). Despite the small number of studies, the results showed high heterogeneity (I2 = 97.0%; P < 0.001). The forest plot was shown in Figure 5. No publication bias was found by visual inspection of the funnel plot. Sensitivity analysis revealed that the omission of the study by Salehi and Mashayekhi (2009) significantly altered the result, suggesting instability. To draw more reliable conclusions, future studies should increase the sample size.
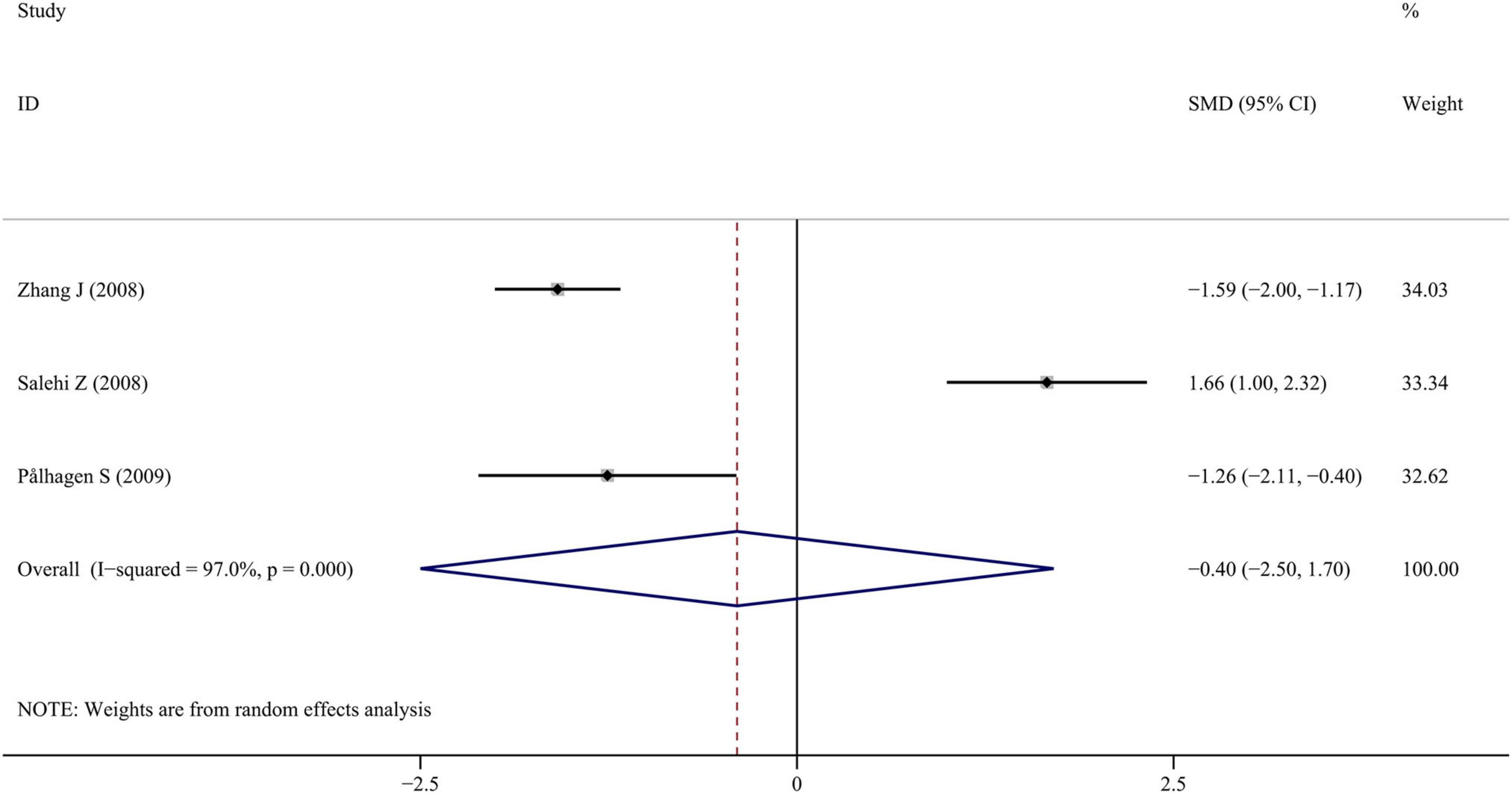
Figure 5. Meta-analysis of studies on CSF BDNF in PD patients and controls. CI, confidence interval; SMD, standardized mean difference; BDNF, brain-derived neurotrophic factor; PD, Parkinson’s disease; CSF, cerebrospinal fluid.
4 Discussion
Although BDNF has been extensively investigated as a key neurotrophic factor in a variety of diseases, no consensus has been reached regarding its expression levels in the blood and CSF of patients with PD, particularly those with non-motor symptoms. To our knowledge, the present study is the first to comprehensively examine the association between blood BDNF levels and non-motor symptoms of PD, including depression, cognitive impairment, sleep disorders, and others. Through the present systematic review and meta-analysis, it was found that BDNF levels in the blood of PD patients were lower than those in healthy controls. Subgroup analyses suggested that these differences might be influenced by specimen type, ethnicity, and diagnostic criteria. However, there was no difference on CSF BDNF levels between PD and healthy controls with limited sample sizes. Additionally, serum BDNF concentrations did not differ significantly between PD patients with and without depression. Lower serum BDNF levels were associated with cognitive impairment, autonomic nerve dysfunction, and sleep disorders in PD patients.
Differences in blood BDNF levels between PD patients and healthy controls observed in our analysis are consistent with previous findings. Two studies demonstrated that serum BDNF was significantly lower in PD patients (Jiang et al., 2019; Rahmani et al., 2019). One study demonstrated that PD patients had decreased BDNF levels than healthy controls in plasma samples (Chen and Zhang, 2023). In our subgroup analysis, the difference was pronounced in serum but sightly in plasma. The physiological concentration of BDNF in CSF and plasma is substantially lower than that in serum under normal conditions (Castrén and Monteggia, 2021). This phenomenon can be explained by the fact that platelets serve as a primary peripheral reservoir for BDNF, and differences in platelet activation between plasma and serum processing can markedly affect peripheral BDNF measurements (Beura et al., 2022; Bouhaddou et al., 2024). Therefore, structural and functional platelet abnormalities reported in PD may contribute to the observed reduction in serum BDNF levels. In contrast, studies of BDNF levels in CSF were significantly constrained by the invasive procedures and the potential risks involved in lumbar puncture. The three included CSF studies in our analysis exhibited marked heterogeneity and yielded inconclusive results, underscoring the need for cautious interpretation and highlighting the importance of larger, standardized investigations in the future.
Of the thirty-eight studies analyzing blood samples, twenty-eight were conducted in Asia, while the remaining ten were primarily from Western Europe. In the non-Asian subgroup, blood BDNF levels in PD patients did not significantly differ from those in healthy controls. It was worth noting that the proportion of plasma studies was higher in non-Asian subgroup, which may partially account for the observed discrepancies between the two subgroups. In a previous meta-analysis, the association between the BDNF 196 AA + AG genotype and PD was evident in European populations but not in Asian populations, suggesting that interethnic differences in BDNF genotype distribution could influence study outcomes (Lee and Song, 2014). In addition, our analysis indicated that differences in diagnostic criteria may have some effect on the results, although they are unlikely to represent a major source of heterogeneity.
Meta-regression analysis indicated that sample size, age, sex distribution, H–Y scores, disease duration, years of education, and UPDRS-III scores were not significant confounding factors in the present study. However, age, sex proportion and H-Y scores have been identified as confounding factors in previous meta-analyses (Jiang et al., 2019; Rahmani et al., 2019). The discrepancy may be attributed to differences in the number of included studies and the incorporation of Chinese databases in the current analysis. Supporting evidence from individual studies has reported a negative correlation between serum BDNF levels and H–Y scores in PD patients (Huang et al., 2021a). Similarly, a study involving 104 PD patients found inverse associations between BDNF levels and both disease duration and age (Di Lazzaro et al., 2024). Beyond these factors, cognitive performance, motor subtypes, and lifestyle-related variables–such as occupational exposures and caffeine consumption–represent critical variables that warrant detailed consideration and integration in subsequent researches.
Depression, one of the most common non-motor symptoms in PD, is thought to be multi-factorial in etiology. Consistent with previous reports, the present study found no significant difference in serum BDNF levels between PD patients with and without depression (Chen and Zhang, 2023; Rahmani et al., 2019). Nonetheless, several studies have suggested that the Met allele of BDNF Val66Met polymorphism has a significantly moderated relationship with depression (Hosang et al., 2014; Zhao et al., 2018; Zou et al., 2024). Lower peripheral BDNF concentrations were found in major depression disease than controls, supporting a potential link between peripheral BDNF expression and depression (Cavaleri et al., 2023; Kishi et al., 2017; Shi et al., 2020; Tiwari et al., 2022). In contrast, other studies have found no clear association between BDNF genotype and depression (Suktas et al., 2024; Wang et al., 2023). Accordingly, the association between BDNF and depression remains undetermined. Moreover, it has been reported that after 7 weeks of antidepressant treatment, BDNF levels in patients with depression become comparable to those in healthy individuals (Gelle et al., 2021). Due to insufficient data, the present analysis could not account for antidepressant use in PD patients with depression, which may partially explain the lack of significant differences observed between depressed and non-depressed PD groups.
Cognitive impairment is among the most frequent non-motor symptoms of PD, though it is often given greater attention in the advanced stages of the disease. Previous studies have demonstrated that lower BDNF levels was associated with cognitive impairment in Alzheimer’s disease and diabetes mellitus (Anita et al., 2022; He et al., 2024; Kim et al., 2017; Xie et al., 2020). Additionally, the Met allele of BDNF Val66Met polymorphism has been significantly linked to cognitive impairment in PD (Wang et al., 2019; Yin et al., 2019). These findings collectively support the association between decreased BDNF levels and cognitive impairment of PD in the present analysis. It was noteworthy that the severity of cognitive impairment may influence the relationship (Kim et al., 2017; Ng et al., 2019). Indeed, more severe cognitive decline has been associated with greater reductions in serum BDNF levels (Siuda et al., 2017). Beyond cognitive function, our analysis also identified associations between reduced blood BDNF levels and other non-motor symptoms–including RBD, RLS, and autonomic nerve dysfunction–suggesting that impairment of BDNF-mediated neuroprotective pathways may contribute to both the development of non-motor symptoms and the progression of PD.
In addition to the confounding factors addressed in this study, several other variables–often unreported in the included studies–may also influence the results. Dopamine replacement therapy has been documented to influence BDNF expression, particularly L-3,4-dihydroxyphenylalanine (L-DOPA). In a PD mouse model, repeated L-DOPA administration was observed to increase BDNF levels in the dopamine-depleted subthalamic nucleus (Zhang et al., 2006). Moreover, certain antidepressants, including paroxetine and fluoxetine, have been implicated in altering BDNF expression levels (De Foubert et al., 2004; Martínez-Turrillas et al., 2005). Although the majority of included studies employed the Enzyme-Linked Immunosorbent Assay (ELISA) for measurement, the impact on the resulting data may vary depending on the specific type of reagent and manufacturer utilized. Therefore, standardization of experimental protocols and assay conditions remains essential for generating valid and comparable findings.
5 Limitations
There were also some limitations in this study. Firstly, the number of studies included in this analysis is relatively limited, particularly those examining CSF BDNF levels in PD patients. Further investigations are warranted to clarify the relationship between BDNF and non-motor symptoms in PD. Secondly, owing to incomplete or inconsistent reporting in the included studies, several potential sources of heterogeneity could not be fully assessed. Future research should incorporate more comprehensive datasets that include variables such as age at onset, dopamine replacement therapy, and antidepressant use. Additionally, the potential associations between BDNF levels and other non-motor symptoms–such as constipation and olfactory dysfunction–deserve greater attention, as these could contribute to the earlier detection and improved clinical characterization of PD.
6 Conclusion
The current study not only examined the concentrations of BDNF in the blood and CSF of PD patients, but also represents the first meta-analysis to characterize trends in BDNF levels among PD patients with non-motor symptoms. We identified a correlation between reduced BDNF expression and a range of non-motor manifestations in PD. These findings provide an evidence-based foundation for future research into the role of BDNF in PD pathogenesis and highlight the importance of further investigating BDNF alterations in PD patients presenting with non-motor symptoms.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZZ: Data curation, Supervision, Writing – original draft, Conceptualization, Resources, Writing – review & editing, Project administration, Validation, Funding acquisition, Formal analysis. JS: Visualization, Formal analysis, Data curation, Writing – original draft, Methodology, Investigation, Conceptualization, Software. YL: Project administration, Resources, Validation, Data curation, Conceptualization, Writing – review & editing, Supervision. ML: Writing – original draft, Formal analysis, Visualization, Methodology, Investigation, Resources. DT: Visualization, Writing – original draft, Data curation, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1620172/full#supplementary-material
References
Alomari, M., Khalil, H., Khabour, O., Alzoubi, K., and Dersieh, E. (2018). Altered cardiovascular function is related to reduced BDNF in Parkinson’s disease. Exp. Aging Res. 44, 232–245. doi: 10.1080/0361073X.2018.1449589
Anita, N., Zebarth, J., Chan, B., Wu, C., Syed, T., Shahrul, D., et al. (2022). Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; a systematic review and meta-analysis. Brain Behav. Immun. 100, 55–69. doi: 10.1016/j.bbi.2021.11.005
Azevedo, L., Pereira, J., Silva Santos, R., Rocha, N., Teixeira, A., Christo, P., et al. (2022). Acute exercise increases BDNF serum levels in patients with Parkinson’s disease regardless of depression or fatigue. Eur. J. Sport Sci. 22, 1296–1303. doi: 10.1080/17461391.2021.1922505
Baiano, C., Barone, P., Trojano, L., and Santangelo, G. (2020). Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 35, 45–54. doi: 10.1002/mds.27902
Beura, S., Panigrahi, A., Yadav, P., and Singh, S. (2022). Role of platelet in Parkinson’s disease: Insights into pathophysiology & theranostic solutions. Ageing Res. Rev. 80:101681. doi: 10.1016/j.arr.2022.101681
Bouhaddou, N., Mabrouk, M., Atifi, F., Bouyahya, A., and Zaid, Y. (2024). The link between BDNF and platelets in neurological disorders. Heliyon 10:e39278. doi: 10.1016/j.heliyon.2024.e39278
Brockmann, K., Apel, A., Schulte, C., Schneiderhan-Marra, N., Pont-Sunyer, C., Vilas, D., et al. (2016). Inflammatory profile in LRRK2-associated prodromal and clinical PD. J. Neuroinflammation 13:122. doi: 10.1186/s12974-016-0588-5
Castrén, E., and Monteggia, L. (2021). Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry 90, 128–136. doi: 10.1016/j.biopsych.2021.05.008
Cavaleri, D., Moretti, F., Bartoccetti, A., Mauro, S., Crocamo, C., Carrà, G., et al. (2023). The role of BDNF in major depressive disorder, related clinical features, and antidepressant treatment: Insight from meta-analyses. Neurosci. Biobehav. Rev. 149:105159. doi: 10.1016/j.neubiorev.2023.105159
Chen, B., Li, Q., Liu, A., and Zhou, X. (2015). Expression and significance of brain-derived neurotrophic factor, soluble tumour necrosis factor receptor 1 and S-100B in serum of patients with Parkinson’s disease. Chin. J. Gerontol. 35, 931–932. doi: 10.3969/j.issn.1005-9202.2015.04.029
Chen, Z., and Zhang, H. (2023). A meta-analysis on the role of brain-derived neurotrophic factor in Parkinson’s disease patients. Adv. Clin. Exp. Med. 32, 285–295. doi: 10.17219/acem/154955
Colucci-D’Amato, L., Speranza, L., and Volpicelli, F. (2020). Neurotrophic Factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 21:7777. doi: 10.3390/ijms21207777
Cong, S., Xiang, C., Zhang, S., Zhang, T., Wang, H., and Cong, S. (2022). Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci. Biobehav. Rev. 141:104749. doi: 10.1016/j.neubiorev.2022.104749
Costa, C., Oliveira, G., Fonseca, A., Lana, R., Polese, J. C., and Pernambuco, A. P. (2019). Levels of cortisol and neurotrophic factor brain-derived in Parkinson’s disease. Neurosci. Lett. 708:134359. doi: 10.1016/j.neulet.2019.134359
De Foubert, G., Carney, S., Robinson, C., Destexhe, E., Tomlinson, R., Hicks, C., et al. (2004). Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience 128, 597–604. doi: 10.1016/j.neuroscience.2004.06.054
Di Lazzaro, G., Picca, A., Boldrini, S., Bove, F., Marzetti, E., Petracca, M., et al. (2024). Differential profiles of serum cytokines in Parkinson’s disease according to disease duration. Neurobiol. Dis. 190:106371. doi: 10.1016/j.nbd.2023.106371
GBD 2016 Parkinson’s Disease Collaborators. (2018). Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 17, 939–953. doi: 10.1016/S1474-4422(18)30295-3
Gelle, T., Samey, R., Plansont, B., Bessette, B., Jauberteau-Marchan, M., Lalloué, F., et al. (2021). BDNF and pro-BDNF in serum and exosomes in major depression: Evolution after antidepressant treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 109:110229. doi: 10.1016/j.pnpbp.2020.110229
He, W., Chang, F., Wang, T., Sun, B., Chen, R., and Zhao, L. (2024). Serum brain-derived neurotrophic factor levels in type 2 diabetes mellitus patients and its association with cognitive impairment: A meta-analysis. PLoS One 19:e0297785. doi: 10.1371/journal.pone.0297785
Hernández-Vara, J., Sáez-Francàs, N., Lorenzo-Bosquet, C., Corominas-Roso, M., Cuberas-Borròs, G., Lucas-Del Pozo, S., et al. (2020). BDNF levels and nigrostriatal degeneration in “drug naïve” Parkinson’s disease patients. An “in vivo” study using I-123-FP-CIT SPECT. Parkinsonism Relat. Disord. 78, 31–35. doi: 10.1016/j.parkreldis.2020.06.037
Hosang, G., Shiles, C., Tansey, K., McGuffin, P., and Uher, R. (2014). Interaction between stress and the BDNF Val66Met polymorphism in depression: A systematic review and meta-analysis. BMC Med. 12:7. doi: 10.1186/1741-7015-12-7
Hou, J., Ye, L., Ou, L., Huang, G., Ye, Z., and Huang, W. (2021). Correlation between serum MIF, BDNF and urine AD7C-NTP expression levels and cognitive impairment in patients with Parkinson’s dementia. West China Med. J. 2021, 1665–1669. doi: 10.3969/j.issn.1672-3511.2021.11.021
Hu, H. (2021). Relationship between serum BDNF, UA levels and cognitive function in elderly patients with Parkinson’s disease. Clin. Med. 41, 34–36. doi: 10.19528/j.issn.1003-3548.2021.07.011
Huang, G., Qin, B., Lu, F., Liu, H., Chen, H., Wei, L., et al. (2022). Relationship of severity of white matter lesions with BDNF and TNF-α in patients with Parkinson’s disease. Chin J. Geriatric. Heart Brain Vessel Dis. 24, 1173–1177.
Huang, Y., Huang, C., Zhang, Q., Wu, W., and Sun, J. (2021a). Serum BDNF discriminates Parkinson’s disease patients with depression from without depression and reflect motor severity and gender differences. J. Neurol. 268, 1411–1418. doi: 10.1007/s00415-020-10299-3
Huang, Y., Yun, W., Zhang, M., Luo, W., and Zhou, X. (2018). Serum concentration and clinical significance of brain-derived neurotrophic factor in patients with Parkinson’s disease or essential tremor. J. Int. Med. Res. 46, 1477–1485. doi: 10.1177/0300060517748843
Huang, Y., Zhang, Q., Huang, C., Wu, W., and Sun, J. (2021b). Association of decreased serum BDNF with restless legs syndrome in Parkinson’s disease patients. Front. Neurol. 12:734570. doi: 10.3389/fneur.2021.734570
Jiang, L., Zhang, H., Wang, C., Ming, F., Shi, X., and Yang, M. (2019). Serum level of brain-derived neurotrophic factor in Parkinson’s disease: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 88, 168–174. doi: 10.1016/j.pnpbp.2018.07.010
Jin, H., Shen, H., Liu, C., Wang, L., Mao, C., Chen, J., et al. (2023). Decreased serum BDNF contributes to the onset of REM sleep behavior disorder in Parkinson’s disease patients. Neurosci. Lett. 812:137380. doi: 10.1016/j.neulet.2023.137380
Ju, X., Wang, W., Qu, Q., Da, R., Li, P., and Song, W. (2018). Relationship between serum BDNF level and Parkinson disease depression. J. Neurosci. Mental Health 18, 691–693. doi: 10.3969/j.issn.1009-6574.2018.10.002
Khalil, H., Alomari, M., Khabour, O., Al-Hieshan, A., and Bajwa, J. (2017). The association between physical activity with cognitive function and brain-derived neurotrophic factor in people with Parkinson’s disease: A pilot study. J. Aging Phys. Act 25, 646–652. doi: 10.1123/japa.2016-0121
Kim, B., Lee, S., Graham, P., Angelucci, F., Lucia, A., Pareja-Galeano, H., et al. (2017). Peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease and mild cognitive impairment: A comprehensive systematic review and meta-analysis. Mol. Neurobiol. 54, 7297–7311. doi: 10.1007/s12035-016-0192-9
Kishi, T., Yoshimura, R., Ikuta, T., and Iwata, N. (2017). Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiatry 8:308. doi: 10.3389/fpsyt.2017.00308
Lee, Y., and Song, G. G. (2014). BDNF 196 G/A and 270 C/T polymorphisms and susceptibility to Parkinson’s disease: A meta-analysis. J. Mot. Behav. 46, 59–66. doi: 10.1080/00222895.2013.862199
Leite Silva, A., Gonçalves de Oliveira, R. W., Diógenes, G. P., de Castro Aguiar, M. F., Sallem, C. C., Lima, M. P. P., et al. (2023). Premotor, nonmotor and motor symptoms of Parkinson’s disease: A new clinical state of the art. Ageing Res. Rev. 84:101834. doi: 10.1016/j.arr.2022.101834
Li, Q., and Zhong, P. (n.d.). Correlation between serum BDNF, NFL and S100B levels with cognitive impairment in Parkinson’s disease. Correlation between serum BDNF, NFL and S100B levels with cognitive impairment in Parkinson’s disease.
Li, X., Fan, G., Lu, J., and Hu, J. (2019). Correlation between serum neuro-specific enolase and cognitive dysfunction in elderly patients with Parkinson’s Disease. Chin. J. Gerontol. 39, 3455–3458.
Lin, X., Wang, Z., Zhou, D., Chen, M., Liu, H., and Chen, H. (2021). Correlation between serum levels of epidermal growth factor and brain-derived neurotrophic factor and cognitive impairment in patients with Parkinson disease. Chin. Foreign Med. Res. 19, 90–92. doi: 10.14033/j.cnki.cfmr.2021.25.028
Liu, J., Huang, Y., and Gao, Z. (2015). The correlation between serum brain-derived neurotrophic factor and cognitive dysfunction in elderly patients with Parkinson’s disease. Chin. J. Gerontol. 35, 6193–6194.
Liu, L., Kang, T., Wang, W., Wei, J., and Du, F. (2024). Study on the relationship between serum BDNF, IGF-1 and CysC levels and autonomic nerve dysfunction in patients with Parkinson’s disease. Prog. Modern Biomed. 24, 4268–4270. doi: 10.13241/j.cnki.pmb.2024.22.019
Liu, Z., and Liu, H. (2023). Correlation of the levels of BDNF, IGF-1, CRP and TNF-α in serum with the dyskinesia in Parkinson’s disease. Correlation of the levels of BDNF, IGF-1, CRP and TNF-α in serum with the dyskinesia in Parkinson’s disease. Guilin: Guilin Medical University.
Ma, Z., Xu, Y., Lian, P., Wu, Y., Liu, K., Zhang, Z., et al. (2024). Alpha-synuclein fibrils inhibit activation of the BDNF/ERK signaling Loop in the mPFC to Induce Parkinson’s disease-like alterations with depression. Neurosci. Bull. 41, 951–969. doi: 10.1007/s12264-024-01323-x
Martínez-Turrillas, R., Del Río, J., and Frechilla, D. (2005). Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology 49, 1178–1188. doi: 10.1016/j.neuropharm.2005.07.006
Ng, T., Ho, C., Tam, W., Kua, E., and Ho, R. (2019). Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 20:257. doi: 10.3390/ijms20020257
Nikitina, M., Bragina, E., Nazarenko, M., and Alifirova, V. (2024a). The association of single-nucleotide polymorphism rs6265 of the brain-derived neurotrophic factor gene with clinical features in Parkinson’s disease. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 124, 82–88. doi: 10.17116/jnevro202412407182
Nikitina, M., Koroleva, E., Brazovskaya, N., Boyko, A., Levchuk, L., Ivanova, S., et al. (2024b). Associations of serum neuromarkers with clinical features of Parkinson’s disease. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 124, 145–152. doi: 10.17116/jnevro2024124041145
Numakawa, T., and Kajihara, R. (2025). The role of brain-derived neurotrophic factor as an essential mediator in neuronal functions and the therapeutic potential of its mimetics for neuroprotection in neurologic and psychiatric disorders. Molecules 30:848. doi: 10.3390/molecules30040848
Palasz, E., Wysocka, A., Gasiorowska, A., Chalimoniuk, M., Niewiadomski, W., and Niewiadomska, G. (2020). BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 21:1170. doi: 10.3390/ijms21031170
Pålhagen, S., Qi, H., Mårtensson, B., Wålinder, J., Granérus, A., and Svenningsson, P. (2010). Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson’s disease and major depression. J. Neurol. 257, 524–532. doi: 10.1007/s00415-009-5353-6
Peng, X., Deng, J., Li, Q., and Cai, M. (2019). The correlation between serum uric acid level and cognitive function, inflammatory factors and neurocytokines in patients with Parkinson’s disease. Chin. J. Diffic. Compl. Cas. 18, 119–122. doi: 10.3969/j.issn.1671-6450.2019.02.003
Quan, Y., Wang, J., Wang, S., and Zhao, J. (2020). Association of the plasma long non-coding RNA MEG3 With Parkinson’s disease. Front. Neurol. 11:532891. doi: 10.3389/fneur.2020.532891
Rahmani, F., Saghazadeh, A., Rahmani, M., Teixeira, A., Rezaei, N., Aghamollaii, V., et al. (2019). Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res. 1704, 127–136. doi: 10.1016/j.brainres.2018.10.006
Rocha, N., Ferreira, J., Scalzo, P., Barbosa, I., Souza, M., Christo, P. P., et al. (2018). Circulating levels of neurotrophic factors are unchanged in patients with Parkinson’s disease. Arq. Neuropsiquiatr. 76, 310–315. doi: 10.1590/0004-282X20180035
Salehi, Z., and Mashayekhi, F. (2009). Brain-derived neurotrophic factor concentrations in the cerebrospinal fluid of patients with Parkinson’s disease. J. Clin. Neurosci. 16, 90–93. doi: 10.1016/j.jocn.2008.03.010
Scalzo, P., Kümmer, A., Bretas, T., Cardoso, F., and Teixeira, A. (2010). Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 257, 540–545. doi: 10.1007/s00415-009-5357-2
Schaeffer, E., Roeben, B., Granert, O., Hanert, A., Liepelt-Scarfone, I., Leks, E., et al. (2022). Effects of exergaming on hippocampal volume and brain-derived neurotrophic factor levels in Parkinson’s disease. Eur. J. Neurol. 29, 441–449. doi: 10.1111/ene.15165
Schapira, A., Chaudhuri, K., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. doi: 10.1038/nrn.2017.62
Shi, Y., Luan, D., Song, R., and Zhang, Z. (2020). Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 41, 40–51. doi: 10.1016/j.euroneuro.2020.09.633
Siuda, J., Patalong-Ogiewa, M., Żmuda, W., Targosz-Gajniak, M., Niewiadomska, E., Matuszek, I., et al. (2017). Cognitive impairment and BDNF serum levels. Neurol. Neurochir. Pol. 51, 24–32. doi: 10.1016/j.pjnns.2016.10.001
Suktas, A., Ekalaksananan, T., Aromseree, S., Bumrungthai, S., Songserm, N., and Pientong, C. (2024). Genetic polymorphism involved in major depressive disorder: A systemic review and meta-analysis. BMC Psychiatry 24:716. doi: 10.1186/s12888-024-06195-z
Sun, G. (2011). The comparison of serum BDNF levels in early, middle and latestages of Parkinson’s disease cases. J. Clin. Med. Pract. 15, 110–111.
Szymura, J., Kubica, J., Wiecek, M., and Pera, J. (2020). The immunomodulary effects of systematic exercise in older adults and people with Parkinson’s disease. J. Clin. Med. 9:184. doi: 10.3390/jcm9010184
Tekşen, Y., Gündüz, M., Berikten, D., Özatik, F., and Aydın, H. (2024). Peganum harmala L. seed extract attenuates anxiety and depression in rats by reducing neuroinflammation and restoring the BDNF/TrkB signaling pathway and monoamines after exposure to chronic unpredictable mild stress. Metab Brain Dis 39, 1523–1541. doi: 10.1007/s11011-024-01416-6
Tiwari, S., Qi, L., Wong, J., and Han, Z. (2022). Association of peripheral manifestation of brain-derived neurotrophic factor with depression: A meta-analysis. Brain Behav. 12:e32581. doi: 10.1002/brb3.2581
Ventriglia, M., Zanardini, R., Bonomini, C., Zanetti, O., Volpe, D., Pasqualetti, P., et al. (2013). Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed. Res. Int. 2013:901082. doi: 10.1155/2013/901082
Wang, Q., Liu, J., Guo, Y., Dong, G., Zou, W., and Chen, Z. (2019). Association between BDNF G196A (Val66Met) polymorphism and cognitive impairment in patients with Parkinson’s disease: A meta-analysis. Braz. J. Med. Biol. Res. 52:e8443. doi: 10.1590/1414-431X20198443
Wang, X., Zhou, C., Li, Y., Yang, H., Sun, X., Li, S., et al. (2024). Sex-dependent associations of serum BDNF, glycolipid metabolism and cognitive impairments in Parkinson’s disease with depression: A comprehensive analysis. J. Neural Transm. 131, 1047–1057. doi: 10.1007/s00702-024-02802-1
Wang, Y., and Liu, H. (n.d.). Correlation between serum brain-derived nerve growth factor and uric acid levels and Parkinson’s disease. Correlation between serum brain-derived nerve growth factor and uric acid levels and Parkinson’s disease.
Wang, Y., Li, O., Li, N., Sha, Z., Zhao, Z., and Xu, J. (2023). Association between the BDNF Val66Met polymorphism and major depressive disorder: A systematic review and meta-analysis. Front. Psychiatry 14:1143833. doi: 10.3389/fpsyt.2023.1143833
Wang, Y., Liu, H., Du, X., Zhang, Y., Yin, G., Zhang, B., et al. (2017). Association of low serum BDNF with depression in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 41, 73–78. doi: 10.1016/j.parkreldis.2017.05.012
Wang, Y., Liu, H., Zhang, B., Soares, J., and Zhang, X. (2016). Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 29, 66–71. doi: 10.1016/j.parkreldis.2016.05.023
Wu, H. (2018). Correlation between serum uric acid level and neurotrophy, nerve injury and systemic oxidative stress response in patients with Parkinson’s disease. J. Hainan Med. Univ. 24, 1806–1809. doi: 10.13210/j.cnki.jhmu.20180928.005
Wu, Q., Yu, M., and Liu, H. (2022). Correlation between serum BDNF, Val66Met gene polymorphism and clinical symptoms in early Parkinson’s disease. J. Apoplexy Nerv. Dis. 39, 425–429. doi: 10.19845/j.cnki.zfysjjbzz.2022.0108
Xiao, Y. (2016). Correlation between serum BDNF level and cognitive dysfunction in elderly patients with Parkinson. Med. J. Chin. People‘s Health 28, 48–49. doi: 10.3969/j.issn.1672-0369.2016.19.024
Xie, B., Zhou, H., Liu, W., Yu, W., Liu, Z., Jiang, L., et al. (2020). Evaluation of the diagnostic value of peripheral BDNF levels for Alzheimer’s disease and mild cognitive impairment: Results of a meta-analysis. Int. J. Neurosci. 130, 218–230. doi: 10.1080/00207454.2019.1667794
Xie, Y. (2017). Relationship between serum epidermal growth factor level and cognitive disorder in patients with Parkinson’s disease. Pract. J. Cardiac. Cerebral Pneumal. Vasc. Dis. 25, 49–52.
Xu, P., Zhu, X., Zhou, S., Li, G., and Yu, M. (2024). Study on the relationship between the levels of serum homocysteine, brain-derived neurotrophic factor and α-synuclein and the leukodystrophy in patients with Parkinson’s disease. Pract. J. Clin. Med. 21, 71–74.
Ye, X., Li, T., Li, X., Yang, L., and Li, C. (2016). Study on changes of the levels of EGF, BDNF in serum and their relations with cognitive impairment in patients with Parkinson’s disease. J. Brain Nerv. Dis. 2016, 406–408.
Yin, Y., Su, X., Pan, L., and Li, C. (2019). BDNF Val66Met polymorphism and cognitive impairment in Parkinson’s disease-a meta-analysis. Neurol. Sci. 40, 1901–1907. doi: 10.1007/s10072-019-03907-2
Zhang, J., Sokal, I., Peskind, E., Quinn, J., Jankovic, J., Kenney, C., et al. (2008). CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J. Clin. Pathol. 129, 526–529. doi: 10.1309/W01Y0B808EMEH12L
Zhang, T., Xu, W., and Zhou, S. (2021). Correlation between changes of serum uric acid and functional affective disorders, neurotransmitters and cytokines in patients with Parkinson’s disease. Chin Mod. Doc. 59, 38–41.
Zhang, X., Andren, P., and Svenningsson, P. (2006). Repeated l-DOPA treatment increases c-fos and BDNF mRNAs in the subthalamic nucleus in the 6-OHDA rat model of Parkinson’s disease. Brain Res. 1095, 207–210. doi: 10.1016/j.brainres.2006.04.019
Zhao, L., Li, H., and Wang, S. (2021). Correlation between the expression of miR-543-3p and miR-124 and the levels of IGF-1, BDNF and NGF in the serum of patients with Parkinson’s disease. Chin. J. Integr. Med. Cardio-Cerebrovas. Dis. 19, 1748–1751. doi: 10.12102/j.issn.1672-1349.2021.10.039
Zhao, M., Chen, L., Yang, J., Han, D., Fang, D., Qiu, X., et al. (2018). BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J. Affect. Disord. 227, 226–235. doi: 10.1016/j.jad.2017.10.024
Zhao, Y., Zuo, B., Jia, J., Jv, C., Zhang, Y., Cai, H., et al. (2023). Correlation between serum levels of S100β, NSE, and BDNF and cognitive impairments in patients with Parkinson disease. Chin. J. Clin. Med. 30, 652–657.
Zhao, Z., and Xu, Z. (n.d.). The study of brain dopamine transporter, the level of proBDNF in serum and intestinal flora’s changes in patients with Parkinson’s disease. The study of brain dopamine transporter, the level of proBDNF in serum and intestinal flora’s changes in patients with Parkinson’s disease.
Zhou, L., Wong, K., and Xie, H. (2025). Modulation of intestinal inflammation and protection of dopaminergic neurons in Parkinson’s disease mice through a probiotic formulation targeting NLRP3 inflammasome. J. Neuroimmune Pharmacol. 20:9. doi: 10.1007/s11481-024-10163-5
Zhou, X., Yang, D., Zhang, M., Lei, R., and Ren, L. (2023). Assessment of serum PARK2, GFAP and BDNF expression on disease staging and cognitive function in elderly patients with Parkinson’s disease. Chin. J. Gerontol. 43, 2142–2145. doi: 10.3969/j.issn.1005-9202.2023.09.028
Keywords: brain-derived neurotrophic factor, Parkinson’s disease, non-motor symptom, blood, cerebrospinal fluid, meta-analysis
Citation: Zhao Z, Sun J, Liu Y, Liu M and Tong D (2025) Association of brain-derived neurotrophic factor in blood and cerebrospinal fluid with Parkinson’s disease and non-motor symptoms of Parkinson’s disease: a systematic review and meta-analysis of 6655 participants. Front. Aging Neurosci. 17:1620172. doi: 10.3389/fnagi.2025.1620172
Received: 29 April 2025; Accepted: 02 September 2025;
Published: 19 September 2025.
Edited by:
Dongning Su, Capital Medical University, ChinaReviewed by:
Andreas Wree, University of Rostock, GermanyXin Geng, First Affiliated Hospital of Kunming Medical University, China
Copyright © 2025 Zhao, Sun, Liu, Liu and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhen Zhao, MTcxMjI0MTQyQHFxLmNvbQ==
 Zhenzhen Zhao
Zhenzhen Zhao Jiahui Sun
Jiahui Sun Youhong Liu
Youhong Liu