- 1 Department of Sports Science, Zhejiang University, Hangzhou, Zhejiang, China
- 2 Sport Science School, Beijing Sport University, Beijing, China
- 3 College of Physical Education, Hangzhou Normal University, Hangzhou, Zhejiang, China
- 4 School of Physical Education, Da Lian University, Dalian, Liaoning, China
Objective: The purpose of this study was to conduct a systematic review and meta-analysis to evaluate the effects of exercise on postural control, gait, and muscle strength in older adults with diabetic peripheral neuropathy (DPN).
Research Design: Systematic review and meta-analysis.
Methods: An extensive literature search was performed in PubMed, EBSCO, Web of Science and Cochrane Library from database inception to 30 September 2023. The inclusion criteria were exercise intervention on postural control, gait characteristics, and muscle strength in older adults with DPN. Two reviewers independently extracted data and assessed the quality of studies by Cochrane Risk of Bias.
Results: The literature search elicited a total of 523 references, 23 articles were included in this systematic review and meta-analyses. Exercise could effectively decrease the Centre of Pressure (COP) path (SMD = −0.38, 95%CI = −0.77
Conclusion: Exercise improves postural control, gait speed, and muscle strength in older adults with DPN, reducing fall risk and enhancing lower limb strength, though evidence on stride length improvement is limited.
Systematic Review Registration: identifier CRD42023436799.
1 Introduction
Diabetic Peripheral Neuropathy (DPN), a prevalent chronic complication of diabetes, significantly impacts the quality of life in approximately 50% of diabetic patients (Sloan et al., 2021). The pathogenesis of DPN is multifaceted, primarily attributed to prolonged hyperglycemia and metabolic dysregulation, leading to sensory and motor nerve damage (Sloan et al., 2021). Notably, individuals with DPN exhibit a 2.3-fold higher risk of falls compared to diabetic patients without DPN (Reeves et al., 2021; Kruse et al., 2010), and a staggering 15-fold increase relative to their healthy counterparts (Khan et al., 2021a; Mustapa et al., 2016). Clinical manifestations of DPN include distal sensory abnormalities, neuropathic pain, muscle weakness, and motor dysfunction, collectively contributing to gait disturbances and impaired postural control (Sloan et al., 2021). These impairments substantially elevate fall risk, with potentially severe consequences such as fractures and intracranial hemorrhages, which not only impose significant economic burdens but are also associated with increased mortality rates (Gupta et al., 2023).
A complex interaction of factors affects the increased risk of falls among older adults with DPN. This is attributed to the glycation of skeletal muscle proteins as well as axonal degeneration and segmental demyelination of the peripheral motor nerve (Reeves et al., 2021), leading to lower extremity motor impairments and a loss of sensory feedback in the feet (Reeves et al., 2021). DPN causes pain, diminished muscle quality, diminished peripheral sensation, unstable gait, impaired balance, and motor dysfunction, ultimately resulting in increased fall risk (Khan et al., 2021b). In particular, postural instability and gait imbalance in DPN mainly contribute to high fall incidence (Wang et al., 2022). People with DPN usually exhibit a conservative gait strategy with worse gait speed and step length (Reeves et al., 2021). Reeves et al. reported the strongest correlations with individuals’ self-perceived unsteadiness were with gait velocity, stride length and severity of DPN (Reeves et al., 2017). Otherwise, it is possibly due to the part absence of peripheral sensation and the delaying of neuromuscular control this could result in balance impairment and a high risk of falls in the medial-lateral (ML) dynamic sway (Brown et al., 2015), as a key indicator to distinguish among people with and without DPN (Reeves et al., 2017).
Exercise intervention, as a first-line non-pharmacological treatment strategy for diabetic peripheral neuropathy (DPN), holds significant potential in enhancing patients’ postural control, gait function, and muscle strength (Johnson and Takemoto, 2019). These interventions include balance training (Song et al., 2011; Lee et al., 2013; Grewal et al., 2015), resistance training (Melai et al., 2014; Sartor et al., 2014), aerobic training (Abdelaal and El-Shamy, 2022; Zhao et al., 2021), multicomponent exercise (Perrin et al., 2021; Waheed et al., 2021), and Tai Chi, which can effectively control blood glucose levels and thereby reverse motor dysfunction caused by neuropathy (Sloan et al., 2021). Improving balance ability in older adults is often a primary goal of fall prevention interventions (Blodgett et al., 2022). Research by Ahmad and colleagues further confirmed that an eight-week sensorimotor training intervention significantly improved patients’ balance and proprioception (Ahmad et al., 2019). Aerobic training not only enhances neural structure and function but also alleviates neuropathic signs and symptoms (Perrin et al., 2021; Dixit et al., 2016). Additionally, physical exercise can reduce pain and/or numbness caused by neuropathy, ultimately improving instability and mobility (Li and Hondzinski, 2012). A review noted that exercise combined with dietary interventions can induce systemic and cellular changes, thereby ameliorating complications associated with DPN (Enders et al., 2023). Although exercise can improve balance, reduce fear of falling, and enhance the quality of life (Lima et al., 2021), there is currently no consensus on the extent to which exercise improves postural control, gait characteristics, and muscle strength in older adults with DPN. Furthermore, it remains unclear whether these improvements contribute to reducing the risk of falls in this population.
This study conducted a systematic review and meta-analysis of randomized controlled trials to assess the outcomes of exercise interventions on fall risk factors including postural control, gait and muscle strength in older adults with DPN.
2 Methods
2.1 Protocol and registration
The review study followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 statement (Page et al., 2021). A protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) ID: CRD42023436799.
2.2 Eligibility criteria
The PICOS (populations, interventions, comparator interventions, outcomes and study design) framework guided the eligibility criteria selection (Cochrane Collaboration, 2024).
2.2.1 Populations
The diagnosis of DSPN follows an exclusion-based approach, with diagnostic criteria encompassing the following key points: (1) a confirmed history of diabetes; (2) onset of neuropathy at or after the diagnosis of diabetes; (3) presence of clinical symptoms of neuropathy (e.g., pain, numbness, paresthesia) accompanied by at least one abnormal finding in five neurological examinations (ankle reflex, vibration sensation, pressure sensation, temperature sensation, and pinprick sensation); in the absence of clinical symptoms, at least two abnormal findings are required; (4) exclusion of other potential causes of neuropathy, including neurotoxic medications (e.g., chemotherapeutic agents), vitamin B12 deficiency, cervical or lumbar spine disorders (e.g., compression, stenosis, degenerative changes), cerebral infarction, chronic inflammatory demyelinating polyneuropathy, hereditary neuropathies, vasculitis, infections (e.g., acquired immunodeficiency syndrome), and metabolic neurotoxicity secondary to renal insufficiency.
2.2.2 Interventions
The included interventions in this study primarily encompass aerobic exercise, anaerobic training, resistance exercise, whole-body vibration training, balance exercise, sensorimotor training, foot-ankle functional exercise, Tai Chi, yoga, dance training, as well as combinations of two or more of the aforementioned exercise modalities. Exercise combined with other non-exercise interventions, such as co-administration of medication, acupuncture, electrical stimulation, heat application, or physical therapy, or lifestyle modifications, have been excluded. Moreover, studies with fully supervised exercise programs or those involving short-term exercise interventions (<1 week) have also been excluded.
2.2.3 Comparator interventions
The control group interventions may include no exercise, routine foot care, health education, or regular physical activity interventions.
2.2.4 Outcomes
The outcome of the study was one or more of the following: (1) postural control: COP sway path; (2) gait parameters: speed, stride length, step cycle time, cadence, etc.; (3) muscle strength.
2.2.5 Studies design
This study included RCTs on exercise interventions for older adults with DPN in all settings (community, hospitals and institutions). It is acceptable that this literature only considered exercise as the exposure or intervention factor.
2.3 Information sources and search strategy
Three-step search for relevant randomized controlled trials (RCTs) as recommended by the Cochrane Handbook for Systematic Reviews of Interventions was conducted (Cochrane Collaboration, 2024). Four electronic databases (PubMed, Web of Science, EBSCO, Cochrane Library) were searched for articles published up to 9 September 2023. Then, searching was also done in published trial articles. Medical Subject Headings (MeSH) terms and keywords were chosen based on study design (“Randomized Controlled Trials”), exposure (“Exercise” OR “Training” OR “Physical activity”), outcomes (“Postural Control” OR “Gait Performance” OR “Biomechanics”) and participants (“Diabetic Peripheral Neuropathy”). The full search strategy for PubMed can be found in the online Supplementary Appendix B. Reference lists of included studies were also searched for relevant articles.
2.4 Study selection
All returned titles were screened by the first author (DW) to exclude duplicate or non-relevant studies. The abstract of each remaining study was then independently reviewed by DW and XP during the literature search. Then the full texts of the remaining studies were independently reviewed by the two authors against the inclusion and exclusion criteria. Disagreements were discussed and consensus was reached among the authors in all cases. All studies in the systematic review were eligible for inclusion in the meta-analyses.
2.5 Data collection process and data items
We collected data on authors, year of publication, number of participants allocated to the intervention and control groups, participant-based information (age, gender, BMI, duration of diabetes, HbA1c), type and duration of exercise intervention for the experimental and control groups (time, frequency), outcome measures, and study duration. This study gathered the outcomes including RCTs in exercise intervention, encompassing statistical metrics of continuous variables such as sample size, mean, standard deviation, and others for each study.
2.6 Study risk of bias assessment
Two authors (DW and XP) independently assessed the risk of bias at the study level of included RCTs following the Cochrane Risk of Bias Tool (RoB 2) (Sterne et al., 2019). The seven items considered for the risk of bias included: the randomization process, bias arising from period and carryover effects, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result and overall bias. Open and apply the macro using the Excel tool (website of Cochrane Methods Bias: https://www.riskofbias.info/) to assess the risk of bias for each article (Liu et al., 2021).
2.7 Synthesis methods
This study employed RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) software to perform meta-analysis, subgroup analysis, and generate forest plots. The extracted data were all continuous variables, expressed as mean difference (MD) and their 95% confidence intervals (CI). If the units were inconsistent, standardized mean difference (SMD) was used. Heterogeneity of outcome measures was assessed using the I2 statistic and p-values. If heterogeneity was low (I2 ≤ 50%, p ≤ 0.01), a fixed-effects model was applied; if heterogeneity was high (I2 > 50%, p > 0.01), a random-effects model was used (Higgins et al., 2003). Subgroup analyses focused on three key factors: postural control, gait, and muscle strength. Each factor was further subdivided based on different variables to minimize heterogeneity in the study. If significant heterogeneity persisted, sensitivity analysis was conducted to identify its sources. If the sources of heterogeneity could not be determined, descriptive analysis was performed. The significance level ɑ for the pooled effect size was set at 0.05.
3 Results
3.1 Study selection
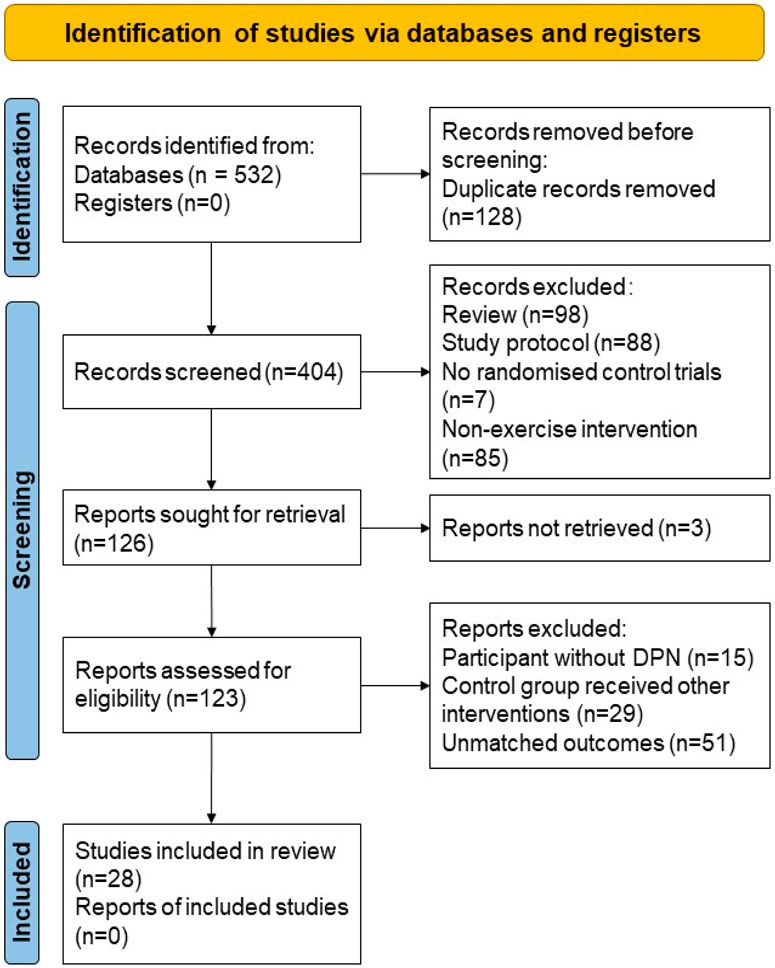
After the initial search, a total of 532 articles were included. After removing 128 duplicate articles, 280 articles were excluded based on title and abstract screening. Further full-text retrieval, reading, and quality assessment led to the exclusion of 98 articles that did not meet the inclusion criteria. Ultimately, 23 articles were included in the study. The process of article inclusion and exclusion is illustrated in Figure 1. The characteristics of the study participants, intervention, control, and outcome measures were shown in the review (Table 1).
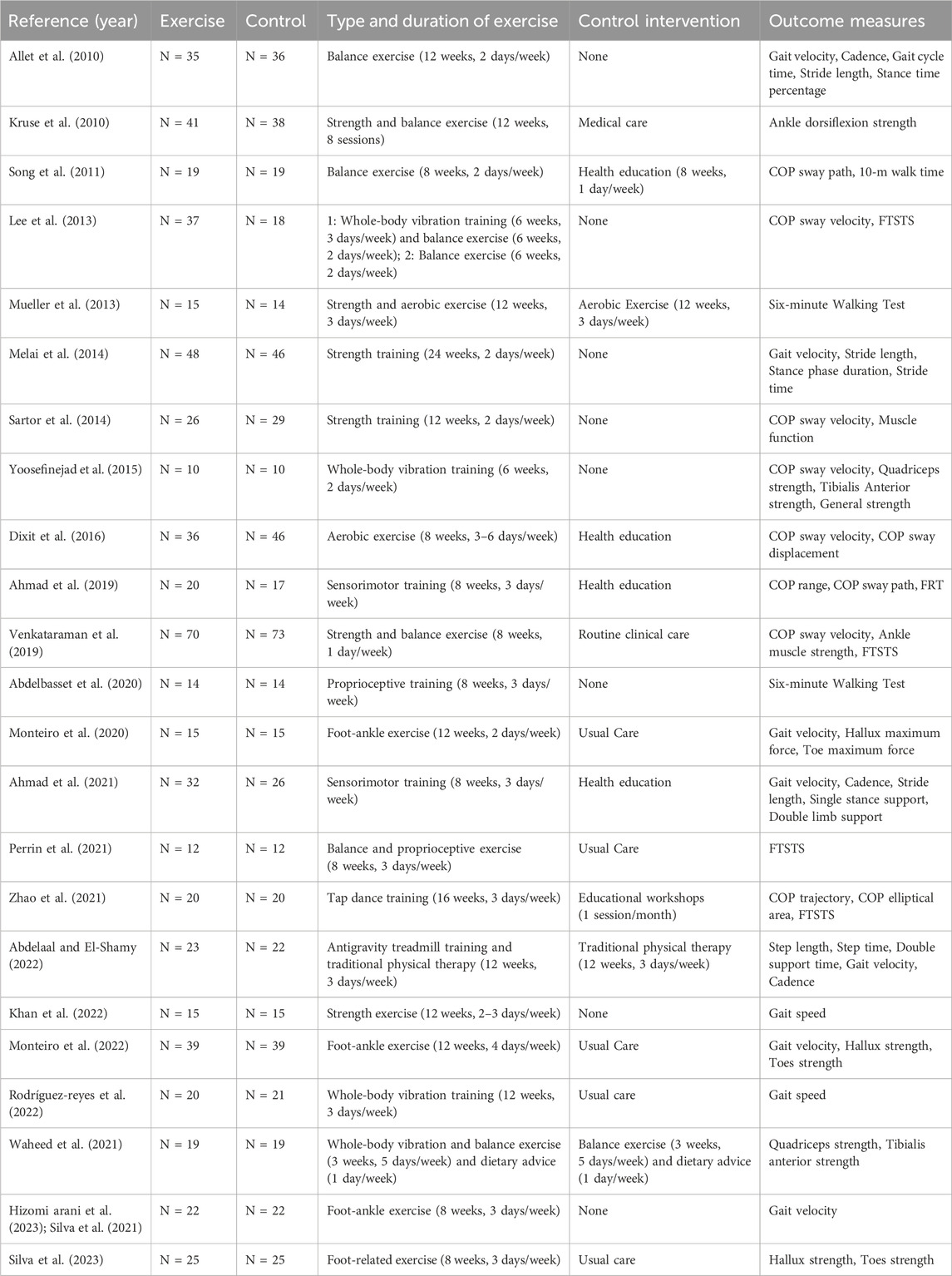
Table 1. Characteristics of the included studies, including type and duration of exercise, control intervention, and outcome measure.
3.2 Risk of bias in the included studies
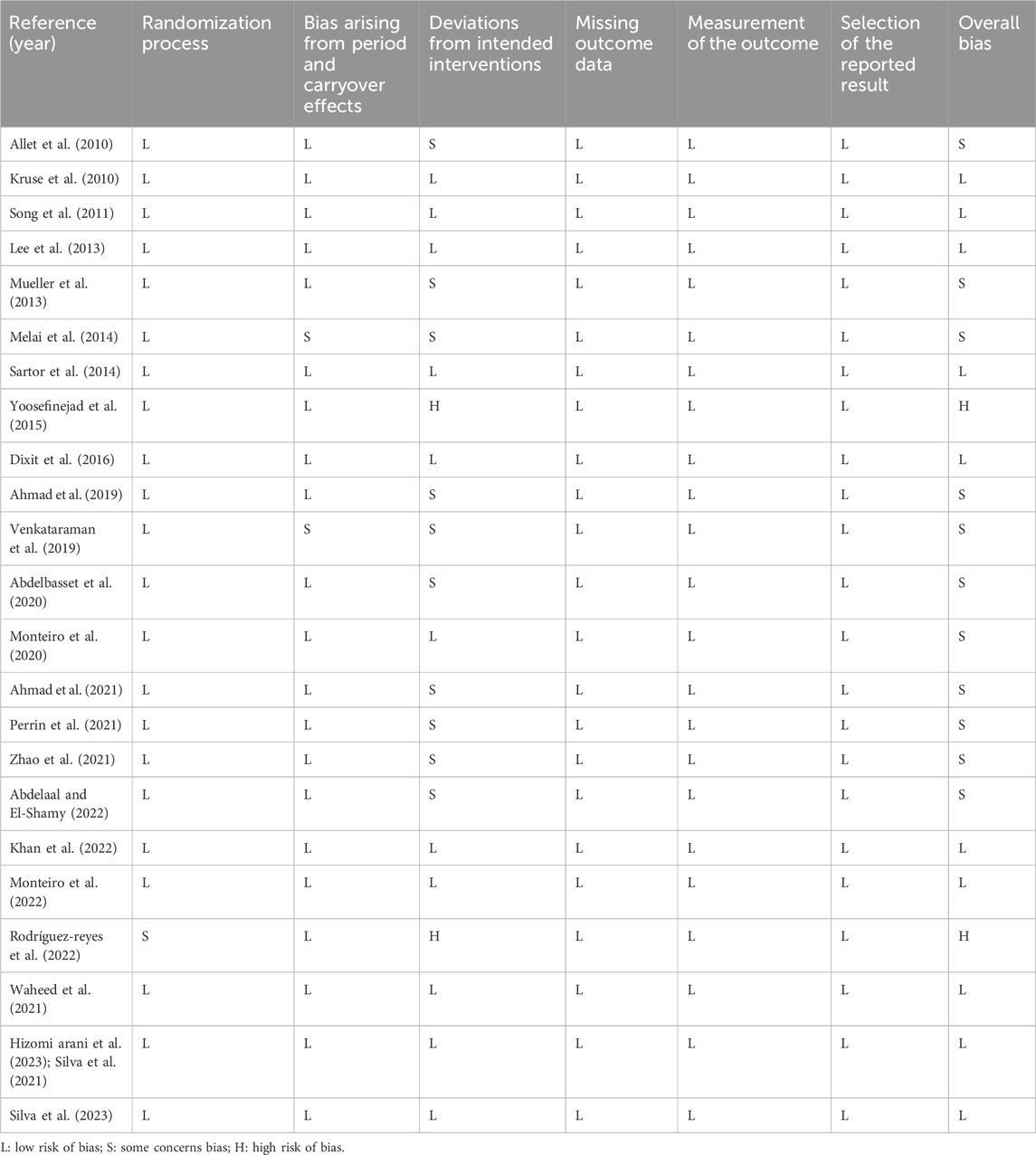
The 10 studies included in this research all exhibited a low risk in the randomization process (Kruse et al., 2010; Song et al., 2011; Lee et al., 2013; Sartor et al., 2014; Waheed et al., 2021; Dixit et al., 2016; Khan et al., 2022; Monteiro et al., 2022; Hizomi arani et al., 2023; Silva et al., 2023), with no instances of missing data. Among these, one study was an open-label randomized controlled trial (Rodrigues et al., 2022), resulting in a high risk in intervention allocation; two studies did not implement allocation concealment for participants or assessors (Melai et al., 2014; Venkataraman et al., 2019), leading to some risk in intervention allocation; and two studies failed to conceal allocation from treatment providers, leading to a high risk of bias in intervention compliance (Rodrigues et al., 2022; Yoosefinejad et al., 2015). The ten studies have not clearly described the concealment of the allocation of treatment and testing personnel, introducing some risk to intervention adherence (Melai et al., 2014; Abdelaal and El-Shamy, 2022; Zhao et al., 2021; Perrin et al., 2021; Waheed et al., 2021; Ahmad et al., 2019; Venkataraman et al., 2019; Allet et al., 2010; Mueller et al., 2013; Abdelbasset et al., 2020). The risk of bias assessment results for the included studies are presented in Table 2.
3.3 Outcome measures
3.3.1 COP sway path
There were three studies regarding the COP sway path after exercise in the AP and ML directions (Figure 2). Overall, it was shown that exercise could effectively decrease the sway path of postural control (SMD = −0.38, 95%CI = −0.77
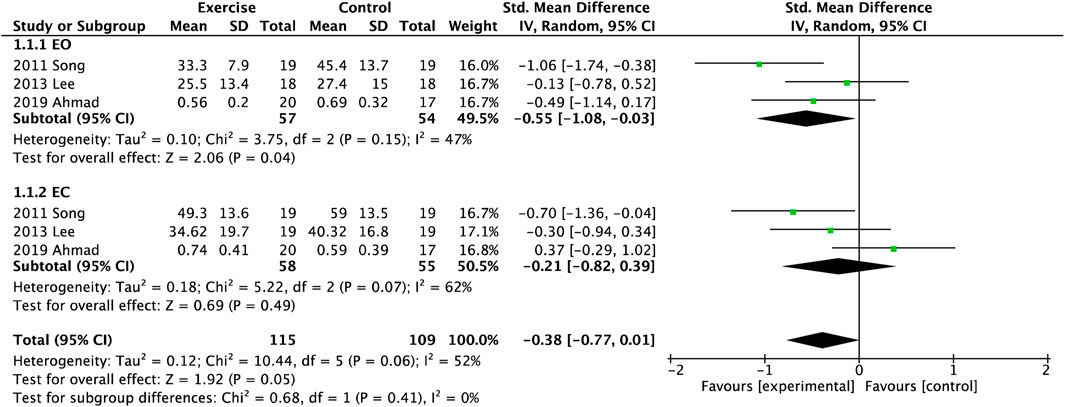
Figure 2. Forest plot of meta-analyses showing the effect of centre of pressure (COP) sway path with open eye (EO) and closed eye (EC).
3.3.2 Gait characteristics
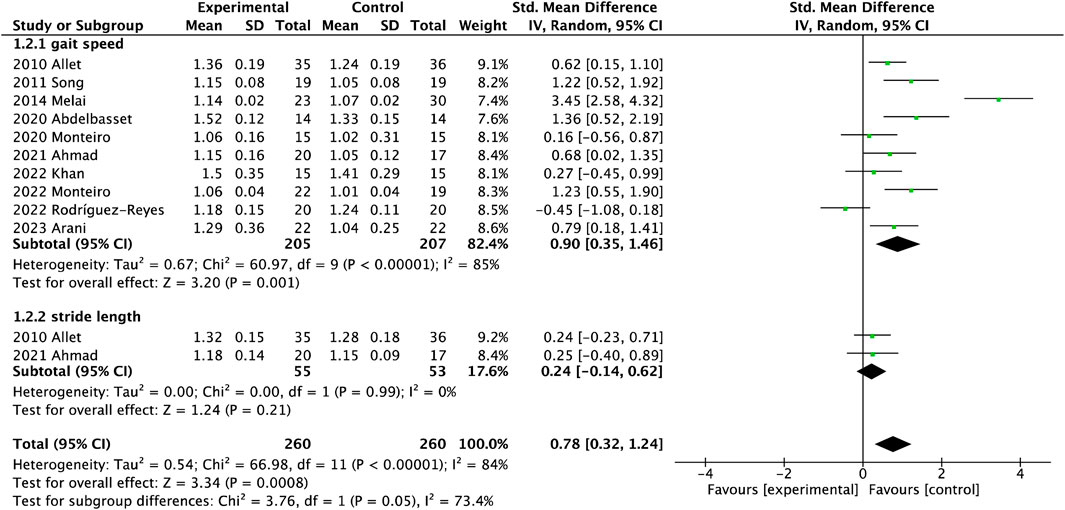
There were ten studies on gait speed involving 412 participants and three studies on stride length involving 145 participants in Figure 3-1.2.1. Gait performance was measured with the gait velocity and stride length. Gait velocity had significant differences between the interventional and control groups (MD = 0.08, 95%CI = 0.05
3.3.3 Muscle strength
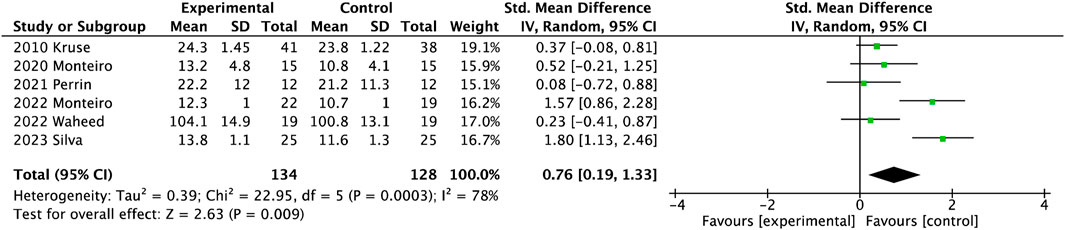
As shown in Figure 4, six studies involving 262 participants presented the muscle strength of lower limbs. The muscle strength of the lower extremity was significantly reduced after exercise (SMD = 0.76, 95%CI = 0.19
4 Discussion
Exercise, as a clinical rehabilitation tool, can partially restore sensorimotor impairments accompanied by neuropathic symptoms and promote physical function. This review aims to evaluate the effectiveness of exercise interventions on postural control, gait characteristics, and muscle strength in older adults with DPN, and to provide evidence-based exercise recommendations.
4.1 Effect of exercise on postural control
Impaired Balance is a strong risk factor for falls with a medium to large effect size (Chantanachai et al., 2021). In previous studies, these methods of balance testing had a high heterogeneity within or across settings (Perell et al., 2001), such as COP, TUG, BBS, OLS, and so on (Blodgett et al., 2022; Perell et al., 2001). Objective evaluation of postural control is usually based on the analysis COP using a force platform (Blodgett et al., 2022; Quijoux et al., 2020), as the gold standard for evaluation of balance. The velocity and sway area of COP were the best features for discriminating between fallers and non-fallers (Perell et al., 2001), and had a high correlation with the severity of neuropathy (Brown et al., 2015; Perell et al., 2001). In this review, three studies (Melai et al., 2014; Enders et al., 2023) included meta-analysis to estimate postural control between experimental and control groups and observed that could significantly decrease AP sway amplitude with EO and EC after balance training. Although studies presented stability in the ML direction as a key factor to differentiate high fall risk among older population (Brown et al., 2015), Figure 2 showed only COP sway path with EC had improved compared with EO.
Otherwise, five studies with other exercises were not included in the meta-analysis (Sartor et al., 2014; Zhao et al., 2021; Dixit et al., 2016; Venkataraman et al., 2019; Yoosefinejad et al., 2015). Dixit and coworkers only found that eight-week aerobic exercise could reduce COP sway velocity along the x-axis and increase ML displacement on foam with EC (Dixit et al., 2016). Zhao et al. also validated the effect of aerobic exercise significantly affected COP trajectory and elliptical area (Zhao et al., 2021). A study described a relatedly lower COP velocity measured by plantar pressure system after 12-week strength training (Sartor et al., 2012), but the number and quality of each study were relatively less. However, a meta-analysis reported that balance exercise intervention changed the COP parameters in either eyes or closed condition among older adults (age > 60), while resistance and multi-component exercise did not (Low et al., 2017). What are the possible explanations for the relatively fewer changes observed in COP sway indicators of ML direction? A study showed that ML sway path length/velocity did not change in older adults after balance exercises (Low et al., 2017), similar to this review. Moreover, another review showed that ML data were found to be more discriminatory than AP features (Quijoux et al., 2020). The possible reasons were the small sample size and inconsistencies in the data collection protocols, leading to high heterogeneity (69%) of the ML sway path.
In conclusion, exercise interventions revealed a moderate to high effect on balance performance parameters compared to the control group, and balance training significantly improved postural control to a greater extent compared to other exercises. Balance exercise, in this review, included regular stability training, sensorimotor training, proprioception training, and combined training above. A review presented that sensorimotor training also plays a crucial role in targeting balance control and existing sensory and motor signs and symptoms of DPN (Streckmann et al., 2022).
4.2 Effect of exercise on gait performance
It is well known that gait performance, a natural daily activity heavily reliant on the synergy between the nervous and musculoskeletal systems, can be easily affected by the pathological process in older adults with DPN (Wang et al., 2022). Adults with DPN usually adopt a conservation gait with lower gait velocity, shorter stride length, longer stride time and stance time, compared with non-neuropathy diabetes (Wang et al., 2022). A study showed that exercise could significantly improve gait velocity compared to a control group, especially balance exercise (Allet et al., 2010). Some described that velocity and stride length effectively increased after 8-week sensorimotor exercise in older adults with DPN regardless of age (Reeves et al., 2021), similar to this review. In general, studies reported that lower walking speeds accompanied by increased falling risk in older adults, significantly discriminate between fallers and non-fallers (Reeves et al., 2017). Based on meta-analysis, balance exercise could more greatly increase gait velocity under both self-gait speed and fast-gait speed conditions than resistance training. Otherwise, this review reported that stride length only had an increase trend after exercise interventions. There was a potential factor that the sample size was small only 75 in the interventional group and 70 in the control group. Another reason was that cadence contributed 80% whereas stride length only contributed 20% to this change of gait velocity, resulting in difficulty regulating stride length (Allet et al., 2010).
4.3 Effect of exercise on muscle strength
Diabetes is responsible for the deterioration of muscle strength and becomes more severe as DPN progresses, leading to altered gait biomechanics, impaired stability and increased fall risks (Orlando et al., 2022). Some suggested that reduced strength of the knee and ankle may cause a disturbance in perturbation response in balance, potentially increasing the risk of falling. Although these results had a low level of quality and evidence, in this review, exercise could increase muscle strength and joint mobility in people with DPN, especially after foot-ankle functional training. Regarding the foot-ankle exercise, the exercise protocol is designed to consist of the same set: warm-up exercise, strengthening of the intrinsic foot muscles, strengthening of the extrinsic foot muscles, and functional exercise (e.g., balance and gait training), to manage the musculoskeletal complications related to diabetes (Silva et al., 2023; Monteiro et al., 2020). The observed phenomenon of reducing foot-ankle joint mobility and intrinsic muscle strength may be attributed to symptoms of distal peripheral neuropathy (Mustapa et al., 2016; Wang et al., 2022). Strength combined with functional training provided a better effect on increasing muscle strength of the lower extremity, compared to resistance training alone. Although resistance exercise was an effective intervention to combat muscle loss and delay some neurological symptoms (Melai et al., 2014; Venkataraman et al., 2019), people with neuropathy should be wary of weight-bearing exercise because of plantar loading links to potential foot ulcer development (Li and Hondzinski, 2012). Combinational exercises could more greatly improve metabolic control of the organism than aerobic and resistance training, similar to the previous study (Orlando et al., 2022). Improving foot functionality has a positive impact on people’s overall physical activity levels and quality of life (Streckmann et al., 2022). Foot-ankle functional training significantly increased ankle joint ranges of motion including dorsiflexion and plantarflexion in older adults with DPN. That is the foot-ankle exercise program focused mainly on the foot joints of muscle strength and mobility in gait or dynamic activities.
4.4 Limitations of the study
This review has some limitations. The primary limitation is the focus on short-term outcomes, without accounting for the potential long-term benefits or risks associated with the interventions. Moreover, this study did not conduct subgroup analyses based on exercise types or exercise prescriptions (such as intensity, duration, frequency, etc.), and failed to provide detailed guidance for the implementation of specific exercise programs in the daily rehabilitation of DPN patients. Furthermore, although 23 studies were included in this review, the exercise modalities assessed were not evenly distributed. Most studies focused on balance training, followed by multi-component and foot-ankle functional exercises. In contrast, strength training and whole-body vibration training were represented by fewer studies. This uneven distribution may contribute to increased heterogeneity across the meta-analysis and limit the precision of the confidence intervals. Therefore, clinicians should interpret the results with caution when considering them for decision-making.
5 Conclusion
Exercise is a fundamental intervention for older adults with DPN and significantly improves physical activity, measured by postural control, gait characteristics and muscle strength. Exercise could enhance postural control under the open eye and closed eye to prevent or reduce the risk of falls. Gait is a component of ability and skill for daily life. Exercise could effectively decrease the gait speed in walking, but did not improve the stride length with low-quality evidence. This study found that the muscle strength in the lower limbs was significantly enhanced after exercise among older adults with DPN.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XP: Data curation, Investigation, Validation, Visualization, Writing – original draft. DW: Data curation, Investigation, Software, Validation, Writing – original draft. FZ: Visualization, Writing – review and editing, Data curation, Validation. WL: Writing – review and editing, Conceptualization, Methodology, Supervision. BG: Methodology, Supervision, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Research and Development Program of Zhejiang Province, 2022C03148.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2025.1507232/full#supplementary-material
References
Abdelaal, A., and El-Shamy, S. (2022). Effect of antigravity treadmill training on gait and balance in patients with diabetic polyneuropathy: a randomized controlled trial. F1000Research 11, 52. doi:10.12688/f1000research.75806.3
Abdelbasset, W. K., Alrawaili, S. M., Nambi, G., Yassen, E., Moawd, S. A., and Ahmed, S. A. (2020). Therapeutic effects of proprioceptive exercise on functional capacity, anxiety, and depression in patients with diabetic neuropathy: a 2-month prospective study. Clin. Rheumatol. 39 (10), 3091–3097. doi:10.1007/s10067-020-05086-4
Ahmad, I., Noohu, M. M., Verma, S., Singla, D., and Hussain, M. E. (2019). Effect of sensorimotor training on balance measures and proprioception among middle and older age adults with diabetic peripheral neuropathy. Gait Posture 74, 114–120. doi:10.1016/j.gaitpost.2019.08.018
Ahmad, I., Verma, S., Noohu, M. M., and Ejaz, M. (2021). Effect of sensorimotor training on spatiotemporal parameters of gait among middle and older age adults with diabetic peripheral neuropathy. Somatosens. and Mot. Res. 38 (3), 230–240. doi:10.1080/08990220.2021.1955671
Allet, L., Armand, S., Aminian, K., Pataky, Z., Golay, A., de Bie, R. A., et al. (2010). An exercise intervention to improve diabetic patients’ gait in a real-life environment. Gait Posture 32 (2), 185–190. doi:10.1016/j.gaitpost.2010.04.013
Blodgett, J. M., Ventre, J. P., Mills, R., Hardy, R., and Cooper, R. (2022). A systematic review of one-legged balance performance and falls risk in community-dwelling adults. Ageing Res. Rev. 73, 101501. doi:10.1016/j.arr.2021.101501
Brown, S. J., Handsaker, J. C., Bowling, F. L., Boulton, A. J. M., and Reeves, N. D. (2015). Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care 38 (6), 1116–1122. doi:10.2337/dc14-1982
Chantanachai, T., Sturnieks, D. L., Lord, S. R., Payne, N., Webster, L., and Taylor, M. E. (2021). Risk factors for falls in older people with cognitive impairment living in the community: systematic review and meta-analysis. Ageing Res. Rev. 71, 101452. doi:10.1016/j.arr.2021.101452
Cochrane Collaboration (2024). Cochrane handbook for systematic reviews of interventions. Available online at: https://training.cochrane.org/handbook.
Dixit, S., Maiya, A., Shastry, B. A., and Guddattu, V. (2016). Analysis of postural control during quiet standing in a population with diabetic peripheral neuropathy undergoing moderate intensity aerobic exercise training: a single blind, randomized controlled trial. Am. J. Phys. Med. and Rehabilitation 95, 516–524. doi:10.1097/PHM.0000000000000426
Enders, J., Elliott, D., and Wright, D. E. (2023). Emerging nonpharmacologic interventions to treat diabetic peripheral neuropathy. Antioxidants and Redox Signal. 38 (13-15), 989–1000. doi:10.1089/ars.2022.0158
Grewal, G. S., Schwenk, M., Lee-Eng, J., Parvaneh, S., Bharara, M., Menzies, R. A., et al. (2015). Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: a randomized controlled trial. Gerontology 61 (6), 567–574. doi:10.1159/000371846
Gupta, G., Maiya, G. A., Bhat, S. N., Hande, H. M., and Mayya, S. S. (2023). Functional fitness and risk of falling in older adults with diabetic neuropathy. Phys. and Occup. Ther. 41, 538–555. doi:10.1080/02703181.2023.2187104
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Br. Med. J. 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hizomi Arani, R., Fakhri, F., Shams, A., and Zahedi, M. (2023). Effect of an exercise program on the balance, gait, vibration sense, and cardiometabolic parameters among patients with diabetic peripheral neuropathy: a randomized controlled trial. SN Compr. Clin. Med. 5 (1), 129. doi:10.1007/s42399-023-01470-8
Johnson, C. E., and Takemoto, J. K. (2019). A review of beneficial low-intensity exercises in diabetic peripheral neuropathy patients. J. Pharm. and Pharm. Sci. 22 (1), 22–27. doi:10.18433/jpps30151
Khan, K. S., Christensen, D. H., Nicolaisen, S. K., Gylfadottir, S. S., Troels, S. J., Nielsen, J. S., et al. (2021a). Falls and fractures associated with type 2 diabetic polyneuropathy: a cross-sectional nationwide questionnaire study. J. Diabetes Investigation 12 (10), 1827–1834. doi:10.1111/jdi.13542
Khan, K. S., Overgaard, K., Tankisi, H., Karlsson, P., Devantier, L., Gregersen, S., et al. (2022). Effects of progressive resistance training in individuals with type 2 diabetic polyneuropathy: a randomised assessor-blinded controlled trial. Diabetologia 65 (4), 620–631. doi:10.1007/s00125-021-05646-6
Khan, K. S., Pop-Busui, R., Devantier, L., Kristensen, A. G., Tankisi, H., Dalgas, U., et al. (2021b). Falls in individuals with type 2 diabetes; a cross-sectional study on the impact of motor dysfunction, postural instability and diabetic polyneuropathy. Diabet. Med. 38 (9), e14470. doi:10.1111/dme.14470
Kruse, R. L., LeMaster, J. W., and Madsen, R. W. (2010). Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys. Ther. 90 (11), 1568–1579. doi:10.2522/ptj.20090362
Lee, K., Lee, S., and Song, C. (2013). Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. J. Exp. Med. 231 (4), 305–314. doi:10.1620/tjem.231.305
Li, L., and Hondzinski, J. M. (2012). Select exercise modalities may reverse movement dysfunction because of peripheral neuropathy. Exerc. Sport Sci. Rev. 40 (3), 133–137. doi:10.1097/JES.0b013e31825f7483
Lima, RADO., Piemonte, G. A., Nogueira, C. R., and Nunes-Nogueira, V. D. S. (2021). Efficacy of exercise on balance, fear of falling, and risk of falls in patients with diabetic peripheral neuropathy: a systematic review and meta-analysis. Archives Endocrinol. Metabolism 65 (2), 198–211. doi:10.20945/2359-3997000000337
Liu, J., Liu, C., and Hua, C. (2021). Risk bias assessment tool RoB2 (revised version 2019) for randomized controlled trial: an interpretation. Chin. J. Evidence-based Med. 21 (6), 737–744.
Low, D. C., Walsh, G. S., and Arkesteijn, M. (2017). Effectiveness of exercise interventions to improve postural control in older adults: a systematic review and meta-analyses of centre of pressure measurements. Sports Med. 47 (1), 101–112. doi:10.1007/s40279-016-0559-0
Melai, T., Schaper, N. C., Ijzerman, T. H., Willems, P. J. B., de Lange, T. L. H., Meijer, K., et al. (2014). Strength training affects lower extremity gait kinematics, not kinetics, in people with diabetic polyneuropathy. J. Appl. Biomechanics 30 (2), 221–230. doi:10.1123/jab.2013-0186
Monteiro, R. L., Ferreira, JSSP., Silva, E. Q., Cruvinel-Júnior, R. H., Veríssimo, J. L., Bus, S. A., et al. (2022). Foot–ankle therapeutic exercise program can improve gait speed in people with diabetic neuropathy: a randomized controlled trial. Sci. Rep. 12 (1), 7561. doi:10.1038/s41598-022-11745-0
Monteiro, R. L., Ferreira, JSSP., Silva, E. Q., Donini, A., Cruvinel-Júnior, R. H., Verissímo, J. L., et al. (2020). Feasibility and preliminary efficacy of a foot-ankle exercise program aiming to improve foot-ankle functionality and gait biomechanics in people with diabetic neuropathy: a randomized controlled trial. Sensors (Basel) 20 (18), 5129. doi:10.3390/s20185129
Mueller, J. M., Tuttle, L. J., Lemaster, J. W., Strube, M. J., McGill, J. B., Hastings, M. K., et al. (2013). Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Archives Phys. Med. Rehabilitation 94 (5), 829–838. doi:10.1016/j.apmr.2012.12.015
Mustapa, A., Justine, M., Mustafah, N. M., Jamil, N., and Manaf, H. (2016). Postural control and gait performance in the diabetic peripheral neuropathy: a systematic review. BioMed Res. Int. 2016, 9305025. doi:10.1155/2016/9305025
Orlando, G., Balducci, S., Boulton, A. J. M., Degens, H., and Reeves, N. D. (2022). Neuromuscular dysfunction and exercise training in people with diabetic peripheral neuropathy: a narrative review. Diabetes Res. Clin. Pract. 183, 109183. doi:10.1016/j.diabres.2021.109183
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372, n71. doi:10.1136/bmj.n71
Perell, K. L., Nelson, A., Goldman, R. L., Luther, S. L., Prieto-Lewis, N., and Rubenstein, L. Z. (2001). Fall risk assessment measures: an analytic review. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 56 (12), M761–M766. doi:10.1093/gerona/56.12.m761
Perrin, B. M., Southon, J., McCaig, J., Skinner, I., Skinner, T. C., and Kingsley, M. I. C. (2021). The effect of structured exercise compared with education on neuropathic signs and symptoms in people at risk of neuropathic diabetic foot ulcers: a randomized clinical trial. Med. Kaunas. 58 (1), 59. doi:10.3390/medicina58010059
Quijoux, F., Vienne-Jumeau, A., Bertin-Hugault, F., Zawieja, P., Lefèvre, M., Vidal, P. P., et al. (2020). Center of pressure displacement characteristics differentiate fall risk in older people: a systematic review with meta-analysis. Ageing Res. Rev. 62, 101117. doi:10.1016/j.arr.2020.101117
Reeves, N. D., Brown, S. J., Petrovic, M., Boulton, A. J. M., and Vileikyte, L. (2017). How does self-perceived unsteadiness influence balance and gait in people with diabetes? Preliminary observations. Diabetes Care 40 (5), e51–e52. doi:10.2337/dc16-2183
Reeves, N. D., Orlando, G., and Brown, S. J. (2021). Sensory-motor mechanisms increasing falls risk in diabetic peripheral neuropathy. Med. Kaunas. 57 (5), 457. doi:10.3390/medicina57050457
Rodrigues, G. M., Paixão, A., Arruda, T., de Oliveira, B. R. R., Maranhão Neto, G. A., Marques Neto, S. R., et al. (2022). Anodal transcranial direct current stimulation increases muscular strength and reduces pain perception in women with patellofemoral pain. J. Strength Cond. Res. 36 (2), 371–378. doi:10.1519/JSC.0000000000003473
Rodríguez-reyes, G., García-Ulloa, A. C., Hernández-Jiménez, S., Alessi-Montero, A., Núñez Carrera, L., Rojas-Torres, F., et al. (2022). Effect of whole-body vibration training on transcutaneous oxygen levels of the foot in patients with type 2 diabetes: a randomized controlled trial. J. Biomechanics 139, 110871. doi:10.1016/j.jbiomech.2021.110871
Sartor, C. D., Hasue, R. H., Cacciari, L. P., Butugan, M. K., Watari, R., Pássaro, A. C., et al. (2014). Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: results of a randomized controlled trial. BMC Musculoskelet. Disord. 15, 137. doi:10.1186/1471-2474-15-137
Sartor, C. D., Watari, R., Pássaro, A. C., Picon, A. P., Hasue, R. H., and Sacco, I. C. N. (2012). Effects of a combined strengthening, stretching and functional training program versus usual-care on gait biomechanics and foot function for diabetic neuropathy: a randomized controlled trial. BMC Musculoskelet. Disord. 13, 36. doi:10.1186/1471-2474-13-36
Silva, E. Q., Santos, D. P., Beteli, R. I., Monteiro, R. L., Ferreira, JSSP., Cruvinel-Junior, R. H., et al. (2021). Feasibility of a home-based foot–ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: randomised controlled FOotCAre (FOCA) Trial II. Sci. Rep. 11 (1), 12404. doi:10.1038/s41598-021-91901-0
Silva, E. Q., Veríssimo, J. L., Ferreira, JSSP., Cruvinel-Júnior, R. H., Monteiro, R. L., Suda, E. Y., et al. (2023). Effects of a home-based foot–ankle exercise program with educational booklet for foot dysfunctions in people with diabetic neuropathy: results of the FOCA-II randomized controlled clinical trial. Appl. Sci. 13, 1423. doi:10.3390/app13031423
Sloan, G., Selvarajah, D., and Tesfaye, S. (2021). Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17 (7), 400–420. doi:10.1038/s41574-021-00496-z
Song, C. H., Petrofsky, J. S., Lee, S. W., Lee, K. J., and Yim, J. E. (2011). Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol. and Ther. 13 (8), 803–811. doi:10.1089/dia.2011.0036
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Br. Med. J. 366, l4898. doi:10.1136/bmj.l4898
Streckmann, F., Balke, M., Cavaletti, G., Toscanelli, A., Bloch, W., Décard, B. F., et al. (2022). Exercise and neuropathy: systematic review with meta-analysis. Sports Med. 52 (5), 1043–1065. doi:10.1007/s40279-021-01596-6
Venkataraman, K., Tai, B. C., Khoo, E. Y. H., Tavintharan, S., Chandran, K., Hwang, S. W., et al. (2019). Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: a randomised controlled trial. Diabetologia 62 (12), 2200–2210. doi:10.1007/s00125-019-04979-7
Waheed, A., Azharuddin, M., Ahmad, I., and Noohu, M. (2021). Whole-body vibration, in addition to balance exercise, shows positive effects for strength and functional ability in patients with diabetic peripheral neuropathy: a single-blind randomized controlled trial. J. Diabetology 12, 456–463. doi:10.4103/jod.jod_47_21
Wang, Z., Peng, S., Zhang, H., Sun, H., and Hu, J. (2022). Gait parameters and peripheral neuropathy in patients with diabetes: a meta-analysis. Front. Endocrinol. 13, 891356. doi:10.3389/fendo.2022.891356
Yoosefinejad, A. K., Shadmehr, A., Olyaei, G., Talebian, S., Bagheri, H., and Mohajeri-Tehrani, M. R. (2015). Short-term effects of the whole-body vibration on the balance and muscle strength of type 2 diabetic patients with peripheral neuropathy: a quasi-randomized-controlled trial study. J. Diabetes and Metabolic Disord. 14, 45. doi:10.1186/s40200-015-0173-y
Zhao, Y., Cai, K., Wang, Q., Hu, Y., Wei, Y., and Gao, H. (2021). Effect of tap dance on plantar pressure, postural stability and lower body function in older patients at risk of diabetic foot: a randomized controlled trial. BMJ Open Diabetes Res. and Care 9 (1), e001909. doi:10.1136/bmjdrc-2020-001909
Keywords: diabetic peripheral neuropathy, gait characteristics, muscle strength, postural control, fall prevention
Citation: Pang X, Wang D, Zhang F, Guo B and Liu W (2025) Exploring the exercise for enhancing postural control, gait, and muscle strength in older adults with diabetic peripheral neuropathy: a systematic review and meta-analysis. Front. Aging 6:1507232. doi: 10.3389/fragi.2025.1507232
Received: 07 October 2024; Accepted: 16 April 2025;
Published: 29 April 2025.
Edited by:
Wiktoria Staśkiewicz-Bartecka, Medical University of Silesia, PolandReviewed by:
Agata Kiciak, Faculty of Public Health in Bytom Medical University of Silesia in Katowice, PolandIvan Julian-Rochina, University of Valencia, Spain
Copyright © 2025 Pang, Wang, Zhang, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Guo, Z3VvYmluQGRsdS5lZHUuY24=; Wenming Liu, bGl1d2VubWluZ0B6anUuZWR1LmNu
† These authors share first authorship
 Xiangsheng Pang
Xiangsheng Pang Dongmei Wang2
†
Dongmei Wang2
† Wenming Liu
Wenming Liu