- 1College of Sport Sciences, Qufu Normal University, Qufu, Shandong, China
- 2College of Rehabilitation Sciences, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 3Central Lab, Shanghai Key Laboratory of Pathogenic Fungi Medical Testing, Shanghai Pudong New Area People’s Hospital, Shanghai, China
Abstract: Alzheimer’s disease (AD), the most commonly diagnosed form of senile dementia worldwide, is closely associated with aging and distinct neuropathological features. Recent studies highlight that up to 90% of individuals, either preclinical or clinical, diagnosed with vascular pathology in the context of AD exhibit thickening and hyalinization of the media in small and medium-sized cerebral vessels. Exercise has emerged as a potential, non-pharmaceutical, and cost-effective intervention for the prevention and treatment of AD. However, there is limited research exploring the effects of high-intensity interval training (HIIT) on cerebrovascular function in AD.
Methods: Four-month-old female C57BL/6 J mice and APP/PS1 transgenic mice were initially acclimated to a standard diet for 1 week. The two groups were then divided into sedentary and exercise cohorts, with the exercise group engaging in a 6-week HIIT regimen. Post-intervention, hippocampal specimens were collected for analysis. Aβ and Tau protein levels were measured to assess AD pathology, while cognitive function was evaluated using the eight-arm radial maze and BDNF mRNA expression. Additionally, markers of cerebrovascular function-including VEGF, EPO, eNOS, GPR68, and ET-1-were examined, and HIF-1α was utilized to assess the hippocampal response to AD pathology.
Results: HIIT significantly reduced reference memory errors (p = 0.025) and markedly upregulated Bdnf mRNA expression (p < 0.001) specifically in APP/PS1 mice. Furthermore, HIIT significantly decreased protein levels of AD pathological markers p-TAU (p = 0.001) and APP (p = 0.002) in APP/PS1 mice. HIIT significantly increased the mRNA (p < 0.001) and protein (p = 0.003) levels of EPO and Vegfa mRNA (p < 0.001) levels to stimulate pro-angiogenic signal in APP/PS1 mice. HIIT also significantly increased both the mRNA and proteins levels of eNOS expression (p < 0.001) while decreasing the mRNA and proteins levels of ET-1 (p < 0.001) and GPR68 (p < 0.001) to enhance vasodilation in APP/PS1 mice. Finally, HIIT significantly increased HIF-1α expression at both protein and mRNA levels (p < 0.001), independent of genotype.
Conclusion: HIIT ameliorates cognitive function and reduces hallmark AD pathology. This positive effect is potentially mediated through cerebral microangiogenesis, cerebrovascular function regulation, and hypoxic metabolism. HIIT represents a promising non-pharmacological strategy for targeting multiple aspects of AD pathophysiology.
1 Introduction
Alzheimer’s disease (AD) is the most common cause of dementia globally, with its prevalence increasing alongside global aging (Weller and Budson, 2018). This progressive neurodegenerative disease is characterized by memory loss and multiple cognitive impairments. In the earliest phases of AD, accumulated amyloid β (Aβ) deposits on the walls of cerebral vasculature, coupled with the spread of tau pathology, are observed (Scheltens et al., 2021; Wójtowicz et al., 2020). A promising treatment strategy in clinical trials is poised to develop the anti-amyloid β, anti-tau, and anti-inflammatory targeted drugs (Scheltens et al., 2021). As individuals age, central arterial stiffness and increased arterial pulsation are associated with higher cerebrovascular resistance, reduced cerebral blood flow, and cerebrovascular dysfunction, which are major risk factors for dementia (Tomoto and Zhang, 2024). Notably, 80%–90% of preclinical or clinical people have been diagnosed cerebral vascular amyloidosis (CAA) via neuroimaging technique (Dorner et al., 2024; Charidimou et al., 2022), characterized as one of the biomarkers of AD, before the development of classical significant cognitive decline (Graff-Radford et al., 2021). In the rodent AD model, previous research has revealed that early morphological abnormalities (Zhang et al., 2018; Lifshitz et al., 2012) and microvascular degeneration spread in the subregions of the hippocampus and cortex via the whole-brain analysis of the angioarchitecture (Quintana et al., 2021). Furthermore, declines in regional cerebral blood flow (CBF) and diminished functional and structural connectivity have been observed, both linked to cerebrovascular function (Wiesmann et al., 2017). Evidence suggests that vascular and endothelial dysfunctions play critical roles in the progression of AD, significantly exacerbating underlying neurodegenerative processes (Palmer et al., 2025; Stelzer and Lima-Filho, 2024).
Erythropoietin (EPO) and vascular endothelial growth factor (VEGF) are two vasoactive molecules crucial for brain development due to their trophic effects. These molecules respond to neuronal damage and confer neuroprotective and neurorestorative benefits to the central nervous system, and mitigate brain injury (Urena-Guerrero et al., 2020; Shim and Madsen, 2018). In the context of the brain, these molecules primarily contribute to cell survival, neural regeneration, neurogenesis, and vascular remodeling (Shibuya, 2014; Kaur et al., 2022), suggesting potential novel therapeutic targeted strategies for AD treatment. Endothelial nitric oxide synthase (eNOS) is a calmodulin-dependent enzyme that produces nitric oxide (NO). This signaling molecule plays a crucial role in neuronal survival, synaptic plasticity, vascular smooth muscle relaxation, and endothelial cell permeability in the brain (de la Monte et al., 2000). A deficiency of eNOS can damage the blood-brain barrier (BBB) and cause insufficient mild chronic perfusion, which resulted in cerebrovascular injury in middle-aged mice (Chen X. et al., 2022). G-protein coupled receptor 68 (GPR68), also known as OGR1, functions as a cellular sensor of acidification (Ludwig et al., 2003). It has been reported to be expressed in endothelial cells of small-diameter arteries and serves as the flow sensor (Xu et al., 2018). It is also expressed in the human aortic smooth muscle cells to regulate the signaling of cAMP-cyclooxygenase-2 (COX2)-prostaglandin I2 (PGI2) for vasodilatation (Sisignano et al., 2021). Endothelin-1 (ET-1) is widely distributed in microvascular endothelial cells, neurons, and glial cells in the central nervous system (CNS) (Li et al., 2018). It was identified as the most potent vasoconstrictor peptide with proliferative, prooxidative, and proinflammatory properties (Kuwaki et al., 1997), which reduces CBF (Chen et al., 2013).
Hypoxia-inducible factor-1α (HIF-1α) is a nuclear transcription factor that responds to oxygen availability by binding to hypoxia response elements (HREs) and activating downstream transcription factors (Yang et al., 2022), such as EPO, VEGF, eNOS, GPR68, and ET-1. These factors promote erythropoiesis, angiogenesis, and vasodilation to overcome hypoxia (McLaren et al., 2007). Regarding the regulation of vascular factors, HIF bounds to EPO 5’ hypoxic response element, and EPO gene transcription increases under the condition of anemia or hypoxia (Shih et al., 2018). Inhibiting or interfering with the activation of HIF-1α reduces the expression of VEGF at both the protein and mRNA levels from 6 h onwards (Wang et al., 2019). Transient transfection of HIF-1α in the endothelial cells was reported to result in a 2.7-fold increase in NOS promoter activity (Palmer et al., 1998). ET-1 is a major contributor to vascular inflammatory remodeling, likely through HIF-1-dependent activation of NF-κB (Singh and Prakash, 2017). Under hypoxia, HIF-1α can bind to the promoter of GPR81 to increase its activity (de Valliere et al., 2016). Therefore, HIF-1α may be a critical molecule for regulating these factors and cerebral vascular function in AD.
Exercise has been shown to induce beneficial changes in cerebrovascular health (Nishijima et al., 2016; Whitaker et al., 2020). It enhances cerebral blood oxygen supply, improves antioxidant signaling pathways, and increases cerebral blood flow (CBF), thereby potentially enhancing synaptic plasticity and cognition for the treatment and prevention of AD (Bliss et al., 2021; De la Rosa et al., 2020). High-intensity interval training (HIIT) is an exercise pattern of alternating periods of high-intensity aerobic exercise with light recovery exercise or no exercise (Taylor et al., 2019), which is superior to moderate-intensity continuous training (MICT) in cardiometabolic processes and physical performance (Gripp et al., 2021; Miguet et al., 2020). Recent evidences have shown the beneficial effect HIIT on the AD -like pathology. For instance, it promoted Aβ and p-Tau clearance by regulating astrocytic metabolism (Feng et al., 2023) and mitochondrial energy metabolism (Liu et al., 2022). In the aspect of cerebrovascular function regulation, acute HIIT was reported to increase the de/oxygenated hemoglobin and cerebrovascular reactivity and decrease the middle cerebral artery blood and velocity, thereby improving dynamic cerebral autoregulation (Whitaker et al., 2020; Komiyama et al., 2020). Several research also indicate that HIIT better maintains post-exercise CBF and velocity compared to MICT, supporting sustained brain function (Coates et al., 2023; Whitaker et al., 1985). Furthermore, HIIT stimulates cerebral microangiogenesis in wild-type mice (Morland et al., 2017), enhancing cerebrovascular plasticity. Collectively, these findings suggest HIIT may reduce cerebrovascular risk, although little research has reported the role of HIIT in CAA no matter to say the AD-related cerebral pathology. However, the mechanism links between HIIT and cerebrovascular protection remains poorly understood.
In this study, vascular-related factors were explored in the hippocampus to test the hypothesis that HIIT mitigates AD-related pathology by enhancing cerebral microvascular function, with a focus on molecules of angiogenesis (EPO and VEGF), cerebrovascular regulation (eNOS, ET-1, and GPR68), and hypoxic adaptation (HIF-1α).
2 Materials and methods
2.1 Animals
28 female 4-month-old C57BL/6J and APP/PS1 mice, weighing 20–23 g, were procured from the Shanghai south model organism (China). After 1 week of acclimation the mice were randomly divided into a control group (n = 7), APP/PS1 group (n = 7), control + HIIT group (n = 7), and APP/PS1 + HIIT group (n = 7). Random numbers were generated using the standard = RAND () function in excel. three to four mice per group were housed in IVC cages under controlled conditions (22 C–24 C, 12:12 light-dark cycle) with ad libitum access to food and water, supplemented with environmental enrichment. Daily welfare monitoring included assessments of body weight, fur condition, behavior, and social interactions, with predefined humane endpoints (>20% weight loss, severe lethargy, or inability to eat/drink). Following the last behavioral testing, mice were humanely euthanized via sodium pentobarbital (150 mg/kg i.p., in 0.9% saline). The hippocampus was rapidly dissected on a pre-cooled crushed ice box, following standard procedures, and then stored at −80 C. The study complied with ARRIVE Guidelines 2.0, the Institutional Animal Ethics Committee (IAEC), and the Ethical Committee for Science Research at Qufu Normal University (Approval No. 2020-0001). The suffering and number of animals used was minimized without compromising the power of the experimental design.
2.2 HIIT paradigm
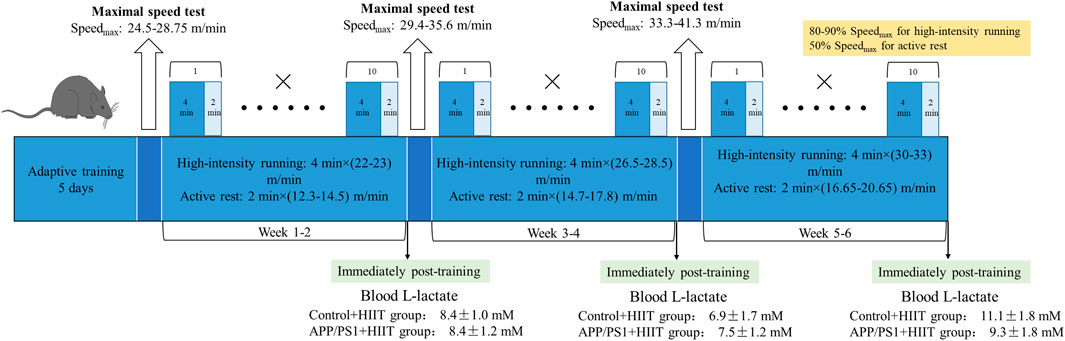
Mice were familiarized with five bouts of treadmill running (4 min × (20-22) m/min) and active rest (2 min × 10 m/min) for 5 days and accepted HIIT training. After familiarization, the mice completed a 6-week HIIT. They were exposed to the HIIT exercise protocol for five consecutive days each week. This exercise regime has been previously described (Hu et al., 2021). Each session consisted of warm-up (10 min × 10 m/min), followed by 10 bouts of 4 min high-intensity running (4 min × (80-90)% Speedmax), and separated by active rest (2 min × 50 % Speedmax). Running took place on a treadmill at a 0°. The maximal speed test for mice was respectively performed at the end of adaptive training, the second and the fourth week of training, and the speed of interval training. The beginning speed of mice was 10 m/min for lasting 10 min to warm up. Then the speed was increased by 2 m/min every 2 min until exhaustion. In the first and second weeks, the HIIT group completed warm-up (10 min × 10 m/min) and then 10 bouts of high-intensity running (4 min × (22-23) m/min), and separated by active rest (2 min × (12.3-14.5) m/min) (the blood L-lactate was 8.4 ± 1.0 mM in control + HIIT group and 8.4 ± 1.2 mM in APP/PS1 + HIIT group immediately post-training). In the third and fourth weeks, the HIIT group completed warm-up and then 10 bouts of high-intensity running (4 min × (26.5-28.5) m/min), and separated by active rest (2 min × (14.7-17.8) m/min) (the blood L-lactate was 6.9 ± 1.7 mM in control + HIIT group and 7.5 ± 1.2 mM in APP/PS1+HIIT group immediately post-training). In the fifth and sixth weeks, the HIIT group completed warm-up and then 10 bouts of high-intensity running (4 min × (30-33) m/min), separated by active rest (2 min × (16.65-20.65) m/min) (the blood L-lactate was 11.1 ± 1.8 mM in control + HIIT group and 9.3 ± 1.8 mM in APP/PS1 + HIIT group immediately post-training) (Figure 1).
2.3 Eight-arm radial maze test
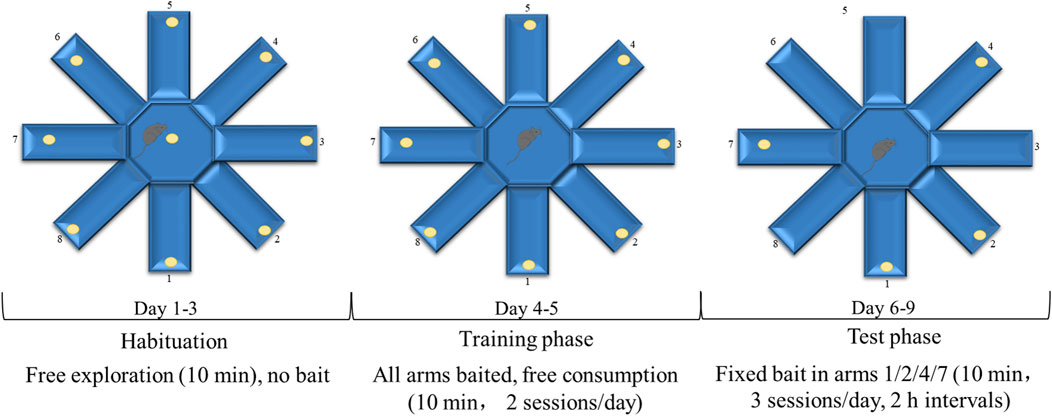
At the end of the 6-week training, all mice performed the eight-arm radial maze test to evaluate cognitive function under strictly blinded conditions, with all experimenters unaware of genotype and treatment assignments. Animals were randomized by an independent researcher to maintain blinding integrity throughout data collection and analysis. The radial eight-arm maze apparatus comprised eight sidearms (50 cm long × 10 cm wide × 30 cm high) extending from an octagonal central area. 2 days before the test, the mice were weighed and placed on a restricted diet (2–3 g, adjusted based on body weight) daily to maintain their body weights at 85%. The maze consisted of eight arms, and four to five food baits per arm were distributed throughout the arms and central region of the maze. During the initial training period from the first to the third day, the seven animals in each group were allowed unrestricted feeding and free exploration for 10 min in the maze to get familiar with the environment. From the fourth and fifth day, each animal was trained individually. One food bait was placed in each arm and the animal was free to consume as desired for 10 min. From the sixth to the ninth day, one bait was placed in arms 1, 2, 4, and 7. Each mouse was allowed to freely move around the maze and consume the pellets in each arm for 10 min daily. The number of food arms first were entered by the mice without record until re-entering the arm where the bait was previously placed (the total number of entries into baited arms during a single trial - 1), recording as a working memory error. The number of entering the arm where food has not been placed (the total number of entries into arms without food pellets throughout the testing phase) was recorded as a reference memory error. After each test, wipe the inner wall of the maze with 75% ethanol to eliminate residual odor interference. The record was verified by two blinded observers (inter-rater reliability >90%), with maze surfaces sanitized between trials and unmotivated trials excluded per predefined criteria. Three training sessions were conducted each day, with breaks of more than 2 h between sessions (Figure 2). This protocol has been previously described (Braida et al., 2002).
2.4 L-lactate measurements
Blood lactate was detected by Lactate-Scout (EKF Co., Germany) immediately at the end of second, fourth and sixth week of training. Seven mice in each group were collected tail tip blood immediately at the end of training.
2.5 Western blotting
The hippocampus of each mouse was washed with pre-cooling PBS. The sample was homogenated at 14,000 × g for 5 min in RIPA Lysis Buffer (No. P0013B, Beyotime, Shanghai, China), which contained phosphatase and protease inhibitors, with 1 mM PMSF (No. ST506, Beyotime, Shanghai, China), and the supernatant was collected. Material from vitro experiments was processed similarly. Proteins (30 μg) in SDS were loaded on a 10%–12.5% polyacrylamide gradient gel. The gel was then transferred to a 0.22 μm PVDF membrane (Epizyme Biotech, Shanghai, China). The membranes were blocked with Protein Free Rapid Blocking Buffer (No. PS108P Epizyme Biotech, Shanghai, China) for 10 min, followed by an overnight incubation at 4 °C with gentle shaking using primary antibodies. After washing, the membranes were incubated with secondary antibodies for 1 h at room temperature. The primary antibodies used include HIF-1α (1:1000, No. AF1009, Affinity, USA), EPO (1:1000, No. AF5190, Affinity, USA), GPR68 (1:1000, No. AF0723, Affinity, USA), VEGFA (1:5000, No. 26157-1-AP, Proteintech, USA), ET-1 (1:1000, No. 12191-1-AP, Proteintech, USA), eNOS (1:5000, No. 27120-1-AP, Proteintech, USA), p-TAU (1:1000, No. 28866-1-AP, Proteintech, USA), APP (1:1000, No. 25524-1-AP, Proteintech, USA). Quantification of the band density was performed using ImageJ. Protein levels were normalized to the band intensity of β-ACTIN (1:1000, No. 4970S, Cell Signaling Technology, USA).
2.6 RNA extraction and Real-Time PCR (RT-PCR)
Total RNA was prepared from the rested hippocampal tissues using TRIZOL (Ambion, USA). The RNA quality was assessed on a NanoDrop 2000 (Thermo, USA), where the 260/280 ratio was obtained. Samples with a ratio of 1.8–2.0 were processed for gene analysis. Reverse transcription was performed using PrimeScript™RT Master Mix (No. RR036A, Takara) according to the manufacturer’s protocol. Real-time PCR was performed using SYBR® Premix Ex Taq II (No. RR820A, Takara) and StepOnePlus Real-Time PCR System (Applied Biosystems, CA, USA). The mRNA levels of Bdnf (Forward TCATACTTCGGTTGCATGAAGG, Reverse ACACCTGGGTAGGCCAAGTT), Epo (Forward ACTCTCCTTGCTACTGATTCCT, Reverse ATCGTGACATTTTCTGCCTCC), Vegfa (Forward CTGCCGTCCGATTGAGACC, Reverse CCCCTCCTTGTACCACTGTC), Nos1 (Forward CTGGTGAAGGAACGGGTCAG, Reverse CCGATCATTGACGGCGAGAAT), Gpr68 (Forward TATCTTGCCCCATCGACCACA, Reverse AGTACCCGAAGTAGAGGGACA), Edn1 (Forward GCACCGGAGCTGAGAATGG, Reverse GTGGCAGAAGTAGACACACTC), Hif1α (Forward ACCTTCATCGGAAACTCCAAAG, Reverse CTGTTAGGCTGGGAAAAGTTAGG) were normalized to the internal loading control of Gapdh (Forward AGGTCGGTGTGAACGGATTTG, Reverse TGTAGACCATGTAGTTGAGGTCA) and determined with the comparative ΔΔCT method.
2.7 Statistics
All data are presented as means ± SE. Comparisons of two groups were performed using an unpaired Student’s t-test. A p value of 0.05 or less was considered statistically significant. Comparisons of multiple groups were performed using the two-way analysis of variance (two-way ANOVA) with the following main effect: genotype (Control group or APP/PPS1 group) or exercise (APP/PPS1 group or APP/PPS1 + HIIT group), as well as genotype × exercise interaction. If significant interactions were observed, post hoc simple effect tests were performed. All calculations and the graph construction were performed using SPSS 20.0 and GraphPad software (La Jolla, CA, USA).
3 Results
3.1 The effect of HIIT on the cognitive function of APP/PS1 mice
To evaluate whether HIIT could ameliorate cognitive deficits in APP/PS1 mice, we first assessed spatial memory by using the eight-arm radial maze and synaptic plasticity by measuring the Bdnf mRNA levels. The working memory errors in control group, APP/PS1 group, control + HIIT group, and APP/PS1 + HIIT group was 5.00 ± 0.87, 8.86 ± 1.10, 4.57 ± 0.72, and 6.57 ± 1.09, respectively. The reference memory errors in control group, APP/PS1 group, control + HIIT group, and APP/PS1 + HIIT group was 5.57 ± 1.00, 9.14 ± 0.67, 4.57 ± 0.69 and 9.14 ± 0.67, respectively. As shown in Figures 3A,B, the genotype × exercise interaction was not significant in both working memory errors and reference memory errors. The main effect of genotype was significant in working memory errors (F (1,24) = 9.339, p = 0.005, η2 = 0.280) and reference memory errors (F (1,24) = 10.695, p = 0.003, η2 = 0.308). The main effect of exercise was significant in reference memory errors (F (1,24) = 5.695, p = 0.025, η2 = 0.192) but not significant in working memory errors (F (1,24) = 2.006, p = 0.170, η2 = 0.077).
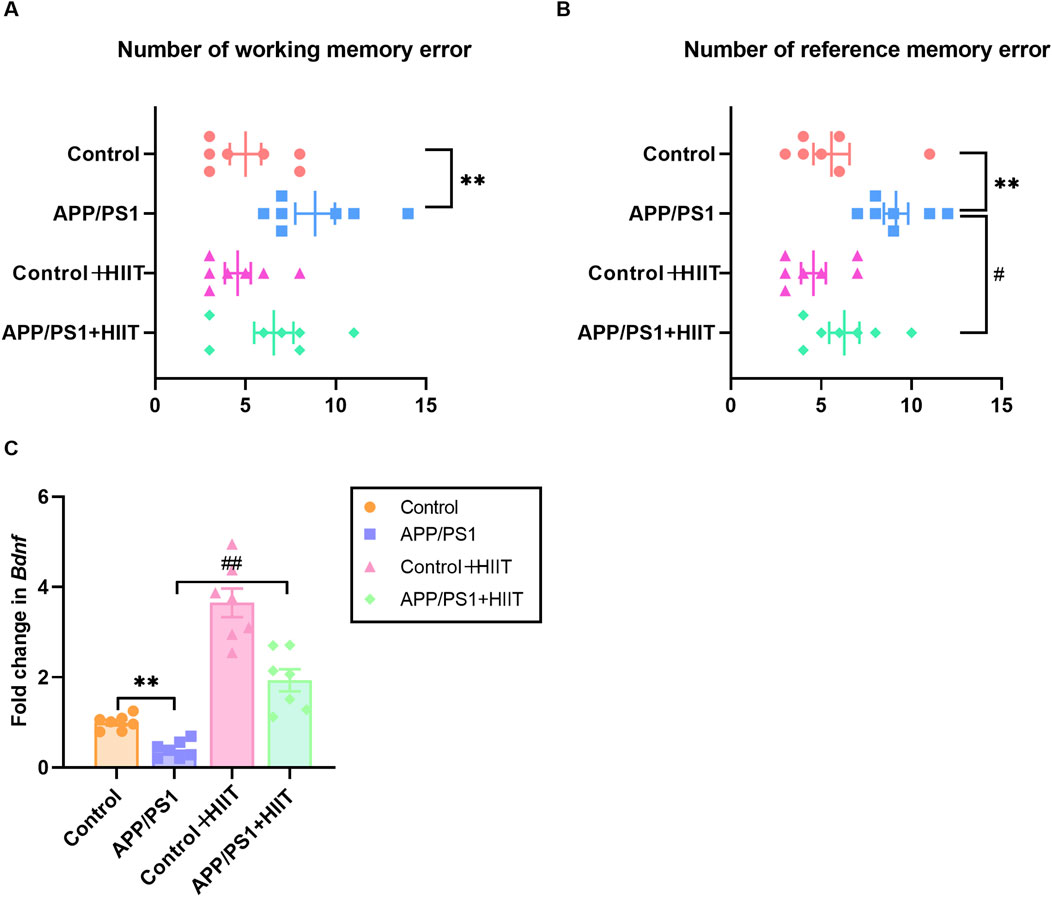
Figure 3. The effect of HIIT on the memory and Bdnf expression in APP/PS1 mice. (A) the number of working memory errors in an eight-arm radial maze. (B) the number of reference memory errors in an eight-arm radial maze. (C) the mRNA expression of Bndf. Gapdh is an internal control for quantification. Data are displayed as mean ± SE (n = 7). *: p < 0.05, p < 0.01 APP/PS1 group versus Control group; #: p < 0.05, p < 0.01 APP/PS1 + HIIT group versus APP/PS1 group.
In terms of Bdnf expression, a significant genotype × exercise interaction was observed (F (1,24) = 7.328, p = 0.012, η2 = 0.234). The main effect of genotype (F (1,24) = 31.406, p < 0.001, η2 = 0.567) and exercise (F (1,24) = 102.958, p < 0.001, η2 = 0.811) were significant. Then the simple effect test was conducted. Compared with the APP/PS1 group, HIIT significantly increased the mRNA levels of Bdnf (F (1,24) = 31.406, p < 0.001, η2 = 0.567) (Figure 3C). These results indicate that while HIIT may have limited effects on working memory, it can significantly improve reference memory and upregulate BDNF expression in AD mice, potentially supporting synaptic maintenance.
3.2 The effect of HIIT on the pathological markers in APP/PS1 mice
P-TAU and APP have demonstrated specificity to AD versus non-AD neurodegenerative diseases, which were critical for clinical diagnosis and eligibility for therapies. To determine whether cognitive improvements were associated with reduced AD pathology, we examined these two proteins. A significant genotype × exercise interaction was observed in p-TAU (F (1,24) = 8.868, p = 0.007, η2 = 0.270) and APP (F (1,24) = 4.410, p = 0.046, η2 = 0.155). The main effect of genotype was significant in p-TAU (F (1,24) = 19.178, p < 0.00, η2 = 0.444) and APP (F (1,24) = 45.204, p < 0.001, η2 = 0.653). The main effect of exercise was significant in p-TAU (F = 4.706,p = 0.040, η2 = 0.164) and APP (F (1,24) = 7.432, p = 0.012, η2 = 0.236). Then the simple effect test was conducted. Compared with the APP/PS1 group, HIIT significantly decreased the protein expression levels of p-TAU (F (1,24) = 13.247, p = 0.001, η2 = 0.356) and APP (F (1,24) = 11.646, p = 0.002, η2 = 0.327) (Figures 4A–C). The findings demonstrate that HIIT can mitigate hallmark AD pathology.
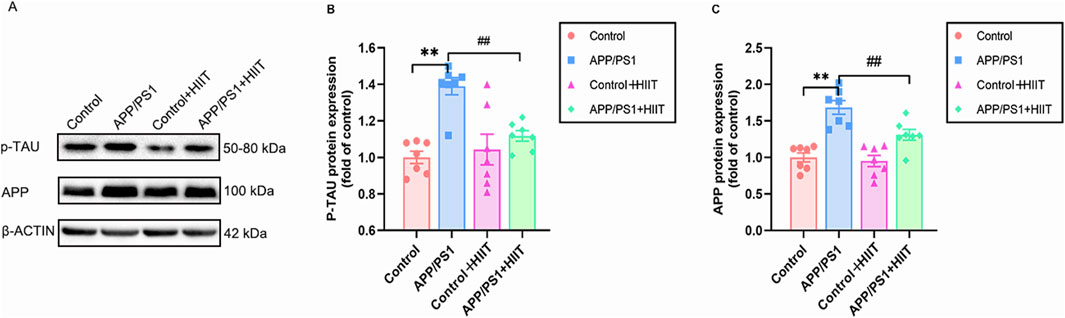
Figure 4. The effect of HIIT on the expression of p-TAU and APP in AD. (A) the Western blot image of p-TAU and APP. (B) quantification of p-TAU protein expression in each group. (C) quantification of APP protein expression in each group. β-ACTIN is a loading control. Data are expressed as mean ± SE (n = 7). *: p < 0.01 APP/PS1 group versus Control group; #: p < 0.01 APP/PS1+ HIIT group versus APP/PS1 group.
3.3 The effect of HIIT on the cerebral microangiogenesis in APP/PS1 mice
Then changes of EPO and VEGF were detect to assess whether HIIT promotes cerebrovascular remodeling via angiogenesis under the condition of AD, depicted in Figure 5. In the protein levels, the genotype × exercise interaction was not significant in EPO (F (1,24) = 0.164, p = 0.690, η2 = 0.007) and VEGFA (F (1,24) = 1.379, p = 0.252, η2 = 0.054). The main effect of genotype was significant in VEGFA (F (1,24) = 4.623, p = 0.042, η2 = 0.162). The main effect of exercise was significant in EPO (F (1,24) = 25.341, p < 0.001, η2 = 0.514) (Figures 5B,C). In the mRNA levels, the genotype × exercise interaction was significant in Epo (F (1,24) = 14.207, p = 0.001, η2 = 0.372). The main effect of genotype was significant in Vegfa (F (1,24) = 9.617, p = 0.005, η2 = 0.286) and Epo (F (1,24) = 26.436, p < 0.001, η2 = 0.524). The main effect of exercise was significant in Vegfa (F (1,24) = 48.249, p < 0.001, η2 = 0.668) and Epo (F (1,24) = 108.010, p < 0.001, η2 = 0.818). Then the simple effect test was conducted for Epo. Compared with the APP/PS1 group, HIIT significantly increased the Epo mRNA levels (F (1,24) = 26.436, p < 0.001, η2 = 0.524) (Figures 5D,E), suggesting that HIIT potently stimulates pro-angiogenic signaling, particularly in APP/PS1 mice.
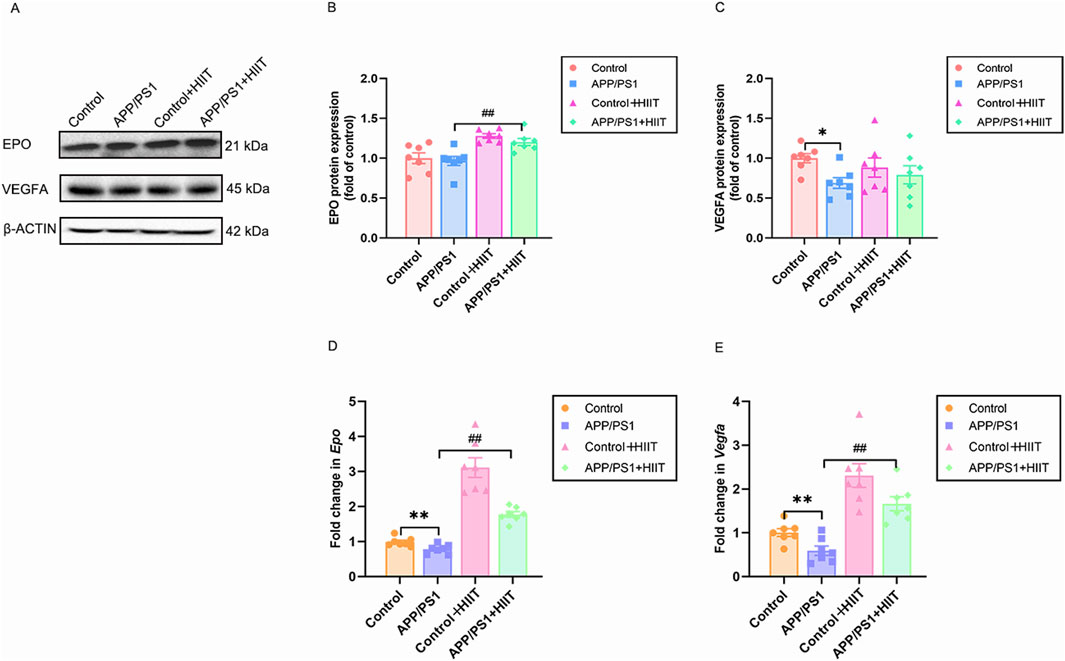
Figure 5. The effect of HIIT on the cerebral microangiogenesis in APP/PS1 mice. (A) the Western blot image of EPO and VEGFA. (B) quantification of EPO protein expression in each group. (C) quantification of VEGFA protein expression in each group. (D) the mRNA expression of Epo is shown for each of the four groups. (E) the mRNA expression of Vegfa is shown for each of the four groups. β-ACTIN and Gapdh are internal controls for quantification. Data displayed as mean ± SE (n = 7). *: p < 0.05, p < 0.01 APP/PS1 group versus Control group; #: p < 0.01 APP/PS1 + HIIT group versus APP/PS1 group.
3.4 The effect of HIIT on the cerebrovascular regulation of APP/PS1 mice
eNOS and GPR68 are vasodilatory factors and ET-1 is vasoconstrictor factors. We then examined these indicters to investigate how HIIT might improve cerebral blood flow. In the protein levels, the genotype × exercise interaction was significant in eNOS (F (1,24) = 26.193, p < 0.001, η2 = 0.522), GPR68 (F (1,24) = 16.324, p < 0.001, η2 = 0.405), and ET-1 (F (1,24) = 21.026, p < 0.001, η2 = 0.467). The main effect of genotype was significant in eNOS (F (1,24) = 29.220, p < 0.001, η2 = 0.549). While, the main effect of genotype was not significant in GPR68 (F (1,24) = 0.237, p = 0.631, η2 = 0.010) and ET-1 (F (1,24) = 0.256, p = 0.617, η2 = 0.011). The main effect of exercise was significant in eNOS (F (1,24) = 36.058, p < 0.001, η2 = 0.600), GPR68(F(1,24) = 7.849, p = 0.010, η2 = 0.246), and ET-1 F (1,24) = 4.518, p = 0.044, η2 = 0.158). Then the simple effect test was conducted. Compared with the APP/PS1 group, HIIT significantly increased the eNOS expresssion (F (1,24) = 61.858, p < 0.001, η2 = 0.720) and decreased the protein expression of GPR68 (F (1,24) = 23.406, p < 0.001, η2 = 0.494) and ET-1 (F (1,24) = 22.519, p < 0.001, η2 = 0.484) (Figures 6A–D). In the mRNA levels, genotype × exercise interaction was significant in Nos1 (F (1,24) = 4.373, p = 0.047, η2 = 0.154), Gpr68 (F (1,24) = 10.656, p = 0.003, η2 = 0.307), Edn1 (F (1,24) = 6.491, p = 0.018, η2 = 0.213). The main effect of genotype was significant in Nos1 (F (1,24) = 57.828, p < 0.001, η2 = 0.707), Gpr68 (F (1,24) = 14.774, p = 0.001, η2 = 0.381), and Edn1 (F (1,24) = 18.494, p < 0.001, η2 = 0.435). The main effect of exercise was significant in Nos1 (F (1,24) = 85.963, p < 0.001, η2 = 0.782), Gpr68 (F (1,24) = 11.134, p = 0.003, η2 = 0.317), and Edn1 (F (1,24) = 40.524, p < 0.001, η2 = 0.628). Then the simple effect test was conducted. Compared with the APP/PS1 group, HIIT significantly increased the Nos1 expresssion (F (1,24) = 57.828, p < 0.001, η2 = 0.707) and decreased the mRNA levels of Gpr68 (F (1,24) = 23.406, p < 0.001, η2 = 0.494) and Edn1 (F (1,24) = 18.494, p < 0.001, η2 = 0.435) (Figures 6E–G). These coordinated changes in vascular regulators suggest that HIIT promotes cerebrovascular dilation.
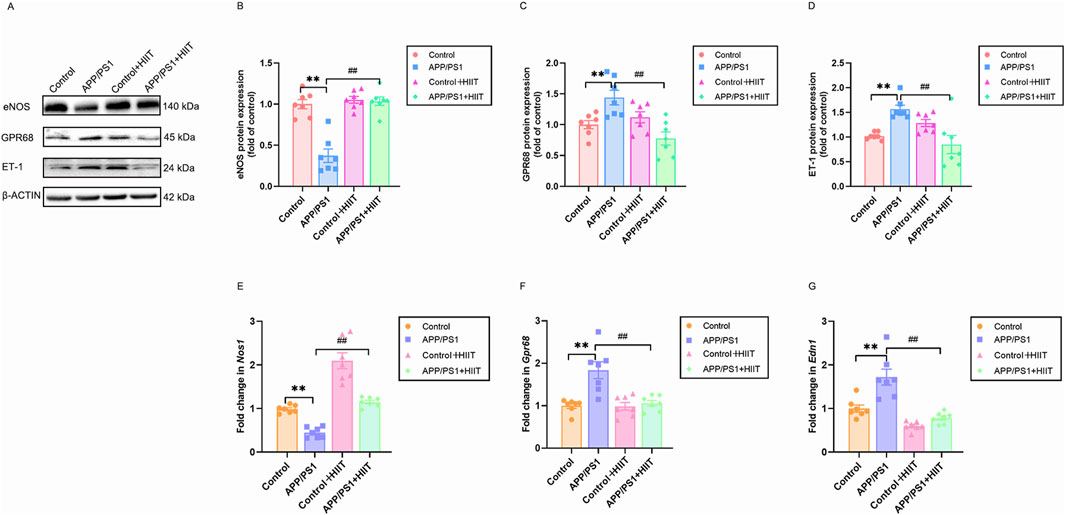
Figure 6. The effect of HIIT on the cerebrovascular regulation of APP/PS1 mice. (A) the Western blot image of eNOS, GPR68, and ET-1. (B) quantification of eNOS in each group. (C) quantification of GPR81 in each group. (D) quantification of ET-1 in each group. (E) the mRNA expression of Nos1. (F) the mRNA expression of Gpr68. (G) the mRNA expression of Edn1. β-ACTIN and Gapdh are internal controls for quantification. Data are displayed as mean ± SE (n = 7). *: p < 0.01 APP/PS1 group versus Control group; #: p < 0.01 APP/PS1 + HIIT group versus APP/PS1 group.
3.5 The effect of HIIT on the HIF-1α expression in APP/PS1 mice
Since HIF-1α is a potential upstream regulator of the observed vascular changes, we detected its protein and mRNA expression, as shown in Figure 7. Genotype × exercise interaction was not significant in protein levels (F (1,24) = 3.863, p = 0.061, η2 = 0.139) and mRNA levels (F (1,24) = 0.779, p = 0.386, η2 = 0.031). The main effect of genotype was not significant in protein levels (F (1,24) = 2.127, p = 0.158, η2 = 0.081) and mRNA levels (F (1,24) = 1.531, p = 0.228, η2 = 0.060). However, the main effect of exercise was significant in protein levels (F (1,24) = 59.871, p < 0.001, η2 = 0.714) and mRNA levels (F (1,24) = 21.687, p < 0.001, η2 = 0.475) (Figures 7A–C). The result suggests that HIIT-induced HIF-1α activation may represent a fundamental mechanism driving the observed vascular improvements, independent of AD pathology.
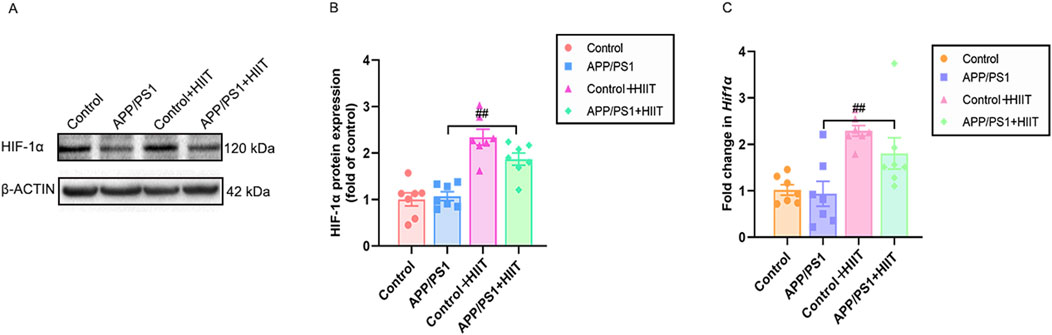
Figure 7. The effect of HIIT on the HIF-1α expression in APP/PS1 mice. (A) the Western blot image of HIF-1α. (B) quantification of HIF-1 α in each group. (C) the mRNA expression of Hif-1α. β-ACTIN and Gapdh are internal controls for quantification. Data displayed as mean ± SE (n = 7). #: p < 0.05, p < 0.01 APP/PS1 + HIIT group versus APP/PS1 group.
4 Discussion
In AD, TAU becomes abnormally aggregated into amyloid filaments featuring cross-β structures, which, along with diminished clearance in the brain, contributes to the process known as amyloidogenesis-the formation of amyloid fibrils (Parra Bravo et al., 2024; Annadurai et al., 2021). The phosphorylation process plays an essential role in TAU protein’s physiological function, which is related to amyloid metabolism and synaptic integrity (Wesenhagen et al., 2022). APP is hydrolyzed to generate Aβ, a key component of amyloid plaques (Akasaka-Manya and Manya, 2020), which is a pathological feature of AD. In our results, we found that APP/PS1 significantly increased both the TAU protein phosphorylation and APP levels. These results imply that hyperphosphorylated TAU promotes amyloid plaques and neurofibrillary tangles formation, which deteriorates AD development (Naseri et al., 2019). Meanwhile, overproducing APP fragments triggers typical Aβ pathology, neuroinflammation, and memory impairment in an age-dependent manner (Saito et al., 2014). Moreover, accumulation of mutant APP has been reported to be responsible for reduced MAP2, dendritic spines, and hippocampal-based impaired learning and memory in 12-month-old mice (Manczak et al., 2018). These results hint that excessive P-TAU and APP drive the pathology of AD and contribute to the reduction of synaptic strength, synaptic loss, and neurodegeneration in this AD model. In comparison, HIIT inhibited the expression of p-TAU and APP in the hippocampus of APP/PS1 mice, indicating its capacity to mitigate AD-like pathology and potentially improve cognitive function.
Given that TAU and APP aggregation correlate with the severity of cognitive impairment in AD patients (Stevens and Brown, 2015), we assessed memory in mice. We found the increase of reference memory error and the working memory errors in the APP/PS1 mice, suggesting the short- and long-term memory impairment. BDNF critically supports hippocampal long-term potentiation and synaptic efficacy, which is positively associated with memory (Lu et al., 2014). We also detected the mRNA expression of Bndf to evaluate whether the cognitive impairment involved molecular levels. Bdnf mRNA levels were reduced in APP/PS1 mice versus wild-type littermates, suggesting neuroplasticity impairment triggered by the APP/PS1 mutation. In contrast, HIIT significantly reduced the reference memory error and enhanced the mRNA expression of Bndf in APP/PS1 mice, indicating potential recovery of cognitive function and neuroplasticity. However, HIIT had no significant effect on working memory errors. This limited effect may be attributed to the duration of HIIT in our study. For example, longer interventions of HIIT (e.g., 8-week (Feng et al., 2023) or 10-week (Liu et al., 2022)) have shown broader cognitive benefits in AD models, such as learning and memory and a desire for exploration.
EPO is linked to cerebrovascular events and ameliorates cognitive deficits by reducing oxidative stress, mitigating inflammatory responses, enhancing ATP generation, activating serotonergic signaling pathways, and facilitating the clearance of Aβ plaques in the hippocampus of AD mice (Dara et al., 2019; Shim et al., 2022). It was reported that there is a decline in the hippocampus of aging rats (Li et al., 2016). Our results indicated that the mRNA expression of Epo decreased, whereas there were no changes in the protein levels in APP/PS1 mice compared to the wildtype. We speculated that the inconsistent results could be explained by the following factors: the maintenance of EPO protein levels may represent an endogenous compensation or protection mechanism during AD pathology; the pathological environment of AD (such as Aβ accumulation) may inhibit protein degradation pathways, leading to a reduced turnover rate of EPO protein, thereby maintaining its steady-state levels even when mRNA levels decrease; The inconsistency between protein and gene transcription suggests that the regulation of EPO expression may occur at the post-transcriptional or post-translational levels. After a 6-week HIIT intervention, both the mRNA and protein expression of EPO were significantly increased in the APP/PS1 + HIIT group compared to the APP/PS1 group. The results demonstrated that HIIT can effectively reverse the pathological inhibition of EPO expression in the APP/PS1 mouse model, simultaneously increasing its mRNA transcription levels and protein synthesis efficiency.
VEGF is also a classical vasoactive molecule involved in neurogenesis (During and Cao, 2006). Aberrant VEGF signaling may lead to impaired brain capillary function and reduced blood flow, contributing to cognitive decline (Ali and Bracko, 2022). Our results indicated that both mRNA and protein expression of VEGF were reduced in APP/PS1 mice, suggesting impaired cerebrovascular function. These findings were consistent with those of Tsartsalis et al., who observed decreased immunostaining of VEGF in vascular endothelial cells of AD patients, along with disorders of angiogenesis and compromised BBB integrity (Tsartsalis et al., 2024). Previous studies have demonstrated that exercise-induced hippocampal neurogenesis (Fabel et al., 2003) and cerebral microangiogenesis (Morland et al., 2017; Ding et al., 2006) depended on the VEGF. However, our findings indicate that only mRNA expression increased in the AD-trained mice. This divergence between transcriptional activation and translational output suggests that post-transcriptional regulatory mechanisms might predominantly control VEGF homeostasis in the AD brain during exercise intervention. Moreover, the discrepancy could also stem from differences in exercise patterns, duration, intensity, and the organism’s physiological state (Vital et al., 2014). In summary, these findings imply that HIIT improves cerebrovascular remodeling in the pathological state of AD by mainly stimulating EPO.
Vasculopathy in AD brain regions, particularly in white matter vessels, correlates with reduced eNOS expression levels in cerebral vessels (de la Monte et al., 2000). Our findings revealed that protein levels of eNOS and mRNA levels of Nos1 were decreased in APP/PS1 mice, indicating possible vasculopathy in the hippocampal region. Concurrently, the reduced production of NO, the end-product of eNOS, could exacerbate endothelial inflammation and vasoconstriction, resulting in damage (!!! INVALID CITATION), implying that APP/PS1 mutation may lead to reduced cerebral perfusion in the rodent model. Supporting this notion, studies in eNOS knockout mice showed an increased perfusion response to whisker stimulations in the barrel cortex alongside reduced glutamine levels in the frontal cortex, hippocampus, parahippocampal region, and cerebellum. These changes led to classical AD-like pathological manifestations, including Aβ accumulation and memory deficits (Hariharan et al., 2019). This is because chronic reduced cerebrovascular eNOS and NO could impair vasodilation responses and diminish the capacity to remove respiratory waste products and toxins from the extracellular space (de la Monte et al., 2000). Consequently, the impairment in working and reference memory observed in APP/PS1 mice is likely associated with their reduced eNOS levels. Following the exercise intervention, the mRNA expression of Nos1 significantly increased in the wild-type mice. Moreover, the expression levels of eNOS and Nos1 in the APP/PS1 mice were restored or nearly reached those seen in normal wild-type mice. Previous studies have shown that running exercise markedly enhances eNOS activity in the rat cortex, resulting in improved cerebrovascular function. This includes upregulation of proangiogenic factors, as well as increases in the length, volume, and surface area of capillaries, and a decrease in antiangiogenic factors (Zang et al., 2023). Hence, our results hinted that this exercise regimen promoted eNOS expression to facilitate vasodilation of hippocampal cerebrovascular structures, thereby improving perfusion.
In the stroke brain, overexpression of GPR68 exerted neuroprotection and was able to alleviate the neuronal damage via inhibiting the protein kinase C (PKC) activation (Wang et al., 2020). In our study, however, we observed abnormally elevated levels of GPR68 protein and mRNA in APP/PS1 mice, suggesting that its upregulation may represent a compensatory protective mechanism against neuronal damage. Following exercise intervention, GPR68 levels returned to those of the control group. We speculated that HIIT ameliorates AD pathology by mitigating stressors such as acidosis and inflammation (Yu et al., 2025), which would otherwise sustain compensatory GPR68 upregulation. This implies that prolonged GPR68 activation is unnecessary, given its dual roles: activation in acidic microenvironments (Ludwig et al., 2003) and induction of pro-inflammatory cytokine release (Ichimonji et al., 2010). Additionally, HIIT may provide more potent neuroprotective molecules like BDNF, potentially restoring GPR68 expression to physiological levels or reducing its functional demand.
The elevated ET-1 levels have been reported to increase BBB permeability and correlate with the degree of cerebral hypoperfusion in patients with AD and related dementia (D'Orleans-Juste et al., 2019). Our results showed that both protein and mRNA expression of ET-1 were significantly increased in the hippocampus of APP/PS1 mice, implying enhanced cerebral vasoconstriction in AD. This finding aligns with the established role of vascular dysregulation in AD pathogenesis. The elevated ET-1 expression likely reflects endothelial dysfunction, a recognized hallmark of AD progression. Furthermore, we found that HIIT reduced the protein levels of ET-1 and the mRNA expression of Edn1 compared to the APP/PS1 mice, suggesting that physical activity may inhibit ET-1 release, thereby improving vascular perfusion. This mechanism may promote cerebrovascular dilation and enhance CBF, consequently optimizing oxygen and nutrient delivery to brain tissues. In summary, HIIT contributes to cerebrovascular repair through positive regulation of vascular function, ultimately ameliorating brain health in AD.
Pre-clinical findings indicated an important role for the endogenous oxygen sensing machinery, particularly HIF and its target genes, in proper cognitive function (Gruneberg et al., 2016). During the development of AD pathology, dysregulated HIF-1α activation may exacerbate cerebral hypoperfusion (Porel et al., 2024). HIF-1α is implicated in the production of pro-inflammatory cytokines and Aβ, further contributing to neuronal damage (Alexander et al., 2022; Jung et al., 2023). Conversely, HIF-1α may support synaptic plasticity and neuronal survival by counteracting the toxic effects of Aβ and inhibiting p-TAU (Wang et al., 2021). In our study, we found no significant difference in HIF-1α protein or mRNA expression in the hippocampus between control mice and APP/PS1 mice. Similar to our results, Maria et al. found no changes in the mRNA expression of HIF-1α in APP/PS1 mice from 3 to 12 months of age (de Lemos et al., 2013). Sabrina et al. also reported unchanged HIF-1α expression in nuclear extracts from the cortex of 5xFAD transgenic mice (Petralla et al., 2024). This lack of change may be attributed to several factors: (1) Early hypoxia in AD might be compensated by alternative pathways, preventing significant fluctuations in HIF-1α levels; (2) the regulation of HIF-1α through post-translational modifications, such as acetylation, provides another layer of complexity in its stabilization (3) Differences in AD mouse models (e.g., specific mutations and disease stages examined); may influence whether or not a robust hypoxia response is triggered. For example, one potential compensatory mechanism involves the interaction between NO and HIF-1α, which can prevent HIF-1α degradation in astrocytes, initiating a response to hypoxia (Shi et al., 2016). Furthermore, chronic hypoxia in AD brains can promote inflammasome formation (Jung et al., 2023), which in turn may maintain HIF-1α expression, potentially establishing a detrimental feedback loop contributing to vascular damage. Furthermore, acetylation can stabilize HIF-1α protein levels without affecting mRNA expression, enhancing its stability during inflammation. This suggests that similar mechanisms may function under hypoxic conditions in AD to maintain HIF-1α levels (Chen Q. et al., 2022). Consistent with the notion of model-dependent effects, HIF-1α conversely upregulation has been reported in the hippocampus and cortex of other AD models, such as SAMP8 (Shang et al., 2024) and 3x-Tg AD mice (Jung et al., 2023).
Interestingly, prior research has demonstrated that exercise can induce tissue hypoxia in organs like the heart (Xu et al., 2024) and small intestine (Wu et al., 2020) for metabolic adaptation. There is limited evidence concerning the role of HIF-1α in the brain during exercise. Our findings indicated that HIIT significantly enhanced both protein and mRNA expression of HIF-1α in the hippocampus of APP/PS1 training mice compared to sedentary APP/PS1 controls. This HIIT-induced upregulation of HIF-1α may indicate an enhanced metabolic adaptation to hypoxia in the context of AD. On the one hand, elevated HIF-1α can promote the expression of factors essential for angiogenesis (e.g., EPO, VEGF) and regulate cerebrovascular function (e.g., eNOS and ET-1), thereby improving CBF and oxygen delivery to meet metabolic demands, particularly under hypoxic stress. On the other hand, upregulating HIF-1α by exercise may promote neuroprotection in APP/PS1 mice, potentially by enhancing BDNF expression and rescuing synaptic loss (Guo et al., 2015).
5 Conclusion
Physical activity, particularly HIIT, has been shown to induce beneficial changes that are essential for maintaining cognitive function and overall brain health. These benefits arise from various mechanisms, including improving cerebrovascular function like enhanced blood oxygen supply and cerebral perfusion. Our research first observed that 6-week HIIT was conducive to cerebrovascular health through promoting cerebral microangiogenesis and modulating cerebrovascular function. It also enhanced the HIF-1α expression to modulate the hypoxic metabolism environment for AD, which may act as an upstream regulator of vascular factor release and angiogenesis signaling. Further mechanistic studies are needed to confirm its regulatory role. Collectively, these HIIT-induced adaptations ultimately contribute to a reduction in APP and TAU protein levels in AD. These findings highlight HIIT as a promising non-pharmacological strategy for mitigating cerebrovascular dysfunction in AD models. However, clinical application requires validation in human studies.
6 Limitations
Firstly, the number of animals in each group in this experiment is relatively small. Our animal model only applied to the female mice, which lacked evidence of the role of HIIT on the male AD mice. However, this limitation does not affect the overall conclusion. Future studies should aim to expand the sample size for each group to further ensure the vascular protective effect of HIIT on the AD of different genders. Secondly, we adopted the 6-week duration of HIIT, which was insufficient to trigger an obvious long-term exercise dose effect. Further study may include multiple exercise patterns to compare the differences between those exercises in AD treatment as possible. Thirdly, in this study, we identified for the first time that HIIT promotes brain health in AD by enhancing cerebral blood vessel function. The use of neuroimaging technologies and histological experiment to observe cerebrovascular changes will be necessary to further elucidate the effects of HIIT on AD. Lack of direct vascular and Aβ morphological data weakens mechanistic conclusions. Finally, although the HIF-1α levels in the hippocampus did not significantly change in AD mice, they were enhanced by exercise. This study also did not establish a causal relationship between the exercise-induced increase in HIF-1α expression and various vascular factors associated with the pathological changes in AD. Given the potential association between HIF-1α and these factors, it is essential to employ genetic tools to knock out or knock down HIF-1α in rodent models. This approach could clarify the regulatory effects of HIF-1α on cerebral vascular metabolism in AD, providing deeper insights into the molecular mechanisms by which exercise improves cerebrovascular function and identifying molecular targets for exercise interventions. Notably, we selected 4-month-old APP/PS1 mice as a model for the early stages of AD. Further research is necessary to investigate the effects of HIIT on AD pathology across both early and late stages, as this may help to elucidate the underlying mechanisms through which exercise prevents and treats AD, ultimately aiding in the formulation of specific exercise strategies tailored for the disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Ethical Committee for Science Research at Qufu Normal University (Approval No. 2020-0001). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LZ: Funding acquisition, Writing – original draft. MC: Conceptualization, Investigation, Methodology, Writing – original draft. ZL: Methodology, Validation, Writing – original draft. QW: Methodology, Validation, Writing – original draft. TZ: Validation, Writing – original draft. JH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was sponsored by Shanghai Pudong Science and Technology Commission Science and Technology Development Special Fund for People’s Livelihood Research (Medical and Health) (PKJ2023-Y16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akasaka-Manya, K., and Manya, H. (2020). The role of APP O-Glycosylation in Alzheimer's Disease. Biomolecules 10 (11), 1569. doi:10.3390/biom10111569
Alexander, C., Li, T., Hattori, Y., Chiu, D., Frost, G. R., Jonas, L., et al. (2022). Hypoxia Inducible Factor-1α binds and activates γ-secretase for Aβ production under hypoxia and cerebral hypoperfusion. Mol. Psychiatry 27 (10), 4264–4273. doi:10.1038/s41380-022-01676-7
Ali, M., and Bracko, O. (2022). VEGF paradoxically reduces cerebral blood flow in Alzheimer's Disease mice. Neurosci. Insights 17, 26331055221109254. doi:10.1177/26331055221109254
Annadurai, N., De Sanctis, J. B., Hajduch, M., and Das, V. (2021). Tau secretion and propagation: perspectives for potential preventive interventions in Alzheimer's disease and other tauopathies. Exp. Neurol. 343, 113756. doi:10.1016/j.expneurol.2021.113756
Bliss, E. S., Wong, R. H., Howe, P. R., and Mills, D. E. (2021). Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow. Metab. 41 (3), 447–470. doi:10.1177/0271678X20957807
Braida, D., Pozzi, M., Cavallini, R., and Sala, M. (2002). 3,4 methylenedioxymethamphetamine (ecstasy) impairs eight-arm radial maze performance and arm entry pattern in rats. Behav. Neurosci. 116 (2), 298–304. doi:10.1037//0735-7044.116.2.298
Charidimou, A., Boulouis, G., Frosch, M. P., Baron, J. C., Pasi, M., Albucher, J. F., et al. (2022). The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 21 (8), 714–725. doi:10.1016/S1474-4422(22)00208-3
Chen, Y., Imai, H., Ito, A., and Saito, N. (2013). Novel modified method for injection into the cerebrospinal fluid via the cerebellomedullary cistern in mice. Acta Neurobiol. Exp. (Wars). 73 (2), 304–311. doi:10.55782/ane-2013-1938
Chen, X., Chen, L., Lin, G., Wang, Z., Kodali, M. C., Li, M., et al. (2022a). White matter damage as a consequence of vascular dysfunction in a spontaneous mouse model of chronic mild chronic hypoperfusion with eNOS deficiency. Mol. Psychiatry 27 (11), 4754–4769. doi:10.1038/s41380-022-01701-9
Chen, Q., Cui, K., Zhao, Z., Xu, X., Liu, Y., Shen, Y., et al. (2022b). LPS stimulation stabilizes HIF-1α by enhancing HIF-1α acetylation via the PARP1-SIRT1 and ACLY-Tip60 pathways in macrophages. Faseb J. 36 (7), e22418. doi:10.1096/fj.202200256R
Coates, A. M., Joyner, M. J., Little, J. P., Jones, A. M., and Gibala, M. J. (2023). A perspective on high-intensity interval training for performance and health. Sports Med. 53 (Suppl. 1), 85–96. doi:10.1007/s40279-023-01938-6
D'Orleans-Juste, P., Akide Ndunge, O. B., Desbiens, L., Tanowitz, H. B., and Desruisseaux, M. S. (2019). Endothelins in inflammatory neurological diseases. Pharmacol. Ther. 194, 145–160. doi:10.1016/j.pharmthera.2018.10.001
Dara, T., Vatanara, A., Sharifzadeh, M., Khani, S., Vakilinezhad, M. A., Vakhshiteh, F., et al. (2019). Improvement of memory deficits in the rat model of Alzheimer's disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn Mem. 166, 107082. doi:10.1016/j.nlm.2019.107082
De la Rosa, A., Olaso-Gonzalez, G., Arc-Chagnaud, C., Millan, F., Salvador-Pascual, A., García-Lucerga, C., et al. (2020). Physical exercise in the prevention and treatment of Alzheimer's disease. J. Sport Health Sci. 9 (5), 394–404. doi:10.1016/j.jshs.2020.01.004
de Lemos, M. L., de la Torre, A. V., Petrov, D., Brox, S., Folch, J., Pallas, M., et al. (2013). Evaluation of hypoxia inducible factor expression in inflammatory and neurodegenerative brain models. Int. J. Biochem. Cell Biol. 45 (7), 1377–1388. doi:10.1016/j.biocel.2013.04.011
de la Monte, S. M., Sohn, Y. K., Etienne, D., Kraft, J., and Wands, J. R. (2000). Role of aberrant nitric oxide synthase-3 expression in cerebrovascular degeneration and vascular-mediated injury in Alzheimer's disease. Ann. N. Y. Acad. Sci. 903, 61–71. doi:10.1111/j.1749-6632.2000.tb06351.x
de Valliere, C., Cosin-Roger, J., Simmen, S., Atrott, K., Melhem, H., Zeitz, J., et al. (2016). Hypoxia positively regulates the expression of pH-Sensing G-Protein-Coupled receptor OGR1 (GPR68). Cell Mol. Gastroenterol. Hepatol. 2 (6), 796–810. doi:10.1016/j.jcmgh.2016.06.003
Ding, Y. H., Li, J., Zhou, Y., Rafols, J. A., Clark, J. C., and Ding, Y. (2006). Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc Res. 3 (1), 15–23. doi:10.2174/156720206775541787
Dorner, M., Tyndall, A., Hainc, N., von Kanel, R., Neumann, K., Euler, S., et al. (2024). Neuropsychiatric symptoms and lifelong mental activities in cerebral amyloid angiopathy - a cross-sectional study. Alzheimers Res. Ther. 16 (1), 196. doi:10.1186/s13195-024-01519-3
During, M. J., and Cao, L. (2006). VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr. Alzheimer Res. 3 (1), 29–33. doi:10.2174/156720506775697133
Fabel, K., Fabel, K., Tam, B., Kaufer, D., Baiker, A., Simmons, N., et al. (2003). VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18 (10), 2803–2812. doi:10.1111/j.1460-9568.2003.03041.x
Feng, S., Wu, C., Zou, P., Deng, Q., Chen, Z., Li, M., et al. (2023). High-intensity interval training ameliorates Alzheimer's disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization. Theranostics 13 (10), 3434–3450. doi:10.7150/thno.81951
Graff-Radford, J., Yong, K. X. X., Apostolova, L. G., Bouwman, F. H., Carrillo, M., Dickerson, B. C., et al. (2021). New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 20 (3), 222–234. doi:10.1016/s1474-4422(20)30440-3
Gripp, F., Nava, R. C., Cassilhas, R. C., Esteves, E. A., Magalhães, C. O. D., Dias-Peixoto, M. F., et al. (2021). HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur. J. Appl. physiology 121 (1), 159–172. doi:10.1007/s00421-020-04502-6
Gruneberg, D., Montellano, F. A., Plaschke, K., Li, L., Marti, H. H., and Kunze, R. (2016). Neuronal prolyl-4-hydroxylase 2 deficiency improves cognitive abilities in a murine model of cerebral hypoperfusion. Exp. Neurol. 286, 93–106. doi:10.1016/j.expneurol.2016.10.001
Guo, C., Zhang, Y. X., Wang, T., Zhong, M. L., Yang, Z. H., Hao, L. J., et al. (2015). Intranasal deferoxamine attenuates synapse loss via up-regulating the P38/HIF-1α pathway on the brain of APP/PS1 transgenic mice. Front. Aging Neurosci. 7, 104. doi:10.3389/fnagi.2015.00104
Hariharan, A., Jing, Y., Collie, N. D., Zhang, H., and Liu, P. (2019). Altered neurovascular coupling and brain arginine metabolism in endothelial nitric oxide synthase deficient mice. Nitric oxide Biol. Chem. 87, 60–72. doi:10.1016/j.niox.2019.03.006
Hu, J., Cai, M., Shang, Q., Li, Z., Feng, Y., Liu, B., et al. (2021). Elevated lactate by high-intensity interval training regulates the hippocampal BDNF expression and the mitochondrial quality control System. Front. Physiol. 12, 629914. doi:10.3389/fphys.2021.629914
Ichimonji, I., Tomura, H., Mogi, C., Sato, K., Aoki, H., Hisada, T., et al. (2010). Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 299 (4), L567–L577. doi:10.1152/ajplung.00415.2009
Jung, E., Kim, Y. E., Jeon, H. S., Yoo, M., Kim, M., Kim, Y. M., et al. (2023). Chronic hypoxia of endothelial cells boosts HIF-1α-NLRP1 circuit in Alzheimer's disease. Free Radic. Biol. and Med. 204, 385–393. doi:10.1016/j.freeradbiomed.2023.05.011
Kaur, D., Behl, T., Sehgal, A., Singh, S., Sharma, N., Badavath, V. N., et al. (2022). Unravelling the potential neuroprotective facets of erythropoietin for the treatment of Alzheimer's disease. Metab. Brain Dis. 37 (1), 1–16. doi:10.1007/s11011-021-00820-6
Komiyama, T., Tanoue, Y., Sudo, M., Costello, J. T., Uehara, Y., Higaki, Y., et al. (2020). Cognitive impairment during high-intensity exercise: influence of cerebral blood flow. Med. Sci. Sports Exerc 52 (3), 561–568. doi:10.1249/MSS.0000000000002183
Kuwaki, T., Kurihara, H., Cao, W. H., Kurihara, Y., Unekawa, M., Yazaki, Y., et al. (1997). Physiological role of brain endothelin in the central autonomic control: from neuron to knockout mouse. Prog. Neurobiol. 51 (5), 545–579. doi:10.1016/s0301-0082(96)00063-9
Li, X., Chen, Y., Shao, S., Tang, Q., Chen, W., Chen, Y., et al. (2016). Oxidative stress induces the decline of brain EPO expression in aging rats. Exp. Gerontol. 83, 89–93. doi:10.1016/j.exger.2016.07.012
Li, W., Abdul, Y., Ward, R., and Ergul, A. (2018). Endothelin and diabetic complications: a brain-centric view. Physiol. Res. 67 (Suppl. 1), S83-S94–S94. doi:10.33549/physiolres.933833
Lifshitz, V., Weiss, R., Benromano, T., Kfir, E., Blumenfeld-Katzir, T., Tempel-Brami, C., et al. (2012). Immunotherapy of cerebrovascular amyloidosis in a transgenic mouse model. Neurobiol. Aging 33 (2), 432 e1–432. doi:10.1016/j.neurobiolaging.2011.01.006
Liu, Q., Fu, X., Han, R., Liu, X., Zhao, X., and Wei, J. (2022). Neuroprotective effect of HIIT against GFAP hypertrophy through mitochondrial dynamics in APP/PS1 mice. Oxidative Med. Cell. Longev. 2022, 1764589. doi:10.1155/2022/1764589
Lu, B., Nagappan, G., and Lu, Y. (2014). BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 220, 223–250. doi:10.1007/978-3-642-45106-5_9
Ludwig, M. G., Vanek, M., Guerini, D., Gasser, J. A., Jones, C. E., Junker, U., et al. (2003). Proton-sensing G-protein-coupled receptors. Nature 425 (6953), 93–98. doi:10.1038/nature01905
Manczak, M., Kandimalla, R., Yin, X., and Reddy, P. H. (2018). Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 27 (8), 1332–1342. doi:10.1093/hmg/ddy042
McLaren, A. T., Marsden, P. A., Mazer, C. D., Baker, A. J., Stewart, D. J., Tsui, A. K., et al. (2007). Increased expression of HIF-1alpha, nNOS, and VEGF in the cerebral cortex of anemic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292 (1), R403–R414. doi:10.1152/ajpregu.00403.2006
Miguet, M., Fearnbach, N. S., Metz, L., Khammassi, M., Julian, V., Cardenoux, C., et al. (2020). Effect of HIIT versus MICT on body composition and energy intake in dietary restrained and unrestrained adolescents with obesity. Appl. physiology, Nutr. metabolism = Physiologie appliquee, Nutr. metabolisme 45 (4), 437–445. doi:10.1139/apnm-2019-0160
Morland, C., Andersson, K. A., Haugen, O. P., Hadzic, A., Kleppa, L., Gille, A., et al. (2017). Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 15557. doi:10.1038/ncomms15557
Naseri, N. N., Wang, H., Guo, J., Sharma, M., and Luo, W. (2019). The complexity of tau in Alzheimer's disease. Neurosci. Lett. 705, 183–194. doi:10.1016/j.neulet.2019.04.022
Nishijima, T., Torres-Aleman, I., and Soya, H. (2016). Exercise and cerebrovascular plasticity. Prog. Brain Res. 225, 243–268. doi:10.1016/bs.pbr.2016.03.010
Palmer, L. A., Semenza, G. L., Stoler, M. H., and Johns, R. A. (1998). Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am. J. Physiol. 274 (2), L212–L219. doi:10.1152/ajplung.1998.274.2.L212
Palmer, J. A., Kaufman, C. S., Whitaker-Hilbig, A. A., and Billinger, S. A. (2025). APOE4 carriers display loss of anticipatory cerebrovascular regulation across the Alzheimer's disease continuum. Alzheimers Dement. 21 (5), e70229. doi:10.1002/alz.70229
Parra Bravo, C., Naguib, S. A., and Gan, L. (2024). Cellular and pathological functions of tau. Nat. Rev. Mol. Cell Biol. 25 (11), 845–864. doi:10.1038/s41580-024-00753-9
Petralla, S., Saveleva, L., Kanninen, K. M., Oster, J. S., Panayotova, M., Fricker, G., et al. (2024). Increased expression of Transferrin receptor 1 in the brain cortex of 5xFAD mouse model of Alzheimer's Disease is associated with activation of HIF-1 signaling pathway. Mol. Neurobiol. 61 (9), 6383–6394. doi:10.1007/s12035-024-03990-3
Porel, P., Bala, K., and Aran, K. R. (2024). Exploring the role of HIF-1α on pathogenesis in Alzheimer's disease and potential therapeutic approaches. Inflammopharmacology 33, 669–678. doi:10.1007/s10787-024-01585-x
Quintana, D. D., Anantula, Y., Garcia, J. A., Engler-Chiurazzi, E. B., Sarkar, S. N., Corbin, D. R., et al. (2021). Microvascular degeneration occurs before plaque onset and progresses with age in 3xTg AD mice. Neurobiol. aging 105, 115–128. doi:10.1016/j.neurobiolaging.2021.04.019
Saito, T., Matsuba, Y., Mihira, N., Takano, J., Nilsson, P., Itohara, S., et al. (2014). Single App knock-in mouse models of Alzheimer's disease. Nat. Neurosci. 17 (5), 661–663. doi:10.1038/nn.3697
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397 (10284), 1577–1590. doi:10.1016/s0140-6736(20)32205-4
Shang, C., Su, Y., Ma, J., Li, Z., Wang, P., Ma, H., et al. (2024). Huanshaodan regulates microglial glucose metabolism reprogramming to alleviate neuroinflammation in AD mice through mTOR/HIF-1α signaling pathway. Front. Pharmacol. 15, 1434568. doi:10.3389/fphar.2024.1434568
Shi, Q., Liu, X., Wang, N., Zheng, X., Fu, J., and Zheng, J. (2016). Nitric oxide from brain microvascular endothelial cells may initiate the compensatory response to mild hypoxia of astrocytes in a hypoxia-inducible factor-1α dependent manner. Am. J. Transl. Res. 8 (11), 4735–4749.
Shibuya, M. (2014). VEGF-VEGFR signals in health and disease. Biomol. Ther. Seoul. 22 (1), 1–9. doi:10.4062/biomolther.2013.113
Shih, H. M., Wu, C. J., and Lin, S. L. (2018). Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 117 (11), 955–963. doi:10.1016/j.jfma.2018.03.017
Shim, J. W., and Madsen, J. R. (2018). VEGF signaling in neurological disorders. Int. J. Mol. Sci. 19 (1), 275. doi:10.3390/ijms19010275
Shim, K. H., Ha, S., Choung, J. S., Choi, J. I., Kim, D. Y., Kim, J. M., et al. (2022). Therapeutic effect of erythropoietin on Alzheimer's Disease by activating the serotonin pathway. Int. J. Mol. Sci. 23 (15), 8144. doi:10.3390/ijms23158144
Singh, M., and Prakash, A. (2017). Possible role of endothelin receptor against hyperhomocysteinemia and beta-amyloid induced AD type of vascular dementia in rats. Brain Res. Bull. 133, 31–41. doi:10.1016/j.brainresbull.2017.02.012
Sisignano, M., Fischer, M. J. M., and Geisslinger, G. (2021). Proton-Sensing GPCRs in health and disease. Cells 10 (8), 2050. doi:10.3390/cells10082050
Stelzer, G. T., and Lima-Filho, R. A. S. (2024). Amyloid-beta as a key player in cerebrovascular dysfunction in Alzheimer's Disease. J. Neurosci. 44 (27), e0663242024. doi:10.1523/JNEUROSCI.0663-24.2024
Stevens, L. M., and Brown, R. E. (2015). Reference and working memory deficits in the 3xTg-AD mouse between 2 and 15-months of age: a cross-sectional study. Behav. brain Res. 278, 496–505. doi:10.1016/j.bbr.2014.10.033
Taylor, J. L., Holland, D. J., Spathis, J. G., Beetham, K. S., Wisloff, U., Keating, S. E., et al. (2019). Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog. Cardiovasc Dis. 62 (2), 140–146. doi:10.1016/j.pcad.2019.01.004
Tomoto, T., and Zhang, R. (2024). Arterial aging and cerebrovascular function: impact of aerobic exercise training in older adults. Aging Dis. 15 (4), 1672–1687. doi:10.14336/ad.2023.1109-1
Tsartsalis, S., Sleven, H., Fancy, N., Wessely, F., Smith, A. M., Willumsen, N., et al. (2024). A single nuclear transcriptomic characterisation of mechanisms responsible for impaired angiogenesis and blood-brain barrier function in Alzheimer's disease. Nat. Commun. 15 (1), 2243. doi:10.1038/s41467-024-46630-z
Urena-Guerrero, M. E., Castaneda-Cabral, J. L., Rivera-Cervantes, M. C., Macias-Velez, R. J., Jarero-Basulto, J. J., Gudino-Cabrera, G., et al. (2020). Neuroprotective and neurorestorative effects of epo and VEGF: perspectives for new therapeutic approaches to neurological diseases. Curr. Pharm. Des. 26 (12), 1263–1276. doi:10.2174/1381612826666200114104342
Vital, T. M., Stein, A. M., de Melo Coelho, F. G., Arantes, F. J., Teodorov, E., and Santos-Galduroz, R. F. (2014). Physical exercise and vascular endothelial growth factor (VEGF) in elderly: a systematic review. Arch. Gerontol. Geriatr. 59 (2), 234–239. doi:10.1016/j.archger.2014.04.011
Wang, H., Niu, F., Fan, W., Shi, J., Zhang, J., and Li, B. (2019). Modulating effects of preconditioning exercise in the expression of ET-1 and BNP via HIF-1α in ischemically injured brain. Metab. Brain Dis. 34 (5), 1299–1311. doi:10.1007/s11011-019-00450-z
Wang, T., Zhou, G., He, M., Xu, Y., Rusyniak, W. G., Xu, Y., et al. (2020). GPR68 is a neuroprotective proton receptor in brain ischemia. Stroke 51 (12), 3690–3700. doi:10.1161/STROKEAHA.120.031479
Wang, Y. Y., Huang, Z. T., Yuan, M. H., Jing, F., Cai, R. L., Zou, Q., et al. (2021). Role of hypoxia inducible Factor-1α in Alzheimer's Disease. J. Alzheimers Dis. 80 (3), 949–961. doi:10.3233/jad-201448
Weller, J., and Budson, A. (2018). Current understanding of Alzheimer's disease diagnosis and treatment. F1000Res 7, F1000 Faculty Rev-1161. doi:10.12688/f1000research.14506.1
Wesenhagen, K. E. J., Tijms, B. M., Boonkamp, L., Hoede, P. L., Goossens, J., Dewit, N., et al. (2022). P-tau subgroups in AD relate to distinct amyloid production and synaptic integrity profiles. Alzheimers Res. Ther. 14 (1), 95. doi:10.1186/s13195-022-01038-z
Whitaker, A. A., Aaron, S. E., Kaufman, C. S., Kurtz, B. K., Bai, S. X., Vidoni, E. D., et al. (1985)2022). Cerebrovascular response to an acute bout of low-volume high-intensity interval exercise and recovery in young healthy adults. J. Appl. Physiol. 132 (1), 236–246. doi:10.1152/japplphysiol.00484.2021
Whitaker, A. A., Alwatban, M., Freemyer, A., Perales-Puchalt, J., and Billinger, S. A. (2020). Effects of high intensity interval exercise on cerebrovascular function: a systematic review. PLoS One 15 (10), e0241248. doi:10.1371/journal.pone.0241248
Wiesmann, M., Zerbi, V., Jansen, D., Lutjohann, D., Veltien, A., Heerschap, A., et al. (2017). Hypertension, cerebrovascular impairment, and cognitive decline in aged AβPP/PS1 mice. Theranostics 7 (5), 1277–1289. doi:10.7150/thno.18509
Wójtowicz, S., Strosznajder, A. K., Jeżyna, M., and Strosznajder, J. B. (2020). The novel role of PPAR alpha in the brain: promising target in therapy of Alzheimer's Disease and other neurodegenerative disorders. Neurochem. Res. 45 (5), 972–988. doi:10.1007/s11064-020-02993-5
Wu, D., Cao, W., Xiang, D., Hu, Y. P., Luo, B., and Chen, P. (2020). Exercise induces tissue hypoxia and HIF-1α redistribution in the small intestine. J. Sport Health Sci. 9 (1), 82–89. doi:10.1016/j.jshs.2019.05.002
Xu, J., Mathur, J., Vessières, E., Hammack, S., Nonomura, K., Favre, J., et al. (2018). GPR68 senses flow and is essential for vascular physiology. Cell 173 (3), 762–75.e16. doi:10.1016/j.cell.2018.03.076
Xu, L., Yang, M., Wei, A., Wei, Z., Qin, Y., Wang, K., et al. (2024). Aerobic exercise-induced HIF-1α upregulation in heart failure: exploring potential impacts on MCT1 and MPC1 regulation. Mol. Med. 30 (1), 83. doi:10.1186/s10020-024-00854-3
Yang, Y., Lu, H., Chen, C., Lyu, Y., Cole, R. N., and Semenza, G. L. (2022). HIF-1 interacts with TRIM28 and DNA-PK to release paused RNA polymerase II and activate target gene transcription in response to hypoxia. Nat. Commun. 13 (1), 316. doi:10.1038/s41467-021-27944-8
Yu, F., Jia, D., and Wang, R. (2025). Proton-Sensing G protein-coupled receptors and their potential role in exercise regulation of arterial function. Biomolecules 15 (6), 813. doi:10.3390/biom15060813
Zang, Q., Wang, S., Qi, Y., Zhang, L., Huang, C., Xiu, Y., et al. (2023). Running exercise improves spatial learning and memory ability and enhances angiogenesis in the cerebral cortex via endogenous nitric oxide. Behav. brain Res. 439, 114243. doi:10.1016/j.bbr.2022.114243
Keywords: high-intensity interval training, Alzheimer’s disease, pathological biomarkers, neuroplasticity, cerebrovascular function
Citation: Zhu L, Cai M, Lu Z, Wang Q, Zhai T and Hu J (2025) The impact of high-intensity interval training on cerebrovascular function in the APP/PS1 mice. Front. Aging 6:1647628. doi: 10.3389/fragi.2025.1647628
Received: 16 June 2025; Accepted: 03 October 2025;
Published: 14 October 2025.
Edited by:
Brenna Osborne, University of Copenhagen, DenmarkReviewed by:
Na Zhao, East China Normal University, ChinaHarshal Sawant, Marshall University, United States
Copyright © 2025 Zhu, Cai, Lu, Wang, Zhai and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyun Hu, aHVqaW5neXVuQHNocGRwaC5jb20=
 Lei Zhu
Lei Zhu Ming Cai
Ming Cai Zhe Lu1
Zhe Lu1 Jingyun Hu
Jingyun Hu