- IRCCS Centro Neurolesi "Bonino-Pulejo", Messina, Italy
Background: Cognitive dysfunction is a common impairment observed in individuals with Multiple Sclerosis (MS), affecting key domains such as attention, information processing speed, memory, and executive functions. This deficit is typically identified through comprehensive neuropsychological assessments, which represent the gold standard for cognitive evaluation. In recent years, increasing efforts have been made to integrate neuropsychological findings with advanced neuroimaging techniques to better understand the neural substrates of cognitive dysfunction. Among these, Diffusion Tensor Imaging (DTI) has emerged as a valuable tool for investigating microstructural changes in white matter (WM) that may underlie cognitive deficits in MS. However, despite its clinical utility, the pathophysiological mechanisms contributing to cognitive impairment, particularly in subjects with Relapsing–Remitting MS (RRMS), remain complex and not yet fully understood. This review focuses on studies investigating WM alterations measured by DTI and their correlation with cognitive dysfunction as assessed through neuropsychological testing.
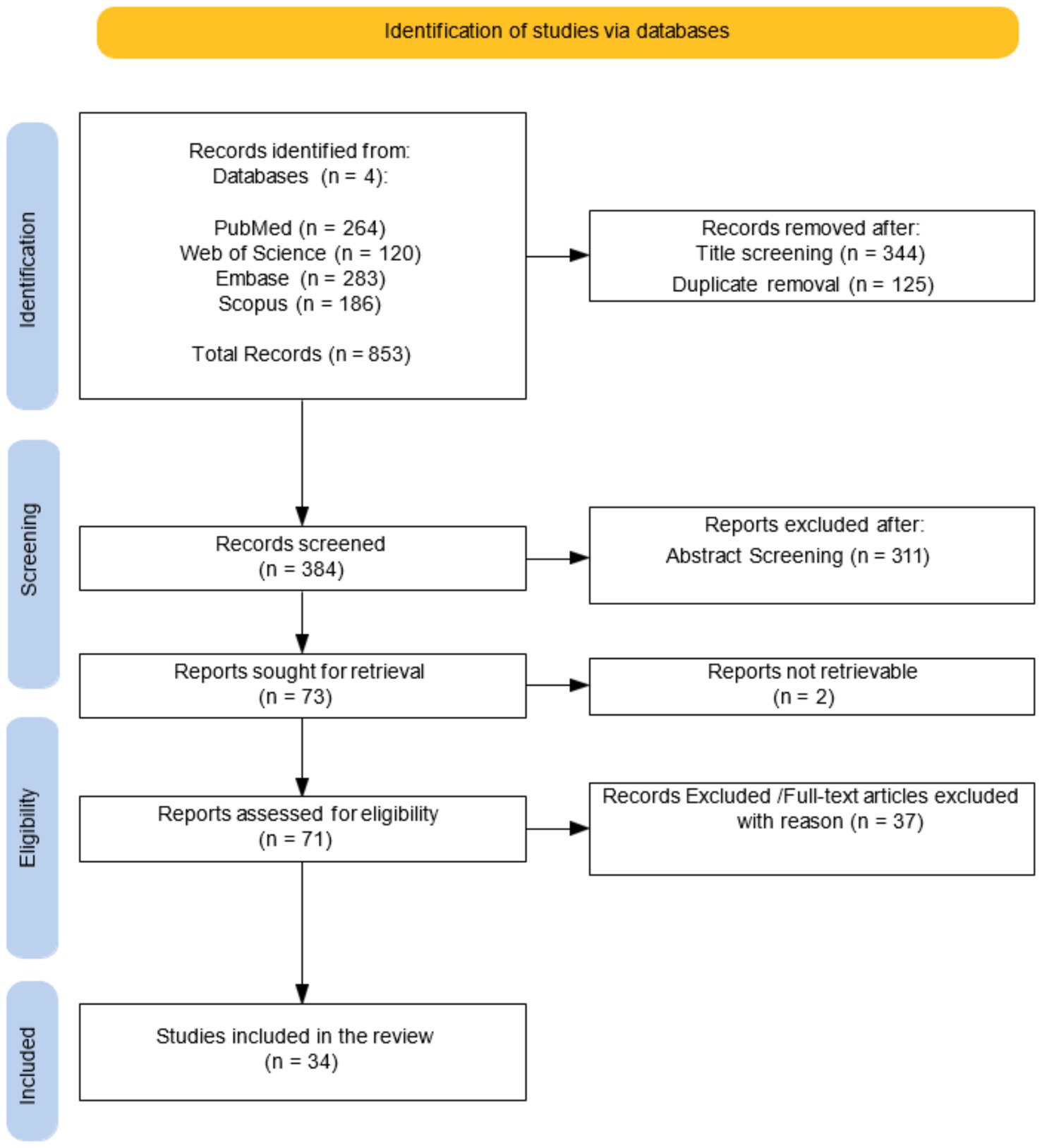
Method: Papers were identified by searching in PubMed, Embase, Web of Science and Scopus databases from 2002 - the years, of first related published article, − to December 2024. From the initial 853, we included only 34 studies that met to eligibility criteria.
Results: In subjects with RRMS, WM alterations, assessed through DTI, were found to correlate with cognitive dysfunction, as measured by standardized neuropsychological tests. These alterations were observed both in global WM and in specific regions, including the corpus callosum, thalamus, hippocampus, cerebellar structure, cingulum, and cerebral fascicles.
Conclusion: These findings underscore the relevance of integrating neuropsychological assessment with advanced neuroimaging techniques, such as DTI, to enhance our understanding of cognitive impairment in RRMS. DTI-derived measures of WM integrity show promise as potential biomarkers of cognitive dysfunction, while cognitive profiling can help localize underlying neuropsychological damage. This integrated approach may improve early detection of cognitive alterations and support the development of targeted therapeutic strategies aimed at preserving cognitive functioning in individuals with MS.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251073195, Identifier CRD420251073195.
1 Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory, neurodegenerative disease that damages the brain and spinal cord by causing demyelination. It is marked by neurological symptoms and signs which contribute to physical disability (motor, sensory, and visual deficits, etc.), cognitive dysfunction, and fatigue even in the early stages of the disease. According to an estimate from the MS International Federation of Atlas, approximately 2.8 million people worldwide live with a diagnosis of MS. Globally, MS prevalence is 35.9 cases per 100,000 residents, with a higher incidence in women (Walton et al., 2020). Cognitive dysfunction is a major contributor to MS-related disability, with prevalence estimates ranging from 40 to 65% during the course of the disease (Chiaravalloti and DeLuca, 2008). Its frequency and severity may increase over time, becoming more evident in cases of MS progressive form. The presence of cognitive dysfunction is associated with a worse prognosis, reduced social participation, and lower quality of life (Rao et al., 1991; Amato et al., 2006). The pathophysiological mechanisms underlying cognitive dysfunction in MS have a multifactorial etiology, e.g., alterations in nerve conduction due to demyelination processes; axonal damage within cognitive networks; failure of compensatory mechanisms due to brain injury (Prins et al., 2015). Magnetic resonance imaging (MRI) studies have consistently highlighted correlations between the progression of cognitive dysfunction in MS and increased tissue damage, brain atrophy, and alterations in functional connectivity, particularly within the prefrontal cortex and limbic system (Zhang et al., 2021; Keser et al., 2018).
Among advanced MRI modalities, Diffusion Tensor Imaging (DTI) has emerged as a valuable tool for investigating organization and microstructural brain alterations that underlie cognitive dysfunction in MS. DTI allows for the in vivo assessment of white matter (WM) integrity by quantifying the diffusion properties of water molecules within neural tissues (Lope-Piedrafita, 2018). This technique is particularly sensitive to subtle tissue damage that may not be detectable with conventional MRI, including abnormalities in both WM lesions and apparently normal-appearing brain tissue (NABT), as well as in cortical and deep grey matter regions (Hori et al., 2022; Kolasa et al., 2019).
Key DTI-derived metrics include fractional anisotropy (FA), which reflects the directionality of water diffusion and serves as an indicator of WM fiber integrity (Beaulieu, 2002); radial diffusivity (RD), which represents the average diffusion of water molecules perpendicular to the principal fiber orientation and is particularly sensitive to myelin integrity (Song et al., 2002); axial diffusivity (AD), reflecting water diffusion parallel to the main fiber direction and often associated with axonal integrity (Song et al., 2003); mean diffusivity (MD), reflecting the overall magnitude of water diffusion and indicative of tissue density and extracellular space (Alexander et al., 2007); and the apparent diffusion coefficient (ADC), which quantifies the ease of water molecule movement within tissue and is often increased in areas of microstructural damage (Bihan et al., 2001).
Alterations in these parameters have been associated with demyelination, axonal loss, and disruption of cellular membranes, thereby providing insights into the microstructural substrates of cognitive dysfunction in MS (Andersen et al., 2018; Sijens et al., 2006). Importantly, abnormalities detected in NABT through DTI may precede the formation of new lesions or occur concurrently in homologous contralateral regions, suggesting a diffuse and dynamic pathological process (Bao et al., 2022).
Rao et al. (1989) suggest that cognitive dysfunction is caused by a disruption of cortico-subcortical circuits, which connect the frontal cortices to the thalamus and basal ganglia. However, other studies have reported that posterior brain areas and the corpus callosum (CC) may also play a role in cognitive impairment (Mesaros et al., 2009; Granberg et al., 2015). Furthermore, it has been suggested that a reduction in information processing speed may be associated with difficulties in sensory and motor functions (Dineen et al., 2009; Roosendaal et al., 2009).
Although the so-called “clinical–radiological paradox” describes the imperfect correlation between radiological findings and clinical symptoms (Rocca et al., 2015; Sumowski and Leavitt, 2013), cognitive dysfunction is frequently observed in MS subjects, affecting multiple domains such as attention, information processing speed (IPS), memory, and executive control (De Meo et al., 2021; Lugosi et al., 2024). The Symbol Digit Modality Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) are among the most used tests for evaluating cognitive deficits associated with lesions in specific brain areas in MS, measuring attention, concentration, and information processing speed (Matias-Guiu et al., 2018; Berrigan et al., 2022). Despite its clinical relevance, the underlying mechanisms of cognitive dysfunction in MS remain complex and not fully understood.
This review was conducted to clarify the current state of knowledge regarding the correlation between WM damage, as detected through DTI, and cognitive dysfunction assessed by neuropsychological evaluation.
2 Materials and method
2.1 Search strategy
A protocol for this systematic review was registered in PROSPERO (2025 ID: CRD420251073195). The review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The PRISMA flow diagram was employed to provide a visual overview of the study selection process, encompassing the stages of identification, screening, eligibility, and inclusion. The PRISMA guidelines were followed to identify and analyse DTI studies investigating WM and cognitive function in MS patients. This systematic review did not involve human nor animal data collection.
Papers were identified (from September 2024 to December 2024) by searching in PubMed, Embase, Web of Science and Scopus database from 2002 - the years, of first related published article, − to December 2024. The search combined the following terms (“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields]) OR “multiple sclerosis”[All Fields] OR (“sclerosis”[All Fields] AND “multiple”[All Fields]) OR “sclerosis multiple”[All Fields]) AND (“diffusion tensor imaging”[MeSH Terms] OR (“diffusion”[All Fields] AND “tensor”[All Fields] AND “imaging”[All Fields]) OR “diffusion tensor imaging”[All Fields]) AND (“cognition”[MeSH Terms] OR “cognition”[All Fields] OR “cognitions”[All Fields] OR “cognitive”[All Fields] OR “cognitively”[All Fields] OR “cognitives”[All Fields]).
2.2 Data extraction
Two independent reviewers (AM and CS) screened titles and abstracts and extracted relevant data from the included studies. Extracted information included the authorship, year of publication, country of origin, study design, sample size, sex proportion, age, Expanded Disability Status Scale (EDSS) scores, disease duration, MRI device and strength, MRI acquisition sequence, MRI analysis software, cognitive test scores, and the main findings regarding the correlation between DTI measurements and neuropsychological evaluation in people with MS. The search results were imported into Excel software, where the two researchers independently removed duplicates and screened titles and abstracts based on predefined eligibility criteria. Studies that utilized DTI modalities in MS and assessed cognitive domains using standardized neuropsychological measures were identified and subjected to a comprehensive full-text review. We consider standardized neuropsychological instruments, validated tests that reliably assess cognitive domains, allowing for comparisons across participants and studies. In this context, cognitive performance refers to individuals’ abilities across different cognitive domains, such as memory, attention, processing speed, and executive functions. The agreement between the two independent reviewers was evaluated using Cohen’s kappa statistic. A kappa value greater than 0.61 was considered indicative of substantial agreement, ensuring a robust assessment of inter-rater reliability during the data extraction process.
2.3 Study selection
These researchers read the full-text articles deemed suitable for the study and performed data collection to reduce the risk of bias. Any disagreements among the researchers were resolved by consulting a senior reviewer (VLB).
Studies were included after they fulfilled the following criteria: (a) use of DTI; (b) studies assessed the relationship between WM damage and cognitive functions (c); study with only RRMS subjects (d) studies using standardized neuropsychological measures (e); presence of a control group (f); observational design, including case–control, cross–sectional, and cohort studies (either retrospective or prospective); (g) original articles written in English.
The exclusion criteria were: (a) reviews, single case studies, conference abstracts, protocols, editorials, letters, book chapters, case reports, and case series (b); pharmacological studies (c); paediatric onset study (d); studies with methodological ambiguities o insufficiently described methods (e); studies with incomplete, insufficient, or missing data.
A comprehensive search of databases yielded 853 initial studies: 264 articles from PubMed, 283 from Embase, 120 from Web of Science, 186 articles from Scopus. After screening the title database and removing duplicate studies, 384 publications were initially identified. On these, 71 studies were selected based on RRMS subjects. Following an accurate revision of full manuscripts, 34 articles satisfied the inclusion/exclusion criteria (Figure 1).
2.4 PECO evaluation
We employed a PECO (Population, Exposure, Comparison, Outcome) framework to structure our research question. The population consisted of adult patients (≥18 years) diagnosed with RRMS. The exposure was defined as WM microstructural alterations assessed through DTI parameters, including FA, MD, AD, RD and ADC. The comparison group included age- and sex-matched healthy controls with no history of neurological or psychiatric disorders. The outcome was cognitive performance, evaluated using a standardized battery of neuropsychological tests. This approach enabled us to explore the relationship between WM damage and cognitive functioning in RRMS subjects.
2.5 Assess the quality of included studies - risk of bias
The risk of bias in controlled studies was independently assessed by A. M. and C. S. any disagreements during this process, as well as during previous stages, were resolved through consultation with V. L. B., who provided the final decision. For all the selected study we used the Cochrane tool for non-randomized controlled studies-of exposures (ROBINS-E) tool (Higgins et al., 2024) (Figure 2), which comprises seven domains: (i) bias due to confounding, (ii) bias arising from measurement of the exposure, (iii) bias in selection of participants into the study (or into the analysis), (iv) bias due to post-exposure interventions, (v) bias due to missing data, (vi) bias arising from measurement of the outcome, (vii) bias in selection of reported result.
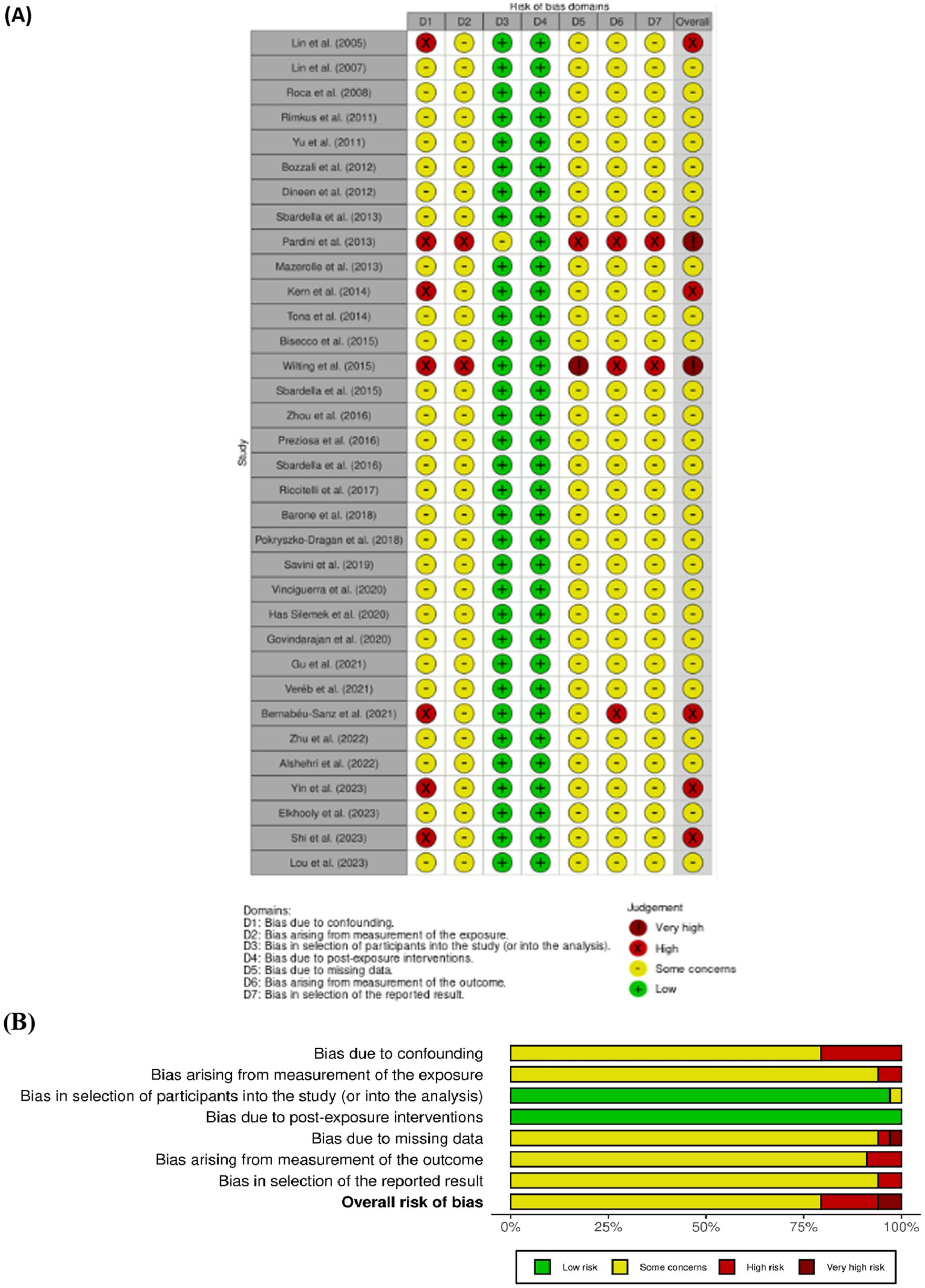
Figure 2. (A, B) ROBINS-E, summary of the risk of bias assessment and depicts distribution of bias concerns across the included studies.
3 Result
3.1 Study characteristics
The main characteristics of all selected studies are summarized in Table 1. Of the 34 studies included, 20 were conducted in Europe (Pokryszko-Dragan et al., 2018; Bisecco et al., 2015; Bernabéu-Sanz et al., 2021; Has Silemek et al., 2020; Vinciguerra et al., 2021; Veréb et al., 2022; Sbardella et al., 2013, 2015, 2016; Savini et al., 2019; Tona et al., 2014; Riccitelli et al., 2019; Wilting et al., 2016; Bozzali et al., 2013; Pardini et al., 2014; Preziosa et al., 2016; Dineen et al., 2012; Barone et al., 2018; Lin et al., 2005, 2008), 7 in America (Rimkus et al., 2011; Mazerolle et al., 2013; Yu et al., 2012; Kern et al., 2014; Roca et al., 2008; Elkhooly et al., 2023; Govindarajan et al., 2021), 6 in China (Shi et al., 2023; Gu et al., 2022; Zhu et al., 2022; Zhou et al., 2016; Yin et al., 2023; Luo et al., 2024), and 1 in Australia (Alshehri et al., 2022).
The studies collectively included data from 1,460 individuals with RRMS, of whom 72% were female. The average age was 35.7 ± 6.68 years, and the mean disease duration was 9.99 ± 6.68 years.
3.1.1 Correlation between diffusion tensor imaging metrics and cognitive function
This section describes the relationship between alteration in DTI parameters and cognitive performance according to specific anatomical structures, including global WM and network connectivity, CC and commissure, thalamus, hippocampus, cerebellum, cingulum, and cerebral fascicles. This structure-oriented analysis provides a more detailed understanding of microstructural WM changes in cognitive outcomes in individuals with RRMS.
3.1.2 Global WM and network connectivity
Nine studies showed a significant correlation between DTI parameters and cognitive performance, emphasizing a consistent association between WM integrity and cognition in RRMS. Alshehri et al. (2022) found that total Fractional Anisotropy Total Brain White Matter (FA-TBWM) showed moderate positive correlations with total Audio Recorded Cognitive Screen (tARCS) (r = 0.41, p ≤ 0.05), memory (r = 0.41, p ≤ 0.05), fluency (r = 0.35, p ≤ 0.05), and attention (r = 0.41, p ≤ 0.05) and positive but non-significant correlation with SDMT scores. Conversely, Mean Diffusivity Total Brain White Matter (MD-TBWM) correlated negatively with memory (r = −0.37, p ≤ 0.05) and fluency (r = −0.39, p ≤ 0.05), while RD-TBWM showed stronger negative correlations with tARCS (r = −0.43, p ≤ 0.01), memory (r = −0.47, p ≤ 0.01), and fluency (r = −0.45, p ≤ 0.01). Enhanced structural connectivity within the default mode network (DMN) showed a positive correlation with improved performance in verbal memory (VLMT) and spatial memory (BVMT), as well as in attention tasks (PASAT and TAP). A higher global structural strength was associated with better performance in SDMT (r = 0.46, p = 0.007) and BVMT (Sum 1–3: r = 0.55, p = 0.001 and Recall: r = 0.53, p = 0.002) across most functional networks (Yeo networks), particularly in DMN. Similarly, VLMT scores were positively correlated with a higher global structural strength within the nodes of the DMN and the limbic network (p < 0.05) (Has Silemek et al., 2020). Global Efficiency (GE) of FA-weighted networks correlated strongly with SDMT, indicating that greater damage corresponded to worse cognitive performance. In RRMS subjects with cognitive dysfunction, GE within the DMN was strongly associated with SDMT (r = 0.87, R2 = 0.76, p < 0.001), while this correlation was significantly weaker in cognitively preserved RRMS subjects (Savini et al., 2019). Veréb et al. (2022) found that reduced rightward lateralization of DMN areas, particularly in the angular gyrus and inferior parietal lobule p < 0.005, was correlated with better scores on the Brief Visuospatial Memory Test-Revised (BVMT-R) (r = −0.52, p < 0.023). Vinciguerra et al. (2021) reported moderate correlations between peak skeletonized mean diffusivity (PSMD) and verbal memory (SRT-LTS: r = −0.35, p = 0.02, SRT-CLTR: r = −0.37, p = 0.016), visual memory (SPART): r = −0.28, p = 0.02 and verbal fluency (WLG): r = 0.25, p = 0.04. As well as strong associations between both MD and FA of the WM skeleton (R2 = 0.30, p = 0.012) and SDMT (R2 = 0.54, p < 0.001), confirming the relationship between WM microstructural integrity and processing speed. In individuals with RRMS, KFA values of both U-fibre lesions (UFLs) and non-UFLs showed positive correlations with Digit Span Test (DST) (r = 0.371, p < 0.02) and Symbol Digit Modalities Test (SDMT) scores (r = −0.399, p < 0.02). Additionally, increased magnetic susceptibility (mrQSM) in UFLs was negatively correlated with Montreal Cognitive Assessment (MoCA) scores (r = −0.372, p = 0.021), suggesting a link between lesion burden and global cognitive decline. U-fibre lesion volume also showed a negative correlation with SDMT performance (r = −0.335, p = 0.024) (Luo et al., 2024). Elevated MK in lesions correlated with reduced MoCA and SDMT scores, whereas FA in T1 and T2 lesions showed a relationship with MoCA and SDMT scores. MD values showed a negative correlation with cognitive scores, suggesting a link between WM alterations and cognitive function (Zhu et al., 2022). Finally, the KFA values of PLWM linked to Contrast Enhancement Lesions (CELs) showed a positive correlation with SDMT scores. Furthermore, in PLWM associated with iron rim lesions (IRLs), both KFA and MD values were significantly correlated with DST scores: KFA showed a positive correlation (r = 0.443, p = 0.021), while MD showed a negative correlation (r = −0.518, p = 0.006). These findings suggest that microstructural damage in IRL-associated regions is linked to reduced working memory performance in RRMS (Shi et al., 2023).
Overall, these findings highlight a consistent relationship between WM microstructural integrity and cognitive performance in RRMS, indicating that widespread and network-specific alterations, particularly within the DMN and limbic circuits, contribute to deficits in processing speed, memory, and executive functioning.
3.2 Corpus callosum and commissure
Seventeen studies reported significant associations between DTI metrics of the CC and cognitive performance in RRMS. FA measures in the genu and splenium of the CC showed positive correlation with SDMT performance (r = 0.33, p = 0.022 for the genu; r = 0.38, p = 0.009 for the splenium) and negative correlation with SDMT performance and negative correlation with Apparent Diffusion Coefficient (ADC) in the splenium (r = −0.29, p = 0.046) (Pokryszko-Dragan et al., 2018). Streamlined tractography analyses further revealed a positive association between SDMT scores and FA in occipital callosal fibres (r = 0.52, p = 0.003) (Bernabéu-Sanz et al., 2021). FA and RD were correlated with SDMT (FA: r = 0.46, p = 0.02; RD: r = −0.37, p = 0.07) and the Hopkins Verbal Learning Test–Delayed Recall (HVLT-DR) performance (r = 0.45, p = 0.03). Additionally, FA in the corpus callosum correlated with Logical Memory II (r = 0.41, p = 0.05), and RD with Stroop performance (r = −0.39, p = 0.06) (Rimkus et al., 2011). Similar FA, MD, AD, and RD measures have all shown a positive correlation with performance on the PASAT-2 s (GM/ICV ratio, r = 0.47, p = 0.005) (Sbardella et al., 2013, 2015). Lower FA and higher MD, RD, and AD values have been consistently associated with poorer SDMT performance and slower response times, including on tasks such as the Simple Reaction Time (SRT) and Semantic Search Reaction Time (SSRT) and Semantic Search Reaction Time (p < 0.05) (Mazerolle et al., 2013). Other research has shown that reduced SDMT and PASAT scores are linked to increased MD and AD values (PASAT: r = 0.26, p < 0.0001; SDMT: r = −0.20, p = 0.01) (Riccitelli et al., 2019), as well as elevated average ADCa values in relation to PASAT performance (r = −0.53, p = 0.0012) (Lin et al., 2005, 2008). Moreover, decreased FA in callosal fibers has been associated with impairments on Rey Auditory Verbal Learning Test (RAVLT), PASAT, and most notably, the SDMT (Yu et al., 2012). Positive correlations have also been observed between anterior commissure microstructural integrity and PASAT-3 s scores in CC areas (Bozzali et al., 2013). Govindarajan et al. (2021) did not find a significant correlation between FA and cognitive performance. However, MD, RD, AD were negatively correlated with SDMT scores (p < 0.05), in addition to correlations between MD, AD, and reaction time on the Detection task (DET). Barone et al. (2018) further stratified RRMS subjects into Cluster in based on FA and CC volume, showing that Cluster 2 (mild CC damage) had significant deficits in verbal fluency, executive function, processing speed measured with Word List Generation test (WLG), SDMT, STROOP-C (p = 0.05 vs. Cluster 1; p = 0.004 vs. HC), and Cluster 3 (patients with the most severe CC damage) had lowest scores in this valuation and also in SRTD for verbal memory (p = 0.05 vs. Cluster 1; p = 0.02 vs. HC). Similarly, heightened MD and reduced FA in the splenium of the CC were associated with deficits in visual memory, attention, and executive functions score of 10/36 Spatial Recall Test (10/36 SRT), SDMT, PASAT and Wisconsin Card Sorting Test (WCST) instead for splenium of the CC the decreased FA correlated with 10/36 Spatial Recall Test, SDMT and PASAT, and increased MD with WCST, SDMT and PASAT. Also, in Forceps Major and Minor decreased FA correlated with SDMT and PASAT, 10/36 SRT and MD increased with 10/36 SRT, SDMT and PASAT (Preziosa et al., 2016). Finally, Dineen et al. (2012) showed that a reduction of fornix FA had a significant correlation with low scores in BVRT (Benton Visual Retention Test) (R2 = 0.31, p = 0.008).
Overall, these findings suggest that microstructural disruption within the CC, reflected by decreased FA and increased diffusivity, likely represents impaired interhemispheric communication, contributing to reduced information processing speed and global cognitive efficiency. The observed correlations highlight the central role of callosal integrity in sustaining distributed cognitive networks in RRMS.
3.2.1 Thalamus
A total of ten studies have reported correlations between the microstructural characteristics of thalamocortical WM pathways and cognitive alterations in RRMS subjects. ADC measurements have shown a strong correlation with SDMT scores (r = −0.30, p = 0.041) (Pokryszko-Dragan et al., 2018). Bisecco et al. (2015) found that attention, spatial and verbal memory, verbal fluency, and executive functions, measured with several tests BRB-N, SRT, 10/36, SDMT, PASAT 2 and 3, Word List Generation (WLG), Wisconsin Card Sorting Test (WCST), were predicted by lesion volume or MD within corticothalamic tracts. Verbal memory correlated with MD in bilateral fronto-caudate-radial (F-CDR) pathways, spatial memory related to MD in the left thalamo-temporal (T–T) pathway, and verbal fluency was linked to both FA in the occipital-caudate-radial (O-CDR) pathway and MD in the left fronto-thalamic (F-T) pathway. Executive functions were associated with MD in the right F-CDR pathway. Bernabéu-Sanz et al. (2021) found that SDMT scores were positively correlated with FA (r = 0.40, p = 0.03) and negatively with MD in bilateral thalamic projections to the frontal (left: r = 0.55, p = 0.002; right: r = 0.56, p = 0.002), parietal (left: r = 0.58, p < 0.001; right: r = 0.55, p = 0.001), temporal (left: r = 0.55, p = 0.002; right: r = 0.57, p = 0.001), and occipital cortices (left: r = 0.52, p = 0.003). Tona et al. (2014) found that increased thalamic functional connectivity was inversely related to PASAT performance (p < 0.05), whereas no significant correlations were found between FA, MD, or thalamic volumes and PASAT. Zhou et al. (2016) found a negative relationship between AD in the prefrontal corticothalamic tract and PASAT performance. In line with these findings, Wilting et al. (2016) showed that FA and MD values in the thalamus were significantly related to processing speed, cognitive flexibility measured by SDMT, and TMT (TMT-B: FA r = 0.308, p = 0.007; MD r = 0.242, p = 0.034). Additionally, MD values in the thalamus were significantly correlated with overall cognitive impairment. Moreover, Yin et al. (2023) validated a negative relationship between thalamic MD and SDMT performance (r = −0.419, p = 0.003). Mazerolle et al. (2013) offered additional evidence, associating diminished FA and elevated MD, RD, and AD in thalamic radiations with lower SDMT scores and extended reaction times in both simple and semantic tasks. In addition, Preziosa et al. (2016) showed that decreased FA of thalamic radiation and increased MD were correlated with visual memory (10/36 SRT r = −0.53, p < 0.001), attention, and processing speed such as SDMT and PASAT (r = 0.63, p < 0.001 for thalamic atrophy). Recently, Elkhooly et al. (2023) noted stable patterns between FA in distinct corticothalamic pathways and SDMT performance (r = 0.441, p = 0.015), highlighting the importance of thalamocortical connectivity in cognitive function in RRMS people. In summary, these findings suggest that microstructural alterations within thalamocortical pathways, as reflected by diffusion metrics such as FA, MD, and ADC, are strongly associated with cognitive decline in RRMS, particularly affecting processing speed, memory, and executive functions.
3.2.2 Hippocampus
Regarding the role of hippocampal structures, Gu et al. (2022) found a significant negative correlation between MD in the left hippocampus and MoCA scores. Additionally, Tona et al. (2014) identified a significant inverse relationship between FC in the right hippocampus and PASAT-2 s performance (p < 0.05). These findings underscore the critical role of hippocampal integrity and commissural pathways in supporting cognitive function in MS.
3.2.3 Cerebellar structure
Significant correlations have been observed between cognitive abilities and microstructural characteristics of cerebellar WM in 2 studies. Elkhooly et al. (2023) reported significant negative correlations between mean diffusivity (MD) values and SDMT scores, particularly in white matter regions such as the superior cerebellar peduncle (r = −0.512, p = 0.004), the left corticothalamic tract (r = −0.441, p = 0.015), and the right medial lemniscus (r = −0.454, p = 0.012). In contrast, fractional anisotropy (FA) values in these regions showed positive correlations with SDMT scores, suggesting that preserved microstructural integrity in cerebellar efferent pathways may support faster cognitive processing. Likewise, Riccitelli et al. (2019) noted that decreased FA in the middle and superior Cerebellar Peduncle (CP) and RD increased in left middle, inferior, and superior CP were significantly with poorer SDMT performance (p < 0.05) while Sbardella et al. (2013) showed positive associations between FA, MD, and PASAT-2 s scores across various WM tracts as the cerebral peduncles (p < 0.05). These studies emphasize the role of cerebellar and brainstem WM structures in cognitive efficiency while highlighting their susceptibility to cognitive impairments associated with RRMS subjects.
3.2.4 Cingulum and cerebral fascicles
Five selected studies investigated the correlations between cognitive test performance and the microstructural integrity of the cingulate and cerebral WM fasciculi. SDMT scores, significant positive correlations with streamline integrity and negative with AD in the right inferior fronto-occipital fasciculus (IFO) (Bernabéu-Sanz et al., 2021). In the same study, Multiple Ability Test subtests were associated with streamlines in the left uncinate fasciculus (UF) r = 0.57, p = 0.001 and left inferior longitudinal fasciculus (ILF) (ILF; r = 0.477, p = 0.009), right cingulum (r = 0.38, p = 0.04), along with FA and AD in the fornix in relation to episodic memory performance. Kern et al. (2014) found associations between better WM integrity and higher scores in neuropsychological assessment. In particular, processing speed was positively associated with greater integrity of the UF integrity, with FA-RD relationship drove the association, and a similar pattern was observed for spatial memory. Sbardella et al. (2013) MD, AD, and RD showed positive correlations with PASAT 2 s scores in cingulum (p < 0.05) confirmed in subsequent studies (Sbardella et al., 2016). The comprehensive analysis by Preziosa et al. (2016) found that decreased FA of the left superior longitudinal fasciculus (SLF) (r = 0.71, p < 0.001) and right cingulate were predictive of global cognitive dysfunction defined as having abnormal performance on at least two tests of the BRB-N battery and WCST. In SLF, Inferior fronto-occipital fasciculus (IFOF) and right cingulate increased MD and decreased FA correlated with worse visual memory (10/36 SRT), attention and processing speed (SDMT and PASAT) and executive function (WCST). Moreover, in the UF increased MD correlated with worse scores in SDMT, PASAT, 10/36 SRT while decreased FA with worse WCST.
DTI parameters as MD, RD, and AD were negatively correlated with SDMT scores in the left corticospinal tract (p < 0.05). MD was also negatively correlated with DET scores in the right superior occipital-frontal fasciculus (p < 0.05). RD showed a significant negative correlation with DET scores in the left superior longitudinal fasciculus, with correlations observed for AD and RD in small clusters in the right superior occipital-frontal fasciculus (Govindarajan et al., 2021).
In summary, the evidence consistently indicates that microstructural disruptions within the cingulate and major cerebral WM fasciculi, particularly the SLF, UF, ILF, IFOF, and cingulum, are closely linked to cognitive impairment in RRMS. Alterations in diffusion metrics (FA, MD, AD, RD) across these tracts are systematically associated with deficits in processing speed, memory, attention, and executive functioning, emphasizing the critical contribution of fronto-limbic and associative pathways to cognitive integrity in this population.
3.2.5 Other cerebral WM tracts
Five studies described the connection between WM and cognitive functioning in different neuropsychological measures. ADC values were positively correlated with performance on the Multiple Errands Test – Hospital Version (MET) (r = 0.72, p = 0.01) and the Hotel Task (r = 0.68, p = 0.02), particularly in the fronto-lateral (FL) region (Roca et al., 2008). Furthermore, a positive correlation between FA and PASAT 2 s scores was observed in the internal capsule. Similarly, Preziosa et al. (2016) reported that decreased FA in corona radiata correlated with poorer performance on SDMT and PASAT and 10/36 SRT, while increased MD in the same region was linked to 10/36 SRT.
In a previous study, Sbardella et al. (2013) showed positive associations between FA, MD, and PASAT-2 s scores across various WM tracts as the internal and external capsule (p < 0.05).
Direct associations were identified between the connectivity of the dentate gyrus with the frontal cortex and PASAT performance (Sbardella et al., 2016). Additionally, reductions in FA were significantly correlated with performance on the SDMT, RAVLT, and PASAT, with the strongest association found for the SDMT. In associative fibers such as the cingulum and the superior longitudinal fasciculus, FA reductions correlated with SDMT and PASAT. Similarly, in projection fibers such as the corona radiata and the internal capsule, significant correlations were found with SDMT, RAVLT, and PASAT (p < 0.01) (Yu et al., 2012).
These studies collectively demonstrate that disruptions in WM microstructure, particularly within projection (e.g., corona radiata, internal capsule, cerebral peduncles) and associative tracts (e.g., cingulum, superior longitudinal fasciculus), are strongly associated with cognitive impairment in RRMS. Alterations in diffusion metrics, including FA, MD, AD, and RD, consistently correlate with deficits in processing speed, memory, and executive functioning, highlighting the crucial role of widespread fronto-subcortical and cerebellar connections in supporting cognitive efficiency.
Across studies, alterations in WM microstructural integrity, particularly as measured by FA, MD, and RD, were consistently associated with cognitive performance in individuals with RRMS. Higher FA in key commissural tracts, such as the CC, and in subcortical structures, including the thalamus and hippocampus, generally correlated with better processing speed, attention, memory, and executive function. Conversely, increased MD and RD, especially in regions affected by U-fiber or other focal lesions, were associated with poorer cognitive outcomes.
A comprehensive overview of these correlations is provided in Supplementary Table 1 (overview of reported correlations), while structure-specific associations between DTI metrics and cognitive performance are detailed in Supplementary Table 2.
3.3 Risk of bias
The Cochrane Risk of Bias Assessment Tool (ROBINS-E) (Barone et al., 2018) was used to assess the risk of bias of the articles included in this review. Figure 2 shows the summary of the risk of bias assessment, while the graph depicts the distribution of bias concerns across the included studies. Most of the studies reviewed were observational and cross-sectional, and therefore, they were reported as having “some concerns” about bias (Lin et al., 2008; Roca et al., 2008; Yu et al., 2012; Bozzali et al., 2013; Sbardella et al., 2013, 2015, 2016). Common issues included inadequate adjustment for confounding variables, non-random selection of participants, use of retrospective designs, or data collection at a single point in time. Fewer studies were rated as having “high” risk of bias indicating more serious problems (Kern et al., 2014; Bernabéu-Sanz et al., 2021; Yin et al., 2023; Shi et al., 2023). These issues included a lack of clarity in measuring exposures and outcomes, poor communication of methods, and insufficient control for differences between participants that could influence the results. Two studies (Pardini et al., 2014; Wilting et al., 2016) were rated as having a “very high risk of bias,” reflecting significant methodological limitations such as heterogeneous outcome measures, inadequate sample representativeness, and incomplete reporting.
4 Discussion
The findings summarized in this review underscore the critical role of WM integrity, as measured by DTI, in sustaining cognitive function in individuals with RRMS. Across numerous studies, alterations in DTI metrics, particularly FA, but also MD, and RD, have consistently been associated with impairment in cognitive domains, including attention, memory, executive function, and processing speed. Notably, global measures WM microstructural abnormalities have been linked to poorer performance on tasks assessing sustained attention, vigilance, and working memory, reinforcing the relevance of diffuse structural changes in the cognitive dysfunction observed in RRMS (Alshehri et al., 2022; Vinciguerra et al., 2021). Increased MD and RD, particularly in regions affected by U-fiber lesions, were associated with reduced performance, suggesting that microstructural damage plays a pivotal role in disrupting cognitive processing.
Region-specific analyses provide additional insight into the neural substrates underlying cognitive dysfunction. The CC, a major commissural tract essential for interhemispheric communication, emerged as a consistent predictor of cognitive outcomes, with higher FA in the genu and splenium correlating with better outcomes in attentive subtests, processing speed and executive function (Pokryszko-Dragan et al., 2018; Bernabéu-Sanz et al., 2021). Similarly, thalamic microstructural changes, particularly in thalamic radiations, were strongly associated with cognitive flexibility (Bisecco et al., 2015; Wilting et al., 2016), pointing to the role of subcortical relay structures in MS-related cognitive deficits. The hippocampus, a critical hub for memory consolidation, also demonstrated significant DTI alterations, with increased MD correlating negatively with global cognitive performance (Gu et al., 2022). Functional disconnection within the hippocampus was also associated with working memory impairments (Tona et al., 2014), reaffirming its centrality in cognitive performance in MS subjects. Additionally, alterations involving the cerebellar peduncles, particularly the superior cerebellar peduncle, were associated with processing speed information, suggesting that cerebellar-cortical circuits contribute to cognitive efficiency beyond motor coordination (Elkhooly et al., 2023; Riccitelli et al., 2019). In addition to these key structures, the integrity of associative and projection fibers, including the cingulum, corona radiata, and inferior fronto-occipital fasciculus, was also linked to cognitive performance, notably in tasks involvingspeed and memory (Sbardella et al., 2013; Bernabéu-Sanz et al., 2021). In general, the integrity of interhemispheric connections and connecting structures is crucial; consequently, the presence of lesions and deterioration may lead to greater cognitive deficits related to MS. Further, these findings together support a network-based view of cognition in MS, in which distributed WM disruptions, rather than isolated lesions, may better explain the variability in cognitive decline. The use of DTI appears to be emerging as a potential standard in the management and monitoring of disease progression, as it can detect microstructural damage not visible through conventional MRI scans. These microstructural changes may evolve into more severe lesions or clinical manifestations. Therefore, early identification could facilitate timely interventions and the implementation of preventative strategies. In the case of RRMS, DTI may also anticipate the formation of active lesions or help identify the neuroanatomical basis of otherwise unexplained symptoms. WM damage disrupts brain networks and can have significant repercussions on cognitive functioning. Interestingly, even when no correlation is observed between DTI findings and cognitive performance, perhaps due to compensatory factors such as cognitive reserve, education level, disease duration, or age, these cases might still indicate areas of potential vulnerability. This provides an opportunity for early intervention and cognitive enhancement, before clinical deterioration becomes evident.
A combined and in-depth use of DTI alongside neuropsychological evaluation could improve the detection of disturbances not captured by conventional assessments. Furthermore, it could help explain subjective cognitive complaints or cognitive fatigue that are not attributable to other conditions but may stem from subtle WM alterations.
Several methodological limitations should be considered when interpreting the findings of this review. Primarily, the predominance of observational and cross-sectional studies among the DTI metrics and cognitive outcomes. Furthermore, considerable heterogeneity in MRI acquisition protocols (e.g., variations in field strength, diffusion encoding directions, and preprocessing pipelines) and in the selection and administration of cognitive assessment tools reduced the overall comparability and reproducibility of findings across studies. Such variability may affect both the sensitivity of diffusion measures and the strength of associations with cognitive performance. In addition, the potential influence of publication bias and selective reporting must be acknowledged, as studies reporting statistically significant or positive results are more likely to be published, thereby skewing the available evidence. Many studies also employed relatively small sample sizes, limiting statistical power and reducing the generalizability of their conclusions to broader populations. Given the substantial heterogeneity across studies, encompassing differences in study design, participant demographics, imaging methodologies (including the specific DTI metrics used), cognitive assessment approaches, and reported outcomes, a quantitative meta-analysis was not deemed appropriate. The lack of standardized reporting formats and consistent outcome measures further hindered the reliable aggregation of data. Moreover, variations in methodological rigor across the included studies may have introduced bias, further compromising the validity of potential pooled effect estimates. By explicitly addressing these sources of variability, we provide a more nuanced interpretation of the literature and contextualize differences observed across studies. Consequently, a qualitative synthesis was prioritized, thus enabling a more comprehensive andcontext-sensitive evaluation of the extant evidence base. This approach highlighted both converging findings and critical gaps in the literature. Therefore, in individuals with RRMS, WM integrity, assessed by DTI parameters, may represent a valuable biomarker for cognitive performance. Likewise, comprehensive cognitive assessments can provide insights into the specific brain regions associated with cognitive dysfunction. The connection between microstructural changes in network connectivity and key brain regions, such as global WM, hippocampus, cingulate, cerebral fascicles, and particularly interhemispheric WM, such as CC, thalamus, and cerebellar structure, and cognitive deficits underscores the link between structural connectivity and cognitive function. These findings highlight the potential of DTI as a valuable tool for assessing cognitive decline in MS and for guiding interventions aimed at preserving cognitive abilities in affected individuals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Data curation, Investigation, Writing – original draft. CS: Supervision, Writing – original draft. GC: Investigation, Visualization, Writing – original draft. GD’A: Supervision, Writing – review & editing, CR: Visualization, Writing – review & editing. ES: Supervision, Writing – review & editing. AQ: Supervision, Writing – review & editing. VLB: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Italian Ministry of Health on NextGenerationEU PNRR funds (Missione: M6/Componente: C2 Investimento: 2.1 Valorizzazione, CUP: I43C22000650007, Grant No. PNRR-POC-2022-12376360).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1661821/full#supplementary-material
References
Alexander, A. L., Lee, J. E., Lazar, M., and Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. doi: 10.1016/j.nurt.2007.05.011
Alshehri, A., Al-Iedani, O., Arm, J., Gholizadeh, N., Billiet, T., Lea, R., et al. (2022). Neural diffusion tensor imaging metrics correlate with clinical measures in people with relapsing-remitting MS. Neuroradiol. J. 35, 592–599. doi: 10.1177/19714009211067400
Amato, M. P., Zipoli, V., and Portaccio, E. (2006). Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J. Neurol. Sci. 245, 41–46. doi: 10.1016/j.jns.2005.08.019
Andersen, O., Hildeman, A., Longfils, M., Tedeholm, H., Skoog, B., Tian, W., et al. (2018). Diffusion tensor imaging in multiple sclerosis at different final outcomes. Acta Neurol. Scand. 137, 165–173. doi: 10.1111/ane.12797
Bao, J., Tu, H., Li, Y., Sun, J., Hu, Z., Zhang, F., et al. (2022). Diffusion tensor imaging revealed microstructural changes in Normal-appearing white matter regions in relapsing-remitting multiple sclerosis. Front. Neurosci. 16:837452. doi: 10.3389/fnins.2022.837452
Barone, S., Caligiuri, M. E., Valentino, P., Cherubini, A., Chiriaco, C., Granata, A., et al. (2018). Multimodal assessment of normal-appearing corpus callosum is a useful marker of disability in relapsing-remitting multiple sclerosis: an MRI cluster analysis study. J. Neurol. 265, 2243–2250. doi: 10.1007/s00415-018-8980-y
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15, 435–455. doi: 10.1002/nbm.782
Bernabéu-Sanz, Á., Morales, S., Naranjo, V., and Sempere, Á. P. (2021). Contribution of gray matter atrophy and white matter damage to cognitive impairment in mildly disabled relapsing-remitting multiple sclerosis patients. Diagnostics (Basel) 11:578. doi: 10.3390/diagnostics11030578
Berrigan, L. I., LeFevre, J. A., Rees, L. M., Berard, J. A., Francis, A., Freedman, M. S., et al. (2022). The symbol digit modalities test and the paced auditory serial addition test involve more than processing speed. Mult. Scler. Relat. Disord. 68:104229. doi: 10.1016/j.msard.2022.104229
Bihan, D. L., Mangin, J., Poupon, C., Clark, C. A., Pappata, S., Molko, N., et al. (2001). Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 13, 534–546. doi: 10.1002/jmri.1076
Bisecco, A., Rocca, M. A., Pagani, E., Mancini, L., Enzinger, C., Gallo, A., et al. (2015). Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum. Brain Mapp. 36, 2809–2825. doi: 10.1002/hbm.22809
Bozzali, M., Spanò, B., Parker, G. J., Giulietti, G., Castelli, M., Basile, B., et al. (2013). Anatomical brain connectivity can assess cognitive dysfunction in multiple sclerosis. Mult. Scler. 19, 1161–1168. doi: 10.1177/1352458512474088
Chiaravalloti, N. D., and DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. Lancet Neurol. 7, 1139–1151. doi: 10.1016/S1474-4422(08)70259-X
De Meo, E., Portaccio, E., Giorgio, A., Ruano, L., Goretti, B., Niccolai, C., et al. (2021). Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol. 78, 414–425. doi: 10.1001/jamaneurol.2020.4920
Dineen, R. A., Bradshaw, C. M., Constantinescu, C. S., and Auer, D. P. (2012). Extra-hippocampal subcortical limbic involvement predicts episodic recall performance in multiple sclerosis. PLoS One 7:e44942. doi: 10.1371/journal.pone.0044942
Dineen, R. A., Vilisaar, J., Hlinka, J., Bradshaw, C. M., Morgan, P. S., Constantinescu, C. S., et al. (2009). Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132, 239–249. doi: 10.1093/brain/awn275
Elkhooly, M., Bao, F., Raghib, M., Millis, S., and Bernitsas, E. (2023). Role of white matter in cognitive impairment among relapsing remitting multiple sclerosis patients. Mult. Scler. Relat. Disord. 79:105030. doi: 10.1016/j.msard.2023.105030
Govindarajan, S. T., Liu, Y., Parra Corral, M. A., Bangiyev, L., Krupp, L., Charvet, L., et al. (2021). White matter correlates of slowed information processing speed in unimpaired multiple sclerosis patients with young age onset. Brain Imaging Behav. 15, 1460–1468. doi: 10.1007/s11682-020-00345-z
Granberg, T., Martola, J., Bergendal, G., Shams, S., Damangir, S., Aspelin, P., et al. (2015). Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: results of a 17-year longitudinal study. Mult. Scler. 21, 1151–1158. doi: 10.1177/1352458514560928
Gu, X. Q., Liu, Y., Gu, J. B., Li, L. F., Fu, L. L., and Han, X. M. (2022). Correlations between hippocampal functional connectivity, structural changes, and clinical data in patients with relapsing-remitting multiple sclerosis: a case-control study using multimodal magnetic resonance imaging. Neural Regen. Res. 17, 1115–1124. doi: 10.4103/1673-5374.324855
Has Silemek, A. C., Fischer, L., Pöttgen, J., Penner, I. K., Engel, A. K., Heesen, C., et al. (2020). Functional and structural connectivity substrates of cognitive performance in relapsing remitting multiple sclerosis with mild disability. NeuroImage: Clinical 25:102177. doi: 10.1016/j.nicl.2020.102177
Higgins, J. P. T., Morgan, R. L., Rooney, A. A., Taylor, K. W., Thayer, K. A., Silva, R. A., et al. (2024). A tool to assess risk of Bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 186:108602. doi: 10.1016/j.envint.2024.108602
Hori, M., Maekawa, T., Kamiya, K., Hagiwara, A., Goto, M., Takemura, M. Y., et al. (2022). Advanced diffusion MR imaging for multiple sclerosis in the brain and spinal cord. Magn. Reson. Med. Sci. 21, 58–70. doi: 10.2463/mrms.rev.2021-0091
Kern, K. C., Gold, S. M., Lee, B., Montag, M., Horsfall, J., O'Connor, M. F., et al. (2014). Thalamic-hippocampal-prefrontal disruption in relapsing-remitting multiple sclerosis. Neuroimage Clin. 8, 440–447. doi: 10.1016/j.nicl.2014.12.015
Keser, Z., Hasan, K. M., Mwangi, B., Younes, K., Khayat-Khoei, M., Kamali, A., et al. (2018). Quantitative limbic system mapping of main cognitive domains in multiple sclerosis. Front. Neurol. 9:132. doi: 10.3389/fneur.2018.00132
Kolasa, M., Hakulinen, U., Brander, A., Hagman, S., Dastidar, P., Elovaara, I., et al. (2019). Diffusion tensor imaging and disability progression in multiple sclerosis: a 4-year follow-up study. Brain Behav. 9:e01194. doi: 10.1002/brb3.1194
Lin, X., Tench, C. R., Morgan, P. S., and Constantinescu, C. S. (2008). Use of combined conventional and quantitative MRI to quantify pathology related to cognitive impairment in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 79, 437–441. doi: 10.1136/jnnp.2006.112177
Lin, X., Tench, C. R., Morgan, P. S., Niepel, G., and Constantinescu, C. S. (2005). 'Importance sampling' in MS: use of diffusion tensor tractography to quantify pathology related to specific impairment. J. Neurol. Sci. 237, 13–19. doi: 10.1016/j.jns.2005.04.019
Lope-Piedrafita, S. (ed.). (2018). “Diffusion tensor imaging (DTI)” in Methods in molecular biology (New York, NY: Humana Press), 103–116.
Lugosi, K., Engh, M. A., Huszár, Z., Hegyi, P., Mátrai, P., Csukly, G., et al. (2024). Domain-specific cognitive impairment in multiple sclerosis: a systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 11, 564–576. doi: 10.1002/acn3.51976
Luo, D., Peng, Y., Zhu, Q., Zheng, Q., Luo, Q., Han, Y., et al. (2024). U-fiber diffusion kurtosis and susceptibility characteristics in relapsing-remitting multiple sclerosis may be related to cognitive deficits and neurodegeneration. Eur. Radiol. 34, 1422–1433. doi: 10.1007/s00330-023-10114-3
Matias-Guiu, J. A., Cortés-Martínez, A., Montero, P., Pytel, V., Moreno-Ramos, T., Jorquera, M., et al. (2018). Structural MRI correlates of PASAT performance in multiple sclerosis. BMC Neurol. 18:214. doi: 10.1186/s12883-018-1223-0
Mazerolle, E. L., Wojtowicz, M. A., Omisade, A., and Fisk, J. D. (2013). Intra-individual variability in information processing speed reflects white matter microstructure in multiple sclerosis. Neuroimage Clin 2, 894–902. doi: 10.1016/j.nicl.2013.06.012
Mesaros, S., Rocca, M. A., Riccitelli, G., Pagani, E., Rovaris, M., Caputo, D., et al. (2009). Corpus callosum damage and cognitive dysfunction in benign MS. Hum. Brain Mapp. 30, 2656–2666. doi: 10.1002/hbm.20692
Pardini, M., Bergamino, M., Bommarito, G., Bonzano, L., Luigi Mancardi, G., and Roccatagliata, L. (2014). Structural correlates of subjective and objective memory performance in multiple sclerosis. Hippocampus 24, 436–445. doi: 10.1002/hipo.22237
Pokryszko-Dragan, A., Banaszek, A., Nowakowska-Kotas, M., Jeżowska-Jurczyk, K., Dziadkowiak, E., Gruszka, E., et al. (2018). Diffusion tensor imaging findings in the multiple sclerosis patients and their relationships to various aspects of disability. J. Neurol. Sci. 391, 127–133. doi: 10.1016/j.jns.2018.06.007
Preziosa, P., Rocca, M. A., Pagani, E., Stromillo, M. L., Enzinger, C., Gallo, A., et al. (2016). Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: a multicenter study. Hum. Brain Mapp. 37, 1627–1644. doi: 10.1002/hbm.23125
Prins, M., Schul, E., Geurts, J., van der Valk, P., Drukarch, B., and van Dam, A. M. (2015). Pathological differences between white and grey matter multiple sclerosis lesions. Ann. N. Y. Acad. Sci. 1351, 99–113. doi: 10.1111/nyas.12841
Rao, S. M., Leo, G. J., Bernardin, L., and Unverzagt, F. (1991). Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41, 685–691. doi: 10.1212/wnl.41.5.685
Rao, S. M., Leo, G. J., Haughton, V. M., St Aubin-Faubert, P., and Bernardin, L. (1989). Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 39, 161–166. doi: 10.1212/wnl.39.2.161
Riccitelli, G. C., Pagani, E., Rodegher, M., Colombo, B., Preziosa, P., Falini, A., et al. (2019). Imaging patterns of gray and white matter abnormalities associated with PASAT and SDMT performance in relapsing-remitting multiple sclerosis. Mult. Scler. 25, 204–216. doi: 10.1177/1352458517743091
Rimkus, C.d. M., Junqueira, T.d. F., Lyra, K. P., Jackowski, M. P., Machado, M. A., Miotto, E. C., et al. (2011). Corpus callosum microstructural changes correlate with cognitive dysfunction in early stages of relapsing-remitting multiple sclerosis: axial and radial diffusivities approach. Mult. Scler. Int. 2011:304875. doi: 10.1155/2011/304875
Roca, M., Torralva, T., Meli, F., Fiol, M., Calcagno, M., Carpintiero, S., et al. (2008). Cognitive deficits in multiple sclerosis correlate with changes in fronto-subcortical tracts. Mult. Scler. 14, 364–369. doi: 10.1177/1352458507084270
Rocca, M. A., Amato, M. P., De Stefano, N., Enzinger, C., Geurts, J. J., Penner, I. K., et al. (2015). Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 14, 302–317. doi: 10.1016/S1474-4422(14)70250-9
Roosendaal, S. D., Geurts, J. J., Vrenken, H., Hulst, H. E., Cover, K. S., Castelijns, J. A., et al. (2009). Regional DTI differences in multiple sclerosis patients. NeuroImage 44, 1397–1403. doi: 10.1016/j.neuroimage.2008.10.026
Savini, G., Pardini, M., Castellazzi, G., Lascialfari, A., Chard, D., D'Angelo, E., et al. (2019). Default mode network structural integrity and cerebellar connectivity predict information processing speed deficit in multiple sclerosis. Front. Cell. Neurosci. 13:21. doi: 10.3389/fncel.2019.00021
Sbardella, E., Petsas, N., Tona, F., Prosperini, L., Raz, E., Pace, G., et al. (2013). Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One 8:e63250. doi: 10.1371/journal.pone.0063250
Sbardella, E., Tona, F., Petsas, N., Upadhyay, N., Piattella, M. C., Filippini, N., et al. (2015). Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 21, 1681–1692. doi: 10.1177/1352458514568826
Sbardella, E., Upadhyay, N., Tona, F., Prosperini, L., De Giglio, L., Petsas, N., et al. (2016). Dentate nucleus connectivity in adult patients with multiple sclerosis: functional changes at rest and correlation with clinical features. Mult. Scler. 23, 546–555. doi: 10.1177/1352458516657438
Shi, Z., Pan, Y., Yan, Z., Ding, S., Hu, H., Wei, Y., et al. (2023). Microstructural alterations in different types of lesions and their perilesional white matter in relapsing-remitting multiple sclerosis based on diffusion kurtosis imaging. Mult. Scler. Relat. Disord. 71:104572. doi: 10.1016/j.msard.2023.104572
Sijens, P. E., Irwan, R., Potze, J. H., Mostert, J. P., De Keyser, J., and Oudkerk, M. (2006). Relationships between brain water content and diffusion tensor imaging parameters (apparent diffusion coefficient and fractional anisotropy) in multiple sclerosis. Eur. Radiol. 16, 898–904. doi: 10.1007/s00330-005-0033-0
Song, S., Sun, S., Ju, W., Lin, S., Cross, A. H., and Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 20, 1714–1722. doi: 10.1016/j.neuroimage.2003.07.005
Song, S. K., Sun, S. W., Ramsbottom, M. J., Chang, C., Russell, J., and Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17, 1429–1436. doi: 10.1006/nimg.2002.1267
Sumowski, J. F., and Leavitt, V. M. (2013). Cognitive reserve in multiple sclerosis. Mult. Scler. 19, 1122–1127. doi: 10.1177/1352458513498834
Tona, F., Petsas, N., Sbardella, E., Prosperini, L., Carmellini, M., Pozzilli, C., et al. (2014). Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology 271, 814–821. doi: 10.1148/radiol.14131688
Veréb, D., Kovács, M. A., Kocsis, K., Tóth, E., Bozsik, B., Király, A., et al. (2022). Functional connectivity lateralisation shift of resting state networks is linked to visuospatial memory and white matter microstructure in relapsing-remitting multiple sclerosis. Brain Topogr. 35, 268–275. doi: 10.1007/s10548-021-00881-x
Vinciguerra, C., Giorgio, A., Zhang, J., Nardone, V., Brocci, R. T., Pastò, L., et al. (2021). Peak width of skeletonized mean diffusivity (PSMD) and cognitive functions in relapsing-remitting multiple sclerosis. Brain Imaging Behav. 15, 2228–2233. doi: 10.1007/s11682-020-00394-4
Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R. A., et al. (2020). Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult. Scler. 26, 1816–1821. doi: 10.1177/1352458520970841
Wilting, J., Rolfsnes, H. O., Zimmermann, H., Behrens, M., Fleischer, V., Zipp, F., et al. (2016). Structural correlates for fatigue in early relapsing remitting multiple sclerosis. Eur. Radiol. 26, 515–523. doi: 10.1007/s00330-015-3857-2
Yin, F., Yan, Z., Li, Y., Ding, S., Wang, X., Shi, Z., et al. (2023). Multimodal investigation of deep gray matter nucleus in patients with multiple sclerosis and their clinical correlations: a multivariate pattern analysis study. J Pers Med 13:1488. doi: 10.3390/jpm13101488
Yu, H. J., Christodoulou, C., Bhise, V., Greenblatt, D., Patel, Y., Serafin, D., et al. (2012). Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. NeuroImage 59, 3713–3722. doi: 10.1016/j.neuroimage.2011.10.053
Zhang, J., Cortese, R., De Stefano, N., and Giorgio, A. (2021). Structural and functional connectivity substrates of cognitive impairment in multiple sclerosis. Front. Neurol. 12:671894. doi: 10.3389/fneur.2021.671894
Zhou, F., Gong, H., Chen, Q., Wang, B., Peng, Y., Zhuang, Y., et al. (2016). Intrinsic functional plasticity of the Thalamocortical system in minimally disabled patients with relapsing-remitting multiple sclerosis. Front. Hum. Neurosci. 10:2. doi: 10.3389/fnhum.2016.00002
Keywords: multiple sclerosis, relapsing-remitting multiple sclerosis, diffusion tensor imaging, cognition, neuropsychology, white matter
Citation: Mirabile A, Susinna C, Cipriano GL, D’Aleo G, Rifici C, Sessa E, Quartarone A and Lo Buono V (2025) Correlation of cognitive dysfunctions and diffusion tensor MRI measures in subjects with RRMS. Front. Aging Neurosci. 17:1661821. doi: 10.3389/fnagi.2025.1661821
Reviewed by:
Antonio Carotenuto, University of Naples Federico II, ItalyAntonia Lefter, University of Antwerp, Belgium
Copyright © 2025 Mirabile, Susinna, Cipriano, D’Aleo, Rifici, Sessa, Quartarone and Lo Buono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Susinna, Y2FybGEuc3VzaW5uYUBpcmNjc21lLml0
 Alessio Mirabile
Alessio Mirabile Carla Susinna
Carla Susinna Giovanni Luca Cipriano
Giovanni Luca Cipriano Viviana Lo Buono
Viviana Lo Buono