- 1Institute of Physical Education, Xinxiang Medical University, Xinxiang, China
- 2Key Laboratory of Movement Disorders, The Third Affiliated Hospital of Xinxiang Medical University, Xinxiang, China
- 3Department of Neurology, The Third Affiliated Hospital of Xinxiang Medical University, Xinxiang, China
- 4Department of Vascular Surgery, The First Affiliated Hospital of Xinxiang Medical University, Weihui, China
- 5Institute of Rehabilitation, Xinxiang Medical University, Xinxiang, China
Background: Excessive daytime sleepiness (EDS), which is common in Parkinson’s disease (PD), has been reported to exacerbate gait disturbance in patients with PD, but there is a lack of objective assessment, as well as an unknown specific mechanism. The purpose of our study is to explore the relationship between EDS and gait parameters.
Methods: Sixty-one patients with PD were recruited and divided into the EDS group (n = 29) and the non-EDS group (n = 32) based on the scores of the Epworth Sleepiness Scale (ESS). The gait metrics of the two groups were then assessed by wearable devices and compared under various walking scenarios.
Results: Compared with the non-EDS group, the EDS group showed significantly shorter step lengths and stride lengths, slower walk speed and gait speed, reduced shank-max forward swing and sagittal angular velocity, and increased phase coordination indices and mean duration of turns. Pearson correlation analysis revealed a significant association between ESS scores and various gait parameters. Furthermore, multiple linear regression analysis confirmed that EDS is an independent factor influencing gait in patients with PD.
Conclusion: EDS was independently associated with gait disturbances in patients with PD, suggesting that EDS symptoms warrant serious attention in clinical practice.
1 Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder that predominantly affects the elderly. It is characterized by motor symptoms, such as bradykinesia, resting tremor, and abnormal gait, and non-motor symptoms, including mood disorders, insomnia, autonomic dysfunctions, and cognitive impairments (Tanner and Ostrem, 2024). Notably, up to 50% of PD patients experience excessive daytime sleepiness (EDS; Abbott et al., 2005), which is often associated with disease progression and dopaminergic drugs, especially dopaminoagonists (Montastruc et al., 2001). It refers to the significant episodes during the day when patients struggle to remain awake and alert, resulting in an uncontrollable need for sleep or inadvertent lapses into sleep (Sateia, 2014). Furthermore, gait disruption is one of the most prevalent motor complaints among PD patients, which can be exacerbated by EDS (Höglund et al., 2015; Chen et al., 2025). Previous studies on the relationship between EDS and gait have used scales to assess gait. Currently, none of the objective research focuses on the relationship between EDS and gait abnormalities. Recent advances in wearable sensor technology have enabled researchers to measure various aspects of gait, including speed and movement patterns (Liu et al., 2022). This makes it possible to objectively assess and record gait impairment in PD patients (Pulliam et al., 2018; Hinchliffe et al., 2024; Borzì et al., 2025).
In this study, a wearable device equipped with inertial sensors was employed to measure the temporal and spatial gait characteristics of PD patients with and without EDS during the Timed Up and Go (TUG) paradigm and 5-meter straight walking paradigm, aiming at investigating the relationship between EDS and gait parameters.
2 Materials and methods
2.1 Participants
Sixty-one patients with PD were recruited at the outpatient clinic of the Department of Neurology at the Third Affiliated Hospital of Xinxiang Medical University between April 1, 2023, and February 1, 2025. The diagnosis was made according to the MDS criteria for PD (Postuma et al., 2015). The patients with PD included were those at the Hoehn-Yahr (HY) stage ranging from 1 to 2.5. All participants provided written informed consent prior to enrollment in the study. Patients who cannot complete the gait test and those with secondary Parkinsonism syndrome or other superimposed syndromes will be excluded. The study conforms to the ethical guidelines set forth by the Declaration of Helsinki. The study was authorized by the Ethics Committee of the Third Affiliated Hospital of Xinxiang Medical University (approval number K2022-072-01).
2.2 Demographic information and clinical evaluations
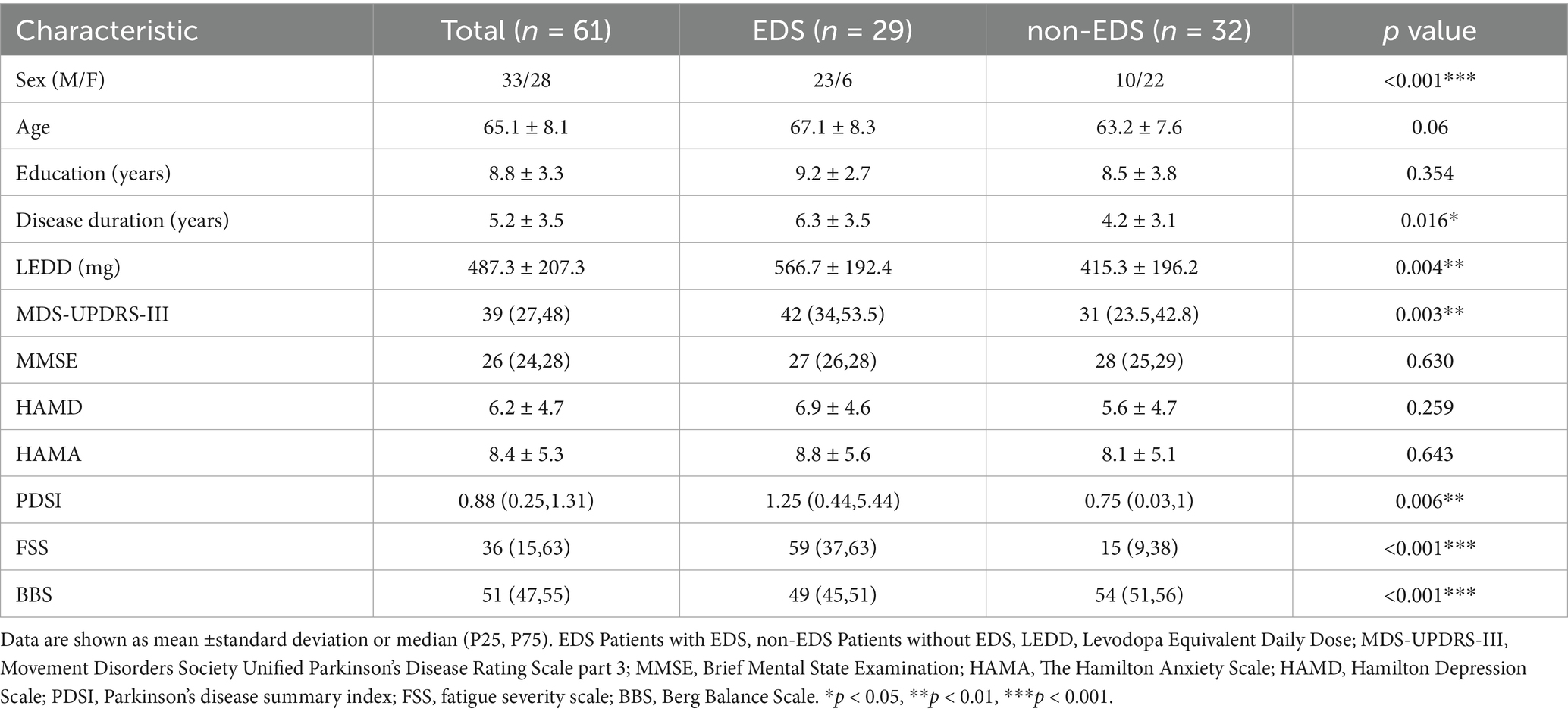
The demographic data collected included gender, age, height, education, levodopa equivalent daily dose (LEDD), disease duration, past illness, and surgical history. The severity of motor symptoms was evaluated by the MDS-Unified Parkinson’s Disease Rating Scale Part 3 (MDS-UPDRS-III). The severity of EDS was assessed by the Epworth Sleepiness Scale (ESS), ranging from 0 to 24, with higher scores indicating more severe EDS. EDS patients are defined as patients with an ESS score of more than 10 points (Johns, 1991). The psychological conditions and cognitive functions of the patients were assessed by the Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD) and Minimum Mental State Examination (MMSE), respectively. The patients’ quality of life (QoL) was evaluated by the Parkinson’s Disease Questionnaire-39 (PDQ-39), and the QoL burden was reflected by the PDQ-39 summary index (PDSI) (Jenkinson et al., 1997). The fatigue level was evaluated by the fatigue severity scale (FSS). The balance was evaluated by the Berg Balance Scale (BBS).
2.3 Gait assessments
Gait analysis was performed using the GYENNO MATRIX Wearable Movement and Gait Quantitative Evaluation System, as previously validated (Cai et al., 2023). Sensors are mounted on 10 regions of the body, specifically the chest, waist, left and right wrists, left and right thighs, left and right calves, and left and right feet, and are employed to collect motion data, including trajectories, accelerations, and angular velocities, from these regions, as well as for the detection of the balance index in the walking state: Phase coordination index (PCI; Plotnik et al., 2007; Plotnik et al., 2009). This system is helpful for clinicians to assess movement with objective data. This system has two detecting modes: TUG and 5-meter straight walking. Participants were instructed to walk at a normal speed during the TUG test and at their maximum walking speed for the 5-meter straight walking test, which were used to represent both normal and rapid gait patterns in daily life. These gait measurements have been widely used in previous studies, and presented well efficiency (Gildner et al., 2019; Wang and Zou, 2022). Gait measurements were carried out during the “on-period,” and assessors ensured the safety of participants while they completed the gait tasks.
2.4 Statistical analysis
Continuous variables conforming to a normal distribution were presented as mean ± standard deviation (SD), while non-normally distributed variables were characterized by median values accompanied by interquartile range (IQR). Continuous variables were analysed between groups with Student’s t-test for parametric data and Mann–Whitney U test for nonparametric data, determined through distribution normality assessments. Categorical variables were evaluated using chi-square or Fisher’s exact tests. To account for multiple testing, two-sided p values were adjusted using the Benjamini-Hochberg (B/H) method to control the false discovery rate (FDR). An association was considered statistically significant if the corresponding B/H-adjusted p value was less than 0.05, indicating an FDR of 5%. The relationship between ESS ratings and gait metrics was examined by Pearson correlation. In the multiple linear regression analysis, the EDS (categorical variable) was set as the independent variable and the gait parameters as the dependent variable, adjusting for the factors of age, sex, disease duration, LEDD and FSS scores. The difference was statistically significant when p < 0.05. The statistical analysis was performed with IBM SPSS Statistics 27.0.
3 Results
3.1 Demographic information and clinical characteristics
Twenty-nine of the 61 patients with PD (47.5%) were classsified into the EDS group. No statistically significant difference was found in age and education years between the two groups. In our study, males were more likely to have EDS which is also in accordance with previous reports (Feng et al., 2021). The patients in the EDS group had a longer duration of the disease, and took more medication than the non-EDS group. Moerover, the MDS-UPDRS-III score, PDSI score, FSS score, and BBS score of patients in the EDS group were significantly higher than those in the non-EDS group (Table 1).
3.2 Gait parameters in different walking paradigms
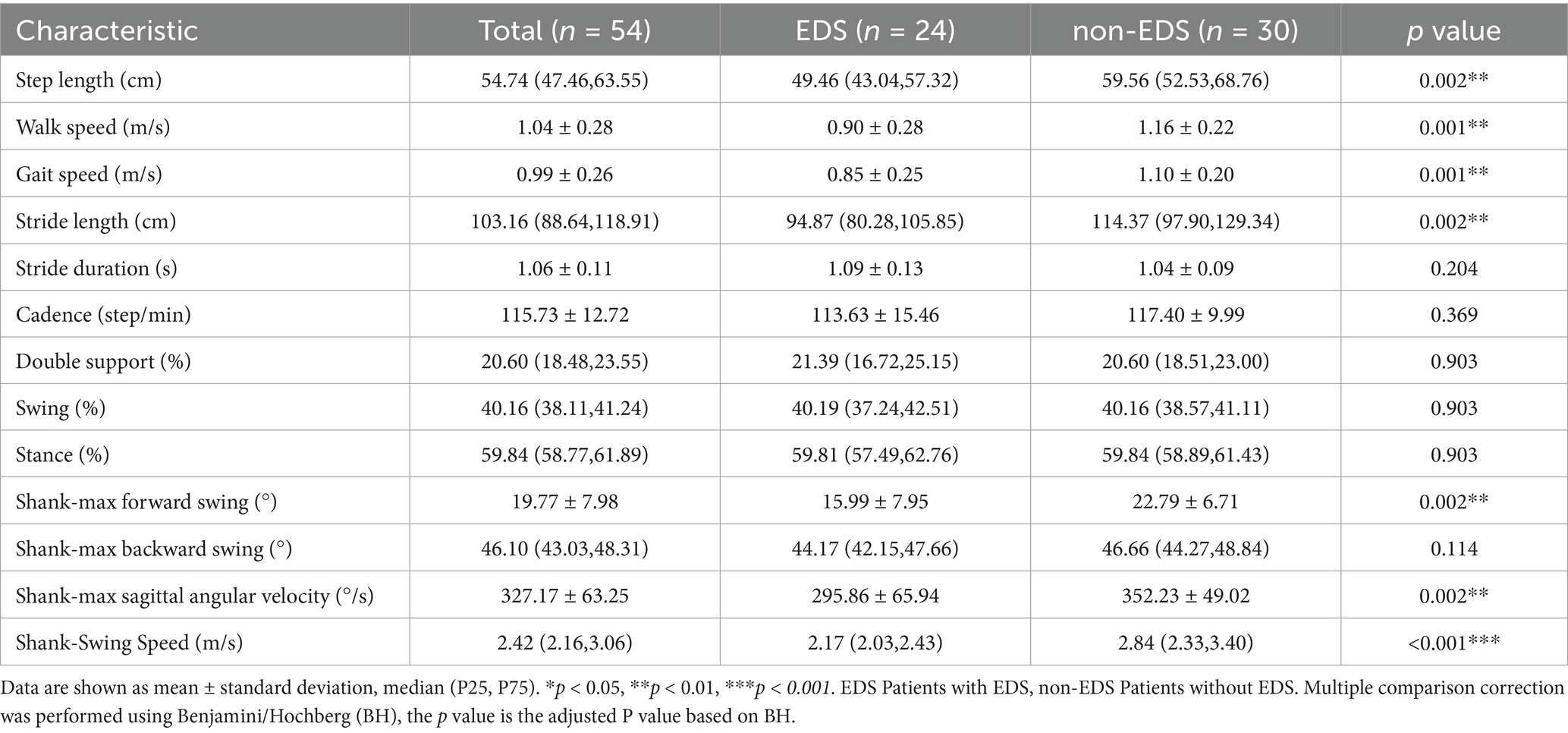
In the task of TUG, the patients in the EDS group had shorter step length and stride length, slower walk speed and more reduced shank-max forward swing, shank-max sagittal angular velocity and mean angular velocity, compared to non-EDS group (Table 2 and Figure 1).
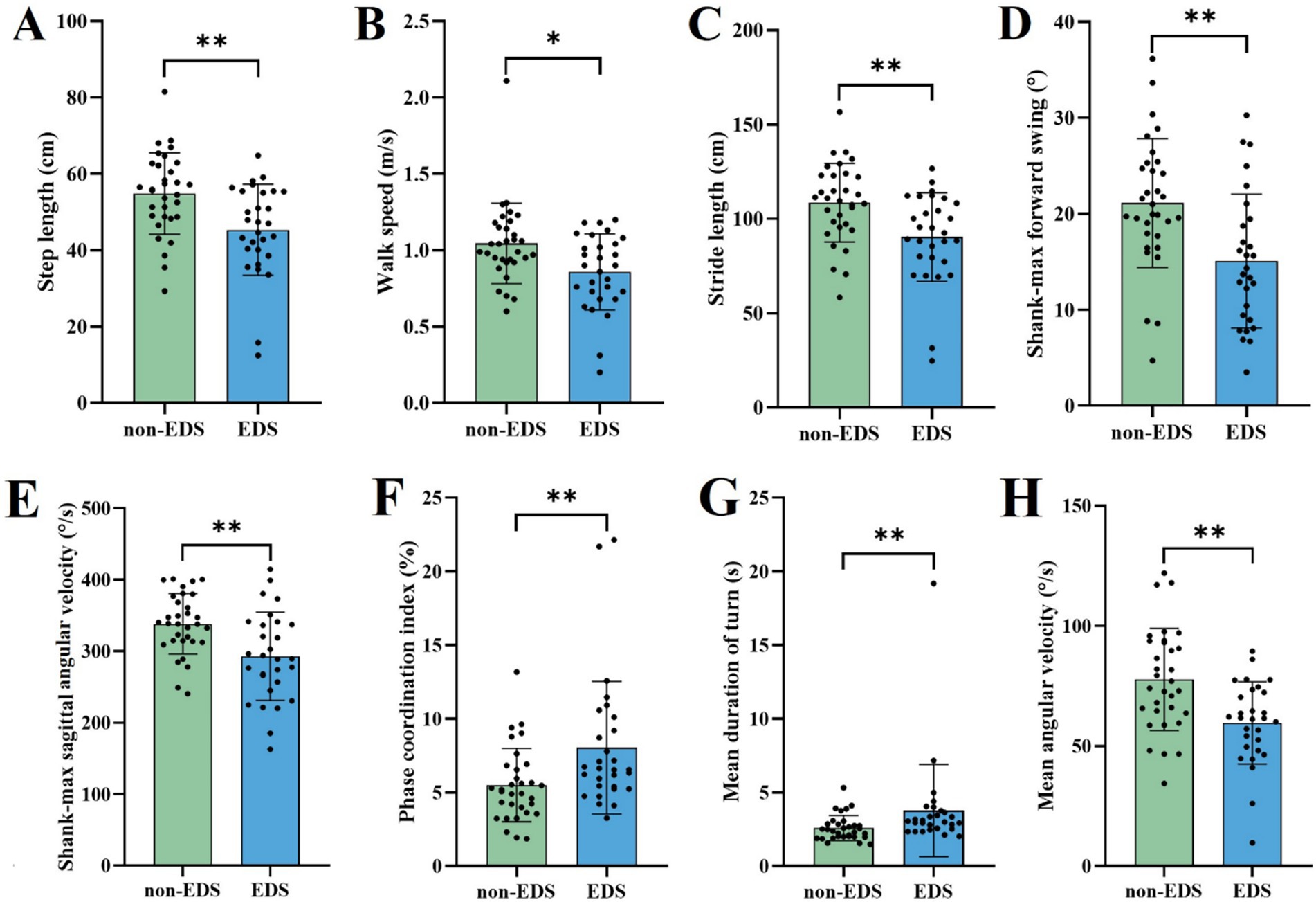
Figure 1. Comparison of gait parameters with significant differences in the TUG between different EDS states. EDS Patients with EDS, non-EDS Patients without EDS. *p < 0.05, **p < 0.01, ***p < 0.001. Multiple comparison correction was performed using Benjamini/Hochberg (BH).
In the 5-meter straight walking, EDS group had shorter step length, stride length, smaller shank-max forward swing, shank-max sagittal angular velocity and shank—swing speed compared to non-EDS group, consistent with the TUG paradigm (Table 3 and Figure 2).

Figure 2. Comparison of gait parameters with significant differences in the 5-meter straight walking between different EDS states. EDS Patients with EDS, non-EDS Patients without EDS. *p < 0.05, **p < 0.01, ***p < 0.001. Multiple comparison correction was performed using Benjamini/Hochberg (BH).
3.3 The correlation of EDS with gait parameters
There was a significant correlation between the TUG gait parameters and the 5-meter straight walking parameters and ESS scores in PD patients. In TUG, ESS scores were negatively correlated with step length (r = −0.26, p < 0.05), stride length (r = −0.26, p < 0.05), shank-max forward swing (r = −0.341, p < 0.01), shank-max sagittal angular velocity (r = −0.273, p < 0.05), and mean angular velocity (r = −0.358, p < 0.01) and positively correlated with phase coordination index (r = 0.253, p < 0.05). In 5-meter straight walking, the ESS scores were also associated with step length (r = −0.319, p < 0.05), walk speed (r = −0.355, p < 0. 01), gait speed (r = −0.375, p < 0.01), stride length (r = −0.326, p < 0. 05), shank-max forward swing (r = −0.331, p < 0.05), shank-max sagittal angular velocity (r = −0.353, p < 0.01), and shank-swing speed (r = −0.431, p < 0.01).
3.4 The multiple linear regression analysis of EDS and gait parameters
To investigate the relationship between EDS and individual gait parameters and control for the effects of confounding factors (including age, sex, disease duration, LEDD, and FSS score), multiple linear regression analysis were performed in this study. The unadjusted crude model showed that EDS had a significant effect on step length (B = −9.507, p = 0.002), walk speed (B = −0.188, p = 0.006) and stride length (B = −18.201, p = 0.002)in TUG, and step length (B = −12.266, p < 0.001), walk speed (B = −0.265, p < 0.001), and stride length (B = −22.681, p < 0.001) in the 5-meter straight walking, were all significant. Furthermore, after adjusting for age, disease duration, gender, LEDD and FSS score, EDS still had a significant effect on step length (B = −10.417, p = 0.009), walk speed (B = −0.232, p = 0.01) and stride length (B = −20.162, p = 0.01) in TUG, and step length (B = −12.195, p = 0.007), walk speed (B = −0.219, p = 0.018) and stride length (B = −21.858, p = 0.008) in the 5-meter straight walking, remained significant (Table 4).

Table 4. Multiple linear regression analysis of EDS and gait parameters before and after model adjustment.
4 Discussion
In this study, nearly half of the patients with PD exhibited EDS, and male patients and those with a longer disease duration were more likely to present with EDS. These results are consistent with previous reports (Mengdie et al., 2022; Chahine et al., 2017; Liu et al., 2021). The LEDD of patients in the EDS group was significantly higher than patients in the non-EDS group, which is common in reports related to EDS. EDS is more common in PD patients taking higher doses of Dopaminoagonists and levodopa (Liu et al., 2022). Even most antiparkinsonian medications (Koller et al., 2005; Hauser et al., 2014) can induce or aggravate EDS due to their sedative effects (Arnulf and Leu-Semenescu, 2009). However, some drugs can also improve EDS, such as piribedil (Eggert et al., 2014) and selegiline (Gallazzi et al., 2021). Therefore, it is very important to carefully assess EDS and select reasonable drugs for patients.
Longitudinal studies on PD have identified several risk factors for EDS, including age, gender, and disease duration (Zhu et al., 2016; Tholfsen et al., 2015). Additionally, we found that there was a significant difference in FSS scores between the two groups at baseline. So we adjusted these risk factors that might affect the outcome. By quantifying gait parameters more objectively, we found that PD patients with EDS exhibited more severe gait impairment. Specifically, EDS may be associated with the deterioration of both normal walking gait and fast walking gait in PD patients. Even the effect of EDS on gait remained significant after adjusting for confounders such as sex, age, disease duration, LEDD and FSS. The results are consistent with previous scale-only studies showing that EDS is associated with a wider range of motor and nonmotor PD features including axial/postural/gait deficits, depression, and pain (Höglund et al., 2015). One reason may be that EDS is often accompanied by cognitive impairments such as poor concentration (Bohnen et al., 2012), memory loss and executive dysfunction (Gasa et al., 2013). This cognitive dysfunction affects the patient’s gait in the early stages of PD (Rochester et al., 2017). During walking, cognitive functions play an important role in gait planning, maintenance of balance, and perception of and response to the environment. Impaired cognitive function may lead to gait abnormalities such as disorientation and delayed reaction time when walking, increasing the risk of falls. The observed sensory integration delays may arise from attentional deficits associated with sleep disturbances. Effective postural control fundamentally relies on the central nervous system’s capacity to synchronize visual cues, vestibular signals, and proprioceptive feedback in real-time (Teasdale and Simoneau, 2001; Ouchi et al., 1999); this sensory integration requires a high degree of attention, especially as the efficiency of sensory inputs decreases with age, which may affect gait performance (Tyagi et al., 2017). Although previous studies have attributed EDS-related gait deficiency to impaired attention or executive function, we found comparable MMSE scores between groups, which seems contradictory. However, the MMSE primarily assesses general cognition and lacks sensitivity to executive dysfunction in specific domains (Hausdorff, 2005). In PD patients, gait control relies greatly on prefrontal-mediated processes that cannot be captured by the MMSE.
Step length shortening is consistent with the “sequence effect” of PD, in which there is a gradual decay in amplitude of movement, which is usually associated with basal ganglia dysfunction. EDS may exacerbate this phenotype through nigrostriatal dopamine depletion, as animal models show that sleep deprivation accelerates the loss of dopaminergic neurons (Parhizkar et al., 2023).
Importantly, gait disturbances caused by EDS are a direct threat to patient safety and quality of life. Both gait speed and stride length, which are predictors of falls in older adults (Kyrdalen et al., 2019), are significantly reduced in PD patients with EDS, who are at very high risk of falling (Fasano et al., 2017; Allen et al., 2013). Falls frequently lead to fractures (Kalilani et al., 2016), hospitalization (Paul et al., 2017), functional decline, significantly reducing patients’ independence and quality of life (Rascol et al., 2015). Therefore, early detection of EDS provides a critical window for intervention to mitigate future gait deterioration and fall risk. We recommend emphasizing the management of EDS in early PD, including nonpharmacological therapies such as repetitive transcranial magnetic stimulation that may improve both Sleep problems and motor function (Zhang et al., 2022).
While the use of scales and direct observation by clinicians is still common in routine assessments, a growing number of studies has demonstrated the added value of wearable inertial sensors for objective gait analysis in patients with PD (Pulliam et al., 2018; Ricci et al., 2020; Isaacson et al., 2019; Dai et al., 2021; Perez-Ibarra et al., 2020; Rigas et al., 2012; Demrozi et al., 2020; Mariani et al., 2013). On this basis, we used the Wearable Movement and Gait Quantitative Assessment System to obtain accurate quantitative gait parameters during the TUG and the 5-meter straight walking task. We aimed to provide an objective and intuitive assessment of how EDS affects gait function in patients with PD.
There are certain restrictions on this study. The sample size of the study was relatively small. Consequently, the reliability and generalizability of the results may be limited. To enhance the statistical significance of the findings, future research should consider increasing the sample size. Using only the MMSE as a cognitive assessment tool is insufficient, and future research could incorporate more tests, especially for specific cognitive functions such as attention and integration. This study did not analyse patients for the specific type of medication they were using and only focused on patients with PD in the early stages of the disease, future studies should include detailed medications and patients in all periods of time. Additionally, due to the cross-sectional nature of this study, it was not possible to establish a causal relationship between gait impairment and EDS. Future investigations could adopt a longitudinal study design to better understand the long-term effects of EDS on gait function by tracking changes over time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Third Affiliated Hospital of Xinxiang Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YX: Data curation, Methodology, Writing – original draft, Conceptualization, Writing – review & editing, Formal analysis. MZ: Writing – review & editing, Data curation, Writing – original draft. YG: Writing – original draft, Writing – review & editing, Methodology, Supervision. PT: Writing – review & editing, Writing – original draft, Data curation. SL: Methodology, Writing – review & editing, Writing – original draft, Supervision. HX: Project administration, Funding acquisition, Writing – review & editing, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Henan Province (grant number 232300420061), the Joint Construction Project of Medical Science and Technology Research Plan of Henan Province (grant number LHGJ20230499), the Joint Construction Project of Medical Science and Technology Research Plan of Henan Province (grant number LHGJ20230549), the National Foreign Expert Project (grant number G2023026014L), the Joint Construction Project of Medical Science and Technology Research Plan of Henan Province (grant number LHGJ20240510), the Key Research and Development Program of Henan Province (grant number 25111310700), and the Science and Technology Research Project of Henan Province (grant number 252102310279).
Acknowledgments
Thanks for the help from Professor Feng-Tao Liu for the review of the study and manuscript. Thanks for the help from Professor Yan-Wei Li for the help in recruiting patients. We acknowledge the collaboration of the patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, R. D., Ross, G. W., White, L. R., Tanner, C. M., Masaki, K. H., Nelson, J. S., et al. (2005). Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65, 1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d
Allen, N. E., Schwarzel, A. K., and Canning, C. G. (2013). Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis. 2013:906274. doi: 10.1155/2013/906274
Arnulf, I., and Leu-Semenescu, S. (2009). Sleepiness in Parkinson's disease. Parkinsonism Relat. Disord. 15, S101–S104. doi: 10.1016/S1353-8020(09)70792-8
Bohnen, N. I., Müller, M. L., Kotagal, V., Koeppe, R. A., Kilbourn, M. R., Gilman, S., et al. (2012). Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J. Cereb. Blood Flow Metab. 32, 1609–1617. doi: 10.1038/jcbfm.2012.60
Borzì, L., Demrozi, F., Bacchin, R. A., Turetta, C., Sigcha, L., Rinaldi, D., et al. (2025). Freezing of gait detection: the effect of sensor type, position, activities, datasets, and machine learning model. J. Parkinsons Dis. 15, 163–181. doi: 10.1177/1877718X241302766
Cai, G., Shi, W., Wang, Y., Weng, H., Chen, L., Yu, J., et al. (2023). Specific distribution of digital gait biomarkers in Parkinson's disease using body-worn sensors and machine learning. J. Gerontol. A Biol. Sci. Med. Sci. 78, 1348–1354. doi: 10.1093/gerona/glad101
Chahine, L. M., Amara, A. W., and Videnovic, A. (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson's disease from 2005 to 2015. Sleep Med. Rev. 35, 33–50. doi: 10.1016/j.smrv.2016.08.001
Chen, M., Guo, Y., Zhang, X., Zhao, M., Zheng, T., Song, J., et al. (2025). Impact of excessive daytime sleepiness on the progression of freezing of gait in de novo Parkinson's disease: a cohort study. Neurol. Sci. 46, 723–731. doi: 10.1007/s10072-024-07738-8
Dai, H., Cai, G., Lin, Z., Wang, Z., and Ye, Q. (2021). Validation of inertial sensing-based wearable device for tremor and bradykinesia quantification. IEEE J. Biomed. Health Inform. 25, 997–1005. doi: 10.1109/JBHI.2020.3009319
Demrozi, F., Bacchin, R., Tamburin, S., Cristani, M., and Pravadelli, G. (2020). Toward a wearable system for predicting freezing of gait in people affected by Parkinson's disease. IEEE J. Biomed. Health Inform. 24, 2444–2451. doi: 10.1109/JBHI.2019.2952618
Eggert, K., Öhlwein, C., Kassubek, J., Wolz, M., Kupsch, A., Ceballos-Baumann, A., et al. (2014). Influence of the nonergot dopamine agonist piribedil on vigilance in patients with Parkinson disease and excessive daytime sleepiness (PiViCog-PD): an 11-week randomized comparison trial against pramipexole and ropinirole. Clin. Neuropharmacol. 37, 116–122. doi: 10.1097/WNF.0000000000000041
Fasano, A., Canning, C. G., Hausdorff, J. M., Lord, S., and Rochester, L. (2017). Falls in Parkinson's disease: a complex and evolving picture. Movement Disord. 32, 1524–1536. doi: 10.1002/mds.27195
Feng, F., Cai, Y., Hou, Y., Ou, R., Jiang, Z., and Shang, H. (2021). Excessive daytime sleepiness in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 85, 133–140. doi: 10.1016/j.parkreldis.2021.02.016
Gallazzi, M., Mauri, M., Bianchi, M. L., Riboldazzi, G., Princiotta Cariddi, L., Carimati, F., et al. (2021). Selegiline reduces daytime sleepiness in patients with Parkinson's disease. Brain Behav. 11:e01880. doi: 10.1002/brb3.1880
Gasa, M., Tamisier, R., Launois, S. H., Sapene, M., Martin, F., Stach, B., et al. (2013). Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J. Sleep Res. 22, 389–397. doi: 10.1111/jsr.12039
Gildner, T. E., Snodgrass, J. J., Evans, C., and Kowal, P. (2019). Associations between physical function and subjective well-being in older adults from low- and middle-income countries: results from the study on global AGEing and adult health (SAGE). J. Aging Phys. Act. 27, 213–221. doi: 10.1123/japa.2016-0359
Hausdorff, J. M. (2005). Gait variability: methods, modeling and meaning. J. Neuroeng. Rehabil. 2:19. doi: 10.1186/1743-0003-2-19
Hauser, R. A., Schapira, A. H., Barone, P., Mizuno, Y., Rascol, O., Busse, M., et al. (2014). Long-term safety and sustained efficacy of extended-release pramipexole in early and advanced Parkinson's disease. Eur. J. Neurol. 21, 736–743. doi: 10.1111/ene.12375
Hinchliffe, C., Rehman, R. Z. U., Pinaud, C., Branco, D., Jackson, D., Ahmaniemi, T., et al. (2024). Evaluation of walking activity and gait to identify physical and mental fatigue in neurodegenerative and immune disorders: preliminary insights from the IDEA-FAST feasibility study. J. Neuroeng. Rehabil. 21:94. doi: 10.1186/s12984-024-01390-1
Höglund, A., Broman, J. E., Pålhagen, S., Fredrikson, S., and Hagell, P. (2015). Is excessive daytime sleepiness a separate manifestation in Parkinson's disease? Acta Neurol. Scand. 132, 97–104. doi: 10.1111/ane.12378
Isaacson, S. H., Boroojerdi, B., Waln, O., McGraw, M., Kreitzman, D. L., Klos, K., et al. (2019). Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson's disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat. Disord. 64, 132–137. doi: 10.1016/j.parkreldis.2019.01.025
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R., and Hyman, N. (1997). The Parkinson's disease questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 26, 353–357. doi: 10.1093/ageing/26.5.353
Johns, M. W. (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545. doi: 10.1093/sleep/14.6.540
Kalilani, L., Asgharnejad, M., Palokangas, T., and Durgin, T. (2016). Comparing the incidence of falls/fractures in Parkinson's disease patients in the US population. PLoS One 11:e0161689. doi: 10.1371/journal.pone.0161689
Koller, W., Guarnieri, M., Hubble, J., Rabinowicz, A. L., and Silver, D. (2005). An open-label evaluation of the tolerability and safety of Stalevo (carbidopa, levodopa and entacapone) in Parkinson's disease patients experiencing wearing-off. J. Neural Transm. 112, 221–230. doi: 10.1007/s00702-004-0184-1
Kyrdalen, I. L., Thingstad, P., Sandvik, L., and Ormstad, H. (2019). Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiotherapy Res. Int. 24:e1743. doi: 10.1002/pri.1743
Liu, H., Li, J., Wang, X., Huang, J., Wang, T., Lin, Z., et al. (2022). Excessive daytime sleepiness in Parkinson's disease. Nat. Scie. Sleep 14, 1589–1609. doi: 10.2147/NSS.S375098
Liu, M., Luo, Y.-J., Gu, H.-Y., Wang, Y.-M., Liu, M.-H., Li, K., et al. (2021). Sex and onset-age-related features of excessive daytime sleepiness and night-time sleep in patients with Parkinson’s disease. BMC Neurol. 21:165. doi: 10.1186/s12883-021-02192-x
Liu, R., Wang, Z., Qiu, S., Zhao, H., Wang, C., Shi, X., et al. (2022). A wearable gait analysis and recognition method for Parkinson's disease based on error state Kalman filter. IEEE J. Biomed. Health Inform. 26, 4165–4175. doi: 10.1109/JBHI.2022.3174249
Mariani, B., Jiménez, M. C., Vingerhoets, F. J., and Aminian, K. (2013). On-shoe wearable sensors for gait and turning assessment of patients with Parkinson's disease. IEEE Trans. Biomed. Eng. 60, 155–158. doi: 10.1109/TBME.2012.2227317
Mengdie, H., Lijie, Z., and Xinling, Y. (2022). Recent advance in Parkinson’s disease with excessive daytime sleepiness. Chin. J. Pract. Nerv. Dis. 25, 1030–1034. doi: 10.12083/SYSJ.220576
Montastruc, J. L., Brefel-Courbon, C., Senard, J. M., Bagheri, H., Ferreira, J., Rascol, O., et al. (2001). Sleep attacks and antiparkinsonian drugs: a pilot prospective pharmacoepidemiologic study. Clin. Neuropharmacol. 24, 181–183. doi: 10.1097/00002826-200105000-00013
Ouchi, Y., Okada, H., Yoshikawa, E., Nobezawa, S., and Futatsubashi, M. (1999). Brain activation during maintenance of standing postures in humans. Brain 122, 329–338.
Parhizkar, S., Gent, G., Chen, Y., Rensing, N., Gratuze, M., Strout, G., et al. (2023). Sleep deprivation exacerbates microglial reactivity and aβ deposition in a TREM2-dependent manner in mice. Sci. Transl. Med. 15:eade6285. doi: 10.1126/scitranslmed.ade6285
Paul, S. S., Harvey, L., Canning, C. G., Boufous, S., Lord, S. R., Close, J. C., et al. (2017). Fall-related hospitalization in people with Parkinson's disease. Eur. J. Neurol. 24, 523–529. doi: 10.1111/ene.13238
Perez-Ibarra, J. C., Siqueira, A. A. G., and Krebs, H. I. (2020). Identification of gait events in healthy subjects and with Parkinson's disease using inertial sensors: an adaptive unsupervised learning approach. IEEE Trans. Neural Syst. Rehabilit. Eng. 28, 2933–2943. doi: 10.1109/TNSRE.2020.3039999
Plotnik, M., Giladi, N., and Hausdorff, J. M. (2007). A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson's disease. Exp. Brain Res. 181, 561–570. doi: 10.1007/s00221-007-0955-7
Plotnik, M., Giladi, N., and Hausdorff, J. M. (2009). Bilateral coordination of gait and Parkinson's disease: the effects of dual tasking. J. Neurol. Neurosurg. Psychiatry 80, 347–350. doi: 10.1136/jnnp.2008.157362
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Movement Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Pulliam, C. L., Heldman, D. A., Brokaw, E. B., Mera, T. O., Mari, Z. K., and Burack, M. A. (2018). Continuous assessment of levodopa response in Parkinson's disease using wearable motion sensors. IEEE Trans. Biomed. Eng. 65, 159–164. doi: 10.1109/TBME.2017.2697764
Rascol, O., Perez-Lloret, S., Damier, P., Delval, A., Derkinderen, P., Destée, A., et al. (2015). Falls in ambulatory non-demented patients with Parkinson's disease. J. Neural Transm. 122, 1447–1455. doi: 10.1007/s00702-015-1396-2
Ricci, M., Di Lazzaro, G., Pisani, A., Mercuri, N. B., Giannini, F., and Saggio, G. (2020). Assessment of motor impairments in early untreated Parkinson's disease patients: the wearable electronics impact. IEEE J. Biomed. Health Inform. 24, 120–130. doi: 10.1109/JBHI.2019.2903627
Rigas, G., Tzallas, A. T., Tsipouras, M. G., Bougia, P., Tripoliti, E. E., Baga, D., et al. (2012). Assessment of tremor activity in the Parkinson's disease using a set of wearable sensors. IEEE Trans. Inform. Technol. Biomed. 16, 478–487. doi: 10.1109/TITB.2011.2182616
Rochester, L., Galna, B., Lord, S., Yarnall, A. J., Morris, R., Duncan, G., et al. (2017). Decrease in Aβ42 predicts dopa-resistant gait progression in early Parkinson disease. Neurology 88, 1501–1511. doi: 10.1212/WNL.0000000000003840
Sateia, M. J. (2014). International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394. doi: 10.1378/chest.14-0970
Tanner, C. M., and Ostrem, J. L. (2024). Parkinson's disease. N. Engl. J. Med. 391, 442–452. doi: 10.1056/NEJMra2401857
Teasdale, N., and Simoneau, M. (2001). Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture 14, 203–210. doi: 10.1016/S0966-6362(01)00134-5
Tholfsen, L. K., Larsen, J. P., Schulz, J., Tysnes, O. B., and Gjerstad, M. D. (2015). Development of excessive daytime sleepiness in early Parkinson disease. Neurology 85, 162–168. doi: 10.1212/WNL.0000000000001737
Tyagi, S., Perera, S., and Brach, J. S. (2017). Balance and mobility in community-dwelling older adults: effect of daytime sleepiness. J. Am. Geriatr. Soc. 65, 1019–1025. doi: 10.1111/jgs.14735
Wang, L., and Zou, B. (2022). The association between gait speed and sleep problems among Chinese adults aged 50 and greater. Front. Neurosci. 16:855955. doi: 10.3389/fnins.2022.855955
Zhang, X., Zhuang, S., Wu, J., Wang, L., Mao, C., Chen, J., et al. (2022). Effects of repetitive transcranial magnetic stimulation over right dorsolateral prefrontal cortex on excessive daytime sleepiness in patients with Parkinson's disease. Sleep Med. 100, 133–138. doi: 10.1016/j.sleep.2022.08.003
Keywords: Parkinson’s disease, excessive daytime sleepiness, gait, gait assessment, wearable sensors
Citation: Xie Y, Zhao M, Guo Y, Tian P, Liu S and Xing H (2025) Excessive daytime sleepiness and gait disturbances in patients with Parkinson’s disease. Front. Aging Neurosci. 17:1626247. doi: 10.3389/fnagi.2025.1626247
Edited by:
Robert Petersen, College of Medicine, Central Michigan University, United StatesReviewed by:
Donato Melchionda, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, ItalyMatilde Bertoli, University Children’s Hospital Basel, Switzerland
Copyright © 2025 Xie, Zhao, Guo, Tian, Liu and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxia Xing, eGh4d2gwMkAxNjMuY29t
 Yibo Xie1,2
Yibo Xie1,2 Hongxia Xing
Hongxia Xing