- Orthopedic Trauma, China-Japan Friendship Hospital, Beijing, China
Background: Hip fractures are common in elderly patients, with some experiencing contralateral fractures. Even so, information on predictors of hip fractures in elderly adults is lacking. In this study, we investigated risk factors for contralateral hip fractures after surgical treatment of primary fractures.
Methods: This was a prospective cohort study of 115 patients aged ≥65 years with low-energy hip fractures. The clinical parameters evaluated included age, sex, bone mineral density (BMD), T score, and hip flexor strength deficit. Patients were stratified into two groups: those with (n = 12) and those without contralateral fractures (n = 103).
Results: Contralateral fractures occurred in 10.4% of the patients. Logistic regression revealed that age (OR = 1.08), reduced BMD (OR = 0.33), lower T score (OR = 1.45), and hip flexor imbalance (OR = 2.2) were significant predictors.
Conclusion: A multimodal approach that integrates anti-osteoporosis therapy and targeted rehabilitation may reduce contralateral fracture risk in elderly patients.
Introduction
The increasing prevalence of osteoporotic hip fractures in aging populations has drawn significant attention to contralateral hip fractures, which occur in 6.8%–16.0% of patients with osteoporotic hip fractures (Lau et al., 2014; Zidrou et al., 2023; Ryg et al., 2009). While previous research has focused predominantly on demographic and skeletal factors (Lau et al., 2014; Rathbun et al., 2017; Zhao et al., 2024), emerging evidence underscores the importance of biomechanical contributors, particularly postural instability and muscle weakness (Lau et al., 2014; Rathbun et al., 2017; Zhao et al., 2024; Gottschall and Kram, 2005; Akalan et al., 2016). The aim of this study was to combine biomechanical (hip flexor strength) and skeletal (BMD) parameters to identify multifactorial predictors of contralateral fractures. To our knowledge, this is the first prospective cohort study to integrate both biomechanical (hip flexor strength) and skeletal (BMD) parameters in the prediction of contralateral hip fractures.
Study objectives and design
The investigation focused on systematically evaluating biomechanical, biochemical, and functional parameters, including 1) bone quality metrics (BMD and T scores via DXA); 2) neuromuscular functional capacity (hip flexor strength quantified through isokinetic dynamometry); 3) clinical outcomes (Harris Hip Scores); and 4) demographic/operative characteristics. A standardized data collection protocol was implemented across perioperative hospitalization and postdischarge follow-up (6-month intervals) to ensure longitudinal validity.
Materials and methods
Consecutive patients who underwent surgical management for low-energy hip fractures at our Level I Trauma Center (January 2022-December 2023) and met the following criteria were included: 1. Age ≥65 years with low-energy trauma (fall from standing height); 2. Radiographically confirmed intertrochanteric (AO/OTA 31-A; n = 55) or femoral neck fractures (Garden II-IV; n = 60); and 3. Independent ambulation status prior to the fracture. Furthermore, patients were excluded if they met any of the following criteria: nonsurgical candidate, pathological fracture, neurological/musculoskeletal comorbidity, or visual impairment (best-corrected acuity <20/40). Postoperative Protocol: All patients received standardized anti-osteoporosis therapy and procedure-specific rehabilitation. In particular, in cases of PFNA fixation (n = 72), protected weight-bearing was implemented for ≥6 weeks, and in cases of arthroplasty (n = 43), immediate full weight-bearing post quadriceps-strength assessment (MMT grade IV) was implemented.
Ethical compliance statement
This study was approved by the Ethics Committee of China-Japan Friendship Hospital (Approval No. 2023-KY-145), and written informed consent was obtained from all participants. All procedures involving human subjects were conducted in strict accordance with the ethical standards of the Declaration of Helsinki (2013 revision), the International Council for Harmonisation (ICH) Guidelines for Good Clinical Practice (GCP), and institutional review board (IRB) policies. Patient confidentiality and data anonymity were rigorously maintained throughout the study.
Outcome assessment
Operative protocol
Surgical procedures followed standard protocols for intertrochanteric fractures (PFNA fixation) and femoral neck fractures (hemiarthroplasty/THA).
Postoperation protocol
All enrolled patients were routinely administered analgesics, anticoagulants and anti-osteoporosis medications according to standardized protocols. On postoperative day 2, targeted rehabilitation exercises focusing on quadriceps femoris activation, with particular emphasis on the vastus medialis oblique (VMO), were initiated. Patients were permitted to commence partial weight-bearing ambulation with full assistive support only after achieving grade IV muscle strength in the quadriceps femoris, as assessed by manual muscle testing (MMT).
Follow-up
At the 6-month postoperative follow-up, comprehensive functional assessments were performed. Hip abductor function was evaluated using the standardized Trendelenburg test (Hardcastle and Nade, 1985), with positive findings (inability to maintain pelvic alignment during a single-leg stance), resulting in study exclusion. Hip flexor strength, represented by peak torque (PT), was measured using the BIODEX SYSTEM 4 PRO (Biodex Medical Systems, Inc., Shirley, NY) under (Gade et al., 2021) the supervision of licensed physical therapists. The testing protocol involved a unilateral stance with rapid contralateral leg elevation, measuring peak torque (ft-lb) and automatically calculating torque deficit percentages. Deficit severity was categorized as follows: 1) 1%–10%, within normal limits; 2) 11%–20%, indicating the need for rehabilitation; and 3) >20%, representing significant functional impairment. All measurements were recorded immediately postassessment to ensure data integrity and reliability.
Study groups
The study population was stratified into two comparative cohorts on the basis of fracture characteristics: Group A (n = 12) consisted of patients presenting with contralateral hip fractures, and Group B (n = 103) comprised patients without contralateral involvement. A comprehensive comparative analysis was conducted between these cohorts. The variables in this analysis comprised multiple demographic and clinical parameters, including but not limited to age distribution, sex ratio, fracture classification according to the AO/OTA system, bone mineral density (BMD) in the hip region, T scores derived from dual-energy X-ray absorptiometry (DEXA) scans, length of hospital stay, Harris Hip Score (HHS) for functional assessment, and isokinetic measurements of hip flexor peak torque with corresponding deficit calculations.
Statistical analysis
The sample size of 115 was determined on the basis of a power analysis (α = 0.05, β = 0.20) to detect a 15% difference between groups. All the statistical analyses were performed using PASW Statistics 21.0 software (IBM Corp., Armonk, NY, United States). Intergroup comparisons between Group A and Group B were conducted for multiple variables. Continuous variables, including age, time interval from injury to surgical intervention, length of hospital stay, bone mineral density (BMD), T score, Harris hip score (HHS), and isokinetic measurements of hip flexor peak torque (with associated deficit values), were analyzed using independent samples t tests following the confirmation of a normal distribution by means of Shapiro–Wilk tests. Categorical variables, such as sex distribution and fracture classification, were evaluated using Pearson’s chi-square (χ2) test or Fisher’s exact test, as appropriate. Missing data, which accounted for less than 5% of the total dataset, were excluded from the analysis under the assumption of being missing completely at random (MCAR). Variables demonstrating statistically significant differences (p < 0.05) in the univariate analysis were subsequently incorporated into multivariate logistic regression models to identify independent predictors. A two-tailed p value of <0.05 was considered statistically significant for all analyses.
Results
Consecutive patients (N = 115) enrolled while 3 patients were excluded due to their request to withdraw consent. Contralateral fractures occurred in 10.4% of the patients (12/115) at a mean latency of 14.3 ± 3.8 months. Compared with the patients in Group B, those in Group A (contralateral fractures) were significantly older (84.3 vs. 79.1 years, p = 0.015), had a lower BMD (0.68 vs. 0.77 g/cm2, p = 0.021), and had higher proportion of cases of hip flexor deficit (22.5% vs. 14.6%, p = 0.032) (Table 1). Multivariate analysis revealed that age, T score, BMD, and hip flexor deficit were independent risk factors (Table 3).
Postoperative outcomes and fracture epidemiology
The mean hospitalization duration was 8.7 ± 3.2 days (range: 3–22) in the prospective cohort, and the injury-to-surgery interval was 1.8 ± 0.9 days (range: 1–3), with no intergroup differences (p > 0.05). Systematic follow-up over 6–20 months revealed a contralateral fracture incidence of 10.4% (12/115), indicating a balanced sex ratio, comprising 6 males and 6 females, representing an equal proportion (50%) in Group A. Fracture patterns were comparable between intertrochanteric (10.9%, 6/55) and femoral neck fractures (10.0%, 6/60). Contralateral hip fractures occurred at a mean latency of 14.3 ± 3.8 months postoperatively (range: 9–20).
Surgical and rehabilitation protocols
All patients received standardized anti-osteoporosis therapy (calcium/alfacalcidol) and procedure-specific rehabilitation:
• PFNA fixation (n = 72): Protected weight-bearing for ≥6 weeks with progressive quadriceps strengthening.
• Arthroplasty (n = 43): Immediate full weight-bearing upon achieving MMT grade IV quadriceps strength. Postoperative hip mobility was restricted to −10°–90° during the first month, supplemented by supervised isometric exercises initiated within 24 h postoperatively.
Comparative analysis of risk factors
Univariate analysis revealed significant disparities between the contralateral fracture (Group A, n = 12) and control (Group B, n = 103) cohorts (Table 1).
No significant differences were observed between Group A and Group B in terms of sex, length of hospital stay, fracture type, Harris score, or involved hip flexor peak torque (Table 2).
The logistic regression analysis revealed that age, lower T scores, reduced BMD, and greater hip flexor deficit were significant risk factors for contralateral hip fractures. These findings highlight the importance of addressing both skeletal and biomechanical factors in the prevention and management of contralateral hip fractures in elderly patients (Table 3). Although univariate analysis suggested a trend toward higher contralateral fracture rates in males (33.3% vs. 28.2%), this association did not reach statistical significance in multivariate modeling (p = 0.603).
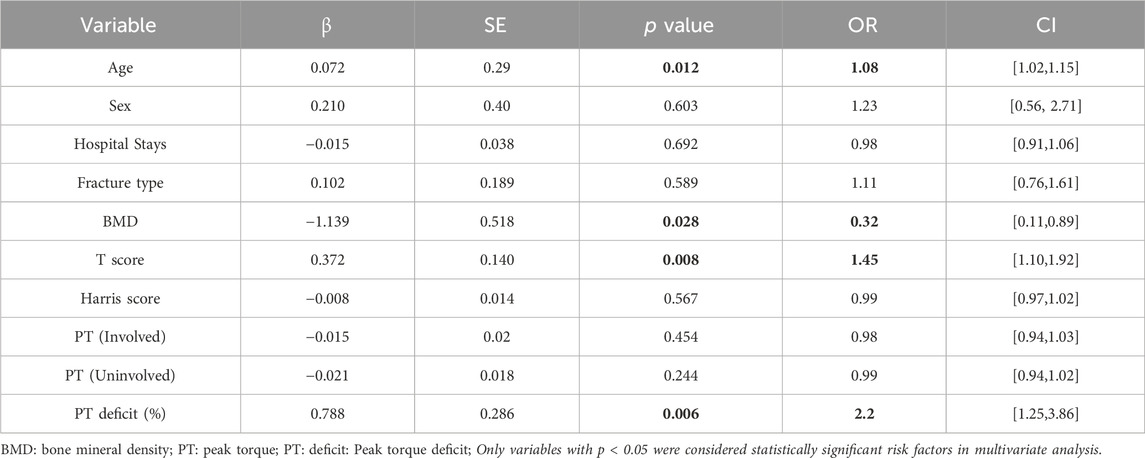
Table 3. Multivariate logistic regression analysis of independent risk factors for contralateral hip fractures.
Kaplan-Meier analysis revealed a cumulative contralateral fracture incidence of 10.4% at 20 months (Figure 1).
The Kaplan-Meier curve illustrates the cumulative incidence of contralateral hip fractures over a 20-month follow-up period in elderly patients following surgical treatment of primary hip fractures. The x-axis represents time in months, and the y-axis shows the cumulative survival probability (i.e., the proportion of patients without contralateral fractures). The curve demonstrates a gradual decline in survival probability, with a cumulative incidence of 10.4% at 20 months. Censored data points (indicated by “+” symbols) represent patients who experienced a contralateral hip fracture. This analysis highlights the temporal pattern of contralateral fracture risk, emphasizing the need for extended monitoring and secondary prevention strategies beyond the first-year post-surgery.
Factors associated with contralateral hip fractures
Bone mineral density (BMD) measurements revealed a mean of 0.68 ± 0.11 in Group A and 0.77 ± 0.09 in Group B (t = 2.15, p = 0.03), whereas T scores of −3.1 ± 0.8 in Group A and −2.0 ± 0.7 in Group B indicated more severe osteoporosis in patients with contralateral fractures (t = 4.94, p < 0.001). The musculoskeletal assessment revealed that the percent deficit in hip flexor strength was significantly greater in Group A (22.5 ± 5.3) than in Group B (14.6 ± 4.1); t = −2.01, p = 0.047), suggesting greater bilateral muscle imbalance in patients with contralateral fractures.
Discussion
The synergistic effect of advanced age and reduced BMD underscores the critical role of osteoporosis management in contralateral fracture prevention (Leslie and Morin, 2014; Gregg et al., 2000; Ryg et al., 2009), as evidenced by our findings (Group A: BMD 0.68 vs. Group B: 0.77 g/cm2, p = 0.03). A lower T score (−3.1 vs. −2.0, p < 0.001) aligns with prior evidence that age-related bone loss exacerbates fracture risk (Fujita et al., 2022). The mean latency period of 14.3 months postoperatively highlights a critical window for secondary prevention, potentially linked to delayed bone remodeling and altered weight-bearing mechanics (Ramisetty et al., 2015; Rathbun et al., 2017). Extended follow-up protocols beyond 12 months may improve monitoring efficacy. Notably, hip flexor strength imbalance (OR = 2.2) emerged as a stronger biomechanical predictor, suggesting targeted rehabilitation to mitigate gait instability and fall risk (Fujita et al., 2022; Gade et al., 2021; Akalan et al., 2016).
The findings of this study provide critical insights into the multifactorial nature of contralateral hip fractures in elderly patients, highlighting several key areas for clinical consideration and future research.
Age/BMD interaction
The significant correlation between advanced age and reduced bone mineral density (BMD) underscores the synergistic effect of these factors in increasing contralateral hip fracture risk (Leslie and Morin, 2014; Gregg et al., 2000; Ryg et al., 2009; Zhao et al., 2024; Vochteloo et al., 2012; Fujita et al., 2022). Our data show that in patients with contralateral fractures, compared with those of the controls, the BMD values of the patients are significantly lower (0.68 ± 0.11 g/cm2), and the mean age of the patients is significantly higher (84.3 ± 5.1 years). This finding aligns with those reported in the literature indicating that age-related bone loss exacerbates fracture susceptibility, particularly in the proximal femur (Leslie and Morin, 2014; Gregg et al., 2000; Ryg et al., 2009; Fujita et al., 2022). The observed T score disparity (−3.1 ± 0.8 vs. −2.0 ± 0.7, p < 0.001) further emphasizes the need for aggressive osteoporosis management in this population.
Temporal pattern of contralateral fractures
The mean latency period of 14.3 ± 3.8 months for contralateral fractures suggests a critical window for secondary prevention (Zhu et al., 2014). This temporal pattern may reflect the combined effects of postsurgical bone remodeling, altered weight-bearing mechanics, and potential delays in achieving optimal BMD through pharmacological intervention (Zhao et al., 2024; Vochteloo et al., 2012). The clustering of contralateral hip fractures between 9 and 20 months postoperatively warrants the consideration of extended monitoring protocols beyond the conventional 12-month follow-up period (Ryg et al., 2009; Zhao et al., 2024; Vochteloo et al., 2012; Fujita et al., 2022).
Biomechanical factors
The significantly greater hip flexor peak torque deficit in the contralateral fracture group (22.5% ± 5.3% vs. 14.6% ± 4.1%, p = 0.047) highlights the biomechanical consequences of muscular imbalance. This deficit may contribute to altered gait patterns and increased fall risk, creating a vicious cycle of instability and fracture susceptibility (OR = 2.2) (Zhao et al., 2024; Fujita et al., 2022; Zhu et al., 2014). The integration of targeted hip flexor strengthening into rehabilitation protocols may mitigate these risks (Fujita et al., 2022; Methenitis et al., 2016).
Protocol-specific rehabilitation outcomes
Our protocol-specific approach, which differentiates between PFNA fixation and arthroplasty patients, demonstrated the importance of tailored rehabilitation strategies (Kahn et al., 2013; Zhao et al., 2024; Vochteloo et al., 2012; Fujita et al., 2022; Parker and Handoll, 2010; Bhandari et al., 2003). The immediate weight-bearing protocol for arthroplasty patients, contingent upon achieving MMT grade IV quadriceps strength, appeared to facilitate earlier functional recovery without compromising surgical outcomes (Fujita et al., 2022; Bhandari et al., 2003). Conversely, the protected weight-bearing regimen for PFNA patients likely contributed to the observed fracture consolidation rates (Zhao et al., 2024; Parker and Handoll, 2010; Bhandari et al., 2003; Zhu et al., 2014).
Multivariate regression analysis of risk factors
Preliminary multivariate analysis revealed three independent predictors of contralateral fracture risk (Zhao et al., 2024; Zhu et al., 2014): age ≥80 years (OR = 1.08, 95% CI: 1.02, 1.15), BMD≤0.5 (OR = 0.32, 95% CI: 0.11, 0.89), T score ≤ −2.5 (OR = 1.45, 95% CI: 1.10–1.92), and hip flexor deficit ≥20% (OR = 2.2, 95% CI: 1.25–3.86). These findings underscore the multifactorial nature of contralateral hip fracture risk and the need for comprehensive risk assessment tools (Leslie and Morin, 2014; Zidrou et al., 2023; Ryg et al., 2009; Zhao et al., 2024; Fujita et al., 2022; Kanis et al., 2008; Compston et al., 2017).
Longitudinal analysis of functional recovery
The Harris Hip Score (HHS), while widely used, has notable limitations, including subjectivity in patient-reported outcomes, insensitivity to subtle functional changes (Ramisetty et al., 2015; Wamper et al., 2010), and inadequate assessment of physical activity levels, particularly muscle strength (Wamper et al., 2010). These limitations underscore the need for complementary assessment tools in high-risk populations. The lack of significant HHS differences between groups (Table 2) likely reflects its limited sensitivity for detecting subtle functional deficits, as previously documented (Ramisetty et al., 2015; Wamper et al., 2010). Consequently, researchers and clinicians should consider alternative or supplementary scoring systems when evaluating hip-related outcomes.
Subgroup analysis based on fracture classification
The comparable contralateral fracture rates between intertrochanteric (10.9%) and femoral neck fractures (10.0%) suggest that fracture morphology may be less predictive of contralateral fracture risk than systemic factors such as BMD and muscular function. However, the small subgroup sizes and single-center study design limit definitive conclusions, warranting larger-scale investigations (Zhao et al., 2024; Vochteloo et al., 2012). Potential measurement errors in hip flexor strength assessment and a follow-up period limited to 20 months, which may limit generalizability.
Clinical implications
These findings not only advance risk stratification for contralateral hip fractures but also highlight broader implications for geriatric musculoskeletal health. The interplay between muscle imbalance and bone fragility may extend to other fragility fractures (e.g., vertebral or distal radius fractures), suggesting a unified framework for sarcopenia-osteoporosis management in aging populations. Lack of appropriate physical therapy after the first fracture is a significant risk factor for shorter intervals between first and second hip fractures in geriatric patients (Saglam et al., 2024). Furthermore, integrating biomechanical biomarkers (Antico et al., 2012) (e.g., muscle deficit quantification) with systemic inflammatory profiles could catalyze interdisciplinary collaborations spanning orthopedics, geriatrics, and rehabilitation medicine. To bridge current evidence gaps (Koot et al., 1996; Salo and Logomarsino, 2011; Liu, 2014; Methenitis et al., 2016), future research should prioritize three avenues: 1) cost-effectiveness analyses of population-level osteoporosis screening informed by fracture biomechanical risk, 2) development of AI-driven predictive models that synthesize radiological, biochemical, and functional mobility data, and 3) clinical trials evaluating whether neuromuscular re-education protocols—successful in stroke rehabilitation—can be adapted to mitigate post-fracture imbalance. Such translational efforts may redefine preventive care paradigms beyond traditional bone-centric approaches.
Data availability statement
The datasets generated and/or analyzed during the current study are available in the figshare repository, https://doi.org/10.6084/m9.figshare.28620911. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of China-Japan Friendship Hospital (Approval No. 2023-KY-145). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HY: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review and editing. YY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review and editing. RG: Data curation, Methodology, Software, Validation, Writing – review and editing. LS: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. FS: Writing – review and editing. YC: Funding acquisition, Project administration, Resources, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National High Level Hospital Clinical Research Funding (No. 2023-NHLHCRF-YYPPLC-ZR-12), the Elite Medical Professionals Project of China-Japan Friendship Hospital (NO. ZRJY2023-QM29), and the China-Japan Friendship Hospital Self-selected Project (NO. 2023-HX-46).
Acknowledgments
We thank the staff of China-Japan Friendship Hospital for their assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akalan, N. E., Kuchimov, S., Apti, A., Temelli, Y., and Nene, A. (2016). Weakening iliopsoas muscle in healthy adults May induce stiff knee pattern. Acta Orthop. Traumatol. Turc 50 (6), 642–648. doi:10.1016/j.aott.2016.03.007
Antico, A., Tampoia, M., Tozzoli, R., and Bizzaro, N. (2012). Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 12 (2), 127–136. doi:10.1016/j.autrev.2012.07.007
Bhandari, M., Devereaux, P. J., Swiontkowski, M. F., Tornetta, P., Obremskey, W., Koval, K. J., et al. (2003). Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. JBJS Am. 85 (9), 1673–1681. doi:10.2106/00004623-200309000-00004
Compston, J., Cooper, A., Cooper, C., Gittoes, N., Gregson, C., Harvey, N., et al. (2017). UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 12 (1), 43. doi:10.1007/s11657-017-0324-5
Fujita, T., Takegami, Y., Ando, K., Sakai, Y., Nakashima, H., Takatsu, S., et al. (2022). Risk factors for second hip fracture in elderly patients: an age, sex, and fracture type matched case-control study. Eur. J. Orthop. Surg. Traumatol. 32 (3), 437–442. doi:10.1007/s00590-021-02996-0
Gade, G. V., Jørgensen, M. G., Ryg, J., Masud, T., Jakobsen, L. H., and Andersen, S. (2021). Development of a multivariable prognostic prediction model for 1-year risk of falling in a cohort of community-dwelling older adults aged 75 years and above (PREFALL). BMC Geriatr. 21 (1), 402. doi:10.1186/s12877-021-02346-z
Gottschall, J. S., and Kram, R. (2005). Energy cost and muscular activity required for leg swing during walking. J. Appl. Physiol. 99 (1), 23–30. doi:10.1152/japplphysiol.01190.2004
Gregg, E. W., Pereira, M. A., and Caspersen, C. J. (2000). Physical activity, falls and fractures among older adults: a review of the epidemiologic evidence. J. Am. Geriatr. Soc. 48 (8), 883–893. doi:10.1111/j.1532-5415.2000.tb06884.x
Hardcastle, P., and Nade, S. (1985). The significance of the Trendelenburg test. J. Bone Jt. Surg. Br. 67-B (5), 741–746. doi:10.1302/0301-620X.67B5.4055873
Kahn, S. K., Rushton, S. P., Dosani, A., Gray, A. C., and Deehan, D. J. (2013). Factors influencing length of stay and mortality after first and second hip fractures: an event modeling analysis. J. Orthop. Trauma 27 (2), 82–86. doi:10.1097/BOT.0b013e3182519114
Kanis, J. A., Johnell, O., Oden, A., Johansson, H., and McCloskey, E. (2008). FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19 (4), 385–397. doi:10.1007/s00198-007-0543-5
Koot, V. C. M., Kesselaer, S. M., Clevers, G. J., de Hooge, P., Weits, T., and van der Werken, C. (1996). Evaluation of the Singh index for measuring osteoporosis. JBJS Br. 78 (5), 831–834. doi:10.1302/0301-620X.78B5.0780831
Lau, J. C., Ho, K. W., and Sadiq, S. (2014). Patient characteristics and risk of subsequent contralateral hip fracture after surgical management of first fracture. Injury 45 (10), 1620–1623. doi:10.1016/j.injury.2014.05.030
Leslie, W. D., and Morin, S. N. (2014). Osteoporosis epidemiology 2013: implications for diagnosis, risk assessment, and treatment. Curr. Opin. Rheumatol. 26 (4), 440–446. doi:10.1097/BOR.0000000000000064
Liu, S. (2014). Risk factors for the second contralateral hip fracture in elderly patients: a systematic review and meta-analysis. Clin. Rehabil. 28 (7), 15–16. doi:10.1177/0269215514527593
Methenitis, S. K., Zaras, N. D., Spengos, K. M., Stasinaki, A. N. E., Karampatsos, G. P., Georgiadis, G. V., et al. (2016). Role of muscle morphology in jumping, sprinting, and throwing performance in participants with different power training duration experience. J. Strength Cond. Res. 30 (3), 807–817. doi:10.1519/JSC.0000000000001147
Parker, M. J., and Handoll, H. H. (2010). Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst. Rev. (9), CD000093. doi:10.1002/14651858.CD000093.pub5
Ramisetty, N., Kwon, Y., and Mohtadi, N. (2015). Patient-reported outcome measures for hip preservation surgery-a systematic review of the literature. J. Hip Preserv Surg. 2 (1), 15–27. PMID: 27011811; PMCID: PMC4718480. doi:10.1093/jhps/hnv002
Rathbun, A. M., Magaziner, J., Shardell, M. D., Orwig, D. L., Sterling, R., Hawkes, W., et al. (2017). Older men who sustain a hip fracture experience greater declines in bone mineral density at the contralateral hip than non-fracture comparators. Osteoporos. Int. 28 (10), 2741–2748. doi:10.1007/s00198-017-4280-0
Ryg, J., Rejnmark, L., Overgaard, S., Brixen, K., and Vestergaard, P. (2009). Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J. Bone Min. Res. 24 (7), 1299–1307. doi:10.1359/jbmr.090207
Saglam, S., Arican, M., Karaduman, Z. O., Yucel, M. O., Degirmenci, E., and Uludag, V. (2024). The characteristics and outcomes of contralateral non-concurrent hip fractures: a retrospective Study in geriatric patients. Med. Kaunas. 60 (6), 928. doi:10.3390/medicina60060928
Salo, A., and Logomarsino, J. V. (2011). Relationship of vitamin D status and cardiometabolic risk factors in children and adolescents. Pediatr. Endocrinol. Rev. 9 (1), 456–462. PMID: 22423523.
Vochteloo, A. J., Borger van der Burg, B. L., Röling, M. A., van Leeuwen, D. H., van den Berg, P., Niggebrugge, A. H. P., et al. (2012). Contralateral hip fractures and other osteoporosis-related fractures in hip fracture patients: incidence and risk factors. An observational cohort study of 1,229 patients. Arch. Orthop. Trauma Surg. 132 (8), 1191–1197. doi:10.1007/s00402-012-1520-9
Wamper, K. E., Sierevelt, I. N., Poolman, R. W., Bhandari, M., and Haverkamp, D. (2010). The Harris hip score: do ceiling effects limit its usefulness in orthopedics? Acta Orthop. 81 (6), 703–707. PMID: 21110703; PMCID: PMC3216080. doi:10.3109/17453674.2010.537808
Zhao, L., Tian, S., Sha, W., Wang, L., and Xu, Y. (2024). Analysis of the clinical characteristics and risk factors associated with contralateral hip fracture after initial hip fracture in elderly patients: a retrospective cohort study. Sci. Rep. 14 (1), 14292. doi:10.1038/s41598-024-65165-3
Zhu, Y., Chen, W., Sun, T., Zhang, Q., Liu, S., and Zhang, Y. (2014). Epidemiological characteristics and outcome in elderly patients sustaining non-simultaneous bilateral hip fracture: a systematic review and meta-analysis. Geriatr. Gerontol. Int. 14 (3), 11–18. doi:10.1111/ggi.12368
Keywords: hip fracture, osteoporosis, bone mineral density, muscle imbalance, rehabilitation, geriatric trauma
Citation: Yang H, Yuan Y, Ge R, Shi L, Si F and Chen Y (2025) Risk factors and predictors of contralateral hip fracture after surgical treatment in elderly patients. Front. Aging 6:1633184. doi: 10.3389/fragi.2025.1633184
Received: 22 May 2025; Accepted: 06 August 2025;
Published: 18 August 2025.
Edited by:
You Shilong, China Medical University, ChinaReviewed by:
Daniela Burguêz, Hospital São Lucas da PUCRS, BrazilSönmez Sağlam, Duzce Universitesi Tip Fakultesi, Türkiye
Copyright © 2025 Yang, Yuan, Ge, Shi, Si and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Chen, Y3l6cnloQDE2My5jb20=
 Huan Yang
Huan Yang Yusong Yuan
Yusong Yuan Ruidong Ge
Ruidong Ge

