- 1Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX, United States
- 3Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 4Department of Bioengineering, University of Texas at Arlington, Arlington, TX, United States
- 5Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 6Vanderbilt Memory and Alzheimer’s Center, Vanderbilt University Medical Center, Nashville, TN, United States
- 7Department of Orthopaedic Surgery, Vanderbilt University Medical Center, Nashville, TN, United States
- 8Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
Sedentary behavior has been associated with poor health outcomes, especially in older adulthood. Given that sedentary behavior is a highly prevalent, modifiable health behavior, there has been a recent increased interest in examining how sedentary behavior relates to cognition and brain health. The current body of literature is limited and mixed. The purpose of this systematic review was to examine the associations of sedentary behavior with cognition and brain health in older adults across the cognitive spectrum. This study was pre-registered with PROSPERO (CRD42023477868). Six comprehensive databases were searched with pre-registered search terms. A total of 33 studies were included. Overall, results indicated that greater sedentary behavior was associated with worse cognition and brain health, although associations varied based on differences in measurement and classification of sedentary behavior. We discuss next steps and implications for future research.
1 Introduction
Engaging in regular physical activity (PA) is a well-known behavioral strategy to maintain cognition and brain health (Won et al., 2021), and reduce risk for Alzheimer’s disease and related dementias (ADRD) (Iso-Markku et al., 2022). While a robust body of literature links participation in physical activity and exercise to improved cognition and brain health (Barella et al., 2010; Iso-Markku et al., 2018; Gogniat et al., 2022; Erickson et al., 2019), there has been significantly less research interest in sedentary behavior. Sedentary behavior is typically defined as any waking behavior characterized by an energy expenditure ≤1.5 metabolic equivalents (METs), while in a sitting, reclining or lying posture (Sedentary Behaviour Research Network, 2012). Sedentary behavior can also be operationalized into many different components including total time, breaks, and bouts amongst others (Tremblay et al., 2017). A distinct class of behaviors from physical activity and characterized by low energy expenditure (Biddle et al., 2004), sedentary behavior may be a lifestyle risk factor independently related to cognitive function and brain health in older adulthood. Greater sedentary time in older adulthood has been associated with several poor health outcomes including increased risk for cardiovascular disease, stroke, and all-cause mortality (Hajduk and Chaudhry, 2016; Wu et al., 2023). A prior systematic review in older adults aged 60 and older showed evidence from a small study (N = 649) that the average English older adult spends almost 9 h per day sedentary when measured objectively (Harvey et al., 2013). Evidence also shows that sitting for 12 h/day increased all-cause dementia risk by 63% (Raichlen et al., 2023). The high prevalence of sedentary behavior in older adulthood (Matthews et al., 2008) also lends importance to understanding the biological mechanisms by which it may accelerate risk for age-related cognitive decline and neurodegeneration.
Despite some evidence showing that sedentary time is associated with worse cognition in older adulthood, associations across studies are mixed. Results from various studies suggest that greater sedentary behavior is associated with worse global cognition (Wu et al., 2020), in addition to domain-specific associations, such as poorer executive function (Kesse-Guyot et al., 2012; Coelho et al., 2020) and memory (Bakrania et al., 2018). Other studies, however, show no impact of sedentary behavior on cognition (Yan et al., 2020; Maasakkers et al., 2020; Falck et al., 2017). Given the inconsistencies in study findings, there is a critical need to systematically examine the current literature to better understand the nature of these associations and what additional factors (e.g., measurement, study characteristics) may be driving these effects. Significant heterogeneity exists across studies in the measurement of sedentary behavior. Historically, sedentary behavior was measured via self-report, which may be faulty due to bias and unreliability in individuals with memory impairment (VandeBunte et al., 2022). The recent advent of wearable devices (e.g., wrist, thigh, hip) acquires more objective data, but presents some challenges including comparing across devices and setting appropriate activity cutpoints (Tremblay et al., 2017).
In addition to cognitive function, prior literature also supports the connection between sedentary behavior and brain structure and function in aging. For example, greater sedentary behavior has been associated with medial temporal lobe thinning (Siddarth et al., 2018), white matter atrophy (Arnardottir et al., 2016) and hyperintensities (Bronas et al., 2019), and lower cerebral blood flow (Zlatar et al., 2014). However, the pathophysiological mechanisms underlying these associations are poorly understood. Linking sedentary behavior to specific pathological brain changes would strengthen our understanding and inform prevention and intervention strategies aimed at improving brain health outcomes among older adults, which would be particularly important for those at risk for ADRD.
Given the recency of this literature base, there have been few systematic reviews in this area. A systematic review from Falck et al. (2017) included eight studies and concluded that sedentary behavior was associated with reduced cognitive function over the lifespan. Another systematic review on sedentary behavior and cognition with 13 studies was inconclusive (Olanrewaju et al., 2020). There have been far fewer reviews examining sedentary behavior and brain health. One prior review found a tentative association between habitual sedentary behavior and structural white matter (Maasakkers et al., 2022). Taken together, there is a need to provide updated information from a larger pool of studies that include updated sedentary behavior methodology, larger and more representative samples, and longitudinal follow-up to further understand these connections.
A PECO (Population, Exposure, Comparator, Outcome) framework (Morgan et al., 2018) was utilized to define the scope of this study. The purpose of this systematic review was to synthesize the current literature on associations between sedentary behavior (E) and cognition (O) AND sedentary behavior (E) and brain structure and function (O) in older adulthood (P) compared to those who do not engage in increased sedentary behavior (C) to provide a comprehensive understanding of associations that exist. We also examined the current literature in the context of different methodologies employed, outcomes measured, and risk of bias within studies. Overall, we hypothesized that greater sedentary behavior would be related to worse cognition and poor brain health. We also hypothesized that these relationships may vary based on the sedentary behavior mode of measurement (objective vs. subjective report) and the outcomes evaluated (e.g., comprehensive neuropsychological evaluation vs. cognitive screener; structural neuroimaging vs. functional neuroimaging).
2 Method
The current systematic review was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). This systematic review (CRD42023477868) was pre-registered on November 14, 2023 with PROSPERO International Prospective Register of Systematic Reviews and can be accessed at the following website: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023477868.
2.1 Eligibility criteria
Inclusion criteria were the following: (1) peer-reviewed publications, (2) available in English, (3) cross-sectional and cohort/observational studies, (4) older adults ages 60 and older with or without cognitive impairment, (5) measurement of sedentary behavior at baseline, (6) objective cognition outcomes acquired by a validated assessment measure, (7) brain health outcomes measured via structural (volume, thickness, surface area, diffusion tensor imaging) and/or functional (fMRI, functional connectivity, cerebral blood flow) neuroimaging.
Exclusion criteria were the following: (1) study not available in English, (2) Intervention studies unless sedentary behavior and cognition/brain health are reported cross-sectionally at baseline, (3) studies that do not examine the associations of sedentary behavior with a cognition or brain health outcome, (4) participants younger than 60 years old, (5) participants with reported psychopathology or neurological disorders (e.g., depression, Parkinson’s Disease) other than Alzheimer’s disease.
2.2 Information sources
A search was conducted using EBSCOhost (MEDLINE, Academic Search Premier), Ovid (PsycINFO), ProQuest (PSYCArticles), PubMed, and Sedentary Behavior Research Database (SBRD) databases. The initial search began on December 8, 2023, and therefore all articles published prior to this date were eligible to be included in the search. Following this, reference lists from pertinent studies, reviews, and meta-analyses were manually searched by study authors (MAG, JW) for studies that may have not been captured in the original search. This search strategy was developed and pre-registered prior to beginning the search by MAG, JW, and SG.
2.3 Search strategy
An identical search strategy was applied to each database and included the following: (“older adults” OR “geriatrics” OR “aging” OR “seniors” OR “elderly” OR “healthy aging” OR “MCI” OR “dementia” OR “Alzheimer’s disease) AND (“sedentary behavior” OR “inactivity” OR “sitting” OR “low activity”) AND (“neuroimaging” OR “brain volume” OR “brain change” OR “MRI” OR “white matter” OR “connectivity” OR “PET” OR “cerebral blood flow” OR “cortical” OR “cognition” OR “memory” OR “thinking”). These search terms were developed by MAG, SG, and JW.
2.4 Study selection
Eligibility was assessed using the previously discussed criteria (see Eligibility Criteria). Following the initial search, study titles and abstracts were reviewed for preliminary determination of eligibility (i.e., sedentary behavior studies in older adults) by MAG. Studies that met the initial eligibility were then reviewed in detail to make a final decision on eligibility (MAG, JW, AA, CC). The corresponding authors were contacted when the data presented was insufficient to be able to determine final eligibility. When discrepancies assessment arose, MAG and JW discussed the studies until a consensus was reached.
2.5 Data collection process
A data extraction form was developed using Microsoft Excel and was consistent with the Cochrane Consumers and Communication Data Extraction Template (Ryan and Hill, 2019). Several authors (AA, CC, AV, JW, MAG) extracted the data, and discussions regarding data collection procedures (i.e., inclusion of demographic information, classification of outcomes) were routinely conducted via bi-weekly group meetings. All extracted data was verified by a second rater. The list of variables to be coded included the following: age, sex, education level, cognitive status (e.g., normal cognition, mild cognitive impairment, dementia), type of sedentary behavior (e.g., self-report, objectively measured, etc.), cognitive outcome (e.g., global cognition, memory, executive functions, processing speed, etc.), brain health outcome (e.g., volume, thickness, white matter integrity, functional connectivity, etc.), and bias in individual studies. For cohort studies where baseline sedentary behavior was related to cognition or brain health outcomes over time, follow-up time was extracted.
2.6 Risk of bias in individual studies
In order to assess risk of bias within individual studies, the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used1, which uses 14 criteria to evaluate the methodological quality of cohort longitudinal and cross-sectional studies. Only one criterion: “Were the outcome assessors blinded to the exposure status of participants?” was not evaluated, as it was not applicable for observational studies with no status manipulation.
3 Results
3.1 Study selection
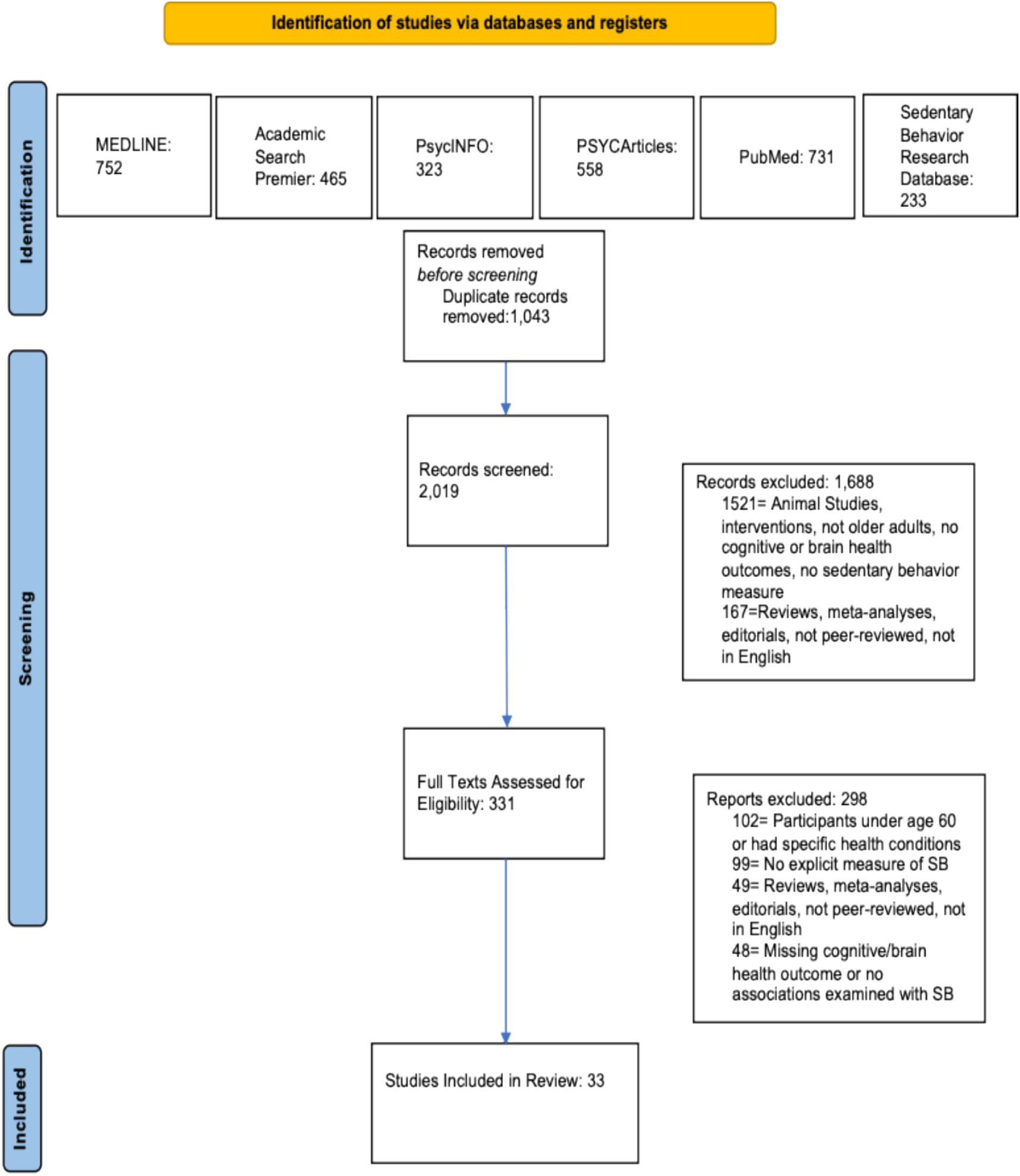
The initial search results from MEDLINE, PsycInfo, PsycArticles, Academic Search Premier, PubMed, and Sedentary Behavior Research Database returned 3,062 records. After removal of duplicates, 2,019 unique records remained. These 2,019 unique entries were initially screened using title and abstract review, and 1,688 records were excluded because based on this screen. Following this, 331 records were assessed for full eligibility using the criteria listed above (see Eligibility Criteria), and 298 records were excluded. No new records were included from the manual reference search. Final inclusion was 33 studies. This information is presented in a flowchart (see Figure 1) based on the PRISMA template (Page et al., 2021).
3.2 Study characteristics
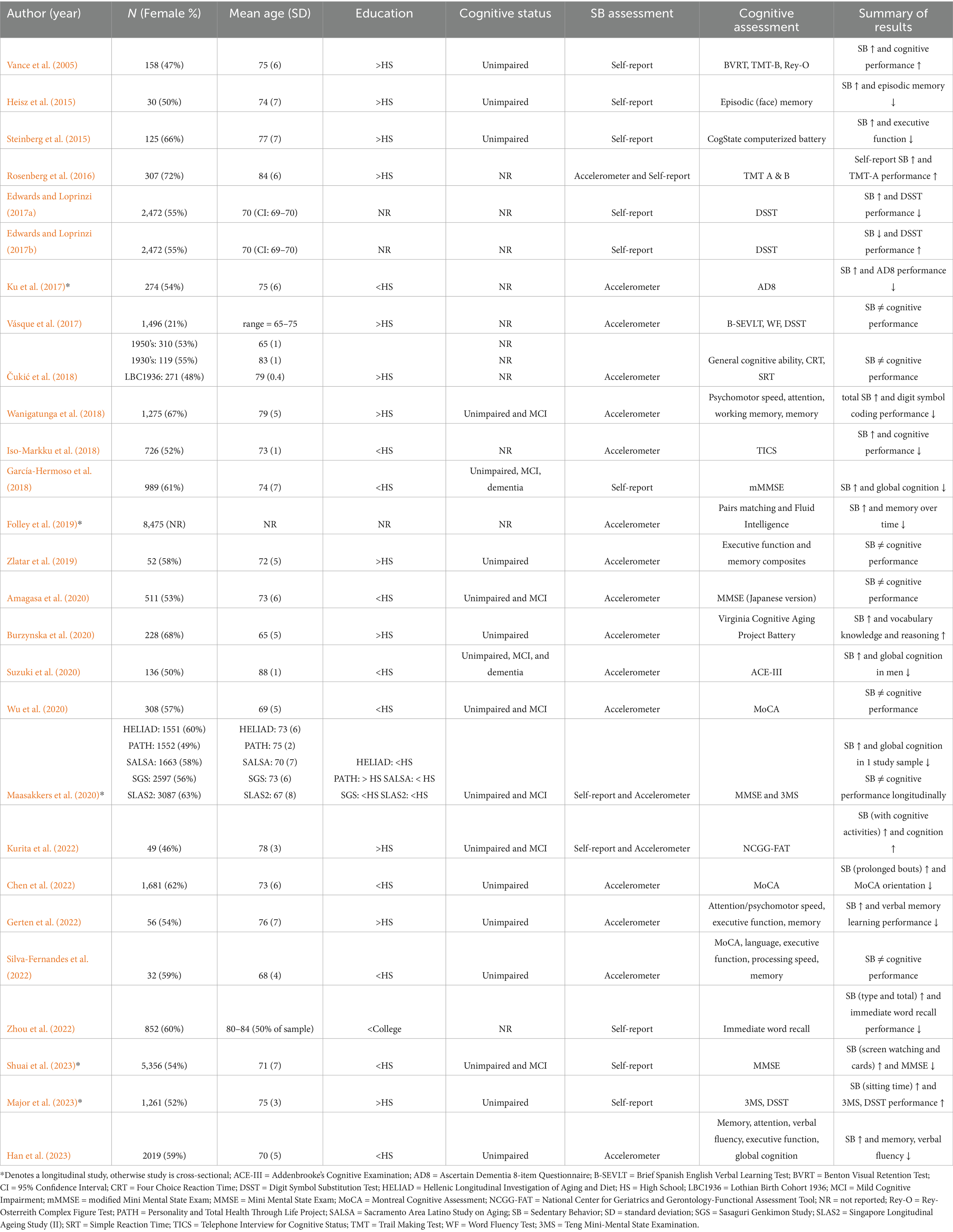
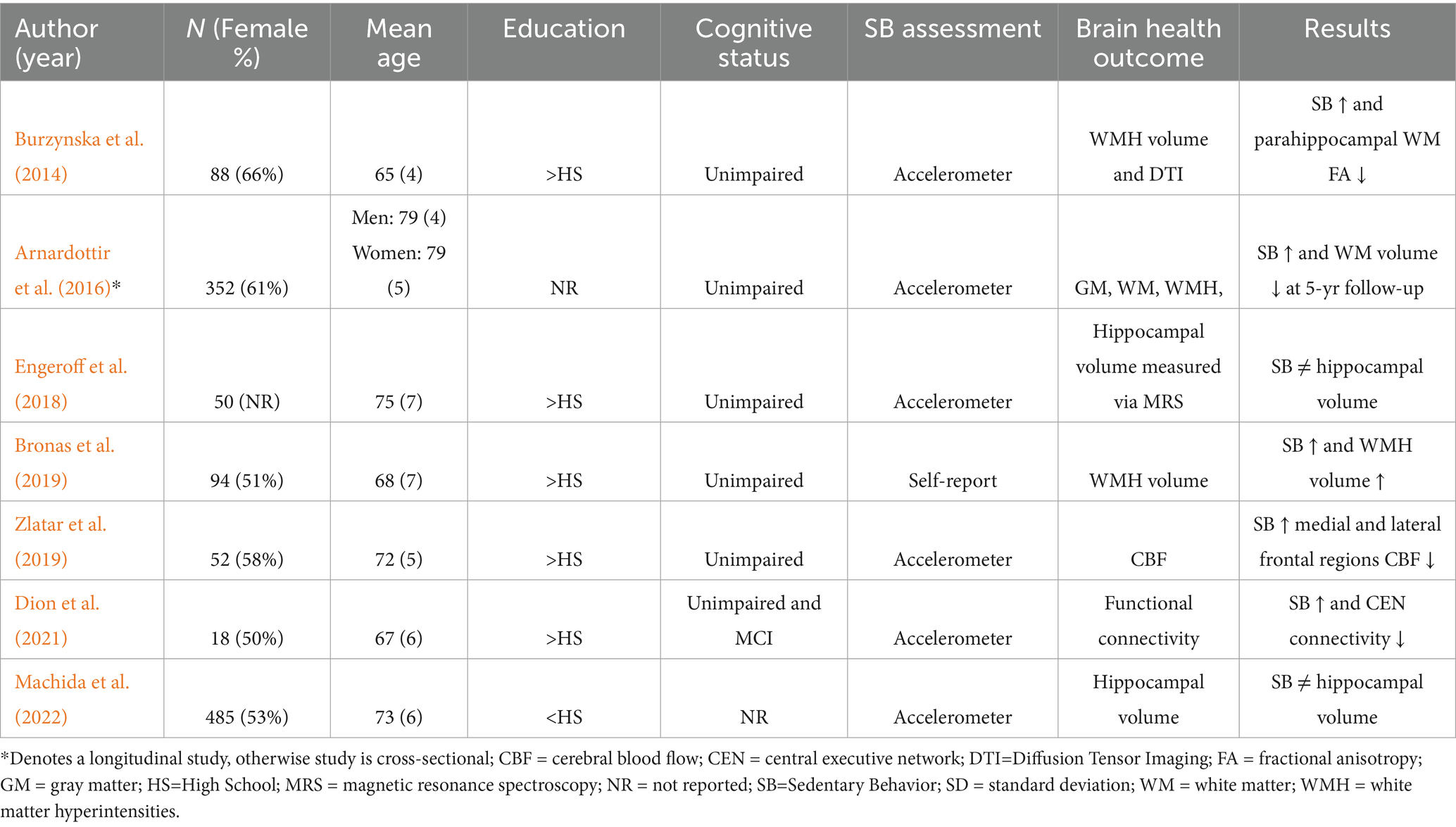
Study characteristics for cognition (Table 1) and brain health (Table 2) were presented separately. Detailed study information including sample size, sample demographics (e.g., sex, age, education), cognitive status, and sedentary behavior are presented in Table 1 (Iso-Markku et al., 2018; Wu et al., 2020; Vance et al., 2005; Heisz et al., 2015; Steinberg et al., 2015; Rosenberg et al., 2016; Edwards and Loprinzi, 2017a; Edwards and Loprinzi, 2017b; Ku et al., 2017; Vásquez et al., 2017; Čukić et al., 2018; Wanigatunga et al., 2018; García-Hermoso et al., 2018; Folley et al., 2019; Zlatar et al., 2019; Amagasa et al., 2020; Burzynska et al., 2020; Suzuki et al., 2020; Maasakkers et al., 2020; Kurita et al., 2022; Chen et al., 2022; Gerten et al., 2022; Silva-Fernandes et al., 2022; Zhou et al., 2022; Shuai et al., 2023; Major et al., 2023; Han et al., 2023) and Table 2 (Arnardottir et al., 2016; Bronas et al., 2019; Zlatar et al., 2019; Burzynska et al., 2014; Engeroff et al., 2018; Dion et al., 2021; Machida et al., 2022). Two included studies had overlapping participants (Edwards and Loprinzi, 2017a; Edwards and Loprinzi, 2017b).
The 33 included studies resulted in a total of 43,577 participants (M = 1,321, SD = 2,375, range = 18–10,450). The sample displayed some variability in age (M = 73, SD = 5, range = 65–88) and was, on average, gender balanced (% female; M = 56%, SD = 9%, range 21–72%). Of the 28 studies that reported education level, 57% reported a majority completing high school or higher, while 43% reported a majority of the sample completing less than high school. Only 23 studies explicitly reported on the cognitive status of the sample. Most studies utilized cognitively healthy samples (n = 14), and nine studies included participants with mild cognitive impairment (MCI) and/or dementia.
Regarding measurement of sedentary behavior, a majority of studies (n = 20) used purely objective measures, followed by subjective measures (n = 10), and then combination of both objective and subjective methods (n = 3). Of the studies that used an objective measure of sedentary behavior and reported the device location, the majority of studies used a hip-worn device placement (n = 16), with one study using a wrist-worn device and one study using a thigh-worn device (n = 5 did not put wear location but hip is suspected based on device type). The most common devices utilized were versions of the Actigraph (GT3X, GT3X+, GTM1; n = 14), and the majority of studies focused on total sedentary time using <100 counts per minute (n = 13). In studies that utilized a subjective measure of sedentary behavior (n = 13), all studies included participant self-report of sedentary behavior. Of these 13 studies that utilized some sort of self-report, some (n = 6) used a validated measure with several questions (e.g., Sedentary Behavior Questionnaire (Rosenberg et al., 2010)), while the rest utilized 1–3 individual questions (n = 7).
Among studies that reported a cognitive outcome (n = 27), the most commonly used assessment was a single-domain cognitive measure or composite scores (e.g., executive function composite; n = 11), followed by a cognitive screening tool (e.g., Mini Mental Status Exam; [MMSE]) (n = 10), and finally, fewer studies included more comprehensive neuropsychological batteries (n = 6). A majority of the studies with cognitive outcomes (n = 15/27) reported a negative association between sedentary behavior and cognitive performance, indicating that greater sedentary time was associated with worse cognitive performance. Five studies reported positive associations between sedentary behavior and cognitive performance, and seven studies reported no or mixed associations between sedentary behavior and cognitive performance.
Of the studies that reported brain structure and function outcomes (n = 7), the most common outcome measured was white matter hyperintensities (WMH; n = 3), followed by hippocampal volume (n = 2), and functional neuroimaging outcomes (n = 2). All brain imaging studies reported scanner strength: most studies utilized a 3 T scanner (n = 5) while two studies utilized a 1.5 T scanner. Five of the brain health studies reported negative associations between sedentary behavior and measures of brain health, while two studies reported no association between sedentary behavior and hippocampal volume.
Most studies were cross-sectional (n = 27/33) with only 1/6 longitudinal studies belonging to the brain health category. A majority of the longitudinal studies (n = 5/6) reported negative associations between sedentary behavior and cognitive/brain health outcomes. The mean follow-up time for longitudinal studies was 3.6 years. One study (Zlatar et al., 2019) contained both cognitive and brain health outcomes, and those results are presented separately in both tables. Two studies with cognitive outcomes utilized participants from the same sample (Edwards and Loprinzi, 2017a; Edwards and Loprinzi, 2017b).
3.3 Risk of bias results
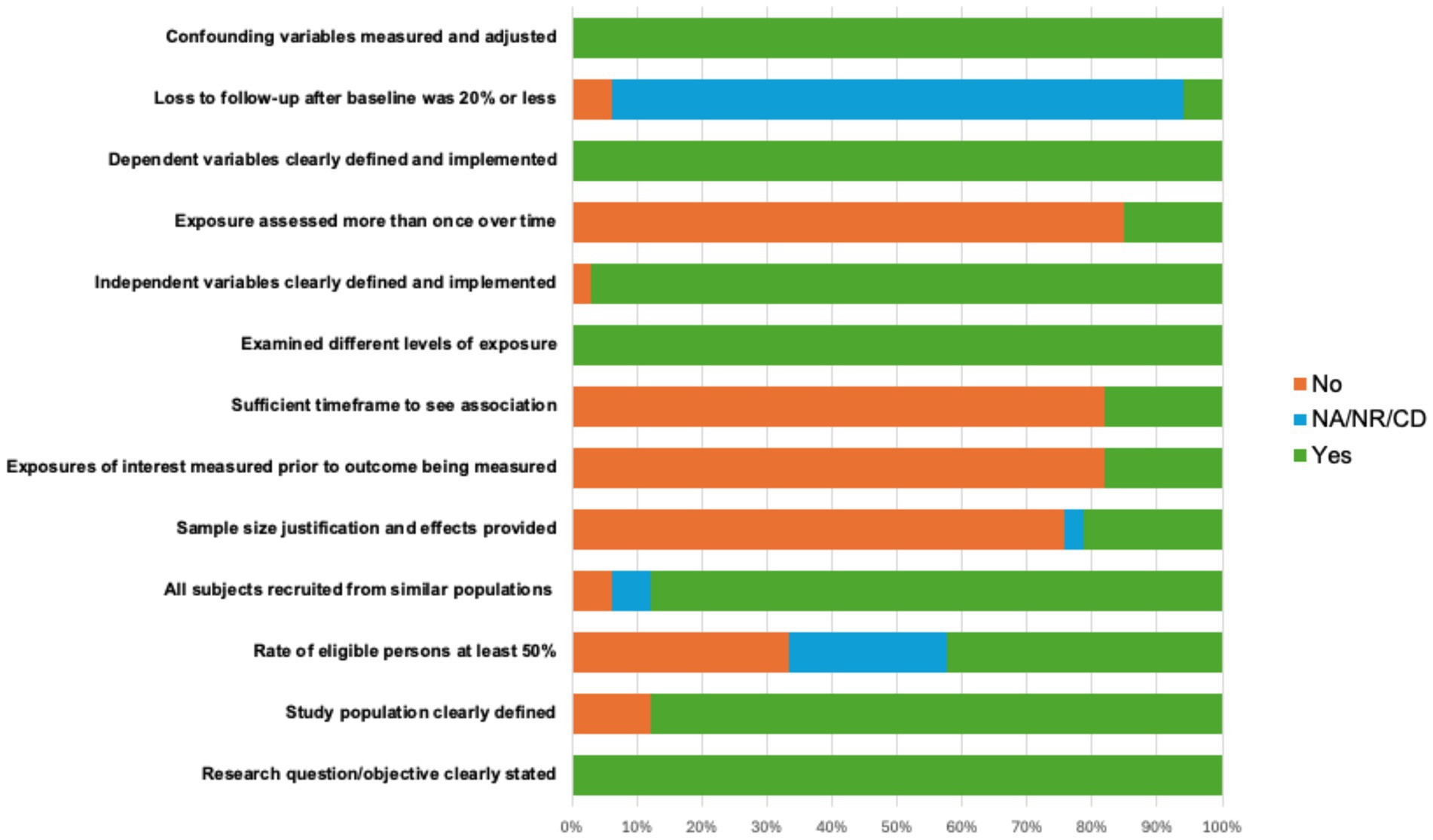
The risk of bias (ROB) assessment revealed consistent, reasonable methodological standards for the majority of studies, with 73% of the studies rated overall as “good” and 27% of the studies scoring “fair” taking into account their methodological rigor. Nearly all studies included clear statement of the research objective (100%), defined the study population (88%), recruited subjects from similar populations (88%), defined independent (97%) and dependent variables (100%), examined different levels of exposure of the independent variable (100%), and adequately addressed confounding variables (100%). Across all studies, lower endorsed ROB categories included providing sample size justification (21%) and obtaining participation rate above 50% of the total eligible sample (42%). In addition, due to most of the studies being cross-sectional, time-dependent categories such as assessing exposure more than once (15%) or providing sufficient time frame to observe associations (18%) were less frequent. ROB metrics are depicted in detail in Figure 2.
3.4 Synthesis of results
3.5 Sedentary behavior and cognition
As mentioned above, most of the studies in this systematic review contained cognitive outcomes (vs brain health outcomes). The majority of studies indicated that there was a negative association between sedentary behavior and cognition (Iso-Markku et al., 2018; Heisz et al., 2015; Steinberg et al., 2015; Edwards and Loprinzi, 2017a; Edwards and Loprinzi, 2017b; Ku et al., 2017; Wanigatunga et al., 2018; García- Hermoso et al., 2018; Folley et al., 2019; Suzuki et al., 2020; Chen et al., 2022; Gerten et al., 2022; Zhou et al., 2022; Shuai et al., 2023; Han et al., 2023), while the rest of the studies reported mixed or no effect (Wu et al., 2020; Vásquez et al., 2017; Čukić et al., 2018; Zlatar et al., 2019; Amagasa et al., 2020; Maasakkers et al., 2020; Silva-Fernandes et al., 2022), or positive effects (Vance et al., 2005; Rosenberg et al., 2016; Burzynska et al., 2020; Kurita et al., 2022; Major et al., 2023). A majority of the studies assessed cognition using a cognitive screening tool or a more limited cognitive battery. Of the five studies reporting positive associations between sedentary behavior and cognition, two studies utilized self-report of sedentary behavior, two utilized self-report and objective measurement, and only one used objective measurement. Five studies were longitudinal (Ku et al., 2017; Folley et al., 2019; Maasakkers et al., 2020; Shuai et al., 2023; Major et al., 2023). Of these five, most found a negative association between sedentary behavior and cognition (3/5), while 1 study found no association, and 1 found a positive association (see Table 1).
3.6 Sedentary behavior and brain health
Seven studies investigated the associations of sedentary behavior with brain structure and function measured via magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS). Specifically, observational studies reported that greater sedentary behavior was associated with increases in WM damage (Bronas et al., 2019), deterioration of microstructure organization (Burzynska et al., 2014), decrease in regional CBF (Zlatar et al., 2019), and reduction in functional network connectivity (Dion et al., 2021) in older adult samples. Furthermore, a longitudinal study found that high sedentary behavior at baseline was associated with reduction in WM volume at 5-year follow-up (Arnardottir et al., 2016). In contrast, no associations of sedentary behavior with hippocampal volume were observed. In terms of sedentary behavior measurements, all the studies used objective measurement of sedentary behavior using accelerometry, except one study (Bronas et al., 2019), which used self-reported measurement of sedentary behavior (see Table 2).
4 Discussion
4.1 Measurement of sedentary behavior
Our systematic review of the literature suggests that accelerometry is the most popular method of sedentary behavior measurement. The majority of the studies, especially in the most recent studies, assessed the associations of sedentary behavior with cognition (n = 15) utilizing an accelerometer. This is likely because accelerometry technology and validation for use of sedentary behavior in older adulthood has improved (Heesch et al., 2018), and this methodology is becoming more easy to implement. A minority of studies utilized self-report measures only (n = 9), mostly with validated questionnaires, or combined self-report and accelerometry methods (n = 3). Surprisingly, almost all studies except for one that examined brain health outcomes utilized an accelerometer. It is important to note that self-report has some important considerations, as older adults, particularly with cognitive impairment, pay not be as accurate in their reporting (VandeBunte et al., 2022).
Studies that used accelerometry varied in the devices used, the placement of devices, and the data processing cutpoints applied to classify sedentary level cut-offs, which has well-documented implications for the accuracy of capturing activity level, particularly in older adults (Schrack et al., 2016), and using wrist-worn devices (Wu et al., 2020). Compared to placing the accelerometer device on the wrist or hip, thigh device placement is considered the gold standard for the measurement of sedentary behavior because it captures positional information and postural changes (Kozey-Keadle et al., 2011). Thigh-worn devices were only utilized in one study reviewed in this investigation (Čukić et al., 2018). Future research should consider how device type, processing methodology, and placement in interpretation of findings. In addition, as physical activity and sedentary behavior are interrelated but separate behaviors (Dogra and Stathokostas, 2012), study results may differ based on whether physical activity level was adjusted for in analyses. As the field evolves in identifying best practices, future work might consider the importance of developing standardization procedures.
4.2 Classification of sedentary behavior
Many studies with self-report of sedentary behavior differed in the way sedentary behavior was categorized, and this could be a potential explanation for why results were not always consistent. Studies that examined specific domains of sedentary behavior may be better equipped to disentangle these discrepancies, as cognitively stimulating sedentary activities may be less detrimental or even helpful to cognition. For example, Major et al. (2023). found that higher amounts of sedentary reading time was positively associated with cognition, while participants who increased their TV watching time in particular, had significantly lower global cognition. Shuai et al. (2023) found that longer screen watching and playing cards was related to better global cognition, while other forms of sedentary behavior that did not involve screen time or playing games were associated with worse global cognition. In addition, not only did the category of sedentary behavior appear to sometimes be differentially related to cognition, but also perhaps the type as well. For example, Chen et al. (2022) found that sedentary time that accumulated in prolonged bouts, but not total sedentary time, was inversely associated with cognitive orientation ability among older adults. These examples demonstrate the importance of specifying the type of sedentary behavior as another important methodological consideration.
5 Conclusion and future directions
We systematically searched and reviewed 33 studies, and these studies generally had a low RoB. Like all systematic reviews, there were limitations to our search including the inability to include all possibly relevant databases, broad search terms, ability of the study team to only include studies that were written/translated into English, and the possibility of publication bias in included studies. However, the results of this systematic review suggest that overall, greater sedentary behavior is negatively associated with cognition and with brain health (see Figure 3 for a conceptual summary diagram). Researchers should consider several methodological factors including how sedentary behavior was measured (objective vs. subjective) and classified (e.g., sedentary time watching TV, working). Additional research is needed to better understand how the type and/or quantification of sedentary behavior (e.g., cognitively stimulating vs. not; total time vs. bouts vs. duration) influences its associations with cognition and brain health, as this may have an impact on the directionality of results (Wanders et al., 2021). Few studies in this review utilized both self-report and accelerometer measurement of sedentary behavior, although this may not be feasible or reliable in an older adult population with cognitive impairment. In addition, few studies had longitudinal measures of cognitive and brain health outcomes, limiting our ability to understand the direction of these associations over time. Given that there is evidence that the negative effects of sedentary behavior are likely cumulative (Diaz et al., 2017) and may be like other lifestyle factors in midlife that are critical for cognitive aging (Barnes and Yaffe, 2011; Livingston et al., 2024), more longitudinal research is needed to determine directionality and draw stronger conclusions. Finally, we sought to examine these associations across the cognitive spectrum from healthy aging to ADRD. Unfortunately, few studies included participants with MCI, and even fewer with dementia. Future work should consider including participants with a wider range of cognitive abilities to assess whether these associations may differ based on cognitive status.
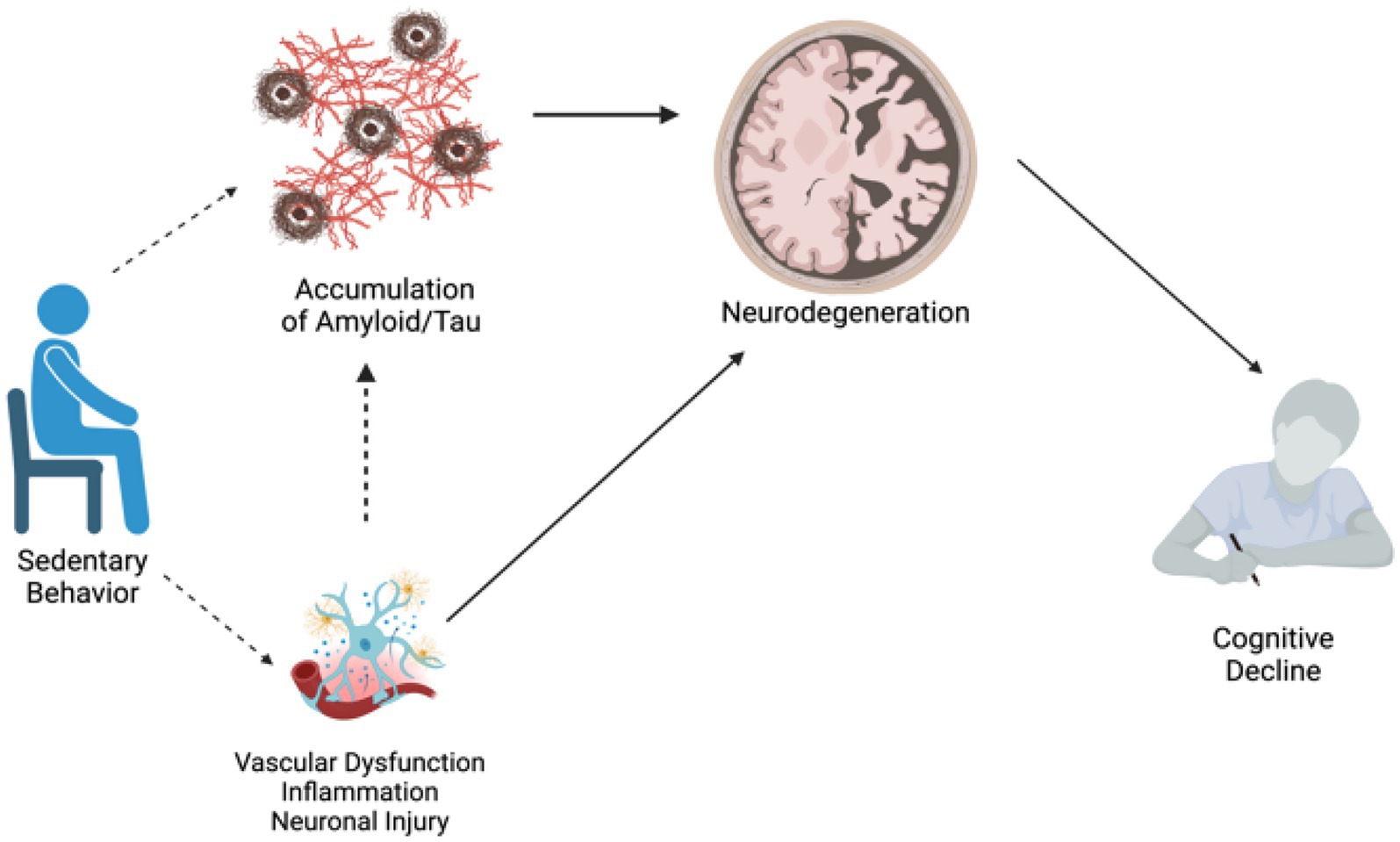
Figure 3. Conceptual diagram: potential pathways connecting sedentary behavior to AD risk. Dotted lines represent pathways to be tested, solid lines represent pathways with strong literature support.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
MAG: Formal analysis, Data curation, Writing – review & editing, Project administration, Methodology, Writing – original draft, Investigation, Conceptualization, Supervision, Resources. JW: Supervision, Conceptualization, Writing – review & editing, Data curation, Writing – original draft, Methodology. CC: Data curation, Formal analysis, Visualization, Writing – review & editing, Writing – original draft. AA: Writing – review & editing, Writing – original draft, Data curation. AV: Formal analysis, Writing – review & editing, Visualization. SG: Writing – review & editing, Conceptualization, Methodology. AW: Writing – review & editing. ABZ: Writing – review & editing. KC: Writing – review & editing. KF: Writing – review & editing. BS: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Network of Excellence in Neuroscience Clinical Trials (University of Pittsburgh CRS): NINDS 2U24NS107166–06 (MAG); K01-AG083223 (KMF), T32- HL082610 (ABZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Amagasa, S., Inoue, S., Murayama, H., Fujiwara, T., Kikuchi, H., Fukushima, N., et al. (2020). Associations of sedentary and physically-active behaviors with cognitive-function decline in community-dwelling older adults: compositional data analysis from the NEIGE study. J. Epidemiol. 30, 503–508. doi: 10.2188/jea.JE20190141
Arnardottir, N. Y., Koster, A., Domelen, D. R. V., Brychta, R. J., Caserotti, P., Eiriksdottir, G., et al. (2016). Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: age, gene/environment susceptibility-Reykjavik study. Behav. Brain Res. 296, 118–124. doi: 10.1016/j.bbr.2015.09.005
Bakrania, K., Edwardson, C. L., Khunti, K., Bandelow, S., Davies, M. J., and Yates, T. (2018). Associations between sedentary behaviors and cognitive function: cross-sectional and prospective findings from the UK biobank. Am. J. Epidemiol. 187, 441–454. doi: 10.1093/aje/kwx273
Barella, L. A., Etnier, J. L., and Chang, Y. K. (2010). The immediate and delayed effects of an acute bout of exercise on cognitive performance of healthy older adults. J. Aging Phys. Act. 18, 87–98. doi: 10.1123/japa.18.1.87
Barnes, D. E., and Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. doi: 10.1016/s1474-4422(11)70072-2
Biddle, S. J., Gorely, T., Marshall, S. J., Murdey, I., and Cameron, N. (2004). Physical activity and sedentary behaviours in youth: issues and controversies. J. R. Soc. Promot. Heal. 124, 29–33. doi: 10.1177/146642400312400110
Bronas, U. G., Steffen, A., Dion, C., et al. (2019). Sedentary time and white matter Hyperintensity volume in older adults. Med. Sci. Sports Exerc. 51, 1613–1618. doi: 10.1249/MSS.0000000000001957
Burzynska, A. Z., Chaddock-Heyman, L., Voss, M. W., Wong, C. N., Gothe, N. P., Olson, E. A., et al. (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One 9:e107413. doi: 10.1371/journal.pone.0107413
Burzynska, A. Z., Voss, M. W., Fanning, J., Salerno, E. A., Gothe, N. P., McAuley, E., et al. (2020). Sensor-measured sedentariness and physical activity are differentially related to fluid and crystallized abilities in aging. Psychol. Aging 35, 1154–1169. doi: 10.1037/pag0000580
Chen, S., Chen, T., Honda, T., Nofuji, Y., Kishimoto, H., and Narazaki, K. (2022). Associations of objectively-measured sedentary time and patterns with cognitive function in non-demented Japanese older adults: a cross-sectional study. Int. J. Environ. Res. Public Health 19. doi: 10.3390/ijerph19041999
Coelho, L., Hauck, K., McKenzie, K., Copeland, J. L., Kan, I. P., Gibb, R. L., et al. (2020). The association between sedentary behavior and cognitive ability in older adults. Aging Clin. Exp. Res. 32, 2339–2347. doi: 10.1007/s40520-019-01460-8
Čukić, I., Shaw, R., Der, G., Chastin, S. F. M., Dontje, M. L., Gill, J. M. R., et al. (2018). Cognitive ability does not predict objectively measured sedentary behavior: evidence from three older cohorts. Psychol. Aging 33, 288–296. doi: 10.1037/pag0000221
Diaz, K. M., Howard, V. J., Hutto, B., Colabianchi, N., Vena, J. E., Safford, M. M., et al. (2017). Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults: a national cohort study. Ann. Intern. Med. 167:465. doi: 10.7326/m17-0212
Dion, C., Tanner, J. J., Crowley, S. J., Wiggins, M. E., Marecchi, T., Ding, M., et al. (2021). Functional connectivity of key resting state networks and objectively measured physical activity in older adults with joint pain: a pilot study. Exp. Gerontol. 153. doi: 10.1016/j.exger.2021.111470
Dogra, S., and Stathokostas, L. (2012). Sedentary behavior and physical activity are independent predictors of successful aging in middle-aged and older adults. J. Aging Res. 2012, 1–8. doi: 10.1155/2012/190654
Edwards, M. K., and Loprinzi, P. D. (2017a). Combined associations of sedentary behavior and cardiorespiratory fitness on cognitive function among older adults. Int. J. Cardiol. 229, 71–74. doi: 10.1016/j.ijcard.2016.11.264
Edwards, M. K., and Loprinzi, P. D. (2017b). The association between sedentary behavior and cognitive function among older adults may be attenuated with adequate physical activity. J. Phys. Act. Health 14, 52–58. doi: 10.1123/jpah.2016-0313
Engeroff, T., Füzéki, E., Vogt, L., Fleckenstein, J., Schwarz, S., Matura, S., et al. (2018). Is objectively assessed sedentary behavior, physical activity and cardiorespiratory fitness linked to brain plasticity outcomes in old age? Neuroscience 388, 384–392. doi: 10.1016/j.neuroscience.2018.07.050
Erickson, K. I., Hillman, C., Stillman, C. M., Ballard, R. M., Bloodgood, B., Conroy, D. E., et al. (2019). Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 51, 1242–1251. doi: 10.1249/MSS.0000000000001936
Falck, R. S., Davis, J. C., and Liu-Ambrose, T. (2017). What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 51, 800–811. doi: 10.1136/bjsports-2015-095551
Folley, S., Zhou, A., Llewellyn, D. J., and Hyppönen, E. (2019). Physical activity, APOE genotype, and cognitive decline: exploring gene-environment interactions in the UK biobank. J. Alzheimer Disease 71, 741–750. doi: 10.3233/JAD-181132
García-Hermoso, A., Ramírez- Vélez, R., Celis- Morales, C. A., Olloquequi, J., and Izquierdo, M. (2018). Can physical activity attenuate the negative association between sitting time and cognitive function among older adults? A mediation analysis. Exp. Gerontol. 106, 173–177. doi: 10.1016/j.exger.2018.03.002
Gerten, S., Engeroff, T., Fleckenstein, J., Füzéki, E., Matura, S., Pilatus, U., et al. (2022). Deducing the impact of physical activity, sedentary behavior, and physical performance on cognitive function in healthy older adults. Front. Aging Neurosci. 13:777490. doi: 10.3389/fnagi.2021.777490
Gogniat, M. A., Robinson, T. L., Jean, K. R., and Stephen, M. L. (2022). Physical activity moderates the association between executive function and functional connectivity in older adults. Aging Brain. 2:100036. doi: 10.1016/j.nbas.2022.100036
Hajduk, A. M., and Chaudhry, S. I. (2016). Sedentary behavior and cardiovascular risk in older adults: a scoping review. Curr. Cardiovasc. Risk Rep. 10:5. doi: 10.1007/s12170-016-0485-6
Han, X., Song, L., Li, Y., Dong, Y., Liu, R., Han, Q., et al. (2023). Accelerometer-measured sedentary behavior patterns, brain structure, and cognitive function in dementia-free older adults: a population-based study. J. Alzheimers Dis. 96, 657–668. doi: 10.3233/JAD-230575
Harvey, J., Chastin, S., and Skelton, D. (2013). Prevalence of sedentary behavior in older adults: a systematic review. IJERPH. 10, 6645–6661. doi: 10.3390/ijerph10126645
Heesch, K. C., Hill, R. L., Aguilar-Farias, N., van Uffelen, J. G. Z., and Pavey, T. (2018). Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. Int. J. Behav. Nutr. Phys. Act. 15:119. doi: 10.1186/s12966-018-0749-2
Heisz, J. J., Vandermorris, S., Wu, J., McIntosh, A. R., and Ryan, J. D. (2015). Age differences in the association of physical activity, sociocognitive engagement, and TV viewing on face memory. Health Psychol. 34, 83–88. doi: 10.1037/hea0000046
Iso-Markku, P., Kujala, U. M., Knittle, K., Polet, J., Vuoksimaa, E., and Waller, K. (2022). Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br. J. Sports Med. 56, 701–709. doi: 10.1136/bjsports-2021-104981
Iso-Markku, P., Waller, K., Vuoksimaa, E., Vähä-Ypyä, H., Lindgren, N., Heikkilä, K., et al. (2018). Objectively measured physical activity profile and cognition in Finnish elderly twins. Alzheimer’s Dementia (New York, N Y). 4, 263–271. doi: 10.1016/j.trci.2018.06.007
Kesse-Guyot, E., Charreire, H., Andreeva, V. A., Touvier, M., Hercberg, S., Galan, P., et al. (2012). Cross-sectional and longitudinal associations of different sedentary behaviors with cognitive performance in older adults. PLoS One 7:e47831. doi: 10.1371/journal.pone.0047831
Kozey-Keadle, S., Libertine, A., Lyden, K., Staudenmayer, J., and Freedson, P. S. (2011). Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 43, 1561–1567. doi: 10.1249/MSS.0b013e31820ce174
Ku, P. W., Liu, Y. T., Lo, M. K., Chen, L. J., and Stubbs, B. (2017). Higher levels of objectively measured sedentary behavior is associated with worse cognitive ability: two-year follow-up study in community-dwelling older adults. Exp. Gerontol. 99, 110–114. doi: 10.1016/j.exger.2017.09.014
Kurita, S., Doi, T., Tsutsumimoto, K., Nakakubo, S., Ishii, H., and Shimada, H. (2022). Development of a questionnaire to evaluate older adults’ total sedentary time and sedentary time with cognitive activity. J. Geriatr. Psychiatry Neurol. 35, 392–399. doi: 10.1177/08919887211006468
Livingston, G., Huntley, J., Liu, K. Y., Costafreda, S. G., Selbæk, G., Alladi, S., et al. (2024). Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet 404, 572–628. doi: 10.1016/S0140-6736(24)01296-0
Maasakkers, C. M., Claassen, J. A. H. R., Gardiner, P. A., Olde Rikkert, M. G. M., Lipnicki, D. M., Scarmeas, N., et al. (2020). The association of sedentary behaviour and cognitive function in people without dementia: a coordinated analysis across five cohort studies from COSMIC. Sports Med. 50, 403–413. doi: 10.1007/s40279-019-01186-7
Maasakkers, C. M., Weijs, R. W. J., Dekkers, C., Gardiner, P. A., Ottens, R., Olde Rikkert, M. G. M., et al. (2022). Sedentary behaviour and brain health in middle-aged and older adults: a systematic review. Neurosci. Biobehav. Rev. 140. doi: 10.1016/j.neubiorev.2022.104802
Machida, M., Takamiya, T., Amagasa, S., Murayama, H., Fujiwara, T., Odagiri, Y., et al. (2022). Objectively measured intensity-specific physical activity and hippocampal volume among community-dwelling older adults. J. Epidemiol. 32, 489–495. doi: 10.2188/jea.JE20200534
Major, L., Simonsick, E. M., Napolitano, M. A., and DiPietro, L. (2023). Domains of sedentary behavior and cognitive function: the health, aging, and body composition study, 1999/2000 to 2006/2007. J. Gerontol. A Biol. Sci. Med. Sci. 78, 2035–2041. doi: 10.1093/gerona/glad020
Matthews, C. E., Chen, K. Y., Freedson, P. S., Buchowski, M. S., Beech, B. M., Pate, R. R., et al. (2008). Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am. J. Epidemiol. 167, 875–881. doi: 10.1093/aje/kwm390
Morgan, R. L., Whaley, P., Thayer, K. A., and Schünemann, H. J. (2018). Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 121, 1027–1031. doi: 10.1016/j.envint.2018.07.015
Olanrewaju, O., Stockwell, S., Stubbs, B., and Smith, L. (2020). Sedentary behaviours, cognitive function, and possible mechanisms in older adults: a systematic review. Aging Clin. Exp. Res. 32, 969–984. doi: 10.1007/s40520-019-01457-3
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrowm, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ :n71. doi: 10.1136/bmj.n71
Raichlen, D. A., Aslan, D. H., Sayre, M. K., Bharadwaj, P. K., Ally, M., Maltagliati, S., et al. (2023). Sedentary behavior and incident dementia among older adults. JAMA 330, 934–940. doi: 10.1001/jama.2023.15231
Rosenberg, D. E., Bellettiere, J., Gardiner, P. A., Villarreal, V. N., Crist, K., and Kerr, J. (2016). Independent associations between sedentary behaviors and mental, cognitive, physical, and functional health among older adults in retirement communities. J. Gerontol. A Biol. Sci. Med. Sci. 71, 78–83. doi: 10.1093/gerona/glv103
Rosenberg, D. E., Norman, G. J., Wagner, N., Patrick, K., Calfas, K. J., and Sallis, J. F. (2010). Reliability and validity of the sedentary behavior questionnaire (SBQ) for adults. J. Phys. Act. Health 7, 697–705. doi: 10.1123/jpah.7.6.697
Ryan, R., and Hill, S. (2019). Supporting implementation of Cochrane methods in complex communication reviews: resources developed and lessons learned for editorial practice and policy. Health Res Policy Sys. 17:32. doi: 10.1186/s12961-019-0435-0
Schrack, J. A., Cooper, R., Koster, A., Shiroma, E. J., Murabito, J. M., Rejeski, W. J., et al. (2016). Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. GERONA. 71, 1039–1048. doi: 10.1093/gerona/glw026
Sedentary Behaviour Research Network (2012). Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 37, 540–542. doi: 10.1139/h2012-024
Shuai, Z., Jingya, Z., Qing, W., Qiong, W., Chen, D., Guodong, S. Prof., et al. (2023). Associations between sedentary duration and cognitive function in older adults: a longitudinal study with 2-year follow-up. J. Nutr. Health Aging 27, 656–662. doi: 10.1007/s12603-023-1963-4
Siddarth, P., Burggren, A. C., Eyre, H. A., Small, G. W., and Merrill, D. A. (2018). Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults SD Ginsberg, ed. PLoS One 13:e0195549. doi: 10.1371/journal.pone.0195549
Silva-Fernandes, A., Cruz, S., Moreira, C. S., Pereira, D. R., Sousa, S. S., Sampaio, A., et al. (2022). Processing speed mediates the association between physical activity and executive functioning in elderly adults. Front. Psychol. 13:958535. doi: 10.3389/fpsyg.2022.958535
Steinberg, S. I., Sammel, M. D., Harel, B. T., Schembri, A., Policastro, C., Bogner, H. R., et al. (2015). Exercise, sedentary pastimes, and cognitive performance in healthy older adults. Am. J. Alzheimers Dis. Other Dement. 30, 290–298. doi: 10.1177/1533317514545615
Suzuki, K., Niimura, H., Kida, H., Eguchi, Y., Kitashima, C., Takayama, M., et al. (2020). Increasing light physical activity helps to maintain cognitive function among the community-dwelling oldest old population: a cross-sectional study using actigraph from the Arakawa 85+ study. Geriatr Gerontol Int 20, 773–778. doi: 10.1111/ggi.13967
Tremblay, M. S., Aubert, S., Barnes, J. D., Saunders, T. J., Carson, V., Latimer-Cheung, A. E., et al. (2017). Sedentary behavior research network (SBRN) – terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 14:75. doi: 10.1186/s12966-017-0525-8
Vance, D. E., Wadley, V. G., Ball, K. K., Roenker, D. L., and Rizzo, M. (2005). The effects of physical activity and sedentary behavior on cognitive health in older adults. J. Aging Phys. Act. 13, 294–313. doi: 10.1123/japa.13.3.294
VandeBunte, A., Gontrum, E., Goldberger, L., Fonseca, C., Djukic, N., You, M., et al. (2022). Physical activity measurement in older adults: wearables versus self-report. Front Digit Health. 4:869790. doi: 10.3389/fdgth.2022.869790
Vásquez, E., Strizich, G., Isasi, C. R., Echeverria, S. E., Sotres-Alvarez, D., Evenson, K. R., et al. (2017). Is there a relationship between accelerometer-assessed physical activity and sedentary behavior and cognitive function in US Hispanic/Latino adults? The Hispanic community health study/study of Latinos (HCHS/SOL). Prev. Med. 103, 43–48. doi: 10.1016/j.ypmed.2017.07.024
Wanders, L., Bakker, E. A., Van Hout, H. P. J., Eijsvogels, T. M., Hopman, M. T., Visser, L. N., et al. (2021). Association between sedentary time and cognitive function: a focus on different domains of sedentary behavior. Prev. Med. 153:106731. doi: 10.1016/j.ypmed.2021.106731
Wanigatunga, A. A., Manini, T. M., Cook, D. R., Katula, J., Fielding, R. A., Kramer, A. F., et al. (2018). Community-based activity and sedentary patterns are associated with cognitive performance in mobility-limited older adults. Front. Aging Neurosci. 10:341. doi: 10.3389/fnagi.2018.00341
Won, J., Callow, D. D., Pena, G. S., Gogniat, M. A., Kommula, Y., Arnold-Nedimala, N. A., et al. (2021). Evidence for exercise-related plasticity in functional and structural neural network connectivity. Neurosci. Biobehav. Rev. 131, 923–940. doi: 10.1016/j.neubiorev.2021.10.013
Wu, J., Fu, Y., Chen, D., Zhang, H., Xue, E., Shao, J., et al. (2023). Sedentary behavior patterns and the risk of non-communicable diseases and all-cause mortality: a systematic review and meta-analysis. Int. J. Nurs. Stud. 146:104563. doi: 10.1016/j.ijnurstu.2023.104563
Wu, Z. J., Wang, Z. Y., Hu, B. Q., Zhang, X. H., Zhang, F., Wang, H. L., et al. (2020). Relationships of accelerometer-based measured objective physical activity and sedentary behaviour with cognitive function: a comparative cross-sectional study of China’s elderly population. BMC Geriatr. 20:149. doi: 10.1186/s12877-020-01521-y
Yan, S., Fu, W., Wang, C., Mao, J., Liu, B., Zou, L., et al. (2020). Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl. Psychiatry 10:112. doi: 10.1038/s41398-020-0799-5
Zhou, W., Webster, K. E., Veliz, P. T., and Larson, J. L. (2022). Profiles of sedentary behaviors in the oldest old: findings from the National Health and aging trends study. Aging Clin. Exp. Res. 34, 2071–2079. doi: 10.1007/s40520-022-02157-1
Zlatar, Z. Z., Hays, C. C., Mestre, Z., Campbell, L. M., Meloy, M. J., Bangen, K. J., et al. (2019). Dose-dependent association of accelerometer-measured physical activity and sedentary time with brain perfusion in aging. Exp. Gerontol. 125:110679. doi: 10.1016/j.exger.2019.110679
Keywords: sedentary behavior, cognition, Alzheimer’s disease, brain health, older adult
Citation: Gogniat MA, Won J, Cruz C, Aranda A, Verma A, Gujral S, Weinstein AM, Zaheed AB, Cole KR, Full KM and Snitz BE (2025) Sedentary behavior, cognition, and brain health in older adults: a systematic review. Front. Aging Neurosci. 17:1622049. doi: 10.3389/fnagi.2025.1622049
Edited by:
Yih-Kuen Jan, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Pablo Martinez Amezcua, Johns Hopkins University, United StatesKei Shing Ng, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2025 Gogniat, Won, Cruz, Aranda, Verma, Gujral, Weinstein, Zaheed, Cole, Full and Snitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marissa A. Gogniat, Z29nbmlhdG1hQHVwbWMuZWR1
 Marissa A. Gogniat
Marissa A. Gogniat Junyeon Won
Junyeon Won Carlos Cruz2,4
Carlos Cruz2,4 Aryan Verma
Aryan Verma Swathi Gujral
Swathi Gujral Andrea M. Weinstein
Andrea M. Weinstein