- 1Department of Rehabilitation and Treatment, Hubei Rehabilitation Hospital, Wuhan, China
- 2School of Public Finance and Taxation, Central University of Finance and Economics, Beijing, China
- 3Department of Rehabilitation Medicine, Beijing Jishuitan Hospital Guizhou Hospital, Guizhou Provincial Orthopedics Hospital, Guiyang, China
Background: Repetitive transcranial magnetic stimulation (rTMS) has emerged as a promising neuromodulatory approach for alleviating sleep disturbances and depressive symptoms in Parkinson’s disease (PD), yet direct comparisons of different stimulation frequencies remain scarce.
Objective: To evaluate and rank the efficacy of three rTMS frequencies (1 Hz, 5 Hz, and 10 Hz), each combined with conventional therapy, on sleep disorders and depression in PD patients, thereby informing clinical decision-making.
Methods: We conducted a systematic search for randomized controlled trials (RCTs) in PubMed, Embase, the Cochrane Library, Web of Science, ProQuest, China National Knowledge Infrastructure, Wanfang, and the Chinese Scientific and Journal Database. A network meta-analysis was performed to compare the effects of different frequencies of rTMS (1 Hz, 5 Hz, and 10 Hz) on sleep disorders and depression in PD patients.
Results: Thirty-one RCTs involving 1,977 PD patients met inclusion criteria. Compared with conventional treatment alone, adjunctive 5 Hz and 10 Hz rTMS produced significant improvements in both Pittsburgh Sleep Quality Index (PSQI) and Parkinson’s Disease Sleep Scale (PDSS). Although 1 Hz rTMS yielded numerically greater PSQI and PDSS improvements than conventional therapy, these differences did not reach statistical significance, nor did differences between the three stimulation frequencies. In terms of depressive symptoms, all three frequencies (1 Hz, 5 Hz, and 10 Hz) significantly reduced HAMD scores versus standard care, with head-to-head comparisons indicating superior efficacy of 10 Hz over 1 Hz and 5 Hz. The Surface Under the Cumulative Ranking area (SUCRA) consistently identified 10 Hz rTMS as the most effective frequency for PSQI, PDSS, and HAMD outcomes.
Conclusion: Adjunctive rTMS at 1 Hz, 5 Hz, and 10 Hz each confer benefits for sleep and mood in PD patients, but 10 Hz stimulation appears to offer the greatest overall improvement. These findings support the preferential use of 10 Hz rTMS when targeting non-motor symptoms in Parkinson’s disease.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/recorddashboard, identifier CRD42024614337.
1 Introduction
Parkinson’s disease (PD), which primarily affects middle-aged and older adults, is the world’s second most common neurodegenerative disorder (Rocca, 2018; GBD 2016 Neurology Collaborators., 2019). In addition to its hallmark motor symptoms such as tremor, rigidity and bradykinesia, PD is also characterized by a wide range of non-motor impairments, including mood disturbances, cognitive decline and sleep disorders (Hendricks and Khasawneh, 2021; Kirmani et al., 2021). The exact pathogenesis of PD remains unclear; current evidence suggests that non-motor symptoms predominantly arise from diminished dopaminergic transmission within the midbrain–limbic and midbrain–cortical systems (Moore et al., 2008). Most individuals with PD experience sleep problems early in the disease course or even before overt motor signs appear (Barone et al., 2009). Common sleep disorders in PD include rapid eye movement sleep behavior disorder (RBD), insomnia, restless legs syndrome, sleep-related breathing disturbances and excessive daytime sleepiness (Chahine et al., 2017). These disturbances may result from side effects of dopaminergic medications, neurodegenerative changes in brain structures that regulate sleep and nocturnal motor symptoms (French and Muthusamy, 2016). The regulation of sleep relies on the comprehensive function of multiple brain regions and various neurotransmitters, including dopamine, serotonin, norepinephrine, and other PD-related neurotransmitters (Stefani and Högl, 2020). These neurotransmitters not only regulate sleep disorders but may also be associated with cognitive dysfunction in PD (Yeung and Cavanna, 2014; Maggi et al., 2021; Malhotra, 2022). Emotional disorders involve depression, anxiety, etc. Recent studies have shown that there is a strong correlation between sleep quality and depressive and anxious emotions in PD patients, and the severity of sleep disorders is related to the degree of depression (Kay et al., 2018; Rana et al., 2018). Depression is another prevalent non-motor feature of PD and often has an insidious onset that precedes typical motor manifestations (Langston, 2006; Simonetta-Moreau, 2014). In patients with PD, depressive episodes frequently co-occur with anxiety, irritability, sadness and pessimism about the future, all of which can worsen sleep disturbances (van der Hoek et al., 2011). Depression and sleep disorders frequently co-occur in PD, each exacerbating the other in a self-perpetuating cycle. The close link between sleep dysfunction and depression leads to loss of mobility, reduced functional independence and severe impairments in mood and daily quality of life (Hariz and Forsgren, 2011; Zhao et al., 2021). These challenges also place a heavy burden on families and healthcare systems, since the global economic cost of PD reached an estimated $52 billion in 2017 and is projected to exceed $80 billion by 2040 as populations continue to age (Dorsey et al., 2018). Current management of PD relies mainly on dopaminergic pharmacotherapy, but long-term use of these agents carries risks of adverse effects and may even accelerate neurodegeneration (Jiménez-Urbieta et al., 2015; Seppi et al., 2019). It is therefore critical to explore non-pharmacological interventions that carry fewer risks and can help alleviate both sleep disturbances and depression in PD patients.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique grounded in electromagnetic induction; a pulsed magnetic field is applied to the skull surface to induce weak electrical currents in targeted brain regions (Chail et al., 2018). Depending on the frequency of the stimulation pulses, rTMS is classified as either low-frequency (≤1 Hz, low frequency rTMS, LF-rTMS) or high-frequency (>1 Hz, high frequency rTMS, HF-rTMS) stimulation (Xia et al., 2022). Previous studies have demonstrated that its therapeutic effects arise from bidirectional modulation of cortical excitability: low-frequency rTMS reduces excitability, while high-frequency rTMS enhances it (Cirillo et al., 2017). However, direct comparisons across frequencies are scarce, so this study employed a network meta-analysis (NMA) to evaluate how different rTMS frequencies affect sleep disorders and depression in PD patients and to identify the optimal stimulation frequency for clinical use.
2 Materials and methods
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Evaluation and Meta-Analysis statement (PRISMA) guidelines (Hutton et al., 2015) and the Cochrane Handbook for Systematic Reviews of Interventions to ensure methodological rigor. The protocol was registered with PROSPERO under registration number CRD42024614337.
2.1 Search strategy
Systematic searches were conducted on PubMed, EMBASE, Cochrane Library, Web of Science, ProQuest, China National Knowledge Infrastructure, Wanfang Database and Chinese Scientific and Journal Database (VIP) before February 2025 for randomized controlled trials (RCTs) on the effects of rTMS stimulation on sleep disorders and depression in PD patients. Using a combination of logical connective, medical MeSH, and free-text terms, search terms included: “Parkinson’s Disease,” “Parkinson,” “Parkinson’s,” “Parkinsonism,” “Repetitive Transcranial Magnetic Stimulation,” “Transcranial Magnetic Stimulation,” “rTMS,” “Sleep,” “Sleep disorders,” “Depression.” See Supplementary Table 1 for detailed search strategies for PubMed.
2.2 Inclusion criteria
Inclusion criteria were defined according to the PICOS framework (Population, Interventions, Comparators, Outcomes, and Study Design).
1. Population: Participants diagnosed with PD who reported sleep disorders, were over 18 years of age, of any gender, and provided informed consent.
2. Interventions: Experimental groups received low-frequency rTMS (LF-rTMS) or high-frequency rTMS (HF-rTMS); all interventions were given in addition to standard care, which encompassed conventional antiparkinsonian medications or routine rehabilitation.
3. Comparators: Different frequencies of rTMS, no stimulation, or sham stimulation (the latter referring to the absence of effective magnetic stimulation with only the sound simulated); all comparators were given in addition to standard care, which encompassed conventional antiparkinsonian medications or routine rehabilitation.
4. Outcomes: Primary outcomes included the Pittsburgh Sleep Quality Index (PSQI), Parkinson’s Disease Sleep Scale (PDSS), and Hamilton Rating Scale for Depression (HAMD); secondary outcomes comprised adverse events.
5. Study design: Only randomized controlled trials (RCTs) in human participants were eligible.
2.3 Exclusion criteria
1. Sleep disorders were not attributable to PD; (2) data on any primary or secondary outcomes were unavailable; (3) the full text could not be retrieved; (4) the study was a duplicate publication.
2.4 Study selection
All retrieved records were first imported into EndNoteX9 for duplicate removal. Two independent reviewers then screened titles and abstracts to exclude studies that did not meet the inclusion criteria. The full text of all remaining articles was read and assessed for eligibility, and any disagreements were resolved by a third reviewer (S.W.H).
2.5 Data extraction
Data extraction was performed independently by two investigators (X.Y and L.Y.J). For each included study, we recorded the first author, year and country of publication, sample size, participant age, intervention type, stimulation parameters, stimulation site and outcome measures. All data were entered into an Excel spreadsheet and cross-checked by both investigators; any discrepancies were adjudicated by a third investigator (S.W.H). When multiple reports used the same data set, we selected the publication with the higher quality score or, if scores were equal, the larger sample size.
2.6 Quality assessment
The quality of the included studies was assessed by two independent reviewers (W.H.L and H.X) using the Cochrane Risk of Bias Tool 2.0 (RoB 2.0) (Sterne et al., 2019) and the Physiotherapy Evidence Database (PEDro) scale. The evaluation of RoB 2.0 encompasses the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. The risk of bias in each domain can be categorized into three levels: “low risk,” “some concerns,” and “high risk.” If the assessment results in all domains are “low risk,” then the overall risk of bias is considered “low risk”; if some domains have “some concerns” and none have “high risk,” then the overall risk of bias is “some concerns”; if even one domain is rated “high risk,” the overall risk of bias is considered “high risk.” The PEDro scale consists of 11 items, with the first item not contributing to the total score, which totals 10 points. Studies with a score of ≥6 (6/10) are considered “good” quality, 4–5 are “fair” quality, and <4 are “poor” quality. Any disagreements in the assessment process were decided by a third investigator (S.W.H).
2.7 Statistical analysis
Network meta-analysis was conducted in Stata 16.0. Because all outcomes were continuous variables measured on the same scale, we used weighted mean differences (WMD) and 95 percent confidence intervals as effect sizes. We visualized comparisons in a network evidence diagram in which each node represents an intervention (node size proportional to total sample size) and each connecting line represents a direct comparison (line thickness proportional to number of studies). Consistency between direct and indirect evidence was assessed via ring inconsistency testing; a 95% CI for the inconsistency factor that included zero indicated good agreement. Pairwise comparative forest plots were generated to display intervention effects; effect sizes lying on one side of the null line with confidence intervals that did not cross 0 were considered statistically significant. We calculated Surface Under the Cumulative Ranking area (SUCRA) to rank interventions. The SUCRA value ranges from 0 to 100, where higher values indicate superior intervention efficacy and lower values correspond to diminished effectiveness. Finally, used funnel plots to evaluate publication bias and other small-study effects. Subgroup and sensitivity analyses were conducted to assess the robustness of our findings. Studies were stratified into two subgroups based on total pulse count (≤600 pulses and >600 pulses); Sensitivity analyses were conducted after excluding studies with sample sizes <10, PEDro scores < 6, and Hoehn & Yahr stages (H&Y) > 3. Publication bias and small-study effects were evaluated using funnel plots in Stata 16.0.
3 Results
3.1 Study selection
The initial search yielded 5303 records. After removing 1332 duplicates, 3 971 records remained. Title and abstract screening excluded 3862 records, leaving 109 articles for full-text review; 78 of these were excluded and 31 trials were included in the network meta-analysis.
Figure 1 depicts the screening and selection process of the articles.
3.2 Characteristics of the included studies
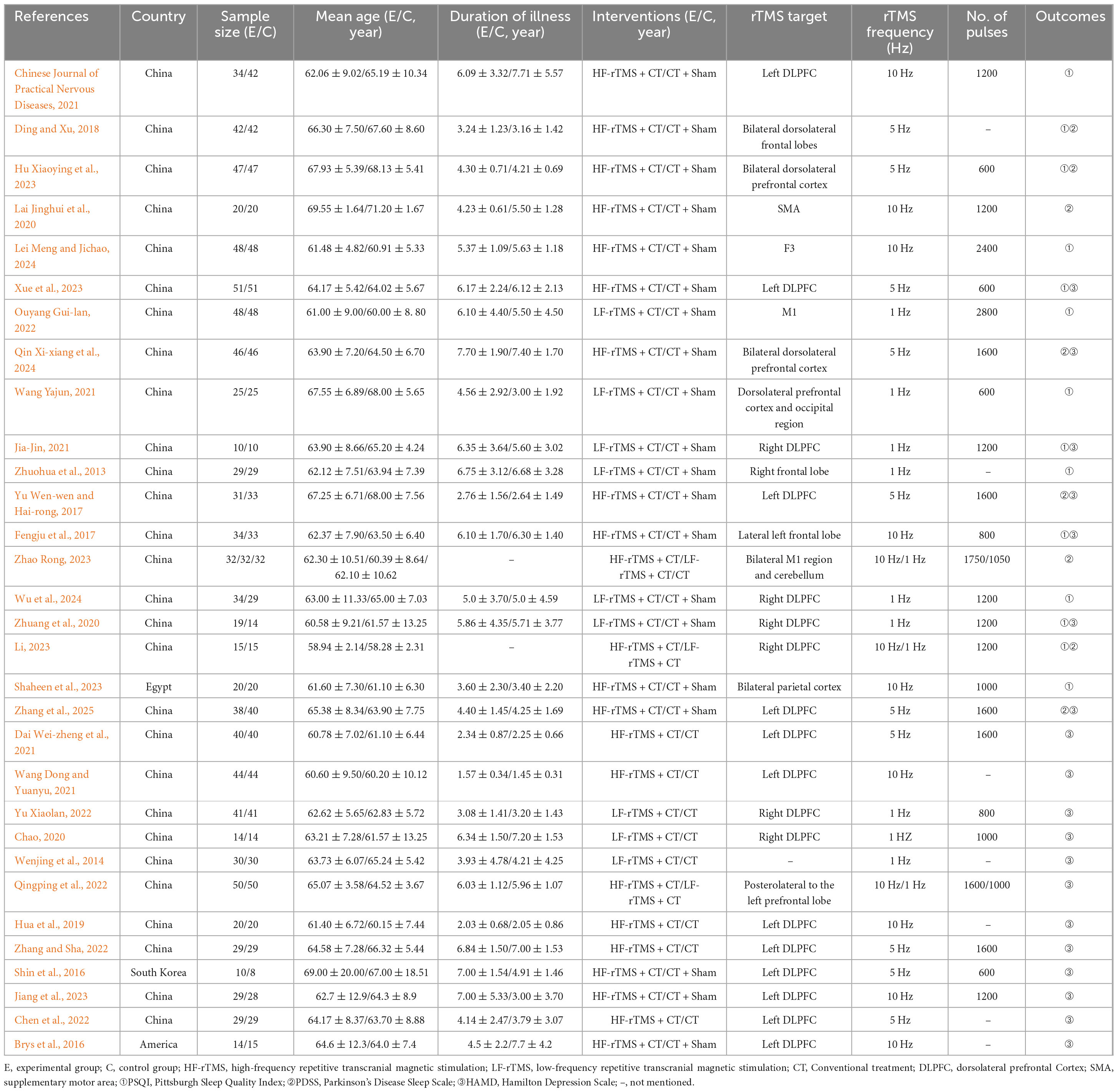
In total, 1977 Parkinson’s disease patients were enrolled. Twenty-four trials were published in Chinese and seven in English, with publication dates ranging from 2013 to 2024. In the control arms, one trial combined rehabilitation training with sham stimulation and the remainder combined medication with sham stimulation. In the experimental arms, 12 trials applied low-frequency rTMS at 1 Hz; 10 trials applied high-frequency rTMS at 5 Hz; and 12 trials applied high-frequency rTMS at 10 Hz. 14 studies reported PSQI, 8 studies reported PDSS only, and 19 studies reported HAMD. Detailed characteristics of the included studies are shown in Table 1. (Moore et al., 2008; Zhuohua et al., 2013; Wenjing et al., 2014; Brys et al., 2016; Shin et al., 2016; Fengju et al., 2017; Yu Wen-wen and Hai-rong, 2017; Ding and Xu, 2018; Hua et al., 2019; Chao, 2020; Lai Jinghui et al., 2020; Zhuang et al., 2020; Chinese Journal of Practical Nervous Diseases, 2021; Dai Wei-zheng et al., 2021; Jia-Jin, 2021; Wang Dong and Yuanyu, 2021; Wang Yajun, 2021; Chen et al., 2022; Ouyang Gui-lan, 2022; Qingping et al., 2022; Yu Xiaolan, 2022; Zhang and Sha, 2022; Hu Xiaoying et al., 2023; Jiang et al., 2023; Li, 2023; Shaheen et al., 2023; Xue et al., 2023; Zhao Rong, 2023; Lei Meng and Jichao, 2024; Qin Xi-xiang et al., 2024; Wu et al., 2024; Zhang et al., 2025).
3.3 Quality evaluation
Risk of bias was assessed using the Cochrane Risk of Bias 2.0 tool. Eighteen trials (58.1%) described their randomization process; four (12.9%) reported allocation concealment; eight (25.8%) reported blinding of participants and personnel; and 17 (54.8%) reported blinding of outcome assessment. All research data were complete. Detailed results of the risk-of-bias assessment are provided in Figure 2. Regarding PEDro scores, all studies scored >4 (median 6; range 4–9). See Supplementary Table 2 for details.
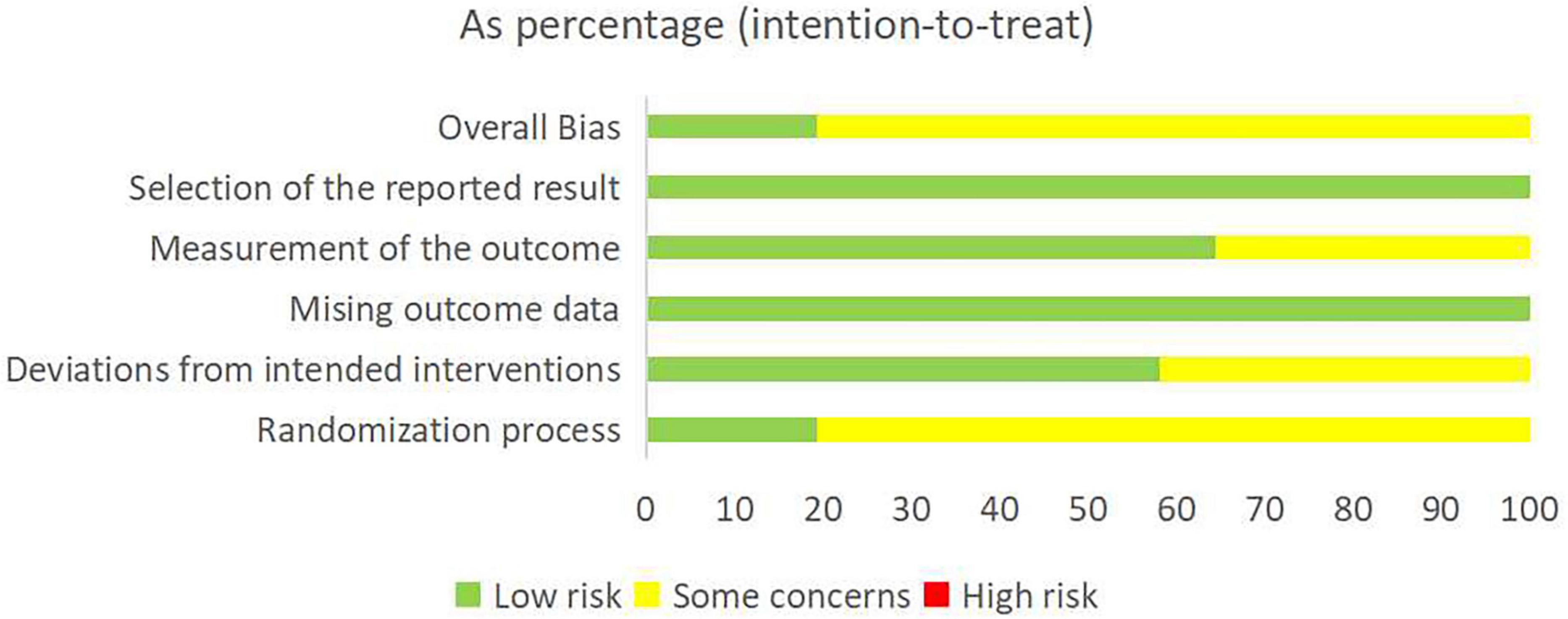
Figure 2. Risk bias assessment plot (green, yellow, and red indicate low, moderate, and high risk levels).
3.4 Network of evidence
Network geometry for each outcome is shown in Figures 3–5. Figure 3A illustrates the four interventions compared on PSQI (with the largest sample in the conventional treatment node); Figure 4A shows the PDSS network of four interventions; and Figure 5A depicts the HAMD network.
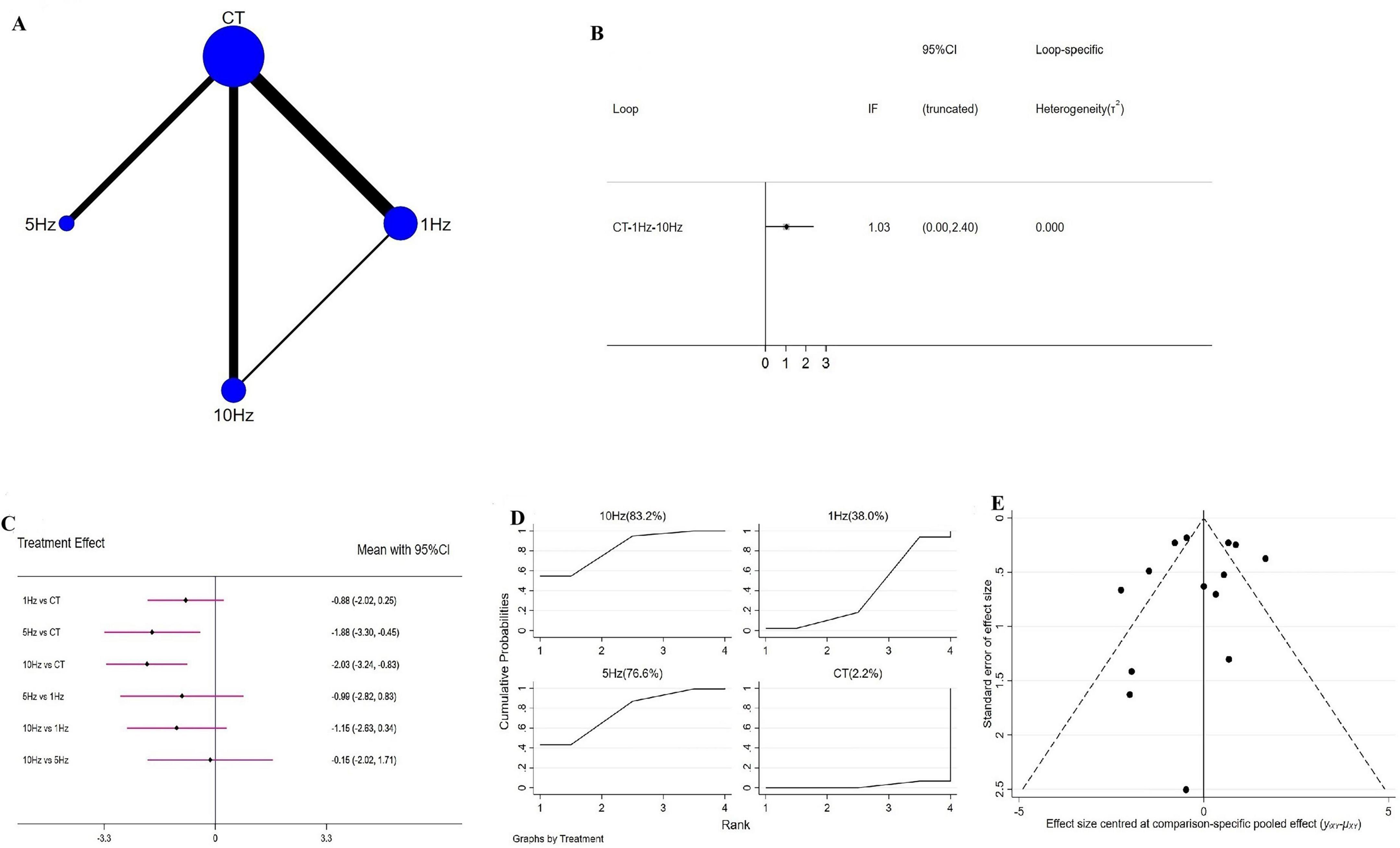
Figure 3. Network meta-analysis results for Pittsburgh Sleep Quality Index (PSQI). (A) Network plot; (B) ring inconsistencies (X-axis, effect size, 0 represents no effect; Y-axis, closed loop between interventions, the horizontal line range represents the 95% confidence interval; diamond represents point estimate); (C) forest plot (X-axis, effect size, with 0 representing no effect; Y-axis, comparison between interventions, with the red line range indicating the 95% confidence interval; diamonds representing pooled effect sizes); (D) the figure of ranking probability (X-axis, ranking position (Rank), Y-axis, representing Cumulative Probability); (E) funnel plot (X-axis, effect size, Y-axis, standard error of effect size, and black dots represent the results of each study).
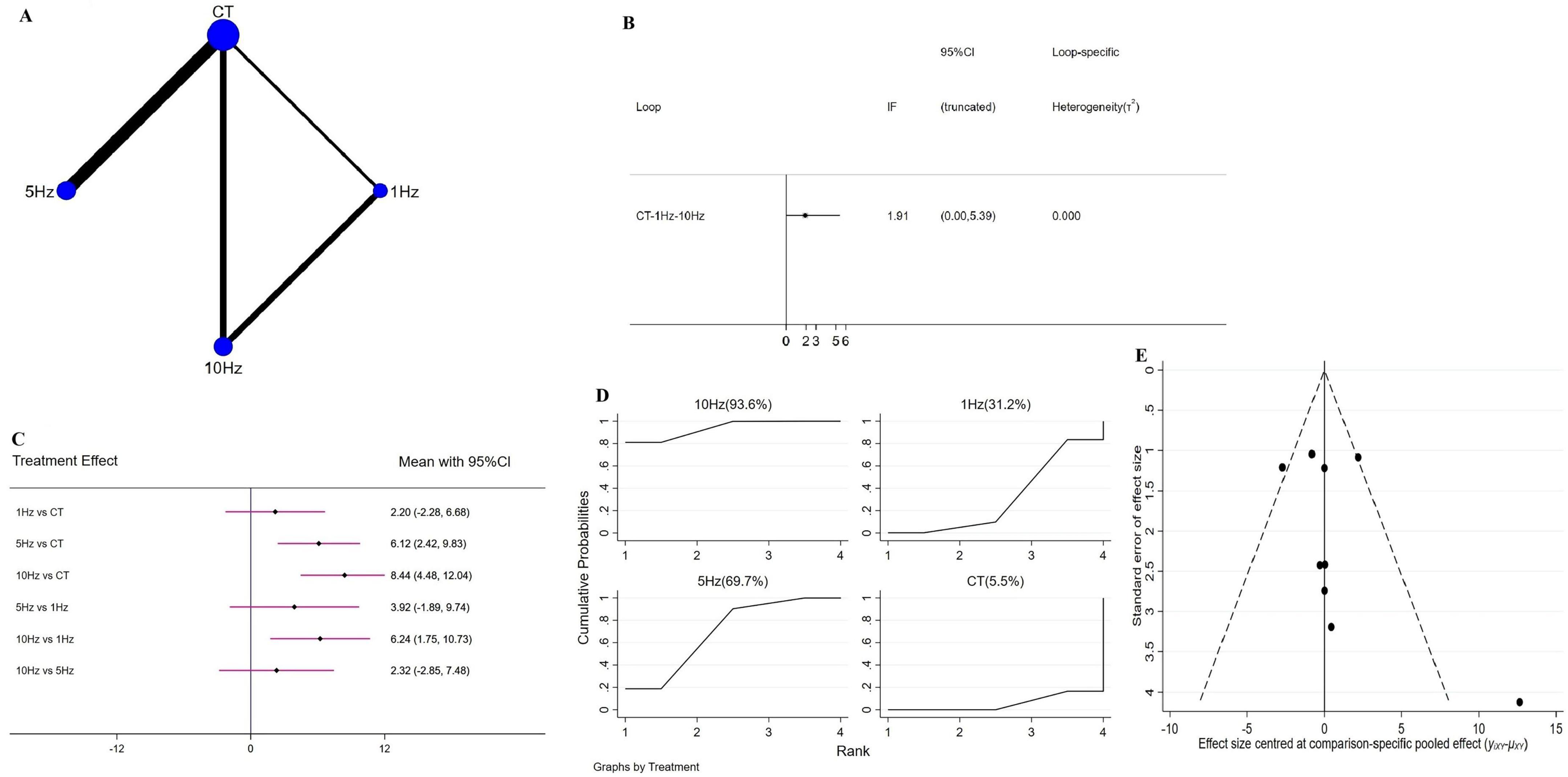
Figure 4. Network meta-analysis results for Parkinson’s Disease Sleep Scale (PDSS). (A) Network plot; (B) ring inconsistencies (X-axis, effect size, 0 represents no effect; Y-axis, closed loop between interventions, the horizontal line range represents the 95% confidence interval; diamond represents point estimate); (C) forest plot (X-axis, effect size, with 0 representing no effect; Y-axis, comparison between interventions, with the red line range indicating the 95% confidence interval; diamonds representing pooled effect sizes); (D) the figure of Ranking probability (X-axis, ranking position (Rank), Y-axis, representing Cumulative Probability); (E) funnel plot (X-axis, effect size, Y-axis, standard error of effect size, and black dots represent the results of each study).

Figure 5. Network meta-analysis results for Hamilton Depression Scale (HAMD). (A) Network plot; (B) ring inconsistencies (X-axis, effect size, 0 represents no effect; Y-axis, closed loop between interventions, the horizontal line range represents the 95% confidence interval; diamond represents point estimate); (C) forest plot (X-axis, effect size, with 0 representing no effect; Y-axis, comparison between interventions, with the red line range indicating the 95% confidence interval; diamonds representing pooled effect sizes); (D) the figure of Ranking probability (X-axis, ranking position (Rank), Y-axis, representing Cumulative Probability); (E) funnel plot (X-axis, effect size, Y-axis, standard error of effect size, and black dots represent the results of each study).
3.4.1 rTMS for PSQI
A total of 14 RCTs evaluated the effects of rTMS on PSQI, encompassing four interventions-1 Hz rTMS, 5 Hz rTMS, 10 Hz rTMS, and conventional treatment (CT)-and forming a closed intervention loop. Loop inconsistency testing demonstrated good agreement across studies (IF = 1.03, 95% CI = 0.00 to 2.40) (Figure 3B), permitting use of a consistency model. Compared with CT, both 5 Hz (WMD = −1.88, 95% CI = −3.30 to −0.45) and 10 Hz rTMS (WMD = −2.03, 95% CI = −3.24 to −0.83) significantly improved PSQI, whereas 1 Hz rTMS (WMD = −0.88, 95% CI = −2.02 to 0.25) produced a non-significant reduction (Figure 3C); no pairwise differences among frequencies reached significance. SUCRA ranking indicated that 10 Hz rTMS had the highest probability of being the optimal intervention (SUCRA = 83.2%), followed by 5 Hz (76.6%), 1 Hz (38.0%) and CT (2.2%) (Figure 3D). Sensitivity analysis-excluding studies with sample sizes <10-yielded identical SUCRA orderings, reaffirming 10 Hz rTMS as the most effective. Pairwise comparisons displayed in forest plots likewise mirrored the overall findings (Supplementary Figure 5).
3.4.2 rTMS for PDSS
A total of eight RCTs evaluated the effect of rTMS on PDSS, comprising four interventions, 1 Hz rTMS, 5 Hz rTMS, 10 Hz rTMS, and conventional treatment (CT), which together formed a closed intervention loop. Loop inconsistency testing demonstrated good agreement (IF = 1.91, 95% CI = 0.00 to −5.39) (Figure 4B), permitting analysis via a consistency model. Compared with CT, both 5 Hz (WMD = 6.12, 95% CI = 2.42 to 9.83) and 10 Hz rTMS (WMD = 8.44, 95% CI = 4.84 to 12.04) produced significant improvements in PDSS scores, whereas 1 Hz rTMS (WMD = 2.20, 95% CI = −2.28 to 6.68) yielded a non-significant reduction. In head-to-head comparisons, 10 Hz outperformed 1 Hz (WMD = 6.24, 95% CI = 1.75 to 10.73), with no other pairwise differences reaching significance (Figure 4C). SUCRA ranking indicated that 10 Hz rTMS was the most likely optimal intervention (93.6%), followed by 5 Hz (69.7%), 1 Hz (31.2%), and CT (5.5%) (Figure 4D). No sensitivity analysis was performed for this outcome, as all included studies had sample sizes ≥10.
3.4.3 rTMS for HAMD
A total of 18 RCTs assessed rTMS effects on HAMD, using the same four interventions–1 Hz rTMS, 5 Hz rTMS, 10 Hz rTMS, and CT–which also formed a closed network. Loop inconsistency testing again showed good agreement (IF = 0.46, 95 CI = 0.00 to 1.86) (Figure 5B), justifying use of a consistency model. Versus CT, each rTMS frequency 1 Hz (WMD = −2.70, 95% CI = −4.67 to −0.74), 5 Hz (WMD = −2.60, 95% CI = −4.10 to −1.11) and 10 Hz (WMD = −4.28, 95% CI = −5.99 to −2.56) significantly reduced HAMD scores, with no significant differences in pairwise frequency comparisons (Figure 5C). SUCRA ranking identified 10 Hz as the best intervention (97.2%), followed by 5 Hz (58.5%), 1 Hz (44.3%), and CT (0.0%) (Figure 5D). Sensitivity analysis–excluding studies with sample sizes <10–yielded the same SUCRA order and mirrored the overall forest-plot results (Supplementary Figure 6).
3.5 Subgroup
Previous studies have demonstrated that varying the number of rTMS pulses may elicit dose-dependent remodeling of neuronal networks in PD patients (Anil et al., 2023). Accordingly, we stratified analyses into two subgroups based on total pulse count: 600-pulse and >600-pulse.
3.5.1 600 pulse subgroup
In the 600-pulse PSQI subgroup, three RCTs comparing 1 Hz and 5 Hz rTMS were pooled using a consistency model. Relative to conventional treatment (CT), 5 Hz rTMS significantly reduced PSQI scores (WMD = −1.72, 95% CI = −2.18 to −1.27), whereas 1 Hz rTMS was inferior to CT (WMD = 1.12, 95% CI = 0.39 to 1.85), likely reflecting the subgroup’s small sample size. A direct comparison confirmed superior PSQI improvement with 5 Hz versus 1 Hz (WMD = −2.84, 95% CI = −3.71 to −1.98). SUCRA ranking designated 5 Hz as the optimal intervention (SUCRA = 100.0%), followed by CT (49.9%) and 1 Hz (0.1%) (Supplementary Figure 1). Only one RCT has examined 600-pulse rTMS for PDSS, and literature on 600-pulse rTMS effects on HAMD is similarly limited; thus, subgroup analyses for these outcomes were not performed.
3.5.2 Subgroup with >600 pulses
In the >600-pulse PSQI subgroup, ten RCTs comparing 1 Hz and 10 Hz rTMS in PD patients were pooled using a consistency model after demonstrating good network agreement (p > 0.05). Both 1 Hz (WMD = −1.72, 95% CI = −2.87 to −0.58) and 10 Hz rTMS (WMD = −2.09, 95% CI = −3.51 to −0.68) yielded significant PSQI improvements versus conventional treatment (CT), with no significant difference observed between the two frequencies (WMD = −0.37, 95% CI = −2.00 to 1.26). SUCRA ranking placed 10 Hz first (83.3%), followed by 1 Hz (66.5%) and CT (0.2%) (Supplementary Figure 2).
In the >600-pulse PDSS subgroup, seven RCTs involving 1 Hz, 5 Hz and 10 Hz rTMS were analyzed under a consistency model (loop inconsistency p > 0.05). Both 5 Hz (WMD = 6.12, 95% CI = 1.79 to 10.44) and 10 Hz rTMS (WMD = 9.25, 95% CI = 5.43 to 13.8) significantly enhanced PDSS scores compared to CT, whereas 1 Hz (WMD = 0.55, 95% CI = −4.69 to 5.79) did not. Head-to-head comparisons showed 10 Hz to be superior to 1 Hz (WMD = 8.70, 95% CI = 2.61 to 14.79), with no other pairwise differences reaching significance. SUCRA ranked 10 Hz highest (95.1%), then 5 Hz (69.6%), 1 Hz (21.3%) and CT (14.1%) (Supplementary Figure 3).
In the >600-pulse HAMD subgroup, eleven RCTs assessing 1 Hz, 5 Hz and 10 Hz rTMS formed a closed network with good consistency (P > 0.05). All three frequencies significantly reduced HAMD scores versus CT (1 Hz WMD = −2.14, 95% CI = −2.88 to −1.40, 5 Hz WMD = −2.40, 95% CI = −2.95 to −1.85 and 10 Hz WMD = −3.97, 95% CI = −4.54 to −3.40). Pairwise analyses revealed that 10 Hz outperformed both 1 Hz (WMD = −1.83, 95% CI = −2.46 to −1.19) and 5 Hz (WMD = 1.57, 95% CI = −2.37 to −0.77), while the latter two did not differ significantly. SUCRA indicated 10 Hz as the optimal intervention (100.0%), followed by 5 Hz (56.9%), 1 Hz (43.1%) and CT (0.0%) (Supplementary Figure 4).
3.6 Publication bias
Publication bias, evaluated via funnel plots for all outcomes, was broadly symmetrical, with only a few studies lying outside the funnel and suggesting minimal bias (Figures 3E–5E).
3.7 Sensitivity analyses
After excluding studies with a PEDro score of less than 6, the research results remained unchanged (Supplementary Figures 7–9). There was only one study with an H-Y stage >3, and even after excluding it, the research results remained unchanged (Supplementary Figures 10, 11). This indicates that the results of this study are relatively stable and reliable.
3.8 Adverse reactions
Eight studies provided detailed accounts of adverse effects: 13 patients experienced transient headaches that resolved with rest and were able to complete the protocol, and six patients reported transient dizziness, which likewise subsided after resting (Supplementary Table 3).
4 Discussion
This study employed a NMA to evaluate and compare the effects of rTMS at various frequencies, combined with conventional therapy, on sleep disorders and depressive symptoms in patients with Parkinson’s disease. Compared with conventional therapy alone, all rTMS frequencies significantly improved PSQI, PDSS, and HAMD scores, with 10 Hz rTMS appearing to be the most effective for both sleep and mood. We then stratified stimulation by pulse count (600 pulses vs. >600 pulses). In the 600-pulse group, which did not include 10 Hz stimulation, 5 Hz rTMS yielded the greatest benefit; in the >600-pulse subgroup, 10 Hz rTMS produced the most pronounced improvements.
This study employed a NMA to assess and compare the effects of different rTMS frequencies, each combined with conventional therapy, on sleep disorders in PD patients. The findings demonstrated that, relative to conventional treatment alone, all rTMS frequencies significantly improved PSQI, PDSS and HAMD scores, with 10 Hz rTMS plus standard therapy emerging as the most effective intervention for each outcome. There are certain differences in the findings regarding the impact on sleep between this study and that of Cristini et al. (2025). Cristini’s research revealed that LF-rTMS can enhance subjective sleep quality in PD patients, yet the evidence for HF-rTMS improving sleep quality is insufficient. This discrepancy may stem from Cristini’s study not precisely categorizing HF-rTMS by frequency, potentially leading to interference from mixing different frequency groups. Additionally, the patients in the HF-rTMS group in that study had relatively mild sleep issues, which could have contributed to a ceiling effect. Regarding depression, a previous meta-analysis (Zhou et al., 2018) indicated that 5 Hz rTMS is most effective in alleviating depressive symptoms. Upon comparison, we found that the studies included in that meta-analysis were self-controlled before-and-after designs, and the number of included studies was limited. Currently, it is believed that abnormal discharges in the subthalamic nucleus (STN) of PD patients are transmitted through the cortical–striatal–thalamic circuit, leading to disruptions in the sleep–wake cycle. Repetitive TMS is one of the most widely applied neurostimulation modalities: high-frequency rTMS (HF-rTMS), defined as stimulation above 1 Hz has been shown to induce long-term excitatory effects (Valero-Cabré et al., 2017), whereas low-frequency rTMS (LF-rTMS), defined as 1 Hz or below, is expected to produce inhibitory effects and elicit long-term depression (Romero et al., 2002).
Low frequency rTMS reduces sleep fragmentation by attenuating abnormal beta oscillations (20–30 Hz) in the thalamus, subthalamic nucleus (STN) and motor cortex via long-term depression (LTD) (Chen et al., 1997). Simultaneous stimulation of the prefrontal cortex (PFC) increases δ-wave (1–4 Hz) power and prolongs slow-wave sleep. LF-rTMS also upregulates striatal dopamine D2-receptor expression, enhances dopaminergic signaling, and alleviates Parkinson’s disease-associated REM sleep behavior disorder (RBD) (Ahmed et al., 2012). Moreover, cortical rTMS promotes the release of dopamine and pineal melatonin, increases brain serotonin and norepinephrine levels, and elevates serum GABA–neurotransmitters critical to the sleep–wake cycle–thereby improving sleep quality and reducing daytime somnolence (Strafella et al., 2003; Feng et al., 2019). High-frequency (HF) rTMS activates the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) via long-term potentiation (LTP), inhibits the noradrenergic arousal system in the locus coeruleus (LC), and ameliorates excessive daytime sleepiness (Lefaucheur et al., 2020). Prior studies have shown that HF-rTMS over the parietal lobe enhances deep sleep and sleep efficiency while reducing nocturnal awakenings in PD patients, suggesting the parietal cortex as a key target for deepening subsequent sleep by decreasing Stage I and increasing Stage IV sleep (van Dijk et al., 2009). HF-rTMS also augments cortical excitability, improves cerebral blood flow, and promotes endogenous dopamine release, thereby modulating excitation within the direct and indirect striatal–pallidal pathways, which may further alleviate sleep disturbances (Chou et al., 2015; Qin et al., 2018). The superior efficacy of 10 Hz rTMS observed here may reflect dose-dependent neuroplastic changes: higher pulse counts strengthen neural network connectivity and induce sustained synaptic potentiation, enhancing neuromodulatory potential (Zhou et al., 2018; Anil et al., 2023). This dose dependency is supported by our subgroup analysis, which indicates that stimulation dosage differentially affects outcomes in PD patients.
In our network meta-analysis of depressive symptoms, hyporeactivity of the left DLPFC has been implicated in PD-related depression (Mottaghy et al., 2002). Clinical protocols therefore aim to increase left DLPFC excitability while inhibiting right DLPFC activity: LF-rTMS to the right DLPFC reduces cortical excitability, diminishing negative affect and trans-synaptically activating the hypoactive left DLPFC (Grimm et al., 2008). Conversely, HF-rTMS elicits release of dopamine, serotonin (5-HT), glutamate, and brain-derived neurotrophic factor (BDNF). Because depression in PD involves deficits in dopaminergic and serotonergic systems, rTMS may improve mood through dual-transmitter regulation (Strafella et al., 2001). HF-rTMS targeting the DLPFC also modulates prefrontal–limbic functional connectivity (e.g., amygdala, ACC) by enhancing local neuronal excitability, inhibiting aberrant default mode network (DMN) activity, and strengthening frontal regulation of limbic regions (Lefaucheur et al., 2020). Furthermore, the observed correlation between sleep quality and mood, namely, PD patients with poor sleep exhibit more severe depression than those with normal sleep, suggests that amelioration of sleep disturbances may contribute to improvements in depressive symptoms. Patients with neurodegenerative diseases often exhibit higher rates of depression than the general population (Tandberg et al., 1998). Dopaminergic dysfunction is hypothesized to underlie the strong association between poor sleep quality and depression severity in Parkinson’s disease (PD). In healthy individuals, sleep deprivation elicits a compensatory increase in central dopamine levels; however, PD-related dopamine deficits may impair this adaptive response, thereby exacerbating depressive symptoms (Kay et al., 2018). Moreover, improvements in HAMD anxiety scores have been positively correlated with PSQI improvements, suggesting that enhanced sleep quality is associated with reduced anxiety (Huang et al., 2018).
The use of dopaminergic drugs may also affect the efficacy of rTMS. Previous studies (Fierro et al., 2008) found that 10 Hz rTMS only enhances cortical inhibition during drug withdrawal in PD patients, whereas the improvement in cortical inhibition during medication use is comparable to that of the drugs themselves. All subjects included in this study were on dopaminergic drugs during the trial period. The reason for the divergence may be related to the large number of subjects included in this study–all of whom were randomized controlled trials–as well as differences in intervention methods and targets. Fierro et al. (2008) used 10 Hz, 500-pulse stimulation over the M1 region, while most studies in this review used 10 Hz, 1200-pulse stimulation, with the stimulation targets mostly being the DLPFC, which may also account for the differences in results. The stage of PD is another factor affecting the efficacy of rTMS. Flamez et al. (2016) found that LF-rTMS did not significantly improve motor function in PD patients, possibly because all subjects in their study were late-stage PD patients (H&Y ≥ 3). In this study, most subjects in the included literature were in H&Y stages 1–3 (only one was a late-stage patient; after sensitivity analysis, the results remained unchanged; see Supplementary Figures 10, 11), which also explains the differences between this study’s results and those of previous studies. Meanwhile, previous studies (Cong et al., 2022) have also shown that the degree of sleep disturbance and depression in PD patients is positively correlated with H&Y stage. Late-stage PD patients have extensive neurodegenerative lesions, and local stimulation may not be able to regulate distant pathological networks.
Regarding adverse events reported in this study, only a small number of subjects experienced transient dizziness, headache, or scalp numbness, which resolved after rest and allowed them to complete the trial. This indicates the safety of rTMS treatment for PD patients and supports its clinical application. The funnel plot results showed overall symmetry but with a small number of scatter points outside the funnel, so the findings should be interpreted with caution, and more high-quality studies are needed for future verification.
5 Limitation
Several limitations should be acknowledged. First, rTMS target regions and pulse counts varied across the included studies, limiting the generalizability of our findings. Second, previous studies (Chung et al., 2019) have indicated that high-level estrogen exposure during HF-rTMS stimulation can enhance the neuroplasticity effect of the prefrontal cortex, suggesting that gender may also influence stimulation outcomes. This study includes a mixed-gender sample from the literature, making subgroup analysis impossible. Third, the severity of sleep disturbance correlates positively with age in PD, yet all participants in the analyzed studies were over 60 years old, precluding age-stratified subgroup analyses. Therefore, future research can focus on more personalized designs for rTMS stimulation targets, pulse counts, gender, and age to provide references for clinical applications.
6 Conclusion
In summary, this analysis demonstrates the potential of different rTMS frequencies to ameliorate sleep disturbances and depressive symptoms in PD patients. Notably, 10 Hz rTMS emerged as the most effective intervention for both outcomes. These results provide clinicians and researchers with valuable guidance for managing non-motor symptoms in PD.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YX: Writing – review & editing, Writing – original draft, Data curation, Software. HW: Methodology, Investigation, Supervision, Writing – review & editing, Conceptualization. XH: Validation, Data curation, Methodology, Supervision, Conceptualization, Writing – review & editing. WS: Investigation, Conceptualization, Data curation, Software, Methodology, Writing – review & editing. YL: Validation, Writing – original draft, Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Guizhou Provincial Science and Technology Support Programme Project (NO:[2023]general179).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1623917/full#supplementary-material
References
Ahmed, M. A., Darwish, E. S., Khedr, E. M., El Serogy, Y. M., and Ali, A. M. (2012). Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J. Neurol. 259, 83–92. doi: 10.1007/s00415-011-6128-4
Anil, S., Lu, H., Rotter, S., and Vlachos, A. (2023). Repetitive transcranial magnetic stimulation (rTMS) triggers dose-dependent homeostatic rewiring in recurrent neuronal networks. PLoS Comput. Biol. 19:e1011027. doi: 10.1371/journal.pcbi.1011027
Barone, P., Antonini, A., Colosimo, C., Marconi, R., Morgante, L., Avarello, T. P., et al. (2009). The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 24, 1641–1649. doi: 10.1002/mds.22643
Brys, M., Fox, M. D., Agarwal, S., Biagioni, M., Dacpano, G., Kumar, P., et al. (2016). Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology 87, 1907–1915. doi: 10.1212/WNL.0000000000003279
Chahine, L. M., Amara, A. W., and Videnovic, A. (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med. Rev. 35, 33–50. doi: 10.1016/j.smrv.2016.08.001
Chail, A., Saini, R. K., Bhat, P. S., Srivastava, K., and Chauhan, V. (2018). Transcranial magnetic stimulation: A review of its evolution and current applications. Industrial Psychiatry J. 27, 172–180. doi: 10.4103/ipj.ipj_88_18
Chao, Z. (2020). Clinical study on low-frequency repetitive transcranial magnetic stimulation for the treatment of Parkinson’s disease. Jiangsu: Suzhou University.
Chen, J., Xu, P., Guo, X., and Zou, T. (2022). Comparative analysis of the effects of escitalopram, pramipexole, and transcranial magnetic stimulation on depression in patients with Parkinson disease: An open-label randomized controlled trial. Clin. Neuropharmacol. 45, 84–88. doi: 10.1097/WNF.0000000000000507
Chen, R., Classen, J., Gerloff, C., Celnik, P., Wassermann, E. M., Hallett, M., et al. (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–1403. doi: 10.1212/wnl.48.5.1398
Chinese Journal of Practical Nervous Diseases. (2021). Effect of high frequency repetitive transcranial magnetic stimulation in left dorsolateral prefrontal cortex on patients with Parkinson’s disease. Chinese J. Pract. Nervous Dis. 24, 686–691. doi: 10.12083/SYSJ.2021.14.004
Chou, Y. H., Hickey, P. T., Sundman, M., Song, A. W., and Chen, N. K. (2015). Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 72, 432–440. doi: 10.1001/jamaneurol.2014.4380
Chung, S. W., Thomson, C. J., Lee, S., Worsley, R. N., Rogasch, N. C., Kulkarni, J., et al. (2019). The influence of endogenous estrogen on high-frequency prefrontal transcranial magnetic stimulation. Brain Stimul. 12, 1271–1279. doi: 10.1016/j.brs.2019.05.007
Cirillo, G., Di Pino, G., Capone, F., Ranieri, F., Florio, L., Todisco, V., et al. (2017). Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 10, 1–18. doi: 10.1016/j.brs.2016.11.009
Cong, S., Xiang, C., Zhang, S., Zhang, T., Wang, H., and Cong, S. (2022). Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci. Biobehav. Rev. 141:104749. doi: 10.1016/j.neubiorev.2022.104749
Cristini, J., Medina-Rincon, A., Van Roy, A., Seo, F., Moncion, K., Carrier, J., et al. (2025). Non-invasive brain stimulation to enhance sleep quality and architecture in Parkinson’s disease: A systematic review and Bayesian network meta-analysis. Sleep Med. Rev. 82:102117. doi: 10.1016/j.smrv.2025.102117
Dai Wei-zheng, W. M., Fu Mao-lin, Y. Y., and He Wen-qin, R. Z. (2021). Observation on clinical effects of pramipexole combined with high-frequency repetitive transcranial magnetic stimulation (rTMS) in the treatment of patients suffered from early Parkinson’s disease (PD)and depression. Military Med. J. Southeast China 23, 592–595. doi: 10.3969/j.issn.1672-271X.2021.06.007
Ding, L. T. X., and Xu, C. H. F. (2018). Effect of repetitive transcranial magnetic stimulation on plasma glu and gaba levels in patients with Parkinson’s sleep disorder. China Foreign Med. Treatment 37:22. doi: 10.16662/j.cnki.1674-0742.2018.26.007
Dorsey, E. R., Sherer, T., Okun, M. S., and Bloem, B. R. (2018). The emerging evidence of the parkinson pandemic. J. Parkinson’s Dis. 8, S3–S8. doi: 10.3233/JPD-181474
Feng, J., Zhang, Q., Zhang, C., Wen, Z., and Zhou, X. (2019). The Effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain Behav. 9:e01206. doi: 10.1002/brb3.1206
Fengju, Z., Xiaoxue, W., and Xinxin, L. (2017). The therapeutic effect of repetitive transcranial magnetic stimulation combined with cognitive-behavioral therapy on non motor symptoms of Parkinson’s disease. Chinese J. Pract. Nervous Dis. 20, 61–63. doi: 10.3969/j.issn.1673-5110.2017.09.024
Fierro, B., Brighina, F., D’Amelio, M., Daniele, O., Lupo, I., Ragonese, P., et al. (2008). Motor intracortical inhibition in PD: L-DOPA modulation of high-frequency rTMS effects. Exp. Brain Res. 184, 521–528. doi: 10.1007/s00221-007-1121-y
Flamez, A., Cordenier, A., De Raedt, S., Michiels, V., Smetcoren, S., Van Merhaegen-Wieleman, A., et al. (2016). Bilateral low frequency rTMS of the primary motor cortex may not be a suitable treatment for levodopa-induced dyskinesias in late stage Parkinson’s disease. Parkinsonism Relat. Disord. 22, 54–61. doi: 10.1016/j.parkreldis.2015.11.009
French, I. T., and Muthusamy, K. A. (2016). A review of sleep and its disorders in patients with Parkinson’s disease in relation to various brain structures. Front. Aging Neurosci. 8:114. doi: 10.3389/fnagi.2016.00114
GBD 2016 Neurology Collaborators. (2019). Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 18, 459–480. doi: 10.1016/S1474-4422(18)30499-X
Grimm, S., Beck, J., Schuepbach, D., Hell, D., Boesiger, P., Bermpohl, F., et al. (2008). Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 63, 369–376. doi: 10.1016/j.biopsych.2007.05.033
Hariz, G. M., and Forsgren, L. (2011). Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol. Scand. 123, 20–27. doi: 10.1111/j.1600-0404.2010.01344.x
Hendricks, R. M., and Khasawneh, M. T. (2021). A systematic review of Parkinsons disease cluster analysis research. Aging Dis. 12, 1567–1586. doi: 10.14336/AD.2021.0519
Hu Xiaoying, L. H., Zhang Tong, F. C., and Ruiping, W. (2023). Clinical observation of bilateral dorsolateral prefrontal rTMS stimulation in patients with Parkinson’s disease. Chinese J. Pract. Nervous Dis. 26, 443–447. doi: 10.12083/SYSJ.221518
Hua, S., Zeshuai, W., Fang, Z., and Xinzhou, J. (2019). A research of repetitive transcranial magnetic stimulation and escitalopram in the treatment of patients with Par-kinson’s disease and depression. Chinese J. Pract. Nervous Dis. 22, 1819–1825. doi: 10.12083/SYSJ.2019.16.305
Huang, Z., Li, Y., Bianchi, M. T., Zhan, S., Jiang, F., Li, N., et al. (2018). Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study. Brain Stimul. 11, 1103–1109. doi: 10.1016/j.brs.2018.05.016
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Jia-Jin, W. (2021). The effect of low-frequency repetitive transcranial magnetic stimulation on the clinical symptoms and sleep structure of Parkinson’s disease in the dorsolateral prefrontal cortex of the right side. Jiangsu: Suzhou University.
Jiang, S., Zhan, C., He, P., Feng, S., Gao, Y., Zhao, J., et al. (2023). Neuronavigated repetitive transcranial magnetic stimulation improves depression, anxiety and motor symptoms in Parkinson’s disease. Heliyon 9:e18364. doi: 10.1016/j.heliyon.2023.e18364
Jiménez-Urbieta, H., Gago, B., de la Riva, P., Delgado-Alvarado, M., Marin, C., and Rodriguez-Oroz, M. C. (2015). Dyskinesias and impulse control disorders in Parkinson’s disease: From pathogenesis to potential therapeutic approaches. Neurosci. Biobehav. Rev. 56, 294–314. doi: 10.1016/j.neubiorev.2015.07.010
Kay, D. B., Tanner, J. J., and Bowers, D. (2018). Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 8:e00967. doi: 10.1002/brb3.967
Kirmani, B. F., Shapiro, L. A., and Shetty, A. K. (2021). Neurological and neurodegenerative disorders: Novel concepts and treatment. Aging Dis. 12, 950–953. doi: 10.14336/AD.2021.0530
Lai Jinghui, C. Y., Xia Min, Q. L., Wen Jing, Y. L., and Renxiong, Z. (2020). Effects of high-frequency Rtms on limb movement and sleep in patients with Parkinson’s disease. World J. Sleep Med. 7, 1861–1863. doi: 10.3969/j.issn.2095-7130.2020.11.001
Langston, J. W. (2006). The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann. Neurol. 59, 591–596. doi: 10.1002/ana.20834
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., et al. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin. Neurophysiol. 131, 474–528. doi: 10.1016/j.clinph.2019.11.002
Lei Meng, L. H., and Jichao, G. (2024). Impact of high -frequency repetitive transcr anial magnetic stimulation combined with drug therapy on autonomic nervous function, cognitive function and sleep quality in patients with Parkinson’s disease. Clin. Med. Eng. 31, 1029–1030. doi: 10.3969/j.issn.1674-4659.2024.09.1029
Li, X. (2023). Application of repetitive transcranial magnetic stimulation with different frequencies in the treatment of Parkinson’s sleep disorder. Med. Sci. 45, 232–235. doi: 10.3760/cma.j.issn.0254-1424.2023.03.008
Maggi, G., Trojano, L., Barone, P., and Santangelo, G. (2021). Sleep disorders and cognitive dysfunctions in Parkinson’s disease: A meta-analytic study. Neuropsychol. Rev. 31, 643–682. doi: 10.1007/s11065-020-09473-1
Malhotra, R. K. (2022). Neurodegenerative disorders and sleep. Sleep Med. Clin. 17, 307–314. doi: 10.1016/j.jsmc.2022.02.009
Moore, R. Y., Whone, A. L., and Brooks, D. J. (2008). Extrastriatal monoamine neuron function in Parkinson’s disease: An 18F-dopa PET study. Neurobiol. Dis. 29, 381–390. doi: 10.1016/j.nbd.2007.09.004
Mottaghy, F. M., Keller, C. E., Gangitano, M., Ly, J., Thall, M., Parker, J. A., et al. (2002). Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 115, 1–14. doi: 10.1016/s0925-4927(02)00032-x
Ouyang Gui-lan, M. H. Y. H. (2022). Clinical study of repetitive transcranial magnetic stimulation in the treatment of neuropathic pain associated with Parkinson’s disease. J. Gannan Med. Univer. 42, 805–808. doi: 10.3969/j.issn.1001-5779.2022.08.004
Qin Xi-xiang, L. W., Mai Yong-jia, X. Y., and Zi-qing, M. O. (2024). Clinical efficacy of repetitive transcranial magnetic stimulation and pramipexole in mid and late Parkinson’s disease with depression. J. Guangdong Med. Univer. 42, 186–189. doi: 10.3969/j.issn.1005-4057.2024.02.013
Qin, B., Chen, H., Gao, W., Zhao, L. B., Zhao, M. J., Qin, H. X., et al. (2018). Effectiveness of high-frequency repetitive transcranial magnetic stimulation in patients with depression and Parkinson’s disease: A meta-analysis of randomized, controlled clinical trials. Neuropsychiatr. Dis. Treat. 14, 273–284. doi: 10.2147/NDT.S156695
Qingping, S., Lianhong, H., Wei, X., Shuying, G., Cute, C., Enyu, C., et al. (2022). Comparison of clinical effects of low frequency and high frequency repetitive transcranial magnetic stimulation in the treatment of patients with Parkinson disease. Chinese Foreign Med. Res. 20, 14–17. doi: 10.14033/j.cnki.cfmr.2022.22.004
Rana, A. Q., Qureshi, A., Shamli Oghli, Y., Saqib, Y., Mohammed, B., Sarfraz, Z., et al. (2018). Decreased sleep quality in Parkinson’s patients is associated with higher anxiety and depression prevalence and severity, and correlates with pain intensity and quality. Neurol. Res. 40, 696–701. doi: 10.1080/01616412.2018.1462880
Rocca, W. A. (2018). The burden of Parkinson’s disease: A worldwide perspective. Lancet Neurol. 17, 928–929. doi: 10.1016/S1474-4422(18)30355-7
Romero, J. R., Anschel, D., Sparing, R., Gangitano, M., and Pascual-Leone, A. (2002). Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin. Neurophysiol. 113, 101–107. doi: 10.1016/s1388-2457(01)00693-9
Seppi, K., Ray Chaudhuri, K., Coelho, M., Fox, S. H., Katzenschlager, R., Perez Lloret, S., et al. (2019). Update on treatments for nonmotor symptoms of Parkinson’s disease-An evidence-based medicine review. Mov. Disord. 34, 180–198. doi: 10.1002/mds.27602
Shaheen, H. A., Gomaa, M., Maarouf, M. M., and Daker, L. I. (2023). Exploring the effect of transcranial magnetic stimulation on quality of sleep in Parkinson’s disease. Egypt. J. Neurol. Psychiatr. Neurosurg. 59:173. doi: 10.1186/s41983-023-00771-y
Shin, H. W., Youn, Y. C., Chung, S. J., and Sohn, Y. H. (2016). Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson’s disease. J. Neurol. 263, 1442–1448. doi: 10.1007/s00415-016-8160-x
Simonetta-Moreau, M. (2014). Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann. Phys. Rehabil. Med. 57, 530–542. doi: 10.1016/j.rehab.2014.08.003
Stefani, A., and Högl, B. (2020). Sleep in Parkinson’s disease. Neuropsychopharmacology 45, 121–128. doi: 10.1038/s41386-019-0448-y
Sterne, J., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898
Strafella, A. P., Paus, T., Barrett, J., and Dagher, A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001
Strafella, A. P., Paus, T., Fraraccio, M., and Dagher, A. (2003). Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126, 2609–2615. doi: 10.1093/brain/awg268
Tandberg, E., Larsen, J. P., and Karlsen, K. (1998). A community-based study of sleep disorders in patients with Parkinson’s disease. Mov. Disord. 13, 895–899. doi: 10.1002/mds.870130606
Valero-Cabré, A., Amengual, J. L., Stengel, C., Pascual-Leone, A., and Coubard, O. A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 83, 381–404. doi: 10.1016/j.neubiorev.2017.10.006
van der Hoek, T. C., Bus, B. A., Matui, P., van der Marck, M. A., Esselink, R. A., and Tendolkar, I. (2011). Prevalence of depression in Parkinson’s disease: Effects of disease stage, motor subtype and gender. J. Neurol. Sci. 310, 220–224. doi: 10.1016/j.jns.2011.07.007
van Dijk, K. D., Møst, E. I., Van Someren, E. J., Berendse, H. W., and van der Werf, Y. D. (2009). Beneficial effect of transcranial magnetic stimulation on sleep in Parkinson’s disease. Mov. Disord. 24, 878–884. doi: 10.1002/mds.22462
Wang Dong, W. J., and Yuanyu, R. (2021). Clinical efficacy of high-frequency repetitive transcranial magnetic stimulation in patients with Parkinson’s disease complicated with depression. J. Int. Psychiatry 48, 65–69. doi: 10.13479/j.cnki.jip.2021.01.019
Wang Yajun, L. X. (2021). Effect of pramipexole combined with rtms on sleep disturbance in patients with parkinson’s disease. J. Shandong Sec. Med. Univer. 43, 172–174. doi: 10.16846/j.issn.1004-3101.2021.03.004
Wenjing, Z., Kun, N., Yuhu, Z., Huigen, H., Shaofang, L., and Ruiping, G. (2014). Observation of the effect of repetitive transcranial magnetic stimulation in treating insomnia patients with Parkinson’s disease. J. Nurs. 21, 28–30. doi: CNKI:SUN:NFHL.0.2014-23-010
Wu, J., Zhuang, S., Zhang, X., Wang, L., Ma, X., Jin, H., et al. (2024). Objective sleep enhancement in Parkinson’s disease: A sham-controlled trial of low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex. Parkinsonism Relat. Disord. 126:107050. doi: 10.1016/j.parkreldis.2024.107050
Xia, Y., Xu, Y., Li, Y., Lu, Y., and Wang, Z. (2022). Comparative efficacy of different repetitive transcranial magnetic stimulation protocols for stroke: A network meta-analysis. Front. Neurol. 13:918786. doi: 10.3389/fneur.2022.918786
Xue, L., Siyuan, Shaopu, W., Qi, G., Dongsheng, L., Weiwei, Q., et al. (2023). The effect of repetitive transcranial magnetic stimulation on sleep and plasma orexin-A levels in patients with advanced Parkinson’s disease. Chin. J. Phys. Med. Rehabil. 45, 232–235. doi: 10.3760/cma.j.issn.0254-1424.2023.03.008
Yeung, E., and Cavanna, A. E. (2014). Sleep attacks in patients with parkinson’s disease on dopaminergic medications: A systematic review. Mov. Disord. Clin. Pract. 1, 307–316. doi: 10.1002/mdc3.12063
Yu Wen-wen, and Hai-rong, S. (2017). Clinical investigation of repetitive transcranial magnetic stimulation on treating depression and sleep disorder in patients with Parkinson’s disease in early stage. J. Clin. Neurol. 30, 341–345. doi: 10.3969/j.issn.1004-1648.2017.05.007
Yu Xiaolan, C. H. (2022). Clinical effect and prognosis analysis of repeated transcranial Mag-netic stimulation in the treatment of patients with Parkinson’s disease and insomnia. China Foreign Med. Treatment 41, 20–24. doi: 10.16662/j.cnki.1674-0742.2022.04.020
Zhang, K., and Sha, L. (2022). Effectiveness of high-frequency repetitive transcranial magnetic stimulation on the depression, anxiety and quality of life of Parkinson disease patients. Acta Academiae Medicinae Xuzhou 42, 885–888. doi: 10.3969/j.issn.2096-3882.2022.12.005
Zhang, X., Jing, C., and Xueling, Z. (2025). Clinical efficacy of repetitive transcranial magnetic stimulation combined with Entacapone-Levodopa-carbidopa on motor and non-motor symptoms in Parkinson’s disease: A randomized controlled trial. Chin. General Pract. 28, 581–593. doi: 10.12114/j.issn.1007-9572.2024.0411
Zhao Rong, Y. C. (2023). Clinical efficacy of high-low frequency interactive rTMS in the treatment of mid-stage Parkinson’s disease. J. Yan’an Univer. 21, 59–63. doi: 10.19893/j.cnki.ydyxb.2023-0076
Zhao, N., Yang, Y., Zhang, L., Zhang, Q., Balbuena, L., Ungvari, G. S., et al. (2021). Quality of life in Parkinson’s disease: A systematic review and meta-analysis of comparative studies. CNS Neurosci. Ther. 27, 270–279. doi: 10.1111/cns.13549
Zhou, L., Guo, Z., Xing, G., Peng, H., Cai, M., Chen, H., et al. (2018). Antidepressant effects of repetitive transcranial magnetic stimulation over prefrontal cortex of Parkinson’s disease patients with depression: A meta-analysis. Front. Psychiatry 9:769. doi: 10.3389/fpsyt.2018.00769
Zhuang, S., Wang, F. Y., Gu, X., Wu, J. J., Mao, C. J., Gui, H., et al. (2020). Low-frequency repetitive transcranial magnetic stimulation over right dorsolateral prefrontal cortex in Parkinson’s disease. Parkinson’s Dis. 2020:7295414. doi: 10.1155/2020/7295414
Keywords: Parkinson, sleep disorder, depression, repetitive transcranial magnetic stimulation, meta-analysis
Citation: Xia Y, Wan H, Hu X, Sun W and Li Y (2025) Effects of different frequencies of repetitive transcranial magnetic stimulation on sleep disorders and depression in patients with Parkinson’s disease: a systematic review and network meta-analysis. Front. Aging Neurosci. 17:1623917. doi: 10.3389/fnagi.2025.1623917
Received: 07 May 2025; Accepted: 25 August 2025;
Published: 08 September 2025.
Edited by:
Suwarna Chakraborty, Johns Hopkins University, United StatesReviewed by:
Koji Ishikuro, Toyama University Hospital, JapanValentino Racki, University of Rijeka, Croatia
Zhen Ni, National Institutes of Health (NIH), United States
Copyright © 2025 Xia, Wan, Hu, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Li, eWpsMjAyMTAyMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yuan Xia
Yuan Xia Haili Wan
Haili Wan Xin Hu1†
Xin Hu1† Yongjie Li
Yongjie Li