Abstract
Background:
With the gradual aging of the global population, early identification and intervention are crucial for mitigating the negative impact of cognitive decline on patients and the healthcare system. This study aimed to develop and validate a prognostic scoring system for predicting cognitive decline in adults aged 50 and over in Shanghai, China.
Methods:
This community-based longitudinal study included 1,032 participants aged 50 and older with normal cognitive function at baseline. Of them, 986 participants were followed up for 2 years. Complete data from 864 individuals were randomly divided into derivation (n = 686) and validation (n = 178) cohorts and used to generate a prognostic scoring model. Sociodemographic and behavioral characteristics, comorbidities, and biochemical factors were collected from all participants. The least absolute shrinkage and selection operator (LASSO) logistic regression method was used to identify significant predictors. A multivariable logistic regression model was developed and validated using derivation and validation cohorts.
Results:
Of the thirteen variables initially selected, nine (age, gender, smoking, tea drinking history, hypertension, diabetes mellitus, coronary artery disease (CAD), hyperlipidemia, cerebral hemorrhage, and decline in daily function) were included in the final model. The nomogram-based scoring system showed moderate discriminatory power, with the area under the curve (AUC) of 0.65 and 0.67 in the training and validation sets, respectively, and good calibration.
Conclusion:
The developed prognostic scoring system provides a practical tool for predicting cognitive decline among adults aged 50 and older in Shanghai, China. The moderate discriminatory power and good calibration suggest that the model can effectively guide early interventions. Future research should validate the model in diverse populations and explore additional risk factors to enhance its predictive accuracy.
Introduction
Cognitive decline is a pressing public health issue, particularly in China, where the aging population is expanding at an unprecedented rate (Lunenfeld and Stratton, 2013; Bai and Lei, 2020). Cognitive disorders may range from mild cognitive impairment (MCI) to severe dementia and have far-reaching implications not only for affected individuals but also for their families, caregivers, and the healthcare system in general (Aerts et al., 2017). With the continuous aging of the population, the prevalence of cognitive decline is currently on the rise (Prince et al., 2015; Bhatti et al., 2019). Patients with MCI are at a greater risk of developing Alzheimer’s disease (AD) or related dementia (Campbell et al., 2013). Recent advancements in the treatment of early AD with agents such as lecanemab, a humanized IgG1 monoclonal antibody that binds with high affinity to Aβ soluble protofibrils, further emphasize the need for early detection of MCI or preclinical AD stages. Such early screening, coupled with the timely initiation of pharmacological treatments, is crucial to mitigating the impact of the condition (Sabbagh et al., 2020).
Cognitive decline may be influenced by an interplay of various risk factors (Rao et al., 2023), including sociodemographic characteristics (age and gender), history of smoking and alcohol consumption, and various comorbidities such as hypertension, diabetes, dyslipidemia, etc (Okusaga et al., 2013; Han et al., 2023). Furthermore, the incidence of cognitive impairment accelerates from the sixth decade (Prince et al., 2024). At the same time, the major modifiable risk factors (e.g., hypertension, diabetes, dyslipidemia, obesity, smoking, depression, hearing loss, physical inactivity) are highly prevalent and actionable in late midlife (Rolandi et al., 2020), a window during which targeted management and closer follow-up can realistically alter next-visit risks. Therefore, developing a prognostic scoring system for this particular age group (adults aged 50 years and above) is crucial and will align with local screening and chronic disease management programs that typically begin in this age range.
However, despite extensive research, predicting cognitive decline remains challenging because traditional methods often fail to account for the intricate interrelationships between these factors (Hampel et al., 2011; Salthouse, 2011), underscoring the need for a more sophisticated and tailored prognostic approach.
Most existing studies and models are based on Western populations, which may have different risk profiles, lifestyle factors, and healthcare environments than those in China. However, China’s socio-demographic and cultural context and specific health-related characteristics necessitate a localized approach to predicting and managing cognitive decline (Wang et al., 2020; Chen et al., 2022). Therefore, there is a growing understanding that there is a need for a prognostic model specifically designed to cater to the unique characteristics of China’s population (Bao et al., 2022; van der Flier et al., 2023). Recently, several dementia/cognitive-impairment risk models have been developed or validated in Chinese cohorts (Hu et al., 2021; Hu et al., 2022; Zhou et al., 2020). While these studies advance the field, important gaps remain for frontline use. Many models rely on assessments or biomarkers that are not routinely available in primary care, report limited or no external validation, provide incomplete calibration or clinical utility evaluation (e.g., decision curve analysis), or are anchored to follow-up structures that differ from those seen in real-world clinics. In addition, most prior models target time-to-event outcomes that require precise onset dates. In contrast, routine services in many Chinese settings ascertain impairment status at discrete visits, leaving the onset time interval censored. Therefore, formulating prediction as a fixed-horizon (“next-visit”) risk task using indicators commonly available at the index visit, and evaluating both discrimination and calibration with transparent and reproducible methods, is imperative to address this practice-relevant scenario.
This study aims to generate and validate a prognostic scoring model for cognitive decline in adults aged 50 and older admitted to a tertiary care center in China. Healthcare providers can use such tools to identify individuals at high risk and implement timely interventions.
Methods
Study design
This community-based longitudinal study included a retrospective data analysis on a cohort of middle-aged and older adults from the Shanghai Brain Health Foundation (SHBHF2016001). Initially, the cohort included 1,032 individuals ≥50 years old with normal cognitive function and no signs of MCI or dementia at the time of recruitment. The study targeted adults aged 50 years or older to reflect the rising incidence of cognitive impairment in late midlife and the high prevalence of modifiable risk factors that are actionable in routine care. The intended use of the model is risk stratification at the index visit to estimate the probability of meeting criteria for cognitive impairment at the subsequent scheduled assessment (fixed-horizon prediction), rather than estimating time-to-event.
Over the course of 2 years, 986 participants remained in the study, while 46 participants were lost to follow-up. Of them, 122 participants had missing information for the variables included in the least absolute shrinkage and selection operator (LASSO) model. Finally, 864 participants constituted the final sample for analysis.
This study was approved by the Ethics Committee of the Shanghai Mental Health Center (No. 2018-11R, Date: 2018-07-27). Data collection commenced following ethical approval and written informed consent from all participants.
Data collection
Candidate predictors: types, measurement, units, and preprocessing. Candidate predictors were pre-specified from routinely available clinical information at the index visit and grouped as follows.
(a) Demographics and history. Age (years; continuous), sex (female/male), years of formal education (years), marital status (married/other), and self-reported physician diagnoses of hypertension, diabetes, dyslipidaemia, coronary heart disease, stroke/TIA, and depression (yes/no). Medication classes (antihypertensives, antidiabetics, statins, antidepressants, anticholinergics) were extracted from the medication list (yes/no for each class).
(b) Lifestyle factors. Smoking (never/former/current); alcohol intake (never/occasional/weekly/daily; converted to standard drinks/week); tea consumption (never/≤3 cups/week/≥4 cups/week); physical activity from the short-form IPAQ expressed as MET-minutes/week (continuous) and categorized (low/moderate/high) for sensitivity checks; sleep duration (hours/night; continuous).
(c) Vital signs and anthropometrics. Systolic and diastolic blood pressure (mmHg) measured using a calibrated automated sphygmomanometer after ≥5 min seated rest; two readings 1–2 min apart, averaged. Heart rate (beats/min) recorded simultaneously. Height (cm) via wall stadiometer (no shoes) and weight (kg) via digital scale (light clothing); body mass index (BMI, kg/m2) computed as weight/height2. Waist circumference measured midway between the lowest rib and the iliac crest (cm) at end-expiration.
(d) Cognitive and functional measures at index visit. Baseline global cognition by the Montreal Cognitive Assessment (MoCA) Chinese version (0–30). Basic and instrumental activities of daily living (ADL/IADL) recorded using validated Chinese instruments; summary scores used as continuous predictors (higher = greater impairment). Depressive symptoms screened with PHQ-9 (0–27). Hearing/vision difficulty recorded (none/mild/moderate/severe) by self-report of functional limitation.
(e) Laboratory/biochemical indicators. Venous blood was drawn after an overnight fast (8–12 h) and analyzed in the hospital central laboratory accredited under routine internal and external quality control procedures. Units and methods were as follows:
-
Fasting plasma glucose (mmol/L; hexokinase method); HbA1c (%; HPLC, NGSP/DCCT-aligned).
-
Lipid profile: total cholesterol, LDL-C, HDL-C, triglycerides (all mmol/L; enzymatic colorimetric assays).
-
Serum creatinine (μmol/L; enzymatic); eGFR (mL/min/1.73 m2; CKD-EPI equation).
-
ALT/AST (U/L; kinetic method).
-
High-sensitivity C-reactive protein (hs-CRP) (mg/L; immunoturbidimetric); homocysteine (μmol/L; enzymatic cycling).
-
Uric acid (μmol/L; uricase); TSH (mIU/L; chemiluminescent immunoassay); vitamin B12 (pmol/L; chemiluminescence).
Preprocessing and standardization. To ensure comparability, all laboratory values were harmonized to SI units. If any values were recorded in conventional units, they were converted using standard factors before analysis. Right-skewed biomarkers (e.g., triglycerides, hs-CRP, homocysteine) were natural-log transformed. Continuous predictors were then standardized (z-scores: mean 0, SD 1) using parameters estimated from the training set; the same parameters were applied to the validation set to avoid information leakage. Categorical variables were dummy-encoded (one-hot). Outliers were handled via winsorisation at the 1st/99th percentiles within the training data before standardization. Missing values (when present) were imputed within the resampling/training folds only using simple, predictor-wise imputation (median for continuous, mode for categorical) to avoid leakage; the trained imputation parameters were then applied to the corresponding validation folds. No outcome information was used in any preprocessing step.
Cognitive status at follow-up was assessed using the Montreal Cognitive Assessment (MoCA), with a conventional threshold of <26 indicating cognitive impairment. MoCA is a screening instrument (not a diagnostic gold standard); therefore, some misclassification is expected. Since any residual misclassification is likely non-differential with respect to baseline predictors, its primary effect would be to attenuate effect sizes and modestly reduce discrimination, yielding conservative performance estimates for our model. Follow-up data were collected over two years to assess the participants’ cognitive status (Nasreddine et al., 2005).
The development of cognitive decline was considered a dependent variable. Independent variables included sociodemographic factors (age, sex, education, marital status, family history of dementia), behavioral characteristics (smoking, alcohol use, tea consumption), BMI, self-reported comorbidities (decline in daily living activities, sleep problems, DM, HTN, CAD, cerebral hemorrhage, cerebral infarction, depression, TBI) and biochemical parameters such as complete blood count parameters, levels of ALT, AST, total bilirubin, total cholesterol, LDL, HDL and triglycerides.
Statistical analysis
STATA software v.14.2 was used for the analysis. Continuous variables were summarized as means and standard deviations (SD), while categorical variables were presented as proportions. The dataset was divided into derivation and validation cohorts. A prognostic scoring system was developed to predict the risk of cognitive decline using the derivation cohort and subsequently validated internally with the validation cohort.
For the model development, univariable logistic regression analysis was performed using all variables. The entire dataset was randomly split into training and validation sets at an 8:2 ratio.
Development of the model
Variable selection
The LASSO regression was done to select the variables to obtain a subset of variables that minimizes the error for a group of quantitative response variables (Musoro et al., 2014).
Feature selection and hyperparameter tuning
A L1-penalized logistic regression (LASSO) was used to perform simultaneous shrinkage and variable selection for fixed-horizon risk prediction. All continuous predictors were standardized to a mean of zero and a variance of one prior to modeling; categorical variables were dummy-encoded. The regularization parameter (λ) was chosen exclusively via stratified 10-fold cross-validation within the training data, optimizing binomial deviance. To favor parsimony, when applicable, the one-standard-error rule was adopted, selecting the most regularized model whose cross-validated deviance lay within one standard error of the minimum. No hypothesis testing or p-value thresholds were used to select predictors at any stage; the active set of variables was determined solely by the cross-validated L1 penalty (Musoro et al., 2014). Model coefficients were then refit on the full training set at the selected λ and evaluated.
Finalizing model
The multivariable logistic regression was done based on the variables identified through LASSO regression. The final set of predictors was determined by LASSO with λ selected via stratified 10-fold cross-validation. All variables were presented as adjusted odds ratios (aORs) with 95% confidence intervals (CIs).
Sample size and model complexity
To control overfitting, the pragmatic “events-per-parameter” (EPP) rule of thumb was followed, targeting at least 20 outcome events per model degree of freedom. The derivation (training) set comprised n = 686 participants. The observed 2-year incidence of cognitive decline in the overall cohort was ≈28.4% (95% CI: 25.4%–31.5%), implying approximately E ≈ 195 events in the derivation set (0.284 × 686). Under an EPP ≥ 20 criterion, the derivation set supports about E/20 ≈9–10 effective parameters. The final penalized model included 9 degrees of freedom (one per retained predictor), thus satisfying the EPP ≥ 20 target. In addition, LASSO with stratified 10-fold cross-validation was used to select λ and shrink coefficients, further mitigating optimism beyond the EPP safeguard.
Construction of nomogram-scoring system
A nomogram-scoring system was constructed to present the model using the “nomolog” package in STATA. Nomogram generation and calculation of predicted probability in a logistic regression model involved the following steps:
i. Scores were generated using the logit regression coefficients;
ii. The possible scores (α1 xi) were plotted for each variable (X1.N).
iii. The constant value (α0) was obtained.
iv. The probability of event (p) was calculated:
Total points = TP = α1 X1+ α2 X2+…
Discrimination and calibration of the model
Receiver Operating Characteristic (ROC) analysis was performed by using the predicted probability of the event (p) as the test variable and the final true outcome as the gold standard variable. The area under the curve (AUC) was generated to explain the discriminatory ability of the nomogram-based model. The analysis was re-run in the validation set.
The calibration curve with the observed frequency was plotted against the predicted probability of cognitive decline to check calibration and identify any considerable deviation from 450 lines of a perfect fit in the training and validation sets (Collins et al., 2015).
Results
The baseline characteristics of study participants are summarized in Table 1. The average age of patients in the training and validation cohorts was 70.0 (7.4) and 70.6 (7.8) years, respectively. Both cohorts had a higher percentage of female patients. There was a higher prevalence of hypertension in the training and validation cohorts (67.1 and 74.7%, respectively). Of 864 patients, 246 (28.5%; 95% CI: 25.4–31.5%) experienced cognitive decline during the 2-year follow-up period. The dataset was randomly split into training and validation sets at a 8:2 ratio (686 in the training set and 178 in the validation set).
TABLE 1
| Characteristics | Training set, n (%) N = 686 |
Validation set, n (%) N = 178 |
| Age of the patient | ||
| Mean age ( ± SD) | 70.0 (7.41) | 70.55 (7.80) |
| Gender of the patient | ||
| Male | 238 (34.7) | 64 (36.0) |
| Female | 448 (65.3) | 114 (64.0) |
| Marital status | ||
| Never married | 99 (14.5) | 21 (11.8) |
| Currently married | 568 (83.0) | 152 (85.4) |
| Widowed/separated/divorced | 17 (2.5) | 5 (2.8) |
| Educational qualification | ||
| No formal education | 20 (2.9) | 6 (3.4) |
| Primary | 55 (8.0) | 12 (6.7) |
| Secondary | 186 (27.1) | 53 (29.8) |
| Higher secondary | 225 (32.8) | 57 (32.0) |
| Graduate | 104 (15.2) | 26 (14.6) |
| Post-graduate | 96 (14.0) | 24 (13.5) |
| Smoking | ||
| No | 561 (81.8) | 144 (80.9) |
| Yes | 125 (18.2) | 34 (19.1) |
| Alcohol user | ||
| No | 587 (85.6) | 146 (82.0) |
| Yes | 99 (14.4) | 32 (18.0) |
| Tea drinker | ||
| No | 348 (50.7) | 97 (54.5) |
| Yes | 338 (49.3) | 81 (45.5) |
| Hypertension | ||
| Present | 460 (67.1) | 133 (74.7) |
| Absent | 226 (32.9) | 45 (25.3) |
| Diabetes mellitus | ||
| Present | 181 (26.4) | 54 (30.3) |
| Absent | 505 (73.6) | 124 (69.7) |
| Coronary artery disease | ||
| Present | 123 (17.9) | 42 (23.6) |
| Absent | 563 (82.1) | 136 (76.4) |
| Hyperlipidemia | ||
| Present | 304 (44.3) | 74 (41.6) |
| Absent | 382 (55.7) | 104 (58.4) |
| Cerebral hemorrhage | ||
| Present | 18 (2.6) | 5 (2.8) |
| Absent | 668 (97.4) | 173 (97.2) |
| Cerebral infarction | ||
| Present | 163 (23.8) | 43 (24.2) |
| Absent | 523 (76.2) | 135 (75.8) |
| Depression | ||
| Present | 74 (10.8) | 19 (10.7) |
| Absent | 608 (89.2) | 158 (89.3) |
| Decline in daily function | ||
| Present | 26 (3.8) | 8 (4.5) |
| Absent | 660 (96.2) | 170 (95.5) |
| Physiological and biochemical parameters | ||
| Body mass index (BMI in kg/m2) mean (±SD) | 24.07 (3.54) | 24.47 (3.42) |
| Red cell count (RBC) mean (±SD) | 4.39 (0.61) | 4.39 (0.67) |
| Blood platelets mean (±SD) | 2.08 (0.64) | 2.01 (0.56) |
| Mean platelet volume mean (±SD) | 9.25 (1.07) | 9.26 (1.16) |
| Lymphocyte count mean (±SD) | 2.15 (0.74) | 2.11 (0.70) |
| Hemoglobin concentration mean ( ± SD) | 13.23 (1.8) | 13.23 (2.02) |
| Alanine aminotransferase mean (±SD) | 22.02 (11.3) | 26.11 (16.65)) |
| Total protein mean (±SD) | 7.6 (0.45) | 7.6 (4.57) |
| Aspartate aminotransferase mean (±SD) | 23.15 (7.30) | 25.72 (10.57) |
| HDL cholesterol mean (±SD) | 1.37 (0.37) | 1.37 (0.36) |
| LDL cholesterol mean (±SD) | 3.09 (0.91) | 2.99 (1.12) |
| Total cholesterol mean (±SD) | 5.04 (1.02) | 5.02 (1.20) |
| Triglycerides mean (±SD) | 1.66 (1.00) | 1.85 (1.58) |
Baseline characteristics of the study participants.
A total of 686 patients were included in the training set, of whom 190 (27.7%) had cognitive impairment and 496 (72.3%) did not. The overall mean age was 70.0 ± 7.41 years, with patients exhibiting cognitive impairment being slightly older (71.11 ± 7.54 years) than those without (69.56 ± 7.32 years). Females comprised 65.3% of the cohort, with a higher proportion among those with cognitive impairment (69.5%) compared to those without (63.7%). Regarding marital status, 83.0% were currently married, 14.5% were never married, and 2.5% were widowed, separated, or divorced. In terms of educational qualifications, the most common level was higher secondary (32.8%), followed by graduate (15.2%) and post-graduate (14.0%). Most patients were non-smokers (81.8%) and non-alcohol users (85.6%); however, tea consumption was reported by 49.3% of the total sample, with a lower proportion among those with cognitive impairment (40.0%) compared to those without (52.8%). Comorbid conditions were common, with hypertension present in 67.1%, diabetes mellitus in 26.4%, coronary artery disease in 17.9%, and hyperlipidemia in 44.3% of patients. Cerebral hemorrhage and infarction were reported in 2.6 and 23.8% of patients, respectively, while depression was noted in 10.8% and a decline in daily function in 3.8%. Physiologically, the overall body mass index was 24.07 ± 3.54 kg/m2, the red cell count 4.39 ± 0.61, and hemoglobin concentration 13.23 ± 1.80 g/dL. Biochemical parameters were as follows: alanine aminotransferase (22.02 ± 11.30 U/L), aspartate aminotransferase (23.15 ± 7.30 U/L), HDL cholesterol (1.37 ± 0.37 mmol/L), LDL cholesterol (3.09 ± 0.91 mmol/L), total cholesterol (5.04 ± 1.02 mmol/L), and triglycerides (1.66 ± 1.00 mmol/L).
Development of a nomogram-based model
Based on the lambda value selected by EBIC (Figure 1), 13 variables (age, gender, smoking, alcohol, tea drinking history, hypertension, diabetes mellitus, CAD, hyperlipidemia, cerebral hemorrhage, cerebral infarction, depression, decline in daily function) were chosen for the nomogram-based model. Multivariable logistic regression was then performed using the set of selected variables. As shown in Table 2, the results of the analysis showed that age (OR:1.03, 95% CI: 1.01–1.05; P = 0.020), female gender (OR: 2.09, 95% CI: 1.25–3.51; P = 0.005), smoking (OR: 2.31 95% CI: 1.27–4.20; P = 0.006), history of tea consumption (OR:1.55, 95% CI: 1.07–2.24, P = 0.020), absence of hyperlipidemia (OR:0.60, 95% CI: 0.41–0.86; P = 0.005), cerebral hemorrhage (OR:3.03, 95% CI: 1.12–5.68; P = 0.029), and decline in the daily function (OR: 2.45, 95% CI: 1.06–5.68; P = 0.036) were all risk factors that may predict cognitive decline, and were selected for the final nomogram-based model (Table 2).
FIGURE 1

LASSO (Least Absolute Shrinkage and Selection Operator) coefficient profile plots for variables associated with cognitive decline. The x-axis represents the log-transformed lambda values, and the y-axis shows the coefficients of predictors included in the model. Variables retained as significant predictors are shown with nonzero coefficients.
TABLE 2
| Characteristics | Adjusted OR | S.E. | 95% CI | P-value |
| Age of the patient | 1.03 | 0.013 | 1.01–1.05 | 0.020* |
| Gender | ||||
| Females | 2.09 | 0.55 | 1.25–3.51 | 0.005* |
| Males | Ref | – | – | - |
| Smoking | ||||
| Non-smoker | Ref | – | – | - |
| Smoker | 2.31 | 0.71 | 1.27–4.20 | 0.006* |
| History of tea consumption | ||||
| Regular drinker | Ref | – | – | - |
| Non-drinker | 1.55 | 0.29 | 1.07–2.24 | 0.020* |
| Diabetes mellitus | ||||
| Absent | Ref | – | – | - |
| Present | 1.42 | 0.28 | 0.97–2.10 | 0.074 |
| Coronary artery disease | ||||
| Absent | Ref | – | – | - |
| Present | 1.51 | 0.33 | 0.98–2.33 | 0.064 |
| Hyperlipidemia | ||||
| Present | Ref | – | – | - |
| Absent | 0.60 | 0.11 | 0.41–0.86 | 0.005* |
| Cerebral hemorrhage | ||||
| Absent | Ref | – | – | - |
| Present | 3.03 | 1.53 | 1.12–5.68 | 0.029* |
| Decline in the daily function | ||||
| Absent | Ref | – | – | - |
| Present | 2.45 | 1.05 | 1.06–5.68 | 0.036* |
Multivariable logistic regression for the predictors of cognitive decline based on training set (N = 686).
OR: odds ratio; S.E: standard error; CI: confidence interval; Ref: reference category.
*p-value < 0.05 i.e., statistically significant.
Nomogram-scoring system
The final model was presented as a scoring system (Figure 2). The selected variables in the nomogram-based model were arranged one by one on a horizontal plane in the scoring system, ranging from 0 to 10 at the bottom. The total score ranged from 0 to 29.3, and the scores of individual variables are provided in Table 3.
FIGURE 2

Nomogram scoring system developed to predict the risk of cognitive decline among older adults in Shanghai, China. Each predictor corresponds to a score based on its presence or value, with the total score providing the estimated probability of cognitive decline.
TABLE 3
| Characteristics | Range or status | Score (points) |
| Age of the patient (in years) | ≥95 | 10.0 |
| 89–94 | 9.4 | |
| 83–88 | 8.8 | |
| 77–82 | 8.2 | |
| 72–76 | 7.5 | |
| 66–71 | 6.9 | |
| 60–65 | 6.3 | |
| 54–59 | 5.7 | |
| Cerebral hemorrhage | Present | 4.0 |
| Absent | 0.0 | |
| Smoking | Smoker | 3.0 |
| Non-smoker | 0.0 | |
| Decline in daily function | Present | 3.3 |
| Absent | 0.0 | |
| Gender | Females | 2.7 |
| Males | 0.0 | |
| Coronary artery disease | Present | 1.5 |
| Absent | 0.0 | |
| Tea drinking | Non-drinker | 1.6 |
| Tea drinker | 0.0 | |
| Hyperlipidemia | Present | 1.9 |
| Absent | 0.0 | |
| Diabetes mellitus | Present | 1.3 |
| Absent | 0.0 |
Scoring system for prediction of cognitive decline amongst older adults.
The nomogram calculates the total score for each individual by summing the points assigned to each risk factor, and the corresponding probability of cognitive decline can be determined based on the total score. For example, a total score of 15 points corresponds to a probability of approximately 0.50 (50%) for cognitive decline. Higher total scores indicate a higher likelihood of cognitive decline, allowing clinicians to interpret and apply the prognostic model in clinical settings easily.
Discrimination and calibration
The nomogram-based model’s discriminatory power in the training set was 0.65 (95% CI: 0.61–0.70) (Figure 3). Similar discriminatory power was detected for the validation set (0.67; 95% CI: 0.58–0.75) (Figure 4). Figures 5, 6 illustrate the calibration plot for predicted probabilities versus observed outcomes, demonstrating the model’s predictive performance in both the derivation set and the validation set. The points represent grouped predicted probabilities, along with their corresponding observed frequencies, and 95% CIs (vertical bars). The blue smoother line closely follows the diagonal reference line, indicating a good agreement between predictions and observed outcomes. This suggests that the model demonstrates acceptable calibration with no significant deviations across the probability range.
FIGURE 3

Receiver Operating Characteristic (ROC) curve for the derivation cohort. The curve illustrates the trade-off between sensitivity and specificity of the prediction model, with an area under the curve (AUC) of 0.65 indicating moderate discriminatory power.
FIGURE 4

Receiver Operating Characteristic (ROC) curve for the validation cohort. The curve demonstrates the performance of the prediction model on the validation dataset, with an area under the curve (AUC) of 0.67, reflecting moderate accuracy.
FIGURE 5
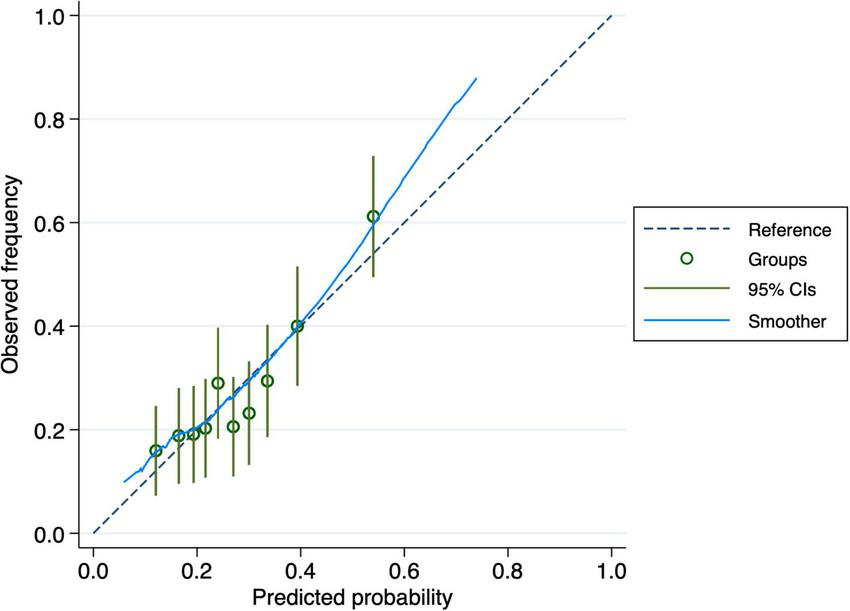
Calibration curve for the derivation cohort. The plot compares observed frequencies of cognitive decline (y-axis) with predicted probabilities (x-axis), with the dashed line representing perfect calibration. The 95% confidence intervals (vertical bars) indicate variability within each group.
FIGURE 6

Calibration curve for the validation cohort. The plot assesses the agreement between observed and predicted probabilities of cognitive decline. The dashed line represents perfect calibration, and the blue smoothed curve shows the actual calibration performance with 95% confidence intervals.
Discussion
The current study aimed to develop and validate a prognostic scoring system to predict cognitive decline in individuals aged 50 and older in Shanghai, China. The final model included nine variables: age, gender, smoking, tea-drinking history, hypertension, diabetes mellitus, CAD, hyperlipidemia, cerebral hemorrhage, and decline in daily function. The model demonstrated moderate discriminatory power and good calibration in the training and validation sets, indicating its robustness and potential utility in clinical and community settings. The model and findings were consistent with those of models developed in the existing literature (Hu et al., 2021; Hu et al., 2022; Zhou et al., 2020).
The results showed that age and gender are significant predictors of cognitive decline, consistent with the previous research. Numerous studies have highlighted the strong association between aging and cognitive deterioration and identified age ss the most significant predictor (Dufouil et al., 1996; Schönknecht et al., 2005; Ma et al., 2020; Yang et al., 2023). The gender differences observed in this study also reflect the findings from other research, indicating that females are at a higher risk of cognitive decline, possibly due to hormonal differences and longer life expectancy (Sohn et al., 2018; Rahman et al., 2019; Levine et al., 2021; Verhagen et al., 2022). These associations may be explained by several mechanisms and existing theoretical frameworks. Age emerged as the most significant predictor of cognitive decline, a finding consistent with the natural aging process. Aging is associated with a cumulative burden of oxidative stress, inflammatory processes, and cellular senescence, all contributing to neuronal damage and cognitive impairment (Liguori et al., 2018). The longer life expectancy of women, coupled with the hormonal changes that occur post-menopause, may explain why gender also emerged as a significant predictor, with females showing a higher risk of cognitive decline (Han et al., 2023).
The impact of various behavioral factors on cognitive health has been extensively studied. This study demonstrated a higher risk of cognitive decline among smokers, which is consistent with previous research (Anstey et al., 2007; Peters et al., 2008; Sabia et al., 2012). Nicotine and other harmful substances in cigarettes are known to cause vascular damage and reduce cerebral blood flow, leading to neurodegeneration (Mazzone et al., 2010). Smoking also increases oxidative stress and inflammation, which can accelerate cognitive decline (Peters et al., 2008). This aligns with existing literature that consistently finds a strong association between smoking and various forms of cognitive impairment (Ford et al., 1996; Liu et al., 2017).
The literature has been less consistent on the impact of tea drinking on cognitive health. Several studies suggested a protective effect due to the antioxidants present in tea (Ng et al., 2008; Sargent et al., 2020; Wei et al., 2023). In agreement with these observations, the findings of this study indicate that non-tea drinkers are at a slightly higher risk of cognitive decline.
The role of comorbid conditions such as hypertension, diabetes, CAD, and hyperlipidemia in cognitive decline has been well-documented. These conditions affect vascular health, which is crucial for maintaining cognitive function (Alagiakrishnan et al., 2006; Duron and Hanon, 2008; Cohen et al., 2009). This study supports such associations, showing that hypertension, diabetes, CAD, and hyperlipidemia are all significantly associated with cognitive deterioration, further highlighting the importance of managing cardiovascular health to prevent mental decline. All these conditions are known to impair blood flow to the brain, leading to ischemia (Alagiakrishnan et al., 2006; Duron and Hanon, 2008; Cohen et al., 2009). Hypertension and diabetes can cause microvascular damage, which affects the brain’s white matter and leads to cognitive deficits (van Sloten et al., 2020). The relation between hyperlipidemia and cognitive decline can be due to the formation of atherosclerotic plaques, which limit blood flow to the brain (Duong et al., 2021). The significant impact of cerebral hemorrhage on cognitive decline observed in our study is also in line with previous research (Potter et al., 2021; El Husseini et al., 2023). Cerebral hemorrhage directly damages brain tissue and disrupts normal brain function (Ungvari et al., 2017). The presence of cerebral hemorrhage as a significant predictor in this model highlights the severe and immediate effects of such vascular events on cognition.
Contrary to some studies that did not find a link between depression and cognitive decline, the initial model in this study included depression as a predictor (Kim, 2022). However, it was excluded from the final model due to a lack of statistical significance, suggesting that while depression may be related to cognitive decline, its impact may not be as strong as other factors in our population.
The decline in daily function that was more frequently reported in older patients with cognitive decline in the present cohort may be considered both a symptom and a predictor of cognitive decline (Cipriani et al., 2020; Dubbelman et al., 2020; Oveisgharan et al., 2024). As cognitive abilities diminish, individuals are less able to perform daily activities, and this reduction in daily function can further exacerbate cognitive deterioration through reduced mental and physical activity.
Strengths and limitations of the study
The main goal of the study was not to replace existing models but to provide a parsimonious, deployable tool tailored to routine Chinese clinical workflows where outcomes are assessed at discrete visits and detailed onset times are unavailable. By specifying a fixed-horizon risk target, relying on readily obtainable predictors, and reporting calibration and simple decision-support thresholds, the study complemented prior work and enabled head-to-head external validation against existing Chinese models in future studies.
The current study involved a substantial number of participants, which increases the statistical power and generalizability of our results. A two-year period allowed for capturing changes in cognitive function over time, providing a more dynamic understanding of cognitive decline. A wide range of acquired sociodemographic, behavioral, comorbid, and biochemical data allowed for a thorough analysis of multiple risk factors. Using LASSO logistic regression and EBIC for model selection ensured that the most relevant predictors were identified without overfitting. The model was internally validated with an external cohort, adding robustness. The developed nomogram-based scoring system provides a practical tool that can be readily used in clinical and community settings to identify patients at high risk of cognitive decline.
This study has some limitations. The main limitation is that the outcome was defined using a screening tool (MoCA < 26) rather than a clinical diagnosis. While MoCA has good sensitivity and acceptable specificity for detecting cognitive impairment, misclassification is still possible. Non-differential outcome misclassification would likely bias the results toward the null and slightly lower the AUC, meaning our estimates of discrimination are probably conservative. Future validation against confirmed clinical outcomes (and exploring education-adjusted thresholds) will help determine any effects on calibration and net benefit. Discrimination was modest (AUC around 0.65 in the derivation set and about 0.67 in the validation set), which may limit its use in standalone clinical decisions. This probably reflects our baseline-only predictor set and the use of a screening test to define cognitive impairment at follow-up, both of which can reduce the apparent ability to distinguish risks. The validation was internal to the available cohort and does not replace independent external validation across different sites and case-mixes. Therefore, generalizability remains uncertain. Predictors included only demographics, medical history, and routine blood measures to maximize feasibility and equity in primary care settings. The exclusion of APOE genotyping and neuroimaging likely limited the maximum discrimination and may partly explain the moderate AUC.
The study only included patients from a single community in Shanghai. Future studies should include participants from other regions or countries with different demographic and cultural characteristics to improve generalizability. Additionally, the study relied on the self-reporting of behavioral and comorbid conditions, which may have resulted in recall or social desirability bias, potentially affecting the accuracy of the information. Although the study is longitudinal, some variables were only measured at baseline, limiting the ability to capture changes in these factors over time and their impact on cognitive decline. The model’s discriminatory power was moderate (AUC around 0.65–0.67), suggesting that there may be other unmeasured factors influencing cognitive decline that were not captured in this study.
Implications for clinical practice
Despite limitations, the reported findings have significant implications for clinical practice and public health policy. The developed prognostic scoring system provides a practical and reliable tool for healthcare providers to assess the risk of cognitive decline in middle-aged and older adults on time, enabling them to implement targeted interventions that delay or prevent the onset of cognitive decline. Healthcare providers can use the model to screen patients during routine visits, particularly focusing on older adults with multiple risk factors such as advanced age, smoking history, and cardiovascular comorbidities. Early intervention strategies, such as cognitive training, lifestyle modifications, and managing cardiovascular risk factors, can be more effectively applied to patients at high risk.
The reported predictions are intended to guide care planning, not to diagnose dementia. In practice, a “high-risk” classification can trigger proportionate actions that are already available in routine settings: (i) optimization of vascular and metabolic risk factors; (ii) review and reduction of anticholinergic burden and other potentially inappropriate medications; (iii) evaluation and correction of hearing and vision deficits; (iv) screening and management of depression, sleep problems, and social isolation; and (v) closer interval follow-up or referral for formal cognitive assessment when indicated. Framed this way, risk stratification supports targeted prevention and earlier evaluation beyond generic lifestyle advice, aligning with pragmatic resource allocation in busy clinics.
Additionally, the detailed scoring system in this study enables clinicians to design care plans tailored to each patient’s individual risk profile. For instance, a smoker with hypertension and diabetes may receive a tailored intervention plan that includes smoking cessation support, blood pressure control, and diabetes management. Personalized interventions can be more effective in mitigating risk factors and slowing the progression of cognitive decline.
Moreover, by identifying high-risk populations, health systems can prioritize resource allocation to areas and groups most in need, such as cognitive health programs and preventive services. This targeted approach can maximize the impact of available resources, improve population health outcomes, and reduce the burden on the healthcare system.
This study highlights the importance of lifestyle factors, including smoking cessation and cardiovascular health, in preventing cognitive decline. Public health campaigns can use these findings to educate the community about the importance of healthy living and its impact on cognitive health. Increasing public awareness can motivate individuals to adopt healthier behaviors and seek regular health check-ups.
While this study provides a robust framework for predicting cognitive decline, further research is necessary to enhance and expand upon these findings. Future studies should include diverse populations from different geographic regions and cultural backgrounds to validate and potentially refine the prognostic scoring system. This will ensure the model is generalizable and applicable to various populations, increasing its utility and effectiveness. Although this study followed participants for two years, longer follow-up periods are essential to capture the long-term progression of cognitive decline. Extended follow-up can provide deeper insights into the trajectory of cognitive changes and the impact of various risk factors over time. Further studies are needed to elucidate the underlying mechanisms linking the identified risk factors with cognitive decline. Understanding the biological and physiological pathways of cognitive decline can inform the development of more targeted and effective interventions. Integrating technology, such as mobile health applications and wearable devices, can facilitate the continuous monitoring of risk factors and cognitive function. Future research should explore the feasibility and effectiveness of using technology to enhance early detection and intervention strategies. Research should also focus on the impact of policy changes informed by our findings. Evaluating the effectiveness of public health interventions and resource allocation strategies based on the prognostic model can provide valuable feedback for continuous improvement.
Conclusion
This study successfully developed and validated a practical prognostic scoring system for predicting cognitive decline among middle-aged and older adults in Shanghai, integrating key sociodemographic, behavioral, and comorbid factors. The model demonstrates moderate discriminatory power and good calibration, offering a valuable tool for early identification and intervention. Future research should focus on validating the model across diverse populations and exploring additional risk factors to enhance predictive accuracy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Shanghai Mental Health Centre (Approval No. 2018-11R, Date: 2018-07-27). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. ZQ: Formal analysis, Methodology, Validation, Writing – review & editing. YJ: Formal analysis, Methodology, Resources, Validation, Visualization, Writing – review & editing. HY: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Validation.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. Huangpu District Dapuqiao Community Health Center, Shanghai, China (2022KJCX040); The 2023 Shanghai “Rising Stars in Medicine” Youth Medical Talent Training and Funding Program (SHWSRS(2024)_70).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aerts L. Heffernan M. Kochan N. A. Crawford J. D. Draper B. Trollor J. N. et al (2017). Effects of MCI subtype and reversion on progression to dementia in a community sample.Neurology882225–2232. 10.1212/WNL.0000000000004015
2
Alagiakrishnan K. McCracken P. Feldman H. (2006). Treating vascular risk factors and maintaining vascular health: Is this the way towards successful cognitive ageing and preventing cognitive decline?Postgrad. Med. J.82101–105. 10.1136/pgmj.2005.035030
3
Anstey K. J. von Sanden C. Salim A. O’Kearney R. (2007). Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies.Am. J. Epidemiol.166367–378. 10.1093/aje/kwm116
4
Bai C. Lei X. (2020). New trends in population aging and challenges for China’s sustainable development.China Econ. J.133–23. 10.1080/17538963.2019.1700608
5
Bao J. Zhou L. Liu G. Tang J. Lu X. Cheng C. et al (2022). Current state of care for the elderly in China in the context of an aging population.Biosci. Trends16107–118. 10.5582/bst.2022.01068
6
Bhatti G. K. Reddy A. P. Reddy P. H. Bhatti J. S. (2019). Lifestyle modifications and nutritional interventions in aging-associated cognitive decline and Alzheimer’s disease.Front. Aging Neurosci.11:369. 10.3389/fnagi.2019.00369
7
Campbell N. L. Unverzagt F. LaMantia M. A. Khan B. A. Boustani M. A. (2013). Risk factors for the progression of mild cognitive impairment to dementia.Clin. Geriatr. Med.29873–893. 10.1016/j.cger.2013.07.009
8
Chen X. Lee C. Huang H. (2022). Neighborhood built environment associated with cognition and dementia risk among older adults: A systematic literature review.Soc. Sci. Med.292:114560. 10.1016/j.socscimed.2021.114560
9
Cipriani G. Danti S. Picchi L. Nuti A. Fiorino M. D. (2020). Daily functioning and dementia.Dement. Neuropsychol.1493–102. 10.1590/1980-57642020dn14-020001
10
Cohen R. A. Poppas A. Forman D. E. Hoth K. F. Haley A. P. Gunstad J. et al (2009). Vascular and cognitive functions associated with cardiovascular disease in the elderly.J. Clin. Exp. Neuropsychol.3196–110. 10.1080/13803390802014594
11
Collins G. S. Reitsma J. B. Altman D. G. Moons K. G. M. (2015). Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement.Ann. Intern. Med.16255–63. 10.7326/M14-0697
12
Dubbelman M. A. Jutten R. J. Tomaszewski Farias S. E. Amariglio R. E. Buckley R. F. Visser P. J. et al (2020). Decline in cognitively complex everyday activities accelerates along the Alzheimer’s disease continuum.Alzheimer’s Res. Therapy12:138. 10.1186/s13195-020-00706-2
13
Dufouil C. Fuhrer R. Dartigues J. F. Alpérovitch A. (1996). Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration.Am. J. Epidemiol.144634–641. 10.1093/oxfordjournals.aje.a008974
14
Duong M. T. Nasrallah I. M. Wolk D. A. Chang C. C. Y. Chang T.-Y. (2021). Cholesterol, atherosclerosis, and APOE in Vascular Contributions To Cognitive Impairment And Dementia (VCID): Potential mechanisms and therapy.Front. Aging Neurosci.13:647990. 10.3389/fnagi.2021.647990
15
Duron E. Hanon O. (2008). Vascular risk factors, cognitive decline, and dementia.Vasc. Health Risk Manag.4363–381. 10.2147/vhrm.s1839
16
El Husseini N. Katzan I. L. Rost N. S. Blake M. L. Byun E. Pendlebury S. T. et al (2023). Cognitive impairment after ischemic and hemorrhagic stroke: A scientific statement from the american heart association/american stroke association.Stroke54e272–e291. 10.1161/STR.0000000000000430
17
Ford A. B. Mefrouche Z. Friedland R. P. Debanne S. M. (1996). Smoking and cognitive impairment: A population-based study.J. Am. Geriatr. Soc.44905–909. 10.1111/j.1532-5415.1996.tb01858.x
18
Hampel H. Prvulovic D. Teipel S. Jessen F. Luckhaus C. Frölich L. et al (2011). The future of Alzheimer’s disease: The next 10 years.Prog. Neurobiol.95718–728. 10.1016/j.pneurobio.2011.11.008
19
Han S.-L. Liu D.-C. Tan C.-C. Tan L. Xu W. (2023). Male- and female-specific reproductive risk factors across the lifespan for dementia or cognitive decline: A systematic review and meta-analysis.BMC Med.21:457. 10.1186/s12916-023-03159-0
20
Hu M. Gao Y. Kwok T. C. Y. Shao Z. Xiao L. D. Feng H. (2022). Derivation and validation of the cognitive impairment prediction model in older adults: A national cohort study. Front. Aging Neurosci.14:755005. 10.3389/fnagi.2022.755005
21
Hu M. Shu X. Yu G. Wu X. Välimäki M. Feng H. (2021). A risk prediction model based on machine learning for cognitive impairment among Chinese community-dwelling elderly people with normal cognition: Development and validation study. J. Med. Internet. Res.23:e20298. 10.2196/20298
22
Kim D. (2022). Effects of depression on changes in cognitive function in older adults: A fixed-effects model analysis using the korean longitudinal study of aging (KLoSA).Alzheimer Dis. Assoc. Disord.36319–326. 10.1097/WAD.0000000000000531
23
Levine D. A. Gross A. L. Briceño E. M. Tilton N. Giordani B. J. Sussman J. B. et al (2021). Sex differences in cognitive decline among US adults.JAMA Netw. Open4:e210169. 10.1001/jamanetworkopen.2021.0169
24
Liguori I. Russo G. Curcio F. Bulli G. Aran L. Della-Morte D. et al (2018). Oxidative stress, aging, and diseases.Clin. Interv. Aging13757–772. 10.2147/CIA.S158513
25
Liu J. Shang S. Li P. Deng M. Chen C. Jiang Y. et al (2017). Association between current smoking and cognitive impairment depends on age: A cross-sectional study in Xi’an. China.Med. Clin.149203–208. 10.1016/j.medcli.2017.02.033
26
Lunenfeld B. Stratton P. (2013). The clinical consequences of an ageing world and preventive strategies.Best Pract. Res. Clin. Obstet. Gynaecol.27643–659. 10.1016/j.bpobgyn.2013.02.005
27
Ma Y. Liang L. Zheng F. Shi L. Zhong B. Xie W. (2020). Association between sleep duration and cognitive decline.JAMA Netw. Open3:e2013573. 10.1001/jamanetworkopen.2020.13573
28
Mazzone P. Tierney W. Hossain M. Puvenna V. Janigro D. Cucullo L. (2010). Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: Expanding the awareness of smoking toxicity in an underappreciated area.Int. J. Environ. Res. Public Health74111–4126. 10.3390/ijerph7124111
29
Musoro J. Z. Zwinderman A. H. Puhan M. A. ter Riet G. Geskus R. B. (2014). Validation of prediction models based on lasso regression with multiply imputed data.BMC Med. Res. Methodol.14:116. 10.1186/1471-2288-14-116
30
Nasreddine Z. S. Phillips N. A. Bédirian V. Charbonneau S. Whitehead V. Collin I. et al (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment.J. Am. Geriatr. Soc.53695–699. 10.1111/j.1532-5415.2005.53221.x
31
Ng T.-P. Feng L. Niti M. Kua E.-H. Yap K.-B. (2008). Tea consumption and cognitive impairment and decline in older Chinese adults.Am. J. Clin. Nutr.88224–231. 10.1093/ajcn/88.1.224
32
Okusaga O. Stewart M. C. W. Butcher I. Deary I. Fowkes F. G. R. Price J. F. (2013). Smoking, hypercholesterolaemia and hypertension as risk factors for cognitive impairment in older adults.Age Ageing42306–311. 10.1093/ageing/afs193
33
Oveisgharan S. Wang T. Barnes L. L. Schneider J. A. Bennett D. A. Buchman A. S. (2024). The time course of motor and cognitive decline in older adults and their associations with brain pathologies: A multicohort study.Lancet Healthy Long.5e336–e345. 10.1016/S2666-7568(24)00033-3
34
Peters R. Poulter R. Warner J. Beckett N. Burch L. Bulpitt C. (2008). Smoking, dementia and cognitive decline in the elderly, a systematic review.BMC Geriatr.8:36. 10.1186/1471-2318-8-36
35
Potter T. Lioutas V.-A. Tano M. Pan A. Meeks J. Woo D. et al (2021). Cognitive impairment after intracerebral hemorrhage: A systematic review of current evidence and knowledge gaps.Front. Neurol.12:716632. 10.3389/fneur.2021.716632
36
Prince J. B. Davis H. L. Tan J. Muller-Townsend K. Markovic S. Lewis D. M. G. et al (2024). Cognitive and neuroscientific perspectives of healthy ageing.Neurosci. Biobehav. Rev.161:105649. 10.1016/j.neubiorev.2024.105649
37
Prince M. J. Wu F. Guo Y. Gutierrez Robledo L. M. O’Donnell M. Sullivan R. et al (2015). The burden of disease in older people and implications for health policy and practice.Lancet385549–562. 10.1016/S0140-6736(14)61347-7
38
Rahman A. Jackson H. Hristov H. Isaacson R. S. Saif N. Shetty T. et al (2019). Sex and gender driven modifiers of Alzheimer’s: The role for estrogenic control across age, race, medical, and lifestyle risks.Front. Aging Neurosci.11:315. 10.3389/fnagi.2019.00315
39
Rao R. V. Subramaniam K. G. Gregory J. Bredesen A. L. Coward C. Okada S. et al (2023). Rationale for a multi-factorial approach for the reversal of cognitive decline in Alzheimer’s disease and MCI: A review.Int. J. Mol. Sci.24:1659. 10.3390/ijms24021659
40
Rolandi E. Zaccaria D. Vaccaro R. Abbondanza S. Pettinato L. Davin A. et al (2020). Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: A population-based study.Alzheimers Res. Ther.12:94. 10.1186/s13195-020-00661-y
41
Sabbagh M. N. Boada M. Borson S. Chilukuri M. Doraiswamy P. M. Dubois B. et al (2020). Rationale for early diagnosis of Mild Cognitive Impairment (MCI) supported by emerging digital technologies.J. Prevent. Alzheimer’s Dis.7158–164. 10.14283/jpad.2020.19
42
Sabia S. Elbaz A. Dugravot A. Head J. Shipley M. Hagger-Johnson G. et al (2012). Impact of smoking on cognitive decline in early old age: The Whitehall II cohort study.Arch. Gen. Psychiatry69627–635. 10.1001/archgenpsychiatry.2011.2016
43
Salthouse T. A. (2011). Neuroanatomical substrates of age-related cognitive decline.Psychol. Bull.137753–784. 10.1037/a0023262
44
Sargent A. Watson J. Topoglu Y. Ye H. Suri R. Ayaz H. (2020). Impact of tea and coffee consumption on cognitive performance: An fNIRS and EDA study.Appl. Sci.10:2390. 10.3390/app10072390
45
Schönknecht P. Pantel J. Kruse A. Schröder J. (2005). Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects.Am. J. Psychiatry1622071–2077. 10.1176/appi.ajp.162.11.2071
46
Sohn D. Shpanskaya K. Lucas J. E. Petrella J. R. Saykin A. J. Tanzi R. E. et al (2018). Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer’s disease.Sci. Rep.8:7490. 10.1038/s41598-018-25377-w
47
Ungvari Z. Tarantini S. Kirkpatrick A. C. Csiszar A. Prodan C. I. (2017). Cerebral microhemorrhages: Mechanisms, consequences, and prevention.Am. J. Physiol. Heart Circ. Physiol.312H1128–H1143. 10.1152/ajpheart.00780.2016
48
van der Flier W. M. de Vugt M. E. Smets E. M. A. Blom M. Teunissen C. E. (2023). Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia.Nat. Aging3494–505. 10.1038/s43587-023-00404-2
49
van Sloten T. T. Sedaghat S. Carnethon M. R. Launer L. J. Stehouwer C. D. A. (2020). Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression.Lancet Diab. Endocrinol.8325–336. 10.1016/S2213-8587(19)30405-X
50
Verhagen C. Janssen J. Biessels G. J. Johansen O. E. Exalto L. G. (2022). Females with type 2 diabetes are at higher risk for accelerated cognitive decline than males: CAROLINA-COGNITION study.Nutr. Metab. Cardiovasc. Dis.32355–364. 10.1016/j.numecd.2021.10.013
51
Wang J. Xiao L. D. Wang K. Luo Y. Li X. (2020). Cognitive impairment and associated factors in rural elderly in North China.J. Alzheimers Dis.771241–1253. 10.3233/JAD-200404
52
Wei C. Zhang J. Chen N. Xu Z. Tang H. (2023). Does frequent tea consumption provide any benefit to cognitive function in older adults? Evidence from a national survey from China in 2018.Front. Public Health11:1269675. 10.3389/fpubh.2023.1269675
53
Yang Y. Wang D. Hou W. Li H. (2023). Cognitive decline associated with aging.Adv. Exp. Med. Biol.141925–46. 10.1007/978-981-99-1627-6_3
54
Zhou J. Lv Y. Mao C. Duan J. Gao X. Wang J. et al (2020). Development and validation of a nomogram for predicting the 6-year risk of cognitive impairment among Chinese older adults. J. Am. Med. Direct Assoc.21, 864–871.e6. 10.1016/j.jamda.2020.03.032
Summary
Keywords
cognitive impairment, China, prognosis, scoring system, aging population
Citation
Zhang N, Meng L, Qian Z, Jin Y and Yan H (2025) Development and validation of a prognostic scoring system for cognitive decline in adults aged 50 and older in China. Front. Aging Neurosci. 17:1664943. doi: 10.3389/fnagi.2025.1664943
Received
13 July 2025
Accepted
08 September 2025
Published
23 September 2025
Volume
17 - 2025
Edited by
Vahid Rashedi, University of Social Welfare and Rehabilitation Sciences, Iran
Reviewed by
Giuseppe Murdaca, University of Genoa, Italy
Shoukang Zou, Sichuan University, China
Yongfei Dong, Sichuan University, China
Updates
Copyright
© 2025 Zhang, Meng, Qian, Jin and Yan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yan, yanhua_candy@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.