- 1The Third Unit, The Department of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 2Beijing Institute of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China
Background: Sarcopenia, physical activity (PA), and sedentary behavior are associated with metabolic dysfunction-associated steatotic liver disease (MASLD). The study aimed to evaluate the effects of sarcopenia and PA on the presence and severity of MASLD.
Methods: This cross-sectional study analyzed data from the 2017-2018 National Health and Nutrition Examination Survey (NHANES). Hepatic steatosis and liver fibrosis were assessed by vibration-controlled transient elastography (VCTE). Sarcopenia was defined based on the Foundation for the National Institutes of Health criteria. PA and sedentary behavior were evaluated using the Global Physical Activity Questionnaire (GPAQ).
Results: Among 1,831 participants, 664 were diagnosed with MASLD, including 482 with severe steatosis and 89 with significant fibrosis. The prevalence of sarcopenia in the MASLD and non-MASLD populations was 11.7% and 3.8%, respectively. Multivariable-adjusted models demonstrated that sarcopenia significantly increased the risk of MASLD (OR 2.45; 95% CI: 1.33–4.52), severe steatosis (OR 2.56; 95% CI: 1.40–4.66), and significant fibrosis (OR 6.10; 95% CI: 2.08–17.84). Additionally, individuals with sarcopenia and low PA had a 7.91-fold increased risk of developing significant fibrosis (OR, 7.91; 95% CI: 1.42–44.16, P = 0.022). Sarcopenia and prolonged sedentary behavior further increased the risk of MASLD (OR 3.75; 95% CI: 1.60–8.76), severe steatosis (OR 17.58; 95% CI: 1.93–159.79), and significant fibrosis (OR 4.32; 95% CI: 1.31–14.31).
Conclusion: Patients with sarcopenia should increase physical activity and reduce sedentary time to decrease the risk and progression of MASLD. Increasing muscle mass and strength through resistance exercise to reduce the risk of significant fibrosis in sarcopenia patients.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as the most rapidly increasing chronic liver disease worldwide, affecting more than 30% of the global population (Miao et al., 2024; Younossi et al., 2024). MASLD encompasses liver conditions ranging from metabolic dysfunction-associated steatotic liver (MASL), metabolic dysfunction-associated steatohepatitis (MASH), liver fibrosis, and cirrhosis (Targher et al., 2024). The presence of MASLD is closely linked to cardiometabolic risk factors like obesity, type 2 diabetes, and metabolic syndrome (Targher et al., 2021). Sarcopenia, as defined by the Global Leadership Initiative on Sarcopenia (GLIS), is a progressive and systemic skeletal muscle disease characterized by a reduction in both muscle mass and strength (Kirk et al., 2024). Notably, sarcopenia and MASLD may exacerbate each other’s progression through shared mechanisms, including insulin resistance, chronic inflammation, and hormonal imbalance (Merz and Thurmond, 2020; Altajar and Baffy, 2020). The process of muscle loss results in changes to muscle-related factors, such as elevated myostatin and reduced irisin levels, which exacerbate insulin resistance and systemic inflammation via the endocrine effects, influencing the progression of metabolic diseases (Polyzos et al., 2023).
Physical activity (PA) also plays a critical role in preventing and managing sarcopenia and MASLD, enhancing metabolic health, muscle mass, and function (Piercy et al., 2018; Jamali et al., 2022). PA has been proven effective in preventing MASLD and delaying its progression. Notably, engaging in moderate to vigorous PA was associated with a reduced risk of mortality in patients with MASLD (Golbidi et al., 2012; Kim et al., 2021). Furthermore, prolonged sedentary behavior significantly increased the risk of developing diabetes and cardiovascular diseases (Grøntved and Hu, 2011). PA guidelines emphasized that reducing sedentary behavior can benefit nearly everyone (Piercy et al., 2018). It has been proven that sarcopenia, physical inactivity, and prolonged sedentary behavior were risk factors for MASLD (Kim et al., 2022; Chun et al., 2023; Kim et al., 2020; Harring et al., 2023). Importantly, individuals with sarcopenia often have less PA, potentially further exacerbating their risk for liver steatosis and fibrosis. Despite increasing evidence for the independent effects of sarcopenia and physical activity on MASLD, the combined influence of these factors on disease severity remains poorly understood (Kim et al., 2020; Harring et al., 2023; Franco et al., 2024; Golabi et al., 2020). It is essential for developing integrated strategies to mitigate the burden of MASLD in individuals with sarcopenia.
Therefore, this study aimed to explore the association between sarcopenia, physical activity, and MASLD severity using data from the NHANES (National Health and Nutrition Examination Survey). We hypothesized that physical inactivity and prolonged sedentary behavior increase the risk of MASLD and liver fibrosis in individuals with sarcopenia.
2 Methods
2.1 Subjects and study design
This retrospective study analyzed the data from NHANES 2017-2018, which employed a national, multistage, and stratified sampling design to obtain a representative sample of the U.S. population. NHANES 2017-2018 was the first to use Fibroscan measurements of CAP and VCTE to assess liver steatosis and fibrosis. This approach was adopted due to the extensive evaluation of elastography for its accuracy in assessing hepatic steatosis and fibrosis. MASLD was diagnosed based on the EASL-EASD-EASO Clinical Practice Guidelines, which require the presence of hepatic steatosis through imaging techniques (vibration-controlled transient elastography), combined with at least one of the five defined cardiometabolic risk factors (Rinella et al., 2023). Hepatic steatosis was quantified using the controlled attenuation parameter (CAP), with steatosis defined as a median CAP value of ≥248 dB/m. Moderate and severe steatosis were further classified with CAP values of ≥268 dB/m and ≥280 dB/m, respectively (Tracker, 2024). Significant liver fibrosis was defined as a median liver stiffness measurement (LSM) of ≥8 kPa. The NHANES study was ethically approved by the Ethics Review Board of the National Center for Health Statistics, and all participants provided written informed consent.
2.2 Clinical and laboratory assessments
Variables were categorized using previously established methods. Race was divided into five groups: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and others. Educational status was classified as college or non-college graduate, while marital status was categorized as married or other. Smoking status was defined as never smoked, former smoker, or current smoker.
Body Mass Index (BMI) was calculated by dividing weight (kg) by height squared (m2). Obesity was defined as a BMI >25 kg/m2 for Asians and >30 kg/m2 for other races (WHO Expert Consultation, 2004). Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, a diastolic blood pressure of ≥90 mm Hg, and current treatment with antihypertensive medications (Whelton et al., 2018). Diabetes mellitus was defined as a fasting blood glucose level of ≥126 mg/dL, glycated hemoglobin (HbA1c) of ≥6.5%, or the use of hypoglycemic drugs or insulin (American Diabetes Association, 2018).
2.3 Sarcopenia definition
Sarcopenia was defined using appendicular lean mass (ALM) measured by dual-energy X-ray absorptiometry (DXA), which quantifies lean mass in all four limbs to determine total ALM. The diagnostic criteria for sarcopenia followed the guidelines of the Foundation for the National Institutes of Health (FNIH), defined as an ALM/BMI <0.789 for men and <0.512 for women (Studenski et al., 2014).
2.4 Physical activity
Detailed physical activity (PA) data were collected using the global physical activity questionnaire including leisure-time physical activity (LTPA), work-related physical activity (WPA), transportation-related physical activity (TPA), and sedentary behavior (sitting time) (Armstrong and Bull, 2006). Each type of PA was assessed for weekly frequency (days per week), duration (minutes), and intensity (vigorous vs. moderate). Total PA time was calculated as the sum of vigorous activity time and 2 times spent in moderate activity. The total PA was defined as the total of LTPA, WPA, and TPA. According to the 2018 guidelines, PA was categorized into 0-149 min/week and ≥150 min/week (Piercy et al., 2018). To investigate sedentary behavior, we assessed total sitting time by the reported hours per day in a typical week. Sedentary behavior was defined as total sitting time≥ 420 min/d (Kim et al., 2022).
2.5 Statistical analysis
All analyses accounted for the weights of each variable provided in the NHANES database, given the complex survey design of NHANES. Continuous variables were described as weighted means with standard error (SE), and categorical variables were presented as weighted frequencies with weighted percentages. Multivariate logistic regression models were used after adjusting for potential confounding factors, including age, sex, race, education, marital status, smoking status, T2DM, hypertension, hyperlipidemia, ALT, AST, triglycerides, and total cholesterol. Missing data in key variables were handled using multiple imputation by chained equations (MICE), a method commonly used for handling missing data in complex survey designs. The extent of missing data in key variables was minimal, with no variable exhibiting more than 6% missing data. All statistical analyses were conducted using R software (version 4.3.3; R Foundation for Statistical Computing, Vienna, Austria), with a two-sided p-value of <0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics of the study population
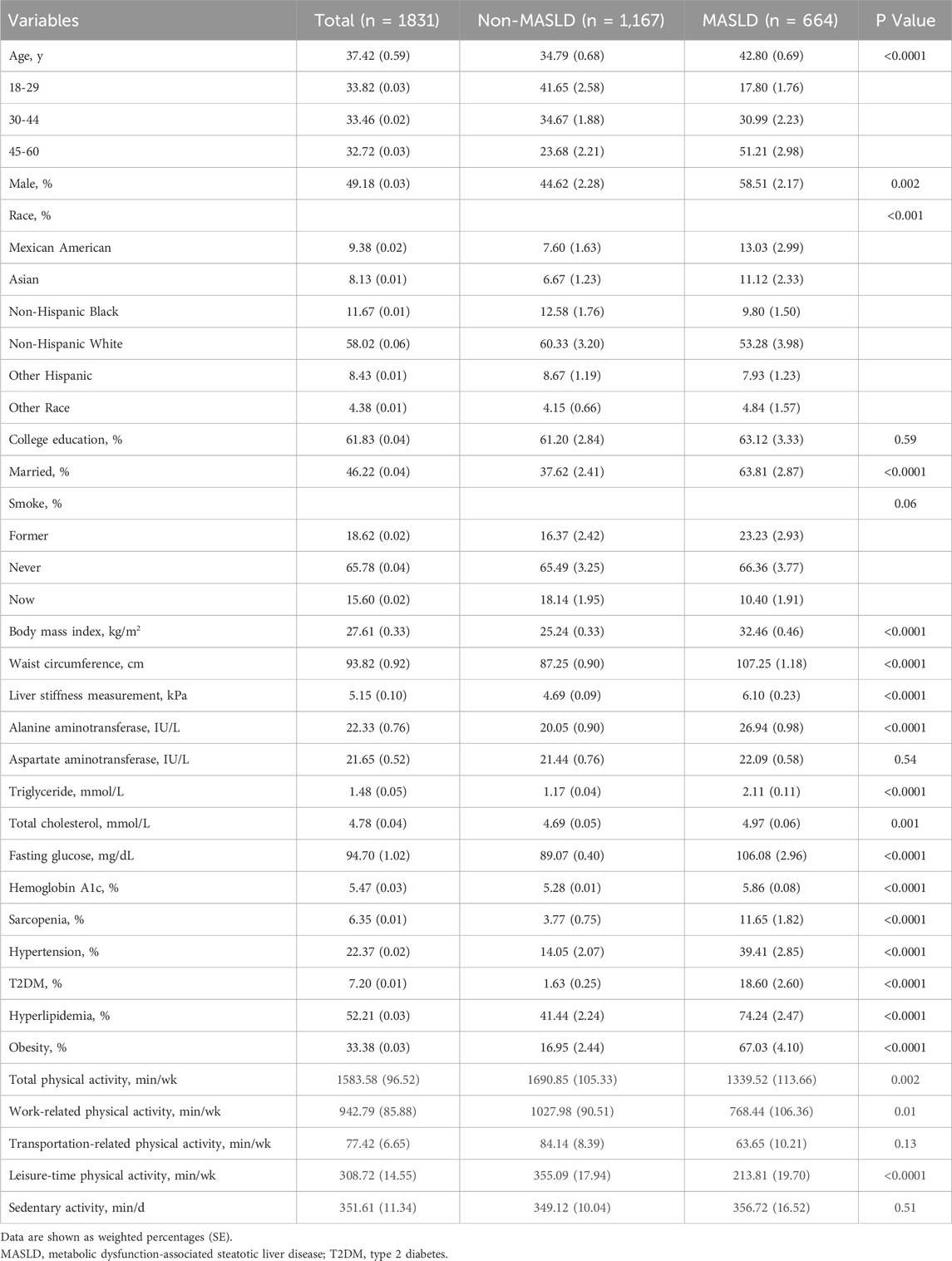
After excluding individuals who did not meet the inclusion criteria, 1,831 participants were included in the study (Figure 1). The baseline characteristics were detailed in Table 1. The mean age of the participants was 37 years, and males accounted for 49.18%. The mean BMI was 27.61 kg/m2. The majority of the participants were non-Hispanic whites (58.02%) and non-Hispanic blacks (11.67%).
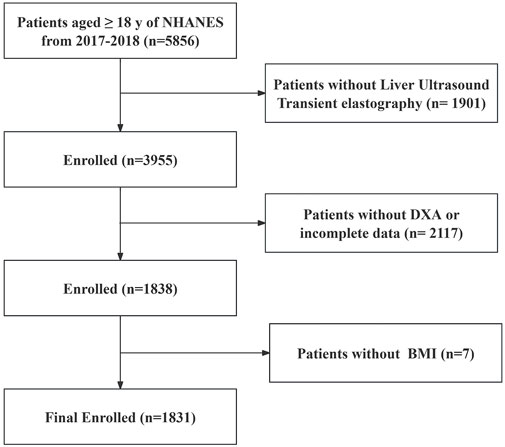
Figure 1. Flowchart of the study population. According to the exclusion criteria of the study, 4,025 adults were excluded from the 5,856 adults, and a total of 1831 patients were finally analyzed. NHANES, National Health and Nutrition Examination Survey; MASLD, Metabolic dysfunction-associated steatotic liver disease; BMI, Body mass index; DXA, Dual-energy X-ray absorptiometry.
3.2 Prevalence of MASLD, MASLD-Related severe steatosis, and significant fibrosis
The prevalence of MASLD in the study cohort was 36.3%. Compared to the non-MASLD population, patients with MASLD were older, more males, and exhibited elevated clinical parameters, including higher BMI, WC, LSM, ALT, TG, TC, HbA1c, and fasting glucose. Additionally, the prevalence of T2DM, hypertension, hyperlipidemia, and obesity were significantly higher in patients with MASLD compared to those without (Table 1).
The prevalence of severe steatosis among patients with MASLD was 72.6%. Compared with patients with non-severe steatosis, those with severe steatosis had significantly higher rates of hypertension, diabetes, and obesity (Supplementary Table S1). Furthermore, 13.4% of the MASLD population had significant fibrosis. The prevalence of hypertension, diabetes, and obesity was also higher in the significant fibrosis group compared to those with early fibrosis (Supplementary Table S2).
3.3 Association between sarcopenia and the severity of MASLD
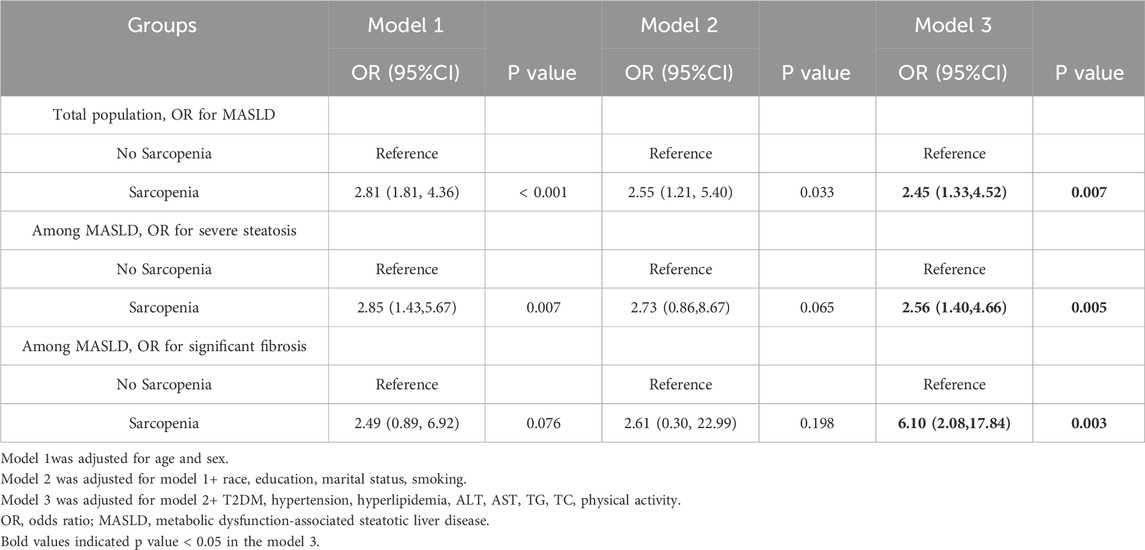
The prevalence of sarcopenia was significantly higher in individuals with MASLD compared to those without MASLD (11.7% vs. 3.8%). The strongest association observed was between sarcopenia and significant fibrosis in MASLD patients. After adjusting for potential confounders, sarcopenia was independently associated with a 6.10-fold increased risk of significant fibrosis (OR = 6.10, 95% CI: 2.08–17.84, P = 0.003) (Table 2; Figure 2). This finding underscores the critical role of sarcopenia in liver fibrosis. Sarcopenia was more prevalent among MASLD patients with significant fibrosis compared to those without significant fibrosis (21.8% vs. 10.0%). Multivariable logistic regression analysis also revealed that sarcopenia was independently associated with a 2.45-fold increased risk of MASLD (OR = 2.45, 95% CI: 1.33–4.52, P = 0.007).
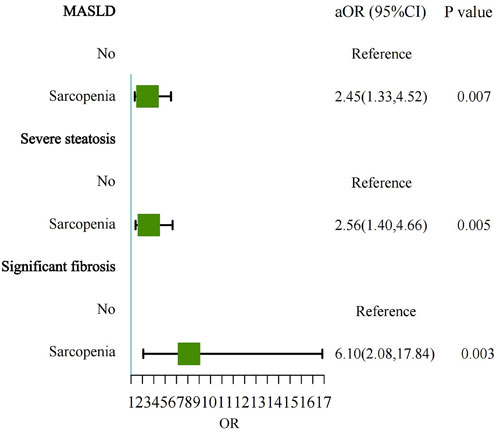
Figure 2. Forest plot showing the multivariable-adjusted risk of sarcopenia for MASLD, severe steatosis, and significant fibrosis. MASLD, Metabolic dysfunction-associated steatotic liver disease; aOR, adjusted odds ratio.
Similarly, sarcopenia was more prevalent among individuals with severe steatosis compared to those with mild to moderate steatosis (13.9% vs. 5.4%). In the multivariable model, sarcopenia was independently associated with a 2.56-fold increased risk of severe steatosis (OR = 2.56, 95% CI: 1.40–4.66, P = 0.005).
3.4 Association between physical activity and the severity of MASLD
Higher physical activity was associated with a reduced risk of MASLD. Individuals engaging in total physical activity of ≥150 min/wk had a significantly reduced risk of MASLD (OR = 0.51, 95% CI: 0.31–0.84, P = 0.011). Similarly, individuals participating in ≥150 min/wk of recreational physical activity had a 51% (OR = 0.49, 95% CI: 0.38–0.63, P < 0.001), and those ≥150 min/wk transportation-related physical activity had a 32% (OR = 0.68, 95% CI: 0.48–0.97, P = 0.035) lower risk of MASLD (Table 3). However, no significant association was observed between physical activity and the severity of steatosis. Additionally, those who reported ≥420 min/d of sedentary activity had a 2.12-fold increased risk of significant fibrosis (OR = 2.12, 95% CI: 1.01–4.44, P = 0.046). (Figure 3).
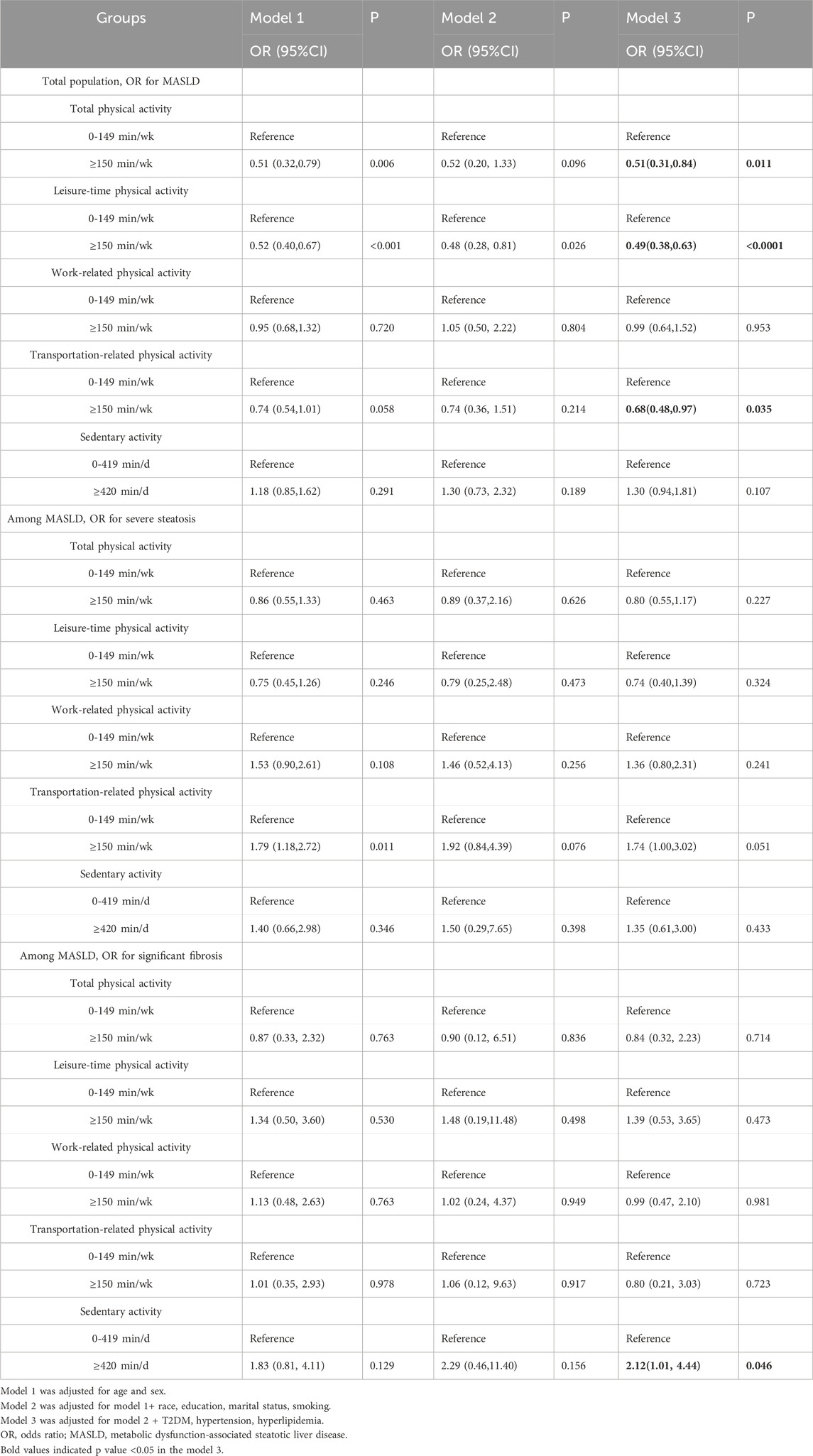
Table 3. Multivariate ORs of physical activity for MASLD, severe steatosis, and significant fibrosis.
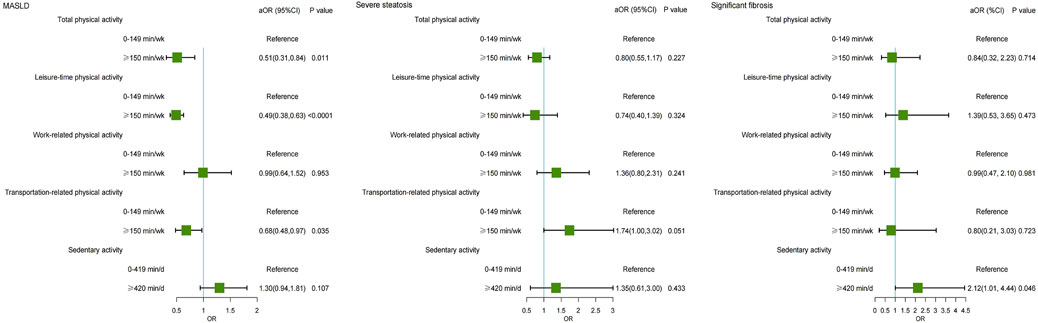
Figure 3. Forest plot showing the multivariable-adjusted risk of physical activity for MASLD, severe steatosis, and significant fibrosis. MASLD, Metabolic dysfunction-associated steatotic liver disease; aOR, adjusted odds ratio.
3.5 Association between sarcopenia, physical activity, and the severity of MASLD
Table 4 further investigated the combined effect of sarcopenia and physical activity on the severity of MASLD. Compared to individuals without sarcopenia, those with sarcopenia and high physical activity had a 2.15-fold increased risk of MASLD (OR = 2.15, 95% CI: 1.03–4.49, P = 0.042). Similarly, patients with sarcopenia who reported prolonged sedentary activity (≥420 min/d) exhibited a 3.75-fold increased risk of MASLD (OR = 3.75, 95% CI: 1.60–8.76, P = 0.005). While sarcopenia combined with physical activity showed no statistically significant effect on the severity of steatosis, patients with sarcopenia and prolonged sedentary activity significantly increased the risk of severe steatosis, with an OR of 17.58 (OR = 17.58, 95% CI: 1.93–159.79, P = 0.014). Additionally, compared to those without sarcopenia, individuals with sarcopenia and low physical activity had a 7.91-fold increased risk of developing significant fibrosis (OR = 7.91, 95% CI: 1.42–44.16, P = 0.022). Furthermore, patients with sarcopenia and prolonged sedentary activity had a 4.32 times higher risk of significant fibrosis (OR = 4.32, 95% CI: 1.31–14.31, P = 0.020). (Figure 4).
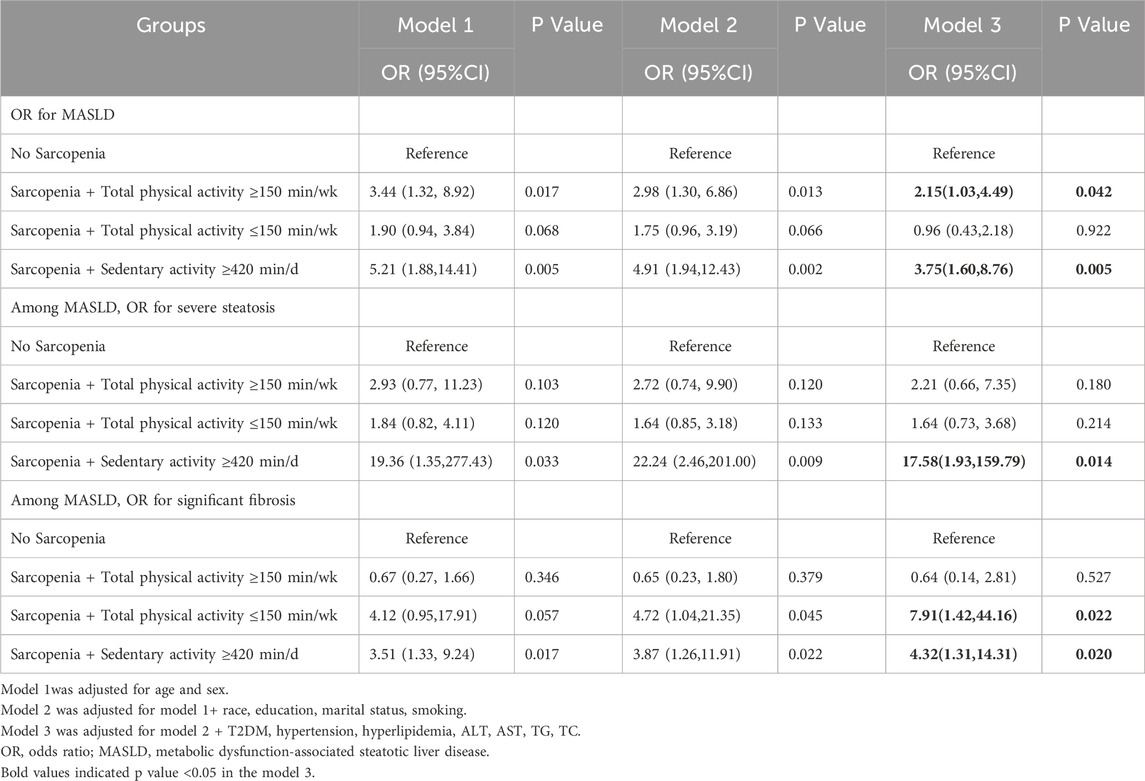
Table 4. Multivariate ORs of sarcopenia and physical activity for MASLD, severe steatosis, and significant fibrosis.
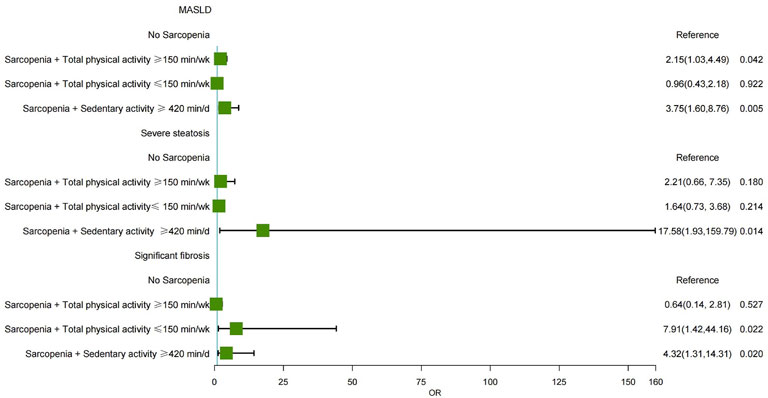
Figure 4. Forest plot showing the multivariable-adjusted risk of sarcopenia and physical activity for MASLD, severe steatosis, and significant fibrosis. MASLD, Metabolic dysfunction-associated steatotic liver disease; aOR, adjusted odds ratio.
3.6 Sensitivity analysis
Sensitivity analyses were conducted across various subgroups stratified by sex, age, race, marital status, education level, smoking, hypertension, T2DM, obesity, and hyperlipidemia to assess potential interactions with sarcopenia. Among these factors, only obesity significantly interacted with sarcopenia in the prevalence of MASLD (P = 0.016) (Supplementary Table S3).
4 Discussion
Our study highlights a significant association between sarcopenia, physical inactivity, and prolonged sedentary behavior with MASLD progression. While previous studies have demonstrated a significant association between sarcopenia and MASLD, our study is one of the first to explore the combined effects of sarcopenia, physical activity, and sedentary behavior on MASLD progression. Sarcopenic patients who were physical inactive were more likely to have significant fibrosis, while those with prolonged sedentary time were associated with a higher risk of developing and progressing MASLD. This finding suggested the need for targeted interventions to increase physical activity (≥150 min/wk) and reduce sedentary behavior (≤420 min/d) among sarcopenic patients as a potential strategy to mitigate MASLD progression. These findings provide a novel perspective on the importance of addressing both muscle mass and activity levels as part of a comprehensive strategy to manage MASLD.
Previous studies have demonstrated a significant association between sarcopenia and MASLD, which can be attributed to several physiological mechanisms. First, skeletal muscle serves as a primary target tissue for insulin. Reducing muscle mass can exacerbate insulin resistance, leading to increased lipolysis and the accumulation of free fatty acids in the liver, thereby promoting the occurrence and progression of MASLD (Utzschneider and Kahn, 2006). Insulin activates the mammalian target of rapamycin (mTOR), 4E-binding protein 1 (4EBP1), and ribosomal S6 kinase 1 (S6K1). These proteins are involved in protein synthesis, maintenance of muscle mass, and skeletal muscle anabolic metabolism (Fujita et al., 2007). Additionally, elevated levels of chronic inflammation markers, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), along with reduced secretion of muscle-derived factors, may further contribute to hepatic steatosis and liver inflammation. TNF-α contributed to hepatic lipid accumulation by promoting de novo lipogenesis (DNL). It also stimulates nuclear factor kappa B (NF-κB), a key transcription factor for pro-inflammatory cytokines, which facilitates the progression of NAFLD and muscle catabolism (Beyer et al., 2012; Han et al., 2022). Myostatin, a muscle-related protein, has also been implicated in liver fibrosis progression by activating hepatic stellate cells (Kuchay et al., 2022; Miller et al., 2011; Delogu et al., 2019). Myostatin mediates Smad 2/3 activation, which suppresses myogenesis and protein synthesis by inhibiting the Akt-mediated mTOR signaling pathway, ultimately leading to muscle atrophy. Furthermore, it promotes muscle protein degradation through FoxO-dependent activation of the ubiquitin-proteasome pathway and autophagy (Han et al., 2013). In this study, our findings demonstrated that sarcopenia significantly associated with MASLD, severe steatosis, and significant fibrosis, with ORs of 2.45 (95% CI: 1.33–4.52), 2.56 (95% CI: 1.40–4.66), and 6.10 (95% CI: 2.08–17.84), respectively. These results are consistent with previous research, including a meta-analysis of 19 studies that demonstrated a significant association between sarcopenia and NAFLD, NASH, and significant fibrosis, with pooled ORs of 1.33 (95% CI: 1.20-1.48), 2.42 (95% CI: 1.27-3.57), and 1.56 (95% CI: 1.34-1.78), respectively (Cai et al., 2020). These findings further verify the robustness of our results and indicate the potential prevalence of sarcopenia as a critical risk factor in the progression of liver disease. However, the higher OR for significant fibrosis observed in our study. One potential explanation was demographic differences: while the meta-analysis included a mix of older populations and patients with advanced liver disease, our study focused on adults in the United States. Additionally, differences in sarcopenia diagnostic criteria may explain discrepancies; while we used the FNIH definitions, other studies often incorporate muscle strength metrics, such as grip strength, which may influence effect sizes. Our study defined sarcopenia using the FNIH criteria. Specifically, the FNIH criteria primarily emphasize lean mass, which might lead to higher prevalence estimates of sarcopenia in certain populations, particularly younger adults, whereas EWGSOP definitions yield lower prevalence rates due to their focus on functional impairments more characteristic of older populations.
Numerous studies have established a strong association between PA and MASLD. The 2018 American Physical Activity Guidelines recommend that adults engage in at least 150 min/week of moderate-intensity PA, with additional health benefits observed when exceeding 300 min/week (Piercy et al., 2018). PA has beneficial effects on MASLD through various mechanisms, including the activation of uncoupling protein-1, peroxisome proliferator-activated receptor γ (PPAR-γ), adipocytokines, and branched-chain amino acids, which collectively reduce insulin resistance, promote lipolysis, and exert anti-inflammatory and antioxidant effects (Hughes et al., 2021; Babu et al., 2022; Thorp and Stine, 2020; Su et al., 2023). Conversely, sedentary behavior negatively impacts metabolic processes, increasing the risk of health issues such as obesity, diabetes, insulin resistance, and metabolic syndrome (Hamilton et al., 2007). Previous research has shown that engaging in more than 300 min/week of leisure-time PA significantly reduces the association of NAFLD and significant fibrosis in the general population, with ORs of 0.51 (95% CI: 0.40-0.65) and 0.41 (95% CI: 0.22-0.74), respectively. In contrast, sedentary behavior exceeding 8 h/day significantly increases the risk of NAFLD, with an OR of 1.44 (95% CI: 1.01-2.05) (Kim et al., 2022). Additionally, a Korean multicenter study involving 11,690 NAFLD patients found that PA exceeding 600 MET min/week was protective against fibrosis, sarcopenia, and cardiovascular disease. However, fibrosis was assessed using non-invasive serological markers (Chun et al., 2023). Our results indicated that total, leisure-time, and transportation-related PA exceeding 150 min/wk significantly reduce the association with MASLD, while sedentary behavior exceeding 420 min/d increased the association with the development of significant fibrosis in MASLD patients. Notably, our results demonstrated a substantial increase in the risk of severe steatosis among individuals with sarcopenia combined with prolonged sedentary activity, with an odds ratio of 17.58 (95% CI: 1.93–159.79). We acknowledge that the wide confidence interval reflects statistical uncertainty, likely influenced by the small sample size in this subgroup of sarcopenia and sedentary activity ≥420 min/d. However, no significant effect was observed on the risk of severe steatosis. A combined analysis of sarcopenia and physical activity showed that individuals with sarcopenia and prolonged sedentary activity were associated with the development of MASLD and severe fibrosis compared to those without sarcopenia. However, individuals with sarcopenia who engaged in high physical activity were still associated with MASLD, suggesting that while physical activity may decrease the negative effects of sarcopenia, it does not completely balance the elevated risk associated with sarcopenia. Interestingly, individuals with sarcopenia and low-intensity physical activity were associated with a significant association of fibrosis progression in patients with MASLD. These results highlight the need for early identification and management of sarcopenia in patients with MASLD, as well as the promotion of increased physical activity and reduced sedentary behavior. Therefore, it is recommended that individuals with sarcopenia increase their physical activity levels by incorporating both aerobic and resistance exercise. Current guidelines suggest a minimum of 150 min of moderate-intensity aerobic exercise per week, along with resistance training based on established exercise prescription principles, which should target major muscle groups at least 1-2 times per week. Additionally, progressive resistance exercises, tailored to the individual’s capacity, can help improve muscle mass and strength, while aerobic exercises enhance cardiovascular health and metabolic function.
This study had several limitations that should be considered when interpreting the findings. First, the NHANES data used in this study were cross-sectional, limiting our ability to infer causality. Second, physical activity and sedentary time were assessed via self-reported data using the GPAQ, which may introduce recall and reporting biases. Third, the study population was restricted to individuals aged 18-59 years, while sarcopenia is more prevalent in older adults aged 60 years and above. Fourth, there were more recent definitions of sarcopenia (e.g., EWGSOP, AWGS), the FNIH criteria were specifically used in this study because there is no indicator of grip strength in the NHANES data. Fifth, while the study sample is representative of the United States population, the small sample size of certain ethnic groups may affect the generalizability of the results to these populations. Sixth, DXA-derived ALM adjusted for BMI, as applied using the FNIH criteria, may overestimate muscle mass in obese individuals. Finally, despite adjusting for multiple covariates, the possibility of residual confounding by unmeasured variables cannot be entirely ruled out.
5 Conclusion
In conclusion, the study emphasized the pivotal role of sarcopenia and physical activity in the progression of MASLD. Sarcopenia is associated with an increased risk of severe steatosis and significant fibrosis, while physical activity demonstrates a protective effect against MASLD, with a more limited impact on disease severity. These findings underscore the importance of early screening for sarcopenia in patients with MASLD, as timely targeting interventions to improve muscle mass and metabolic health. From a prevention perspective, integrating exercise programs that combine resistance and aerobic exercise into routine clinical practice could benefit patients with MASLD, particularly those with sarcopenia. Such interventions not only improve muscle strength and function but also mitigate the synergistic effects of sarcopenia and sedentary behavior in promoting liver fibrosis. Further longitudinal studies are warranted to explore the mechanisms underlying these associations and to identify effective strategies for mitigating the impact of sarcopenia and inactivity in MASLD management.
Data availability statement
The datasets presented in this study can be found in online repositories. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board (Protocol number: 2018–01; 2021–05). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW: Writing – original draft, Data curation, Formal analysis. XL: Writing – original draft, Data curation, Formal analysis. JZ: Writing – original draft, Data curation, Formal analysis. YZ: Supervision, Writing – review and editing. LQ: Methodology, Writing – review and editing. JZ: Writing-original draft, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study has received funding by National Science and Technology Major Project of China (2023ZD0508703); Capitals’ Funds for Health Improvement and Research (2022-2Z-2187 and 2024-2-1151); Beijing high-level Public Health Technical Talent Construction Project (03-23); National Science and Technology Major Project of China (2023ZD0508703); Scientific Research Project of Beijing Youan Hospital (BJYAYY-YN2022-17); Beijing Hospitals Authority Clinical Medicine Development of special funding support (YGLX202339).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2025.1573170/full#supplementary-material
References
Altajar, S., and Baffy, G. (2020). Skeletal muscle dysfunction in the development and progression of nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 8 (4), 414–423. doi:10.14218/JCTH.2020.00065
American Diabetes Association (2018). 2. Classification and diagnosis of diabetes: Standards of medical Care in diabetes-2018. Diabetes Care 41 (Suppl. 1), S13–s27. doi:10.2337/dc18-S002
Armstrong, T., and Bull, F. (2006). Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 14 (2), 66–70. doi:10.1007/s10389-006-0024-x
Babu, A. F., Csader, S., MäNNISTö, V., Tauriainen, M. M., Pentikäinen, H., Savonen, K., et al. (2022). Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci. Rep. 12 (1), 6485. doi:10.1038/s41598-022-10481-9
Beyer, I., Mets, T., and Bautmans, I. (2012). Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 15 (1), 12–22. doi:10.1097/MCO.0b013e32834dd297
Cai, C., Song, X., Chen, Y., and Yu, C. (2020). Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol. Int. 14 (1), 115–126. doi:10.1007/s12072-019-09964-1
Chun, H. S., Lee, M., Lee, H. A., Oh, S. Y., Baek, H. J., Moon, J. W., et al. (2023). Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 21 (2), 358–369.e12. doi:10.1016/j.cgh.2021.12.043
Delogu, W., Caligiuri, A., Provenzano, A., Rosso, C., Bugianesi, E., Coratti, A., et al. (2019). Myostatin regulates the fibrogenic phenotype of hepatic stellate cells via c-jun N-terminal kinase activation. Dig. Liver Dis. 51 (10), 1400–1408. doi:10.1016/j.dld.2019.03.002
Franco, I., Bianco, A., Bonfiglio, C., Curci, R., Campanella, A., and Osella, A. R. (2024). Leisure-time physical activity, time spent sitting and risk of non-alcoholic fatty liver disease: a cross-sectional study in puglia. J. Gen. Intern Med. 39 (14), 2788–2796. doi:10.1007/s11606-024-08804-9
Fujita, S., Rasmussen, B. B., Cadenas, J. G., Drummond, M. J., Glynn, E. L., Sattler, F. R., et al. (2007). Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56 (6), 1615–1622. doi:10.2337/db06-1566
Golabi, P., Gerber, L., Paik, J. M., Deshpande, R., de Avila, L., and Younossi, Z. M. (2020). Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2 (6), 100171. doi:10.1016/j.jhepr.2020.100171
Golbidi, S., Mesdaghinia, A., and Laher, I. (2012). Exercise in the metabolic syndrome. Oxid. Med. Cell Longev. 2012, 349710. doi:10.1155/2012/349710
GrøNTVED, A., and Hu, F. B. (2011). Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. Jama 305 (23), 2448–2455. doi:10.1001/jama.2011.812
Hamilton, M. T., Hamilton, D. G., and Zderic, T. W. (2007). Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56 (11), 2655–2667. doi:10.2337/db07-0882
Han, H. Q., Zhou, X., Mitch, W. E., and Goldberg, A. L. (2013). Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 45 (10), 2333–2347. doi:10.1016/j.biocel.2013.05.019
Han, J. W., Kim, D. I., Nam, H. C., Chang, U. I., Yang, J. M., and Song, D. S. (2022). Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis. Clin. Mol. Hepatol. 28 (2), 219–231. doi:10.3350/cmh.2021.0082
Harring, M., Golabi, P., Paik, J. M., Shah, D., Racila, A., Cable, R., et al. (2023). Sarcopenia among patients with nonalcoholic fatty liver disease (NAFLD) is associated with advanced fibrosis. Clin. Gastroenterol. Hepatol. 21 (11), 2876–2888.e5. doi:10.1016/j.cgh.2023.02.013
Hughes, A., Dahmus, J., Rivas, G., Hummer, B., Chen See, J. R., Wright, J. R., et al. (2021). Exercise training reverses gut dysbiosis in patients with biopsy-proven nonalcoholic steatohepatitis: a proof of concept study. Clin. Gastroenterol. Hepatol. 19 (8), 1723–1725. doi:10.1016/j.cgh.2020.08.063
Jamali, T., Raasikh, T., Bustamante, G., Sisson, A., Tandon, P., Duarte-Rojo, A., et al. (2022). Outcomes of exercise interventions in patients with advanced liver disease: a systematic review of randomized clinical trials. Official J. Am. Coll. Gastroenterology | ACG 117 (10), 1614–1620. doi:10.14309/ajg.0000000000001883
Kim, D., Konyn, P., Cholankeril, G., and Ahmed, A. (2022). Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin. Gastroenterol. Hepatol. 20 (6), e1438–e1455. doi:10.1016/j.cgh.2021.06.029
Kim, D., Murag, S., Cholankeril, G., Cheung, A., Harrison, S. A., Younossi, Z. M., et al. (2021). Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 19 (6), 1240–1247.e5. doi:10.1016/j.cgh.2020.07.023
Kim, D., Vazquez-Montesino, L. M., Li, A. A., Cholankeril, G., and Ahmed, A. (2020). Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 72 (5), 1556–1568. doi:10.1002/hep.31158
Kirk, B., Cawthon, P. M., Arai, H., Ávila-Funes, J. A., Barazzoni, R., Bhasin, S., et al. (2024). The conceptual definition of sarcopenia: delphi consensus from the global leadership initiative in sarcopenia (GLIS). Age Ageing 53 (3), afae052. doi:10.1093/ageing/afae052
Kuchay, M. S., MartíNEZ-Montoro, J. I., Kaur, P., Fernández-García, J. C., and Ramos-Molina, B. (2022). Non-alcoholic fatty liver disease-related fibrosis and sarcopenia: an altered liver-muscle crosstalk leading to increased mortality risk. Ageing Res. Rev. 80, 101696. doi:10.1016/j.arr.2022.101696
Merz, K. E., and Thurmond, D. C. (2020). Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 10, 785–809. doi:10.1002/cphy.c190029
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y., and Zheng, M. H. (2024). Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 35 (8), 697–707. doi:10.1016/j.tem.2024.02.007
Miller, A. M., Wang, H., Bertola, A., Park, O., Horiguchi, N., Ki, S. H., et al. (2011). Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 54 (3), 846–856. doi:10.1002/hep.24517
Piercy, K. L., Troiano, R. P., Ballard, R. M., Carlson, S. A., Fulton, J. E., Galuska, D. A., et al. (2018). The physical activity guidelines for Americans. Jama 320 (19), 2020–2028. doi:10.1001/jama.2018.14854
Polyzos, S. A., Vachliotis, I. D., and Mantzoros, C. S. (2023). Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 147, 155676. doi:10.1016/j.metabol.2023.155676
Rinella, M. E., Lazarus, J. V., Ratziu, V., Francque, S. M., Sanyal, A. J., Kanwal, F., et al. (2023). A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79 (6), 1542–1556. doi:10.1016/j.jhep.2023.06.003
Studenski, S. A., Peters, K. W., Alley, D. E., Cawthon, P. M., McLean, R. R., Harris, T. B., et al. (2014). The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69 (5), 547–558. doi:10.1093/gerona/glu010
Su, P., Chen, J. G., and Tang, D. H. (2023). Exercise against nonalcoholic fatty liver disease: possible role and mechanism of lipophagy. Life Sci. 327, 121837. doi:10.1016/j.lfs.2023.121837
Targher, G., Byrne, C. D., and Tilg, H. (2024). MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut 73 (4), 691–702. doi:10.1136/gutjnl-2023-330595
Targher, G., Tilg, H., and Byrne, C. D. (2021). Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6 (7), 578–588. doi:10.1016/S2468-1253(21)00020-0
Thorp, A., and Stine, J. G. (2020). Exercise as medicine: the impact of exercise training on nonalcoholic fatty liver disease. Curr. Hepatol. Rep. 19, 402–411. doi:10.1007/s11901-020-00543-9
Tracker, F. (2024). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol., doi:10.1016/j.jhep.2024.04.031
Utzschneider, K. M., and Kahn, S. E. (2006). Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. & Metabolism 91 (12), 4753–4761. doi:10.1210/jc.2006-0587
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 71 (19), e127–e248. doi:10.1016/j.jacc.2017.11.006
WHO Expert Consultation (2004). Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363 (9403), 157–163. doi:10.1016/S0140-6736(03)15268-3
Keywords: metabolic dysfunction-associated steatotic liver disease, physical activity, sarcopenia, NHANES, liver fibrosis
Citation: Wei X, Liu X, Zhao J, Zhang Y, Qiu L and Zhang J (2025) Association of sarcopenia and physical activity on the severity of metabolic dysfunction-associated steatotic liver disease among United States adults: NHANES 2017 - 2018. Front. Aging 6:1573170. doi: 10.3389/fragi.2025.1573170
Received: 11 February 2025; Accepted: 21 April 2025;
Published: 13 May 2025.
Edited by:
Caroline Sarah Stokes, Humboldt University of Berlin, GermanyReviewed by:
Francesco Corica, University of Messina, ItalyTülin Düger, Hacettepe University, Türkiye
Copyright © 2025 Wei, Liu, Zhao, Zhang, Qiu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang, emp5b3VhbkBjY211LmVkdS5jbg==
†ORCID: Jing Zhang, orcid.org/0000-0002-3082-8330
‡These authors have contributed equally to this work and share first authorship
 Xiaodie Wei1‡
Xiaodie Wei1‡ Yang Zhang
Yang Zhang Jing Zhang
Jing Zhang