- Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, United States
Rapamycin, an antibiotic discovered in the 1970s from Streptomyces hygroscopicus on Easter Island (Rapanui), has become a critical tool in biomedical research. Initially recognized for its potent antifungal and immunosuppressive properties, rapamycin has recently gained significant attention for anti-aging therapy and seizure treatment via mTOR pathway inhibition. The mechanistic target of the rapamycin (mTOR) pathway is an evolutionarily conserved metabolic signaling cascade that regulates cell division, growth, and survival. There is growing evidence that mTOR pathway activity accelerates aging and the development of age-related diseases including cancer, atherosclerosis, diabetes, and declining immune function. Therefore physicians and “biohackers” are using mTOR inhibition via rapamycin (and rapamycin analogs) off-label for prevention of age-related conditions despite not being widely recognized as a treatment by the broader clinical community. Currently, rapamycin (i.e., sirolimus and everolimus) is FDA approved for the prevention of transplant organ rejection and for anti-seizure therapy in Tuberous Sclerosis Complex (TSC; caused by variants in TSC1 or 2). We aim to summarize the mTOR pathway, the impact rapamycin has on the mTOR pathway, and the state of rapamycin use in the field of aging and longevity. Importantly, we will discuss the gaps in knowledge, pitfalls, and potential for the use of rapamycin to prevent aging/age-related disease and discuss the lessons learned from achieving FDA approval of evirolimus for TSC-related seizures after many years of off-label use.
Introduction
Rapamycin in anti-aging and longevity research
Aging is defined as an intrinsic, progressive decline in physiological function that increases vulnerability to disease and death (Kirkwood, 2005; Elsevier, 2005; Vijg and Campisi, 2008; López-Otín et al., 2013). This process is characterized by cellular senescence, genomic instability, mitochondrial dysfunction, and loss of proteostasis. Researchers have long pursued interventions to delay or reverse aspects of aging and caloric restriction (CR) as a potential intervention. Initial findings demonstrated that reduced nutrient intake extended lifespan in rodents (McCay et al., 1935). Since then, CR has extended lifespans across experimental models (e.g., yeast, flies, and rodents) including with significant results in primates. However, evidence for lifespan extension by CR in humans is unclear (McCay et al., 1935; Colman et al., 2009; Mattison et al., 2012; Swindell, 2012; Longo et al., 2015; Mihaylova et al., 2023). These findings prompted the search for CR-mimetic compounds that engage similar molecular pathways without the need for chronic CR (Longo et al., 2015; Madeo et al., 2019). The effects of CR converge on nutrient-sensing pathways and therefore, the mTOR pathway and its inhibition by rapamycin has emerged as a leading CR mimetic.
mTOR complex 1 (mTORC1), the master kinase within the mTOR pathway, regulates cell growth, protein synthesis, and metabolism, and its activity increases with age, contributing to age-related pathologies (Kennedy and Lamming, 2016; Mannick and Lamming, 2023). By inhibiting mTORC1, rapamycin mimics the biochemistry of nutrient scarcity achieved by CR. Thus, suppression of mTORC1 is theorized to shift cellular activity from anabolic processes toward maintenance and repair pathways, promoting autophagy, and improved proteostasis–mechanisms associated with lifespan extension (Harrison et al., 2009; Johnson et al., 2013; Longo et al., 2015; Papadopoli et al., 2019). Translating these findings to humans remains uncertain, as the complexity of human aging and lack of validated endpoints complicate implementation (Lee et al., 2021). Some early clinical studies suggest that short-term rapamycin or analogs (rapalogs) may improve aspects of immune function in older adults (Mannick et al., 2014). However, this study relied on serologic responses to influenza vaccinations as a marker of enhanced immune function. Such markers have limited predictive value for broader immunocompetence, especially in aging populations where vaccines elicit only a weak to modest stimulus of CD8 T-cells (McElhaney et al., 2020; Riese et al., 2022). Broader measures of immunocompetence including T cell repertoire diversity, innate immune activity, and real-world infection resistance remain underexplored in human rapamycin trials (Mannick and Lamming, 2023; Lee et al., 2024). Further, the long-term effects and safety of chronic mTOR inhibition in healthy humans and whether rapamycin can truly “slow” human aging or prevent age-related diseases without unacceptable side effects is unknown.
The mTOR pathway and the function of rapamycin
The mTOR pathway was first identified through the purification of the FKBP12–rapamycin complex from mammalian cells, revealing a protein (RAFT1) homologous to yeast TOR (Target of Rapamycin) proteins (Sabatini et al., 1994). Additional discoveries in yeast identified TOR as a conserved nutrient sensing kinase, establishing the pathway’s role in regulating cell growth in response to environmental cues (Heitman et al., 1991). Together, these findings positioned the mammalian target of rapamycin (mTOR; now called “mechanistic target of rapamycin”) as a master regulator of cell growth integrating signals from growth factors, nutrients, and energy status to control protein synthesis, lipid metabolism, and autophagy (Sabatini et al., 1994; Sabatini, 2006; Saxton and Sabatini, 2017; Papadopoli et al., 2019) (Figure 1). Additional work has demonstrated mTOR’s critical role in aging and disease. Hyperactive mTOR signaling has been implicated in many age-related conditions–cancer, type 2 diabetes, neurodegeneration–and in the aging process itself (Johnson et al., 2013; López-Otín et al., 2013; Longo et al., 2015; Saxton and Sabatini, 2017). Notably, mTOR pathway activity is elevated in many tissues with age and correlates with a decline in clearance of damaged proteins and organelles (Guo et al., 2022; Dai et al., 2023; Mannick and Lamming, 2023). These observations have provided support for mTOR inhibition as a potential mechanism to slow aging. Indeed, rapamycin was the first small molecule shown to extend murine lifespan (Harrison et al., 2009).
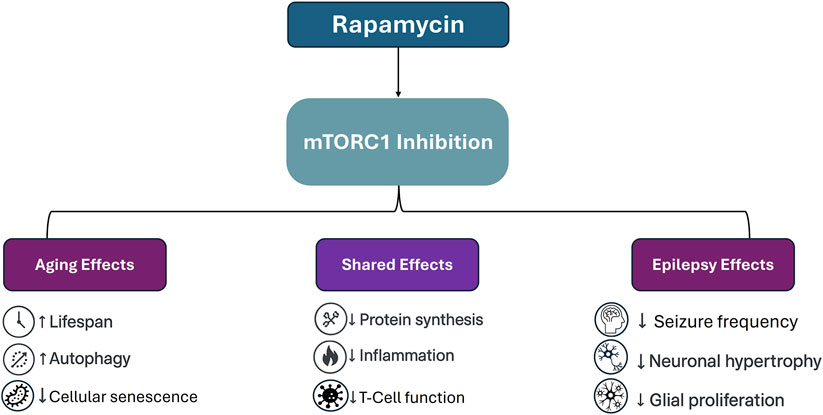
Figure 1. Schematic representation of rapamycin’s effects via mTORC1 inhibition across aging and epilepsy. While some outcomes such as reduced inflammation and suppressed protein synthesis are shared across both applications, others diverge significantly. In aging models, mTOR inhibition is associated with increased autophagy and delayed senescence, whereas in epilepsy, therapeutic benefits include seizure suppression and reversal of cortical hypertrophy and glial overgrowth.
Rapamycin’s purported geroprotective effects are often attributed to its ability to induce autophagy, a cellular recycling process responsible for degrading protein aggregates and other damage-associated molecular patterns (DAMPs) (Rubinsztein et al., 2011; Arensman and Eng, 2018; Zinecker and Simon, 2022; Szőke et al., 2023; Tabibzadeh, 2023). mTORC1 normally inhibits autophagy by phosphorylating components of the Unc-51-like autophagy-activating kinases 1 (ULK1 complex), and its inhibition by rapamycin removes this suppression and initiates autophagosome formation (Kim et al., 2011; Park et al., 2023). Online proponents of anti-aging interventions claim that rapamycin-induced autophagy promotes longevity by maintaining proteostasis and reducing “toxic burden” in post-mitotic cells which is not based in formal geroscience and lacks precise biological definition or clinical validation (Zimmermann et al., 2021; Szőke et al., 2023; Healthspan, 2025). Further, while autophagy may suppress tumor initiation by clearing damaged cellular components, it can also support the survival and growth of established tumors (Marsh et al., 2020). Thus, autophagy can suppress or enhance cancer growth depending on the cellular microenvironment and disease stage. Thus, enhancing autophagy in aging populations with elevated cancer risks and an unknown genetic background may inadvertently promote oncogenesis (Park et al., 2019; Li et al., 2020).
In addition to autophagy induction, mTOR pathway inhibition alters immune regulation through multiple mechanisms. In clinical settings, immunosuppressive mechanisms increase infection risk and impair wound healing- especially in otherwise healthy individuals without clinical manifestations that outweigh the risk of side effects (Lee et al., 2024). Indeed, both mice and humans administered rapamycin for prevention of immunoscenescense, developed glucose intolerance, hyperlipidemia, and testicular atrophy (Deutsch et al., 2007; Houde et al., 2010; Wilkinson et al., 2012). In transplant patients, long-term rapamycin caused metabolic and hematological complications (Hudson et al., 2024). These findings indicate that rapamycin may not be a universal anti-aging solution. Claims of rapamycin as a broadly applicable geroprotector should therefore be tempered by a careful evaluation of risk, mechanism, and both clinical and genetic context.
Discussion
Preclinical and clinical data: promises and challenges
Rapamycin administration initiated in mid-life extends lifespan by 9%–14% in mice and is associated with delayed onset of age-related pathologies (e.g., malignancies and neurodegeneration (Harrison et al., 2009; Wilkinson et al., 2012). In transgenic models predisposed to Alzheimer’s-like pathology, rapamycin prevented memory deficits and reduced cognitive decline (Spilman et al., 2010).
Rapamycin’s claimed benefits in animal models are not limited to aging but extend to models of neurological disorders. In mouse models of Tuberous Sclerosis Complex (TSC), where mTOR pathway hyperactivation is a hallmark, rapamycin prevented seizures, reduced mortality, and rescued neuropathology (i.e., glial proliferation and disrupted cortical architecture). Similar effects were observed in GATOR1-related models such as Nprl3 knockout mice, reinforcing the potential of mTOR inhibition to alter epileptogenesis (Zeng et al., 2008; Iffland et al., 2022). Indeed, rapamycin can be effective at preventing seizures when delivered in utero or postnatally (Zeng et al., 2008; Parker et al., 2013; Iffland et al., 2022).
In contrast, studies on rapamycin in aging are more nuanced and context dependent. Transient, short-term rapamycin treatment in early adulthood improved late-life health outcomes in mice, extending lifespan in both sexes at low doses, but only in males at higher doses (Bitto et al., 2016). Further, late-onset rapamycin treatment in Drosophila did not increase lifespan, further emphasizing the temporal specificity of its effects (Schinaman et al., 2019). Interestingly, intermittent late-life administration of rapamycin has been shown to extend lifespan in both sexes, underscoring the importance of timing and dosing strategy (Arriola Apelo et al., 2016; Moel et al., 2025). The recently published PEARL trial demonstrated that low-dose intermittent rapamycin was well tolerated over 1 year and resulted in modest changes in biomarkers of biological aging, though long-term clinical benefits remain to be established. These discrepancies in lifespan extension across dosing paradigms may also depend on other factors including genetic background, dosing regimen, and timing of administration (Wilkinson et al., 2012; Bitto et al., 2016; Schinaman et al., 2019). While mTOR remains a compelling target in aging research, current animal data do not support rapamycin as a reliable intervention for extending lifespan. Thus, caution is warranted when extrapolating these findings to clinical care.
Across diverse preclinical systems, rapamycin and its analogs have some promise of delaying aging and preventing age-related diseases. While rapamycin robustly extends lifespan in nearly all murine studies (Mannick and Lamming, 2023), its translational efficacy in humans remains unclear, in part, due to the absence of standardized pharmacodynamic biomarkers. In many aging studies, surrogate biomarkers of mTORC1 inhibition, such as phosphorylated ribosomal protein S6, are either underreported or inconsistently applied, making it difficult to determine if outcomes truly reflect effective mTOR inhibition (Lee et al., 2024). Without reliable standardized biomarkers, rapamycin’s benefits remain speculative. Comparisons across studies are confounded by limited known cell-type specific differences, differences in response due to (epi)genetic background, and effects on common geriatric diseases (Mannick et al., 2014; Saxton and Sabatini, 2017; Reifsnyder et al., 2020).
A systematic review evaluated the effects of rapamycin and its derivatives on aging-related physiological changes and diseases and found improvements in the immune, cardiovascular, and integumentary systems but not in the endocrine, muscular, or neurological systems (Lee et al., 2024). Interestingly, this contrasts with robust neurological effects observed in preclinical models of epilepsy associated with mTOR pathway hyperactivating variants (“mTORopathies”) where rapamycin prevented seizures and corrected structural abnormalities (Zeng et al., 2008; Iffland et al., 2022).
The success of rapamycin in mTORopathy models stems from clearly defined molecular etiology and robust biomarkers. Specifically, highly penetrant mutations driving mTOR hyperactivation renders the pathway an actionable target. In contrast, aging is a heterogeneous and multifactorial process without a single dominant pathway, and most preclinical studies do not incorporate genetic stratification or polygenic risk scores. This may explain why mTOR inhibition yields robust disease-modifying effects in monogenic epilepsy models but produces inconsistent outcomes in aging research. Understanding rapamycin’s efficacy in epilepsy may inform how to refine translational models of aging.
However, long-term mTOR inhibition is accompanied by significant side effects. In epilepsy cohorts and transplant populations, chronic rapamycin or everolimus use is associated with mucosal ulcers, impaired wound healing, delayed tissue repair, and increased infection risk (Crino, 2016; Peterson et al., 2016; Hudson et al., 2024). Metabolic disturbances are also common, including elevated cholesterol and triglyceride levels (French et al., 2016; Lee et al., 2024). These effects are mechanistically attributed not only to mTORC1 inhibition but also the unintended suppression of mTORC2. Rapamycin induced mTORC2 inhibition has been shown to induce insulin resistance, highlighting a mechanistic trade-off between metabolic side effects and longevity benefits (Lamming et al., 2012). In female patients, hormonal side effects such as dysmenorrhea, menstrual irregularities, and ovarian dysfunction have been reported (Canpolat et al., 2018). These findings are largely derived from populations using rapamycin chronically at immunosuppressive doses. However, emerging data suggest that low-dose or intermittent rapamycin regimens may be more readily tolerated and are currently under investigation in multiple clinical trials (Kaeberlein et al., 2023; Konopka and Lamming, 2023; Mannick and Lamming, 2023; Hudson et al., 2024). Nonetheless, until long-term data are available, caution is warranted–particularly when considering the use of rapamycin in otherwise healthy individuals. The ethical implications of exposing such populations to even low levels of immunosuppression remain unresolved and merit careful deliberation and scrutiny.
Drug interactions and systemic tolerability represent key barriers to translating rapamycin’s preclinical success into routine clinical use. Rapamycin and its analogs are metabolized by cytochrome P450 enzyme CYP3A and are sensitive to pharmacokinetic interactions. Interestingly, cannabidiol (CBD) is a potent CYP3A inhibitor that increases circulating levels of mTOR inhibitors, raising the risk of toxicity (Ebrahimi-Fakhari et al., 2020; Wray et al., 2023). Given the widespread use of CBD and other cannabis products, it may be beneficial to study the interaction of these two drugs in the context of anti-aging therapy.
The clinical relevance of the above concerns was illustrated in the EXIST-3 trial, a multicenter phase III study evaluating adjunctive everolimus in patients with treatment resistant epilepsy due to TSC. Everolimus significantly reduced seizure frequency, with approximately 40% of patients in the high-dose group achieving a ≥50% reduction in seizures compared to only 15% in the placebo group (French et al., 2016). This outcome led to regulatory approval of everolimus for TSC-associated seizures. However, the EXIST-III trial also exposed the limitations of rapamycin therapy. Complete seizure freedom was rare and withdrawal of therapy frequently led to seizure recurrence indicating that mTOR inhibition is suppressive rather than curative (Sadowski et al., 2022). Importantly, adverse events including stomatitis, infections, hyperlipidemia, and cytopenias were common in the EXIST-III and other trials (Krueger et al., 2013; French et al., 2016). While these side effects were classified as “manageable” in trial settings, they may be less tolerable in individuals without overt disease who are taking rapamycin for aesthetic or anti-aging purposes. These findings offer valuable lessons for aging research: successful translation depends not only on targeting a relevant pathway but on doing so in populations where that pathway plays a central, actionable role.
Ethical considerations regarding off-label accessibility of rapamycin
As rapamycin gains popularity for its anti-aging potential, online longevity clinics have emerged offering access to the drug with minimal medical oversight. This semi-regulated availability raises ethical concerns regarding patient safety, misinformation, and the potential for serious harm. This is best illustrated by the widely publicized case of tech entrepreneur Bryan Johnson, who undertook an elaborate self-directed anti-aging regimen involving rapamycin, metformin, and over 100 daily supplements. Despite extensive physiological tracking, Johnson ultimately discontinued rapamycin and expressed regret over its use citing side effects such as elevated blood glucose, susceptibility to infection, and impaired healing (The Economic Times, 2025). This case highlights the risks of bypassing peer-reviewed science in favor of anecdotal “biohacking” culture. Clinical literature has long documented rapamycin-associated toxicities that mirror the complaints reported by Johnson and others (Peterson et al., 2016; Hudson et al., 2024; Lee et al., 2024). The use of such a powerful immunosuppressant outside established indications, especially in otherwise healthy individuals, demands stronger ethical scrutiny and public education.
Lastly, while the FDA does not recognize aging as a disease, there is growing interest in approving therapeutics that enhance healthspan, or delay aging-related decline. However, FDA approvals are structured around specific, diagnosable indications, rather than generalized syndromes. Should rapamycin or related compounds demonstrate efficacy, they would be approved for specific indicatons (e.g., Alzhiemer’s) rather than aging per se under the current approval standards. Nonetheless, even within this evolving framework, it is important to note that most off-label prescribing–despite it being common clinical practice–rarely achieves FDA approval, as only about 30% of off-label prescribing is supported by adequate scientific evidence despite any clinically observed positive outcomes (Gupta and Nayak, 2014). These regulatory and evidentiary constraints must be considered when evaluating rapamycin’s future clinical and research trajectory.
Equity, access, and ethical use in research
The rise of online rapamycin clinics has also introduced serious equity concerns. These services are often inaccessible to lower-income individuals, exacerbating existing disparities in health and longevity. While some clinics offer rapamycin for as little as $64 per month, that figure excludes substantial additional costs—membership fees typically range from $124/month to over $700 per six-month cycle, limiting access to affluent consumers (author observations). As a result, the promise of rapamycin may be disproportionately realized by wealthier populations, further entrenching health inequities.
Another concern is the potential diversion of limited drug supply away from populations with approved, medically necessary, indications such as organ transplant recipients and individuals with epilepsy, TSC, and other mTORopathies. The emergence of online private pharmacies dispensing rapamycin mirrors the dynamics seen with GLP-1 agonists like semaglutide, where surging off-label use prompted both shortages and regulatory intervention. The FDA has already cracked down on unauthorized compounding and distribution of GLP-1 analogs, which may foreshadow tighter oversight of rapamycin dispensing in the future (U.S.Food and Drug Administration, 2025).
Rapamycin has been in therapeutic use for over a decade to treat seizures in individuals with variants in genes coding regulators of the mTOR pathway (Parker et al., 2013; French et al., 2016; Moloney et al., 2023). These findings underscore that rapamycin’s benefits are effective in monogenic disorders where mTOR hyperactivation is the dominant underlying pathology (Krueger et al., 2013; Iffland and Crino, 2017). These trials were predicated on pre-clinical data where phospho-S6 levels were used as a surrogate marker of mTORC1 activation to assay resected brain tissue specimens and experimental models for mTOR pathway hyperactivation (Meikle et al., 2008; Crino, 2015; Levitin et al., 2023). Unfortunately, phospho-S6 has not proven to be a consistent clinical biomarker, making it difficult to ascertain how the extent of mTOR inhibition, rapamycin blood levels, and reduction in seizures correlate. Thus, in an even more complex and dynamic biological process like aging, biomarkers will be even more challenging to deploy clinically. To begin addressing this, standard guidelines should be implemented to ensure consistency of reported data across studies. Indeed, while some studies include pharmacokinetic measurements (Mannick et al., 2014), these data are often not reported with the results, and many other studies looking at the impact of rapamycin on aging/longevity omit measurements of rapamycin levels, degree of mTOR inhibition, or other mTOR signaling-related biomarkers (Kraig et al., 2018; Mannick et al., 2018; Chung et al., 2019; Kaeberlein et al., 2023).
Another useful approach includes cross-species pharmacokinetic/pharmacodynamic (PK/PD) studies and the use of large-animal aging models to bridge the gap between murine data and human physiology. Further, trials should incorporate genetic stratification and population-specific endpoints, identifying subgroups (e.g., elderly adults with metabolic risk) most likely to benefit from intervention. Lastly, clinical trials must move beyond lifespan alone to assess validated healthspan outcomes such as immune resilience, frailty indices, and neurocognitive performance that many reveal the usefulness of rapamycin for aging-related diseases. Without these strategies, rapamycin’s promise will remain confined to experimental models, unable to meet the ethical, clinical, and scientific standards required for widespread human use.
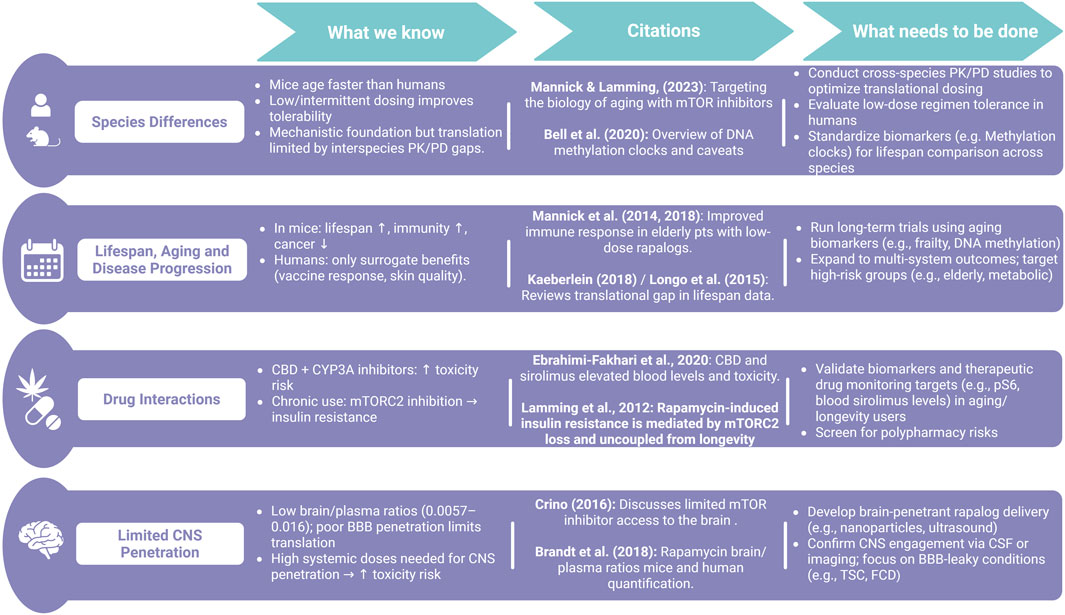
Figure 2. Summary of translational challenges and research gaps in applying rapamycin from animal models to humans. While preclinical studies show promising effects on lifespan and disease, translation is limited by species differences, drug interactions, poor CNS penetration, and inconsistent blood-based surrogate biomarkers that bypass established S6 phospho-targets.
Author contributions
KR: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review and editing. PI: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NIH NINDS RO1NS131223 to PI.
Acknowledgments
We would like to thank the reckless biohackers and wellness influencers around the world who inspired this work. Figure 2 created in BioRender https://BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arensman, M. D., and Eng, C. H. (2018). Self-digestion for lifespan extension: enhanced autophagy delays aging. Mol. Cell 71 (4), 485–486. doi:10.1016/j.molcel.2018.08.002
Arriola Apelo, S. I., Pumper, C. P., Baar, E. L., Cummings, N. E., and Lamming, D. W. (2016). Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 71 (7), 876–881. doi:10.1093/gerona/glw064
Bitto, A., Ito, T. K., Pineda, V. V., LeTexier, N. J., Huang, H. Z., Sutlief, E., et al. (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351. doi:10.7554/eLife.16351
Canpolat, M., Gumus, H., Kumandas, S., Coskun, A., and Per, H. (2018). The use of rapamycin in patients with tuberous sclerosis complex: long-term results. Epilepsy & Behav. 88, 357–364. doi:10.1016/j.yebeh.2018.09.020
Chung, C. L., Lawrence, I., Hoffman, M., Elgindi, D., Nadhan, K., Potnis, M., et al. (2019). Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. GeroScience 41 (6), 861–869. doi:10.1007/s11357-019-00113-y
Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., Beasley, T. M., et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Sci. (New York, N.Y.) 325 (5937), 201–204. doi:10.1126/science.1173635
Crino, P. B. (2015). mTOR signaling in epilepsy: insights from malformations of cortical development. Cold Spring Harb. Perspect. Med. 5 (4), a022442. doi:10.1101/cshperspect.a022442
Crino, P. B. (2016). The mTOR signalling cascade: paving new roads to cure neurological disease. Nat. Rev. Neurol. 12 (7), 379–392. doi:10.1038/nrneurol.2016.81
Dai, D.-F., Kang, P., and Bai, H. (2023). The mTOR signaling pathway in cardiac aging. J. Cardiovasc. Aging 3 (3), 24–N/A. doi:10.20517/jca.2023.10
Deutsch, M. A., Kaczmarek, I., Huber, S., Schmauss, D., Beiras-Fernandez, A., Schmoeckel, M., et al. (2007). Sirolimus-associated infertility: case report and literature review of possible mechanisms. Am. J. Transplant. 7 (10), 2414–2421. doi:10.1111/j.1600-6143.2007.01929.x
Ebrahimi-Fakhari, D., Agricola, K. D., Tudor, C., Krueger, D., and Franz, D. N. (2020). Cannabidiol elevates mechanistic target of rapamycin inhibitor levels in patients with tuberous sclerosis complex. Pediatr. Neurol. 105, 59–61. doi:10.1016/j.pediatrneurol.2019.11.017
Elsevier (2005). Handbook of the biology of aging. Available online at: https://shop.elsevier.com/books/handbook-of-the-biology-of-aging/masoro/978-0-12-088387-5 (Accessed May 23, 2025).
French, J. A., Lawson, J. A., Yapici, Z., Ikeda, H., Polster, T., Nabbout, R., et al. (2016). Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388 (10056), 2153–2163. doi:10.1016/S0140-6736(16)31419-2
Guo, J., Huang, X., Dou, L., Yan, M., Shen, T., Tang, W., et al. (2022). Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 7 (1), 391–40. doi:10.1038/s41392-022-01251-0
Gupta, S. K., and Nayak, R. P. (2014). Off-label use of medicine: perspective of physicians, patients, pharmaceutical companies and regulatory authorities. J. Pharmacol. & Pharmacother. 5 (2), 88–92. doi:10.4103/0976-500X.130046
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460 (7253), 392–395. doi:10.1038/nature08221
Healthspan (2025). Rapamycin prescription online: doctor-Prescribed for anti-aging (no date) healthspan. Available online at: https://gethealthspan.com/protocols/rapamycin-program (Accessed May 12, 2025).
Heitman, J., Movva, N. R., and Hall, M. N. (1991). Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253 (5022), 905–909. doi:10.1126/science.1715094
Houde, V. P., Brûlé, S., Festuccia, W. T., Blanchard, P. G., Bellmann, K., Deshaies, Y., et al. (2010). Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 59 (6), 1338–1348. doi:10.2337/db09-1324
Hudson, J., Kaeberlein, T., Mahal, A., Wong, N., Ghorbanifarajzadeh, M., Radella, F., et al. (2024). Evaluation of off-label rapamycin use on oral health. GeroScience 46 (5), 4135–4146. doi:10.1007/s11357-024-01221-0
Iffland, P. H., and Crino, P. B. (2017). Focal cortical dysplasia: gene mutations, cell signaling, and therapeutic implications. Annu. Rev. Pathology 12, 547–571. doi:10.1146/annurev-pathol-052016-100138
Iffland, P. H., Everett, M. E., Cobb-Pitstick, K. M., Bowser, L. E., Barnes, A. E., Babus, J. K., et al. (2022). NPRL3 loss alters neuronal morphology, mTOR localization, cortical lamination and seizure threshold. Brain 145, 3872–3885. doi:10.1093/brain/awac044
Johnson, S. C., Rabinovitch, P. S., and Kaeberlein, M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature 493 (7432), 338–345. doi:10.1038/nature11861
Kaeberlein, T. L., Green, A. S., Haddad, G., Hudson, J., Isman, A., Nyquist, A., et al. (2023). Evaluation of off-label rapamycin use to promote healthspan in 333 adults. GeroScience 45 (5), 2757–2768. doi:10.1007/s11357-023-00818-1
Kennedy, B. K., and Lamming, D. W. (2016). The mechanistic Target of Rapamycin: the grand conducTOR of metabolism and aging. Cell metab. 23 (6), 990–1003. doi:10.1016/j.cmet.2016.05.009
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. cell Biol. 13 (2), 132–141. doi:10.1038/ncb2152
Kirkwood, T. B. L. (2005). Understanding the odd science of aging. Cell 120 (4), 437–447. doi:10.1016/j.cell.2005.01.027
Konopka, A. R., and Lamming, D. W.RAP PAC InvestigatorsEVERLAST Investigators (2023). Blazing a trail for the clinical use of rapamycin as a geroprotecTOR. GeroScience 45 (5), 2769–2783. doi:10.1007/s11357-023-00935-x
Kraig, E., Linehan, L. A., Liang, H., Romo, T. Q., Liu, Q., Wu, Y., et al. (2018). A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: immunological, physical performance, and cognitive effects. Exp. Gerontol. 105, 53–69. doi:10.1016/j.exger.2017.12.026
Krueger, D. A., Wilfong, A. A., Holland-Bouley, K., Anderson, A. E., Agricola, K., Tudor, C., et al. (2013). Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann. Neurology 74 (5), 679–687. doi:10.1002/ana.23960
Lamming, D. W., Ye, L., Katajisto, P., Goncalves, M. D., Saitoh, M., Stevens, D. M., et al. (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Sci. (New York, N.y.) 335 (6076), 1638–1643. doi:10.1126/science.1215135
Lee, D. J. W., Kuerec, A. H., and Maier, A. B. (2024). Targeting ageing with rapamycin and its derivatives in humans: a systematic review. Lancet Healthy Longev. 5 (2), e152–e162. doi:10.1016/S2666-7568(23)00258-1
Lee, M. B., Hill, C. M., Bitto, A., and Kaeberlein, M. (2021). Antiaging diets: separating fact from fiction. Science 374 (6570), eabe7365. doi:10.1126/science.abe7365
Levitin, M. O., Rawlins, L. E., Sanchez-Andrade, G., Arshad, O. A., Collins, S. C., Sawiak, S. J., et al. (2023). Models of KPTN-related disorder implicate mTOR signalling in cognitive and overgrowth phenotypes. Brain 146 (11), 4766–4783. doi:10.1093/brain/awad231
Li, Z., Tian, X., Ji, X., Wang, J., Chen, H., Wang, D., et al. (2020). ULK1-ATG13 and their mitotic phospho-regulation by CDK1 connect autophagy to cell cycle. PLOS Biol. 18 (6), e3000288. doi:10.1371/journal.pbio.3000288
Longo, V. D., Antebi, A., Bartke, A., Barzilai, N., Brown-Borg, H. M., Caruso, C., et al. (2015). Interventions to slow aging in humans: are we ready? Aging Cell 14 (4), 497–510. doi:10.1111/acel.12338
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2013). The hallmarks of aging. Cell 153 (6), 1194–1217. doi:10.1016/j.cell.2013.05.039
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J., and Kroemer, G. (2019). Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29 (3), 592–610. doi:10.1016/j.cmet.2019.01.018
Mannick, J. B., Del Giudice, G., Lattanzi, M., Valiante, N. M., Praestgaard, J., Huang, B., et al. (2014). mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6 (268), 268ra179. doi:10.1126/scitranslmed.3009892
Mannick, J. B., and Lamming, D. W. (2023). Targeting the biology of aging with mTOR inhibitors. Nat. aging 3 (6), 642–660. doi:10.1038/s43587-023-00416-y
Mannick, J. B., Morris, M., Hockey, H. U. P., Roma, G., Beibel, M., Kulmatycki, K., et al. (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 10 (449), eaaq1564. doi:10.1126/scitranslmed.aaq1564
Marsh, T., Kenific, C. M., Suresh, D., Gonzalez, H., Shamir, E. R., Mei, W., et al. (2020). Autophagic degradation of NBR1 restricts metastatic outgrowth during mammary tumor progression. Dev. Cell 52 (5), 591–604.e6. doi:10.1016/j.devcel.2020.01.025
Mattison, J. A., Roth, G. S., Beasley, T. M., Tilmont, E. M., Handy, A. M., Herbert, R. L., et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys: the NIA study. Nature 489 (7415), 318–321. doi:10.1038/nature11432
McCay, C. M., Crowell, M. F., and Maynard, L. A. (1935). The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J. Nutr. 10 (1), 63–79. doi:10.1093/jn/10.1.63
McElhaney, J. E., Verschoor, C. P., Andrew, M. K., Haynes, L., Kuchel, G. A., and Pawelec, G. (2020). The immune response to influenza in older humans: beyond immune senescence. Immun. & Ageing 17 (1), 10. doi:10.1186/s12979-020-00181-1
Meikle, L., Pollizzi, K., Egnor, A., Kramvis, I., Lane, H., Sahin, M., et al. (2008). Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 28 (21), 5422–5432. doi:10.1523/JNEUROSCI.0955-08.2008
Mihaylova, M. M., Chaix, A., Delibegovic, M., Ramsey, J. J., Bass, J., Melkani, G., et al. (2023). When a calorie is not just a calorie: diet quality and timing as mediators of metabolism and healthy aging. Cell metab. 35 (7), 1114–1131. doi:10.1016/j.cmet.2023.06.008
Moel, M., Harinath, G., Lee, V., Nyquist, A., Morgan, S. L., Isman, A., et al. (2025). Influence of rapamycin on safety and healthspan metrics after one year: PEARL trial results. Aging (Albany NY) 17 (4), 908–936. doi:10.18632/aging.206235
Moloney, P. B., Kearney, H., Benson, K. A., Costello, D. J., Cavalleri, G. L., Gorman, K. M., et al. (2023). Everolimus precision therapy for the GATOR1-related epilepsies: a case series. Eur. J. Neurology 30 (10), 3341–3346. doi:10.1111/ene.15975
Papadopoli, D., Boulay, K., Kazak, L., Pollak, M., Mallette, F. A., Topisirovic, I., et al. (2019). mTOR as a central regulator of lifespan and aging. F1000Research 8, F1000 Faculty Rev-998–998. doi:10.12688/f1000research.17196.1
Park, J.-M., Lee, D.-H., and Kim, D.-H. (2023). Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun. 14 (1), 2994. doi:10.1038/s41467-023-38401-z
Park, J. S., Lee, D. H., Lee, Y. S., Oh, E., Bae, K. H., Oh, K. J., et al. (2019). Dual roles of ULK1 (unc-51 like autophagy activating kinase 1) in cytoprotection against lipotoxicity. Autophagy 16 (1), 86–105. doi:10.1080/15548627.2019.1598751
Parker, W. E., Orlova, K. A., Parker, W. H., Birnbaum, J. F., Krymskaya, V. P., Goncharov, D. A., et al. (2013). Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci. Transl. Med. 5 (182), 182ra53. doi:10.1126/scitranslmed.3005271
Peterson, D. E., O'Shaughnessy, J. A., Rugo, H. S., Elad, S., Schubert, M. M., Viet, C. T., et al. (2016). Oral mucosal injury caused by mammalian target of rapamycin inhibitors: emerging perspectives on pathobiology and impact on clinical practice. Cancer Med. 5 (8), 1897–1907. doi:10.1002/cam4.761
Reifsnyder, P. C., Te, A., and Harrison, D. E. (2020). Differential effects of rapamycin on glucose metabolism in nine inbred strains. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 75 (1), 50–57. doi:10.1093/gerona/glz157
Riese, P., Trittel, S., Akmatov, M. K., May, M., Prokein, J., Illig, T., et al. (2022). Distinct immunological and molecular signatures underpinning influenza vaccine responsiveness in the elderly. Nat. Commun. 13 (1), 6894. doi:10.1038/s41467-022-34487-z
Rubinsztein, D. C., Mariño, G., and Kroemer, G. (2011). Autophagy and aging. Cell 146 (5), 682–695. doi:10.1016/j.cell.2011.07.030
Sabatini, D. M. (2006). mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6 (9), 729–734. doi:10.1038/nrc1974
Sabatini, D. M., Erdjument-Bromage, H., Lui, M., Tempst, P., and Snyder, S. H. (1994). RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78 (1), 35–43. doi:10.1016/0092-8674(94)90570-3
Sadowski, K., Sijko, K., Domańska-Pakieła, D., Borkowska, J., Chmielewski, D., Ulatowska, A., et al. (2022). Antiepileptic effect and safety profile of rapamycin in pediatric patients with tuberous sclerosis complex. Front. Neurology 13, 704978. doi:10.3389/fneur.2022.704978
Saxton, R. A., and Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell 168 (6), 960–976. doi:10.1016/j.cell.2017.02.004
Schinaman, J. M., Rana, A., Ja, W. W., Clark, R. I., and Walker, D. W. (2019). Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila. Sci. Rep. 9, 7824. doi:10.1038/s41598-019-44106-5
Spilman, P., Podlutskaya, N., Hart, M. J., Debnath, J., Gorostiza, O., Bredesen, D., et al. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of alzheimer’s disease. PLoS ONE 5 (4), e9979. doi:10.1371/journal.pone.0009979
Swindell, W. R. (2012). Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res. Rev. 11 (2), 254–270. doi:10.1016/j.arr.2011.12.006
Szőke, K., Bódi, B., Hendrik, Z., Czompa, A., Gyöngyösi, A., Haines, D. D., et al. (2023). Rapamycin treatment increases survival, autophagy biomarkers and expression of the anti-aging klotho protein in elderly mice. Pharmacol. Res. & Perspect. 11 (3), e01091. doi:10.1002/prp2.1091
Tabibzadeh, S. (2023). Role of autophagy in aging: the good, the bad, and the ugly. Aging Cell 22 (1), e13753. doi:10.1111/acel.13753
The Economic Times (2025). Has Bryan Johnson’s anti-aging experiment backfired? Biohacker spending $2 million-a-year admits to a costly misstep. Mumbai, India: The Economic Times. Available online at: https://economictimes.indiatimes.com/magazines/panache/has-bryan-johnsons-anti-aging-experiment-backfired-biohacker-spending-2-million-a-year-admits-to-a-costly-misstep/articleshow/120036487.cms?from=mdr (Accessed May 6, 2025).
U.S.Food and Drug Administration (2025). “FDA’s concerns with unapproved GLP-1 drugs used for weight loss,”. Silver Spring, MD, United States FDA. Available online at: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fdas-concerns-unapproved-glp-1-drugs-used-weight-loss (Accessed May 12, 2025).
Vijg, J., and Campisi, J. (2008). Puzzles, promises and a cure for ageing. Nature 454 (7208), 1065–1071. doi:10.1038/nature07216
Wilkinson, J. E., Burmeister, L., Brooks, S. V., Chan, C. C., Friedline, S., Harrison, D. E., et al. (2012). Rapamycin slows aging in mice. Aging cell 11 (4), 675–682. doi:10.1111/j.1474-9726.2012.00832.x
Wray, L., Berwaerts, J., Critchley, D., Hyland, K., Chen, C., Thai, C., et al. (2023). Pharmacokinetic drug-drug interaction with coadministration of cannabidiol and everolimus in a phase 1 healthy volunteer trial. Clin. Pharmacol. Drug Dev. 12 (9), 911–919. doi:10.1002/cpdd.1262
Zeng, L.-H., Xu, L., Gutmann, D. H., and Wong, M. (2008). Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. neurology 63 (4), 444–453. doi:10.1002/ana.21331
Zimmermann, A., Madreiter-Sokolowski, C., Stryeck, S., and Abdellatif, M. (2021). Targeting the mitochondria-proteostasis Axis to delay aging. Front. Cell Dev. Biol. 9, 656201. doi:10.3389/fcell.2021.656201
Keywords: aging, sirolimus, epilepsy, everolimus, mTOR
Citation: Roark KM and Iffland PH II (2025) Rapamycin for longevity: the pros, the cons, and future perspectives. Front. Aging 6:1628187. doi: 10.3389/fragi.2025.1628187
Received: 13 May 2025; Accepted: 05 June 2025;
Published: 20 June 2025.
Edited by:
Jianhua Zhang, University of Alabama at Birmingham, United StatesReviewed by:
Dudley Lamming, University of Wisconsin-Madison, United StatesCopyright © 2025 Roark and Iffland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip H. Iffland II, cGlmZmxhbmRAc29tLnVtYXJ5bGFuZC5lZHU=
 Kelley M. Roark
Kelley M. Roark Philip H. Iffland II
Philip H. Iffland II