Abstract
Objective:
Peritoneal dialysis (PD) patients demonstrate distinct iron homeostasis imbalances. However, the relationship between brain iron and cognitive impairment in this population remains poorly elucidated.
Methods:
This study enrolled 52 PD patients and 49 healthy controls (HCs). Quantitative susceptibility mapping (QSM) was employed to quantify cerebral iron deposition. Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) and a comprehensive neuropsychological test battery. Dose–response relationships between iron metabolism parameters and cognitive performance were analyzed using generalized additive models (GAMs).
Results:
PD patients exhibited significantly higher iron deposition in the left amygdala and right putamen compared to HCs. Serum ferritin (SF) demonstrated an approximately inverted U-shaped relationship with MoCA scores, with an inflection point at 258.4 μg/L (p < 0.001). Every 100 μg/L increase in SF beyond this threshold was associated with a 3.1-point decrease in MoCA score. Iron deposition in the left amygdala showed significant correlations with scores on the Digit Symbol Test (DST), Self-Rating Depression Scale (SDS), Self-Rating Anxiety Scale (SAS), and Verbal Fluency Test (VFT), but exhibited no direct association with peripheral iron metabolism parameters.
Conclusion:
In peritoneal dialysis patients, abnormal cerebral iron deposition predominantly localizes to limbic-basal ganglia regions. Iron accumulation in the left amygdala may specifically mediate the development of multi-domain cognitive impairment. QSM represents a sensitive technique for early detection of pathological iron accumulation.
1 Introduction
Patients with chronic kidney disease (CKD) exhibit a significantly higher prevalence of cognitive impairment compared to the general population, with underlying mechanisms involving multifactorial interactions such as uremic toxin accumulation, inflammatory responses, oxidative stress, and cerebrovascular lesions (Drew et al., 2019; Viggiano et al., 2020). As a renal replacement therapy for end-stage renal disease (ESRD), peritoneal dialysis (PD) partially clears metabolic waste products; however, the incidence of cognitive impairment in PD patients remains notably elevated (22.3–74.5%), severely compromising treatment adherence and quality of life (Kalirao et al., 2011; Liu et al., 2015; Shea et al., 2016). In recent years, the role of iron metabolism dysregulation in neurodegenerative disorders has garnered increasing attention (Wu et al., 2023).
Iron is an essential element for neurotransmitter synthesis and myelination, and its metabolic imbalance can induce oxidative stress damage, leading to neuronal apoptosis and synaptic dysfunction (Munoz and Humeres, 2012). Serum ferritin (SF) is a reliable marker for assessing peripheral iron stores. Notably, iron metabolism disorders exhibit a dose-dependent impact on cognition—moderate iron levels sustain neuronal plasticity, whereas overload triggers neuroinflammatory cascades through microglial activation (Kim et al., 2023; Liu et al., 2025; Nunez and Hidalgo, 2019). Compared to hemodialysis (HD) patients, PD patients demonstrate distinct iron dysregulation patterns: (1) Chronic peritoneal exposure to high-glucose dialysate induces local oxidative stress and upregulates hepcidin expression, leading to intestinal iron absorption inhibition and reticuloendothelial iron sequestration (Yamaji et al., 2004). PD-associated protein loss (particularly transferrin) exacerbates functional iron deficiency, necessitating frequent intravenous iron supplementation that perpetuates an “iron overload-inflammation-hepcidin elevation” vicious cycle (Niikura et al., 2019; Zou et al., 2021); (2) Residual renal function differences influence iron regulation: Compared to HD counterparts, PD patients retain stronger residual glomerular filtration function during the early-to-middle stages of treatment (Moist et al., 2000; Sinnakirouchenan and Holley, 2011), which theoretically supports their greater urinary iron excretion capacity. However, this regulatory effect on systemic iron load may be counterbalanced by peritoneal iron loss (Niikura et al., 2019). This unique iron homeostasis imbalance potentially facilitates cerebral iron accumulation through blood–brain barrier permeability alterations, a phenomenon that has been overlooked in existing research. Nevertheless, current studies reveal significant discrepancies regarding iron metabolism-cognitive impairment associations: In Alzheimer’s disease populations, elevated SF levels show significant negative correlation with MMSE score decline (Li et al., 2024). Conversely, higher SF values were found to be associated with higher cognitive function in infants aged 1–3 years (Parkin et al., 2020). Therefore, the role of iron metabolism in cognitive impairment remains to be explored.
The advent of Quantitative Susceptibility Mapping (QSM) technology has revolutionized research on cerebral iron deposition. Compared to conventional T2-weighted imaging, QSM leverages phase information to quantitatively resolve tissue susceptibility differences, enabling precise discrimination between paramagnetic substances such as iron and calcium, with a spatial resolution of 1 mm3 (Liu et al., 2015; Wang et al., 2022). This technique significantly enhanced sensitivity in detecting iron deposition within deep gray matter nuclei (e.g., basal ganglia and hippocampus)(Ning et al., 2019). Studies have established significant correlations between iron accumulation in regions such as the putamen and caudate nucleus and impairments in executive function and memory (Modica et al., 2015; Zhi et al., 2024). Moreover, the high sensitivity of QSM enables the detection of early subclinical iron deposition changes, offering a predictive biomarker for cognitive decline. For instance, in patients with CKD stages 1–4, increased susceptibility values in the putamen have been observed to precede the onset of abnormalities in conventional neuropsychological tests (Wang et al., 2023). This temporal precedence highlights QSM’s potential as a prospective tool for stratifying cognitive risk in PD patients, facilitating early intervention before overt neuropsychological deficits emerge. However, a critical knowledge gap persists in PD research, where the absence of QSM-based investigations severely hinders the development of personalized iron management strategies for PD populations.
Although existing studies have revealed the role of iron metabolism dysregulation in CKD-related cognitive impairment, critical gaps persist in research targeting PD populations: (1) Most investigations focus on HD patients, overlooking the unique iron homeostasis alterations in PD patients caused by peritoneal glucose exposure and albumin loss; (2) The association between cerebral iron deposition and domain-specific cognitive deficits (such as working memory and processing speed) in PD patients remains incompletely understood. (3) Clinical observations have revealed that PD patients commonly exhibit dysregulation of iron metabolism. Long-term peritoneal dialysis may contribute to systemic iron overload, while the association between peripheral blood levels of ferritin and cerebral iron deposition remains inconclusive.
Here, we performed QSM analyses to compare the magnetic susceptibility of the regional brain between patients with PD and healthy controls (HC). By integrating neuropsychological assessments, we systematically investigate the associations among iron metabolism index, cerebral iron deposition, and cognitive impairment in PD patients. This study combined iron metabolism, QSM, and neuropsychological data to elucidate the neuropathological mechanisms of systemic iron overload and central nervous system deposition in cognitive impairment.
2 Materials and methods
2.1 Study population
This cross-sectional study was approved by the Research Ethics Committee of the First Affiliated Hospital, Anhui Medical University (Ethics approval number: PJ2024-06-63). All participants involved in this study provided written informed consent in accordance with the Declaration of Helsinki.
From June 2024 to February 2025, patients who underwent clinical evaluation, laboratory tests, and multimodal neuroimaging assessments at the Department of Nephropathy of The First Affiliated Hospital of Anhui Medical University were enrolled in this study. Furthermore, Fifty-two HCs matched for gender, age, and years of education were recruited from the local community, with three participants excluded due to cerebral infarction or claustrophobia. All patients fulfilled the following inclusion criteria: aged 18–80 years, undergoing continuous ambulatory peritoneal dialysis (CAPD) with standard lactate-based peritoneal dialysis solution (containing 15 or 25 g glucose, 5.38 g sodium chloride, calcium chloride, 0.051 g magnesium chloride, and 4.48 g sodium lactate per 1,000 mL), stable dialysis duration >3 months, and right-handedness. Exclusion criteria were as follows: (1) History of craniocerebral injury or other neurological disorders (e.g., traumatic brain injury, intracranial space-occupying lesions); (2) Prior history of impaired consciousness, affective disorders, or neuropsychological conditions (e.g., depression, anxiety disorders, schizophrenia); (3) Documented substance dependence or abuse (including alcohol, medications, or illicit drugs); (4) Patients with renal transplantation, acute infections, heart failure, or severe hepatic dysfunction; (5) Inability to complete neuropsychological assessments or cranial magnetic resonance imaging (MRI). All imaging data were independently evaluated by two experienced radiologists blinded to participants’ clinical and cognitive profiles. Participants exhibiting suboptimal image quality, severe white matter disease, cerebral infarction (excluding lacunar infarcts), or intracranial hemorrhage were excluded from subsequent analyses.
2.2 Clinical and biochemical assessments
Clinical and laboratory data of patients were obtained using standardized forms. The following indicators were extracted: Demographic data, including age, gender, years of education, and dialysis duration; Physical examination variables, including systolic blood pressure (SBP), diastolic blood pressure (DBP), height, and body weight with dry abdomen; Health history, including the history of diabetes and peritonitis, which was collected from the electronic medical records of our hospital; and laboratory variables from serum, urine, and peritoneal dialysis fluid, including white blood cell count, hemoglobin, serum albumin, blood urea nitrogen (BUN), creatinine, uric acid, residual glomerular filtration rate (rGFR), C-reactive protein (CRP), serum iron metabolism indicators, alkaline phosphatase, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, calcium-phosphorus product, serum bicarbonate concentration, intact parathyroid hormone (iPTH), etc. Standard laboratory methods were used to measure the dialysate/plasma creatinine ratio (D/P Cr), total creatinine clearance (CCr), and total Kt/V on an automated chemical analyzer. The specific details are shown in Table 1. Residual renal function, expressed as rGFR, was calculated using the following formula:rGFR = 1/2[urine Cr(μmol/L)/serum Cr(μmol/L) + urine urea(mmol/L)/serum urea(mmol/L)] × urine volume(mL)/1,440 (Nolph et al., 1993). These values were obtained through the peritoneal equilibrium test (PET). Patients should receive standard CAPD treatment before the PET, and the nocturnal peritoneal dialysis fluid should be retained in the abdominal cavity for 8–12 h. No blood biochemical tests were performed on the control subjects.
Table 1
| Variables | All (n = 52) | PD-MCI (n = 34) | PD-CN (n = 18) | t/χ2/z | P value |
|---|---|---|---|---|---|
| Age (years) | 53 (47, 57) | 54 (50, 57) | 48 (42, 56) | −2.265 | 0.023 |
| Female [n (%)] | 33 (63.4) | 21 (61.8) | 12 (66.7) | 0.122 | 0.727 |
| Education (years) | 8 (6, 9) | 7 (6, 8) | 10 (8, 12) | −3.895 | <0.001 |
| Dialysis period (months) | 23 (12, 61) | 27 (13, 69) | 20 (12, 52) | −1.059 | 0.290 |
| BMI (kg/m2) | 23.17 ± 2.91 | 23.52 ± 2.84 | 22.51 ± 3.02 | −1.185 | 0.242 |
| Diabetes [n (%)] | 11 (21.1) | 8 (23.5) | 3 (16.7) | - | 0.727 |
| Incidence of peritonitis [n (%)] | 14 (26.9) | 7 (20.6) | 7 (38.9) | - | 0.197 |
| Systolic blood pressure (mmHg) | 142.83 ± 17.59 | 142.12 ± 18.96 | 144.17 ± 15.11 | 0.396 | 0.694 |
| Diastolic blood pressure (mmHg) | 91.35 ± 12.17 | 88.82 ± 12.08 | 96.11 ± 11.15 | 2.124 | 0.039 |
| Laboratory data | |||||
| White blood cell count (109/L) | 6.74 ± 2.29 | 7.04 ± 2.19 | 6.17 ± 2.43 | −1.311 | 0.196 |
| Hemoglobin (g/L) | 100.79 ± 17.80 | 101.21 ± 19.25 | 100.00 ± 15.17 | −0.230 | 0.819 |
| Albumin (g/L) | 37.85 ± 3.35 | 38.13 ± 3.50 | 37.33 ± 3.07 | −0.810 | 0.422 |
| Alkaline phosphatase (U/L) | 83.35 (63.25, 105.93) | 84.7 (65.5, 102.0) | 77.3 (62.4, 123.1) | −0.115 | 0.908 |
| LDH (U/L) | 229.25 (193.08, 264.35) | 236.0 (205.4, 266.9) | 210.85 (187.3, 251.3) | −1.135 | 0.256 |
| BUN (mmol/L) | 21.39 (17.19, 24.01) | 21.39 (17.57, 24.29) | 21.18 (16.48, 23.28) | −0.529 | 0.597 |
| Uric acid (μmol/L) | 434.48 ± 80.79 | 450.20 ± 86.43 | 404.78 ± 60.41 | −1.984 | 0.053 |
| Serum creatinine (umol/L) | 968.31 ± 260.30 | 949.06 ± 282.34 | 1004.66 ± 215.37 | 0.729 | 0.469 |
| rGFR ml/min/1.73m2 | 1.37 (0, 5.10) | 2.24 (0.00, 5.62) | 0.57 (0.00, 3.28) | −1.188 | 0.235 |
| Total cholesterol (mmol/L) | 4.56 ± 0.99 | 4.51 ± 1.11 | 4.66 ± 0.74 | 0.522 | 0.604 |
| Triglyceride (mmol/L) | 1.58 (1.07, 2.79) | 1.67 (1.06, 3.54) | 1.42 (1.08, 2.66) | −0.616 | 0.538 |
| LDL-C (mmol/L) | 2.99 ± 0.72 | 2.92 ± 0.75 | 3.14 ± 0.66 | 1.029 | 0.308 |
| HDL-C (mmol/L) | 1.17 ± 0.30 | 1.16 ± 0.33 | 1.18 ± 0.24 | 0.238 | 0.813 |
| iPTH (pg/ml) | 258 (101, 396) | 316.72 ± 211.71 | 271.98 ± 278.73 | −0.649 | 0.520 |
| Calcium-phosphorus product | 3.85 ± 0.95 | 4.03 ± 0.89 | 3.53 ± 1.01 | −1.848 | 0.071 |
| Bicarbonate (mmol/L) | 24.96 ± 2.62 | 24.97 ± 2.68 | 24.94 ± 2.57 | −0.037 | 0.970 |
| Serum iron (umol/L) | 11.04 ± 4.16 | 10.68 ± 4.11 | 11.71 ± 4.27 | 0.849 | 0.400 |
| Serum ferritin (ug/L) | 159.75 (98.68, 327.38) | 337.05 (281.60, 440.20) | 257.05 (243.10, 271.90) | −3.789 | <0.001 |
| Transferrin(g/L) | 1.82 (1.66, 2.04) | 1.84 (1.67, 2.21) | 1.81 (1.63, 1.88) | −1.000 | 0.317 |
| Transferrin saturation (%) | 22.28 ± 11.51 | 30.01 ± 6.64 | 23.52 ± 4.39 | −3.728 | <0.001 |
| TIBC (umol/L) | 41.01 (38.33, 46.71) | 41.85 (38.49, 48.99) | 40.55 (36.82, 43.11) | −1.164 | 0.245 |
| CRP (mg/L) | 1.79 (0.54, 5.68) | 2.17 (1.00, 5.61) | 0.87 (0.43, 5.70) | −1.173 | 0.241 |
| D/P Cr at 4 h | 0.62 ± 0.11 | 0.60 ± 0.08 | 0.66 ± 0.13 | 1.843 | 0.071 |
| Total CCr (L/week) | 50.95 (45.31, 59.49) | 50.71 (45.61, 55.41) | 52.06 (39.57, 63.83) | −0.115 | 0.908 |
| Total Kt/V | 1.94 (1.43, 2.16) | 1.95 (1.38, 2.16) | 1.94 (1.50, 2.23) | −0.144 | 0.885 |
| Peritoneal function | 1.728 | 0.189 | |||
| High/high average transport (n (%)) | 17 (32.7) | 9 (26.5) | 8 (44.4) | ||
| Low/low average transport (n (%)) | 35 (67.3) | 25 (73.5) | 10 (55.6) | ||
Demographical and clinical biochemical data for the peritoneal dialysis patients included in the study.
Values are presented as means ± SD or interquartile range or percentages. PD-MCI, peritoneal dialysis with mild cognitive impairment; PD-CN, peritoneal dialysis with normal cognition; BMI, body mass index; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; rGFR, residual glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TIBC, total iron binding capacity; CRP, c-reactive protein; iPTH, intact parathyroid hormone; D/P Cr, dialysate/plasma creatinine ratio; CCr, creatinine clearance rete; Kt/V, urea clearance index.
2.3 Cognitive and behavioral assessments
Neurological examinations were conducted for all participants by two senior investigators in a quiet environment one day prior to MRI examinations. Cognitive function was evaluated using the Beijing-Chinese version of the MoCA, a validated cognitive screening instrument, as the primary outcome measure (Lu et al., 2011). It assesses seven domains: visuospatial/executive function, naming, attention, abstraction, language, delayed memory, and orientation, with a total score ranging from 0 to 30 points (the higher the score, the better the function). To mitigate educational bias, an additional point was added to the final score for individuals with educational attainment <12 years. All participants of mild cognitive impairment (MCI) were diagnosed in adherence to the Petersen criteria, which encompass: (1) subjective memory complaints, (2) MoCA score <26 (adjusted for educational attainment if necessary), (3) normal function in daily life, and (4) absence of dementia (Marcos et al., 2016). In addition, A standardized neuropsychological battery was administered to evaluate emotional status, cognitive processing speed, attentional capacity, executive function, and language abilities: (1) Emotional profiling: self-rating anxiety scale (SAS) and self-rating depression scale (SDS) were utilized to quantify anxiety and depressive symptoms; (2) Processing speed: symbol digit modalities test (SDMT) assessed visual-motor information processing efficiency. The outcome measure is the number of correct symbol-digit pairings completed within a fixed time period. (3) Attention metrics: digit span test (forward and backward versions) measured auditory-verbal working memory and attentional control. (4) Executive function: trail making test (TMT) evaluated cognitive flexibility, while stroop color-word test quantified inhibitory control. The core outcome measure of the former is the total time (in seconds) required to complete the trail-making task, while the outcome indicator of the latter is the total time (in seconds) needed to finish all items under each condition. (5)Language competence: verbal fluency test (VFT)-Fruit Category was employed to assess semantic retrieval and lexical access.
2.4 Imaging protocol
MRI images of each subject were acquired on a 3.0 T MRI system (Siemens Healthcare, Erlangen, Germany) equipped with a 64 - channel head matrix coil. All participants were instructed to maintain strict supine positioning throughout scanning procedures, with foam padding and noise-reducing earplugs applied to minimize motion artifacts and acoustic interference. Quantitative susceptibility mapping was obtained using a three-dimensional multi-echo gradient echo sequence with the following parameters: echo time (TE) 6.3 ms, repetition time (TR) 35 ms, field of view (FOV) 220 × 192 mm2, flip angle 20°, acquisition matrix 416 × 291, slice thickness 2.0 mm with 0.4 mm interslice gap, 72 contiguous slices, and total acquisition time 5.3 min. Structural imaging employed a T1-weighted 3D brain volume sequence (MPRAGE) with optimized parameters: TE 2.9 ms, TR 2300 ms, FOV 256 × 240 mm2, flip angle 9°, isotropic matrix 256 × 256, 1.0 mm slice thickness with 0.5 mm gap, 208 slices, achieving full brain coverage in 5.1 min. Additional FLAIR imaging was acquired using the following protocol: TE 98 ms, TR 8000 ms, FOV 220 × 200 mm2, flip angle 150°, matrix size 320 × 224, 5.0 mm slice thickness with 1.0 mm gap, 24 slices, completed within 98 s.
2.5 Quantitative susceptibility mapping processing and standardization protocol
The QSM data were reconstructed and analyzed using the SuscEptibility mapping PIpeline toolfor phAse images (SEPIA) toolbox (Chan and Marques, 2021) in the MATLAB program (MathWorks, Natick, MA). First, non-brain tissues were removed from the raw phase and magnitude images using the Brain Extraction Tool (BET) with a fractional intensity threshold of 0.3. Phase unwrapping was subsequently performed using a Laplacian-based algorithm to resolve phase discontinuities. Local background magnetic field contributions were corrected via the Laplacian Boundary Value (LBV) method. The susceptibility distribution map was reconstructed using an inverse filter with a k-space threshold of 0.15 to suppress high-frequency noise artifacts. Spatial smoothing was applied using a Gaussian kernel with a full width at half maximum (FWHM) of 2 mm to enhance signal-to-noise ratio (SNR).
The subsequent processing steps were performed using SPM12 based on MATLAB 2017a. The reconstructed QSM images were spatially normalized to the Montreal Neurological Institute (MNI) standard space through a one-step registration approach. The specific workflow consisted of two phases: (1) The Normalise (Estimate) module in SPM12 was employed to perform non-linear registration between the magnitude map of the first echo sequence from individual subjects and the tissue probability map (TPM) in MNI space, during which the deformation matrix was generated and preserved; (2) The Normalize (Write) module was then applied to propagate the preserved deformation field onto the QSM images, achieving spatial normalization of QSM data into MNI space (Figure 1). Finally, all normalized QSM images were resliced to achieve isotropic spatial resolution of 2 × 2 × 2 mm3 through voxel interpolation.
Figure 1

Flowchart of QSM image reconstruction and spatial normalization processing. BET, brain extraction tool; MNI, montreal neurological institute; SEPIA, SuscEptibility mapping PIpeline toolfor phAse images; QSM, quantitative susceptibility mapping.
The normalized susceptibility maps were aligned to the automated anatomical Labeling (AAL) parcellation template, and voxel-wise magnetic susceptibility values within each of the 116 brain regions were automatically extracted using code written in MATLAB R2017a. Regional susceptibility characteristics were quantified as the arithmetic mean of voxel values per ROI. To evaluate the relationship between magnetic susceptibility values and cognitive performance, we selected iron-rich subcortical nuclei as ROIs, guided by prior research findings and accounting for the anatomical limitations of the AAL template (Haacke et al., 2005; Moon et al., 2016). The ROIs in this study included the thalamus, caudate nucleus, globus pallidus, putamen, hippocampus, and amygdala.
2.6 Statistical analysis
All statistical analyses were performed using R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org), EmpowerStats (version 3.0; X&Y Solutions Inc., Boston, MA; https://www.empowerstats.com), and SPSS software (version 25.0; IBM Corp., Armonk, NY, United States). Normality of data distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables conforming to a normal distribution were expressed as mean ± standard deviation (SD), whereas skewed variables were reported as median with interquartile range (IQR). Categorical variables were summarized as frequencies and percentages.
For group comparisons, independent two-sample t-tests and Mann–Whitney U tests were applied to evaluate differences in normally and non-normally distributed continuous variables, respectively, between the PD and HC groups. Pearson’s chi-square test was utilized for categorical variables. For multiple comparisons, tests were corrected using the Bonferroni method (p < 0.05/116 = 0.0004).
Multivariable linear regression models were constructed to examine the association between MoCA scores and ferritin levels. Generalized additive models (GAM) with smooth curve fitting were employed to explore potential curve relationships between MoCA scores and ferritin. To investigate threshold effects in the MoCA-ferritin correlation, subgroup analyses were conducted using stratified multivariable regression models, incorporating interaction terms to assess variability across predefined subgroups (e.g., age, gender, or BMI). The correlation was analyzed using Pearson’s correlation coefficient for normally distributed data or Spearman’s rank correlation coefficient for non-normally distributed data between the mean QSM values of ROIs, cognitive scores, and laboratory data. However, to mitigate the elevated risk of type I errors arising from multiple comparisons, false discovery rate (FDR) correction methodology was implemented throughout the analytical process. The statistically significant p value was set at 0.05.
3 Results
3.1 Demographics and clinical characteristics
This study enrolled 52 patients undergoing peritoneal dialysis, categorized into peritoneal dialysis with mild cognitive impairment (PD-MCI) (n = 34) and peritoneal dialysis with normal cognition (PD-CN) (n = 18) groups based on cognitive function assessments. Demographic and clinical characteristics are summarized in Tables 1, 2. Significant intergroup differences were observed in demographic profiles: the PD-MCI group exhibited a higher median age (p = 0.023) and shorter educational attainment (median 7 years vs. 10 years, p < 0.001). Gender distribution (61.8% females in PD-MCI vs. 66.7% in PD-CN) and other baseline characteristics, including BMI, diabetes prevalence, and dialysis duration, showed no significant differences (all p > 0.05).
Table 2
| Variables | PD (n = 52) | HC (n = 49) | P value |
|---|---|---|---|
| Age (years) | 50.83 ± 7.90 | 49.37 ± 8.12 | 0.359 |
| Female [n (%)] | 34 (64.2) | 34 (69.4) | 0.575 |
| Education (years) | 8 (6, 9) | 9 (8, 9) | 0.224 |
| SAS (score) | 38.75 (32.50, 41.25) | 27.50 (26.25, 31.25) | <0.001 |
| SDS (score) | 42.50 (37.50, 51.25) | 27.50 (25.00, 32.50) | <0.001 |
| Cognitive screening | |||
| MoCA (score) | 23 (17, 27) | 27 (25, 28) | <0.001 |
| SDMT (score) | 21.55 ± 15.54 | 39.49 ± 15.45 | <0.001 |
| DST-forwards (score) | 6 (5, 8) | 9 (9, 10) | <0.001 |
| DST-backwards (score) | 4 (3, 5) | 6 (5, 7) | <0.001 |
| Stroop-word (s) | 33.16 (23.65, 43.67) | 26.96 (22.19, 32.51) | 0.006 |
| Stroop-color (s) | 47.74 (37.57, 66.01) | 39.17 (34.86, 46.07) | 0.004 |
| Stroop-interference (s) | 80.13 (65.00, 106.89) | 66.00 (58.75, 81.00) | 0.003 |
| TMT-A (s) | 68.59 (49.10, 82.38) | 62.00 (45.00, 84.00) | 0.547 |
| TMT-B (s) | 83.82 (55.02, 100.48) | 84.00 (67.00, 131.00) | 0.127 |
| VFT (score) | 10 (8, 12) | 12 (10, 15) | 0.001 |
Neuropsychological data for the peritoneal dialysis patients and healthy controls included in the study.
Values are presented as means ± SD or interquartile range or percentages. PD, peritoneal dialysis; HC: healthy control; SAS, self-rating anxiety scale; SDS, self-rating depression scale; MoCA, montreal cognitive assessment; SDMT, symbol digit modalities test; DST, digit span test; TMT, trail making test; VFT, verbal fluency test.
Laboratory analyses revealed marked disparities in iron metabolism parameters: serum ferritin levels were significantly elevated in the PD-MCI group (p < 0.001), with correspondingly higher transferrin saturation (p < 0.001). Hemodynamically, the PD-CN group demonstrated higher diastolic blood pressure (p = 0.039). Although the PD-MCI group showed a trend toward higher uric acid levels and calcium-phosphorus product values, these differences approached but did not reach statistical significance (p = 0.053 and p = 0.071, respectively). No significant intergroup variations were detected in the remaining laboratory and clinical parameters, including inflammatory markers (CRP), peritoneal transport characteristics, and nutritional indices (all p > 0.05).
As shown in Table 2, patients undergoing PD exhibited multidimensional neuropsychological impairments compared to HC. The two groups showed no statistically significant differences in age, gender distribution, or years of education (all p > 0.05). However, neuropsychological assessments revealed the following: (1) Emotional dimensions: The PD group scored significantly higher on the SAS and SDS than the HC group (both p < 0.001). (2) Global cognitive function: The PD group demonstrated significantly lower MoCA scores (p < 0.001). (3) Processing speed: The SDMT scores were markedly reduced in the PD group (p < 0.001). (4) Working memory: Both forward and backward Digit Span Test scores were significantly diminished in the PD group (both p < 0.001). (5). Executive function: The Stroop Test revealed significantly prolonged completion times for word naming, color naming, and interference tasks in the PD group. (6). Verbal fluency: The PD group showed significantly lower scores on the VFT. Notably, no significant differences were observed between the two groups in completion times for TMT-A or TMT-B. These findings suggest that PD patients experience broad cognitive impairment, with pronounced deficits in information processing speed, working memory, executive function, and emotional regulation, while visual search and task-switching abilities remain relatively preserved.
3.2 Association between SF and cognitive function
This study employed a two-stage regression analysis to investigate factors influencing MoCA scores in peritoneal dialysis patients. Univariate linear regression analysis revealed significant associations between MoCA scores and age (β = −0.348, 95% CI: −0.554 to −0.143, p = 0.001), years of education (β = 1.191, 95% CI: 0.485 to 1.896, p = 0.001), serum ferritin (β = −0.040, 95% CI: −0.054 to −0.025, p < 0.001), and transferrin saturation (β = −0.310, 95% CI: −0.567 to −0.053, p = 0.019) (Supplementary Table 1). The borderline significant variable, dialysis duration (p = 0.052), was included in the multivariate model for adjustment. Following multivariate linear regression analysis, age (β = −0.204, 95% CI: −0.400 to −0.007, p = 0.042) and serum ferritin (β = −0.031, 95% CI: −0.046 to −0.017, p < 0.001) retained independent statistical significance (Table 3). Specifically, a 100 μg/L increase in serum ferritin correlated with a 3.1-point decline in MoCA scores. However, the associations with education years (p = 0.614) and transferrin saturation (p = 0.512) were attenuated in the adjusted model. These findings indicate that advanced age and iron overload are independent risk factors for cognitive impairment in peritoneal dialysis patients.
Table 3
| Variables | β (95%CI) | P value |
|---|---|---|
| Age (years) | −0.204 (−0.400, −0.007) | 0.042 |
| Education (years) | 0.187 (−0.554, 0.928) | 0.614 |
| Dialysis period(months) | −0.028 (−0.069, 0.013) | 0.181 |
| Serum ferritin (ug/L) | −0.031 (−0.046, −0.017) | <0.001 |
| Transferrin saturation (%) | −0.072 (−0.293, 0.148) | 0.512 |
Multivariable analysis of the relationship between MoCA scores and clinical indicators in peritoneal dialysis patients.
MoCA: montreal cognitive assessment.
The generalized additive models with smooth curve fits were employed to delineate the nonlinear relationship between SF and MoCA scores, revealing a distinct inflection point at 258.4 μg/L (Figure 2). Subsequent threshold effect analysis using a two-piecewise linear regression model (Table 4) demonstrated divergent associations: above this threshold (SF ≥ 258.4 μg/L), each 1 μg/L increase in SF corresponded to a 0.053-point decline in MoCA scores (95% CI, −0.069 to −0.037). Conversely, below this threshold, each 1 μg/L increase in SF is linked to a slight increase of approximately 0.015 points in MoCA scores (95% CI, −0.018 to 0.047). Serum ferritin and MoCA scores appear to exhibit an inverted U-shaped relationship. Likelihood ratio tests confirmed significant improvement in model fit for the piecewise model compared to the standard linear model (p < 0.001), indicating superior data representation. The area between the upper and lower dashed lines is represented as 95% CI. Each point shows the magnitude of the SF and is connected to form a continuous line. Analyses were adjusted for age, educational attainment, dialysis duration, and transferrin saturation.
Figure 2
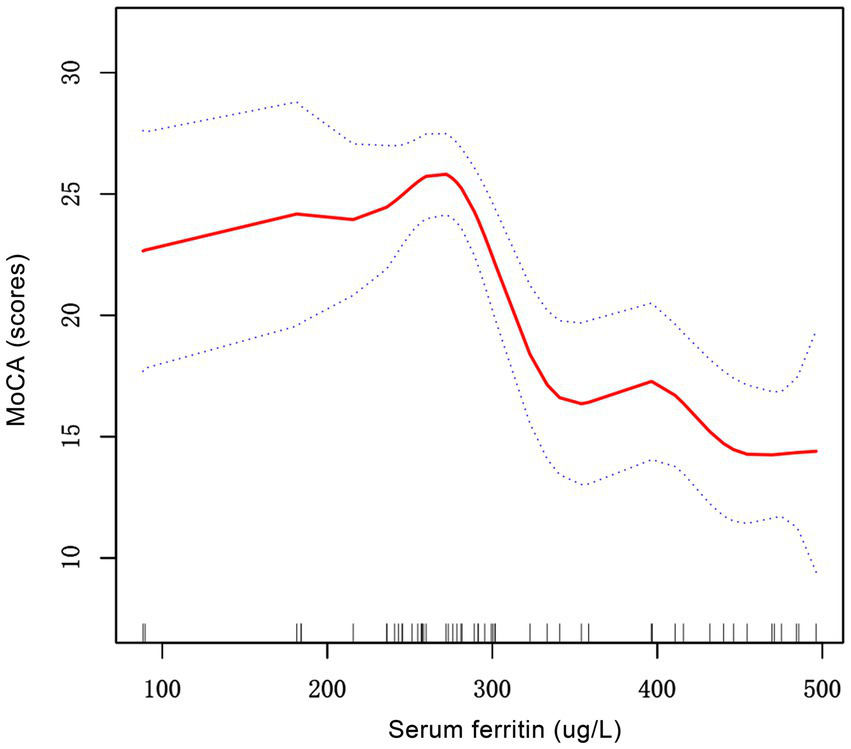
The correlation between serum ferritin and MoCA scores in patients with PD patients. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. MoCA, montreal cognitive assessment; PD, peritoneal dialysis.
Table 4
| MoCA | β (95% CI), P value |
|---|---|
| Ferritin | |
| Model 1 | |
| One line effect | −0.036 (−0.050, −0.022), <0.001 |
| Model 2 | |
| Turning point(K) | 258.4 |
| <258.4 slope1 | 0.015 (−0.018, 0.047), 0.374 |
| >258.4 slope2 | −0.053 (−0.069, −0.037), <0.001 |
| slope2-1 | −0.068 (−0.108, −0.028), 0.002 |
| Model fit value at K | 25.7 (24.0, 27.3) |
| Log-likelihood ratio test | <0.001 |
Threshold effect analysis of serum ferritin on MoCA scores.
The model adjusted for age, education, dialysis period, and transferrin saturation. MoCA: montreal cognitive assessment.
Subgroup analyses were performed to assess the robustness and consistency of the SF-MoCA score relationship across potential effect modifiers, including age, gender, BMI, educational attainment, dialysis duration, diabetes, hypertension, and peritoneal transport status. Detailed results were reported in Supplementary Table 2. All interaction tests demonstrated non-significant effects (all P for interaction>0.05), indicating that the inverse association between SF and MoCA scores remained stable regardless of these stratification variables.
3.3 Increased regional brain iron in PD compared with controls
Following Bonferroni correction, significant intergroup differences in iron deposition were observed among PD and HC across specific brain regions (Table 5). The PD group exhibited significantly higher susceptibility values in the left amygdala, and right putamen compared to HC (p < 0.005/116 = 0.0004). No statistically significant differences were detected in other regions, including the hippocampus, caudate nucleus, globus pallidus, or thalamus (all p > 0.05/116 = 0.0004).
Table 5
| ROI | HC (n = 49) | PD (n = 52) | Effect size (d/Δ) | P value* |
|---|---|---|---|---|
| Hippocampus_L | −0.0014 ± 0.009 | −0.0021 ± 0.010 | −0.074 | 0.713 |
| Hippocampus_R | −0.0050 ± 0.008 | −0.0046 ± 0.010 | 0.045 | 0.824 |
| Amygdala_L | −0.0280 (−0.0465, −0.0164) | −0.0140 (−0.0236, 0.0022) | 0.669 | 0.0002 |
| Amygdala_R | −0.0267 (−0.0468, −0.0123) | −0.0153 (−0.0274, −0.0065) | 0.379 | 0.017 |
| Caudate_L | 0.0071 ± 0.016 | 0.0066 ± 0.013 | −0.031 | 0.878 |
| Caudate_R | 0.0141 (0.0068, 0.0293) | 0.0120 (−0.0007, 0.0178) | −0.279 | 0.099 |
| Putamen_L | 0.0080 ± 0.019 | 0.0206 ± 0.017 | 0.689 | 0.001 |
| Putamen_R | 0.0124 ± 0.019 | 0.0268 ± 0.018 | 0.771 | 0.0002 |
| Pallidum_L | 0.0636 ± 0.032 | 0.0762 ± 0.038 | 0.358 | 0.076 |
| Pallidum_R | 0.0765 (0.0468, 0.0973) | 0.0755 (0.0562, 0.0919) | 0.165 | 0.625 |
| Thalamus_L | −0.0071 (−0.0181, −0.0006) | −0.0022 (−0.0082, 0.0056) | 0.552 | 0.006 |
| Thalamus_R | −0.0079 ± 0.011 | −0.0037 ± 0.010 | 0.381 | 0.059 |
Comparison of susceptibility (ppm) in selected structures among two groups.
Values are presented as means ± SD or interquartile range or percentages. *For the comparison among two groups (Bonferroni-corrected P < 0.05/116 = 0.0004). ROI: region of interest; PD: peritoneal dialysis; HC: healthy control.
3.4 Correlation analysis of regional brain iron with cognitive and iron metabolism indicators
The MoCA total score was significantly correlated with the susceptibility values of the left amygdala (Figure 3A). Subsequently, the correlation analysis revealed distinct correlation patterns between magnetic susceptibility values in the left amygdala and multidimensional cognitive assessments (Figures 3B–F and Supplementary Table 3). Specifically, the left amygdala exhibited significant positive correlations with SAS (r = 0.297, p = 0.004, FDR corrected) and SDS (r = 0.295, p = 0.004, FDR corrected), while demonstrating negative correlations with DST-forwards, DST-backwards, and VFT (r = −0.371, p < 0.001; r = −0.226, p = 0.019, and r = −0.216, p = 0.022, FDR corrected, respectively). However, the right putamen demonstrated no significant correlations with cognition function measures (all p > 0.05).
Figure 3

Correlation analysis between the susceptibility of left amygdala and neuropsychological data. (A) MoCA score; (B) SAS score; (C) SDS score; (D) DST-forwards score; (E) DST-backwards score; (F) VFT score. p values were corrected for false discovery rate. MoCA: montreal cognitive assessment; SAS: self-rating anxiety scale; SDS: self-rating depression scale; DST: digit span test; VFT: verbal fluency test.
Moreover, the susceptibility values in the left amygdala showed no significant correlation with hemoglobin, serum iron, ferritin, or transferrin saturation in peritoneal dialysis patients (all p > 0.05) (Supplementary Figures 1A–D).
4 Discussion
This study systematically investigates, for the first time, the synergistic mechanism between iron metabolism abnormalities and region-specific cerebral iron deposition in cognitive impairment among peritoneal dialysis patients. Through multidimensional neuropsychological assessments combined with quantitative susceptibility mapping, two pivotal findings emerge: First, serum ferritin exhibits a inverted U-shaped relationship with global cognitive function, with its critical threshold effect (258.4 μg/L) establishing a basis for clinical iron overload management. Second, iron deposition in the left amygdala demonstrates specific associations with attention, emotional regulation, and language competence. These spatial distribution patterns contrast with Alzheimer’s disease-related pathological signatures (Zhi et al., 2024), suggesting a distinct neural regulatory mechanism underlying uremia-associated cognitive decline.
The present study revealed that 65% of PD patients exhibited cognitive impairment, a finding highly consistent with recent research by Bras et al. in European populations (Bras et al., 2024). However, a striking discrepancy was observed when compared with the 15% CI prevalence reported in Western populations by Noumann’s team (Neumann et al., 2018) which may be attributed to heterogeneity in assessment tools. Our multidimensional neuropsychological battery evaluation demonstrated significant impairments across multiple cognitive domains, particularly in executive function, processing speed, and language abilities. Notably, despite these pervasive cognitive deficits, PD therapy remains clinically feasible through its user-friendly home-based protocol (requiring only 3–4 daily exchanges), automated device support (e.g., APD cycler), and comprehensive medical team guidance (Mujais and Story, 2006). This suggests that PD implementation may have a relatively low cognitive threshold requirement for successful treatment adherence.
Iron, a pivotal regulator that mediates neurotoxic effects, has been closely associated with cognitive impairment through pathological iron accumulation. Studies demonstrate that iron overload induces neuronal damage via oxidative stress, inflammatory cascades, and ferroptosis pathways (Li et al., 2023; Xie et al., 2023). The heightened expression of transferrin receptor 1 (TfR1) within the central nervous system potentially facilitates iron ion transport across the blood–brain barrier (BBB), leading to cerebral iron deposition and subsequent neurodegenerative pathologies (Li et al., 2012; Zhang et al., 2021). Elevated serum ferritin levels exhibit significant correlations with cognitive impairment disorders including Alzheimer’s disease and cerebral hemorrhage, underscoring its neurotoxic potential (Lan et al., 2021; Li et al., 2024). Additionally, there is also evidence indicating that improvements in SF levels are associated with enhanced cognitive abilities, particularly in working memory, language processing, and executive functions (Raz et al., 2022; Rosell-Diaz et al., 2024). However, it is crucial to acknowledge that this association between peripheral ferritin and cognitive function stands in contrast to the broader body of research in Alzheimer’s disease. Most studies investigating Alzheimer’s disease have failed to detect consistent correlations between peripheral iron parameters (such as serum ferritin, transferrin saturation, and serum iron) and cognitive decline (Milward et al., 2010; Wang et al., 2025). Instead, compelling evidence in Alzheimer’s disease and other neurodegenerative disorders (e.g., Parkinson’s disease, vascular dementia) highlights that regional cerebral iron accumulation is the key pathological correlate of cognitive impairment (Moon et al., 2016; Guan et al., 2022). Current study only reveals a marked inverse correlation between serum ferritin concentrations and cognitive performance in peritoneal dialysis population. Multivariate regression analysis identifies both age and serum ferritin as independent risk factors for cognitive impairment. However, when using the Generalized Additive Model and curve fitting for curve analysis, an inverted U-shaped relationship was identified between serum ferritin levels and MoCA scores in peritoneal dialysis patients and the β value are low. Therefore, further research is necessary to determine the clinical significance of these findings. Mechanistically, iron accumulation may impair cognition through three synergistic pathways: (1) Iron-mediated free radical generation exacerbating oxidative damage (Munoz and Humeres, 2012); (2) Microglial activation triggering pro-inflammatory cytokine release (e.g., IL-6, TNF-α), thereby disrupting synaptic plasticity (Wang et al., 2023); (3) Synergistic interaction with uremic toxins to accelerate β-amyloid deposition. Clinically, the threshold of serum ferritin ≥258.4 μg/L shows promise as an early warning indicator for cognitive decline, with dynamic monitoring enabling timely interventions. Nevertheless, large-scale prospective studies remain imperative to validate these findings.
Previous studies have demonstrated that iron metabolism disorders represent one of the critical mechanisms underlying neurological complications in patients with CKD (Agarwal, 2011). ESRD patients with chronic anemia often require treatment with erythropoietin (EPO) and iron supplementation, which may predispose them to systemic iron overload. Research involving hemodialysis patients has revealed a significant increase in iron deposition in brain regions such as the caudate, which is associated with cognitive decline (Chai et al., 2015). Due to differences in treatment modalities (e.g., continuous solute clearance and hemodynamic stability), the iron metabolism characteristics of PD patients may differ from those observed in hemodialysis patients. Animal studies indicate that the accumulation of uremic toxins can upregulate iron transporters, such as transferrin receptor 1, in the brain, thereby promoting iron deposition across the blood–brain barrier (Deguchi et al., 2006). Nevertheless, the specific pattern of iron deposition in distinct brain regions and its association with cognitive impairment in PD patients remains poorly understood. The present study revealed that the left amygdala and right putamen in PD patients exhibited significantly higher values compared to healthy controls, with the former showing a negative correlation with MoCA scores. These findings suggest that cognitive impairment in peritoneal dialysis patients may be mechanistically linked to iron dysregulation in limbic structures, particularly the amygdala. As a core structure of the limbic system, the abnormal deposition of iron in the amygdala may lead to the generation of free radicals, potentially impairing cognitive function through the following mechanisms. First, excessive iron ions catalyze the production of free radicals, disrupting synaptic plasticity and neurotransmitter balance (e.g., 5-HT and GABA systems), thereby directly affecting emotion regulation and memory consolidation (Thielen et al., 2019). Second, disrupted iron homeostasis may exacerbate anxiety and depressive-like behaviors by modulating the GABAergic neurotransmitter system to enhance aberrant activation of the amygdala-prefrontal neural circuitry, while simultaneously suppressing hippocampal-dependent cognitive processes (Alves et al., 2022; Arora et al., 2024; Zhang et al., 2023).
The precise relationship between peripheral iron metabolism and cerebral iron metabolism has not been fully elucidated. This study also found no significant correlation between magnetic susceptibility values in the left amygdala and levels of hemoglobin, serum iron, ferritin, or transferrin saturation in peritoneal dialysis patients, suggesting that cerebral iron metabolism and systemic iron homeostasis may be regulated through independent mechanisms. First, the integrity of the blood–brain barrier may be compromised in chronic kidney disease, potentially decoupling brain iron deposition from peripheral iron parameters (Faucher et al., 2023). Simultaneously, the expression of key cerebral iron regulatory proteins, such as hepcidin and transferrin receptors, exhibits region-specific patterns within the central nervous system (Zheng and Monnot, 2012). Amygdala-specific iron metabolism pathways may maintain local iron equilibrium via the autophagy-lysosomal system (Xu et al., 2017). Furthermore, neuroinflammation triggered by the accumulation of uremic toxins may activate the iron transport system within microglia, leading to localized iron deposition independent of systemic iron status (Hamed, 2019; Xu et al., 2017).
The present study has several limitations that warrant attention. Firstly, due to the small sample size, we should approach the interpretation and application of our findings with caution. Secondly, the cross-sectional design makes it difficult to establish the causal relationship between iron metabolism indicators and cognitive impairment. In particular, the microinflammatory state commonly existing in peritoneal dialysis patients may simultaneously affect the redistribution of iron metabolism and the permeability of the blood–brain barrier. Longitudinal studies are required to observe the process of iron deposition. Third, systematic measurements of key regulatory factors such as hepcidin and IL-6 were not performed, potentially missing important mediating variables in the inflammation-iron metabolism interaction. In addition, due to the exclusion of non-dialysis CKD patients and HD patients, we cannot clarify the direction of PD’s impact on cerebral iron deposition, nor can we determine if the iron deposition in the left amygdala and right putamen is a PD-specific finding or a common change in ESRD. Meanwhile, we cannot rule out the possibility of similar iron deposition in non-dialysis CKD/HD patients. Cross-sectional studies will be conducted in the future to compare the patterns of cerebral iron deposition among these three groups of patients and longitudinal studies will be carried out to track changes in iron metabolism in non-dialysis CKD patients as their disease progresses, in order to clarify the association between PD and iron abnormalities. Finally, while the AAL template was usually used for brain segmentation, its limitations in defining anatomical boundaries of deep nuclei (e.g., globus pallidus, substantia nigra) may introduce partial volume effect errors.
5 Conclusion
In this study, QSM was employed to quantitatively analyze iron deposition changes in the hippocampus, amygdala, caudate nucleus, putamen, globus pallidus, and thalamus. The results demonstrated that the severity of PD-MCI correlates with cerebral iron deposition. Specifically, magnetic susceptibility values in the left amygdala showed a negative correlation with cognitive function assessed by the MoCA. These findings suggest that magnetic susceptibility in the left amygdala, which reflects regional iron deposition, is closely associated with cognitive impairment in PD patients and may hold potential as a candidate imaging indicator for exploring the neuropathological mechanisms of cognitive decline in this population.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the First Affiliated Hospital, Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DL: Methodology, Investigation, Conceptualization, Writing – original draft, Formal analysis, Writing – review & editing. YY: Writing – original draft, Writing – review & editing, Investigation, Conceptualization, Methodology, Formal analysis. SJ: Investigation, Writing – review & editing, Methodology. JY: Methodology, Writing – review & editing, Investigation. LY: Writing – review & editing, Methodology, Investigation. NF: Resources, Writing – review & editing, Methodology, Project administration, Investigation. ZS: Project administration, Writing – review & editing, Methodology, Investigation, Resources. ZC: Resources, Project administration, Investigation, Writing – review & editing, Methodology. HX: Writing – review & editing, Project administration, Methodology, Investigation, Resources. YZ: Methodology, Investigation, Writing – review & editing, Data curation, Formal analysis. JF: Data curation, Writing – review & editing, Formal analysis, Methodology, Investigation. XQ: Investigation, Writing – review & editing, Visualization, Validation. HW: Writing – original draft, Conceptualization, Supervision, Validation, Writing – review & editing. YW: Conceptualization, Supervision, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all participants and their families who were involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1660734/full#supplementary-material
References
1
Agarwal A. K. (2011). (2021). Iron metabolism and management: focus on chronic kidney disease. Kidney Int. Suppl.11, 46–58. doi: 10.1016/j.kisu.2020.12.003
2
Alves J. de Sa C. N. de Lima R. Quillfeldt J. A. Dalmaz C. (2022). Effects of early life adversities upon memory processes and cognition in rodent models. Neuroscience497, 282–307. doi: 10.1016/j.neuroscience.2022.04.023
3
Arora I. Mal P. Arora P. Paul A. Kumar M. (2024). Gabaergic implications in anxiety and related disorders. Biochem. Biophys. Res. Commun.724:150218. doi: 10.1016/j.bbrc.2024.150218
4
Bras A. C. Marques J. Fernandes V. Ferreira A. C. (2024). Cognitive dysfunction screening in peritoneal dialysis patients: a cross-sectional study. Indian J. Nephrol.34, 357–362. doi: 10.25259/ijn_378_23
5
Chai C. Yan S. Chu Z. Wang T. Wang L. Zhang M. et al . (2015). Quantitative measurement of brain iron deposition in patients with haemodialysis using susceptibility mapping. Metab. Brain Dis.30, 563–571. doi: 10.1007/s11011-014-9608-2
6
Chan K. S. Marques J. P. (2021). Sepia-susceptibility mapping pipeline tool for phase images. NeuroImage227:117611. doi: 10.1016/j.neuroimage.2020.117611
7
Deguchi T. Isozaki K. Yousuke K. Terasaki T. Otagiri M. (2006). Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier. J. Neurochem.96, 1051–1059. doi: 10.1111/j.1471-4159.2005.03550.x
8
Drew D. A. Weiner D. E. Sarnak M. J. (2019). Cognitive impairment in ckd: pathophysiology, management, and prevention. Am. J. Kidney Dis.74, 782–790. doi: 10.1053/j.ajkd.2019.05.017
9
Faucher Q. van der Made T. K. De Lange E. Masereeuw R. (2023). Blood-brain barrier perturbations by uremic toxins: key contributors in chronic kidney disease-induced neurological disorders?Eur. J. Pharm. Sci.187:106462. doi: 10.1016/j.ejps.2023.106462
10
Guan X. Guo T. Zhou C. Wu J. Zeng Q. Li K. et al . (2022). Altered brain iron depositions from aging to Parkinson's disease and Alzheimer's disease: A quantitative susceptibility mapping study. NeuroImage1:119683. doi: 10.1016/j.neuroimage.2022.119683
11
Haacke E. M. Cheng N. Y. House M. J. Liu Q. Neelavalli J. Ogg R. J. et al . (2005). Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging23, 1–25. doi: 10.1016/j.mri.2004.10.001
12
Hamed S. A. (2019). Neurologic conditions and disorders of uremic syndrome of chronic kidney disease: presentations, causes, and treatment strategies. Expert. Rev. Clin. Pharmacol.12, 61–90. doi: 10.1080/17512433.2019.1555468
13
Kalirao P. Pederson S. Foley R. N. Kolste A. Tupper D. Zaun D. et al . (2011). Cognitive impairment in peritoneal dialysis patients. Am. J. Kidney Dis.57, 612–620. doi: 10.1053/j.ajkd.2010.11.026
14
Kim S. Sharma C. Jung U. J. Kim S. R. (2023). Pathophysiological role of microglial activation induced by blood-borne proteins in Alzheimer's disease. Biomedicine11:383. doi: 10.3390/biomedicines11051383
15
Lan T. Y. Cui D. Y. Liu T. T. Pan J. Y. Wang X. (2021). Correlation of serum ferritin levels with neurological function-related indices and cognitive dysfunction in patients with cerebral hemorrhage. Clin. Neuropathol.40, 333–340. doi: 10.5414/NP301368
16
Li Y. Duan L. Shi Y. Qi J. Li T. (2024). Diagnostic accuracy of combined measurement of serum homocysteine, c-reactive protein, and serum ferritin in mild cognitive impairments and alzheimer's disease. J. Coll. Physicians Surg. Pak.34, 1303–1308. doi: 10.29271/jcpsp.2024.11.1303
17
Li Y. Wang B. Yang J. Liu R. Xie J. Wang J. (2023). Iron overload causes ferroptosis but not apoptosis in mo3.13 oligodendrocytes. Neurochem. Res.48, 830–838. doi: 10.1007/s11064-022-03807-6
18
Li Q. Zhou L. Shen Y. (2012). Recent research progresses of transferrin receptor 1 in central nervous system. Sheng Li Ke Xue Jin Zhan43, 188–192.
19
Liu C. Li W. Tong K. A. Yeom K. W. Kuzminski S. (2015). Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J. Magn. Reson. Imaging42, 23–41. doi: 10.1002/jmri.24768
20
Liu G. L. Pi H. C. Hao L. Li D. D. Wu Y. G. Dong J. (2015). Vitamin d status is an independent risk factor for global cognitive impairment in peritoneal dialysis patients. PLoS One10:e143782. doi: 10.1371/journal.pone.0143782
21
Liu Z. Shen X. Li M. Liu P. Ge Z. Jin J. (2025). Exploring the nexus: how ferroptosis, microglia, and neuroinflammation converge in ischemic stroke pathogenesis. Mol. Neurobiol.62, 8965–8972. doi: 10.1007/s12035-025-04815-7
22
Lu J. Li D. Li F. Zhou A. Wang F. Zuo X. et al . (2011). Montreal cognitive assessment in detecting cognitive impairment in chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol.24, 184–190. doi: 10.1177/0891988711422528
23
Marcos G. Santabarbara J. Lopez-Anton R. De-la-Camara C. Gracia-Garcia P. Lobo E. et al . (2016). Conversion to dementia in mild cognitive impairment diagnosed with dsm-5 criteria and with petersen's criteria. Acta Psychiatr. Scand.133, 378–385. doi: 10.1111/acps.12543
24
Milward E. A. Bruce D. G. Knuiman M. W. Divitini M. L. Cole M. Inderjeeth C. A. et al . (2010). A cross-sectional community study of serum iron measures and cognitive status in older adults. J Alzheimer's Dis20, 617–623. doi: 10.3233/JAD-2010-1402
25
Modica C. M. Zivadinov R. Dwyer M. G. Bergsland N. Weeks A. R. Benedict R. H. (2015). Iron and volume in the deep gray matter: association with cognitive impairment in multiple sclerosis. AJNR Am. J. Neuroradiol.36, 57–62. doi: 10.3174/ajnr.A3998
26
Moist L. M. Port F. K. Orzol S. M. Young E. W. Ostbye T. Wolfe R. A. et al . (2000). Predictors of loss of residual renal function among new dialysis patients. J. Am. Soc. Nephrol.11, 556–564. doi: 10.1681/ASN.V113556
27
Moon Y. Han S. H. Moon W. J. (2016). Patterns of brain iron accumulation in vascular dementia and alzheimer's dementia using quantitative susceptibility mapping imaging. J Alzheimer's Dis51, 737–745. doi: 10.3233/JAD-151037
28
Mujais S. Story K. (2006). Peritoneal dialysis in the us: evaluation of outcomes in contemporary cohorts. Kidney Int. Suppl.2011, S21–S26. doi: 10.1038/sj.ki.5001912
29
Munoz P. Humeres A. (2012). Iron deficiency on neuronal function. Biometals25, 825–835. doi: 10.1007/s10534-012-9550-x
30
Neumann D. Mau W. Wienke A. Girndt M. (2018). Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int.93, 430–438. doi: 10.1016/j.kint.2017.07.022
31
Niikura T. Maruyama Y. Nakashima S. Matsuo N. Tanno Y. Ohkido I. et al . (2019). Hepcidin/ferritin ratios differ among non-dialyzed chronic kidney disease patients, and patients on hemodialysis and peritoneal dialysis. Ther. Apher. Dial.23, 341–346. doi: 10.1111/1744-9987.12773
32
Ning N. Liu C. Wu P. Hu Y. Zhang W. Zhang L. et al . (2019). Spatiotemporal variations of magnetic susceptibility in the deep gray matter nuclei from 1 month to 6 years: a quantitative susceptibility mapping study. J. Magn. Reson. Imaging49, 1600–1609. doi: 10.1002/jmri.26579
33
Nolph K. D. Moore H. L. Prowant B. Meyer M. Twardowski Z. J. Khanna R. et al . (1993). Cross sectional assessment of weekly urea and creatinine clearances and indices of nutrition in continuous ambulatory peritoneal dialysis patients. Perit. Dial. Int.13, 178–183.
34
Nunez M. T. Hidalgo C. (2019). Noxious iron-calcium connections in neurodegeneration. Front. Neurosci.13:48. doi: 10.3389/fnins.2019.00048
35
Parkin P. C. Koroshegyi C. Mamak E. Borkhoff C. M. Birken C. S. Maguire J. L. et al . (2020). Association between serum ferritin and cognitive function in early childhood. J. Pediatr.217, 189–191. doi: 10.1016/j.jpeds.2019.09.051
36
Raz S. Koren A. Levin C. (2022). Associations between red blood cell indices and iron status and neurocognitive function in young adults: evidence from memory and executive function tests and event-related potentials. Ann. N. Y. Acad. Sci.1517, 300–313. doi: 10.1111/nyas.14877
37
Rosell-Diaz M. Santos-Gonzalez E. Motger-Alberti A. Gallardo-Nuell L. Arnoriaga-Rodriguez M. Coll-Martinez C. et al . (2024). Lower serum ferritin levels are associated with worse cognitive performance in aging. J. Nutr. Health Aging28:100190. doi: 10.1016/j.jnha.2024.100190
38
Shea Y. F. Lam M. F. Lee M. S. Mok M. Y. Lui S. L. Yip T. P. et al . (2016). Prevalence of cognitive impairment among peritoneal dialysis patients, impact on peritonitis and role of assisted dialysis. Perit. Dial. Int.36, 284–290. doi: 10.3747/pdi.2014.00247
39
Sinnakirouchenan R. Holley J. L. (2011). Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv. Chronic Kidney Dis.18, 428–432. doi: 10.1053/j.ackd.2011.09.001
40
Thielen J. W. Gancheva S. Hong D. Rohani R. S. Chen B. Apostolopoulou M. et al . (2019). Higher gaba concentration in the medial prefrontal cortex of type 2 diabetes patients is associated with episodic memory dysfunction. Hum. Brain Mapp.40, 4287–4295. doi: 10.1002/hbm.24702
41
Viggiano D. Wagner C. A. Martino G. Nedergaard M. Zoccali C. Unwin R. et al . (2020). Mechanisms of cognitive dysfunction in ckd. Nat. Rev. Nephrol.16, 452–469. doi: 10.1038/s41581-020-0266-9
42
Wang X. Bakulski K. M. Karvonen-Gutierrez C. A. Park S. K. Morgan D. Jackson B. P. et al . (2025). Blood essential trace elements and Alzheimer's disease biomarkers in midlife. Front. Aging Neurosci.30:1539749. doi: 10.3389/fnagi.2025.1539749
43
Wang H. Liu X. Song L. Yang W. Li M. Chen Q. et al . (2023). Dysfunctional coupling of cerebral blood flow and susceptibility value in the bilateral hippocampus is associated with cognitive decline in nondialysis patients with ckd. J. Am. Soc. Nephrol.34, 1574–1588. doi: 10.1681/ASN.0000000000000185
44
Wang C. Martins-Bach A. B. Alfaro-Almagro F. Douaud G. Klein J. C. Llera A. et al . (2022). Phenotypic and genetic associations of quantitative magnetic susceptibility in uk biobank brain imaging. Nat. Neurosci.25, 818–831. doi: 10.1038/s41593-022-01074-w
45
Wang M. Tang G. Zhou C. Guo H. Hu Z. Hu Q. et al . (2023). Revisiting the intersection of microglial activation and neuroinflammation in alzheimer's disease from the perspective of ferroptosis. Chem. Biol. Interact.375:110387. doi: 10.1016/j.cbi.2023.110387
46
Wu Q. Ren Q. Meng J. Gao W. J. Chang Y. Z. (2023). Brain iron homeostasis and mental disorders. Antioxidants12:997. doi: 10.3390/antiox12111997
47
Xie J. Lv H. Liu X. Xia Z. Li J. Hong E. et al . (2023). Nox4-and tf/tfr-mediated peroxidation and iron overload exacerbate neuronal ferroptosis after intracerebral hemorrhage: involvement of eaat3 dysfunction. Free Radic. Biol. Med.199, 67–80. doi: 10.1016/j.freeradbiomed.2023.02.015
48
Xu H. Wang Y. Song N. Wang J. Jiang H. Xie J. (2017). New progress on the role of glia in iron metabolism and iron-induced degeneration of dopamine neurons in parkinson's disease. Front. Mol. Neurosci.10:455. doi: 10.3389/fnmol.2017.00455
49
Yamaji Y. Nakazato Y. Oshima N. Hayashi M. Saruta T. (2004). Oxidative stress induced by iron released from transferrin in low ph peritoneal dialysis solution. Nephrol. Dial. Transplant.19, 2592–2597. doi: 10.1093/ndt/gfh278
50
Zhang G. Lv S. Zhong X. Li X. Yi Y. Lu Y. et al . (2023). Ferroptosis: a new antidepressant pharmacological mechanism. Front. Pharmacol.14:1339057. doi: 10.3389/fphar.2023.1339057
51
Zhang N. Yu X. Xie J. Xu H. (2021). New insights into the role of ferritin in iron homeostasis and neurodegenerative diseases. Mol. Neurobiol.58, 2812–2823. doi: 10.1007/s12035-020-02277-7
52
Zheng W. Monnot A. D. (2012). Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol. Ther.133, 177–188. doi: 10.1016/j.pharmthera.2011.10.006
53
Zhi Y. Huang T. Liu S. Li M. Hu H. Liang X. et al . (2024). Correlation between iron deposition and cognitive function in mild to moderate alzheimer's disease based on quantitative susceptibility mapping. Front. Aging Neurosci.16:1485530. doi: 10.3389/fnagi.2024.1485530
54
Zou L. X. Sun L. Hua R. X. Wu Y. (2021). Serum hepcidin-25 and all-cause mortality in patients undergoing maintenance hemodialysis. Int. J. Gen. Med.14, 3153–3162. doi: 10.2147/IJGM.S313777
Summary
Keywords
peritoneal dialysis, serum ferritin, iron deposition, cognitive impairment, quantitative susceptibility mapping
Citation
Li D, Yin Y, Jiang S, Yin J, Yu L, Feng N, Sun Z, Chen Z, Xu H, Zhou Y, Fang J, Qi X, Wang H and Wu Y (2025) Regional high iron deposition is linked with cognitive impairments in peritoneal dialysis: a quantitative susceptibility mapping study. Front. Aging Neurosci. 17:1660734. doi: 10.3389/fnagi.2025.1660734
Received
06 July 2025
Accepted
15 September 2025
Published
24 September 2025
Volume
17 - 2025
Edited by
Claudia Fiorillo, University of Florence, Italy
Reviewed by
Nadja Schroder, Federal University of Rio Grande do Sul, Brazil
Jun Li, Shanghai Jiao Tong University, China
Updates
Copyright
© 2025 Li, Yin, Jiang, Yin, Yu, Feng, Sun, Chen, Xu, Zhou, Fang, Qi, Wang and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggui Wu, wuyonggui@medmail.com.cn; Haibao Wang, wanghaibao916@163.com
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.