- 1Department of Developmental Cell Biology, Key Laboratory of Cell Biology, Ministry of Public Health, China Medical University, Shenyang, China
- 2Department of Environmental Health, School of Public Health, China Medical University, Shenyang, China
- 3Department of Neurology, The First People' s Hospital of Shenyang, Shenyang, Liaoning, China
- 4Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, China
- 5Department of Pharmaceutical Toxicology, School of Pharmacy, China Medical University, Shenyang, China
- 6Key Laboratory of Medical Electrophysiology, Ministry of Education & Medical Electrophysiological Key Laboratory of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, China
1 Introduction
The rapid aging of the global population represents one of the most significant social challenges of the twenty first century, accompanied by a sharp increase in age-related diseases (Kaizu and Miyata, 2025; Reinehr et al., 2024). Among these, the progressive decline of the nervous system plays a central role, impairing mobility, perception, autonomic regulation, and cognition, thereby reducing the quality of life and independence of the elderly (Huang et al., 2025). Substantial research indicates that neuro inflammation is a key mechanism in neurological disorders and cognitive aging (Marino et al., 2022; Wang et al., 2025).
Extensive epidemiological and clinical evidence has demonstrated significant gender differences in the incidence, progression, and treatment responses of many age-related diseases, with females often exhibiting greater vulnerability or distinct clinical manifestations (Migliore et al., 2021). Biological sex is thus a fundamental variable influencing disease susceptibility. Foundational studies confirm that immune responses and neuro inflammatory pathways show sexual dimorphism (Shabab et al., 2017), shaped by sex hormones, sex chromosome genes, and sociocultural factors.
Understanding gender differences in neuro inflammation during aging is crucial for elucidating disparities in neurodegenerative diseases and for developing gender-specific therapeutic strategies. This requires attention not only to molecular mechanisms but also to hormonal dynamics and immune-neural interactions. This article focuses on astrocytes, sex hormones, and the gut-brain axis in modulating gender differences in neuro inflammation, aiming to inform clinical research and targeted therapies (Table 1).
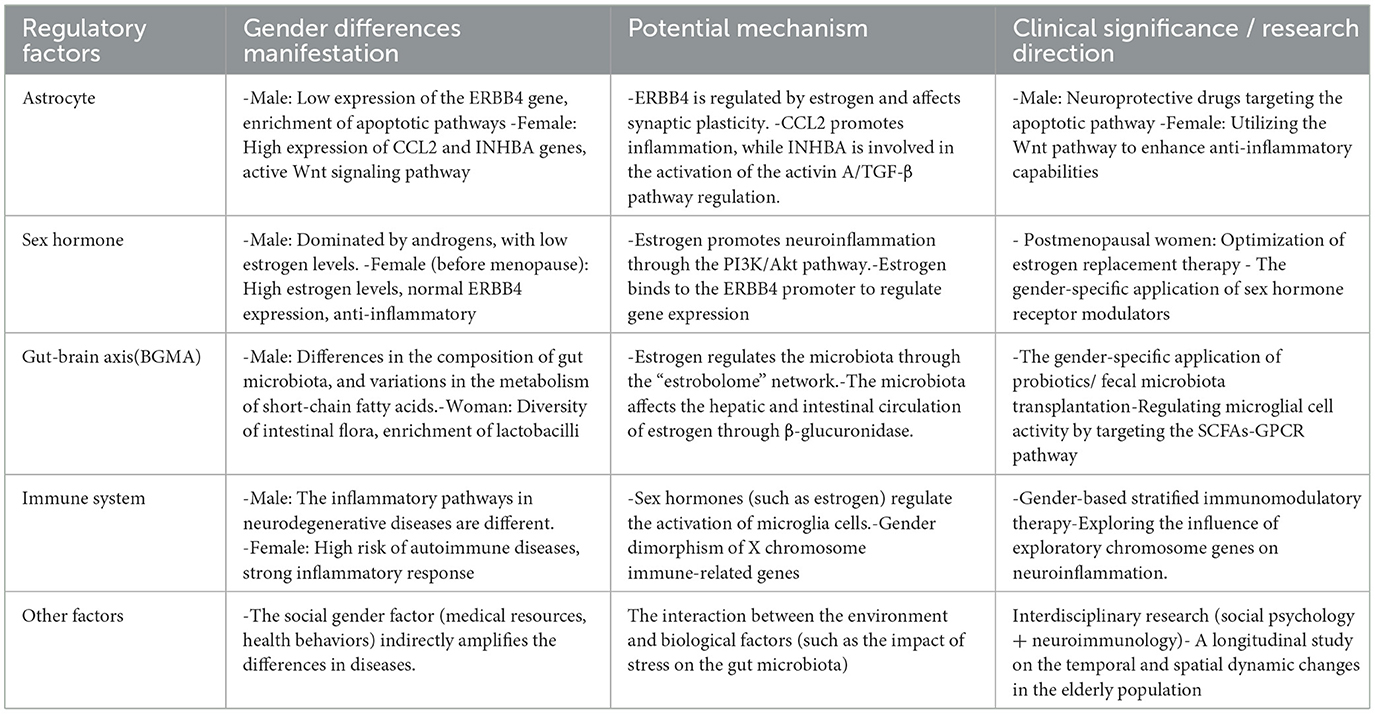
Table 1. Gender differences, potential mechanisms and clinical significance in the regulation of neuroinflammation.
2.1 Astrocytes
Astrocytes can be categorized into homeostatic astrocytes and reactive astrocytes, forming an integral component of the innate immunity within the central nervous system (CNS) (Tyurikova, 2024). These cells are capable of producing a variety of cytokines, chemokines, and other immune factor reservoirs (Wiese et al., 2012; Jensen et al., 2013; Farina et al., 2007). Astrocytes engage in complex intercellular communication with neurons, oligodendrocytes, microglia, and other cell types in the CNS, including bidirectional crosstalk with microglia during neuroinflammatory responses (Singh, 2022). The neuroinflammation mediated by astrocytes plays a significant role in neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease. Notably, there are sex-specific differences in the gene expression profiles of astrocytes involved in the regulation of neuro inflammation.
During the modulation of neuro inflammation, male-specific alterations in astrocytes have been observed, such as a significant downregulation of the ERBB4 gene, which encodes a receptor tyrosine kinase essential for neurodevelopment and synaptic plasticity. Abnormal expression of this gene may trigger synaptic dysfunction and neuroinflammation (Santiago et al., 2022). In contrast, female-specific changes include the upregulation of the CCL2 (C-C motif chemokine ligand 2) gene and the INHBA (inhibin subunit beta A) gene. CCL2, which encodes a monocyte chemoattractant protein-1, serves as a crucial regulatory factor in neuro inflammation (Posillico et al., 2021), exhibiting significantly higher expression levels during neuroinflammatory modulation. INHBA encodes a subunit of activin A, a member of the transforming growth factor beta (TGF-β) superfamily, which is involved in the regulation of neuroinflammation (Wu et al., 2021).
Furthermore, a notable enrichment of apoptosis and programmed cell death processes is present in male astrocytes, contrasting with alterations in Wnt signaling and cell cycle transition pathways observed in female astrocytes. This indicates profound differences between the sexes in their neuroinflammatory responses (Soudy et al., 2025). Investigating these sex-specific cellular mechanisms may enhance our understanding of the role of sex in neurobiology and provide new avenues for personalized medicine. For instance, drug development targeting apoptotic pathways in male astrocytes could potentially improve prognosis in male patients suffering from neurological disorders (Jan and Chaudhry, 2019). Concurrently, leveraging the protective mechanisms associated with female astrocytes, future research could explore how modulation of Wnt signaling pathways might augment cellular anti-inflammatory capacities, thereby offering new therapeutic strategies for the repair of the nervous system.
In addition to astrocytes, other neural cell types also play critical roles in the gender-specific regulation of neuro inflammation. Microglia, the resident immune cells of the CNS, display marked sexual dimorphism in their density, morphology, and activation thresholds (Li et al., 2024). Female microglia tend to mount stronger pro-inflammatory responses, which has been linked to the higher incidence of autoimmune and neuro inflammatory disorders in women, whereas male microglia may exhibit greater resilience to certain inflammatory challenges (Gildawie et al., 2020). Oligodendrocytes and their progenitor cells also contribute to sex differences, as they are essential for myelination and remyelination after injury (Long et al., 2021). Evidence suggests that estrogen and androgens can differentially regulate oligodendrocyte survival and maturation, thereby influencing white matter integrity in a sex-dependent manner (Zahaf et al., 2023).
2.2 Sex hormones
Sex hormones are a class of significant chemical substances secreted by endocrine glands, primarily consisting of estrogens, progestogens, and androgens (McEwen and Milner, 2017). These hormones play a crucial role in the regulation of neuro inflammation, particularly estrogen. Intuitively, the marked differences in estrogen and androgen levels between males and females result in gender-specific variations in the regulatory roles of sex hormones in neuro inflammation (Lu et al., 2025). However, the mechanisms through which sex hormones influence neuro inflammation are notably complex and extensive (Amanollahi et al., 2023), including effects on the activation of microglial cells and influences on the gut microbiota, the latter of which will be elaborated upon in the context of the gut-brain axis.
Sex hormones exert regulatory effects on numerous genes associated with neuro inflammatory responses. Taking the previously mentioned astrocytic ERBB4 gene as an example, this gene is regulated by estrogen, with its promoter region containing an estrogen response element half-site overlapping a binding site for activator protein-1 (Zhu et al., 2006). Upon stimulation by estrogen, this regulatory element enhances the binding of estrogen receptors to the intracellular domain of ERBB4 (4ICD), thereby modulating the expression of ERBB4. Given that estrogen levels in males are significantly lower than in females, ERBB4 is markedly underexpressed in males compared to females, subsequently promoting neuroinflammation; conversely, the normal expression of ERBB4 in females serves an anti-inflammatory function. Nevertheless, the decline in estrogen levels in aging females, particularly post-menopause, may increase the risk of neuroinflammation, indirectly corroborating the higher incidence of neuroinflammatory-related diseases (such as neurodegenerative disorders) in females compared to males (Bianco et al., 2023).
A bidirectional regulatory network exists between sex hormones and neuroinflammatory genes. For instance, as previously discussed, INHBA plays a critical role in follicular development and hormonal regulation in the ovaries, while the overexpression of INHBA can decrease the secretion of activin and estradiol while simultaneously increasing the secretion of inhibin and progesterone (Wu et al., 2021). Therefore, the INHBA gene is involved both in the regulatory processes of sex hormones and in mechanisms of neuroinflammation and neuroprotection. This illustrates the complexity of the body's regulatory processes concerning neuroinflammation, highlighting the pivotal interactions among various factors (Wu et al., 2021).
Additionally, estrogen plays an important role in neurogenesis, particularly evident in the dentate gyrus region of the hippocampus, where its neuroprotective effects are mediated through the enhancement of the PI3K/Akt signaling pathway (Harms et al., 2001).
Taken together, current evidence indicates that sex hormones exert dual and context-dependent effects on neuroinflammation. Estrogen is generally regarded as anti-inflammatory and neuroprotective, as seen in Alzheimer's disease and Parkinson's disease, where it reduces excessive microglial activation and supports neuronal survival (Wu et al., 2016). However, under certain pathological conditions, estrogens may also enhance immune activation, thereby exerting pro-inflammatory actions (Dragin et al., 2017). In contrast, androgens are more often associated with immunosuppressive and anti-inflammatory roles, although the supporting data remain less consistent (Gubbels bupp and Jorgensen, 2018). Clinical observations further highlight these dynamics: in multiple sclerosis, which disproportionately affects women, elevated estrogen levels during pregnancy are associated with symptomatic improvement, while disease activity often rebounds postpartum (Voskuhl and Momtazee, 2017); in Alzheimer's disease, the postmenopausal decline in estrogen contributes to the increased female susceptibility (Ali et al., 2023). These findings suggest that sex hormones are not uniformly protective or harmful but rather act in a disease- and stage-specific manner, reinforcing the need for carefully designed, sex-stratified therapeutic strategies.
2.3 Gut-brain axis
The diversity of gut microbiota is considered to be associated with gender, which is a significant factor influencing the intestinal microbiome, resulting in disparities in the composition and types of gut microbiota between males and females at birth (Rio et al., 2024). These differences in gut microbial composition also lead to gender-specific variations in responses and susceptibilities to related diseases.
The Brain-Gut Microbiome Axis (BGMA) plays a crucial role in brain-related disorders. Short-chain fatty acids (SCFAs) in the gut activate microglial cells through G protein-coupled receptors (GPCRs) (Kartjito et al., 2023). When the intestinal barrier function is compromised due to dysbiosis, abnormal immune system responses interact with the nervous system, ultimately leading to a state of “neuroinflammation”(Loh et al., 2024).
The immune system plays a pivotal role in the bidirectional communication between the gut microbiota and the brain (Yoo et al., 2020). BGMA regulation by the immune system is a particularly intriguing mechanism contributing to gender-related differences in health risks. Gut microbiota and sex steroids jointly modulate immune function, and further research on specific bacterial metabolites that regulate immunity could inform dietary interventions for preventing or treating autoimmune diseases (Rizzetto et al., 2018). Gender differences in microglial characteristics may also differentially influence sensitivity to pro-inflammatory and anti-inflammatory products of the microbiome (Kodama and Gan, 2019).
Sex hormones influence the brain-gut microbiome axis at multiple levels, including the central nervous system, enteric nervous system, and enteroendocrine cells. Numerous studies have demonstrated the significant role of sex hormones in the formation and regulation of gut microbiota (Kaliannan et al., 2018; Sakamuri et al., 2023). Specifically, the effects of sex hormones are reflected both in the absolute abundance of certain bacterial taxa and in the relative proportions of microbial communities. For example, estrogens selectively increase the quantity of Lactobacillus species, while also reshaping the overall microbial composition by enhancing community diversity in females compared with males (Siddiqui et al., 2022). In addition, sex hormone levels modulate functional microbial outputs such as SCFA production, thereby indirectly influencing inflammatory and neuroimmune pathways (Dalile et al., 2019). Conversely, the microbiome plays a crucial role by modulating sex hormones. Circulating estrogen levels are significantly regulated by the microbiome, and when the microbiome is disrupted, estrogen levels decrease accordingly (Baker et al., 2017). The gut microbiota regulates the enterohepatic circulation of estrogen through β-glucuronidase, forming the “estrobolome” network (Baker et al., 2017; Plottel and Blaser, 2011); estrogen metabolites, in turn, selectively promote the proliferation of specific bacteria (such as Lactobacillus), revealing a positive feedback mechanism (Kwa et al., 2016). Androgens (particularly testosterone) similarly affect the activity of the gut-brain axis, partly promoting the conversion of estradiol (Zuloaga et al., 2020). Thus, it is evident that gut microbiota and sex hormones jointly act on the regulation of neuroinflammation, a process exhibiting gender differences.
During the aging process, significant changes occur in the gut microbiota, accompanied by a decrease in sex hormones. Therefore, it seems worthwhile to further explore how the interactions between sex hormones and gut microbiota, as well as their regulation of neuroinflammation, may change. In clinical treatment, it is noteworthy to consider gender-specific therapeutic effects of gut microbiota interventions (such as probiotics/fecal microbiota transplantation).
3 Conclusion
Gender disparities in neuroinflammation highlight the need for precise, gender-specific therapies, particularly in the elderly. Future studies should use organoid models and gender-stratified trials to test interventions targeting astrocytes, sex hormones, and gut microbiota. The immune system remains central, with BGMA–hormone regulation requiring deeper investigation. Social factors, such as healthcare access and behaviors, also amplify disparities.
Spatiotemporal dynamics of neuroinflammation in aging demand longitudinal studies, while interactions among astrocytes, microglia, and peripheral immune cells can now be dissected with single-cell sequencing. Advances in genomics and epigenetics further identify sex-specific genes and networks as therapeutic targets. Despite challenges in translating animal findings and modeling hormone dynamics, interdisciplinary efforts will be key to progress.
Author contributions
J-WS: Writing – original draft. X-LM: Conceptualization, Writing – review & editing. YY: Writing – original draft. Y-TW: Writing – review & editing. H-XD: Writing – review & editing. RF: Conceptualization, Supervision, Writing – review & editing. KZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. ML: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. W-XQ: Conceptualization, Funding acquisition, Supervision, Writing review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Open Fund of the Medical Electrophysiology Key Laboratory (Southwest Medical University) (KeyME-2024-05) and the General Program of the Liaoning Provincial Department of Education (LJKMZ20221156).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CNS, Central Nervous System; CCL2, C-C motif Chemokine Ligand 2; INHBA, Inhibin Subunit Beta A; TGF-β, Transforming Growth Factor Beta; WNT, Wingless/Integrated; BGMA, Brain-Gut Microbiome Axis; SCFAs, Short-Chain Fatty Acids; GPCRs, G Protein-Coupled Receptors.
References
Ali, N., Sohail, R., Jaffer, S. R., Siddique, S., Kaya, B., Atowoju, I., et al. (2023). The role of estrogen therapy as a protective factor for alzheimer's disease and dementia in postmenopausal women: a comprehensive review of the literature. Cureus 15:e43053. doi: 10.7759/cureus.43053
Amanollahi, M., Jameie, M., Heidari, A., and Rezaei, N. (2023). The dialogue between neuroinflammation and adult neurogenesis: mechanisms involved and alterations in neurological diseases. Mol. Neurobiol. 60, 923–959. doi: 10.1007/s12035-022-03102-z
Baker, J. M., Al-nakkash, L., and Herbst-kralovetz, M. M. (2017). Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. doi: 10.1016/j.maturitas.2017.06.025
Bianco, A., Antonacci, Y., and Liguori, M. (2023). Sex and gender differences in neurodegenerative diseases: challenges for therapeutic opportunities. Int. J. Mol. Sci. 24:6354. doi: 10.3390/ijms24076354
Dalile, B., Van oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
Dragin, N., Nancy, P., Villegas, J., Roussin, R., Le panse, R., and Berrih-aknin, S. (2017). Balance between estrogens and proinflammatory cytokines regulates chemokine production involved in thymic germinal center formation. Sci. Rep. 7:7970. doi: 10.1038/s41598-017-08631-5
Farina, C., Aloisi, F., and Meinl, E. (2007). Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145. doi: 10.1016/j.it.2007.01.005
Gildawie, K. R., ORSO, R., Peterzell, S., Thompson, V., and Brenhouse, H. C. (2020). Sex differences in prefrontal cortex microglia morphology: impact of a two-hit model of adversity throughout development. Neurosci. Lett. 738:135381. doi: 10.1016/j.neulet.2020.135381
Gubbels bupp, M. R., and Jorgensen, T. N. (2018). Androgen-Induced Immunosuppression. Front Immunol 9:794. doi: 10.3389/fimmu.2018.00794
Harms, C., Lautenschlager, M., Bergk, A., Katchanov, J., Freyer, D., Kapinya, K., et al. (2001). Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J. Neurosci. 21, 2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001
Huang, L., Liu, M., Li, Z., Li, B., Wang, J., and Zhang, K. (2025). Systematic review of amyloid-beta clearance proteins from the brain to the periphery: implications for Alzheimer's disease diagnosis and therapeutic targets. Neural. Regen. Res. 20, 3574–3590. doi: 10.4103/NRR.NRR-D-24-00865
Jan, R., and Chaudhry, G. E. (2019). Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 9, 205–218. doi: 10.15171/apb.2019.024
Jensen, C. J., Massie, A., and De keyser, J. (2013). Immune players in the CNS: the astrocyte. J. Neuroimmun. Pharmacol. 8, 824–839. doi: 10.1007/s11481-013-9480-6
Kaizu, Y., and Miyata, K. (2025). Post-hip fracture knee pain in older adults: a narrative review. Aging Adv. 2, 62–66. doi: 10.4103/AGINGADV.AGINGADV-D-24-00022
Kaliannan, K., Robertson, R. C., Murphy, K., Stanton, C., Kang, C., Wang, B., et al. (2018). Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6:205. doi: 10.1186/s40168-018-0587-0
Kartjito, M. S., Yosia, M., Wasito, E., Soloan, G., Agussalim, A. F., and Basrowi, R. W. (2023). Defining the relationship of gut microbiota, immunity, and cognition in early life-a narrative review. Nutrients 15:2642. doi: 10.3390/nu15122642
Kodama, L., and Gan, L. (2019). Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends Mol. Med. 25, 741–749. doi: 10.1016/j.molmed.2019.05.001
Kwa, M., Plottel, C. S., Blaser, M. J., and Adams, S. (2016). The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Nat. Cancer Inst. 108:djw029. doi: 10.1093/jnci/djw029
Li, Q., Li, B., Liu, L., Wang, K. J., Liu, M. Y., Deng, Y., et al. (2024). Monocytes release cystatin F dimer to associate with Aβ and aggravate amyloid pathology and cognitive deficits in Alzheimer's disease. J. Neuroinflamm. 21, 125. doi: 10.1186/s12974-024-03119-2
Loh, J. S., Mak, W. Q., Tan, L. K. S., Ng, C. X., Chan, H. H., Yeow, S. H., et al. (2024). Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Sig. Transduct Target Ther. 9:37. doi: 10.1038/s41392-024-01743-1
Long, K. L. P., Breton, J. M., Barraza, M. K., Perloff, O. S., and Kaufer, D. (2021). Hormonal regulation of oligodendrogenesis i: effects across the lifespan. Biomolecules 11:283. doi: 10.3390/biom11020283
Lu, J., Xian, T.-J., Li, C.-J., and Wang, Y. (2025). The estrogen–brain interface in neuroinflammation: a multidimensional mechanistic insight. Front. Aging Neurosci. Vol.17–2025. doi: 10.3389/fnagi.2025.1671552
Marino, M., Mele, E., Pastorino, G. M. G., Meccariello, R., Operto, F. F., Santoro, A., et al. (2022). Neuroinflammation: molecular mechanisms and therapeutic perspectives. Cent. Nerv. Syst. Agents Med. Chem. 22, 160–174. doi: 10.2174/1871524922666220929153215
McEwen, B. S., and Milner, T. A. (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 95, 24–39. doi: 10.1002/jnr.23809
Migliore, L., Nicolì, V., and Stoccoro, A. (2021). Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines 9:652. doi: 10.3390/biomedicines9060652
Plottel, C. S., and Blaser, M. J. (2011). Microbiome and malignancy. Cell Host Microbe 10, 324–335. doi: 10.1016/j.chom.2011.10.003
Posillico, C. K., Garcia-hernandez, R. E., and Tronson, N. C. (2021). Sex differences and similarities in the neuroimmune response to central administration of poly I:C. J. Neuroinflamm. 18:193. doi: 10.1186/s12974-021-02235-7
Reinehr, S., Köseoglu, A. E., Qin, W., Tsai, T., Dick, H.B., and Joachim, S. C. (2024). Mechanisms of age-related ocular diseases: a comprehensive review with an emphasis on glaucoma. Aging Adv. 1:42–51. doi: 10.4103/AGINGADVANCES.AGINGADV-D-24-00001
Rio, P., Caldarelli, M., Chiantore, M., Ocarino, F., Candelli, M., Gasbarrini, A., et al. (2024). Immune cells, gut microbiota, and vaccines: a gender perspective. Cells 13:526. doi: 10.3390/cells13060526
Rizzetto, L., Fava, F., Tuohy, K. M., and Selmi, C. (2018). Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J. Autoimmun. 92, 12–34. doi: 10.1016/j.jaut.2018.05.008
Sakamuri, A., Bardhan, P., Tummala, R., Mauvais-jarvis, F., Yang, T., Joe, B., et al. (2023). Sex hormones, sex chromosomes, and microbiota: identification of Akkermansia muciniphila as an estrogen-responsive microbiota. Microbiota Host 1:e230010. doi: 10.1530/mah-23-0010
Santiago, J. A., Quinn, J. P., and Potashkin, J. A. (2022). Sex-specific transcriptional rewiring in the brain of Alzheimer's disease patients. Front. Aging Neurosci. 14:1009368. doi: 10.3389/fnagi.2022.1009368
Shabab, T., Khanabdali, R., Moghadamtousi, S. Z., Kadir, H. A., and Mohan, G. (2017). Neuroinflammation pathways: a general review. Int. J. Neurosci. 127, 624–633. doi: 10.1080/00207454.2016.1212854
Siddiqui, R., Makhlouf, Z., Alharbi, A. M., Alfahemi, H., and Khan, N. A. (2022). The gut microbiome and female health. Biology 11:1683. doi: 10.3390/biology11111683
Singh, D. (2022). Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer's disease. J. Neuroinflamm. 19:206. doi: 10.1186/s12974-022-02565-0
Soudy, M., Bars, S. L., and Glaab, E. (2025). Sex-dependent molecular landscape of Alzheimer's disease revealed by large-scale single-cell transcriptomics. Alzheimers Dement 21, e14476. doi: 10.1002/alz.14476
Tyurikova, O. (2024). Morphological and physiological features of human cerebral cortical astrocytes in regenerative medicine: a narrative review. Regen. Med. Rep. 1, 52–58. doi: 10.4103/REGENMED.REGENMED-D-24-00003
Voskuhl, R., and Momtazee, C. (2017). Pregnancy: effect on multiple sclerosis, treatment considerations, and breastfeeding. Neurotherapeutics 14, 974–984. doi: 10.1007/s13311-017-0562-7
Wang, Y. T., Li, Q., Liu, J. C., Chen, C., Ding, H. X., Zha, X., et al. (2025). Cystatin F-a key player in central nervous system disease. J. Neuroinflamm. 22:203. doi: 10.1186/s12974-025-03526-z
Wiese, S., Karus, M., and Faissner, A. (2012). Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 3:120. doi: 10.3389/fphar.2012.00120
Wu, B., Zhang, S., Guo, Z., Bi, Y., Zhou, M., Li, P., et al. (2021). The TGF-β superfamily cytokine Activin-A is induced during autoimmune neuroinflammation and drives pathogenic Th17 cell differentiation. Immunity 54, 308-323. doi: 10.1016/j.immuni.2020.12.010
Wu, S,. Y., CHEN, Y,. W., TSAI, S,. F., WU, S,. N., SHIH, Y,. H., JIANG-SHIEH, Y. F., et al. (2016). Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K(+) channel. Sci. Rep. 6:22864. doi: 10.1038/srep22864
Yoo, J. Y., Groer, M., Dutra, S. V. O., Sarkar, A., and Mcskimming, D. I. (2020). Gut Microbiota and Immune System Interactions. Microorganisms 8.1587. doi: 10.3390/microorganisms8101587
Zahaf, A., Kassoussi, A., Hutteau-hamel, T., Mellouk, A., Marie, C., Zoupi, L., et al. (2023). Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination. Nat. Commun. 14:1592. doi: 10.1038/s41467-023-36846-w
Zhu, Y., Sullivan, L. L., Nair, S. S., Williams, C. C., Pandey, A. K., Marrero, L., et al. (2006). Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res. 66, 7991–7998. doi: 10.1158/0008-5472
Keywords: neuro inflammation, gender differences, astrocytes, sex hormones, gut-brain axis, aging
Citation: Shi J-W, Meng X-L, Ye Y, Wang Y-T, Ding H-X, Qi W-X, Feng R, Zhang K and Lei M (2025) Regulatory mechanisms of neuro inflammation from a gender perspective: interactions among astrocytes, sex hormones, and the gut-brain axis. Front. Aging Neurosci. 17:1675694. doi: 10.3389/fnagi.2025.1675694
Received: 29 July 2025; Accepted: 03 September 2025;
Published: 24 September 2025.
Edited by:
Yu-Min Kuo, National Cheng Kung University, TaiwanCopyright © 2025 Shi, Meng, Ye, Wang, Ding, Qi, Feng, Zhang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Feng, cmZlbmdAY211LmVkdS5jbg==; Ke Zhang, a3poYW5nQGNtdS5lZHUuY24=; Ming Lei, bGVpbWluZ0Bzd211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jia-Wei Shi1,2†
Jia-Wei Shi1,2† Rui Feng
Rui Feng Ke Zhang
Ke Zhang Ming Lei
Ming Lei